- 1Department of Standardization of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 2Department of Standardization of Chinese Medicine, Guangdong Provincial Academy of Chinese Medicine, Guangzhou, China
- 3State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Background: The Appraisal of Guidelines Research and Evaluation (AGREE) II instrument has been widely used in the methodological quality assessment of clinical practice guidelines (CPG). Chinese medicine CPGs have unique characteristics which distinguish them from those of Western medicine, e.g. syndrome differentiation, on which treatments are based. As such, certain domains and items in AGREE II are unsuitable for assessing TCM CPGs. Therefore, it is necessary to adjust and supplement the description and rating section of some items of the AGREE Ⅱinstrument.
Purpose: To adjust and expand AGREE II according to characteristics of TCM clinical practice guidelines.
Methods: A research working group was established, consisting of a core working group and an expert consensus group, before a systematic literature search performed to screen for TCM guidelines. Two researchers evaluated the quality of the included guidelines using AGREE Ⅱ and later proposed adjustments to some items of AGREE Ⅱ and supplementary comments, which were applicable to TCM CPGs, and drafted an initial version of AGREE Ⅱ for TCM. Suggestions from literature on development and evaluation of TCM CPGs were solicited and integrated into the revised version, which 16 experts were then invited to advise on. When the experts reached a consensus, their comments to the draft were adopted by the core group into the final version.
Results: After evaluating the included TCM guidelines, the two researchers offered adjustments and supplementary comments for AGREE Ⅱ Items 1, 7, 10, 11, 12, 15, and 18, and drafted an initial version of AGREE Ⅱ for TCM. Combining suggestions from the literature on development and quality evaluation of TCM clinical guidelines, the core working group modified AGREE Ⅱ items 2, 4, 5, 8, 9, 13, 20, and 21, then proposed the revised version of AGREE Ⅱ for TCM, on which was advised by a group of experts, before consensus on improvements was reached. The results of the first round of expert surveys showed strong agreement, and experts’ opinions were adopted into the final version of AGREE Ⅱ for TCM.
Conclusion: Based on the characteristics of the TCM CPGs, we adjustment and expansion were made to create AGREE II for TCM. This version is suitable for the assessment of methodological quality of TCM CPGs, capable of providing content support for the standardization of procedures and methods of formulating TCM CPGs.
1 Introduction
Clinical practice guidelines (CPGs) are recommendations for providing patients with the optimal healthcare services (Institute of Medicine, 2011). They are the core technical standard in the field of medicine. Traditional Chinese medicine (TCM) and Western medicine have long complemented each other in promoting the health of the people in China, which has become a significant advantage of “health services with Chinese characteristics”. In recent years, with the Central government’s promotion of TCM, there has been a proliferation of CPGs for TCM, of which, however, the quality varies. Moreover, the development of some guidelines lacked scientific normativity (Huang et al., 2018). Studies pointed out that there remains a wide gap between the methodological quality of TCM clinical guidelines and the international ones. Problems in quality existing requires effective measures (Yao, 2016). Additionally, rate of application for clinical guidelines is not promising, and some guidelines do not serve as a guidance for clinical practice (Wu et al., 2016).
Appropriate methodology and strict formulation strategy are not only crucial for drafting quality guidelines, but also conducive for implementing the guidelines’ recommendations within (Burgers et al., 2004). Guideline evaluation is an effective way to improve guideline quality and provide necessary reference for clinical application. Many researchers believe that scientifically valid methods for rational evaluation of guidelines are needed so that recommendations can be made for revising or updating guideline (Jiang and Chen 2016; Li et al., 2016; Huang et al., 2018). The Appraisal of Guidelines for Research and Evaluation instrument, second edition (AGREE Ⅱ) (The AGREE Next Steps Consortiu, 2009) is recognized as the international “gold standard” of guideline evaluation (Brouwers et al., 2012). At present, it is widely used in the field of guideline development and evaluation.
Unlike current medical guidelines, TCM guidelines have distinctive characteristics, including, for example, treatment based on syndrome differentiation, Chinese Medicine formulas and traditional therapy, etc. The prescription of medication in TCM is based on the results of syndrome differentiation. There is a clear corresponding relationship between the etiology and pathogenesis, syndrome differentiation and treatment formulas. This direct cause-and-effect relationship should be presented in the TCM guidelines. The content of ancient texts and the experience of famous TCM practitioners are often essential in the formation of TCM guidelines. The above contents specific to TCM are not focused on in the AGREE II instrument. Therefore, we believe it is necessary to adjust and supplement the description and rating section of some items of the AGREE Ⅱinstrument to be applicable to the evaluation of TCM guidelines. Study showed that existing domestic and international guideline evaluation instruments (including AGREE II) are not directly applicable to TCM guidelines due to certain limitations. For example, a 7-point scale is used in some instruments, but scoring criteria for each value is not described; some evaluation fields do not conform to conditions in China; some items do not apply to the field of TCM (Bai et al., 2020a). At present, there is no relevant research on whether AGREE II is applicable to TCM CPGs. In only one study, AGREE II was used to evaluate the methodological quality of TCM CPGs, but there was no further research on its applicability to evaluating TCM CPGs (Yao, 2016). A guideline quality evaluation system in line with the characteristics of TCM may not only guide the process and methods of formulating guidelines regarding TCM diagnosis and treatment, but also promote scientific development of methodology of formulating TCM clinical practices. In this study, we attempted to adjust and expand the description and rating section of some items of the AGREE Ⅱinstrument to cover the methodological quality assessment of TCM CPGs based on their characteristics, which may promote the accurate assessment of TCM CPGs, and the presentation of opinions that help improve the procedures and methods for TCM CPGs.
2 Methods
2.1 Establishing a research working group
Firstly, we established a research working group for this study. It consisted of: 1) A core working group, including professionals in guideline methodology, evidence-based medicine, and clinicians, who were responsible for searching literature, establishing initial items, and collecting and statistically analyzing questionnaires, and writing reports. Each member was required to have experience using AGREE II to evaluate guidelines; 2) An expert consensus group, including guideline methodology experts, evidence-based medicine experts, and clinical experts, who were responsible for evaluating and screening the evaluation instrument’s items.
2.2 Guideline evaluation
2.2.1 Systematic search for guidelines
On 26 March 2020, we performed a systematic literature search from inception till now with the keywords “Guideline*“, “recommendation*“, “statement”, “regulation”, “consensus”, “Chinese medicine”, “Chinese herb” and “TCM”. We searched seven computerized databases [Embase, PubMed and Web of Science, CBM (Chinese Biomedical Literature Database), CNKI (China National Knowledge Infrastructure) and Wanfang Data]; four guideline databases [NGC(National Guideline Clearinghouse), GIN (Guidelines International Network) and NICE (National Institute for Health and Care Excellence)]; one standard database (National public service platform for standard information); and four online book malls.
2.2.2 Guideline screening
The inclusion criteria are as follows: 1) Literature that meets the definition of guideline in AGREE II (CPGs were systematically developed statements to assist practitioner and patient decisions about appropriate healthcare for specific clinical circumstances) (Woolf et al., 1999); 2) Article titles containing “TCM guideline”, or the names of publishing organizations containing “Chinese Medicine”; 3) Issued by official organizations. Exclusion criteria include: 1) abstracts; 2) bibliographic guidelines; 3) outdated, translated or adapted versions of guidelines; 4) acupuncture guidelines. We classified all retrieved guidelines by publishing organizations. In the first round of guideline screening, one guideline from each organization on different specialties would be selected, to ensure that the specialties of each guide were as different as much as possible. Then we analyzed if any specialties were missed. In the second round, we screened guidelines, issued by organizations having published more than two guidelines, for ones that focused on specialties left out in the first round. For the guidelines published by the same institution that are combined into books, only one of the guidelines for a particular specialty are selected.
2.2.3 Evaluation of guideline quality
Two trained reviewers from the core working group (Xiuli Xie, Yangyang Wang) who were familiar with guideline development methodology independently apply AGREE Ⅱ to evaluate the eligible CPGs’ methodological quality. The reviewers analyzed which TCM guideline characteristics were different from modern medical guidelines. Finally, based on the evaluation of the TCM guidelines experience, the two reviewers suggested adjustments and supplementary comments to some items of AGREE Ⅱ, and drafted an initial version of AGREE Ⅱ for TCM.
2.3 Systematic search for literature on TCM clinical guideline development and evaluation
2.3.1 Literature retrieval
On 21 May 2020, we performed a systematic literature search with the keywords “Guideline*“, “Chinese medicine”, “Chinese herb”, “TCM”, “development”, “evaluation” and “methodology”. The search date range began with each database’s inception date. We used four Chinese computerized databases: CBM, CNKI and Wanfang Data.
2.3.2 Literature screening
The inclusion criteria are literature on the development and evaluation of TCM clinical guidelines, including but not limited to the literature about evaluation by AGREE II. A total of 40 articles were included. We extracted the results of TCM clinical guideline quality evaluations in all the literature to establish a database, giving special attention to any material on deficiencies in TCM clinical guidelines and difficulties about the quality of TCM clinical guidelines. We also refer to the difficulties and solutions of establishing quality evaluation instruments (Bai et al., 2020b), applicability evaluation instruments (Bai et al., 2020c) and guideline reporting standards (Xia, 2019) for TCM guidelines in the study on how to make the evaluation instruments and reporting standards conform to TCM’s characteristics. All material in the database was classified according to the AGREE Ⅱ items.
The members of the core working group held a meeting to discuss the initial version of AGREE Ⅱ for TCM Further suggestions were proposed for adjustments and additions to improve the initial version, which were adopted into a revised version.
2.4 Expert consensus
Expert consensus method was applied, consisting of one or two rounds of expert investigation in the form of electronic questionnaire. The composition of the expert group is shown in Supplementary File S3. If experts’ opinions were convergent after the first round of the survey, the second round of investigation would be skipped. We asked experts to score the importance of each item. For the evaluation index, we adopted Likert’s five scale scoring method (Dawes, 2008), which were “strongly agree” “agree”, “somewhat agree”, “disagree” and “strongly disagree”, assigned 5, 4, 3, two and one points, respectively.
If not agreeing to certain content of the items completely, they would be invited to offer advice to modify it. Next, questionnaires were collected and sorted. The team members would then optimize the evaluation items’ content according to the results and drafted the final version of AGREE Ⅱ for TCM based on the expert consensus reached.
2.5 Statistical analysis
We analyzed expert consensus using the degree of expert positivity and authority (Zeng, 1996). Expert positivity coefficient was the recovery rate for effective questionnaires. The principles of judging a questionnaire invalid were as follows: 1) Unqualified answers accounted for a large proportion of the questionnaire. For example, if all evaluated items in a questionnaire received full points, it would be judged invalid. 2) The percentage of missing answers to key variables in a questionnaire exceeded 15%.
Experts’ degree of authority is expressed by the expert authority coefficient (Cr), which is generally determined by two factors: one is the basis for experts to make judgments on problems, as represented by Ca, and the other is the coefficient of experts’ familiarity with indicators, represented by Cs. Authority equals to half of the sum of judgment coefficient and familiarity, that is:
The authority of experts is primarily based on self-evaluation. There is a functional relationship between experts’ authority and the prediction accuracy, with the latter increases along with the former. Furthermore, evaluation basis involves four dimensions: theoretical analysis, practical experience, knowledge from peers, and intuition. Each dimension is divided into three levels: high, medium and low, according to the degree of influence on expert judgment. The values are as follows (Zeng, 1996): theoretical analysis (0.3, 0.2, 0.1), practical experience (0.5, 0.4, 0.3), knowledge from peers (0.1, 0.1, 0.1) and intuition (0.1, 0.1, 0.1, 0.1). The degree of familiarity can be divided into five grades: 1, 0.8, 0.6, 0.4, and 0.2.
Statistical analysis was conducted in Statistical Package for the Social Sciences (SPSS) 17.0. Concentration degree for experts’ opinions was represented by arithmetic mean, grade sum and full score rate; coordination degree for experts’ opinions was represented by a variation coefficient and a coordination coefficient.
3 Results
3.1 Establishing the initial version
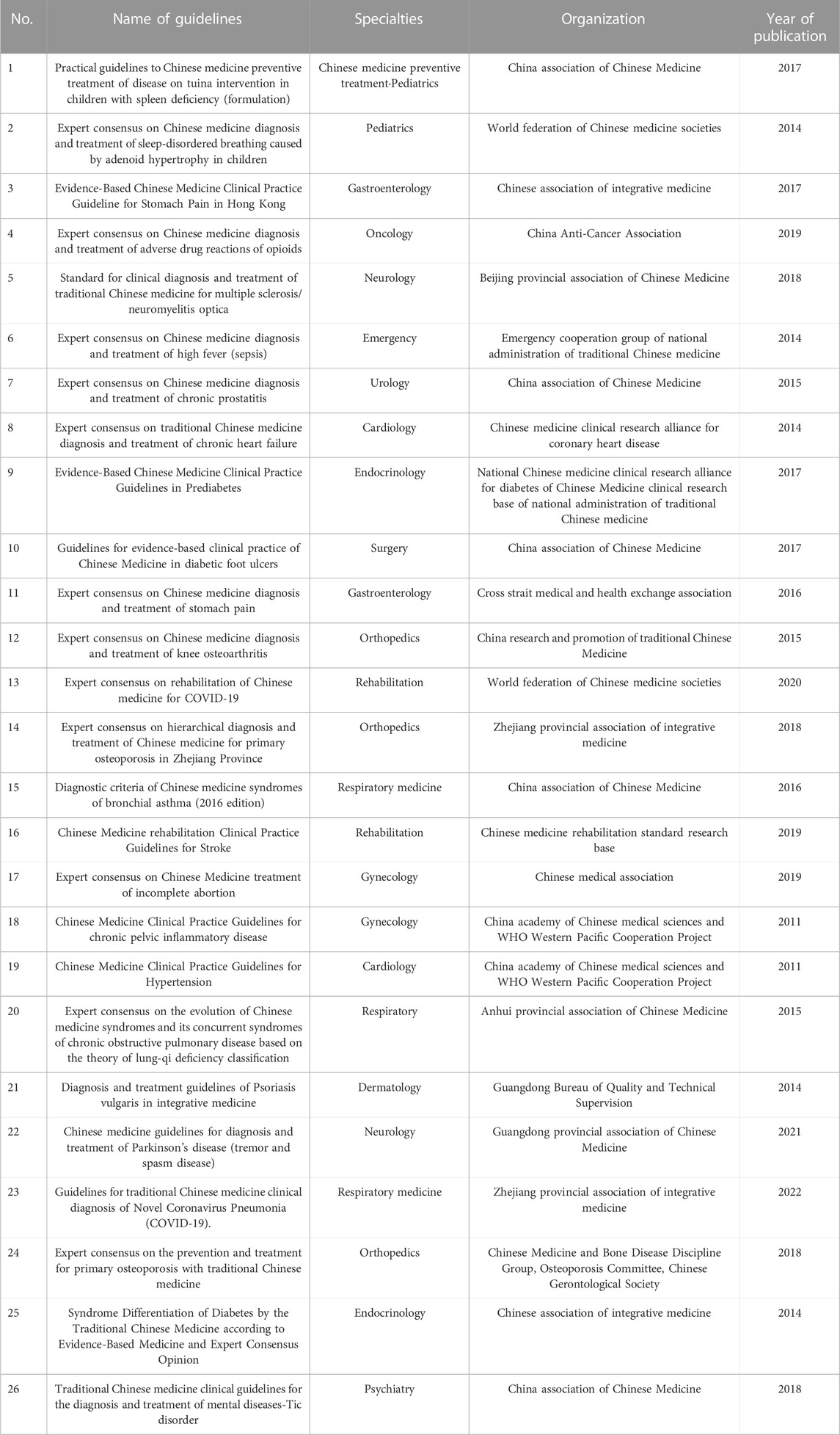
A total of 518 sets of guidelines were included in this study, of which 17 sets were published in English and 501 were published in Chinese. They were developed by 19 organizations. After analysis, similarity was found among guidelines issued by a same organization, in terms of their development method, reporting structure and form. Therefore, it was unnecessary to include guidelines issued by a same organization,, while the comprehensiveness of the clinical specialties involved, and year of publication involved should be taken into consideration. We selected guidelines published in the recent 10 years whenever possible. Eleven organizations published only one guideline, while some published more than two, with the organization that published the most TCM guidelines being China association of Chinese medicine, having issued 465 guidelines. Numbers of guidelines developed by different organizations are shown in Supplementary File S1. After two rounds of screening, we eventually included a total of 26 sets of guidelines for evaluation (Chen et al., 2017; Sun et al., 2014; Linda et al., 2017; Geng et al., 2019; Fang and Wang, 2018; Liu et al., 2014; Li et al., 2015; Mao and Zhu, 2014; Fang et al., 2017; Wang and Xue, 2015; Li 2016; Chen et al., 2015; Li and Zhang, 2020; Yao et al., 2018; Li and Wang, 2016; Lin et al., 2019; Integrative Medicine Group of the Eighth Committee of the Chinese Medical Association, Family Planning Branch, 2019; China association of Chinese Medicine, 2011a; China association of Chinese Medicine, 2011b; Key Research Unit of COPD Lung-Qi Deficiency Syndrome et al., 2015; Guangdong Bureau of Quality and Technical Supervision, 2014; Guangdong Provincial Association of Chinese Medicine, 2021; Jiaxing Standard Quality Construction Promotion Association, 2021; Chinese Medicine and Bone Disease Discipline Group et al., 2015; Guo et al., 2014; China Association of Chinese Medicine, 2018) (Supplementary File S2). Based on the evaluation of the TCM guideline experience, the two evaluators offered adjustments and supplementary comments which were applicable to evaluating TCM CPGs for the “user’s manual description” section and “how to rate” section of some items of the AGREE Ⅱ instrument. The following items are included: Domain 1 (Item 1), Domain 3 (Items 7, 10, 11, 12), Domain 4 (Item 15), and Domain 5 (Item 18) (Table 1). We then drafted the initial version of AGREE Ⅱ for TCM.
3.2 Improving the initial version of AGREE Ⅱ for TCM
Based on the initial version of AGREE Ⅱ for TCM and the literature on TCM clinical guideline development and quality evaluation, the core working group improved the initial version of AGREE Ⅱ for TCM. After discussion, modification was proposed to AGREE Ⅱ Instrument Domain 1 (Items 1, 2), Domain 2 (Items 4, 5), Domain 3 (Items 8, 13), and Domain 5 (Item 20) after discussion (Table 2). Meanwhile, the AGREE Ⅱ Instrument entailed a 7-point rating table, but there were no specific scoring criteria for each point, which could introduce subjectivity, and prolong time of evaluation. The core working group agreed to add details on evaluation rules and deduction criteria in the “Evaluation” part of each item.
3.3 Expert consensus for the revised version
3.3.1 The experts
The expert group’s primary responsibility was to reach a consensus on the items. Sixteen members were invited into the expert group. They were professionals in TCM or evidence-based medicine from around the world (Beijing, Guangzhou, Shenzhen, Lanzhou, Canada, etc.), and are representative in terms of expertise regionally. Among them, 11 were male (68.8%), and five female experts (31.3%). Average age was 32.9 ± 5.21 years (range: 27–46 years), and average length of time invested in related professional fields was 7.5 ± 4.47 years (range: 2–20 years).
3.3.2 Experts’ positivity coefficient and authority
We collected 16 questionnaires, with a responding rate of 100%. None of the questionnaires were invalid, and the experts’ positivity coefficient was 100%.
Generally, experts’ authority coefficient is no less than 0.70, which is acceptable (Li 2001). All experts in this study had authority coefficients no less than 0.838, indicating that they had strong authority on the investigation content.
3.3.3 Concentration of experts’ opinions
The indicators of experts’ opinion concentration include arithmetic mean, full score ratio and grade sum. The meanings of the three indicators are as follows: arithmetic mean is the mean of the evaluation index scores. A mean score of an item is less than or equal to three indicates that this item is unreasonable, and should be revised according to the expert’s opinion before reaching another round of consensus. Full score ratio (Ki) refers to the percentage of experts who “strongly agree” with an item. Ki ≤ 30% indicates that experts deem that the item offers little contribution to the greater evaluation system, and it is one of the conditions for adjusting the items’ content. When an item’s Ki = 0, the item should be deleted. The grade sum (Si) represents the total expert recognition score for an item; it represents the item’s necessity to the greater evaluation system.
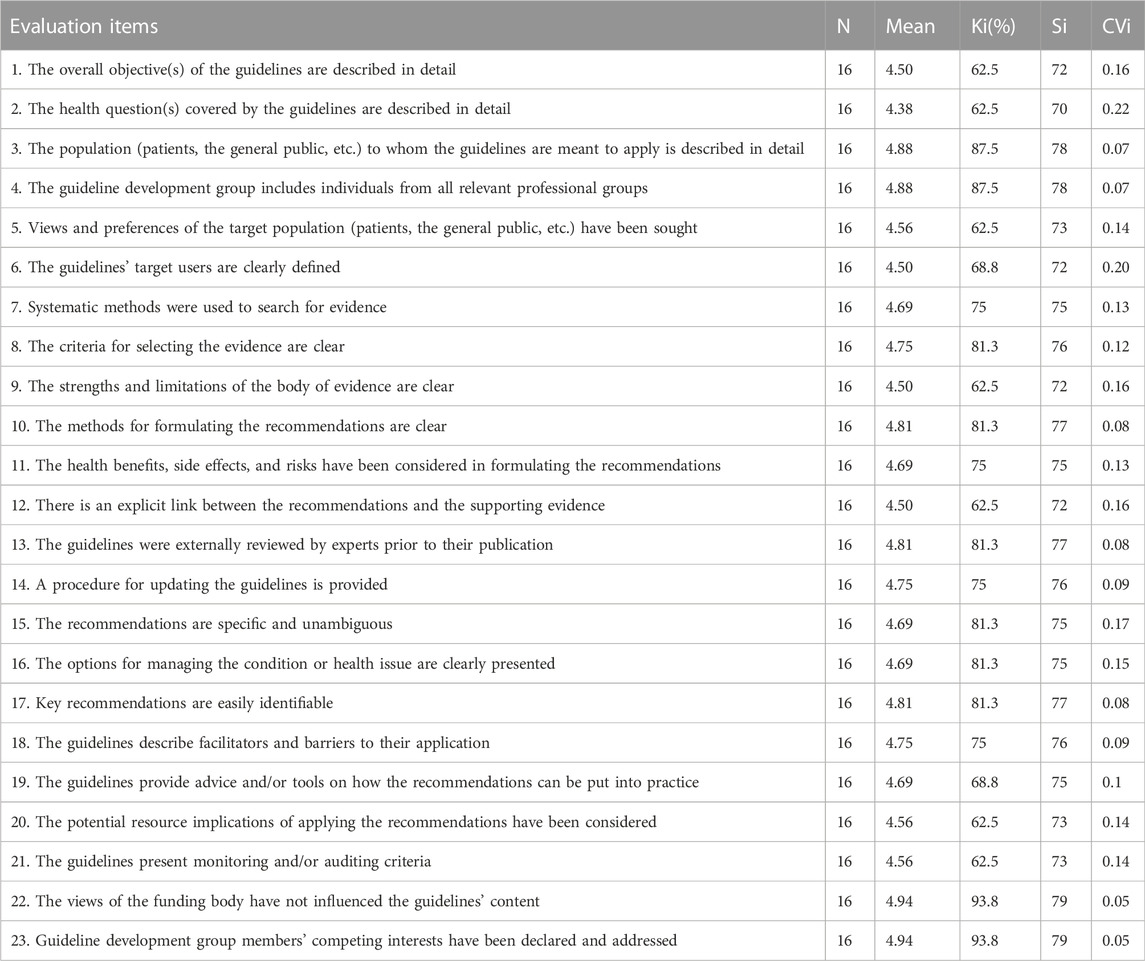
The results of the first round of expert surveys show that experts are in strong agreement for all items. The arithmetic mean of all items is greater than or equal to 4.38, the Ki is greater than or equal to 62.5%, and the Si is greater than or equal to 70 (Table 3).
3.3.4 Degree of coordination in experts’ opinions
3.3.4.1 Coefficient of variation
This shows the experts’ fluctuation degree (or coordination degree) for the relative importance of an item. The smaller the CVI, the more coordinated the experts’ evaluation of an item’s importance. The results showed that each item’s Cvi fluctuated between 0.05 and 0.22, the Cvi was small, and the consistency was high (Table 3).
3.3.4.2 Coefficient of coordination (kendall coefficient)
The coefficient of coordination indicates the overall consistency of all experts’ opinions on the importance of all items. We tested the Kendall coordination coefficient, and determined that the coordination coefficient was 0.10, p < 0.05. We considered this coordination coefficient statistically significant, with expert consistency and reliable results (Table 3).
3.4 The final version of AGREE Ⅱ for TCM
After the first round of investigation, each item in this evaluation system received strong approval from the experts, and the experts’ degree of authority was high. Therefore, a second-round investigation was unnecessary. The experts proposed modifying AGREE Ⅱ Instrument Domain 2 (Items 6), Domain 4 (Items 16) and Domain 5 (Items 19) (Supplementary File S4). Some experts offered suggestions on the expression of some of the items. After being deliberated by the core working group, the expert opinions were adopted to improve the item without affecting the items’ core contents. Finally, under the framework of AGREE II, we finalized the AGREE Ⅱ for TCM.
This new version of AGREE Ⅱ tailored for TCM incorporates the structure, scoring method and main content of the items from AGREE II. The evaluation instrument includes six domains (Scope and Purpose, Stakeholder Involvement, Rigor of Development, Presentation Clarity, Applicability and Editorial Independence), with 23 items in total, and two overall assessment items. We modified and supplied content for the description and rating of several items from AGREE II. This included adjustments and expansion to Items 1, 4, 5, 7, 8, 10, 11, 12, 15, and 19 so that the instrument would be more suitable for clinical TCM guidelines. When providing evaluation examples for each item, we tried to list as many examples from TCM CPGs as possible in items 1, 2, 6, 13, 15, 16, 18, and 20. A total of 16 items were included. We list the full content of all adjusted and expanded items in Supplementary File S4. Any content which has been added to or expanded from AGREE II is underlined in each item. In the “rating” section, we provided detailed explanations on how to score, specifying elements corresponding to each score. A score of one should be given when the information that is relevant to the item is poorly or not reported, or if the authors state explicitly that criteria were not met. A score of seven should be given where all scoring criteria have been met. Two to three points will be deducted if information related to a criterium under an Item is incomplete. Points will be deducted cumulatively according to how many criteria are not met.
4 Discussion
In this study, we develop the AGREE Ⅱfor TCM instrument to cover the methodological quality assessment of TCM CPGs based on their characteristics. Currently, adaptation and extension studies of AGREE II have been conducted in several fields, including AGREE -S for surgical interventions (Logullo et al., 2022) and AGREE -China for the evaluation of Chinese CPGs (Wang et al., 2018), etc. No extension studies of AGREE Ⅱ for TCM are available yet.
The AGREE Ⅱfor TCM instrument was developed in an accurate and transparent manner in accordance with international standard methods. Methods of guideline evaluation, literature analysis and expert consensus were used during the development of AGREE Ⅱ for TCM. We conducted a systematic search of TCM clinical guidelines, which were classified according to their publishing organizations. Then, we analyzed any characteristics which were different from modern medical guidelines by combining them with each item of AGREE II. Finally, we proposed which contents should be included as items in the new evaluation system. This ensured that the adjusted evaluation system would reflect TCM characteristics as much as possible, and highlighted TCM guidelines’ unique contents and development methods. Distinguishing this part of the study from other TCM guideline quality evaluation studies (Yao, 2016) is that the purpose of conducting TCM guideline evaluation in this study is not to evaluate the quality of TCM guidelines. Rather, it is to understand these items’ applicability to TCM guidelines through evaluation.
By searching the literature on the development and evaluation of TCM guidelines, we collected all problems reported in the literature. After several discussions, we integrated them, and improved the initial version. Based on literature integration and group discussion, we improved them according to the expert opinions and reached an expert consensus. This is how we produced the final version of AGREE Ⅱ for TCM. The research methods above can be used to catalog opinions on TCM guidelines, from content reports to development methodology, and to attain a high degree of expert consensus. This ensures that the research results are scientific, comprehensive and applicable.
During the adjustment and expansion of AGREE II, we added the requirements for, and examples of, TCM guidelines in the description and rating section of some items of the AGREE II instrument to develop the AGREE II for TCM instrument. For example, we used Item one to evaluate whether the purpose of the guidelines was clear, as it was proposed that “the treatment purpose and advantages of the guideline should be described in detail. If the disease in the guidelines is based on Western medicine, its corresponding TCM name needs to be reported. If the disease in the guidelines is based in TCM, its corresponding category of diseases in Western medicine must be reported too”. In terms of highlighting the advantages of TCM, we point out the advantages of TCM in the treatment of a disease must proposed, such as preventing disease recurrence or reducing the side effects of Western medicine. In the context of the widespread use of modern medicine, it is beneficial to explain the therapeutic advantages of TCM in the TCM guidelines to clarify the timing and promote the use of TCM. Item four requires that the development team include experts of TCM or integrated Chinese and Western medicine. Development staff specialization is one of the most important measures for ensuring TCM guidelines’ professionalism, and ensuring that the ideas and methods of guideline development are more compatible to TCM; Item seven requires that systematic methods be applied to retrieve evidence, and information resources include ancient Chinese medicine literature databases such as Chinese medical dictionary and modern Chinese electronic literature databases such as CNKI, Wanfang, VIP and CBM. The keywords must include the TCM disease name corresponding to the disease, such as “traditional Chinese medicine, Chinese patent medicine, herbal medicine “, etc. This item is used to evaluate the rationality and comprehensiveness of the retrieval of TCM diagnosis and treatment guidelines. This is done by evaluating whether the retrieval database and keywords have TCM elements; Item 12 requires that there be a clear relationship between the recommendations and supporting evidence, and that there be a clear corresponding relationship between the etiology and pathogenesis, syndrome differentiation and treatment principles, treatment formulas or Chinese patent medicine in the TCM guidelines. The composition of the treatment formulas in the recommendation must have the same name and composition as those in the supporting evidence. The corresponding relationship between TCM theory, principle, method, prescription and medicine must also be considered when formulating recommendations.
AGREE Ⅱ for TCM does not add and delete any item from AGREE II. Rather, only some content related to TCM is added in the “Description” and “how to rate” section, describing specific scoring criteria for evaluation and improving the instruments’ operability. It not only retains the scientificity and rigor of AGREE II, but also highlights the characteristics of TCM, and supplements and improves AGREE II. Applying this evaluation system to the evaluation of TCM CPGs can accurately and scientifically reflect the quality of TCM guidelines, while promoting the normative formulation and clinical application of TCM CPGs. We are planning a validation study of this instrument to see how it works with people outside of this research project. We will promote and disseminate it to more TCM guideline development organizations and hope to continue to modify and improve the AGREE Ⅱfor TCM instrument in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical stetement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the [patients/ participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
HL designed the study and reviewed the manuscript. XX collected and analyzed the data and wrote the manuscript. YW assisted with data analysis and manuscript preparation.
Funding
This study was supported by Guangdong Provincial Hospital of Chinese Medicine’s Specific Research Fund for TCM Science and Technology (No. YN2020QN17/No. YK2013BINOI/YN2019QL17); Collaborative Innovation Team of Guangzhou University of Chinese Medicine "Double First Class" and High-level University Discipline (2021XK08); special project of the State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2021ZZ02).
Acknowledgments
The authors would like to thank the participants who took part in the expert consensus. Without them, this study would not have been possible. AGREE II for TCM does not add and delete any item from AGREE II. The results of this study are only for academic communication and not for commercial use.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1057920/full#supplementary-material
References
Bai, X., Liu, J., Guo, Y., Feng, X., Yang, S., Fang, S., et al. (2020b). Quality evaluation recommendation list and interpretation of traditional Chinese medicine clinical practice guidelines. China J. Chin. Materia Medica 45, 1600–1605. doi:10.19540/j.cnki.cjcmm.20191230.502
Bai, X., Liu, J., Guo, Y., Feng, X., Fang, S., Yang, S., et al. (2020a). Consideration on establishing evaluation system of clinical practice guidelines in traditional Chinese medicine. China J. Chin. Materia Medica 45, 1596–1599. doi:10.19540/j.cnki.cjcmm.20191230.503
Bai, X., Liu, J., Guo, Y., Yang, S., Fang, S., Guang, Y., et al. (2020c). Applicability evaluation recommendation list and interpretation of traditional Chinese medicine clinical practice guidelines. China J. Chin. Materia Medica 45, 1606–1610. doi:10.19540/j.cnki.cjcmm.20191230.504
Brouwers, M. C., Kiio, M. E., Browman, G. P., Burgers, J. S., Cluzeau, F., Feder, G., et al. (2012). The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. J. Clin. Epidemiol. 65, 526–534. doi:10.1016/j.jclinepi.2011.10.008
Burgers, J. S., Fervers, B., Haugh, M., Brouwers, M., Browman, G., Philip, T., et al. (2004). International assessment of the quality of clinical practice guidelines in oncology using the appraisal of guidelines and research and evaluation Instrument. J. Clin. Oncol. 22, 2000–2007. PMID: 15143093. doi:10.1200/JCO.2004.06.157
Chen, W., Liu, X., Tong, P., and Zhan, H. (2015). Expert consensus on Chinese medicine diagnosis and treatment of knee osteoarthritis (2015 edition). J. Traditional Chin. Orthop. Traumatology 27, 4–5.
Chen, X., Wu, Y., Wang, J., and Yu, X. (2017). Practical guidelines to Chinese medicine preventive treatment of disease on tuina intervention in children with spleen deficiency (formulation). J. Pediatr. Traditional Chin. Med. 13, 5–8. doi:10.16840/j.issn1673-4297.2017.02.02
China association of Chinese Medicine (2011a). “Chinese Medicine Clinical Practice Guidelines for chronic pelvic inflammatory disease,” in China association of Chinese Medicine.Evidence-based guidelines of clinical practice in Chinese medicine for internal medicine (Beijing: China press of traditional Chinese medicine), 227–249.
China association of Chinese Medicine (2011b). “Chinese medicine clinical practice guidelines for hypertension,” in China association of Chinese Medicine.Evidence-based guidelines of clinical practice in Chinese medicine for specific disease (Beijing: China press of traditional Chinese medicine), 107–125.
China association of Chinese Medicine (2018). T/CACM 1133-2018 Traditional Chinese medicine clinical guidelines for the diagnosis and treatment of mental diseases-Tic disorder. Beijing: China press of traditional Chinese medicine.
Chinese Medicine and Bone Disease Discipline GroupOsteoporosis CommitteeChinese Gerontological Society (2015). Expert consensus on the prevention and treatment for primary osteoporosis with traditional Chinese medicine. Chin. J. Osteoporos. 21, 1023–1028. doi:10.3969/j.issn.1006-7108.2015.09.001
Dawes, J. (2008). Do data characteristics change according to the number of scale points used? An experiment using 5-point, 7-point and 10-point scales. Int. J. Mark. Res. 50, 61–104. doi:10.1177/147078530805000106
Fang, C., Tong, X., Duan, J., Ni, Q., Wei, J., Xie, C., et al. (2017). Evidence-based Chinese medicine clinical practice guidelines in prediabetes. J. Traditional Chin. Med. 58, 268–272. doi:10.13288/j.11-2166/r.2017.03.023
Fang, Y., and Wang, S. (2018). Standard for clinical diagnosis and treatment of traditional Chinese medicine for multiple sclerosis/neuromyelitis optica. J. Cap. Med. Univ. 39, 833–835.
Geng, G., Jia, L., Jia, Y., Jiang, Y., Li, J., Li, P., et al. (2019). Expert consensus on Chinese medicine diagnosis and treatment of adverse drug reactions of opioids. Chin. J. Clin. Oncol. 46, 321–323.
Guangdong Bureau of Quality and Technical Supervision (2014). DB44/T 1423-2014 Diagnosis and treatment guidelines of Psoriasis vulgaris in integrative medicine. Guangzhou: Guangdong Institute of Standardization.
Guangdong provincial association of Chinese Medicine (2021). T/GDACM 0103-2021 Chinese medicine guidelines for diagnosis and treatment of Parkinson's disease (tremor and spasm disease). Guangzhou: Guangdong provincial association of Chinese Medicine.
Guo, J., Chen, H., Song, J., Wang, J., Zhao, L., and Tong, X. (2014). Syndrome differentiation of diabetes by the traditional Chinese medicine according to evidence-based medicine and expert consensus opinion. Evid. Based. Complement. Altern. Med. 2014, 492193. doi:10.1155/2014/492193
Huang, Q., Chen, Y., Jiang, S., Wang, X., and Wang, Q. (2018). The standardization development of clinical practice guidelines in China under the background of deepening medical reform. Chin. Health Qual. Manag. 25, 43–45. doi:10.13912/j.cnki.chqm.2018.25.4.14
Institute of Medicine (2011). Clinical Practice Guidelines we can trust. Washington, DC: National Academies Press.
Integrative Medicine Group of the Eighth Committee of the Chinese Medical Association, Family Planning Branch (2019). Expert consensus on Chinese Medicine treatment of incomplete abortion. China J. Traditional Chin. Med. Pharm. 34, 3625–3629.
Jiang, Y., and Chen, K. (2016). Development and quality evaluation of evidence-based clinical practice guidelines of Chinese medicine. Chin. J. Integr. Traditional West. Med. 36, 11–15.
Jiaxing Standard Quality Construction Promotion Association (2021). T/JX 043-2022 Guidelines for traditional Chinese medicine clinical diagnosis of Novel Coronavirus Pneumonia (COVID-19). Jiaxing, Zhejiang Province, China: Jiaxing Standard Quality Construction Promotion Association.
Key Research Unit of COPD Lung-Qi Deficiency SyndromeState Administration of Traditional Chinese MedicineAnhui Provincial Association of Traditional Chinese MedicineChinese Medicine Pulmonary Disease Committee (2015). Expert consensus on the evolution of Chinese medicine syndromes and its concurrent syndromes of chronic obstructive pulmonary disease based on the theory of lung-qi deficiency classification. Chin. J. Integr. Traditional West. Med. Intensive Crit. Care 22, 113–114.
Li, H., Wang, B., and Zhao, B. (2015). Expert consensus on Chinese medicine diagnosis and treatment of chronic prostatitis. Beijing J. Traditional Chin. Med. 34, 412–415. doi:10.16025/j.1674-1307.2015.05.024
Li, H., Xie, X., Wang, Y., Cai, H., Chen, Y., and Lu, C. (2016). Implementation and evaluation of revised clinical practice guideline of traditional Chinese medicine(integrated traditional Chinese and Western medicine). China J. Traditional Chin. Med. Pharm. 31, 5119–5123.
Li, J. (2016). Expert consensus on Chinese medicine diagnosis and treatment of stomach pain. J. Traditional Chin. Med. 57, 87–90. doi:10.13288/j.11-2166/r.2016.01.023
Li, J., and Wang, Z. (2016). Diagnostic criteria of Chinese medicine syndromes of bronchial asthma (2016 edition). J. Traditional Chin. Med. 57, 1978–1980. doi:10.13288/j.11-2166/r.2016.22.022
Li, J., and Zhang, H. (2020). Expert consensus on rehabilitation of Chinese medicine for COVID-19. Acta Chin. Med. 35, 681–688. doi:10.16368/j.issn.1674-8999.2020.04.154
Li, L. (2001). Statistical applications of social research. Beijing: Social sciences academic press.
Lin, Z., Xue, X., Jiang, Y., You, Y., Wang, J., Zhan, Z., et al. (2019). Chinese medicine rehabilitation clinical practice guidelines for stroke. Rehabil. Med. 29 (6-9), 15.
Linda, Z., Nan-nan, S., Liang, D., Chi, Z. T., Bacon, N. G., Xu-dong, T., et al. (2017). Evidence-based Chinese medicine clinical practice guideline for stomach pain in Hong Kong. Chin. J. Integr. Med. 23, 793–800. doi:10.1007/s11655-016-2586-y
Liu, Q., Zhang, X., Kong, L., Yao, W., and Xue, G. (2014). Expert consensus on Chinese medicine diagnosis and treatment of high fever (sepsis). J. Emerg. Traditional Chin. Med. 23, 1961–1963.
Logullo, P., Florez, I. D., Antoniou, G. A., Markar, S., López-Cano, M., Silecchia, G., et al. (2022). AGREE-S: AGREE II extension for surgical interventions - united European gastroenterology and European association for endoscopic surgery methodological guide. United Eur. Gastroenterol. J. 10 (4), 425–434. doi:10.1002/ueg2.12231
Mao, J., and Zhu, M. (2014). Expert consensus on traditional Chinese medicine diagnosis and treatment of chronic heart failure. J. Traditional Chin. Med. 55, 1258–1260. doi:10.13288/j.11-2166/r.2014.14.026
Sun, S., Ma, Y., Qiao, J., Duan, Y., and Wei, H. (2014). Expert consensus on Chinese medicine diagnosis and treatment of sleep-disordered breathing caused by adenoid hypertrophy in children. World J. Sleep Med. 1 (313), 316–320.
The AGREE Next Steps Consortium (2009). Appraisal of guidelines for research & evaluation II. Hamilton: The AGREE Research Trust.
Wang, J., Wang, Q., Wang, X., Jin, X., Zhang, B., and Gao, X. (2018). Development and initial validation of a clinical practice guideline evaluation system in China. Shanghai Med. J. 41 (06), 321–326.
Wang, J., and Xue, Y. (2015). Guidelines for evidence-based clinical practice of Chinese Medicine in diabetic foot ulcers. Chin. J. Surg. Integr. Traditional West. Med. 21, 540–543.
Woolf, S. H., Grol, R., Hutchinson, A., Eccles, M., and Grimshaw, J. (1999). Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ 318, 527–530. PMID: 10024268; PMCID: PMC1114973. doi:10.1136/bmj.318.7182.527
Wu, M., Zhang, S., Zhou, S., Zhang, Y., and Li, X. (2016). Use and demand of clinical practice guidelines in China. Chin. J. Med. Libr. Inf. Sci. 25, 37–42.
Xia, Y. (2019). Research on reporting standard of clinical practice guidelines for traditional Chinese medicine based on RIGHT statement. dissertation/master's thesis (Guangzhou: Guangzhou University of Chinese Medicine).
Yao, L. (2016). The methodology quality of clinical practice guidelines for traditional Chinese medicine. dissertation/master's thesis (Lanzhou: Lanzhou University).
Yao, X., Shi, X., Wang, J., Fang, S., Yin, H., Shi, S., et al. (2018). Expert consensus on hierarchical diagnosis and treatment of Chinese medicine for primary osteoporosis in Zhejiang Province. Zhejiang J. Traditional Chin. ‘[Med. 53, 237–241. doi:10.13633/j.cnki.zjtcm.2018.04.002
Keywords: agree Ⅱ, adjust and expand, guidelines, quality, evaluation
Citation: Xie X, Wang Y and Li H (2023) AGREE II for TCM: Tailored to evaluate methodological quality of TCM clinical practice guidelines. Front. Pharmacol. 13:1057920. doi: 10.3389/fphar.2022.1057920
Received: 30 September 2022; Accepted: 01 December 2022;
Published: 12 January 2023.
Edited by:
Juei-Tang Cheng, Chang Jung Christian University, TaiwanReviewed by:
Nora Ibargoyen-Roteta, Basque Foundation for Health Innovation and Research, SpainChen Weil, Beijing University of Chinese Medicine, China
Copyright © 2023 Xie, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, bGlodWl0Y21AMTI2LmNvbQ==
 Xiuli Xie
Xiuli Xie Yangyang Wang
Yangyang Wang Hui Li1,2,3*
Hui Li1,2,3*