- 1Observational Health Data Sciences and Informatics, New York, NY, United States
- 2Observational Health Data Analytics, Janssen R&D, Titusville, NJ, United States
- 3Department of Biostatistics, University of California, Los Angeles, Los Angeles, CA, United States
- 4Quality Use of Medicines and Pharmacy Research Centre, Clinical and Health Sciences, University of South Australia, Adelaide, SA, Australia
- 5School of Public Health and Community Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 6College of Pharmacy, Riyadh Elm University, Riyadh, Saudi Arabia
- 7Department of Biomedical Informatics, Columbia University, New York, NY, United States
- 8Centre for Statistics in Medicine, NDORMS, University of Oxford, Oxford, United Kingdom
- 9Department of Medical Informatics, Erasmus University Medical Center, Rotterdam, Netherlands
- 10O’Brien Institute for Public Health, Faculty of Medicine, University of Calgary, Calgary, AB, Canada
- 11Division of Medical Sciences, University of Manchester, Manchester, United Kingdom
- 12Department of Human Genetics, University of California, Los Angeles, Los Angeles, CA, United States
A Corrigendum on
Vaccine safety surveillance using routinely collected healthcare data—An empirical evaluation of epidemiological designs
by Schuemie MJ, Arshad F, Pratt N, Nyberg F, Alshammari TM, Hripcsak G, Ryan P, Prieto-Alhambra D, Lai LYH, Li X, Fortin S, Minty E and Suchard MA (2022). Front. Pharmacol. 13:893484. doi: 10.3389/fphar.2022.893484
In the published article, there was an error in Figure 4 and Figure 5 as published. After publication, the authors found that the positive control imputation multiplication was accidentally applied twice, meaning the intended multiplication of 1.25, 2, and 4 actually were 1.252 = 1.5625, 22 = 4, and 42 = 16, respectively. This means the type 2 error in Figure 4 and the time to 50% sensitivity in Figure 5 were underestimated. The corrected Figure 4 and Figure 5 appear below.
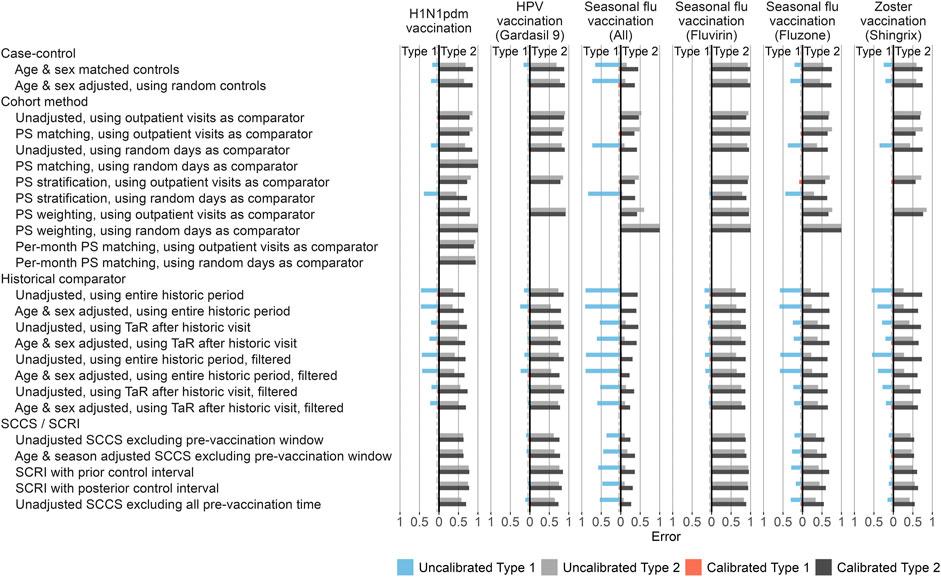
FIGURE 4. Type 1 and 2 error before and after empirical calibration. For each method variation and vaccine group, the type 1 and 2 error before and after empirical calibration in the Optum EHR database are shown. The x-axis indicates the type 1 error (higher values to the left) and type 2 error (higher values to the right), based on the (calibrated) one-sided p-value. The dashed line indicates nominal type 1 error of 5%. HPV = Human papillomavirus, PS = Propensity Score, SCCS = Self-Controlled Case Series, SCRI = Self-Controlled Risk Interval, TaR = Time-at-Risk.
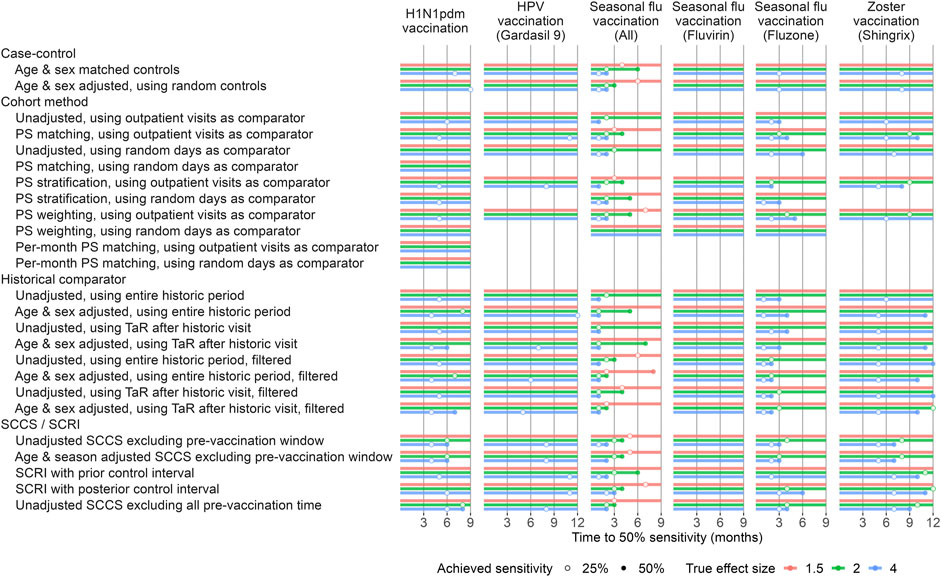
FIGURE 5. Time to 50% sensitivity after calibration. For each method variation and vaccine group, the number of months of data needed to achieve 50% sensitivity based on the calibrated MaxSPRT in the Optum EHR database are shown, stratified by true effect size of the positive controls. HPV = Human papillomavirus, PS = Propensity Score, SCCS = Self-Controlled Case Series, SCRI = Self-Controlled Risk Interval, TaR = Time-at-Risk.
In the published article the same error was present in the Supplementary Material. This means the type 2 error and the time to 50% sensitivity were underestimated. The correct Supplementary Data Sheet S1 can be found in the Supplementary Materials.
The authors apologize for these errors and state that they does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1088973/full#supplementary-material
Keywords: vaccine safely, routinely collected data, adverse event, surveillance, methods
Citation: Schuemie MJ, Arshad F, Pratt N, Nyberg F, Alshammari TM, Hripcsak G, Ryan P, Prieto-Alhambra D, Lai LYH, Li X, Fortin S, Minty E and Suchard MA (2022) Corrigendum: Vaccine safety surveillance using routinely collected healthcare data—An empirical evaluation of epidemiological designs. Front. Pharmacol. 13:1088973. doi: 10.3389/fphar.2022.1088973
Received: 03 November 2022; Accepted: 04 November 2022;
Published: 24 November 2022.
Edited and reviewed by:
Carlos Alves, University of Coimbra, PortugalCopyright © 2022 Schuemie, Arshad, Pratt, Nyberg, Alshammari, Hripcsak, Ryan, Prieto-Alhambra, Lai, Li, Fortin, Minty and Suchard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martijn J. Schuemie, c2NodWVtaWVAb2hkc2kub3Jn
 Martijn J. Schuemie
Martijn J. Schuemie Faaizah Arshad
Faaizah Arshad Nicole Pratt
Nicole Pratt Fredrik Nyberg
Fredrik Nyberg Thamir M Alshammari
Thamir M Alshammari George Hripcsak
George Hripcsak Patrick Ryan
Patrick Ryan Daniel Prieto-Alhambra
Daniel Prieto-Alhambra Lana Y. H. Lai
Lana Y. H. Lai Xintong Li
Xintong Li Stephen Fortin
Stephen Fortin Evan Minty
Evan Minty Marc A. Suchard1,3,12
Marc A. Suchard1,3,12