
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 07 December 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1078303
This article is part of the Research TopicOvarian Cancer Targeted Medication: PARP Inhibitors, Anti-Angiogenic Drugs, Immunotherapy, and MoreView all 13 articles
Ovarian cancer is among the most common malignant tumors in gynecology and is characterized by insidious onset, poor differentiation, high malignancy, and a high recurrence rate. Numerous studies have shown that poly ADP-ribose polymerase (PARP) inhibitors can improve progression-free survival (PFS) in patients with BRCA-mutated ovarian cancer. With the widespread use of BRCA mutation and PARP inhibitor (PARPi) combination therapy, the side effects associated with BRCA mutation and PARPi have garnered attention worldwide. Mutations in the BRCA gene increase KEAP1-NRF2 ubiquitination and reduce Nrf2 content and cellular antioxidant capacity, which subsequently produces side effects such as cardiovascular endothelial damage and atherosclerosis. PARPi has hematologic toxicity, producing thrombocytopenia, fatigue, nausea, and vomiting. These side effects not only reduce patients’ quality of life, but also affect their survival. Studies have shown that natural phytochemicals, a class of compounds with antitumor potential, can effectively prevent and treat the side effects of chemotherapy. Herein, we reviewed the role of natural phytochemicals in disease prevention and treatment in recent years, including sulforaphane, lycopene, catechin, and curcumin, and found that these phytochemicals have significant alleviating effects on atherosclerosis, nausea, and vomiting. Moreover, these mechanisms of action significantly correlated with the side-effect-producing mechanisms of BRCA mutations and PARPi. In conclusion, natural phytochemicals may be effective in alleviating the side effects of BRCA mutant ovarian cancer cells and PARP inhibitors.
Ovarian cancer is among most common malignancies in gynecology (Bookman et al., 2009). Two hundred thousand women worldwide are diagnosed with ovarian cancer each year, 70% of whom are intermediate to advanced cases, with a mortality rate of 62.5%. High-grade plasmacytoma is a common type of ovarian cancer that arises from ovarian epithelial cells. It is poorly differentiated, highly malignant, and has a high recurrence rate (Colombo et al., 2019). According to treatment guidelines, ovarian cancer is treated chiefly with platinum drugs in combination with paclitaxel or with the anti-angiogenic drug bevacizumab alone (Perren et al., 2011). Platinum drugs are key to treating platinum-sensitive recurrent ovarian cancer; however, as the number of recurrences increases, this type of ovarian cancer becomes resistant to platinum drugs (Foley et al., 2013). The median progression-free survival (PFS) for bevacizumab was 19.0 months, slightly higher than the median PFS in the standard treatment group (17.3 months), as noted by the European Society of Medical Oncology and the International Society for Gynecologic Cancer meetings (Perren et al., 2011). Although rational treatment significantly prolongs patient survival, 7080% of patients experience relapse or further disease progression after first-line treatment (Lorusso et al., 2020). PARPi, an inhibitor of polyadenosine diphosphate ribose polymerase, extends PFS to approximately 56 months in patients with platinum-resistant, BRCA-deficient, or refractory ovarian cancer by affecting the self-replication of ovarian cancer cells, providing a new approach for the maintenance treatment of ovarian cancer patients (Mirza et al., 2019; Vanacker et al., 2021).
Numerous studies have demonstrated that phytochemicals extracted from foods have antitumor potential. Audesh et al. found that some phytochemicals extracted from fruits have significant inhibitory effects on human ovarian teratoma cells (PA-1) at their respective IC50 concentrations (Li et al., 2021). Phytochemicals have been extensively studied to inhibit the development of ovarian cancer, and interfere with cancer cells along with antioxidant and anti-inflammatory effects (Pundir et al., 2021). Islam et al. demonstrated that the antioxidant and anti-inflammatory effects of phytochemicals were effective in preventing some side effects of chemotherapy (Islam et al., 2021). Chemotherapy plays a very important role in ovarian cancer treatment, but its side effects also seriously affect patients’ quality of life, and symptomatic supportive treatment to alleviate these side effects will further increase the burden on the patient’s body. In contrast to drugs, various types of phytochemicals, such as phenols, terpenoids, and sulfur-containing compounds, are distributed in numerous fruits and vegetables consumed daily (Roy and Datta, 2019). The use of phytochemicals as an alternative to drugs would reduce the patient’s fear and organismal burden of oncologic chemotherapy and improve patient compliance.
This study reviewed the mitigating effects of phytochemicals on PARPi side effects and the prevention of pathological changes caused by BRCA mutations. We further clarified the mechanisms by which phytochemicals alleviate the side effects of synergistic lethal treatment regimens.
Poly (adenosine diphosphate ribose) polymerase (PARP) is a cleavage substrate for the core members of apoptosis, caspases. PARP is also involved in damage repair after DNA breaks (Wei and Yu, 2016). PARP1 plays more than 90% of the total role of the PARP family, and PARP1 is active in base excision repair (BER) (Durkacz et al., 1980), DNA single-strand breaks (SSB) (Haince et al., 2008), DNA double-strand breaks (DSBs), and replication fork damage (Haince et al., 2008). After DNA damage, PARP1 recognizes the damage through the zinc finger structural domain and orientates to the nick for ADP ribosylation based on nicotinamide adenine dinucleotide (NAD+), forming PARP-1-ADP ribose branched chain, which reduces the binding of PARP1 to DNA and dissociates from DNA to participate in DNA repair (Bürkle, 2001; Lord and Ashworth, 2017), PARP2 is similar to PARP1 in function, but it acts on different substrates (Kutuzov et al., 2020), and PARP2 is crucial in the repair of SSBs. It is by damaging DNA and thus affecting mitosis that cisplatin treats ovarian cancer. Thus, PARP plays a key role in apoptosis and repair of platinum-induced DNA damage in ovarian cancer cells (Hoeijmakers, 2001; Damia and Broggini, 2019).
PARPi competes with NAD+ for the PARP active site, thereby inhibiting the formation of poly (ADP-ribose) polymers; when single-strand damage occurs in DNA molecules, the repair is mainly accomplished by PARP and DNA ligase IIIa (Murai et al., 2012; Murai et al., 2014). PARPi can bind specifically to the NAD+ binding site of PARP1 (and/or PARP2), resulting in a significant reduction in DNA-PARP dissociation, maintaining PARP binding to DNA, thus perpetuating the DNA-PARP complex and inhibiting subsequent repair. This process is known as “trapping” of the DNA-PARP complex (Murai et al., 2012). The persistence of the complex on a single strand of DNA allows for the accumulation of large amounts of single-stranded broken DNA and thus DSBs, causing cell death. To resolve these barriers and restore the cell cycle, functional homologous recombination (HR) must be utilized (Bunting et al., 2010).
Breast cancer susceptibility gene (BRCA) participates in DNA repair and is present in the human body as a tumor suppressor gene (Prakash et al., 2015). Carriers of BRCA1 and BRCA2 germline mutations have a 54% and 23% risk, respectively, of developing ovarian cancer (Ramus and Gayther, 2009; Milne and Antoniou, 2011). First, BRCA proteins act through the HR process to protect humans (Bryant et al., 2005). HR ensures that the cellular repair of DSBs in the S-phase is precise and error-free (Farmer et al., 2005). The function of BRCA1 in HR is to cleave DSB 5′–3′, leaving an overhanging 3′. HR is an essential method of DNA double-strand break repair. The HR repair pathway is purportedly blocked by BRCA (BRCA1/2) mutations. In this case, the DSB repair mechanism is no longer stable and the DNA damage repair function of the cell is greatly reduced. Therefore, cancer cells damaged by platinum cannot be repaired (Bryant et al., 2005; D’Andrea, 2018; Li et al., 2020). This suggests that BRCA plays a role in the repair of DSBs (Gudmundsdottir and Ashworth, 2006). The application of platinum-based drugs after BRCA mutations can inhibit DNA replication in ovarian cancer cells (Birkbak et al., 2012).
If PARPi is used in the presence of BRCA mutations in ovarian or breast cancer cells, then, it will further inhibit DNA break repair due to HR defects, and the cells will be unable to repair DSBs leading to cell death, a synergistic lethal phenomenon (Rottenberg et al., 2008; Srinivasan et al., 2017). This phenomenon destabilizes the tumor genome, which can counteract the tumor cell proliferation and effectively increase the patients’ survival time. Therefore, PARPi induces cell death in HR-deficient cells as a primary approach for the treatment of ovarian cancer (Noordermeer and van Attikum, 2019; Curtin and Szabo, 2020) as shown in Figure 1.
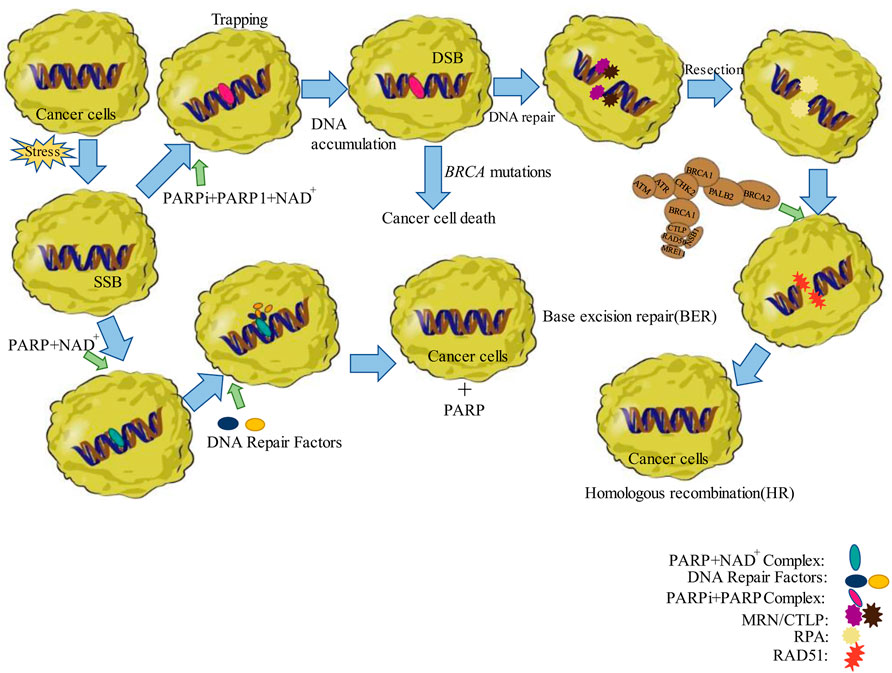
FIGURE 1. Schematic representation of the functions of PARP and BRCA in DNA repair. The left side shows base excision repair. Right side shows the “trapping” of the DNA-PARP complex and DNA homologous recombination repair. Abbreviations: DSB, double-strand break; MRN/CTLP, DNA damage sensor; NAD+, nicotinamide adenine dinucleotide; RAD51, restriction-associated site DNA51; RPA, replication proteinase A; SSB, single-strand break.
The use of PARPi in patients with BRCA-deficient ovarian cancer has had notable success, but the use of PARPi induces discomfort in ovarian cancer patients. For example, the hematologic toxicity produced by niraparib (ZEJULA), a highly absorbed, highly permeable drug, should not be underestimated. Berek observed in 553 patients who added niraparib, that about 33% developed thrombocytopenia and 13% developed anemia (Berek et al., 2018). Meanwhile, data published by LaFargue et al. (2019) showed that the probability of fatigue in the first month after niraparib was approximately 32.4%, vomiting was about 19.6%, and nausea was up to 61.9%. Most of the fatigue was due to ischemia and decreased platelet count. Olaparib (Lynparza), a low permeability, low absorption drug, is highly susceptible to hypertension, with a 48% chance of nausea and vomiting. The side effects of PARPi seriously affect patients’ quality of life (Munroe and Kolesar, 2016; Paik, 2021).
Phytochemicals have strong antioxidant properties and are commonly used for skin care. However, numerous studies have shown that saffron, cyclic adenosine phosphate, and curcumin from ginger can reduce the incidence of some chemotherapy side effects, such as nausea, vomiting, and anemia, at the sites shown in Table 1.
Crocin, a carotenoid present in the stigma of saffron, improves collagen-induced platelet aggregation and adhesion and A23187-mediated endogenous production of ROS and H2O2 in platelet mitochondria (Thushara et al., 2013; Yaribeygi et al., 2018). Pourmasoumi et al. (2019) reported significant decreases in diastolic blood pressure, body weight, and other factors associated with cardiovascular disease (CVD) in 622 individuals taking Crocin. Javandoost et al. (2017) found that adding Crocin was associated with a significant increase in high-density lipoprotein (HDL) levels during an 8-week Crocin intervention. The addition of Crocin to PARPi not only reduces oxidative stress but also prevents the reduction of platelets and increases blood pressure. Crocin also reduces HDL production, which can reduce the prevalence of CVD in several ways.
The high content of cyclic adenosine phosphate in jujube can dilate blood vessels, provide nutrients to the heart muscle and increase its contractility, induce the expression of erythropoietin, and stimulate hematopoiesis in the body (Chen and Tsim, 2020). The increase in hematopoietic parameters after cancer treatment in mice with jujube further suggests that jujube can ameliorate anemia in cancer patients (Periasamy et al., 2020). Improvement in anemia results in patients feeling less fatigued. The cyclic adenosine phosphate in dates may reduce the discomfort experienced by patients after niraparib administration.
The use of ginger as an antiemetic is well-known in China and is used in traditional Chinese medicine, where ginger is rich in curcumin, curcumin, gingerols, and curcuminoids (Ahmed et al., 2021). These active substances influence gastrointestinal motility and promote gastric emptying, while they affect the central nervous system by mediating the 5-hydroxytryptamine-3 of 5-hydroxytryptamine (5-HT), reducing nausea and vomiting (Nocerino et al., 2021). Marx et al. (2017) conducted a double-blind randomized intervention with ginger in 51 patients, identifying less fatigue in the intervention group (p = 0.006) from the three chemotherapy cycles, especially in the third cycle. Subsequently, Crichton et al. (2019) found through a meta-analysis that ginger supplementation not only had a significant effect in suppressing nausea and vomiting but also reduced the likelihood of fatigue by approximately 80%. Therefore, the administration of ginger in the treatment of ovarian cancer with PARPi can reduce PARPi side effects in patients.
Saffron, cyclic adenosine phosphate, and curcumin have a significant inhibitory effect on the side effects caused by niraparib and olaparib, as shown in Figure 2. In the future, a rational combination could reduce the pain associated with treatment of ovarian cancer patients and increase their quality of life.
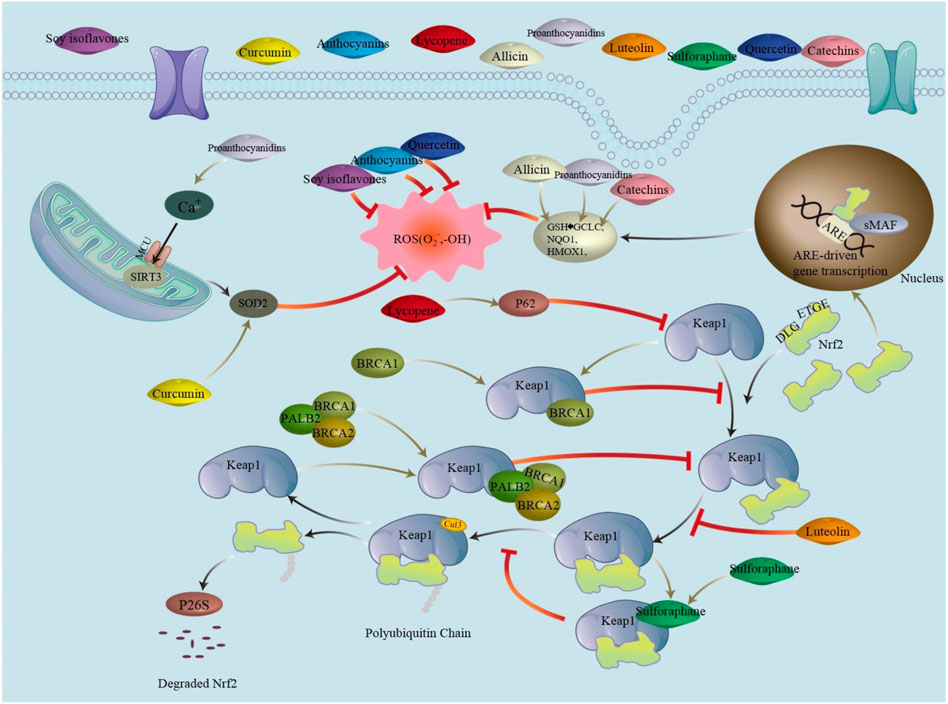
FIGURE 2. Nrf2/Keap1 is a signaling pathway in which Nrf2 binds to Keap1 via ETGE and DLG, ubiquitinating it, which is then degraded by the proteasome. Phytochemicals can promote nuclear translocation of Nrf2 by mediating the Keap1-Nrf2 complex. Binding ARE after forming a heterodimer with sMAF activates transcriptional production of downstream antioxidant enzymes. Phytochemicals can also affect ROS production by acting directly on ROS. Abbreviations: ARE, antioxidant response element; BRCA (1/2), breast cancer 1/2; CUL3, cullin3; DLG/ETGE, nrf2 structural domain; Keap1, recombinant kelch like ech associated protein1; MCU, mitochondrial calcium uniporter; Nrf2, nuclear factor erythroid 2-related factor 2; PALB2, partner and localizer of brca2; ROS, reactive oxygen species; SIRT3, silence regulatory protein3; sMAF, specific macrophage arming factor; SOD2, superoxide dismutase.
Mutations or deletions of BRCA in normal individuals significantly increase the risk of developing cancers, such as ovarian cancer (Sekine et al., 2021). However, diseases beyond ovarian or breast cancer are associated with BRCA, and analysis excluding causes of cancer death found that survival was also significantly lower in individuals with mutations or deletions in BRCA (Mai et al., 2009).
Many survival studies on BRCA gene deletions have sufficiently demonstrated that cardiovascular-related diseases are another critical cause of death in individuals with BRCA mutations or deletions (Arts-de Jong et al., 2014; Lammert et al., 2022). Sajjad et al. (2017) studied 401 cancer-free female BRCA1/2 mutation carriers and found that BRCA mutation carriers were at increased risk of cardiovascular disease compared to the general population. Zhou et al. noted that BRCA gene deletion causes cardiac diseases including ischemic heart disease, atherosclerosis, and other myocardial diseases (Arts-de Jong et al., 2014). Atherosclerosis is a major cause of aortic disease, peripheral vascular-related diseases, coronary heart disease, and cerebral infarction (Alexander et al., 2021; Shea et al., 2021). Therefore, addressing atherosclerosis is key to preventing cardiovascular diseases caused by BRCA defects.
Atherosclerosis has been extensively studied, and through the analysis of causative factor ranking, endothelial dysfunction has been established as the factor of atherosclerosis (Gimbrone and García-Cardeña, 2016), and apoptosis of endothelial cells plays a crucial role in the occurrence of endothelial dysfunction (Xu et al., 2021), thus, can be suggested that endothelial cell injury plays a driving role in atherosclerosis (Zheng et al., 2017). Therefore, inhibiting endothelial cell apoptosis in atherosclerosis can help prevent atherosclerosis development (Gimbrone and García-Cardeña, 2016).
Low-density lipoprotein (LDL) represents the beginning of the atherosclerotic response when it enters the subendothelial space from the endothelium by cellular action and is deposited in the subintima of the vessel where it is oxidized by ROS (Porter et al., 2013). Oxidation of LDL by ROS results in the formation of oxidized low-density lipoprotein (Ox-LDL), which is accompanied by endothelial destruction, binding of Ox-LDL to the scavenger receptors of macrophages, and intracellular accumulation of Ox-LDL after phagocytosis by vascular smooth muscle cells, resulting in the formation of foam cells (Porter et al., 2013). ROS cause endothelial cell apoptosis and atherosclerosis. Therefore, ROS can be used as both a marker of early atherosclerosis and as an entry point to control atherosclerosis (Panieri and Santoro, 2015). In Korea, Lee et al. (2021) used zearalenone (ZEN) to treat endothelial cells, and the rise of ROS after the activation of cytoplasmic calcium by ZEN further accelerated the apoptosis of endothelial cells, verifying that the difficulty in solving atherosclerosis lies in the treatment of LDL and ROS.
BRCA1 regulates ROS as a newly identified Nrf2 (antioxidant transcription factor) binding protein (Vurusaner et al., 2012). In 2006, PALB2 was identified as a protein that interacts with BRCA2 (Xia et al., 2006). BRCA1, BRCA2, and PALB2 are involved in regulating the activity of Keap1 (KELCH-like ECH-associated protein 1)-mediated ubiquitination of Nrf2, thereby regulating the amount of Nrf2, and E3 ubiquitin ligase (cullin3) is a critical enzyme in the ubiquitination reaction, with Keap1 as its recognition subunit (Song et al., 2021). Japanese researchers have found that the ETGE and DLG motifs in the Neh2 structural domain of Nrf2 can bind to the Kelch structural domain of Keap1. ETGE of Nrf2 is bound to the Keap1 dimer using what is known as a hinge, while the Cul3-Rbx1 complex is stably bound to Keap1 using a DLG latching motif (Tong et al., 2006), forming KEAP1-NRF2, which lays the foundation for ubiquitination. Ubiquitinated Nrf2 is then transported to the 26S proteasome to be degraded and destroyed (Zhang et al., 2004). Laboratory analysis of the transfected gene revealed that cells with deletion of the BRCA1/2 gene are more sensitive to oxidative stress (Fridlich et al., 2015). BRCA1 has an ETGE-like structure, competitively inhibits KEAP1-NRF2 ubiquitination, and increases Nrf2 content by binding to the ETGE-binding site of Keap1 (Zhou et al., 2021). Amino acids 9-44 of PALB2 determine its linkage to BRCA1 (Gardini et al., 2014). It was also found that PALB2 is linked to BRCA2 in the N-terminal domain, and it is worth noting that PALB2 has a highly conserved ETGE-type Keap1 binding motif, which shares the same site of action as Keap1 and Nrf2 (Xia et al., 2007). Thus, PALB2 can participate in the binding process between Nrf2 and Keap1, compete with Nrf2 for Keap1, inhibit KEAP1-NRF2, and stabilize Nrf2. As Nrf2 mediates the antioxidant response, PALB2 causes Nrf2 to remain in the nucleus to reduce the level of ROS in the cell and the rate of exit from the nucleus (Ma et al., 2012; Gorrini et al., 2013). In the absence of BRCA1/2 or PALB2, KEAP1-NRF2 is not inhibited, ubiquitination of Nrf2 results in high ROS production, and regulating the Keap1 pathway to inhibit endothelial apoptosis and is an essential means of alleviating atherosclerosis from the root (Kobayashi et al., 2004; Singh et al., 2013).
The prevention of cardiovascular-related diseases through phytochemicals has garnered substantial public interest. Several phytochemicals have been shown to act as cardiovascular disease preventers in cells, animals, and human populations. Examples include sulfur-containing compounds, terpenoids, and polyphenols, the action points of which are listed in Table 1.
Sulforaphane (SFN), a natural isothiocyanate compound with excellent antioxidant properties, is abundant in cruciferous vegetables and is produced by the breakdown of glucose by endogenous mustard enzymes (Kaiser et al., 2021). Considering the antioxidant properties of SFN, Asif et al. (2022) found that SFN preferentially acts on c151 in Keap1 cysteine residues. In the cytoplasm, Nrf2 binds to Keap1 first due to high ETGE binding, followed by partial binding of DLG, and cullin3 recognizes Keap1 binding immediately, followed by ubiquitination and degradation of Nrf2. If Nrf2 binds to Keap1 and then SFN is added, SFN acts on c151 on Keap1, disrupting the binding of Keap1 to cullin3. Immobilization is prevented and Keap1 cannot continue to participate in the cycle to bind newly generated Nrf2 (Kobayashi et al., 2009). The reduction in Keap1 allows newly generated Nrf2 to enter the nucleus, where it binds to antioxidant response elements (ARE) to activate antioxidant responses, causing a reduction in ROS (Dinkova-Kostova et al., 2017). The regulation of Nrf2 by SFN effectively reduces endothelial cell injury, thus explaining its reduction in atherosclerosis and its role in combating cardiovascular disease (Dana and Alejandro, 2022).
Glutathione is among the most studied cellular antioxidants. However, orally supplemented glutathione is hydrolyzed and oxidized by intestinal enzymes. Acetylcysteine (NAC) is a precursor of glutathione, and oral supplementation with NAC increases glutathione levels in the body after conversion in the liver (Schmitt et al., 2015). After NAC supplementation, glutathione peroxidase (GPX) activity is enhanced to convert reduced glutathione (GSH) to oxidized glutathione (GSSG), thereby protecting cells from ROS damage (Kwon, 2021). Allicin, also known as diallyl thiosulfate, is a sulfur-containing compound. When allicin was substituted for NAC in intervention studies, researchers also found enhanced GPX activity, which may indicate that allicin, a natural phytochemical, has specific antioxidant effects that counteract ROS production, and thus could be considered for the prevention of atherosclerosis caused by BRCA deficiency (Hasan et al., 2006; Catanzaro et al., 2022).
Lycopene (LYC), a terpene fat-soluble natural pigment widely found in tomatoes, watermelon, carrots, and other red fruits and vegetables, can be an effective antioxidant because of its powerful ability to scavenge free radicals LYC induces autophagic degradation of Keap1 by increasing the expression of autophagic protein p62 (Ulasov et al., 2021). When Nrf2 dissociates from Keap1, then nuclear ectopic and binds to ARE in the nucleus to induce the expression of antioxidants downstream of the pathway to avoid oxidative cell death (Baird and Yamamoto, 2020; Wang et al., 2020). Since LYC intervention in rats results in a decrease in LDL and triglycerides and an increase in HDL, it was demonstrated that LYC is an anti-atherogenic phytochemical (Bentzon et al., 2014; Wong, 2014). ROS production was significantly decreased by LYC supplementation, which inhibited endothelial cell injury caused by BRCA deletion or mutation (Roy and Datta, 2021). This further demonstrated that LYC is essential for preventing atherosclerosis caused by BRCA deficiency.
Luteolin, also known as phytoalexin, is among the more common terpene antioxidants in nature that reduces free radical activity, prevents ROS damage to cells, and has a surprising effect on BRCA-deficient cancers (Gong et al., 2018). Lutein is an essential nutrient and one of the most common antioxidants found in egg yolks. Furthermore, Mitra et al. (2021) recently noted that dark-colored greens are usually high in luteins, such as kale, spinach, and lettuce. A recent study reconfirmed that the two parts of the carbon chain of lutein are hydrophilic (HO-) and hydrophobic (CH2−), respectively (Nakamura and Sugiura, 2022). Moreover, the hydrophilic part of lutein remains on both sides of the cell membrane, whereas the hydrophobic part is in the phospholipid molecule layer, which allows lutein to bind tightly to the cell membrane lipids and increase the stability of the cell membrane (Algan et al., 2022). Conversely, luteolin activates extracellular regulated protein kinase (ERK), allowing Nrf2 phosphorylation and cleavage of the Nfr2/Keap1 complex. This causes nuclear translocation of Nrf2 to bind to the DNA regulatory region of ARE. It induces the expression of antioxidant genes and reduces intracellular ROS levels (Ahn and Kim, 2021). Luteolin can be expressed as an antioxidant that reduces the oxidative response of LDL and inhibits the development of atherosclerosis (Hajizadeh-Sharafabad et al., 2021; Ramanna and Somu, 2021). This suggests that luteolin can inhibit atherosclerosis, thereby preventing the development of CVD.
Polyphenols significantly impact human health and are known as the “seventh nutrient.” Their role in lowering antioxidant LDL and blood cholesterol has been extensively studied (Abdal Dayem et al., 2016). Vegetables such as spinach, broccoli, and cabbage have high polyphenol contents (Zeb, 2021). Cherries, blueberries, and other dark fruits also have relatively high polyphenol contents. Polyphenols are a natural component of cocoa beans, and the high polyphenol content in black beans contributes to their unique flavor (Yang et al., 2018). Interestingly, Khan et al. (2021) reported that polyphenols not only prevent CVD, but also mediate BRCA1/2 expression. Polyphenols can be divided into flavonoids and phenolic compounds, the most common of which are catechins, proanthocyanidins, quercetin, soy isoflavones, anthocyanins, and curcumin.
The antioxidant capacity of catechins is even higher than that of vitamin E. Numerous studies have demonstrated that catechins can increase the activity of antioxidant enzymes (SOD2 and GPX), thus inhibiting the oxidation of LDL to Ox-LDL (Chen et al., 2020; Ahmadi et al., 2022; Dal and Yilmaz, 2022). Japanese researchers observed that LDL oxidation was prolonged in the catechin group by administering 1 g of catechin in capsule form to 19 healthy men in a double-blind crossover trial (Suzuki-Sugihara et al., 2016). The reduction in Ox-LDL levels led to a significant decrease in the probability of atherosclerosis and effectively prevented CVD caused by BRCA mutations.
Proanthocyanidins comprise varying amounts of catechins, epicatechin, and gallic acid, which are abundant in grapes and are converted into anthocyanins in plants. Proanthocyanidins play a role in CVD by preventing lipid peroxidation through calcium-dependent NO release, vasorelaxation, and the inhibition of Ox-LDL production (de la Iglesia et al., 2010). Proanthocyanidins reduce intracellular ROS production by increasing the NRF2/Keap1 ratio, increasing SOD2 expression, and inhibiting oxidase expression (NOX4 and iNOS) (Kowalska et al., 2021). In addition, proanthocyanidin supplementation can prevent ROS production from BRCA defects (Xian et al., 2019). This reduces the risk of atherosclerosis due to BRCA defects.
Quercetin is found at high levels in daily life in sea buckthorn, hawthorn, and buckwheat sticks. Its antioxidant capacity is 20 times that of vitamin C and 50 times that of vitamin E. This is due to the good scavenging ability of the o-diphenol hydroxyl group for superoxide anion (O2-) and hydroxyl radical (-OH), reducing the production of oxidative stress ROS because the action of the o-diphenol hydroxyl group maintains biofilm integrity (Chu, 2022), and reduces necrosis of vascular endothelial cells. The reduction in ROS leads to the inhibition of LDL oxidation, reducing the risk of atherosclerosis and other cardiovascular diseases (Deng et al., 2020). Concurrently, quercetin inhibits the production of platelet lipoxygenase and cyclooxygenase, which leads to the release of thrombolytic and vascular membrane-protective mediators from the endothelium to counteract thrombosis.
Anthocyanins are glycosylated anthocyanins that are widely distributed in black, red, and purple plant foods, such as black rice, mulberry, and eggplant, which have powerful antioxidant capacity (Bagchi et al., 2004). Anthocyanins are more substantial than common antioxidants, such as vitamin E, catechins, and quercetin, in scavenging free radicals. They have many phenolic hydroxyl groups, which can directly scavenge many free radicals by oxidizing and releasing electrons to maintain redox balance (Dangles and Fenger, 2018). At the same time, anthocyanins reduce the production of ROS by further activating the activity of SOD2 and GPX to reduce oxidative stress damage (Tian et al., 2019). In addition, it prevents the death of vascular endothelial cells and improves arterial blood-vessel stiffness. In patients with cardiovascular diseases deficient in BRCA, supplementation with anthocyanins may improve the risk of related diseases (Speciale et al., 2020).
Estrogen secretion increases in ovarian cancer patients (Langdon et al., 2020). When estrogen levels are elevated, the structure of soy isoflavones becomes similar to that of estrogen. Therefore, soy isoflavones prevent estrogen from binding to the receptor, thus acting as estrogen antagonists (Kim, 2021). Moreover, soy isoflavones, similar to quercetin, can contribute to the antioxidant response by providing hydrogen atoms to inhibit the production of reactive oxygen radicals and reduce the level of ROS (Syamala et al., 2021). Su et al. conducted a logistic regression analysis of 500 patients with ovarian cancer and 500 normal subjects (mean age, 59 years) in southern China. They found that moderate intake of soy foods activated cellular autophagy, reduced the risk of ovarian cancer, and increased the sensitivity to carboplatin (Runlin et al., 2022). A Korean study investigated 5509 people at high risk of ovarian cancer and found a relationship between metabolism and soy isoflavone intake, with soy isoflavones being inversely associated with LDL in men and women and negatively associated with the incidence of metabolic syndrome in women. From these data, it can be concluded that soy isoflavone supplementation can inhibit metabolism-induced ROS and LDL production (Woo et al., 2019). Therefore, it is necessary to provide soy isoflavone supplementation to people with BRCA mutations, especially to patients with BRCA ovarian cancer.
Curcumin is a representative phenolic compound and, as a natural compound that can be extracted from the ginger family, deserves our attention as it mediates histone acetyltransferase activity to regulate acetylation of DSB sites, thus reducing the aggregation of critical non-homologous end-joining factors to DSB sites and achieving PARPi sensitization (Ogiwara et al., 2013). Surprisingly, curcumin promotes the increase of ROS in tumor cells, causing tumor cell death (Mortezaee et al., 2019); however, in normal cells, curcumin downregulates the antioxidant response of miR-125b to reduce cell death (Schwertheim et al., 2017). When treating ovarian cancer patients with BRCA mutations, adjuvant treatment with curcumin can be considered, not only to increase synergistic lethality, but also to prevent the side effects of PARPi and CVD caused by BRCA mutations.
Phytochemicals, such as sulfur-containing compounds, terpenoids, and polyphenols, which regulate the production of ROS and the levels of HDL and LDL in different ways to prevent atherosclerosis caused by BRCA mutations and thus prevent CVD, are shown in Figure 2.
PARPi and BRCA mutations play a significant role in the treatment of ovarian cancer. Clinicians are increasingly concerned about the side effects associated with PARPi and BRCA mutations. Phytochemicals, mostly derived from fruits and vegetables, have a high safety profile and are easily accessible, and therefore, patients have high compliance. In this study, we sorted out the principles of phytochemicals in antioxidants and maintenance of metabolic substance balance. We found that phytochemicals such as sulfur-containing compounds, polyphenols, and terpenoids can modulate the development of atherosclerosis, a key pathological change in the process of CVD caused by BRCA mutations, by mediating Keap1-Nrf2, free radicals, and LDL. In addition, phytochemicals can reduce the common clinical side effects of phytochemicals in reducing nausea and vomiting, relieving fatigue, and reducing hematotoxicity by modulating 5-HT, stimulating erythropoietin secretion, and antioxidant substances. We conclude that phytochemicals can inhibit the pathological changes caused by BRCA mutations and alleviate the side effects caused by PARPi by summarizing the relevant mechanisms. However, studies on phytochemicals that reduce the side effects of ovarian cancer treatment in animals are lacking, and natural phytochemicals are expected to gain wide usage in the clinical treatment of ovarian cancer.
CW writes the manuscript, PG and SL searches for articles, JX creates the images, WT provides valuable professional advice and guidance, JL gives language guidance, and LZ helps revise the manuscript.
Yunnan Provincial Department of Science and Technology-Kunming Medical University Applied Basic Research Joint Special Project (202001AY070001-076); Yunnan Provincial Science and Technology Department Major Science and Technology Special Program (202102AE090027-3); Kunming Medical University Innovation Fund (2022S312).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdal Dayem, A., Choi, H. Y., Yang, G. M., Kim, K., Saha, S. K., and Cho, S. G. (2016). The anti-cancer effect of polyphenols against breast cancer and cancer stem cells: Molecular mechanisms. Nutrients 8 (9), 581. doi:10.3390/nu8090581
Ahmadi, A., Jamialahmadi, T., and Sahebkar, A. (2022). Polyphenols and atherosclerosis: A critical review of clinical effects on LDL oxidation. Pharmacol. Res. 184, 106414. doi:10.1016/j.phrs.2022.106414
Ahmed, S. H. H., Gonda, T., and Hunyadi, A. (2021). Medicinal chemistry inspired by ginger: Exploring the chemical space around 6-gingerol. RSC Adv. 11 (43), 26687–26699. doi:10.1039/d1ra04227k
Ahn, Y. J., and Kim, H. (2021). Lutein as a modulator of oxidative stress-mediated inflammatory diseases. Antioxidants 10 (9), 1448. doi:10.3390/antiox10091448
Alexander, Y., Osto, E., Schmidt-Trucksäss, A., Shechter, M., Trifunovic, D., Duncker, D. J., et al. (2021). Endothelial function in cardiovascular medicine: A consensus paper of the European society of cardiology working groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc. Res. 117 (1), 29–42. doi:10.1093/cvr/cvaa085
Algan, A. H., Gungor-Ak, A., and Karatas, A. (2022). Nanoscale delivery systems of lutein: An updated review from a pharmaceutical perspective. Pharmaceutics 14 (9), 1852. doi:10.3390/pharmaceutics14091852
Arts-de Jong, M., Maas, A. H., Massuger, L. F., Hoogerbrugge, N., and de Hullu, J. A. (2014). BRCA1/2 mutation carriers are potentially at higher cardiovascular risk. Crit. Rev. Oncol. Hematol. 91 (2), 159–171. doi:10.1016/j.critrevonc.2014.01.008
Asif, M., Kala, C., Gilani, S. J., Imam, S. S., Mohamad, T., Naaz, F., et al. (2022). Protective effects of isothiocyanates against alzheimer's disease. Curr. Tradit. Med. 8 (3), 1–10. doi:10.2174/2215083807666211109121345
Bagchi, D., Sen, C., Bagchi, M., and Atalay, M. (2004). Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry. 69 (1), 75–80. doi:10.1023/b:biry.0000016355.19999.93
Baird, L., and Yamamoto, M. (2020). The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 40 (13), 000999-20. doi:10.1128/mcb.00099-20
Bentzon, J. F., Otsuka, F., Virmani, R., and Falk, E. (2014). Mechanisms of plaque formation and rupture. Circ. Res. 114 (12), 1852–1866. doi:10.1161/circresaha.114.302721
Berek, J. S., Matulonis, U. A., Peen, U., Ghatage, P., Mahner, S., Redondo, A., et al. (2018). Safety and dose modification for patients receiving Niraparib. Ann. Oncol. 29 (8), 1784–1792. doi:10.1093/annonc/mdy181
Birkbak, N. J., Wang, Z. C., Kim, J. Y., Eklund, A. C., Li, Q., Tian, R., et al. (2012). Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2 (4), 366–375. doi:10.1158/2159-8290.Cd-11-0206
Bookman, M. A., Brady, M. F., McGuire, W. P., Harper, P. G., Alberts, D. S., Friedlander, M., et al. (2009). Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A phase III trial of the gynecologic cancer intergroup. J. Clin. Oncol. 27 (9), 1419–1425. doi:10.1200/jco.2008.19.1684
Bryant, H. E., Schultz, N., Thomas, H. D., Parker, K. M., Flower, D., Lopez, E., et al. (2005). Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434 (7035), 913–917. doi:10.1038/nature03443
Bunting, S. F., Callén, E., Wong, N., Chen, H. T., Polato, F., Gunn, A., et al. (2010). 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141 (2), 243–254. doi:10.1016/j.cell.2010.03.012
Bürkle, A., and Burkle, A. (2001). Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays 23 (9), 795–806. doi:10.1002/bies.1115
Catanzaro, E., Canistro, D., Pellicioni, V., Vivarelli, F., and Fimognari, C. (2022). Anticancer potential of allicin: A review. Pharmacol. Res. 177, 106118. doi:10.1016/j.phrs.2022.106118
Chen, J., and Tsim, K. W. K. (2020). A review of edible jujube, the ziziphus jujuba fruit: A heath food supplement for anemia prevalence. Front. Pharmacol. 11, 593655. doi:10.3389/fphar.2020.593655
Chen, Y., She, Y., Shi, X., Zhang, X., Wang, R., and Men, K. (2020). Green tea catechin: Does it lower blood cholesterol? IOP Conf. Ser. Earth Environ. Sci. 559, 012027. doi:10.1088/1755-1315/559/1/012027
Chu, A. J. (2022). Quarter-century explorations of bioactive polyphenols: Diverse health benefits. Front. Biosci. 27 (4), 134. doi:10.31083/j.fbl2704134
Colombo, N., Sessa, C., du Bois, A., Ledermann, J., McCluggage, W. G., McNeish, I., et al. (2019). ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 30 (5), 672–705. doi:10.1093/annonc/mdz062
Crichton, M., Marshall, S., Marx, W., McCarthy, A. L., and Isenring, E. (2019). Efficacy of ginger (zingiber officinale) in ameliorating chemotherapy-induced nausea and vomiting and chemotherapy-related outcomes: A systematic review update and meta-analysis. J. Acad. Nutr. Diet. 119 (12), 2055–2068. doi:10.1016/j.jand.2019.06.009
Curtin, N. J., and Szabo, C. (2020). Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 19 (10), 711–736. doi:10.1038/s41573-020-0076-6
D'Andrea, A. D. (2018). Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair (Amst) 71, 172–176. doi:10.1016/j.dnarep.2018.08.021
Dal, H. Ö. G., and Yilmaz, Y. (2022). “Use of tea catechins in foods as a functional ingredient,” in Tea as a food ingredient (Boca Raton, Florida, United States: CRC Press), 241–257.
Damia, G., and Broggini, M. (2019). Platinum resistance in ovarian cancer: Role of DNA repair. Cancers (Basel) 11 (1), 119. doi:10.3390/cancers11010119
Dana, A.-H., and Alejandro, S.-P. (2022). Role of sulforaphane in endoplasmic reticulum homeostasis through regulation of the antioxidant response. Life Sci. 299, 120554. doi:10.1016/j.lfs.2022.120554
Dangles, O., and Fenger, J.-A. (2018). The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 23 (8), 1970. doi:10.3390/molecules23081970
de la Iglesia, R., Milagro, F. I., Campión, J., Boqué, N., and Martínez, J. A. (2010). Healthy properties of proanthocyanidins. Biofactors 36 (3), 159–168. doi:10.1002/biof.79
Deng, Q., Li, X. X., Fang, Y., Chen, X., and Xue, J. (2020). Therapeutic potential of quercetin as an antiatherosclerotic agent in atherosclerotic cardiovascular disease: A review. Evid. Based. Complement. Altern. Med. 2020, 5926381. doi:10.1155/2020/5926381
Dinkova-Kostova, A. T., Fahey, J. W., Kostov, R. V., and Kensler, T. W. (2017). KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 69, 257–269. doi:10.1016/j.tifs.2017.02.002
Durkacz, B. W., Omidiji, O., Gray, D. A., and Shall, S. (1980). (ADP-ribose)n participates in DNA excision repair. Nature 283 (5747), 593–596. doi:10.1038/283593a0
Farmer, H., McCabe, N., Lord, C. J., Tutt, A. N., Johnson, D. A., Richardson, T. B., et al. (2005). Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434 (7035), 917–921. doi:10.1038/nature03445
Foley, O. W., Rauh-Hain, J. A., and del Carmen, M. G. (2013). Recurrent epithelial ovarian cancer: An update on treatment. Oncol. Willist. Park 27 (4), 288–298.
Fridlich, R., Annamalai, D., Roy, R., Bernheim, G., and Powell, S. N. (2015). BRCA1 and BRCA2 protect against oxidative DNA damage converted into double-strand breaks during DNA replication. DNA Repair (Amst) 30, 11–20. doi:10.1016/j.dnarep.2015.03.002
Gardini, A., Baillat, D., Cesaroni, M., and Shiekhattar, R. (2014). Genome-wide analysis reveals a role for BRCA1 and PALB2 in transcriptional co-activation. Embo J. 33 (8), 890–905. doi:10.1002/embj.201385567
Gimbrone, M. A., and García-Cardeña, G. (2016). Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118 (4), 620–636. doi:10.1161/circresaha.115.306301
Gong, X., Smith, J. R., Swanson, H. M., and Rubin, L. P. (2018). Carotenoid lutein selectively inhibits breast cancer cell growth and potentiates the effect of chemotherapeutic agents through ROS-mediated mechanisms. Molecules 23 (4), 905. doi:10.3390/molecules23040905
Gorrini, C., Baniasadi, P. S., Harris, I. S., Silvester, J., Inoue, S., Snow, B., et al. (2013). BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 210 (8), 1529–1544. doi:10.1084/jem.20121337
Gudmundsdottir, K., and Ashworth, A. (2006). The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 25 (43), 5864–5874. doi:10.1038/sj.onc.1209874
Haince, J. F., McDonald, D., Rodrigue, A., Déry, U., Masson, J. Y., Hendzel, M. J., et al. (2008). PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J. Biol. Chem. 283 (2), 1197–1208. doi:10.1074/jbc.M706734200
Hajizadeh-Sharafabad, F., Tarighat-Esfanjani, A., Ghoreishi, Z., and Sarreshtedari, M. (2021). Lutein supplementation combined with a low-calorie diet in middle-aged obese individuals: Effects on anthropometric indices, body composition and metabolic parameters. Br. J. Nutr. 126 (7), 1028–1039. doi:10.1017/S0007114520004997
Hasan, N., Yusuf, N., Toossi, Z., and Islam, N. (2006). Suppression of Mycobacterium tuberculosis induced reactive oxygen species (ROS) and TNF-alpha mRNA expression in human monocytes by allicin. FEBS Lett. 580 (10), 2517–2522. doi:10.1016/j.febslet.2006.03.071
Hoeijmakers, J. H. (2001). Genome maintenance mechanisms for preventing cancer. Nature 411 (6835), 366–374. doi:10.1038/35077232
Islam, S. U., Ahmed, M. B., Ahsan, H., Lee, Y.-S., Shehzad, A., Sonn, J. K., et al. (2021). An update on the role of dietary phytochemicals in human skin cancer: New insights into molecular mechanisms. Antioxidants 10 (5), 916. doi:10.3390/antiox9100916
Javandoost, A., Afshari, A., Nikbakht-Jam, I., Khademi, M., Eslami, S., Nosrati, M., et al. (2017). Effect of crocin, a carotenoid from saffron, on plasma cholesteryl ester transfer protein and lipid profile in subjects with metabolic syndrome: A double blind randomized clinical trial. ARYA Atheroscler. 13 (5), 245–252.
Kaiser, A. E., Baniasadi, M., Giansiracusa, D., Giansiracusa, M., Garcia, M., Fryda, Z., et al. (2021). Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers 13 (19), 4796. doi:10.3390/cancers13194796
Khan, H., Labanca, F., Ullah, H., Hussain, Y., Tzvetkov, N. T., Akkol, E. K., et al. (2021). Advances and challenges in cancer treatment and nutraceutical prevention: The possible role of dietary phenols in BRCA regulation. Phytochem. Rev. 21, 385–400. doi:10.1007/s11101-021-09771-3
Kim, I.-S. (2021). Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 10 (7), 1064. doi:10.3390/antiox10071064
Kobayashi, A., Kang, M. I., Okawa, H., Ohtsuji, M., Zenke, Y., Chiba, T., et al. (2004). Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24 (16), 7130–7139. doi:10.1128/mcb.24.16.7130-7139.2004
Kobayashi, M., Li, L., Iwamoto, N., Nakajima-Takagi, Y., Kaneko, H., Nakayama, Y., et al. (2009). The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 29 (2), 493–502. doi:10.1128/mcb.01080-08
Kowalska, K., Dembczyński, R., Gołąbek, A., Olkowicz, M., and Olejnik, A. (2021). ROS modulating effects of lingonberry (vaccinium vitis-idaea L.) polyphenols on obese adipocyte hypertrophy and vascular endothelial dysfunction. Nutrients 13 (3), 885. doi:10.3390/nu13030885
Kutuzov, M. M., Belousova, E. A., Ilina, E. S., and Lavrik, O. I. (2020). Impact of PARP1, PARP2 & PARP3 on the base excision repair of nucleosomal DNA. Adv. Exp. Med. Biol. 1241, 47–57. doi:10.1007/978-3-030-41283-8_4
Kwon, Y. (2021). Possible beneficial effects of n-acetylcysteine for treatment of triple-negative breast cancer. Antioxidants 10 (2), 169. doi:10.3390/antiox10020169
LaFargue, C. J., Dal Molin, G. Z., Sood, A. K., and Coleman, R. L. (2019). Exploring and comparing adverse events between PARP inhibitors. Lancet. Oncol. 20 (1), e15–e28. doi:10.1016/s1470-2045(18)30786-1
Lammert, J., Basrai, M., Struck, J., Hartmann, O., Engel, C., Bischoff, S. C., et al. (2022). Associations of plasma bioactive adrenomedullin levels with cardiovascular risk factors in BRCA1/2 mutation carriers. Geburtshilfe Frauenheilkd. 82 (6), 601–609. doi:10.1055/a-1811-2164
Langdon, S. P., Herrington, C. S., Hollis, R. L., and Gourley, C. (2020). Estrogen signaling and its potential as a target for therapy in ovarian cancer. Cancers 12 (6), 1647. doi:10.3390/cancers12061647
Lee, H.-J., Oh, S.-Y., and Jo, I. (2021). Zearalenone induces endothelial cell apoptosis through activation of a cytosolic Ca2+/ERK1/2/p53/Caspase 3 signaling pathway. Toxins 13 (3), 187. doi:10.3390/toxins13030187
Li, H., Liu, Z. Y., Wu, N., Chen, Y. C., Cheng, Q., and Wang, J. (2020). PARP inhibitor resistance: The underlying mechanisms and clinical implications. Mol. Cancer 19 (1), 107. doi:10.1186/s12943-020-01227-0
Li, L., Mangali, S., Kour, N., Dasari, D., Ghatage, T., Sharma, V., et al. (2021). Syzygium cumini (jamun) fruit-extracted phytochemicals exert anti-proliferative effect on ovarian cancer cells. J. Cancer Res. Ther. 17 (6), 1547–1551. doi:10.4103/jcrt.JCRT_210_20
Lord, C. J., and Ashworth, A. (2017). PARP inhibitors: Synthetic lethality in the clinic. Science 355 (6330), 1152–1158. doi:10.1126/science.aam7344
Lorusso, D., Ceni, V., Daniele, G., Salutari, V., Pietragalla, A., Muratore, M., et al. (2020). Newly diagnosed ovarian cancer: Which first-line treatment? Cancer Treat. Rev. 91, 102111. doi:10.1016/j.ctrv.2020.102111
Ma, J., Cai, H., Wu, T., Sobhian, B., Huo, Y., Alcivar, A., et al. (2012). PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function. Mol. Cell. Biol. 32 (8), 1506–1517. doi:10.1128/mcb.06271-11
Mai, P. L., Chatterjee, N., Hartge, P., Tucker, M., Brody, L., Struewing, J. P., et al. (2009). Potential excess mortality in BRCA1/2 mutation carriers beyond breast, ovarian, prostate, and pancreatic cancers, and melanoma. PLoS One 4 (3), e4812. doi:10.1371/journal.pone.0004812
Marx, W., McCarthy, A. L., Ried, K., McKavanagh, D., Vitetta, L., Sali, A., et al. (2017). The effect of a standardized ginger extract on chemotherapy-induced nausea-related quality of life in patients undergoing moderately or highly emetogenic chemotherapy: A double blind, randomized, placebo controlled trial. Nutrients 9 (8), 867. doi:10.3390/nu9080867
Milne, R. L., and Antoniou, A. C. (2011). Genetic modifiers of cancer risk for BRCA1 and BRCA2 mutation carriers. Ann. Oncol. 22 (1), i11–i17. doi:10.1093/annonc/mdq660
Mirza, M. R., Åvall Lundqvist, E., Birrer, M. J., dePont Christensen, R., Nyvang, G. B., Malander, S., et al. (2019). Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): A randomised, phase 2, superiority trial. Lancet. Oncol. 20 (10), 1409–1419. doi:10.1016/s1470-2045(19)30515-7
Mitra, S., Rauf, A., Tareq, A. M., Jahan, S., Emran, T. B., Shahriar, T. G., et al. (2021). Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 154, 112328. doi:10.1016/j.fct.2021.112328
Mortezaee, K., Salehi, E., Mirtavoos-mahyari, H., Motevaseli, E., Najafi, M., Farhood, B., et al. (2019). Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 234 (8), 12537–12550. doi:10.1002/jcp.28122
Munroe, M., and Kolesar, J. (2016). Olaparib for the treatment of BRCA-mutated advanced ovarian cancer. Am. J. Health. Syst. Pharm. 73 (14), 1037–1041. doi:10.2146/ajhp150550
Murai, J., Huang, S. Y., Das, B. B., Renaud, A., Zhang, Y., Doroshow, J. H., et al. (2012). Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 72 (21), 5588–5599. doi:10.1158/0008-5472.Can-12-2753
Murai, J., Huang, S. Y., Renaud, A., Zhang, Y., Ji, J., Takeda, S., et al. (2014). Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 13 (2), 433–443. doi:10.1158/1535-7163.Mct-13-0803
Nakamura, M., and Sugiura, M. (2022). Serum lutein and zeaxanthin are inversely associated with high-sensitivity C-reactive protein in non-smokers: The mikkabi study. Antioxidants 11 (2), 259. doi:10.3390/antiox11020259
Nocerino, R., Cecere, G., Micillo, M., De Marco, G., Ferri, P., Russo, M., et al. (2021). Efficacy of ginger as antiemetic in children with acute gastroenteritis: A randomised controlled trial. Aliment. Pharmacol. Ther. 54 (1), 24–31. doi:10.1111/apt.16404
Noordermeer, S. M., and van Attikum, H. (2019). PARP inhibitor resistance: A tug-of-war in BRCA-mutated cells. Trends Cell Biol. 29 (10), 820–834. doi:10.1016/j.tcb.2019.07.008
Ogiwara, H., Ui, A., Shiotani, B., Zou, L., Yasui, A., and Kohno, T. (2013). Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor. Carcinogenesis 34 (11), 2486–2497. doi:10.1093/carcin/bgt240
Paik, J. (2021). Olaparib: A review as first-line maintenance therapy in advanced ovarian cancer. Target. Oncol. 16 (6), 847–856. doi:10.1007/s11523-021-00842-1
Panieri, E., and Santoro, M. M. (2015). ROS signaling and redox biology in endothelial cells. Cell. Mol. Life Sci. 72 (17), 3281–3303. doi:10.1007/s00018-015-1928-9
Periasamy, S., Wu, W. H., Chien, S. P., Liu, C. T., and Liu, M. Y. (2020). Dietary ziziphus jujuba fruit attenuates colitis-associated tumorigenesis: A pivotal role of the NF-κB/IL-6/JAK1/STAT3 pathway. Nutr. Cancer 72 (1), 120–132. doi:10.1080/01635581.2019.1615515
Perren, T. J., Swart, A. M., Pfisterer, J., Ledermann, J. A., Pujade-Lauraine, E., Kristensen, G., et al. (2011). A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 365 (26), 2484–2496. doi:10.1056/NEJMoa1103799
Porter, S. A., Pedley, A., Massaro, J. M., Vasan, R. S., Hoffmann, U., and Fox, C. S. (2013). Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: The framingham heart study. Arterioscler. Thromb. Vasc. Biol. 33 (1), 139–146. doi:10.1161/atvbaha.112.300075
Pourmasoumi, M., Hadi, A., Najafgholizadeh, A., Kafeshani, M., and Sahebkar, A. (2019). Clinical evidence on the effects of saffron (crocus sativus L.) on cardiovascular risk factors: A systematic review meta-analysis. Pharmacol. Res. 139, 348–359. doi:10.1016/j.phrs.2018.11.038
Prakash, R., Zhang, Y., Feng, W., and Jasin, M. (2015). Homologous recombination and human health: The roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb. Perspect. Biol. 7 (4), a016600. doi:10.1101/cshperspect.a016600
Pundir, M., Sharma, A., and Kumar, J. (2021). Phytochemicals used as inhibitors in the treatment of ovarian cancer: A mini-review. Mater. Today Proc. 48, 1620–1625. doi:10.1016/j.matpr.2021.09.505
Ramanna, M. K., Somu, L., L, S., and Prasad T, K. (2021). A comparative study on efficacy of lutein and atorvastatin on lipid profile and lipoprotein (A) in hypercholesterolemic male wistar rats. Biomed. Pharmacol. J. 14 (1), 503–511. doi:10.13005/bpj/2151
Ramus, S. J., and Gayther, S. A. (2009). The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol. Oncol. 3 (2), 138–150. doi:10.1016/j.molonc.2009.02.001
Rottenberg, S., Jaspers, J. E., Kersbergen, A., van der Burg, E., Nygren, A. O., Zander, S. A., et al. (2008). High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. U. S. A. 105 (44), 17079–17084. doi:10.1073/pnas.0806092105
Roy, M., and Datta, A. (2019). “Fundamentals of phytochemicals,” in Cancer genetics and therapeutics (New York, United States: Springer), 49–81.
Roy, M., and Datta, A. (2021). “Phytochemicals in ROS mediated epigenetic modulation of cancer,” in Handbook of oxidative stress in cancer: Mechanistic aspects (New York, United States: Springer), 1–18.
Runlin, W., Xiang, P., Juan, L., and Shu, Y. (2022). Soybean isoflavones activating autophagy and improving the chemosensitivity of carboplatin to ovarian cancer cells. J. Biomater. tissue Eng. 12 (9), 1805–1812. doi:10.1166/jbt.2022.3108
Sajjad, M., Fradley, M., Sun, W., Kim, J., Zhao, X., Pal, T., et al. (2017). An exploratory study to determine whether BRCA1 and BRCA2 mutation carriers have higher risk of cardiac toxicity. Genes (Basel) 8 (2), 59. doi:10.3390/genes8020059
Schmitt, B., Vicenzi, M., Garrel, C., and Denis, F. M. (2015). Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 6, 198–205. doi:10.1016/j.redox.2015.07.012
Schwertheim, S., Wein, F., Lennartz, K., Worm, K., Schmid, K. W., and Sheu-Grabellus, S.-Y. (2017). Curcumin induces G2/M arrest, apoptosis, NF-κB inhibition, and expression of differentiation genes in thyroid carcinoma cells. J. Cancer Res. Clin. Oncol. 143 (7), 1143–1154. doi:10.1007/s00432-017-2380-z
Sekine, M., Nishino, K., and Enomoto, T. (2021). Differences in ovarian and other cancers risks by population and BRCA mutation location. Genes (Basel) 12 (7), 1050. doi:10.3390/genes12071050
Shea, S., Navas-Acien, A., Shimbo, D., Brown, E. R., Budoff, M., Bancks, M. P., et al. (2021). Spatially weighted coronary artery calcium score and coronary heart disease events in the multi-ethnic study of atherosclerosis. Circ. Cardiovasc. Imaging 14 (1), e011981. doi:10.1161/circimaging.120.011981
Singh, K. K., Shukla, P. C., Quan, A., Al-Omran, M., Lovren, F., Pan, Y., et al. (2013). BRCA1 is a novel target to improve endothelial dysfunction and retard atherosclerosis. J. Thorac. Cardiovasc. Surg. 146 (4), 949–960. doi:10.1016/j.jtcvs.2012.12.064
Song, M.-Y., Lee, D.-Y., Chun, K.-S., and Kim, E.-H. (2021). The role of NRF2/KEAP1 signaling pathway in cancer metabolism. Int. J. Mol. Sci. 22 (9), 4376. doi:10.3390/ijms22094376
Speciale, A., Saija, A., Bashllari, R., Molonia, M. S., Muscarà, C., Occhiuto, C., et al. (2020). Anthocyanins as modulators of cell redox-dependent pathways in non-communicable diseases. Curr. Med. Chem. 27 (12), 1955–1996. doi:10.2174/0929867325666181112093336
Srinivasan, G., Sidhu, G. S., Williamson, E. A., Jaiswal, A. S., Najmunnisa, N., Wilcoxen, K., et al. (2017). Synthetic lethality in malignant pleural mesothelioma with PARP1 inhibition. Cancer Chemother. Pharmacol. 80 (4), 861–867. doi:10.1007/s00280-017-3401-y
Suzuki-Sugihara, N., Kishimoto, Y., Saita, E., Taguchi, C., Kobayashi, M., Ichitani, M., et al. (2016). Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 36 (1), 16–23. doi:10.1016/j.nutres.2015.10.012
Syamala, S., Sreepriya, M., and Sudhandiran, G. (2021). “Soy isoflavones, mitochondria and cell fate,” in Mitochondrial physiology and vegetal molecules (Cambridge, Massachusetts, United States: Academic Press), 625–643. doi:10.1016/b978-0-12-821562-3.00046-0
Thushara, R. M., Hemshekhar, M., Santhosh, M. S., Jnaneshwari, S., Nayaka, S. C., Naveen, S., et al. (2013). Crocin, a dietary additive protects platelets from oxidative stress-induced apoptosis and inhibits platelet aggregation. Mol. Cell. Biochem. 373 (1-2), 73–83. doi:10.1007/s11010-012-1476-7
Tian, X., Xin, H., Paengkoum, P., Paengkoum, S., Ban, C., and Sorasak, T. (2019). Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on nutrient utilization, rumen fermentation, plasma antioxidant capacity, and mammary gland gene expression in dairy goats. J. Anim. Sci. 97 (3), 1384–1397. doi:10.1093/jas/sky477
Tong, K. I., Katoh, Y., Kusunoki, H., Itoh, K., Tanaka, T., and Yamamoto, M. (2006). Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 26 (8), 2887–2900. doi:10.1128/mcb.26.8.2887-2900.2006
Ulasov, A. V., Rosenkranz, A. A., Georgiev, G. P., and Sobolev, A. S. (2021). Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 291, 120111. doi:10.1016/j.lfs.2021.120111
Vanacker, H., Harter, P., Labidi-Galy, S. I., Banerjee, S., Oaknin, A., Lorusso, D., et al. (2021). PARP-inhibitors in epithelial ovarian cancer: Actual positioning and future expectations. Cancer Treat. Rev. 99, 102255. doi:10.1016/j.ctrv.2021.102255
Vurusaner, B., Poli, G., and Basaga, H. (2012). Tumor suppressor genes and ROS: Complex networks of interactions. Free Radic. Biol. Med. 52 (1), 7–18. doi:10.1016/j.freeradbiomed.2011.09.035
Wang, S., Wu, Y. Y., Wang, X., Shen, P., Jia, Q., Yu, S., et al. (2020). Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through p62-triggered autophagic Keap1 degradation. Aging (Albany NY) 12 (9), 8167–8190. doi:10.18632/aging.103132
Wei, H., and Yu, X. (2016). Functions of PARylation in DNA damage repair pathways. Genomics Proteomics Bioinforma. 14 (3), 131–139. doi:10.1016/j.gpb.2016.05.001
Wong, N. D. (2014). Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 11 (5), 276–289. doi:10.1038/nrcardio.2014.26
Woo, H. W., Kim, M. K., Lee, Y. H., Shin, D. H., Shin, M. H., and Choi, B. Y. (2019). Habitual consumption of soy protein and isoflavones and risk of metabolic syndrome in adults ≥ 40 years old: A prospective analysis of the Korean multi-rural communities cohort study (MRCohort). Eur. J. Nutr. 58 (7), 2835–2850. doi:10.1007/s00394-018-1833-8
Xia, B., Dorsman, J. C., Ameziane, N., de Vries, Y., Rooimans, M. A., Sheng, Q., et al. (2007). Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat. Genet. 39 (2), 159–161. doi:10.1038/ng1942
Xia, B., Sheng, Q., Nakanishi, K., Ohashi, A., Wu, J., Christ, N., et al. (2006). Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol. Cell 22 (6), 719–729. doi:10.1016/j.molcel.2006.05.022
Xian, D., Lai, R., Song, J., Xiong, X., and Zhong, J. (2019). Emerging perspective: Role of increased ROS and redox imbalance in skin carcinogenesis. Oxid. Med. Cell. Longev. 2019, 8127362. doi:10.1155/2019/8127362
Xu, S., Ilyas, I., Little, P. J., Li, H., Kamato, D., Zheng, X., et al. (2021). Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 73 (3), 924–967. doi:10.1124/pharmrev.120.000096
Yang, Q. Q., Gan, R. Y., Ge, Y. Y., Zhang, D., and Corke, H. (2018). Polyphenols in common beans (Phaseolus vulgaris L.): Chemistry, analysis, and factors affecting composition. Compr. Rev. Food Sci. Food Saf. 17 (6), 1518–1539. doi:10.1111/1541-4337.12391
Yaribeygi, H., Mohammadi, M. T., and Sahebkar, A. (2018). Crocin potentiates antioxidant defense system and improves oxidative damage in liver tissue in diabetic rats. Biomed. Pharmacother. 98, 333–337. doi:10.1016/j.biopha.2017.12.077
Zeb, A. (2021). “Phenolic AntioxidantsAntioxidants in vegetables,” in Phenolic antioxidants in foods: Chemistry, biochemistry and analysis (Cham: Springer International Publishing), 131–148.
Zhang, D. D., Lo, S. C., Cross, J. V., Templeton, D. J., and Hannink, M. (2004). Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 24 (24), 10941–10953. doi:10.1128/mcb.24.24.10941-10953.2004
Zheng, B., Yin, W. N., Suzuki, T., Zhang, X. H., Zhang, Y., Song, L. L., et al. (2017). Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol. Ther. 25 (6), 1279–1294. doi:10.1016/j.ymthe.2017.03.031
Keywords: phytochemicals, ovarian cancer, PARP, BRCA, PARP inhibitors
Citation: Wang C, Gao P, Xu J, Liu S, Tian W, Liu J and Zhou L (2022) Natural phytochemicals prevent side effects in BRCA-mutated ovarian cancer and PARP inhibitor treatment. Front. Pharmacol. 13:1078303. doi: 10.3389/fphar.2022.1078303
Received: 24 October 2022; Accepted: 28 November 2022;
Published: 07 December 2022.
Edited by:
Jing Wang, Xiangya School of Medicine, Central South University, ChinaReviewed by:
Quan Du, Peking University, ChinaCopyright © 2022 Wang, Gao, Xu, Liu, Tian, Liu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Zhou, emx5eXNAYWxpeXVuLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.