
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 17 November 2022
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1043217
This article is part of the Research TopicNew Insights into Molecular Mechanisms and Targeted Therapy for Gastrointestinal TumorsView all 9 articles
 Caiyun Nie1,2,3,4
Caiyun Nie1,2,3,4 Weifeng Xu1,2,3,4
Weifeng Xu1,2,3,4 Huifang Lv1,2,3,4
Huifang Lv1,2,3,4 Xiaohui Gao5
Xiaohui Gao5 Guofeng Li6
Guofeng Li6 Beibei Chen1,2,3,4
Beibei Chen1,2,3,4 Jianzheng Wang1,2,3,4
Jianzheng Wang1,2,3,4 Yingjun Liu7
Yingjun Liu7 Jing Zhao1,2,3,4
Jing Zhao1,2,3,4 Yunduan He1,2,3,4
Yunduan He1,2,3,4 Saiqi Wang1,2,3,4
Saiqi Wang1,2,3,4 Xiaobing Chen1,2,3,4*
Xiaobing Chen1,2,3,4*Background: There is currently still a lack of effective therapeutic manner after the failure of first-line therapy for patients with advanced or metastatic gastric cancer. The present study aimed to evaluate the clinical efficacy and safety of different treatment strategies as second-line or above therapy for patients with advanced or metastatic gastric cancer.
Methods: This was an observational multicenter real-world study. From January 2018 to December 2020, advanced or metastatic gastric cancer patients who have failed prior therapy were enrolled and treated with chemotherapy, anti-angiogenic TKIs (tyrosine kinase inhibitors) + chemotherapy or TKIs + ICIs (immune checkpoint inhibitors). In this study, progression free survival (PFS) was the primary end-point. Other evaluation indicators were objective response rate (ORR), disease control rate (DCR), overall survival (OS) and drug toxicities.
Results: 162 patients were enrolled, of which 61 patients received chemotherapy, 47 patients received TKIs plus chemotherapy, and 54 patients received TKIs + ICIs. No statistically significant difference existed in ORR among groups (16.4% vs. 19.1% vs. 18.5%, p = 0.924). Patients who received TKIs plus chemotherapy obtained better DCR compared with the chemotherapy group (78.7% vs. 54.1%, p = 0.008), and simultaneously, the median PFS (3.3 m vs. 2.8 m, p = 0.001) and OS (8.0 m vs. 5.8 m, p = 0.005) in TKIs plus chemotherapy group were superior to chemotherapy group. Consistent results were observed in subgroup analysis, including sex, age, ECOG, number of metastatic sites and treatment line. No statistically differences were found between TKIs + ICIs and the chemotherapy group concerning DCR (63.0% vs. 54.1%, p = 0.336), median PFS (3.0 m vs. 2.8 m, p = 0.051) and OS (5.2 m vs. 5.8 m, p = 0.260). Different treatment manner present a special spectrum of adverse events (AEs), and the incidence of Grade 3–4 AEs were 31.1%, 38.3% and 18.5%, respectively.
Conclusion: Compared with chemotherapy, anti-angiogenic TKIs plus chemotherapy demonstrated superior second-line or above therapeutic efficacy for advanced or metastatic gastric cancer with well tolerated toxicity. However, TKIs + ICIs failed to demonstrate a clinical advantage over chemotherapy.
Cancer epidemiological data showed that the incidence of gastric cancer is increasing year by year in the world (Sung et al., 2021). With the deepening of understanding of the pathogenesis and molecular changes of gastric cancer, as well as the progress of new comprehensive treatment methods, the overall treatment level of gastric cancer and the prognosis of patients has been significantly improved in recent years (Korfer et al., 2021; Hogner and Moehler, 2022). However, a considerable proportion of patients with gastric cancer are in the advanced or metastatic stage at the time of initial diagnosis. There is still no effective treatment option for advance or metastatic gastric cancer, which is a bottleneck restricting the long-term survival of gastric cancer patient (Hackshaw et al., 2020; Parisi et al., 2020).
Currently, platinum- and fluorouracil-based chemotherapy is the recommended first-line treatment regimen for advanced or metastatic gastric cancer (Koizumi et al., 2008; Luo et al., 2010; Lu et al., 2018). The main scheme of the second-line treatment is single drug chemotherapy with paclitaxel and irinotecan or a combination of two drug chemotherapy schemes (Hawkes et al., 2011; Hironaka et al., 2013; Shitara et al., 2017). However, the therapeutic effect of gastric cancer second-line treatment is not satisfactory, and the median overall survival (OS) is less than 1 year (Hironaka et al., 2013).
Anti-angiogenic therapy and immunotherapy provide new treatment options for patients with advanced or metastatic gastric cancer. Based on the results of the RAINBOW, ATTRACTION-02 and KEYNOTE-059 clinical trial, anti-angiogenic ramucirumab combined with chemotherapy and immunotherapy were approved by the FDA for the second-line and third-line treatment of metastatic gastric cancer (Wilke et al., 2014; Kang et al., 2017; Fuchs et al., 2018), respectively.
Some small-sample retrospective studies suggested that anti-angiogenic small-molecule multi-targeted tyrosine kinase inhibitors (TKIs) combined with chemotherapy, immune checkpoint inhibitors (ICIs) plus anti-angiogenesis may be potentially effective combination therapy strategies (Gao et al., 2022; Li et al., 2022; Tang et al., 2022). The present study aimed to evaluate the clinical efficacy and safety of chemotherapy, anti-angiogenic TKIs + chemotherapy and TKIs + ICIs as second-line or above therapy options for advanced or metastatic gastric cancer.
From January 2018 to December 2020, patients with advanced or metastatic gastric cancer who have failed prior treatment in 3 institutions across China were enrolled in this study, including the Affiliated Cancer Hospital of Zhengzhou University, First Affiliated Hospital of Henan University of Science and Technology and First Affiliated Hospital of Henan University of CM. The criteria included: 1) advanced and metastatic gastric cancer; 2) failed from prior treatment; 3) based on RECIST v1.1, the presence of at least one measurable lesion.
In this observational multicenter real-world study, the patients received chemotherapy, anti-angiogenic TKIs + chemotherapy or TKIs + ICIs therapy as second-line and beyond until progressive disease, death or intolerable toxicity occurs. In the chemotherapy regimen group, the patients received irinotecan or paclitaxel monotherapy. Irinotecan was administered intravenously at a dose of 125 mg/m2 on d1 and d8 every 3 weeks. The paclitaxel was given intravenously, the dosage was 80 mg/m2 on d1, d8 and d 15 every 4 weeks. In the TKIs + chemotherapy regimen group, the patients received apatinib or anlotinib plus chemotherapy. Apatinib was administered orally, the dosage was 250 mg daily and anlotinib was administered at a dose of 10 mg once a day from d1 to d14 for 3 weeks. The chemotherapy was given at the recommended dose, including irinotecan or paclitaxel. In the TKIs + ICIs therapy regimen, apatinib or anlotinib was given at the same dose as the TKIs + chemotherapy regimen group and combined with ICIs. Concering ICIs, programmed cell death protein 1 (PD-1) inhibitor was administered intravenously at the recommended dose, including sintilimab, camrelizumab or tislelizumab.
After every two cycles of treatment, imaging examinations were performed with CT or MRI. The clinical response was evaluated as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to RECIST version 1.1 response evaluation criteria in solid tumors. The ratio of CR plus PR was objective response rate (ORR), and the ratio of CR, PR, plus SD was disease control rate (DCR). The drug toxicities were evaluated using the Common Terminology Criteria for Adverse Events (version 4.0).
Differences between groups were compared by Pearson’s chi-squared test or Fisher’s exact test. The follow-up period ends on 31 December 2021. Progression free survival (PFS) was calculated from using chemotherapy, TKIs + chemotherapy or TKIs + ICIs therapy to disease progression or death. Overall survival (OS) was calculated from using chemotherapy, TKIs + chemotherapy or TKIs + ICIs therapy to the death of the patient or end of follow-up. Kaplan-Meier method and the log-rank test were used to perform survival and prognostic analysis. Subgroup analysis of impact indicators for PFS was carried out by Cox proportional hazards model, including sex, age, ECOG, number of metastatic sites and treatment line. SPSS 22.0 software (SPSS Inc., IL, US) was used for statistical analysis, and p < 0.05 was defined as statistically significant.
A total of 162 patients were included. Table 1 summarized the baseline clinicopathological and treatment characteristics of patients. The median age of patients in this study was 55 years (range 25–80), with 117 (72.2%) male patients and 45 (27.8%) female patients. ECOG PS was 0–1 in 115 (71.0%) patients and the other 47 (29.0%) patients were ECOG PS 2. The tumor site in 69.1% patients was gastric, and the other 30.9% patients were gastroesophageal junction (GEJ) adenocarcinoma. The common metastatic sites included lymph node (66.0%), liver (44.4%), peritoneum (28.4%), lung (21.0%) and other sites (35.2%). 113 (69.8%) patients had 1 or 2 metastatic sites, and the metastatic sites in other 49 (30.2%) patients were 3 or more. All the 162 patients had undergone previous systematic therapy, 66 (40.7%) received chemotherapy, TKIs + chemotherapy or TKIs + ICIs therapy as second-line therapy, and as third-fourth line therapy in the other 96 (59.3%) patients. In this study, patients received the guideline-recommended first-line treatment regimen. For HER2-negative patients, first-line chemotherapy is based on fluorouracil in combination with platinum and/or taxane. For HER2-positive patients, first-line therapy is chemotherapy combined with trastuzumab targeted therapy.
Sixty-one patients received chemotherapy, of which paclitaxel and irinotecan were given in 29 (47.5%) and 32 (52.5%) patients, respectively. Forty-seven patients received anti-angiogenic TKIs combined with chemotherapy. In this group, 26 (55.3%) patients received apatinib plus irinotecan therapy, 14 (29.8%) patients received anlotinib plus paclitaxel therapy and the other 7 (14.9%) patients received anlotinib plus irinotecan regimen (Table 2). Fifty-four patients received anti-angiogenic TKIs combined with ICIs. Apatinib plus sintilimab was given in 25 (46.3%) patients, the other 29 (53.7%) patients received anlotinib plus ICIs, including sintilimab (11, 20.4%), camrelizumab (10, 18.5%) and tislelizumab (8, 14.8%). No statistically significant differences existed in the baseline clinicopathological characteristics among chemotherapy, TKIs + chemotherapy and TKIs + ICIs groups.
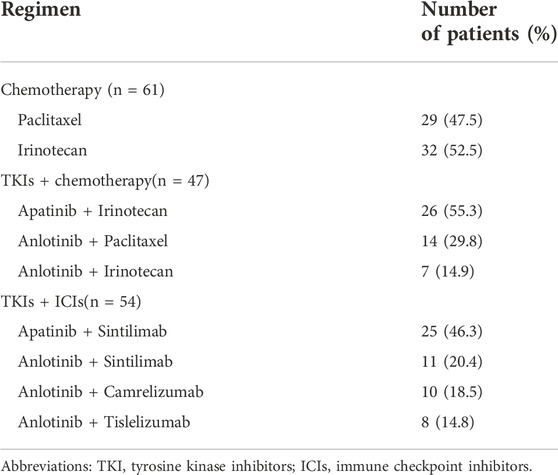
TABLE 2. Regimens given in chemotherapy, TKIs + chemotherapy and TKIs + ICIs for patients with advanced or metastatic gastric cancer.
In this study, no patient achieved CR after treatment. After treatment, 29 patients obtained PR, 75 patients and 58 patients had SD and PD, respectively. The ORR and DCR in the present study were 17.9% (29/162) and 64.2% (104/162), respectively (Table 3). In the chemotherapy group, after treatment, 10 patients obtained PR, 23 and 28 patients had SD and PD, respectively. The ORR and DCR in the chemotherapy group were 16.4% (10/61) and 54.1% (33/61), respectively. In the TKIs + chemotherapy group, after treatment, 9 patients obtained PR, 28 and 10 patients had SD and PD, respectively. The ORR and DCR in the TKIs + chemotherapy group were 19.1% (9/47) and 78.7% (37/47), respectively. In the TKIs + ICIs group, after treatment, 10 patients obtained PR, 24 and 20 patients had SD and PD, respectively. The ORR and DCR in the TKIs + ICIs group were 18.5% (10/54) and 63.0% (34/54), respectively. The waterfall plot of the best response change after treatment from baseline was shown in Figure 1. There was no statistically significant difference in ORR among groups (16.4% vs. 19.1% vs. 18.5%, p = 0.924). Patients who received TKIs plus chemotherapy obtained better DCR compared with the chemotherapy group (78.7% vs. 54.1%, p = 0.008). However, no statistically significant difference was found in DCR between the TKIs + ICIs group and the chemotherapy group (63.0% vs. 54.1%, p = 0.336).
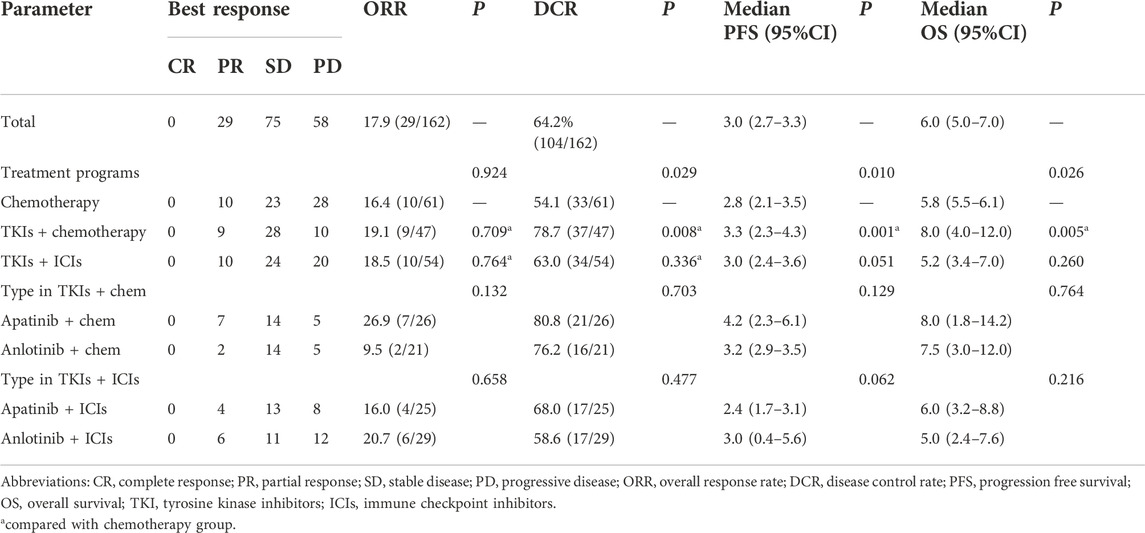
TABLE 3. Efficacy of chemotherapy, TKIs + chemotherapy and TKIs + ICIs in patients with advanced or metastatic gastric cancer.
In the general population, the median PFS and median OS were 3.0 months (95% CI = 2.7–3.3) and 6.0 months (95% CI = 5.0–7.0), respectively (Figures 2A,B). The median PFS in the chemotherapy, TKIs + chemotherapy and TKIs + ICIs population were 2.8 months (95% CI = 2.1–3.5), 3.3 months (95% CI = 2.3–4.3) and 3.0 months (95% CI = 2.4–3.6), respectively (p = 0.010; Figure 3A). The median OS in the three groups were 5.8 months (95% CI = 5.5–6.1), 8.0 months (95% CI = 4.0–12.0) and 5.2 months (95% CI = 3.4–7.0), respectively (p = 0.026; Figure 3B). The median PFS (3.3 m vs. 2.8 m, p = 0.001) and OS (8.0 m vs. 5.8 m, p = 0.005) in TKIs plus chemotherapy group were superior to the chemotherapy group. Consistent results were observed in subgroup analysis, including sex, age, ECOG, number of metastatic sites and treatment line (Figure 4). However, no significant difference was found in PFS (p = 0.051) and OS (p = 0.260) between the patients who received TKIs + ICIs therapy and chemotherapy (Figure 5). In the TKIs + chemotherapy group, no statistically significant differences were found in ORR, DCR, PFS and OS concering treatment type (apatinib plus chemotherapy vs. anlotinib plus chemotherapy; Figures 3C,D). Similar results were also found in the TKIs plus ICIs population (Figures 3E,F).
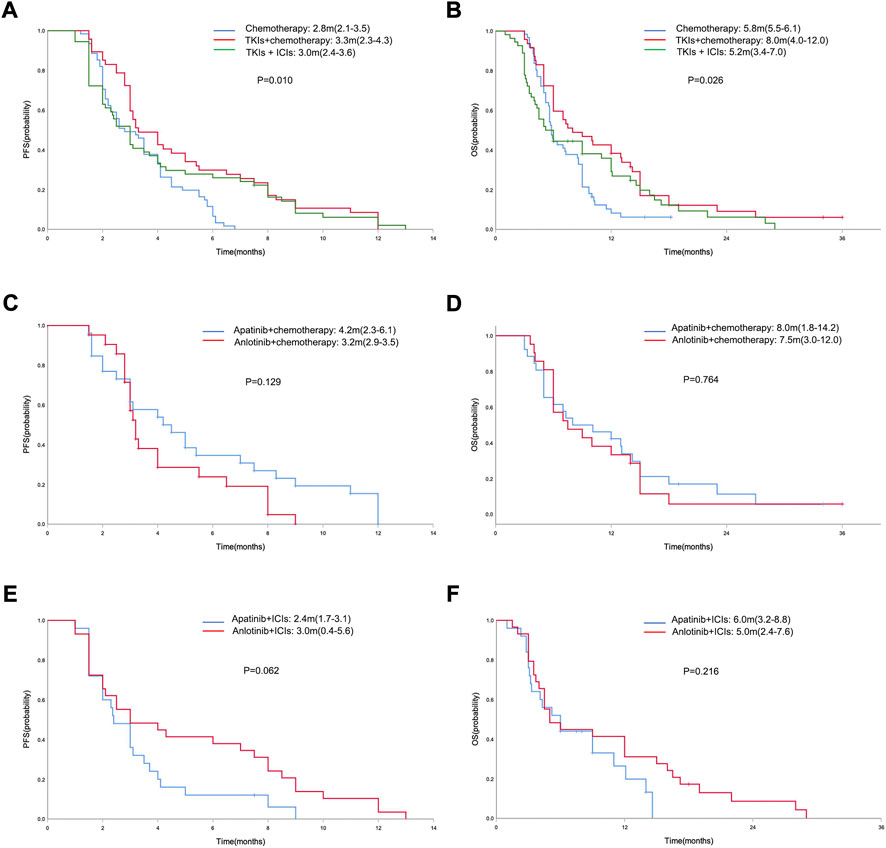
FIGURE 3. Kaplan-Meier curve of PFS (A) and OS (B) in the chemotherapy, TKIs + chemotherapy and TKIs + ICIs population. Kaplan-Meier curve of PFS (C) and OS (D) in the TKIs + chemotherapy group with respect to treatment type (apatinib plus chemotherapy vs. anlotinib plus chemotherapy). Kaplan-Meier curve of PFS (E) and OS (F) in the TKIs + ICIs group with respect to treatment type (apatinib plus ICIs vs. anlotinib plus ICIs).
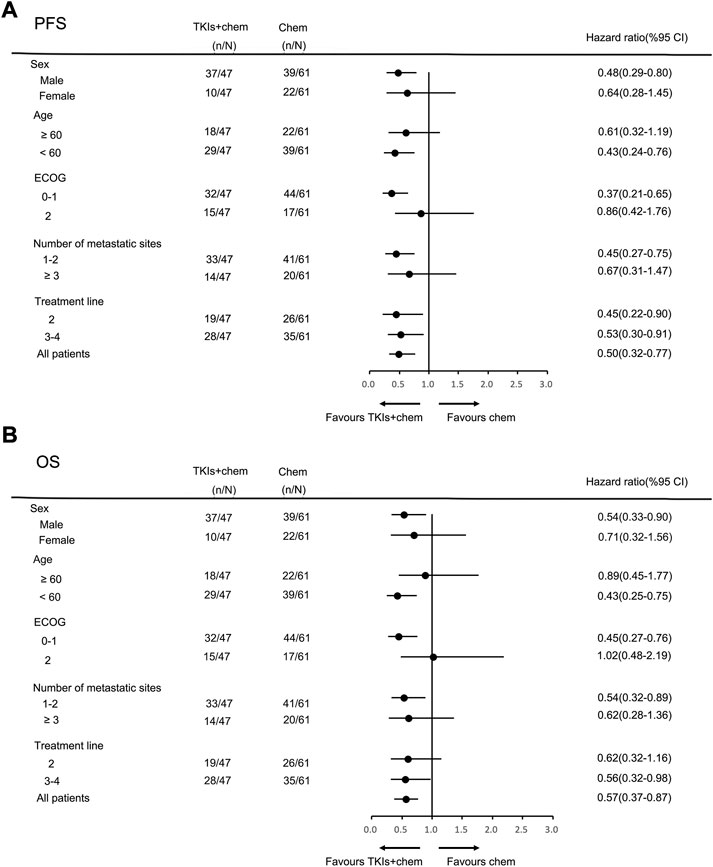
FIGURE 4. Subgroup analysis of PFS (A) and OS (B) according to clinicopathologic factors in TKIs + chemotherapy group.
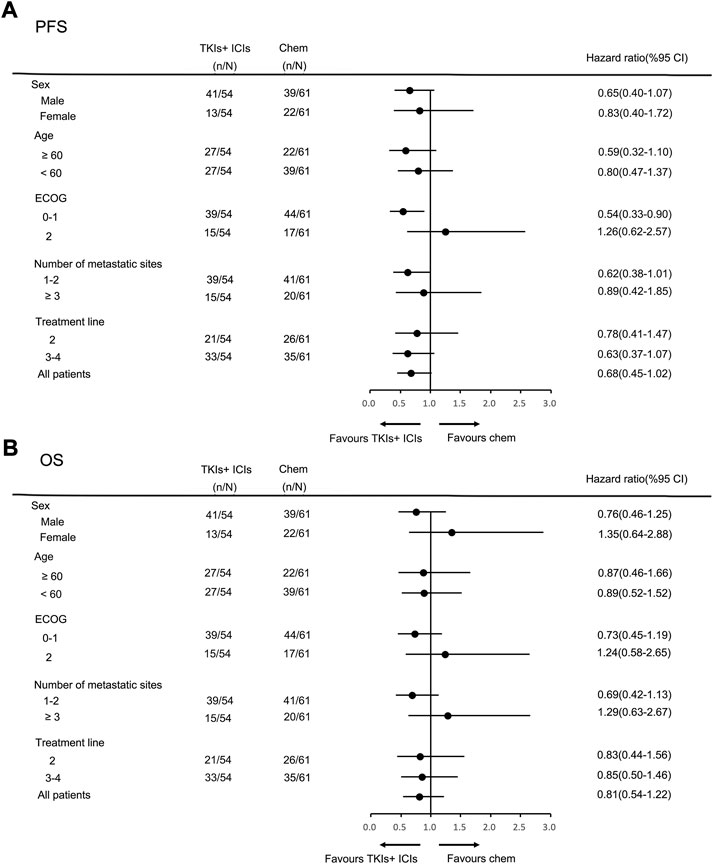
FIGURE 5. Subgroup analysis of PFS (A) and OS (B) according to clinicopathologic factors in TKIs + ICIs group.
Safety data of the chemotherapy, TKIs + chemotherapy and TKIs + ICIs groups were consistent with the known safety profiles of relevant drugs (Table 4). No unexpected side effects or treatment-related death were observed. Most of the adverse events were grade 1–2 in severity and the incidence of Grade 3–4 AEs were 31.1%, 38.3% and 18.5%, respectively. Different treatment manners present with special spectrum of adverse events. The incidence of hematological toxicity was higher in the chemotherapy and TKIs + chemotherapy group. Grade 3–4 hematological AEs in chemotherapy and TKIs + chemotherapy group were decreased white blood count (n = 6, 9.8%; n = 5, 10.6%), decreased neutrophil count (n = 5, 8.2%; n = 5, 10.6%), anemia (n = 2, 3.3%; n = 1, 2.1%) and increased ALT/AST (n = 1, 1.6%; n = 1, 2.1%). In TKIs + chemotherapy and TKIs + ICIs groups, the most common apatinib or anlotinib related non-hematological AEs were secondary hypertension, hand-foot syndrome and proteinuria. Grade 3–4 secondary hypertension occurred in 1 (2.1%) and 5 (9.3%) patients, and grade 3–4 hand-foot syndrome occurred in 3 (6.4%) and 3 (5.6%) patients, respectively. 4 patients in TKIs + chemotherapy and 3 patients in TKIs + ICIs group had gum bleeding, but no severe bleeding events occurred. The most common ICIs -related adverse events in TKIs + ICIs group were rash, pneumonitis and hypothyroidism. Grade 3–4 rash and pneumonitis occurred in 1 (1.9%) and 1 (1.9%) patient, respectively.
The cytotoxic chemotherapy drugs represented by irinotecan and taxanes are still the cornerstone of second-line treatment for metastatic gastric cancer, but these traditional chemotherapy drugs have entered a bottleneck period. The randomized and phase III WJOG 4007 clinical study compared the efficacy of irinotecan and paclitaxel in patients with advanced or metastatic gastric cancer after failure of prior fluoropyrimidine plus platinum chemotherapy. The median PFS of paclitaxel and irinotecan was only 3.6 months and 2.3 months (Hironaka et al., 2013). Therefore, It is necessary to find new therapeutic targets and corresponding treatment methods to further improve the prognosis of patients.
In the era of precision medicine, targeted therapy and immunotherapy have achieved promising clinical efficacy. Based on the results of phase 3 ToGA study, trastuzumab combined with chemotherapy has become the standard treatment option for human epidermal growth factor receptor-2 (HER2) positive metastatic gastric cancer (Bang et al., 2010). However, the positive rate of HER-2 in Chinese patients is only 12–13%, and simultaneously the value of continuous use of trastuzumab beyond progression (TBP) as second-line therapy is still controversial (Al-Shamsi et al., 2016; Ter Veer et al., 2018). Until now, the approved anti-angiogenic drugs for metastatic gastric cancer include ramucirumab and apatinib. Phase III RAINBOW study showed that the anti vascular endothelial growth factor receptor 2 (VEGFR2)_monoclonal antibody ramucirumab in combination with chemotherapy significantly improved the OS compared with single-agent paclitaxel chemotherapy (9.63 vs. 7.36 months) (Wilke et al., 2014). However, ramucirumab has not yet been marketed in China. Another phase III clinical study evaluated the efficacy of anti VEGFR-2 small molecule tyrosine kinase inhibitor apatinib, which demonstrated that apatinib significantly improved the median PFS of patients (2.6 vs. 1.8 months) (Li et al., 2016). The prospective ATTRACTION-02 and KEYNOTE-059 studies confirmed the efficacy of immunotherapy as third-line treatment option for metastatic gastric cancer. Table 5 summarized the clinical second-line or above therapy research data for metastatic gastric cancer (Kang et al., 2017). Overall, the current efficacy of traditional chemotherapy drugs has reached a plateau. In the second-line of metastatic gastric cancer, the median PFS of chemotherapy was 2.3–3.6 months. Compared with chemotherapy, anti-angiogenic targeted therapy and immunotherapy provide new strategies for the late-line treatment of metastatic gastric cancer. However, there is still a huge unmet clinical demand.
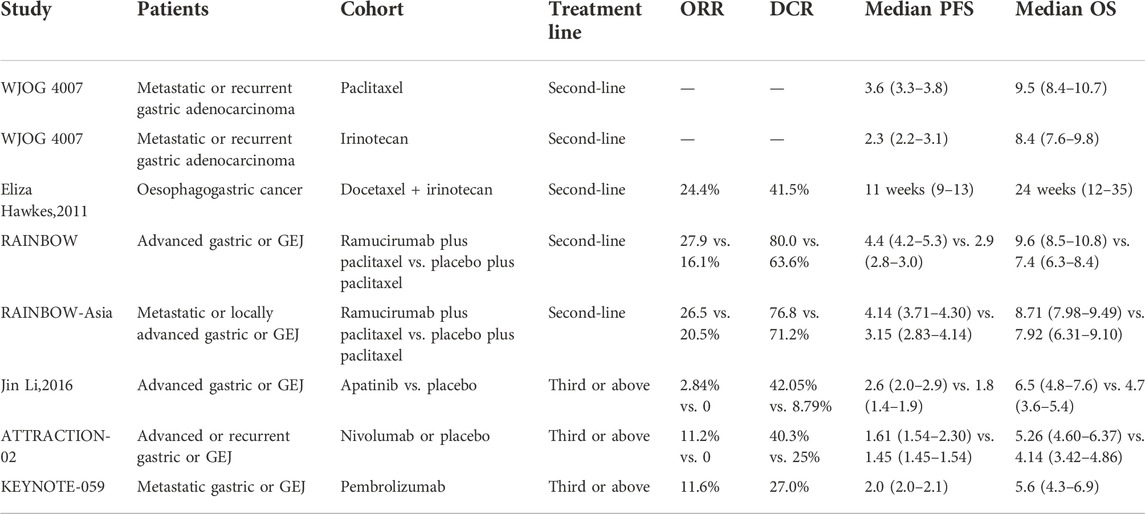
TABLE 5. Summary of clinical research data on second-line or above therapy for advanced or metastatic gastric cancer.
Some recent studies suggest that anti-angiogenic targeted TKIs combined with chemotherapy or immunotherapy have shown a good coordination effect and have become a new exploration strategy. Our present study enrolled 162 patients with advanced or metastatic gastric cancer and evaluating the efficacy and safety of chemotherapy, TKIs + chemotherapy and TKIs + ICIs. To our knowledge, this is the first and largest real-world study comparing clinical efficacy of traditional chemotherapy and novel combination therapy strategy. Although no significant difference was found in ORR between chemotherapy and TKIs + chemotherapy, patients who received TKIs plus chemotherapy obtained better DCR (78.7% vs. 54.1%) and superior survival. Median PFS of 3.3 months and median OS of 8.0 months were obtained in TKIs plus chemotherapy group. In RAINBOW-Asia study, which also evaluated the efficacy of anti-angiogenic ramucirumab combined with chemotherapy, the DCR was 76.8%, median PFS and OS were 4.14 and 8.71 months, respectively (Xu et al., 2021). Unlike the RAINBOW-Asia study, which enrolled all patients receiving second-line therapy, the proportion of patients who received TKIs plus chemotherapy as second-line therapy in our present study was only 40.4%, the other 59.6% patients as third to fourth-line treatment. Unlike monoclonal antibody anti-angiogenic drugs, small-molecule multi-targeted TKIs can target multiple receptor sites simultaneously, including VEGFR, c-Kit, c-Met, fibroblast growth factor receptor (FGFR) and platelet-derived growth factor receptor (PDGFR). However, monotherapy with TKIs has shown limited efficacy in most cancers. We reviewed relevant literature, and there are few basic studies on the mechanism of action of anti-angiogenic TKIs combined with chemotherapy. Preclinical studies have shown that anti-angiogenic agents can normalize blood vessels in tumor tissue, thus sustaining the therapeutic efficacy of chemotherapy (Lennernas et al., 2003; Wang et al., 2021a). Several recent studies have confirmed the efficacy of TKIs plus chemotherapy in non-small cell lung cancer (NSCLC), ovarian cancer, and etc., (Bondarenko et al., 2015; Lan et al., 2018; Nie et al., 2021). Our present study confirmed the value of anti-angiogenic TKIs plus chemotherapy for metastatic gastric cancer. We will continue to pay attention to reports of basic research and clinical trails related to anti-angiogenic TKIs combined with chemotherapy in metastatic gastric cancer.
Anlotinib was not approved for the treatment of gastric cancer. There are two reasons for why we use this agent. First, all the 162 patients had undergone previous systematic therapy, 40.7% received chemotherapy, TKIs + chemotherapy or TKIs + ICIs therapy as second-line therapy, and as third-fourth line therapy in the other 59.3% patients. Therefore, some of the patients who received anlotinib in this study were patients who failed from apatinib treatment. Second, a small number of patients developed intolerable toxicity after receiving apatinib, so they were switched to anlotinib treatment. Although anlotinib was not approved, several retrospective and real-world studies have confirmed the efficacy of anlotinib in metastatic gastric cancer. And simultaneously, before the treatment, we conducted adequate communication and informed notification.
The phase Ib REGONIVO study evaluated the antitumor activity of nivolumab plus regorafenib in the gastric and colorectal cancer cohort. The median PFS of gastric and colorectal cancer patients was 7.9 and 5.6 months, and the 12-month PFS rate reached 41.8% and 22.4%, respectively (Fukuoka et al., 2020). It was considered that anti-angiogenesis therapy was a potential method to reverse immunotherapy resistance. Some clinical studies also explored the efficacy of bevacizumab combined with a PD-1 inhibitor, but the results could not confirm the value of bevacizumab combined with immunotherapy (Moore et al., 2021). This study suggests for the first time that TKIs plus ICIs may become a novel and effective treatment strategy for gastrointestinal tumors. Basic research has shown that anti-angiogenic TKIs can relieve the immunosuppression caused by Treg and TAM cells on T cells by inhibiting colony-stimulating factor 1 receptor (CSF1R) and VEGFR to enhance the efficacy of immunotherapy (Bai et al., 2021; Doleschel et al., 2021). Several previous studies have confirmed the value of anti-angiogenic TKIs plus immunotherapy in renal cell carcinoma, colorectal cancer, NSCLC etc (Atkins et al., 2018; Wang et al., 2021b). However, the efficacy of anti-angiogenic TKIs plus immunotherapy in gastric cancer is still controversial. REGONIVO study results show that the ORR of regorafenib combined with nivolumab in the treatment of gastric cancer is as high as 44%. The LEAP-005 trial showed that the ORR of lenvatinib plus pembrolizumab in patients with previously treated gastric cancer was only 10%. The JVDF trial evaluated the efficacy of ramucirumab plus pembrolizumab in patients with previously treated advanced gastroesophageal cancer, the ORR was only 7% and the median PFS was 2.5 months (Herbst et al., 2019). In our present study, compared with chemotherapy, TKIs plus ICIs as second-line or above therapy did not significantly improve ORR, DCR, median PFS and OS in gastric cancer. And thus, our present study did not support the application of anti-angiogenic TKIs plus ICIs for metastatic gastric cancer.
From previous clinical studies, there is no evidence that the combination of anti-angiogenic TKIs plus chemotherapy or immunotherapy will increase the proportion of adverse events (Wang et al., 2020; Laccetti et al., 2021). The most common adverse events in the chemotherapy and TKIs + chemotherapy group were hematological toxicity. Adding TKIs on basis of chemotherapy did not increase the risk of all grade and grade 3–4 of decreased white blood or neutrophil count, anemia and decreased platelet. Our present study also had limitations because this was an observational study rather than a randomized controlled clinical study. Thus, future validation clinical trials would be needed to confirm the value of TKIs + chemotherapy and TKIs + ICIs as second-line and beyond therapy for patients with metastatic gastric cancer.
In conclusion, these data confirm that compared with chemotherapy, TKIs plus chemotherapy provide a superior second-line or above therapeutic strategy in patients with advanced or metastatic gastric cancer with well tolerated toxicity. However, TKIs + ICIs failed to demonstrate a clinical advantage over chemotherapy.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by This study was carried out in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Affiliated Cancer Hospital of Zhengzhou University. Written informed consent was obtained from all patients. The patients/participants provided their written informed consent to participate in this study.
All authors contributed to the manuscript. CN and XC designed the study, and drafted the paper. WX, HL, XG, GL, and BC collected and analyzed the data. CN, JW YL, JZ, YH, and SW performed statistical analysis.
This work was supported by Medical Science and Technique Foundation of Henan Province (Nos. 212102310623, LHGJ20210172, 222102310424), Young and Middle-aged Health and Technology Innovation Leading Talent Project of Henan Province (No. YXKC2020008), 1000 Talents Program of Central plains (No. 204200510023), the Sate Key Laboratory of Esophageal Cancer Prevention & Treatment (No. Z2020000X).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Shamsi, H. O., Fahmawi, Y., Dahbour, I., Tabash, A., Rogers, J. E., Mares, J. E., et al. (2016). Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: The MD anderson experience. J. Gastrointest. Oncol. 7 (4), 499–505. doi:10.21037/jgo.2016.06.16
Atkins, M. B., Plimack, E. R., Puzanov, I., Fishman, M. N., McDermott, D. F., Cho, D. C., et al. (2018). Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: A non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet. Oncol. 19 (3), 405–415. doi:10.1016/S1470-2045(18)30081-0
Bai, H., Wang, J., Phan, C. U., Chen, Q., Hu, X., Shao, G., et al. (2021). Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat. Commun. 12 (1), 759. doi:10.1038/s41467-021-21071-0
Bang, Y. J., Van Cutsem, E., Feyereislova, A., Chung, H. C., Shen, L., Sawaki, A., et al. (2010). Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376 (9742), 687–697. doi:10.1016/S0140-6736(10)61121-X
Bondarenko, I. M., Ingrosso, A., Bycott, P., Kim, S., and Cebotaru, C. L. (2015). Phase II study of axitinib with doublet chemotherapy in patients with advanced squamous non-small-cell lung cancer. BMC Cancer 15, 339. doi:10.1186/s12885-015-1350-6
Doleschel, D., Hoff, S., Koletnik, S., Rix, A., Zopf, D., Kiessling, F., et al. (2021). Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J. Exp. Clin. Cancer Res. 40 (1), 288. doi:10.1186/s13046-021-02043-0
Fuchs, C. S., Doi, T., Jang, R. W., Muro, K., Satoh, T., Machado, M., et al. (2018). Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4 (5), e180013. doi:10.1001/jamaoncol.2018.0013
Fukuoka, S., Hara, H., Takahashi, N., Kojima, T., Kawazoe, A., Asayama, M., et al. (2020). Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: An open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J. Clin. Oncol. 38 (18), 2053–2061. doi:10.1200/JCO.19.03296
Gao, S., Li, X., Shi, W., Huo, L., and Liu, H. (2022). Efficacy of the low dose apatinib plus chemotherapy on advanced gastric carcinoma. J. Oncol. 2022, 3009494. doi:10.1155/2022/3009494
Hackshaw, M. D., Bui, C. L., Ladner, A., Tu, N., Islam, Z., Ritchey, M. E., et al. (2020). Review of survival, safety, and clinical outcomes in HER2+ metastatic gastric cancer following the administration of trastuzumab. Cancer Treat. Res. Commun. 24, 100189. doi:10.1016/j.ctarc.2020.100189
Hawkes, E., Okines, A. F., Papamichael, D., Rao, S., Ashley, S., Charalambous, H., et al. (2011). Docetaxel and irinotecan as second-line therapy for advanced oesophagogastric cancer. Eur. J. Cancer 47 (8), 1146–1151. doi:10.1016/j.ejca.2010.12.021
Herbst, R. S., Arkenau, H. T., Santana-Davila, R., Calvo, E., Paz-Ares, L., Cassier, P. A., et al. (2019). Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): A multicohort, non-randomised, open-label, phase 1a/b trial. Lancet. Oncol. 20 (8), 1109–1123. doi:10.1016/S1470-2045(19)30458-9
Hironaka, S., Ueda, S., Yasui, H., Nishina, T., Tsuda, M., Tsumura, T., et al. (2013). Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J. Clin. Oncol. 31 (35), 4438–4444. doi:10.1200/JCO.2012.48.5805
Hogner, A., and Moehler, M. (2022). Immunotherapy in gastric cancer. Curr. Oncol. 29 (3), 1559–1574. doi:10.3390/curroncol29030131
Kang, Y. K., Boku, N., Satoh, T., Ryu, M. H., Chao, Y., Kato, K., et al. (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390 (10111), 2461–2471. doi:10.1016/S0140-6736(17)31827-5
Koizumi, W., Narahara, H., Hara, T., Takagane, A., Akiya, T., Takagi, M., et al. (2008). S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet. Oncol. 9 (3), 215–221. doi:10.1016/S1470-2045(08)70035-4
Korfer, J., Lordick, F., and Hacker, U. T. (2021). Molecular targets for gastric cancer treatment and future perspectives from a clinical and translational point of view. Cancers 13 (20), 5216. doi:10.3390/cancers13205216
Laccetti, A. L., Garmezy, B., Xiao, L., Economides, M., Venkatesan, A., Gao, J., et al. (2021). Combination antiangiogenic tyrosine kinase inhibition and anti-PD1 immunotherapy in metastatic renal cell carcinoma: A retrospective analysis of safety, tolerance, and clinical outcomes. Cancer Med. 10 (7), 2341–2349. doi:10.1002/cam4.3812
Lan, C. Y., Wang, Y., Xiong, Y., Li, J. D., Shen, J. X., Li, Y. F., et al. (2018). Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): A phase 2, single-arm, prospective study. Lancet. Oncol. 19 (9), 1239–1246. doi:10.1016/S1470-2045(18)30349-8
Lennernas, B., Albertsson, P., Lennernas, H., and Norrby, K. (2003). Chemotherapy and antiangiogenesis-drug-specific, dose-related effects. Acta Oncol. 42 (4), 294–303. doi:10.1080/02841860310001835
Li, J., Qin, S., Xu, J., Xiong, J., Wu, C., Bai, Y., et al. (2016). Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34 (13), 1448–1454. doi:10.1200/JCO.2015.63.5995
Li, L. H., Chen, W. C., and Wu, G. (2022). Feasibility and tolerance of apatinib plus PD-1 inhibitors for previously treated advanced gastric cancer: A real-world exploratory study. Dis. Markers 2022, 4322404. doi:10.1155/2022/4322404
Lu, Z., Zhang, X., Liu, W., Liu, T., Hu, B., Li, W., et al. (2018). A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer 21 (5), 782–791. doi:10.1007/s10120-018-0809-y
Luo, H. Y., Xu, R. H., Wang, F., Qiu, M. Z., Li, Y. H., Li, F. H., et al. (2010). Phase II trial of XELOX as first-line treatment for patients with advanced gastric cancer. Chemotherapy 56 (2), 94–100. doi:10.1159/000305256
Moore, K. N., Bookman, M., Sehouli, J., Miller, A., Anderson, C., Scambia, G., et al. (2021). Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: Placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J. Clin. Oncol. 39 (17), 1842–1855. doi:10.1200/JCO.21.00306
Nie, C., Lv, H., Xing, Y., Chen, B., Xu, W., Wang, J., et al. (2021). The efficacy and safety of apatinib treatment for patients with advanced or recurrent biliary tract cancer: A retrospective study. BMC Cancer 21 (1), 189. doi:10.1186/s12885-021-07907-4
Parisi, A., Porzio, G., and Ficorella, C. (2020). Multimodality treatment in metastatic gastric cancer: From past to next future. Cancers (Basel) 12 (9), E2598. doi:10.3390/cancers12092598
Shitara, K., Takashima, A., Fujitani, K., Koeda, K., Hara, H., Nakayama, N., et al. (2017). Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): An open-label, randomised, non-inferiority, phase 3 trial. Lancet. Gastroenterol. Hepatol. 2 (4), 277–287. doi:10.1016/S2468-1253(16)30219-9
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, Z., Wang, Y., Yu, Y., Cui, Y., Liang, L., Xu, C., et al. (2022). Neoadjuvant apatinib combined with oxaliplatin and capecitabine in patients with locally advanced adenocarcinoma of stomach or gastroesophageal junction: A single-arm, open-label, phase 2 trial. BMC Med. 20 (1), 107. doi:10.1186/s12916-022-02309-0
Ter Veer, E., van den Ende, T., Creemers, A., de Waal, L., van Oijen, M. G. H., and van Laarhoven, H. W. M. (2018). Continuation of trastuzumab beyond progression in HER2-positive advanced esophagogastric cancer: A meta-analysis. Acta Oncol. 57 (12), 1599–1604. doi:10.1080/0284186X.2018.1503421
Wang, K., Chen, Q., Liu, N., Zhang, J., and Pan, X. (2021). Recent advances in, and challenges of, anti-angiogenesis agents for tumor chemotherapy based on vascular normalization. Drug Discov. Today 26 (11), 2743–2753. doi:10.1016/j.drudis.2021.07.024
Wang, Y., Shi, X., Qi, Q., Ye, B., and Zou, Z. (2021). Safety of anlotinib capsules combined with PD-1 inhibitor camrelizumab in the third-line treatment of advanced non-small-cell lung cancer and their effect on serum tumor markers. J. Healthc. Eng. 2021, 2338800. doi:10.1155/2021/2338800
Wang, Z. M., Zhang, S. L., Yang, H., Zhuang, R. Y., Guo, X., Tong, H. X., et al. (2020). Efficacy and safety of anlotinib, a multikinase angiogenesis inhibitor, in combination with epirubicin in preclinical models of soft tissue sarcoma. Cancer Med. 9 (10), 3344–3352. doi:10.1002/cam4.2941
Wilke, H., Muro, K., Van Cutsem, E., Oh, S. C., Bodoky, G., Shimada, Y., et al. (2014). Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet. Oncol. 15 (11), 1224–1235. doi:10.1016/S1470-2045(14)70420-6
Xu, R. H., Zhang, Y., Pan, H., Feng, J., Zhang, T., Liu, T., et al. (2021). Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): A randomised, multicentre, double-blind, phase 3 trial. Lancet. Gastroenterol. Hepatol. 6 (12), 1015–1024. doi:10.1016/S2468-1253(21)00313-7
Keywords: gastric cancer, chemotherapy, anti-angiogenesis, immunotherapy, tyrosine kinase inhibitors
Citation: Nie C, Xu W, Lv H, Gao X, Li G, Chen B, Wang J, Liu Y, Zhao J, He Y, Wang S and Chen X (2022) Tailoring second-line or above therapy for patients with advanced or metastatic gastric cancer: A multicenter real-world study. Front. Pharmacol. 13:1043217. doi: 10.3389/fphar.2022.1043217
Received: 13 September 2022; Accepted: 08 November 2022;
Published: 17 November 2022.
Edited by:
Tao Zhang, Shanghai Jiao Tong University, ChinaReviewed by:
Miaozhen Qiu, Sun Yat-sen University Cancer Center (SYSUCC), ChinaCopyright © 2022 Nie, Xu, Lv, Gao, Li, Chen, Wang, Liu, Zhao, He, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobing Chen, emx5eWNoZW54YjA4MDdAenp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.