- 1Division of Pharmacology, Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy
- 2Section of Anatomy and Histology, Department of Neuroscience, Biomedicine and Movement Sciences, University of Verona, Verona, Italy
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease associated with motor neuron degeneration, progressive paralysis and finally death. Despite the research efforts, currently there is no cure for ALS. In recent years, multiple epigenetic mechanisms have been associated with neurodegenerative diseases. A pathological role for histone hypoacetylation and the abnormal NF-κB/RelA activation involving deacetylation of lysines, with the exclusion of lysine 310, has been established in ALS. Recent findings indicate that the pathological acetylation state of NF-κB/RelA and histone 3 (H3) occurring in the SOD1(G93A) murine model of ALS can be corrected by the synergistic combination of low doses of the AMP-activated kinase (AMPK)-sirtuin 1 pathway activator resveratrol and the histone deacetylase (HDAC) inhibitors MS-275 (entinostat) or valproate. The combination of the epigenetic drugs, by rescuing RelA and the H3 acetylation state, promotes a beneficial and sexually dimorphic effect on disease onset, survival and motor neurons degeneration. In this mini review, we discuss the potential of the epigenetic combination of resveratrol with HDAC inhibitors in the ALS treatment.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease associated with motor neurons (MNs) degeneration, muscle weakness, fasciculations, muscle atrophy, swallowing and speech disabilities, paralysis and finally death (van Es et al., 2017). Death is usually caused by respiratory failure and occurs in 2–4 years after the onset (Huisman et al., 2011; Wittie et al., 2013; Oskarsson et al., 2018). ALS occurs with an incidence of 1–2 cases in 100,000 individuals per year, with about 90% of cases being sporadic and 10% characterized as familial (Oskarsson et al., 2018). More than 20 genes have been associated with familial ALS, of which the ones encoding for chromosome nine open reading frame 72 (C9orf72), superoxide dismutase 1 (SOD1), Fused in sarcoma (FUS), and TAR DNA-binding protein 43 (TDP-43), account for most of the cases (Chia et al., 2018).
However, despite decades of research, the mechanisms underlying ALS pathogenesis remain unclear. ALS appears as a multifactorial disease where several processes operate simultaneously. These include oxidative stress, glutamate excitotoxicity, mitochondrial dysfunction, inflammatory response, impairment of axonal transport, impaired protein homeostasis and transcriptional dysregulation (Giribaldi et al., 2013; Mejzini et al., 2019; Bendotti et al., 2020; De Marchi et al., 2021).
Epidemiological studies and clinical observations have shown evidence of sexual dimorphism in ALS (Vegeto et al., 2020). Men display higher risk of developing ALS, with a male/female ratio reported between 1 and 3, depending on geographic area and population considered (Manjaly et al., 2010). Although the overall survival is similar in both sexes, the disease appears earlier in men (Blasco et al., 2012). Moreover, the ALS phenotype is different in males and females, with a predominance of limb onset in men and bulbar onset in women (Blasco et al., 2012).
Currently, no cure is available for ALS and the molecules tested, alone or in combinations, in animal models and in patients did not lead to real improvements (Mitsumoto et al., 2014; Wobst et al., 2020). The only therapeutic drugs approved for ALS treatment are riluzole, a glutamate receptor antagonist approved in 1995, and edavarone, a free radical scavenger approved by the FDA in 2017 (Oskarsson et al., 2018; Yoshino 2019; Wobst et al., 2020). Riluzole has been shown in clinical trials to prolong median survival from 11.8 to 14.8 months, postponing the use of surrogate approaches, such as tracheotomy and mechanical ventilation (Lacomblez et al., 1996; Miller et al., 2012). Edavarone modestly slows the rate of disease progression and prolongs the tracheostomy-free survival in ALS patients (Writing Group and Edaravone (MCI-186) ALS 19 Study Group. 2017; Okada et al., 2018).
The pathogenic role of anomalous acetylation of NF-κB/RelA and histones in ALS
In the central nervous system (CNS), the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) transcription factors play a pivotal role in a number of physiological processes including neurogenesis (Zhang and Hu, 2012), neuritogenesis (Gutierrez and Davies, 2011), learning and memory (Kaltschmidt and Kaltschmidt, 2015). On the other hand, NF-κB dysregulation has been associated to neurodegenerative mechanisms occurring in pathological conditions such as stroke, epilepsy, Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Srinivasan and Lahiri, 2015; Bellucci et al., 2020).
The NF-κB family is composed by five different members [p50/p105 (NF-κB1), p52/p100 (NF-κB2), p65 (RelA), c-Rel and RelB] that combine to form the transcriptionally active dimer (Gilmore and Wolenski 2012; Lanzillotta et al., 2015). Neurotoxic stimuli, including ischemia (Inta et al., 2006), glutamate (Pizzi et al., 2002), β-amyloid (Pizzi et al., 2005), or 1-methyl-4-phenylpyridinium (MPP+) (Ghosh et al., 2007; Sarnico et al., 2008) induce the activation of p50/RelA dimer and the transcription of pro-apoptotic factors, such as Bim and Noxa. The opposite effects exerted by the NF-κB dimer p50/RelA on neuron survival can rely on changes in the acetylation state of RelA within the nuclear p50/RelA (Lanzillotta et al., 2010; Sarnico et al., 2012). In particular, a specific pro-apoptotic acetylation profile of nuclear RelA, involving the general deacetylation of lysines but a site-specific acetylation at the lysine 310 [acRelA(K310)], occurs in lethal ischemia but not in protective preconditioning brain ischemia (Lanzillotta et al., 2010). The correction of RelA acetylation by pharmacological HDAC modulation (see below) reduced both the brain infarct volume and the neurological deficits (Lanzillotta et al., 2013; Faggi et al., 2018).
A pathogenic role for the activation of NF-κB/RelA has been suggested also for ALS.
Major genetic risk factors linked to ALS, including mutations in genes encoding for SOD1, OPTN, TBK1, TDP-43 and FUS, activate the NF-κB pathway (Källstig et al., 2021). NF-κB/RelA levels were elevated in mutant SOD1 MNs and astrocytes cellular model of ALS (Prell et al., 2014; Ikiz et al., 2015; Yin et al., 2018). Interestingly, Yin and colleagues reported that the MNs vulnerability to the conditioned medium obtained from mutant SOD1 astrocytes was dependent on the activation of the phosphorylated form of RelA, a modification known to promote RelA acetylation at the K310 residue (Yin et al., 2018). NF-κB/RelA was increased with disease progression in the SOD1(G93A) and TDP-43 mouse models of ALS (Swarup et al., 2011; Frakes et al., 2014). Neuron-specific expression of super-repressor form of the inhibitory kB proteins (IκB-SR) ameliorated behavioral and pathologic phenotypes in three mouse models of ALS carrying either human mutated TDP-43 or SOD1 transgenes (Dutta et al., 2020). Furthermore, NF-κB inhibition by administration of withaferin A alleviated disease symptoms in TDP-43 mice (Swarup et al., 2011) and extended lifespan of SOD1(G93A) and SOD1(G37R) mouse models of ALS (Frakes et al., 2014; Patel et al., 2015). In addition, levels of NF-κB/RelA were higher also in the spinal cord of ALS patients when compared with age-matched healthy subjects (Jiang et al., 2005; Swarup et al., 2011). Notably, as previously found in ischemic brain neurons (Lanzillotta et al., 2010; Lanzillotta et al., 2013), the pro-apoptotic acetylation profile acRelA(K310) was observed also in the lumbar spinal cord of SOD1(G93A) mice (Schiaffino et al., 2018; Bankole et al., 2022). These results suggest that NF-κB signaling may represent a unique therapeutic target for ALS disease. The beneficial effect of a therapeutic approach inhibiting NF-κB/RelA could be enormously enhanced by switching-off the p50/RelA pro-inflammatory/pro-apoptotic activity without modifying the dimer effects on neurogenesis, neuritogenesis and cell survival.
Histone acetylation at lysine residues is an important epigenetic mechanism regulating chromatin folding and the accessibility of transcription factors to their target genes (Eberharter and Becker 2002). Altered histone acetylation has been associated with reduced neuronal survival and pathological CNS conditions, including stroke, PD, AD and Huntington’s disease (Konsoula and Barile, 2012; Uzdensky and Demyanenko 2021). A growing body of evidence suggests a possible role for a dysregulation of epigenetic mechanisms, including histone acetylation, also in the occurrence and progression of ALS (Jimenez-Pacheco et al., 2017; Bennett et al., 2019; Zhang et al., 2022). For example, overexpression of FUS or TDP-43 in yeast ALS proteinopathy models resulted in histone hypo- and hyperacetylation, respectively, suggesting that each proteinopathy may correspond to a specific alteration of histone acetylation (Chen K et al., 2018). Moreover, histone acetylation was reduced in the spinal cord of SOD1(G93A) and Tg FUS+/+ mouse models of ALS (Schiaffino et al., 2018; Rossaert et al., 2019; Bankole et al., 2022). In light of these considerations, histone acetylation appears as a potential target for ALS treatment.
The acetylation state of NF-κB/RelA and histones results from the opposing activity of histones acetyltransferases (HATs) and histone deacetylases (HDACs).
Until now, eighteen mammalian HDACs have been characterized and grouped into four major classes according to their homology with yeast HDACs (Shukla and Tekwani, 2020). The Class I, II, and IV are known as classical HDACs and use zinc as cofactor. Class III HDACs, commonly known as sirtuins (SIRT 1–7), are NAD dependent and are involved in regulation of metabolism, stress and aging. Members of class I HDAC (HDAC 1, 2, 3 and 8) are most involved in the regulation of acetylation state of NF-κB/RelA (Chen and Greene, 2004). A body of evidence showed an unbalance of HATs and HDACs activity in patients as well as in preclinical models of ALS (Schmalbach and Petri, 2010; Lazo-Gómez et al., 2013; Jimenez-Pacheco et al., 2017; Shukla and Tekwani, 2020; Klingl et al., 2021). HDAC1 silencing or treatment with pan-HDAC inhibitors exert a protective role against wild-type or pathological mutant TDP-43 toxicity, suggesting TDP-43 acetylation as a new potential therapeutic target (Sanna et al., 2020). While data from expression analysis of HDAC isoforms in post-mortem brain and spinal cord tissue of ALS patients remain controversial (Janssen et al., 2010; Dios et al., 2019), recent findings suggest that increasing HDACs activity might exert a protective role in ALS. This is the case of the class IIa HDAC 4, whose expression in skeletal muscle of an ALS mouse model is responsible for compensatory reinnervation (Pigna et al., 2019).
The anomalous acetylation of NF-κB/RelA and histones can be corrected by the association of resveratrol and HDAC inhibitors
We reported that the pathological acetylation profile of RelA and histones in brain ischemia can be corrected by the synergistic combination of low doses of the epigenetic drugs resveratrol and the HDAC inhibitor MS-275 (entinostat) (Lanzillotta et al., 2013).
Resveratrol is a polyphenol stilbene widely investigated for the prevention or treatment of different diseases thanks to its anti-aging, anti-inflammatory, anti-oxidant and anti-tumorigenic properties (Rauf et al., 2017; Parrella et al., 2020; Zhang et al., 2021). Among its multiple mechanisms of action, the molecule is able to activate the class III NAD + -dependent HDAC SIRT1 (Lee et al., 2019) and AMP-activated protein kinase (AMPK), a serine–threonine kinase acting as a key metabolic and stress sensor/effector (Ruderman et al., 2010).
The synthetic benzamide MS-275 has been shown to inhibit class I HDAC (HDAC 1–3) with excellent pharmacokinetic properties (Simonini et al., 2006; Khan et al., 2008). The molecule is in clinical trials for the treatment of different types of cancer (Wang et al., 2022).
The use of resveratrol and MS-275 promoted a synergistic neuroprotection in primary cortical neurons exposed to oxygen and glucose deprivation (OGD) and in mice subjected to transient middle cerebral artery occlusion (tMCAO) (Lanzillotta et al., 2013). Similarly, a single treatment with resveratrol and MS-275 reduced stroke-mediated brain injury and inflammation in mice subjected to permanent MCAO (Mota et al., 2020).
The beneficial effects of the combination of resveratrol and MS-275 are mediated by the reversion of the mismatch of RelA acetylation state by respectively reducing the acetylation at the K310 via SIRT1 activation and enhancing the RelA general acetylation (Lanzillotta et al., 2013). The drug combination also reverted the histone H3 deacetylation produced by the ischemic injury (Lanzillotta et al., 2013). Of note, the drug effect was sustained by the resveratrol-promoted AMPK activation that, by increasing generation of acetyl-CoA, can support HAT activity (Lanzillotta et al., 2013). Moreover, AMPK could also corroborate SIRT1 activation by resveratrol via induction of NAD+, the fundamental co-factor for class III HDACs (Ruderman et al., 2010).
The substitution of MS-275 with valproate (VPA), an antiepileptic/mood stabilizer endowed with inhibitory activity for class I and class IIa HDACs (Perucca, 2002; Gurvich et al., 2004), in association with resveratrol exerted synergistic neuroprotection in the OGD cellular model of brain ischemia, by correcting the pathological acetylation state of RelA and reverting the histone H3 deacetylation (Faggi et al., 2018). Moreover, a single intraperitoneal administration of the association of resveratrol and VPA synergistically reduced infarct volume and neurological deficits in the tMCAO mouse model of ischemic stroke (Faggi et al., 2018). Interestingly, VPA increased RelA general acetylation in vivo (Chen S et al., 2018).
Resveratrol and many HDAC inhibitors have been individually studied also against ALS (Chuang et al., 2009; Carrera-Juliá et al., 2020; Shukla and Tekwani, 2020; Klingl et al., 2021; Novak et al., 2021). The major effects of resveratrol and the main HDAC inhibitors tested individually or in combination in ALS preclinical models or patients are reported in Table 1 and described in the following sections.
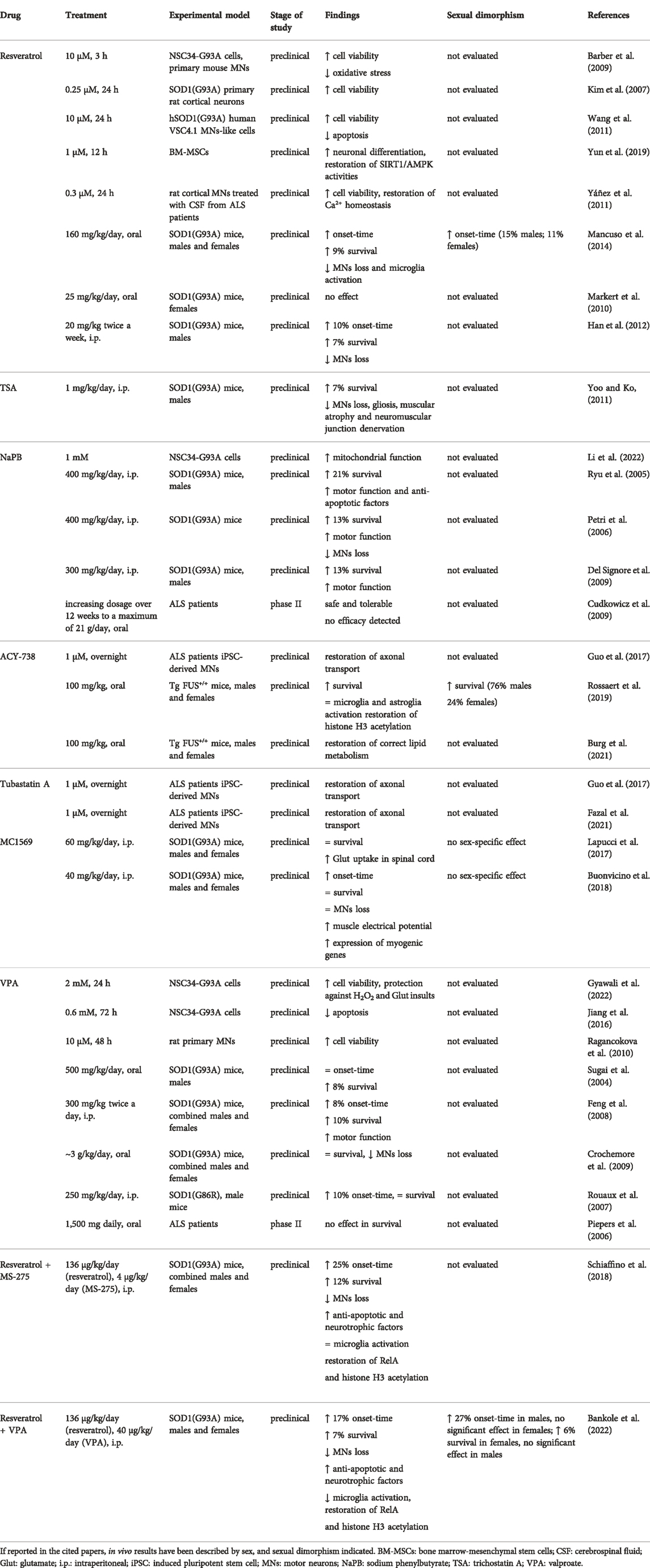
TABLE 1. Effect of resveratrol and the main HDAC inhibitors administered individually or in combination in ALS animal models and patients.
Effect of resveratrol in ALS preclinical models and patients
A protective effect of resveratrol in ALS was demonstrated in neuronal cell lines expressing the SOD1(G93A) mutant (Kim et al., 2007; Barber et al., 2009; Wang et al., 2011). Interestingly, it has been reported that bone marrow mesenchymal stem cells from ALS patients displayed down-regulation of AMPK/SIRT1 signalling, which was rescued by treatment with resveratrol (Yun et al., 2019). In addition, resveratrol prevented the neurotoxic effect of cerebrospinal fluid (CSF) from ALS patients on cultured MNs (Yáñez et al., 2011).
Dietary treatment of SOD1(G93A) male and female mice with resveratrol (160 mg/kg/day) delayed disease onset, extended lifespan of approximately 10%, and preserved MNs survival (Mancuso et al., 2014). Interestingly, resveratrol delayed disease onset in a sexually dimorphic fashion, postponing symptoms onset of 2 weeks in males and 1 week in females (corresponding to a delay of approximately 15% and 11%, respectively).
Another study reported that dietary resveratrol at the dose 25 mg/kg/day did not promote functional effects in SOD1(G93A) female mice (Markert et al., 2010). Conversely, Han and colleagues reported that intraperitoneal administration of resveratrol at the dose 20 mg/kg twice a week delayed disease onset, extended survival of 7% and reduced MNs loss in SOD1(G93A) male mice (Han et al., 2012).
Effect of HDAC inhibitors in ALS preclinical models and patients
Intraperitoneal treatment of SOD1(G93A) male mice with 1 mg/kg/day of trichostatin A (TSA), a pan-HDAC inhibitor of all zinc-dependent HDACs (Khan et al., 2008), promoted an increase of 7% in lifespan, together with a reduction of MNs loss, gliosis, muscular atrophy and neuromuscular junction denervation (Yoo and Ko, 2011). However, studies in human lymphoblasts in vitro pointed out genotoxic effects of TSA, raising doubts about a possible clinical use of the molecule (Olaharski et al., 2006).
Sodium phenylbutyrate (NaPB) is an inhibitor of HDAC class I and IIa (Kusaczuk et al., 2015). A recent study has shown the beneficial effect of NaPB in improving mitochondrial bioenergetics in a cellular model of ALS (Li et al., 2022). NaPB injected intraperitoneally at the dose of 400 mg/kg/day, prolonged the survival by 21%, ameliorated motor function and promoted expression of anti-apoptotic genes in SOD1(G93A) male mice (Ryu et al., 2005). The effect of NaPB on SOD1(G93A) mice was confirmed in another study, where the molecule injected intraperitoneally at the same dose significantly increased motor function, extended survival by 13% and attenuated MNs loss (Petri et al., 2006). In a third study, daily intraperitoneal treatment of SOD1(G93A) male mice with NaPB at the dose 300 mg/kg improved survival by approximately 13% while ameliorating body weight loss and grip strength (Del Signore et al., 2009). A phase II clinical trial studying the effect of an increasing dosage of NaPB over 12 weeks to a maximum of 21 g/day, reported the safety and tolerability of the drug, but did not evaluate its efficacy in ALS (Cudkowicz et al., 2009).
ACY-738 is a novel HDAC inhibitor selective for HDACs 1, 2, 3 and 6 (Mithraprabhu et al., 2013; Jochems et al., 2014). Treatment with ACY-738 rescued axonal transport deficits in MNs expressing FUS mutation derived from induced pluripotent stem cells (iPSCs) from ALS patients (Guo et al., 2017). Recently Van Den Bosch and colleagues have shown that the oral treatment of the ALS transgenic mouse model Tg FUS+/+ (both males and females) with ACY-738 (100 mg/kg) slowed down the disease progression, and improved the lifespan (Rossaert et al., 2019). Interestingly, the authors reported a larger survival extension in males (76% in males, 24% in females) (Rossaert et al., 2019). The beneficial effect of the molecule was associated with a mitigation of lipid metabolism alterations and a restoration of global histone acetylation, but not with a reduction of astrocytosis nor microgliosis (Rossaert et al., 2019; Burg et al., 2021).
Tubastatin A is a highly selective inhibitor of HDAC 6 (Butler et al., 2010) which has been investigated in different neurological disease animal models (Shen et al., 2020). Similarly to ACY-738, pharmacological inhibition of HDAC 6 by tubastatin A reverted axonal transport deficits in ALS patient-derived MNs with mutations for FUS and TDP-43 (Guo et al., 2017; Fazal et al., 2021).
MC1569 is a novel selective inhibitor of the HDAC class II (Mai et al., 2005). Treatment of SOD1(G93A) male and female mice with MC1568 (60 mg/kg/day, i.p.) restored glutamate uptake capacity in spinal cord, but did not increase lifespan (Lapucci et al., 2017). In a second study, i.p. treatment of SOD1(G93A) male and female mice with the drug at 40 mg/kg/day promoted early improvement of motor performances that disappeared at later stages of disease (Buonvicino et al., 2018). The transient motor improvement was coupled with increased skeletal muscle electrical potentials and muscle expression of myogenic genes, but not with a protection of MNs from neurodegeneration (Buonvicino et al., 2018). No evidence of sex-specific effect was found.
VPA treatment reduced neurotoxicity in motor neuron cellular models of ALS (Ragancokova et al., 2010; Jiang et al., 2016; Gyawali et al., 2022). In vivo, oral administration of VPA at the antiepileptic dose of approximately 500 mg/kg/day increased lifespan by 8% without delaying the disease onset in SOD1(G93A) male mice (Sugai et al., 2004). Feng and colleagues reported that VPA treatment (300 mg/kg twice a day, i.p.) in SOD1(G93A) male and female mice delayed motor deficits onset by 8%, improved lifespan by 10%, and had beneficial effects on motor dysfunction (Feng et al., 2008). In another study, oral treatment of SOD1(G93A) male and female mice with the drug at antiepileptic dose slowed down MNs loss without significantly improving lifespan (Crochemore et al., 2009). VPA, when administered intraperitoneally at the antiepileptic dose of 250 mg/kg/day to SOD1(G86R) male mice, delayed the disease onset of 10%, but failed in improving mean survival (Rouaux et al., 2007). VPA efficacy has been investigated also in phase II clinical trials for ALS, but VPA-treated subjects (1,500 mg daily) did not show a difference in survival or disease progression rate compared to placebo-treated patients (Piepers et al., 2006).
The association of resveratrol and HDAC inhibitors in the ALS treatment
It has been shown the resveratrol and HDAC inhibitors at very low doses can synergize in promoting neuroprotection in the SOD1(G93A) mouse model of ALS (Schiaffino et al., 2018; Bankole et al., 2022).
When administered to combined male and female SOD1(G93A) mice, the association resveratrol (136 μg/kg/day) and MS-275 (4 μg/kg/day) delayed symptoms’ onset by 3 weeks and prolonged lifespan by 2 weeks, corresponding to an increase of 25% and 12%, respectively (Schiaffino et al., 2018). Furthermore, the treatment rescued MNs, and increased the levels of anti-apoptotic B-cell lymphoma-extra large (Bcl-xL) and neurotrophic Brain-Derived Neurotrophic Factor (BDNF) in the lumbar spinal cord, without modifying microglia activation (Schiaffino et al., 2018).
In a similar fashion, the treatment of SOD1(G93A) male and female mice with the association resveratrol (136 μg/kg/day) and VPA (40 μg/kg/day) promoted a significant improvement in motor performances, the delay of disease onset, and longer survival (Bankole et al., 2022). Moreover, the epigenetic drugs protected MNs from neurodegeneration, reduced immunoreactivity of microglia, and increased expression of Bcl-xL and BDNF levels in the lumbar spinal cord (Bankole et al., 2022).
In accordance with studies on brain ischemia models (Lanzillotta et al., 2013; Faggi et al., 2018), the beneficial effects promoted by the association of resveratrol and HDAC inhibitors was coupled to the rescue of RelA and the histone 3 acetylation state, and of AMPK activation (Schiaffino et al., 2018; Bankole et al., 2022), indicating a mechanism of action based on the reversion of the mismatch of RelA and histone acetylation also in ALS.
It is important to note that resveratrol can also modify the acetylation status of other proteins potentially involved in ALS pathogenesis. For example, resveratrol was able to deacetylate p53 and the peroxisome proliferator-activated receptor gamma coactivator1alpha (PGC1-α) in preclinical models of ALS (Kim et al., 2007; Mancuso et al., 2014). Both the proteins have been involved in mechanisms of MNs death (Ranganathan and Bowser, 2010; Lazo-Gómez et al., 2013), and their deacetylation has been associated with neuroprotection (Hasegawa and Yoshikawa, 2008; Panes et al., 2022). Therefore, it can be speculated that other mechanisms, besides the modulation of RelA and histone acetylation, may support the beneficial action of this pharmacological association.
Figure 1 depicts the mechanisms responsible for NF-κB/RelA and H3 histone acetylation upon treatment with combination of resveratrol and HDAC inhibitors in ALS mice.
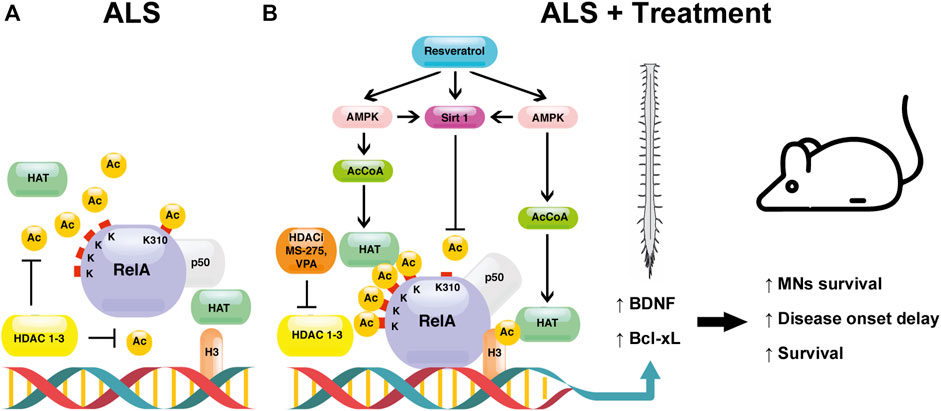
FIGURE 1. The proposed mechanism underlying the effect of the combination between resveratrol and HDAC inhibitors (HDACi) [MS-275 or valproate (VPA)] in ALS. (A) In the spinal cord of the SOD1(G93A) ALS mouse model, NF-κB/RelA is present in its aberrantly acetylated form, consisting of a hypoacetylated state with the exception of the residue K310 (ALS). In this pathological condition, the acetylation of histone H3 is decreased. (B) The treatment of SOD1(G93A) mice with the combination resveratrol/HDAC inhibitors reverts the aberrant RelA acetylation (ALS + treatment). HDAC inhibitors, by blocking HDAC activity, induce HAT-mediated acetylation of both RelA and H3 histone. Resveratrol activates the class III NAD+ -dependent HDAC SIRT1, promoting the deacetylation of RelA at residue K310. Moreover, resveratrol stimulates AMPK pathway, leading to an increase of NAD+ and AcCoA levels, enhancing the activation of SIRT1 and HATs, respectively. The modulation of RelA and H3 acetylation in the nucleus promotes the transcription of the anti-apoptotic Bcl-xL and the neurotrophic BDNF factors. Altogether, the treatment leads to a protection of MNs, a delay of symptoms’ onset, and longer survival.
The SOD1(G93A) mouse model displayed a sexually dimorphic behavior in response to the association of resveratrol and VPA (Bankole et al., 2022). Specifically, in accordance with a previous study investigating the effect of resveratrol in SOD1(G93A) mice (Mancuso et al., 2014), males showed positive outcomes in the early phases of the disease (onset delay of 27%). Conversely, only in females the epigenetic drugs reduced motor deficits in a later phase of the disease and prolonged survival by 6%. These findings suggest a possible action of resveratrol and VPA on specific sex-related molecular targets. For example, it is plausible that these compounds could potentiate the neuroprotective effect of female sex steroids in vivo (Bankole et al., 2022). In support of this, both resveratrol and VPA are endowed with estrogenic properties (Stempin et al., 2013; Qasem, 2020).
Conclusion
In conclusions, recent evidence supports that the pathological RelA acetylation and histone hypoacetylation may represent an appealing pharmacological target for ALS treatment. The correction of pathological acetylation state in the SOD1(G93A) ALS model has been achieved by the synergistic combination of resveratrol with the class I HDAC inhibitors MS-275 and VPA (Schiaffino et al., 2018; Bankole et al., 2022).
The doses of the epigenetic drugs active in combination were extremely low, in contrast to those reported in ALS studies employing individual molecules, where the modulation of the enzymatic activity of HDAC requires a very high concentration. Administration of individual molecules promoted a delay of disease onset and an extension of lifespan sometimes comparable, or even better than that achieved by the association resveratrol and MS-275 or VPA (Schiaffino et al., 2018; Bankole et al., 2022), but at doses several folds higher. In some cases the beneficial outcomes promoted by individual-molecule treatment were overshadowed by severe side effects.
The low drug doses used in the association could minimize possible side or off-target effects and modulate better the neuroprotective action. For example, preclinical and clinical studies investigating the use of VPA in ALS treatment failed to provide real effective results. If VPA protected MNs likely via inhibition of class I HDACs, the drug did not avoid denervation of neuromuscular junction at late stages, possibly because of concomitant inhibition of class IIa HDAC 4 at the high doses used (Boutillier et al., 2019; Pigna et al., 2019). The fact that VPA and resveratrol impinge on different molecular targets (class I HDACs, SIRT1 and AMPK), and their low doses, could reduce off-target effects and lead to better outcomes.
In light of these considerations, we advocate further research on different ALS models and clinical trials aimed to investigate the synergistic effect and the mechanism of action of the combination of resveratrol with various HDAC inhibitors. It is worth noting that, in an effort to target different ALS mechanisms, some of the described HDAC inhibitors have been already successfully tested in combination with other molecules, including riluzole (Del Signore et al., 2009), the catalytic antioxidant AEOL 10150 (Petri et al., 2006), and the mood stabilizer lithium (Feng et al., 2008). Therefore, the combination of resveratrol with HDAC inhibitors could be also tested with the additional compounds above mentioned, and potentially others.
Finally, future studies focusing on the effects of these compounds on sex-related molecular targets will be necessary to define sex-specific treatment strategies aimed to improve therapeutic options for ALS patients.
Author contributions
EP performed literature search, wrote the original draft and edited the manuscript. VP, IS, MMG and CG edited the manuscript and prepared the figure and the table. MB, OB, and RM edited the manuscript. MP conceived the study, provided funding, and edited the manuscript.
Funding
An Institutional Grant of University of Brescia (ex 60%) supported this work. VP Researcher Fellowship is covered by Fondazione Cariplo–Giovani Ricercatori—Research Support GR 2018–0391. OB INVITE Doctoral Fellowship is covered by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 754345.
Acknowledgments
We are thankful to Marco Veronese from University of Verona for the help in the figure preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bankole, O., Scambi, I., Parrella, E., Muccilli, M., Bonafede, R., Turano, E., et al. (2022). Beneficial and sexually dimorphic response to combined HDAC inhibitor valproate and AMPK/SIRT1 pathway activator resveratrol in the treatment of ALS mice. Int. J. Mol. Sci. 23 (3), 1047. doi:10.3390/ijms23031047
Barber, S. C., Higginbottom, A., Mead, R. J., Barber, S., and Shaw, P. J. (2009). An in vitro screening cascade to identify neuroprotective antioxidants in ALS. Free Radic. Biol. Med. 46 (8), 1127–1138. doi:10.1016/j.freeradbiomed.2009.01.019
Bellucci, A., Bubacco, L., Longhena, F., Parrella, E., Faustini, G., Porrini, V., et al. (2020). Nucle-ar factor-κb dysregulation and α-synuclein pathology: Critical interplay in the pathogenesis of Parkinson's disease. Front. Aging Neurosci. 12, 68. doi:10.3389/fnagi.2020.00068
Bendotti, C., Bonetto, V., Pupillo, E., Logroscino, G., Al-Chalabi, A., Lunetta, C., et al. (2020). Focus on the heterogeneity of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 21 (7-8), 485–495. doi:10.1080/21678421.2020.1779298
Bennett, S. A., Tanaz, R., Cobos, S. N., and Torrente, M. P. (2019). Epigenetics in amyotrophic lateral sclerosis: A role for histone post-translational modifications in neurodegenerative disease. Transl. Res. 204, 19–30. doi:10.1016/j.trsl.2018.10.002
Blasco, H., Guennoc, A. M., Veyrat-Durebex, C., Gordon, P. H., Andres, C. R., Camu, W., et al. (2012). Amyotrophic lateral sclerosis: A hormonal condition? Amyotroph. Lateral Scler. 13 (6), 585–588. doi:10.3109/17482968.2012.706303
Boutillier, A. L., Tzeplaeff, L., and Dupuis, L. (2019). The dark side of HDAC inhibition in ALS. EBioMedicine 41, 38–39. doi:10.1016/j.ebiom.2019.02.039
Buonvicino, D., Felici, R., Ranieri, G., Caramelli, R., Lapucci, A., Cavone, L., et al. (2018). Effects of class II-selective histone deacetylase inhibitor on neuromuscular function and disease progression in SOD1-ALS mice. Neuroscience 379, 228–238. doi:10.1016/j.neuroscience.2018.03.022
Burg, T., Rossaert, E., Moisse, M., Van Damme, P., and Van Den Bosch, L. (2021). Histone deacetylase inhibition regulates lipid home-ostasis in a mouse model of amyotrophic lateral sclerosis. Int. J. Mol. Sci. 22 (20), 11224. doi:10.3390/ijms222011224
Butler, K. V., Kalin, J., Brochier, C., Vistoli, G., Langley, B., and Kozikowski, A. P. (2010). Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J. Am. Chem. Soc. 132 (31), 10842–10846. doi:10.1021/ja102758v
Carrera-Juliá, S., Moreno, M. L., Barrios, C., de la Rubia Ortí, J. E., and Drehmer, E. (2020). Antioxidant alternatives in the treatment of amyotrophic lateral sclerosis: A comprehensive review. Front. Physiol. 11, 63. doi:10.3389/fphys.2020.00063
Chen, K., Bennett, S. A., Rana, N., Yousuf, H., Said, M., Taaseen, S., et al. (2018). Neurodegenerative disease proteinopathies are connected to distinct histone post-translational modification landscapes. ACS Chem. Neurosci. 9 (4), 838–848. doi:10.1021/acschemneuro.7b00297
Chen, L. F., and Greene, W. C. (2004). Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell. Biol. 5 (5), 392–401. doi:10.1038/nrm1368
Chen, S., Ye, J., Chen, X., Shi, J., Wu, W., Lin, W., et al. (2018). Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J. Neuroinflammation 15 (1), 150. doi:10.1186/s12974-018-1193-6
Chia, R., Chiò, A., and Traynor, B. J. (2018). Novel genes associated with amyotrophic lateral sclerosis: Diagnostic and clinical implica-tions. Lancet. Neurol. 17 (1), 94–102. doi:10.1016/S1474-4422(17)30401-5
Chuang, D. M., Leng, Y., Marinova, Z., Kim, H. J., and Chiu, C. T. (2009). Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 32 (11), 591–601. doi:10.1016/j.tins.2009.06.002
Crochemore, C., Virgili, M., Bonamassa, B., Canistro, D., Pena-Altamira, E., Paolini, M., et al. (2009). Long-term dietary administration of valproic acid does not affect, while retinoic acid decreases, the lifespan of G93A mice, a model for amyotrophic lateral sclerosis. Muscle Nerve 39 (4), 548–552. doi:10.1002/mus.21260
Cudkowicz, M. E., Andres, P. L., Macdonald, S. A., Bedlack, R. S., Choudry, R., Brown, R. H., et al. (2009). Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph. Lateral Scler. 10 (2), 99–106. doi:10.1080/17482960802320487
De Marchi, F., Munitic, I., Amedei, A., Berry, J. D., Feldman, E. L., Aronica, E., et al. (2021). Interplay between immunity and amyotrophic lateral sclerosis: Clinical impact. Neurosci. Biobehav. Rev. 127, 958–978. doi:10.1016/j.neubiorev.2021.06.027
Del Signore, S. J., Amante, D. J., Kim, J., Stack, E. C., Goodrich, S., Cormier, K., et al. (2009). Combined riluzole and sodium phenylbutyrate therapy in transgenic amyotrophic lateral sclerosis mice. Amyotroph. Lateral Scler. 10 (2), 85–94. doi:10.1080/17482960802226148
Dios, A. M., Babu, S., Granucci, E. J., Mueller, K. A., Mills, A. N., Alshikho, M. J., et al. (2019). Class I and II histone deacetylase expression is not altered in human amyotrophic lateral sclerosis: Neuropathological and positron emission tomography molecular neuroimaging evidence. Muscle Nerve 60 (4), 443–452. doi:10.1002/mus.26620
Dutta, K., Thammisetty, S. S., Boutej, H., Bareil, C., and Julien, J. P. (2020). Mitigation of ALS pathology by neuron-specific inhibition of nuclear factor kappa B signaling. J. Neurosci. 40 (26), 5137–5154. doi:10.1523/JNEUROSCI.0536-20.2020
Eberharter, A., and Becker, P. B. (2002). Histone acetylation: A switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 3 (3), 224–229. doi:10.1093/embo-reports/kvf053
Faggi, L., Pignataro, G., Parrella, E., Porrini, V., Vinciguerra, A., Cepparulo, P., et al. (2018). Synergistic association of valproate and resveratrol reduces brain injury in ischemic stroke. Int. J. Mol. Sci. 19 (1), E172. pii. doi:10.3390/ijms19010172
Fazal, R., Boeynaems, S., Swijsen, A., De Decker, M., Fumagalli, L., Moisse, M., et al. (2021). HDAC6 inhibition restores TDP-43 pathology and axonal transport defects in human motor neurons with TARDBP mutations. EMBO J. 40 (7), e106177. doi:10.15252/embj.2020106177
Feng, H. L., Leng, Y., Ma, C. H., Zhang, J., Ren, M., and Chuang, D. M. (2008). Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience 155 (3), 567–572. doi:10.1016/j.neuroscience.2008.06.040
Frakes, A. E., Ferraiuolo, L., Haidet-Phillips, A. M., Schmelzer, L., Braun, L., Miranda, C. J., et al. (2014). Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 81 (5), 1009–1023. doi:10.1016/j.neuron.2014.01.013
Ghosh, A., Roy, A., Liu, X., Kordower, J. H., Mufson, E. J., Hartley, D. M., et al. (2007). Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 104 (47), 18754–18759. doi:10.1073/pnas.0704908104
Gilmore, T. D., and Wolenski, F. S. (2012). NF-ΚB: Where did it come from and why? Immunol. Rev. 246, 14–35. doi:10.1111/j.1600-065X.2012.01096.x
Giribaldi, F., Milanese, M., Bonifacino, T., Anna Rossi, P. I., Di Prisco, S., Pittaluga, A., et al. (2013). Group I metabotropic glutamate autoreceptors induce abnormal glutamate exocytosis in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology 66, 253–263. doi:10.1016/j.neuropharm.2012.05.018
Guo, W., Naujock, M., Fumagalli, L., Vandoorne, T., Baatsen, P., Boon, R., et al. (2017). HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 8 (1), 861. doi:10.1038/s41467-017-00911-y
Gurvich, N., Tsygankova, O. M., Meinkoth, J. L., and Klein, P. S. (2004). Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 64 (3), 1079–1086. doi:10.1158/0008-5472.can-03-0799
Gutierrez, H., and Davies, A. M. (2011). Regulation of neural process growth, elaboration and structural plasticity by NF-κB. Trends Neurosci. 34 (6), 316–325. doi:10.1016/j.tins.2011.03.001
Gyawali, A., Latif, S., Choi, S. H., Hyeon, S. J., Ryu, H., and Kang, Y. S. (2022). Monocarboxylate transporter functions and neuroprotective effects of valproic acid in experimental models of amyotrophic lateral sclerosis. J. Biomed. Sci. 29 (1), 2. doi:10.1186/s12929-022-00785-3
Han, S., Choi, J. R., SoonShin, K., and Kang, S. J. (2012). Resveratrol upregulated heat shock proteins and extended the survival of G93A-SOD1 mice. Brain Res. 1483, 112–117. doi:10.1016/j.brainres.2012.09.022
Hasegawa, K., and Yoshikawa, K. (2008). Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J. Neurosci. 28 (35), 8772–8784. doi:10.1523/JNEUROSCI.3052-08.2008
Huisman, M. H., de Jong, S. W., van Doormaal, P. T., Weinreich, S. S., Schelhaas, H. J., van der Kooi, A. J., et al. (2011). Population based epidemiology of amyotrophic lateral sclerosis using capture-recapture methodology. J. Neurol. Neurosurg. Psychiatry 82 (10), 1165–1170. doi:10.1136/jnnp.2011.244939
Ikiz, B., Alvarez, M. J., Ré, D. B., Le Verche, V., Politi, K., Lotti, F., et al. (2015). The regulatory machinery of neurodegeneration in in vitro models of amyotrophic lateral sclerosis. Cell. Rep. 12 (2), 335–345. doi:10.1016/j.celrep.2015.06.019
Inta, I., Paxian, S., Maegele, I., Zhang, W., Pizzi, M., Spano, P., et al. (2006). Bim and Noxa are candidates to mediate the deleterious ef-fect of the NF-kappa B subunit RelA in cerebral ischemia. J. Neurosci. 26 (50), 12896–12903. doi:10.1523/JNEUROSCI.3670-06.2006
Janssen, C., Schmalbach, S., Boeselt, S., Sarlette, A., Dengler, R., and Petri, S. (2010). Differential histone deacetylase mRNA expression patterns in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 69 (6), 573–581. doi:10.1097/NEN.0b013e3181ddd404
Jiang, H. Z., Wang, S. Y., Yin, X., Jiang, H. Q., Wang, X. D., Wang, J., et al. (2016). Downregulation of homer1b/c in SOD1 G93A models of ALS: A novel mechanism of neuroprotective effect of lithium and valproic acid. Int. J. Mol. Sci. 17 (12), 2129. doi:10.3390/ijms17122129
Jiang, Y. M., Yamamoto, M., Kobayashi, Y., Yoshihara, T., Liang, Y., Terao, S., et al. (2005). Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Ann. Neurol. 57 (2), 236–251. doi:10.1002/ana.20379
Jimenez-Pacheco, A., Franco, J. M., Lopez, S., Gomez-Zumaquero, J. M., Magdalena Leal-Lasarte, M., Caballe-ro-Hernandez, D. E., et al. (2017). Epigenetic mechanisms of gene regulation in amyotrophic lateral sclerosis. Adv. Exp. Med. Biol. 978, 255–275. doi:10.1007/978-3-319-53889-1_14
Jochems, J., Boulden, J., Lee, B. G., Blendy, J. A., Jarpe, M., Mazitschek, R., et al. (2014). Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology 39 (2), 389–400. doi:10.1038/npp.2013.207
Källstig, E., McCabe, B. D., and Schneider, B. L. (2021). The links between ALS and NF-κB. Int. J. Mol. Sci. 22 (8), 3875. doi:10.3390/ijms22083875
Kaltschmidt, B., and Kaltschmidt, C. (2015). NF-KappaB in long-term memory and structural plasticity in the adult mammalian brain. Front. Mol. Neurosci. 24 (8), 69. doi:10.3389/fnmol.2015.00069
Khan, N., Jeffers, M., Kumar, S., Hackett, C., Boldog, F., Khramtsov, N., et al. (2008). Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem. J. 409 (2), 581–589. doi:10.1042/BJ20070779
Kim, D., Nguyen, M. D., Dobbin, M. M., Fischer, A., Sananbenesi, F., Rodgers, J. T., et al. (2007). SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 26 (13), 3169–3179. doi:10.1038/sj.emboj.7601758
Klingl, Y. E., Pakravan, D., and Van Den Bosch, L. (2021). Opportunities for histone deacetylase inhibition in amyotrophic lateral sclerosis. Br. J. Pharmacol. 178 (6), 1353–1372. doi:10.1111/bph.15217
Konsoula, Z., and Barile, F. A. (2012). Epigenetic histone acetylation and deacetylation mechanisms in experimental models of neurodegenerative disorders. J. Pharmacol. Toxicol. Methods 66 (3), 215–220. doi:10.1016/j.vascn.2012.08.001
Kusaczuk, M., Bartoszewicz, M., and Cechowska-Pasko, M. (2015). Phenylbutyric acid: Simple structure - multiple effects. Curr. Pharm. Des. 21 (16), 2147–2166. doi:10.2174/1381612821666150105160059
Lacomblez, L., Bensimon, G., Leigh, P. N., Guillet, P., and Meininger, V. (1996). Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 347, 1425–1431. doi:10.1016/s0140-6736(96)91680-3
Lanzillotta, A., Pignataro, G., Branca, C., Cuomo, O., Sarnico, I., Benarese, M., et al. (2013). Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol. Dis. 49, 177–189. doi:10.1016/j.nbd.2012.08.018
Lanzillotta, A., Porrini, V., Bellucci, A., Benarese, M., Branca, C., Parrella, E., et al. (2015). NF-κB in innate neuroprotection and age-related neurodegenerative diseases. Front. Neurol. 6, 98. doi:10.3389/fneur.2015.00098
Lanzillotta, A., Sarnico, I., Ingrassia, R., Boroni, F., Branca, C., Benarese, M., et al. (2010). The acetylation of RelA in Lys310 dictates the NF-κB-dependent response in post-ischemic injury. Cell. Death Dis. 1, e96. doi:10.1038/cddis.2010.76
Lapucci, A., Cavone, L., Buonvicino, D., Felici, R., Gerace, E., Zwergel, C., et al. (2017). Effect of Class II HDAC inhibition on glutamate transporter expression and survival in SOD1-ALS mice. Neurosci. Lett. 656, 120–125. doi:10.1016/j.neulet.2017.07.033
Lazo-Gómez, R., Ramírez-Jarquín, U. N., Tovar-Y-Romo, L. B., and Tapia, R. (2013). Histone deacetylases and their role in motor neuron degeneration. Front. Cell. Neurosci. 7, 243. doi:10.3389/fncel.2013.00243
Lee, S. H., Lee, J. H., Lee, H. Y., and Min, K. J. (2019). Sirtuin signaling in cellular senescence and aging. BMB Rep. 52 (1), 24–34. doi:10.5483/BMBRep.2019.52.1.290
Li, X., Dong, L., Li, A., Yi, J., Brotto, M., and Zhou, J. (2022). Butyrate ameliorates mitochondrial respiratory capacity of the motor-neuron-like cell line NSC34-g93a, a cellular model for ALS. Biomolecules 12 (2), 333. doi:10.3390/biom12020333
Mai, A., Massa, S., Pezzi, R., Simeoni, S., Rotili, D., Nebbioso, A., et al. (2005). Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. J. Med. Chem. 48 (9), 3344–3353. doi:10.1021/jm049002a
Mancuso, R., del Valle, J., Modol, L., Martinez, A., Granado-Serrano, A. B., Ramirez-Núñez, O., et al. (2014). Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 11 (2), 419–432. doi:10.1007/s13311-013-0253-y
Manjaly, Z. R., Scott, K. M., Abhinav, K., Wijesekera, L., Ganesalingam, J., Goldstein, L. H., et al. (2010). The sex ratio in amyotrophic lateral sclerosis: A population based study. Amyotroph. Lateral Scler. 11 (5), 439–442. doi:10.3109/17482961003610853
Markert, C. D., Kim, E., Gifondorwa, D. J., Childers, M. K., and Milligan, C. E. (2010). A single-dose resveratrol treatment in a mouse model of amyotrophic lateral sclerosis. J. Med. Food 13 (5), 1081–1085. doi:10.1089/jmf.2009.0243
Mejzini, R., Flynn, L. L., Pitout, I. L., Fletcher, S., Wilton, S. D., and Akkari, P. A. (2019). ALS genetics, mechanisms, and therapeutics: Where are we now? Front. Neurosci. 13, 1310. doi:10.3389/fnins.2019.01310
Miller, R. G., Mitchell, J. D., and Moore, D. H. (2012). Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2012 (3), CD001447. doi:10.1002/14651858.CD001447.pub3
Mithraprabhu, S., Khong, T., Jones, S. S., and Spencer, A. (2013). Histone deacetylase (HDAC) inhibitors as single agents induce multiple myeloma cell death principally through the inhibition of class I HDAC. Br. J. Haematol. 162 (4), 559–562. doi:10.1111/bjh.12388
Mitsumoto, H., Brooks, B. R., and Silani, V. (2014). Clinical trials in amyotrophic lateral sclerosis: Why so many negative tri-als and how can trials be improved? Lancet. Neurol. 13 (11), 1127–1138. doi:10.1016/S1474-4422(14)70129-2
Mota, M., Porrini, V., Parrella, E., Benarese, M., Bellucci, A., Rhein, S., et al. (2020). Neuroprotective epi-drugs quench the inflammatory response and microglial/macrophage activation in a mouse model of permanent brain ischemia. J. Neuroinflammation 17 (1), 361. doi:10.1186/s12974-020-02028-4
Novak, V., Rogelj, B., and Župunski, V. (2021). Therapeutic potential of polyphenols in amyotrophic lateral sclerosis and frontotemporal dementia. Antioxidants (Basel) 10 (8), 1328. doi:10.3390/antiox10081328
Okada, M., Yamashita, S., Ueyama, H., Ishizaki, M., Maeda, Y., and Ando, Y. (2018). Long-term effects of edaravone on survival of patients with amyotrophic lateral sclerosis. eNeurologicalSci 11, 11–14. doi:10.1016/j.ensci.2018.05.001
Olaharski, A. J., Ji, Z., Woo, J. Y., Lim, S., Hubbard, A. E., Zhang, L., et al. (2006). The histone deacetylase inhibitor trichostatin a has genotoxic effects in human lymphoblasts in vitro. Toxicol. Sci. 93 (2), 341–347. doi:10.1093/toxsci/kfl068
Oskarsson, B., Gendron, T. F., and Staff, N. P. (2018). Amyotrophic lateral sclerosis: An update for 2018. Mayo Clin. Proc. 93 (11), 1617–1628. doi:10.1016/j.mayocp.2018.04.007
Panes, J. D., Wendt, A., Ramirez-Molina, O., Castro, P. A., and Fuentealba, J. (2022). Deciphering the role of PGC-1α in neurological disorders: From mitochondrial dysfunction to synaptic failure. Neural Regen. Res. 17 (2), 237–245. doi:10.4103/1673-5374.317957
Parrella, E., Gussago, C., Porrini, V., Benarese, M., and Pizzi, M. (2020). From preclinical stroke models to humans: Polyphenols in the prevention and treatment of stroke. Nutrients 13 (1), 85. doi:10.3390/nu13010085
Patel, P., Julien, J. P., and Kriz, J. (2015). Early-stage treatment with Withaferin A reduces levels of misfolded superoxide dismutase 1 and extends lifespan in a mouse model of amyotrophic lateral sclerosis. Neurotherapeutics 12 (1), 217–233. doi:10.1007/s13311-014-0311-0
Perucca, E. (2002). Pharmacological and therapeutic properties of valproate: A summary after 35 years of clinical experi-ence. CNS Drugs 16 (10), 695–714. doi:10.2165/00023210-200216100-00004
Petri, S., Kiaei, M., Kipiani, K., Chen, J., Calingasan, N. Y., Crow, J. P., et al. (2006). Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 22 (1), 40–49. doi:10.1016/j.nbd.2005.09.013
Piepers, S., Veldink, J. H., de Jong, S. W., van der Tweel, I., van der Pol, W. L., Uijtendaal, E. V., et al. (2006). Randomized sequential trial of valproic acid in amyotrophic lateral sclerosis. Ann. Neurol. 66 (2), 227–234. doi:10.1002/ana.21620
Pigna, E., Simonazzi, E., Sanna, K., Bernadzki, K. M., Proszynski, T., Heil, C., et al. (2019). Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis. EBioMedicine 40, 717–732. doi:10.1016/j.ebiom.2019.01.038
Pizzi, M., Boroni, F., Bianchetti, A., Moraitis, C., Sarnico, I., Benarese, M., et al. (2002). Expression of functional NR1/NR2B-type NMDA receptors in neuronally differentiated SK-N-SH human cell line. Eur. J. Neurosci. 16 (12), 2342–2350. doi:10.1046/j.1460-9568.2002.02403.x
Pizzi, M., Sarnico, I., Boroni, F., Benarese, M., Steimberg, N., Mazzoleni, G., et al. (2005). NF-kappaB factor c-Rel mediates neuroprotection elicited by mGlu5 receptor agonists against amyloid beta-peptide toxicity. Cell. Death Differ. 12 (7), 761–772. doi:10.1038/sj.cdd.4401598
Prell, T., Lautenschläger, J., Weidemann, L., Ruhmer, J., Witte, O. W., and Grosskreutz, J. (2014). Endoplasmic reticulum stress is accompanied by activation of NF-κB in amyotrophic lateral sclerosis. J. Neuroimmunol. 270 (1-2), 29–36. doi:10.1016/j.jneuroim.2014.03.005
Qasem, R. J. (2020). The estrogenic activity of resveratrol: A comprehensive review of in vitro and in vivo evidence and the potential for endocrine disruption. Crit. Rev. Toxicol. 50 (5), 439–462. doi:10.1080/10408444.2020.1762538
Ragancokova, D., Song, Y., Nau, H., Dengler, R., Krampfl, K., and Petri, S. (2010). Modulation of synaptic transmission and analysis of neuroprotective effects of valproic Acid and derivates in rat embryonic motoneurons. Cell. Mol. Neurobiol. 30 (6), 891–900. doi:10.1007/s10571-010-9518-8
Ranganathan, S., and Bowser, R. (2010). p53 and cell cycle proteins participate in spinal motor neuron cell death in ALS. Open Pathol. J. 4, 11–22. doi:10.2174/1874375701004010011
Rauf, A., Imran, M., Suleria, H. A. R., Ahmad, B., Peters, D. G., and Mubarak, M. S. (2017). A comprehensive review of the health perspectives of resveratrol. Food Funct. 8 (12), 4284–4305. doi:10.1039/c7fo01300k
Rossaert, E., Pollari, E., Jaspers, T., Van Helleputte, L., Jarpe, M., Van Damme, P., et al. (2019). Restoration of histone acetylation ameliorates disease and metabolic abnormalities in a FUS mouse model. Acta Neuropathol. Commun. 7 (1), 107. doi:10.1186/s40478-019-0750-2
Rouaux, C., Panteleeva, I., René, F., Gonzalez de Aguilar, J. L., Echaniz-Laguna, A., Dupuis, L., et al. (2007). Sodium valproate exerts neuroprotective effects in vivo through CREB-binding protein-dependent mechanisms but does not improve survival in an amyotrophic lateral sclerosis mouse model. J. Neurosci. 27 (21), 5535–5545. doi:10.1523/JNEUROSCI.1139-07.2007
Ruderman, N. B., Xu, X. J., Nelson, L., Cacicedo, J. M., Saha, A. K., Lan, F., et al. (2010). AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 298 (4), E751–E760. doi:10.1152/ajpendo.00745.2009
Ryu, H., Smith, K., Camelo, S. I., Carreras, I., Lee, J., Iglesias, A. H., et al. (2005). Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J. Neurochem. 93 (5), 1087–1098. doi:10.1111/j.1471-4159.2005.03447.x
Sanna, S., Esposito, S., Masala, A., Sini, P., Nieddu, G., Galioto, M., et al. (2020). HDAC1 inhibition ameliorates TDP-43-induced cell death in vitro and in vivo. Cell. Death Dis. 11 (5), 369. doi:10.1038/s41419-020-2580-3
Sarnico, I., Branca, C., Lanzillotta, A., Porrini, V., Benarese, M., Spano, P. F., et al. (2012). NF-κB and epigenetic mechanisms as integrative regulators of brain resilience to anoxic stress. Brain Res. 1476, 203–210. doi:10.1016/j.brainres.2012.04.013
Sarnico, I., Boroni, F., Benarese, M., Sigala, S., Lanzillotta, A., Battistin, L., et al. (2008). Activation of NF-kappaB p65/c-Rel dimer is associated with neuroprotection elicited by mGlu5 receptor agonists against MPP(+) toxicity in SK-N-SH cells. J. Neural Transm. 115 (5), 669–676. doi:10.1007/s00702-007-0007-2
Schiaffino, L., Bonafede, R., Scambi, I., Parrella, E., Pizzi, M., and Mariotti, R. (2018). Acetylation state of RelA modulated by epigenetic drugs prolongs survival and induces a neuroprotective effect on ALS murine model. Sci. Rep. 8 (1), 12875. doi:10.1038/s41598-018-30659-4
Schmalbach, S., and Petri, S. (2010). Histone deacetylation and motor neuron degeneration. CNS Neurol. Disord. Drug Targets 9 (3), 279–284. doi:10.2174/187152710791292684
Shen, S., Svoboda, M., Zhang, G., Cavasin, M. A., Motlova, L., McKinsey, T. A., et al. (2020). Structural and in vivo characterization of tubastatin A, a widely used histone deacetylase 6 inhibitor. ACS Med. Chem. Lett. 11 (5), 706–712. doi:10.1021/acsmedchemlett.9b00560
Shukla, S., and Tekwani, B. L. (2020). Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front. Pharmacol. 11, 537. doi:10.3389/fphar.2020.00537
Simonini, M. V., Camargo, L. M., Dong, E., Maloku, E., Veldic, M., Costa, E., et al. (2006). The benzamide MS-275 is a potent, long-lasting brain region-selective inhibitor of histone deacetylases. Proc. Natl. Acad. Sci. U. S. A. 103 (5), 1587–1592. doi:10.1073/pnas.0510341103
Srinivasan, M., and Lahiri, D. K. (2015). Significance of NF-κB as a pivotal therapeutic target in the neurodegenerative pa-thologies of Alzheimer's disease and multiple sclerosis. Expert Opin. Ther. Targets 19 (4), 471–487. doi:10.1517/14728222.2014.989834
Stempin, S., Andres, S., Bumke Scheer, M., Rode, A., Nau, H., Seidel, A., et al. (2013). Valproic acid and its derivatives enhanced estrogenic activity but not androgenic activity in a structure dependent manner. Reprod. Toxicol. 42, 49–57. doi:10.1016/j.reprotox.2013.07.019
Sugai, F., Yamamoto, Y., Miyaguchi, K., Zhou, Z., Sumi, H., Hamasaki, T., et al. (2004). Benefit of valproic acid in suppressing disease progression of ALS model mice. Eur. J. Neurosci. 20 (11), 3179–3183. doi:10.1111/j.1460-9568.2004.03765.x
Swarup, V., Phaneuf, D., Dupré, N., Petri, S., Strong, M., Kriz, J., et al. (2011). Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor κB-mediated pathogenic pathways. J. Exp. Med. 208 (12), 2429–2447. doi:10.1084/jem.20111313
Uzdensky, A. B., and Demyanenko, S. (2021). Histone acetylation and deacetylation in ischemic stroke. Neural Regen. Res. 16 (8), 1529–1530. doi:10.4103/1673-5374.303024
van Es, M. A., Hardiman, O., Chio, A., Al-Chalabi, A., Pasterkamp, R. J., Veldink, J. H., et al. (2017). Amyotrophic lateral sclerosis. Lancet 4390 (10107), 2084–2098. doi:10.1016/S0140-6736(17)31287-4
Vegeto, E., Villa, A., Della Torre, S., Crippa, V., Rusmini, P., Cristofani, R., et al. (2020). The role of sex and sex hormones in neurodegenerative diseases. Endocr. Rev. 41 (2), bnz005–319. doi:10.1210/endrev/bnz005
Wang, C., Lin, Y., Zhu, H., Zhou, Y., Mao, F., Huang, X., et al. (2022). Efficacy and safety profile of histone deacetylase inhibitors for metastatic breast cancer: A meta-analysis. Front. Oncol. 12, 901152. doi:10.3389/fonc.2022.901152
Wang, J., Zhang, Y., Tang, L., Zhang, N., and Fan, D. (2011). Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. 503 (3), 250–255. doi:10.1016/j.neulet.2011.08.047
Wittie, M., Nelson, L. M., Usher, S., Ward, K., and Benatar, M. (2013). Utility of capture-recapture methodology to assess completeness of amyotrophic lateral sclerosis case ascertainment. Neuroepidemiology 40 (2), 133–141. doi:10.1159/000342156
Wobst, H. J., Mack, K. L., Brown, D. G., Brandon, N. J., and Shorter, J. (2020). The clinical trial landscape in amyotrophic lateral sclerosis-Past, present, and future. Med. Res. Rev. 40 (4), 1352–1384. doi:10.1002/med.21661
Writing GroupEdaravone (MCI-186) ALS 19 Study Group (2017). Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet. Neurol. 16 (7), 505–512. doi:10.1016/S1474-4422(17)30115-1
Yáñez, M., Galán, L., Matías-Guiu, J., Vela, A., Guerrero, A., and García, A. G. (2011). CSF from amyotrophic lateral sclerosis patients produces glutamate independent death of rat motor brain cortical neurons: Protection by resveratrol but not riluzole. Brain Res. 1423, 77–86. doi:10.1016/j.brainres.2011.09.025
Yin, X., Wang, S., Qi, Y., Wang, X., Jiang, H., Wang, T., et al. (2018). Astrocyte elevated gene-1 is a novel regulator of astrogliosis and excitatory amino acid transporter-2 via interplaying with nuclear factor-κB signaling in astrocytes from amyotrophic lateral sclerosis mouse model with hSOD1G93A mutation. Mol. Cell. Neurosci. 90, 1–11. doi:10.1016/j.mcn.2018.05.004
Yoo, Y. E., and Ko, C. P. (2011). Treatment with trichostatin A initiated after disease onset delays disease progression and increases survival in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 231 (1), 147–159. doi:10.1016/j.expneurol.2011.06.003
Yoshino, H. (2019). Edaravone for the treatment of amyotrophic lateral sclerosis. Expert Rev. Neurother. 19 (3), 185–193. doi:10.1080/14737175.2019.1581610
Yun, Y. C., Jeong, S. G., Kim, S. H., and Cho, G. W. (2019). Reduced sirtuin 1/adenosine monophosphate-activated protein kinase in amyotrophic lateral sclerosis patient-derived mesenchymal stem cells can be restored by resveratrol. J. Tissue Eng. Regen. Med. 13 (1), 110–115. doi:10.1002/term.2776
Zhang, L. X., Li, C. X., Kakar, M. U., Khan, M. S., Wu, P. F., Amir, R. M., et al. (2021). Resveratrol (rv): A pharmacological review and call for further research. Biomed. Pharmacother. 143, 112164. doi:10.1016/j.biopha.2021.112164
Zhang, S., Cooper-Knock, J., Weimer, A. K., Shi, M., Moll, T., Marshall, J. N. G., et al. (2022). Genome-wide identification of the genetic basis of amyotrophic lateral sclerosis. Neuron 110 (6), 992–1008.e11. e11. doi:10.1016/j.neuron.2021.12.019
Keywords: amyotrophic lateral sclerosis (ALS), NF-κB/RelA, histone acetylation, resveratrol, histone deacetylase (HDAC) inhibitors, epigenetic drugs, sexual dimorphism
Citation: Parrella E, Porrini V, Scambi I, Gennari MM, Gussago C, Bankole O, Benarese M, Mariotti R and Pizzi M (2022) Synergistic association of resveratrol and histone deacetylase inhibitors as treatment in amyotrophic lateral sclerosis. Front. Pharmacol. 13:1017364. doi: 10.3389/fphar.2022.1017364
Received: 11 August 2022; Accepted: 06 October 2022;
Published: 21 October 2022.
Edited by:
Morena Zusso, University of Padua, ItalyReviewed by:
Caroline Rouaux, INSERM U1118 Mécanismes Centraux et Périphériques de la Neurodégénérescence, FranceCarlos Batthyany, Institut Pasteur de Montevideo, Uruguay
Copyright © 2022 Parrella, Porrini, Scambi, Gennari, Gussago, Bankole, Benarese, Mariotti and Pizzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vanessa Porrini, di5wb3JyaW5pQHVuaWJzLml0
 Edoardo Parrella
Edoardo Parrella Vanessa Porrini
Vanessa Porrini Ilaria Scambi
Ilaria Scambi Michele M. Gennari
Michele M. Gennari Cristina Gussago
Cristina Gussago Oluwamolakun Bankole
Oluwamolakun Bankole Marina Benarese
Marina Benarese Raffaella Mariotti
Raffaella Mariotti Marina Pizzi
Marina Pizzi