
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 30 September 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1023314
Aim: To evaluate the clinical efficacy and safety of Xiaoaiping injection combined with chemotherapy in the treatment of advanced gastric cancer by meta-analysis.
Methods: Seven databases, including China National Knowledge Infrastructure (CNKI), Wanfang Database, VIP Database, Cochrane Library, PubMed, Embase, and Web of Science, were searched by computer for randomized controlled clinical trials of Xiaoaiping injection combined with chemotherapy in the treatment of gastric cancer. Risk of bias assessment and meta-analysis were performed by Review Manager 5.3 software.
Results: There were 16 articles that met the inclusion criteria, with a total of 1,236 patients, 617 in the observation group and 619 in the control group. The results of meta-analysis showed that the observation group was better than chemotherapy alone control group in RR [OR = 1.86, p < 0.00001]; disease control rate (DCR) [OR = 2.45, p < 0.00001]; Karnofsky performance status (KPS) score [OR = 3.21, p < 0.00001] or [MD = 7.73, p = 0.001]. In terms of biochemical indicators, Xiaoaiping significantly reduced inflammation factors level, including tumor necrosis factor alpha (TNF-α) [MD = −15.00, p < 0.00001]; interleukin-6 (IL-6) [MD = −13.00, p < 0.00001]; C-reaction protein (CRP) [MD = −5.80, p < 0.00001]. Xiaoaiping could enhance immune function, significantly reducing myeloid-derived suppressor cells (MDSCs) [MD = −6.20, p < 0.00001] and Treg [MD = −1.70, p < 0.00001]. Xiaoaiping injection combined with chemotherapy could significantly decrease tumor markers, including carcinoembryonic antigen (CEA) [MD = −11.64, p < 0.00001]; CA199 [MD = −33.57, p = 0.02]; CA242 [MD = −20.66, p < 0.00001]; CA125 [MD = −12.50, p = 0.0005]. In the comparison of adverse reactions, the incidence rate of Xiaoaiping injection group was significantly lower than that of control group. The funnel plot showed that the left and right sides are basically symmetrical, and it can be considered that there is no obvious publication bias.
Conclusion: Xiaoaiping injection combined with chemotherapy has better curative effect and less adverse reactions in the treatment of gastric cancer. However, limited by the quality of the included studies, more high-quality studies are still needed to be verified.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022353842], identifier [CRD42022353842].
Gastric cancer is a common malignant tumor of digestive tract, and its morbidity and mortality are high among malignant tumors, which seriously affects the quality of life and physical health of patients (Parkin et al., 1999; Chen et al., 2016). Clinically, it is more common in patients with advanced gastric cancer, which basically loses the technique of radical surgery, and needs to be intervened by anti-tumor drugs such as radiotherapy and chemotherapy to prolong the life cycle and improve the quality of life (Smyth et al., 2020; Thrift and El-Serag 2020; Sung et al., 2021). Clinical strategies for the treatment of gastric cancer include XELOX (oxaliplatin + xeloda), FOLFOX6 regimen (oxaliplatin + leucovorin + 5-fluorouracil), SOX (oxaliplatin + seggio), etc. Patients often cannot tolerate the adverse reactions of radiotherapy and chemotherapy due to low immune function (Johnston and Beckman 2019). Chinese patent medicine may have the effect of increasing the efficacy of radiotherapy and chemotherapy and reducing adverse reactions. In recent years, a variety of Chinese patent medicine injections combined with chemotherapy have shown good efficacy and less side effects in the treatment of gastric cancer (Liu et al., 2015). Xiaoaiping injection is a Chinese patent medicine preparation made of Marsdenia tenacissima (Roxb.) Wight et Arn extract. It has the functions of clearing away heat and detoxifying, resolving phlegm and softening the pain (Chen and Liu 2004; Chen et al., 2021). It had pharmacological effects such as anti-tumor, antihypertensive and antiasthmatic, and immune regulation, clinically used for gastric cancer, lung cancer, esophageal cancer, liver cancer, and other diseases (Chen and Liu 2004). Modern pharmacological research showed the mechanisms of anti-tumor were related with promoting tumor cell apoptosis, inhibiting tumor cell proliferation and tumor blood vessel growth, and regulating immunity, etc. In this study, combined with clinical practice, the conventional chemotherapy + Xiaoaiping injection was used as the observation group, and the conventional chemotherapy was used as the control group. The application of Xiaoaiping injection provides references and suggestions.
The protocol for this review and meta-analysis has been registered on the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42022353842.
The research object is the randomized controlled trials (RCTs) of Xiaoaiping injection in the treatment of gastric cancer published at home and abroad. Seven databases were searched, including China National Knowledge Infrastructure (CNKI), Wanfang, VIP, Cochrane Library, PubMed, Embase and Web of Science. The search period is from inception until July 2022. The search terms included (Gastric Cancer) AND (Xiaoaiping Injection or Xiaoaiping).
1) RCT; 2) Subjects: gastric cancer confirmed by histopathological examination and other imaging data; 3) Group: observation group and control group; 4) Intervention measures: The observation group was treated with Xiaoaiping injection combined with chemotherapy for gastric cancer, and the control group was treated with chemotherapy alone, with unlimited dose and course of treatment; 5) Outcome indicators: the efficacy rate must be included.
1) Non-RCTs; 2) duplicate publications, conference papers, dissertations, etc.; 3) no control group; 4) animal experiments; 5) interventions that are not Xiaoaiping injections, but other formulation type, such as tablets, capsules, etc.; 6) Literature for non-gastric cancer patients.
Two experienced researchers independently read and screened the literature according to the inclusion and exclusion criteria and extracted data from the final included literature. The bias risk of the included studies was evaluated according to the Cochrane Handbook’s Bias Risk Assessment Tool for RCTs. In case of disagreement, consensus was reached with the help of a third author, who comprehensively analyzed and guided whether to include or not, and finally determined the included literature, read the full text of the included literature in detail, and extracted information such as the authors, allocation methods, intervention measures, and outcome indicators.
Review Manager 5.3 software was used for statistical analysis and bias risk assessment. There are four evaluation criteria for the efficacy of drug: complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). The remission rate (RR) was calculated as CR + PR, and the disease control rate (DCR) was calculated as CR + PR + SD. When performing a meta-analysis, the heterogeneity test was first carried out. p > 0.05 and I2 < 50% indicated the favorable homogeneity, so the fixed effect model was used to analyze. p < 0.05 and I2 > 50% indicated the poor homogeneity, so the random effects model was used for analysis. The count data were analyzed by odds ratio (OR); the measurement data were evaluated by mean difference (MD), and each response was expressed with 95% confidence interval (CI), and a forest plot was drawn. Finally, funnel plots were drawn to objectively and quantitatively assess the publication bias of the studies.
16 RCTs were finally included through computer searching, manual screening, and full text reading. The literature screening process is shown in Figure 1. A total of 1,236 patients were included in the 16 RCTs, including 617 cases in the observation group and 619 cases in the control group. Basic characteristics of the included literature were shown in Table 1. The overall bias risk assessment was shown in Figure 2.
16 RCTs (Huo and Chen 2009; Liu and Zhu 2012; Saifuding et al., 2012; Gao et al., 2015; Lin et al., 2015; Ma 2015; Xiong et al., 2015; Zhang and Li 2015; Deng et al., 2016; Li and Ran 2016; Zheng et al., 2017b; Guo et al., 2018; Wang and Chen 2018; Yan et al., 2018; Ge et al., 2019; Ruan et al., 2021) included 1,236 patients reported RR (RR = CR + PR). The results were shown in Figure 3. Heterogeneity test showed p = 0.81, I2 = 0.0%. Therefore, the fixed-effects model was used for analysis. The meta-analysis results showed that RR of the experimental group was significantly higher than that of the control group [OR = 1.86, 95% CI (1.48, 2.35), Z = 5.27, p < 0.00001].
14 literatures (Liu and Zhu 2012; Saifuding et al., 2012; Gao et al., 2015; Lin et al., 2015; Xiong et al., 2015; Zhang and Li 2015; Deng et al., 2016; Li and Ran 2016; Zheng et al., 2017b; Guo et al., 2018; Wang and Chen 2018; Yan et al., 2018; Ge et al., 2019; Ruan et al., 2021) included 1,128 patients reported DCR (DCR = CR + PR + SD). The results showed in Figure 4. The heterogeneity test showed p = 0.83, I2 = 0.0%, so the fixed effect model was used for analysis. The meta-analysis results showed that DCR of the experimental group was significantly higher than that of the control group [OR = 2.45, 95%CI (1.84, 3.27), Z = 6.12, p < 0.00001].
In those studies (Liu and Zhu 2012; Saifuding et al., 2012; Lin et al., 2015; Ma 2015; Zhang and Li 2015; Deng et al., 2016; Zheng et al., 2017b; Guo et al., 2018; Ge et al., 2019; Ruan et al., 2021), the KPS score is expressed by the number of cases of score improvement, while less literature reports specific scores (Huo and Chen 2009; Saifuding et al., 2012). The results showed in Figure 5. The heterogeneity test in Figure 5A showed p = 0.91, I2 = 0.0%. Therefore, the fixed-effects model was used for analysis. The meta-analysis results showed that KPS of the experimental group was significantly higher than that of the control group [OR = 3.21, 95% CI (2.30, 4.48), Z = 6.87, p < 0.00001]. The heterogeneity test in Figure 5B showed p = 0.06, I2 = 72.0%. Therefore, the random effects model was used for analysis. The meta-analysis results showed that KPS of the experimental group was significantly higher than that of the control group [MD = 7.73, 95% CI (3.05, 12.42), Z = 3.24, p=0.001].
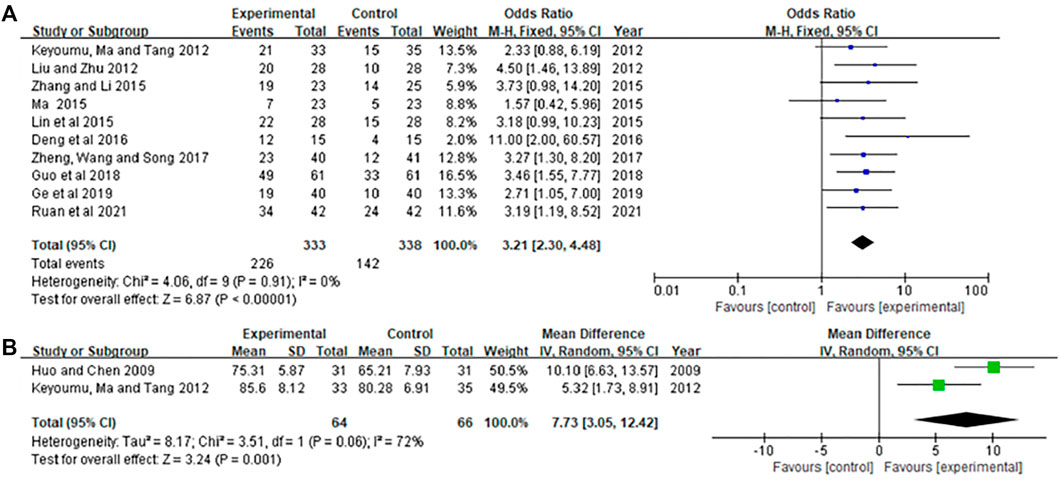
FIGURE 5. Meta-analysis of KPS. (A) KPS as a dichotomous variable, (B) KPS as a continuous variable.
Three included studies (Guo et al., 2018; Wang and Chen 2018; Xiong et al., 2015) reported the level of TNF-α in serum of patients, and one literature (Wang and Chen 2018) reported the levels of serum IL-6 and CRP in patients. The results are shown in Figure 6. Figure 6A Heterogeneity test showed p = 0.14, I2 = 48.0%. The results of the meta-analysis using the fixed-effects model and MD showed that Xiaoaiping injection could significantly reduce TNF-α levels [MD = −15.00, 95% CI (−17.62, -12.38), Z = 11.23, p < 0.00001]. Figures 6B,C showed that Xiaoaiping injection significantly reduced serum IL-6 [MD = −13.00, 95% CI (−15.78, -10.30), Z = 9.44, p < 0.00001]; CRP [MD = −5.80, 95% CI (−7.21, −4.39), Z = 8.04, p < 0.00001].
Three literatures (Guo et al., 2018; Wang and Chen 2018; Ruan et al., 2021) reported immune function, one of the articles (Wang and Chen 2018) reported the levels of MDSCs and Treg in patients, two articles (Guo et al., 2018; Ruan et al., 2021) reported patient CD3+, CD4+, CD8+ levels. The results are shown in Figure 7. Figures 7A,B results showed that Xiaoaiping injection significantly reduces MDSCs [MD = −6.20, 95% CI (−7.19, −5.21), Z = 12.32, p < 0.00001], Treg [MD = −1.70, 95% CI (−1.92, −1.48), Z = 15.21, p < 0.00001]. The heterogeneity test in Figure 7C suggested that the random effects model was used for analysis, and the results showed that Xiaoaiping injection combined with chemotherapy could increase the immune function of the body, but the difference was not statistically significant CD3+ [MD = 12.29, 95% CI (−0.63, 25.22), Z = 1.86, p = 0.06], CD4+ [MD = 8.41, 95% CI (−1.12, 17.95), Z = 1.73, p = 0.08], CD8+ [MD = 4.32, 95% CI (−4.64, 13.29), Z = 0.94, p = 0.34].
In the included studies, two literatures (Ge et al., 2019; Li and Ran 2016) detected the levels of tumor markers in the serum of patients, of which two literatures (Ge et al., 2019; Li and Ran 2016) reported the levels of CEA and CA199, one literature (Li and Ran 2016) reported the levels of CA242, and one literature (Ge et al., 2019) reported CA125 levels. The results are shown in Figure 8. Xiaoaiping injection combined with chemotherapy could significantly reduce the levels of serum tumor markers, including CEA [MD = −11.64, 95% CI (−15.07, −8.21), Z = 6.65, p < 0.00001], CA199 [MD = −33.57, 95% CI (−60.84, −6.29), Z = 2.41, p = 0.02], CA242 [MD = −20.66, 95% CI (−23.07, −18.25), Z = −16.77, p < 0.00001], CA125 [MD = −12.50, 95% CI (−19.53, −5.47), Z = 3.48, p = 0.0005].
16 RCTs reported adverse reactions, including leukopenia, hand-foot syndrome, decreased hemoglobin, decreased platelets, nausea and vomiting, oral mucositis, abnormal liver and kidney function, peripheral neurotoxicity and other 14 adverse reactions (Figure 9). Heterogeneity test for neutropenic markers revealed I2 > 50%, and random effects model was used for analysis. No obvious heterogeneity was found in the remaining 13 adverse reactions, so a fixed effect model was used for analysis. Meta-analysis results showed that Xiaoaiping injection combined with chemotherapy could reduce OR for the four adverse events, including hemoglobin [OR = 1.01, 95% CI (0.65, 1.57), p = 0.96], diarrhea [OR = 0.67, 95% CI (0.40, 1.14), p = 0.14], anemia [OR = 0.59, 95% CI (0.29, 1.19), p = 0.14], feeling abnormal [OR = 1.02, 95% CI (0.44, 2.39), p = 0.96], but the difference was not statistically significant. Xiaoaiping injection combined with chemotherapy could significantly reduce OR for the 10 adverse events, including leukopenia [OR = 0.32, 95% CI (0.24, 0.44, p < 0.00001], hand-foot syndrome [OR = 0.43, 95% CI (0.30, 0.63), p < 0.0001], thrombocytopenia [OR = 0.43, 95% CI (0.31, 0.59), p < 0.00001], sick and vomit [OR = 0.60, 95% CI (0.43, 0.84), p = 0.003], oral mucositis [OR = 0.61, 95% CI (0.40, 0.91), p = 0.02], abnormal liver function [OR = 0.69, 95% CI (0.51, 0.94), p = 0.02], abnormal kidney function [OR = 0.37, 95% CI (0.18, 0.75), p = 0.006], neutropenia [OR = 0.20, 95% CI (0.07, 0.54), p = 0.002], peripheral neurotoxicity [OR = 0.65, 95% CI (0.40, 1.06), p = 0.08], fatigue [OR = 0.44, 95% CI (0.20, 0.95), p = 0.04].

FIGURE 9. Meta-analysis of adverse reactions. (A) Leukopenia; (B) Hand-foot syndrome; (C) Decreased hemoglobin; (D) Thrombocytopenia; (E) Nausea and vomiting; (F) Oral mucositis; (G) Abnormal liver function; (H) Abnormal renal function; (I) Neutrophil decrease; (J) diarrhea; (K) peripheral neurotoxicity; (L) Anemia; (M) Fatigue; (N) paresthesia.
A funnel plot was drawn according to the disease response rate and disease control rate, and the results are shown in Figure 10. It can be seen from the figure that the studies are basically symmetrical on the left and right, suggesting that the included studies can be considered to have no obvious publication bias.
Gastric cancer is the most common malignant tumor of the digestive system. In recent years, the detection methods have been continuously improved, but the detection of early gastric cancer is still low. It is the most commonly used method for the treatment of gastric cancer, but the patient’s immune function is affected (Xue et al., 2014). There are many adverse reactions after chemotherapy, such as leukopenia, thrombocytopenia, liver and kidney damage, oral mucositis, etc. Traditional Chinese medicine therapy has certain advantages in improving the quality of life of patients undergoing chemotherapy, and has been clinically recognized. Xiaoaiping injection is a Chinese patent medicine that completely retains the active ingredients of the medicine by adopting low-temperature extraction, bioseparation and high-tech ion exchange extraction and other modern Chinese medicine preparation processes. Xiaoaiping injection mainly included polysaccharides, C-21 steroidal saponins, organic acids and alkaloids etc., which had the effect of clearing away heat and detoxifying, resolving phlegm and softening firmness. It had pharmacological effects such as anti-tumor, antihypertensive, and antiasthmatic, and immune regulation, clinically used for gastric cancer, lung cancer, esophageal cancer, liver cancer, and other diseases (Chen and Liu 2004). Reports showed that Xiaoaiping injection, from the extract of M. tenacissima (Roxb.) Wight et Arn., had definite anti-tumor effects, and the mechanisms were related with promoting tumor cell apoptosis, inhibiting tumor cell proliferation and tumor blood vessel growth, and regulating immunity, etc. Li et al. (2016) found that M. tenacissima and its active ingredients could treat human Burkitt leukemia by inhibiting the proliferation of tumor cells and promoting cell apoptosis. Experimental study showed that the combined use of Xiaoaiping and cisplatin significantly promoted apoptosis inhibited the proliferation, migration and erosion of tumor cells, and significantly improved the anti-tumor efficacy of cisplatin (Zheng et al., 2016; Zheng et al., 2017a). Wang et al. (2010) found that M. tenacissima preparation (Xiaoaiping injection) could inhibit the proliferation of ovarian cancer Caoy-3 cells and arrest the cell cycle in G0/G1 phase, and its mechanism was related to the inhibition of PI3K/Akt signaling pathway. Xiaoaiping injection may also inhibit tumor development by inducing gastric cancer cells to differentiate into normal cells (Li et al., 2001). In addition, it found that Xiaoaiping injection exerts anti-tumor effect by regulating the expression of vascular endothelial growth factor receptor 2 (VEGFR2) through PI3K/Akt signaling pathway (Wang et al., 2016). It showed that Xiaoaiping could also reduce the drug resistance of tumor cells (Han et al., 2016), whose mechanism was related to down-regulation of VEGF, basic fibroblast growth factor (bFGF), etc (Ding et al., 2016). The C21 steroidal saponin Tenacissimoside A of M. tenacissima extract could act on HepG2/Dox tumor cells, prevent the expression of P-glycoprotein, reduce the drug resistance of tumor cells, and enhance their sensitivity to drugs (Huang et al., 2013). Other study also reported the extracts and main components regulated the immunity in order to play anti-tumor effect (Chen et al., 2010; Xing et al., 2011; Huang et al., 2013; Han et al., 2017). The results of this meta-analysis found that Xiaoaiping injection combined with chemotherapy has a good effect on advanced gastric cancer, and the improvement mechanism is related to inhibiting inflammatory response, improving immunity, and reducing the expression of tumor markers.
In the results of the quality assessment of the included literature, seven studies (Guo et al., 2018; Wang and Chen 2018; Saifuding et al., 2012; Ruan et al., 2021; Gao et al., 2015; Zheng et al., 2017b; Ge et al., 2019) mentioned use of random number tables, but nine studies (Huo and Chen 2009; Liu and Zhu 2012; Lin et al., 2015; Ma 2015; Xiong et al., 2015; Zhang and Li 2015; Deng et al., 2016; Li and Ran 2016; Yan et al., 2018) did not mention of how the random number sequence is generated. None of the 16 studies detailed the assignment method, which may lead to an increased risk of selection bias. At the same time, all studies did not blind the participants and reviewers, and were prone to subjective interference during the implementation process, and lacked the ability to evaluate the objectivity of results. The interventions included conventional chemotherapy regimens such as SOX and XELOX. The dosage of Xiaoaiping injection includes: 40 ml/d (Guo et al., 2018; Wang and Chen 2018; Zhang and Li 2015; Gao et al., 2015), 60 ml/d (Saifuding et al., 2012; Lin et al., 2015; Ma 2015; Yan et al., 2018), 80 ml/d (Liu and Zhu 2012; Xiong et al., 2015; Deng et al., 2016; Li and Ran 2016; Ge et al., 2019), 100 ml/d (Ruan et al., 2021). What’s more, the treatment time of Xiaoaiping injection combined with chemotherapy is usually 3 weeks as a course, with two consecutive courses. The dosage and intervention time of the drugs are not completely consistent, which may affect the outcome indicators. The outcome indicators of included study reported clinical efficacy and adverse reactions, and 11 literatures reported KPS score. In addition, some studies also detected the levels of serum inflammatory factors and tumor markers in patients with gastric cancer, and some studies reported the immune function of patients with gastric cancer, which were used to carry out meta-analysis. The results were basically consistent with the anti-tumor effect mechanisms of Xiaoaiping injection.
Xiaoaiping injection combined with chemotherapy regimen in the treatment of advanced gastric cancer can achieve better clinical efficacy in terms of improving the effective rate and the quality of life, also reducing the incidence of adverse reactions. Since most of the studies did only observe the clinical efficacy and adverse reaction-related indicators, but not observe or report the biochemical indicators in serum or plasma. It is necessary to design the mechanism-related reports of clinical studies in the future, which will provide reference for the treatment of gastric cancer. At the same time, due to the limited literature included and the low methodological quality in this study, it is needed about more prospective, high-quality, large-sample, multi-center randomized controlled trials in the future (Hu et al., 2008).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
All authors made a substantial contribution to this work. XQZ and YZC developed the review protocol, also contributed to the conception and design of the review. RAC and CYS read and screened abstracts and titles of potentially relevant studies, and were also responsible for extracting data. JH were responsible for rating the quality of the papers. XQZ drafted the manuscript. YZC, RAC, and JH critically reviewed the draft and suggested amendments before submission.
This work was supported by the Jiangsu Provincial Department of Education [Jiangsu Government Scholarship for Overseas Study No. (2019)43], and Science and Technology Project of Nantong City (No. JCZ19092).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
bFGF, basic fibroblast growth factor; CEA/CA, carcinoembryonic antigen; CNKI, China National Knowledge Infrastructure; CR, complete remission; CI, confidence interval; CRP, C-reaction protein; DCR, disease control rate; FOIFOX, oxaliplatin + leucovorin + 5-fluorouracil; IL-6, interleukin-6; KPS, Karnofsky performance status; MDSCs, myeloid-derived suppressor cells; MD, mean difference; OR, odds ratio; PR, partial remission; PD, progressive disease; PROSPERO, prospective register of systematic reviews; RCTs, randomized controlled trials; RR, remission rate; SOX, seggio + oxaliplatin capsules; SD, stable disease; TNF-α, tumor necrosis factor alpha; TP, paclitaxel + cisplatin; Treg, regulatory T cells; VEGFR2, vascular endothelial growth factor receptor 2; XAPI, Xiaoaiping injection; XELOX, xeloda + oxaliplatin.
Chen, B., Li, C. P., Ouyang, J., and Shao, X. Y. (2010). Effect of extractive of marsdenia tenacissima on human normal immunocytes and hemopoietic stem cells in vitro. Chin. Clin. Oncol. 15 (10), 887–890. doi:10.3969/j.issn.1009-0460.2010.10.006
Chen, C. H., and Liu, A. X. Application of traditional Chinese medicine in tumor treatment. Henan Tradit. Chin. Med., 2004 24 (03):74–75. doi:10.16367/j.issn.1003-5028.2004.03.058
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer statistics in China, 2015. Ca. Cancer J. Clin. 66 (2), 115–132. doi:10.3322/caac.21338
Chen, W. X., Niu, Y. F., Kang, Y., Huang, L., Ma, C. Z., and Zhao, A. G. (2021). Research progress of anti-tumor Chinese patent medicine injection. Chin. J. Gerontology 41 (23), 5439–5444. doi:10.3969/j.issn.1005-9202.2021.23.067
Deng, W. Y., Xu, Y. F., Li, N., and Luo, S. X. (2016). Observation on clinical effects of Xiaoaiping injection combined with TP regimen in advanced gastric cancer. Chin. J. Geriatric Care 14 (06), 51–52. doi:10.3969/j.issn.1672-4860.2016.06.020
Ding, J. Y., Huang, J., Kou, J. Y., Zhang, Z., Zheng, W. Z., and Li, J. (2016). The effect of xiaoaiping combined thermochemistry on the gefitinib resistant lung adenocarcinoma cell line A549: An experimental study. Chin. J. General Pract. 14 (01), 32–34. doi:10.16766/j.cnki.issn.1674-4152.2016.01.010
Gao, L., Lu, L. Q., Hong, C. J., and Wang, Y. X. (2015). Analysis of Xiaoaiping injection combined with XELOX regimen in the treatment of advanced gastric cancer. Chin. Archives Traditional Chin. Med. 33 (05), 1259–1261. doi:10.13193/j.issn.1673-7717.2015.05.075
Ge, Y. N., Zhang, G. Z., Liu, Z. J., and Zheng, Z. D. (2019). The clinical efficacy and adverse effects of Xiaoaiping injection combined with S-1 capsule in the treatment of elderly patients with advanced gastric cancer. Anti-tumor Pharm. 9 (06), 892–896. doi:10.3969/j.issn.2095-1264.2019.06.12
Guo, P. Z., Feng, L. Y., Yang, X. M., Zhang, Z. Y., Lin, Y. Y., and Huang, L. X. (2018). Clinical effect of Xiaoaiping assisted XELOX scheme in treatment of advanced gastric cancer patients and effect on serum TNF-α and immune function. World Latest Med. Inf. 18 (59), 19–21. doi:10.19613/j.cnki.1671-3141.2018.59.009
Han, S. Y., Zheng, W. X., He, X. R., Zhao, C., Jiang, S. T., Pang, L. N., et al. (2016). Xiaoaiping injection combined with gefitnib inhibits resistant non-small cell lung cancer xenografts H460 and H1975. Chin. J. Pharmacol. Toxicol. 30 (01), 44–52. doi:10.3867/j.issn.1000.3002.201.6.01.00
Han, L., Leng, C. Y., Li, W. H., Feng, H. L., Yu, S. H., and Zhang, H. (2017). Study on in-vitro and in-vivo antitumor activity of extracts from caulis Marsdenia tenacissimae. Traditional Chin. Drug Res. Clin. Pharmacol. 28 (01), 51–55. doi:10.19378/j.issn.1003-9783.2017.01.011
Hu, Y. J., Shen, X. L., Lu, H. L., Zhang, Y. H., Huang, X. A., Fu, L. C., et al. (2008). Tenacigenin B derivatives reverse P-glycoprotein-mediated multidrug resistance inHepG2/Dox cells. J. Nat. Prod. 71 (6), 1049–1051. doi:10.1021/np070458f
Huang, Z., Wang, Y., Chen, J., Wang, R., and Chen, Q. (2013). Effect of Xiaoaiping injection on advanced hepatocellular carcinoma in patients. J. Tradit. Chin. Med. 33 (1), 34–38. doi:10.1016/s0254-6272(13)60097-7
Huo, Y., and Chen, G. (2009). Clinical observation of Xiaoaiping combined with chemotherapy in the treatment of advanced gastric cancer. Chin. Community Dr. 11 (18), 138. doi:10.3969/j.issn.1007-614x.2009.18.170
Johnston, F. M., and Beckman, M. (2019). Updates on management of gastric cancer. Curr. Oncol. Rep. 21 (8), 67. doi:10.1007/s11912-019-0820-4
Li, M. Q., Shen, J. H., Xu, B., and Chen, J. (2001). The mechanism of laboratory research for xiaoaiping treating SGC 7901 gastric carcinoma cellular strains. J. Interventional Radiology 10 (04), 228–231. doi:10.3969/j.issn.1008-794X.2001.04.013
Li, D., Li, C., Song, Y., Zhou, M., Sun, X., Zhu, X., et al. (2016). Marsdenia tenacssima extract and its functional components inhibits proliferation and induces apoptosis of human Burkitt leukemia/lymphoma cells in vitro and in vivo. Leuk. Lymphoma 57 (2), 419–428. doi:10.3109/10428194.2015.1043546
Li, N., and Ran, J. B. (2016). Clinical efficacy of Xiaoaiping combined with CPT-11 in the treatment of elderly patients with advanced gastric cancer. Hebei Med. 22 (02), 198–201. doi:10.3969/j.issn.1006-6233.2016.02.008
Lin, Q., Chen, M. C., Xu, X. M., Xu, Z. Q., Liu, S. X., and Zhou, S. L. (2015). Observation on the efficacy of XELOX regimen combined with Xiaoaiping injection in the treatment of advanced gastric cancer. Chin. J. Integr. Traditional West. Med. Dig. 23 (06), 435–437. doi:10.3969/j.issn.1671-038X.2015.06.18
Liu, H. D., and Zhu, Z. Y. (2012). Study of Xiaoaiping injection combined with chemotherapy on treatment of advanced gastric cancer. Hebei Med. 18 (12), 1704–1707. doi:10.3969/j.issn.1006-6233.2012.12.013
Liu, J., Zhao, Q. N., Qiao, X., Song, L. J., Yu, X. R., Wu, M. Y., et al. (2015). Research progress of Chinese traditional medicines for gastric cancer. Anti-tumor Pharm. 5 (06), 410–413. doi:10.3969/j.issn.2095-1264.2015.05.03
Ma, Y. J. (2015). Efficacy observation of Xiaoaiping injection combined with SOX regimen in the treatment of advanced gastric cancer. Electron. J. Clin. Med. Literature 2 (32), 6731–6732. doi:10.16281/j.cnki.jocml.2015.32.127
Parkin, D. M., Pisani, P., and Ferlay, J. (1999). Global cancer statistics. Ca. Cancer J. Clin. 49 (1), 33–64. doi:10.3322/canjclin.49.1.33
Ruan, X. J., Jia, J., Liu, H. L., Wang, F., Liu, Y. F., and Zhang, X. (2021). Clinical observation of Xiaoaiping injection combined with SOX chemotherapy for advanced gastric cancer. Chin. J. Clin. Ration. Drug Use 14 (22), 13–16. doi:10.15887/j.cnki.13-1389/r.2021.22.005
Saifuding, Y. K. M., Ma, L. Y., and Tang, Y. (2012). Clinical observation of Xiaoaiping injection combined with chemotherapy on the treatment of advanced gastric cancer. J. Basic Clin. Oncol. 25 (05), 397–399. doi:10.3969/j.issn.1673-5412.2012.05.010
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., and Lordick, F. (2020). Gastric cancer. Lancet 396 (10251), 635–648. doi:10.1016/S0140-6736(20)31288-5
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Thrift, A. P., and El-Serag, H. B. (2020). Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 18 (3), 534–542. doi:10.1016/j.cgh.2019.07.045
Wang, J. Y., and Chen, J. T. (2018). Clinical research on the complementary treatment of Xiaoaiping injection on patients with advanced gastric cancer. Chin. J. Operative Proced. General Surg. Electron. Ed. 12 (06), 503–506. doi:10.3877/cma.j.issn.1674-3946.2018.06.017
Wang, C., Li, S. Y., Wang, X. B., Dong, X., and Liu, C. Y. (2010). Inhibition of cell growth of human ovarian cancer by Xiaoaiping injection via Akt signal pathway. Chin. J. Inf. Traditional Chin. Med. 17 (S1), 28–30. CNKI:SUN:XXYY.0.2010-S1-016.
Wang, M. J., Du, D. Y., Fan, W., Zhang, C., Liu, Y., Fan, J. H., et al. (2016). Effects and mechanisms of Xiao-Ai-Ping injection on angiogenesis. Yao Xue Xue Bao 51 (2), 309–315. doi:10.16438/j.0513-4870.2015-0988
Xing, W. X., TANg, T., Gu, N., Guo, S. C., and Wu, Y. T. (2011). The preliminary study on the anti-tumor actions and their mechanisms of Marsdenia tenacissima. Health Res. 31 (01), 12–16. doi:10.3969/j.issn.1674-6449.2011.01.004
Xiong, L., Meng, Y. X., and Li, D. (2015). Observation on the effect of Xiaoaiping injection in chemotherapy patients with advanced gastric cancer. Shandong Med. J. 55 (14), 71–72. doi:10.3969/j.issn.1002-266X.2015.14.027
Xue, Y. F., Ren, Z. H., and Shen, Y. L. (2014). Effects of chemotherapy on immune-related functions in elderly patients with gastric cancer. Chin. J. Gerontology 34 (02), 492–493. doi:10.3969/j.issn.1005-9202.2014.02.098
Yan, X., Zhang, Q. X., Li, P., and Qu, F. J. (2018). Efficacy and safety of Xiaoaiping combined with XELOX regimen chemotherapy in the treatment of advanced metastatic gastric adenocarcinoma. Chin. J. Postgraduates Med. 41 (05), 413–417. doi:10.3760/cma.j.issn.1673-4904.2018.05.008
Zhang, H., and Li, X. L. (2015). Efficacy of Xiaoaiping injection combined with XELOX regimen in the treatment of elderly patients with advanced gastric cancer. Jiangsu Med. J. 41 (06), 642–644. doi:10.19460/j.cnki.0253-3685.2015.06.008
Zheng, A., Li, T., Chen, Y., Fang, J., Zhang, Y., and Feng, J. (2016). Inhibitory effect of a Chinese medicine Xiaoaiping combined with cisplatin on the proliferation, invasion and apoptosis in ovarian cancer HO-8910 PM cells in vitro and in vivo. Zhonghua Zhong Liu Za Zhi 38 (1), 11–16. Chinese. doi:10.3760/cma.j.issn.0253-3766.2016.01.003
Zheng, A. W., Chen, Y. Q., Fang, J., Zhang, Y. L., and Jia, D. D. (2017a). Xiaoaiping combined with cisplatin can inhibit proliferation and invasion and induce cell cycle arrest and apoptosis in human ovarian cancer cell lines. Biomed. Pharmacother. 89, 1172–1177. doi:10.1016/j.biopha.2017.03.012
Keywords: Xiaoaiping injection, advanced gastric cancer, meta-analysis, systemic review, updated
Citation: Zhou XQ, Chang YZ, Shen CY, Han J and Chang RA (2022) Xiaoaiping injection combined with chemotherapy for advanced gastric cancer: An updated systematic review and meta-analysis. Front. Pharmacol. 13:1023314. doi: 10.3389/fphar.2022.1023314
Received: 19 August 2022; Accepted: 07 September 2022;
Published: 30 September 2022.
Edited by:
Yuanliang Yan, Central South University, ChinaCopyright © 2022 Zhou, Chang, Shen, Han and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ren An Chang, Q2hhbmdyZW5hbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.