
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 26 October 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1028356
This article is part of the Research Topic Neuropharmacology of Neuro –degenerative, -logical, -psychiatric disorders View all 6 articles
 Komal Latif1
Komal Latif1 Aman Ullah2
Aman Ullah2 Anastasiia D. Shkodina3,4*
Anastasiia D. Shkodina3,4* Dmytro I. Boiko5
Dmytro I. Boiko5 Zakia Rafique1
Zakia Rafique1 Badrah S. Alghamdi6,7
Badrah S. Alghamdi6,7 Mohamed A. Alfaleh8,9
Mohamed A. Alfaleh8,9 Ghulam Md. Ashraf10*
Ghulam Md. Ashraf10*Given the high whittling down rates, high costs, and moderate pace of new medication, revelation, and improvement, repurposing “old” drugs to treat typical and uncommon illnesses is progressively becoming an appealing proposition. Drug repurposing is the way toward utilizing existing medications in treating diseases other than the purposes they were initially designed for. Faced with scientific and economic challenges, the prospect of discovering new medication indications is enticing to the pharmaceutical sector. Medication repurposing can be used at various stages of drug development, although it has shown to be most promising when the drug has previously been tested for safety. We describe strategies of drug repurposing for Parkinson’s disease, which is a neurodegenerative condition that primarily affects dopaminergic neurons in the substantia nigra. We also discuss the obstacles faced by the repurposing community and suggest new approaches to solve these challenges so that medicine repurposing can reach its full potential.
Drug reprofiling history goes back to 1950; however, Ted T Ashburn and Karl B. Thar were the first to introduce the inception of drug repositioning in 2004 (Langedijk et al., 2015). Initially, people were unaware of this term, although this method was practiced in the late 1990s with the repositioning of thalidomide (Emanuel Almeida Moreira de Oliveira1, 2018). It is a fact that traditional drug development is complicated and tiresome (Gupta et al., 2021). Drug reprofiling or redirecting is a very attractive, economical, and time-saving process because this approach includes adding newer indications to the previously existing drugs (Steinhagen, 2011). This approach has succeeded in reducing the total period of drug development on average by 3–12 years, as shown in Figure 1. According to one of the studies in recent years, more than 30% of the US Food and Drug Administration (FDA)-approved drugs and vaccines have undergone the drug repurposing process (Jin and Wong, 2014). This tremendous achievement has opened the doors for researchers and drug developers interested in drug repurposing (Schonfeld, 2014).
Currently, many pharmaceutical firms are involved in drug research and development and are looking for innovative and economic approaches to treat diseases in better ways (Khanna, 2012). Such firms have allocated enormous proportions of money for research and development to support drug discovery and development. In recent years, it has been observed that research and development budgets have been significant (Mizushima, 2011). The massive success in repositioning sildenafil (Viagra), one of Pfizer’s products, has proved the landmark in drug repositioning (Dhir et al., 2020). The phase I clinical trial of sildenafil had a minimal effect against angina pectoris (primarily indication) with marked penile erection (Lue, 2000). Later, in 1998, researchers considered sildenafil to be the only regimen for erectile dysfunction and marketed it in the U.S under the brand name Viagra (Srinath and Kotwal, 1999).
Similarly, thalidomide was initially withdrawn from clinical use and was later rediscovered for its secondary action (Bartlett et al., 2004). Thalidomide was developed as a sedative and recommended to pregnant women to treat morning sickness, but this drug caused severe birth skeletal abnormalities in children (Vargesson, 2019). Thalidomide was banned due to its side effects, but later on, it was rediscovered as an inhibitor of TNF-α and was used to treat the condition erythema nodosum laprosum (ENL) (Ashburn and Thor, 2004a). It is also antiangiogenic, which led to its use as an anticancer agent for treating multiple myeloma (Gillies, 2016). Hence, there is always a possibility of repurposing and rediscovering a drug (Fetro and Scherman, 2020). Ramosetron is another drug that was initially used as an antiemetic (Desai et al., 2013). Later, it was reprofiled for irritable bowel syndrome because of its side effect, constipation (Graul et al., 2009). Therefore, drug repurposing includes scientific recreation of pharmacological activities of current drugs (Ashburn and Thor, 2004b).
Parkinson’s disease is a condition that still has a lot of unclear questions about its treatment despite a long history of its study (Seppi et al., 2019). More and more drugs that have a pharmacodynamic effect on the components of the pathogenesis of PD are undergoing clinical trials in order to optimize modern therapy and search for new ways to use known drugs (Deleu et al., 2002).
Due to its associated issues, the failure of traditional drug discovery has diverted the focus toward drug reprofiling (coined as drug repurposing, drug repositioning, drug re-tasking, or therapeutic switching), which is less time-consuming, cost-practical, and more effective. As the pharmacologist and Nobel laureate James Black said, “The most fruitful basis for the discovery of a new drug is to start with an old drug” (Pantziarka et al., 2018).
Reprofiling has an extra advantage over the traditional approach as new approaches overcome major drug discovery problems (Ashburn and Thor, 2004a). The survey report of expenses utilized in reprofiling in 2007 concluded that the cost to reprofile a drug averages $8.4 million (Agrawal, 2015). The success rate of reprofiled drugs is also higher than that of the traditional approach because of the established profiles of these compounds (Pushpakom et al., 2019). According to a survey report published in 2007, only 25% of drugs from phase II and 65% from phase III clinical trials reached the market compared to new molecular entities, which are 10% and 50% (Pantziarka et al., 2018). In addition, the complete picture of the successful report from the preclinical stage to the approved status is reported in Table 1 as drug reprofiling has additional significance over standard drugs because the repurposed drug already has a different test for various toxicity and side effects (Polamreddy and Gattu, 2019). These drugs have already passed through clinical trials, which reduces the development cost for prescriptions (Sun et al., 2016). According to a recent report based on a survey of 30 pharmaceutical industries and biotechnology companies, introducing a drug again as repurposed averages $8.4 million, while the price for research and development of a new 101 molecule is very high, averaging $41.3 million (Naylor et al., 2015). They also have a higher success rate than the original drugs because of known and tested information regarding their pharmacology, formulation stability, potential toxicity, safety, and adverse effects (Wen et al., 2015). However, introducing a new drug to the market requires clinical trials, scrutinizing tests on different models, and might be a waste of time, money, and effort (Califf, 2006). Instead of submitting a new drug to the market, using medications with known indications is more favorable (Rask-Andersen et al., 2011). Developing a new drug costs roughly one billion dollars, while reprofiling takes 60% less time than developing a novel drug and is less costly (Silverdale et al., 2005a; Silveira et al., 2019a).
Companies like Pfizer, Novartis, Eli Lilly, Biovista, and SOM Biotech are involved in the drug reprofiling process (Sekhon, 2013). Due to its associated issues, traditional drug discovery failures have shifted the focus toward drug reprofiling, which is not a cost-effective, time-saving, and more effective technique (Parvathaneni et al., 2019). According to the pharmacologist and Nobel laureate James Black, “The most fruitful basis for discovering a new drug is to start with an old drug” (Pantziarka et al., 2018).
Drug re-profiling has an extra advantage compared to the traditional approach because the new methods overcome the significant problems of drug discovery (Ashburn and Thor, 2004a). According to a survey report in 2007, the average expense in reprofiling was $8.4 million (Agrawal, 2015). The established profiles of these compounds achieved the success rate of reprofiled drugs higher than that of the traditional approach (Tobinick, 2009). The survey report published in 2007 showed that only 25% of drugs from phase II and 65% from phase III clinical trials reached the market compared to new molecular entities, which are 10% and 50% (Pantziarka et al., 2018). A complete picture of the success report from preclinical to clinical trials is reported in Table 2 (Polamreddy and Gattu, 2019).
PD is the second most known neurodegenerative disease (Seppi et al., 2019). Approximately 7–10 million humans around the globe are affected by this disease, i.e., approximately one percent of the world population (Ismail et al., 2019). In North America, 0.075 million newly diagnosed individuals are added up each year to this count (Crippa et al., 2019). James Parkinson, in 1817, published an essay, “Shaking Palsy” (Pandey, 2012). Later, William Rutherford Sanders, in 1876, was the first to use the term “Parkinson” in the medical panorama (Lewis et al., 2020).
Bradykinesia is the principal feature of PD along with other motor deficits, i.e., rest tremors, gait, postural instabilities, agitation, swallowing disturbances, and slurred speech (Pandey, 2012). Non-motor co-morbidities include cognitive disorders, neuropsychological disorders, sleep disorders, orthostatic hypotension, constipation, bladder dysfunction, and sexual dysfunction (Poewe and Mahlknecht, 2009; Shkodina et al., 2022). The central issue of currently available treatment is motor response fluctuation or on–off treatment (Emanuel and Karen, 2018). Another problem encountered after a few years of treatment was patients complaining of the wear-off effect (Pandey, 2012). The exact etiology of PD remains a challenge for researchers as about 85% of idiopathic PD and only 15% are caused by a mutation in specific genes responsible for altering functions of various proteins (Kalinderi et al., 2016). The proposed etiologies are thought to arise in genetically sensitive individuals or might have environmental impacts on the molecular levels, such as insecticides, other toxins, or teratogenic causes (Emanuel and Karen, 2018). Rotenone, an insecticide and toxin MPTP, was used to induce PD in animal studies (Emanuel and Karen, 2018). PD is a neurodegenerative disorder of aging individuals with predominantly slow degradation of dopaminergic neurons in the substantia nigra pars compacta part of the brain (involved in motor function), which subsequently results in a decline in levels of the neurotransmitter dopamine in the striatum (Maiti et al., 2017). Synuclein (Lewy bodies) aggregation in the brain is the hallmark of PD isolated in 1997 (Baba et al., 1998). α-Synuclein is an essential protein, and its aggregation results in motor deficits (George and Brundin, 2015). Its post-translational modification, such as oligomerization or false synuclein aggregation, causes PD (Stoker et al., 2018). Molecular alteration and underlying causes of PD are evaluated in different studies (Zhou et al., 2008). Protein kinases and signaling pathways that are linked, tested, and assessed for relation in PD are phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) and leucine-rich repeat kinase 2 (LRRK2) (Alessi, Sammler, 2022). PINK1 and LRRK2 with associated protein kinase B (AKT) and c-Jun N-terminal kinase (JNK) signaling pathways have proven to be strong footings in PD (Mehdi et al., 2016). α-Synuclein (SNCA) proteins are produced by soma cells and play a prime role in the pathophysiology of PD (Stefanis, 2012). Usually, α-synuclein is distributed in the axon and stays in nerve terminals (Uchihara and Giasson, 2016). They function as the maintenance of synaptic balance and transmission of nerve impulses (Bendor et al., 2013). Synuclein is a protein with three domains: the (amino) N-terminal domain, hydrophobic domain, and (carboxyl) C-terminal domain (Jagannatha Rao, 2007). The hydrophobic domain, also known as NAC, is essential for the conversion of synuclein to an oligomer; in addition, it is believed to mediate a conformational change to the random coil to the beta-sheet structure upon aggregation (Uversky and Eliezer, 2009). The presence of the NAC region in α-synuclein discriminates it from beta- and gamma synuclein, and it is responsible for the induction of accumulation of these proteins (Brás and Outeiro, 2021).
Cellular homeostasis involves protein degradation through the ubiquitin–proteasomal system (UPS) and different types of autophagy (Tolosa, 2010). Chaperone-mediated autophagy (LeWitt et al., 2007) pathways are involved in α-synuclein elimination under normal conditions (Tolosa, 2010). The SNCA sequence at the 95–99 residue VKKDQ configuration resembles the lysosomal surface receptor LAMP-2A (Tolosa, 2010). However, due to mutation in α-synuclein, binding and autophagy through lysosomes are disturbed, and they begin to oligomerize and aggregate within neurons (Gorman, 2008). Accumulation of α-synuclein in a considerable amount results in Lewy bodies, and neurons gradually become less functional and disappear as in PD pathogenesis, the neuron count in the substantia nigra is decreased (Fahn, 2003). Mutations in LRRK2 genes also play a significant role in PD pathogeneses (Rocha et al., 2022). It has domains like protein kinase and GTPase, the later environment being dominant in pathological changes (Taylor and Alessi, 2020). Phosphorylation of a group of RAB proteins by LRRK2 causes radical changes in essential aspects of autophagy and lysosomal physiology (Alessi and Sammler, 2022). LRRK2 mutations encompass almost all PD categories, like familial PD, idiopathic late-onset PD, autosomal dominantly inherited PD, and sporadic PD (Mehdi et al., 2016).
The second most typical cause of falling recessive PD is an alteration in PTEN-induced PINK1, commonly termed DJ-1 (Balestrino and Schapira, 2020). It is responsible for handling mitochondrial DNA levels, ATP production, calcium handling, and regulating free radical generation, and alteration in these functions can lead to apoptosis (Schapira, 2008). This change in PINK1 causes a reduction in the kinase activity related to atypical PD and causes the early age onset and slow progression of the disease (Valente et al., 2004). Alteration in PINK1 functionalities is also linked to familial juvenile PD around 1–8% (Myhre et al., 2008).
One of the molecular pathways of PD pathogenesis is oxidative stress, which is caused by the accumulation of reactive oxygen species (ROS) because of a deficiency in antioxidant systems that leads to cell death, including apoptosis, parthanatos, necroptosis, and autophagic cell death (Trist et al., 2019). Some genetic risk factors are also associated with mitochondrial dysfunction in dopaminergic neurons, which makes a significant contribution to the development of oxidative stress in PD (Dias et al., 2013). This complexity and multidimensionality of the pathogenesis of PD make it difficult to find an appropriate drug therapy (Krüger et al., 2017).
There are currently no disease-modifying treatments for PD, and dopaminergic medications constitute the mainstay of treatment (Stoker et al., 2018). Preparations of levodopa, the precursor of dopamine, are the most widely utilized, and they are given in combination with a dopa-decarboxylase inhibitor to reduce some of the side effects, such as nausea (Deleu et al., 2002). Ropinirole and rotigotine, which are dopamine agonists, are also used (LeWitt et al., 2007). Endogenous dopamine metabolism can be slowed using monoamine oxidase B inhibitors like rasagiline and selegiline, as well as catechol-O methyltransferase (COMT) inhibitors like entacapone (Chen and Swope, 2007). Treatments for PD can restore dopaminergic function in the striatum, resulting in improvements in motor symptoms (Calabresi et al., 2000). They do not, however, cure many non-motor symptoms, which are very disabling for many individuals (Pfeiffer, 2016). Some non-motor symptoms, such as postural hypotension and neuropsychiatric issues, may be exacerbated by therapy in a few cases (Worth, 2013).
The majority of people who receive dopamine replacement medication suffer aberrant involuntary movements, such as L-DOPA-induced dyskinesia (Myhre et al., 2008). It is debilitating, and there is only one drug that can help, amantadine (Buck and Ferger, 2010). Repurposing compounds that have been shown to be safe in humans at phase II or higher can be a very efficient way to get new therapies to patients quickly (Schein, 2020). Repurposing avoids many high-risk phases of the drug development process (Rudrapal et al., 2020). During repurposing, development for a further indication at phase IIa is significantly less costly, takes as little as 4 years, and has an ∼3,000 times higher chance of reaching patients than a novel drug (Singh et al., 2020). We focus on historical and modern techniques to discover possible repurposed medications, propose mechanisms to prioritize the testing of new compounds, and highlight hurdles, particularly in the translation from preclinical testing to phase II clinical proof-of-concept studies (Crippa et al., 2019).
Antidyskinetic effects of NMDA antagonists were described in animal models of PD, including the MPTP-lesioned non-human primate, around 30 years after the discovery of amantadine (NHP) (Jakowec and Petzinger, 2004). These findings prompted a re-evaluation of amantadine’s effects in PD, and two separate groups reported a reduction in L-DOPA-induced dyskinesia in patients on amantadine in 1998, arguing for the drug’s usage as an antidyskinetic agent (Myhre et al., 2008). The off-label use of immediate-release amantadine has been shown to provide significant relief of LID in up to one-third of patients (Vijverman and Fox, 2014). In some individuals, long-term amantadine use, at least, to date, in the immediate release form may be compromised by tachyphylaxis, which has been reported to occur as early as 6 months of usage (Deleu et al., 2002). Long-term use does, however, provide clinical benefits for many patients (Wolf et al., 2010). Amantadine is also poorly tolerated because of cognitive issues, like confusion and hallucinations, and some non-cognitive side effects, like ankle edema (Jackson et al., 2009). It is not appropriate for individuals with renal failure (deVries et al., 2019). Relatively better tolerability of the extended-release amantadine is observed by the once-daily dosing at night, but long-term clinical use is yet required to confirm this proposition (Sharma et al., 2018).
With a better understanding of the CB1 cannabinoid receptor’s role in the control of basal ganglia transmission, another possible repurposing candidate was identified in the mid-1990s (Johnston et al., 2019). Indeed, the CB1 agonist nabilone, which is used to treat chemotherapy-related nausea, was demonstrated to diminish LID in NHPs that had been lesioned with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Johnston et al., 2019). These findings, however, have not led to widespread usage of nabilone in LID due to non-efficacy concerns (Johnston et al., 2019). It was hypothesized that focusing on it would alter firing patterns and lower LID in a way that had previously been validated, albeit more invasively, with deep-brain stimulation (Heumann et al., 2014). As a result of this idea, the anticonvulsant levetiracetam was identified as a potential repurposing candidate (Bezard et al., 2004). In the MPTP-NHP model, levetiracetam activates SV2A and exhibits strong antidyskinetic efficacy (Johnston et al., 2019). However, because the drug was poorly tolerated in the PD patient population, these improvements could not be converted into effectiveness in phase II trials (Wong et al., 2011). Nabilone and levetiracetam are two examples of repurposing drugs that emphasize the relevance of efficacy and tolerability (Crippa et al., 2019).
Exenatide, a well-known diabetic medication for type 2 diabetes and a glucagon-like peptide-1 (GLP-1) agonist, and nilotinib, a tyrosine kinase inhibitor, have both recently been repurposed and tested in PD patients (Fletcher et al., 2021). At the same time, nilotinib is used to treat chronic myelogenous leukemia; thus, data on their safety and tolerability in patient populations already exist, which has aided their advancement through clinical studies, which have shown promising results (Athauda and Foltynie, 2018). In toxin-based mouse models of nigrostriatal degeneration, exenatide has been demonstrated to have neuroprotective and neurorestorative effects, enhancing motor function, behavior, learning, and memory (Athauda and Foltynie, 2015). Nilotinib has been shown to improve misfolded α-synuclein, making it a promising candidate for lowering SNCA levels via autophagy (Pagan et al., 2016). PD has been linked to higher levels of c-abl, which is thought to enhance the phosphorylation and aggregation of SNCA (Lindholm et al., 2016). Furthermore, an increase in the c-abl activity reduces the action of parkin, a key protein in mitochondrial biogenesis whose mutations cause familial PD (Brahmachari et al., 2017). Nilotinib has been shown to attenuate exogenously expressed SNCA levels in mice and reduce SNCA-induced nigral degeneration (Wong and Krainc, 2017). However, because there was no placebo group in this study and significant baseline differences between the two small groups, it was impossible to comment on any potential clinical benefits of the medicine (Espay et al., 2020). Despite the promising results of preclinical research and the fact that another trial (NILO-PD) is now underway in the United States, there is no convincing evidence of nilotinib’s efficacy in PD patients (Stoker et al., 2018).
The central nervous system (CNS) is the most important and crucial area for drug repositioning due to its complicated pathophysiology, complex anatomy, and extra barriers that make it difficult to understand (Messick et al., 1985). So the exact mechanism of action of already established drugs for CNS disorders is not clearly understood (Gilroy et al., 2004). CNS is being researched continuously to understand receptor profiling and the mode of action of already developed and marketed drugs to address these problems (Anighoro et al., 2014). The prevalence rate of neurodegenerative disorders is much more in the world population (Chandra et al., 2006). Still, the drug discovery and development of these disorders is shallow and does not meet the needs of the people (Ekins et al., 2019). So to cope with this world’s worst dilemma, it is the need of the hour to discover new therapies (Ashburn and Thor, 2004a). Drug repositioning can address this issue by finding new drug therapies and better combinations of drugs for increasing efficacy and decreasing side effects (Sun et al., 2016). This review discusses the historical and current status of FDA-approved repositioned medicines for PD, focusing on new approaches to identify potential drugs that can be repurposed and identifying their mechanism of action. We know that PD is the second most prevalent neurodegenerative disorder (Seppi et al., 2019). According to the previous literature review, more than 6 million people are affected by PD worldwide (Nadim et al., 2020). There is an intense demand to find therapies that will prevent and slow the extension of this progressive and chronic condition, which significantly affects the patient’s quality of life (Athauda and Foltynie, 2018). The already established treatment regimens for PD had some direct side effects, so new agent development through repositioning is inevitable due to the ease of work, reduced cost, and evolution time (Nussbaum, 2002). FDA-approved repositioned drugs for PD are mentioned in Table 3.
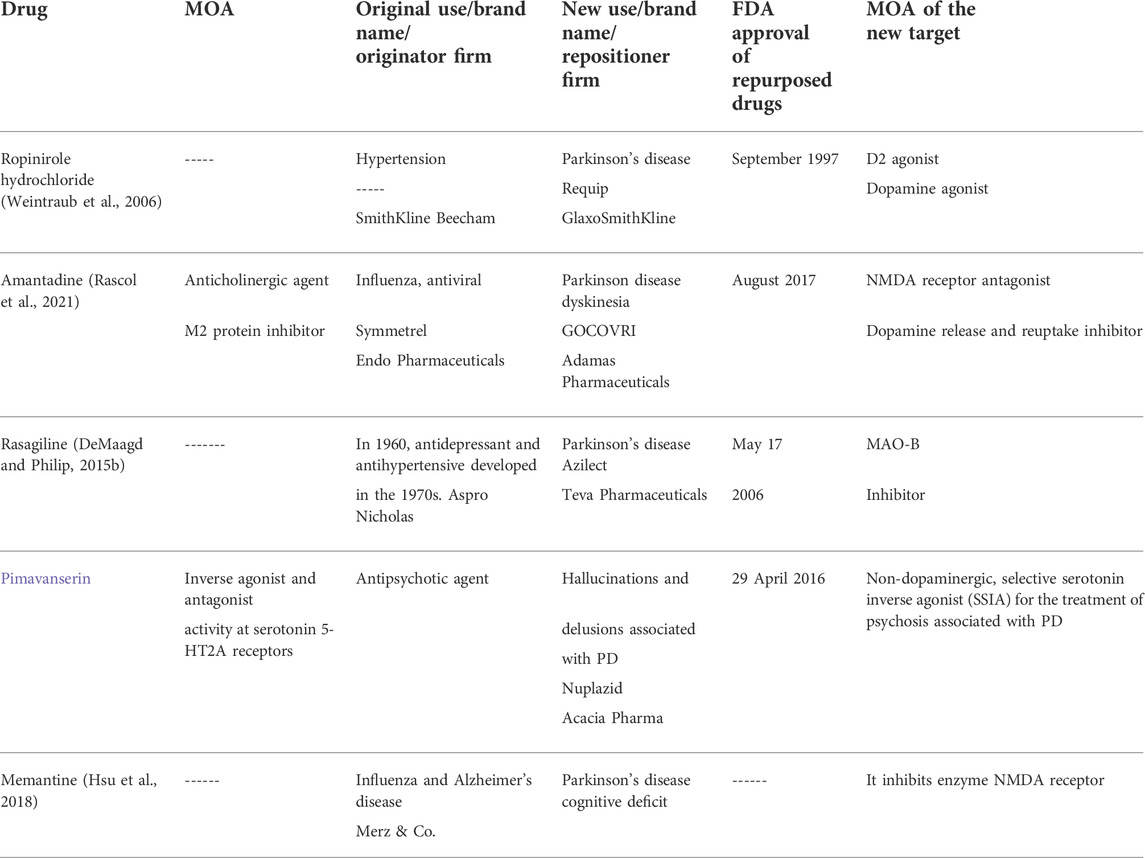
TABLE 3. Repurposed drugs reported in the literature with FDA-approved status for the treatment of Parkinson’s disease.
Ropinirole hydrochloride is one of the several ergoline D-2 receptor agonists (Kaye and Nicholls, 2000). SmithKline Beecham first developed it for hypertension; then, it was repositioned by GSK and approved by the FDA in 1997 for early and later PD (Zesiewicz and Hauser, 1999). Dopamine agonist drugs act by mimicking levodopa in the brain and improve problems associated with levodopa use (Antonini and Tolosa, 2009). Levodopa is the principal drug used for PD treatment (Fiala et al., 2003). Although it is the most potent therapy, its side effects include dyskinesias (involuntary muscle movement) and “on–off” symptoms, which are troublesome in long-term use (Thanvi et al., 2007). Alternatives that delay or reduce exposure to levodopa have been explored to improve the patient’s quality of life and reduce the risk of side effects (Schapira, 2005). To address levodopa-induced dyskinesia, ropinirole was successively repositioned for PD (Nyholm, 2003). Nowadays, according to NICE guidelines, dopamine agonists and monoamine oxidase B (MAO-B) inhibitors may be used for the correction of motor deficits in case it does not impact the quality of life (Stocchi et al., 2015).
The FDA approved amantadine in October 1966 as a prophylactic agent against influenza (Douglas, 1982). The exact mechanism by which it exerts its antiviral activity is unknown. However, it is believed to prevent the release of viral nucleic acid into the host cell by inhibiting the M2 viral protein (Skehel et al., 1978). During the 2009 pandemic flu season, the Centers for Disease Control and Prevention (CDC) found flu samples 100% resistant to amantadine (Dapat et al., 2012). This drug was accidentally discovered to be reducing symptoms of PD in 1969 (Hubsher et al., 2012). Amantadine hydrochloride (the antidyskinetic agent) was repositioned by Adamas Pharmaceuticals and approved by the FDA for treating dyskinesia in PD patients receiving levodopa-based therapy (Sharma et al., 2018). In August 2017, the FDA had approved the first and only drug for treating dyskinesia in PD patients (Chen et al., 2020). Amantadine treats dyskinesia by blocking the NMDA receptor, thus decreasing the inactivation of dopamine and blocking presynaptic dopamine reuptake, and prolonging its adequate time (Schaeffer et al., 2014). These repurposed molecules have proven safe in humans and can be a highly efficient method of rapidly bringing new treatments to patients (O'Connor and Roth, 2005). Repurposing bypasses many high-risk phases of the drug development process (Shineman et al., 2014).
In early 1970, Aspro Nicholas first invented and patented rasagiline for hypertension (Entzeroth and Ratty, 2017). But in mid-2006, while identifying a potential repurposing candidate in the case of PD, the MAO inhibitor role of rasagiline was discovered (Guay, 2006). Rasagiline was identified as an MAO-B inhibitor effective as monotherapy (Fiedorowicz and Swartz, 2004). MAO-B inhibitors can be prescribed as adjuvant therapy for motor symptoms, and it is supposed that they have a lower risk of hallucinations than dopamine agonists (Oertel and Schulz, 2016). Since the accumulation of SNCA aggregates leads to an increase in oxidative stress, mitochondrial dysfunction, and apoptosis, the use of rasagiline in PD is pathogenetically determined (Dias et al., 2013). It has been researched to have a powerful neuroprotective function: regulation of the mitochondrial apoptosis system, maintenance of the mitochondrial function, and increased expression of antioxidant enzyme genes (Naoi et al., 2020). The management of PD is relatively easy at the initial stages of the disease, where all dopamine-mimetic dopamine and drugs and amantadine or selegiline (or an antimuscarinic agent if the tremor is the main problem) can be very productive (Birkmayer and Riederer, 2012). As the disease progresses and these agents become insufficient, levodopa can be added (DeMaagd and Philip, 2015a). They compensate for the primary deficiency in PD and the decreased dopamine levels in the brain (Wu and Hallett, 2013).
PD is a complicated disease, and until now, there are no disease-modifying treatments for PD (Warren Olanow and Kieburtz, 2010). Supportive therapies exist, like physiotherapy, medication (dopamine), and surgery, in rare cases, but still, there is a need for safer and more effective pharmacological treatments for psychosis in PD (Maiti et al., 2017). Figure 2 illustrates a complete list of drugs tested in clinical trials.
PD is a progressive concern in our society. There are, however, still numerous obstacles on the way to discovering methods for a cure. This literature review intended to give an overview of PD repositioned drugs that are currently in clinical trials and approved and are in use for PD. The aforementioned tables have comprehensive descriptions, including MOA and original, repurposed indications for each drug. We have demonstrated drugs that have already been repurposed and are suitable for PD, emphasizing the importance of finding disease-modifying therapies for PD. New drugs that have, in their pharmacodynamic effects, directed at the components of the pathogenesis of PD can be successfully studied as such therapy.
KL and AU: conceptualization, writing—original draft, and visualization. ZR and BA: writing—original draft. AS and DB: writing–review and editing. MA and GA: writing—original draft, writing—review and editing, supervision, and project administration.
This research work was funded by the Institutional Fund Projects under the grant no. IFPDP-10-22. Therefore, the authors gratefully acknowledge technical and financial support from the Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia. The work in Ukrainian institutions was supported by Poltava State Medical University (research project nos. 0121U108235 and 0119U102848).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aaseth, J., Dusek, P., and Roos, P. M. (2018). Prevention of progression in Parkinson's disease. Biometals 31 (5), 737–747. doi:10.1007/s10534-018-0131-5
Agrawal, P. (2015). Advantages and challenges in drug Re-profiling. J. Pharmacovigil. 383, s2. doi:10.4172/2329-6887.s2-e002
Alquraan, L., Alzoubi, K. H., Hammad, H., Rababa'h, S. Y., and Mayyas, F. (2019). Omega-3 fatty acids prevent post-traumatic stress disorder-induced memory impairment. Biomolecules 9 (3), E100. doi:10.3390/biom9030100
Anighoro, A., Bajorath, J., and Rastelli, G. (2014). Polypharmacology: Challenges and opportunities in drug discovery.discovery: Miniperspective. J. Med. Chem. 57 (19), 7874–7887. doi:10.1021/jm5006463
Antonini, A., and Tolosa, E. (2009). Apomorphine and levodopa infusion therapies for advanced Parkinson's disease: Selection criteria and patient management. Disease: Selection criteria and patient management. Expert Rev. Neurother. 9 (6), 859–867. doi:10.1586/ern.09.48
Ashburn, T. T., and Thor, K. B. (2004). Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3 (8), 673–683. doi:10.1038/nrd1468
Ashburn, T. T., and Thor, K. B. (2004). Drug repositioning: Identifying and developing new uses for existing, 3, 673–683.drugsNat. Rev. Drug Discov.8
Athauda, D., and Foltynie, T. (2018). Drug repurposing in Parkinson's disease. CNS Drugs 32 (8), 747–761. doi:10.1007/s40263-018-0548-y
Athauda, D., and Foltynie, T. (2015). The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 11 (1), 25–40. doi:10.1038/nrneurol.2014.226
Baba, M., Nakajo, S., Tu, P-H., Tomita, T., Nakaya, K., Lee, V., et al. (1998). Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am. J. Pathol. 152 (4), 879–884.
Balestrino, R., and Schapira, A. (2020). Parkinson disease. Eur. J. Neurol. 27 (1), 27–42. doi:10.1111/ene.14108
Bartlett, J. B., Dredge, K., and Dalgleish, A. G. (2004). The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat. Rev. Cancer 4 (4), 314–322. doi:10.1038/nrc1323
Bell, S. M., Barnes, K., Clemmens, H., Al-Rafiah, A. R., Al-Ofi, E. A., Leech, V., et al. (2018). Ursodeoxycholic acid improves mitochondrial function and redistributes Drp1 in fibroblasts from patients with either sporadic or familial alzheimer's disease. J. Mol. Biol. 430 (21), 3942–3953. doi:10.1016/j.jmb.2018.08.019
Bendor, J. T., Logan, T. P., and Edwards, R. H. (2013). The function of α-synuclein. Neuron 79 (6), 1044–1066. doi:10.1016/j.neuron.2013.09.004
Bezard, E., Hill, M. P., Crossman, A. R., Brotchie, J. M., Michel, A., Grimée, R., et al. (2004). Levetiracetam improves choreic levodopa-induced dyskinesia in the MPTP-treated macaque. improves choreic levodopa-induced dyskinesia in the MPTP-treated macaque. Eur. J. Pharmacol. 485 (1-3), 159–164. doi:10.1016/j.ejphar.2003.11.065
Birkmayer, W., and Riederer, P. (2012). Parkinson’s disease: Biochemistry, clinical pathology, and treatment.
Bortolanza, M., Nascimento, G. C., Socias, S. B., Ploper, D., Chehin, R. N., Raisman-Vozari, R., et al. (2018). Tetracycline repurposing in neurodegeneration: Focus on Parkinson's disease. J. Neural Transm. 125 (10), 1403–1415. doi:10.1007/s00702-018-1913-1
Bourque, M., Morissette, M., and Di Paolo, T. (2019). Repurposing sex steroids and related drugs as potential treatment for Parkinson's disease. Neuropharmacology 147, 37–54. doi:10.1016/j.neuropharm.2018.04.005
Brahmachari, S., Karuppagounder, S. S., Ge, P., Lee, S., Dawson, V. L., Dawson, T. M., et al. (2017). c-Abl and Parkinson's Disease: Mechanisms and Therapeutic Potential. Parkinson’s disease: mechanisms and therapeutic potential. J. Park. Dis. 7 (4), 589–601. doi:10.3233/JPD-171191
Brás, I. C., and Outeiro, T. F. (2021). Alpha-Synuclein: Mechanisms of release and pathology progression in synucleinopathies.n synucleinopathies. Cells. Cells 10 (2), 375. doi:10.3390/cells10020375
Buck, K., and Ferger, B. (2010). L-DOPA-induced dyskinesia in Parkinson's disease: A drug discovery perspective.perspective. Drug Discov. Today 15 (19-20), 867–875. doi:10.1016/j.drudis.2010.08.014
Calabresi, P., Centonze, D., and Bernardi, G. (2000). Electrophysiology of dopamine in normal and denervated striatal neurons. striatal neurons. Trends Neurosci. 23, S57–S63. doi:10.1016/s1471-1931(00)00017-3
Califf, R. M. (2006). Clinical trials bureaucracy: Unintended consequences of well-intentioned policy. Clin. Trials 3 (6), 496–502. doi:10.1177/1740774506073173
Cankaya, S., Cankaya, B., Kilic, U., Kilic, E., and Yulug, B. (2019). The therapeutic role of minocycline in Parkinson's disease. Drugs Context 8, 212553. doi:10.7573/dic.212553doi:212553
Chandra, V., Pandav, R., Laxminarayan, R., Tanner, C., Manyam, B., Rajkumar, S., et al. Neurological disorders. Dis. control priorities. 2006: 21.
Chen, J-F., Cunha, R. A., and istradefylline for treatment of Parkinson’s disease, (2020). The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson's disease. Purinergic Signal. 16, 167–174. doi:10.1007/s11302-020-09694-2
Chen, J. J., and Swope, D. M. (2007). Pharmacotherapy for Parkinson's disease. Pharmacother. Hum. Pharmacol. Drug Ther. 27 (12P2), 161S-173S–73S. doi:10.1592/phco.27.12part2.161S
Crippa, J. A. S., Hallak, J. E. C., Zuardi, A. W., Guimaraes, F. S., Tumas, V., and Dos Santos, R. G. (2019). Is cannabidiol the ideal drug to treat non-motor Parkinson's disease symptoms? Eur. Arch. Psychiatry Clin. Neurosci. 269 (1), 121–133. doi:10.1007/s00406-019-00982-6
da Silva, T. M., Munhoz, R. P., Alvarez, C., Naliwaiko, K., Kiss, A., Andreatini, R., et al. (2008). Depression in Parkinson's disease: A double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J. Affect. Disord. 111 (2-3), 351–359. doi:10.1016/j.jad.2008.03.008
Dapat, I. C., Dapat, C., Baranovich, T., Suzuki, Y., Kondo, H., Shobugawa, Y., et al. (2012). Genetic characterization of human influenza viruses in the pandemic (2009-2010) and post-pandemic (2010-2011) periods in Japan. characterization of human influenza viruses in the pandemic (2009–2010) and post-pandemic (2010–2011) periods in Japan. PloS one 7 (6), e36455. doi:10.1371/journal.pone.0036455
Deleu, D., Northway, M. G., and Hanssens, Y. Clinical pharmacokinetic and pharmacodynamic properties of drugs used in the treatment of Parkinson's disease. properties of drugs used in the treatment of Parkinson’s disease. Clin. Pharmacokinet. 380 2002; 41, (4): 261–309. doi:10.2165/00003088-200241040-00003
Delgobo, M., Agnes, J. P., Gonçalves, R. M., dos Santos, V. W., Parisotto, E. B., Zamoner, A., et al. (2019). N-acetylcysteine and alpha-lipoic acid improve antioxidant defenses and decrease oxidative stress, inflammation and serum lipid levels in ovariectomized rats via estrogen-independent mechanisms. J. Nutr. Biochem. 67, 190–200. doi:10.1016/j.jnutbio.2019.02.012
DeMaagd, G., and Philip, A. (2015). Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P Trans. 40 (8), 504–532.
DeMaagd, G., and Philip, A. (2015). Parkinson’s disease and its management: Part 3: Nondopaminergic and nonpharmacological treatment options. P Trans. 40 (10), 668–679.
Desai, S., Santosh, M., Annigeri, R., Santoshi, V., and Rao, R. (2013). Comparison of the antiemetic effect of ramosetron with the combination of dexamethasone and ondansetron in middle ear surgery: A double-blind, randomized clinical study. Ramosetron with the combination of dexamethasone and ondansetron in middle ear surgery: A double-blind, randomized clinical study. Saudi J. Anaesth. 7 (3), 254–258. doi:10.4103/1658-354X.115328
deVries, T., Dentiste, A., Di Lea, C., Pichette, V., and Jacobs, D. (2019). Effects of renal impairment on the pharmacokinetics of once-daily amantadine extended-release tablets. Pharmacokinetics of once-daily amantadine extended-release tablets. CNS drugs 33 (8), 783–789. doi:10.1007/s40263-019-00651-1
Dhir, N., Jain, A., Mahendru, D., Prakash, A., and Medhi, B. (2020). Drug repurposing and orphan disease therapeutics. Drug Repurposing-Hypothesis. Mol. Aspects Ther. Appl. IntechOpen.
Dias, V., Junn, E., and Mouradian, M. M. (2013). The role of oxidative stress in Parkinson's disease. J. Park. Dis. 3 (4), 461–491. doi:10.3233/JPD-130230
Eberling, J., Pivirotto, P., Bringas, J., Steiner, J. P., Kordower, J. H., Chu, Y., et al. (2002). The immunophilin ligand GPI-1046 does not have neuroregenerative effects in MPTP-treated monkeys. Exp. Neurol. 178 (2), 236–242. doi:10.1006/exnr.2002.8023
Ekins, S., Puhl, A. C., Zorn, K. M., Lane, T. R., Russo, D. P., Klein, J. J., et al. (2019). Exploiting machine learning for end-to-end drug discovery and development. for end-to-end drug discovery and development. Nat. Mat. 18 (5), 435–441. doi:10.1038/s41563-019-0338-z
Emanuel Almeida Moreira de Oliveira1, K. L. L. (2018). Drug repositioning: Concept, classification, methodology, and importance in rare/orphans and neglected diseases. J. Appl. Pharm. Sci., 157–165.
Emanuel, A. MdO., and Karen, Luise Lang (2018). Drug repositioning: Concept, classification, methodology, and importance in rare/orphans and neglected diseases. J. Appl. Pharm. Sci., 157–165.
Entzeroth, M., and Ratty, A. K. (2017). Monoamine oxidase inhibitors—Revisiting a therapeutic principle. Open J. Depress. 6 (02), 31–68. doi:10.4236/ojd.2017.62004
Espay, A. J., Hauser, R. A., and Armstrong, M. J. (2020). The narrowing path for nilotinib and other potential disease-modifying therapies for Parkinson disease. Disease-modifying therapies for Parkinson disease. JAMA Neurol. 77 (3), 295–297. doi:10.1001/jamaneurol.2019.3983
Fahn, S. (2003). Description of Parkinson's disease as a clinical syndrome. Ann. N. Y. Acad. Sci. Acad. Sci. 991, 1–14. doi:10.1111/j.1749-6632.2003.tb07458.x
Fetro, C., and Scherman, D. (2020). Drug repurposing in rare diseases: Myths and reality. Therapie 75 (2), 157–160. doi:10.1016/j.therap.2020.02.006
Fiala, K. H., Whetteckey, J., and Manyam, B. V. (2003). Malignant melanoma and levodopa in Parkinson's disease: Causality or coincidence? Park. Relat. Disord. 9 (6), 321–327. doi:10.1016/s1353-8020(03)00040-3
Fiedorowicz, J. G., and Swartz, K. L. (2004). The role of monoamine oxidase inhibitors in current psychiatric practice. J. Psychiatr. Pract. 10 (4), 239–248. doi:10.1097/00131746-200407000-00005
Fletcher, E. J., Kaminski, T., Williams, G., and Duty, S. (2021). Drug repurposing strategies of relevance for Parkinson's disease. Parkinson’s disease. Pharmacol. Res. Perspect. 9 (4), e00841. doi:10.1002/prp2.841
George, S., and Brundin, P. (2015). Immunotherapy in Parkinson's disease: Micromanaging alpha-synuclein aggregation. Aggregation. J. Park. Dis. 5 (3), 413–424. doi:10.3233/JPD-150630
Gilroy, D. W., Lawrence, T., Perretti, M., and Rossi, A. G. (2004). Inflammatory resolution: New opportunities for drug discovery. Drug discovery. Nat. Rev. Drug Discov. 3 (5), 401–416. doi:10.1038/nrd1383
Gorman, A. M. Neuronal cell death in neurodegenerative diseases: Recurring themes around protein handling. J. Cell. Mol. Med. 2008; 12, (6a):2263–2280. doi:10.1111/j.1582-4934.2008.00402.x
Graul, A. I., Revel, L., Barrionuevo, M., Cruces, E., Rosa, E., Verges, C., et al. (2009). The year's new drugs & biologics - 2008. biologics—2008. Drug News Perspect. 22 (1), 7–29. doi:10.1358/dnp.2009.22.1.1303754
Guay, D. R. (2006). Rasagiline (TVP-1012): A new selective monoamine oxidase inhibitor for Parkinson's disease. Am. J. Geriatr. Pharmacother. 4 (4), 330–346. doi:10.1016/j.amjopharm.2006.12.001
Gupta, R., Srivastava, D., Sahu, M., Tiwari, S., Ambasta, R. K., and Kumar, P. (2021). Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 25(3), 1315–1360. doi:10.1007/s11030-021-10217-3
Heumann, R., Moratalla, R., Herrero, M. T., Chakrabarty, K., Drucker‐Colín, R., Garcia‐Montes, J. R., et al. (2014). Dyskinesia in Parkinson's disease: Mechanisms and current non‐pharmacological interventions. J. Neurochem. 130 (4), 472–489. doi:10.1111/jnc.12751
Hsu, W-Y., Lane, H-Y., and Lin, C-H. (2018). Medications used for cognitive enhancement in patients with schizophrenia, bipolar disorder, Alzheimer’s disease, and Parkinson’s disease. Front. Psychiatry 9, 91. doi:10.3389/fpsyt.2018.00091
Hubsher, G., Haider, M., and Okun, M. (2012). Amantadine: The journey from fighting flu to treating Parkinson disease. Neurology 78 (14), 1096–1099. doi:10.1212/WNL.0b013e31824e8f0d
Ismail, N. H., Du, M., Martinez, D., and He, Z. (2019). Multivariate multi-step deep learning time series approach in forecasting Parkinson's disease future severity progression. Proceedings of the 10th ACM International Conference on Bioinformatics, Computational Biology and Health Informatics.
Jackson, S., Jansen, P., and Mangoni, A. (2009). Prescribing for elderly patients. John Wiley & Sons. NJ, USA,
Jagannatha Rao, K. (2007). Studies on alpha-synuclein aggregation and the effect of dietary curcumin derivative: Relevance to Parkinson’s disease. Mysuru, Karnataka: University of Mysore.
Jakowec, M. W., and Petzinger, G. M. (2004). 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned model of Parkinson's disease, with emphasis on mice and nonhuman primates. of Parkinson's disease, with emphasis on mice and nonhuman primates. Comp. Med. 54 (5), 497–513.
Jin, G., and Wong, S. T. (2014). Toward better drug repositioning: Prioritizing and integrating existing methods into efficient pipelines. Methods into efficient pipelines. Drug Discov. Today 19 (5), 637–644. doi:10.1016/j.drudis.2013.11.005
Johnston, T. H., Lacoste, A. M., Visanji, N. P., Lang, A. E., Fox, S. H., and Brotchie, J. M. (2019). Repurposing drugs to treat l-DOPA-induced dyskinesia in Parkinson's disease. treat l-DOPA-induced dyskinesia in Parkinson's disease. Neuropharmacology 147, 11–27. doi:10.1016/j.neuropharm.2018.05.035
Kalinderi, K., Bostantjopoulou, S., and Fidani, L. (2016). The genetic background of Parkinson's disease: Current progress and future prospects. Current progress and future prospects. Acta Neurol. Scand. 134 (5), 314–326. doi:10.1111/ane.12563
Kamatani, N., Kushiyama, A., Toyo-Oka, L., and Toyo-Oka, T. (2019). Treatment of two mitochondrial disease patients with a combination of febuxostat and inosine that enhances cellular ATP. J. Hum. Genet. 64 (4), 351–353. doi:10.1038/s10038-018-0558-0
Kaye, C. M., and Nicholls, B. (2000). Clinical pharmacokinetics of ropinirole. Clin. Pharmacokinet. 39 (4), 243–254. doi:10.2165/00003088-200039040-00001
Khanna, I. (2012). Drug discovery in pharmaceutical industry: Productivity challenges and trends. Drug Discov. Today 17 (19-20), 1088–1102. doi:10.1016/j.drudis.2012.05.007
Krüger, R., Klucken, J., Weiss, D., Tönges, L., Kolber, P., Unterecker, S., et al. (2017). Classification of advanced stages of Parkinson's disease: Translation into stratified treatments.advanced stages of Parkinson’s disease: Translation into stratified treatments. J. Neural Transm. 124 (8), 1015–1027. doi:10.1007/s00702-017-1707-x
Langedijk, J., Mantel-Teeuwisse, A. K., Slijkerman, D. S., and Schutjens, M. H. (2015). Drug repositioning and repurposing: Terminology and definitions in literature. Repurposing: Terminology and definitions in literature. Drug Discov. Today 20 (8), 1027–1034. doi:10.1016/j.drudis.2015.05.001
Lewis, P. A., Plun-Favreau, H., Rowley, M., and Spillane, J. (2020). Pierre D. And the first photographs of Parkinson's disease Parkinson's disease. Mov. Disord. 35 (3), 389–391. doi:10.1002/mds.27965
LeWitt, P. A., Boroojerdi, B., MacMahon, D., Patton, J., and Jankovic, J. (2007). Overnight switch from oral dopaminergic agonists to transdermal rotigotine patch in subjects with Parkinson disease. dopaminergic agonists to transdermal rotigotine patch in subjects with Parkinson disease. Clin. Neuropharmacol. 30 (5), 256–265. doi:10.1097/wnf.0b013e318154c7c4
Lindholm, D., Pham, D. D., Cascone, A., Eriksson, O., Wennerberg, K., and Saarma, M. (2016). c-Abl Inhibitors Enable Insights into the Pathophysiology and Neuroprotection in Parkinson's Disease. enable insights into the pathophysiology and neuroprotection in Parkinson’s disease. Front. Aging Neurosci. Neurosci. 8, 254. doi:10.3389/fnagi.2016.00254
Liss, B., and Striessnig, J. (2019). The potential of L-type calcium channels as a drug target for neuroprotective therapy in Parkinson's disease. Annu. Rev. Pharmacol. Toxicol. 59 (7), 263–289. doi:10.1146/annurev-pharmtox-010818-021214
Lue, T. F. (2000). Erectile dysfunction. N. Engl. J. Med. 342 (24), 1802–1813. doi:10.1056/NEJM200006153422407
Magistrelli, L., and Comi, C. (2019). Beta2-Adrenoceptor agonists in Parkinson’s disease and other synucleinopathies. J. Neuroimmune Pharmacol. 15, 74–81. doi:10.1007/s11481-018-09831-0
Maiti, P., Manna, J., and Dunbar, G. L. (2017). Current understanding of the molecular mechanisms in Parkinson's disease: Targets for potential treatments. Transl. Neurodegener. 6 (1), 1–35.
Mehdi, S. J., Rosas-Hernandez, H., Cuevas, E., Lantz, S. M., Barger, S. W., Sarkar, S., et al. (2016). Protein kinases and Parkinson's disease. Kinases and Parkinson's disease. Int. J. Mol. Sci. 17 (9), E1585. doi:10.3390/ijms17091585
Messick, J. M., Newberg, L. A., Nugent, M., and Faust, R. J. (1985). Principles of neuroanesthesia for the nonneurosurgical patient with CNS pathophysiology nonneurosurgical patient with CNS pathophysiology. Anesth. Analg. 64 (2), 143–174. doi:10.1213/00000539-198502000-00008
Mizushima, T. (2011). Drug discovery and development focusing on existing medicines: Drug re-profiling strategy. Profiling strategy. J. Biochem. 149 (5), 499–505. doi:10.1093/jb/mvr032
Myhre, R., Steinkjer, S., Stormyr, A., Nilsen, G. L., Zayyad, H. A., Horany, K., et al. (2008). Significance of the parkin and PINK1 gene in Jordanian families with incidences of young-onset and juvenile parkinsonism. BMC Neurol. 8 (1), 1–11.
Nadim, R., Tang, J., Dilmohamed, A., Yuan, S., Wu, C., Bakre, A. T., et al. (2020). Influence of periodontal disease on risk of dementia: A systematic literature review and a meta-analysis. Disease on risk of dementia: A systematic literature review and a meta-analysis. Eur. J. Epidemiol. 35 (9), 821–833. doi:10.1007/s10654-020-00648-x
Nagayama, H., Kano, O., Murakami, H., Ono, K., Hamada, M., Toda, T., et al. (2019). Effect of istradefylline on mood disorders in Parkinson's disease. J. Neurol. Sci. 396, 78–83. doi:10.1016/j.jns.2018.11.005
Naoi, M., Maruyama, W., and Shamoto-Nagai, M. (2020). Rasagiline and selegiline modulate mitochondrial homeostasis, intervene apoptosis system and mitigate α-synuclein cytotoxicity in disease-modifying therapy for Parkinson’s disease. J. Neural Transm. 127 (2), 131–147. doi:10.1007/s00702-020-02150-w
Naylor, D. M., Kauppi, D., and Schonfeld, J. (2015). Therapeutic drug repurposing, repositioning and rescue. Drug Discov. 57.
Nussbaum, A. D. KaM. (2002). Emerging therapies in the pharmacological treatment of Parkinson’s disease. Adis Int. Ltd.
Nyholm, D. (2003). Pharmacotherapy for Parkinson's disease-observations and innovations. Acta Univ. Ups.
O'Connor, K. A., and Roth, B. L. (2005). Finding new tricks for old drugs: An efficient route for public-sector drug discovery. Drug discovery. Nat. Rev. Drug Discov. 4 (12), 1005–1014. doi:10.1038/nrd1900
Oertel, W., and Schulz, J. B. (2016). Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 139, 325–337. doi:10.1111/jnc.13750
Ogawa, N. (1994). Levodopa and dopamine agonists in the treatment od Parkinson's disease: Advantages and disadvantages. Neuroscience 34 (3), 20–28. doi:10.1159/000119538
Ogawa, N. Levodopa and dopamine agonists in the treatment of Parkinson’s disease: Advantages and disadvantages. Eur. Neurol. 1994;34(Suppl. 3):20–28. doi:10.1159/000119538
Pagan, F., Hebron, M., Valadez, E. H., Torres-Yaghi, Y., Huang, X., Mills, R. R., et al. (2016). Nilotinib Effects in Parkinson's disease and Dementia with Lewy bodies. in Parkinson’s disease and dementia with Lewy bodies. J. Park. Dis. 6 (3), 503–517. doi:10.3233/JPD-160867
Palmer, C., Coronel, R., Bernabeu-Zornoza, A., and Liste, I. (2019). Therapeutic application of stem cell and gene therapy in Parkinson’s disease. Berlin, Germnay; Springer Nature Singapore, 159–171.
Pantziarka, P., Pirmohamed, M., and Mirza, N. (2018). New uses for old drugs. BMJ 361, k2701. doi:10.1136/bmj.k2701
Parmar, M., Torper, O., and Drouin-Ouellet, J. (2019). Cell-based therapy for Parkinson's disease: A journey through decades toward the light side of the force. Eur. J. Neurosci. 49 (4), 463–471. doi:10.1111/ejn.14109
Parvathaneni, V., Kulkarni, N. S., Muth, A., and Gupta, V. (2019). Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 24 (10), 2076–2085. doi:10.1016/j.drudis.2019.06.014
Pfeiffer, R. F. (2016). Non-motor symptoms in Parkinson's disease. Park. Relat. Disord. 22, S119–S122. doi:10.1016/j.parkreldis.2015.09.004
Poewe, W., and Mahlknecht, P. (2009). The clinical progression of Parkinson's disease. related disorders. Park. Relat. Disord. 15, S28–S32. doi:10.1016/S1353-8020(09)70831-4
Polamreddy, P., and Gattu, N. (2019). The drug repurposing landscape from 2012 to 2017: Evolution, challenges, and possible solutions. Challenges, and possible solutions. Drug Discov. Today 24 (3), 789–795. doi:10.1016/j.drudis.2018.11.022
Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A., et al. (2019). Drug repurposing: Progress, challenges and recommendations. Progress, challenges and recommendations. Nat. Rev. Drug Discov. 18 (1), 41–58. doi:10.1038/nrd.2018.168
Rao, S. CaJ., and Rao, J. (2009). Rivastigmine in Parkinson’s disease dementia. Expert Opin. Drug Metab. Toxicol. 5, 941–955. doi:10.1517/17425250903105420
Rascol, O., Fabbri, M., and Poewe, W. (2021). Amantadine in the treatment of Parkinson's disease and other movement disorders. Lancet. Neurol. 20 (12), 1048–1056. doi:10.1016/S1474-4422(21)00249-0
Rask-Andersen, M., Almén, M. S., and Schiöth, H. B. (2011). Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 10 (8), 579–590. doi:10.1038/nrd3478
Rocha, E. M., Keeney, M. T., Di Maio, R., De Miranda, B. R., and Greenamyre, J. T. (2022). LRRK2 and idiopathic Parkinson's disease. Parkinson’s disease. Trends Neurosci. 45, 224–236. doi:10.1016/j.tins.2021.12.002
Rudrapal, M., Khairnar, S. J., and Jadhav, A. G. (2020). Drug Repurposing (DR): An Emerging Approach in Drug Discovery. Drug Repurposing - Hypothesis, Molecular Aspects and Therapeutic Applications, 1–20.
Santa-Cecilia, F. V., Leite, C. A., Del-Bel, E., and Raisman-Vozari, R. (2019). The neuroprotective effect of doxycycline on neurodegenerative diseases. Neurotox. Res. 35, 981–986. doi:10.1007/s12640-019-00015-z
Savitt, D., and Jankovic, J. (2019). Targeting α-synuclein in Parkinson’s disease: Progress towards the development of disease-modifying therapeutics. Drugs 79 (8), 797–810. doi:10.1007/s40265-019-01104-1
Schaeffer, E., Pilotto, A., and Berg, D. (2014). Pharmacological strategies for the management of levodopa-induced dyskinesia in patients with Parkinson's disease. induced dyskinesia in patients with Parkinson’s disease. CNS drugs 28 (12), 1155–1184. doi:10.1007/s40263-014-0205-z
Schapira, A. (2005). Present and future drug treatment for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry Neurosurg. Psychiatry 76 (11), 1472–1478. doi:10.1136/jnnp.2004.035980
Schapira, A. H. (2008). Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet. Neurol. 7 (1), 97–109. doi:10.1016/S1474-4422(07)70327-7
Schein, C. H. (2020). Repurposing approved drugs on the pathway to novel therapies. Med. Res. Rev. 40 (2), 586–605. doi:10.1002/med.21627
Schonfeld, D. S. NaJ. M. (2014). Therapeutic drug repurposing, repositioning and rescue. Drug Discov. World Winter.
Sekhon, B. S. (2013). Repositioning drugs and biologics: Retargeting old/existing drugs for potential new therapeutic applications. J. Pharm. Educ. Res. 4 (1).
Seppi, K., Ray Chaudhuri, K., Coelho, M., Fox, S. H., Katzenschlager, R., Perez Lloret, S., et al. (2019). Update on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. Mov. Disord. 34 (2), 180–198. doi:10.1002/mds.27602
Sharma, V. D., Lyons, K. E., and Pahwa, R. Amantadine extended-release capsules for levodopa-induced dyskinesia in patients with Parkinson's disease. dyskinesia in patients with Parkinson’s disease. Ther. Clin. Risk Manag. 2018;14:665.665, 673. doi:10.2147/TCRM.S144481
Shineman, D. W., Alam, J., Anderson, M., Black, S. E., Carman, A. J., Cummings, J. L., et al. (2014). Overcoming obstacles to repurposing for neurodegenerative disease. obstacles to repurposing for neurodegenerative disease. Ann. Clin. Transl. Neurol. 1 (7), 512–518. doi:10.1002/acn3.76
Shkodina, A. D., Tarianyk, K. A., Boiko, D. I., Zehravi, M., Akter, S., Md Ashraf, G., et al. (2022). Cognitive and affective disturbances in patients with Parkinson's disease: Perspectives for classifying of motor/neuropsychiatric subtypes. affective disturbances in patients with Parkinson's disease: Perspectives for classifying of motor/neuropsychiatric subtypes. Neurosci. Lett. 781, 136675. doi:10.1016/j.neulet.2022.136675doi:136675
Silveira, C., MacKinley, J., Coleman, K., Li, Z., Finger, E., Bartha, R., et al. (2019). Ambroxol as a novel disease-modifying treatment for Parkinson’s disease dementia: Protocol for a single-centre, randomized, double-blind, placebo-controlled trial. BMC Neurol. 19 (1), 1–10.
Silveira, C. R. A., MacKinley, J., Coleman, K., Li, Z., Finger, E., Bartha, R., et al. (2019). Ambroxol as a novel disease-modifying treatment for Parkinson's disease dementia: Protocol for a single-centre, randomized, double-blind, placebo-controlled trial. BMC Neurol. 19 (1), 20. doi:10.1186/s12883-019-1252-3
Silverdale, M. A., Nicholson, S., Crossman, A., and Brotchie, J. (2005). Topiramate reduces levodopa-induced dyskinesia in the MPTP-lesioned marmoset model of Parkinson's disease. dyskinesia in the MPTP‐lesioned marmoset model of Parkinson's disease. Mov. Disord. 20 (4), 403–409. doi:10.1002/mds.20345
Silverdale, M. A., Nicholson, S. L., Crossman, A. R., and Brotchie, J. M. (2005). Topiramate reduces levodopa- induced dyskinesia in the MPTP-lesioned marmoset model of Parkinson's disease. Mov. Disord. 20 (4), 403–409. doi:10.1002/mds.20345
Singh, T. U., Parida, S., Lingaraju, M. C., Kesavan, M., Kumar, D., and Singh, R. K. (2020). Drug repurposing approach to fight COVID-19 approach to fight COVID-19. Pharmacol. Rep. 72, 1479–1508. doi:10.1007/s43440-020-00155-6
Skehel, J. J., Hay, A. J., and Armstrong, J. A. (1978). On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. amantadine hydrochloride. J. Gen. Virol. 38 (1), 97–110. doi:10.1099/0022-1317-38-1-97by
Smith, M. D., and Peall, K. J. (2018). Repurposed drugs for use in Parkinson's disease. J. Neurol. 265 (3), 728–730. doi:10.1007/s00415-018-8772-4
Srinath, N., and Kotwal, S. V. (1999). Sildenafil-Oral medication for erectile dysfunction-a review. Med. J. Armed Forces India J. Armed Forces India 55 (3), 233–236. doi:10.1016/S0377-1237(17)30452-5
Stefanis, L. α-Synuclein in Parkinson's disease. Cold Spring Harb. Perspect. Med. 446 2012;2, a009399, doi:10.1101/cshperspect.a0093992): doi:a009399
Steinhagen, H. (2011). The evolution of drug discovery: From traditional medicines to modern drugs. By enrique raviña. Drugs. By enrique raviña. ChemMedChem 6 (9), 1746–1747. doi:10.1002/cmdc.201100321
Stocchi, F., Fossati, C., and Torti, M. (2015). Rasagiline for the treatment of Parkinson’s disease: An update. Expert Opin. Pharmacother. 16 (14), 2231–2241. doi:10.1517/14656566.2015.1086748
Stoker, T. B., Torsney, K. M., and Barker, R. A. (2018). Emerging treatment approaches for Parkinson's disease. Front. Neurosci. 12, 693. doi:10.3389/fnins.2018.00693
Styczynska-Soczka, K., Zechini, L., and Zografos, L. (2017). Validating the predicted effect of astemizole and ketoconazole using a Drosophila model of Parkinson's disease. Assay. Drug Dev. Technol. 66315 (3), 106–112. doi:10.1089/adt.2017.776
Sun, W., Sanderson, P. E., and Zheng, W. (2016). Drug combination therapy increases successful drug repositioning. repositioning. Drug Discov. Today 21 (7), 1189–1195. doi:10.1016/j.drudis.2016.05.015
Sun, Y., Pham, A. N., and Waite, T. D. (2018). Mechanism underlying the effectiveness of deferiprone in alleviating Parkinson's disease symptoms. ACS Chem. Neurosci. 9 (5), 1118–1127. doi:10.1021/acschemneuro.7b00478
Taylor, M., and Alessi, D. R. (2020). Advances in elucidating the function of leucine-rich repeat protein kinase-2 in normal cells and Parkinson's disease. kinase-2 in normal cells and Parkinson's disease. Curr. Opin. Cell Biol. 63, 102–113. doi:10.1016/j.ceb.2020.01.001
Thanvi, B., Lo, N., and Robinson, T. (2007). Levodopa-induced dyskinesia in Parkinson's disease: Clinical features, pathogenesis, prevention and treatment. Features, pathogenesis, prevention and treatment. Postgrad. Med. J. 83 (980), 384–388. doi:10.1136/pgmj.2006.054759
Tobinick, E. L. (2009). The value of drug repositioning in the current pharmaceutical market. Drug News Perspect. 22 (2), 119–125. doi:10.1358/dnp.2009.22.2.1303818
Tolosa, A. H. V. SaE. Molecular and clinical prodrome of Parkinson disease: Implications for treatment. Nature reviews | neurology. 2010: [Anthonay at el.].
Tong, H., Zhang, X., Meng, X., Lu, L., Mai, D., and Qu, S. (2018). Simvastatin inhibits activation of NADPH oxidase/p38 MAPK pathway and enhances expression of antioxidant protein in Parkinson disease models. Front. Mol. Neurosci. 11, 165. doi:10.3389/fnmol.2018.00165
Trist, B. G., Hare, D. J., and Double, K. L. (2019). Oxidative stress in the aging substantia nigra and the etiology of Parkinson's disease. of Parkinson's disease. Aging Cell 18 (6), e13031. doi:10.1111/acel.13031
Uchihara, T., and Giasson, B. I. (2016). Propagation of alpha-synuclein pathology: Hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. And yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 131 (1), 49–73. doi:10.1007/s00401-015-1485-1
Uversky, V. N., and Eliezer, D. (2009). Biophysics of Parkinson's disease: Structure and aggregation of alpha-synuclein.synuclein. Current protein and peptide science. Curr. Protein Pept. Sci. 10 (5), 483–499. doi:10.2174/138920309789351921
Valente, E. M., Salvi, S., Ialongo, T., Marongiu, R., Elia, A. E., Caputo, V., et al. (2004). PINK1 mutations are associated with sporadic early-onset parkinsonism. associated with sporadic early‐onset parkinsonism. Ann. Neurol. 56 (3), 336–341. doi:10.1002/ana.20256
Vargesson, N. (2019). The teratogenic effects of thalidomide on limbs. J. Hand Surg. Eur. Vol. 44 (1), 88–95. doi:10.1177/1753193418805249
Vijverman, A-C., and Fox, S. H. (2014). New treatments for the motor symptoms of Parkinson’s disease. Expert Rev. Clin. Pharmacol. 7 (6), 761–777. doi:10.1586/17512433.2014.966812
Warren Olanow, C., and Kieburtz, K. (2010). Defining disease‐modifying therapies for PD—A road map for moving forward. Mov. Disord. 25 (12), 1774–1779. doi:10.1002/mds.23288
Weber, M., Chang, W. L., Breier, M. R., Yang, A., Millan, M. J., and Swerdlow, N. R. (2010). The effects of the dopamine D2 agonist sumanirole on prepulse inhibition in rats. Eur. Neuropsychopharmacol. 20 (6), 421–425. doi:10.1016/j.euroneuro.2010.02.011
Weintraub, D., Siderowf, A. D., Potenza, M. N., Goveas, J., Morales, K. H., Duda, J. E., et al. (2006). Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch. Neurol. 63 (7), 969–973. doi:10.1001/archneur.63.7.969
Wen, H., Jung, H., and Li, X. (2015). Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. Issues: Opportunities and challenges. AAPS J. 17 (6), 1327–1340. doi:10.1208/s12248-015-9814-9
Wolf, E., Seppi, K., Katzenschlager, R., Hochschorner, G., Ransmayr, G., Schwingenschuh, P., et al. (2010). Long‐term antidyskinetic efficacy of amantadine in Parkinson's disease. Mov. Disord. 25 (10), 1357–1363. doi:10.1002/mds.23034
Wong, K. K., Alty, J. E., Goy, A. G., Raghav, S., Reutens, D. C., and Kempster, P. A. (2011). A randomized, double-blind, placebo-controlled trial of levetiracetam for dyskinesia in Parkinson's disease.‐ blind, placebo‐controlled trial of levetiracetam for dyskinesia in Parkinson's disease. Mov. Disord. 26 (8), 1552–1555. doi:10.1002/mds.23687
Wong, Y. C., and Krainc, D. (2017). α-Synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Strategies. Nat. Med. 23 (2), 1–13. doi:10.1038/nm.4269
Worth, P. F. (2013). When the going gets tough: How to select patients with Parkinson's disease for advanced therapies. Advanced therapies. Pract. Neurol. 13 (3), 140–152. doi:10.1136/practneurol-2012-000463
Wu, T., and Hallett, M. (2013). The cerebellum in Parkinson’s disease. Brain 136 (3), 696–709. doi:10.1093/brain/aws360
Yssel, J. D., O'Neill, E., Nolan, Y. M., Connor, T. J., and Harkin, A. (2018). Treatment with the noradrenaline re-uptake inhibitor atomoxetine alone and in combination with the α2-adrenoceptor antagonist idazoxan attenuates loss of dopamine and associated motor deficits in the LPS inflammatory rat model of Parkinson's disease. Brain Behav. Immun. 69, 456–469. doi:10.1016/j.bbi.2018.01.004
Zesiewicz, T. A., and Hauser, R. A. (1999). Ropinirole in the treatment of Parkinson’s disease. Expert Opin. Investig. Drugs investigational drugs 8 (5), 697–710. doi:10.1517/13543784.8.5.697
Keywords: central nervous system, drug repurposing, Parkinson’s disease, drug discovery, neurodegeneration
Citation: Latif K, Ullah A, Shkodina AD, Boiko DI, Rafique Z, Alghamdi BS, Alfaleh MA and Ashraf GM (2022) Drug reprofiling history and potential therapies against Parkinson’s disease. Front. Pharmacol. 13:1028356. doi: 10.3389/fphar.2022.1028356
Received: 26 August 2022; Accepted: 03 October 2022;
Published: 26 October 2022.
Edited by:
Philippe De Deurwaerdere, Université de Bordeaux, FranceReviewed by:
Hamadjida Adjia, Université de Montréal, CanadaCopyright © 2022 Latif, Ullah, Shkodina, Boiko, Rafique, Alghamdi, Alfaleh and Ashraf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghulam Md. Ashraf, YXNocmFmLmdtQGdtYWlsLmNvbQ==; Anastasiia D. Shkodina, QWQuc2hrb2RpbmFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.