- 1College of Life Science, Zhejiang Chinese Medical University, Hangzhou, China
- 2College of Basic Medical Sciences, Hangzhou, China
Background: Acute ischemic stroke (AIS) is associated with high morbidity, mortality, and disability. Clinical trials have shown that Honghua class injections (HCIs) combined with WM achieve better clinical efficacy than WM alone. In this study, we performed a Bayesian network meta-analysis (NMA) of randomized controlled trials (RCTs) to evaluate the efficacy of different HCIs combined with WM in treating AIS.
Methods: First, the inclusion and exclusion criteria were established. From inception to 1 June 2022, a systematic literature search was conducted in multiple databases for the treatment of AIS with HCIs, including Honghua injection (HI), Safflower Yellow injection (SYI), Guhong injection (GHI), and Danhong injection (DHI). Subsequently, OpenBUGS 3.2.3 was applied to conduct a Bayesian algorithm, and Stata 16.0 was used to prepare the graphs. Multidimensional cluster analysis was performed using the “scatterplot3d” package in R 3.6.1 software.
Results: In this NMA, a total of 120 eligible RCTs were included, involving 12,658 patients, and evaluating the clinical effectiveness rates, activities of daily living (ADL), hemorheological indexes, and adverse reactions (ADRs). DHI + WM was the best intervention for improving the clinical effectiveness rate. Moreover, cluster analysis demonstrated that DHI + WM and SYI + WM had better comprehensive therapeutic effects. As most of the included RCTs did not monitor ADRs, the safety of the HCIs remains to be further explored.
Conclusion: DHI + WM and SYI + WM probably have a better clinical efficacy on AIS patients. Nevertheless, due to the limitation of this NMA, this conclusion may be biased. High-quality RCTs should be performed to validate our findings.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42021229599
1 Introduction
Stroke is the leading cause of death in China and the second-most prevalent cause of death worldwide (Benjamin et al., 2018; Zhou et al., 2019). The prevalence of stroke in China has been increasing continuously since 2006, with ∼13 million stroke patients. Ischemic stroke (IS) has the highest incidence, accounting for ∼80% of all stroke patients (Benjamin et al., 2018). Due to its high morbidity, mortality, and disability, stroke has become a major disease that seriously endangers human health. At present, the treatment of acute ischemic stroke (AIS) mainly includes thrombolysis, intervention, anti-platelet aggregation, anticoagulation, lowering the levels of fibrinogen and blood lipids, expansion of blood capacity, and neuroprotection. Notably, the most effective treatment is ultra-early thrombolysis (Ospel et al., 2020). However, due to the strict treatment time window of thrombolysis, the population in which this treatment is carried out is extremely limited in China (Zhu et al., 2020). Therefore, it is necessary to explore other effective therapeutic methods. Traditional Chinese medicine (TCM) has several advantages, such as multiple targets, good synergy, and low side effects, widely used in treating complex diseases (Chen et al., 2017). TCM-related injections (TCMIs) are often combined with western medicine (WM) for the treatment of acute diseases in Chinese clinics and show instant effectiveness and high bioavailability (Li et al., 2017). The detailed pharmacology information, composition and the extraction procedure of HCIs have been stated unambiguously, shown in Supplementary Material S1.
In TCM theory, AIS is usually related to blood stasis syndrome and its treatment strategy involves the promotion of blood circulation and removal of blood stasis (Tang et al., 2012; Wang et al., 2020b). Honghua (Asteraceae, Carthamus, Carthamus tinctorius L.) is commonly used in the treatment of ischemic cardiovascular and cerebrovascular diseases, as it has a good effect on promoting blood circulation and removing blood stasis, which was described in the Compendium of Materia Medica (Ming Dynasty, ∼500 years ago) (Cao et al., 2014; Bai et al., 2020). Moreover, Hydroxysafflor yellow A is the main active component of Honghua (Bai et al., 2020). TCMIs contain an extract from Honghua, including Honghua injection (HI), Safflower Yellow injection (SYI), Guhong injection (GHI), and Danhong injection (DHI). Herein, we call them Honghua class injections (HCIs). All HCIs have been approved by the State Pharmaceutical Administration of China. HCIs combined with WM are commonly adopted in treating AIS and achieve a good clinical effect (Li et al., 2015b; Du et al., 2018; Feng et al., 2018).
Compared with traditional meta-analyses, network meta-analysis (NMA) can synthesize multiple interventions and perform direct or indirect comparisons for the same disease (Guo et al., 2020). Moreover, it can help to evaluate and rank the efficacy of different treatments (Catalá-López et al., 2014). Existing studies demonstrated that HCIs combined with WM achieved better clinical efficacy than WM alone in the treatment of AIS. However, there is a lack of clinical trials comparing HCIs directly. Therefore, it is necessary to evaluate and compare the efficacy of various HCIs by NMA. In this study, four HCIs, including HI, SYI, GHI, and DHI were selected as adjuvant therapies for AIS, which were all combined with conventional WM treatments. Subsequently, we applied a Bayesian NMA to explore the comparative effectiveness and safety between different HCIs combined with WM against AIS, providing a reference for clinical practice.
2 Methods
This NMA has been registered on the International Prospective Register of Systematic Review (PROSPERO) platform (CRD42021229599). This NMA study was conducted strictly according to the guidelines based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) as shown in Supplementary Material S2 (Moher et al., 2009).
2.1 Search strategy
All RCTs focusing on HCIs against AIS literatures were searched electronically from the following seven databases, Cochrane Library, PubMed, China National Knowledge Infrastructure Database (CNKI), China Biomedical Literature Service System (SinoMed), and Wan-Fang Database. All database searches were conducted on studies dating from inception to 1 June 2022, with no restrictions on language. The Medical Subject Heading (MeSH) terms and free-text keywords were utilized, including “acute ischemic stroke (MeSH Terms),” “ischemic stroke,” “acute ischemic stroke,” “stoke,” “brain infarction,” “acute cerebral infarction,” “cerebral infarction,” “brain embolism,” “cerebrovascular disorders,” “Honghua injection,” “Safflower Yellow injection,” “Guhong injection,” “Danhong injection,” and “randomized controlled trial (Publication Type).” In addition, there were no restrictions on the blinding methods, publication year, and language. Furthermore, the specific retrieval strategy is shown in Supplementary Material S3.
2.2 Inclusion criteria
2.2.1 Patient populations
All included cases were diagnosed with AIS. This study only recruited patients within 2 weeks of onset. There were no restrictions on the age, gender, race, and severity of disease.
2.2.2 Interventions and comparators
The interventions of experiment groups were HCIs (HI, SYI, GHI, or DHI) combined with WM treatments. The control group only received WM therapy. Conventional WM treatment, including anti-platelet aggregation; anticoagulation; lipid-lowering; correction of water, electrolyte disorders, and acid-base imbalance; improvement of cerebral circulation and using neuroprotective agent. The dosage and duration of treatment was not restricted.
2.2.3 Outcomes
The primary outcome was the clinical effectiveness rate, according to the National Institute of Health Stroke Scale (NIHSS) evaluation: a reduction of 91%–100%, 46%–90%, and 18%–45% corresponds to “basic cure,” “notable progress,” and “progress” respectively, which defined the effectiveness (Wang et al., 2018).
The secondary outcomes were the activities of daily living (ADLs), using Barthel Index Scale Scores; hemorheological indexes, including low shear blood viscosity (LBV), high shear blood viscosity (HBV), plasma viscosity (PV), fibrinogen (FIB) levels; and adverse reactions (ADRs), including adverse drug events (ADEs).
2.2.4 Study design
Randomized controlled trials (RCTs) of HCIs combined with WM in the treatment of AIS. RCTs were not restricted by language, country, publication date, or stage.
2.3 Exclusion criteria
The RCTs that met one of the following conditions were excluded: 1) RCTs that did not meet the criteria for clinical efficacy evaluation; 2) thrombolytic therapy; 3) interventions involving a combination therapy with Chinese herbal medicine, acupuncture or other TCM injections; 4) the full text of the study was unavailable; 5) incorrect or incomplete data; 6) duplicate reports; and 7) no related outcomes.
2.4 Data extraction
All the articles were managed by NoteExpress software (Tongji University Library, Shanghai, China) and selected by two independent reviewers by excluding irrelevant articles, reviews, and animal experiments. Two reviewers independently extracted the eligible research data using Excel (Microsoft, United States) and the collected the information as follows: 1) sample size in each group, sex, age, and duration of disease; 2) intervention (the types of HCIs, dose, and course of treatment); 3) outcome indicators: clinical effectiveness rate, ADL, LBV, HBV, PV, FIB levels, and ADRs/ADEs; 4) factors to evaluate the risk of bias.
2.5 Quality assessment
In this study, we applied the Cochrane bias risk assessment tool to assess the methodological quality of the included RCTs (Higgins et al., 2011). The tool assessment items were as follows: random sequence generation, allocation concealment, blinding of participants and researchers, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. There were two independent investigators (LL and CS) who assessed the quality of the included RCTs. Any disagreement was resolved by a third researcher (HW) or by consensus. RevMan 5.4 software was applied to generate the risk of bias diagram for quality evaluation.
2.6 Statistical analysis
Bayesian modeling was performed using OpenBUGS 3.2.3, (MRC Biostatistics Unit, Cambridge, UK) (Owen et al., 2020). Each chain in the OpenBUGS program has 50,000 iterations, with the first 20,000 iterations being burn-in tests to remove the initial value effect. The Odds ratio (OR) and mean difference (MD) with a 95% credible interval (CI) were used to estimate the binary results and continuous data, respectively (Da Costa et al., 2013; Pateras et al., 2018). If the ORs did not include 1 and MDs did not cover 0, the difference between the two groups was deemed significant (Da Costa et al., 2013; Pateras et al., 2018).
The software, Stata 16.0 (College Station, TX, United States) was applied to generate graphs, carry out the publication bias test, and consistency test. In the network graphs, nodes represented interventions, and the link lines indicated direct comparisons. For each outcome, the probability of the intervention was ranked using the surface under the cumulative ranking area (SUCRA), a larger area under the curve indicated a better cure (Zhou et al., 2020). Based on SUCRA value, R 3.6.1 software (Mathsoft, Cambridge, United States) was applied to perform the cluster analysis using K-means method (Guo et al., 2020). In addition, “scatterplot3d” package was used for multidimensional cluster analysis. The comprehensive curative effects of HCIs in two or three outcomes were evaluated.
The publication bias was assessed using a comparison-adjusted funnel plot (Song et al., 2010). Publication bias did not exist if the funnel plots were symmetrical (Debray et al., 2018). Moreover, as this NMA had no closed loops, the overall consistency test could not be performed. However, a local consistency test was performed. It was determined that there were no local inconsistencies in this study when p > 0.05.
3 Results
3.1 Literature selection
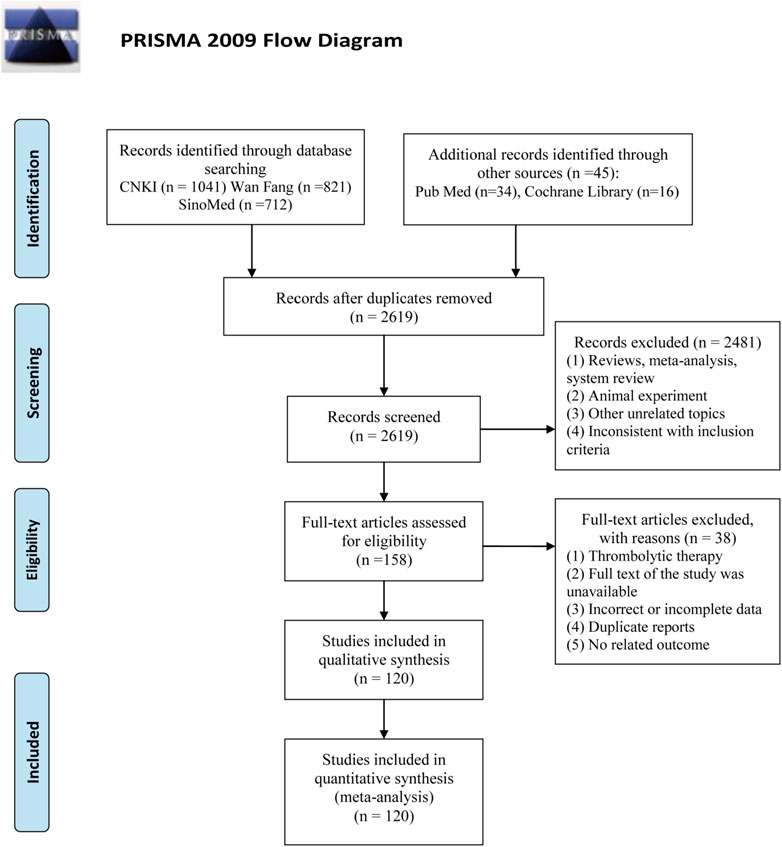
Using the search strategy, a total of 2,669 articles were retrieved. After removing duplicates, 2,619 articles were obtained. Subsequently, 2,481 articles were excluded on account of being reviews, meta-analyses, systematic reviews, animal experiments, and other irrelevant literature, or on account of having inconsistencies in inclusion criteria. A total of 158 articles were evaluated for qualification. Next, 38 articles were excluded for the following reasons: thrombolytic therapy, full text of the study was unavailable, incorrect or incomplete data, duplicate reports, and no related outcomes. Finally, 120 RCTs were eligible for inclusion in this NMA (Figure 1).
3.2 Study characteristics
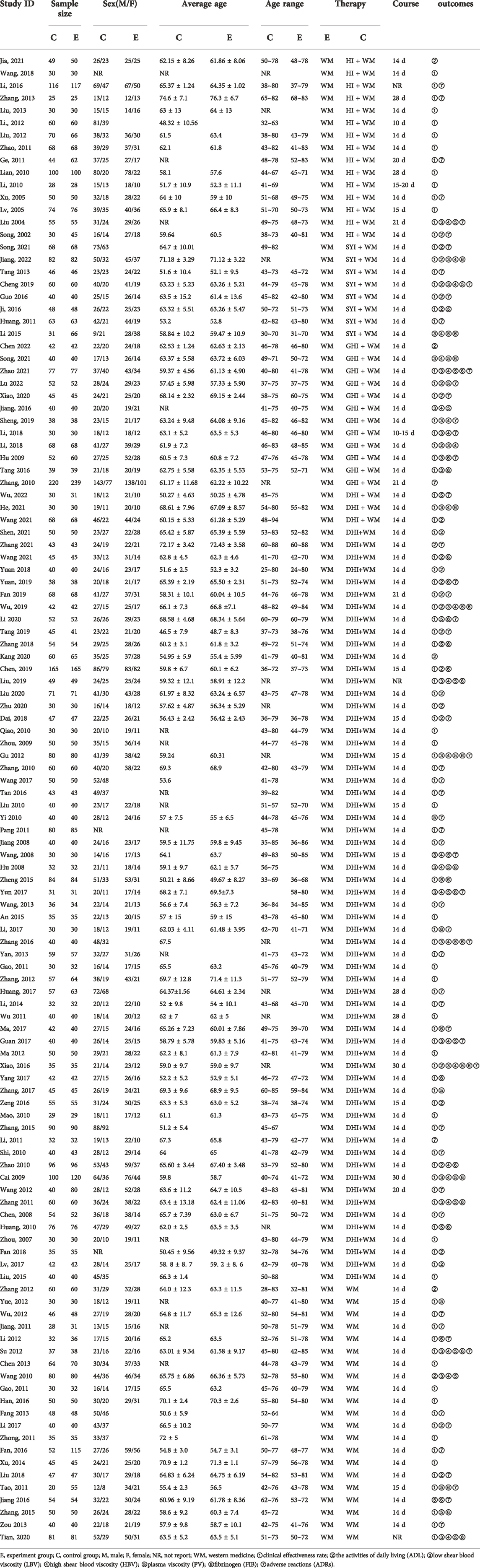
Four types of HCIs were incorporated, including HI, SYI, GHI, and DHI. Overall, the 120 RCTs involved 12,658 patients (6,185 in the control group and 6,473 in the experiment group). A total of four comparisons were evaluated: HI + WM vs. WM (n = 15), SYI + WM vs. WM (n = 8), GHI + WM vs. WM (n = 12), and DHI + WM vs. WM (n = 85). The control groups were treated with WM, which primarily contained aspirin, anticoagulants, neuroprotectants, etc. The characteristic details of the RCTs were shown in Table 1, and the included literatures are described in Supplementary Material S4. The network graphs of the four HCIs with different outcomes were shown in Figure 2.
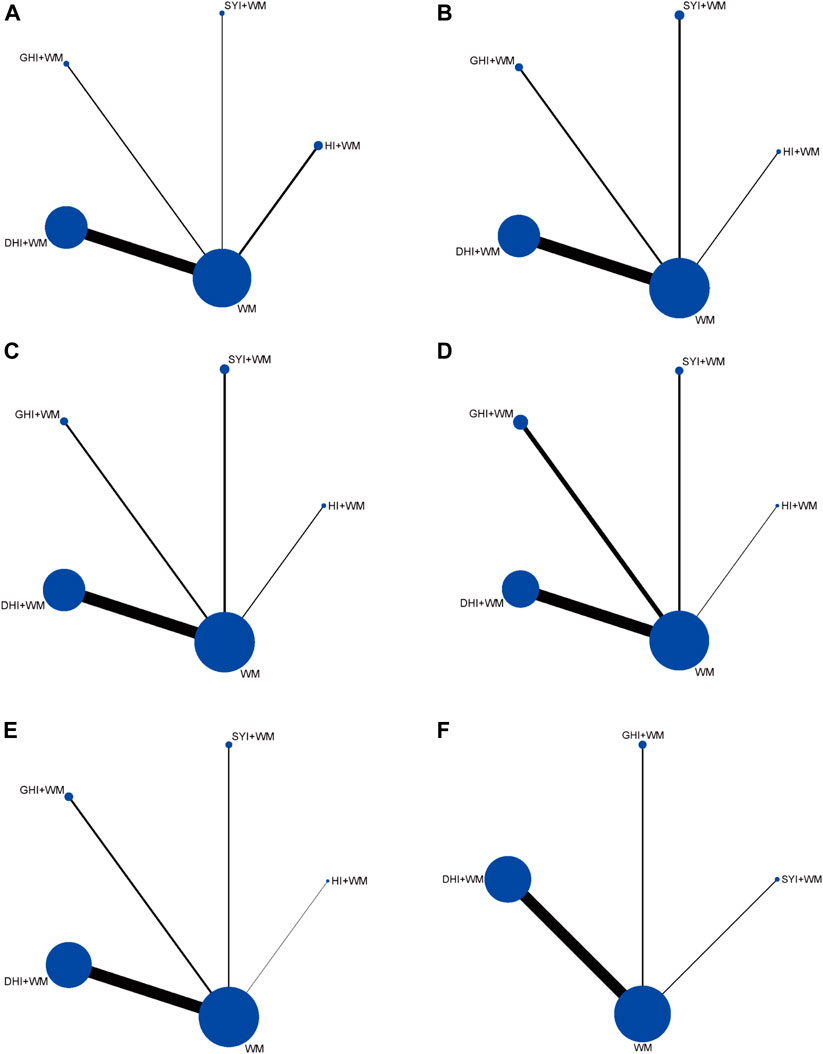
FIGURE 2. Network diagrams of the outcomes. (A) Clinical effectiveness rate; (B) ADL function; (C) LBV; (D) HBV; (E) PV; and (F) FIB.
3.3 Quality evaluation
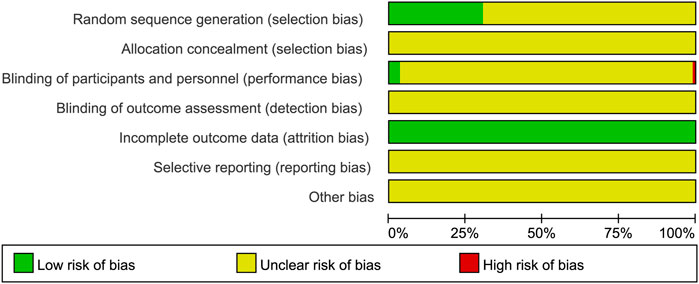
The risk of bias in RCTs included in this NMA was assessed using a tool developed by the Cochrane Collaboration (Higgins et al., 2011). In 35 RCTs, random sequences were generated using a random number table, considered as low risk in terms of selection bias; the allocation concealment was not clear. In terms of performance bias, four RCTs mentioned blinding, evaluated as low risk, and one RCT clearly pointed out that blinding was not used, considered to be high risk. In all RCTs, complete data were available, and the attrition bias was evaluated as low risk. Moreover, reporting bias and other bias were not clear. The risk of bias for the RCTs included in this NMA is described in Figure 3.
3.4 Outcomes
3.4.1 Clinical effectiveness rates
A total of 104 RCTs compared the clinical effectiveness rates: HI + WM vs. WM (n = 14), SYI + WM vs. WM (n = 7), GHI + WM vs. WM (n = 8), and DHI + WM vs. WM (n = 75). As shown in Figure 4A, HCIs + WM treatments had significantly higher clinical effectiveness rates than WM alone: HI + WM (OR = 0.26, 95% CI: 0.19, 0.36), SYI + WM (OR = 0.26, 95% CI: 0.15, 0.40), GHI + WM (OR = 0.3, 95% CI: 0.18, 0.46), and DHI + WM (OR = 0.25, 95% CI: 0.22, 0.29). In addition, the results of a pairwise comparison of the four injections were as follows: HI + WM vs. SYI + WM (OR = 1.09, 95% CI: 0.60,1.81), HI + WM vs. GHI + WM (OR = 0.93, 95% CI: 0.52,1.56), HI + WM vs. DHI + WM (OR = 1.06, 95% CI: 0.73, 1.47), SYI + WM vs. GHI + WM (OR = 0.91, 95% CI: 0.43, 1.64), and GHI + WM vs. DHI + WM (OR = 1.2, 95% CI: 0.73, 1.88), indicating there were no significantly differences in the pairwise comparisons of the four types of HCI.
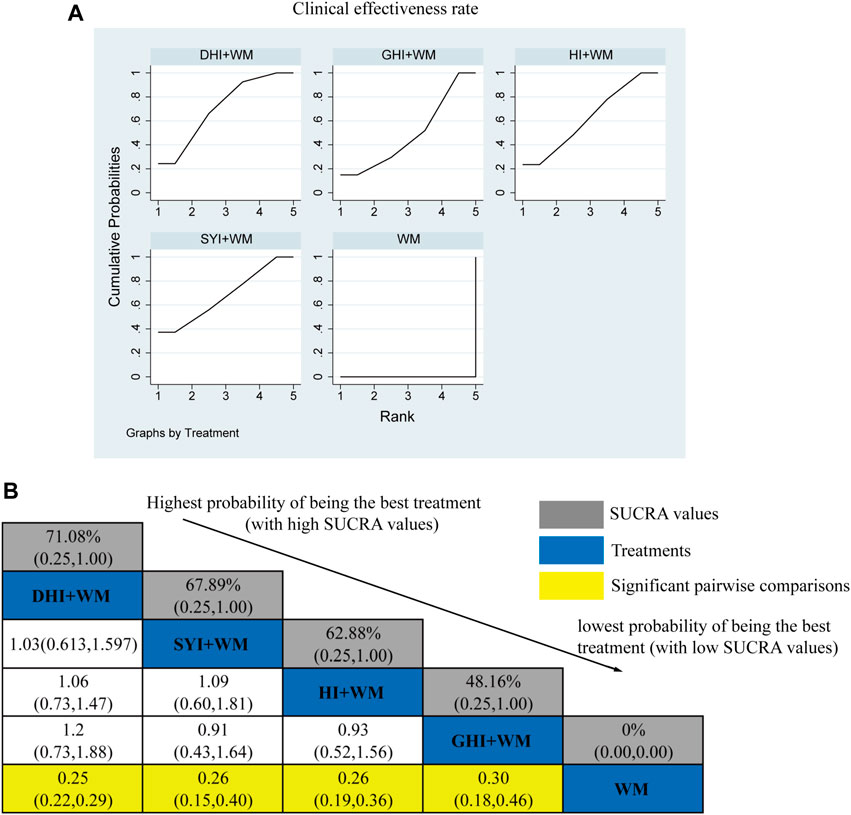
FIGURE 4. Relative effect sizes of the clinical effectiveness rate according to NWA. (A) SUCRA graph for the clinical effectiveness rate. (B) SUCRA values and ORs with 95% CIs of clinical effectiveness rates. Significant pairwise comparisons are highlighted in yellow.
The SUCRA is shown in Figures 4A,B, DHI + WM (71.08%) >SYI + WM (67.89%) >HI + WM (62.88%) >GHI + WM (48.16%) >WM (0%). According to the SUCRA, DHI + WM treatment was the best intervention for improving the clinical effectiveness rate.
3.4.2 ADL Function
A total of 36 RCTs investigated ADL: HI + WM vs. WM (n = 2), SYI + WM vs. WM (n = 5), GHI + WM vs. WM (n = 4), and DHI + WM vs. WM (n = 23). Figure 5B shows the comparisons of SYI + WM vs. WM (MD = −17.68, 95% CI: −25.52,−9.83) and DHI + WM vs. WM (MD = −15.36, 95% CI: 19.26, −11.45). There were no significantly differences in the pairwise comparisons of other types of HCI.
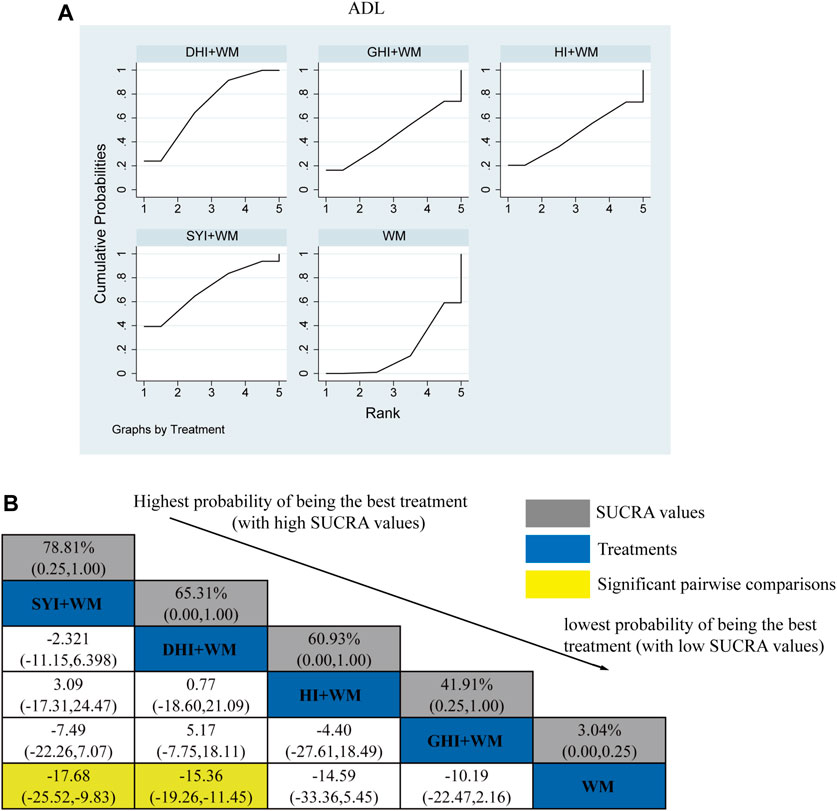
FIGURE 5. Relative effect sizes of the ADL according to NWA. (A) SUCRA graph for ADL. (B) SUCRA values and MDs with 95% CIs of the ADL. The significant pairwise comparisons are highlighted in yellow.
The SUCRA is shown in Figures 5A,B, SYI + WM (78.81%), DHI + WM (65.31%), HI + WM (60.93%), and GHI + WM (41.91%), respectively. Therefore, SYI + WM treatment was probably the best effective intervention for improving theADL.
3.4.3 LBV level
A total of 27 RCTs investigated the LBV: HI + WM vs. WM (n = 1), SYI + WM vs. WM (n = 3), GHI + WM vs. WM (n = 8), and DHI + WM vs. WM (n = 15). Figure 6C shows the comparisons of GHI + WM vs. WM (MD = 1.14, 95% CI: 0.59, 1.67) and DHI + WM vs. WM (MD = 0.56, 95% CI: 0.15, 0.98).
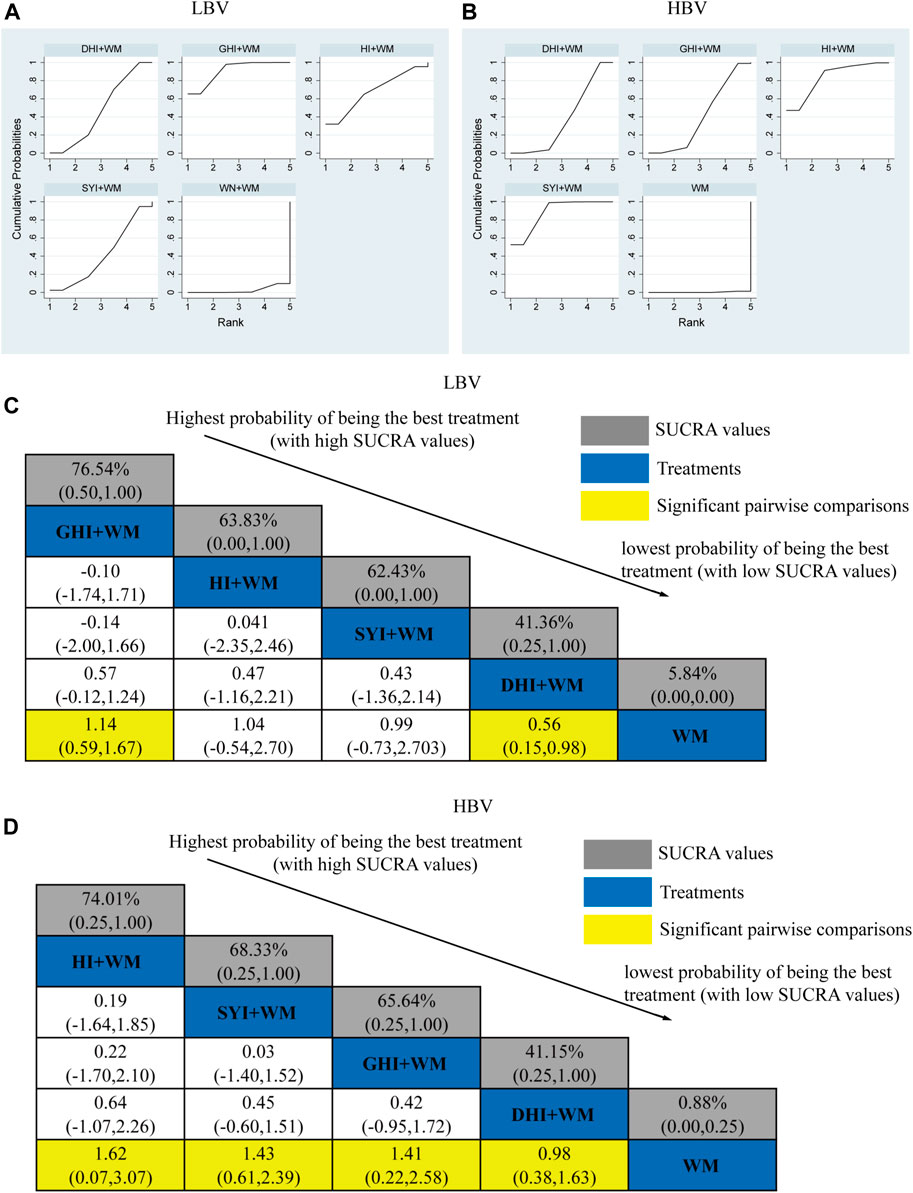
FIGURE 6. Relative effect sizes of the LBV and HBV according to NWA. (A,B) SUCRA graphs of the outcomes. (C,D) SUCRA values and MDs with 95% CIs. The significant pairwise comparisons are highlighted in yellow.
The SUCRA is shown in Figures 6A,C, GHI + WM (76.54%) > HI + WM (63.83%) > SYI + WM (62.43%) > DHI + WM (41.36%). According to the ranking of SUCRA, the GHI + WM (76.54%) treatment was probably to be the best intervention in neurological impairment.
3.4.4 HBV level
A total of 26 RCTs investigated the HBV level: HI + WM vs. WM (n = 1), SYI + WM vs. WM (n = 3), GHI + WM vs. WM (n = 6), and DHI + WM vs. WM (n = 16). All CHIs combined with WM achieved a better effect in HBV than using WM alone. The significant results have been shown in Figure 6D, HI + WM vs. WM (MD = 1.62, 95% CI: 0.07, 3.07), SYI + WM vs. WM (MD = 1.43, 95% CI: 0.61, 2.39), GHI + WM vs. WM (MD = 1.41, 95% CI: 0.22, 2.58), and DHI + WM vs. WM (MD = 0.98, 95% CI: 0.38, 1.63).
The SUCRA is shown in Figures 6B,D, HI + WM (74.01%), SYI + WM (68.33%), GHI + WM (65.64%), and DHI + WM (41.15%), respectively. According to the SUCRA probabilities, the HI + WM treatment appeared to be the best interventions for HBV.
3.4.5 PV level
A total of 33 RCTs investigated the PV: HI + WM vs. WM (n = 1), SYI + WM vs. WM (n = 3), GHI + WM vs. WM (n = 4), and DHI + WM vs. WM (n = 25). The statistically significant results are shown in Figure 7C, DHI + WM vs. WM (MD = 0.38, 95% CI: 0.20, 0.57).
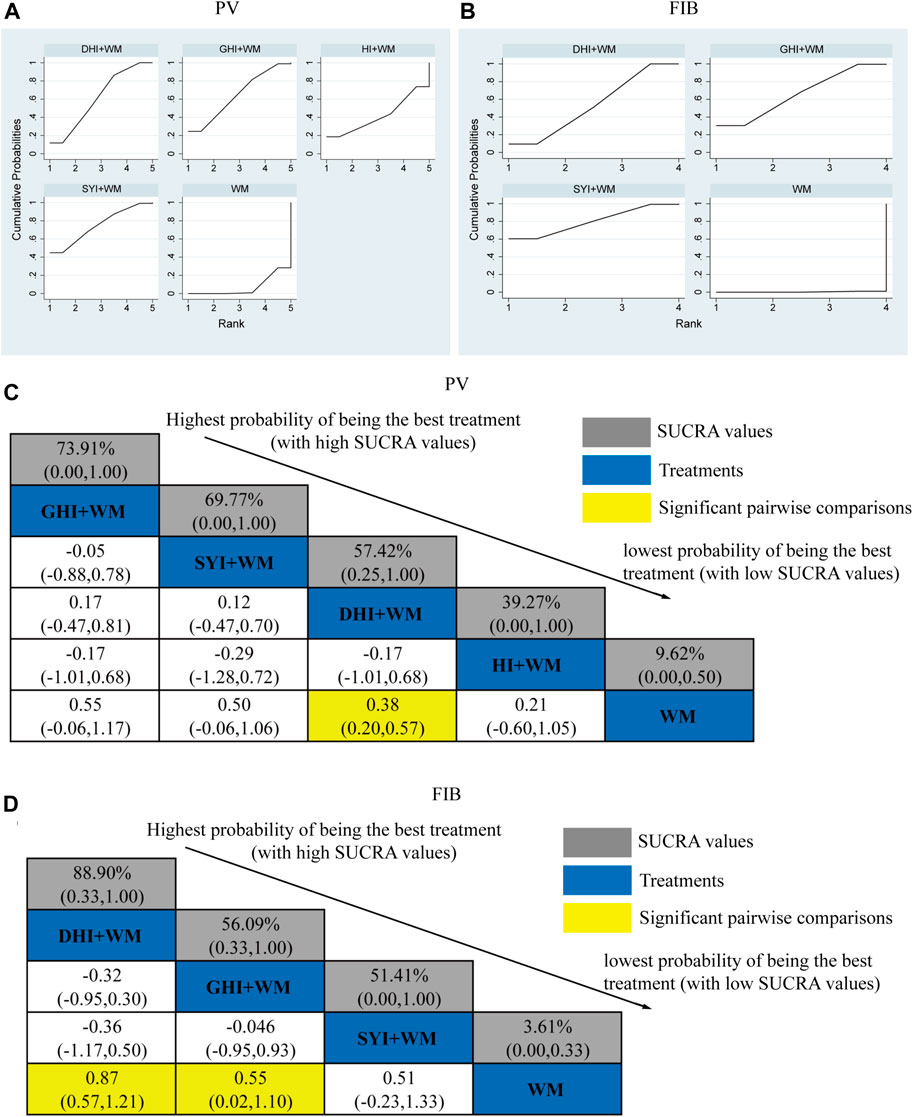
FIGURE 7. Relative effect sizes of PV and FIB according to NWA. (A,B) SUCRA graphs of the outcomes. (C,D) SUCRA values and MDs with 95% CIs. Significant pairwise comparisons are highlighted in yellow.
The SUCRA is shown in Figures 7A,C, GHI + WM (73.91%), SYI + WM (69.77%), DHI + WM (57.42%), and HI + WM (39.27%). GHI + WM treatment was probably to be the best intervention in plasma viscosity. However, GHI + WM vs. WM (MD = 0.55, 95% CI: −0.06, 1.17) and SYI + WM vs. WM (MD = 0.50, 95% CI: −0.06, 1.06), indicating there was no significant difference in the pairwise comparisons.
3.4.6 FIB level
A total of 33 RCTs investigated the FIB level: HI + WM vs. WM (n = 0), SYI + WM vs. WM (n = 2), GHI + WM vs. WM (n = 4), and DHI + WM vs. WM (n = 27). The statistically significant results are shown in Figure 7D, DHI + WM vs. WM (MD = 0.87, 95% CIs: 0.57, 1.2), and GHI + WM vs. WM (MD = 0.55, 95% CIs: 0.02, 1.10).
The SUCRA is shown in Figures 7B,D, DHI + WM (88.90%), GHI + WM (56.09%), and SYI + WM (51.41%). Accordingly, DHI + WM treatment was the best intervention in decreasing the FIB level.
3.5 Cluster analysis
When cluster analysis was performed to four HCIs that reported the clinical effectiveness rate and ADL, we found SYI + WM, DHI + WM and HI + WM were classified into the same category, indicating they exerted similar effectiveness (Figure 8A). In addition, we performed multidimensional cluster analysis on interventions with more than 1 included RCTs to further evaluate the comprehensive efficacy of HCIs. All the results demonstrated DHI + WM and SYI + WM might have better therapeutic effects (Figures 8B–E).
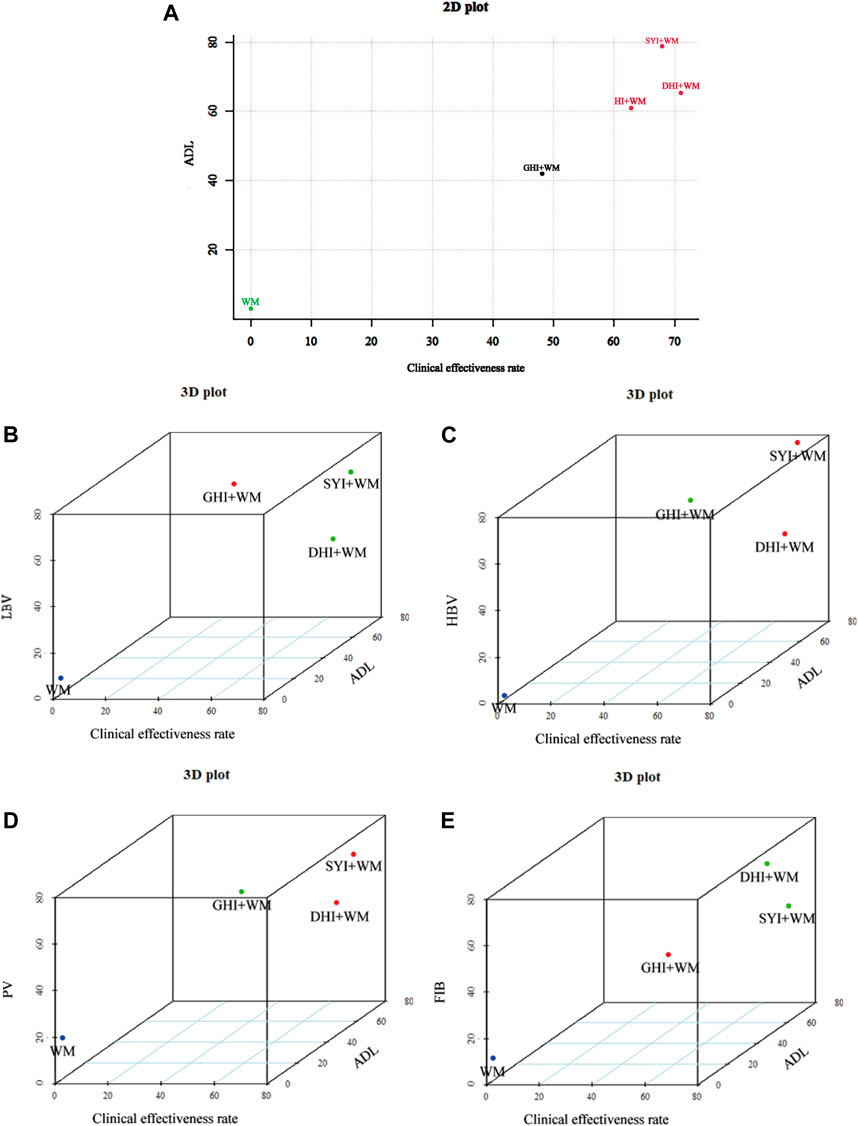
FIGURE 8. Cluster analysis plots. (A) Cluster analysis for clinical effectiveness rate (X axis) and ADL (Y axis). (B) Cluster analysis for clinical effectiveness rate (X axis), ADL (Y axis) and LBV (Z axis). (C) Cluster analysis for clinical effectiveness rate (X axis), ADL (Y axis) and HBV (Z axis). (D) Cluster analysis for clinical effectiveness rate (X axis), ADL (Y axis) and PV (Z axis). (E) Cluster analysis for clinical effectiveness rate (X axis), ADL (Y axis) and FIB (Z axis). Interventions with the same color belong to the same cluster.
3.6 Safety evaluation
Of the 120 RCTs, 59 trials investigated the ADRs/ADEs, involving a total of 5,749 patients. Overall, 2,778 cases in the control group (WM), and 2,971 cases in the experiment group (HCIs + WM) were included.
In terms of the safety evaluation of HI, six RCTs were included, involving 674 patients, 320 cases in the WM group and 354 cases in the HI + WM group. There were three cases of ADRs that occurred in the HI + WM group: pruritus (2 cases) and skin flushing (1 case).
In terms of the safety evaluation of SYI, five RCTs were included, involving 554 patients, 277 cases in the WM group and 277 cases in the SYI + WM group. There were seven cases of ADRs that occurred in the SYI + WM group: rash (2 cases), gastrointestinal reactions (4 cases), such as nausea, vomiting and diarrhea, and gingival bleeding (1 case). Five cases of ADRs occurred in the WM group: gastrointestinal reactions (3 cases) and gingival bleeding (2 cases).
In terms of the safety evaluation of GHI, seven RCTs were included, involving 1055 patients, 514 cases in the WM group, and 541 cases in the GHI + WM group. There were 23 cases of ADRs in the GHI + WM group: 10 cases of gastrointestinal reactions, such as nausea, vomiting, and diarrhea, cases of headache or dizziness (5 cases), rash (3 cases), vasculitis (1 case), lethargy (2 cases), and mild fever (2 cases). 29 cases of ADRs occurred in the WM group: gastrointestinal reactions (11 cases), headache or dizziness (8 cases), rash (1 case), gingival bleeding (2 cases), vasculitis (2 cases), 5 cases of lethargy, and mild abnormal liver function (2 cases).
In terms of the safety evaluation of DHI, 41 RCTs were included, involving 3,847 patients, 1,848 cases in the WM group, and 1,999 cases in the DHI + WM group. There were 53 cases of ADRs that had occurred in the DHI + WM group: gastrointestinal reactions (18 cases), headache or dizziness (19 cases), rash (7 cases), vasculitis (1 case), fatigue (1 case), 1 case of hypotension, mild palpitation (2 cases), limb pain (2 cases), and cough (2 cases). 64 cases of ADRs occurred in the WM group: gastrointestinal reactions (17 cases), headache or dizziness (19 cases), rash (9 cases), vasculitis (2 cases), fatigue (1 case), hypotension (3 cases), mild palpitation (3 cases), epigastric discomfort (4 cases), limb pain (3 cases), and cough (3 cases).
3.7 Publication bias
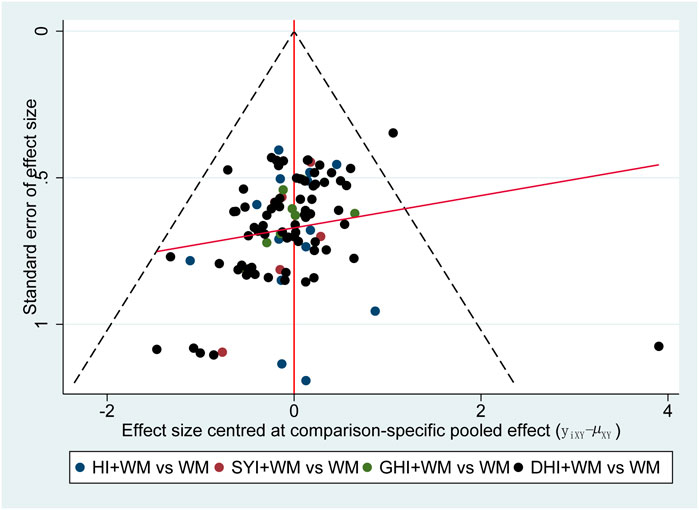
The funnel plot of the clinical effectiveness rate was not quite symmetrical, indicating the potential publication bias in this NMA (Figure 9).
3.8 Consistency test
In this NMA, as there were no closed loops, an overall consistency test was not possible. All the evidences about these contrasts were obtained from the trials, which directly compare them. The local consistency test showed that p > 0.05, indicating that there was no local inconsistency in this study.
4 Discussion
We performed a NMA on four common HCIs and compared the outcomes to determine the most appropriate choice, providing some references for the clinical treatment of AIS. This NMA included 120 RCTs involving 12,658 patients, evaluating the clinical effectiveness rate, ADL, LBV HBV, PV, FIB levels, and ADRs after the application of four HCIs combined with WM and WM alone. The clinical effectiveness rate, evaluated by neurological function recovery, was considered as the primary outcome. According to the ranking of SUCRA, DHI + WM treatment was the best intervention for improving the clinical effectiveness rate, while SYI + WM was the best treatment for ADL. Moreover, multidimensional cluster analysis demonstrated that DHI + WM and SYI + WM might have a better comprehensive therapeutic effect.
DHI is extracted from Danshen (Salvia miltiorrhiza Bunge, Lamiaceae, Salviae miltiorrhizae radix et rhizoma) and Honghua, widely used in the treatment of AIS. The main active components of DHI are danshensu, protocatechuic aldehyde, safflower yellow A, and salvianolic acid (Jiang et al., 2015; Liu et al., 2019). The clinical trial has shown that the value of DHI in treating AIS with blood stasis syndrome is comprehensively evaluated as Grade A (Cui et al., 2021). Moreover, pharmacological studies have shown that DHI can protect the blood-brain barrier after AIS (Zeng et al., 2021a), improve the energy metabolism of cells in the ischemic area (Zeng et al., 2021b), enhance the mitochondrial function (Orgah et al., 2019), exert anti-inflammatory, anti-apoptotic, and antioxidant effects (Wang et al., 2020a), thereby improving neurological impairment. SYI is extracted from Honghua, its main component is safflower yellow. A clinical trial on IS has shown that SYI may treat AIS by alleviating the inflammation reaction of patients (Li et al., 2015a). The main active component of safflower yellow is hydroxysafflower yellow A (HYSA), with antioxidant activity. Pharmacological studies have demonstrated that HYSA plays an important role in anti-atherosclerosis (Xue et al., 2021), protecting neurons from excitotoxic damage (Wang et al., 2016), dilating cerebral vessels, and improving the cerebrovascular permeability (Sun et al., 2018; Li et al., 2022).
Atherosclerosis is the pathological basis of IS. Abnormal hemorheological indexes, especially the increase of plasma viscosity and fibrinogen levels, will accelerate the pathological process of atherosclerosis and lead to stroke. Furthermore, a previous study has shown that hemorheological disturbances affect cognitive functions of IS patients (Velcheva and Nikolova, 2008). Ameliorating hemorheology is of great significance to the prognosis of stroke (Resch, 1992). In this NMA, we found that GHI had the best effect in reducing PV and LBV and DHI had the best effect in reducing FIB. As there was only one RCT in HI treatment, it could not prove that the curative effect of HI was better than other HCIs. GHI comprised safflower aqueous extract and aceglutamide. Several studies have shown that the mechanisms of GHI in treating IS were related to inhibiting inflammation and reducing neuronal apoptosis (Wang et al., 2022; Zhang et al., 2022). During inflammation, the hemorheological system is impaired (Pretorius, 2018). The regulation of hemodynamics by GHI may be related to its anti-inflammatory effects.
In addition to the clinical efficacy, the safety of CHIs in treating AIS is also particularly important. In this NMA, 59 RCTs investigated the ADRs/ADEs (HI 6 RCTs; SYI five RCTs; GHI seven RCTs and DHI 41 RCTs), more than 50% of RCTs did not report safety. However, more than 50% of RCTs did not report them. Therefore, we were unable to make the accurate conclusions of HCIs’ safety. Previous studies had shown that most ADRs of DHI were mild and moderate. Post-marketing safety monitoring and re-evaluation studies of DHI with 30,888 cases show that the incidence of ADRs/ADEs was 3.50‰ (Li et al., 2015b). Unfortunately, there is a lack of large samples and high-quality safety research on other HCIs post-marketing. Since there are few studies focused on safety assessments in this NMA, further experimental and clinical evidence is required to verify the safety of these HCIs. Moreover, in order to improve the safety of clinical applications of TCMIs, a research system and post-marketing safety surveillance and re-evaluation should be established.
This study is a NMA based on RCTs, using a Bayesian algorithm, which has some advantages. A comprehensive multi-platform literature search was conducted for this study and strict inclusion and exclusion criteria were formulated. Importantly, this is the first NMA to evaluate the clinical efficacy of HCIs + WM in treating AIS. We also analyzed the changes of hemorheological indexes and the safety of HCIs, which has certain significance for guiding the treatment of AIS.
However, this study still has some limitations. First, the current NMA-included RCTs were observed to have a selection bias and performance bias; there was a lack of information regarding allocation concealment and blinding of participants or personnel in most RCTs. Second, apart from DHI + WM, there were few eligible RCTs for other interventions, which might lead to bias of the outcomes. Third, most of the included RCTs were single-center studies, and the patients included in the studies may be regionalized. Additionally, all of the RCTs in this NMA were conducted among Chinese population. Whether the curative effect is affected by race or region is still unclear. Importantly, clinical heterogeneity might happen due to the diversity of conventional WM treatment and the different time of onset, dosage and the course of treatment. Moreover, few eligible RCTs reported follow-up results; the recurrence and mortality rates of patients treated with HCI + WM are not well understood. In view of the above limitations, RCTs should be conducted more standardized, clearly defined in terms of populations, interventions, comparators, outcomes, and study designs (PICOS). In order to accurately evaluate the efficacy and safety of TCMIs and promote their application in the real-world clinical practice, the methodological quality of RCTs should be improved.
5 Conclusion
In conclusion, based on the results of this Bayesian NMA, HCIs combined with WM treatments significantly improve the therapeutic effect, and DHI and SYI combined with WM treatments are probably preferred among HCIs for the treatment of AIS. However, due to the limitations, this conclusion may be biased. Importantly, high-quality, multicenter, and double-blind RCTs should be performed in the future to validate our findings. Additionally, we need to improve the quality of TCMI security assessment in RCTs, strictly monitor the ADRs/ADEs of TCMI, and standardize medication.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
LL and HW contributed to conception and design of the study. LL, CS, ZL, and XW conducted the systematic review and extracted the data. LL and JY performed the statistical analysis. LL and CS wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81874366, No. 82174066) and Postdoctoral Science Foundation of Zhejiang Province (No. ZJ2020026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1010533/full#supplementary-material
References
Bai, X., Wang, W., Fu, R., Yue, S., Gao, H., Chen, Y., et al. (2020). Therapeutic potential of hydroxysafflor yellow A on cardio-cerebrovascular diseases. Front. Pharmacol. 11, 01265. doi:10.3389/fphar.2020.01265
Benjamin, E. J., Virani, S. S., Callaway, C. W., Chamberlain, A. M., Chang, A. R., Cheng, S., et al. (2018). Heart disease and stroke statistics-2018 update: A report from the American heart association. Circulation 137, e67–e492. doi:10.1161/CIR.0000000000000558
Cao, J., Chen, Z., Zhu, Y., Li, Y., Guo, C., Gao, K., et al. (2014). Huangqi-Honghua combination and its main components ameliorate cerebral infarction with Qi deficiency and blood stasis syndrome by antioxidant action in rats. J. Ethnopharmacol. 155, 1053–1060. doi:10.1016/j.jep.2014.05.061
Catalá-López, F., Tobías, A., Cameron, C., Moher, D., and Hutton, B. (2014). Network meta-analysis for comparing treatment effects of multiple interventions: An introduction. Rheumatol. Int. 34, 1489–1496. doi:10.1007/s00296-014-2994-2
Chen, H. S., Qi, S. H., and Shen, J. G. (2017). One-Compound-Multi-Target: Combination prospect of natural compounds with thrombolytic therapy in acute ischemic stroke. Curr. Neuropharmacol. 1, 134–156. doi:10.2174/1570159x14666160620102055
Cui, X., Han, S., Jin, X. L., Wang, Z. F., Zhang, Q., and Xie, Y. M. (2021). Clinical comprehensive evaluation of Danhong Injection in treatment of stroke with blood stasis syndrome. Zhongguo Zhong Yao Za Zhi 46, 6096–6104. doi:10.19540/j.cnki.cjcmm.20210930.506
Da Costa, B. R., Nüesch, E., Rutjes, A. W., Johnston, B. C., Reichenbach, S., Trelle, S., et al. (2013). Combining follow-up and change data is valid in meta-analyses of continuous outcomes: A meta-epidemiological study. J. Clin. Epidemiol. 66, 847–855. doi:10.1016/j.jclinepi.2013.03.009
Debray, T. P. A., Moons, K. G. M., and Riley, R. D. (2018). Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests. Res. Synth. Methods 9, 41–50. doi:10.1002/jrsm.1266
Du, Y., Zhong, L., Wu, S., and Wang, X. (2018). Comparative study of the effects of Danhong injection with different doses on ischemic stroke: A substudy of hospital-based Danhong injection registry. J. Traditional Chin. Med. 38, 917–925. doi:10.1016/s0254-6272(18)30992-0
Feng, W. W., Zhang, Y., Tang, J. F., Zhang, C. E., Dong, Q., Li, R. Y., et al. (2018). Combination of chemical fingerprinting with bioassay, a preferable approach for quality control of Safflower Injection. Anal. Chim. Acta 1003, 56–63. doi:10.1016/j.aca.2017.11.069
Guo, S., Wu, J., Ni, M., Jia, S., Zhang, J., Zhou, W., et al. (2020). Comparative efficacy of danshen class injections for treating acute coronary syndrome: A multidimensional bayesian network meta-analysis of randomized controlled trials. Front. Pharmacol. 11, 1260. doi:10.3389/fphar.2020.01260
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Jiang, X., Lv, B., Li, P., Ma, X., Wang, T., Zhou, Q., et al. (2015). Bioactivity-integrated UPLC/Q-TOF-MS of Danhong injection to identify NF-κB inhibitors and anti-inflammatory targets based on endothelial cell culture and network pharmacology. J. Ethnopharmacol. 174, 270–276. doi:10.1016/j.jep.2015.08.026
Li, J. P., Liu, Y., Guo, J. M., Shang, E. X., Zhu, Z. H., Zhu, K. Y., et al. (2017). A comprehensive strategy to evaluate compatible stability of Chinese medicine injection and infusion solutions based on chemical analysis and bioactivity assay. Front. Pharmacol. 8, 833. doi:10.3389/fphar.2017.00833
Li, L. J., Li, Y. M., Qiao, B. Y., Jiang, S., Li, X., Du, H. M., et al. (2015a). The value of safflower yellow injection for the treatment of acute cerebral infarction: A randomized controlled trial. Evid. Based. Complement. Altern. Med. 2015, 478793. doi:10.1155/2015/478793
Li, X. L., Tang, J. F., Li, W. X., Li, C. X., Zhao, T., Zhao, B. C., et al. (2015b). Postmarketing safety surveillance and reevaluation of Danhong injection: Clinical study of 30888 cases. Evid. Based. Complement. Altern. Med. 2015, 610846. doi:10.1155/2015/610846
Li, Y., Liu, X. T., Zhang, P. L., Li, Y. C., Sun, M. R., Wang, Y. T., et al. (2022). Hydroxysafflor yellow A blocks HIF-1α induction of NOX2 and protects ZO-1 protein in cerebral microvascular endothelium. Antioxidants (Basel) 11, 728. doi:10.3390/antiox11040728
Liu, S., Wang, K., Duan, X., Wu, J., Zhang, D., Liu, X., et al. (2019). Efficacy of danshen class injection in the treatment of acute cerebral infarction: A bayesian network meta-analysis of randomized controlled trials. Evid. Based. Complement. Altern. Med. 2019, 5814749. doi:10.1155/2019/5814749
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 151, 264–269. doi:10.7326/0003-4819-151-4-200908180-00135
Orgah, J., Ren, J., Liu, X., Orgah, E., Gao, X., Zhu, Y. J. R. N., et al. (2019). Danhong injection facilitates recovery of post-stroke motion deficit via Parkin-enhanced mitochondrial function. Restor. Neurol. Neurosci. 37, 375–395. doi:10.3233/RNN-180828
Ospel, J. M., Menon, B. K., Demchuk, A. M., Almekhlafi, M. A., Kashani, N., Mayank, A., et al. (2020). Clinical course of acute ischemic stroke due to medium vessel occlusion with and without intravenous alteplase treatment. Stroke 51, 3232–3240. doi:10.1161/STROKEAHA.120.030227
Owen, R. K., Bujkiewicz, S., Tincello, D. G., and Abrams, K. R. (2020). Multivariate network meta-analysis incorporating class effects. BMC Med. Res. Methodol. 20, 184. doi:10.1186/s12874-020-01025-8
Pateras, K., Nikolakopoulos, S., and Roes, K. (2018). Data-generating models of dichotomous outcomes: Heterogeneity in simulation studies for a random-effects meta-analysis. Stat. Med. 37, 1115–1124. doi:10.1002/sim.7569
Pretorius, E. (2018). Erythrocyte deformability and eryptosis during inflammation, and impaired blood rheology. Clin. Hemorheol. Microcirc. 69, 545–550. doi:10.3233/CH-189205
Song, F., Parekh, S., Hooper, L., Loke, Y. K., Ryder, J., Sutton, A. J., et al. (2010). Dissemination and publication of research findings: An updated review of related biases. Health Technol. Assess. 14, 1–193. iii, ix-xi. doi:10.3310/hta14080
Sun, Y., Xu, D., Qin, Z., Wang, P., Hu, B., Yu, J., et al. (2018). Protective cerebrovascular effects of hydroxysafflor yellow A (HSYA) on ischemic stroke. Eur. J. Pharmacol. 818, 604–609. doi:10.1016/j.ejphar.2017.11.033
Tang, H., Gu, B. L., and Zhou, X. M. (2012). Study on the scores of blood stasis syndrome of acute ischemic stroke and its correlation with TOAST subtypes. Zhongguo Zhong Xi Yi Jie He Za Zhi 32, 1500–1502.
Velcheva, I., and Nikolova, G. (2008). Hemorheological disturbances and cognitive function in patients with cerebrovascular disease. Clin. Hemorheol. Microcirc. 39, 397–402. doi:10.3233/ch-2008-1108
Wang, G., Fang, B., Yu, X., and Li, Z. (2018). Interpretation of 2018 guidelines for the early management of patients with acute ischemic stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 30, 289–295. doi:10.3760/cma.j.issn.2095-4352.2018.04.001
Wang, S., Yu, L., Sun, G., Liu, Y., Hu, W., Liu, Y., et al. (2020a). Danhong injection protects hemorrhagic brain by increasing peroxiredoxin 1 in aged rats. Front. Pharmacol. 11, 346. doi:10.3389/fphar.2020.00346
Wang, X., Ma, Z., Fu, Z., Gao, S., Yang, L., Jin, Y., et al. (2016). Hydroxysafflor yellow A protects neurons from excitotoxic death through inhibition of NMDARs. ASN Neuro 8, 1759091416642345. doi:10.1177/1759091416642345
Wang, Y., Wu, H., Han, Z., Sheng, H., Wu, Y., Wang, Y., et al. (2022). Guhong injection promotes post-stroke functional recovery via attenuating cortical inflammation and apoptosis in subacute stage of ischemic stroke. Phytomedicine. 99, 154034. doi:10.1016/j.phymed.2022.154034
Wang, Y., Zhang, L., Pan, Y. J., Fu, W., Huang, S. W., Xu, B., et al. (2020b). Investigation of invigorating qi and activating blood circulation prescriptions in treating qi deficiency and blood stasis syndrome of ischemic stroke patients: Study protocol for a randomized controlled trial. Front. Pharmacol. 11, 892. doi:10.3389/fphar.2020.00892
Xue, X., Deng, Y., Wang, J., Zhou, M., Liao, L., Wang, C., et al. (2021). Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine. 91, 153694. doi:10.1016/j.phymed.2021.153694
Zeng, M., Zhou, H., He, Y., Du, H., Yin, J., Hou, Y., et al. (2021a). Danhong injection enhances the therapeutic effect of mannitol on hemispheric ischemic stroke by ameliorating blood-brain barrier disruption. Biomed. Pharmacother. 142, 112048. doi:10.1016/j.biopha.2021.112048
Zeng, M., Zhou, H., He, Y., Wang, Z., Shao, C., Yin, J., et al. (2021b). Danhong injection alleviates cerebral ischemia/reperfusion injury by improving intracellular energy metabolism coupling in the ischemic penumbra. Biomed. Pharmacother. 140, 111771. doi:10.1016/j.biopha.2021.111771
Zhang, J., Zhou, R., Cao, G., Zhang, Y., Xu, H., and Yang, H. (2022). Guhong injection prevents ischemic stroke-induced neuro-inflammation and neuron loss through regulation of C5ar1. Front. Pharmacol. 13, 818245. doi:10.3389/fphar.2022.818245
Zhou, D., Xie, L., Wang, Y., Wu, S., Liu, F., Zhang, S., et al. (2020). Clinical efficacy of tonic traditional Chinese medicine injection on acute cerebral infarction: A bayesian network meta-analysis. Evid. Based. Complement. Altern. Med. 2020, 8318792. doi:10.1155/2020/8318792
Zhou, M., Wang, H., Zeng, X., Yin, P., Zhu, J., Chen, W., et al. (2019). Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet 394, 1145–1158. doi:10.1016/S0140-6736(19)30427-1
Keywords: Honghua class injections, acute ischemic stroke, network meta-analysis, Bayesian, randomized controlled trials, comparative efficacy
Citation: Li L, Shao C, Liu Z, Wu X, Yang J and Wan H (2022) Comparative efficacy of Honghua class injections for treating acute ischemic stroke: A Bayesian network meta-analysis of randomized controlled trials. Front. Pharmacol. 13:1010533. doi: 10.3389/fphar.2022.1010533
Received: 03 August 2022; Accepted: 31 August 2022;
Published: 28 September 2022.
Edited by:
Mingbao Lin, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Zhaojun Chen, Beijing University of Chinese Medicine Third Affiliated Hospital, ChinaKe Wei, Hunan University of Chinese Medicine, China
Copyright © 2022 Li, Shao, Liu, Wu, Yang and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiehong Yang, amllaG9uZ3lAeWVhaC5uZXQ=; Haitong Wan, d2h0b25nQDEyNi5jb20=
†These authors share first authorship
 Lan Li
Lan Li Chongyu Shao
Chongyu Shao Zheting Liu
Zheting Liu Xiaolong Wu
Xiaolong Wu Jiehong Yang2*
Jiehong Yang2* Haitong Wan
Haitong Wan