- 1Department of Family Medicine, Taipei Medical University Hospital, Taipei, Taiwan
- 2Department of Education, Center for Evidence-Based Medicine, Taipei Medical University Hospital, Taipei, Taiwan
- 3Department of Surgical Oncology, Fox Chase Cancer Center, Philadelphia, PA, United States
- 4Section of Neurosurgery, Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 5Department of Internal Medicine, Lower Bucks Hospital, Bristol, PA, United States
- 6Department of Internal Medicine, Taipei Medical University Hospital, Taipei, Taiwan
Proton pump inhibitors (PPI), one of the most commonly prescribed medications, carry a myriad of adverse events. For colorectal cancer (CRC) patients, it still remains unclear whether the concurrent use of proton pump inhibitors (PPI) would negatively affect chemotherapy. PubMed, Medline, Embase, and Cochrane Library were searched from inception to 10 June 2022, to identify relevant studies involving CRC patients receiving chemotherapy and reporting comparative survival outcomes between PPI users and non-users. Meta-analyses were performed using random-effects models. We identified 16 studies involving 8,188 patients (PPI = 1,789; non-PPI = 6,329) receiving either capecitabine-based or fluorouracil-based regimens. The overall survival (HR, 1.02; 95% CI, 0.91 to 1.15; I2 = 0%) and progression-free survival (HR, 1.15; 95% CI, 0.98 to 1.35; I2 = 29%) were similar between PPI users and non-users in patients taking capecitabine-based regimens, with low statis-tical heterogeneity. Although the subgroup analysis indicated that early-stage cancer patients taking capecitabine monotherapy with concurrent PPI had a significantly higher disease progression rate (HR, 1.96; 95% CI, 1.21 to 3.16; I2 = 0%) than those who did not use PPIs, both groups had comparable all-cause mortality (HR, 1.31; 95% CI, 0.75 to 2.29; I2 = 0%). On the other hand, there was little difference in both OS and PFS in both early- and end-stage patients taking capecitabine combination therapy between PPI users and non-users. Conversely, the use of concomitant PPI in patients taking fluorouracil-based regimens contributed to a marginally significant higher all-cause mortality (HR, 1.18; 95% CI, 1.00 to 1.40; I2 = 74%), but with high statistical heterogeneity. In conclusion, PPI has little survival influence on CRC patients treated with capecitabine-based regimens, especially in patients taking capecitabine combination therapy. Thus, it should be safe for clinicians to prescribe PPI in these patients. Although patients treated with fluorouracil-based regimens with concomitant PPI trended toward higher all-cause mortality, results were subject to considerable heterogeneity.
Systematic Review Registration: identifier https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022338161
Introduction
Proton pump inhibitor (PPI) is a ubiquitous medication among clinicians’ armamentarium and is generally, but not exclusively, prescribed for gastroesophageal reflux disease, peptic ulcer disease, prevention of NSAID-associated ulcers, and a pivotal part of H. pylori eradication (Strand et al., 2017). PPI is also the most commonly used gastric acid suppressants in cancer patients. In addition, in the National Comprehensive Cancer Network (NCCN) guidelines for antiemesis recommended the use of either histamine-2 blocker (H2-blocker) or PPI in the management of dyspepsia in cancer patients undergoing chemotherapy (Berger et al., 2017). However, long-term use of PPI carries a myriad of adverse events, including infection, chronic kidney disease, hypomagnesemia, osteoporotic fractures (Haastrup et al., 2018), and, most notably, dysbiosis effects (Routy et al., 2018), which are well-recognized for the disruption of gut microbiota with subsequent impairment of the effectiveness of immune checkpoint inhibitors and chemotherapy in cancer patients (Roy and Trinchieri, 2017). For instance, PPI has been shown to pose detrimental effects on non-small cell lung cancer patients taking either immunotherapy (Hopkins et al., 2022) or chemotherapy (Chalabi et al., 2020), and urothelial carcinoma patients taking immune checkpoint inhibitors (Hopkins et al., 2020).
However, for colorectal cancer (CRC) patients, it still remains unclear whether the concurrent use of PPI negatively affects chemotherapy as various studies provided conflicting findings. There is concern that with concomitant use of oral chemotherapy agents and PPI, the former would be less effective. Although a post hoc analysis (Kichenadasse et al., 2021) of the N016966 trial (Saltz et al., 2008) suggested that there was no difference in both overall survival (OS) and progression-free survival (PFS) between PPI users and non-users in patients taking capecitabine combined with oxaliplatin (CapeOx), Sun et al. (2016) reported a significant increase in disease recurrence in patients with concomitant PPI, with a 5-year recurrence free survival rate of 74% for PPI users as opposed to 83% for non-users. On the other hand, Wang et al. (2017) showed PPI possessed a positive survival influence on patients receiving FOLFOX chemotherapy, demonstrating PPI increases chemosensitivity in CRC cells arguing that an acidic microenvironment may increase chemoresistance.
A recent systematic review (Patel et al., 2021) suggested that concomitant use of PPI in patients taking capecitabine may result in poorer oncologic outcomes. However, studies included in this review contained contradictory results. For example, Kim et al. (2021) concluded that PPI had no negative impact on capecitabine-based chemotherapy while Wong et al. (2019) found the use of PPI contributed to a significantly higher risk of disease recurrence. Another systematic review (Viñal et al., 2020) attempted to address the same issues, but the regimen was limited to capecitabine, and a meta-analysis has yet to be undertaken. Although a pooled analysis investigated the effect of PPI on fluoropyrimidine chemotherapy (Kichenadasse et al., 2021), a systematic review process was not conducted, and two questions remained unanswered: 1) the effect of PPI on capecitabine-based chemotherapy; 2) the influence of PPI on fluoropyrimidine monotherapy. Due to conflicting results, with several areas of uncertainty in between clinical studies, we are motivated to conduct this systematic review and meta-analysis to delve further into PPI’s influence on the effectiveness of chemotherapy in CRC patients, which could be useful in further studies to understand CRC tumor microenvironment and tumor recurrences.
Materials and methods
We performed this present systematic review and meta-analysis according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2022) and the subsequent results were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and Meta-analysis Of Observational Studies in Epidemiology guidelines (MOOSE) (Supplementary Method S1, S2). The study was registered on PROS-PERO (CRD42022338161).
Study selection
PubMed, Medline, Embase, and the Cochrane Library were searched, from inception up until 10 June 2022. Three investigators (S.S.W, E.A, and M.A) independently identified relevant studies, and discrepancies were addressed by reaching a consensus with the senior reviewers (Y.C.C and K.Y.C). Search details are presented inSupplementary Method S3.
Eligibility criteria
The three predefined criteria for evidence selection were as follows: 1) Randomized controlled trials (RCTs), prospective or retrospective cohort studies; 2) studies involving adult patients aged over 18 with colorectal cancers receiving chemotherapy; 3) studies reporting at least one comparative survival outcome, either overall survival (OS) or progression-free survival (PFS) between PPI users and non-users irrespective of indications.
Data extraction
Two investigators (T.H.W and Y.S.L) independently extracted relevant information from eligible articles, including 1) first author’s name with publication year, 2) study type, 3) country, 4) cancer stage, 5) chemotherapeutic regimen, 6) sample size, 7) history of prior chemotherapy, 8) PPI users, 9) PPI using window, 10) age, 11) Eastern Cooperative Oncology Group (ECOG) performance status, and 12) duration of follow up.
Quality assessment
Two investigators (Y.C and R.B) independently completed a critical appraisal of included literature by using the Cochrane Risk of Bias tool 2.0 (Sterne et al., 2019) for RCTs, and the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) (Sterne et al., 2016) tool for non-RCTs. Any discrepancy was addressed through discussion with the third investigator (Y.N.K).
Main outcomes and statistical analysis
The unadjusted hazard ratio (HR) for OS and PFS were extracted directly from included studies for subsequent pooled analysis. When studies did not report the HR but presented Kaplan-Meier survival curves instead, we acquired an estimated HR from the curves through a well-established method (Parmar et al., 1998) by using a calculation spreadsheet developed by Tierney and colleagues (Tierney et al., 2007). All estimated effects were presented with a 95% confidence interval (CI). Meta-analyses were conducted using RStudio with the “meta” package (Supplementary Method S4). The pooled estimate was based on random-effects with the restricted maximum likelihood (REML) (Harville, 1977) method due to inevitable between-trial variance. Heterogeneity was assessed using I-square (Higgins et al., 2003), with values of I2 < 25%, 25% < I2 <50%, and I2 > 50% indicating low, moderate, and high heterogeneity, respectively. Pre-specified sensitivity analyses included subgroup analyses based on cancer stage, different treatment modification in both capecitabine-based and fluorouracil-based regimens as well as history of prior chemotherapy, and exclusion of studies subject to critical risk of bias. Determination of statistical significance in these analyses followed common threshold (p < 0.05).
Publication bias
For analyses with more than 10 comparisons, a funnel plot was created to qualitatively detect publication bias. We also applied Egger’s test to quantitatively assess significant small study effects, with p <0.05 indicating a positive Egger’s test. When publication bias was suspected according to the Egger’s test, we performed a sensitivity analysis using the trim-and-fill method to impute potentially missing studies and re-estimated the overall effect estimates (Duval and Tweedie, 2000; Peters et al., 2007).
Results
After the systematic review, we identified 38,624 references, with 53 studies for full-text inspection, of which 37 studies did not meet the eligibility criteria (Supplementary Result S1). In the end, a total of 16 studies were included in qualitative and quantitative syntheses (Figure 1).
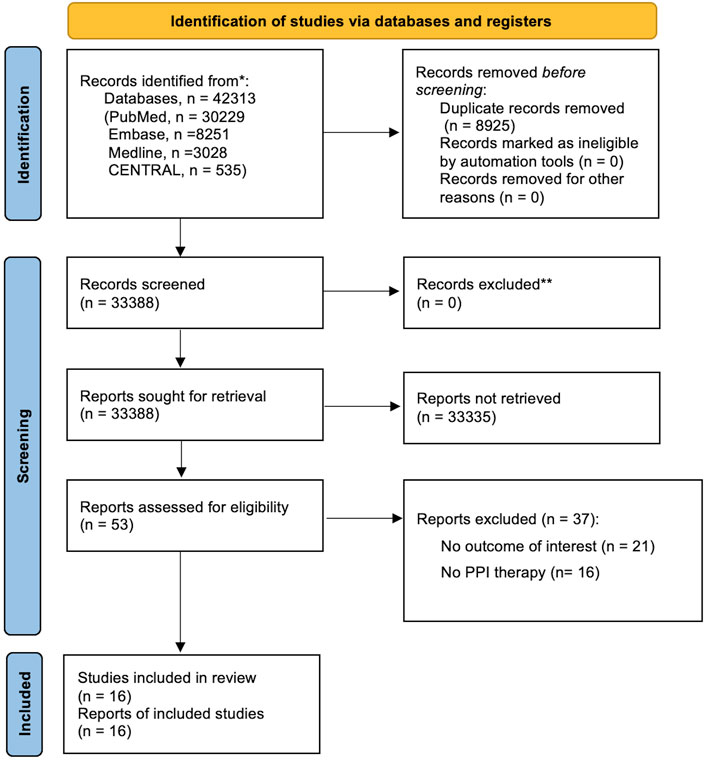
FIGURE 1. PRISMA flowchart diagram. We initially extracted a total of 42,313 potential references, including 30,229 from PubMed, 8,521 from Embase, 3,028 from Medline, and 535 from CENTRAL. After a duplicate exclusion, 33,388 studies were identified. Screening the titles and abstracts yielded 53 full-text articles, the eligibility of which was assessed. 37 studies were excluded after reading whole texts owing to reasons elaborated in Supplementary Results S1 in the following section. Eventually, 16 studies fulfilled the eligibility criteria and were included for qualitative and quantitative syntheses. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of included studies
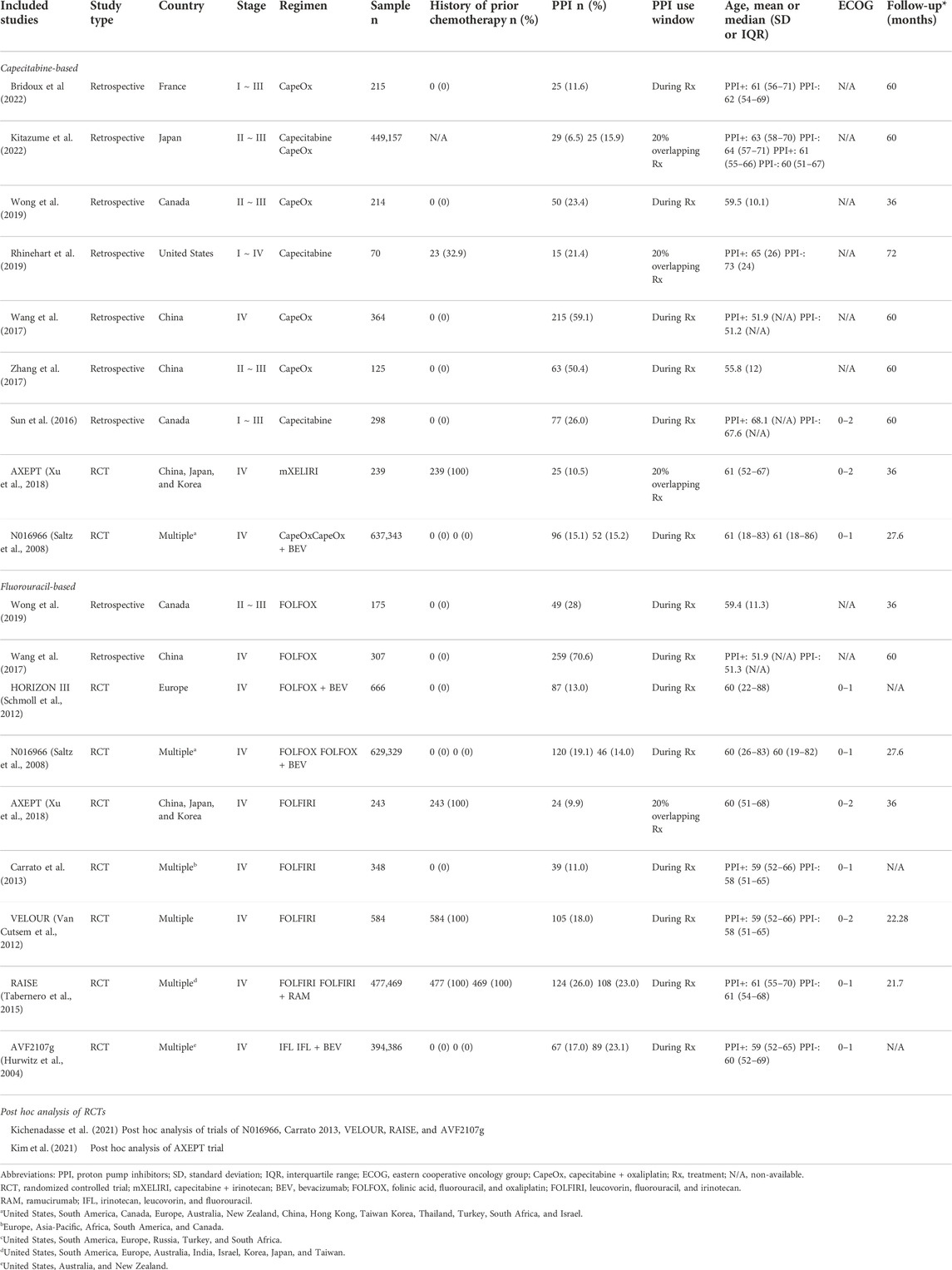
A total of 8,118 patients (PPI = 1,789; non-PPI = 6,329) 295 enrolled between 2000 and 2022 were included in the present 296 study (Table 1). Seven are retrospective studies (Sun et al., 2016; Wang et al., 2017; Zhang et al., 2017; Rhinehart et al., 2019; Wong et al., 2019; Bridoux et al., 2022; Kitazume et al., 2022), Kim et al. (2021) is a post hoc analyses of AXEPT (Xu et al., 2018) trial, and Kichenadasse et al. (2021) is another post hoc analysis of 6 trials, including HORIZON III (Schmoll et al., 2012), N016966 (Saltz et al., 2008), Carrato 2013 (Carrato et al., 2013), VELOUR (Van Cutsem et al., 2012), RAISE (Tabernero et al., 2015), and AVF2107g (Hurwitz et al., 2004). Two post hoc analyses (Kichenadasse et al., 2021; Kim et al., 2021) were included for quantitative analysis, while the RCTs (Hurwitz et al., 2004; Saltz et al., 2008; Schmoll et al., 2012; Van Cutsem et al., 2012; Carrato et al., 2013; Tabernero et al., 2015; Xu et al., 2018) were included for qualitative analysis. Five studies (Sun et al., 2016; Zhang et al., 2017; Wong et al., 2019; Bridoux et al., 2022; Kitazume et al., 2022) enrolled early-stage patients, 8 studies (Hurwitz et al., 2004; Saltz et al., 2008; Schmoll et al., 2012; Van Cutsem et al., 2012; Carrato et al., 2013; Tabernero et al., 2015; Wang et al., 2017; Xu et al., 2018) investigated end-stage patients, and 1 study (Rhinehart et al., 2019) included patients of all stages. Of note, the population enrolled in AXEPT (Xu et al., 2018) trial was Asian only, and included patients from China, Japan, and Korea. Regarding the chemotherapeutic regimens, 9 studies (Saltz et al., 2008; Sun et al., 2016; Wang et al., 2017; Zhang et al., 2017; Rhinehart et al., 2019; Wong et al., 2019; Kim et al., 2021; Bridoux et al., 2022; Kitazume et al., 2022) investigated capecitabine-based regimens, of which, 3 studies (Sun et al., 2016; Rhinehart et al., 2019; Kitazume et al., 2022) examined capecitabine monotherapy, and a total of 7 studies (Saltz et al., 2008; Wang et al., 2017; Zhang et al., 2017; Xu et al., 2018; Wong et al., 2019; Bridoux et al., 2022; Kitazume et al., 2022) investigated capecitabine combination therapy, including regimens of capecitabine plus oxaliplatin (CapeOx), CapeOx plus bevacizumab (BEV), and capecitabine plus irinotecan (mXELIRI). There were another 9 studies (Hurwitz et al., 2004; Saltz et al., 2008; Schmoll et al., 2012; Van Cutsem et al., 2012; Carrato et al., 2013; Tabernero et al., 2015; Wang et al., 2017; Xu et al., 2018; Wong et al., 2019) that concentrated on fluorouracil-based regimens, with 4 studies (Saltz et al., 2008; Schmoll et al., 2012; Wang et al., 2017; Wong et al., 2019) investigating FOLFOX-based regimens, another 4 studies (Van Cutsem et al., 2012; Carrato et al., 2013; Tabernero et al., 2015; Xu et al., 2018) examining regimens of leucovorin plus continuous infusion of fluorouracil plus irinotecan, with or without ramucirumab (FOLFIRI-based regimens), and one other study (Hurwitz et al., 2004) that delved into regimens of irinotecan plus leucovorin plus bolus injection of fluorouracil, with or without BEV (IFL-based regimen). Detailed eligibility criteria for the included studies are elaborated in Supplementary Table S1. The sources of risk of bias mostly arise from bias due to confounding and the classification of interventions (Supplementary Figure S1). No study was evaluated as critical risk of bias. Supplementary Result S2 provides the detailed protocol for the ROBINS-I assessment and elaboration for each domain).
Meta-analysis of capecitabine-based chemotherapy
Our meta-analysis demonstrated that there was no significant difference in survival outcomes for both OS (HR, 1.02; 95% CI, 0.91 to 1.15; I2 = 0%; Figure 2) and PFS (HR, 1.15; 95% CI, 0.98 to 1.35; I2 = 29%; Figure 2) between concomitant PPI-users and non-users. Although scatters in the funnel appeared asymmetrical through the visualization (Figure 2), eggers’ test (Supplementary Result S3; p = 0.09) suggests there was no publication bias in the OS. However, regarding PFS, the funnel plot was asymmetrical and the subsequent eggers’ test detected potential small study effects (Supplementary Result S3; p = 0.008). Therefore, we performed a trim-and-fill analysis with 5 fictive studies being imputed, and notably, the effect estimate (HR, 1.01; 95% CI, 0.84 to 1.22; Supplementary Figure S2) did not alter significantly.
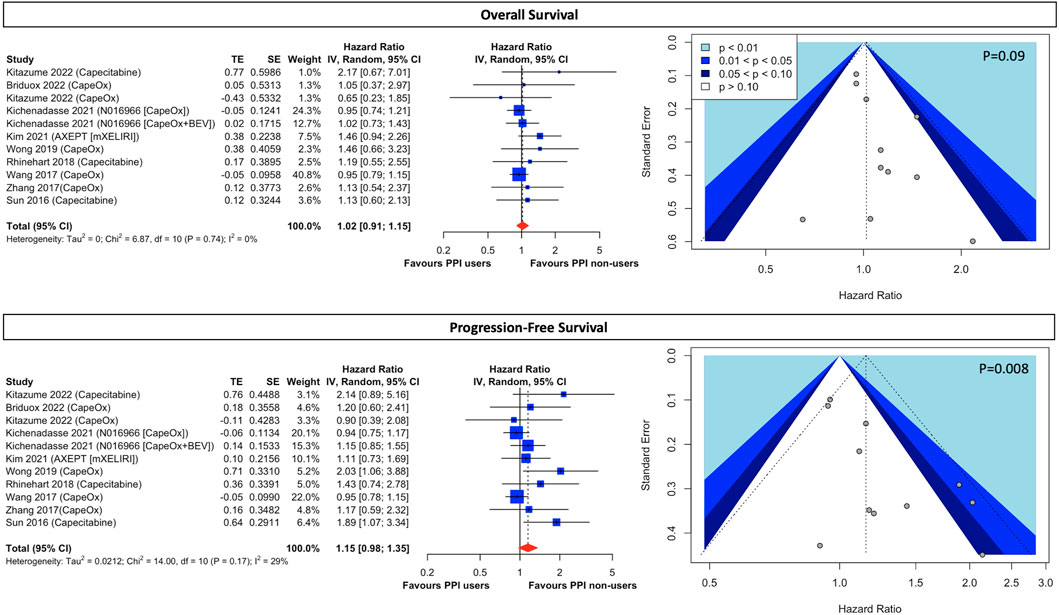
FIGURE 2. Forest plot and funnel plot of comparative overall survival and progression-free survival between PPI users and non-users in CRC cancer patients treated with capecitabine-based regimens The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95%. PPI, proton pump inhibitors; CI, confidential interval; CRC, colorectal cancers; CapeOx, capecitabine plus oxaliplatin; mXELIRI, capecitabine plus irinotecan; BEV, bevacizumab.
Although results were presented with low statistical heterogeneity, conceptual heterogeneity was determined to be inevitable as cancer stages and the capecitabine-based regimens were diverse as shown in Table 1. Thus, the subgroup analysis for different cancer stages and chemotherapeutic regimens indicated that early-stage cancer patients taking capecitabine monotherapy with concurrent PPI had a significantly higher disease progression rate (HR, 1.96; 95% CI, 1.21 to 3.16; I2 = 0%; Figure 3) than those who did not use PPIs. However, both groups had comparable all-cause mortality (HR, 1.31; 95% CI, 0.75 to 2.29; I2 = 0%; Figure 3). On the other hand, there was little difference in both OS and PFS in both early- and end-stage patients taking combination therapy, including regimens of CapeOx, CapeOx plus BEV, and mXELIRI, between PPI users and non-users (Figure 3). Other prespecified sensitivity analyses (Supplementary Result S4) showed that baseline PPI use had a neutral influence on survival outcomes, irrespective of the PPI administration window (Supplementary Figure S3), and the history of prior chemotherapy (Supplementary Figure S4).
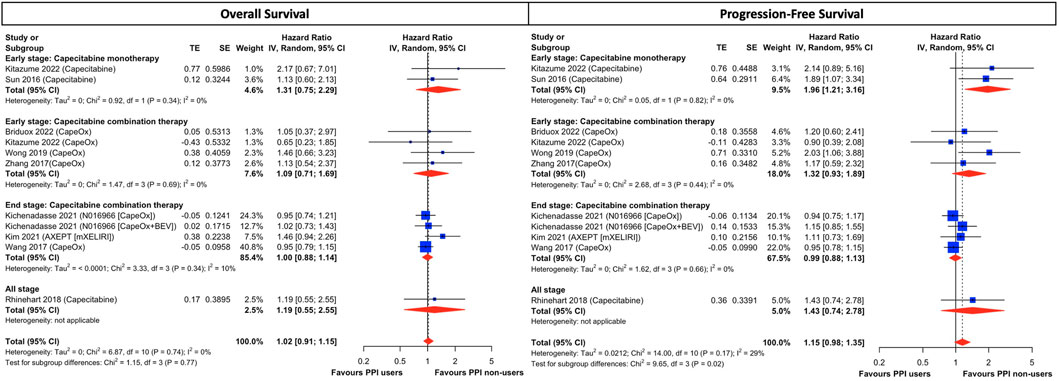
FIGURE 3. Subgroup analysis of cancer stage and treatment combination in CRC patients treated with capecitabine-based regimens. The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95%. PPI, proton pump inhibitors; CI, confidential interval; CRC, colorectal cancers; CapeOx, capecitabine plus oxaliplatin; mXELIRI, capecitabine plus irinotecan; BEV, bevacizumab.
Meta-analysis of FU-based chemotherapy
For patients receiving 5FU-based chemotherapeutic regimens, the use of concomitant PPI is associated with a marginally significant 18% higher rate of all-cause mortality (HR, 1.18; 95% CI, 1.00 to 1.40; I2 = 74%; Figure 4) and an insignificant 15% higher rate of disease progression (HR, 1.12; 95% CI, 0.93 to 1.34; I2 = 77%; Figure 4), however, with high statistical heterogeneity. Although assessment of publication bias through the visualization of the funnel plots in both OS (Figure 4) alluded to potential asymmetry, Eggers’ tests (Supplementary Result S5) are insignificant in both OS (p = 0.66) and PFS (p = 0.60). Prespecified subgroup analysis (Supplementary Result S6) of regimen modifications shows the use of baseline PPI contributes to significantly higher all-cause mortality (HR, 1.36; 95% CI, 1.04 to 1.76; I2 = 70%; Supplementary Figure S5) but insignificant disease progression (HR, 1.26; 95% CI, 0.96 to 1.65; I2 = 71%; Supplementary Figure S5) in FOLFIRI-treated patients. Albeit no survival difference between PPI users and non-users in patients treated with FOLFOX-based and IFL-based therapy, the results are still subject to considerable heterogeneity (Supplementary Figure S5). In the post hoc exploratory analyses (Supplementary Result S7), with the exclusion of Wong 2019 and Wang 2017 due to their retrospective nature and the exclusion of AXEPT owing to their limited enrollment of an Asian population, both FOLFIRI-treated and FOLFOX-treated patients taking PPI were associated with significantly lower OS and PFS (Supplementary Figure S6) than those without PPI, with a significant decrease in statistical heterogeneity.
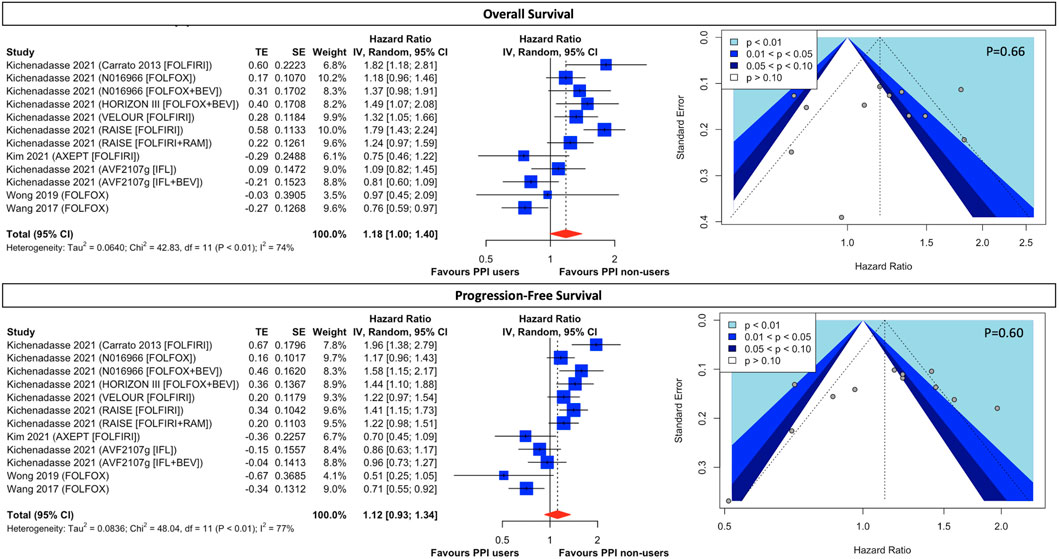
FIGURE 4. Forest plot and funnel plot of comparative overall survival and progression-free survival between PPI users and non-users in CRC cancer patients treated with fluorouracil-based regimens. The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95%. PPI, proton pump inhibitors; CI, confidential interval; CRC, colorectal cancers; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; FOLFIRI, leucovorin, fluorouracil, and irinotecan; RAM, ramucirumab; IFL, irinotecan, leucovorin, and fluorouracil.
Meta-analysis using adjusted HR
Supplementary Table S3 provides variables introduced into the multivariate models for adjusted outcomes for respective studies. Moreover, Supplementary Results S8 provides the results of the meta-analysis using adjusted HR in both OS and PFS (Supplementary Figures S7–S11). It appears that there is no significant discrepancy between the unadjusted and adjusted outcomes.
Discussion
Our study demonstrated that the survival difference was negligible between PPI users and non-users in CRC patients taking capecitabine-based chemotherapeutic regimens, regardless of cancer stages, PPI administration window and history of prior chemotherapy. The only exception was early-stage patients taking capecitabine mono-therapy with concomitant PPI, who suffered from a significantly higher risk of disease progression but comparable overall survival, compared to those without PPI. Capecitabine is an orally available prodrug that is activated through a three-step enzymatic metabolic process, initially to 5′-deoxy-5-flu- orocytidine (5′-DFCR), then to 5′-deoxy- 5-fluorouridine (5′-DFUR), and finally to 5-FU by thymidine phosphorylase in tumor tissue (Reigner et al., 2001). Our findings resonated with a recent cross-over RCT (van Doorn et al., 2022), which found that capecitabine was not negatively affected by the co-administration of esomeprazole and which surprisingly showed that the half-life of capecitabine exposure was actually pro-longed following PPI treatment. Notably, 82% patients enrolled in that RCT were CRC with ECOG <2 taking either capecitabine monotherapy or in combination with oxaliplatin and/or bevacizumab, similar to our study cohort. Moreover, Sekido et al. (2019) showed that rabeprazole did not significantly affect the pharmacokinetics of capecitabine, 5′-DFCR, 5′-DFUR, and 5-FU. Our meta-analysis in combination with these two aforementioned pharmacokinetics studies apparently contradict previous postulation (Cheng et al., 2019) that suggested increased intragastric pH levels inflicted by PPI should subsequently impair the dissolution and absorption of capecitabine, which in turn exerted detrimental effects on patients receiving capecitabine-based regimens. In fact, in vitro data (Röhss et al., 2004; Wishart et al., 2018) also did not support this hypothesis as the dissolution of capecitabine is similar between pH levels 2 and 6.8 with the intragastric pH level needing to reach 8.8 to become ionized before it is poorly absorbed, which is beyond PPI’s scope. Although significant asymmetry was detected in the funnel plot of PFS, it should not be interpreted abruptly as publication bias because there is a myriad of possible sources for this, including poor methodological quality, artefactual, chance, and true heterogeneity, responsible for the asymmetry (Sterne et al., 2011). In our case, the conceptual heterogeneity may be the main culprit for the observed asymmetry in the PFS since the subgroup analysis of cancer stages and regimen modifications demonstrated significant quantitative interaction between the subgroups (test for sub-group difference, p = 0.02; Figure 3) and the source of heterogeneity came from early-stage patients taking capecitabine monotherapy. The subgroup analysis showed the PFS remained comparable between PPI users and non-users in both early- and end-stage patients taking capecitabine combination therapy, but it was significantly lower in patients taking monotherapy with concomitant PPI. Consequently, from both a clinical and a statistical perspective, quantitative interaction between subgroups supports the idea of conceptual heterogeneity accounting for the asymmetry in the funnel plot. Even if small study effects existed to cause funnel asymmetry, based on the trim-and-fill analysis (Supplementary Figure S1), the overall effect estimates would not be altered significantly. The quantitative interaction between monotherapy and combination therapy implies to some degree that there are unknown interactions between PPI and capecitabine or PPI and CRC per se, which merit further investigation. Notwithstanding, although capecitabine monotherapy and combination therapy are considered to be standard postoperative adjuvant chemo-therapy for early-stage CRC (Benson et al., 2020; Benson et al., 2021), the efficacy of combination therapy has proved to be superior to monotherapy (Twelves et al., 2005; Haller et al., 2011). Thus, from a clinical perspective, it should be safe for oncologists and clinicians to prescribe PPI in patients taking capecitabine-based regimens as, theoretically, combination regimens would be prioritized and not be negatively affected by PPI.
As opposed to our study, a post hoc analysis of TRIO013/LOGiC trial (Hecht et al., 2016), conducted by Chu et al. (2017), indicated that concurrent use of PPI in human epidermal growth factor receptor-2 (HER-2) gastroesophageal cancer patients taking CapeOx regimen was associated with a higher all-cause mortality and a greater disease progression rate. This trial is considered a landmark study that supports the idea of detrimental effects of PPIs on capecitabine. However, two “infrequently mentioned” post hoc analyses of RCT, Yang et al. (2017) and Roberto et al. (2020), demonstrated PPI use during the capecitabine treatment window was not associated with decreased efficacy in advanced gastric and gastrointestinal cancers, respectively. Such variations in conclusions between Chu et al. (2017) and Yang et al. (2017) and Roberto et al. (2020) may have arisen due to the distinctive feature of the TRIO013 trial (Hecht et al., 2016), which enrolled patients with HER-2 overexpression. Studies have recognized the pivotal role of ethnicity in responses to the treatment in HER-2 amplification (Killelea et al., 2015; Yi et al., 2016) and convincing data has also suggested that the response to lapatinib, a tyrosine kinase inhibitor of HER-2, be different across ethnic groups (Hecht et al., 2016). Thus, different treatment response due to ethnicity among the cohorts may have confounded the true influence of PPI on the efficacy of capecitabine. Of note, patients in Roberto et al. (2020) were not limited to HER-2 amplification but, unfortunately, the detailed enrollment of Yang et al. (2017) remains unknown as Yang et al. (2017) was only presented as a conference abstract. Since every cancer type contains its own exclusive histopathologic profile and presents distinctive responses to chemotherapy, it is apparent that more studies are required to delve into this special population for a robust conclusion to be reached. We did not include these trials as they did not meet the eligibility criteria but we have briefly summarized the characteristics of these studies in Supplementary Table 4 as a reference for readers.
Regarding CRC patients taking FU-based regimens, those with concomitant use of PPI trend toward lower OS and PFS, compared to those without the use of PPI. However, effect estimates not only failed to reach statistical significance but were subject to considerable statistical heterogeneity, even in the pre-specified subgroup analysis of regimen modifications. We attempted to address the between-study variance by conducting post hoc exploratory analysis as follows. Firstly, in patients treated with FOLFOX, the most compelling source of variance is study design, with Wong et al. and Wang et al. being retrospective studies while the other 3 being RCTs. Secondly, in FOLFIRI-treated patients, although the association of PPI with significantly higher all-cause mortality suffered from residual high statistical heterogeneity, we can easily identify AXEPT (Rhinehart et al., 2019) as an outlier, alluding to it as a source of variance and qualitative interaction. Of note, the feature that distinguished AXEPT from the other three trials, Carroto et al., VELOUR, and RAISE, is the patient population. Patients enrolled in AXEPT were limited to those of Asian descent as the study was conducted in China, Korea, and Japan, contrasting with the other 3 studies which were mainly undertaken in western countries. With the removal of AXEPT from our exploratory analysis, the hazardous effects of PPI on FOLFIRI-treated patients appeared to be more consistent. We postulated that there may have been an interaction between ethnicity and treatment response, suggesting a biologic and pharmacogenetic difference between the Asian and non-Asian groups. Non-etheless, readers should bear in mind that instead of prespecified analysis, these were post hoc exploratory analysis, which may inevitably introduce a false interpretation of heterogeneity (Higgins et al., 2022). Thus, more studies are needed for further clarification. On the other hand, although IFL regimens share a similar combination to FOLIFRI, patients treated with IFL-based regimens experienced little survival difference between PPI users and non-users. A prospective study has already concluded that PPI did not alter the pharmacokinetics of irinotecan. Moreover, since the difference between IFL and FOLFIRI resides in the ad-ministration of fluorouracil, which is used as a bolus injection in the former and as a 48-h continuous infusion in the latter regimen, the detrimental effects of PPI on FOLFIRI instead of IFL indicate a possible interaction between fluorouracil administration and antitumor response.
The present study has various strengths. Firstly, although two systematic reviews (Viñal et al., 2020; Patel et al., 2021) attempted to address the same issues, their study design did not allow quantitative syntheses to be conducted. Secondly, two areas of uncertainty, which were proposed by Kichenadasse et al. (2021) as limitations for their study, were well-addressed: 1) the effect of PPI on capecitabine-based chemotherapy; 2) the influence of PPI on fluoropyrimidine monotherapy. Despite above mentioned novelties provided by our study, we acknowledge that our study contains several limitations. Firstly, the dose and types of PPI were not well elucidated, with this pertinent information lacking. The capability of acid suppression varied significantly in different variants of PPI (Röhss et al., 2004). In addition, it has been shown that PPI dosage exhibits a positive correlation with the magnitude and duration of gastric acid suppression (Lind et al., 2000). Secondly, the scare of information on the timing of PPI intake may hinder the assessment of association between PPI and chemotherapy, especially oral intake of capecitabine, as it takes at least 3 h for intragastric pH to attain its maximal elevation following the consumption of PPI (Hunfeld et al., 2012). Thirdly, the indications of the use of PPI were insufficient, which may have introduced unmeasured confounding bias. Last but not least, although we exhausted every chemotherapeutic regimen in current literature to explore the effect of PPI on chemotherapy, various target therapies, such as encorafenib, cetuximab, and panitumumab, used in combination with chemotherapy for stage IV CRC are still not available for investigation.
Conclusion
The use of PPI has little survival influence on CRC patients treated with capecitabine combination therapy, regardless of cancer stages. Although early-stage patients taking capecitabine monotherapy with concomitant PPI were associated with significantly higher disease progression, the all-cause mortality remained comparable between PPI users and non-users. Thus, it should be safe for clinicians to prescribe PPI in these patients, especially in those patients taking capecitabine combination therapy. Conversely, both FOLFOX-treated and FOLFIRI-treated patients taking concomitant PPI trended toward higher all-cause mortality and greater disease progression, however, with considerable heterogeneity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
W-YL: Conceptualization, wiring–original draft preparation, wiring–review and editing; S-SW: Data curation, investigation, formal analysis, wiring–review and editing; Y-NK: Methodology, software, formal analysis, wiring–review and editing; AP: Conceptualization, writing–original draft preparation, visualization, writing–review and editing; YC: Investigation, methodology, formal analysis, visualization; C-HH: Data curation, formal analysis, writing–review and editing; RB: Conceptualization, writing–review and editing; EA-L: Investigation, writing–review and editing; MA: Investigation, writing–review and editing; T-HW: Data curation; Y-SL: Data curation; Y-CC: Conceptualization, supervision, investigation, writing–re-view and editing; K-YC: Conceptualization, supervision, investigation, methodology, software, formal analysis, writing–original draft preparation, writing–review and editing.
Acknowledgments
Our team would like to thank Mr. Tim Stubbings for proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1048980/full#supplementary-material
References
Benson, A. B., Venook, A. P., Al-Hawary, M. M., Arain, M. A., Chen, Y. J., Ciombor, K. K., et al. (2021). Colon cancer, version 2.2021, NCCN clinical practice guidelines in Oncology. J. Natl. Compr. Canc. Netw. 19 (3), 329–359. doi:10.6004/jnccn.2021.0012
Benson, A. B., Venook, A. P., Al-Hawary, M. M., Arain, M. A., Chen, Y. J., Ciombor, K. K., et al. (2020). NCCN guidelines insights: Rectal cancer, version 6.2020. J. Natl. Compr. Canc. Netw. 18 (7), 806–815. doi:10.6004/jnccn.2020.0032
Berger, M. J., Ettinger, D. S., Aston, J., Barbour, S., Bergsbaken, J., Bierman, P. J., et al. (2017). NCCN guidelines insights: Antiemesis, version 2.2017. J. Natl. Compr. Canc. Netw. 15 (7), 883–893. doi:10.6004/jnccn.2017.0117
Bridoux, M., Le Deley, M. C., Bertrand, N., Simon, N., Sylla, D., Mirabel, X., et al. (2022). Effects of proton pump inhibitors intake during chemoradiotherapy for rectal cancer: A retrospective cohort study. J. Gastrointest. Cancer. doi:10.1007/s12029-022-00825-z
Carrato, A., Swieboda-Sadlej, A., Staszewska-Skurczynska, M., Lim, R., Roman, L., Shparyk, Y., et al. (2013). Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: A randomized, phase III trial. J. Clin. Oncol. 31 (10), 1341–1347. doi:10.1200/JCO.2012.45.1930
Chalabi, M., Cardona, A., Nagarkar, D. R., DhAwAhir ScAlA, A., Rittmeyer, A., et al. (2020). Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 31 (4), 525–531. doi:10.1016/j.annonc.2020.01.006
Cheng, V., Lemos, M., Hunter, N., Badry, N., and Lemos, J. (2019). Concomitant use of capecitabine and proton pump inhibitors - is it safe? J. Oncol. Pharm. Pract. 25 (7), 1705–1711. doi:10.1177/1078155219846952
Chu, M. P., Hecht, J. R., Slamon, D., Wainberg, Z. A., Bang, Y. J., Hoff, P. M., et al. (2017). Association of proton pump inhibitors and capecitabine efficacy in advanced gastroesophageal cancer: Secondary analysis of the TRIO-013/LOGiC randomized clinical trial. JAMA Oncol. 3 (6), 767–773. doi:10.1001/jamaoncol.2016.3358
Duval, S., and Tweedie, R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56 (2), 455–463. doi:10.1111/j.0006-341x.2000.00455.x
Haastrup, P. F., Thompson, W., Søndergaard, J., and Jarbøl, D. E. (2018). Side effects of long-term proton pump inhibitor use: A review. Basic Clin. Pharmacol. Toxicol. 123 (2), 114–121. doi:10.1111/bcpt.13023
Haller, D. G., Tabernero, J., Maroun, J., de Braud, F., Price, T., Van Cutsem, E., et al. (2011). Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J. Clin. Oncol. 29 (11), 1465–1471. doi:10.1200/JCO.2010.33.6297
Harville, D. A. (1977). Maximum likelihood approaches to variance component estimation and to related problems. J. Am. Stat. Assoc. 72 (358), 320–338. doi:10.1080/01621459.1977.10480998
Hecht, J. R., Bang, Y. J., Qin, S. K., Chung, H. C., Xu, J. M., Park, J. O., et al. (2016). Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC--A randomized phase III trial. J. Clin. Oncol. 34 (5), 443–451. doi:10.1200/JCO.2015.62.6598
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
J. P. T. T. J. Higgins, J. Chandler, M. Cumpston, T. Li, M. J. Page, and V. A. Welch (Editors) (2022). Cochrane Handbook for systematic reviews of interventions (Cochrane) version 6.3. Available from: www.trainingcochraneorg/handbook. 2022.
Hopkins, A. M., Kichenadasse, G., Karapetis, C. S., Rowland, A., and Sorich, M. J. (2020). Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin. Cancer Res. 26 (20), 5487–5493. doi:10.1158/1078-0432.CCR-20-1876
Hopkins, A. M., Kichenadasse, G., McKinnon, R. A., Abuhelwa, A. Y., Logan, J. M., Badaoui, S., et al. (2022). Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: Post hoc analysis of IMpower150. Br. J. Cancer 126 (1), 42–47. doi:10.1038/s41416-021-01606-4
Hunfeld, N. G., Touw, D. J., Mathot, R. A., van Schaik, R. H., and Kuipers, E. J. (2012). A comparison of the acid-inhibitory effects of esomeprazole and rabeprazole in relation to pharmacokinetics and CYP2C19 polymorphism. Aliment. Pharmacol. Ther. 35 (7), 810–818. doi:10.1111/j.1365-2036.2012.05014.x
Hurwitz, H., Fehrenbacher, L., Novotny, W., Cartwright, T., Hainsworth, J., Heim, W., et al. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350 (23), 2335–2342. doi:10.1056/NEJMoa032691
Kichenadasse, G., Miners, J. O., Mangoni, A. A., Karapetis, C. S., Hopkins, A. M., and Sorich, M. J. (2021). Proton pump inhibitors and survival in patients with colorectal cancer receiving fluoropyrimidine-based chemotherapy. J. Natl. Compr. Canc. Netw. 19 (9), 1037–1044. doi:10.6004/jnccn.2020.7670
Killelea, B. K., Yang, V. Q., Wang, S. Y., Hayse, B., Mougalian, S., Horowitz, N. R., et al. (2015). Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: Results from the national cancer data base. J. Clin. Oncol. 33 (36), 4267–4276. doi:10.1200/JCO.2015.63.7801
Kim, S. Y., Lee, J. S., Kang, J., Morita, S., Park, Y. S., Sakamoto, J., et al. (2021). Proton pump inhibitor use and the efficacy of chemotherapy in metastatic colorectal cancer: A post hoc analysis of a randomized phase III trial (AXEPT). Oncologist 26 (6), e954–e962. doi:10.1002/onco.13735
Kitazume, Y., Kawazoe, H., Uozumi, R., Yoshizawa, T., Iihara, H., Fujii, H., et al. (2022). Proton pump inhibitors affect capecitabine efficacy in patients with stage II–III colorectal cancer: A multicenter retrospective study. Sci. Rep. 12 (1), 6561. doi:10.1038/s41598-022-10008-2
Lind, T., Rydberg, L., Kylebäck, A., Kyleback, A., Jonsson, A., HasselGren, G., et al. (2000). Esomeprazole provides improved acid control vs. omeprazole in patients with symptoms of gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 14 (7), 861–867. doi:10.1046/j.1365-2036.2000.00813.x
Parmar, M. K., Torri, V., and Stewart, L. (1998). Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 17 (24), 2815–2834. doi:10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
Patel, A., Spychalski, P., Antoszewska, M., Regula, J., and Kobiela, J. (2021). Proton pump inhibitors and colorectal cancer: A systematic review. World J. Gastroenterol. 27 (44), 7716–7733. doi:10.3748/wjg.v27.i44.7716
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R., and Rushton, L. (2007). Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat. Med. 26 (25), 4544–4562. doi:10.1002/sim.2889
Reigner, B., Blesch, K., and Weidekamm, E. (2001). Clinical pharmacokinetics of capecitabine. Clin. Pharmacokinet. 40 (2), 85–104. doi:10.2165/00003088-200140020-00002
Rhinehart, H. E., Phillips, M. A., Wade, N., and Baran, A. (2019). Evaluation of the clinical impact of concomitant acid suppression therapy in colorectal cancer patients treated with capecitabine monotherapy. J. Oncol. Pharm. Pract. 25 (8), 1839–1845. doi:10.1177/1078155218818237
Roberto, M., Romiti, A., Mazzuca, F., Milano, A., D'Antonio, C., Lionetto, L., et al. (2020). Combination therapy of high-dose rabeprazole plus metronomic capecitabine in advanced gastro-intestinal cancer: A randomized phase II trial. Cancers (Basel) 12 (11), 3084. doi:10.3390/cancers12113084
Röhss, K., Lind, T., and Wilder-Smith, C. (2004). Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux symptoms. Eur. J. Clin. Pharmacol. 60 (8), 531–539. doi:10.1007/s00228-004-0804-6
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillere, R., et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359 (6371), 91–97. doi:10.1126/science.aan3706
Roy, S., and Trinchieri, G. (2017). Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 17 (5), 271–285. doi:10.1038/nrc.2017.13
Saltz, L. B., Clarke, S., Díaz-Rubio, E., Scheithauer, W., Figer, A., Wong, R., et al. (2008). Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 26 (12), 2013–2019. doi:10.1200/JCO.2007.14.9930
Schmoll, H. J., Cunningham, D., Sobrero, A., Karapetis, C. S., Rougier, P., Koski, S. L., et al. (2012). Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: A double-blind, randomized phase III study (HORIZON III). J. Clin. Oncol. 30 (29), 3588–3595. doi:10.1200/JCO.2012.42.5355
Sekido, M., Fujita, K. I., Kubota, Y., Ishida, H., Takahashi, T., Ohkuma, R., et al. (2019). Rabeprazole intake does not affect systemic exposure to capecitabine and its metabolites, 5'-deoxy-5-fluorocytidine, 5'-deoxy-5-fluorouridine, and 5-fluorouracil. Cancer Chemother. Pharmacol. 83 (6), 1127–1135. doi:10.1007/s00280-019-03837-y
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savovic, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. doi:10.1136/bmj.i4919
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Sterne, J. A. C., Sutton, A. J., Ioannidis, J. P. A., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002. doi:10.1136/bmj.d4002
Strand, D. S., Kim, D., and Peura, D. A. (2017). 25 Years of proton pump inhibitors: A comprehensive review. Gut Liver 11 (1), 27–37. doi:10.5009/gnl15502
Sun, J., Ilich, A. I., Kim, C. A., Chu, M. P., Wong, G. G., Ghosh, S., et al. (2016). Concomitant administration of proton pump inhibitors and capecitabine is associated with increased recurrence risk in early stage colorectal cancer patients. Clin. Colorectal Cancer 15 (3), 257–263. doi:10.1016/j.clcc.2015.12.008
Tabernero, J., Yoshino, T., Cohn, A. L., Obermannova, R., Bodoky, G., Garcia-Carbonero, R., et al. (2015). Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised double-blind, multicentre, phase 3 study. Lancet. Oncol. 6, 499–508. doi:10.1016/S1470-2045(15)70127-0
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S., and Sydes, M. R. (2007). Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16. doi:10.1186/1745-6215-8-16
Twelves, C., Wong, A., Nowacki, M. P., Abt, M., Burris, H., Carrato, A., et al. (2005). Capecitabine as adjuvant treatment for stage III colon cancer. N. Engl. J. Med. 352 (26), 2696–2704. doi:10.1056/NEJMoa043116
Van Cutsem, E., Tabernero, J., Lakomy, R., Prenen, H., Prausova, J., Macarulla, T., et al. (2012). Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 30 (28), 3499–3506. doi:10.1200/JCO.2012.42.8201
van Doorn, L., Heersche, N., de Man, F. M., de Bruijn, P., Bijl, I., Oomen-de Hoop, E., et al. (2022). Effect of the proton pump inhibitor esomeprazole on the systemic exposure of capecitabine: Results of A randomized crossover trial. Clin. Pharmacol. Ther. 111 (2), 455–460. doi:10.1002/cpt.2444
Viñal, D., Rodriguez-Salas, N., Perez-Wert, P., Higuera, O., Ghanem, I., and Feliu, J. (2020). Efficacy of capecitabine when used concomitantly with proton pump inhibitors in cancer patients: A systematic review. Clin. Transl. Oncol. 22 (8), 1288–1294. doi:10.1007/s12094-019-02254-0
Wang, X., Liu, C., Wang, J., Fan, Y., Wang, Z., and Wang, Y. (2017). Proton pump inhibitors increase the chemosensitivity of patients with advanced colorectal cancer. Oncotarget 8 (35), 58801–58808. doi:10.18632/oncotarget.18522
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., et al. (2018). DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 46 (D1), D1074–D1082. doi:10.1093/nar/gkx1037
Wong, G. G., Ha, V., Chu, M. P., Dersch-Mills, D., Ghosh, S., Chambers, C. R., et al. (2019). Effects of proton pump inhibitors on FOLFOX and CapeOx regimens in colorectal cancer. Clin. Colorectal Cancer 18 (1), 72–79. doi:10.1016/j.clcc.2018.11.001
Xu, R-H., Muro, K., Morita, S., Iwasa, S., Han, S. W., Wang, W., et al. (2018). Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet. Oncol. 19 (5), 660–671. doi:10.1016/S1470-2045(18)30140-2
Yang, J. Y. S. H., Sandler, R. S., Stürmer, T., Funk, M. J., and Lund, J. L. (2017). Does proton-pump inhibitor use diminish capecitabine efficacy in advanced cancer patients? Pharmacoepidemiol. Drug Saf. 26, 222–223.
Yi, J. H., Kang, J. H., Hwang, I. G., Ahn, H. K., Baek, H. J., Lee, S. I., et al. (2016). A retrospective analysis for patients with HER2-positive gastric cancer who were treated with trastuzumab-based chemotherapy: In the perspectives of ethnicity and histology. Cancer Res. Treat. 48 (2), 553–560. doi:10.4143/crt.2015.155
Keywords: colorectal cancer, proton pump inhibitor (PPI), chemotherapy, systematic review, meta–analysis
Citation: Lin W-Y, Wang S-S, Kang Y-N, Porpiglia AS, Chang Y, Huang C-H, Bhimani R, Abdul-Lattif E, Azmat M, Wang T-H, Lin Y-S, Chang Y-C and Chi K-Y (2022) Do proton pump inhibitors affect the effectiveness of chemotherapy in colorectal cancer patients? A systematic review with meta-analysis. Front. Pharmacol. 13:1048980. doi: 10.3389/fphar.2022.1048980
Received: 20 September 2022; Accepted: 25 November 2022;
Published: 12 December 2022.
Edited by:
Luis Abel Quiñones, University of Chile, ChileReviewed by:
Luis Andrés López-Fernández, Instituto de Investigación Sanitaria Gregorio Marañón, SpainKathryn Elisa Burns, The University of Auckland, New Zealand
Patricia Esperon, Universidad de la República, Uruguay
Copyright © 2022 Lin, Wang, Kang, Porpiglia, Chang, Huang, Bhimani, Abdul-Lattif, Azmat, Wang, Lin, Chang and Chi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Cheng Chang, eXVjaGVuZ2NoYW5nMDYzMEBnbWFpbC5jb20=; Kuan-Yu Chi, a3Vhbnl1aGlwcG9AZ21haWwuY29t
†These authors have contributed equally to this work
 Wan-Ying Lin1†
Wan-Ying Lin1† Yi-No Kang
Yi-No Kang Yu Chang
Yu Chang Kuan-Yu Chi
Kuan-Yu Chi