- 1Departmen of Cardiology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Departmen of Oncology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Beijing University of Chinese Medicine, Beijing, China
Ginseng (Panax ginseng C.A.Mey.) is the dry root and rhizome of the Araliaceae ginseng plant. It has always been used as a tonic in China for strengthening the body. Cardiovascular disease is still the main cause of death in the world. Some studies have shown that the functional components of ginseng can regulate the pathological process of various cardiovascular diseases through different mechanisms, and its formulation also plays an irreplaceable role in the clinical treatment of cardiovascular diseases. Therefore, this paper elaborates the current pharmacological effects of ginseng functional components in treating cardiovascular diseases, summarizes the adverse reactions of ginseng, and sorts out the Chinese patent medicines containing ginseng formula which can treat cardiovascular diseases.
Introduction
Cardiovascular-related disease still remains a major public health problem with high morbidity and mortality in some countries. According to the “Global Health Estimates Report 2022” released by WHO (World Health Statistics, 2022), due to population growth and longer life expectancy, the total number of deaths from non-communicable diseases has increased, and about 33.2 million people worldwide died from cancer, cardiovascular disease, diabetes and chronic respiratory diseases. The “China Cardiovascular Health and Disease Report 2021 Summary” also pointed out that the prevalence and mortality of cardiovascular diseases in China are still on the rise, cardiovascular disease in rural and urban areas accounted for 46.74% and 44.26% of the cause of death respectively, and the burden is still increasing (Chinese Cardiovascular Health and Disease Report Writing Group, 2022). According to a 2020 report by the American Heart Association (AHA), cardiovascular disease kills about 850,000 people and costs more than $300 billion a year, and its main risk factors are high cholesterol, smoking, and diabetes (Virani et al., 2020).
Relevant studies have shown that the occurrence and development of cardiovascular disease CVD is closely related to a variety of pathological processes. For example, coronary artery disease (CAD) is caused by cholesterol deposits and inflammation that damage the coronary arteries, leading to atherosclerosis which results in insufficient oxygen and blood supply. The pathological mechanism depends on dyslipidemia (Borén et al., 2020), inflammation of blood vessels (Ahluwalia et al., 2013), disorder of endothelial function (Davies et al., 1993; Gutiérrez et al., 2013), infiltration and turnover of macrophages (also known as macrophage polarization) (Stöger et al., 2012), etc. Platelets can cause blood clot problems and play an important role in a variety of cardiovascular diseases. During myocardial ischemia, chemicals such as adenosine, bradykinin, lactate, reactive oxygen species (ROS) and histamine are released to stimulate nerve receptors and produce angina symptoms, while the ischemia-reperfusion period will aggravate the accumulation of ROS and cause DNA damage, leading to cardiomyocyte apoptosis (Rezende et al., 2019). Hypertension is emerging as one of the major risk factors for cardiovascular and renovascular disease and also becoming a major risk factor for death worldwide (Ezzati et al., 2002). The main pathological links involved in the occurrence of hypertension include increased endothelin (ET), decreased nitric oxide (NO)/nitric oxide synthase (NOS), and imbalance of renin-angiotensin-aldosterone system (Zhang, 2011). Endothelial dysfunction, arginase activation, decreased NO bioavailability, and increased vascular stiffness are also important factors in leading hypertension (Ryoo et al., 2011; Konukoglu and Uzun, 2017). Another study found that essential hypertension is closely related to an increase in ROS and cell death mediated by defective mitochondrial oxidative phosphorylation (Zhang et al., 2014). Cardiac Arrhythmia is a common cardiovascular disease in clinic. It is a group of disorders that cause abnormal heart beat frequency and/or rhythm due to the origin conduction disorder of cardiac electrophysiology. Arrhythmias is classified as impulse formation abnormality, impulse conduction abnormality, tachyarrhythmias and bradyarrhythmias (Liu, 2021). There are 3.7 million sudden cardiac deaths in the world every year, and a considerable part is caused by severe arrhythmia (Kuriachan et al., 2015). According to the epidemiological survey of sudden cardiac death in China, more than 80% of sudden cardiac death events are caused by malignant arrhythmia every year (Chen et al., 2020). Heart failure (HF) is a serious public health problem and the leading cause of death from cardiovascular disease worldwide. It is a complex clinical syndrome caused by abnormal changes in cardiac structure and function due to various reasons, resulting in dysfunction of ventricular systolic and diastolic functions which mainly manifesting as dyspnea, fatigue, and fluid retention (Heart Failure Group of Cardiology, 2018). According to the “China Cardiovascular Disease Report 2020,” there are 8.9 million heart failure patients in China, The China Heart Failure Patient Registration Study (China-HF) shows that the mortality rate of hospitalized patients with heart failure is 4.1% (China Cardiovascular Health and Disease Report Writing Group, 2021), the 5-year survival rate of heart failure patients is comparable to that of malignant tumors (Kannel, 2000). However, with the wider application of drug therapy, patients can benefit from it, but at the same time, some toxic and side effects have gradually become prominent. For example, long-term use of lipid-lowering drugs will have adverse reactions of myopathy and liver. Aspirin and warfarin often cause gastric mucosal damage, severe intracranial hemorrhage, and even death. Calcium channel blocker (CCB) can cause blurred vision and eye pain. Diltiazem can cause auditory hallucinations and vision loss in patients. Verapamil can cause vertigo symptoms (Yi, 2013). Side effects of antihypertensive drugs include gastrointestinal reactions, nervous system toxicity, etc. Antiarrhythmic drugs themselves also have proarrhythmic effects (Liao and Yang, 2008).
The World Health Organization defines the functional components of herbal medicines as “the components that have a therapeutic effect in herbal medicines.” Traditional Chinese Medicine (TCM) has played an important role in preventing cardiovascular diseases by applying herbal medicine based on its unique theory and experience (Hao et al., 2017). Several preclinical studies have confirmed that ginseng and its main functional components are involved in the treatment and prevention of cardiovascular disease, and have the potential to reduce cardiovascular risk factors. From 1976 to 1978, Chinese researchers isolated and identified three saponins from ginseng, namely panaxadiol, panaxatriol and oleanolic acid saponins (Li and Teng, 1978; Chen et al., 2002). Subsequently, ginseng polysaccharides, volatile oil and other components were continuously explored. This article aims to clarify the pharmacological mechanism of ginseng functional components in the prevention and treatment of cardiovascular disease, and to sort out the clinical application of ginseng’s formulation in detail.
Pharmacological effects of ginsenosides in treating cardiovascular diseases
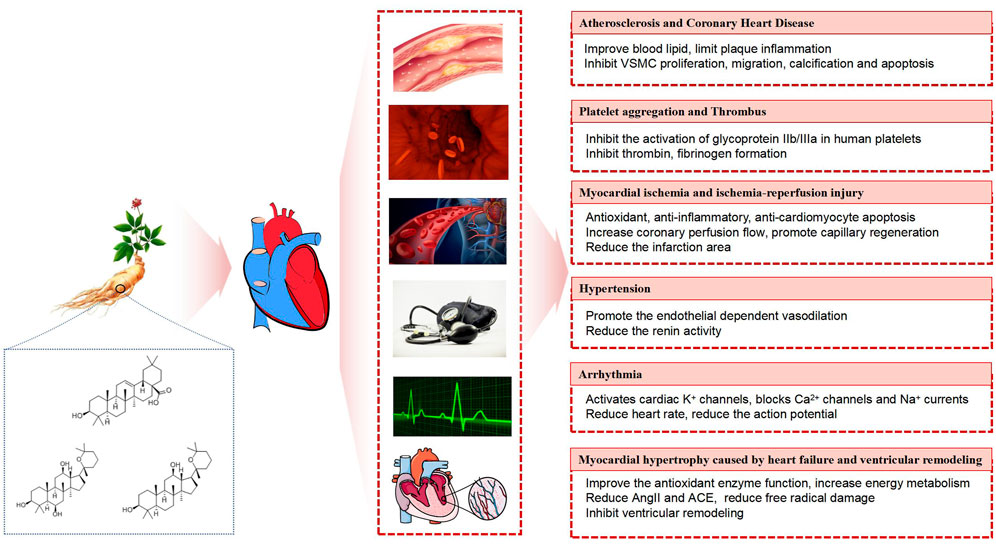
Ginsenosides belong to triterpenoid saponins and are mainly divided into three types: protopanaxadiol type, such as ginsenoside Ra1, Rbl, Rb2, Rc, Rd, etc. protopanaxatriol type, such as ginsenoside Re, Rf, Rgl, Rg2, Rhl, etc. Oleanolic acid type, such as ginsenoside RO, Rh3, etc (Chu and Zhang, 2009; Cao et al., 2012) (Figure 1). In one study, a variety of ginsenosides at different sites of ginseng were analyzed using ultra performance liquid chromatography-quadrupole time of flight /mass spectrometry (UPLC-QTOF/MS), and then multivariate analysis was performed on the dataset, according to the results, ginsenosides such as Ra1 and Ra2, are unique among the roots of ginseng (Lee et al., 2017a).
Atherosclerosis and coronary heart disease
Ginseng has shown to involve in the atherosclerotic gene regulation with anti-inflammatory effects and results in the changes in improving in lipid profile (Duan et al., 2017; Cholesterol Treatment Trialists' (CTT) Collaboration O'Connell et al., 2015). Studies have found that protopanaxadiol and protopanaxatriol saponins can improve the lipid profile by reducing the concentrations of cholesterol, triglycerides, low-density lipoprotein and free fatty acids in plasma, while increasing the total concentration of high-density lipoprotein (Deng et al., 2017). This lipid metabolism-modulating effect of ginseng supplementation was also validated in a meta-analysis (Hernández-García et al., 2019). Ginsenosides can also reduce the progression of atherosclerosis by inhibiting the expression of VCAM-1 and enhancing α-smooth muscle actin (α-SMA) (Zhang et al., 2004). In addition to improving blood lipid levels, ginseng extract can also reduce superoxide dismutase (SOD) and catalase (CAT) levels (Kim and Park, 2003). Studies have shown that ginsenoside compounds K and Rb1 are potential active components to restore TNF-α-induced oxidative stress and oxidized low-density lipoprotein (ox-LDL)-induced inflammation and apoptosis (Zhou et al., 2017a; Lu et al., 2019). In animal models, ginsenosides Rg3 and Rb1 are thought to inhibit vascular smooth muscle cell (VSMC) proliferation, migration, calcification, and induce apoptosis (Guo et al., 2018a; Nanao-Hamai et al., 2019). Ginsenoside Re (G-Re) can affect platelet-derived growth factor-BB (PDGF-BB)-induced proliferation of VSMCs by regulating the eNOS/NO/cGMP pathway through G0/G1 cell cycle arrest (Gao et al., 2019). Another study found that ginsenosides Rb1 and Rg1 could inhibit the apoptosis process in atherosclerosis model by increasing autophagy (Zhou et al., 2018; Yang et al., 2018).
Ginseng can also reduce atherosclerosis, or limit intraplaque inflammatory responses by modulating macrophage polarization in order to prevent the occurrence of atherosclerosis (Guo et al., 2018b; Zhang et al., 2018). Recently, a study has proposed that the anti-inflammatory effects of several ginsenosides (Rg3, Rb1, and Rg1) may be due to inducing M2 polarization of macrophages and microglia, which in turn helps to inhibit inflammatory progression and promote inflammation resolution (Im, 2020). By inhibiting the expression of NF-ĸB and JNK, ginsenosides can not only reduce the production of inflammatory cytokines such as VCAM-1 and ICAM-1, but also reduce the number of macrophages, thereby controlling the size of atherosclerotic lesions (Su et al., 2016). A study has shown that ginsenosides can reduce the expression of interleukin, inhibit the expression of NF-ĸB/p65, and exert anti-inflammatory effects (Sun et al., 2013; Zhang et al., 2008). Ginsenoside Rg3 can reduce the expression of cell adhesion molecules and pro-inflammatory cytokines in blood vessels, inhibit the expression of TNF-α, and has anti-inflammatory and anti-atherosclerotic effects (Hien et al., 2010). Another study found that ginsenoside Rb1 can significantly inhibit inflammation, oxidative stress and apoptosis by inhibiting the production of ROS and MDA, reducing the expression levels of IL-6, IL-1, ICAM-1, VCAM-1, VEGF, MMP-2, and MMP-9 (Zhou et al., 2017b). Based on the above research results, it can be found that ginseng has a good effect in treating and preventing atherosclerosis and coronary heart disease caused by inflammation and lipid profile.
Platelet aggregation and thrombus
The existing research results show that ginsenosides can exert antiplatelet and anticoagulant activities through various mechanisms. Ginsenoside Rg1 was found to improve the aggregation of platelets and formation of arterial thrombus, which may be achieved by regulating the ERK/Akt signaling pathway (Zhou et al., 2014). What’s more, ginsenoside Rg3 has also been found by two other studies to inhibit the platelet aggregation process through the key signaling pathways such as ERK2 and cAMP (Lee et al., 2008; Kwon, 2018a). Ginsenoside Rg3 can also achieve antithrombotic effect by inhibiting the formation of thrombin (Jeong et al., 2017). For 20(S)-ginsenoside Rg3, its antiplatelet mechanism may be related to the inhibition of glycoprotein IIb/IIIa activation in human platelets (Kwon, 2018b). In another study, ginsenoside Ro was shown to inhibit the binding of fibrinogen to αIIb/β3 in human platelets (Shin et al., 2016). A study evaluated the synergistic effects of different ginsenosides to assess whether structural modifications affect their antiplatelet activity. Ginsenoside Rp3 was prepared from the structural modification of Re by means of reduction with hydrogenation. Rp1 was prepared from other ginsenosides (Rg3, 2h-Rg3, Rg5, and Rk1) with the same method that was used for Rp3 production. 2H-Rg3 is called Dihydroxy-G-Rg3, which is chemically derived from Rg3 by means of reduction with hydrogenation. It was found that G-Rp3 was shown to be effective in inhibiting platelet aggregation by exerting synergistic effects with G-Rp1 and 2H-Rg3 (Irfan et al., 2020). Therefore, the research on ginsenosides will help to develop new antiplatelet and antithrombotic drugs in the cardiovascular system.
Myocardial ischemia and ischemia-reperfusion injury
Ginseng total saponin has antioxidant and anti-inflammatory effects, so it can inhibit oxidative stress and reduce myocardial damage (Aravinthan et al., 2015). For example, the combination of ginsenoside Rb3 and Rb2 has a protective effect on myocardial ischemia-reperfusion injury, and its mechanism may be related to anti-inflammatory response, improve oxidative stress and resist cardiomyocyte apoptosis (Liu et al., 2020a). Studies have found that ginsenoside Rbl can reduce serum aspartate transaminase (AST), Lactic dehydrogenase (LDH) and creatine kinase in myocardial tissue, protect the heart by resisting inflammatory and apoptotic damage (Zheng et al., 2017a). Ginsenoside Rg3 also has similar efficacy, which may be achieved by regulating Akt/eNOS and Bcl/BAx signal transduction pathways (Wang et al., 2015; Zhang et al., 2016). Ginsenosides can protect cardiomyocytes from hypoxia/reoxygenation damage by reducing oxidative damage (Li and Liu, 2006), which may be related to reducing intracellular calcium overload (Xu et al., 2005) and inhibiting the activation of JNK signaling pathway (Li et al., 2012; Li et al., 2017; Sun et al., 2019). Ginsenoside Rg1 can also inhibit autophagy in H9c2 cardiomyocytes (Zhang et al., 2012), improve mitochondrial dynamics imbalance (Dong et al., 2016), and promote capillary regeneration in ischemic myocardial tissue (Wang et al., 2005; Zhang and Liu, 2009). Ginseng can also activate the reperfusion injury salvage kinase (RISK) signaling pathway through glucocorticoid receptor/estrogen receptor (GR/ER) in an endothelium nitric oxide synthase (eNOS)-dependent mechanism (Zhou et al., 2011). Another study stated that ginseng can enhance the PI3K/Akt/eNOS pathway, increase the coronary perfusion flow of the heart, and reduce the infarction size (Yi et al., 2010). It is worth mentioning that ginsenoside Rc as well as Rb1 and Re can also inhibit coronary vascular dysfunction (Chai et al., 2005).
Hypertension
There is substantial evidence that ginsenosides are beneficial in the treatment of hypertension, not only does ginseng lower blood pressure, it also acts as a heart protector (Jeon et al., 2000; Nagar et al., 2016). A study shows that ginsenoside Rb3 can reduce oxidative stress in hypertension and protect endothelial function (Wang et al., 2014). In a spontaneously hypertensive rat model, Rg1 has protective effects not only in large arteries but also in small resistance arteries (Chen et al., 2012). Ginsenosides have been found to inhibit arginase, stimulate endothelial nitric oxide synthase coupling, block homocysteine-induced ROS damage, promote endothelium-dependent vasodilation, and achieve the purpose of lowering blood pressure (Shin et al., 2013; Zhou et al., 2005). In addition to the above studies, ginsenoside Rg3 can reduce renin activity by stimulating the expression of iNOS/NO, and also reduce blood pressure (Lee et al., 2016).
Ginseng has been shown to lower blood pressure in several studies, but there have also been reports of raising blood pressure, which may be related to the bidirectional effect. In previous literature, a study found ginsenosides can cause biphasic changes in blood pressure without affecting breathing and heart rate (Takagi et al., 1972). Another study found that large doses of ginsenoside Rb1 can cause an increase in blood pressure, and all other ginsenosides except Rb1 showed biphasic changes (Kaku et al., 1975). Although the effect of ginseng in blood pressure has had conflicting results in previous studies, a recent systematic review of randomized, double-blind, placebo-controlled trials has preliminarily resolved the inconsistencies in ginseng evidence for blood pressure regulation, the results of this study provide optimistic evidence for the efficacy of ginseng in reducing prehypertension, acute hypertension and chronic hypertension (Lee et al., 2017b).
Arrhythmia
Ginsenosides can affect the electrophysiology of cardiomyocytes and are used to regulate arrhythmias. Among them, ginsenoside Re is a major phytosterol of ginseng, which can activate the K+ channel of the heart through the non-genomic pathway of sex hormones (Furukawa et al., 2006), and can also block the Ca2+ channel, reduce the heart rate, reduce the action potential plateau phase, and reduce the P wave amplitude (Jin and Liu, 1994). Several other studies have found that ginsenoside Re can regulate K+ and Ca2+ currents in cardiac electrical activity by inducing NO and cyclic guanosine monophosphate pathways (Bai et al., 2003; Bai et al., 2004; Choi et al., 2009). Ginseng Rg2 has been studied in rat models of calcium chloride-induced arrhythmias, and it has been found to have anti-arrhythmic effects, including shortened duration, mortality, and incidence of malignant arrhythmias, which may be related to inhibition of phosphorylation of Ca2+ (Gou et al., 2020). Experiments have shown that the use of ginsenosides to intervene in arrhythmias can increase the amplitude of the T wave and reduce the amplitude of the QRS wave, thereby restoring the heart rhythm to normal (Chen and Zhang, 2009; Lu et al., 2012). A study found that ginsenoside Rg3 can change the electrocardiogram (ECG) and monophasic action potential (MAP) of Langendorff perfused rabbit heart, shorten the QT interval, and may be related to alleviating the current inhibition of human ether-related genes (hERG) and accelerating the activation process of potassium channels (Zhang et al., 2021). Another study has shown that ginsenosides can also treat arrhythmias by inhibiting the voltage-dependent Na+ current in the myocardium and reducing the amplitude of action potentials (Liu et al., 2019). These studies fully demonstrate the antiarrhythmic therapeutic potential of ginsenosides.
Cardiac hypertrophy due to heart failure and ventricular remodeling
Heart failure is a kind of disease that the impaired heart function or structure causes decreased ventricular filling or decreased ejection function, and the heart output is not enough to meet the needs of the body. There are many causes of heart failure, mainly including the excessive pressure overload during cardiac systole, the excessive volume overload during cardiac diastole, the abnormal energy metabolism of cardiomyocytes, the use of cardiotoxic drugs, and the myocardial fibrosis or ventricular remodeling (Lin et al., 2017).
In improving cardiac systolic and diastolic function, a study has found that ginsenoside Rg3 can significantly inhibit the proliferation of middle vascular smooth muscle cell proliferation, reduce stromal hyperplasia, and enhance vasodilation and vasoconstriction function in elderly rats (Liu et al., 2016). It also has found that ginsenosides can enhance the systolic and diastolic functions of the left ventricle after heart failure (Wang et al., 2008). In terms of regulating the energy metabolism, ginsenoside Rb3 can inhibit mitochondria-mediated apoptosis, upregulate energy metabolism, activate fatty acid oxidation, and exert cardioprotective effects (Chen et al., 2019). The active ingredients of ginseng also have a certain therapeutic effect on heart failure caused by cardiotoxicity. For example, ginsenoside Re can improve myocardial fibrosis and heart failure induced by isoproterenol in rats (Wang et al., 2019). It was found that the expression of p-P70S6K, c-Jun N-terminal kinase 1 and Beclin1 decreased in the ginsenoside Rg1 group. These results showed that ginsenoside Rg1 can reduce the expression of doxorubicin-induced cardiac microtubule-associated protein-light chain 3 and autophagy-related 5, reduce doxorubicin-induced endoplasmic reticulum dilation, and improve cardiac insufficiency by inhibiting endoplasmic reticulum stress and autophagy (Xu et al., 2018). Another study found that ginseng can treat adriamycin-induced heart failure, increase the activity of myocardial glutathione peroxidase (GSH-Px), alleviate mitochondrial damage, reduce the production of ROS, and reduce the amount of ascites (You et al., 2005). In addition, ginsenoside Rbl can improve cardiac function and remodeling in patients with heart failure, and the mechanism may be that ginsenoside can not only reduce β-myosin heavy chain (βMHC), angiotensin I converting enzyme (ACE), angiotensin II (AngII) and atrial natriuretic factor (ANF), but also regulate mitochondrial membrane potential (Zheng et al., 2017b). In addition, the active compounds of ginseng can reduce myocardial hypertrophy and oxidative stress (Tsai et al., 2014), and inhibit cardiac fibrosis and heart failure (Guo et al., 2011; Lo et al., 2017). Some studies have also found that ginsenosides can effectively inhibit the right ventricular hypertrophy induced by monocrotaline in rats, suggesting that it has an anti-ventricular hypertrophy effect (Jiang et al., 2007). This effect has also been found to be dependent on phospho-akt (p-akt) activation and inhibition of p38 mitogen-activated protein kinase (MAPK) (Zhang et al., 2013a), and is often associated with inhibition of vascular mitogenic activity (Qin et al., 2008), both of which have been experimentally verified in vitro and in vivo (Zhang et al., 2019). The contents of functional compounds of ginsenoside are summarized in Table 1.
Pharmacological effects of ginseng polysaccharides in treating cardiovascular diseases
There are many kinds of polysaccharides which are widely distributed. They can be divided into extracellular and intracellular polysaccharides. Among them, plant polysaccharides and microbial polysaccharides are more studied (Jin and Zhang, 1995). In recent years, ginseng polysaccharides have been paid more and more attention as an important component of ginseng to exert pharmacodynamic activity, and ginseng polysaccharides with different structural characteristics and activities have been reported widely. Ginseng polysaccharides are mainly divided into two categories: neutral sugar and acidic pectin (Li and Zhang, 1986). The main active ingredient is ginseng pectin, which is mainly composed of galactosyl, galacturonic acid, arabinose, and rhamnosyl (Zhang et al., 1982; Li and Zhang, 1984). Ginseng polysaccharide has high antioxidant activity, can significantly scavenge hydroxyl radicals and superoxide anions, its mass concentration has a certain dose-effect relationship with antioxidant activity, and is a good natural antioxidant (Zhou et al., 2015). Studies have shown that ginseng polysaccharides can improve oxidative stress injury in cardiomyocytes by inhibiting ROS and apoptosis (Tian et al., 2018). Another study determined the antioxidant activity of ginseng polysaccharides, and the results showed that the antioxidant activity of neutral polysaccharides was higher than that of acid polysaccharides in the aboveground part of ginseng, while the antioxidant activity of acid polysaccharides in the underground part of ginseng was not large (Chen and Huang, 2019). In addition, ginseng polysaccharide can improve its energy metabolism disorder and increase the vitality of mitochondria (Zhang et al., 2013b). A short review proposed that Rb1 can regulate mitochondrial energy metabolism, mitochondrial fission and fusion, apoptosis, oxidative stress and reactive oxygen species release, mitophagy and mitochondrial membrane potential (Zhou et al., 2019). Other studies have found that ginseng polysaccharides can regulate the activities of GSH and SOD enzymes, significantly reduce the expression levels of B cell lymphoma-2 (Bcl-2) and Bcl-2 Assaciated X protein (Bax) in rats, and improve dyslipidemia in rats with coronary heart disease (Wan et al., 2020).
Pharmacological effects of ginseng volatile oil in treating of cardiovascular disease
Ginseng volatile oil has the special aroma of ginseng. Ginseng stems, leaves and flowers have higher level of volatile oils, while ginseng roots have less volatile oils (Chen et al., 1982). The identification of ginseng volatile oil found that there are many more terpenes (Zhang et al., 1994), followed by oxygenated compounds and long-chain alkanes (Sun and Wang, 1997). Volatile oils include compounds such as pentadecane and n-hexadecanoic acid, etc (Yan et al., 1994; Wu et al., 1996). The results of cell experiments suggest that ginseng volatile oil can inhibit the secretion of inflammatory factors such as TNF-α, IL-6 and IL-1β, and inhibit the NF-κB pathway, thereby playing an anti-inflammatory effect (Zuo, 2021). Ginseng volatile oil also has obvious protective effect on ischemic myocardial injury in animals, and can improve blood rheology, anti-platelet aggregation, reduce blood viscosity, and prevent thrombosis (Teng et al., 1989; Zhang, 2016). Ginseng volatile oil can be used for the treatment of coronary heart disease and angina pectoris. It can reduce the content of cardiac troponin I (cTn-I) in serum, reduce the content of Malondialdehyde (MDA), increase the activity of SOD and GSH enzymes, increase the concentration of NO, and protect the myocardium through the mechanism of anti-oxidative damage (Chen, 2015).
Although many studies have proved that ginseng volatile oil has good efficacy on cardiovascular diseases, a recent review concluded that the research on ginseng volatile oil is still in its infancy due to the limitation of the production process (Chen et al., 2022). More large samples and high-quality studies are needed to verify the efficacy of ginseng volatile oil in the treatment of cardiovascular diseases.
Clinical study on the treatment of cardiovascular diseases with ginseng prescriptions
A meta-analysis study using nitrate as a control drug found that ginseng-based drug had a significant effect on symptomatic improvement of angina pectoris and improvement in electrocardiogram (Jia et al., 2012). Another study focused on the efficacy of ginseng prescription in the treatment of patients with coronary angina pectoris, and the study showed that the patients who took the ginseng prescription had greater improvement in ECG results, clinical symptoms and nailfold microcirculation (Yuan et al., 1997). It can be seen that the important role of ginseng-containing prescriptions in the prevention and treatment of cardiovascular diseases should not be ignored. The mechanic and clinical research progress of related drugs is summarized as follows (Table 2).
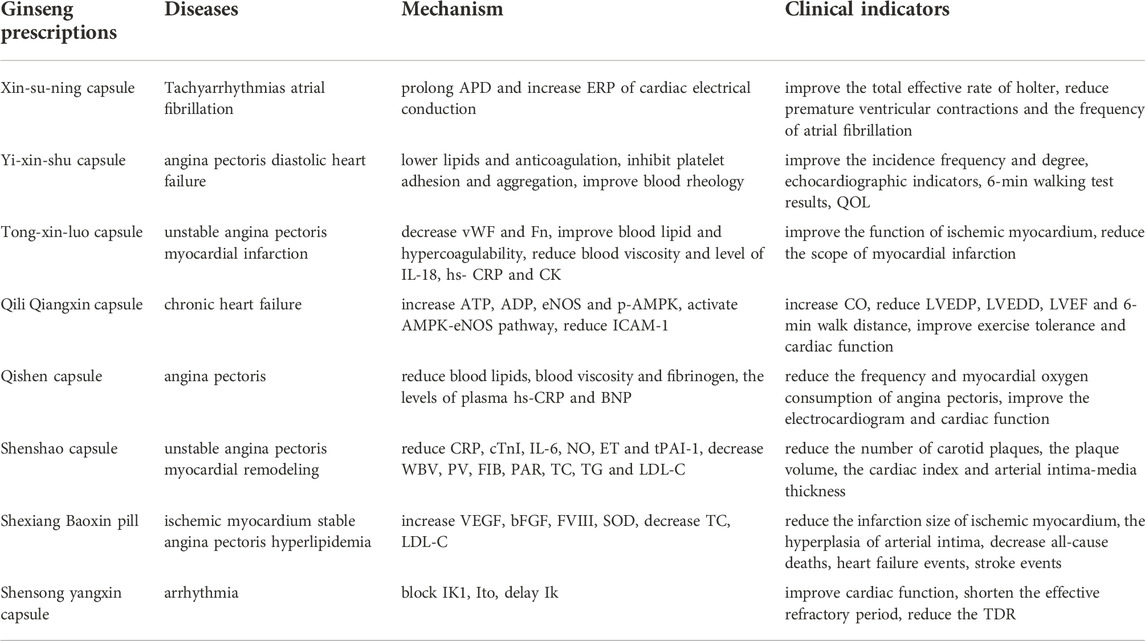
TABLE 2. Mechanism and Clinical Indicators of Ginseng Prescriptions in treating Cardiovascular Diseases.
Xin-su-ning capsule
Xinsuning Capsule can be used to treat tachyarrhythmias. Compared with propafenone and mexiletine hydrochloride, it can effectively improve the total effective rate of holter in patients with premature ventricular contractions, reduce premature ventricular contractions (Wang and Lu, 2008), and significantly improve the clinical symptoms of patients (Yuan, 2000; Song et al., 2022). The frequency of atrial fibrillation was significantly reduced and the incidence of adverse reactions was lower after the addition of Xinsuning Capsules on the basis of conventional western medicine treatment (Li and Zhang, 2015). Xinsuning capsules combined with low-dose betaloc in the treatment of premature ventricular contractions can significantly reduce the number of premature ventricular contractions, maintain ventricular muscle stability, and improve vascular endothelial function and clinical symptoms (Yang, 2020). It is worth noting that Xinsuning can prolong the action potential duration (APD) and increase the effective refractory period (ERP) of cardiac electrical conduction, thereby inhibiting reentry-induced arrhythmias, and has a good antiarrhythmic effect (Jiao and Ding, 2015).
Yi-xin-shu capsule
Yixinshu Capsule is widely used clinically in coronary heart disease, angina pectoris and chronic heart failure. The capsule has the functions of lowering lipids and anticoagulation, inhibiting platelet adhesion and aggregation, and improving blood rheology (Li and Shi, 2013; Wang and Zhi, 2016). Yixinshu Capsule can also improve the symptoms of shortness of breath, fatigue and dry mouth (Cao, 2009; Cao and Xie, 2010). On the basis of conventional western medicine combined with Yixinshu Capsule in the treatment of diastolic heart failure, it was found that the echocardiographic indicators, 6-min walking test results, the quality of life (QOL)were improved, and were better than those of simple western medicine treatment (Zhang and Zhu, 2011). In the treatment of unstable angina pectoris (UA) of CHD, Yixinshu Capsule is better than the control group which only given antiplatelet aggregation and anticoagulant drugs on the frequency of angina pectoris, the degree of angina pectoris and the electrocardiogram (Tao et al., 2009).
Tong-xin-luo capsule
After the application of Tongxinluo Capsules to treat patients with unstable angina pectoris, the plasma Von Willebrand factor (vWF) and fibronectin (Fn) decreased, which can protect vascular endothelial cells (Xiao et al., 2002). Tongxinluo Capsules can also improve the function of ischemic myocardium in patients undergoing percutaneous coronary intervention (PCI) or thrombolysis after acute myocardial infarction (AMI), and can restore the function of part of the viable myocardium, improve myocardial remodeling after AMI (You et al., 2004), significantly improve blood lipid metabolism, reduce blood viscosity, and improve blood hypercoagulability (Wu et al., 2001). Studies have shown that the addition of Tongxinluo Capsules on the basis of western medicine treatment can improve the clinical efficacy of angina pectoris in elderly patients with coronary heart disease, and reduce the serum levels of interleukin-18 (IL-18) and high-sensitivity C-reactive protein (hs- CRP) level (Wang and Li, 2012). Experimental studies have also found that Tongxinluo Capsule can reduce the scope of myocardial infarction after ischemia-reperfusion in rats, reduce the level of plasma CK, reduce the degree of myocardial necrosis, and have a protective effect on ischemia-reperfusion myocardium (Zhao et al., 2000).
Qili Qiangxin capsule
Studies have found that Qili Qiangxin Capsule can significantly increase the left ventricular myocardial contractility and cardiac output (CO), reduce left ventricular end-diastolic pressure (LVEDP), at the same time can increase renal blood flow, effectively improve cardiac function (Liu et al., 2007). Another study found that Qili Qiangxin Capsule can significantly reduce the left ventricular end-diastolic diameter (LVEDD) in patients with chronic heart failure, reduce the plasma vasopressin (AVP) concentration, significantly increase the plasma brain natriuretic peptide (BNP), left ventricular ejection fraction (LVEF) and 6-min walk distance, improve exercise tolerance (Huang, 2010; Wu et al., 2011; Chen and Zhou, 2015). Qili Qiangxin Capsule can significantly increase the content of ATP and ADP in myocardial tissue, reduce the expression of ICAM-1 mRNA, increase eNOS mRNA, and significantly increase the protein expression of p-AMPK. It is suggested that Qiliqiangxin can protect the myocardial capillary endothelium in rats with pressure overload, and its mechanism may be related to the activation of AMPK-eNOS pathway (Zhang et al., 2013c).
Qishen capsule
Qishen Capsule is one of the effective drugs for the treatment of coronary heart disease angina pectoris. It can improve clinical symptoms, reduce blood lipids, blood viscosity and fibrinogen (Shang, 2003), and can also reduce the levels of plasma hs-CRP and BNP (Deng and Liu, 2013), the frequency of angina pectoris and the rate of vasodilator drugs. It also has a significant effect on patients with myocardial infarction complicated by cardiac insufficiency and coronary artery bypass grafting (Wang, 2010), (Yang and Huang, 2013). On the basis of nicorandil as the control group, combined with Qishen Capsule can improve the total effective rate of patients with coronary heart disease, reduce the frequency and myocardial oxygen consumption of angina pectoris, and improve the electrocardiogram and cardiac function indicators (Li et al., 2022).
Shenshao capsule
Shenshao Capsule can reduce the frequency and shorten the duration of angina attacks, and reduce the levels of serum CRP, cTnI and interleukin-6 (IL-6) in patients with unstable angina pectoris. It also has a decreasing effect on indicators such as whole blood viscosity (WBV), plasma viscosity (PV), fibrinogen (FIB) and platelet adhesion rate (PAR) (Liu et al., 2020b). Shenshao Capsule not only has a significant clinical effect in the treatment of coronary heart disease, but also can reduce the dosage of nitroglycerin and the blood lipid level of patients. After treatment, the levels of TC, TG and LDL-C were decreased, and HDL-C was increased (Zhang and Ding, 2021). Because of this, Shenshao Capsule can also reduce the number of carotid plaques, the plaque volume, and arterial intima-media thickness (IMT) (Li, 2015). After using Shenshao Capsule, the cardiac index was reduced, and the levels of nitric oxide (NO), endothelin (ET) and tissue plasminogen activation inhibitor (tPAI-1) in myocardium are both decreased (Li et al., 2010).
Shexiang Baoxin pill
Shexiang Baoxin Pill can significantly reduce the infarction size of ischemic myocardium, and there is a dose-effect relationship (Wang et al., 2004). The expression levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), factor VIII and the surface density of blood vessels in the infarction edge area were significantly increased, suggesting that Shexiang Baoxin Pill can promote the angiogenesis of coronary collaterals (Wang et al., 2002). A prospective, randomized, non-blind controlled clinical trial involving 200 patients with stable angina pectoris also showed that after the treatment of Shexiang Baoxin Pill, the use of nitrates in the treatment group was significantly lower than that before treatment, all-cause deaths, heart failure events, and stroke events also tended to decrease (Zhu et al., 2010). Shexiang Baoxin Pill can also reduce the damage of hyperlipidemia to the artery. It can significantly inhibit the rise of serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) level and increase the concentration of serum SOD. Reduce the hyperplasia of arterial intima, blood damage to the arterial wall, and inhibit the formation of atherosclerosis (Luo et al., 1998).
Shensong Yangxin capsule
Shensong Yangxin Capsule has a broad-spectrum antiarrhythmic effect, and can block inward rectifier potassium current (IK1), instantaneous outward potassium current (Ito) and delayed rectifier potassium current (Ik) in cardiomyocytes to varying degrees. Importantly, Shensong Yangxin Capsules have fewer proarrhythmic side effects (Li et al., 2007), and may also significantly improve clinical symptoms such as palpitation, insomnia, shortness of breath and fatigue in patients with arrhythmia (Chen et al., 2006; Bai and Wang, 2011). In addition, Shensong Yangxin Capsule can play an anti-arrhythmic effect after heart failure, because it can improve cardiac function, and at the same time, it can not only shorten the effective refractory period of the left and right ventricles, but also reduce the transmural dispersion of repolarization (TDR) (Wang et al., 2012).
Adverse reactions of ginseng medication
Although ginseng has a wide range of clinical applications, it should not be abused. The pharmacopoeia stipulates that the single dose of ginseng is 3–9 g. Taking the recommended dose of ginseng will not cause serious adverse reactions. Studies have shown that high doses of ginseng (15 g/d) will lead to ginseng abuse syndrome (GAS), the specific performance is as follows (Siegel, 1979): ①Nervous system: headache, dizziness, fever, restlessness, easy to wake up, insomnia, sweating, euphoria, mania, confusion, cerebral arteritis, mydriasis. ②Cardiovascular system: arrhythmia, palpitations, slow heart rate, high blood pressure, and even heart failure. ③Endocrine and metabolic system: hypokalemia, gynecomastia, breast pain. ④Blood system: neutropenia, gastrointestinal bleeding, uterine bleeding, cerebral hemorrhage. ⑤Digestive system: abdominal pain, nausea and vomiting, intractable. ⑥Respiratory system: shortness of breath, asthma.
In addition, ginseng will also have an effect on other drugs, and it is particularly important to pay attention to clinical use (Ryu and Chien, 1995). There are literature reports of interactions between ginseng and warfarin, which inhibits the pharmacological effects of warfarin and may increase the risk of blood clotting (Smolinske, 1972; Janetzky and Morreale, 1997). Studies have found that ginseng has an inhibitory effect on monoamine oxidase, similar to monoamine oxidase inhibitors (MAOIs), and should be clinically cautiously combined with antidepressants such as phenylcyprotamine and phenelhydrazine (Dai and Yin, 1987). Studies have reported that ginseng can be used only for mild diabetic patients, for moderate and severe patients, when people participate in the combination of insulin or oral hypoglycemic drugs, it will have a synergistic effect, which may lead to low glucose, and the dose of hypoglycemic drugs needs to be reduced (An et al., 2003).
Summary and prospect
This paper mainly focus on the mechanism of ginsenosides, ginseng polysaccharides, and ginseng volatile oil, summarizes the progress of ginseng-containing drugs, lists the adverse reactions of ginseng medication, and expounds the multi-faceted effects of ginseng functional compounds and composition compatibility in cardiovascular diseases. According to the existing research, ginseng and ginseng-containing drugs can treat coronary heart disease by improving inflammation and lowering blood lipid levels, can inhibit the activation of glycoprotein IIb/IIIa in human platelets, it can increase coronary perfusion flow and promote capillary regeneration by antioxidant, anti-inflammatory and anticardiomyocyte apoptosis. Ginseng can also reduce heart rate, lower action potential, and suppress arrhythmias by activating K+ channels, blocking Ca2+ channels and Na+ currents. By improving antioxidant enzyme function, increasing energy metabolism and reducing free radical damage, ginseng can inhibit heart failure and ventricular remodeling. Although the role of ginseng on blood pressure has been controversial in the past, recent studies have shown that ginseng can lower blood pressure by promoting endothelial-dependent vasodilation. It can be seen that ginseng has a good efficacy as a drug for the treatment of cardiovascular diseases and can play a therapeutic role through multiple pathways, which is worth continuing research and development. Besides, there are many related researches on ginsenosides, but relatively few researches on ginseng polysaccharides and volatile oils. Due to the various and complex components of ginseng volatile oils, there are still many unknown unique components of ginseng volatile oils to be separated, identified and developed. At present, there is a lot of clinical evidence for ginseng-containing medicines, but further meta-analysis and quality evaluation are needed to reasonably and clearly explain its therapeutic effects and help the innovative application of botanical medicine.
In order to better explore the natural Chinese herbal medicine represented by ginseng, further develop its functional components, and improve its role in the treatment and prevention of diseases, the following aspects can be achieved: ① Use the theory of traditional Chinese medicine to expand the efficacy and indications of Chinese herbal medicine. Ancient Chinese medicine books are the crystallization of thousands of years of medical practice experience. Tu Youyou was inspired to develop artemisinin from a medical treatise by Ge Hong of the Eastern Jin Dynasty (317–420). Medicine tailored from classic Chinese medicine recipes also play an important role in the fight against COVID-19. ②Pay attention to the combination of ginseng medication. The composition of traditional Chinese medicine prescription is not a simple combination of several drugs, and the rational compatibility and application of rules can improve the clinical efficacy of ginseng. ③Adjust the dosage of Chinese herbal medicine according to the difference of symptoms. The therapeutic effect of Chinese herbal medicine has a dose-effect relationship, and the optimal dose should be selected according to the disease. Taking ginseng as an example, small doses of ginseng are suitable for healthcare people, which can improve physical fitness and enhance disease resistance. Patients with chronic diseases are suitable for medium doses of ginseng, and patients with massive hemorrhagic shock are suitable for large doses of ginseng. ④ Improve the preparation technology of Chinese herbal medicine to promote the development of the industry. There are various types of Chinese herbal preparations in ancient times. In modern times, it is necessary to further use new technologies and methods to design drug delivery systems based on biopharmaceutical characteristics, and to improve the absorption and bioavailability of functional compounds in traditional Chinese medicine preparations, so as to better exert its pharmacological effects.
Author contributions
LL and JH designed the work of review; LH, JH, and QM reviewed the literature available on this topic and wrote the paper; CL, HH, XH, and GY contributed in the scientific writing of the manuscript; PQ and WL polished the formatting of the figures and tables. LD and YD revised the manuscript; JP, YL, QH, JL, and JW defined the framework of the review. All authors approved the paper for publication. LL, JH, and QM contributed equally to this work.
Funding
National Key Research and Development Program (2020YFC2002701): Research on TCM Syndrome Differentiation of Sub-health State, person in charge: JP; State Administration of Traditional Chinese Medicine: The grant number of “Hundreds and Tens of Thousands” Talent Projects for Traditional Chinese Medicine Inheritance and Innovation is (0201000401) (Chief Scientist of Qihuang), person in charge: JW.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahluwalia, N., Andreeva, V. A., Kesse-Guyot, E., and Hercberg, S. (2013). Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 39 (2), 99–110. doi:10.1016/j.diabet.2012.08.007
An, G. H., Geng, X. F., and Ji, M. C. (2003). Interaction between hypoglycemic drugs and other drugs. Chin. J. Clin. Pharmacol. (01), 67–70+74. doi:10.13699/j.cnki.1001-6821.2003.01.016
Aravinthan, A., Kim, J. H., Antonisamy, P., Kang, C. W., Choi, J., Kim, N. S., et al. (2015). Ginseng total saponin attenuates myocardial injury via anti-oxidative and anti-inflammatory properties. J. Ginseng Res. 39 (3), 206–212. doi:10.1016/j.jgr.2014.12.001
Bai, Y. R., and Wang, R. Y. (2011). Observation on the curative effect of Shensong Yangxin Capsule in the treatment of arrhythmia. J. Cardiovasc. Cerebrovasc. Dis. Integr. Traditional Chin. West. Med. 9 (10), 1170–1171. doi:10.3969/j.issn.1672-1349.2011.10.011
Bai, C. X., Sunami, A., Namiki, T., Sawanobori, T., and Furukawa, T. (2003). Electrophysiological effects of ginseng and ginsenoside Re in Guinea pig ventricular myocytes. Eur. J. Pharmacol. 476 (1-2), 35–44. doi:10.1016/s0014-2999(03)02174-5
Bai, C. X., Takahashi, K., Masumiya, H., Sawanobori, T., and Furukawa, T. (2004). Nitric oxide-dependent modulation of the delayed rectifier K+ current and the L-type Ca2+ current by ginsenoside Re, an ingredient of Panax ginseng, in Guinea-pig cardiomyocytes. Br. J. Pharmacol. 142 (3), 567–575. doi:10.1038/sj.bjp.0705814
Borén, J., Chapman, M. J., Krauss, R. M., Packard, C. J., Bentzon, J. F., Binder, C. J., et al. (2020). Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European atherosclerosis society consensus panel. Eur. Heart J. 41 (24), 2313–2330. doi:10.1093/eurheartj/ehz962
Cao, C. H., and Xie, F. (2010). Clinical observation of Yixinshu capsule in treating 102 cases of coronary heart disease and angina pectoris. J. Cardiovasc. Cerebrovasc. Dis. Integr. Traditional Chin. West. Med. 8 (2), 136–137. doi:10.3969/j.issn.1672-1349.2010.02.005
Cao, Z., Zhang, Y. D., and Xu, Y. H. (2012). New progress in research on active ingredients and pharmacological effects of ginseng. Ginseng Res. 24 (2), 39–43. doi:10.3969/j.issn.1671-1521.2012.02.014
Cao, X. J. (2009). Clinical observation of Yixinshu capsule in treating 100 cases of coronary heart disease and angina pectoris. Eval. Analysis Drugs Chin. Hosp. 9 (7), 536–538. doi:10.14009/j.issn.1672-2124.2009.07.003
Chai, H., Zhou, W., Lin, P., Lumsden, A., Yao, Q., and Chen, C. (2005). Ginsenosides block HIV protease inhibitor ritonavir-induced vascular dysfunction of porcine coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 288 (6), H2965–H2971. doi:10.1152/ajpheart.01271.2004
Chen, F., and Huang, G. (2019). Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 125, 906–908. doi:10.1016/j.ijbiomac.2018.12.134
Chen, C. X., and Zhang, H. Y. (2009). Protective effect of ginsenoside Re on isoproterenol-induced triggered ventricular arrhythmia in rabbits. Zhongguo Dang Dai Er Ke Za Zhi 11 (5), 384–388.
Chen, X. D., and Zhou, J. (2015). Combined application of Qili Qiangxin Capsule and trimetazidine in patients with ischemic cardiomyopathy and heart failure. Chin. J. Exp. Prescr. 21 (19), 171–175. doi:10.13422/j.cnki.syfjx.2015190171
Chen, Y. J., Huang, Z., and Li, N. P. (1982). Study on volatile oil of ginseng. China J. Chin. Materia Medica 7 (4).
Chen, Y. J., Dou, D. Q., and Zhao, C. J. (2002). Research on new components, new activities and quality standardization of ginseng. Ginseng Res. 14 (1), 2–19. doi:10.3969/j.issn.1671-1521.2002.01.001
Chen, L. X., Kuang, J. B., and Deng, Y. (2006). Clinical observation of Shensong Yangxin capsule in the treatment of arrhythmia. Int. Med. Health Her. 12 (14), 103–104. doi:10.3760/cma.j.issn.1007-1245.2006.14.053
Chen, H., Yin, J., Deng, Y., Yang, M., Xu, L., Teng, F., et al. (2012). The protective effects of ginsenoside Rg1 against hypertension target-organ damage in spontaneously hypertensive rats. BMC Complement. Altern. Med. 12, 53. doi:10.1186/1472-6882-12-53
Chen, X., Wang, Q., Shao, M., Ma, L., Guo, D., Wu, Y., et al. (2019). Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway. Biomed. Pharmacother. 120, 109487. doi:10.1016/j.biopha.2019.109487
Chen, X., Wang, Y. F., and Zhang, Z. X. (2020). Current status and treatment progress of arrhythmia in China. Chin. J. Res. Hosp. 7 (1), 75198–78201. doi:10.19450/j.cnki.jcrh.2020.01.016
Chen, X. Z., Wang, J., and Fu, D. (2022). Research progress of ginseng active ingredients and preparations in cardiovascular diseases. Clin. Res. TCM 14 (09), 140–144. doi:10.3969/j.issn.1674-7860.2022.09.049
Chen, S. W. (2015). Preclinical study of the new drug "Compound Ginseng Volatile Oil Spray" for the treatment of coronary heart disease and angina pectoris. Jilin Province: Changchun University of Traditional Chinese Medicine.
China Cardiovascular Health and Disease Report Writing Group (2021). China cardiovascular health and disease report 2020 outline. Chin. J. Circulation 36 (6), 521–545. doi:10.3969/j.issn.1000-3614.2021.06.001
Chinese Cardiovascular Health and Disease Report Writing Group (2022). Overview of China cardiovascular health and disease report 2021. Chin. J. Circulation 37 (6), 553–578. doi:10.3969/j.issn.1000-3614.2022.06.001
Choi, S. H., Lee, J. H., Pyo, M. K., Lee, B. H., Shin, T. J., Hwang, S. H., et al. (2009). Mutations Leu427, Asn428, and Leu431 residues within transmembrane domain-I-segment 6 attenuate ginsenoside-mediated L-type Ca(2+) channel current inhibitions. Biol. Pharm. Bull. 32 (7), 1224–1230. doi:10.1248/bpb.32.1224
Cholesterol Treatment Trialists' (CTT) Collaboration O'Connell, R., Voysey, M., Emberson, J., Blackwell, L., Mihaylova, B., Simes, J., et al. (2015). Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174, 000 participants in 27 randomised trials. Lancet 385 (9976), 1397–1405. doi:10.1016/S0140-6736(14)61368-4
Chu, S. F., and Zhang, J. T. (2009). New achievements in ginseng research and its future prospects. Chin. J. Integr. Med. 15 (6), 403–408. doi:10.1007/s11655-009-0403-6
Dai, Y. R., and Yin, Y. (1987). Study on the inhibition effect of B-type monoamine oxidase in traditional Chinese medicine. Chin. J. Geriatrics 06 (1), 27–30. doi:10.3760/cma.j.issn.0254-9026.1987.01.114
Davies, M. J., Gordon, J. L., Gearing, A. J., Pigott, R., Woolf, N., Katz, D., et al. (1993). The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J. Pathol. 171 (3), 223–229. doi:10.1002/path.1711710311
Deng, Y. F., and Liu, J. L. (2013). Effects of Qishen Capsules on hs-CRP and BNP in patients with angina pectoris. China Pract. Med. 8 (25), 150–151. doi:10.3969/j.issn.1673-7555.2013.25.113
Deng, J., Liu, Y., Duan, Z., Zhu, C., Hui, J., Mi, Y., et al. (2017). Protopanaxadiol and protopanaxatriol-type saponins ameliorate glucose and lipid metabolism in type 2 diabetes mellitus in high-fat diet/streptozocin-induced mice. Front. Pharmacol. 8, 506. doi:10.3389/fphar.2017.00506
Dong, G., Chen, T., Ren, X., Zhang, Z., Huang, W., Liu, L., et al. (2016). Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion 26, 7–18. doi:10.1016/j.mito.2015.11.003
Duan, L., Xiong, X., Hu, J., Liu, Y., Li, J., and Wang, J. (2017). Panax notoginseng saponins for treating coronary artery disease: A functional and mechanistic overview. Front. Pharmacol. 8, 702. doi:10.3389/fphar.2017.00702
Ezzati, M., Lopez, A. D., Rodgers, A., Vander Hoorn, S., and Murray, C. J.Comparative Risk Assessment Collaborating Group (2002). Selected major risk factors and global and regional burden of disease. Lancet 360 (9343), 1347–1360. doi:10.1016/S0140-6736(02)11403-6
Furukawa, T., Bai, C. X., Kaihara, A., Ozaki, E., Kawano, T., Nakaya, Y., et al. (2006). Ginsenoside Re, a main phytosterol of Panax ginseng, activates cardiac potassium channels via a nongenomic pathway of sex hormones. Mol. Pharmacol. 70 (6), 1916–1924. doi:10.1124/mol.106.028134
Gao, Y., Zhu, P., Xu, S. F., Li, Y. Q., Deng, J., and Yang, D. L. (2019). Ginsenoside Re inhibits PDGF-BB-induced VSMC proliferation via the eNOS/NO/cGMP pathway. Biomed. Pharmacother. 115, 108934. doi:10.1016/j.biopha.2019.108934
Gou, D., Pei, X., Wang, J., Wang, Y., Hu, C., Song, C., et al. (2020). Antiarrhythmic effects of ginsenoside Rg2 on calcium chloride-induced arrhythmias without oral toxicity. J. Ginseng Res. 44 (5), 717–724. doi:10.1016/j.jgr.2019.06.005
Guo, J., Gan, X. T., Haist, J. V., Rajapurohitam, V., Zeidan, A., Faruq, N. S., et al. (2011). Ginseng inhibits cardiomyocyte hypertrophy and heart failure via NHE-1 inhibition and attenuation of calcineurin activation. Circ. Heart Fail. 4 (1), 79–88. doi:10.1161/CIRCHEARTFAILURE.110.957969
Guo, M., Guo, G., Xiao, J., Sheng, X., Zhang, X., Tie, Y., et al. (2018a). Ginsenoside Rg3 stereoisomers differentially inhibit vascular smooth muscle cell proliferation and migration in diabetic atherosclerosis. J. Cell. Mol. Med. 22 (6), 3202–3214. doi:10.1111/jcmm.13601
Guo, M., Xiao, J., Sheng, X., Zhang, X., Tie, Y., Wang, L., et al. (2018b). Ginsenoside Rg3 mitigates atherosclerosis progression in diabetic apoE-/- mice by skewing macrophages to the M2 phenotype. Front. Pharmacol. 9, 464. doi:10.3389/fphar.2018.00464
Gutiérrez, E., Flammer, A. J., Lerman, L. O., Elízaga, J., Lerman, A., and Fernández-Avilés, F. (2013). Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 34 (41), 3175–3181. doi:10.1093/eurheartj/eht351
Hao, P., Jiang, F., Cheng, J., Ma, L., Zhang, Y., and Zhao, Y. (2017). Traditional Chinese medicine for cardiovascular disease: Evidence and potential mechanisms. J. Am. Coll. Cardiol. 69 (24), 2952–2966. doi:10.1016/j.jacc.2017.04.041
Heart Failure Group of Cardiology (2018). Chinese guidelines for the diagnosis and treatment of heart failure 2018. Chin. J. Cardiovasc. Dis. 46, 760–789. doi:10.3760/cma.j.issn.0253-3758.2018.10.004
Hernández-García, D., Granado-Serrano, A. B., Martín-Gari, M., Naudí, A., and Serrano, J. C. (2019). Efficacy of Panax ginseng supplementation on blood lipid profile. A meta-analysis and systematic review of clinical randomized trials. J. Ethnopharmacol. 243, 112090. doi:10.1016/j.jep.2019.112090
Hien, T. T., Kim, N. D., Kim, H. S., and Kang, K. W. (2010). Ginsenoside Rg3 inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules in human endothelial cells. Pharmazie 65 (9), 699–701.
Huang, B. (2010). Effects of Qili Qiangxin Capsule on cardiac function and plasma brain natriuretic peptide level in patients with chronic systolic heart failure. Chin. J. Exp. Prescr. 16 (16), 191–193. doi:10.3969/j.issn.1005-9903.2010.16.057
Im, D. S. (2020). Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of panax ginseng. Biomolecules 10 (3), 444. doi:10.3390/biom10030444
Irfan, M., Kim, M., and Rhee, M. H. (2020). Anti-platelet role of Korean ginseng and ginsenosides in cardiovascular diseases. J. Ginseng Res. 44 (1), 24–32. doi:10.1016/j.jgr.2019.05.005
Janetzky, K., and Morreale, A. P. (1997). Probable interaction between warfarin and ginseng. Am. J. Health. Syst. Pharm. 54 (6), 692–693. doi:10.1093/ajhp/54.6.692
Jeon, B. H., Kim, C. S., Kim, H. S., Park, J. B., Nam, K. Y., and Chang, S. J. (2000). Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol. Sin. 21 (12), 1095–1100.
Jeong, D., Irfan, M., Kim, S. D., Kim, S., Oh, J. H., Park, C. K., et al. (2017). Ginsenoside Rg3-enriched red ginseng extract inhibits platelet activation and in vivo thrombus formation. J. Ginseng Res. 41 (4), 548–555. doi:10.1016/j.jgr.2016.11.003
Jia, Y., Zhang, S., Huang, F., and Leung, S. W. (2012). Could ginseng-based medicines be better than nitrates in treating ischemic heart disease? A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 20 (3), 155–166. doi:10.1016/j.ctim.2011.12.002
Jiang, Q. S., Huang, X. N., Dai, Z. K., Yang, G. Z., Zhou, Q. X., Shi, J. S., et al. (2007). Inhibitory effect of ginsenoside Rb1 on cardiac hypertrophy induced by monocrotaline in rat. J. Ethnopharmacol. 111 (3), 567–572. doi:10.1016/j.jep.2007.01.006
Jiao, H. C., and Ding, S. W. (2015). “Clinical and basic research of Chinese medicine Xinsuning capsule in the treatment of premature beat,” in The Proceedings of the 2015 Academic Conference of the Heart Disease Branch of the Chinese Society of Traditional Chinese Medicine.
Jin, Z. Q., and Liu, C. M. (1994). Effect of ginsenoside Re on the electrophysiological activity of the heart. Planta Med. 60 (2), 192–193. doi:10.1055/s-2006-959452
Jin, C., and Zhang, S. Z. (1995). Rise and prospect of glycobiology and glycoengineering. Adv. Bioeng. (3), 12–17. doi:10.13523/j.cb.19950306
Kaku, T., Miyata, T., Uruno, T., Sako, I., and Kinoshita, A. (1975). Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. II. Pharmacological part. Arzneimittelforschung. 25 (4), 539–547.
Kannel, W. B. (2000). Incidence and epidemiology of heart failure. Heart fail. Rev. 5 (2), 167–173. doi:10.1023/A:1009884820941
Kim, S. H., and Park, K. S. (2003). Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol. Res. 48 (5), 511–513. doi:10.1016/s1043-6618(03)00189-0
Konukoglu, D., and Uzun, H. (2017). Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 956, 511–540. doi:10.1007/5584_2016_90
Kuriachan, V. P., Sumner, G. L., and Mitchell, L. B. (2015). Sudden cardiac death. Curr. Probl. Cardiol. 40 (4), 133–200. doi:10.1016/j.cpcardiol.2015.01.002
Kwon, H. W. (2018a). Inhibitory effect of 20(S)-Ginsenoside Rg3 on human platelet aggregation and intracellular Ca2+ levels via cyclic adenosine monophosphate dependent manner. Prev. Nutr. Food Sci. 23 (4), 317–325. doi:10.3746/pnf.2018.23.4.317
Kwon, H. W . (2018b). 20(S)-ginsenoside Rg3 inhibits glycoprotein IIb/IIIa activation in human platelets. J. Appl. Biol. Chem. 61 (3), 257–265. doi:10.3839/jabc.2018.037
Lee, W. M., Kim, S. D., Park, M. H., Cho, J. Y., Park, H. J., Seo, G. S., et al. (2008). Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: Critical roles of ERK2 and cAMP. J. Pharm. Pharmacol. 60 (11), 1531–1536. doi:10.1211/jpp/60.11.0015
Lee, K. H., Bae, I. Y., Park, S. I., Park, J. D., and Lee, H. G. (2016). Antihypertensive effect of Korean Red Ginseng by enrichment of ginsenoside Rg3 and arginine-fructose. J. Ginseng Res. 40 (3), 237–244. doi:10.1016/j.jgr.2015.08.002
Lee, J. W., Choi, B. R., Kim, Y. C., Choi, D. J., Lee, Y. S., Kim, G. S., et al. (2017a). Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of panax ginseng by UPLC-QTOF/MS. Molecules 22 (12), 2147. doi:10.3390/molecules22122147
Lee, H. W., Lim, H. J., Jun, J. H., Choi, J., and Lee, M. S. (2017b). Ginseng for treating hypertension: A systematic review and meta-analysis of double blind, randomized, placebo-controlled trials. Curr. Vasc. Pharmacol. 15 (6), 549–556. doi:10.2174/1570161115666170713092701
Li, P., and Liu, Z. X. (2006). Effects of ginsenoside Rb1 on ventricular remodeling in rats with acute myocardial infarction. J. Pract. Cardiovasc. Cerebrovasc. Dis. 14 (2), 118–121. doi:10.3969/j.issn.1008-5971.2006.02.017
Li, C. Y., and Shi, Z. X. (2013). Clinical research progress of Yixinshu capsule in the treatment of coronary heart disease angina pectoris. J. Cardiovasc. Cerebrovasc. Dis. 11 (3), 351–352. doi:10.3969/j.issn.1672-1349.2013.03.052
Li, X. G., and Teng, F. T. (1978). Study on the active ingredients of ginseng—Extraction, separation and identification of ginsenosides and their sapogenins. J. Res. Chin. Pat. Med. (03), 1.
Li, R. Q., and Zhang, Y. S. (1984). Purification and characterization of Panax ginseng C. A. Mey pectin. Chin. J. Pharm. 19 (10), 764–768. doi:10.16438/j.0513-4870.1984.10.009
Li, R. Q., and Zhang, Y. Y. (1986). Structural studies of Panax ginseng C. A. Mey pectin. Chin. J. Pharm. 21 (12), 912–916. doi:10.16438/j.0513-4870.1986.12.006
Li, F. X., and Zhang, C. F. (2015). Clinical study of Xinsuning capsule in the treatment of paroxysmal atrial fibrillation. Front. Med. 55 (26), 95–97. doi:10.3969/j.issn.2095-1752.2015.26.077
Li, N., Wu, X. F., and Ma, K. J. (2007). Effects of Shensong Yangxin Capsules on potassium channels in ventricular myocytes. J. Difficult Dis. 6 (3), 133–137. doi:10.3969/j.issn.1671-6450.2007.03.002
Li, Y. Q., Wen, H. J., and Liu, W. M. (2010). Effects of Shenshao Capsule on myocardial remodeling in atherosclerotic rats. Shandong Med. 50 (4), 21–23. doi:10.3969/j.issn.1002-266X.2010.04.008
Li, J., Shao, Z. H., Xie, J. T., Wang, C. Z., Ramachandran, S., Yin, J. J., et al. (2012). The effects of ginsenoside Rb1 on JNK in oxidative injury in cardiomyocytes. Arch. Pharm. Res. 35 (7), 1259–1267. doi:10.1007/s12272-012-0717-3
Li, Q., Xiang, Y., Chen, Y., Tang, Y., and Zhang, Y. (2017). Ginsenoside Rg1 protects cardiomyocytes against hypoxia/reoxygenation injury via activation of Nrf2/HO-1 signaling and inhibition of JNK. Cell. Physiol. biochem. 44 (1), 21–37. doi:10.1159/000484578
Li, F., Long, Y., Ma, H., Qiang, T., Zhang, G., and Shen, Y. (2022). Promoting the reduction of CO2 to formate and formaldehyde via gas-liquid interface dielectric barrier discharge using a Zn0.5Cd0.5S/CoP/multiwalled carbon nanotubes catalyst. J. Colloid Interface Sci. 46 (6), 880–891. doi:10.1016/j.jcis.2022.04.125
Li, T. C. (2015). Application of Shenshao Capsule in the treatment of unstable angina pectoris. Shandong Med. 55 (38), 34–35. doi:10.3969/j.issn.1002-266X.2015.38.012
Liao, D. N., and Yang, Z. J. (2008). The mechanism of antiarrhythmic drug-induced arrhythmia. Intern. Med. Theory Pract. 3 (4), 232–234. doi:10.16138/j.1673-6087.2008.04.006
Lin, G. W., Wang, J. Y., and Ge, J. B. (2017). Practical internal medicine. J. Sci. Technol. (12), 2. doi:10.16510/j.cnki.kjycb.2017.12.001
Liu, J. X., Ma, X. B., and Wang, Y. H. (2007). Effects of Qili Qiangxin Capsule on cardiac function in dogs with experimental heart failure. J. Difficult Difficult Dis. 6 (3), 141–143. doi:10.3969/j.issn.1671-6450.2007.03.004
Liu, J. J., Li, S. G., Deng, J. J., Ye, M., Zhang, D., Peng, Y., et al. (2016). Effects of ginsenoside Rg3 on vascular structure and function in aged rats. J. Clin. Cardiovasc. Dis. 32 (11), 1154–1158. doi:10.13201/j.issn.1001-1439.2016.11.020
Liu, Z., Song, L., Zhang, P., Cao, Z., Hao, J., Tian, Y., et al. (2019). Ginsenoside Rb1 exerts antiarrhythmic effects by inhibiting INa and ICaL in rabbit ventricular myocytes. Sci. Rep. 9 (1), 20425. doi:10.1038/s41598-019-57010-9
Liu, X., Jiang, Y., Fu, W., Yu, X., and Sui, D. (2020a). Combination of the ginsenosides Rb3 and Rb2 exerts protective effects against myocardial ischemia reperfusion injury in rats. Int. J. Mol. Med. 45 (2), 519–531. doi:10.3892/ijmm.2019.4414
Liu, L. S., Yue, J. B., and Ding, X. (2020b). Clinical study of Shenshao Capsule combined with verapamil in the treatment of unstable angina pectoris. Mod. Med. Clin. 35 (1), 83–87. doi:10.7501/j.issn.1674-5515.2020.01.018
Liu, K. (2021). Advances in diagnosis and treatment of arrhythmia. Chin. J. Prescr. Drugs 19 (9), 23–26. doi:10.3969/j.issn.1671-945X.2021.09.010
Lo, S. H., Hsu, C. T., Niu, H. S., Niu, C. S., Cheng, J. T., and Chen, Z. C. (2017). Ginsenoside Rh2 improves cardiac fibrosis via pparδ-STAT3 signaling in type 1-like diabetic rats. Int. J. Mol. Sci. 18 (7), 1364. doi:10.3390/ijms18071364
Lu, W. J., Zhou, J., and Ma, H. Y. (2012). Effects of astragaloside IV, total ginseng saponins and total American ginseng saponins on arrhythmia in mice induced by toadstool. J. Nanjing Univ. Traditional Chin. Med. 28 (01), 61–64. doi:10.14148/j.issn.1672-0482.2012.01.020
Lu, S., Luo, Y., Zhou, P., Yang, K., Sun, G., and Sun, X. (2019). Ginsenoside compound K protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury via inhibition of nuclear factor-κB, p38, and JNK MAPK pathways. J. Ginseng Res. 43 (1), 95–104. doi:10.1016/j.jgr.2017.09.004
Luo, X. P., Li, Y., and Fan, W. H. (1998). Experimental study on the effect of Shexiang Baoxin pills on reducing the damage of arterial wall caused by hyperlipidemia. China J. Integr. Traditional Chin. West. Med. (8). doi:10.3321/j.issn:1003-5370.1998.08.012
Nagar, H., Choi, S., Jung, S. B., Jeon, B. H., and Kim, C. S. (2016). Rg3-enriched Korean Red Ginseng enhances blood pressure stability in spontaneously hypertensive rats. Integr. Med. Res. 5 (3), 223–229. doi:10.1016/j.imr.2016.05.006
Nanao-Hamai, M., Son, B. K., Komuro, A., Asari, Y., Hashizume, T., Takayama, K. I., et al. (2019). Ginsenoside Rb1 inhibits vascular calcification as a selective androgen receptor modulator. Eur. J. Pharmacol. 859, 172546. doi:10.1016/j.ejphar.2019.172546
Qin, N., Gong, Q. H., Wei, L. W., Wu, Q., and Huang, X. N. (2008). Total ginsenosides inhibit the right ventricular hypertrophy induced by monocrotaline in rats. Biol. Pharm. Bull. 31 (8), 1530–1535. doi:10.1248/bpb.31.1530
Rezende, P. C., Ribas, F. F., Serrano, C. V., and Hueb, W. (2019). Clinical significance of chronic myocardial ischemia in coronary artery disease patients. J. Thorac. Dis. 11 (3), 1005–1015. doi:10.21037/jtd.2019.02.85
Ryoo, S., Berkowitz, D. E., and Lim, H. K. (2011). Endothelial arginase II and atherosclerosis. Korean J. Anesthesiol. 61 (1), 3–11. doi:10.4097/kjae.2011.61.1.3
Ryu, S. J., and Chien, Y. Y. (1995). Ginseng-associated cerebral arteritis. Neurology 45 (4), 829–830. doi:10.1212/wnl.45.4.829
Shang, J. H. (2003). Clinical observation on treating angina pectoris with Qishen capsules. Chin. Pat. Med. 25 (4), 303–304. doi:10.3969/j.issn.1001-1528.2003.04.014
Shin, W., Yoon, J., Oh, G. T., and Ryoo, S. (2013). Korean red ginseng inhibits arginase and contributes to endotheliumdependent vasorelaxation through endothelial nitric oxide synthase coupling. J. Ginseng Res. 37 (1), 64–73. doi:10.5142/jgr.2013.37.64
Shin, J. H., Kwon, H. W., Cho, H. J., Rhee, M. H., and Park, H. J. (2016). Vasodilator-stimulated phosphoprotein-phosphorylation by ginsenoside Ro inhibits fibrinogen binding to αIIb/β3 in thrombin-induced human platelets. J. Ginseng Res. 40 (4), 359–365. doi:10.1016/j.jgr.2015.11.003
Siegel, R. K. (1979). Ginseng abuse syndrome. Problems with the panacea. JAMA 241 (15), 1614–1615. doi:10.1001/jama.1979.03290410046024
Smolinske, S. C. (1972). Dietary supplement-drug interactions. J. Am. Med. Womens Assoc. 54 (4), 191.
Song, X. L., Yang, X. C., and Lu, N. (2022). A multicenter randomized controlled study of Xinsuning capsules in the treatment of phlegm-heat disturbed heart syndrome with premature ventricular contractions. China J. Integr. Med. 42 (4), 438–443. doi:10.7661/j.cjim.20220320.067
Stöger, J. L., Gijbels, M. J., van der Velden, S., Manca, M., van der Loos, C. M., Biessen, E. A., et al. (2012). Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 225 (2), 461–468. doi:10.1016/j.atherosclerosis.2012.09.013
Su, P., Du, S., Li, H., Li, Z., Xin, W., and Zhang, W. (2016). Notoginsenoside R1 inhibits oxidized low-density lipoprotein induced inflammatory cytokines production in human endothelial EA.hy926 cells. Eur. J. Pharmacol. 770, 9–15. doi:10.1016/j.ejphar.2015.11.040
Sun, Y. X., and Wang, H. (1997). Application of variable temperature infrared spectroscopy in extraction of essential oil from ginseng. Synth. Chem. 005 (A10), 759.
Sun, X., Gao, R. L., Lin, X. J., Xu, W. H., and Chen, X. H. (2013). Panax notoginseng saponins induced up-regulation, phosphorylation and binding activity of MEK, ERK, AKT, PI-3K protein kinases and GATA transcription factors in hematopoietic cells. Chin. J. Integr. Med. 19 (2), 112–118. doi:10.1007/s11655-012-1306-4
Sun, J., Yu, X., Huangpu, H., and Yao, F. (2019). Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 109, 254–261. doi:10.1016/j.biopha.2018.09.002
Takagi, K., Saito, H., and Nabata, H. (1972). Pharmacological studies of panax ginseng root: Estimation of pharmacological actions of panax ginseng root. Jpn. J. Pharmacol. 22 (2), 245–249. doi:10.1254/jjp.22.245
Tao, Y. J., Jiang, B. H., and Qiu, H. (2009). Curative effect observation of Yixinshu capsule in the treatment of unstable angina pectoris. J. Cardiovasc. Cerebrovasc. Dis. Integr. Traditional Chin. West. Med. 7 (5), 596–597. doi:10.3969/j.issn.1672-1349.2009.05.046
Teng, C. M., Kuo, S. C., Ko, F. N., Lee, J. C., Lee, L. G., Chen, S. C., et al. (1989). Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim. Biophys. Acta 990 (3), 315–320. doi:10.1016/s0304-4165(89)80051-0
Tian, Y. B., Zhao, D. Q., and Li, X. Y. (2018). Ginseng polysaccharide protects cardiomyocytes from H2O2-induced oxidative stress injury by inhibiting ROS level and apoptosis. J. Central China Normal Univ. Nat. Sci. Ed. 52 (2), 240–247. doi:10.19603/j.cnki.1000-1190.2018.02.014
Tsai, C. C., Chan, P., Chen, L. J., Chang, C. K., Liu, Z., and Lin, J. W. (2014). Merit of ginseng in the treatment of heart failure in type 1-like diabetic rats. Biomed. Res. Int. 2014, 484161. doi:10.1155/2014/484161
Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2020). Heart disease and stroke statistics-2020 update: A report from the American heart association. Circulation 141 (9), e139–e596. doi:10.1161/CIR.0000000000000757
Wan, H. F., Ying, J. N., and Guan, Y. (2020). Protective effect of ginseng polysaccharides on mitochondria in myocardial cells of rats with coronary heart disease. Mod. Food Sci. Technol. 36 (11), 2460–2528. doi:10.13982/j.mfst.1673-9078.2020.11.0510
Wang, G., and Li, J. P. (2012). Effects of Tongxinluo capsules on serum IL-18 and hs-CRP in elderly patients with coronary heart disease and angina pectoris. Guangdong Med. 33 (2), 276–278. doi:10.3969/j.issn.1001-9448.2012.02.053
Wang, C. H., and Lu, J. (2008). Xinsuning capsule in the treatment of 30 cases of viral myocarditis. Shaanxi Tradit. Chin. Med. 29 (10), 1362. doi:10.3969/j.issn.1000-7369.2008.10.068
Wang, H. L., and Zhi, J. C. (2016). Clinical observation of Yixinshu capsule combined with isosorbide dinitrate tablets in the treatment of angina pectoris. Medicine 000 (004), 285. doi:10.16138/j.1671-5837.2016.04.026
Wang, S. S., Li, Y., and Fan, W. H. (2002). Angiogenesis-promoting effect of Shexiang Baoxin Pill on the heart of rats with experimental myocardial infarction. Chin. Pat. Med. 24 (6), 446–449. doi:10.3969/j.issn.1001-1528.2002.06.016
Wang, D. Y., Li, Y., and Fan, W. H. (2004). Effects of Shexiang Baoxin Pill on infarct size and angiogenesis in rats with myocardial infarction. Chin. Pat. Med. 26 (11), 912–915. doi:10.3969/j.issn.1001-1528.2004.11.018
Wang, N. Y., Lu, C. J., and Chen, X. H. (2005). Study on effect of ginsenoside Rg1 in promoting myocardiac vascular endothelial cell regeneration through induction on bone marrow stem cell's migration and differentiation in rabbits of myocardial infarction. China J. Integr. Traditional Chin. West. Med. 25 (10), 916–919. doi:10.3321/j.issn:1003-5370.2005.10.014
Wang, T. X., Yu, X. F., Qu, S. C., et al. (2008). Effect of ginseng Rb group saponins on ventricular remodeling in rats with stress-loaded myocardial hypertrophy and its mechanism of action. J. Lishizhen Med. Materia Medica Res. 19 (7), 1615–1617. doi:10.3969/j.issn.1008-0805.2008.07.032
Wang, X., Duan, H. N., and Hu, J. (2012). Experimental study on the effect of Shensong Yangxin capsule on cardiac function and cardiac electrophysiology. Chin. J. Cardiac Arrhythmia 16 (6), 417–421. doi:10.3760/cma.j.issn.1007-6638.2012.06.004
Wang, Y., Dong, J., Liu, P., Lau, C. W., Gao, Z., Zhou, D., et al. (2014). Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats. Br. J. Pharmacol. 171 (13), 3171–3181. doi:10.1111/bph.12660
Wang, Y., Hu, Z., Sun, B., Xu, J., Jiang, J., and Luo, M. (2015). Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the B-cell lymphoma/B-cell lymphoma-associated X protein pathway. Mol. Med. Rep. 11 (6), 4518–4524. doi:10.3892/mmr.2015.3336
Wang, Q. W., Yu, X. F., Xu, H. L., Zhao, X. Z., and Sui, D. Y. (2019). Ginsenoside Re improves isoproterenol-induced myocardial fibrosis and heart failure in rats. Evid. Based Complement. Altern. Med. 2019, 3714508. doi:10.1155/2019/3714508
Wang, Y. J. (2010). Clinical study of Qishen capsule in the treatment of coronary heart disease and angina pectoris. Chin. J. Traditional Chin. Med. 25 (5), 939–940.
World Health Statistics (2022). The global health observatory. Avaliable at: https://www.who.int/data/gho/publications/world-health-statistics.
Wu, J. Z., Li, X. G., and Yang, J. X. (1996). Comparative study on the volatile oil components of fresh ginseng in Hongshen. Strait Pharm. (1), 5–6.
Wu, X. L., Li, J. B., and Zhang, Y. (2001). Clinical study of Tongxinluo capsule on blood lipid and blood viscosity. China J. Basic Med. Traditional Chin. Med. 7 (5), 32–33. doi:10.3969/j.issn.1006-3250.2001.05.015
Wu, Z. L., Xu, D. L., and Lin, S. (2011). Effects of Qili Qiangxin Capsule on cardiac function and plasma vasopressin in rats with chronic heart failure. J. Difficult Difficult Dis. 10 (2), 120–122. doi:10.3969/j.issn.1671-6450.2011.02.020
Xiao, W. L., Dai, H., and Jiang, Z. A. (2002). Study on the protective effect of Tongxinluo capsule on vascular endothelial cells in patients with unstable angina pectoris. Chin. J. Cardiovasc. Dis. 30 (5), 268. doi:10.3760/j:issn:0253-3758.2002.05.017
Xu, H., Ge, Y. K., and Deng, T. L., (2005). Protective effect of ginsenoside Rb1 on H2O2-induced cardiomyocyte apoptosis in neonatal rats. Chin. Pharmacol. Bull. 21 (7), 803–806. doi:10.3321/j.issn:1001-1978.2005.07.009
Xu, Z. M., Li, C. B., Liu, Q. L., Li, P., and Yang, H. (2018). Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int. J. Mol. Sci. 19 (11), 3658. doi:10.3390/ijms19113658
Yan, J. C., Zhang, H., and Wei, Y. D. (1994). Extraction and analysis of volatile oil from ginseng. J. Analysis Test. 13 (3), 5.
Yang, Q., and Huang, L. (2013). Observation on the efficacy of Qishen Capsules in the treatment of coronary heart disease angina pectoris. China Emerg. Med. 22 (9), 1602. doi:10.3969/j.issn.1004-745X.2013.09.070
Yang, P., Ling, L., Sun, W., Yang, J., Zhang, L., Chang, G., et al. (2018). Ginsenoside Rg1 inhibits apoptosis by increasing autophagy via the AMPK/mTOR signaling in serum deprivation macrophages. Acta Biochim. Biophys. Sin. 50 (2), 144–155. doi:10.1093/abbs/gmx136
Yang, M. (2020). Clinical observation of Xinsuning capsule combined with Betaloc in the treatment of premature ventricular contractions. Guizhou Med. 44 (3), 425–427. doi:10.3969/j.issn.1000-744X.2020.03.033
Yi, X. Q., Li, T., Wang, J. R., Wong, V. K., Luo, P., Wong, I. Y., et al. (2010). Total ginsenosides increase coronary perfusion flow in isolated rat hearts through activation of PI3K/Akt-eNOS signaling. Phytomedicine 17 (13), 1006–1015. doi:10.1016/j.phymed.2010.06.012
Yi, D. L. (2013). Analysis of rational use of cardiovascular drugs in the elderly. Chin. J. Health Nutr. 23 (3), 1376. doi:10.3969/j.issn.1004-7484(s).2013.03.436
You, S. J., Yang, Y. J., and Chen, K. J. (2004). Efficacy and safety of Tongxinluo capsule after revascularization in acute myocardial infarction. J. Difficult Difficult Dis. 3 (4), 193–196. doi:10.3969/j.issn.1671-6450.2004.04.001
You, J. S., Huang, H. F., and Chang, Y. L. (2005). Panax ginseng reduces adriamycin-induced heart failure in rats. Phytother. Res. 19 (12), 1018–1022. doi:10.1002/ptr.1778
Yuan, J., Guo, W., Yang, B., Liu, P., Wang, Q., and Yuan, H. (1997). 116 cases of coronary angina pectoris treated with powder composed of radix ginseng, radix notoginseng and succinum. J. Tradit. Chin. Med. 17 (1), 14–17.
Yuan, S. W. (2000). Observation on 60 cases of tachyarrhythmia in xinsuning capsule. J. Shandong Univ. Traditional Chin. Med. 24 (4), 4. doi:10.3969/j.issn.1007-659X.2000.04.027
Zhang, W., and Ding, J. X. (2021). Clinical effect of Shenshao Capsule in the treatment of coronary heart disease angina pectoris. J. Clin. Ration. Med. 14 (35), 38–39. doi:10.15887/j.cnki.13-1389/r.2021.35.013
Zhang, R., and Liu, Y. F. (2009). Effects of ginsenoside Rg1 on angiogenesis and cardiac function after acute myocardial infarction in rats. Chongqing Med. Sci. 38 (7), 805–807. doi:10.3969/j.issn.1671-8348.2009.07.026
Zhang, Y. L., and Zhu, X. M. (2011). Clinical observation of Yixinshu Capsule in the treatment of diastolic heart failure. J. Cardiovasc. Cerebrovasc. Dis. Integr. Traditional Chin. West. Med. 9 (3), 287–289. doi:10.3969/j.issn.1672-1349.2011.03.015
Zhang, Y. S., Li, R. Q., and Wang, Y. W. (1982). Study on ginseng polysaccharides (I). J. Northeast Normal Univ. Nat. Sci. Ed. 14 (02), 100–107. doi:10.16163/j.cnki.22-1123/n.1982.02.014
Zhang, H. G., Yan, J. C., and Wu, G. X. (1994). Study on fatty acid components in ginseng of Changbai. J. Bethune Med. Univ. 20 (4), 365. doi:10.13481/j.1671-587x.1994.04.027
Zhang, X. M., Qu, S. C., Sui, D. Y., Yu, X. F., and Lv, Z. Z. (2004). Effects of ginsenoside-Rb on blood lipid metabolism and anti-oxidation in hyperlipidemia rats. Zhongguo Zhong Yao Za Zhi 29 (11), 1085–1088. doi:10.3321/j.issn:1001-5302.2004.11.019
Zhang, Y. G., Zhang, H. G., Zhang, G. Y., Fan, J. S., Li, X. H., Liu, Y. H., et al. (2008). Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin. Exp. Pharmacol. Physiol. 35 (10), 1238–1244. doi:10.1111/j.1440-1681.2008.04997.x
Zhang, Z. L., Fan, Y., and Liu, M. L. (2012). Ginsenoside Rg1 inhibits autophagy in H9c2 cardiomyocytes exposed to hypoxia/reoxygenation. Mol. Cell. Biochem. 365 (1-2), 243–250. doi:10.1007/s11010-012-1265-3
Zhang, Y. J., Zhang, X. L., Li, M. H., Iqbal, J., Bourantas, C. V., Li, J. J., et al. (2013a). The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J. Cardiovasc. Pharmacol. 62 (1), 50–57. doi:10.1097/FJC.0b013e31828f8d45
Zhang, D. L., Liang, L. J., and Zhang, J. (2013b). Effects of ginseng polysaccharide on myocardial hypertrophy and myocardial energy metabolism in rats with abdominal aortic constriction. China Mod. Appl. Pharm. 30 (6), 571–575. doi:10.13748/j.cnki.issn1007-7693.2013.06.009
Zhang, J. F., Tang, S. W., and Wang, H. T. (2013c). Effects of Qili Qiangxin Capsule on endothelial injury and energy metabolism in rats with pressure overload heart failure. J. Traditional Chin. Med. 54 (14), 5.
Zhang, Y. S., Chen, X., and Li, Y. (2014). Research progress on the correlation between mitochondrial DNA mutations and essential hypertension. Chin. J. Multiple Organ Dis. Aged (10), 781–787. doi:10.3724/SP.J.1264.2014.000181
Zhang, L. P., Jiang, Y. C., Yu, X. F., Xu, H. L., Li, M., Zhao, X. Z., et al. (2016). Ginsenoside Rg3 improves cardiac function after myocardial ischemia/reperfusion via attenuating apoptosis and inflammation. Evid. Based Complement. Altern. Med. 2016, 6967853. doi:10.1155/2016/6967853
Zhang, X., Liu, M. H., Qiao, L., Zhang, X. Y., Liu, X. L., Dong, M., et al. (2018). Ginsenoside Rb1 enhances atherosclerotic plaque stability by skewing macrophages to the M2 phenotype. J. Cell Mol. Med. 22 (1), 409–416. doi:10.1111/jcmm.13329
Zhang, N., An, X., Lang, P., Wang, F., and Xie, Y. (2019). Ginsenoside Rd contributes the attenuation of cardiac hypertrophy in vivo and in vitro. Biomed. Pharmacother. 109, 1016–1023. doi:10.1016/j.biopha.2018.10.081
Zhang, J., Luo, D., Li, F., Li, Z., Gao, X., Qiao, J., et al. (2021). Ginsenoside Rg3 alleviates antithyroid cancer drug vandetanib-induced QT interval prolongation. Oxid. Med. Cell Longev. 2021, 3520034. doi:10.1155/2021/3520034
Zhang, Hj (2011). Research progress of traditional Chinese and Western medicine on the pathogenesis of essential hypertension. Mod. J. Integr. Traditional Chin. West. Med. 20 (19), 2465–2467. doi:10.3969/j.issn.1008-8849.2011.19.087
Zhang, Y. (2016). Anti-myocardial ischemia mechanism of compound ginseng volatile oil aerosol and its effect on hemorheology. Jilin: Changchun University of Traditional Chinese Medicine.
Zhao, M. Z., Gao, C. M., and Zhang, Y. Y. (2000). Experimental study on the protective effect of Tongxinluo capsule on experimental myocardial ischemia-reperfusion injury. China J. Basic Med. 6 (1), 36–38. doi:10.3969/j.issn.1006-3250.2000.01.013
Zheng, Q., Bao, X. Y., Zhu, P. C., Tong, Q., Zheng, G. Q., and Wang, Y. (2017a). Ginsenoside Rb1 for myocardial ischemia/reperfusion injury: Preclinical evidence and possible mechanisms. Oxid. Med. Cell. Longev. 2017, 6313625. doi:10.1155/2017/6313625
Zheng, X., Wang, S., Zou, X., Jing, Y., Yang, R., Li, S., et al. (2017b). Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp. Anim. 66 (3), 217–228. doi:10.1538/expanim.16-0121
Zhou, W., Chai, H., Lin, P. H., Lumsden, A. B., Yao, Q., and Chen, C. (2005). Ginsenoside Rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 41 (5), 861–868. doi:10.1016/j.jvs.2005.01.054
Zhou, H., Hou, S. Z., Luo, P., Zeng, B., Wang, J. R., Wong, Y. F., et al. (2011). Ginseng protects rodent hearts from acute myocardial ischemia-reperfusion injury through GR/ER-activated RISK pathway in an endothelial NOS-dependent mechanism. J. Ethnopharmacol. 135 (2), 287–298. doi:10.1016/j.jep.2011.03.015
Zhou, Q., Jiang, L., Xu, C., Luo, D., Zeng, C., Liu, P., et al. (2014). Ginsenoside Rg1 inhibits platelet activation and arterial thrombosis. Thromb. Res. 133 (1), 57–65. doi:10.1016/j.thromres.2013.10.032
Zhou, S. S., Wang, Y. Y., and Liu, D. (2015). Optimization of ultrasonic-assisted extraction of polysaccharide from ginseng flowers and its antioxidant activity. Food Sci. 36 (6), 76–81. doi:10.7506/spkx1002-6630-201506014
Zhou, P., Lu, S., Luo, Y., Wang, S., Yang, K., Zhai, Y., et al. (2017). Attenuation of TNF-α-Induced inflammatory injury in endothelial cells by ginsenoside Rb1 via inhibiting NF-κB, JNK and p38 signaling pathways. Front. Pharmacol. 8, 464. doi:10.3389/fphar.2017.00464
Zhou, P., Lu, S., Luo, Y., Wang, S., Yang, K., Zhai, Y., et al. (2017). Attenuation of TNF-α-Induced inflammatory injury in endothelial cells by ginsenoside Rb1 via inhibiting NF-κB, JNK and p38 signaling pathways. Front. Pharmacol. 8, 464. doi:10.3389/fphar.2017.00464
Zhou, P., Xie, W., Luo, Y., Lu, S., Dai, Z., Wang, R., et al. (2018). Inhibitory effects of ginsenoside Rb1 on early atherosclerosis in ApoE-/- mice via inhibition of apoptosis and enhancing autophagy. Molecules 23 (11), 2912. doi:10.3390/molecules23112912
Zhou, P., Xie, W., Sun, Y., Dai, Z., Li, G., Sun, G., et al. (2019). Ginsenoside Rb1 and mitochondria: A short review of the literature. Mol. Cell ProbesMol Cell Probes. 4354, 1101626–1101635. doi:10.1016/j.mcp.2018.12.001
Zhu, H., Luo, X. P., and Wang, L. J. (2010). Evaluation on clinical effect of long-term shexiang baoxin pill administration for treatment of coronary heart disease. China J. Integr. Traditional Chin. West. Med. 30 (5), 474–477.
Keywords: panax ginseng, ginsenoside, herbal medicine, functional components, cardiovascular disease
Citation: Liu L, Hu J, Mao Q, Liu C, He H, Hui X, Yang G, Qu P, Lian W, Duan L, Dong Y, Pan J, Liu Y, He Q, Li J and Wang J (2022) Functional compounds of ginseng and ginseng-containing medicine for treating cardiovascular diseases. Front. Pharmacol. 13:1034870. doi: 10.3389/fphar.2022.1034870
Received: 02 September 2022; Accepted: 24 November 2022;
Published: 02 December 2022.
Edited by:
Karl Tsim, Hong Kong University of Science and Technology, Hong Kong SAR, ChinaReviewed by:
Hoi Huen Chan, Hong Kong Polytechnic University, Hong Kong SAR, ChinaPrabhu Balan, Massey University, New Zealand
Ik-Hyun Cho, Kyung Hee University, South Korea
Copyright © 2022 Liu, Hu, Mao, Liu, He, Hui, Yang, Qu, Lian, Duan, Dong, Pan, Liu, He, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Wang, wangjie0103@126.com
†These authors have contributed equally to this work
 Lanchun Liu
Lanchun Liu Jun Hu1†
Jun Hu1† Qiyuan Mao
Qiyuan Mao Haoqiang He
Haoqiang He Xiaoshan Hui
Xiaoshan Hui Guang Yang
Guang Yang Peirong Qu
Peirong Qu Wenjing Lian
Wenjing Lian Lian Duan
Lian Duan Juhua Pan
Juhua Pan Yongmei Liu
Yongmei Liu Qingyong He
Qingyong He Jie Wang
Jie Wang