Corrigendum: Anticancer applications of phytochemicals in gastric cancer: effects and molecular mechanism
- 1Wujin Institute of Molecular Diagnostics and Precision Cancer Medicine of Jiangsu University, Wujin Hospital Affiliated with Jiangsu University, Chang Zhou, China
- 2Department of Laboratory Medicine, School of Medicine, Jiangsu University, Zhenjiang, China
Gastric cancer (GC) is the fourth most common malignant cancer and is a life-threatening disease worldwide. Phytochemicals have been shown to be a rational, safe, non-toxic, and very promising approach to the prevention and treatment of cancer. It has been found that phytochemicals have protective effects against GC through inhibiting cell proliferation, inducing apoptosis and autophagy, suppressing cell invasion and migration, anti-angiogenesis, inhibit Helicobacter pylori infection, regulating the microenvironment. In recent years, the role of phytochemicals in the occurrence, development, drug resistance and prognosis of GC has attracted more and more attention. In order to better understand the relationship between phytochemicals and gastric cancer, we briefly summarize the roles and functions of phytochemicals in GC tumorigenesis, development and prognosis. This review will probably help guide the public to prevent the occurrence and development of GC through phytochemicals, and develop functional foods or drugs for the prevention and treatment of gastric cancer.
1 Introduction
GC is the fourth most common malignant cancer and the third most common cause of cancer-related death worldwide, with more than 1 million new cases and 769000 deaths annually (Sung et al., 2021). Chemotherapy, radiotherapy and surgery have been recognized as the main therapies for the treatment of gastric cancer, but they have their own disadvantages, such as side effects, toxicity and resistance of anticancer drugs (Khan et al., 2019). In addition, GC is a multicentric and multistep phenomenon which sequentially accumulates molecular and genetic abnormalities. Therefore, it is urgent and necessary to find a multi-stage, more effective and less toxic strategy for the prevention and treatment of gastric cancer (Mao et al., 2020).
Although surgery with or without chemotherapy/radiotherapy as a standard treatment can be an appropriate treatment strategy for gastric cancer, side effects and drug resistance are the two major obstacles to therapy. It has been found that phytochemical agents exhibited significant anticancer activity while causing trivial side effects (Cheshomi et al., 2022).
Phytochemicals have been shown to be a rational, safe, non-toxic, and very promising approach to the prevention and treatment of cancer, especially in high-risk populations (Lu et al., 2016). A rich phytochemical is found in vegetables, spices, fruits, nuts, soy, tea, edible macro-fungi and whole grains, which have a variety of health benefits (Bastos et al., 2010; Al-Ishaq et al., 2020; Mao et al., 2020). Numerous epidemiological investigations and experimental studies have demonstrated that phytochemical is essential to the prevention and management of gastric cancer (Nagata et al., 2002; Bastos et al., 2010; Mao et al., 2020). Phytochemicals have protective effects against GC through various mechanisms, including inhibiting cell proliferation, inducing cell apoptosis and autophagy, suppressing cell invasion and migration, anti-angiogenesis, inhibiting Helicobacter pylori infection, regulating the microenvironment, and other possible mechanisms (Figure 1).
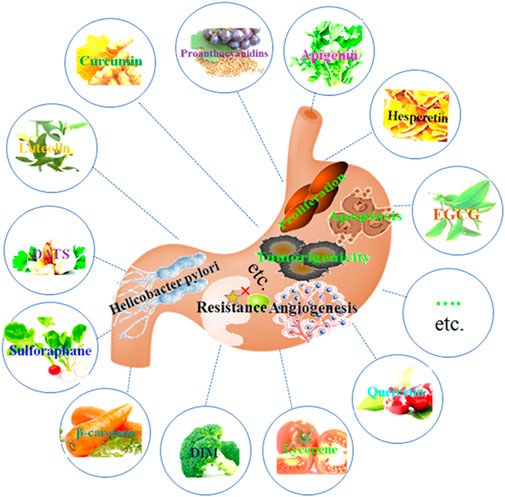
Figure 1. Phytochemicals have protective effects against GC through inhibiting cell proliferation, inducing cell apoptosis and autophagy, suppressing cell invasion and migration, anti-angiogenesis, inhibiting Helicobacter pylori infection, regulating microenvironment, and other possible mechanisms.
The objective of this review is to summarize anti-cancer effects of phytochemicals on GC and discuss the mechanism of action on gastric cancer, and also to show their bioavailability and therapeutic effect on gastric cancer. For the purpose of the review, we used keywords, including gastric cancer and phytochemicals, plant active ingredients, phytochemicals, chemical protection of plants, to retrieve relevant references from 2012 to 2022 in PubMed database. If there are too few references in some part, we will appropriately expand the time span of references.
2 Effects of phytochemicals on the occurrence and development of GC
Numerous epidemiological studies have demonstrated that the intake of phytochemicals is essential to the prevention and treatment of gastric cancer (Mao et al., 2020). GC is a multi-center, multi-step phenomenon, involving a variety of physiological and pathological processes. The effects of phytochemicals in the treatment and prevention of GC have been widely studied, and their mechanism of action has also been studied. We explored the influence of phytochemicals on the main physiological and pathological processes related to gastric cancer.
2.1 Inhibition of GC cell proliferation
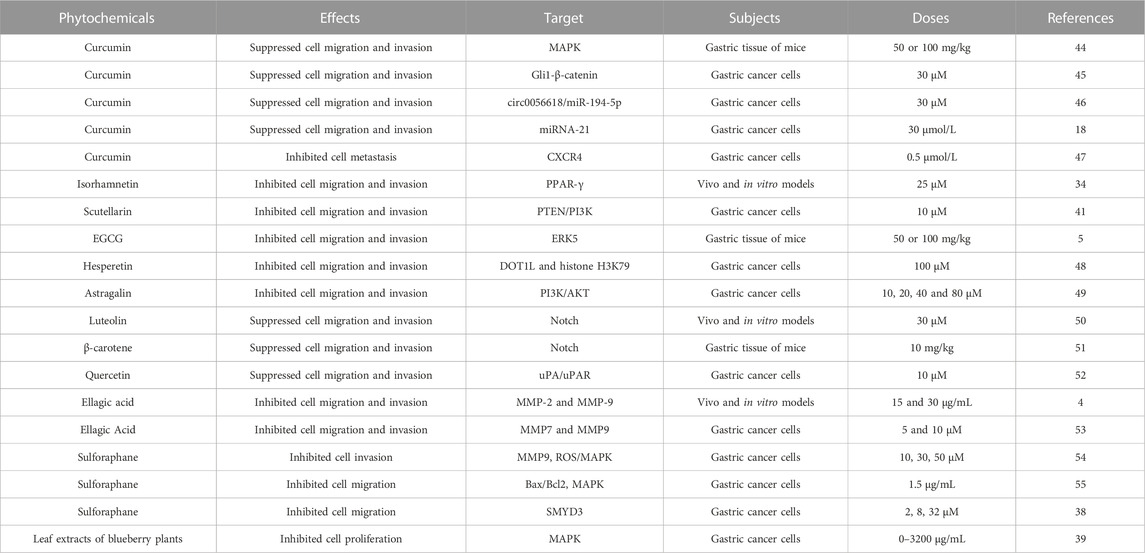
Abnormal cell proliferation is a key step that may promote the occurrence and development of cancer (Kim et al., 2020a; Yang et al., 2020). Numerous studies have confirmed that various phytochemicals can inhibit the proliferation of GC cells and the growth of gastric tumors in mice (Table 1; Figure 2).
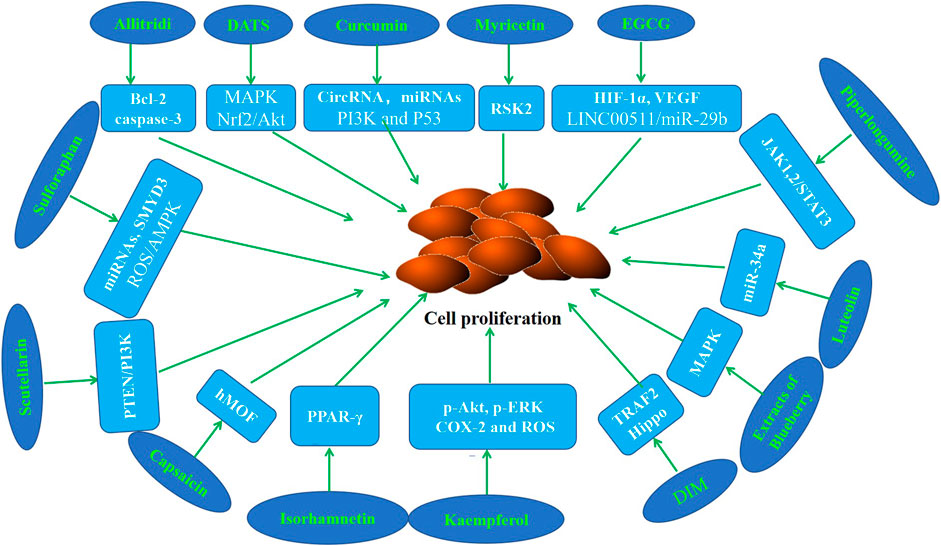
Figure 2. Molecular mechanism of anti-GC effect of representative phytochemicals by inhibiting cell proliferation.
It has been shown by epidemiological evidence that Allitridi reduces the risk of developing malignancies (Sarvizadeh et al., 2021; Rauf et al., 2022). Several studies revealed that Allitridi and Diallyl trisulfide (DATS) inhibit cell proliferation in GC cell lines (Lan and Lu, 2004; Ha et al., 2005; Jiang et al., 2017a). Diallyl trisulfide suppressed tumor growth through the attenuation of Nrf2/Akt and activation of p38/JNK in xenograft mice (Jiang et al., 2017b). Curcumin has garnered attention because of its antiinflammatory, antioxidant, anticancer, and chemopreventive properties. It is reported that curcumin suppresses the proliferation of GC cells by regulating circRNA/miRNA/protein in vivo and in vitro experimental models (Liu et al., 2014; Wang et al., 2017; Fu et al., 2018; Liu et al., 2018; Hassanalilou et al., 2019; Sun et al., 2019). Poncirin is a flavanone glycoside that could inhibit the proliferation of SGC-7901 cells (Zhu et al., 2013). Myricetin is a flavonoid which could inhibit the abnormal proliferation of GC cells by binding with RSK2 (Feng et al., 2015). Epigallocatechin-3-gallate (EGCG), the most abundant and active polyphenol in green tea, has been shown to have anti-inflammatory, anti-oxidant, anti-cancer, and chemopreventive properties. Fu et al. (2019) revealed that EGCG down regulated HIF-1α and VEGF to inhibit the proliferation of GC cells. The data of Zhao et al. (2020) showed that EGCG retarded cell growth of GC in a dose-dependent manner. Piperlongumine, a major component derived from long peppers, has been reported to suppress the proliferation of GC cells (Song et al., 2016). It is reported that kaempferol inhibits the proliferation of GC cell lines and the growth of the tumor xenografts (Song et al., 2015; Liao et al., 2016). Recent studies have revealed that 3,3-diindolylmethane (DIM) has antiproliferation effects in vivo and in vitro GC models (Li et al., 2013; Ye et al., 2021a). Luteolin is a compound of Lonicera japonica Thunb, and has been reported to decrease the viability of cells in the occurrence and development of gastric cancer (Zhou et al., 2018). The study of Xu et al. (2017) reported that the growth inhibition of Galangin and quercetin on the GC cells . Lalitha et al. reported that isorhamnetin inhibits cell proliferation through the modulation of PPAR-γ activation in gastric cancer (Ramachandran et al., 2012). Data of Hamid et al. showed that Elagic acid inhibits the proliferation of GC cells and leads to the reduction of tumor volume in mice (Cheshomi et al., 2022). Sulforaphane is a natural compound of cruciferous vegetables. Sholeh et al. found that significant dose-dependent antiproliferative effects of sulforaphane were observed in GC cells (Choi, 2018; Dong et al., 2018; Kiani et al., 2018; Han et al., 2021). The study of Alejandra et al. demonstrated that the antiproliferative effect of leaf extracts of blueberry plants on GC cells (Ribera-Fonseca et al., 2020). The results of Wang et al. (2016a) showed that capsaicin could suppress cell growth, while changing histone acetylation in GC cells. Scutellarin was found to inhibit GC cell proliferation (Li et al., 2021a). Unfortunately, most of these studies focus on the anti-proliferation study of phytochemicals at the cell line level, and the dosage used is inconsistent, resulting in limited clinical value.
Uncontrolled proliferation of GC cells has been proved to play a critical role in the pathogenesis of gastric cancer. It is generally believed that some phytochemicals possess good effects on cancer prevention and growth. In recent years, there have been many studies involving the inhibition of cell proliferation by phytochemicals in the carcinogenesis and development of gastric cancer. These findings suggested that phytochemicals can be used as a potential means for the prevention and treatment of gastric cancer.
2.2 Inhibition of cell migration and invasion
The ability of cell migration and invasion plays an important role in the occurrence, development, treatment and prognosis of gastric cancer. Some GC patients have lymph node metastasis or even distant metastasis at the first diagnosis, which leads to failure of surgical treatment and affects the prognosis and survival rate of patients (Guo et al., 2021). The enhanced motility and invasiveness afforded by EMT are critical for metastatic initiation of gastric cancer (Li et al., 2019). There is increasing evidence that phytochemicals can inhibit the migration and invasion of GC cells in vivo and in vitro (Table 2).
Curcumin, the major active compound of the plant Curcuma longa, has been shown to inhibit migration and invasion of GC cells (Liang et al., 2015; Liu et al., 2018; Zhang et al., 2020; Li et al., 2021b). The study of Gu et al. (2019) suggested that curcumin inhibits liver metastasis of GC through reducing circulating cancer cells. Lalitha et al. reported that isorhamnetin inhibits cell migration and invasion through the modulation of PPAR-γ activation in gastric cancer (Ramachandran et al., 2012). Scutellarin, a flavonoid plant compound derived from breviscapus, has been found to suppress GC cell migration and invasion (Li et al., 2021a). EGCG suppressed ERK5 activation to reverse tobacco smoke-triggered cell migration and invasion in mice gastric tissues (Lu et al., 2016). The author explored the intervention effect of EGCG in smoke induced GC in vivo and in vitro, which is still an interesting study. Hesperidin decreased the migration and invasion of GC cells by educing the abundance of DOT1L and methylation of histone H3K79 (Wang et al., 2021a). It is reported that Astragalin, a natural flavonoid compound, suppresses GC cells migration and invasion (Wang et al., 2021b). Luteolin significantly inhibited GC cells invasion and migration in a dose-dependent manner via the Notch pathway (Zang et al., 2017a). β-carotene, the carotenoid in fruits and vegetables, suppressed tobacco smoke-triggered cell migration and invasion in mice gastric tissues (Lu et al., 2018). Quercetin inhibited GC cells invasion and migration via the interruption of uPA/uPAR function (Li and Chen, 2018). Study of Hamid and Lim et al. (2019) found that Elagic acid inhibits the invasion and migration of GC cells in vivo and in vitro (Cheshomi et al., 2022). Sulforaphane is a phytochemical found in many cruciferous vegetables. Studies have showed that sulforaphane inhibits cell invasion and migration in human GC cells (Mondal et al., 2016; Dong et al., 2018; Li et al., 2022). The results of Alejandra et al. demonstrated that leaf extracts of blueberry plants suppress the migration of GC cells in vitro (Ribera-Fonseca et al., 2020).
More and more studies showed that phytochemistry can inhibit cell migration and invasion in the process of gastric carcinogenesis and development. These findings suggested that phytochemistry has a good application prospect in the occurrence, progression, prognosis and recurrence of gastric cancer.
2.3 Regulation of cell apoptosis and autophagy
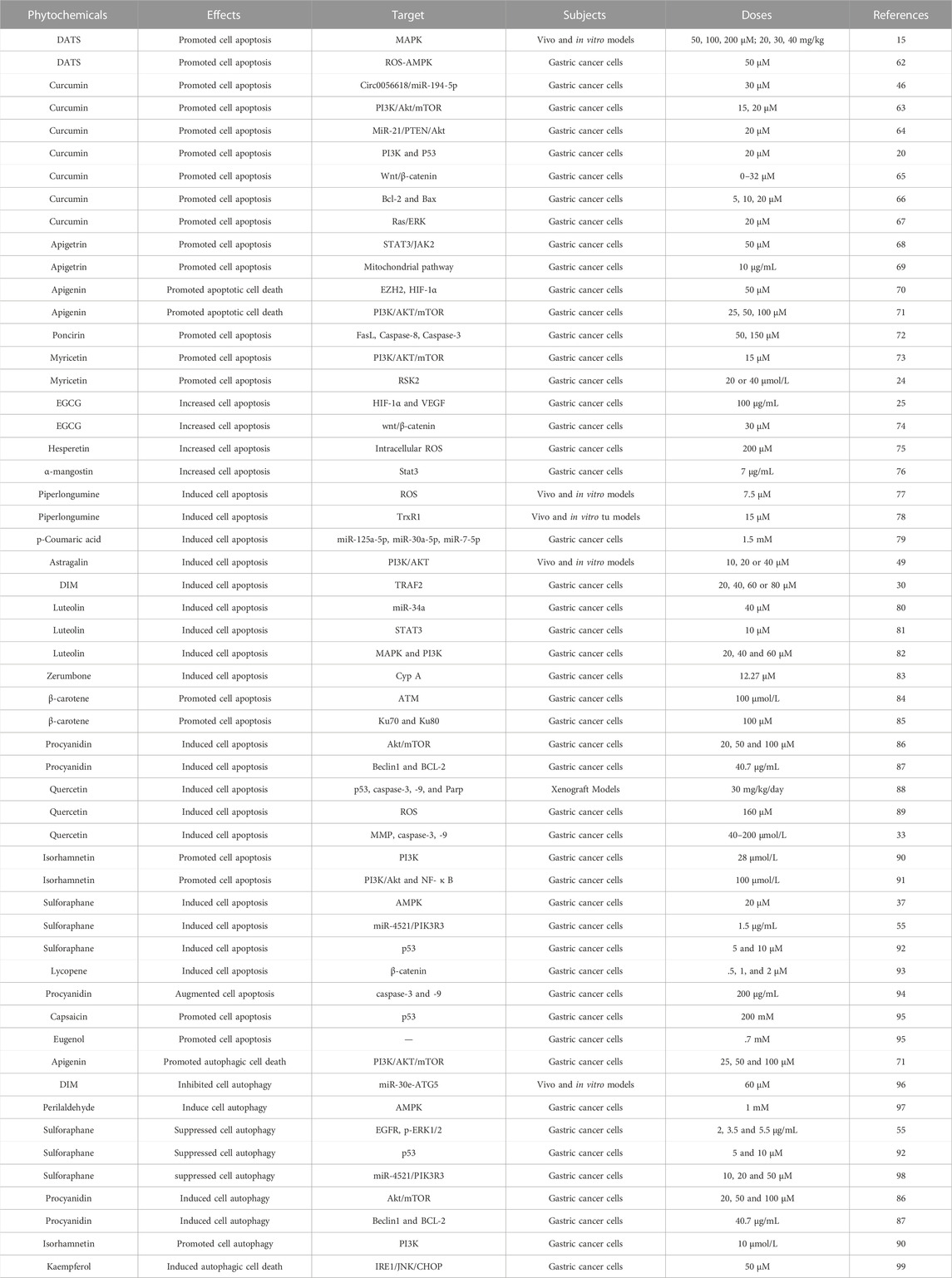
Apoptosis is a highly regulated process of cell death. A series of studies using apoptosis have been proved to be effective in the prevention and treatment of many diseases including cancer (Pistritto et al., 2016; Xu et al., 2019; Berthenet et al., 2020). Cell autophagy is a highly conserved self-defense mechanism (Lu et al., 2022). Autophagy plays a key role in the occurrence, development and prognosis of GC (Cao et al., 2019; Wu et al., 2021; Lu et al., 2022). Induction of cell apoptosis and autophagy has been found maybe a pivotal mechanism of the inhibition of the initiation and the development of gastric cancer. In this section, we focus on the regulatory effects of phytochemicals on apoptosis and autophagy (Table 3; Figure 3).
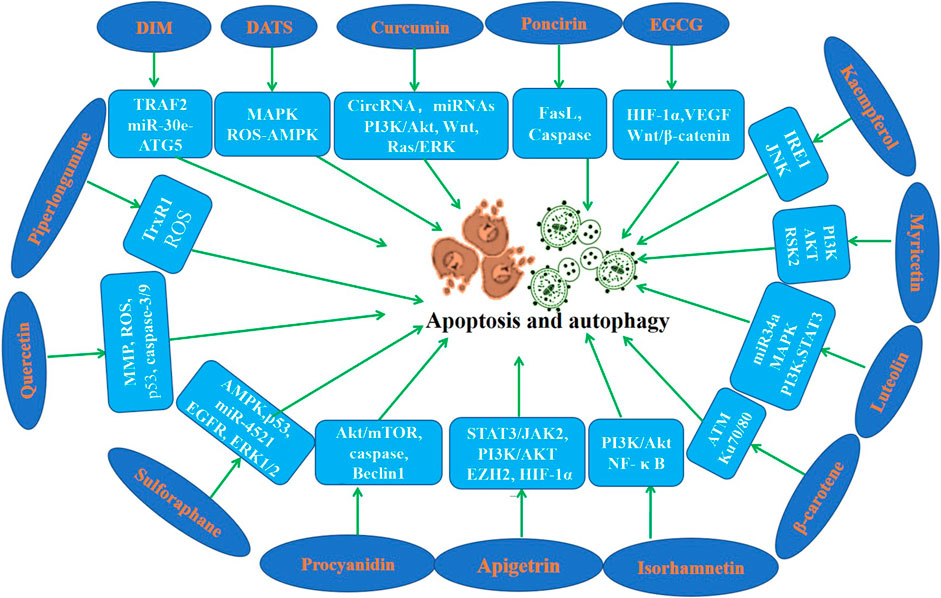
Figure 3. Molecular mechanism of anti-GC effect of representative phytochemicals by regulating apoptosis and autophagy.
DATS has shown its excellent anti GC effect in various studies. DATS promoted cell apoptosis of GC cells in vivo and in vitro (Jiang et al., 2017a; Choi, 2017). Numerous studies have shown that curcumin promotes cell apoptosis of GC cells by regulating circRNA/miRNA/protein in vivo and in vitro (Xue et al., 2014; Cao et al., 2015; Li et al., 2017; Zheng et al., 2017; Fu et al., 2018; Qiang et al., 2019; Li et al., 2021b). However, the bioavailability of curcumin has always been an urgent problem to be solved. We need to find better drug delivery methods, such as nano vesicles or exosomes, which may improve the bioavailability of curcumin. Apigenin enhanced cell apoptosis of GC cells in a time and dose-dependent manner (Chen et al., 2014; Sun et al., 2018). Findings of Seong and Chen et al. indicated that Apigetrin activates apoptotic cell death via HIF-1α, Ezh2 and PI3K/AKT/mTOR in GC cells (Kim et al., 2020b; Kim and Lee, 2021). Poncirin exists in many citrus fruits, and it has been found that it can promote AGS cell apoptosis and play an anti-cancer role (Saralamma et al., 2015). Myricetin is a natural flavonoid found in berries, green tea and nuts, which induces apoptosis of GC cells and exerts anti-GC effects (Feng et al., 2015; Han et al., 2022). Studies demonstrated that EGCG induced GC cells apoptosis in a dose-dependent manner (Yang et al., 2016; Fu et al., 2019). Zhang et al. suggested that hesperidin induces GC cells apoptosis via by increasing the ROS (Zhang et al., 2015). α-Mangosterin, a major xanthone found in the pericarp of mangosteen, can significantly promote apoptosis of GC cells (Shan et al., 2014). Piperlongumine is a natural alkaloid, which induced GC cell apoptosis in vitro and in vivo (Duan et al., 2016; Zou et al., 2016). P-coumaric acid is a phenolic compound abundant in edible plants, which was found to induce apoptosis of GC cells (Jang et al., 2020). It is reported that Astragalin induces apoptosis of GC cells and then exerts its anticancer activity (Wang et al., 2021b). Study have revealed that DIM induced apoptosis of GC cells (Ye et al., 2021a). Luteolin is a natural flavonoid that exists in vegetables, fruits and medicinal herbs, which promotes GC cells apoptosis (Wu et al., 2015; Lu et al., 2017; Song et al., 2017). Zerumbone could induce apoptosis of GC cells through down-regulating CypA (Wang et al., 2016b). Studies found that β-carotene induces apoptosis in AGS cells (Jang et al., 2009; Park et al., 2015). Proanthocyanidins are flavonoids widely found in the skin and seeds of various plants, which have been found to induce apoptosis of GC cells (Nie et al., 2016; Li et al., 2021c). Quercetin is a natural component of natural plants, which induced apoptosis of GC cells in vivo and in vitro (Lee et al., 2016; Xu et al., 2017; Shang et al., 2018). Isorhamnetin induced GC cells apoptosis through PI3K, Akt and NF-κB pathways (Duan et al., 2020; Li et al., 2021d). Sulforaphane significantly enhanced GC cells apoptosis in a dose-dependent manner (Mondal et al., 2016; Choi, 2018; Wang et al., 2021c). Lycopene induced GC cells apoptosis by inhibiting nuclear translocation of β-catenin (Kim et al., 2019a). Anthocyanins isolated from Vitis coignetiae, augmented GC cells apoptosis by activating caspase-3 and caspase-9 (Park et al., 2021). Capsaicin and eugenol induced GC cells apoptosis in the presence or absence of functional p53 (Sarkar et al., 2015). Choi et al. reported that sulforaphane induced GC cells apoptosis by mediating activation of AMPK (Choi, 2018).
According to Seong and colleagues, Apigetrin increased autophagic cell death via HIF-1α, Ezh2 and PI3K/AKT/mTOR in GC cells (Kim et al., 2020b). Ye et al. (2016) reported a novel regulation of GC cells autophagy by DIM in vivo and in vitro models. Perilaldehyde induced autophagy in GC cells and inhibited the growth of gastric cancer (Zhang et al., 2018). Isorhamnetin induced GC cells autophagy via the PI3K pathway (Li et al., 2021d). Sulforaphane also suppressed cell autophagy during the progression of gastric cancer (Mondal et al., 2016; Wang et al., 2021c; Peng and Gu, 2021). Procyanidin exerted anti-cancer activity in GC by regulating autophagy (Nie et al., 2016; Li et al., 2021c). The findings of Tae et al. indicated that kaempferol activates the IRE1/JNK/CHOP signaling to induce autophagic cell death in GC cells (Kim et al., 2018).
Taken together, these findings above illustrated that phytochemistry might be used as a promising candidate against the initiation and progression of GC by mediating cell apoptosis and autophagy.
2.4 Enhancement on chemosensitivity in GC
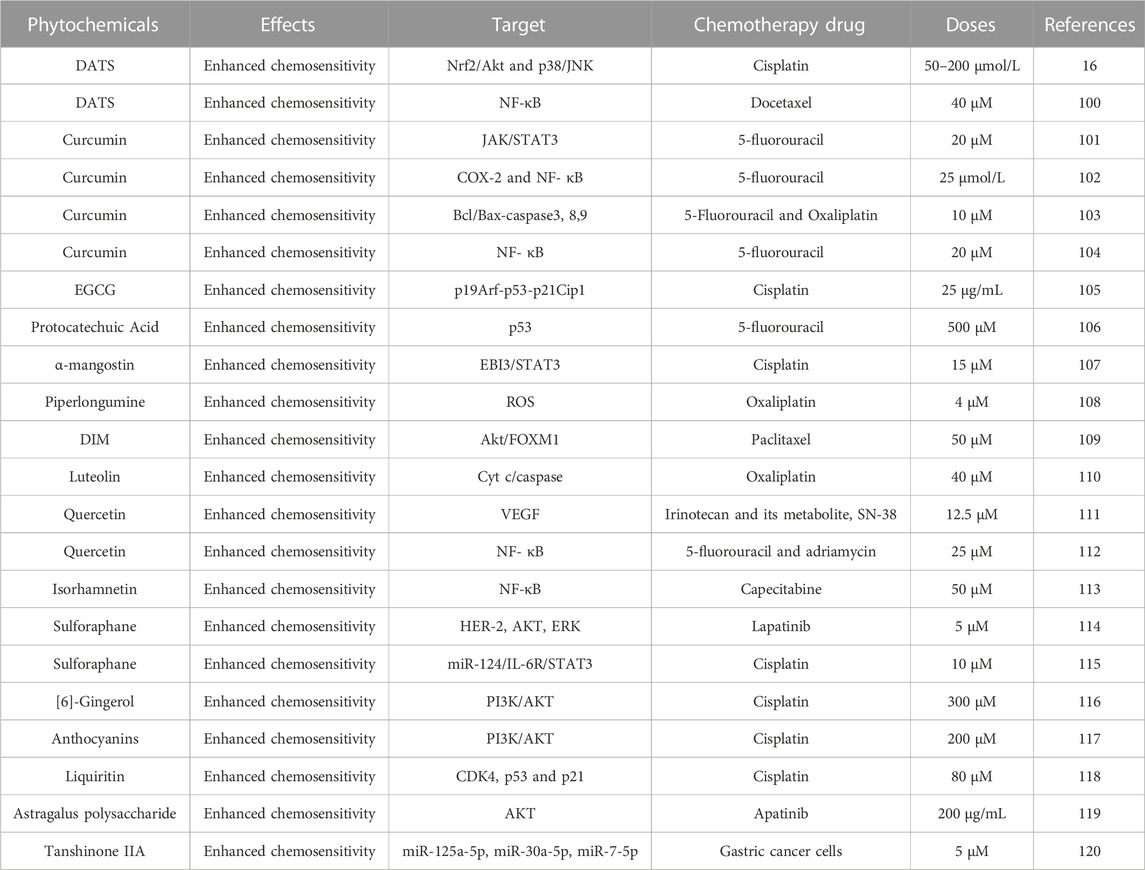
Although great progress has been made in the study of the mechanism of occurrence and development of GC in recent years, surgery with or without chemotherapy is still the appropriate treatment strategy for gastric cancer. However, resistance has become a major problem in the treatment of gastric cancer. In this chapter, we mainly discuss the role of phytochemistry in enhancing the sensitivity of cells to chemotherapy drugs (Table 4).
Studies provided evidences that DATS enhances the sensitivity of GC cells to cisplatin and docetaxel, meanwhile DATS exerts excellent anticancer effects (Pan et al., 2016; Jiang et al., 2017b). Curcumin has shown excellent anticancer effects in a variety of tumors. Studies have found that curcumin enhances the sensitivity of GC cells to first-line chemotherapy drugs such as 5-fluorouracil and oxaliplatin in vitro and in vivo (Kang et al., 2016; Zhou et al., 2016; Yang et al., 2017; Ham et al., 2022). EGCG enhanced the effect of cisplatin on inhibiting GC cells proliferation and inducing cell apoptosis (Xue et al., 2021). Zhang et al. (2019) indicated that protocatechuic acid reduces the dosage of 5-fluorouracil and enhances the chemosensitivity of GC cells to 5-fluorouracil (Motamedi et al., 2020). It is reported that α-mangostin increases the chemosensitivity of GC cells to cisplatin by inactivating the EBI3/STAT3 pathway (Li and Zeng, 2021). These data of Zhang et al. (2019) demonstrated that piperlongumine potentiates the effect of chemotherapy of oxaliplatin in GC cells. The findings of Jin and Park et al. suggested that DIM improves the efficacy of paclitaxel through the Akt/FOXM1 in gastric cancer (Jin et al., 2015). It is elucidated that luteolin potentiated the sensitivity of GC cells to Oxaliplatin through Cytc/caspase (Ren et al., 2020). Studies investigated that quercetin enhances the therapeutic effect of irinotecan/SN-38, 5-fluorouracil and Adriamycin in gastric cancer (Hyun et al., 2018; Lei et al., 2018). Kanjoormana et al. demonstrated that isorhamnetin enhances the anti-GC effects of capecitabine through the NF-κB pathway (Manu et al., 2015). It is reported that sulforaphane might be a promising therapeutic treatment for lapatinib-resistant and cisplatin-resistant gastric cancer (Wang et al., 2016c; Yi et al., 2021). Cisplatin based chemotherapy is a widely used chemotherapy regimen for gastric cancer, [6]-gingerol enhances the sensitivity of GC cells to cisplatin (Luo et al., 2019). Results suggested that anthocyanins enhance anti-GC effects of Cisplatin via inhibiting Akt activity (Lu et al., 2015). Liquiritin circumvented the resistance of cisplatin in cisplatin-resistant GC cells (Wei et al., 2017). Astragalus polysaccharide was reported to enhances the antitumor effects of Apatinib in GC cells (Wu et al., 2018). It is found that tanshinone IIA enhanced the anticancer effect of doxorubicin on drug-resistant GC cells (Xu et al., 2018). Some phytochemicals may exhibit excellent anti-cancer activity in cell and animal research, but their clinical application will be limited because the plants from which these phytochemicals come are uncommon or our body cannot take them regularly.
2.5 Suppression of GC stem cells properties
GC stem cells are a kind of cells with self-renewing and multi-directional differentiation ability. GC stem cells play an critical role in the occurrence, development, heterogeneity, drug resistance, metastasis and recurrence of GC (121, 122). In this chapter, we aim to explore whether phytochemicals can modulate the stemness of GC stem cells to induce a tumorigenic effect.
Ge et al. (2019) found that sulforaphane suppresses the stemness of GC stem cells by inhibiting the Hedgehog pathway. It is reported that Apatinib suppresses GC stem cells properties via inhibiting the Hedgehog pathway (Cao et al., 2021). Low levels of DIM promoted GC progression by activating the Wnt4 pathway to enhance GC cell stemness (Zhu et al., 2016). Sulforaphane regulated GC stem cell properties through the miR-124/IL-6R/STAT3 axis (Wang et al., 2016c). The results of Shen et al. (2016) demonstrated that quercetin inhibits the growth of GC stem cells by inhibiting PI3K/Akt signaling. Constantly exploring phytochemistry that can inhibit stem cell stemness may be a new strategy for prevention and treatment of GC patients with drug resistance, radiotherapy insensitivity and poor prognosis.
2.6 Inhibition of angiogenesis and lymphangiogenesis
Accumulating evidence showed that angiogenesis and lymphangiogenesis play an important role in the occurrence, progression and metastasis of gastric cancer (Da et al., 2015; Zang et al., 2017b; Huang et al., 2017; Da et al., 2019). Studies have found that phytochemicals can prevent and treat GC by inhibiting angiogenesis and lymphatic lineation (Da et al., 2015; Zang et al., 2017b; Huang et al., 2017; Da et al., 2019). Herein, we summarized phytochemicals that inhibit angiogenesis, lymphangiogenesis and analyzed the molecular mechanisms.
It is reported that curcumin inhibits gastric cancer-derived MSC mediate angiogenesis through regulating the NF-κB/VEGF pathway (Huang et al., 2017). Luteolin suppressed angiogenesis by inhibiting the Notch1/VEGF pathway in gastric cancer (Zang et al., 2017b). Tsuboi et al. (2014) found that zerumbone suppresses tumor angiogenesis in gastric cancer. Nitinodine chloride, a natural phytochemical alkaloid, could significantly inhibit angiogenesis of GC in vivo and in vitro (Chen et al., 2012). Curcumin suppressed the lymphangiogenesis of GC cells in vivo and in vitro (Da et al., 2015; Da et al., 2019).
2.7 Modulation of microenvironment and microbiota
In recent years, the relationship between the gut microenvironment and GC has attracted more and more attention (Mao et al., 2020). It was reported that phytochemicals could manage cancers through the modulation of the microenvironment (Mao et al., 2020; Xu et al., 2020). Kim et al. found that β-carotene and lutein inhibit the inflammatory environment around GC cells and oxidative stress, thus preventing the progression of gastric cancer (Kim et al., 2011). Atnip et al. (2020) indicated that anthocyanins suppress the inflammatory environment around GC cells. Gut microbiota also plays an important role in the occurrence, development and prognosis of gastric cancer (Nagano et al., 2019; Qi et al., 2019). Lofgren et al. (2011) reported in 2011 that microbiota may be related to gastric cancer, because mice without specific pathogens are more prone to atrophic gastritis and GC than mice without bacteria. However, there are few reports on the anti-GC effect of phytochemicals through regulating gut microbiota, which may require further elucidation and research.
2.8 Phytochemicals in screening phytochemistry targeting Helicobacter pylori
Accumulating research has proved that Helicobacter pylori infection causes some diseases in stomach and gastric cancer are closely related with it (Kuo et al., 2014; Santos et al., 2015; Ray et al., 2021). Various phytochemicals have shown anti Helicobacter pylori infection efficacy and can be used to prevent the occurrence and development of gastric cancer (Sekiguchi et al., 2008; Haghi et al., 2017).
Santos et al. (2015) and Ray et al. (2021) reported curcumin has a significant intervention effect on the occurrence of GC induced by Helicobacter pylori infection (Haghi et al., 2017). Apigenin has a remarkable ability to inhibit Helicobacter pylori-induced atrophic gastritis and GC progression Apigenin could significantly inhibit the progression of atrophic gastritis and GC induced by Helicobacter pylori (Kuo et al., 2014). The research results of Iwona et al. showed that luteolin can be used for the treatment and prevention of GC infected by Helicobacter pylori (Radziejewska et al., 2021). Studies found that consumption of β-carotene-rich foods may be beneficial to prevent gastric disease induced by helicobacter pylori infection (Kang and Kim, 2017). Similarly, many studies have found that β-carotene has a good application prospect in preventing GC induced by Helicobacter pylori infection (Park et al., 2019a; Kim et al., 2019b; Bae et al., 2021). Quercetin has a protective effect on gastric diseases related to Helicobacter pylori infection (Haghi et al., 2017; Zhang et al., 2017). Lycopene and DATS also have the ability to resist Helicobacter pylori infection (Haghi et al., 2017; Park et al., 2019b).
2.9 Other possible mechanisms
In addition to the above-mentioned modes of action, some phytochemistry also plays a preventive or therapeutic role in the occurrence and development of GC through other modes or mechanisms. DTAS exerted an anticancer effect in GC by regulating the antioxidant enzyme sulfiredoxin (Wang et al., 2019). DATS interfered with the occurrence and development of GC by regulating the activities of quinone oxidoreductase1, FRalpha and calcyclin genes (Li et al., 2002; Kim et al., 2014). Curcumin suppressed GC by inducing DNA demethylation and inhibiting gastrin-mediated acid secretion (Zhou et al., 2017; Tong et al., 2020). Scutellarin suppressed GC by altering lactate dehydrogenase profile, DNA density, mucus content and acidity (Sun and Meng, 2022). Kaempferol, p-Coumaric acid, Astragalin and Tiliroside influence abnormal glycosylation of GC cells, so as to exert the anticancer effect (Radziejewska et al., 2022). DIM suppressed GC via mediated ferroptosis, store-operated calcium entry, gastric cancer-derived mesenchymal stem cells, endogenous hydrogen sulfide biosynthesis (Ye et al., 2020; Ye et al., 2021b; Shi et al., 2021; Ye et al., 2022). It is reported that phytochemicals showed anticancer properties against GC associated with tumor viral infections (Liskova et al., 2021; Sudomova et al., 2021).
3 Summary and the challenges
Phytochemicals, are bioactive compounds that are found in plants such as vegetables, fruits, Chinese herbal medicines, etc. They have elucidated the anticancer activity against GC by adjusting several mechanisms such as inhibitory actions on cell proliferation, migration and invasion, regulating apoptosis and autophagy, enhancing chemosensitivity and blocking infection of Helicobacter pylori. Among them, we found that some phytochemicals have excellent anti GC activity, which can play an intervention effect in multiple processes of gastric cancer, such as proliferation, apoptosis, autophagy, invasion, cancer stem cells properties regulation, helicobacter pylori infection, etc. These excellent phytochemicals include curcumin, sulforaphane, EGCG, DATS, DIM, β-carotene, quercetin, isorhamnetin, luteolin, which are worthy of our in-depth research and development to provide strategies for early prevention and treatment of gastric cancer.
There is not much of phytochemistry really used in clinic and most of phytochemicals that are used in clinic are in an auxiliary role. How to better enhance the function of phytochemicals in GC prevention and treatment is particularly prominent. On one hand, we should devote ourselves to developing effective and safe natural phytochemicals to against gastric cancer. On the other hand, we need to find a more efficient and safer delivery system for phytochemistry in vivo.
In future work, we might deliver phytochemicals through an exosome pathway to improve the bioavailability and targeting of phytochemistry. Or, we might extract phytochemical exosome to effect on GC cells to observe whether they can enhance the anticancer effect and bioavailability. To explore whether phytochemicals can interfere with the development of GC by changing the active components carried by exosomes of GC cells.
Maybe we should pay attention to several aspects in future research. Deliver phytochemicals through an exosome pathway to enhance the bioavailability and targeting of phytochemistry. Extract the exosomes of phytochemicals act on GC cells and observe whether they can enhance the anticancer effect and bioavailability. To explore whether phytochemicals can interfere with the development of GC by changing the active components carried by exosomes of GC cells.
Author contributions
ZL and HQ designed research and wrote the paper. JS, XZ and YZ analyzed data. JJ and YX contributed to the writing and revisions.
Funding
This work was supported by National Natural Science Foundation of China (no. 81602883), project of social development in Zhenjiang (No. SH2021045), Technology Development Project of Jiangsu University (20220516), the Foundation for excellent young teachers of Jiangsu University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Ishaq, R. K., Overy, A. J., and Busselberg, D. (2020). Phytochemicals and gastrointestinal cancer: Cellular mechanisms and effects to change cancer progression. Biomolecules 10 (1), 105. doi:10.3390/biom10010105
Atnip, A., Giusti, M. M., Sigurdson, G. T., Failla, M. L., Chitchumroonchokchai, C., and Bomser, J. A. (2020). The NCI-N87 cell line as a gastric epithelial model to study cellular uptake, trans-epithelial transport, and gastric anti-inflammatory properties of anthocyanins. Nutr. Cancer 72 (4), 686–695. doi:10.1080/01635581.2019.1644354
Bae, S., Lim, J. W., and Kim, H. (2021). β-Carotene inhibits expression of matrix metalloproteinase-10 and invasion in Helicobacter pylori-infected gastric epithelial cells. Molecules 26 (6), 1567. doi:10.3390/molecules26061567
Bastos, J., Lunet, N., Peleteiro, B., Lopes, C., and Barros, H. (2010). Dietary patterns and gastric cancer in a Portuguese urban population. Int. J. Cancer 127 (2), 433–441. doi:10.1002/ijc.25013
Berthenet, K., Castillo Ferrer, C., Fanfone, D., Popgeorgiev, N., Neves, D., Bertolino, P., et al. (2020). Failed apoptosis enhances melanoma cancer cell aggressiveness. Cell Rep. 31 (10), 107731. doi:10.1016/j.celrep.2020.107731
Cao, A. L., Tang, Q. F., Zhou, W. C., Qiu, Y. Y., Hu, S. J., and Yin, P. H. (2015). Ras/ERK signaling pathway is involved in curcumin-induced cell cycle arrest and apoptosis in human gastric carcinoma AGS cells. J. Asian Nat. Prod. Res. 17 (1), 56–63. doi:10.1080/10286020.2014.951923
Cao, W., Li, Y., Sun, H., Yang, C., Zhu, J., Xie, C., et al. (2021). Apatinib suppresses gastric cancer stem cells properties by inhibiting the sonic hedgehog pathway. Front. Cell Dev. Biol. 9, 679806. doi:10.3389/fcell.2021.679806
Cao, Y., Luo, Y., Zou, J., Ouyang, J., Cai, Z., Zeng, X., et al. (2019). Autophagy and its role in gastric cancer. Clin. Chim. Acta 489, 10–20. doi:10.1016/j.cca.2018.11.028
Chen, J., Chen, J., Li, Z., Liu, C., and Yin, L. (2014). The apoptotic effect of apigenin on human gastric carcinoma cells through mitochondrial signal pathway. Tumour Biol. 35 (8), 7719–7726. doi:10.1007/s13277-014-2014-x
Chen, J., Wang, J., Lin, L., He, L., Wu, Y., Zhang, L., et al. (2012). Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol. Cancer Ther. 11 (2), 277–287. doi:10.1158/1535-7163.MCT-11-0648
Cheshomi, H., Bahrami, A. R., Rafatpanah, H., and Matin, M. M. (2022). The effects of ellagic acid and other pomegranate (Punica granatum L.) derivatives on human gastric cancer AGS cells. Hum. Exp. Toxicol. 41, 9603271211064534. doi:10.1177/09603271211064534
Choi, Y. H. (2017). Diallyl trisulfide induces apoptosis and mitotic arrest in AGS human gastric carcinoma cells through reactive oxygen species-mediated activation of AMP-activated protein kinase. Biomed. Pharmacother. 94, 63–71. doi:10.1016/j.biopha.2017.07.055
Choi, Y. H. (2018). ROS-mediated activation of AMPK plays a critical role in sulforaphane-induced apoptosis and mitotic arrest in AGS human gastric cancer cells. Gen. Physiol. Biophys. 37 (2), 129–140. doi:10.4149/gpb_2017026
Da, W., Zhang, J., Zhang, R., and Zhu, J. (2019). Curcumin inhibits the lymphangiogenesis of gastric cancer cells by inhibiton of HMGB1/VEGF-D signaling. Int. J. Immunopathol. Pharmacol. 33, 2058738419861600. doi:10.1177/2058738419861600
Da, W., Zhu, J., Wang, L., and Sun, Q. (2015). Curcumin suppresses lymphatic vessel density in an in vivo human gastric cancer model. Tumour Biol. 36 (7), 5215–5223. doi:10.1007/s13277-015-3178-8
Dong, Q. Q., Wang, Q. T., Wang, L., Jiang, Y. X., Liu, M. L., Hu, H. J., et al. (2018). SMYD3-associated pathway is involved in the anti-tumor effects of sulforaphane on gastric carcinoma cells. Food Sci. Biotechnol. 27 (4), 1165–1173. doi:10.1007/s10068-018-0337-x
Duan, C., Zhang, B., Deng, C., Cao, Y., Zhou, F., Wu, L., et al. (2016). Piperlongumine induces gastric cancer cell apoptosis and G2/M cell cycle arrest both in vitro and in vivo. Tumour Biol. 37 (8), 10793–10804. doi:10.1007/s13277-016-4792-9
Duan, R., Liang, X., Chai, B., Zhou, Y., Du, H., Suo, Y., et al. (2020). Isorhamnetin induces melanoma cell apoptosis via the PI3K/Akt and NF-κB pathways. Biomed. Res. Int. 2020, 1057943. doi:10.1155/2020/1057943
Feng, J., Chen, X., Wang, Y., Du, Y., Sun, Q., Zang, W., et al. (2015). Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol. Cell Biochem. 408 (1-2), 163–170. doi:10.1007/s11010-015-2492-1
Fu, H., Wang, C., Yang, D., Wei, Z., Xu, J., Hu, Z., et al. (2018). Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J. Cell Physiol. 233 (6), 4634–4642. doi:10.1002/jcp.26190
Fu, J. D., Yao, J. J., Wang, H., Cui, W. G., Leng, J., Ding, L. Y., et al. (2019). Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state. Eur. Rev. Med. Pharmacol. Sci. 23 (1), 155–161. doi:10.26355/eurrev_201901_16759
Ge, M., Zhang, L., Cao, L., Xie, C., Li, X., Li, Y., et al. (2019). Sulforaphane inhibits gastric cancer stem cells via suppressing sonic hedgehog pathway. Int. J. Food Sci. Nutr. 70 (5), 570–578. doi:10.1080/09637486.2018.1545012
Gu, X., Zhang, Q., Zhang, W., and Zhu, L. (2019). Curcumin inhibits liver metastasis of gastric cancer through reducing circulating tumor cells. Aging (Albany NY) 11 (5), 1501–1509. doi:10.18632/aging.101848
Guo, Q., Xu, J., Huang, Z., Yao, Q., Chen, F., Liu, H., et al. (2021). ADMA mediates gastric cancer cell migration and invasion via Wnt/β-catenin signaling pathway. Clin. Transl. Oncol. 23 (2), 325–334. doi:10.1007/s12094-020-02422-7
Ha, M. W., Ma, R., Shun, L. P., Gong, Y. H., and Yuan, Y. (2005). Effects of allitridi on cell cycle arrest of human gastric cancer cells. World J. Gastroenterol. 11 (35), 5433–5437. doi:10.3748/wjg.v11.i35.5433
Haghi, A., Azimi, H., and Rahimi, R. (2017). A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. J. Gastrointest. Cancer 48 (4), 314–320. doi:10.1007/s12029-017-9997-7
Ham, I. H., Wang, L., Lee, D., Woo, J., Kim, T. H., Jeong, H. Y., et al. (2022). Curcumin inhibits the cancerassociated fibroblastderived chemoresistance of gastric cancer through the suppression of the JAK/STAT3 signaling pathway. Int. J. Oncol. 61 (1), 85. doi:10.3892/ijo.2022.5375
Han, S. H., Lee, J. H., Woo, J. S., Jung, G. H., Jung, S. H., Han, E. J., et al. (2022). Myricetin induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Heliyon 8 (5), e09309. doi:10.1016/j.heliyon.2022.e09309
Han, S., Wang, Z., Liu, J., Wang, H. D., and Yuan, Q. (2021). miR-29a-3p-dependent COL3A1 and COL5A1 expression reduction assists sulforaphane to inhibit gastric cancer progression. Biochem. Pharmacol. 188, 114539. doi:10.1016/j.bcp.2021.114539
Hassanalilou, T., Ghavamzadeh, S., and Khalili, L. (2019). Curcumin and gastric cancer: A review on mechanisms of action. J. Gastrointest. Cancer 50 (2), 185–192. doi:10.1007/s12029-018-00186-6
Huang, F., Yao, Y., Wu, J., Liu, Q., Zhang, J., Pu, X., et al. (2017). Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-κB/VEGF signaling. Am. J. Transl. Res. 9 (12), 5538–5547.
Hyun, H. B., Moon, J. Y., and Cho, S. K. (2018). Quercetin suppresses CYR61-mediated multidrug resistance in human gastric adenocarcinoma AGS cells. Molecules 23 (2), 209. doi:10.3390/molecules23020209
Jang, M. G., Ko, H. C., and Kim, S. J. (2020). Effects of p-coumaric acid on microRNA expression profiles in SNU-16 human gastric cancer cells. Genes Genomics 42 (7), 817–825. doi:10.1007/s13258-020-00944-6
Jang, S. H., Lim, J. W., and Kim, H. (2009). Mechanism of beta-carotene-induced apoptosis of gastric cancer cells: Involvement of ataxia-telangiectasia-mutated. Ann. N. Y. Acad. Sci. 1171, 156–162. doi:10.1111/j.1749-6632.2009.04711.x
Jiang, X. Y., Zhu, X. S., Xu, H. Y., Zhao, Z. X., Li, S. Y., Li, S. Z., et al. (2017). Diallyl trisulfide suppresses tumor growth through the attenuation of Nrf2/Akt and activation of p38/JNK and potentiates cisplatin efficacy in gastric cancer treatment. Acta Pharmacol. Sin. 38 (7), 1048–1058. doi:10.1038/aps.2016.176
Jiang, X., Zhu, X., Huang, W., Xu, H., Zhao, Z., Li, S., et al. (2017). Garlic-derived organosulfur compound exerts antitumor efficacy via activation of MAPK pathway and modulation of cytokines in SGC-7901 tumor-bearing mice. Int. Immunopharmacol. 48, 135–145. doi:10.1016/j.intimp.2017.05.004
Jin, H., Park, M. H., and Kim, S. M. (2015). 3, 3'-Diindolylmethane potentiates paclitaxel-induced antitumor effects on gastric cancer cells through the Akt/FOXM1 signaling cascade. Oncol. Rep. 33 (4), 2031–2036. doi:10.3892/or.2015.3758
Kang, H., and Kim, H. (2017). Astaxanthin and beta-carotene in Helicobacter pylori-induced gastric inflammation: A mini-review on action mechanisms. J. Cancer Prev. 22 (2), 57–61. doi:10.15430/JCP.2017.22.2.57
Kang, Y., Hu, W., Bai, E., Zheng, H., Liu, Z., Wu, J., et al. (2016). Curcumin sensitizes human gastric cancer cells to 5-fluorouracil through inhibition of the NFκB survival-signaling pathway. Onco Targets Ther. 9, 7373–7384. doi:10.2147/OTT.S118272
Khan, T., Ali, M., Khan, A., Nisar, P., Jan, S. A., Afridi, S., et al. (2019). Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 10 (1), 47. doi:10.3390/biom10010047
Kiani, S., Akhavan-Niaki, H., Fattahi, S., Kavoosian, S., Babaian Jelodar, N., Bagheri, N., et al. (2018). Purified sulforaphane from broccoli (Brassica oleracea var. italica) leads to alterations of CDX1 and CDX2 expression and changes in miR-9 and miR-326 levels in human gastric cancer cells. Gene 678, 115–123. doi:10.1016/j.gene.2018.08.026
Kim, D., Lim, J. W., and Kim, H. (2019). β-Carotene inhibits expression of c-myc and cyclin E in Helicobacter pylori-infected gastric epithelial cells. J. Cancer Prev. 24 (3), 192–196. doi:10.15430/JCP.2019.24.3.192
Kim, M., Kim, S. H., Lim, J. W., and Kim, H. (2019). Lycopene induces apoptosis by inhibiting nuclear translocation of beta-catenin in gastric cancer cells. J. Physiol. Pharmacol. 70 (4). doi:10.26402/jpp.2019.4.11
Kim, S., Kim, W., Kim, D. H., Jang, J. H., Kim, S. J., Park, S. A., et al. (2020). Resveratrol suppresses gastric cancer cell proliferation and survival through inhibition of PIM-1 kinase activity. Arch. Biochem. Biophys. 689, 108413. doi:10.1016/j.abb.2020.108413
Kim, S., Lee, H. G., Park, S. A., Kundu, J. K., Keum, Y. S., Cha, Y. N., et al. (2014). Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One 9 (1), e85984. doi:10.1371/journal.pone.0085984
Kim, S. M., Vetrivel, P., Ha, S. E., Kim, H. H., Kim, J. A., and Kim, G. S. (2020). Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell. J. Nutr. Biochem. 83, 108427. doi:10.1016/j.jnutbio.2020.108427
Kim, T. W., and Lee, H. G. (2021). Apigenin induces autophagy and cell death by targeting EZH2 under hypoxia conditions in gastric cancer cells. Int. J. Mol. Sci. 22 (24), 13455. doi:10.3390/ijms222413455
Kim, T. W., Lee, S. Y., Kim, M., Cheon, C., and Ko, S. G. (2018). Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 9 (9), 875. doi:10.1038/s41419-018-0930-1
Kim, Y., Seo, J. H., and Kim, H. (2011). β-Carotene and lutein inhibit hydrogen peroxide-induced activation of NF-κB and IL-8 expression in gastric epithelial AGS cells. J. Nutr. Sci. Vitaminol. (Tokyo) 57 (3), 216–223. doi:10.3177/jnsv.57.216
Kuo, C. H., Weng, B. C., Wu, C. C., Yang, S. F., Wu, D. C., and Wang, Y. C. (2014). Apigenin has anti-atrophic gastritis and anti-gastric cancer progression effects in Helicobacter pylori-infected Mongolian gerbils. J. Ethnopharmacol. 151 (3), 1031–1039. doi:10.1016/j.jep.2013.11.040
Lan, H., and Lu, Y. Y. (2004). Allitridi induces apoptosis by affecting Bcl-2 expression and caspase-3 activity in human gastric cancer cells. Acta Pharmacol. Sin. 25 (2), 219–225.
Lee, H. H., Lee, S., Shin, Y. S., Cho, M., Kang, H., and Cho, H. (2016). Anti-cancer effect of quercetin in xenograft models with EBV-associated human gastric carcinoma. Molecules 21 (10), 1286. doi:10.3390/molecules21101286
Lei, C. S., Hou, Y. C., Pai, M. H., Lin, M. T., and Yeh, S. L. (2018). Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: In vitro and in vivo studies. J. Nutr. Biochem. 51, 105–113. doi:10.1016/j.jnutbio.2017.09.011
Li, C., Li, J., Li, Y., Li, L., Luo, Y., Li, J., et al. (2021). Isorhamnetin promotes MKN-45 gastric cancer cell apoptosis by inhibiting PI3K-mediated adaptive autophagy in a hypoxic environment. J. Agric. Food Chem. 69 (29), 8130–8143. doi:10.1021/acs.jafc.1c02620
Li, F., Wang, S., and Niu, M. (2021). Scutellarin inhibits the growth and EMT of gastric cancer cells through regulating PTEN/PI3K pathway. Biol. Pharm. Bull. 44 (6), 780–788. doi:10.1248/bpb.b20-00822
Li, H., and Chen, C. (2018). Quercetin has antimetastatic effects on gastric cancer cells via the interruption of uPA/uPAR function by modulating NF-κb, PKC-δ, ERK1/2, and AMPKα. Integr. Cancer Ther. 17 (2), 511–523. doi:10.1177/1534735417696702
Li, R. R., and Zeng, D. Y. (2021). The effects and mechanism of alpha-mangostin on chemosensitivity of gastric cancer cells. Kaohsiung J. Med. Sci. 37 (8), 709–717. doi:10.1002/kjm2.12388
Li, S., Cong, X., Gao, H., Lan, X., Li, Z., Wang, W., et al. (2019). Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J. Exp. Clin. Cancer Res. 38 (1), 6. doi:10.1186/s13046-018-1003-0
Li, S., Khoi, P. N., Yin, H., Sah, D. K., Kim, N. H., Lian, S., et al. (2022). Sulforaphane suppresses the nicotine-induced expression of the matrix metalloproteinase-9 via inhibiting ROS-mediated AP-1 and NF-κB signaling in human gastric cancer cells. Int. J. Mol. Sci. 23 (9), 5172. doi:10.3390/ijms23095172
Li, S., Zhang, L., Li, S., Zhao, H., and Chen, Y. (2021). Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis. Open Life Sci. 16 (1), 937–949. doi:10.1515/biol-2021-0092
Li, W., Zhou, Y., Yang, J., Li, H., Zhang, H., and Zheng, P. (2017). Curcumin induces apoptotic cell death and protective autophagy in human gastric cancer cells. Oncol. Rep. 37 (6), 3459–3466. doi:10.3892/or.2017.5637
Li, X. J., Park, E. S., Park, M. H., and Kim, S. M. (2013). 3, 3'-Diindolylmethane suppresses the growth of gastric cancer cells via activation of the Hippo signaling pathway. Oncol. Rep. 30 (5), 2419–2426. doi:10.3892/or.2013.2717
Li, Y., Lu, X., Tian, P., Wang, K., and Shi, J. (2021). Procyanidin B2 induces apoptosis and autophagy in gastric cancer cells by inhibiting Akt/mTOR signaling pathway. BMC Complement. Med. Ther. 21 (1), 76. doi:10.1186/s12906-021-03225-1
Li, Y., Yang, L., Cui, J. T., Li, W. M., Guo, R. F., and Lu, Y. Y. (2002). Construction of cDNA representational difference analysis based on two cDNA libraries and identification of garlic inducible expression genes in human gastric cancer cells. World J. Gastroenterol. 8 (2), 208–212. doi:10.3748/wjg.v8.i2.208
Liang, Z., Wu, R., Xie, W., Geng, H., Zhao, L., Xie, C., et al. (2015). Curcumin suppresses MAPK pathways to reverse tobacco smoke-induced gastric epithelial-mesenchymal transition in mice. Phytother. Res. 29 (10), 1665–1671. doi:10.1002/ptr.5398
Liao, W., Chen, L., Ma, X., Jiao, R., Li, X., and Wang, Y. (2016). Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 114, 24–32. doi:10.1016/j.ejmech.2016.02.045
Lim, S. C., Hwang, H., and Han, S. I. (2019). Ellagic acid inhibits extracellular acidity-induced invasiveness and expression of COX1, COX2, snail, twist 1, and c-myc in gastric carcinoma cells. Nutrients 11 (12), 3023. doi:10.3390/nu11123023
Liskova, A., Samec, M., Koklesova, L., Brockmueller, A., Zhai, K., Abdellatif, B., et al. (2021). Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 12 (2), 155–176. doi:10.1007/s13167-021-00242-5
Liu, W., Huang, M., Zou, Q., and Lin, W. (2018). Curcumin suppresses gastric cancer biological activity by regulation of miRNA-21: An in vitro study. Int. J. Clin. Exp. Pathol. 11 (12), 5820–5829.
Liu, X., Sun, K., Song, A., Zhang, X., Zhang, X., and He, X. (2014). Curcumin inhibits proliferation of gastric cancer cells by impairing ATP-sensitive potassium channel opening. World J. Surg. Oncol. 12, 389. doi:10.1186/1477-7819-12-389
Lofgren, J. L., Whary, M. T., Ge, Z., Muthupalani, S., Taylor, N. S., Mobley, M., et al. (2011). Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 140 (1), 210–220. doi:10.1053/j.gastro.2010.09.048
Lu, J. N., Lee, W. S., Nagappan, A., Chang, S. H., Choi, Y. H., Kim, H. J., et al. (2015). Anthocyanins from the fruit of vitis coignetiae pulliat potentiate the cisplatin activity by inhibiting PI3K/Akt signaling pathways in human gastric cancer cells. J. Cancer Prev. 20 (1), 50–56. doi:10.15430/JCP.2015.20.1.50
Lu, L., Chen, J., Li, M., Tang, L., Wu, R., Jin, L., et al. (2018). β‑carotene reverses tobacco smoke‑induced gastric EMT via Notch pathway in vivo. Oncol. Rep. 39 (4), 1867–1873. doi:10.3892/or.2018.6246
Lu, L., Chen, J., Tang, H., Bai, L., Lu, C., Wang, K., et al. (2016). EGCG suppresses ERK5 activation to reverse tobacco smoke-triggered gastric epithelial-mesenchymal transition in BALB/c mice. Nutrients 8 (7), 380. doi:10.3390/nu8070380
Lu, L., Liang, Q., Zhang, X., Xu, Y., Meng, D., and Liang, Z. (2022). Autophagy related noncoding RNAs: Emerging regulatory factors of gastric cancer. Cancer Manag. Res. 14, 2215–2224. doi:10.2147/CMAR.S364761
Lu, X., Li, Y., Li, X., and Aisa, H. A. (2017). Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer. Oncol. Lett. 14 (2), 1993–2000. doi:10.3892/ol.2017.6380
Luo, Y., Zha, L., Luo, L., Chen, X., Zhang, Q., Gao, C., et al. (2019). [6]-Gingerol enhances the cisplatin sensitivity of gastric cancer cells through inhibition of proliferation and invasion via PI3K/AKT signaling pathway. Phytother. Res. 33 (5), 1353–1362. doi:10.1002/ptr.6325
Manu, K. A., Shanmugam, M. K., Ramachandran, L., Li, F., Siveen, K. S., Chinnathambi, A., et al. (2015). Isorhamnetin augments the anti-tumor effect of capecitabine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 363 (1), 28–36. doi:10.1016/j.canlet.2015.03.033
Mao, Q. Q., Xu, X. Y., Shang, A., Gan, R. Y., Wu, D. T., Atanasov, A. G., et al. (2020). Phytochemicals for the prevention and treatment of gastric cancer: Effects and mechanisms. Int. J. Mol. Sci. 21 (2), 570. doi:10.3390/ijms21020570
Mondal, A., Biswas, R., Rhee, Y. H., Kim, J., and Ahn, J. C. (2016). Sulforaphene promotes Bax/Bcl2, MAPK-dependent human gastric cancer AGS cells apoptosis and inhibits migration via EGFR, p-ERK1/2 down-regulation. Gen. Physiol. Biophys. 35 (1), 25–34. doi:10.4149/gpb_2015033
Motamedi, Z., Amini, S. A., Raeisi, E., Lemoigne, Y., and Heidarian, E. (2020). Combined effects of protocatechuic acid and 5-fluorouracil on p53 gene expression and apoptosis in gastric adenocarcinoma cells. Turk J. Pharm. Sci. 17 (6), 578–585. doi:10.4274/tjps.galenos.2019.69335
Nagano, T., Otoshi, T., Hazama, D., Kiriu, T., Umezawa, K., Katsurada, N., et al. (2019). Novel cancer therapy targeting microbiome. Onco Targets Ther. 12, 3619–3624. doi:10.2147/OTT.S207546
Nagata, C., Takatsuka, N., Kawakami, N., and Shimizu, H. (2002). A prospective cohort study of soy product intake and stomach cancer death. Br. J. Cancer 87 (1), 31–36. doi:10.1038/sj.bjc.6600349
Nie, C., Zhou, J., Qin, X., Shi, X., Zeng, Q., Liu, J., et al. (2016). Reduction of apoptosis by proanthocyanidin-induced autophagy in the human gastric cancer cell line MGC-803. Oncol. Rep. 35 (2), 649–658. doi:10.3892/or.2015.4419
Pan, Y., Lin, S., Xing, R., Zhu, M., Lin, B., Cui, J., et al. (2016). Epigenetic upregulation of metallothionein 2A by diallyl trisulfide enhances chemosensitivity of human gastric cancer cells to docetaxel through attenuating NF-κB activation. Antioxid. Redox Signal 24 (15), 839–854. doi:10.1089/ars.2014.6128
Park, B., Lim, J. W., and Kim, H. (2019). Lycopene treatment inhibits activation of Jak1/Stat3 and Wnt/β-catenin signaling and attenuates hyperproliferation in gastric epithelial cells. Nutr. Res. 70, 70–81. doi:10.1016/j.nutres.2018.07.010
Park, C., Lee, W. S., Go, S. I., Jeong, S. H., Yoo, J., Cha, H. J., et al. (2021). Apoptotic effects of anthocyanins from vitis coignetiae pulliat are enhanced by augmented enhancer of the rudimentary homolog (ERH) in human gastric carcinoma MKN28 cells. Int. J. Mol. Sci. 22 (6), 3030. doi:10.3390/ijms22063030
Park, Y., Choi, J., Lim, J. W., and Kim, H. (2015). β-Carotene-induced apoptosis is mediated with loss of Ku proteins in gastric cancer AGS cells. Genes Nutr. 10 (4), 467. doi:10.1007/s12263-015-0467-1
Park, Y., Lee, H., Lim, J. W., and Kim, H. (2019). Inhibitory effect of beta-carotene on Helicobacter pylori-induced TRAF expression and hyper-proliferation in gastric epithelial cells. Antioxidants (Basel) 8 (12), 637. doi:10.3390/antiox8120637
Peng, Z. T., and Gu, P. (2021). Sulforaphane suppresses autophagy during the malignant progression of gastric carcinoma via activating miR-4521/PIK3R3 pathway. Hum. Exp. Toxicol. 40 (12), S711–S720. doi:10.1177/09603271211054437
Pistritto, G., Trisciuoglio, D., Ceci, C., Garufi, A., and D'Orazi, G. (2016). Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 8 (4), 603–619. doi:10.18632/aging.100934
Qi, Y. F., Sun, J. N., Ren, L. F., Cao, X. L., Dong, J. H., Tao, K., et al. (2019). Intestinal microbiota is altered in patients with gastric cancer from shanxi province, China. Dig. Dis. Sci. 64 (5), 1193–1203. doi:10.1007/s10620-018-5411-y
Qiang, Z., Meng, L., Yi, C., Yu, L., Chen, W., and Sha, W. (2019). Curcumin regulates the miR-21/PTEN/Akt pathway and acts in synergy with PD98059 to induce apoptosis of human gastric cancer MGC-803 cells. J. Int. Med. Res. 47 (3), 1288–1297. doi:10.1177/0300060518822213
Radziejewska, I., Borzym-Kluczyk, M., and Leszczynska, K. (2021). Luteolin alters MUC1 extracellular domain, sT antigen, ADAM-17, IL-8, IL-10 and NF-κB expression in Helicobacter pylori-infected gastric cancer CRL-1739 cells: A preliminary study. Biomed. Rep. 14 (2), 19. doi:10.3892/br.2020.1395
Radziejewska, I., Supruniuk, K., Tomczyk, M., Izdebska, W., Borzym-Kluczyk, M., Bielawska, A., et al. (2022). p-Coumaric acid, kaempferol, Astragalin and Tiliroside influence the expression of glycoforms in AGS gastric cancer cells. Int. J. Mol. Sci. 23 (15), 8602. doi:10.3390/ijms23158602
Ramachandran, L., Manu, K. A., Shanmugam, M. K., Li, F., Siveen, K. S., Vali, S., et al. (2012). Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor gamma activation pathway in gastric cancer. J. Biol. Chem. 287 (45), 38028–38040. doi:10.1074/jbc.M112.388702
Rauf, A., Abu-Izneid, T., Thiruvengadam, M., Imran, M., Olatunde, A., Shariati, M. A., et al. (2022). Garlic (allium sativum L.): Its chemistry, nutritional composition, toxicity, and anticancer properties. Curr. Top. Med. Chem. 22 (11), 957–972. doi:10.2174/1568026621666211105094939
Ray, A. K., Luis, P. B., Mishra, S. K., Barry, D. P., Asim, M., Pandey, A., et al. (2021). Curcumin oxidation is required for inhibition of Helicobacter pylori growth, translocation and phosphorylation of cag A. Front. Cell Infect. Microbiol. 11, 765842. doi:10.3389/fcimb.2021.765842
Ren, L. Q., Li, Q., and Zhang, Y. (2020). Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. Biomed. Res. Int. 2020, 9396512. doi:10.1155/2020/9396512
Ribera-Fonseca, A., Jimenez, D., Leal, P., Riquelme, I., Roa, J. C., Alberdi, M., et al. (2020). The anti-proliferative and anti-invasive effect of leaf extracts of blueberry plants treated with methyl jasmonate on human gastric cancer in vitro is related to their antioxidant properties. Antioxidants (Basel) 9 (1), 45. doi:10.3390/antiox9010045
Santos, A. M., Lopes, T., Oleastro, M., Gato, I. V., Floch, P., Benejat, L., et al. (2015). Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients 7 (1), 306–320. doi:10.3390/nu7010306
Saralamma, V. V., Nagappan, A., Hong, G. E., Lee, H. J., Yumnam, S., Raha, S., et al. (2015). Poncirin induces apoptosis in AGS human gastric cancer cells through extrinsic apoptotic pathway by up-regulation of fas ligand. Int. J. Mol. Sci. 16 (9), 22676–22691. doi:10.3390/ijms160922676
Sarkar, A., Bhattacharjee, S., and Mandal, D. P. (2015). Induction of apoptosis by eugenol and capsaicin in human gastric cancer AGS cells--elucidating the role of p53. Asian Pac J. Cancer Prev. 16 (15), 6753–6759. doi:10.7314/apjcp.2015.16.15.6753
Sarvizadeh, M., Hasanpour, O., Naderi Ghale-Noie, Z., Mollazadeh, S., Rezaei, M., Pourghadamyari, H., et al. (2021). Allicin and digestive system cancers: From chemical structure to its therapeutic opportunities. Front. Oncol. 11, 650256. doi:10.3389/fonc.2021.650256
Sekiguchi, H., Washida, K., and Murakami, A. (2008). Suppressive effects of selected food phytochemicals on CD74 expression in NCI-N87 gastric carcinoma cells. J. Clin. Biochem. Nutr. 43 (2), 109–117. doi:10.3164/jcbn.2008054
Shan, T., Cui, X. J., Li, W., Lin, W. R., Lu, H. W., Li, Y. M., et al. (2014). α-Mangostin suppresses human gastric adenocarcinoma cells in vitro via blockade of Stat3 signaling pathway. Acta Pharmacol. Sin. 35 (8), 1065–1073. doi:10.1038/aps.2014.43
Shang, H. S., Lu, H. F., Lee, C. H., Chiang, H. S., Chu, Y. L., Chen, A., et al. (2018). Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ. Toxicol. 33 (11), 1168–1181. doi:10.1002/tox.22623
Shen, X., Si, Y., Wang, Z., Wang, J., Guo, Y., and Zhang, X. (2016). Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int. J. Mol. Med. 38 (2), 619–626. doi:10.3892/ijmm.2016.2625
Shi, H., Sun, Y., Ruan, H., Ji, C., Zhang, J., Wu, P., et al. (2021). 3, 3′-diindolylmethane promotes gastric cancer progression via β-TrCP-mediated NF-κB activation in gastric cancer-derived MSCs. Front. Oncol. 11, 603533. doi:10.3389/fonc.2021.603533
Song, B., Zhan, H., Bian, Q., and Gu, J. (2016). Piperlongumine inhibits gastric cancer cells via suppression of the JAK1, 2/STAT3 signaling pathway. Mol. Med. Rep. 13 (5), 4475–4480. doi:10.3892/mmr.2016.5091
Song, H., Bao, J., Wei, Y., Chen, Y., Mao, X., Li, J., et al. (2015). Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 33 (2), 868–874. doi:10.3892/or.2014.3662
Song, S., Su, Z., Xu, H., Niu, M., Chen, X., Min, H., et al. (2017). Luteolin selectively kills STAT3 highly activated gastric cancer cells through enhancing the binding of STAT3 to SHP-1. Cell Death Dis. 8 (2), e2612. doi:10.1038/cddis.2017.38
Sudomova, M., Berchova-Bimova, K., Marzocco, S., Liskova, A., Kubatka, P., and Hassan, S. T. S. (2021). Berberine in human oncogenic herpesvirus infections and their linked cancers. Viruses 13 (6), 1014. doi:10.3390/v13061014
Sun, C., Zhang, S., Liu, C., and Liu, X. (2019). Curcumin promoted miR-34a expression and suppressed proliferation of gastric cancer cells. Cancer Biother Radiopharm. 34 (10), 634–641. doi:10.1089/cbr.2019.2874
Sun, J., and Meng, M. (2022). Chemoprotective effect of scutellarin against gastric cancer in rats: An in vitro and in vivo study. J. Oleo Sci. 71 (7), 1003–1012. doi:10.5650/jos.ess21399
Sun, Q., Lu, N. N., and Feng, L. (2018). Apigetrin inhibits gastric cancer progression through inducing apoptosis and regulating ROS-modulated STAT3/JAK2 pathway. Biochem. Biophys. Res. Commun. 498 (1), 164–170. doi:10.1016/j.bbrc.2018.02.009
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tong, R., Wu, X., Liu, Y., Liu, Y., Zhou, J., Jiang, X., et al. (2020). Curcumin-induced DNA demethylation in human gastric cancer cells is mediated by the DNA-damage response pathway. Oxid. Med. Cell Longev. 2020, 2543504. doi:10.1155/2020/2543504
Tsuboi, K., Matsuo, Y., Shamoto, T., Shibata, T., Koide, S., Morimoto, M., et al. (2014). Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol. Rep. 31 (1), 57–64. doi:10.3892/or.2013.2842
Wang, D., Li, Y., Cui, P., Zhao, Q., Tan, B. B., Zhang, Z. D., et al. (2016). Zerumbone induces gastric cancer cells apoptosis: Involving cyclophilin A. Biomed. Pharmacother. 83, 740–745. doi:10.1016/j.biopha.2016.07.034
Wang, F., Zhao, J., Liu, D., Zhao, T., Lu, Z., Zhu, L., et al. (2016). Capsaicin reactivates hMOF in gastric cancer cells and induces cell growth inhibition. Cancer Biol. Ther. 17 (11), 1117–1125. doi:10.1080/15384047.2016.1235654
Wang, J., Si, L., Wang, G., Bai, Z., and Li, W. (2019). Increased sulfiredoxin expression in gastric cancer cells may Be a molecular target of the anticancer component diallyl trisulfide. Biomed. Res. Int. 2019, 4636804. doi:10.1155/2019/4636804
Wang, L., Chen, X., Du, Z., Li, G., Chen, M., Chen, X., et al. (2017). Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase gamma depletion disrupting cellular bioenergetics. J. Exp. Clin. Cancer Res. 36 (1), 47. doi:10.1186/s13046-017-0513-5
Wang, S. W., Sheng, H., Zheng, F., and Zhang, F. (2021). Hesperetin promotes DOT1L degradation and reduces histone H3K79 methylation to inhibit gastric cancer metastasis. Phytomedicine 84, 153499. doi:10.1016/j.phymed.2021.153499
Wang, X., Li, Y., Dai, Y., Liu, Q., Ning, S., Liu, J., et al. (2016). Sulforaphane improves chemotherapy efficacy by targeting cancer stem cell-like properties via the miR-124/IL-6R/STAT3 axis. Sci. Rep. 6, 36796. doi:10.1038/srep36796
Wang, Y., Wu, H., Dong, N., Su, X., Duan, M., Wei, Y., et al. (2021). Sulforaphane induces S-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells. Sci. Rep. 11 (1), 2504. doi:10.1038/s41598-021-81815-2
Wang, Z., Lv, J., Li, X., and Lin, Q. (2021). The flavonoid Astragalin shows anti-tumor activity and inhibits PI3K/AKT signaling in gastric cancer. Chem. Biol. Drug Des. 98 (5), 779–786. doi:10.1111/cbdd.13933
Wei, F., Jiang, X., Gao, H. Y., and Gao, S. H. (2017). Liquiritin induces apoptosis and autophagy in cisplatin (DDP)-resistant gastric cancer cells in vitro and xenograft nude mice in vivo. Int. J. Oncol. 51 (5), 1383–1394. doi:10.3892/ijo.2017.4134
Wu, H., Huang, M., Liu, Y., Shu, Y., and Liu, P. (2015). Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol. Cancer Res. Treat. 14 (6), 747–755. doi:10.7785/tcrt.2012.500434
Wu, J., Yu, J., Wang, J., Zhang, C., Shang, K., Yao, X., et al. (2018). Astragalus polysaccharide enhanced antitumor effects of Apatinib in gastric cancer AGS cells by inhibiting AKT signalling pathway. Biomed. Pharmacother. 100, 176–183. doi:10.1016/j.biopha.2018.01.140
Wu, Q., Ma, J., Wei, J., Meng, W., Wang, Y., and Shi, M. (2021). lncRNA SNHG11 promotes gastric cancer progression by activating the wnt/β-catenin pathway and oncogenic autophagy. Mol. Ther. 29 (3), 1258–1278. doi:10.1016/j.ymthe.2020.10.011
Xu, X., Lai, Y., and Hua, Z. C. (2019). Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 39 (1), BSR20180992. doi:10.1042/BSR20180992
Xu, X. Y., Zhao, C. N., Cao, S. Y., Tang, G. Y., Gan, R. Y., and Li, H. B. (2020). Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 60 (10), 1693–1705. doi:10.1080/10408398.2019.1588223
Xu, Y. X., Wang, B., and Zhao, X. H. (2017). In vitro effects and the related molecular mechanism of galangin and quercetin on human gastric cancer cell line (SGC-7901). Pak J. Pharm. Sci. 30 (4), 1279–1287.
Xu, Z., Chen, L., Xiao, Z., Zhu, Y., Jiang, H., Jin, Y., et al. (2018). Potentiation of the anticancer effect of doxorubicinin drug-resistant gastric cancer cells by tanshinone IIA. Phytomedicine 51, 58–67. doi:10.1016/j.phymed.2018.05.012
Xue, M., Liu, X., Cheng, B., Rui, X., Wu, M., and Lv, J. (2021). Epigallocatechin gallate enhances inhibition effect of DDP on the proliferation of gastric cancer BGC-823 cells by regulating p19Arf-p53-p21Cip1 signaling pathway. Asian Pac J. Cancer Prev. 22 (4), 1263–1270. doi:10.31557/APJCP.2021.22.4.1263
Xue, X., Yu, J. L., Sun, D. Q., Kong, F., Qu, X. J., Zou, W., et al. (2014). Curcumin induces apoptosis in SGC-7901 gastric adenocarcinoma cells via regulation of mitochondrial signaling pathways. Asian Pac J. Cancer Prev. 15 (9), 3987–3992. doi:10.7314/apjcp.2014.15.9.3987
Yang, C., Du, W., and Yang, D. (2016). Inhibition of green tea polyphenol EGCG((-)-epigallocatechin-3-gallate) on the proliferation of gastric cancer cells by suppressing canonical wnt/β-catenin signalling pathway. Int. J. Food Sci. Nutr. 67 (7), 818–827. doi:10.1080/09637486.2016.1198892
Yang, H., Huang, S., Wei, Y., Cao, S., Pi, C., Feng, T., et al. (2017). Curcumin enhances the anticancer effect of 5-fluorouracil against gastric cancer through down-regulation of COX-2 and NF- κB signaling pathways. J. Cancer 8 (18), 3697–3706. doi:10.7150/jca.20196
Yang, M., Jin, M., Li, K., Liu, H., Yang, X., Zhang, X., et al. (2020). TRAF6 promotes gastric cancer cell self-renewal, proliferation, and migration. Stem Cells Int. 2020, 3296192. doi:10.1155/2020/3296192
Ye, F., Li, X., Sun, K., Xu, W., Shi, H., Bian, J., et al. (2020). Inhibition of endogenous hydrogen sulfide biosynthesis enhances the anti-cancer effect of 3, 3'-diindolylmethane in human gastric cancer cells. Life Sci. 261, 118348. doi:10.1016/j.lfs.2020.118348
Ye, Y., Fang, Y., Xu, W., Wang, Q., Zhou, J., and Lu, R. (2016). 3, 3'-Diindolylmethane induces anti-human gastric cancer cells by the miR-30e-ATG5 modulating autophagy. Biochem. Pharmacol. 115, 77–84. doi:10.1016/j.bcp.2016.06.018
Ye, Y., Li, X., Feng, G., Ma, Y., Ye, F., Shen, H., et al. (2022). 3, 3'-Diindolylmethane induces ferroptosis by BAP1-IP3R axis in BGC-823 gastric cancer cells. Anticancer Drugs 33 (4), 362–370. doi:10.1097/CAD.0000000000001270
Ye, Y., Li, X., Wang, Z., Ye, F., Xu, W., Lu, R., et al. (2021). 3, 3'-Diindolylmethane induces gastric cancer cells death via STIM1 mediated store-operated calcium entry. Int. J. Biol. Sci. 17 (5), 1217–1233. doi:10.7150/ijbs.56833
Ye, Y., Ye, F., Li, X., Yang, Q., Zhou, J., Xu, W., et al. (2021). 3, 3'-diindolylmethane exerts antiproliferation and apoptosis induction by TRAF2-p38 axis in gastric cancer. Anticancer Drugs 32 (2), 189–202. doi:10.1097/CAD.0000000000000997
Yi, H., Li, Z., Liu, X., Dai, S., and Li, S. (2021). Therapeutic mechanism of lapatinib combined with sulforaphane on gastric cancer. Evid. Based Complement. Altern. Med. 2021, 9933274. doi:10.1155/2021/9933274
Zang, M. D., Hu, L., Fan, Z. Y., Wang, H. X., Zhu, Z. L., Cao, S., et al. (2017). Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J. Transl. Med. 15 (1), 52. doi:10.1186/s12967-017-1151-6
Zang, M., Hu, L., Zhang, B., Zhu, Z., Li, J., Zhu, Z., et al. (2017). Luteolin suppresses angiogenesis and vasculogenic mimicry formation through inhibiting Notch1-VEGF signaling in gastric cancer. Biochem. Biophys. Res. Commun. 490 (3), 913–919. doi:10.1016/j.bbrc.2017.06.140
Zhang, J., Wu, D., Vikash,, , Song, J., Wang, J., Yi, J., et al. (2015). Hesperetin induces the apoptosis of gastric cancer cells via activating mitochondrial pathway by increasing reactive oxygen species. Dig. Dis. Sci. 60 (10), 2985–2995. doi:10.1007/s10620-015-3696-7
Zhang, P., Shi, L., Zhang, T., Hong, L., He, W., Cao, P., et al. (2019). Piperlongumine potentiates the antitumor efficacy of oxaliplatin through ROS induction in gastric cancer cells. Cell Oncol. (Dordr) 42 (6), 847–860. doi:10.1007/s13402-019-00471-x
Zhang, S., Huang, J., Xie, X., He, Y., Mo, F., and Luo, Z. (2017). Quercetin from polygonum capitatum protects against gastric inflammation and apoptosis associated with Helicobacter pylori infection by affecting the levels of p38MAPK, BCL-2 and BAX. Molecules 22 (5), 744. doi:10.3390/molecules22050744
Zhang, X., Zhang, C., Ren, Z., Zhang, F., Xu, J., Zhang, X., et al. (2020). Curcumin affects gastric cancer cell migration, invasion and cytoskeletal remodeling through gli1-beta-catenin. Cancer Manag. Res. 12, 3795–3806. doi:10.2147/CMAR.S244384
Zhang, Y., Liu, S., Feng, Q., Huang, X., Wang, X., Peng, Y., et al. (2018). Perilaldehyde activates AMP-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy. J. Cell Biochem. 120, 1716–1725. doi:10.1002/jcb.27491
Zhao, Y., Chen, X., Jiang, J., Wan, X., Wang, Y., and Xu, P. (2020). Epigallocatechin gallate reverses gastric cancer by regulating the long noncoding RNA LINC00511/miR-29b/KDM2A axis. Biochim. Biophys. Acta Mol. Basis Dis. 1866 (10), 165856. doi:10.1016/j.bbadis.2020.165856
Zheng, R., Deng, Q., Liu, Y., and Zhao, P. (2017). Curcumin inhibits gastric carcinoma cell growth and induces apoptosis by suppressing the wnt/β-catenin signaling pathway. Med. Sci. Monit. 23, 163–171. doi:10.12659/msm.902711
Zhou, S., Yao, D., Guo, L., and Teng, L. (2017). Curcumin suppresses gastric cancer by inhibiting gastrin-mediated acid secretion. FEBS Open Bio 7 (8), 1078–1084. doi:10.1002/2211-5463.12237
Zhou, X., Wang, W., Li, P., Zheng, Z., Tu, Y., Zhang, Y., et al. (2016). Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol. Res. 23 (1-2), 29–34. doi:10.3727/096504015X14452563486011
Zhou, Y., Ding, B. Z., Lin, Y. P., and Wang, H. B. (2018). MiR-34a, as a suppressor, enhance the susceptibility of gastric cancer cell to luteolin by directly targeting HK1. Gene 644, 56–65. doi:10.1016/j.gene.2017.10.046
Zhu, X., Luo, F., Zheng, Y., Zhang, J., Huang, J., Sun, C., et al. (2013). Characterization, purification of Poncirin from edible citrus Ougan (Citrus reticulate cv. Suavissima) and its growth inhibitory effect on human gastric cancer cells SGC-7901. Int. J. Mol. Sci. 14 (5), 8684–8697. doi:10.3390/ijms14058684
Zhu, Y., Zhang, B., Gong, A., Fu, H., Zhang, X., Shi, H., et al. (2016). Anti-cancer drug 3, 3'-diindolylmethane activates Wnt4 signaling to enhance gastric cancer cell stemness and tumorigenesis. Oncotarget 7 (13), 16311–16324. doi:10.18632/oncotarget.7684
Keywords: gastric cancer, phytochemicals, prevention, treatment, mechanisms
Citation: Liang Z, Xu Y, Zhang Y, Zhang X, Song J, Qian H and Jin J (2023) Anticancer applications of phytochemicals in gastric cancer: Effects and molecular mechanism. Front. Pharmacol. 13:1078090. doi: 10.3389/fphar.2022.1078090
Received: 24 October 2022; Accepted: 28 December 2022;
Published: 12 January 2023.
Edited by:
Viqar Syed, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Sherif T.S. Hassan, Czech University of Life Sciences Prague, CzechiaCopyright © 2023 Liang, Xu, Zhang, Zhang, Song, Qian and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Jin, amlhbmh1YWppbjg4QHNpbmEuY29t; Zhaofeng Liang, bGlhbmd6aGFvZmVuZ0B1anMuZWR1LmNu; Hui Qian, bHN0bW1tbHRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zhaofeng Liang
Zhaofeng Liang Yumeng Xu2†
Yumeng Xu2† Yue Zhang
Yue Zhang Jiajia Song
Jiajia Song