
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 30 September 2022
Sec. Drug Metabolism and Transport
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1014474
This article is part of the Research TopicClinical Trials in Drug Metabolism and Transport: 2022View all 13 articles
 Fanxi Guo†
Fanxi Guo† Dongying Liu†
Dongying Liu† Yuqing Zhou
Yuqing Zhou Yuanqian Yu
Yuanqian Yu Yidan Xu
Yidan Xu Yuanpeng Zou
Yuanpeng Zou Chongyang Li
Chongyang Li Fengyichi Zhang
Fengyichi Zhang Zugong Yu*
Zugong Yu*Glutamine is an amino acid that is mainly used for the treatment of gastrointestinal diseases in clinic, but there is a lack of such medicine in veterinary clinic, and its research in dogs has never been seen. This study aimed to investigate the pharmacokinetics of single and multiple administration of glutamine (Gln) tablets in Beagles. Twenty-four healthy Beagles were randomly selected for the pharmacokinetic study of a single dose of low (120 mg/kg), medium (240 mg/kg), and high (360 mg/kg) Gln tablets. After 7 days of washout period, six Beagles in the medium group were selected for a multiple-dose pharmacokinetic study, 240 mg/kg twice a day for 7 days. The Gln concentration in plasma was determined by a validated UPLC-MS/MS method. The results of single oral administration of different doses of Gln tablets showed that Cmax, AUC0→t, AUC0→∝ had a certain linear relationship with the dosage. T-tests were performed for single and multiple administration of Tmax, Cmax, t1/2λz, AUC0→t, and AUC0→∝, and the results showed no significant differences (p > 0.05). Therefore, Gln tablets were absorbed quickly by oral administration, and there was no accumulation in Beagles after 7 days of administration.
Gastrointestinal mucosal barrier injury is closely related to gastrointestinal diseases (Turner 2009; Camilleri et al., 2012), and increasing evidence shows that the increase of intestinal permeability plays a major pathogenic role in some intestinal diseases (Zuckerman et al., 1993; Spiller et al., 2000; Marshall et al., 2004; Dunlop et al., 2006). Vomiting and diarrhea caused by gastrointestinal mucosa injury are common in pets (Niu et al., 2021), but the lack of medicine that can effectively repair the intestinal mucosa leads to slow recovery, which affects the health and quality of life of pets. Therefore, medicines that can nourish and repair intestinal mucosal cells are urgently needed in veterinary clinic.
Glutamine (Gln) is an amino acid whose main function is to provide nutrition to gastrointestinal cells and immune cells and maintain the integrity of gastrointestinal mucosa (Ardawi and Newsholme 1983; Burke et al., 1989; Jiang and Fang 2009; Wang and Liu 2009). Therefore, Gln is also suitable for the treatment of diseases related to gastrointestinal mucosal injury in pets. Gln has been widely used in human clinical medicine in a variety of dosage forms, mainly for the treatment of gastritis, gastric ulcer and duodenal ulcer (Griffiths et al., 2000; Anderson and Lalla 2020). A large number of clinical controlled studies have proved the good efficacy of Gln on gastrointestinal diseases (Mochiki et al., 2011; Dai and Meng 2015; Zuo 2015; Yao et al., 2016; Zhou et al., 2019).
Pharmacokinetic studies of Gln in humans (Ziegler et al., 1990; Ward et al., 2003; Zhu et al., 2003; Berg et al., 2005; Tang et al., 2005; Yu et al., 2014; Du et al., 2015; Han et al., 2016; Morris et al., 2022) and rats (Wang et al., 2015) have been reported in some literatures and the results show that oral Gln was rapidly absorbed and metabolized in vivo. However, there was a lack of relevant studies in pets, so pharmacokinetic studies of Gln in dogs are necessary to introduce it into pet clinical practice.
The aim of this study was to clarify the absorption, distribution, metabolism and excretion of Gln tablets in dogs, provide theoretical basis for the introduction of Gln tablets into pet clinical practice, and provide reliable data for the formulation of clinical recommended drug administration program. The pharmacokinetic study of single and multiple oral administration of Gln tablets in dogs was conducted to study the correlation between main pharmacokinetic parameters and dosage, and to compare the similarities and differences between single and multiple administration to investigate the safety of multiple administration.
Twenty-four healthy Beagles (half male and half female) aged from 15 to 17 months, weighing 7.0–10.9 kg, were purchased from Jiangsu Yadong Experimental Animal Research Center. The laboratory animal production license number: SCXK (Su) 2016-0009. All dogs were kept in individual cages and given a daily ration of drug-free feed to maintain proper weight and adequate water. Before each experiment, dogs were fasted for 12 h and water was forbidden for 1 h. In addition, 5 ml of water was fed with a syringe during drug administration, water was fed for 2 h and food for 4 h after drug administration. All animal experiments were conducted in accordance with the guidelines of the Animal Ethics Committee of Nanjing Agricultural University (Nanjing, China).
Gln tablets were produced by Nanjing Jindun Animal Pharmaceutical Co., Ltd., and the production batch number was 20190501. The working standard of Gln and Penciclovir (Internal Standard) were obtained from China National Institutes for Drug Control. Acetonitrile and formic acid were HPLC grade, and ammonium formate was LC/MS grade.
Pharmacokinetics of low (120 mg/kg), medium (240 mg/kg) and high (360 mg/kg) doses of Gln tablets were studied in 24 healthy Beagles, including 6 in the low and high dose groups and 12 in the medium dose group. All animals were weighed and blank plasma samples were collected at −24, −18, −12, and 0 h before administration. The mean value of Gln concentration in blank plasma was used as baseline concentration for correction. Plasma samples were collected at 0.17, 0.33, 0.5, 0.67, 0.83, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 8, 10, 12, and 24 h after oral administration, and the Gln concentration in plasma was measured by UPLC-MS/MS. The concentration of drug-derived Gln was obtained by subtracting the baseline concentration before administration from the total Gln concentration in plasma.
Six Beagles (half males and half females) from the medium-dose group were selected for a pharmacokinetic study of multiple doses (240 mg/kg) after a 1-week cleaning period following a single dose. Multiple oral pharmacokinetic studies were administered twice a day, with an interval of 12 h, for 7 consecutive days (13 times). Blank blood samples were collected at 24, 18, 12, and 0 h before the first dose. The mean glutamine concentration in blank blood samples collected at these times served as the baseline endogenous glutamine concentration. Blood samples were collected at the following time points after the start of dosing: before the 7th dosing (the morning of the 4th day of dosing), before the 9th dosing (the morning of the 5th day of dosing), before the 11th dosing (The morning of the 6th day of administration), 6 h after the 11th administration, before the 12th administration (the evening of the 6th day of administration), before the 13th administration (the morning of the 7th day of administration), and 10, 20, 30, 40, 50 min, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 8, 10, 12, 24 h after the 13th administration. The Gln concentration in plasma was measured by UPLC-MS/MS, and the concentration of drug-derived Gln was obtained by subtracting the baseline concentration before first administration from the total Gln concentration in plasma.
About 3–3.5 ml of blood is collected from the dog’s forelimb vein with a disposable vacuum blood collection tube using heparin (dry powder) anticoagulation. The blood samples were centrifuged at 2,152 ± 5 g for 10 min at 4°C, and the separated plasma was divided into 0.5 ml/part. All blood samples should be centrifuged and aliquoted within 60 min after collection, and the aliquoted plasma should be immediately stored in an ultra-low temperature refrigerator (−80 ± 10°C).
The measurement of plasma FFC levels was performed using a ultra performance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS) device (Waters XEVO TQD, Waters Technologies Co. Ltd., United States) comprised a UPLC separation system and a triple quadrupole mass analyzer.
Separation was carried out using a Waters Acquity UPLC BEH Amide column (100 mm╳2.0 mm, particle size 1.7 µm; Waters Technologies Co., Ltd., Milford United States). The injection volume was 5 µl. The temperature of the column and auto-sampler was set at 35 and 6°C, respectively. The mobile phase was composed of acetonitrile (mobile phase A) and 40 mmol/L ammonium formate mixture (pH = 3.0 with formic acid, mobile phase B) at a flow rate of 0.4 ml/min. The gradient elution was shown in Table 1. This resulted in an overall run time of 5.5 min.
Detection was performed using a TQD triple quadrupole mass spectrometer equipped with an electrospray ionization source in the electrospray positive ion mode (ESI + ). Nitrogen was used as both drying and nebulizing gas. Product ions were detected using the multiple reaction-monitoring (MRM) mode, using argon as collision gas. The capillary voltage and source temperature were optimized at 3.0 kV and 150°C, respectively. The collision energy and cone voltage were optimized for each compound individually. The collision energy varied from 10 to 35 eV, and the cone voltage varied from 23 to 36 V. The MS/MS transition compounds for Gln were 147.2 > 84 and 147.2 > 130 at collision voltages of 10 and 18 eV, respectively. And for Penciclovir (Internal standards) were 254.1 > 135 and 254.1 > 152 at collision voltages of 35 and 18 eV (Liu et al., 2022). Data were collected and processed using the MassLynx software 4.1.
The plasma pretreatment method was acetonitrile and ammonium formate solution for protein precipitation, and penciclovir was used as the internal standard. 100 μl of plasma samples were thawed at room temperature, added 20 μl of water, 200 μl of IS working solution, and 800 μl of initial mobile phase, then vortexed for 2 min and centrifuged at 4°C 14,462 g for 10 min. 780 μl initial mobile phase was added to 20 μl of supernatant liquid, then vortexed for 1 min. Ultimately, the samples were filtered through membrane filters (0.22 μm). The processed samples were injected into UPLC-MS/MS for analysis.
Pharmacokinetic parameters were determined with Phoenix WinNonlin Professional software (Version 8.1) by using non-compartmental analysis. The area under the plasma concentration-time curve (AUC) was calculated by the linear trapezoidal linear interpolation method, and the pharmacokinetic parameters such as the terminal elimination half-life (t1/2λz), the maximum concentration of Gln in plasma (Cmax), the time to reach Cmax (Tmax) were also obtained. All pharmacokinetic results were presented as mean ± SD.
After a single oral dose of low-dose (120 mg/kg), medium-dose (240 mg/kg), and high-dose (360 mg/kg) in Beagles, the mean plasma concentration-time curve of exogenous Gln was shown in Figure 1 and the mean pharmacokinetic parameters (mean ± SD) were shown in Table 2.
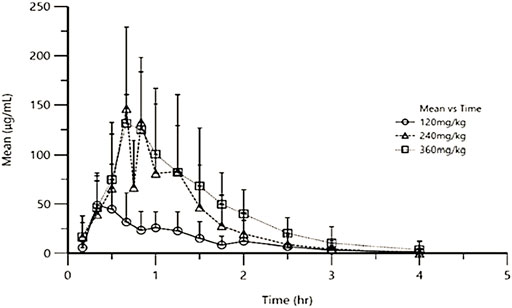
FIGURE 1. Mean exogenous Gln concentration-time curves in Beagles’ plasma after single oral administration of three different doses of Gln tablets.
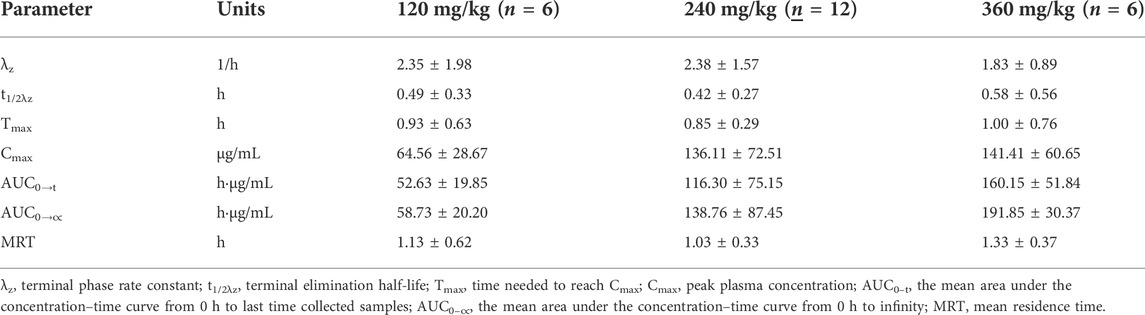
TABLE 2. Mean pharmacokinetic parameters of exogenous Gln in Beagle’ plasma after single oral administration of three different doses of Gln tablets. (mean ± SD).
Taking the dose as the abscissa, and the Cmax, AUC0→t and AUC0→∝ of each tested Beagle as the ordinate, the linear regression was performed. The results were shown in Figure 2. After Spearman rank correlation analysis, the results were shown in Table 3. The results showed that the correlation coefficient r of Cmax-dose was 0.5108 (p < 0.05), the correlation coefficient r of AUC0→t-dose was 0.6725 (p < 0.01), and the correlation coefficient r of AUC0→∝-dose was 0.7463(p<0.01). It showed that Cmax, AUC0→t, AUC0→∝ were positively correlated with the dose, respectively.
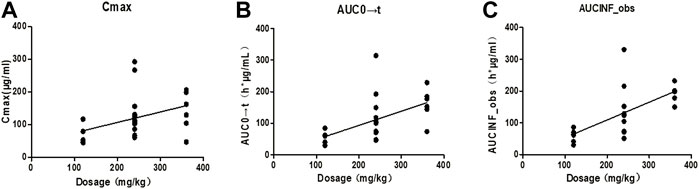
FIGURE 2. Correlation curves of pharmacokinetic parameters and dosage of each Beagle. Note: (A). The correlation curve of Cmax/dose of each Beagle; (B). The correlation curve of AUC0→t/dose of each Beagle; (C). The correlation curve of AUC0→∝/dose of each Beagle.

TABLE 3. Results of ANOVA for the main pharmacokinetic parameters-dosage of three different dosage groups.
Taking the dose as the abscissa and taking the mean of each Cmax, AUC0→t and AUC0→∝ as the ordinate, the linear regression was performed. The results were shown in Figure 3, and the linear regression equation and determination coefficient r2 were shown in Table 4.
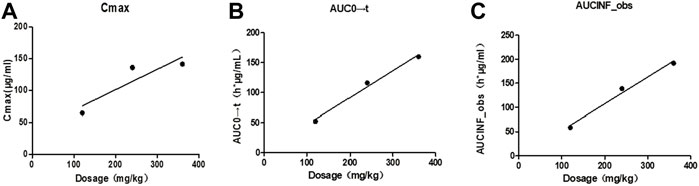
FIGURE 3. Correlation curves of mean pharmacokinetic parameters and dosage of Beagles. Note: (A). The correlation curve of mean Cmax/dose of Beagles; (B). The correlation curve of mean AUC0→t/dose of Beagles; (C). The correlation curve of mean AUC0→∝/dose of Beagles.
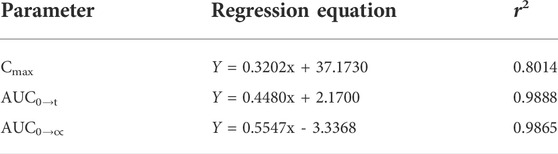
TABLE 4. Linear regression results of the means of the main pharmacokinetic parameters for three dosage groups.
The average pharmacokinetic parameters of 240 mg/kg Gln tablets after single or multiple oral administration of 6 Beagles were shown in Table 5, and the mean Gln concentration in plasma versus time curve was shown in Figure 4. SPSS software was used to conduct t-test on the pharmacokinetic parameters of single and multiple administrations. The results showed that there was no significant difference compared with single administration (p > 0.05).
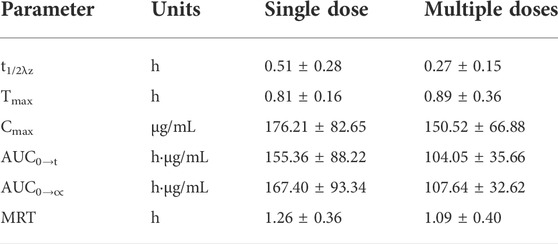
TABLE 5. Comparison of the mean pharmacokinetic parameters of exogenous Gln between single and multiple oral administration of 240 mg/kg Gln tablets in Beagls (mean ± SD, n = 6).
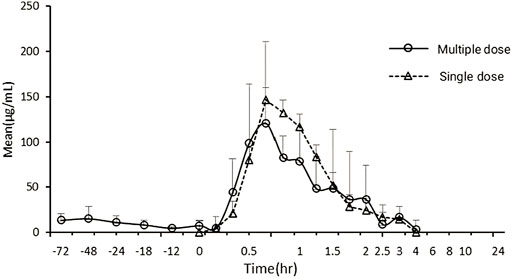
FIGURE 4. Mean exogenous Gln concentration–time curves in Beagles’ plasma after single and multiple oral administration of 240 mg/kg Gln tablets (mean ± SD, n = 6).
As an endogenous substance, the pharmacokinetics of glutamine may be affected by the level of endogenous substances, food, and rhythm changes compared with other drugs (Marzo et al., 1993; Dissanayake 2010; Zhang et al., 2011). This study optimized the experimental design by adjusting for baseline substance levels, administering supra-therapeutic doses, and standardizing dietary management. The methods reported in the literature for the base value subtraction of endogenous substances mainly include the mean value subtraction method (Wang et al., 2015) and the point-to-point subtraction method (Berg et al., 2005; Han et al., 2016). Previous studies by our group (Unpublished prior to submission of this article, but accepted.) showed that the concentration of endogenous Gln in the plasma of Beagles remained basically constant within 48 h, with no circadian rhythm and periodic differences.
Based on the stable endogenous glutamine concentration of beagle dogs, and referring to the guiding principles for human bioavailability and bioequivalence test of pharmaceutical preparations (guiding principles 9,011, Part IV, Chinese Pharmacopoeia, 2020 Edition), blank blood samples were collected at four time points of 24, 18, 12, and 0 h before administration in this study, and the endogenous glutamine concentration was measured, and the average value was calculated as the baseline concentration. The exogenous glutamine concentration was obtained by subtracting the baseline concentration from the total glutamine concentration in blood after administration, and the pharmacokinetics was analyzed with the exogenous glutamine concentration.
In this study, the pharmacokinetics of Gln tablets were tested in dogs with a single oral administration of three dosages, and according to the analysis of exogenous Gln concentration in plasma it can be seen that: after oral administration of glutamine tablets, Tmax was 30–60 min, Cmax were 64.56 ± 28.67 μg/ml (120 mg/kg), 136.11 ± 72.51 μg/ml (240 mg/kg) and 141.41 ± 60.65 μg/ml (360 mg/kg), respectively, and then gradually decreased to the normal range within 90–120 min (120 mg/kg) or 120–240 min (240 mg/kg and 360 mg/kg). According to the report of Ziegler (Ziegler et al., 1990), after oral administration of glutamine 100 mg/kg and300 mg/kg in healthy people, the Tmax was 30–45 min, and the Cmax were 44.89 μg/ml (100 mg/kg) and 88.71 μg/ml (300 mg/kg). And then dropped steadily to the normal range within 90–120 min (100 mg/kg) or 180–240 min (300 mg/kg). From the above results, it can be seen that under the condition of oral low-dose glutamine, the pharmacokinetic behavior in dogs was more similar to that in humans, and the drug-induced Cmax in dogs at high doses was higher than that in humans.
The mean values of Cmax (r2 = 0.8014), AUC0→t (r2 = 0.9888), AUC0→∝ (r2 = 0.9865) showed a certain linear relationship with the administered dose in this test. It was consistent with the results reported by Ziegler that the concentration of glutamine in blood increased in a dose-related after oral glutamine of low and high doses in healthy people (Ziegler et al., 1990).
When glutamine plays a clinical therapeutic role, it is usually administered multiple times (Rotovnik et al., 2017; Arutla et al., 2019; Chang et al., 2019; Zhou et al., 2019). Therefore, it is necessary to clarify the pharmacokinetic characteristics of Gln tablets when they are administered multiple times in dogs, and to investigate whether it will cause accumulated toxicity. The result of the multiple-dose study showed a mean Gln concentration of 8.97 μg/ml at steady state after administration (baseline correction), with an accumulation factor of 1.00. The pharmacokinetic parameters of six dogs who received both single and multiple doses were tested by t test. The results showed that there was no significant difference in Tmax, Cmax, t1/2λz, AUC0→t and AUC0→∝ for single and multiple administrations (p > 0.05). It showed that the absorption, distribution and metabolism of Gln tablets in vivo after repeated administration were similar to those of single administration, and there was no accumulation in vivo.
The results of this study showed that after oral administration of glutamine tablets in dogs, the plasma concentration generally reached a peak value at 30–60 min after administration, with an average peak concentration of 64.56 ± 28.67 μg ml−1 (120 mg kg−1), 136.11 ± 72.51 μg ml−1 (240 mg kg−1) and 141.41 ± 60.65 μg ml−1 (360 mg kg−1), then gradually decreased to the normal range within 90–120 min (120 mg kg−1) or 120–240 min (240 mg kg−1 and 360 mg kg−1).
It has been reported that after oral administration of glutamine 0.1 g/kg (low dose) and 0.3 g/kg (high dose) in adults, the blood concentration of glutamine increases in proportion to the dose. The peak appeared at 30–45 min after the intake of glutamine, and then steadily decreased to the normal range within 90–120 min (low dose) or 180–240 min (high dose). After administration of 0.1 g kg−1 (low dose) and 0.3 g kg−1 (high dose) glutamine, the peak blood concentration of glutamine was 1,028 ± 97 μmol L−1 (150.24 μg ml−1) and 1,328 ± 99 μmol L−1 (194.09 μg ml−1) respectively. The above shows that the pharmacokinetic behavior of glutamine in dogs is similar to that in humans after oral administration.
The dosage regimen of glutamine in the treatment of human gastroenteritis and gastric ulcer is: oral, 0.5 g each time, 2–3 times a day. The body surface area calculation method could be used to convert the human dose to the dog dose, and the dog’s dose was 30–60 mg/kg BW, treatment is repeated at 8 h or 12 h intervals.
Based on available pharmacokinetic data, we speculate that the human dose can be converted into the dog dose for further clinical trials. But the formulation of the final dosing regimen for dogs needs to be based on the results of further clinical trials.
The results of single dose showed that Cmax, AUC0→t, AUC0→∝ had a certain linear relationship with dosage. There were no significant differences in Tmax, Cmax, t1/2λz, AUC0→t and AUC0→∝ between multiple administration and single administration (p > 0.05), indicating no accumulation of oral Gln in the body.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Animal Ethics Committee of Nanjing Agricultural University (Nanjing, China).
FG, DL, and ZY contributed significantly to the conception and design of this experimental protocol. DL, FG, YY, YZ, YX, YZ, CL, and FZ participated in the experiments of pharmacokinetics. DL, YY, and YZ contributed to plasma sample preparation. DL and FG contributed to analysis and interpretation of data for the work. DL, FG, and ZY, edited and reviewed the final version of the article.
This study was supported by the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anderson, P. M., and Lalla, R. V. (2020). Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients 12, 1675. doi:10.3390/nu12061675
Ardawi, M. S., and Newsholme, E. A. (1983). Glutamine metabolism in lymphocytes of the rat. Biochem. J. 212 (3), 835–842. doi:10.1042/bj2120835
Arutla, M., Raghunath, M., Deepika, G., Jakkampudi, A., Murthy, H. V. V., Rao, G. V., et al. (2019). Efficacy of enteral glutamine supplementation in patients with severe and predicted severe acute pancreatitis- A randomized controlled trial. Indian J. Gastroenterol. 38, 338–347. doi:10.1007/s12664-019-00962-7
Berg, A., Rooyackers, O., and Norberg, A. (2005). Elimination kinetics of L-alanyl-L-glutamine in ICU patients. Amino Acids 29, 221–228. doi:10.1007/s00726-005-0230-9
Burke, D. J., Alverdy, J. C., Aoys, E., and Moss, G. S. (1989). Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch. Surg. 124 (12), 1396–1399. doi:10.1001/archsurg.1989.01410120042009
Camilleri, M., Madsen, K., Spiller, R., Greenwood-Van Meerveld, B., Van Meerveld, B. G., and Verne, G. N. (2012). Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 24, 503–512. doi:10.1111/j.1365-2982.2012.01921.x
Chang, S. C., Lai, Y. C., Hung, J. C., and Chang, C. Y. (2019). Oral glutamine supplements reduce concurrent chemoradiotherapy-induced esophagitis in patients with advanced non-small cell lung cancer. Med. Baltim. 98 (8), e14463. doi:10.1097/MD.0000000000014463
Dai, H., and Meng, Q. H. (2015). Clinical effect of glutamine on traumatic stress gastrointestinal ulcers. Chin. J. Clin. Pharmacol. 31 (23), 2287–2289. doi:10.13699/j.cnki.1001-6821.2015.23.003
Dissanayake, S. (2010). Assessing the bioequivalence of analogues of endogenous substances ('endogenous drugs'): Considerations to optimize study design. Br. J. Clin. Pharmacol. 69, 238–244. doi:10.1111/j.1365-2125.2009.03585.x
Du, Q. Q., Wang, T., Wang, Z. J., Jiang, X., and Wang, L. (2015). Rapid determination of glutamine in human plasma by high-performance liquid chromatographic–tandem mass spectrometry and its application in pharmacokinetic studies. J. Chromatogr. Sci. 53 (01), 79–84. doi:10.1093/chromsci/bmu022
Dunlop, S. P., Hebden, J., Campbell, E., Naesdal, J., Olbe, L., Perkins, A. C., et al. (2006). Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 101, 1288–1294. doi:10.1111/j.1572-0241.2006.00672.x
Griffiths, R. D., Jones, C., and Palmer, T. (2000). Six months outcome of critically ill patients given glutamine supplemented parenteral nutrition. Nutrition 2, 145–149. doi:10.1016/S0899-9007(97)00017-8
Han, Y. Z., Jiang, Y., and Lin, M., (2016). Determination of glutamine in human plasma by LC-MS/MS. J. Shenyang Pharm. Univ. 33 (07), 572–576+580. doi:10.14066/j.cnki.cn21-1349/r.2016.07.011
Jiang, X. F., and Fang, R. J. (2009). Advances in studies on nutritive and physiological functions of glutamine. China feed. 11, 31–36. doi:10.15906/j.cnki.cn11-2975/s.2009.11.007
Liu, D. Y., Guo, F. X., Yu, Y. Q., Zhou, Y., Xu, Y., Zou, Y., et al. (2022). A validated UPLC-MS/MS method for quantification of glutamine in plasma and pharmacokinetic study of oral glutamine tablets in healthy Beagles. J. Vet. Pharmacol. Ther. 45, 432–439. doi:10.1111/jvp.13075
Marshall, J. K., Thabane, M., Garg, A. X., Clark, W., Meddings, J., Collins, S. M., et al. (2004). Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 20, 1317–1322. doi:10.1111/j.1365-2036.2004.02284.x
Marzo, A., Rescigno, A., and Arrigoni, M. E. (1993). Some pharmacokinetic considerations about homeostatic equilibrium of endogenous substances. Eur. J. Drug Metab. Pharmacokinet. 18, 215–219. doi:10.1007/BF03188798
Mochiki, E., Ohno, T., Yanai, M., Toyomasu, Y., Andoh, H., and Kuwano, H. (2011). Effects of glutamine on gastrointestinal motor activity in patients following gastric surgery. World J. Surg. 35, 805–810. doi:10.1007/s00268-011-0962-5
Morris, C. R., Kuypers, F. A., Hagar, R., Larkin, S., Lavrisha, L., Saulys, A., et al. (2022). Implications for the metabolic fate of oral glutamine supplementation within plasma and erythrocytes of patients with sickle cell disease: A pharmacokinetics study. Complement. Ther. Med. 64, 102803. doi:10.1016/j.ctim.2022.102803
Niu, H. Y., Zhang, X. Y., and Ma, Q. B., (2021). Investigation and statistical analysis of 2 249 cases of feline diseases in a pet hospital in Nanjing from 2018 to 2019. Animal Husb. Veterinary Med. 53, 115–120.
Rotovnik, K. N., Kompan, L., Žagar, T., and Mrevlje, Z. (2017). Influence of enteral glutamine on inflammatory and hormonal response in patients with rectal cancer during preoperative radiochemotherapy. Eur. J. Clin. Nutr. 71, 671–673. doi:10.1038/ejcn.2017.11
Spiller, R. C., Jenkins, D., Thornley, J. P., Hebden, J. M., WrighT, T., SkinnerM., , et al. (2000). Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47, 804–811. doi:10.1136/gut.47.6.804
Tang, J. L., Zhou, S. W., and Ying, Y., (2005). Study on bioequivalence evaluation of compound glutamine granules in human body. Chin. Pharmacol. Bull. 12, 1509–1513. doi:10.11669/cpj.2015.03.015
Turner, J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809. doi:10.1038/nri2653
Wang, S. P., and Liu, J. H. (2009). Research progress in the physiological function and application of glutamine. J. Anhui Agri 37 (22), 10375–10377. doi:10.13989/j.cnki.0517-6611.2009.22.038
Wang, T., Jiang, X. H., and Wang, L. (2015). HPLC-MS/MS method for determination of glutamine in rat plasma and application to pharmacokinetic study. Chin. Pharm. J. 50 (03), 253–257. doi:10.11669/cpj.2015.03.015
Ward, E., Picton, S., Reid, U., Gardener, C., Smith, M., Henderson, M., et al. (2003). Oral glutamine in paediatric oncology patients: A dose finding study. Eur. J. Clin. Nutr. 57, 31–36. doi:10.1038/sj.ejcn.1601517
Yao, D., Zheng, L., Wang, J., Guo, M., Yin, J., and Li, Y. (2016). Perioperative alanyl-glutamine-supplemented parenteral nutrition in chronic radiation enteritis patients with surgical intestinal obstruction: A prospective, randomized, controlled study. Nutr. Clin. Pract. 31 (2), 250–256. doi:10.1177/0884533615591601
Yu, L., Yang, L., and Wang, T., (2014). Bioequivalence of glutamine capsules in healthy volunteers. Chin. J. Clin. Pharmacol. 30 (11), 1006–1009. doi:10.13699/j.cnki.1001-6821.2014.11.009
Zhang, X., Xie, X. Q., Liu, T. L., and Song, D. M. (2011). Quantitative determination and bioequivalence assessment of the drugs as endogenous compounds. Chin. J. New Drugs 20 (22), 2221–2228.
Zhou, Q. Q., Verne, M. L., Fields, J. Z., Lefante, J. J., Basra, S., Salameh, H., et al. (2019). Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 68, 996–1002. doi:10.1136/gutjnl-2017-315136
Zhu, M. W., Xiao, L. Y., and Liu, N. L., (2003). The pharmacokinetics study of glutamine granules in healthy volunteers. Chin. J. Clin. Nutr. 02, 10–13.
Ziegler, T. R., Benfell, K., Smith, R. J., Young, L. S., Brown, E., Ferrari-BaliviEra, E., et al. (1990). Safety and metabolic effects of L-glutamine administration in humans. JPEN. J. Parenter. Enter. Nutr. 14, 137S–146S. doi:10.1177/0148607190014004201
Zuckerman, M. J., Watts, M. T., Bhatt, B. D., and Ho, H. (1993). Intestinal permeability to [51Cr] EDTA in infectious diarrhea. Dig. Dis. Sci. 38, 1651–1657. doi:10.1007/BF01303174
Keywords: glutamine tablets, pharmacokinetics, multiple administration, single administration, Beagles
Citation: Guo F, Liu D, Zhou Y, Yu Y, Xu Y, Zou Y, Li C, Zhang F and Yu Z (2022) Pharmacokinetic study of single and multiple oral administration of glutamine in healthy Beagles. Front. Pharmacol. 13:1014474. doi: 10.3389/fphar.2022.1014474
Received: 08 August 2022; Accepted: 12 September 2022;
Published: 30 September 2022.
Edited by:
Stanislav Yanev, Bulgarian Academy of Sciences (BAS), BulgariaReviewed by:
Yi Tao, Zhejiang University of Technology, ChinaCopyright © 2022 Guo, Liu, Zhou, Yu, Xu, Zou, Li, Zhang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zugong Yu, eXV6dWdvbmdAbmphdS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.