
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 14 October 2022
Sec. Predictive Toxicology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1026199
This article is part of the Research TopicToxicity Mechanisms, Exposure, Toxicokinetic and Risk Assessment Aspects of Metals, Toxic for Animals and Humans, Volume IIView all 10 articles
 Ola A. Habotta1*†
Ola A. Habotta1*† Xiaoyan Wang2†
Xiaoyan Wang2† Hamzah Othman3
Hamzah Othman3 Abdulrahman A. Aljali4
Abdulrahman A. Aljali4 Mahmoud Gewaily5
Mahmoud Gewaily5 Mahmoud Dawood6,7
Mahmoud Dawood6,7 Asmaa Khafaga8
Asmaa Khafaga8 Amr I. Zaineldin9
Amr I. Zaineldin9 Rajeev K. Singla10,11
Rajeev K. Singla10,11 Bairong Shen10
Bairong Shen10 Heba I. Ghamry12
Heba I. Ghamry12 Eman Elhussieny13
Eman Elhussieny13 Amany El-Mleeh14
Amany El-Mleeh14 Samah F. Ibrahim15
Samah F. Ibrahim15 Ahmed Abdeen16,17*
Ahmed Abdeen16,17*Copper (Cu) could be seriously hazardous when present at excessive levels, despite its vital contribution to various cellular processes. Selenium-enriched yeast (SeY) was reported to improve the health and metabolic status in broiler chicken. Hence, our study was endeavored to illustrate the mitigating efficacy of SeY on Cu-induced hepatic and renal damage. Cobb chicks aged 1 day were allocated into four experimental groups and offered a basal diet, SeY (0.5 mg/kg), CuSO4 (300 mg/kg), or SeY plus CuSO4 in their diets for 42 days. Our results revealed that SeY supplement antagonized significantly the Cu accumulation in livers and kidneys of exposed birds. Marked declines were also detected in the AST, ALT, urea, and creatinine levels, besides marked increases in total protein, glycerides, and cholesterol in the SeY-supplemented group. Moreover, enhancement of cellular antioxidant biomarkers (superoxide dismutase, CAT, GPx, and GSH) along with lowered MDA contents were achieved by SeY in hepatic and renal tissues. Further, SeY exerted a noteworthy anti-inflammatory action as indicated by decreased inflammatory biomarkers (IL-1β and TNF-α) and NO levels in both organs. Noticeable histopathological alterations of both organs further validated the changes in the markers mentioned above. To sum up, our findings indicate that SeY can be considered a potential feed supplement for alleviating Cu-induced hepatic and renal damage in broilers, possibly via activation of antioxidant molecules and lessening the inflammatory stress.
Heavy metals are naturally distributed inorganic compounds, that can be discharged from various sources and negatively affect the health of living organisms (Habotta et al., 2022). Among these hazardous environmental metals, copper (Cu) is an element of concern owing to its wide distribution and high toxicity (Zhao et al., 2018). Cu is widely incorporated in many agrochemicals as pesticides, including cupric sulfate (CuSO4), which is toxic to different living organisms. In addition, increased Cu release to the environment may happen through melting, mining, industrial, and waste removal activities (Yang et al., 2020). Since it is highly soluble in water, CuSO4 can easily disseminate to the environment; therefore, Cu exposure is inevitable to animals via polluted food or water (Liu et al., 2018a). Notably, it contributes substantially to cellular metabolism and numerous physiological processes such as hematopoiesis, mitochondrial respiration, antioxidation, and immunity (Yang et al., 2020). Despite its valuable physiological functions, former studies had reported that excess Cu could evoke toxicity and damage to hepatic, renal, nervous, and digestive systems (Hashem et al., 2021a; Liao et al., 2021). Cu can impair the respiratory enzyme complexes that stimulate the over-generation of highly reactive radicals and cellular oxidative injury (Hashem et al., 2021a).
The metabolism of Cu is controlled principally by the hepatic tissue, where it accumulates upon excess exposure with no noticeable signs. When the exposed Cu overwhelms the hepatic storage capability, hepatocellular lesions are developed together with release into the circulation causing damage to other tissues (Lu et al., 2010). Dietary exposure to CuSO4 elicited notable elevations in serum aminotransferases, alkaline phosphatase together with declines in total protein, albumin, globulins, triglycerides, total cholesterol, low-density lipoprotein-cholesterol, and high-density lipoprotein-cholesterol levels in broilers (Hashem et al., 2021b). Likewise, dietary supplementation of inorganic Cu at a dose of 150 mg/kg significantly decreased liver and meat lipids, cholesterol, plasma lipids, triglycerides and cholesterol, beside increasing plasma AST and ALT in exposed ducks (Attia et al., 2012). Further, Cu-intoxicated chickens had higher serum levels of urea, creatinine, and uric acid in comparison with the control group (Elazab et al., 2021). The deleterious effects of Cu are associated with reactive oxygen species (ROS) formation that surpasses the antioxidant defense system (Liu et al., 2018a). The cellular oxidative stress is accompanied by augmented inflammatory reaction by triggering the proinflammatory mediators (Liu et al., 2018b; Abdeen et al., 2021; Ismail et al., 2022). Liu et al. showed that dietary Cu exposure encouraged oxidative damage and peroxidation of lipid in chicken liver (Liu et al., 2018a). Notable suppression was observed in the antioxidant enzymes with triggered inflammatory responses in immune organs (Yang et al., 2020) and kidneys (Wang et al., 2017) of chicken fed with a Cu-contaminated diet. Further, Cu-induced oxidative damage was reported to trigger mitochondrial fragmentation leading to leakage of cytochrome-c, which in turn facilitates the cell death (Zhao et al., 2018).
Selenium is another essential element crucial for maintaining the intracellular redox balance via scavenging the harmful reactive radicals, thus alleviating cellular oxidative injury (Li et al., 2022). Selenium-enriched yeast (SeY) is an organic form of selenium that is lower in toxicity with higher digestibility and bioavailability than sodium selenide (Arnaut et al., 2021). Yeast cells can bind with selenium’s organic and inorganic forms and incorporate them permanently into their cell structure. Yeast can bioaccumulate and convert inorganic selenium (sodium selenate and sodium selenite) into organic forms (SeY) (Kieliszek et al., 2015). Supplementation of Oreochromis niloticus for SeY over a period of 60 days counteracted hypoproteinemia, elevated serum aminotransferases, urea, and creatinine induced by organophosphorus intoxication (Hassan et al., 2022). Se-enriched yeast reduced creatinine, and blood urea nitrogen levels in the kidneys of chromium-exposed broilers (Zhao et al., 2022). SeY was reported to counteract ochratoxin A-mediated suppression in the levels of antioxidant enzymes and genes in the hepatic and renal tissues of treated chickens (Li et al., 2020b; Li et al., 2020a). Former results unveiled that SeY protected against hepatic and renal oxidative stress and necroptosis elicited by cadmium toxicity in chicken (Wang et al., 2020a; Chen et al., 2021). Furthermore, SeY exerted a remarkable anti-inflammatory effect induced by lead by downregulating the gene transcription levels of inflammatory mediators in skeletal muscles such as interleukin (IL)-1β, IL-4, and IL-10 of exposed chicken (Liu et al., 2019). Similarly, dietary supplementation of chicken by 0.5 mg/kg SeY lessened markedly the expression levels of gene and protein related to hepatic inflammatory markers like iNOS, NF-κB, TNF-α, and prostaglandins (Wang et al., 2020b).
Up to our knowledge, the mitigating efficiency of SeY against Cu-evoked organ injury in broiler chickens has not been investigated. Therefore, this study was designed to explore the potential protective action of SeY as a feed supplement against Cu-induce oxidative and inflammatory stresses in liver and kidney tissues. Hence, this study appraises the protective impact of SeY against excess dietary inclusion of Cu and enriches its application for improvement of health status in young broiler chicks.
Copper (II) sulphate pentahydrate (CuSO4.5H2O; CAS number 7758–99-8) was purchased from Sigma Aldrich Co. (St. Louis, MO, United States). Selenium yeast (Se, 2000 ppm) was provided by Angel Yeast (Hubei, China; purity >99:5%). All other used chemicals and reagents were of high analytical grade.
One day old chicks of Cobb strain broilers were attained from the Faculty of Agriculture, Mansoura University, Egypt. They were raised in clean, well-ventilated floor pens under complete hygienic measures, one replicate per each pen (three birds/replication). Chicks were offered a balanced commercial ration and tap water ad libitum. The ideal temperature was changed; for example, it was set at 32°C for the first week and then dropped by 1°C each following week to reach 25°C. The lighting system was 24 h per day for the first week, then changed to 16 h of light and 8 h of darkness starting on day 7 and lasting until the completion of the experiment. The relative humidity was held between 60 and 70%. The basic diet was developed in accordance with Cobb 500 broilers’ Broiler Performance and Nutrition Supplement. From day 1 through day 10, birds were given a beginning diet; from day 11 through day 22 they were given a growing diet; from day 23 to the completion of the experiment, they were given a finisher diet. At 7 and 14 days old, all birds received vaccinations against Newcastle disease; at 11 and 22 days old, they received vaccinations against Gumboro disease (Giambrone and Clay, 1986). Table 1 lists the feed components and chemical makeup of the basic diet. The experiment design, procedures and techniques were done in accordance with the guidelines of the Institutional Animal Care and Use Committee of Faculty of Veterinary Medicine, Mansoura University (R/133).
The chicks were haphazardly allocated into four groups of 15 each, with 5 replicates in each group (3 birds x 5 replicates), as follows;
1 The first group (Con) received a daily, additive-free basal diet.
2 The second group (Cu) was dietary administered with 300 mg/kg CuSO4 following the method of Cinar et al. (Cinar et al., 2014; Hashem et al., 2021b).
3 The third group (selenium yeast; SeY) received a SeY-supplemented diet at a dose of 0.5 mg/kg (Wang et al., 2020a; Chen et al., 2021).
4 The last group (Cu+SeY) was administered basal diets supplemented with CuSO4/kg diet plus SeY at previously mentioned doses.
All birds were carefully observed for any abnormal signs during the experimental time, which lasted for 42 days. Six randomly chosen birds from each group had blood drawn from their wing veins, which were then centrifuged at 3000 x g for 10 min. Sera were then separated and kept at -20°C in deep freezing until further biochemical analyses. Following the recommendations of the American Veterinary Medical Association (Schaumburg, IL, United States), the birds were killed via cervical dislocation (Leary et al., 2016). Liver and kidney tissue were divided into different portions to evaluate antioxidant enzymes, inflammatory biomarkers, Cu and Se residues in both tissues, and histological alterations.
Following the mineral measurement method (AOAC) (Chemists and Chemists, 1925), 3 ml of concentrated nitric acid and 1.5 ml of concentrated perchloric acid were used to digest 0.5 gm of liver or kidney tissue. The digestion process was then finished by incubating the samples in a water bath adjusted at 53°C/overnight. The resulting mixture was filtered, left to cool to room temperature, and then 20 ml of deionized water was added for dilution. The concentrations of Cu and Se were determined using a flame atomic absorption spectrophotometer (Buck Scientific 210 VGP, Inc., Norwalk, Connecticut, CT, United States).
As directed by the manufacturer, serum samples were utilized to calculate hepatic and renal damage indications. According to Reitman and Frankel, the enzymatic activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were estimated (Reitman and Frankel, 1957). Following the protocol of Lowry et al., the serum level of total protein was examined (Lowry et al., 1951). In addition, Roeschlau et al. (1974) and McGowan et al. (1983) were used to estimate the total serum cholesterol and triglycerides levels, respectively.
According to Coulombe and Favreau’s (Coulombe and Favreau, 1963) and Larsen’s (Larsen, 1972), we evaluated the renal function markers, urea and creatinine.
The peroxidation of lipids was assessed spectrophotometrically by measurement of its secondary metabolite, malondialdehyde (MDA), in liver and kidney homogenates, according to Ohkawa et al. (1979). Depending on the reducing ability of glutathione to 5,5′-dithiobis (2-nitrobenzoic acid) to give yellow-colored 5-thionitrobenzoic acid, glutathione (GSH) was analyzed spectrophotometrically at 405 nm as stated by Ellman (1959).
The activity of superoxide dismutase (SOD) was evaluated depending on the nitroblue tetrazolium dye reduction rate to diformazan following the method established by Sun et al. (1988). Catalase (CAT) activity was assessed based on the depletion of H2O2 into H2O and molecular O at 240 nm, as mentioned by Aebi (1984). Additionally, the evaluation of glutathione peroxidase (GPx) activity was assessed by Paglia and Valentine (1967).
Griess reagent was utilized to estimate nitrite/nitrate (Nitric oxide; NO) levels as stated by Green et al. (1982). The concentrations of IL-1β (Cat number MBS261118) and TNF-α (Cat number MBS2509660) were analyzed using specific ELISA assay kits (MyBioSource, CA, United States).
Liver and kidney specimens were fixed in 10% neutral buffered formalin for 24 h, dehydrated, cleared with xylene, and embedded in molten paraplast. For microscopical examination, a 5 µm thick paraffin section was stained with hematoxylin and eosin.
Obtained data were analyzed by one-way analysis of variance (ANOVA) followed by post hoc Duncan’s multiple range test to decide the significance among groups. Data were displayed as mean ± standard error (SE). At p values <0.05, statistical significance was determined between groups. A multivariate analysis among variables and different treatments was performed through the principal component analysis (PCA) conduction. Together, a clustering heatmap was analyzed by RStudio (R version 4.0.2).
In relation with the control group, significant increases (p < 0.05) were seen in the Cu contents in the liver and kidney of chickens that received dietary CuSO4, as illustrated in Figure 1. Contrarily, the Cu level in the Cu+SeY group was markedly less than (p < 0.05) in the Cu-intoxicated group. Moreover, there was no discernible difference in hepatic Cu levels between the control and SeY groups. SeY group’s renal Cu levels were lower than those of the control group.
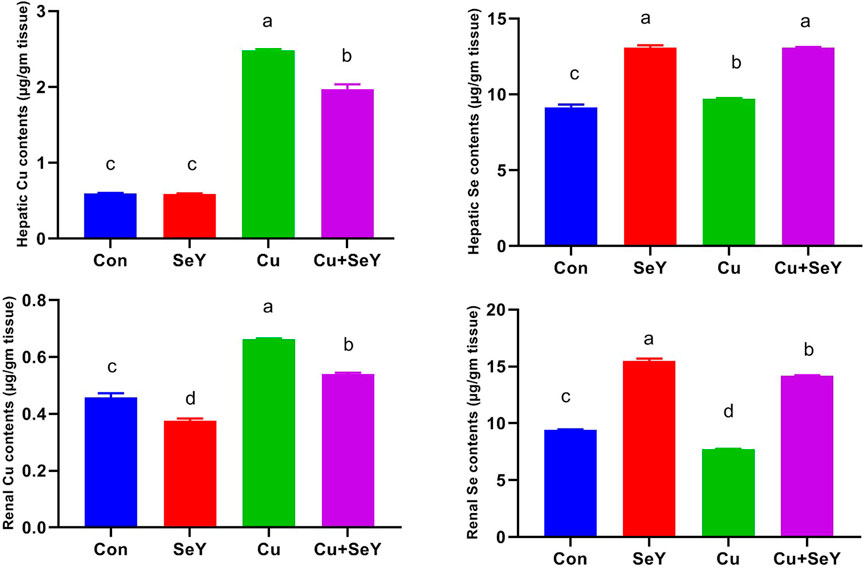
FIGURE 1. The copper (Cu) and selenium (Se) residual levels in the hepatic and renal tissues following dietary exposure to copper sulphate (CuSO4, 300 mg/kg) and/or selenium yeast (SeY, 0.5 mg/kg) in broiler chicken for 42 days. The values were represented as means ± SE (n = 6). Each bar carrying different letters is significantly different (p < 0.05).
Additionally, measurements of the Se content in both organs were made across all groups. The Se contents significantly increased (p < 0.05) in the Cu+SeY and SeY administered groups but notably diminished (p < 0.05) in the Cu-exposed chickens (Figure 1).
The indices of liver integrity, including TP, ALT, and AST were measured in serum samples. Noteworthy rises (p < 0.05) were noticed in ALT and AST activities with marked decreases in TP in respect to the control group. Adversely, dietary supplementation with SeY markedly reversed (p < 0.05) Cu-induced alterations relative to the Cu-treated group (Figure 2). In addition, marked declines (p < 0.05) were noticed in serum levels of TC and TG in the Cu-treated group in relation to the sham group, but the supplement of SeY resulted in prominent rises (p < 0.05) in their level compared to Cu treated only (Figure 2).
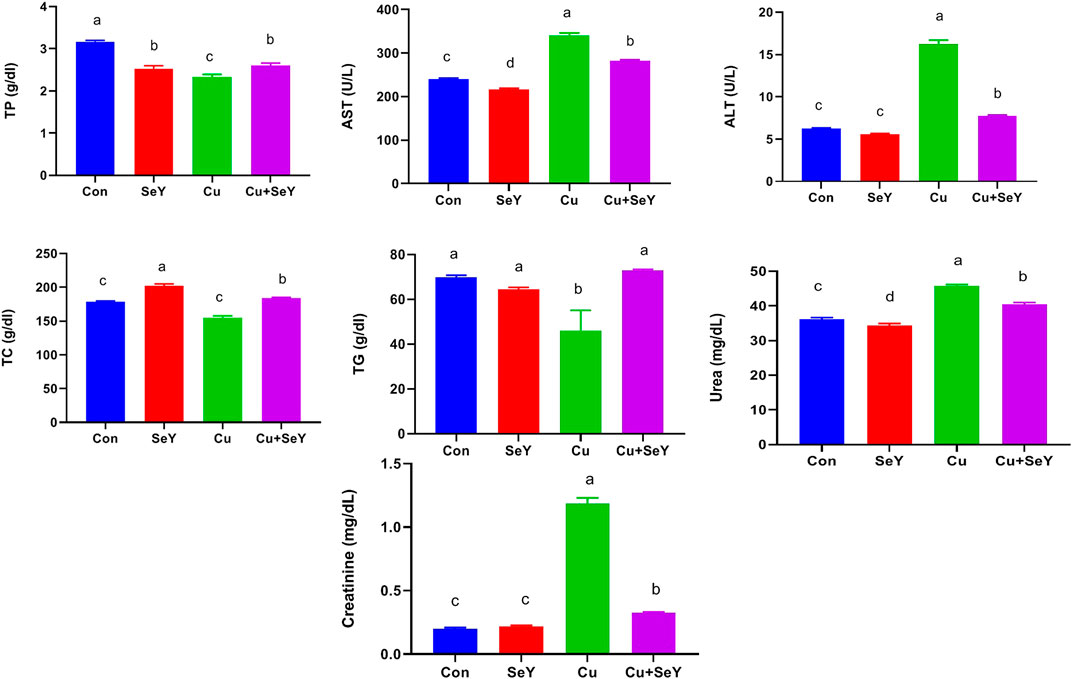
FIGURE 2. Serum biochemical markers following dietary exposure to copper sulphate (CuSO4, 300 mg/kg) and/or selenium yeast (SeY, 0.5 mg/kg) in broiler chicken for 42 days. The values were represented as means ± SE (n = 6). Each bar carrying different letters is significantly different (p < 0.05).
Regarding the effect of SeY on Cu-induced renal functions, serum levels of urea and creatinine were investigated. Our results showed that dietary Cu exposure induced substantial increments (p < 0.05) in urea and creatinine levels in relation to the control. However, their levels notably decreased (p < 0.05) with the combined treatment with Cu plus SeY (Figure 2).
To investigate the impact of Cu and/or SeY on hepatic oxidative stress, enzymatic and non-enzymatic biomarkers were investigated. As shown in Figure 5, significant depletions were noticed in the contents of SOD, CAT, GPx, and GSH (p < 0.05) in the Cu-administered group compared to the control group. These changes were obviously reversed (p < 0.05) after dietary supplementation with SeY. The lipid peroxidation expressed in MDA level showed a substantial rise (p < 0.05) in hepatic tissue of Cu group in relation to the control. The SeY group had a marked lower MDA level (p < 0.05) than that of the group that received Cu only (Figure 3).
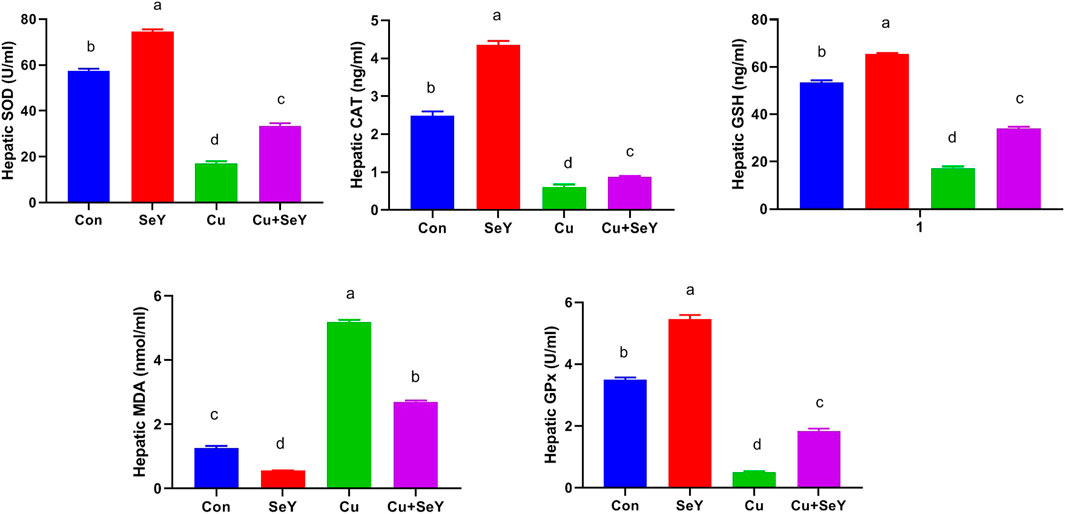
FIGURE 3. Changes in the glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) activities and reduced-glutathione (GSH) and malondaialdehyde (MDA) levels in the liver tissue following dietary exposure to copper sulphate (CuSO4, 300 mg/kg) and/or selenium yeast (SeY, 0.5 mg/kg) in broiler chicken for 42 days. The values were represented as means ± SE (n = 6). Each bar carrying different letters is significantly different (p < 0.05).
The renal contents of SOD, CAT, GPx, and GSH were meaningfully decreased (p < 0.05) in CuSO4-exposed chicken compared to the controls. Contrarily, a remarkable decline (p < 0.05) was detected in renal MDA level in CuSO4 group relative to the sham group. Moreover, the Cu-induced alterations in renal lipid peroxide level and antioxidative biomarkers were counteracted by SeY supplementation compared to the Cu group (Figure 4).
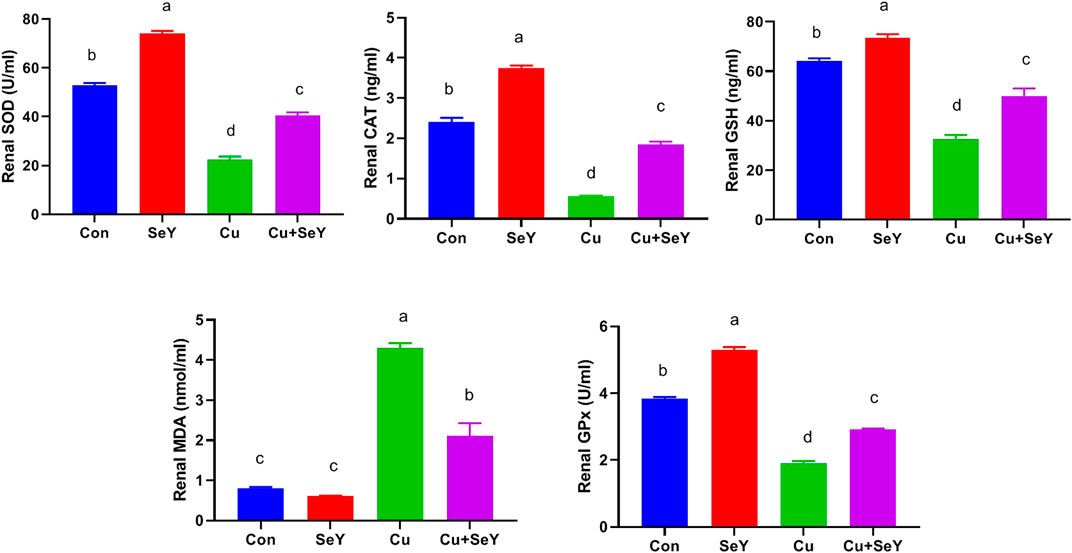
FIGURE 4. Changes in the glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) activities and reduced-glutathione (GSH), and malondaialdehyde (MDA) levels in the renal tissue following dietary exposure to copper sulphate (CuSO4, 300 mg/kg) and/or selenium yeast (SeY, 0.5 mg/kg) in broiler chicken for 42 days. The values were represented as means ± SE (n = 6). Each bar carrying different letters is significantly different (p < 0.05).
As depicted in Figure 5, the CuSO4-exposed group displayed meaningfully higher levels (p < 0.05) of pro-inflammatory cytokines, such as IL-1β and TNF-α, along with NO, in both tissues compared to controls. However, after SeY supplementation, their levels declined (p < 0.05) compared to the Cu-treated group, indicating the anti-inflammatory potential of SeY dietary inclusion.
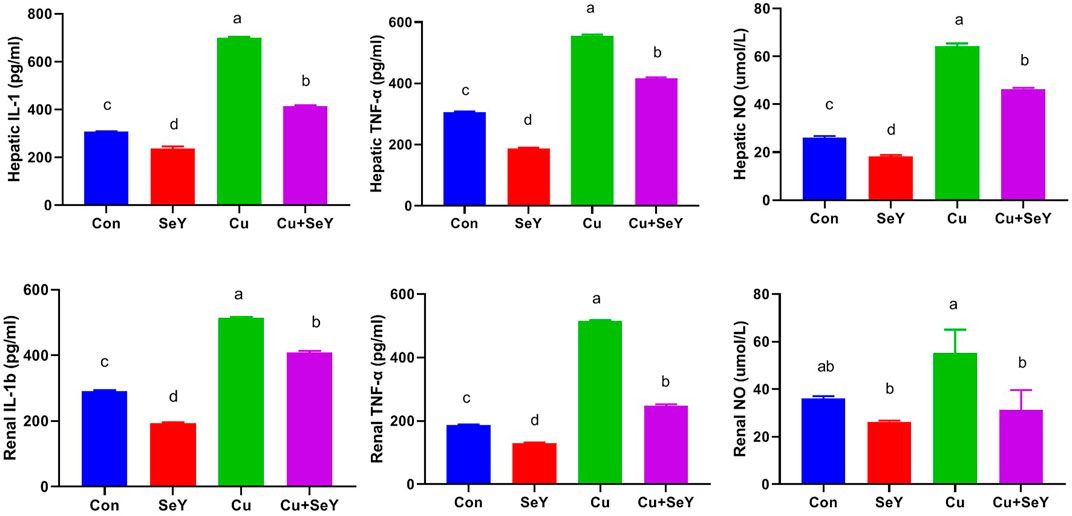
FIGURE 5. Hepatic and renal levels of IL-1β, TNF- α, and NO following dietary exposure to copper sulphate (CuSO4, 300 mg/kg) and/or selenium yeast (SeY, 0.5 mg/kg) in broiler chicken for 42 days. The values were represented as means ± SE (n = 6). Each bar carrying different letters is significantly different (p < 0.05).
Microscopic pictures of hepatic sections from a control group and group that received SeY showed normally arranged hepatocytes in radial plates around central veins with normal sinusoids and portal areas. Further, liver sections in birds received Cu showing portal fibrosis, periportal coagulative necrosis of hepatocytes, and large lymphocyte follicular aggregation. In contrast, mild portal fibrosis with small lymphocytes follicular aggregation was seen in Cu+SeY group (Figure 6).
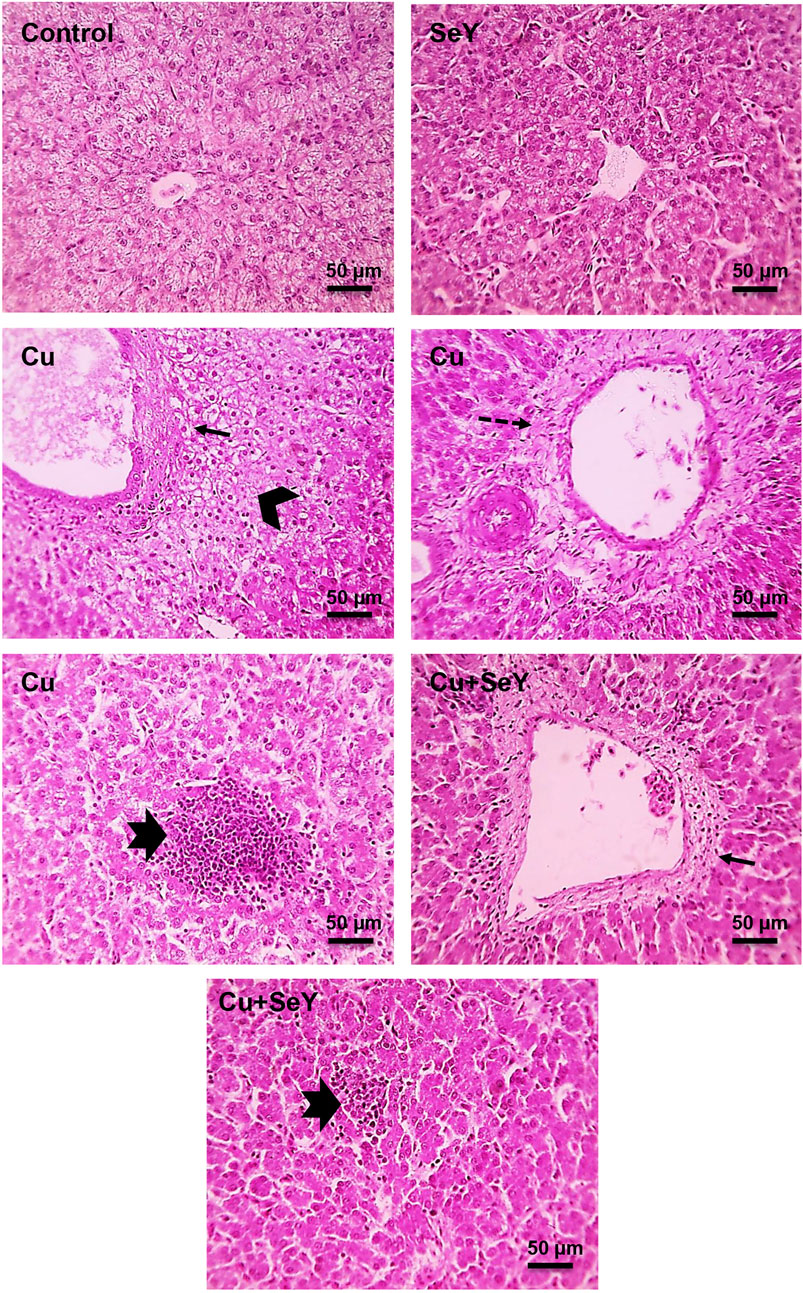
FIGURE 6. Microscopic picture of HE-stained liver sections from Control and SeY groups show normally arranged hepatocytes in radial plates around central veins with normal sinusoids and portal areas. However, liver sections from Cu group show portal fibrosis (thin black arrows), periportal coagulative necrosis of hepatocytes (black arrowheads), large lymphocytes follicular aggregation (thick black arrows). Cu+SeY group exhibited mild portal fibrosis (thin black arrows) with small lymphocytes follicular aggregation (thick black arrows). Bars = 50 µm.
Microscopical screening of kidney sections from control and SeY groups showing normal tubules, glomeruli, and interstitial tissue. However, those from Cu-intoxicated birds exhibited prominent tubular necrosis, few apoptotic cells, congested inter-tubular blood vessels, and severe lymphocytic infiltration. On another hand, mild tubular necrosis, congested inter-tubular blood vessels, and mild lymphocytic aggregation in interstitial tissue were recorded after supplementation of SeY to the Cu-exposed chicks (Figure 7).

FIGURE 7. Microscopic pictures of HE-stained kidney sections from Control group and SeY group show normal tubules, glomeruli, and interstitial tissue. Kidney sections from Cu-treated group exhibit prominent tubular necrosis (thin black arrows), few apoptotic cells (arrowheads), congested inter-tubular blood vessels (red arrows), and large lymphocytes follicular aggregation in interstitial tissue (thick black arrows). Cu+SeY group presents mild tubular necrosis (thin black arrows), congested inter-tubular blood vessels (red arrows), and small lymphocytes follicular aggregation in interstitial tissue (thick black arrows). Bars = 50 µm.
A multivariate analysis (principal component analysis, PCA) was conducted and a smaller set of “summary indices” were elaborated as exhibited in Figure 8A. These data set showed how the studied variables contributed and grouped together along Dimension1 and 2 in response to different treatments. The present PCA indicated that Dimension 1 and 2 had the major contribution (80.7% and 8.2%, respectively). In the same data frame, the PCA score plot revealed that NO, TNF-α, IL-1β, MDA, urea, creatinine, and Cu residues have a tendency to change in the same way in response to Cu toxicity, hence they are grouped oppositely to the Control and SeY groups. Interestingly, the Cu+SeY group is located in the middle between both arms.
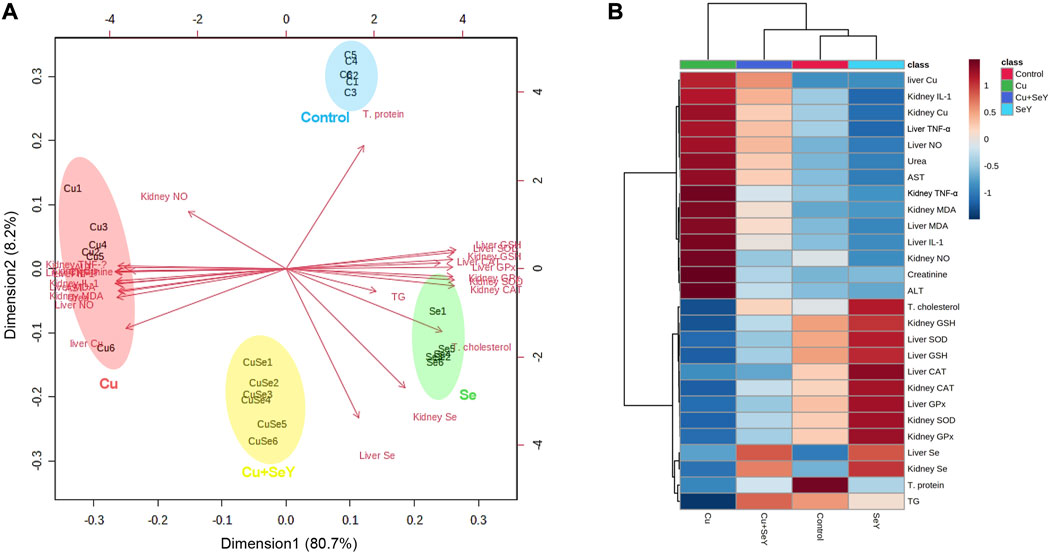
FIGURE 8. Principal components analysis (PCA) and clustering heatmap of SeY versus Cu-induced hepatorenal damage. (A) Score biplot of PCA and variable contribution. (B) Clustering heatmap enables intuitive visualization of all variable concentration values (dark red is the highest and blue represents the lowest values). C1-6, Samples of Control group; CAT, catalase; Cu1-6, Samples of Cu group; CuSe1-6, Samples of Cu+SeY group; GPx, glutathione peroxidase; GSH, reduced-glutathione; MDA, malondialdehyde; Se1-6, Samples of SeY group; SOD, superoxide dismutase; TG, triglycerides; T. protein, total protein.
Moreover, the clustering heatmap seen in Figure 8B summarizes the concentrations of all determined variables in groups. The heatmap shows the variable concentrations in the Cu-exposed birds are negatively correlated to the same corresponding concentrations in other treatments.
The current experiment unveiled that SeY was able to reverse the hepatorenal impairment raised by Cu toxicity via improving organ functions, enhancing the antioxidant enzymatic activity, and lessening the tissue inflammation in growing broiler chicks. The excess Cu which cannot be digested and absorbed can induce liver injury and negatively affects other organs through circulation (Gaetke et al., 2014). In our study, we measured both Cu and Se levels in hepatic and renal tissues. The results revealed that chicken feeding on a Cu-containing diet resulted in increases in its residues in the liver and kidney compared to the control birds. Former studies showed that hepatic Cu retention increased with dietary Cu supplementation (Kim and Kil, 2015; Wu et al., 2020). Another study employed in chickens indicated that the amount of Cu in the kidney increased somewhat as the amount of time exposed to Cu increased (Elazab et al., 2021). Interestingly, the supplementation with SeY conferred a significant antagonistic action against hepatorenal Cu accumulation in chicken. Lui et al. observed that SeY supplementation at a dose of 0.3 mg/kg for 35 days could markedly decrease lead overload in the skeletal muscles of intoxicated chickens (Liu et al., 2019). Additionally, raising chicken on 3 mg/kg SeY for 90 days evoked a noticeable reduction in the accumulation of cadmium in the chicken heart (Ge et al., 2021a). These findings indicated that SeY possesses an efficient metal chelation power via forming inactive complexes with heavy metals and enhancing its excretion with subsequent decreases in their concentrations.
The accumulation of excess Cu in both hepatic and renal tissues resulted in disturbance in these organs’ functions. Our findings showed significant increases in serum transaminases and decreased serum TP levels in the Cu-exposed group, implying hepatotoxicity because the liver is the central location for Cu accumulation. These findings agree with former studies (Wu et al., 2020; Hashem et al., 2021a). This may be endorsed for the hepatic damage that resulted in excess release of hepatic intracellular enzymes into the bloodstream (Atta et al., 2009). Additionally, the observed hypoproteinemia in our results may refer to the impairment in protein synthesis because of liver damage and/or excess protein loss due to renal insufficiency (Al Aboud et al., 2021). These results are well reinforced by the histopathological findings’ periportal coagulative necrosis of hepatocytes. However, supplementation of 450 mg/kg copper proteinate decreased ALT in Bovans laying hens after exposure for 4 weeks, that may be due to the slightly restricted food consumption (Güçlü et al., 2008).
On the contrary, the present study showed that the dietary supplement SeY improved these liver damage biomarkers and decreased the hepatic histological irregularities caused by Cu stress. Similar outcomes were stated by Malyar et al, (2021), who found that selenium-rich Saccharomyces cerevisiae restored the liver function biomarkers in rats kept under high heat stress for 42 days. Moreover, SeY protected against ochratoxin-induced elevations in hepatic AST and ALT in chicken exposed to a contaminated diet for 21 days (Li et al., 2020b). Hence, the modulating effect of SeY on liver function markers indicated its protective effect on the cell membrane of hepatocytes and block the enzyme leak into the blood circulation.
Since the kidney is the leading platform for the excretion of Cu, the renal tubules are vulnerable to Cu harm (Elazab et al., 2021). The analysis of renal function tests unveiled momentous upsurges in serum levels of urea and creatinine in CuSO4-intoxicated birds. As the kidney discharges the nitrogenous end products of the catabolic process, the increases in both biomarkers indicate the impairment in kidney functions (Abdeen et al., 2021; Othman et al., 2021). The histopathological screening of the kidney validated these results characterized by prominent tubular necrosis, few apoptotic cells, congested inter-tubular blood vessels, and large lymphocytes follicular aggregation in the interstitial tissue. These outcomes align with former reports illustrating the adverse renal pathology induced by Cu toxicity in various animal models (Baruah et al., 2018, Hashem et al., 2021b). Wang and colleagues (Wang et al., 2017) reported that chickens exposed to CuSO4 at 300 mg/kg food level for 12 weeks also developed atrophied glomeruli and tubular casts. The tubular cells also experienced degeneration and necrosis. Hence, it could be concluded that Cu impaired both glomerular and tubular functions with deteriorations of overall renal performance. Remarkably, this study’s findings showed that SeY obviously counteracted Cu-encouraged renal dysfunction in exposed chicken. Ge et al, (2021b) reported similar results in cadmium-exposed chicken and co-treated with SeY for 90 days. Also, renal function-related biomarkers (creatinine, urea, and uric acid) displayed significant decreases in the SeY-supplemented chicken related to the ochratoxin-intoxicated group (Li et al., 2020a). This nephroprotective effect of SeY refers to its antioxidant effect, and this was confirmed pathologically by the improved renal histoarchitecture.
Our results also showed that birds supplemented with excess CuSO4 had marked declines in serum TG and TC compared with the controls, which coincides with previous reports (Idowu et al., 2011; Hashem et al., 2021a). The rate-limiting enzyme in the catabolic process of cholesterol 7-hydroxylase was found to be more active when Cu-supplemented meals were administered (Konjufca et al., 1997). Further, adding Cu to the chicken diet diminished the contents of GSH which suppressed the activity of β-methylglutaryl-CoA reductase with a subsequent decrease in the cholesterol level (Kim et al., 1992). Additionally, dietary Cu caused substantial drops in the hepatic lipogenic enzyme activity, 17 beta-estradiol, and plasma lipid levels (Pearce et al., 1983). Therefore, these reductions in TC and TG of Cu-exposed chickens are caused by reduced cholesterol synthesis, increased lipid degradation, or excretion rate. However, different outcomes were reported by earlier studies. Cinar and collaborators did not find any alteration in plasma total cholesterol levels in copper-exposed broilers (Cinar et al., 2014). Additionally, marked decreases were reported in plasma triglycerides and cholesterol in Arbor-Acre unsexed broilers exposed to dietary CuSO4 or copper proteinate at 50, 100, or 150 mg/kg doses for 56 days (Jegede et al., 2011). These differences may endorse breed, diet components, and the investigational strategy. On the other side, the administration of SeY decreased the opposing effect of Cu on lipid metabolism-related markers. This might be attributed to the capacity of SeY to scavenge free radicals and defense against lipid structure peroxidation.
In addition to the induction of hepatorenal impairments in exposed broilers, Cu elicits over-generation hydroxyl radicals and hydrogen peroxide via Fenton and Haber-Weiss reactions (Wang et al., 2018). Many scholars have pointed out that the excess generation of ROS that overwhelm the cellular capacity is one of the hallmarks of heavy metals’ harmful actions (Albarakati et al., 2020; Kassab et al., 2020; Al Aboud et al., 2021). These highly reactive radicals could modify the structure or/and function of cellular molecules. The current findings revealed that chickens exposed to dietary Cu developed an imbalance in their liver and kidney’s oxidant/antioxidant status. SOD can hamper the superoxide anion and convert it into H2O2, which CAT dissociates into water (Albarakati et al., 2020). GPx significantly contributes to the protective function of CAT and is required for the regeneration of GSH. The significant increase in the levels of MDA in both organs indicated that Cu exposure enhanced the formation of OH•, which directly interacted with the polyunsaturated fatty acids in cellular membrane lipid, which led to lipid peroxidation (Wang et al., 2018). Zhao et al. (Zhao et al., 2018) found significant decreases in SOD activity and GSH content in the chicken jejunum in a time-dependent manner after dietary exposure to 300 mg/kg Cu. In another related study, decreases in SOD, CAT, and GPx with increases in MDA in the immune organs were reported in chicken fed on a diet containing Cu at different concentrations for 49 days (Yang et al., 2020). Nevertheless, CuSO4 exposure for 30 days did not evoke significant differences in total antioxidant capacity in chicken jejunum (Zhao et al., 2018).
In contrast, our data demonstrated that SeY could enhance the hepatorenal antioxidant capacity by decreasing MDA levels and restoring the activity of GPx, SOD, CAT, and GSH concentrations. Se is a crucial cofactor for enzymes involved in scavenging ROS and dietary supplements of sodium selenide, and SeY increases birds’ stress tolerance (Arnaut et al., 2021). Li et al. recorded upregulation of hepatic GPx, GLRX2, and MnSOD mRNA expression along with Nrf2 protein expression and its downstream (Keap-1 and HO-1) after dietary inclusion with 0.4 mg/kg SeY in broilers (Li et al., 2020a) suggesting the possible involvement of Nrf2/HO-1/Keap-1 in the protective role of SeY (Li et al., 2020b). Our data were in agreement with those obtained by Cao et al. who reported a reduced aluminum-induced testicular damage in mice after SeY supplementation via modifying the redox status (Cao et al., 2020). Elevated ROS can trigger the production of pro-inflammatory cytokines and different inflammatory cells and the release of inflammatory mediators (AL-Megrin et al., 2020). High dietary Cu exposure has been documented to enhance the IL-1β and TNF-α levels in chicken immune organs (Yang et al., 2020) and liver (Liu et al., 2018a). Concurrently, the existing experiment presented that Cu intoxication caused significant elevations in the IL-1β and TNF-α. Interestingly, the proposed findings indicated that SeY could antagonize the Cu-enhanced hepatic and renal inflammatory responses. Se-rich S. cerevisiae downregulated the hepatic gene expression of IL-6, TNF-α, COX-2, and NF-κB of heat stressed (Malyar et al., 2021) and aluminium-intoxicated (Luo et al., 2018) Wister rats. Supporting a former study (Cao et al., 2020), SeY significantly mitigated aluminum-induced testicular toxicity by decreasing the level of NO and NOS activities. Therefore, we strongly assume that SeY might mitigate hepatorenal inflammation induced by Cu exposure via down-regulation of the inflammatory mediators.
Moreover, the PCA data indicated that all studied variables are clustered into four zones along Dimension1 (80.7% contribution) and Dimension2 (8.2% contribution). Such distribution was depending on different treatments, where, Cu-intoxicated birds were clustered on the left side and could be markedly discriminated from other treated groups confirming the occurrence of Cu toxicity. PCA also confirmed the protective effects of SeY supplementation since the Cu+SeY group has deviated to the midplane between Cu-intoxicated birds and non-intoxicated ones (Control and SeY groups). Moreover, the clustering heatmap summarizes the concentration levels of all measured parameters among different groups. The heatmap suggests that the variable concentrations in the Cu-intoxicated group are negatively correlated to the same corresponding concentrations in other groups. The molecular mechanisms located behind the ameliorative action of SeY toward Cu-stressed chickens are illustrated in Figure 9.
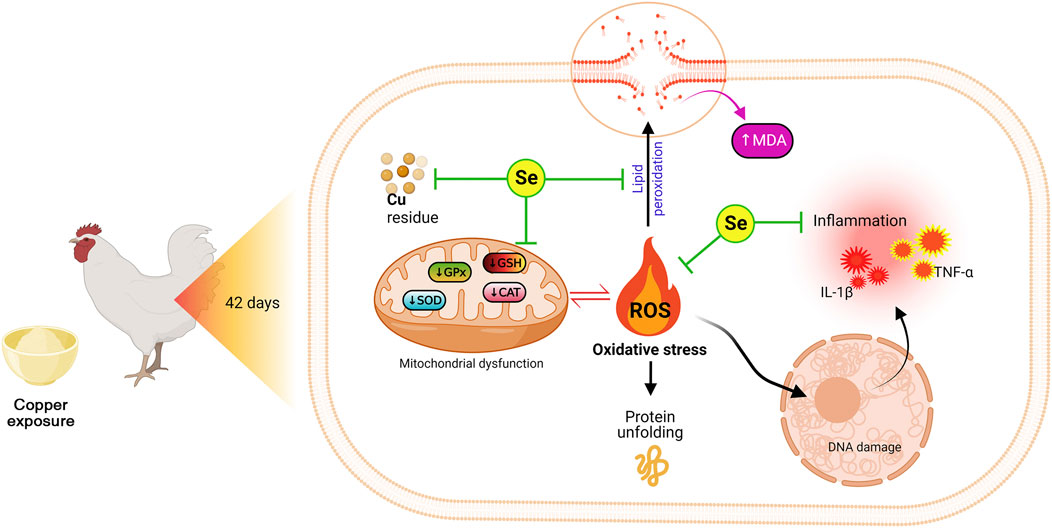
FIGURE 9. The molecular mechanisms located behind the ameliorative action of SeY toward Cu-stressed chickens.
Collectively, this study introduced the alleviating effect of SeY against Cu-induced liver and kidney damage. SeY could reduce the extra-release of inflammatory mediators (IL-1β, TNF-α, and NO) and suppress lipid peroxidation and metal bioaccumulation. These protective mechanisms may be achieved by enhancing the antioxidant enzymatic of SOD, CAT, and GPx, alongside elevating the GSH contents. We strongly suggest that SeY could be a potential feed supplement that offers therapeutic evidence against the Cu-induced liver and kidney injury in chickens. In the future studies, further investigations are required for unveiling the underlying mechanisms for the antagonistic efficacy of Se-Y against heavy metal-induced damage in birds.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by Faculty of Veterinary Medicine Ethical Committee.
OH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing—original draft, Writing—review and editing. XW: Conceptualization, Data curation, Formal analysis, Software, Visualization, Supervision, Validation, Writing—original draft, Writing—review and editing. HO and HG: Conceptualization, Data curation, Formal analysis, Writing—review and editing. AA and AZ: Formal analysis, Resources, Software, Validation, Writing—original draft. MG, AK, EE, and AE-M: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing—original draft. MD, RS, and BS: Data curation, Formal analysis, Software, Visualization, Writing—original draft. SI: Funding acquisition, Resources, Software, Validation, Visualization, Writing—original draft, Writing—review and editing. Ahmed Abdeen: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Visualization, Writing—review and editing.
This research was funded by the Deanship of Scientific Research at King Khalid University through the Large Groups Project under grant number R.G.P2/200/43. This work was also funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R127), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
The authors are grateful for the resources provided by the Deanship of Scientific Research at King Khalid University through the Large Groups Project under grant number R.G.P2/200/43. Authors also acknowledge the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R127), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The appreciation is also extended to the Center of Excellence in Screening of Environmental Contaminants (funded by STDF, Egypt) for the support provided to this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdeen, A., Samir, A., Elkomy, A., Aboubaker, M., Habotta, O. A., Gaber, A., et al. 2021. The potential antioxidant bioactivity of date palm fruit against gentamicin-mediated hepato-renal injury in male albino rats. Biomed. Pharmacother., 143, 112154.doi:10.1016/j.biopha.2021.112154
Aebi, H. (1984). Catalase in vitro. Methods Enzymol. 105, 121–126. doi:10.1016/s0076-6879(84)05016-3
Al Aboud, D., Baty, R. S., Alsharif, K. F., Hassan, K. E., Zhery, A. S., Habotta, O. A., et al. (2021). Protective efficacy of thymoquinone or ebselen separately against arsenic-induced hepatotoxicity in rat. Environ. Sci. Pollut. Res. Int. 28, 6195–6206. doi:10.1007/s11356-020-10955-1
Al-Megrin, W. A., Metwally, D. M., Habotta, O. A., Amin, H. K., Abdel Moneim, A. E., and El-Khadragy, M. (2020). Nephroprotective effects of chlorogenic acid against sodium arsenite-induced oxidative stress, inflammation, and apoptosis. J. Sci. Food Agric. 100, 5162–5170. doi:10.1002/jsfa.10565
Albarakati, A. J. A., Baty, R. S., Aljoudi, A. M., Habotta, O. A., Elmahallawy, E. K., Kassab, R. B., et al. (2020). Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol. Biol. Rep. 47, 2591–2603. doi:10.1007/s11033-020-05346-1
Arnaut, P. R., Da Silva Viana, G., Da Fonseca, L., Alves, W. J., Muniz, J. C. L., Pettigrew, J. E., et al. (2021). Selenium source and level on performance, selenium retention and biochemical responses of young broiler chicks. BMC Vet. Res. 17, 151–213. doi:10.1186/s12917-021-02855-4
Atta, A. H., Fathy, S., Gohar, M., Jan, R., Kamel, G., Mouneir, S. M., et al. (2009). Prolonged administration of high doses of copper nicotinate to rats: Effect on biochemical and cellular constituents of blood and on copper level in serum, liver and muscle. Int. J. Med. Med. Sci. 1, 178–183. doi:10.5897/IJMMS.9000191
Attia, Y. A., Qota, E. M., Zeweil, H. S., Bovera, F., Abd Al-Hamid, A. E., and Sahledom, M. D. 2012. Effect of different dietary concentrations of inorganic and organic copper on growth performance and lipid metabolism of White Pekin male ducks. Br. Poult. Sci., 53, 77–88.doi:10.1080/00071668.2011.650151
Baruah, S., Goswami, S., and Kalita, D. (2018). Haematobiochemical and pathological alterations of chronic copper toxicity in ducks. J. Animal Res. 8, 283–287. doi:10.30954/2277-940X.04.2018.18
Cao, C., Zhang, H., Wang, K., and Li, X. 2020. Selenium-rich yeast mitigates aluminum-mediated testicular toxicity by blocking oxidative stress, inhibiting NO production, and disturbing ionic homeostasis. Biol. Trace Elem. Res., 195, 170–177.doi:10.1007/s12011-019-01820-5
Chemists, A. O. O. A. (1925). Official methods of analysis of the association of official analytical Chemists. California, United States: The Association.
Chen, H., Li, P., Shen, Z., Wang, J., and Diao, L. (2021). Protective effects of selenium yeast against cadmium-induced necroptosis through mir-26a-5p/pten/pi3k/akt signaling pathway in chicken kidney. Ecotoxicol. Environ. Saf. 220, 112387. doi:10.1016/j.ecoenv.2021.112387
Cinar, M., Yildirim, E., Yigit, A. A., Yalcinkaya, I., Duru, O., Kisa, U., et al. 2014. Effects of dietary supplementation with vitamin C and vitamin E and their combination on growth performance, some biochemical parameters, and oxidative stress induced by copper toxicity in broilers. Biol. Trace Elem. Res., 158, 186-96.doi:10.1007/s12011-014-9926-6
Coulombe, J., and Favreau, L. (1963). A new simple semimicro method for colorimetric determination of urea. Clin. Chem. 9, 102–108. doi:10.1093/clinchem/9.1.102
Elazab, S. T., Elshater, N. S., Kishaway, A. T., and Ei-Emam, H. A. (2021). Cinnamon extract and probiotic supplementation alleviate copper-induced nephrotoxicity via modulating oxidative stress, inflammation, and apoptosis in broiler chickens. Animals. 11, 1609. doi:10.3390/ani11061609
Ellman, G. L. 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys., 82, 70-7.doi:10.1016/0003-9861(59)90090-6
Gaetke, L. M., Chow-Johnson, H. S., and Chow, C. K. (2014). Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 88, 1929–1938. doi:10.1007/s00204-014-1355-y
Ge, J., Guo, K., Zhang, C., Talukder, M., Lv, M. W., Li, J. Y., et al. 2021a. Comparison of nanoparticle-selenium, selenium-enriched yeast and sodium selenite on the alleviation of cadmium-induced inflammation via NF-kB/IκB pathway in heart. Sci. Total Environ., 773, 145442.doi:10.1016/j.scitotenv.2021.145442
Ge, J., Liu, L. L., Cui, Z. G., Talukder, M., Lv, M. W., Li, J. Y., et al. 2021b. Comparative study on protective effect of different selenium sources against cadmium-induced nephrotoxicity via regulating the transcriptions of selenoproteome. Ecotoxicol. Environ. Saf., 215, 112135. doi:10.1016/j.ecoenv.2021.112135
Giambrone, J., and Clay, R. P. (1986). Vaccination of day-old broiler chicks against Newcastle disease and infectious bursal disease using commercial live and/or inactivated vaccines. Avian Dis. 30, 557–561. doi:10.2307/1590421
Green, L. C., Wagner, D. A., Glogowski, J., Skipper, P. L., Wishnok, J. S., and Tannenbaum, S. R. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126, 131–138. doi:10.1016/0003-2697(82)90118-x
Güçlü, B. K., Kara, K., Beyaz, L., Uyanik, F., Eren, M., and Atasever, A. 2008. Influence of dietary copper proteinate on performance, selected biochemical parameters, lipid peroxidation, liver, and egg copper content in laying hens. Biol. Trace Elem. Res., 125, 1609.doi:10.1007/s12011-008-8164-1
Habotta, O. A., Elbahnaswy, S., and Ibrahim, I. (2022). Neurotoxicity of singular and combined exposure of Oreochromis niloticus to methomyl and copper sulphate at environmentally relevant levels: Assessment of neurotransmitters, neural stress, oxidative injury and histopathological changes. Environ. Toxicol. Pharmacol. 94, 103935. doi:10.1016/j.etap.2022.103935
Hashem, M. A., Abd El Hamied, S. S., Ahmed, E., Amer, S. A., and El-Sharnouby, M. E. (2021a). Mitigating the growth, biochemical changes, genotoxic and pathological effects of copper toxicity in broiler chickens by supplementing vitamins C and E. Animals. 11, 1811. doi:10.3390/ani11061811
Hashem, M. A., Abd El Hamied, S. S., Ahmed, E. M. A., Amer, S. A., and Hassan, A. M. 2021b. Alleviating effects of vitamins C and E supplementation on oxidative stress, hematobiochemical, and histopathological alterations caused by copper toxicity in broiler chickens. Animals., 11 1739.doi:10.3390/ani11061739
Hassan, M. A., Hozien, S. T., Abdel Wahab, M. M., and Hassan, A. M. 2022. Ameliorative effect of selenium yeast supplementation on the physio-pathological impacts of chronic exposure to glyphosate and or malathion in Oreochromis niloticus. BMC Vet. Res., 18, 159.doi:10.1186/s12917-022-03261-0
Idowu, O., Ajuwon, O., Fafiolu, A., Oso, A., and Akinloye, O. (2011). Modulation of cholesterol and copper residue levels in muscles and blood serum of finishing broiler chickens fed copper and ascorbic acid supplements. Pak. J. Nutr. 10, 781–785. doi:10.3923/pjn.2011.781.785
Ismail, T., Hegazi, E., Nassef, E., Habotta, O. A., and Gewaily, M. S. 2022. The optimized inclusion level of Bacillus subtilis fermented Azolla pinnata in nile tilapia (Oreochromis niloticus) diets: Immunity, antioxidative status, intestinal digestive enzymes and histomorphometry, and disease resistance. Fish. Physiol. Biochem.48, 767, 783. doi:10.1007/s10695-022-01076-2
Jegede, A. V., Oduguwa, O. O., Bamgbose, A. M., Fanimo, A. O., and Nollet, L. 2011. Growth response, blood characteristics and copper accumulation in organs of broilers fed on diets supplemented with organic and inorganic dietary copper sources. Br. Poult. Sci., 52, 133–139.doi:10.1080/00071668.2010.544714
Kassab, R. B., Lokman, M. S., Daabo, H. M., Gaber, D. A., Habotta, O. A., Hafez, M. M., et al. (2020). Ferulic acid influences Nrf2 activation to restore testicular tissue from cadmium-induced oxidative challenge, inflammation, and apoptosis in rats. J. Food Biochem. 44, e13505. doi:10.1111/jfbc.13505
Kieliszek, M., Błażejak, S., Gientka, I., and Bzducha-Wróbel, A. (2015). Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 99, 5373–5382. doi:10.1007/s00253-015-6650-x
Kim, J. W., and Kil, D. Y. (2015). Determination of relative bioavailability of copper in tribasic copper chloride to copper in copper sulfate for broiler chickens based on liver and feather copper concentrations. Animal Feed Sci. Technol. 210, 138–143. doi:10.1016/j.anifeedsci.2015.09.022
Kim, S., Chao, P. Y., and Allen, K. G. (1992). Inhibition of elevated hepatic glutathione abolishes copper deficiency cholesterolemia. FASEB J. 6, 2467–2471. doi:10.1096/fasebj.6.7.1563598
Konjufca, V. H., Pesti, G. M., and Bakalli, R. I. (1997). Modulation of cholesterol levels in broiler meat by dietary garlic and copper. Poult. Sci. 76, 1264–1271. doi:10.1093/ps/76.9.1264
Larsen, K. (1972). Creatinine assay in the presence of protein with LKB 8600 reaction rate analyser. Clin. Chim. Acta. 38, 475–476. doi:10.1016/0009-8981(72)90146-5
Leary, S., Underwood, W., Anthony, R., Corey, D., Grandin, T., Gwaltney-Brant, S., et al. (2016). in AVMA guidelines for the humane slaughter of animals: 2016 edition. Editor A. V. M. Association (Schaumburg, Illinois, United States: AVMA), 64.
Li, K., Cao, Z., Guo, Y., Tong, C., Yang, S., Long, M., et al. (2020a). Selenium yeast alleviates ochratoxin A-induced apoptosis and oxidative stress via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in the kidneys of chickens. Oxid. Med. Cell. Longev. 2020, 4048706. doi:10.1155/2020/4048706
Li, P., Li, K., Zou, C., Tong, C., Sun, L., Cao, Z., et al. (2020b). Selenium yeast alleviates ochratoxin A-induced hepatotoxicity via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in chickens. Toxins 12, 143. doi:10.3390/toxins12030143
Li, X., Hua, J., Wang, S., Hu, Z., Wen, A., and Yang, B. (2022). Genes and signaling pathways involved in the regulation of selenium-enriched yeast on liver metabolism and health of broiler (Gallus gallus). Biol. Trace Elem. Res. 2022, 1–16. doi:10.1007/s12011-022-03150-5
Liao, J., Yang, F., Bai, Y., Yu, W., Qiao, N., Han, Q., et al. (2021). Metabolomics analysis reveals the effects of copper on mitochondria-mediated apoptosis in kidney of broiler chicken (Gallus gallus). J. Inorg. Biochem. 224, 111581. doi:10.1016/j.jinorgbio.2021.111581
Liu, J., Zhao, H., Wang, Y., Shao, Y., Li, J., and Xing, M. (2018a). Alterations of antioxidant indexes and inflammatory cytokine expression aggravated hepatocellular apoptosis through mitochondrial and death receptor-dependent pathways in Gallus gallus exposed to arsenic and copper. Environ. Sci. Pollut. Res. Int. 25, 15462–15473. doi:10.1007/s11356-018-1757-0
Liu, J., Zhao, H., Wang, Y., Shao, Y., Zhang, L., and Xing, M. (2018b). Impacts of simultaneous exposure to arsenic (III) and copper (II) on inflammatory response, immune homeostasis, and heat shock response in chicken thymus. Int. Immunopharmacol. 64, 60–68. doi:10.1016/j.intimp.2018.08.021
Liu, Z., Zhang, F., Lu, P., Zhao, R., Zhang, H., Song, B., et al. (2019). Selenium-yeast alleviated inflammatory damage caused by lead via inhibiting ras/erk pathway and inflammatory factors in chicken skeletal muscles. Biol. Trace Elem. Res. 190, 493–500. doi:10.1007/s12011-018-1558-9
Lowry, O., Rosebrough, N., Farr, A. L., and Randall, R. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. doi:10.1016/s0021-9258(19)52451-6
Lu, L., Wang, R. L., Zhang, Z. J., Steward, F. A., Luo, X., and Liu, B. (2010). Effect of dietary supplementation with copper sulfate or tribasic copper chloride on the growth performance, liver copper concentrations of broilers fed in floor pens, and stabilities of vitamin E and phytase in feeds. Biol. Trace Elem. Res. 138, 181–189. doi:10.1007/s12011-010-8623-3
Luo, J., Li, X., Li, X., He, Y., Zhang, M., Cao, C., et al. 2018. Selenium-Rich Yeast protects against aluminum-induced peroxidation of lipide and inflammation in mice liver. Biometals, 31, 1051–1059.doi:10.1007/s10534-018-0150-2
Malyar, R. M., Naseri, E., Li, H., Ali, I., Farid, R. A., Liu, D., et al. 2021. Hepatoprotective effects of selenium-enriched probiotics supplementation on heat-stressed wistar rat through anti-inflammatory and antioxidant effects. Biol. Trace Elem. Res., 199, 3445–3456.doi:10.1007/s12011-020-02475-3
Ohkawa, H., Ohishi, N., and Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. doi:10.1016/0003-2697(79)90738-3
Othman, M. S., Khaled, A. M., Al-Bagawi, A. H., Fareid, M. A., Ghany, R. A., Habotta, O. A., et al. 2021. Hepatorenal protective efficacy of flavonoids from ocimum basilicum extract in diabetic albino rats: A focus on hypoglycemic, antioxidant, anti-inflammatory and anti-apoptotic activities. Biomed. Pharmacother., 144, 112287. doi:10.1016/j.biopha.2021.112287
Paglia, D. E., and Valentine, W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70, 158–169. doi:10.5555/uri:pii:0022214367900765
Pearce, J., Jackson, N., and Stevenson, M. H. 1983. The effects of dietary intake and of dietary concentration of copper sulphate on the laying domestic fowl: Effects on some aspects of lipid, carbohydrate and amino acid metabolism. Br. Poult. Sci., 24, 337-48.doi:10.1080/00071668308416748
Reitman, S., and Frankel, S. (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28, 56–63. doi:10.1093/ajcp/28.1.56
Sun, Y., Oberley, L. W., and Li, Y. (1988). A simple method for clinical assay of superoxide dismutase. Clin. Chem. 34, 497–500. doi:10.1093/clinchem/34.3.497
Wang, Y., Chen, H., Chang, W., Chen, R., Xu, S., and Tao, D. (2020a). Protective effects of selenium yeast against cadmium-induced necroptosis via inhibition of oxidative stress and MAPK pathway in chicken liver. Ecotoxicol. Environ. Saf. 206, 111329. doi:10.1016/j.ecoenv.2020.111329
Wang, Y., Liu, J., Chen, R., Qi, M., Tao, D., and Xu, S. (2020b). The antagonistic effects of selenium yeast (SeY) on cadmium-induced inflammatory factors and the heat shock protein expression levels in chicken livers. Biol. Trace Elem. Res. 198, 260–268. doi:10.1007/s12011-020-02039-5
Wang, Y., Zhao, H., Liu, J., Shao, Y., Li, J., Luo, L., et al. (2018). Copper and arsenic-induced oxidative stress and immune imbalance are associated with activation of heat shock proteins in chicken intestines. Int. Immunopharmacol. 60, 64–75. doi:10.1016/j.intimp.2018.04.038
Wang, Y., Zhao, H., Shao, Y., Liu, J., Li, J., and Xing, M. (2017). Copper or/and arsenic induce oxidative stress-cascaded, nuclear factor kappa B-dependent inflammation and immune imbalance, trigging heat shock response in the kidney of chicken. Oncotarget 8, 98103–98116. doi:10.18632/oncotarget.21463
Wu, X., Zhu, M., Jiang, Q., and Wang, L. 2020. Effects of copper sources and levels on lipid profiles, immune parameters, antioxidant defenses, and trace element residues in broilers. Biol. Trace Elem. Res., 194, 251–258.doi:10.1007/s12011-019-01753-z
Yang, F., Liao, J., Yu, W., Pei, R., Qiao, N., Han, Q., et al. (2020). Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken. Ecotoxicol. Environ. Saf. 200, 110715. doi:10.1016/j.ecoenv.2020.110715
Zhao, H., Wang, Y., Shao, Y., Liu, J., Liu, Y., and Xing, M. 2018. Deciphering the ionic homeostasis, oxidative stress, apoptosis, and autophagy in chicken intestine under copper(II) stress. Environ. Sci. Pollut. Res. Int., 25, 33172–33182.doi:10.1007/s11356-018-3163-z
Keywords: copper residue, selenium yeast, oxidative stress, inflammatory cytokines, broiler chicken
Citation: Habotta OA, Wang X, Othman H, Aljali AA, Gewaily M, Dawood M, Khafaga A, Zaineldin AI, Singla RK, Shen B, Ghamry HI, Elhussieny E, El-Mleeh A, Ibrahim SF and Abdeen A (2022) Selenium-enriched yeast modulates the metal bioaccumulation, oxidant status, and inflammation in copper-stressed broiler chickens. Front. Pharmacol. 13:1026199. doi: 10.3389/fphar.2022.1026199
Received: 23 August 2022; Accepted: 28 September 2022;
Published: 14 October 2022.
Edited by:
Alex Boye, University of Cape Coast, GhanaReviewed by:
Afrina Mustari, Bangladesh Agricultural University, BangladeshCopyright © 2022 Habotta, Wang, Othman, Aljali, Gewaily, Dawood, Khafaga, Zaineldin, Singla, Shen, Ghamry, Elhussieny, El-Mleeh, Ibrahim and Abdeen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ola A. Habotta, b2xhX2FsaUBtYW5zLmVkdS5lZw==; Ahmed Abdeen, YWhtZWQuYWJkZWVuQGZ2dG0uYnUuZWR1LmVn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.