- Department of General Surgery, Renmin Hospital of Wuhan University, Wuhan, China
Objective: Immune checkpoint inhibitors (ICIs) have recently demonstrated promising performance in improving the prognosis of urological cancer patients. The goal of this meta-analysis was to determine the impact of PPI use on the clinical outcomes of urological cancer patients receiving ICI therapy.
Methods: Before 6 May 2022, the eligible literature was searched using PubMed, EMBASE, Cochrane Library, and Google Scholar. The clinical outcomes were overall survival (OS), progression-free survival (PFS), and objective response rate (ORR).
Results: A total of six articles met the inclusion criteria, and of the 1980 patients with advanced or metastatic urothelial cancers (UC) included. The meta-analysis displayed that PPI use could increase the risk of progression by 50.7% (HR: 1.507, 95% CI: 1.327–1.711, p < 0.001) and death by 58.7% (HR: 1.587, 95% CI: 1.367–1.842, p < 0.001), and reduce the ORR (OR: 0.503, 95% CI: 0.360–0.703, p < 0.001) in UC patients receiving ICIs. No significant heterogeneity and publication bias existed. Sensitivity analysis proved that the results were stable and reliable.
Conclusion: The meta-analysis indicated that concomitant PPI use was significantly associated with low clinical benefit in UC patients.
1 Introduction
Urological cancers, mostly including renal cell carcinoma (RCC), prostate cancer (PC), and urothelial cancer (UC), are the common public health concerns worldwide (Sung et al., 2021). Despite the advances in treatments and techniques for tumors, such as chemotherapy and molecular targeted therapy, the clinical prognosis of urological cancers has not improved considerably over the last 2 decades (Niu et al., 2021). The introduction of immune checkpoint inhibitors (ICIs) has transformed the treatment of a variety of cancers, including urological malignancies. These antibodies act by blocking the checkpoint pathways, which are physiologic mechanisms established to switch off the immune response and prevent autoimmunity (Bimbatti et al., 2022).
UC has the fourth highest rate of mutations of all cancers and is known to be highly antigenic (Kim et al., 2020), whereas RCC has a moderate tumor mutation load but a high frequency of deletion and clonal insertion mutations, which may be linked to neoantigen abundance and CD8+ T cell activation (Carretero-González et al., 2020). These characteristics make the theme appropriate for ICI therapy. In contrast, PC immunogenicity is hampered by a low mutation burden and a highly immunosuppressive microenvironment. As a result, it is deemed a “cold tumor” that is difficult to treat with ICIs (Kim and Koo, 2020). ICIs have been approved for RCC and UC and have been shown to improve patient survival when compared to traditional treatments (Pierantoni et al., 2019; Xu et al., 2020). However, the clinical efficacy of ICIs varies widely amongst sufferers, with only a tiny percentage of the population benefiting from treatment. Furthermore, primary resistance to ICIs is still frequent, and a significant number of patients continue to worsen or relapse as a result of ICI resistance (Sharma et al., 2017; Seto et al., 2019). Regrettably, no perfect biomarker for predicting the efficacy of ICIs exists at this time. Thus, the search for prospective biomarkers that predict its efficacy as well as factors that influence its efficacy is critical for a more targeted selection of treatment populations in clinical practice.
Antacid agents such as proton pump inhibitors (PPIs) and histamine-2-receptor antagonists (H2RAs) are commonly prescribed for extended periods in urological cancer patients. Recent evidence also suggested that PPI usage in patients with advanced NSCLC receiving ICI therapy was associated with an increased mortality risk (Qin et al., 2021; Rizzo et al., 2022; Wei et al., 2022). However, the relationship between antacid use and ICI outcomes in urological cancer patients remains controversial due to a lack of comprehensive evaluations. Therefore, we conducted the first systematic review and meta-analysis to elucidate whether antacid use affects the efficacy of ICI therapy for urological cancer. This will provide evidence for future clinical use of antacids in urological cancers treated with ICIs, thereby maximizing the clinical benefit to patients.
2 Materials and methods
2.1 Literature search strategies
This meta-analysis accompanied the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). The protocol for this meta-analysis is available in PROSPERO (CRD42022332633). On 6 May 2022, PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/), and Cochrane Library (https://www.cochranelibrary.com/) were retrieved. The following Medical Subject Headings (MeSH) terms and their entry terms: “Immune Checkpoint Inhibitors” [Mesh], “Antacids” [Mesh], “Proton Pump Inhibitors” [Mesh], “Histamine H2 Antagonists” [Mesh], as well as the following terms: “omeprazole,” “pantoprazole,” “lansoprazole,” “esomeprazole,” “dexlansoprazole,” “rabeprazole,” “ranitidine” were searched in [All Fields]. Detailed search strategies were shown in Supplementary Table S1. We also searched Google Scholar to uncover gray literature that was not indexed in the previously listed databases, such as presentations and unpublished research data. Furthermore, we also manually retrieved the reference lists of eligible papers.
2.2 Study selection criteria
If articles matched all the following criteria, they were included (Sung et al., 2021). patients diagnosed with urological cancers (Niu et al., 2021); patients treated with ICIs (Bimbatti et al., 2022); patients separated into the antacid use group and non-antacid use group (Kim et al., 2020); provided at least one of the outcomes of interest [multivariable/adjusted overall survival (OS), progression-free survival (PFS), and objective response rate (ORR)]. For retrospective studies, the results of univariable analysis are vulnerable to confounding factors, so we included studies that provided multivariable analysis. Only the article with the most comprehensive data and rigorous methods was chosen when studies reported overlapping patient populations. Meanwhile, the following exclusion criteria were employed: abstract, comments, and case report.
2.3 Data extraction and quality assessment
Data extraction mainly focused on the author, publication year, study region, study period, study type, cancer type, the number of patients, the age of patients, the number of male patients, timing of antacid use, types of ICI treatment, types of antacids, and the outcomes of interest (OS, PFS, and ORR). Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was used to estimate the ORR. The Newcastle-Ottawa Scale (NOS) score was used to estimate the quality of the retrospective studies (Wells et al., 2019). Literature with a score ≥7 was considered to be of high quality. Two authors independently cross-checked all the above steps, and the senior authors (Wenhong Deng and Wang Weixing) addressed any disparities.
2.4 Statistical methods
Stata MP16.0 was used for the statistical analysis. The HR and its 95% CI were used to calculate the influence of antacid use on the risk of survival in cancer patients. The association between ICI efficacy and antacid usage was expressed as an odds ratio (OR) with a 95% CI. The statistical heterogeneity among the studies was determined using the chi-squared test. p > 0.1 and I2 < 50% indicated low heterogeneity where a fixed-effect model was used; otherwise, the random-effect model was adopted. To reduce the influence of heterogeneity on the meta-analysis, a subgroup analysis was performed. Begg’s and Egger’s tests were implemented to assess publication bias. Sensitivity analysis by the leave-one-out method was conducted to estimate the stability of the results. All p values were two-sided with significance set at p < 0.05.
3 Results
3.1 Studies retrieved and characteristics
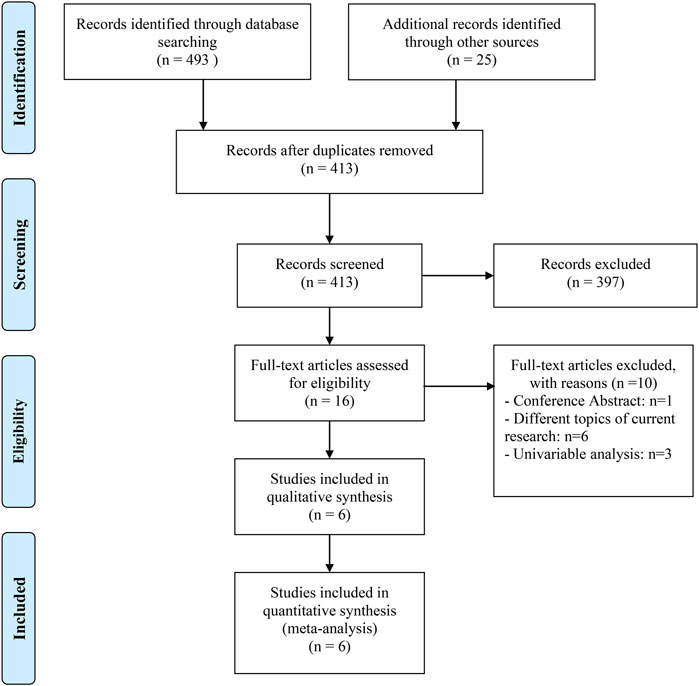
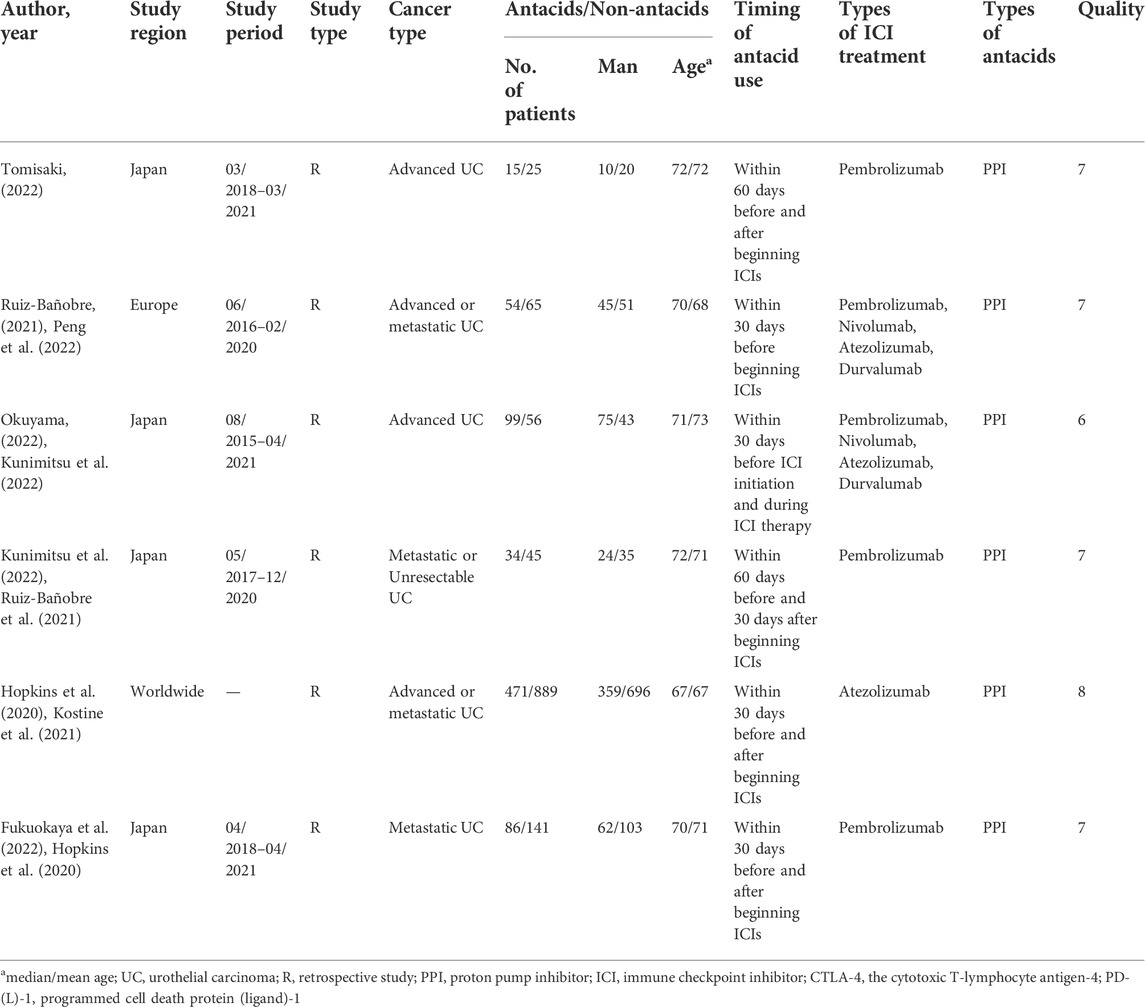
We gathered 518 potentially eligible records and assessed their titles and abstracts to see if they were suitable for inclusion. We discovered that six articles (Hopkins et al., 2020; Ruiz-Bañobre et al., 2021; Fukuokaya et al., 2022; Kunimitsu et al., 2022; Okuyama et al., 2022; Tomisaki et al., 2022) met our criteria for inclusion after carefully reading the full texts of 16 records. The studies on RCC by Peng et al. (2022), Mollica et al. (2022), Kostine et al. (2021) only provided the results of univariate analysis, so they were excluded. Figure 1 depicts the flow diagram for identifying eligible studies. All six articles explored the effects of PPIs on ICI efficacy in patients with advanced or metastatic UC. A total of 1980 patients were included. Of the six retrospective studies, five articles were awarded seven or eight points and were regarded as high quality; one article was awarded six points and was deemed as medium quality. Table 1 shows the baseline characteristics of the included studies as well as the quality evaluation.
3.2 Progression-free survival
Six studies (Hopkins et al., 2020; Ruiz-Bañobre et al., 2021; Fukuokaya et al., 2022; Kunimitsu et al., 2022; Okuyama et al., 2022; Tomisaki et al., 2022), involving 1980 participants (759 who received PPIs and 1221 who did not), explored the impact of concomitant PPI usage on adjusted PFS among UC cancers receiving ICI treatment. As shown in Figure 2A, there was no significant heterogeneity among studies (I2 = 7.4%, p = 0.369), so a fixed-effects model was utilized. Compared with patients without PPI usage, the meta-analysis showed that PPI use could increase the risk of progression by 50.7% (Figure 2A, HR: 1.507, 95% CI: 1.327-1.711, p < 0.001).
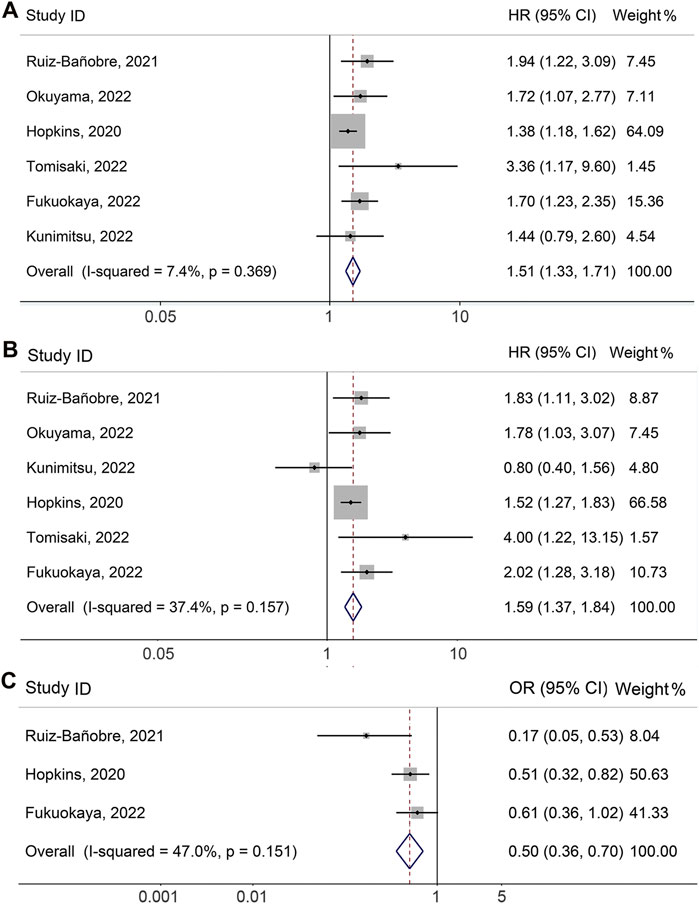
FIGURE 2. Forest plots of HR for correlation of proton pump inhibitor use with adjusted progression-free survival (A) and overall survival (B). Forest plots of OR for correlation of proton pump inhibitor use with adjusted objective response rate (C). OR, odds ratio; HR, hazard ratio; CL, confidence interval.
3.3 Overall survival
\The meta-analysis of adjusted OS was performed on six studies (Hopkins et al., 2020; Ruiz-Bañobre et al., 2021; Fukuokaya et al., 2022; Kunimitsu et al., 2022; Okuyama et al., 2022; Tomisaki et al., 2022) with a total of 1980 participants (759 with PPIs and 1221 without PPIs). Since there was no significant heterogeneity (Figure 2B, I2 = 37.4%, p = 0.157), we applied a fixed-effects model. The meta-analysis revealed that PPI use was related to a shorter OS of UC patients receiving ICIs. PPI usage increased the risk of death by 58.7% (Figure 2B, HR: 1.587, 95% CI: 1.367–1.842, p < 0.001).
3.4 Objective response rate
As shown in Figure 2C, the pooled meta-analysis for multivariable analysis of the ORR included three studies (Hopkins et al., 2020; Ruiz-Bañobre et al., 2021; Fukuokaya et al., 2022) with 1706 urological cancer patients (611 with PPIs and 1095 without PPIs). No significant heterogeneity existed, so a fixed-effects model was implemented (I2 = 47.0%, p = 0.151). The results were consistent with the above finding that concomitant PPI use was associated with lower ORR in patients (OR: 0.503, 95% CI: 0.360–0.703, p < 0.001).
3.5 Publication bias
The Begg’s and Egger’s tests were then performed to investigate publication bias, with the results indicating that there was no evidence of publication bias for adjusted OS (Egger’s test: p = 0.574, Begg’s test: p = 1.000) and adjusted ORR (Egger’s test: p = 0.247, Begg’s test: p = 1.000) across the studies. However, Egger’s test showed a publication bias in adjusted PFS (Egger’s test: p = 0.032, Begg’s test: p = 0.452). Next, the number of missing studies in adjusted PFS was calculated using the trim and fill method. The combined HR was recalculated by including those missing hypothesis studies, which were not found to be significantly altered (HR:1.437, 95% CI: 1.277–1.617; p < 0.001). Thus, the publication bias had little effect, and the result was relatively stable.
3.6 Sensitivity analysis
We also performed a sensitivity analysis via the leave-one-out method to assess the impact of each study on the overall meta-analysis. As shown in Figure 3A, the pooled HR for adjusted PFS was not significantly changed after excluding one study at a time, ranging from 1.489 (95% CI: 1.311–1.692, after omitting Tomisaki, 2022) to 1.697 (95% CI: 1.359–2.076, after omitting Hopkins, 2020). Besides, the pooled HR for adjusted OS also did not significantly differ in the sensitivity analysis. The overall HR ranged from 1.542 (95% CI: 1.317–1.805, after omitting Fukuokaya et al., 2022) to 1.730 (95% CI: 1.336–2.238, after omitting Hopkins et al., 2020) (Figure 3B). Similarly, the pooled OR for adjusted ORR was not significantly different in the sensitivity analysis. The overall OR ranged from 0.439 (95% CI: 0.283–0.679, after omitting Fukuokaya et al., 2022) to 0.553 (95% CI: 0.390–0.784, after omitting Ruiz-Bañobre et al., 2021) (Figure 3C).
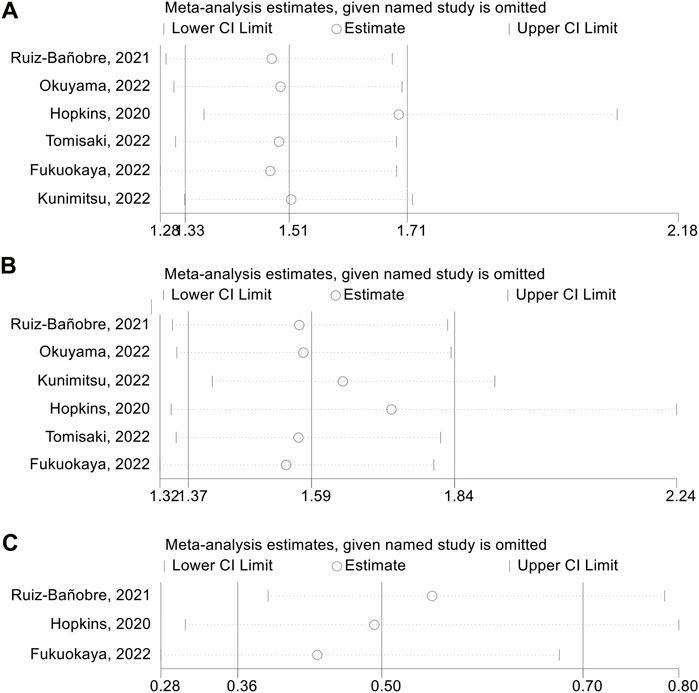
FIGURE 3. Sensitivity analysis of adjusted progression-free survival (A), overall survival (B) and objective response rate (C). CL, confidence interval.
4 Discussion
With the increased use of ICIs in urological tumor therapy, tremendous effort has been made to uncover possible factors that affect its efficacy. Whether PPIs can impact the response to ICIs in UC patients is still being debated. For all we know, this is the first meta-analysis to investigate the relationship between PPIs and ICI efficacy in patients with UC. We synthesized all the available evidence and found concomitant PPI use was significantly associated with low clinical benefit in UC patients treated with ICIs. Our publication bias and sensitivity analyses verified the dependability of our conclusions. Consequently, our study is essential and hopes to provide novel insights into the precise management of PPIs in clinical practice. PPIs should be used with caution before and after ICI treatment in patients with UC.
PPIs were not only used to treat gastrointestinal adverse effects (nausea and vomiting) caused by systemic antineoplastic therapy; they were also used prophylactically for cancer patients taking high-dose glucocorticoids as an antiemetic regimen and with concomitant non-steroidal anti-inflammatory drugs as analgesics. Besides, tumor patients with a history of peptic ulcers or bleeding used PPI prophylaxis to reduce the incidence of stress ulcers (Triadafilopoulos et al., 2013). PPIs have been demonstrated to impact the intestinal microbiota, owing to both altered stomach acidity and direct compounds effects (Imhann et al., 2016; Le Bastard et al., 2018; Maier et al., 2018; Reveles et al., 2018). A significant decrease in bacterial richness and specific bacteria, such as the Bifidobacteriaceae and Ruminococcaceae, as well as a remarkable increase in pathogenic bacteria, were found among PPI users compared to non-users in a study of 1,815 people (Imhann et al., 2016; Reveles et al., 2018). Currently, the impact of microbiota on the response to ICI treatment is receiving increasing attention. Two landmark studies in mice provided the first evidence that the microbiome had a direct impact on ICI effectiveness (Sivan et al., 2015; Vétizou et al., 2015). Prospective studies have also revealed that microbiome diversity and composition were strongly associated with the efficacy of ICIs in patients with RCC (Derosa et al., 2020; Salgia et al., 2020) and NSCLC (Huemer et al., 2019; Hakozaki et al., 2020), among others. Dysbiosis of the gut microbiota reduces the activity of ICIs (Sivan et al., 2015; Vétizou et al., 2015; Huemer et al., 2019; Derosa et al., 2020; Hakozaki et al., 2020; Salgia et al., 2020). Furthermore, several preclinical studies have revealed that PPIs could impair the physiological function of natural killer cells, cytotoxic T-lymphocytes, and polymorphonuclear neutrophils, all of which are implicated in the efficacy of ICIs (Aybay et al., 1995; Zedtwitz-Liebenstein et al., 2002). Thus, PPIs may reduce the efficacy of ICIs by altering the intestinal flora and affecting innate immune cell function.
However, there is also evidence that PPIs not only inhibit tumor growth and enhance chemosensitivity by modulating the acidic environment, but also promote immune responses and prevent tumor immune escape (Peppicelli et al., 2015; Spugnini and Fais, 2017). Esomeprazole has also been shown to inhibit melanoma growth by inactivating NF-κB to downregulate vascular endothelial growth factor-C (VEGF-C) expression (Peppicelli et al., 2013). Notably, no basic research has been conducted on the role of PPI in the development of UC. In the context of ICI treatment, the underlying mechanisms of the effects of PPI on UC are completely unknown and need to be investigated in subsequent experiments.
This article has some inherent restrictions, to be sure. To begin with, this study was essentially a meta-analysis that relied on previously published articles. We did not have sufficient data to perform subgroup analyses based on different types, and doses of PPIs and ICIs, the PPI window respective to ICIs start, etc. Secondly, all included articles in this meta-analysis are retrospective studies with intrinsic limitations of reporting and selection bias. Thus, a larger prospective study should be performed to better understand the relationship between PPI use and ICI efficacy.
5 Conclusion
The meta-analysis suggested that concomitant PPI use was significantly associated with low clinical benefit in UC patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
LZ, CC, WD, and WW conceived and designed the study. LZ, CC, DC, TK, KD, and CL were responsible for the collection and assembly of data, data analysis, and interpretation. LZ, CC, and LL were involved in writing the manuscript. LZ, CC, DC, WD, and WW revised the manuscript. All the work was performed under WD instruction. All authors read and approved the final manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 82172855, 81870442, 82003063)), and Natural Science Foundation of Hubei Province, China (No. 2021CFB365, 2020CFB213).
Acknowledgments
The authors thank all the medical staff who contributed to the maintenance of the medical record database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1018411/full#supplementary-material
References
Aybay, C., Imir, T., and Okur, H. (1995). The effect of omeprazole on human natural killer cell activity. Gen. Pharmacol. 26 (6), 1413–1418. doi:10.1016/0306-3623(94)00301-3
Bimbatti, D., Maruzzo, M., Pierantoni, F., Diminutto, A., Dionese, M., Deppieri, F. M., et al. (2022). Immune checkpoint inhibitors rechallenge in urological tumors: An extensive review of the literature. Crit. Rev. Oncol. Hematol. 170, 103579. doi:10.1016/j.critrevonc.2022.103579
Carretero-González, A., Lora, D., Martín Sobrino, I., Sáez Sanz, I., Bourlon, M. T., Anido Herranz, U., et al. (2020). The value of PD-L1 expression as predictive biomarker in metastatic renal cell carcinoma patients: A meta-analysis of randomized clinical trials. Cancers (Basel) 12 (7), E1945. doi:10.3390/cancers12071945
Derosa, L., Routy, B., Fidelle, M., Iebba, V., Alla, L., Pasolli, E., et al. (2020). Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 78 (2), 195–206. doi:10.1016/j.eururo.2020.04.044
Fukuokaya, W., Kimura, T., Komura, K., Uchimoto, T., Nishimura, K., Yanagisawa, T., et al. (2022). Effectiveness of pembrolizumab in patients with urothelial carcinoma receiving proton pump inhibitors. Urol. Oncol. 12, 346.e1–346346.e8. doi:10.1016/j.urolonc.2022.02.020
Hakozaki, T., Richard, C., Elkrief, A., Hosomi, Y., Benlaïfaoui, M., Mimpen, I., et al. (2020). The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol. Res. 8 (10), 1243–1250. doi:10.1158/2326-6066.CIR-20-0196
Hopkins, A. M., Kichenadasse, G., Karapetis, C. S., Rowland, A., and Sorich, M. J. (2020). Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin. Cancer Res. 26 (20), 5487–5493. doi:10.1158/1078-0432.CCR-20-1876
Huemer, F., Rinnerthaler, G., Lang, D., Hackl, H., Lamprecht, B., and Greil, R. (2019). Association between antibiotics use and outcome in patients with NSCLC treated with immunotherapeutics. Ann. Oncol. 30 (4), 652–653. doi:10.1093/annonc/mdz021
Imhann, F., Bonder, M. J., Vich Vila, A., Fu, J., Mujagic, Z., Vork, L., et al. (2016). Proton pump inhibitors affect the gut microbiome. Gut 65 (5), 740–748. doi:10.1136/gutjnl-2015-310376
Kim, T. J., Cho, K. S., and Koo, K. C. (2020). Current status and future perspectives of immunotherapy for locally advanced or metastatic urothelial carcinoma: A comprehensive review. Cancers 12 (1), E192. doi:10.3390/cancers12010192
Kim, T. J., and Koo, K. C. (2020). Current status and future perspectives of checkpoint inhibitor immunotherapy for prostate cancer: A comprehensive review. Int. J. Mol. Sci. 21 (15), E5484. doi:10.3390/ijms21155484
Kostine, M., Mauric, E., Tison, A., Barnetche, T., Barre, A., Nikolski, M., et al. (2021). Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur. J. Cancer 157, 474–484. doi:10.1016/j.ejca.2021.08.036
Kunimitsu, Y., Morio, K., Hirata, S., Yamamoto, K., Omura, T., Hara, T., et al. (2022). Effects of proton pump inhibitors on survival outcomes in patients with metastatic or unresectable urothelial carcinoma treated with pembrolizumab. Biol. Pharm. Bull. 45 (5), 590–595. doi:10.1248/bpb.b21-00939
Le Bastard, Q., Al-Ghalith, G. A., Grégoire, M., Chapelet, G., Javaudin, F., Dailly, E., et al. (2018). Systematic review: Human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharmacol. Ther. 47 (3), 332–345. doi:10.1111/apt.14451
Maier, L., Pruteanu, M., Kuhn, M., Zeller, G., Telzerow, A., Anderson, E. E., et al. (2018). Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555 (7698), 623–628. doi:10.1038/nature25979
Mollica, V., Santoni, M., Matrana, M. R., Basso, U., De Giorgi, U., Rizzo, A., et al. (2022). Concomitant proton pump inhibitors and outcome of patients treated with nivolumab alone or plus ipilimumab for advanced renal cell carcinoma. Target. Oncol. 17 (1), 61–68. doi:10.1007/s11523-021-00861-y
Niu, X., Zhu, Z., and Bao, J. (2021). Prognostic significance of pretreatment controlling nutritional status score in urological cancers: A systematic review and meta-analysis. Cancer Cell Int. 21 (1), 126. doi:10.1186/s12935-021-01813-2
Okuyama, Y., Hatakeyama, S., Numakura, K., Narita, T., Tanaka, T., Miura, Y., et al. (2022). Prognostic impact of proton pump inhibitors for immunotherapy in advanced urothelial carcinoma. BJUI Compass 3 (2), 154–161. doi:10.1002/bco2.118
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Peng, K., Chen, K., Teply, B. A., Yee, G. C., Farazi, P. A., and Lyden, E. R. (2022). Impact of proton pump inhibitor use on the effectiveness of immune checkpoint inhibitors in advanced cancer patients. Ann. Pharmacother. 56 (4), 377–386. doi:10.1177/10600280211033938
Peppicelli, S., Bianchini, F., Contena, C., Tombaccini, D., and Calorini, L. (2013). Acidic pH via NF-κB favours VEGF-C expression in human melanoma cells. Clin. Exp. Metastasis 30 (8), 957–967. doi:10.1007/s10585-013-9595-4
Peppicelli, S., Bianchini, F., Toti, A., Laurenzana, A., Fibbi, G., and Calorini, L. (2015). Extracellular acidity strengthens mesenchymal stem cells to promote melanoma progression. Cell Cycle 14 (19), 3088–3100. doi:10.1080/15384101.2015.1078032
Pierantoni, F., Maruzzo, M., Gardi, M., Bezzon, E., Gardiman, M. P., Porreca, A., et al. (2019). Immunotherapy and urothelial carcinoma: An overview and future prospectives. Crit. Rev. Oncol. Hematol. 143, 46–55. doi:10.1016/j.critrevonc.2019.08.005
Qin, B. D., Jiao, X. D., Zhou, X. C., Shi, B., Wang, J., Liu, K., et al. (2021). Effects of concomitant proton pump inhibitor use on immune checkpoint inhibitor efficacy among patients with advanced cancer. Oncoimmunology 10 (1), 1929727. doi:10.1080/2162402X.2021.1929727
Reveles, K. R., Ryan, C. N., Chan, L., Cosimi, R. A., and Haynes, W. L. (2018). Proton pump inhibitor use associated with changes in gut microbiota composition. Gut 67 (7), 1369–1370. doi:10.1136/gutjnl-2017-315306
Rizzo, A., Cusmai, A., Giovannelli, F., Acquafredda, S., Rinaldi, L., Misino, A., et al. (2022). Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: A systematic review and meta-analysis. Cancers (Basel) 14 (6), 1404. doi:10.3390/cancers14061404
Ruiz-Bañobre, J., Molina-Díaz, A., Fernández-Calvo, O., Fernández-Núñez, N., Medina-Colmenero, A., Santomé, L., et al. (2021). Rethinking prognostic factors in locally advanced or metastatic urothelial carcinoma in the immune checkpoint blockade era: A multicenter retrospective study. ESMO Open 6 (2), 100090. doi:10.1016/j.esmoop.2021.100090
Salgia, N. J., Bergerot, P. G., Maia, M. C., Dizman, N., Hsu, J., Gillece, J. D., et al. (2020). Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur. Urol. 78 (4), 498–502. doi:10.1016/j.eururo.2020.07.011
Seto, T., Sam, D., and Pan, M. (2019). Mechanisms of primary and secondary resistance to immune checkpoint inhibitors in cancer. Med. Sci. 7 (2), E14. doi:10.3390/medsci7020014
Sharma, P., Hu-Lieskovan, S., Wargo, J. A., and Ribas, A. (2017). Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168 (4), 707–723. doi:10.1016/j.cell.2017.01.017
Sivan, A., Corrales, L., Hubert, N., Williams, J. B., Aquino-Michaels, K., Earley, Z. M., et al. (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350 (6264), 1084–1089. doi:10.1126/science.aac4255
Spugnini, E., and Fais, S. (2017). Proton pump inhibition and cancer therapeutics: A specific tumor targeting or it is a phenomenon secondary to a systemic buffering? Semin. Cancer Biol. 43, 111–118. doi:10.1016/j.semcancer.2017.01.003
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tomisaki, I., Harada, M., Minato, A., Nagata, Y., Kimuro, R., Higashijima, K., et al. (2022). Impact of the use of proton pump inhibitors on pembrolizumab effectiveness for advanced urothelial carcinoma. Anticancer Res. 42 (3), 1629–1634. doi:10.21873/anticanres.15638
Triadafilopoulos, G., Roorda, A. K., and Akiyama, J. (2013). Indications and safety of proton pump inhibitor drug use in patients with cancer. Expert Opin. Drug Saf. 12 (5), 659–672. doi:10.1517/14740338.2013.797961
Vétizou, M., Pitt, J. M., Daillère, R., Lepage, P., Waldschmitt, N., Flament, C., et al. (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350 (6264), 1079–1084. doi:10.1126/science.aad1329
Wei, N., Zheng, B., Que, W., Zhang, J., and Liu, M. (2022). The association between proton pump inhibitor use and systemic anti-tumour therapy on survival outcomes in patients with advanced non-small cell lung cancer: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 88, 3052–3063. doi:10.1111/bcp.15276
Wells, G., Shea, B., and O’Connell, D. (2019). The newcastle-ottawa Scale (NOS) for assessing the quality if nonrandomizes studies in meta-analyses. AvaliableAt: http://wwwohrica/programs/clinical_ epidemiology/oxfordasp.
Xu, W., Atkins, M. B., and McDermott, D. F. (2020). Checkpoint inhibitor immunotherapy in kidney cancer. Nat. Rev. Urol. 17 (3), 137–150. doi:10.1038/s41585-020-0282-3
Keywords: immune checkpoint inhibitors, proton pump inhibitors, urothelial cancer, clinical outcomes, meta-analysis
Citation: Zhang L, Chen C, Chai D, Li C, Kuang T, Liu L, Dong K, Deng W and Wang W (2022) Effects of PPIs use on clinical outcomes of urothelial cancer patients receiving immune checkpoint inhibitor therapy. Front. Pharmacol. 13:1018411. doi: 10.3389/fphar.2022.1018411
Received: 13 August 2022; Accepted: 07 September 2022;
Published: 26 September 2022.
Edited by:
Chen Shi, Huazhong University of Science and Technology, ChinaReviewed by:
Takafumi Yanagisawa, School of Medicine, Jikei University, JapanSha Li, Shanghai Jiao Tong University, China
Copyright © 2022 Zhang, Chen, Chai, Li, Kuang, Liu, Dong, Deng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhong Deng, d2VuaG9uZ2RlbmdAd2h1LmVkdS5jbg==; Weixing Wang, c2F0ZS5sbGl0ZUAxNjMuY29t
†These authors have contributed equally to this work
 Lilong Zhang
Lilong Zhang Chen Chen
Chen Chen Dongqi Chai
Dongqi Chai Chunlei Li
Chunlei Li Tianrui Kuang
Tianrui Kuang Li Liu
Li Liu Keshuai Dong
Keshuai Dong Wenhong Deng
Wenhong Deng Weixing Wang
Weixing Wang