
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 30 November 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1068858
This article is part of the Research TopicTraditional Medicine and Chronic Inflammatory DiseasesView all 16 articles
Asparagus cochinchinensis (Lour.) Merr. (A. cochinchinensis) is a traditional herbal medicine that is used to treat constipation, fever, pneumonia, stomachache, tracheitis, rhinitis, cataract, acne, urticaria. More than 90 compounds have been identified from different structural types in A. cochinchinensis, including steroidal saponins, C21-steroides, lignans, polysaccharides, amino acids, etc. These bioactive ingredients make A. cochinchinensis remarkable for its pharmacological effects on anti-asthma, anti-inflammatory, anti-oxidation, anti-tumor, improving Alzheimer’s disease, neuroprotection, gut health-promoting and so on. Moreover, A. cochinchinensis also plays an important role in food, health product, cosmetic, and other fields. This review focused on the research publications of A. cochinchinensis and aimed to summarize the advances in the botany, traditional uses, phytochemistry, pharmacology, and applications which will provide reference for the further studies and applications of A. cochinchinensis.
A. cochinchinensis is belonging to the genus Asparagus in the family Liliaceae, it is widely distributed in temperate and tropical regions, including China, Japan, Korea, and Vietnam. (Kubota et al., 2012; Pegiou et al., 2019; Pahwa et al., 2022). A. cochinchinensis is one of the most frequently used traditional herbal medicines, with documented cases of its clinical therapeutic effect in many countries. (Sheng., 2022a; Wong et al., 2022). A. cochinchinensis first appeared as a traditional Chinese medicine (TCM) in the earliest Chinese medicinal classic work Shennong’s Classic of Materia Medica (written more than 2000 years ago during the Han Dynasty), it has a long history of medicinal use and its medicinal value has been proved by clinical experience. It was included in the Pharmacopoeia of the People’s Republic of China 1977 edition as a clinical TCM in common use for the first time, and was continuously included until the latest 2020 edition. Dried roots are the main medicinal parts of A. cochinchinensis, it has been commonly used either alone or in combination with other herbal medicines to treat asthma, cough, constipation, thrombosis and inflammatory disease in China for centuries. Many classic formulas containing A. cochinchinensis have been widely used in clinic and have made important contributions to the health of people in China and other traditional medicinal systems in Asia. In addition to its medicinal value, A. cochinchinensis has various commercial applications in health products, food, and cosmetics (Safriani et al., 2022). It is commonly used as a food or nutritional supplement (Siand et al., 2015), cosmetics with whitening and anti-aging effects, and even used as a raw material for fermentation and winemaking (Kim et al., 2017; Topolska et al., 2021). Therefore, its huge potential and broad development prospects are worth exploring.
In the past few decades, A. cochinchinensis has attracted widespread attention as an important herbal medicine. Significant progress on isolation and identification of active constituents in A. cochinchinensis have been made in relevant researches. So far, more than 90 components have been isolated and identified. They mainly include steroidal saponins, C21-steroids, lignans, polysaccharides, and amino acids. At present, A. cochinchinensis has a variety of pharmacological effects and has curative effects in the treatment of asthma, tumor, Alzheimer’s disease, gut diseases, inflammatory diseases (Lee et al., 2009; Lei et al., 2016; Choi et al., 2019; Zhang R. S. et al., 2021). Besides that, medicinal prescription research also has revealed that it functions synergistically in combination with various herbal medicines (Weiying et al., 2006; Jung et al., 2014). With the in-depth exploration of TCM the exploitation and utilization of traditional herbal medicine in the prevention and treatment of various diseases are steadily increasing.
With the current scientific and technological advances and the increasing international recognition of traditional herbal medicine in recent years, research on A. cochinchinensis has made significant progress. However, to the best of our knowledge, there is no review on A. cochinchinensis. It is particularly important and necessary to collate a review on A. cochinchinensis progress in recent years. This is the first review on up to date of A. cochinchinensis research developments in the fields of botany, traditional uses, phytochemistry, pharmacology, and applications. It provides an accurate overview of A. cochinchinensis research and identifies deficiencies in present studies, proposing further research targets. The authors expect this review to encourage further research into the pharmacological effects and mechanisms associated with A. cochinchinensis therapeutic effects and to provide a broader vision and new inspiration for research in current and potential applications of A. cochinchinensis.
A. cochinchinensis is a climbing perennial plant, which has the structural characteristics of pale green stalks, sickle-shaped leaves, pale green axillary flowers, red fruits, and the branches angular or narrowly winged. It usually grows on slopes, roadsides, underwoods, valleys, or wastelands, below 1750 m A. cochinchinensis is usually harvested in autumn and winter, cleaned silt, removed fibrous root, retained tuberous root, boiled in boiling water for 15 min, then peeled and cored, further dried to obtain the medicinal part of A. cochinchinensis. According to the online records of China’s flora (http://www.cn-flora.ac.cn/index.html), the medicinal part of A. cochinchinensis is fusiform, with a swelling in the middle or near the end, which is 3–5 cm long and 1–2 cm thick. A. cochinchinensis’s stem is smooth, often curved or twisted, up to 1–2 m long. A. cochinchinensis’s leafy branches are usually clustered every 3, which are flat or slightly acute triangular due to the keel shape of the midvein, slightly falcate, 0.5–8 cm long, and 1–2 mm wide. Its inflorescence usually has two axillary flowers with alternate petals. The pedicel is 2–6 mm long. The joint is generally located in the middle, the perianth is 2.5–3 mm long, and the female flowers are similar in size to the male flowers. The flowering and fruiting period is generally from May to October. When the fruit matures, it becomes red, with a diameter of 6–7 mm, with only one seed per fruit, as shown in Figure 1.
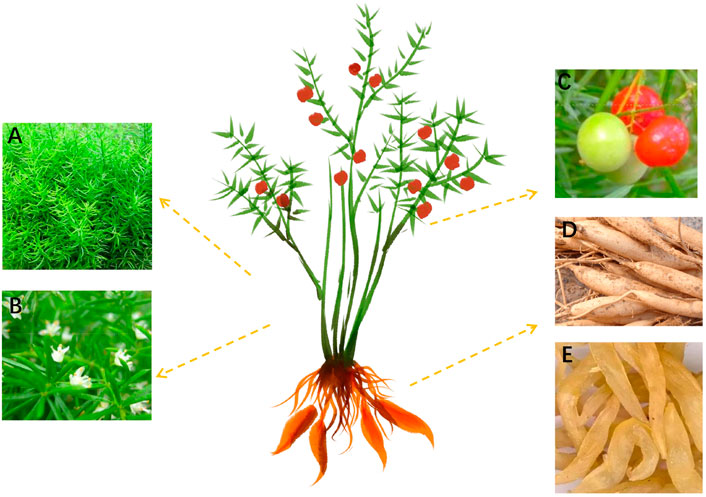
FIGURE 1. A. cochinchinensis plant morphology. (A) The above-ground portion, (B) Flower, (C) Fruits, (D) The underground part, (E) Medicinal part.
A. cochinchinensis has a long history of ethnopharmacological use and is characterized by bitter in taste and cold in nature. Since ancient times, researchers continuously explore and exploited TCM practices (Zhang X. et al., 2021; Wang et al., 2021). Dating back more than 1700 years of history, A. cochinchinensis was first documented in Shennong’s Classic of Materia Medica (Dong Han Dynasty, 25–220 A.D.), which is the earliest classic on TCM. Later, it was listed in many other well-known works on Chinese herb, including “Ming Yi Bie Lu” (Wei and Jin Dynasty, 220–420 A.D.), “Yao Xing Lun” (Tang Dynasty, 618–907 A.D.). In the folk culture, it is often used as a treatment cough, constipation, fever, pneumonia, stomachache, tracheitis, rhinitis, cataract, acne, urticaria and other diseases. In different countries, A. cochinchinensis has different therapeutic effects. It can be combined with other herb medicines to achieve a greater therapeutic effect. In Korea, extracts of formulations composed of A. cochinchinensis and other herbs were shown to have the effect of treating thrombosis (Chang et al., 2005; Lee et al., 2019). In China, the classic prescription composed of A. cochinchinensis (Qisheng pill) contains 114 chemical compounds were identified, including diosgenin, Methyl protodioscin, and ferroic acid, total saponin etc., which can inhibit the occurrence of inflammation, regulate intestinal dysfunction and improve the effect of Alzheimer disease (Xiong et al., 2022). At the same time, the herb formula water decoction composed of A. cochinchinensis can treatment of intestinal diseases, especially alleviate allergic airway inflammation and treat asthma (Luo et al., 2020). This also reflects the different therapeutic effects of A. cochinchinensis in traditional use and the broad application prospects in the future. Therefore, its clinical efficacy and function still need to be further explored.
In the past few decades, A. cochinchinensis have been investigated from a phytochemical perspective. The literature indicates the presence of multiple chemical compounds, predominantly steroidal saponin, C21-steroids, amino acids, lignan, and polysaccharides. To date, more than 90 compounds have been isolated and identified from A. cochinchinensis. These compounds are summarized in Tables 1 and Table 2, and their structures are shown in Figure 2, and Figure 3, and Figure 4.
Steroidal saponins are the major chemical components in A. cochinchinensis (Lee et al., 2015). Thus far, 71 steroidal saponins (1–71) have been isolated from A. cochinchinensis in Table 2. Steroid saponins are mainly composed of steroidal saponins and sugar condensation. They are classified into spirostanol saponins, isosprirostanol saponins, pseudospirostanol saponins and furostanol saponins based on the aglycone component differences. Aglycones are composed of six rings, of which the rutile rings are usually connected in a spiroketal form. The sugar moieties in the ordinary steroidal saponins are attached to the hydroxyl groups at C3. In a word, the structural diversity of different compounds is more reflected in the kind, length of each monosaccharide, the type of glycoside bond at the C3 position, and the position of the substituent.
C21-steroides are steroid derivatives with 21 carbon atoms and are one of the key compounds in A. cochinchinensis. C21 steroids are mostly hydroxyl derivatives with pregnane or its isomers as the basic skeleton. According to the skeleton type, they can be divided into four types, of which 72–79 (Jian et al., 2013; Liu et al., 2021; Zhu et al., 2021) are typical C21-steroides in Figure 3. In addition, there are many hydroxyl and carbonyl groups on the C21-steroid mother nucleus, and most of the carbonyl groups are at C20.
Four kinds of amino acids were isolated from A. cochinchinensis 80–83 (Choi et al., 2019), and their structures are shown in Figure 4. Amino acids are compounds containing both amino and carboxyl groups. In terms of their structure, amino acids are derivatives of carboxylic acid molecules in which amino groups replace the hydrogen in the alkyl group. According to the relative number of amino and carboxyl groups in amino acid molecules, amino acids can be divided into neutral, acidic and basic.
Lignans are a kind of natural compounds synthesized by the polymerization of two-molecular phenylpropanoid derivatives, most of which are free, and a few are glycosides bound to a sugar. At present, a small concentration of lignans 84–85 (Li et al., 2012) was identified from A. cochinchinensis. Compared with other compounds, lignans have less structure. Therefore, future efforts should be made to isolate and characterize lignans in A. cochinchinensis.
In recent years, plant polysaccharides have attracted high research interest due to their unique biological activity and natural origin, with great potential to protect human health. Many natural products are is rich in polysaccharide resources, especially medicinal plant polysaccharides, with long application history and broad development prospects. A. cochinchinensis polysaccharides are mainly comprised of Man, Rha, Glc, Gal, Ara, Xyl, Fru, GlcUA, and GalUA, as shown in Table 3.
In addition to the five major phytochemical compound classes mentioned above, other bioactive constituents have also been isolated from A. cochinchinensis (Zhang et al., 2004; Shi et al., 2009). These include 1-[4-hydroxyphenoxy]-5-[3-methoxy-4-hydroxyphenyl] pent-2-en-3-yne (86), asparenydiol (87),3′-hydroxy-4′-methoxy-4′dehydroxynyasol (88), Nyasol(89), 3″-methoxynyasol(90), 1,3-bis-di-p-hydroxyphenyl-4-penten-1-one(91), trans-coniferyl alcohol(92), Acrylamide(93). The above findings illustrate the wide chemical composition of A. cochinchinensis, which is of immense future research value.
A. cochinchinensis exerts various pharmacological activities, including anti-asthma, anti-inflammation, anti-oxidant, anti-tumor, anti-depressant, neuroprotective, improve Alzheimer’s disease and gut diseases. To illustrate the nature of the active compounds of A. cochinchinensis, the pharmacological effects and potential mechanisms of this plant on the basis of different types of extracts and compounds were summarized in Table 4. A simplified diagram of its pharmacological effects is presented in Figure 5.
Asthma is a common chronic and stubborn respiratory disease, clinically presenting with cough, chest tightness, wheezing, and shortness of breath (Papi et al., 2018). Non-timely treatment will lead to a series of secondary diseases, such as chronic obstructive pulmonary disease and heart failure, which can become life-threatening (Schoettler and Strek, 2020; Miller et al., 2021). At the same time, it also added a serious financial burden to the family (López-Tiro et al., 2022). Therefore, researchers found that the butanol extract of A. cochinchinensis roots, when fermented with Weissella cibaria (BAfW), was found to inhibit the development of asthma development through various potential mechanisms. Choi et al., 2018 alterations in key parameters were measured in ovalbumin (OVA)-challenged Balb/c mice treated with different BAfW dose regimens at three different time points. The results show that when the dosage of A. cochinchinensis fermentation extract was 500 mg, the number of immune cells, OVA-specific immunoglobulin E (Ig E) level, thickness of respiratory enzyme and mucus score decreased significantly in mice, and these parameters could be maintained for 48 h (Choi et al., 2018b). At the same time, researchers explored biomarkers for asthma in OVA-induced asthma mice. The extract of A. cochinchinensis was administered to the model mice at a low concentration of 250 mg/kg and a high concentration of 500 mg/kg, respectively. The changes in their metabolites were observed after administration. The results showed that the immune cells, Ig E serum concentration, the respiratory epithelium’s thickness, and inflammatory cell infiltration in the airway in mice treated with A. cochinchinensis extract recovered significantly. Notably, when assessing the endogenous metabolites, only alanine, glycine, methionine, and tryptophan were significantly recovered after A. cochinchinensis extract treatment, compared with the control group. Therefore, these four metabolites can be used as biomarkers to predict the anti-asthmatic effects (Choi et al., 2019). Moreover, the A. cochinchinensis fermentation extract was shown for the first time to accelerate the recovery from chronic asthma. It prevented airway inflammation and remodeling by restoring the cholinergic regulation of structural cells and inflammatory cells in chronic asthma models. Thus, it can further potentiate the effects of asthma treatments (Choi et al., 2018a). Furthermore, in vitro and in vivo experiments have been conducted to explore the effects of total saponins in A. cochinchinensis extract on asthma. Lipopolysaccharide (LPS) -activated RAW264.7 cells and OVA-induced mice asthma were treated with saponins-rich A. cochinchinensis extract, respectively. The result showed that the concentration of nitric oxide (NO) and mRNA levels of and cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) were significantly decreased in the SEAC/LPS-treated RAW264.7 cells compared with the vehicle/LPS-treated RAW264.7 cells. At the same time, the number of immune cells, infiltration of inflammatory cells and bronchial thickness decreased, meanwhile the levels of interleukin 4 (IL-4), interleukin 13 (IL-13) decreased significantly under the treatment of A. cochinchinensis extract (Sung et al., 2017). In general, A. cochinchinensis extracts can inhibit airway inflammation and remodeling, providing an important natural medicine option for the treatment of asthma.
Inflammation commonly occurs due to the modern lifestyle, and its complications can detrimentally affect people’s health (Yeung et al., 2018; McInnes and Gravallese, 2021). Numerous studies have proved that A. cochinchinensis has anti-inflammatory effect. Previous research by Kim et al., 1998 showed that A. cochinchinensis could inhibit tumor necrosis factor-α (TNF-α) secretion by inhibiting interleukin 1 (IL-1) secretion and that A. cochinchinensis extracts had anti-inflammatory activity in the central nervous system (Kim et al., 1998). Another study showed that the ethanol extract of A. cochinchinensis inhibited acute and chronic inflammation. When the extract was administered at a 200 mg/kg dose, the symptoms of 12-o-tetradecanoyl-phorbol-13-acetate (TPA)-induced mice ear were significantly alleviated. In addition, the skin thickness and tissue weight, inflammatory cytokine production, neutrophil-mediated myeloperoxidase (MPO) activity and histopathological parameters were significantly decreased (Lee et al., 2009). Furthermore, researchers have found that the ethyl acetate extract of A. cochinchinensis was shown to inhibits skin inflammation. In this study, phthalic anhydride (PA) -induced skin inflammation mice were used to identify the effects of A. cochinchinensis ethyl acetate extract on inflammation. The results suggest that ethyl acetate extract of A. cochinchinensis significantly reduced the concentration of Ig E, the surface thickness and number of infiltrating mast cells, and ethyl acetate extract played a key role in the treatment process (Sung et al., 2016). Using in vitro cell experiments, the researchers showed that the A. cochinchinensis ethyl acetate extract could inhibit the LPS stimulated RAW264.7 cell NO production, COX-2 expression, reactive oxygen species (ROS) production, and the inflammatory cytokine cell cycle (Lee et al., 2017). Thus, the above research findings provide strong evidence that A. cochinchinensis extracts may have important medicinal properties for treating specific skin inflammatory diseases. Surprisingly, after fermentation with BAfW, compounds such as protodioscin were significantly enhanced. In addition, a significant suppression was observed in the expression of key members of the iNOS-mediated COX-2 induction pathway and the phosphorylation of mitogen-activated protein kinases. These observations point to the ability to inhibit inflammatory reaction occurrence (Lee et al., 2015). Furthermore, studies have shown that the compound methyl protodioscin in A. cochinchinensis can inhibit the production of pro-inflammatory factors such as interleukin 16 (IL-16), interleukin 8 (IL-8) and TNF-α in lung tissue, suggesting that the compound has therapeutic value for airway inflammatory diseases (Lee et al., 2017a). Additionally, through in vitro cell experiments, the researchers took LPS-induced microglia cell as the study model. They were found that the ethanol extract of A. cochinchinensis at 1.0 μg mL−1 could significantly inhibit the production of NO in microglia cell induced by LPS, so as to play an anti-inflammatory role (Jian et al., 2013). All in all, all these studies have emphasized the potential of A. cochinchinensis extract to inhibit inflammatory reactions.
Anti-oxidants have always played a vital role in people’s health (Milisav et al., 2018; Martemucci et al., 2022). Studies have recently confirmed the anti-oxidant effect of A. cochinchinensis extract. A. cochinchinensis is shown to significantly increase the activities of anti-oxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), nitric oxide synthase (NOS), NO, and glutathione peroxidase (GPX). Liver and kidney hematoxylin and eosin stain sections revealed that D-galactose could cause serious injury, and A. cochinchinensis treatment improved immunity and substantially protected the liver and kidney from oxidative damage in aging mice (Lei et al., 2016). In a similar experiment, compared with the Vitamin C (Vc) positive control group, 0.7 mg⋅mL−1 aqueous root extract of A. cochinchinensis had similar 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and 3-ethylbenzothiazoline-6-sulfonic (ABTS+) scavenging activities, but significantly increased superoxide anion (p < 0.05) and OH scavenging activities (p < 0.01), which suggested strong radical scavenging ability of the aqueous root extract in vitro (Lei et al., 2017). At the same time, the researchers took D-galactose -induced mice as the research object and administered intraperitoneal injection (0.2 ml/20g) to mice for 15 days to make them senile, and further explored the effect of A. cochinchinensis extract on aging mice. The study found that through the detection of mice spleen and plasma, A. cochinchinensis extract could increase the spleen index and the SOD activity, reduces malondialdehyde (MDA) content, inhibits oxidation and slows down aging (Xiong et al., 2011). Additionally, 2,2-diphenyl-1-picropylhydrazine (DPPH) plays an indispensable role in antioxidant process. In a recent study, the fermented A. cochinchinensis root extract’s effects on melanogenic factor levels in human epidermal melanocytes (HEMs) and its anti-tyrosinase activity were analyzed and compared with the unfermented extract. The results showed that the scavenging ability, reducing power, and anti-tyrosinase activity of DPPH in the fermented extract were significantly increased (Wang et al., 2019). Therefore, A. cochinchinensis can be used as a natural anti-oxidant, with broad development and application prospects in the future.
The prevention and treatment of malignant tumors and cancer is a major challenge faced in our modern societies (Liu and Dong, 2021; DiMaio et al., 2022; Mao et al., 2022). With the development of molecular biology and pharmacology, A. cochinchinensis has attracted increasing attention from domestic and foreign medical scholars working in the cancer field. Through in vitro and in vivo experiments, A. cochinchinensis extracts were mainly internalized into tumor cells through phagocytosis, but once they entered the blood, tumor cells would be quickly cleared, further inhibiting the growth and proliferation of tumor cells (Zhang R. S. et al., 2021). Another study found that the compound 3-O-{[β-D-glucopyranosyl-(1→2)]-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranosyl} -(25R)-5β-spirostan-3β-ol mainly exerted its effect on inhibiting the proliferation of human large cell lung cancer cells (NCI-H460) by inducing apoptosis and cell cycle arrest, with an IC50 value of 1.39 μM (Liu et al., 2021). Besides that, the extract of A. cochinchinensis (1–100 mg/ml) dose-dependently not only inhibited the EtOH-induced tumor necrosis TNF-α secretion but also inhibited the EtOH and TNF-α-induced cytotoxicity. In addition, the extract of A. cochinchinensis inhibited the TNF-α -induced apoptosis of Hep G2 cells. Therefore, the above results suggest that A. cochinchinensis may prevent the EtOH-induced cytotoxicity by inhibiting the apoptosis of Hep G2 cells (Koo et al., 2000). These studies will provide a reference for further in-depth clinical application of A. cochinchinensis in cancer treatment.
The risk of depression has greatly increased due to the enormous mental and physical stress people face due to modern, fast-paced lifestyles (Martins and S., 2018; Angeloni and Vauzour, 2019; Payne et al., 2022). The researchers ovariectomized rats and exposed them to a chronic stress reaction state for 4 weeks. They additionally administered A. cochinchinensis extract (1000 and 2000 mg/kg) to observe mental state alterations of the menopausal rats. The results showed that the expression of brain-derived neurotrophic factor (BDNF) and its main receptor tropomyosin receptor kinase B (TrkB) increased in rats. Thus, A. cochinchinensis extract could potentially exert anti-depressant effects (Kim et al., 2020). In addition, another study showed that the A. cochinchinensis extract, activating phosphatase 2 (Shp-2), ERK1/2, and Akt signaling pathways, could directly affect treating depression and nerve protection (Jalsrai et al., 2016). The pathogenesis of Alzheimer’s disease is unclear, but neuroprotection is shown in different studies to prevent and alleviate it. Lee et al., 2018 study showed that phenols, saponins and protodiosgenin in A. cochinchinensis extracts induced enhanced nerve growth factor secretion and decreased intracellular ROS in neurons and microglia cell lines, inhibiting the activity of acetylcholinesterase, thereby improving Alzheimer’s disease (Lee et al., 2018). This study provides novel directions for developing new drugs from A. cochinchinensis, and, more importantly, offers new insights into the treatment of Alzheimer’s disease.
Maintaining a normal gut and digestive tract function is one of the key elements to maintaining good health (Sommer et al., 2017; Fassarella et al., 2021; Nathan et al., 2021). Studies have shown that A. cochinchinensis extract can treat gut damage caused by metal ions. To evaluate such A. cochinchinensis extract effects, the metal ions Drosophila model was used. The results showed that A. cochinchinensis extract can improved the survival rate of Drosophila melanogaster, reduce the mortality of intestinal epithelial cells, and the reduce the intestinal damage caused by metal ions (Zhang L. et al., 2021). At the same time, Kim et al., 2019 found that saponins can increase stool frequency, gastrointestinal transit, mucosal layer thickness, flat luminal surface, and the number of paneth cells, thus playing a role in the treating constipation. Improvements were also observed in the levels of acetylcholine esterase activity, the phosphorylation of myosin light chains, and the expression of muscarinic acetylcholine receptors M2/M3 (Kim et al., 2019). This study provides strong evidence for A. cochinchinensis applications in treating certain gut-related diseases. However, another study showed that polysaccharides in A. cochinchinensis have a role in gut flora regulation. The impact of inulin-type fructan on gut microbiota was investigated by in vitro mediation with human fecal cultures. The results showed that inulin-type fructan was digested by gut microbiota, while the pH value in the A. cochinchinensis neutral polysaccharide (ACNP) fecal culture was greatly decreased. The total short-chain fatty acids, acetic, propionic, i-valeric, and n-valeric acids were significantly increased (Sun et al., 2020). Collectively, inulin-type fructan was shown to regulate gut microbiota beneficially (Vandeputte et al., 2017; Tao et al., 2021). Thus, it has the potential to be used as a dietary supplement or drug to improve health.
As A. cochinchinensis is widely used as a traditional herbal medicine with high medicinal value, its safety profile is very important. A recent study evaluated the hepatotoxicity and nephrotoxicity of A. cochinchinensis toward the livers and kidneys in ICR mice. Female and male ICR mice were orally administered with 150 mg/kg, 300 mg/kg, and 600 mg/kg A. cochinchinensis extract for 14 days, respectively, and the changes in relevant markers (organ weight, urine composition, liver pathology, and kidney pathology) were observed. The results showed that Female and male ICR mice were orally administered with 150 mg/kg, 300 mg/kg, and 600 mg/kg A. cochinchinensis extract for 14 days, respectively, and the changes in relevant markers (organ weight, urine composition, liver pathology, and kidney pathology) were observed (Sung et al., 2017a). Therefore, the saponins in the A. cochinchinensis extract have no specific liver and kidney toxicity, reinforcing the excellent safety profile of A. cochinchinensis.
A. cochinchinensis embodies not only significant medicinal value in the field of TCM but also shows distinctive application value in the fields of pharmaceuticals, health care products, food, cosmetics, and others. These applications are summarized in Figure 6, and A. cochinchinensis patents in pharmaceuticals, foods, health products and cosmetics are listed in Table 5.
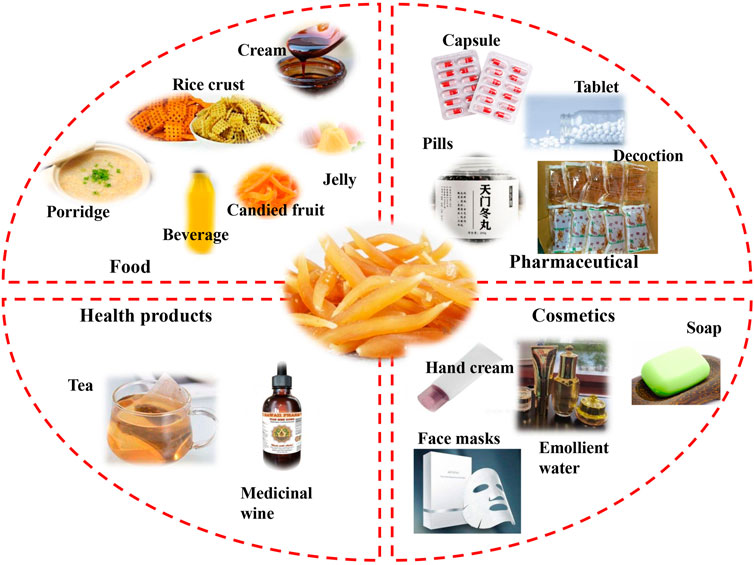
FIGURE 6. The applications of A. cochinchinensis in pharmaceutical, food, health products, and cosmetics.
As mentioned above, A. cochinchinensis contains numerous active compounds having many promising effects in vitro and in vivo, indicating their great potential to for pharmaceutical applications (Ren et al., 2021). The pharmaceutical properties of A. cochinchinensis were recorded well in ancient Chinese medical literature. Nowadays, A. cochinchinensis has a wide range of clinical applications in the respiratory, digestive, urinary system, with diverse uses. Clinically, A. cochinchinensis is often used to treat respiratory diseases such as cough, asthma, and lung cancer. A. cochinchinensis can be used alone, in combination with other pharmaceuticals, or for external use (Hong et al., 2000; Liu et al., 2015). Health care products are becoming highly popular as people pay increasing attention to their physical health (Tabatabai and Sellmeyer, 2021). A. cochinchinensis through self-fermentation or fermentation with other Chinese herbal medicines, is marketed form of functional medicinal wine (Kim et al., 2017; Wuyts et al., 2020). It contributes to lowering blood pressure, blood sugar, and blood lipid, so it is highly sought after by middle-aged and elderly people (Sikand et al., 2015). Moreover, there are functional teas with health-promoting properties (Fu et al., 2018). A. cochinchinensis is also widely used in the food and culinary field. In the folk, people usually use A. cochinchinensis is used as the main raw material to cook porridge or paste, used to relieve cough, expectorant, tonsillitis, dry throat, sore throat, hemoptysis, and treat constipation. It is also processed into A. cochinchinensis candied fruit, that is popular, especially among young people. Certain modern A. cochinchinensis foods products have been patented, such as Radix asparagi and platycodon grandiflorum healthcare rice crust, Rehmannia-radix asparagi beverage and health jelly with algae flavor. Recent studies have shown that long-term consumption of A. cochinchinensis as a traditional edible plant can inhibit the production of pro-inflammatory cytokines interleukin-1 beta (IL-1β) and TNF-α, thereby treating various immune-related diseases (Safriani et al., 2022). Despite the currently limited research on A. cochinchinensis food products, A. cochinchinensis has great potential applications and novel future products in the food field. Interestingly, the extract obtained from fermented A. cochinchinensis is also used as a whitening facial mask and whitening soap, with increasing sales, as it can inhibit the formation of tyrosinase and melanin (Sakuma et al., 1999; Pillaiyar et al., 2017). At the same time, patents granted on A. cochinchinensis show that it has beneficial properties for improving skin aging, skin whitening, reducing skin wrinkles, and moisturizing, among others. Therefore, the potential of further A. cochinchinensis commercial applications in the cosmetics industry should be sought after with increased research efforts.
In this paper, we review the botany, traditional uses, phytochemistry, applications, and pharmacology activities of A. cochinchinensis according to ancient classics and modern researches, and it will provide a new insight for future exploration of A. cochinchinensis. The root of A. cochinchinensis has been widely used to treat cough, fever, pneumonia, stomachache, tracheitis, rhinitis, cataract, acne and urticaria. Meanwhile, the root of A. cochinchinensis has a predominant therapeutic effect in diseases such as sthma, constipation, pneumonia. Interestingly, A. cochinchinensis exerts multiple functions as medicine, food, and cosmetics, which has been widely used as whitening or healthcare product. Up to now, more than 90 compounds have been isolated and identified from A. cochinchinensis. Among these constituents, steroidal saponins represent the main active ingredients. It is expected that more compounds of these categories will be discovered in the future studies. In addition, researches have shown that both extracts and active components of A. cochinchinensis possess a wide range of pharmacological activities, including anti-asthma, anti-inflammatory, anti-oxidation, anti-tumor, improving Alzheimer’s disease, nerve protection, gut health-promoting and so on. These modern pharmacological studies supported most traditional uses of A. cochinchinensis as an indispensable TCM.
However, gaps still exist in the systematic research on A. cochinchinensis. Firstly, reported studies have shown that the main chemical components of A. cochinchinensis is steroidal saponins. While other chemical constituents such as polysaccharides, lignans and amino acids extracted and isolated from A. cochinchinensis are very few compared with steroidal saponins. More chemical constituents must be obtained to explore the relationship between compounds and pharmacological effects in depth. Therefore, new separation and analysis techniques should be developed and implemented to analyze and determine A. cochinchinensis chemical composition comprehensively. Secondly, quality standards have not been adequately set. Since A. cochinchinensis has a wide variety and is easily confused with other varieties, it is very necessary to establish a complete set of quality standards to distinguish these products. This will also contribute to better-protecting people’s health and safety. Thus, it is crucial to establish the A. cochinchinensis quality analytical standards and find the appropriate markers to implement such quality control. At the same time, it is also necessary to conduct systematic and in-depth research on the toxicology of A. cochinchinensis to improve the safety profile of its clinical use. Thirdly, the main part of A. cochinchinensis used for medicinal compound extraction is its dried root, and the other parts are discarded. However, the resources of roots are relatively rare compared to the resources of leaves and fruits. In the future, in-depth research should be conducted on the leaves and fruits of A. cochinchinensis, to explore their value so that the plant can be fully utilized. This reduces the waste of plant resources and might contribute to the development of new drugs, as novel compounds might be discovered in other plant parts. Therefore, we should solve the existing problems as soon as possible, so that the future development of A. cochinchinensis will be better.
In addition, in order to further elucidate the mechanism of A. cochinchinensis in treating diseases, it is essential to establish the internal relationship between chemical components and their pharmacological activities. Pharmacokinetic studies of A. cochinchinensis can also be conducted to try to elucidate its changes including absorption, distribution, metabolism and excretion. This will further elucidate the complex relationship between chemical components and clinical effects to reveal potential mechanism of action. At the same time, A. cochinchinensis can also be used as food and nutritional supplement. People become more aware of their health, edible Chinese herbal medicines with health-promoting and therapeutic effects are becoming very popular. On this basis, in-depth research should be conducted in the fields of A. cochinchinensis health products, food, and cosmetics, which may have broader prospects for future development, providing new idea for A. cochinchinensis research.
To sum up, the root of A. cochinchinensis is an important edible medicinal herb with extensive pharmacological activities and great values in medicine, food, and cosmetics. However, more in-depth and comprehensive studies on clinical utility are needed to determine its safety and availability. Until now, multiple compounds have been discovered in A. cochinchinensis, but what we have done is far from enough. Furthermore, the precise molecular mechanisms of these active ingredients in some diseases still worth further study. Consequently, systematic studies on phytochemistry and bioactivities of A. cochinchinensis will undoubtedly be the key direction of future research. This review should provide an important reference for the development and application of A. cochinchinensis. Siand et al., 2015, Sheng, 2022b.
MW and HK proposed the framework of this paper. SW and ZW drafted the manuscript. SW and WH make tables and figures. BY provided some helpful suggestions in this paper. All authors read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (No.81803686), Supporting Project of National Natural Science Foundation (No.2018PP01), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No.UNPYSCT-2020225), Special Fund Project of Post doctor in Heilongjiang Province (LBH-Q20180), Chief Scientist of Qi-Huang Project of National Traditional Chinese Medicine Inheritance and Innovation “One Hundred Million” Talent Project ([2021] No.7), Heilongjiang Touyan Innovation Team Program ([2019] No.5).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Angeloni, C., and Vauzour, D. (2019). Natural products and neuroprotection. Int. J. Mol. Sci. 20 (22), 5570. doi:10.3390/ijms20225570
Chang, G. T., Min, S. Y., Kim, J. H., Kim, S. H., Kim, J. K., and Kim, C. H. (2005). Anti-thrombic activity of Korean herbal medicine, Dae-Jo-Whan and its herbs. Vasc. Pharmacol. 43 (4), 283–288. doi:10.1016/j.vph.2005.08.014
Chen, J., Yang, F., Guo, H., Wu, F., and Wang, X. (2015). Optimized hydrolysis and analysis of Radix Asparagi polysaccharide monosaccharide composition by capillary zone electrophoresis. J. Sep. Sci. 38 (13), 2327–2331. doi:10.1002/jssc.201500120
Choi, J. Y., Kim, J. E., Park, J. J., Lee, M. R., Song, B. R., Park, J. W., et al. (2018a). The anti-inflammatory effects of fermented herbal roots of Asparagus cochinchinensis in an ovalbumin-induced asthma model. J. Clin. Med. 7 (10), 377. doi:10.3390/jcm7100377
Choi, J. Y., Kim, S. H., Kim, J. E., Park, J. W., Kang, M. J., Choi, H. J., et al. (2019). Four amino acids as serum biomarkers for anti-asthma effects in the ovalbumin-induced asthma mouse model treated with extract of Asparagus cochinchinensis. Lab. Anim. Res. 35 (1), 32–10. doi:10.1186/s42826-019-0033-x
Choi, J. Y., Park, J. W., Kim, J. E., Park, J. J., Lee, M. R., Song, B. R., et al. (2018b). Dose dependence and durability of the therapeutic effects of Asparagus cochinchinensis fermented extract in an ovalbumin-challenged asthma model. Lab. Anim. Res. 34 (3), 101–110. doi:10.5625/lar.2018.34.3.101
DiMaio, D., Emu, B., Goodman, A. L., Mothes, W., and Justice, A. (2022). Cancer microbiology. J. Natl. Cancer Inst. 114 (5), 651–663. doi:10.1093/jnci/djab212
Fassarella, M., Blaak, E. E., Penders, J., Nauta, A., Smidt, H., and Zoetendal, E. G. (2021). Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 70 (3), 595–605. doi:10.1136/gutjnl-2020-321747
Fu, Y., Yang, J. C., Cunningham, A. B., Towns, A. M., Zhang, Y., Yang, H. Y., et al. (2018). A billion cups: The diversity, traditional uses, safety issues and potential of Chinese herbal teas. J. Ethnopharmacol. 222, 217–228. doi:10.1016/j.jep.2018.04.026
Hong, S. J., Fong, J. C., and Hwang, J. H. (2000). Effects of crude drugs on glucose uptake in 3T3-L1 adipocytes. Kaohsiung J. Med. Sci. 16 (9), 445–451.
Jalsrai, A., Numakawa, T., Kunugi, H., Dieterich, D. C., Becker, A., and Becker, A. (2016). The neuroprotective effects and possible mechanism of action of a methanol extract from Asparagus cochinchinensis: In vitro and in vivo studies. Neuroscience 322, 452–463. doi:10.1016/j.neuroscience.2016.02.065
Jian, R., Zeng, K. W., Li, J., Li, N., Jiang, Y., and Tu, P. (2013). Anti-neuroinflammatory constituents from Asparagus cochinchinensis. Fitoterapia 84, 80–84. doi:10.1016/j.fitote.2012.10.011
Jung, K. H., Choi, H. L., Park, S., Lee, G., Kim, M., Min, J. K., et al. (2014). The effects of the standardized herbal formula PM014 on pulmonary inflammation and airway responsiveness in a murine model of cockroach allergen-induced asthma. J. Ethnopharmacol. 155 (1), 113–122. doi:10.1016/j.jep.2014.04.029
Kim, H., Lee, E., Lim, T., Jung, J., and Lyu, Y. (1998). Inhibitory effect of Asparagus cochinchinensis on tumor necrosis factor-alpha secretion from astrocytes. Int. J. Immunopharmacol. 20 (4-5), 153–162. doi:10.1016/s0192-0561(98)00022-8
Kim, H. R., Lee, Y. J., Kim, T. W., Lim, R. N., Hwang, D. Y., Moffat, J. J., et al. (2020). Asparagus cochinchinensis extract ameliorates menopausal depression in ovariectomized rats under chronic unpredictable mild stress. BMC Complement. Med. Ther. 20 (1), 325. doi:10.1186/s12906-020-03121-0
Kim, J. E., Park, J. W., Kang, M. J., Choi, H. J., Bae, S. J., Choi, Y. S., et al. (2019). Anti-inflammatory response and muscarinic cholinergic regulation during the laxative effect of Asparagus cochinchinensis in loperamide-induced constipation of SD rats. Int. J. Mol. Sci. 20 (4), 946. doi:10.3390/ijms20040946
Kim, J. Y., Choi, H. Y., Kim, H. M., Choi, J. H., and Jang, D. S. (2021). A novel cytotoxic steroidal saponin from the roots of Asparagus cochinchinensis. Plants 10 (10), 2067. doi:10.3390/plants10102067
Kim, M., Kim, W. B., Koo, K. Y., Kim, B. R., Kim, D., Lee, S., et al. (2017). Optimal fermentation conditions of hyaluronidase inhibition activity on Asparagus cochinchinensis merrill by Weissella cibaria. J. Microbiol. Biotechnol. 27 (4), 701–708. doi:10.4014/jmb.1611.11051
Koo, H. N., Jeong, H. J., Choi, J. Y., Choi, S. D., Choi, T. J., Cheon, Y. S., et al. (2000). Inhibition of tumor necrosis factor-alpha-induced apoptosis by Asparagus cochinchinensis in Hep G2 cells. J. Ethnopharmacol. 73 (1-2), 137–143. doi:10.1016/s0378-8741(00)00287-7
Kubota, S., Konno, I., and Kanno, A. (2012). Molecular phylogeny of the genus Asparagus (Asparagaceae) explains interspecific crossability between the garden asparagus (A. officinalis) and other Asparagus species. TAG. Theoretical and applied genetics. Theor. Appl. Genet. 124 (2), 345–354. doi:10.1007/s00122-011-1709-2
Lee, D. Y., Choo, B. K., Yoon, T., Cheon, M. S., Lee, H. W., Lee, A. Y., et al. (2009). Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 121 (1), 28–34. doi:10.1016/j.jep.2008.07.006
Lee, H. A., Kim, J. E., Sung, J. E., Yun, W. B., Kim, D. S., Lee, H. S., et al. (2018). Asparagus cochinchinensis stimulates release of nerve growth factor and abrogates oxidative stress in the Tg2576 model for Alzheimer's disease. BMC Complement. Altern. Med. 18 (1), 125. doi:10.1186/s12906-017-1775-3
Lee, H. A., Koh, E. K., Sung, J. E., Kim, J. E., Song, S. H., Kim, D. S., et al. (2017b). Ethyl acetate extract from Asparagus cochinchinensis exerts anti-inflammatory effects in LPS-stimulated RAW264. 7 macrophage cells by regulating COX-2/iNOS, inflammatory cytokine expression, MAP kinase pathways, the cell cycle and anti-oxidant activity. Mol. Med. Rep. 15 (4), 1613–1623. doi:10.3892/mmr.2017.6166
Lee, H. A., Song, B. R., Kim, H. R., Kim, J. E., Yun, W. B., Park, J. J., et al. (2017a). Butanol extracts of Asparagus cochinchinensis fermented with Weissella cibaria inhibit iNOS-mediated COX-2 induction pathway and inflammatory cytokines in LPS-stimulated RAW264.7 macrophage cells. Exp. Ther. Med. 14 (5), 4986–4994. doi:10.3892/etm.2017.5200
Lee, H. J., Park, J. S., Yoon, Y. P., Shin, Y. J., Lee, S. K., Kim, Y. S., et al. (2015). Dioscin and methylprotodioscin isolated from the root of Asparagus cochinchinensis suppressed the gene expression and production of airway MUC5AC mucin induced by phorbol ester and growth factor. Phytomedicine 22 (5), 568–572. doi:10.1016/j.phymed.2015.03.009
Lee, J. H., Lim, H. J., Lee, C. W., Son, K. H., Son, J. K., Lee, S. K., et al. (2015). Evidence-based complementary and alternative medicine, Methyl protodioscin from the roots of Asparagus cochinchinensis attenuates airway inflammation by inhibiting cytokine productioneCAM, 2015, 640846, doi:10.1155/2015/640846
Lee, S. R., Park, H. S., Kim, B. Y., Lee, J. H., Fan, Q., Gaskin, J. F., et al. (2019). An unexpected genetic diversity pattern and a complex demographic history of a rare medicinal herb, Chinese asparagus (Asparagus cochinchinensis) in Korea. Sci. Rep. 9 (1), 9757. doi:10.1038/s41598-019-46275-9
Lei, L., Chen, Y., Ou, L., Xu, Y., and Yu, X. (2017). Aqueous root extract of Asparagus cochinchinensis (Lour.) Merr. Has antioxidant activity in D-galactose-induced aging mice. BMC Complement. Altern. Med. 17 (1), 469. doi:10.1186/s12906-017-1975-x
Lei, L., Ou, L., and Yu, X. (2016). The antioxidant effect of Asparagus cochinchinensis (Lour.) Merr. shoot in D-galactose induced mice aging model and in vitro. J. Chin. Med. Assoc. 79 (4), 205–211. doi:10.1016/j.jcma.2015.06.023
Li, X. N., Chu, C., Cheng, D. P., Tong, S. Q., and Yan, J. Z. (2012). Norlignans from Asparagus cochinchinensis. Nat. Prod. Commun. 7 (10), 1357–1358.
Liang, Z. Z., Aquino, R., De Simone, F., Dini, A., Schettino, O., and Pizza, C. (1988). Oligofurostanosides from Asparagus cochinchinensis. Planta Med. 54 (04), 344–346. doi:10.1055/s-2006-962453
Liu, B., Li, B., Zhou, D., Wen, X., Wang, Y., Chen, G., et al. (2021). Steroidal saponins with cytotoxic effects from the rhizomes of Asparagus cochinchinensis. Bioorg. Chem. 115, 105237. doi:10.1016/j.bioorg.2021.105237
Liu, H., and Dong, Z. (2021). Cancer etiology and prevention principle: "1 + X. Cancer Res. 81 (21), 5377–5395. doi:10.1158/0008-5472.CAN-21-1862
Liu, H., Zheng, Y. F., Li, C. Y., Zheng, Y. Y., Wang, D. Q., Wu, Z., et al. (2015). Discovery of anti-inflammatory ingredients in Chinese herbal formula kouyanqing granule based on relevance analysis between chemical characters and biological effects. Sci. Rep. 5, 18080. doi:10.1038/srep18080
López-Tiro, J., Contreras-Contreras, A., Rodríguez-Arellano, M. E., and Costa-Urrutia, P. (2022). Economic burden of severe asthma treatment: A real-life study. World Allergy Organ. J. 15 (7), 100662. doi:10.1016/j.waojou.2022.100662
Luo, Z., Xu, W., Zhang, Y., Di, L., and Shan, J. (2020). A review of saponin intervention in metabolic syndrome suggests further study on intestinal microbiota. Pharmacol. Res. 160, 105088. doi:10.1016/j.phrs.2020.105088
Mao, J. J., Pillai, G. G., Andrade, C. J., Ligibel, J. A., Basu, P., Cohen, L., et al. (2022). Integrative oncology: Addressing the global challenges of cancer prevention and treatment. Ca. Cancer J. Clin. 72 (2), 144–164. doi:10.3322/caac.21706
Martemucci, G., Portincasa, P., Di Ciaula, A., Mariano, M., Centonze, V., and D'Alessandro, A. G. (2022). Oxidative stress, aging, antioxidant supplementation and their impact on human health: An overview. Mech. Ageing Dev. 206, 111707. doi:10.1016/j.mad.2022.111707
Martins, J., and S, B. (2018). Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomed. Pharmacother. 104, 343–365. doi:10.1016/j.biopha.2018.05.044
McInnes, I. B., and Gravallese, E. M. (2021). Immune-mediated inflammatory disease therapeutics: Past, present and future. Nat. Rev. Immunol. 21 (10), 680–686. doi:10.1038/s41577-021-00603-1
Milisav, I., Ribarič, S., and Poljsak, B. (2018). Antioxidant vitamins and ageing. Subcell. Biochem. 90, 1–23. doi:10.1007/978-981-13-2835-0_1
Miller, R. L., Grayson, M. H., and Strothman, K. (2021). Advances in asthma: New understandings of asthma's natural history, risk factors, underlying mechanisms, and clinical management. J. Allergy Clin. Immunol. 148 (6), 1430–1441. doi:10.1016/j.jaci.2021.10.001
Nathan, N. N., Philpott, D. J., and Girardin, S. E. (2021). The intestinal microbiota: From health to disease, and back. Microbes Infect. 23 (6-7), 104849. doi:10.1016/j.micinf.2021.104849
Pahwa, P., Singh, T., and Goel, R. K. (2022). Anticonvulsant effect of Asparagus racemosus willd. In a mouse model of catamenial epilepsy. Neurochem. Res. 47 (2), 422–433. doi:10.1007/s11064-021-03455-2
Pang, X., Gao, L., Wang, B., Chen, X. J., Zhang, J., Guo, B. L., et al. (2021). New steroidal glycosides from the roots of Asparagus cochinchinensis. J. Asian Nat. Prod. Res. 23 (3), 205–216. doi:10.1080/10286020.2021.1873956
Papi, A., Brightling, C., Pedersen, S. E., and Reddel, H. K. (2018). Asthma. Lancet (London, Engl. 391 (10122), 783–800. doi:10.1016/S0140-6736(17)33311-1
Payne, A., Nahashon, S., Taka, E., Adinew, G. M., and Soliman, K. (2022). Epigallocatechin-3-Gallate (EGCG): New therapeutic perspectives for neuroprotection, aging, and neuroinflammation for the modern age. Biomolecules 12 (3), 371. doi:10.3390/biom12030371
Pegiou, E., Mumm, R., Acharya, P., de Vos, R., and Hall, R. D. (2019). Green and white Asparagus (Asparagus officinalis): A source of developmental, chemical and urinary intrigue. Metabolites 10 (1), 17. doi:10.3390/metabo10010017
Pillaiyar, T., Manickam, M., and Namasivayam, V. (2017). Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 32 (1), 403–425. doi:10.1080/14756366.2016.1256882
Ren, Y., Elkington, B. G., Henkin, J. M., Sydara, K., Kinghorn, A. D., and Soejarto, D. D. (2021). Bioactive small-molecule constituents of Lao plants. J. Med. Plant Res. 15 (12), 540–559. doi:10.5897/jmpr2021.7137
Safriani, N., Zakaria, F. R., Prangdimurti, E., SuwartiVerpoorte, R., and Yuliana, N. D. (2022). Using metabolomics to discover the immunomodulator activity of food plants. Heliyon 8 (5), e09507. doi:10.1016/j.heliyon.2022.e09507
Sakuma, K., Ogawa, M., Sugibayashi, K., Yamada, K., and Yamamoto, K. (1999). Relationship between tyrosinase inhibitory action and oxidation-reduction potential of cosmetic whitening ingredients and phenol derivatives. Arch. Pharm. Res. 22 (4), 335–339. doi:10.1007/BF02979054
Schoettler, N., and Strek, M. E. (2020). Recent advances in severe asthma: From phenotypes to personalized medicine. Chest 157 (3), 516–528. doi:10.1016/j.chest.2019.10.009
Shen, Y., Xu, C. L., Xuan, W. D., Li, H. L., Liu, R. H., Xu, X. K., et al. (2011). A new furostanol saponin from Asparagus cochinchinensis. Arch. Pharm. Res 34 (10), 1587–1591. doi:10.1007/s12272-011-1001-7
Sheng, W. (2022a). The complete chloroplast genome of two traditional medical plants: Asparagus cochinchinensis lour. Merr. And Asparagus dauricus fisch. Ex link. Mitochondrial DNA. B Resour 7 (5), 725–726. doi:10.1080/23802359.2022.2068976
Sheng, W. (2022b). The entire chloroplast genome sequence of Asparagus cochinchinensis and genetic comparison to Asparagus species. Open Life Sci 17 (1), 893–906. doi:10.1515/biol-2022-0098
Shi, J. G., Li, G. Q., Huang, S. Y., Mo, S. Y., Wang, Y., Yang, Y. C., et al. (2004). Furostanol oligoglycosides from Asparagus cochinchinensis. J. Asian Nat. Prod. Res. 6 (2), 99–105. doi:10.1080/1028602031000135576
Shi, Z., Zhang, H., and Zhao, X. (2009). Ultrasonic-assisted precolumn derivatization-HPLC determination of acrylamide formed in Radix Asparagi during heating process. J. Pharm. Biomed. Anal. 49 (4), 1045–1047. doi:10.1016/j.jpba.2008.12.019
Siand, G., Kris-Etherton, P., and Boulos, N. M. (2015). Impact of functional foods on prevention of cardiovascular disease and diabetes. Curr. Cardiol. Rep. 17 (6), 39. doi:10.1007/s11886-015-0593-9
Sommer, F., Anderson, J. M., Bharti, R., Raes, J., and Rosenstiel, P. (2017). The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 15 (10), 630–638. doi:10.1038/nrmicro.2017.58
Sun, Q., Zhu, L., Li, Y., Cui, Y., Jiang, S., Tao, N., et al. (2020). A novel inulin-type fructan from Asparagus cochinchinensis and its beneficial impact on human intestinal microbiota. Carbohydr. Polym. 247, 116761. doi:10.1016/j.carbpol.2020.116761
Sung, J. E., Choi, J. Y., Kim, J. E., Lee, H. A., Yun, W. B., Park, J. J., et al. (2017a). Hepatotoxicity and nephrotoxicity of saponin-enriched extract of Asparagus cochinchinensis in ICR mice. Lab. Anim. Res. 33 (2), 57–67. doi:10.5625/lar.2017.33.2.57
Sung, J. E., Lee, H. A., Kim, J. E., Go, J., Seo, E. J., Yun, W. B., et al. (2016). Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation. Lab. Anim. Res. 32 (1), 34–45. doi:10.5625/lar.2016.32.1.34
Sung, J. E., Lee, H. A., Kim, J. E., Yun, W. B., An, B. S., Yang, S. Y., et al. (2017). Saponin-enriched extract of Asparagus cochinchinensis alleviates airway inflammation and remodeling in ovalbumin-induced asthma model. Int. J. Mol. Med. 40 (5), 1365–1376. doi:10.3892/ijmm.2017.3147
Tabatabai, L. S., and Sellmeyer, D. E. (2021). Nutritional supplements and skeletal health. Curr. Osteoporos. Rep. 19 (1), 23–33. doi:10.1007/s11914-020-00651-x
Tao, C., Zeng, W., Zhang, Q., Liu, G., Wu, F., Shen, H., et al. (2021). Effects of the prebiotic inulin-type fructans on post-antibiotic reconstitution of the gut microbiome. J. Appl. Microbiol. 130 (3), 634–649. doi:10.1111/jam.14827
Topolska, K., Florkiewicz, A., and Filipiak-Florkiewicz, A. (2021). Functional food-consumer motivations and expectations. Int. J. Environ. Res. Public Health 18 (10), 5327. doi:10.3390/ijerph18105327
Vandeputte, D., Falony, G., Vieira-Silva, S., Wang, J., Sailer, M., Theis, S., et al. (2017). Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 66 (11), 1968–1974. doi:10.1136/gutjnl-2016-313271
Wang, G. H., Lin, Y. M., Kuo, J. T., Lin, C. P., Chang, C. F., Hsieh, M. C., et al. (2019). Comparison of biofunctional activity of Asparagus cochinchinensis (Lour.) Merr. Extract before and after fermentation with Aspergillus oryzae. J. Biosci. Bioeng. 127 (1), 59–65. doi:10.1016/j.jbiosc.2018.06.015
Wang, T., Liu, J., Luo, X., Hu, L., and Lu, H. (2021). Functional metabolomics innovates therapeutic discovery of traditional Chinese medicine derived functional compounds. Pharmacol. Ther. 224, 107824. doi:10.1016/j.pharmthera.2021.107824
Weiying, L., Yuanjiang, D., and Baolian, L. (2006). Treatment of the localized neurodermatitis by plum-blossom needle tapping and with the modified yangxue dingfeng tang--a clinical observation of 47 cases. J. traditional Chin. Med. = Chung i tsa chih ying wen pan 26 (3), 181–183.
Wong, K. H., Kong, B. L., Siu, T. Y., Wu, H. Y., But, G. W., Shaw, P. C., et al. (2022). Complete chloroplast genomes of asparagus aethiopicus l., a. densiflorus (kunth) jessop 'myers', and a. cochinchinensis (lour.) merr.: comparative and phylogenetic analysis with congenerics. PloS one 17 (4), e0266376. doi:10.1371/journal.pone.0266376
Wuyts, S., Van Beeck, W., Allonsius, C. N., van den Broek, M. F., and Lebeer, S. (2020). Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 61, 45–52. doi:10.1016/j.copbio.2019.09.023
Xiong, D., Yu, L. X., Yan, X., Guo, C., and Xiong, Y. (2011). Effects of root and stem extracts of Asparagus cochinchinensis on biochemical indicators related to aging in the brain and liver of mice. Am. J. Chin. Med. 39 (4), 719–726. doi:10.1142/S0192415X11009159
Xiong, W., Zhao, X., Xu, Q., Wei, G., Zhang, L., Fan, Y., et al. (2022). Qisheng Wan formula ameliorates cognitive impairment of Alzheimer's disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol. 282, 114598. doi:10.1016/j.jep.2021.114598
Yeung, Y. T., Aziz, F., Guerrero-Castilla, A., and Arguelles, S. (2018). Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 24 (14), 1449–1484. doi:10.2174/1381612824666180327165604
Zhang, H. J., Sydara, K., Tan, G. T., Ma, C., Southavong, B., Soejarto, D. D., et al. (2004). Bioactive constituents from Asparagus cochinchinensis. J. Nat. Prod. 67 (2), 194–200. doi:10.1021/np030370b
Zhang, L., He, F., Gao, L., Cong, M., Sun, J., Xu, J., et al. (2021a). Engineering exosome-like nanovesicles derived from Asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int. J. Nanomedicine 16, 1575–1586. doi:10.2147/IJN.S293067
Zhang, R. S., Liu, Y. Y., Zhu, P. F., Jin, Q., Dai, Z., and Luo, X. D. (2021b). Furostanol saponins from Asparagus cochinchinensis and their cytotoxicity. Nat. Prod. Bioprospect. 11 (6), 651–658. doi:10.1007/s13659-021-00321-0
Zhang, W., and Jin, L. H. (2016). Asparagus cochinchinensis extract alleviates metal ion-induced gut injury in drosophila: an in silico analysis of potential active constituents. Evidence-Based complementary and alternative medicine. 2016, 7603746, doi:10.1155/2016/7603746
Zhang, X., Qiu, H., Li, C., Cai, P., and Qi, F. (2021c). The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci. Trends 15 (5), 283–298. doi:10.5582/bst.2021.01318
Zhu, G. L., Hao, Q., Li, R. T., and Li, H. Z. (2014). Steroidal saponins from the roots of Asparagus cochinchinensis. Chin. J. Nat. Med. 12 (3), 213–217. doi:10.1016/S1875-5364(14)60035-2
Zhu, G. L., Hao, Q., Xing, L., Yang, X. Q., Xie, S. D., Zhao, P., et al. (2021). C21, C22 pregnane glycosides and cytotoxic C27 spriostanol steroids from Asparagus cochinchinesis. Steroids 172, 108874. doi:10.1016/j.steroids.2021.108874
ACNP Asparagus cochinchinensis neutral polysaccharide
ACNVs Asparagus cochinchinensis-derived nanovesicles
Ara Arabinose
BDNF Brain-derived neurotrophic factor
CAT Catalase
COX-2 Cyclooxygenase-2
CZE Capillary zone electrophoresis
DPPH 1,1-diphenyl-2-picrylhydrazyl radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl
Fru Fructose
Gal Galactose
GalUA Galacturonic acid
Glc Glucose
GlcUA Glucuronic acid
GPX Glutathione peroxidase
Ig E Immunoglobulin E
IL-4 Interleukin 4
IL-13 Interleukin 13
IL-1β Interleukin-1 beta
iNOS Inducible nitric oxide synthase
LPS Lipopolysaccharide
Man Mannose
MDA Malondialdehyde
MPO Myeloperoxidase
NGF Nerve growth factor
NO Nitric oxide
NOS Nitric oxide synthase
OVA Ovalbumin
PA Phthalic anhydride
ROS Reactive oxygen species
Rha Rhamnose
SOD Superoxide dismutase
SP Substance P
TCM Traditional Chinese medicines
Tg Transgenic
TNF-α Tumor necrosis factor-α
TPA 12-O-tetradecanoyl-phorbol-13-acetate
TrkB Tropomyosin receptor kinase B
Xyl Xylose
Keywords: Asparagus cochinchinensis (Lour.) Merr, traditional uses, phytochemistry, pharmacology, applications
Citation: Wang M, Wang S, Hu W, Wang Z, Yang B and Kuang H (2022) Asparagus cochinchinensis: A review of its botany, traditional uses, phytochemistry, pharmacology, and applications. Front. Pharmacol. 13:1068858. doi: 10.3389/fphar.2022.1068858
Received: 13 October 2022; Accepted: 15 November 2022;
Published: 30 November 2022.
Edited by:
Zheng Xiang, Liaoning University, ChinaReviewed by:
Xingbin Yin, Beijing University of Chinese Medicine, ChinaCopyright © 2022 Wang, Wang, Hu, Wang, Yang and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haixue Kuang, aHhrdWFuZ0BobGp1Y20ubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.