
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol., 15 December 2022
Sec. Obstetric and Pediatric Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1038090
This article is part of the Research TopicAdvances in Precision Diagnosis and Therapy of Pediatric Rare DiseasesView all 11 articles
Background: Protein glycosylation plays an important role in post-translational modification, which defines a broad spectrum of protein functions. Accordingly, infants with a congenital disorder of glycosylation (CDG) can have N-glycosylation, O-glycosylation, or combined N- and O-glycosylation defects, resulting in similar but different multisystem involvement. CDGs can present notable gastrointestinal and neurologic symptoms. Both protein-losing enteropathy and hypotonia affect the decision of using anesthetics. We reported a case of MPI-CDG with protein-losing enteropathy and muscular hypotonia that underwent different anesthesia approach strategies of vascular access. Here, we highlight why intubation with sevoflurane anesthesia and sparing use of muscle relaxants is the optimal strategy for such a condition.
Case presentation: A 25-month-old girl, weighing 6.6 kg and 64 cm tall, suffered chronic diarrhea, hypoalbuminemia, and hypotonia since birth. Protein-losing enteropathy due to MPI-CDG was documented by whole-exome sequencing. She underwent three sedated surgical procedures in our hospital. The sedation was administered twice by pediatricians with oral chloral hydrate, intravenous midazolam, and ketamine, to which the patient showed moderate to late recovery from sedation and irritability the following night. The most recent one was administered by an anesthesiologist, where endotracheal intubation was performed with sevoflurane as the main anesthetic. The patient regained consciousness immediately after the operation. She had no complications after all three sedation/anesthesia interventions and was discharged 7 days later, uneventful after the third general anesthesia procedure.
Conclusion: We performed safe anesthetic management in a 25-month-old girl with MPI-CDG using sevoflurane under controlled ventilation. She awoke immediately after the procedure. Due to the disease entity, we suggested bypassing the intravenous route to avoid excess volume for drug administration and that muscle relaxant may not be necessary for endotracheal intubation and patient immobilization when performing procedures under general anesthesia in CDG patients.
The provision of safe anesthesia for pediatric patients depends on a clear understanding of the physiologic, pharmacologic, and psychological differences between children and adults (Miller et al., 2009). Practitioners should evaluate several critical pre-operational conditions when planning for pediatric anesthesia. These medical conditions include but are not limited to a patient’s developmental status, the aim of the surgery, preoperative preparation, pharmacology of the candidate drugs, and the possible side effects after administration (Miller et al., 2009). Every step matters; the more information collected, the better the plan can be made. This is particularly important when it comes to dealing with rare diseases. Infants with CDGs present varying levels of involvement of the central nervous system (most often hypotonia and ataxia), dysmorphology, gastrointestinal symptoms including protein-losing enteropathy, and other signs (Kliegman et al., 2016). Some symptoms affect the choice of anesthesia management, while facial dysmorphism/preterm birth raises worries of airway insecurity; both protein-losing enteropathy and hypotonia can affect the decisions made during anesthesia management. To date, the literature on this subject lacks reports on anesthesia management in children with CDGs. To date, only three reports have been published, each regarding CDG types, namely, PMM2-CDG, ALG6-CDG, and STT3B-CDG (Sakai et al., 2017) (Meaudre et al., 2005) (Lehavi et al., 2011). Sakai et al. (2017) suggested the use of neuromuscular monitoring when using rocuronium on CDG patients with hepatic dysfunction and hypotonia. Meaudre et al. (2005) highlighted the complexity of coagulopathy in CDG patients and the perioperative assessment of clotting factors, as part of which practitioners use fresh frozen plasma or a prothrombin complex concentrate to lower hemorrhagic risk during surgery (Altassan et al., 2019). Lehavi et al. (2011) described a nitrous oxide–remifentanil-based anesthesia on a 6-year-old boy (16.2 kg), taking into account concerns about the CDG patient’s hemodynamic status and hepatic function. At present, there are no specific guidelines for managing anesthesia in CDG patients, and more data is required. In response, the present study reports on the anesthesia practices used for a 25-month-old girl suffering from MPI-CDG with protein-losing enteropathy and muscular hypotonia. In our case, we present three approaches to anesthesia management during vascular access for regular albumin infusion, performed by different specialists. We also discuss the particular concerns of the approaches and propose an overall strategy.
The patient was a 25-month-old girl, who weighed 6.6 kg (
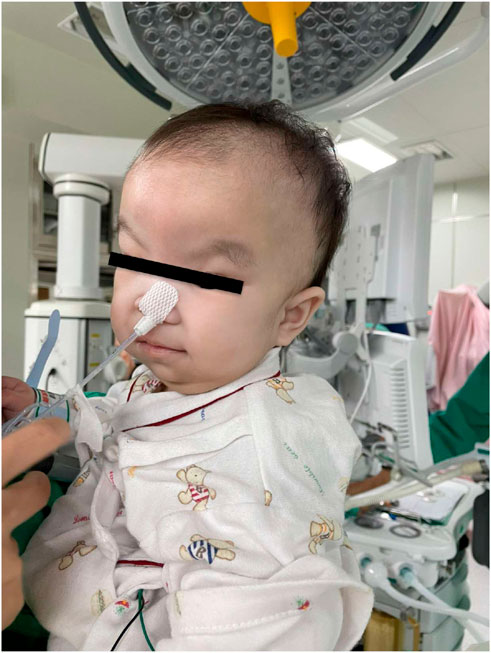
FIGURE 1. Photo of the patient before the port-a-cath exchange. Her dysmorphic features were wide prominent forehead, flat nose, large anterior fontanelle, web neck, and skeletal dysplasia.
The first sedation was administered by pediatricians in the pediatric intensive care unit (PICU) for the purpose of placing a PICC. Routine non-invasive monitoring was established, including BT, BP, HR, SaO2, and RR. Before anesthesia, her vital signs were stable: BT: 36.4°C, BP: 95/74 mmHg, HR: 114/min, SaO2: 100%, and RR: 34/min. The sedation was induced orally using a 3.5 ml 10% chloral hydrate solution (0.5 cc⋅ kg−1), intravenous 0.7 mg midazolam (0.1 mg ⋅ kg−1), and continued on 6 mg ketamine (0.9 mg ⋅ kg−1) seven times, with a total dosage of 42 mg within 169 min. The surgery lasted 215 min; however, we failed to insert the PICC. During the procedure, the addition of ketamine was adjusted according to restlessness to ensure that the patient did not wake up. The patient’s vital signs remained stable, with no bradycardia, hypotension, apnea, or arterial desaturation, during the whole procedure. After awakening from the procedure, there were no other symptoms or discomfort for the patient, except for appearing irritable the following night.
The second sedation was also administered by pediatricians in the PICU to place a PICC. The procedure was performed by a plastic surgeon, who asked for a completely stable patient during the operation. Routine monitoring was established, including BT, BP, HR, SaO2, and RR. Before the anesthesia, her vital signs were stable: BT: 36.2°C, BP: 91/57 mmHg, HR: 120/min, SaO2: 99%, and RR: 30/min. Prior to the procedure, she was induced using 3.4 ml 10% chloral hydrate solution (0.5 cc⋅ kg−1), 0.7 mg midazolam (0.1 mg ⋅ kg−1), and continued on 6 mg ketamine (0.9 mg ⋅ kg−1) five times, with a total dosage of 30 mg within 147 min. The surgery lasted 90 min, anesthesia time was 170 min, and a heart rate over 150/min was used as a sign of awakening and for additional drug dosage supplements. The patient remained stable, and a PICC was successfully placed. During the operation, her vital signs were also stable, with no bradycardia, hypotension, apnea, or arterial desaturation. There were no other complications or discomfort from the patient except for appearing irritable the night after the administration of anesthesia.
The latest anesthesia was administered by anesthesiologists for a port-a-cath exchange in the operating room. At the pre-anesthesia assessment, her anesthesia status was graded as American Society of Anesthesiologists (ASA) Class III for CDG disease entity and possible difficulty in airway establishment. The laboratory examination showed hypoalbuminemia and anemia, and her chest X-ray showed increased non-specific infiltrate in the bilateral lower lungs. Electrocardiography showed normal sinus rhythm. Before the anesthesia, her vital signs were stable with BT: 36.2°C, BP: 89/47 mmHg, HR: 119/min, SaO2: 99%, and RR: 50/min. In order to minimize complications from endotracheal intubation, GlideScope® was used, and the patient was brought to a sniffing position with neck protection. After successful airway establishment, sevoflurane was used as the main anesthesia. After evaluating the patient’s muscle tone, muscle relaxants were not administered. A total of 0.1 mg atropine was given to maintain the heart rate (150–160/min) for an adequate cardiac output. The surgery lasted 60 min, and the anesthesia time lasted 120 min. The patient’s vital signs remained stable and under secured ventilation control, with no bradycardia, hypotension, apnea, or arterial desaturation. After completion of the operation, the patient regained consciousness and was sent back to the PICU, where the ET tube was removed and a nasal cannula was placed for 4 h. No complication or discomfort was observed, and the patient was discharged uneventfully 7 days later.
Three anesthetic interventions were performed in our reported MPI-CDG case. When facing rare diseases, the common obstacles for healthcare professionals include diagnostic delays and lack of information and treatment options. A comprehensive evaluation is particularly critical in situations like this. In this case, the anesthesiologists routinely and thoroughly evaluated the patient’s condition, especially included assessing the patient’s airway condition. While each of the patients’ experiences under anesthetic were fine, it is important to remember that the aim of using anesthesia is to ease the patient for an unbothered surgery.
When managing children under anesthesia, it is important to constantly maintain a secured airway with satisfactory ventilation and oxygenation. A failed airway can cause hypoxia, potentially leading to brain damage and death within minutes (Cook and MacDougall-Davis, 2012). It is currently reported that more than half of critical perioperative events in children are respiratory complications (Habre et al., 2017). Any improvement in preparation (mainly preoxygenation and patient positioning), intubation techniques, and removal of airway devices can minimize perioperative complications. Additionally, capnography monitoring for critical information on ventilation, perfusion, and metabolism is a standard tool that ensures the establishment of a secured endotracheal tube (Soto et al., 2004). In cases in which difficulties with the airway might be expected, it is recommended that intravenous access be prepared beforehand for instant management of potential emergencies, e.g., laryngospasm and bradycardia, where the latter is usually prevented by administering 0.02 mg/kg atropine (Karsli, 2015). In addition, experts recently recommended the use of video laryngoscopy as the first option for patients in which intubation is anticipated to be difficult (Dadure et al., 2019).
The concerns mentioned above were addressed in the third operation. In this case, we anticipated difficult airway management because of dysmorphism (low-set ears, wide eye distance, and some retrognathia) (Roth et al., 2021) and a narrow airway evidenced by X-rays. Preoxygenation and a neutral airway position were established, atropine 0.1 mg was administered through pre-established PICC, and video laryngoscopy (GlideScope®) was used for tracheal intubation. The successful airway establishment and monitoring of ETCO2 not only secured the patient’s status for surgery but also suited the chosen anesthesia route, which is discussed in the next section.
The induction of general anesthesia for children occurs either by inhalation or intravenously (IV). While inhalation induction is the most common technique in young children, there are several conditions for which IV induction is preferred. Whatever the route, it is always necessary to evaluate the children’s history and laboratory findings before planning the anesthetic to be used. We discussed the concerns in three dimensions: patients’ laboratory status, ketamine experience and pharmacokinetics, and IV route drawbacks.
First of all, Saad et al. (2020) strongly highlighted the importance of preoperative malnutrition screening and management for the risk of anesthetic overdose. In our case, the girl has hypoalbuminemia with underlying protein-losing enteropathy. Although supported by intravascular nutrient support, her preoperative albumin levels were all low. The high prevalence of hepatic dysfunction in CDG patients is also noticeable (da Silva et al., 2017) (Schollen et al., 2004), with her transaminases within normal limits but prothrombin time shortened. These laboratory findings affect the pharmacokinetics of anesthetics, in this case, ketamine.
In children, ketamine plays an anesthetic role in short-term procedures. Well-known for its psychodysleptic effects, ketamine is a rapid-acting N-methyl-D-aspartic acid (NMDA) receptor non-competitive antagonist (Kurdi et al., 2014). It also interacts with opioid receptors, monoamine, cholinergic, purinergic, and adrenoreceptor systems, providing both positive and negative modulation in sedation and analgesia (Nowacka and Borczyk, 2019). The benefits include preserving children’s cardio-respiratory stability by enhancing or maintaining a normal skeletal muscle tone (Rosenbaum et al., 2021). However, this does not ensure a secured airway and there may be transient minimal respiratory depression if ketamine is administered too rapidly or in too high a dose. Therefore, pharmacokinetics should always be evaluated on a case-by-case basis. According to the literature (Trevor et al., 2019) (Miller et al., 2009), ketamine onset occurs rapidly due to high lipid solubility and ceases its effect by redistribution to inactive sites at a half-life of 11–16 min. Two aspects of ketamine properties were the foci in our case. For one, it is metabolized in the liver through N-demethylation by the cytochrome CYP3A4 (Dinis-Oliveira, 2017). Second, ketamine is the only intravenous anesthetic that has low protein binding (approximately 12%). In our experience, pediatricians empirically chose IV ketamine with midazolam adjuvant for two sedation episodes. Propofol was not used for her age, under 3, according to the Food and Drug Administration (FDA) of the United States. Although both single and accumulative ketamine dosage were within normal limits (induction 0.5–2 mg/kg, lethal dose 600 mg/kg (Orhurhu1 et al., 2021)), the supplement interval (mean 25 min) indicated a mildly prolonged sedation. We tried to explain this outcome despite the lack of literature on the two foci, liver metabolism and protein binding mentioned above. Given that the girl showed normal transaminase levels, we considered there to be a low risk of hepatic-derived complications in the anesthesia outcome. On the other hand, hypoalbuminemia has a great effect on high protein-binding agents; since ketamine is one of the low protein-binding anesthetics, ketamine pharmacokinetics are rarely studied in hypoalbuminemia. However, as hypoalbuminemia more or less reduces protein-binding and increases the free active fraction of drugs (Pino, 2019), we considered this as the cause of her two prolonged ketamine sedations. Last, ketamine-induced dissociation is a major side effect causing concern in pediatric anesthesia. Although midazolam was used as an adjuvant to reduce ketamine induction dosage, the child showed irritability after both interventions. Therefore, the decision to use ketamine should be made carefully, especially when the child shows abnormal susceptibility.
Residual drugs can remain in the dead space of intravenous lines, which is especially crucial when giving small-volume infusions (
Muscle relaxants, or neuromuscular blocking agents (NMBAs), are commonly used in anesthesia under the indication to facilitate intubation and surgery condition. However, the use of NMBAs should be determined individually (Gueret et al., 2004). NMBAs can have an unexpectedly prolonged effect in patients with hypotonia, and the susceptibility of patients with CDG to non-depolarizing NMBAs remains unclear (Sakai et al., 2017). Although it is not well understood, to the best of our knowledge, trans-synaptic signaling is reduced in CDGs (Frappaolo et al., 2018), the expression of postsynaptic nicotinic acetylcholine receptors with normal function is also reduced (Freeze et al., 2014). This supports our assumption in treating CDG as myasthenia gravis when it comes to NMBAs. For most surgical procedures, administration of NMBAs is not necessary for patients with myasthenia. Adequate relaxation is often reached using inhalation agents alone (Nitahara et al., 2007) (Rocca et al., 2003), in our case, sevoflurane. Even if NMBAs are needed, due to susceptibility concerns (Eisenkraft et al., 1988), it has been suggested that non-depolarizing NMBAs should be used, namely rocuronium or vecuronium with a reversal with sugammadex (Tsukada et al., 2021) (de Boer et al., 2014). However, sugammadex has not received FDA approval for use in children, and to date, there are limited data regarding its administration to pediatric patients (Tobias, 2017). Therefore, due to the patient’s hypotonia and adequate relaxation after administering sevoflurane, additional muscle relaxants were not required to achieve the degree of immobilization required for the surgeon to proceed with the port-a-cath replacement.
The pharmacological characteristics of good sedation and analgesia include ease of application, rapid action, short duration of action, and lack of significant adverse reactions (Norambuena et al., 2013). An experienced and professional practitioner can be a valuable asset when approaching unclear situations (Schulz et al., 2013). The cases presented here raise three crucial concerns of anesthesia, including airway management, choice of drug, and the decision to use a muscle relaxant. Although there is a lack of precise guidelines for anesthesia in CDGs, decisions can still be made by a comprehensive strategy (Figure 2) including evaluating the patient, understanding the underlying mechanisms, and weighing the medical risks and benefits, all to achieve the final purpose—to assure the surgeon and secure the patient.
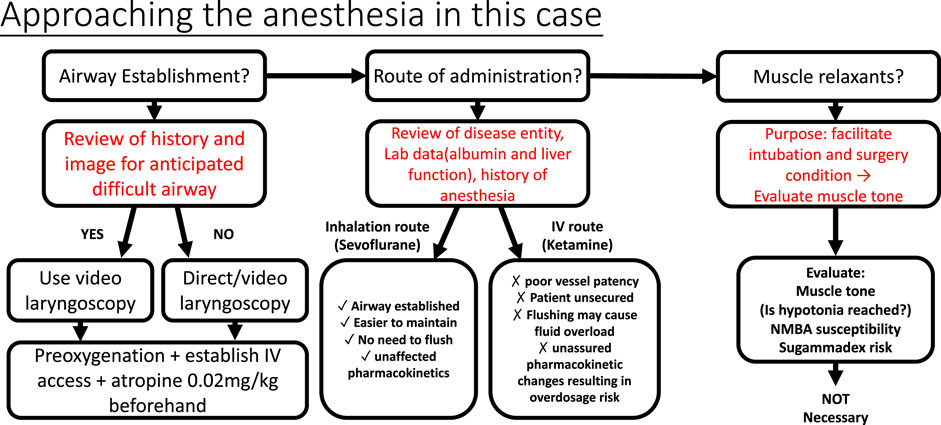
FIGURE 2. Approach to the anesthesia in the case. Secured airway prevents respiratory complications and brain hypoxia in children’s anesthesia. After the review of X-ray and evaluating child appearance, video larnygoscopy was chosen for expected difficult airway intubation. Decision of the route of administration depends on the pros and cons. Hypoalbuminemia increases free active fraction of protein-bound drugs; at the same time, elevated liver transaminases/liver pathology may also interfere with drug metabolism, in this case, ketamine. On the other hand, anesthetic sevoflurane was eliminated directly via lung exhalation, bypassing the liver metabolism. Therefore, the inhalation route outweighed the IV route with its convenience and safety. Muscle relaxants act to facilitate endotracheal intubation and operation; in this case, with hypotonia and the muscle relaxation effect of sevoflurane, NMBAs were not necessary. Sugammadex is a good choice for immediately reversing non-depolarizing muscle relaxants (rocuronium or vecuronium). However, it has not been approved by the USFDA for use in children under two years of age.
This study reports rare clinical experiences in MPI-CDG children’s anesthesia management. Comparing the three anesthetic experiences, we have reviewed the decisions made by two specialists (Table 1). Based on our review of the literature and discussion of recent studies, we suggest that when anesthesia is needed for CDG patients practitioners use inhaled anesthetics instead of the intravenous route and consider not using NMBAs if patients show hypotonia. These findings will help in administering an anesthetic to CDG children in the future and we encourage further research on this subject.

TABLE 1. Comparison of the three anesthetic practices. Notice the durations of both ketamine-induced anesthesia and hypoalbuminemia status, which raise overdose and fluid overload concerns.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Cathay General Hospital IRB (CGH-IRB No. P111017). Written informed consent to participate in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
E-CC contributed to the design, writing, review, and revision of this manuscript. Y-HC and Y-ST contributed to collecting data and drafting the manuscript. C-SW guided E-CC in design, writing and final reviewing the case report. Y-LH and M-JL contributed to the anesthesia management of the case and proofread the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Altassan, R., Péanne, R., Jaeken, J., Barone, R., Bidet, M., Borgel, D., et al. (2019). International clinical guidelines for the management of phosphomannomutase 2-congenital disorders of glycosylation: Diagnosis, treatment and follow up. J. Inherit. Metab. Dis. 42, 5–28. doi:10.1002/jimd.12024
Cook, T. M., and MacDougall-Davis, S. R. (2012). Complications and failure of airway management. Br. J. Anaesth. 109, i68-i85. doi:10.1093/bja/aes393
Cousins, D. (2018). Patients are being underdosed: We need new guidance on small-volume drug infusions. Clin. Pharm. 10. doi:10.1211/CP.2018.20205779
da Silva, D. M., Ferreira, V. D. R., Monticelli, M., Janeiro, P., Videira, P. A., da Silva, P. W., et al. (2017). Liver involvement in congenital disorders of glycosylation (CDG). a systematic review of the literature. J. Inherit. Metab. Dis. 40, 195–207. doi:10.1007/s10545-016-0012-4
Dadure, C., Sabourdin, N., Veyckemans, F., Babre, F., Bourdaud, N., et al. (2019). Management of the child’s airway under anaesthesia: The French guidelines. Anaesth. Crit. Care Pain Med. 38, 681–693. doi:10.1016/j.accpm.2019.02.004
de Boer, H. D., Shields, M. O., and Booij, L. H. D. J. (2014). Reversal of neuromuscular blockade with sugammadex in patients with myasthenia gravis: A case series of 21 patients and review of the literature. Eur. J. Anaesthesiol. 31, 715–721. doi:10.1097/EJA.0000000000000153
Dinis-Oliveira, R. J. (2017). Metabolism and metabolomics of ketamine: A toxicological approach. Forensic Sci. Res. 2, 2–10. doi:10.1080/20961790.2017.1285219
Eisenkraft, J. B., Book, W. J., Mann, S. M., Papatestas, A. E., and Hubbard, M. (1988). Resistance to succinylcholine in myasthenia gravis: A dose-response study. Anesthesiology 69, 760–763. doi:10.1097/00000542-198811000-00021
Frappaolo, A., Sechi, S., Kumagai, T., Karimpour-Ghahnavieh, A., Tiemeyer, M., and Giansanti, M. G. (2018). Modeling congenital disorders of n-linked glycoprotein glycosylation in drosophila melanogaster. Front. Genet. 9, 436. doi:10.3389/fgene.2018.00436
Freeze, H. H., Chong, J. X., Bamshad, M. J., and Ng, B. G. (2014). Solving glycosylation disorders: Fundamental approaches reveal complicated pathways. Am. J. Hum. Genet. 94, 161–175. doi:10.1016/j.ajhg.2013.10.024
Goldstein, S. L., Currier, H., Cd, G., Cosio, C. C., Brewer, E. D., and Sachdeva, R. (2001). Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107, 1309–1312. doi:10.1542/peds.107.6.1309
Goossens, G. A. (2015). Flushing and locking of venous catheters: Available evidence and evidence deficit. Nurs. Res. Pract. 2015, 1–12. doi:10.1155/2015/985686
Gueret, G., Rossignol, B., Kiss, G., Wargnier, J. P., Miossec, A., Spielman, S., et al. (2004).,Is muscle relaxant necessary for cardiac surgery? Anesth. Analg. 99.(5), FF, 1330–1333. doi:10.1213/01.ANE.0000132984.56312-FF
Habre, W., Disma, N., Virag, K., Becke, K., Hansen, T. G., Habre, M. J., et al. (2017). Incidence of severe critical events in paediatric anaesthesia (apricot): A prospective multicentre observational study in 261 hospitals in Europe. Lancet. Respir. Med. 05, 412–425. doi:10.1016/S2213-2600(17)30116-9
Harding, M., Stefka, S., Bailey, M., Morgan, D., and Anderson, A. (2020). Best practice for delivering small-volume intermittent intravenous infusions. J. Infus. Nurs. 43, 47–52. doi:10.1097/NAN.0000000000000355
Holliday, M. A., and Segar, W. E. (1957). The maintenance need for water in parenteral fluid therapy. Pediatrics 19, 823–832. doi:10.1542/peds.19.5.823
Karsli, C. (2015). Managing the challenging pediatric airway: Continuing professional development. Can. J. Anaesth. 62, 1000–1016. doi:10.1007/s12630-015-0423-y
Kliegman, R., Stanton, B., Geme, J., Schor, N., and Behrman, R. (2016). Nelson textbook of pediatrics. Philadelphia: Elsevier.
Kurdi, M. S., Theerth, K. A., and Deva, R. S. (2014). Ketamine: Current applications in anesthesia, pain, and critical care. Anesth. Essays Res. 08, 283–290. doi:10.4103/0259-1162.143110
Lehavi, A., Mandel, H., and Katz, Y. S. (2011). Anesthetic management of a boy with congenital disorder of glycosylation (CDG) i-x. Int. J. Clin. Med. 02, 325–327. doi:10.4236/ijcm.2011.23056
Meaudre, E., Meyrieux, V., Suprano, I., Camboulives, J., and Paut, O. (2005). Anesthesia considerations in carbohydrate-deficient glycoprotein syndrome type i. Paediatr. Anaesth. 15, 905–906. doi:10.1111/j.1460-9592.2005.01671.x
Miller, R. D., Eriksson, L. I., Fleisher, L. A., Wiener-Kronish, J. P., and Young, W. L. (2009). Miller’s anesthesia. Philadelphia: Elsevier Health Sciences.
Nitahara, K., Sugi, Y., Higa, K., Shono, S., and Hamada, T. (2007). Neuromuscular effects of sevoflurane in myasthenia gravis patients. Br. J. Anaesth. 98, 337–341. doi:10.1093/bja/ael368
Norambuena, C., Yañez, J., Flores, V., Puentes, P., Carrasco, P., and Villena, R. (2013). Oral ketamine and midazolam for pediatric burn patients: A prospective, randomized, double-blind study. J. Pediatr. Surg. 48, 629–634. doi:10.1016/j.jpedsurg.2012.08.018
Nowacka, A., and Borczyk, M. (2019). Ketamine applications beyond anesthesia – A literature review. Eur. J. Pharmacol. 860, 172547. doi:10.1016/j.ejphar.2019.172547
Orhurhu1, V. J., Vashisht, R., Claus2, L. E., and Cohen, S. P. (2021). “Ketamine toxicity,” in StatPearls (Tampa, FL, United States: StatPearls Publishing).
Pino, R. M. (2019). “Specific considerations with liver disease - metabolism of anesthetic,” in Clinical anesthesia procedures of the Massachusetts general hospital. Editors R. M. Pino, and Wolters Kluwer. Ninth Edition.
Rocca, G. D., Coccia, C., Diana, L., Pompei, L., Costa, M. G., Rocca, E. T., et al. (2003). Propofol or sevoflurane anesthesia without muscle relaxants allow the early extubation of myasthenic patients. Can. J. Anaesth. 50, 547–552. doi:10.1007/BF03018638
Rosenbaum, S. B., Gupta, V., and Palacios, J. L. (2021). “Ketamine,” in StatPearls (Tampa, FL, United States: StatPearls Publishing).
Roth, D. M., Bayona, F., Baddam, P., and Graf, D. (2021). Craniofacial development: Neural crest in molecular embryology. Head. Neck Pathol. 15, 01–15. doi:10.1007/s12105-021-01301-z
Rout, J., Essack, S., and Brysiewicz, P. (2020). Residual fluid after IV infusion drug administration: Risk of suboptimal dosing. Br. J. Nurs. 29, S6-S8. doi:10.12968/bjon.2020.29.2.S6
Saad, M., Clec’h, B. L., and Dhonneur, G. (2020). Hypoalbuminemia-related prolonged sedation after general anesthesia: A case report. A. A. Pract. 14, e01180. doi:10.1213/XAA.0000000000001180
Sakai, W., Yoshikawa, Y., Tokinaga, Y., and Yamakage, M. (2017). Anesthetic management of a child with phosphomannomutase-2 congenital disorder of glycosylation (pmm2-cdg). JA Clin. Rep. 3, 8. doi:10.1186/s40981-017-0080-y
Schollen, E., Frank, C. G., Keldermans, L., Reyntjens, R., Grubenmann, C. E., Clayton, P. T., et al. (2004). Clinical and molecular features of three patients with congenital disorders of glycosylation type ih (CDG-Ih) (ALG8 deficiency). J. Med. Genet. 41, 550–556. doi:10.1136/jmg.2003.016923
Schulz, C. M., Endsley, M. R., Kochs, E. F., Gelb, A. W., and Wagne, K. J. (2013). Situation awareness in anesthesia: Concept and research. Anesthesiology 118, 729–742. doi:10.1097/ALN.0b013e318280a40f
Selewski, D. T., Cornell, T. T., Lombel, R. M., Blatt, N. B., Han, Y. Y., Mottes, T., et al. (2011). Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 37, 1166–1173. doi:10.1007/s00134-011-2231-3
Soto, R. G., Fu, E. S., Jr, H. V., and Miguel, R. V. (2004). Capnography accurately detects apnea during monitored anesthesia care. Anesth. Analg. 379, 379–382. doi:10.1213/01.ANE.0000131964.67524.E7
Tobias, J. D. (2017). Current evidence for the use of sugammadex in children. Paediatr. Anaesth. 27, 118–125. doi:10.1111/pan.13050
Trevor, A., Katzung, B., and Knuidering-Hall, M. (2019). Katzung & trevor’s pharmacology examination and board review. New York: McGraw-Hill Education.
Keywords: CDG, ketamine, sevoflurane, hypoalbuminemia, neuromuscular blocking agents, hypotonia, intravenous flushing
Citation: Chang E-C, Chang Y-H, Tsai Y-S, Hung Y-L, Li M-J and Wong C-S (2022) Case report: The art of anesthesiology—Approaching a minor procedure in a child with MPI-CDG. Front. Pharmacol. 13:1038090. doi: 10.3389/fphar.2022.1038090
Received: 06 September 2022; Accepted: 25 November 2022;
Published: 15 December 2022.
Edited by:
Jian Gao, Shanghai Children’s Medical Center, ChinaReviewed by:
Wei-Zen Sun, National Taiwan University Hospital, TaiwanCopyright © 2022 Chang, Chang, Tsai, Hung, Li and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Shung Wong, dzgyNTU2QGdtYWlsLmNvbQ==; Min-Jia Li, bWluamlhbGlAbGl2ZW1haWwudHc=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.