
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 09 December 2022
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1026337
This article is part of the Research Topic Hepatocellular carcinoma: from personalized medicine to practical guidelines View all 10 articles
 Jin Lei1†
Jin Lei1† Bowen Chen2†
Bowen Chen2† Meiru Song3†
Meiru Song3† Linzhi Zhang4
Linzhi Zhang4 Xinfeng Zhang3
Xinfeng Zhang3 Xiaoqiang Gao5
Xiaoqiang Gao5 Yinyin Li4
Yinyin Li4 Yinying Lu1,4,6*
Yinying Lu1,4,6* Shi Zuo1,5*
Shi Zuo1,5*Background: Tyrosine kinase inhibitors (TKI) in combination with programmed cell death-1 (PD-1) inhibitors become the potential treatment modality for patients undergoing unresectable hepatocellular carcinoma (uHCC) in the first-line setting. However, the efficacy and safety of this combination regimen in patients after sorafenib failure remains unclear.
Methods: Participants in this study included patients with uHCC after sorafenib failure who received TKI monotherapy (TKI group) or TKI combined with PD-1 inhibitors therapy (combination group) in our center from July 2018 to July 2021. The overall survival (OS) was used to be the primary efficacy endpoint, while progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) were applied to be secondary endpoints. In addition, the adverse events are recorded and evaluated.
Results: Among the 92 patients contained in this work, 50 patients were categorized into the TKI group, while 42 patients were in the combination group. There existed no evident differences between the two groups concerning the ORR (8.0% vs. 9.5%, p = 1.000). However, the DCR in the combined group was better in relative to that in the TKI group (71.4% vs. 50.0%, p = 0.037). In comparison with the TKI group, it was found that the combination group presented notably better median PFS (8.1 months vs. 4.7 months, p = 0.005) and median OS (21.9 months vs. 16.6 months, p = 0.042). According to multivariate analysis, PFS (HR 0.5, 95% CI: 0.3–0.8, p = 0.005) and OS (HR 0.5, 95% CI: 0.3–1.0, p = 0.051) were improved in the combination group in relative to the TKI group after the adjustment for some risk factors. Additionally, the incidence rates of grade ≥1 adverse event in the TKI group and the combination group were 96.0% and 97.6%, respectively. The most normal adverse event in the TKI group was neutropenia (n = 24,48.0%) and the combination group was hypoalbuminemia (n = 23,54.8%). All of these adverse events improved after symptomatic treatment, and no new toxic events were found to occur.
Conclusion: TKI combined with PD-1 inhibitors showed better prognosis with manageable toxicity in uHCC patients after sorafenib failure compared with TKI monotherapy.
According to the latest statistics, primary liver cancer ranks the sixth most normal cancer type globally, with more than 900,000 new cases every year (Sung et al., 2021). Hepatocellular carcinoma (HCC) is found to occupy 85%–90% among all the primary liver cancers (El-Serag and Rudolph, 2007). Due to the early asymptomatic and rapid progress, most patients were diagnosed with advanced-stage disease. Patients who lost the opportunity of local therapy could only choose the best supportive treatment until the emergence of sorafenib brought them hope in 2007 (Llovet et al., 2008). The first-line treatment drugs approved by the Food and Drug Administration (FDA), from sorafenib in 2007 to lenvatinib with non-inferior effect to sorafenib in 2018, and then to atezolizumab plus bevacizumab (A + T) with excellent effect to sorafenib in 2020, have enhanced the prognosis of HCC patients and increased the selectivity of treatment schemes (Kudo et al., 2018; Cheng et al., 2022). The American Gastroenterological Association (AGA) suggests for patients with preserved liver function, A + T can improve the OS of patients with sorafenib but exclude those who are not suitable for immunotherapy and/or are at a high risk of bleeding (Su et al., 2022). Compared with sorafenib, lenvatinib has promising progression-free survival (PFS), but is more prone to hypertension and skin adverse events. The A + T regimen may become the mainstream of the first-line treatment regimen for patients undergoing HCC, but sorafenib will continue to be used to become a first-line therapy for those suffering from HCC for a long period.
As the oral small molecule multityrosine kinase inhibitor (TKI) that can hinder angiogenesis, sorafenib generates an anticancer impact through hindering vascular endothelial growth factor receptor (VEGFR) and fibroblast growth factor receptor (FGFR) (Morse et al., 2019). Although sorafenib significantly prolonged the OS of patients compared with placebo, disease control rate (DCR) was only 43% and PFS approximately 4 months, indicating that more than half of patients did not respond and patients who responded developed resistance in a short time (Llovet et al., 2008; Kudo et al., 2018). In the face of the non-response and high drug resistance rate of sorafenib, active anti-tumor treatment in the back line can benefit the survival of patients. Currently, the second-line treatment approved by FDA includes cabozantinib, regorafenib, pembrolizumab and ramucirumab (HCC patients with AFP>400 ng/ml). These second-line drugs have significantly prolonged OS in HCC patients after sorafenib failure compared with placebo, whereas the lack of head-to-head clinical data limits the level of evidence for second-line treatment options. Clinicians choose second-line treatment schemes mostly based on work experience rather than experimental evidence.
In recent years, PD-1 inhibitors have benefited a variety of cancers. Even though the use of nivolumab and pembrolizumab in HCC patients has promoted the treatment of HCC patients into the era of immunity, the curative effect is not satisfactory (Finn et al., 2020b; Yau et al., 2022). However, TKI combined with PD-1 inhibitors has become the promising treatment option. In KEYNOTE-524, pembrolizumab combined with lenvatinib significantly improved the median OS (22 months) of patients with unresectable HCC(uHCC) (Finn et al., 2020a). In RESCUE, the 18-month survival rate of HCC patients reached 58.1% by camrelizumab combination with apatinib (Xu et al., 2021). Although there are no reports of randomized controlled trials of TKI combined with PD-1 inhibitors compared with TKI monotherapy as the first-line treatment for HCC, in retrospective studies, numerous studies have revealed that the combined treatment of OS and PFS is significantly better than TKI monotherapy (Li et al., 2022; Wu et al., 2022). Nevertheless, it is not clear whether TKI combined with PD-1 inhibitors is better than TKI alone in the second-line treatment. Considering the dilemma of choosing the second-line treatment, and the significant advantages of TKI combined with PD-1 inhibitors in the first-line treatment environment, it is likely to become the best choice for the second-line treatment after sorafenib failure. Additionally, this work attempted to compare the efficacy and safety of TKI monotherapy and TKI in combination with PD-1 inhibitors in HCC patients after sorafenib failure.
This is the retrospective research carried out in the fifth medical center of the General Hospital of the Chinese people’s Liberation Army in China. From July 2018 to July 2021, HCC patients receiving TKI or TKI combined with PD-1 inhibitors as second-line treatment were included. The eligibility criteria included (1) patients diagnosed with uHCC pathologically or by two imaging techniques following the American Association for the Study of Liver Diseases (AASLD) guidelines (Marrero et al., 2018); (2) Child-Pugh class A or B; (3) an Eastern Cooperative Oncology Group (ECOG) scale performance score of 0–1; (4) tumor progression after first-line sorafenib therapy; and (5) at least one measurable tumor lesion. Besides, the exclusion criteria contained: (1) current or a history of another malignant tumor; (2) discontinued sorafenib due to the unacceptable toxicity; and (3) missing data. The approval of this study was obtained from the Chinese registered clinical trial ethics committee, and the implementation scheme was in consistence with the declaration of Helsinki in 1975. Patients are treated according to the dosage and method of TKI or PD-1 inhibitors recommended in the relevant instructions. All included patients were divided into TKI monotherapy group (TKI group) and TKI combined with PD-1 inhibitors treatment group (combination group) using different treatment methods. Demographic characteristics (including age and gender), blood indicators (including liver function, coagulation function, routine blood and tumor markers), and characteristics were collected and evaluated at baseline.
OS was the primary endpoint of this work, which referred to the time interval from initiation of treatment to death from any reason or end of the study, whichever came the first. The secondary endpoints of this work contained progression-free survival (PFS) (determined as the time from the initial dose to the first radiologically confirmed progressive disease (PD) or death from any cause), disease control rate (DCR), and objective response rate (ORR). After treatment initiation, we recorded radiological response by dynamic computed tomography (CT) or magnetic resonance imaging (MRI) at baseline and every 8–12 weeks. The Response Evaluation Criteria in Solid Tumors (RECIST) was adopted for evaluating tumor response. According to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0, we assessed adverse events (AEs).
Categorical data are shown to be the frequency with proportion and explored based on Chi-square test or Fisher’s exact test. With the aim of calculating the PFS and OS and plot the curve, the Kaplan-Meier method was employed. The log-rank test was adopted for comparing the two groups. A 2-tailed p-value ≤0.05 represented statistical significance. Cox proportional hazards models were applied, aiming to explore the correlation between the covariates and PFS or OS. Variables showing p < 0.05 in univariate analysis were subjected to stepwise multivariate analysis. Moreover, all data calculations were conducted by employing R language version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria).
Totally 121 patients with unresectable HCC after failure on sorafenib were screened here from July 2018 to July 2021 in our center. Among them, we excluded 29 patients, containing 9 patients who did not receive treatment as prescribed, 7 patients who were intolerant after receiving sorafenib, 5 patients undergoing liver resection before systemic therapy, 4 patients lacking any effective follow-up, 2 patients with BCLC stage A, and 1 patient without evaluable lesions. Finally, totally 92 patients met the inclusion and exclusion criteria, including 50 in the TKI group and 42 in the combination group. The agents in TKI group included lenvatinib (n = 39, 78.0%), regorafenib (n = 8, 16.0%) and apatinib (n = 3, 6.0%). The main combination therapies included sorafenib plus sintilimab (n = 21, 50.0%) and lenvatinib plus camrelizumab (n = 6, 14.2%) (Supplementary Figure S1). At the time of data cutoff (August 2022), the median duration of follow-up was 19 (95% CI: 16.5–21.4) months. The patients were mainly male (n = 79, 85.9%). The BCLC stage of 80 (87.0%) patients was stage C at the time of enrollment. The etiology was mainly HBV(n = 85,92.4%), and there were 55 patients (60.0%) with extrahepatic metastasis. No significant difference was found in all baseline data between the sorafenib TKI group and the combination group (Table 1).
All patients had at least one follow-up image for radiological tumor response assessment (Table 2). It was found that the ORR rates of the combination group and TKI group were 8.0% and 9.5%, separately. The DCR of the combination group was better than TKI group (71.4% vs. 50.0%, p = 0.037). Efficacy in the combination group was statistically better than that in TKI group in terms of OS [median (95% CI): 21.9 (NE-NE) vs. 16.6 (10.2–23.0) months, p = 0.042] and PFS [median (95% CI):8.1 (5.9–10.3) vs. 4.7 (3.2–6.2) months, p = 0.006] (Figure 1).
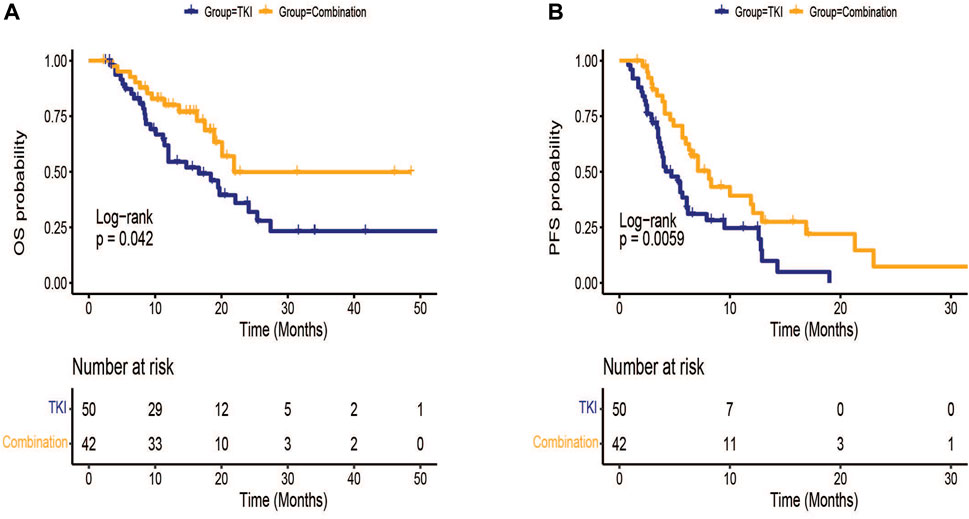
FIGURE 1. Kaplan-Meier survival curves of treatment outcome including (A) overall survival (OS) and (B) progression-free survival(PFS) between TKI group and combination group.
The patients in the TKI group were classified into lenvatinib (n = 39) and other TKI(n = 11) groups. The median OS was 14.7 (95% CI: 6.7–22.7) months in lenvatinib group, while the 13 months survival rate in the control group was 18.4%(95% CI: 3.2–33.6) (p = 0.291)(Figure 2A).The median PFS was 5.5 (95% CI: 3.8–7.2) months in lenvatinib group, while the control group was 3.5 (95% CI: 2.2–4.7) months (p = 0.174) (Figure 2B). In the subgroup analysis in the combination group, there existed no obvious difference in median PFS (8.3 vs. 7.1 months, p = 0.364) and median OS (NE vs. 21.9 months, p = 0.657) between sorafenib in combination with PD-1 inhibitors and lenvatinib in combination with PD-1 inhibitors (Figures 2C,D).
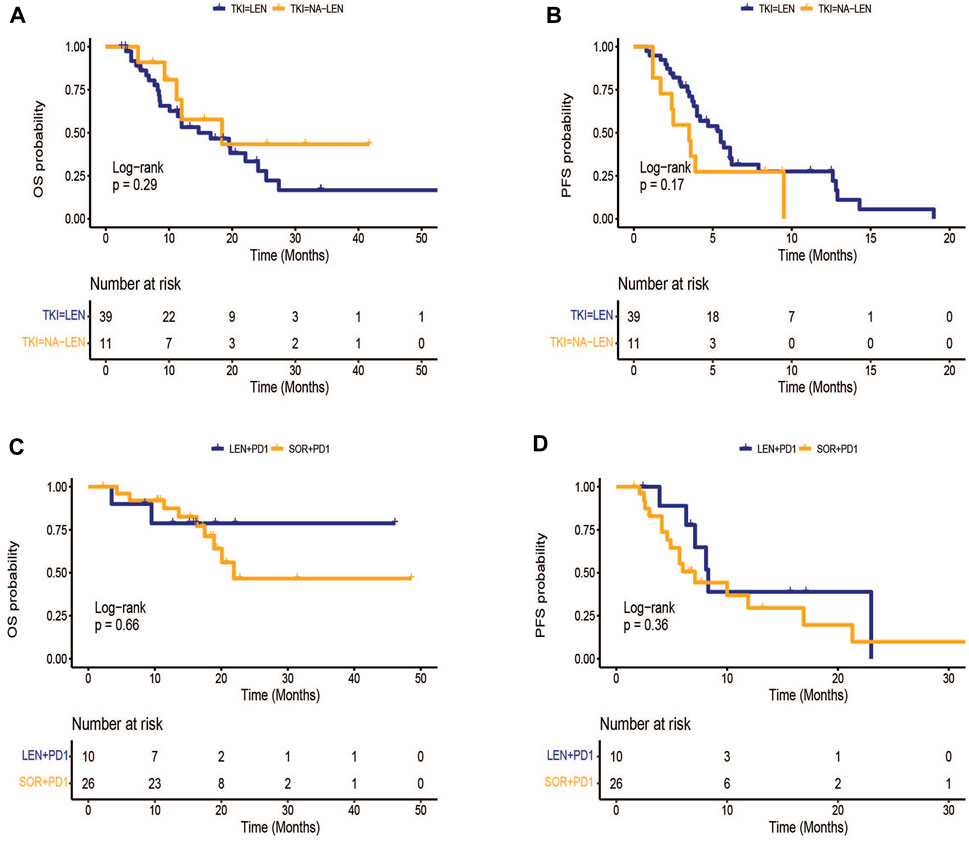
FIGURE 2. Kaplan-Meier survival curves of treatment outcome including (A) overall survival (OS), (B) progression-free survival(PFS) between lenvatinib and other TKI groups, (C) OS, (D) PFS between sorafenib plus PD-1 inhibitors and lenvatinib plus PD-1 inhibitors.
Table 3 shows the factors associated with the patient’s PFS and OS. In univariate analysis, TKI monotherapy, ECOG-PS score 1, and maximum tumor diameter greater than 5 cm were independently related to a shortened PFS, while TKI monotherapy and ECOG-PS score 1 were independently associated for shortened OS. In multivariate analysis, the independently correlated with a shortened PFS included, ECOG-PS score 1 (HR 1.8, 95% CI: 1.1–3.0, p = 0.027) and tumor diameter greater than 5 cm (HR 1.8, 95% CI: 1.1–2.9, p = 0.028), whereas the independently associated with a shortened OS was only ECOG-PS score 1 (HR 1.9, 95% CI: 1.0–3.6, p = 0.021). The combination group had better PFS (HR 0.5, 95% CI: 0.3–0.8, p = 0.005) and prolonged OS (HR 0.5, 95% CI: 0.3–1.0, p = 0.051) compared to the TKI group.
The incidence rates of grade ≥1 adverse event in the TKI group and the combination group were 96.0% and 97.6%, respectively. Obviously, the most common adverse event in the TKI group was neutropenia (n = 24,48.0%) and hypoalbuminemia (n = 23, 54.8%) in the combination group. In addition, the common grade 3–4 adverse events in the TKI group were leukopenia (n = 6, 12.0%), thrombocytopenia (n = 6, 12.0%), hypertension (n = 3, 6.0%), and lymphopenia (n = 3, 6.0%). The common grade 3–4 adverse events in the combined group included lymphopenia (n = 9, 21.4%), leukopenia (n = 3, 9.5%), hypertension (n = 2, 4.7%), and thrombocytopenia (n = 2, 4.7%). In the TKI group, the agent dose was decreased in 1 case due to grade 3 hypertension. In the combination group, two patients discontinued immunotherapy, including 1 with immune-related pneumonitis and 1 with immune-related myocarditis. No patients died due to adverse events (Table 4).
It is acknowledged that this is the first retrospective cohort study comparing treatment response and adverse events between TKI alone and TKI combined with PD-1 inhibitors as a second-line for uHCC. Our findings showed that combination therapy may improve DCR, PFS, and OS in patients in comparison with TKI monotherapy. There existed no statistically significant difference in adverse events between the two groups.
In the IMbrave 150 study, the PFS (6.9 months vs. 4.3 months, p < 0.001) and OS (19.2 months vs. 13.4 months, p < 0.001) of the A + T regimen were significantly prolonged compared with sorafenib monotherapy, and thus the regimen was recommended by the FDA as the standard first-line treatment regimen for uHCC patients (Cheng et al., 2022). The success of this combination therapy has brought novel hope to patients, and the synergistic effect of anti-vascular drugs combined with immune checkpoint inhibitors has already become the focus of patients and doctors. In prospective studies, TKI combined with PD-1 inhibitors (including pembrolizumab plus lenvatinib and camrelizumab plus apatinib) had a promising OS [(Finn et al., 2020a; Xu et al., 2021)]. In the real world, TKI combined with PD-1 inhibitors therapy has obviously prolonged OS in comparison with TKI monotherapy, including lenvatinib plus nivolumab vs. lenvatinib monotherapy (22.9 months vs. 10.3 months, p = 0.01) (Wu et al., 2022), lenvatinib plus camrelizumab vs. lenvatinib monotherapy (not reached vs. 13.9 months, p = 0.02) (Li et al., 2022), and lenvatinib plus sintilimab vs. lenvatinib monotherapy (21.7 months vs. 12.8 months, p = 0.01) (Zhao et al., 2022). Based on the above study, lenvatinib combined with PD-1 inhibitors had a significantly prolonged OS in first-line treatment of uHCC compared with lenvatinib monotherapy. The above studies showed that lenvatinib combined with PD-1 inhibitors significantly prolonged OS in first-line treatment of uHCC compared with lenvatinib monotherapy. In this work, although the TKI of the combination regimen was mainly sorafenib (61.9%) and was used in the second-line treatment of uHCC, the combination regimen also had a better prognosis than TKI monotherapy.
Sorafenib significantly prolongs OS compared to placebo and is widely used worldwide as first-line therapy in uHCC patients (Llovet et al., 2008). Unfortunately, a large number of HCC patients show a poor response to sorafenib or exhibit resistance to sorafenib treatment within 6 months (Chen et al., 2015). Continuing systemic therapy after sorafenib failure is the most effective way to prolong OS. In RESORCE, in patients undergoing HCC who failed sorafenib, continued regorafenib treatment significantly prolonged OS compared with placebo (10.6 months vs. 7.8 months, p < 0.001). The median time to death remained longer in the regorafenib group when survival was evaluated from prior sorafenib (vs. placebo, 26.0 months vs. 19.2 months) (Finn et al., 2018). The benefit of regorafenib for patients after failure of sorafenib was further confirmed in several retrospective clinical studies (Granito et al., 2021a). Recently, many second-line treatment studies have been carried out for HCC patients after sorafenib failure. FDA-approved second-line therapy-targeted drugs that have shown survival benefits in phase 3 clinical trials, including regorafenib (mOS, 10.6 months) (Finn et al., 2018), cabozantinib (mOS, 10.2 months) (Abou-Alfa et al., 2018) and ramucirumab (mOS, 8.5 months) (Zhu et al., 2019). Approved second-line immune monotherapy include nivolumab (mOS, 15.6 months) (El-Khoueiry et al., 2018) and pembrolizumab (mOS, 13.8 months) (Finn et al., 2020b). Additionally, the combination regimen nivolumab plus ipilimumab has not completed a phase 3 clinical trial, but has received FDA accelerated approval in a second-line setting due to long OS (mOS, 22.8 months) (Yau et al., 2020). Furthermore, second-line combination therapy options that are expected to be approved are durvalumab plus tremelimumab (mOS, 18.7 months) (Kelley et al., 2021) and camrelizumab plus apatinib (18 months OS rates, 56.5%) (Xu et al., 2021). Moreover, many second-line drugs that have completed phase 2 clinical trials or have been approved in some countries are booming, including apatinib (mOS, 8.7 months) (Qin et al., 2021), tislelizumab (mOS, 12.4 months) (Ducreux et al., 2021) and camrelizumab (mOS, 13.8 months) (Qin et al., 2020).
Faced with so many second-line treatment options, how to determine the best treatment has become the most perplexing problem. To determine the best second-line treatment regimen, we performed the analysis from different perspectives. First, based on the prospective second-line studies, the combination therapy regimen has a better OS than the monotherapy (targeted therapy or immunotherapy). Nevertheless, such conclusions need to be cautious, because some of the above studies have only completed the phase 2 clinical trials, even if phase 3 clinical trials have been completed but only use placebo as a control. Second, in the real world, controlled trials of second-line drugs only compared single agents and did not screen for superiority, including regorafenib versus nivolumab (Choi et al., 2020), regorafenib versus cabozantinib (Casadei-Gardini et al., 2021) and cabozantinib versus ramucirumab (Trojan et al., 2021). Our results suggest that there is a marginal difference in PFS with lenvatinib compared with other TKI agents (5.5 vs. 3.5 months, p = 0.147). Previously, lenvatinib is superior to sorafenib of PFS in both prospective and retrospective studies (Kudo et al., 2018; Kuo et al., 2021), and thus it may be preferentially recommended in patients who cannot use immunotherapy after sorafenib failure. Certainly, for patients who can use immunotherapy, TKI combined with PD-1 inhibitors as a second-line regimen is a good option in line with our results. Third, the same treatment may exert different effects in different countries or regions. The primary risk factor for non-Japanese Asian patients is HBV, while European and American patients are HCV (El-Serag, 2012). The median OS of HCC patients treated with sorafenib was 10.7 months in Europe, Australasia and the United States, and 6.5 months in China, Taiwan, and South Korea (Llovet et al., 2008; Cheng et al., 2009). Due to the differences in regions and etiologies, although apatinib has been approved to be the second-line treatment in China, the efficacy of this regimen in other countries needs further investigation since the phase 3 clinical trial only included Chinese patients (Qin et al., 2021). Fourth, the current studies on second-line therapy choices for HCC patients are all conducted with sorafenib as a first-line treatment. Intolerance or disease progression due to sorafenib is related to response to second-line therapy. Ramucirumab and pembrolizumab were effective for patients with disease progression after sorafenib treatment, but not for the intolerant to sorafenib (Zhu et al., 2019; Finn et al., 2020b). In RESORCE, patients who were intolerant to sorafenib were excluded from the enrolled patients receiving second-line regorafenib excluded. However, cabozantinib can bring benefits after sorafenib treatment in patients with disease progression or intolerance, and thus it is the only second-line TKI recommended by the AGA for use in patients with sorafenib intolerance (Kelley et al., 2020; Su et al., 2022). Our study also excluded sorafenib-intolerant patients. Therefore, the efficacy of TKI combined with PD-1 inhibitors in sorafenib-intolerant patients in the second-line setting needs to be further explored in follow-up studies.
A comprehensive analysis of the above-mentioned second-line treatment decision-making perspectives, combined with our findings, shows that there is potential value in recommending combination therapy after sorafenib failure. (1) At present, the commonly used second-line single drugs are TKI drugs, and thus there may be cross-resistance with sorafenib which can greatly limit the survival of patients. (2) Commonly used TKI drugs all exert the targeted therapeutic effect of VEGFR, which can not only regulate tumor blood vessels, but also serve as an effective immunomodulatory molecule, affecting TAM, MDSC, Treg cells and effector T cells (Fukumura et al., 2018). Nevertheless, PD-1 inhibitors can restore effector CD8+ T cell function by blocking extensive dephosphorylation between PD-L1 and PD-1, which can impair or abolish the immunosuppressive effects caused by Treg cells and ultimately inhibit tumor growth (Ahn et al., 2018; Granito et al., 2021b). Multiple mouse experiments have demonstrated that TKI combined with PD-1 inhibitors combination therapy can achieve the synergistic effect (Sprinzl et al., 2015; Torrens et al., 2021). (3) Multiple studies have revealed that the toxicity profile and tolerance were similar between TKI monotherapy and combination regimens (Li et al., 2022; Wu et al., 2022). (4) There are many combinations of TKI combined with PD-1 inhibitors, which can avoid the limitations of a certain drug and increase the practicality of the treatment plan. Our subgroup analysis proved that there existed no obvious difference in OS and PFS between sorafeinib combined with PD-1 inhibitors and lenvatinib combined with PD-1 inhibitors, which could also increase the possibility that different combinations may benefit from. However, the mechanism of lenvatinib and sorafenib combined with PD-1 inhibitors is different, the former can specifically reduce the abundance of tumor Treg cells (Torrens et al., 2021), while the latter has the effect of directly inhibiting the activation of M2 macrophages (Sprinzl et al., 2015). The effect of this combination treatment is promising. However, follow-up large-sample and prospective studies need to be performed to explore what kind of combination is more effective and what kind of situation is used.
Several limitations have to be mentioned in this study. First, this study was designed as a retrospective one with the small sample size, which could generate information bias and selection bias. Moreover, we explored multiple second-line TKI or PD-1 inhibitors. The clinical efficacy of specific regimens must be explored in future clinical trials. Third, our study excluded patients with sorafenib intolerance. Thus, the efficacy of TKI in combination with PD-1 inhibitors was not available in these patients.
To conclude, TKI combined with PD-1 inhibitors may benefit more than TKI monotherapy in HCC patients after sorafenib failure. Prospective studies with large samples are required to explore and clarify specific treatment options for patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by China registered clinical trial ethics committee before the study (Approval Number: ChiECRCT20210348). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JL, BC, and MS conducted the experiment and executed most of the data processing and analysis and wrote the manuscript. LZ, XZ, XG, and YL conducted the experiments and analyzed the data. YL and SZ participated in the designing of the experiments and data analysis and guided and supervised the work. All authors read and approved the submitted version.
This study was supported by grants from Science Technology and Innovation Committee of Shenzhen Municipality (2019 N002) of China and Special Fund for Outstanding Young Scientific and Technological Talents of Guizhou Province (Talents of Guizhou Kehe platform [2019]5647).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1026337/full#supplementary-material
Abou-Alfa, G. K., Meyer, T., Cheng, A-L., El-Khoueiry, A. B., Rimassa, L., Ryoo, B-Y., et al. (2018). Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379 (1), 54–63. doi:10.1056/NEJMoa1717002
Ahn, E., Araki, K., Hashimoto, M., Li, W., Riley, J. L., Cheung, J., et al. (2018). Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 115 (18), 4749–4754. doi:10.1073/pnas.1718217115
Casadei-Gardini, A., Rimassa, L., Rimini, M., Yoo, C., Ryoo, B-Y., Lonardi, S., et al. (2021). Regorafenib versus cabozantinb as second-line treatment after sorafenib for unresectable hepatocellular carcinoma: Matching-adjusted indirect comparison analysis. J. Cancer Res. Clin. Oncol. 147 (12), 3665–3671. doi:10.1007/s00432-021-03602-w
Chen, J., Jin, R., Zhao, J., Liu, J., Ying, H., Yan, H., et al. (2015). Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 367 (1), 1–11. doi:10.1016/j.canlet.2015.06.019
Cheng, A-L., Kang, Y-K., Chen, Z., Tsao, C-J., Qin, S., Kim, J. S., et al. (2009). Efficacy and safety of sorafenib in patients in the asia-pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet. Oncol. 10 (1), 25–34. doi:10.1016/S1470-2045(08)70285-7
Cheng, A-L., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T-Y., et al. (2022). Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 76 (4), 862–873. doi:10.1016/j.jhep.2021.11.030
Choi, W-M., Choi, J., Lee, D., Shim, J. H., Lim, Y-S., Lee, H. C., et al. (2020). Regorafenib versus nivolumab after sorafenib failure: Real-world data in patients with hepatocellular carcinoma. Hepatol. Commun. 4 (7), 1073–1086. doi:10.1002/hep4.1523
Ducreux, M., Abou-Alfa, G., Ren, Z., Edeline, J., Li, Z., Assenat, E., et al. (2021). O-1 Results from a global phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with unresectable hepatocellular carcinoma. Ann. Oncol. 32, S217. doi:10.1016/j.annonc.2021.05.005
El-Khoueiry, A. B., Melero, I., Yau, T. C., Crocenzi, T. S., Kudo, M., Hsu, C., et al. (2018). Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): Subanalyses of CheckMate-040. J. Clin. Oncol. 36, 475. doi:10.1200/jco.2018.36.4_suppl.475
El-Serag, H. B. (2012). Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142 (6), 1264–1273. doi:10.1053/j.gastro.2011.12.061
El-Serag, H. B., and Rudolph, K. L. (2007). Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 132 (7), 2557–2576. doi:10.1053/j.gastro.2007.04.061
Finn, R. S., Ikeda, M., Zhu, A. X., Sung, M. W., Baron, A. D., Kudo, M., et al. (2020). Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 38 (26), 2960–2970. doi:10.1200/JCO.20.00808
Finn, R. S., Merle, P., Granito, A., Huang, Y-H., Bodoky, G., Pracht, M., et al. (2018). Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J. Hepatol. 69 (2), 353–358. doi:10.1016/j.jhep.2018.04.010
Finn, R. S., Ryoo, B-Y., Merle, P., Kudo, M., Bouattour, M., Lim, H. Y., et al. (2020). Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J. Clin. Oncol. 38 (3), 193–202. doi:10.1200/JCO.19.01307
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G., and Jain, R. K. (2018). Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 15 (5), 325–340. doi:10.1038/nrclinonc.2018.29
Granito, A., Forgione, A., Marinelli, S., Renzulli, M., Ielasi, L., Sansone, V., et al. (2021). Experience with regorafenib in the treatment of hepatocellular carcinoma. Ther. Adv. Gastroenterol. 14, 17562848211016959. doi:10.1177/17562848211016959
Granito, A., Muratori, L., Lalanne, C., Quarneti, C., Ferri, S., Guidi, M., et al. (2021). Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol. 27 (22), 2994–3009. doi:10.3748/wjg.v27.i22.2994
Kelley, R. K., Ryoo, B-Y., Merle, P., Park, J-W., Bolondi, L., Chan, S. L., et al. (2020). Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: A subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open 5 (4), e000714. doi:10.1136/esmoopen-2020-000714
Kelley, R. K., Sangro, B., Harris, W., Ikeda, M., Okusaka, T., Kang, Y-K., et al. (2021). Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J. Clin. Oncol. 39 (27), 2991–3001. doi:10.1200/JCO.20.03555
Kudo, M., Finn, R. S., Qin, S., Han, K-H., Ikeda, K., Piscaglia, F., et al. (2018). Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391 (10126), 1163–1173. doi:10.1016/S0140-6736(18)30207-1
Kuo, Y-H., Lu, S-N., Chen, Y-Y., Kee, K-M., Yen, Y-H., Hung, C-H., et al. (2021). Corrigendum: Real-World lenvatinib versus sorafenib in patients with advanced hepatocellular carcinoma: A propensity score matching analysis. Front. Oncol. 11, 823960. doi:10.3389/fonc.2021.823960
Li, Q., Cao, M., Yuan, G., Cheng, X., Zang, M., Chen, M., et al. (2022). Lenvatinib plus camrelizumab vs. Lenvatinib monotherapy as first-line treatment for unresectable hepatocellular carcinoma: A multicenter retrospective cohort study. Front. Oncol. 12, 809709. doi:10.3389/fonc.2022.809709
Llovet, J. M., Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J-F., et al. (2008). Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359 (4), 378–390. doi:10.1056/NEJMoa0708857
Marrero, J. A., Kulik, L. M., Sirlin, C. B., Zhu, A. X., Finn, R. S., Abecassis, M. M., et al. (2018). Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatol. Baltim. Md) 68 (2), 723–750. doi:10.1002/hep.29913
Morse, M. A., Sun, W., Kim, R., He, A. R., Abada, P. B., Mynderse, M., et al. (2019). The role of angiogenesis in hepatocellular carcinoma. Clin. Cancer Res. 25 (3), 912–920. doi:10.1158/1078-0432.CCR-18-1254
Qin, S., Li, Q., Gu, S., Chen, X., Lin, L., Wang, Z., et al. (2021). Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. Gastroenterol. Hepatol. 6 (7), 559–568. doi:10.1016/S2468-1253(21)00109-6
Qin, S., Ren, Z., Meng, Z., Chen, Z., Chai, X., Xiong, J., et al. (2020). Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet. Oncol. 21 (4), 571–580. doi:10.1016/S1470-2045(20)30011-5
Sprinzl, M. F., Puschnik, A., Schlitter, A. M., Schad, A., Ackermann, K., Esposito, I., et al. (2015). Sorafenib inhibits macrophage-induced growth of hepatoma cells by interference with insulin-like growth factor-1 secretion. J. Hepatol. 62 (4), 863–870. doi:10.1016/j.jhep.2014.11.011
Su, G. L., Altayar, O., O'Shea, R., Shah, R., Estfan, B., Wenzell, C., et al. (2022). AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology 162 (3), 920–934. doi:10.1053/j.gastro.2021.12.276
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Torrens, L., Montironi, C., Puigvehí, M., Mesropian, A., Leslie, J., Haber, P. K., et al. (2021). Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatol. Baltim. Md) 74 (5), 2652–2669. doi:10.1002/hep.32023
Trojan, J., Mollon, P., Daniele, B., Marteau, F., Martín, L., Li, Y., et al. (2021). Comparative efficacy of cabozantinib and ramucirumab after sorafenib for patients with hepatocellular carcinoma and alpha-fetoprotein ≥ 400 ng/mL: A matching-adjusted indirect comparison. Adv. Ther. 38 (5), 2472–2490. doi:10.1007/s12325-021-01700-2
Wu, W-C., Lin, T-Y., Chen, M-H., Hung, Y-P., Liu, C-A., Lee, R-C., et al. (2022). Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest. New Drugs 40 (4), 789–797. doi:10.1007/s10637-022-01248-0
Xu, J., Shen, J., Gu, S., Zhang, Y., Wu, L., Wu, J., et al. (2021). Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin. Cancer Res. 27 (4), 1003–1011. doi:10.1158/1078-0432.CCR-20-2571
Yau, T., Kang, Y-K., Kim, T-Y., El-Khoueiry, A. B., Santoro, A., Sangro, B., et al. (2020). Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 6 (11), e204564. doi:10.1001/jamaoncol.2020.4564
Yau, T., Park, J-W., Finn, R. S., Cheng, A-L., Mathurin, P., Edeline, J., et al. (2022). Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet. Oncol. 23 (1), 77–90. doi:10.1016/S1470-2045(21)00604-5
Zhao, L., Chang, N., Shi, L., Li, F., Meng, F., Xie, X., et al. (2022). Corrigendum to "lenvatinib plus sintilimab versus lenvatinib monotherapy as first-line treatment for advanced HBV-related hepatocellular carcinoma: A retrospective, real-world study" [heliyon 8 (6) (2022) e09538]. Heliyon 8 (6), e11404. doi:10.1016/j.heliyon.2022.e11404
Zhu, A. X., Kang, Y-K., Yen, C-J., Finn, R. S., Galle, P. R., Llovet, J. M., et al. (2019). Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 20 (2), 282–296. doi:10.1016/S1470-2045(18)30937-9
Keywords: hepatocellular carcinoma, tyrosine kinase inhibitor (EGFR-TKI), programmed death-1 inhibitor, second-line, sorafenib
Citation: Lei J, Chen B, Song M, Zhang L, Zhang X, Gao X, Li Y, Lu Y and Zuo S (2022) TKI or TKI combined with PD-1 inhibitors as second-line treatment for HCC patients after sorafenib failure. Front. Pharmacol. 13:1026337. doi: 10.3389/fphar.2022.1026337
Received: 23 August 2022; Accepted: 28 November 2022;
Published: 09 December 2022.
Edited by:
Anup Kasi, Cancer Center, University of Kansas, United StatesReviewed by:
Alessandro Granito, University of Bologna Department of Medical and Surgical Sciences, ItalyCopyright © 2022 Lei, Chen, Song, Zhang, Zhang, Gao, Li, Lu and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinying Lu, bHV5aW55aW5nMTk3M0AxNjMuY29t; Shi Zuo, ZHJ6dW9zaGlAZ21jLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.