
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 20 October 2022
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1004888
 Qian Ran1,2,3†
Qian Ran1,2,3† Cui Zhang1,2,3†
Cui Zhang1,2,3† Weiguo Wan1,2,3
Weiguo Wan1,2,3 Tianxin Ye1,2,3
Tianxin Ye1,2,3 Ying Zou1,2,3
Ying Zou1,2,3 Zhangchi Liu1,2,3
Zhangchi Liu1,2,3 Yi Yu1,2,3
Yi Yu1,2,3 Junhua Zhang4
Junhua Zhang4 Bo Shen1,2,3*
Bo Shen1,2,3* Bo Yang1,2,3*
Bo Yang1,2,3*Background: Anxiety disorder (AD) is the most common mental disorder, which is closely related to atrial fibrillation (AF) and is considered to be a trigger of AF. Pinocembrin has been demonstrated to perform a variety of neurological and cardiac protective effects through its anti-inflammatory and antioxidant activities. The current research aims to explore the antiarrhythmic effect of pinocembrin in anxiety disorder rats and its underlying mechanisms.
Methods: 60 male Sprague-Dawley rats were distributed into four groups: CTL group: control rats + saline; CTP group: control rats + pinocembrin; Anxiety disorder group: anxiety disorder rats + saline; ADP group: anxiety disorder rats + pinocembrin. Empty bottle stimulation was conducted to induce anxiety disorder in rats for 3 weeks, and pinocembrin was injected through the tail vein for the last 2 weeks. Behavioral measurements, in vitro electrophysiological studies, biochemical assays, ELISA, Western blot and histological studies were performed to assess the efficacy of pinocembrin. In addition, HL-1 atrial cells were cultured in vitro to further verify the potential mechanism of pinocembrin.
Results: After 3 weeks of empty bottle stimulation, pinocembrin significantly improved the exploration behaviors in anxiety disorder rats. Pinocembrin alleviated electrophysiological remodeling in anxiety disorder rats, including shortening the action potential duration (APD), prolonging the effective refractory period (ERP), increasing the expression of Kv1.5, Kv4.2 and Kv4.3, decreasing the expression of Cav1.2, and ultimately reducing the AF susceptibility. These effects may be attributed to the amelioration of autonomic remodeling and structural remodeling by pinocembrin, as well as the inhibition of oxidative stress with upregulation of the nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) pathway.
Conclusion: Pinocembrin can reduce AF susceptibility in anxiety disorder rats induced by empty bottle stimulation, with the inhibition of autonomic remodeling, structural remodeling, and oxidative stress. Therefore, pinocembrin is a promising treatment for AF in patients with anxiety disorder.
Atrial fibrillation (AF) is the most common persistent arrhythmia clinically, involving 2%–4% adults with an estimated 2.3-fold increase due to longevity (Hindricks et al., 2020). AF is often associated with multiple diseases, including heart failure, valvular diseases, cardiomyopathy, and psychiatric disorders, resulting in an increased mortality due to the high incidence of thromboembolism (Lip et al., 2016). Therefore, it is necessary to explore safe and effective preventions and treatments of AF due to the huge economic and mental burden it brings to patients.
Anxiety disorder (AD) is a mixed feeling of restlessness, fear and worry, accompanied by accelerated heart rate and sleep disturbances (Pitsavos et al., 2006). AD is the most common mental disorder, affecting 33.7% of the population, but rarely receives attention from psychiatrists (Bandelow and Michaelis, 2015). Pervious researches have demonstrated that AD increased the incidence of postoperative AF and affected the symptom severity and treatment success rate of AF (Tully et al., 2011; Thompson et al., 2013). AD is considered to be a possible trigger of AF, in which inflammation and oxidative stress play a key role (Severino et al., 2019).
Pinocembrin is a flavonoid extracted from propolis and other plants, and has demonstrated a variety of neurological and cardiac protective effects through its anti-inflammatory and antioxidant activities (Rasul et al., 2013). For instance, pinocembrin ameliorated depression-like behavior and neuronal apoptosis by inhibiting oxidative stress in a mouse model of depression (Wang et al., 2020), and ameliorated cognitive impairment and neurovascular damage in mice with Alzheimer’s disease (Liu et al., 2014). In addition, pinocembrin attenuated myocardial injury and cardiac dysfunction in sepsis rats and heart failure rats by suppressing inflammation and oxidative stress, respectively (Chen et al., 2021; Li et al., 2021). Our previous study has exhibited that pinocembrin relieved AF and ventricular fibrillation (VF) in depression rats (Ye et al., 2020).
Based on the dual neural and cardiac protective effects of pinocembrin, we hypothesized that pinocembrin could ameliorate atrial fibrillation in AD rats. Therefore, the study was designed to explore the effect of pinocembrin on AF in AD rats and explore the potential mechanism.
60 male Sprague-Dawley rats (200 ± 20 g) provided by the Experimental Animal Center of Wuhan University were raised in the barrier environment with free availability of water and food (Animal Ethical Number: 20211003). After 1 week of adaptation, SD rats were randomly distributed into four groups: CTL group: control rats + saline; CTP group: control rats + pinocembrin; AD group: anxiety disorder rats + saline; ADP group: anxiety disorder rats + pinocembrin.
Empty bottle stimulation is a well-established method to induce anxiety disorder in rats (Feng et al., 2018). Briefly, rats in the four groups were allowed to drink water only at 9:00–9:10 and 21:00–21:10 each day for the first week to adjust to the new drinking habits. For the next 2 weeks, rats in the AD group and ADP group were randomly given empty bottles once a day at the two designated times.
Pinocembrin (5 mg/kg) was injected into tail vein in the CTP rats and the ADP rats in the last 2 weeks, and saline was injected into the CTL rats and the AD rats.
After 3 weeks of empty bottle stimulation, the open field experiment was performed. The rats were placed in the center of the open field, which was divided into nine grids on average, for a total of 5 min. The number of central entries, the cumulative time in center, and the number of rearing were recorded.
A 15-min surface electrocardiogram from lead II was recorded from rats anesthetized with pentobarbital sodium (60 mg/kg). The surface electrocardiogram was analyzed by LabChart 8.0 software to obtain heart rate variability, which included time domain parameters (average of RR intervals (average RR), standard deviation of RR intervals (SDRR), and square root of the mean squared differences of successive RR intervals (RMSSD)) and frequency domain parameters (low frequency from 0.2 to 0.75 Hz (LF), high frequency from 0.75 to 2.50 Hz (HF), and the ratio of LF to HF).
The rats were anesthetized 15 min after peritoneal injection of heparin sodium (400 units), then the hearts were removed and immediately connected with the Langerdorff system, which were perfused with HEPES-buffered Tyrode solution (Liu et al., 2018).
The paired platinum-stimulating electrodes (diameter: 0.25 mm; spacing: 1 mm) were placed in the left atrial appendage for programmed stimulation, and the custom-made Ag–AgCl electrodes (diameter: 0.25 mm; spacing: 0.5 mm) were placed in the left atrial appendage 1 cm away from the paired platinum-stimulating electrodes for recording monophasic action potentials (MAPs).
Action potential duration (APD) was obtained by the S1S1 stimulation procedure, which consisted of 10 stimuli at pacing cycle lengths (PCLs) of 250 ms, 200 ms, 150 ms, and 100 ms. The duration of the action potential repolarized to 90% and 50% were recorded.
Effective refractory period (ERP) was stimulated by the S1S2 procedure, which consisted of eight successive stimuli (S1) (cycle length (CL): 200 ms) followed by a preceding stimulus (S2). The interval of S2 was reduced from 100 ms to 1 ms, and the longest S2 interval without capturing the atrium was defined as ERP.
A 50-Hz burst pacing procedure within 6 times was conducted to induce AF, which was defined as rapid irregular atrial rhythm occurring and maintaining for at least 2 seconds (Liu et al., 2018; Ye et al., 2019).
Left atrial tissues were embedded in paraffin and sliced into 5um sections, which were incubated with anti-tyrosine hydroxylase (TH) antibody and anti-growth-associated protein-43 (GAP43) antibody for immunostaining, and Sirius red staining was performed to measure atrial fibrosis.
To further verify the potential mechanisms of pinocembrin, HL-1 cells derived from mice heart atrium were cultured in vitro. Hl-1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium containing 10% fetal bovine serum and 1% penicillin/streptomycin in an incubator containing 5% carbon dioxide at 37°C. The cells were distributed into six groups: (i) CTL group: normal HL-1 cells; (ii) CTL + Pino group: normal HL-1 cells administered with pinocembrin; (iii) CTL + Pino + ML385: normal HL-1 cells administered with pinocembrin and ML385; (iv)H2O2 group: HL-1 cells administered with H2O2; (v) H2O2+Pino group: HL-1 cells administered with pinocembrin and H2O2; (vi) H2O2+Pino + ML385 group: HL-1 cells administered with pinocembrin, ML385 and H2O2. Pinocembrin (25 μM) and ML385 (Nrf2 inhibitor, 5 μM) were added into the medium for 4 h, followed by H2O2 (200 μM) for 3 h to induce oxidative stress.
Frozen left atriums and cell climbing slices were stained with 5 mM DHE at 37°C for 30 min and then observed by fluorescence microscopy to evaluate the fluorescence intensity of ROS.
Inferior venous blood, left atrium and culture supernate were extracted for ELISA and biochemical detection. The blood was centrifuged at 3000 g for 15 min at 4°C to separate the serum. The concentration of norepinephrine (NE) was measured in serum and left atrium.
Biochemical detection was performed to assess the concentration of MDA and the activity of SOD in serum, left atrium, and culture supernate.
Tissues from left atrium were used for western blot, and proteins including Nrf2 (1:1,000; Abcam), HO-1 (1:1,000; Abcam), Cav1.2 (1:1,000, Abcam), Kv1.5 (1:1000, Bioss), Kv4.2 (1:1000, Abcam), Kv4.3 (1:1000, Bioss), Cx40 (1:1000, Bioss), TH (1:1000, Abcam), GAP43 (1:1000, Abcam), TGF-β1 (1:1000, Abcam), Collagen I (1:1000, Bioss), Collagen III (1:1000, Bioss) and GAPDH (1:1000, Servicebio) were measured based on our previous study (Chen et al., 2020).
Continuous variables were presented as means ± standard errors, and categorical variables were presented as counts and percentages (%). Comparisons of continuous variables between two groups were conducted by Student’s t-test, and were conducted by analysis of variance (ANOVA) between multiple groups appropriately. And categorical variables were calculated by the contingency table Pearson’s chi-squared-test or Fisher exact calculation. p < 0.05 was defined statistically significant.
After the empty bottle stimulation, the frequency of central entries, the cumulative time in center, and the number of rearing were significantly reduced in the AD rats compared with the CTL rats, indicating a weakened exploration behavior in the AD rats (Figures Fig1A–D). However, all the behavioral parameters were significantly improved in the ADP rats compared with the AD rats, and no apparent discrepancy was exhibited between the CTL rats and the CTP rats. In order to exclude the influence of dehydration, the concentration of serum creatinine (Cr), urea nitrogen (BUN) and Na+ was measured, and the results showed that there were no significant differences among the four groups (Figures 1E–G).
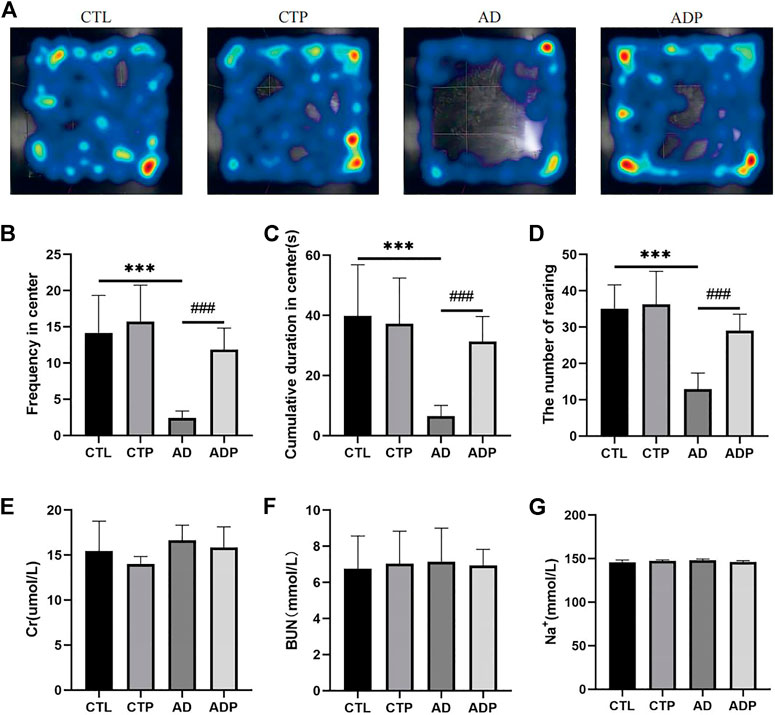
FIGURE 1. Behavioral measurements. (A) Typical images of trajectory after empty bottle stimulation. (B) Frequency of central entries. (C) Cumulative time in center. (D) Number of rearing. n = 15 per group. (E–G) The concentration of creatinine (Cr), urea nitrogen (BUN) and Na+ in serum. *p < 0.05, **p < 0.01, ***p < 0.001 vs CTL; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. AD.
A significant reduction of time domain parameters (average RR, SDRR, and RMSSD) was exhibited in the AD rats compared with the CTL rats (Figures 2A–C). In respect of frequency domain parameters, LF and LF/HF were obviously higher in the AD rats than the CTL rats, while HF was lower in the AD rats (Figures 2D–F). In addition, there was no obvious difference in the HRV parameters between the CTL rats and the CTP rats.

FIGURE 2. Heart rate variability analysis. (A–C) Average RR, SDRR, RMSSD. (D–F) LF, HF and LF/HF. n = 7 per group. *p < 0.05, **p < 0.01, ***p < 0.001 vs. CTL; #p < 0.05, ##p < 0.01, ###p < 0.001 vs AD.
Representative APDs at PCL of 200 ms of the four groups were exhibited in Figure 3A. A significant prolongation of APD50 and APD90 was exhibited in the AD rats compared with the CTL rats, and the prolongation was prevented by pinocembrin in ADP rats (Figures 3B,C). Figure 4A showed the representative ERPs recorded from the four groups. The average ERP of the AD rats was remarkably shortened versus that of the CTL rats, and pinocembrin reversed the change in ADP rats (Figure 4B). In addition, no significant difference was exhibited in both of the APD and ERP between the CTL rats and the CTP rats.
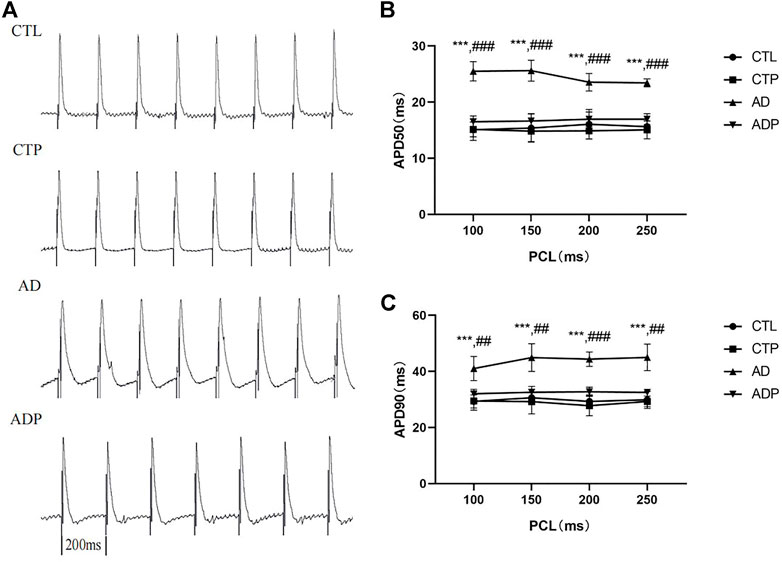
FIGURE 3. Action potential duration. (A) Typical recordings of APD at PCL of 200 ms. (B,C) The spatial dispersions of APD50 and APD90 at PCL of 250 ms, 200 ms, 150 ms, 100 ms. n = 7 per group. *p < 0.05, **p < 0.01, ***p < 0.001 vs. CTL; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. AD.
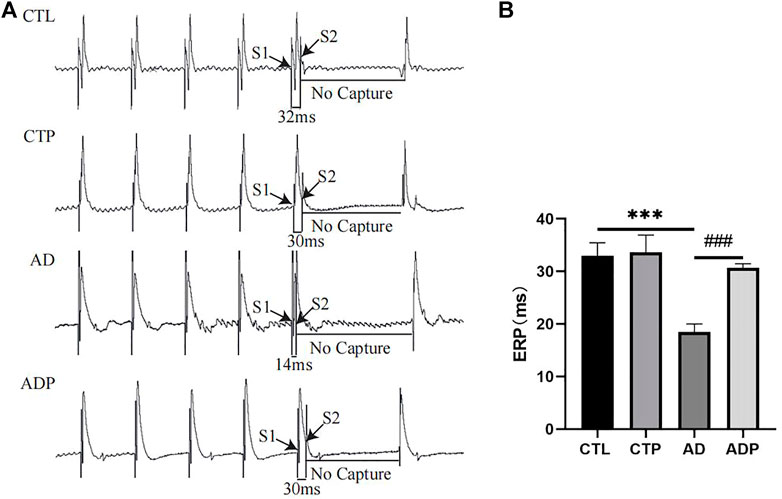
FIGURE 4. Effective refractory period. (A) Typical atrial ERPs at PCL of 200 ms. (B) Average ERP. n = 7 per group. *p < 0.05, **p < 0.01, ***p < 0.001 vs. CTL; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. AD.
As shown in Figure 5A, the burst pacing procedure was conducted to induce AF in the four groups, and AF was only induced in the AD rats and ADP rats. The inductivity of AF in the AD rats was 85.71% (6/7), which was decreased by treatment with pinocembrin in the ADP group (14.29%, 1/7, Figure 5B). The average duration of AF in the AD group was 55.81 ± 46.85s, and AF lasted only 2.16s in the only ADP rat in which AF was successfully induced (Figure 5C).
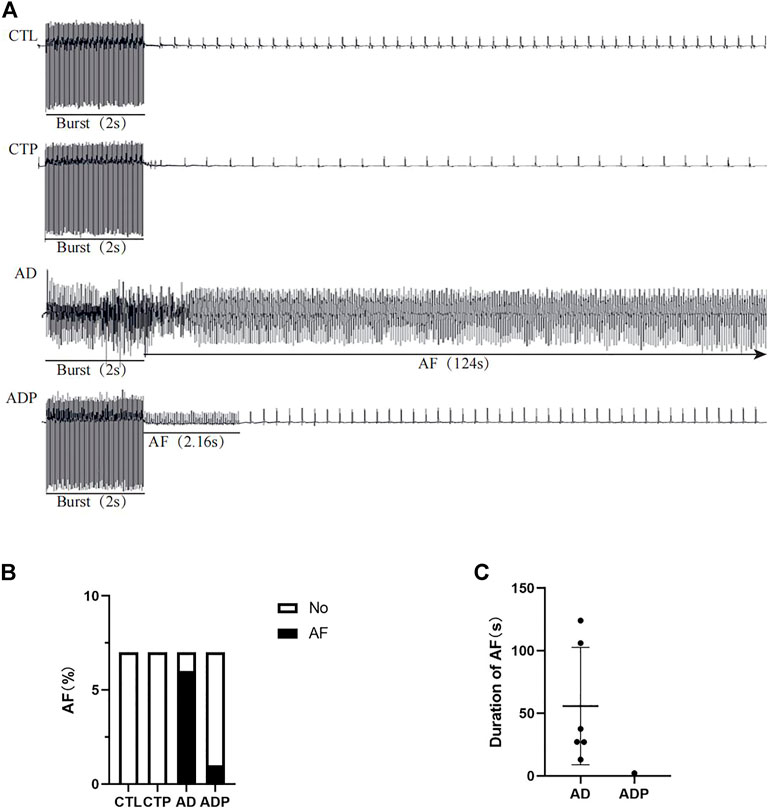
FIGURE 5. AF susceptibility. (A) Typical recordings of AF. (B,C) Inducibility and duration of AFs. n = 7 per group.
To further investigate the mechanisms of atrial electrophysiological abnormalities in AD rats, the content of atrial ion channels was assessed, including Kv1.5, Kv4.2, Kv4.3 and Cav1.2. The expression of Kv1.5, Kv4.2 and Kv4.3 was remarkably diminished, whereas the expression of Cav1.2 was increased in the AD rats versus the CTL rats (Figures 6A,B). In addition, the gap junction channel Cx40 was lower expressed in the AD rats compared with the CTL rats (Figures 6A,B). However, treatment with pinocembrin reversed the abnormal content of ion channels and Cx40 in the ADP rats, and there was no apparent difference between the CTL rats and the CTP rats.
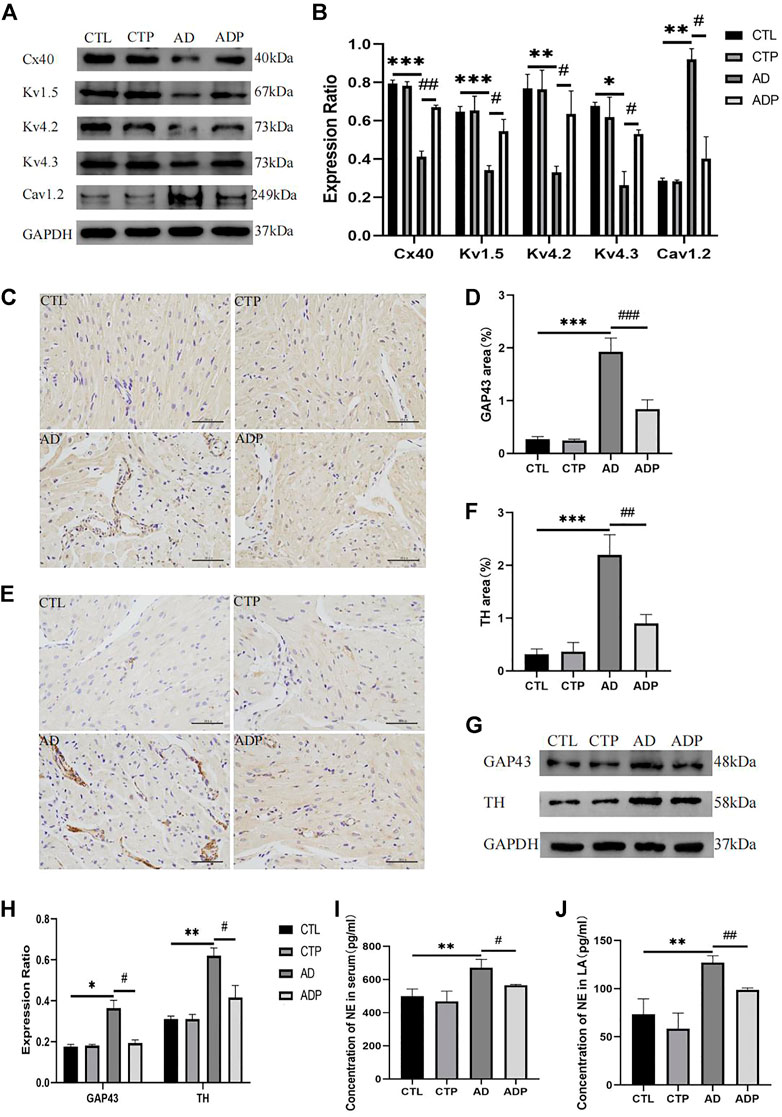
FIGURE 6. Ion channels remodeling, Cx40 expression, and autonomic remodeling. (A,B) Immunoblotting and quantitative analysis of Cx40, Kv1.5, Kv4.2, Kv4.3 and Cav1.2, n = 3 per group (C ,D) Typical images of GAP43 immunohistochemical staining and percentage of the GAP43 area, n = 5 per group. (E,F) Typical images of TH immunohistochemical staining and percentage of the TH area, n = 5 per group. (G,H) Immunoblotting and quantitative analysis of GAP43 and TH, n = 3 per grou4.3p. (I,J) Concentration of NE in serum and LA, n = 5 per group. *p < 0.05, **p < 0.01, ***p < 0.001 vs. CTL; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. AD.
Figures 6C,E presented the typical TH and GAP43 immunohistochemical staining of the four groups. The positive stained area percentage of TH and GAP43 in the AD rats was remarkably expanded compared with the CTL rats, whereas pinocembrin apparently reduced the positive stained area percentage in the ADP rats (Figure 6D, F). The western bolt analysis of TH and GAP43 were exhibited in Figure 6G, which were consistent with the TH and GAP43 immunohistochemical staining. The content of TH and GAP43 was apparently increased in the AD rats compared with the CTL rats, but the content was attenuated by pinocembrin in the ADP rats (Figure 6H). Furthermore, a remarkable increase of NE concentration in serum and left atrium was exhibited in the AD rats, but the increase was suppressed by pinocembrin (Figures 6I, J). In addition, there was no apparent difference between the CTL group and the CTP group.
As shown in Figure 7A remarkably increased atrial fibrosis was exhibited in the AD rats compared with the CTL rats, whereas the atrial fibrosis was obviously diminished in the ADP rats treated with pinocembrin. Furthermore, TGF-β1, collagen I and collagen III were highly expressed in the AD rats, while the content of the three fibrosis related proteins was lowered by pinocembrin in the ADP rats (Figures 7C,D), indicating that pinocembrin could attenuate atrial fibrosis in the AD rats.
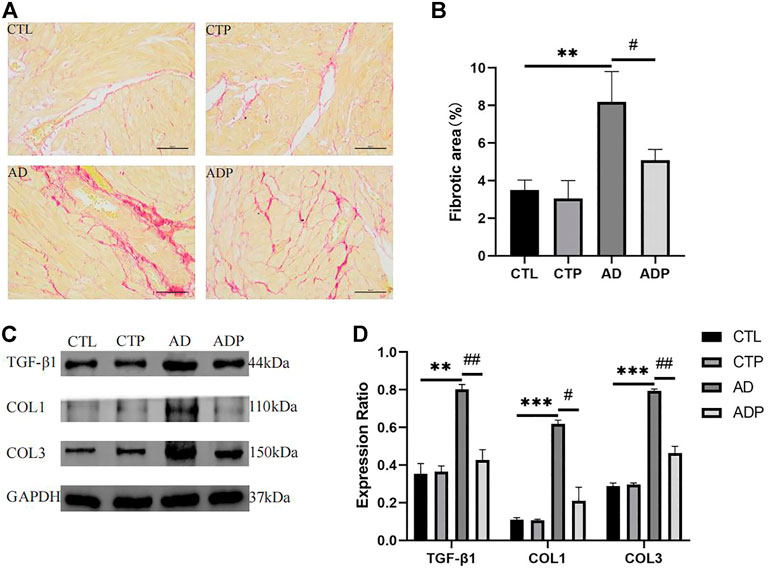
FIGURE 7. Atrial fibrosis. (A,B) Typical images of Sirius red staining and percentage of the fibrosis area, n = 5 per group. (C,D) Immunoblotting and quantitative analysis of TGF-β1, collagen I, and collagen III, n = 3 per group. *p < 0.05, **p < 0.01, ***p < 0.001 vs. CTL; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. AD.
To explore the efficacy of pinocembrin on oxidative stress, the activity of SOD and concentration of MDA in serum and left atrium, and the level of ROS in atrium were detected. Compared with the CTL rats, the fluorescence intensity of ROS and the concentration of MDA were remarkably increased, but the activity of SOD was suppressed in the AD rats (Figures 8A–C). However, these abnormal changes were recovered by pinocembrin in the ADP group.
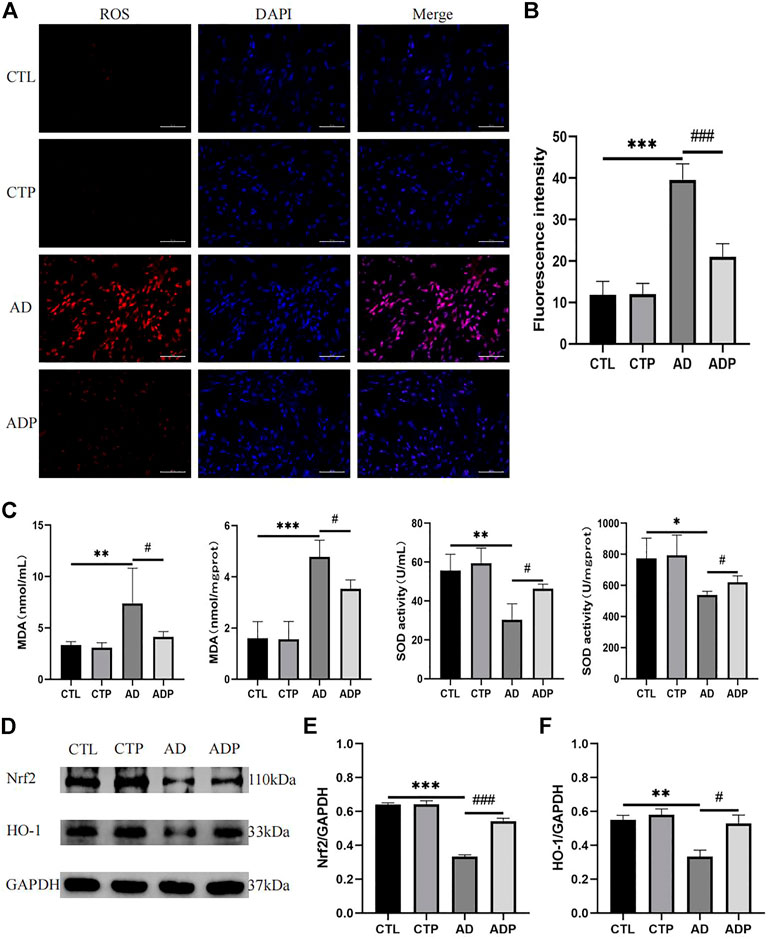
FIGURE 8. Oxidative stress in vivo. (A,B) Typical images and relative fluorescence intensity of ROS in LA, n = 5 per group. (C) The concentration of MDA and activity of SOD in serum and LA, n = 5 per group. (D–F) Immunoblotting and quantitative analysis of Nrf2, HO-1, n = 3 per group.
To further investigate the potential mechanism of pinocembrin on oxidative stress, we explored the Nrf2/HO-1 pathway. As shown in Figure 8D, the content of Nrf2 and HO-1 was lower in the AD rats than the CTL rats, illustrating that the Nrf2/HO-1 pathway was inhibited in the AD rats. However, the Nrf2/HO-1 pathway was upregulated by pinocembrin in the ADP rats with the improved level of Nrf2 and HO-1.
To further demonstrate the antioxidant effect of pinocembrin is dependent on the Nrf2/HO-1 pathway, HL-1 atrial myocytes were cultured as previously reported and treated with H2O2 and Nrf2 inhibitor ML385 in vitro. As shown in Figures 9A–D, after 3 h of H2O2 treatment, ROS accumulation of HL-1 cells and MDA concentration in culture supernate were increased remarkably in the H2O2 group compared with the CTL group, while SOD activity in supernate was decreased significantly. However, ROS accumulation induced by H2O2 was significantly eliminated by the pretreatment of pinocembrin, MDA concentration in the supernate was apparently attenuated, and SOD activity was increased in the H2O2+Pino group. Significantly, these improvements were inhibited by the Nrf2 inhibitor ML385. Correspondingly, in the western blot analysis, pinocembrin improved the significantly decreased content of Nrf2 and HO-1 induced by H2O2, whereas these effects was abrogated by ML385. Therefore, the antioxidant stress effects of pinocembrin were at least partially realized via the Nrf2/HO-1 pathway.
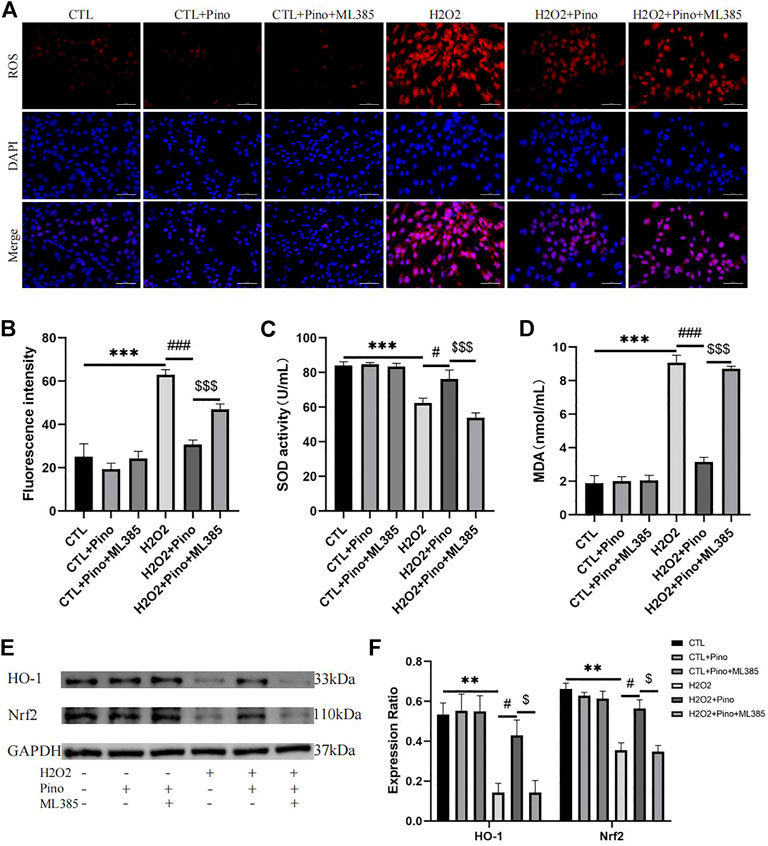
FIGURE 9. Oxidative stress in vitro. (A,B) Typical images and relative fluorescence intensity of ROS in HL-1 cells, n = 5 per group. (C,D) The activity of SOD and concentration of MDA in culture supernate, n = 5 per group. (E,F) Immunoblotting and quantitative analysis of Nrf2, HO-1, n = 3 per group.
In the current study, we demonstrated the efficacy of pinocembrin in reducing the AF susceptibility in anxiety disorder rats. Pinocembrin treatment improved anxiety-like behaviors and decreased atrial autonomic remodeling in anxiety disorder rats. Moreover, pinocembrin improved atrial electrical remodeling in anxiety disorder rats, including shortening of APD, prolongation of ERP, and restoring of ion channels related to action potential repolarization. In addition, pinocembrin improved atrial structural remodeling in anxiety disorder rats, including increased the expression of Cx40 and reduced atrial fibrosis. The improvement of pinocembrin on atrial electrical remodeling and structural remodeling in anxiety disorder rats may be attributed to its antioxidant effect through the Nrf2/HO-1 pathway. Pinocembrin ultimately reduced the AF susceptibility through the above effects in anxiety disorder rats (Figure 10).

FIGURE 10. A summary of the effects of pinocembrin on atrial fibrillation susceptibility in rats with anxiety disorder.
Anxiety disorder and AF often co-occur in patients. Studies with small samples have shown that AD occurred in approximately one-third of patients with AF (Patel et al., 2013; Polikandrioti et al., 2018). A meta-analysis exhibited that AD has made a significant contribution to cardiac events, including an increased risk of cardiovascular mortality, coronary heart disease, and heart failure (Emdin et al., 2016). Patients with AD have been identified to be characterized by prolonged atrial electromechanical delay detected by tissue Doppler echocardiography (Oksuz et al., 2017), which was a herald of AF.
To induce and maintain AF, triggers and substrates are required. Triggers include early afterdepolarization (EAD) and delayed afterdepolarization (DAD), which could induce arrhythmias in the presence of appropriated substrates. Prolonged APD could evoke EAD (Killeen et al., 2007), and intracellular calcium overload could elicit both EAD and DAD (Ohta et al., 1987; Chang et al., 2018). Substrates include uneven prolongation of APD, reduction of conduction velocity, shortening of ERP, and widening of the critical interval of re-excitation (APD-ERP) (Tse et al., 2021). The current study exhibited prolonged APD and shortened ERP in the AD rats, which promoted the occurrence and development of AF, whereas pinocembrin could recover these abnormalities.
The transient outward potassium current (Ito) and the inward calcium current (ICa-L), generated by Kv4.2, Kv4.3 and Cav1.2, respectively, are the two main currents which contribute to the cardiac repolarization in rats. Ito contributes to the early rapid repolarization and the initial part of the platform (Greenstein et al., 2000), and reduced Ito results in prolonged APD (Greenstein et al., 2000). In contrast, genetically increased K+ current in cardiomyocytes could shorten APD, suppress susceptibility to EAD, and prolong ERP (Nuss et al., 1999). ICa-L is the main current in the plateau, and increasd ICa-L prolongs the plateau phase, thus prolonging APD (Anderson, 2004). Sustained activation of ICa-L leads to pathological myocardial remodeling and imbalance of calcium homeostasis, which are closely linked to the arrhythmic phenotype (Zobel et al., 2007). Ultra-rapidly activating (IKur) is generated by potassium current through the Kv1.5 channel, and decreased function of which is associated with AF (Olson et al., 2006; Yang et al., 2010). Our study indicated a significant suppress of Kv1.5, Kv4.2, Kv4.3 and increase of Cav1.2 in the AD rats, which could be reversed by pinocembrin.
Previous study has demonstrated that anxiety disorder can lead to the increased sympathetic function and catecholamine overload, which are important triggers of atrial fibrillation (Severino et al., 2019). In the current study, the positive expression in the TH and GAP43 immunohistochemical staining was increased in AD rats, along with the upregulation of the content of TH and GAP43, indicating that the activity of the cardiac sympathetic nerve was enhanced. HRV is used to assess autonomic nervous function and can be affected by mental factors (Shu et al., 2018). SDRR, RMSSD and HF reflect the parasympathetic activity, while LF reflect the sympathetic activity (Author Anonymous, 1996). In patients with anxiety disorder, RMSSD was suppressed and the LF/HF ratio was enhanced (Christou-Champi et al., 2015). In our study, the AD rats exhibited a reduction of average RR, SDRR, RMSSD, and HF, but an increase of LF and LF/HF, illustrating that the sympathetic nervous system was activated and the parasympathetic nervous system was inhibited. In addition, the serum NE was increased in AD rats. Catecholamine overload can lead to the increased extracellular matrix and abnormal calcium handle, which increases the risk of AF (Boluyt et al., 1995; Nef et al., 2010). However, the imbalance of autonomic nervous function and increased serum NE in AD rats was reversed pinocembrin, demonstrating that pinocembrin suppressed AF susceptibility at least in part by modulating atrial autonomic remodeling.
Atrial fibrosis is a potential substrate of AF. On one hand, fibrosis forms a conduction barrier to block local electrical conduction, on the other hand, fibroblasts interact with cardiomyocytes, promote re-entry and spontaneous ectopic activity (Andrade et al., 2014). Previous study has demonstrated that connexins play a vital role in AF by promoting the conduction of action potential between cardiomyocytes (Chaldoupi et al., 2009). Cx40 is a major connexin in the atria, and cx40 knockout mice exhibited an increased AF susceptibility (Kirchhoff et al., 1998). In our present study, increased atrial fibrosis was found in the AD rats, and the content of TGF-β1, Collagen I and Collagen III was also upregulated, but the expression of cx40 was decreased in the AD rats. However, the increased atrial fibrosis and decreased cx40 were recovered by pinocembrin, indicating that pinocembrin can ameliorate atrial structural remodeling in the AD rats.
Oxidative stress refers to the imbalance between pro-oxidants and antioxidants (Lichtenberg and Pinchuk, 2015). Previous study has illustrated that oxidative stress was increased in anxiety disorder, and antioxidants can ameliorate neurological damage caused by psychiatric disorders (Jin et al., 2014). Our present study revealed that the generation of ROS and the concentration of MDA were increased, but the activity of SOD was reduced in the AD rats, indicating that oxidative stress was intensified in the AD rats. However, those abnormalities were recovered by pinocembrin in the ADP rats.
Oxidative stress can affect the atrial electrical remodeling and thus increase the AF susceptibility. H2O2 can lead to the overload of Na+ and Ca2+ in cardiomyocytes, prolong cardiac action potential and increase early after depolarizations (EADs) (Sovari, 2016). In addition, oxidative stress can reduce the K+ currents, which can be recovered by SOD and NADPH oxidase (NOX) inhibitor apocynin (Shimoni et al., 2005). Moreover, ROS can activate calmodulin-dependent protein kinase II (CaMKII), which leads to the abnormal Ca2+ handling in cardiomyocytes and increases intracellular Ca2+ (Mesubi and Anderson, 2016).
Furthermore, oxidative stress contributes to the atrial structural remodeling and thus provides substrate for the development of AF. Oxidative stress promotes the progression of cardiac fibrosis. NOX2 and NOX4 are major sources of ROS production in the heart. Activation of NOX2 and its downstream JNK/NFAT pathway can promote cardiac fibroblast generation (Fujii et al., 2005), while NOX4 participates in TGF-β-induced cardiac fibroblasts differentiation by activating smad2/3 pathway (Cucoranu et al., 2005). And ROS scavengers (NAC) significantly reduced fibrosis in angiotensin II treated cardiomyocytes by inhibiting TGF-β1/SMADs signaling pathway (Zhu et al., 2020). In addition, oxidative stress can reduce the expression of cx43 in the heart, slowing the conduction velocity and increasing the electrical heterogeneity, which can be reversed by antioxidants (Sovari et al., 2013).
Nrf2/HO-1 is an antioxidant pathway that exhibits endogenous protective effects against damages induced by ROS. In response to stress, Nrf2 separates from its inhibitor Keap1 and transfers into nucleus, promoting the production of antioxidant enzymes represented by HO-1, which could remove heme, produce biliverdin, iron ion and carbon monoxide (Loboda et al., 2016). Previous studies have demonstrated that Nrf2/HO-1 performed protective effects on the nervous system and cardiovascular system. As to Alzheimer’s disease and Parkinson’s disease, oxidative stress exacerbated the progression of which, while inhibition of ROS and promotion of the Nrf2/HO-1 pathway can alleviate the neuronal apoptosis and improve the symptoms (Feng et al., 2016; Li et al., 2018). Nrf2/HO-1 pathway can suppress myocardial apoptosis and reduce the incidence of ventricular arrhythmia induced by myocardial ischemia-reperfusion (Enayati et al., 2018). Our previous study demonstrated that pinocembrin can improve the cardiac function and reduce myocardial apoptosis and cardiac fibrosis in rats with heart failure by promoting Nrf2/HO-1 pathway (Chen et al., 2021). Our study exhibited a remarkable inhibition of the content of Nrf2 and HO-1, but pinocembrin administration improved the Nrf2/HO-1 pathway in the ADP rats. In addition, in H2O2-treated HL-1 cells, pinocembrin promoted the Nrf2/HO-1 pathway, but this promotion was eliminated by ML385, a Nrf2 inhibitor, suggesting that Nrf2 is the therapeutic target of pinocembrin exerting the antioxidant effects.
Pinocembrin can reduce AF susceptibility in anxiety disorder rats, with the inhibition of electrical remodeling, autonomic remodeling, structural remodeling, and oxidative stress. Therefore, pinocembrin is a promising treatment for anxiety disorder patients with AF.
Although the animal study objectively demonstrate pinocembrin is a potentially promising pharmacological candidate, additional studies and clinical trials are required to determine its specific intracellular sites of action and derivative targets in order to fully understand the mechanism of pinocembrin and to further validate its clinical medical applications.
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by the Experimental Animal Center of Wuhan University.
QR and CZ designed this study. QR, TY, YZ, YY, ZL, and JZ performed the experiments. QR and WW collected and analyzed the data. QR drafted the first manuscript, CZ and BS revised the manuscript, BS and BY determined the final manuscript. All authors contributed to the study and approved the final manuscript.
The study was supported by the Nature Science Foundation of China (Nos:81500278, 81800447, 82170316) and the Nature Science Foundation of Hubei Province (No: 2017CFB204).
The authors express their gratitude to Renmin Hospital of Wuhan University, Cardiovascular Research Institute of Wuhan University, and Hubei Key Laboratory of Cardiology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anderson, M. E. (2004). Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends cardiovasc. Med. 14 (4), 152–161. doi:10.1016/j.tcm.2004.02.005
Andrade, J., Khairy, P., Dobrev, D., and Nattel, S. (2014). The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114 (9), 1453–1468. doi:10.1161/CIRCRESAHA.114.303211
Author Anonymous, (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European society of Cardiology and the north American society of pacing and electrophysiology. Circulation 93(5), 1043–1065. doi:10.1161/01.cir.93.5.1043
Bandelow, B., and Michaelis, S. (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 17 (3), 327–335. doi:10.31887/dcns.2015.17.3/bbandelow
Boluyt, M. O., Long, X., Eschenhagen, T., Mende, U., Schmitz, W., Crow, M. T., et al. (1995). Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. Am. J. Physiol. 269, H638–H647. doi:10.1152/ajpheart.1995.269.2.H638
Chaldoupi, S. M., Loh, P., Hauer, R. N., de Bakker, J. M., and van Rijen, H. V. (2009). The role of connexin40 in atrial fibrillation. Cardiovasc. Res. 84 (1), 15–23. doi:10.1093/cvr/cvp203
Chang, P. C., Lu, Y. Y., Lee, H. L., Lin, S. F., Chu, Y., Wen, M. S., et al. (2018). Paradoxical effects of sodium-calcium exchanger inhibition on torsade de Pointes and early afterdepolarization in a heart failure rabbit model. J. Cardiovasc. Pharmacol. 72 (2), 97–105. doi:10.1097/FJC.0000000000000598
Chen, X., Zhang, C., Guo, Y., Liu, X., Ye, T., Fo, Y., et al. (2020). Chronic stimulation of the sigma-1 receptor ameliorates ventricular ionic and structural remodeling in a rodent model of depression. Life Sci. 257, 118047. doi:10.1016/j.lfs.2020.118047
Chen, X., Wan, W., Guo, Y., Ye, T., Fo, Y., Sun, Y., et al. (2021). Pinocembrin ameliorates post-infarct heart failure through activation of Nrf2/HO-1 signaling pathway. Mol. Med. 27 (1), 100. doi:10.1186/s10020-021-00363-7
Christou-Champi, S., Farrow, T. F., and Webb, T. L. (2015). Automatic control of negative emotions: evidence that structured practice increases the efficiency of emotion regulation. Cogn. Emot. 29 (2), 319–331. doi:10.1080/02699931.2014.901213
Cucoranu, I., Clempus, R., Dikalova, A., Phelan, P. J., Ariyan, S., Dikalov, S., et al. (2005). NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 97 (9), 900–907. doi:10.1161/01.RES.0000187457.24338.3D
Emdin, C. A., Odutayo, A., Wong, C. X., Tran, J., Hsiao, A. J., and Hunn, B. H. (2016). Meta-analysis of anxiety as a risk factor for cardiovascular disease. Am. J. Cardiol. 118 (4), 511–519. doi:10.1016/j.amjcard.2016.05.041
Enayati, A., Yassa, N., Mazaheri, Z., Rajaei, M., Pourabouk, M., Ghorghanlu, S., et al. (2018). Cardioprotective and anti-apoptotic effects of Potentilla reptans L. root via Nrf2 pathway in an isolated rat heart ischemia/reperfusion model. Life Sci. 215, 216–226. doi:10.1016/j.lfs.2018.11.021
Feng, C. W., Hung, H. C., Huang, S. Y., Chen, C. H., Chen, Y. R., Chen, C. Y., et al. (2016). Neuroprotective effect of the marine-derived compound 11-dehydrosinulariolide through DJ-1-related pathway in in vitro and in vivo models of Parkinson's disease. Mar. Drugs 14 (10), E187. doi:10.3390/md14100187
Feng, G. K., Ma, X. J., Chen, Y. Y., Bian, G. R., Yang, C., and Gu, B. D. (2018). Effects of chailong jieyu pill on behavior, monoamine neurotransmitters, and corticosteroid receptors in a rat model of anxiety disorder. Evid. Based. Complement. Altern. Med. 2018, 5489215. doi:10.1155/2018/5489215
Fujii, T., Onohara, N., Maruyama, Y., Tanabe, S., Kobayashi, H., Fukutomi, M., et al. (2005). Galpha12/13-mediated production of reactive oxygen species is critical for angiotensin receptor-induced NFAT activation in cardiac fibroblasts. J. Biol. Chem. 280 (24), 23041–23047. doi:10.1074/jbc.M409397200
Greenstein, J. L., Wu, R., Po, S., Tomaselli, G. F., and Winslow, R. L. (2000). Role of the calcium-independent transient outward current I(to1) in shaping action potential morphology and duration. Circ. Res. 87 (11), 1026–1033. doi:10.1161/01.res.87.11.1026
Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomström-Lundqvist, C., et al. (2020). ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42 (5), 373–498.
Jin, H., Kanthasamy, A., Ghosh, A., Anantharam, V., Kalyanaraman, B., and Kanthasamy, A. G. (2014). Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim. Biophys. Acta 1842 (8), 1282–1294. doi:10.1016/j.bbadis.2013.09.007
Killeen, M. J., Thomas, G., Gurung, I. S., Goddard, C. A., Fraser, J. A., Mahaut-Smith, M. P., et al. (2007). Arrhythmogenic mechanisms in the isolated perfused hypokalaemic murine heart. Acta Physiol. 189 (1), 33–46. doi:10.1111/j.1748-1716.2006.01643.x
Kirchhoff, S., Nelles, E., Hagendorff, A., Krüger, O., Traub, O., and Willecke, K. (1998). Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr. Biol. 8 (5), 299–302. doi:10.1016/s0960-9822(98)70114-9
Li, C., Wang, R., Hu, C., Wang, H., Ma, Q., Chen, S., et al. (2018). Pyridoxine exerts antioxidant effects in cell model of Alzheimer's disease via the Nrf-2/HO-1 pathway. Cell. Mol. Biol. 64 (10), 119–124. doi:10.14715/cmb/2018.64.10.19
Li, C., Wan, W., Ye, T., Sun, Y., Chen, X., Liu, X., et al. (2021). Pinocembrin alleviates lipopolysaccharide-induced myocardial injury and cardiac dysfunction in rats by inhibiting p38/JNK MAPK pathway. Life Sci. 277, 119418. doi:10.1016/j.lfs.2021.119418
Lichtenberg, D., and Pinchuk, I. (2015). Oxidative stress, the term and the concept. Biochem. Biophys. Res. Commun. 461 (3), 441–444. doi:10.1016/j.bbrc.2015.04.062
Lip, G. Y., Fauchier, L., Freedman, S. B., Van Gelder, I., Natale, A., Gianni, C., et al. (2016). Atrial fibrillation. Nat. Rev. Dis. Prim. 2, 16016. doi:10.1038/nrdp.2016.16
Liu, R., Li, J. Z., Song, J. K., Zhou, D., Huang, C., Bai, X. Y., et al. (2014). Pinocembrin improves cognition and protects the neurovascular unit in Alzheimer related deficits. Neurobiol. Aging 35 (6), 1275–1285. doi:10.1016/j.neurobiolaging.2013.12.031
Liu, X., Qu, C., Yang, H., Shi, S., Zhang, C., Zhang, Y., et al. (2018). Chronic stimulation of the sigma-1 receptor ameliorates autonomic nerve dysfunction and atrial fibrillation susceptibility in a rat model of depression. Am. J. Physiol. Heart Circ. Physiol. 315 (6), H1521–H1531. doi:10.1152/ajpheart.00607.2017
Loboda, A., Damulewicz, M., Pyza, E., Jozkowicz, A., and Dulak, J. (2016). Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 73 (17), 3221–3247. doi:10.1007/s00018-016-2223-0
Mesubi, O. O., and Anderson, M. E. (2016). Atrial remodelling in atrial fibrillation: CaMKII as a nodal proarrhythmic signal. Cardiovasc. Res. 109 (4), 542–557. doi:10.1093/cvr/cvw002
Nef, H. M., Möllmann, H., Akashi, Y. J., and Hamm, C. W. (2010). Mechanisms of stress (Takotsubo) cardiomyopathy. Nat. Rev. Cardiol. 7 (4), 187–193. doi:10.1038/nrcardio.2010.16
Nuss, H. B., Marbán, E., and Johns, D. C. (1999). Overexpression of a human potassium channel suppresses cardiac hyperexcitability in rabbit ventricular myocytes. J. Clin. Invest. 103 (6), 889–896. doi:10.1172/JCI5073
Ohta, M., Karagueuzian, H. S., Mandel, W. J., and Peter, T. (1987). Acute and chronic effects of amiodarone on delayed afterdepolarization and triggered automaticity in rabbit ventricular myocardium. Am. Heart J. 113, 289–296. doi:10.1016/0002-8703(87)90267-5
Oksuz, F., Yarlioglues, M., Ozturk, S., Kaya, F. D., Oksuz, E., Murat, S. N., et al. (2017). Atrial electromechanical delay analysed by tissue Doppler echocardiography is prolonged in patients with generalised anxiety disorders. Kardiol. Pol. 75 (6), 581–588. doi:10.5603/KP.a2017.0038
Olson, T. M., Alekseev, A. E., Liu, X. K., Park, S., Zingman, L. V., Bienengraeber, M., et al. (2006). Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 15 (14), 2185–2191. doi:10.1093/hmg/ddl143
Patel, D., Mc Conkey, N. D., Sohaney, R., Mc Neil, A., Jedrzejczyk, A., and Armaganijan, L. (2013). A systematic review of depression and anxiety in patients with atrial fibrillation: the mind-heart link. Cardiovasc. Psychiatry Neurol. 2013, 159850. doi:10.1155/2013/159850
Pitsavos, C., Panagiotakos, D. B., Papageorgiou, C., Tsetsekou, E., Soldatos, C., and Stefanadis, C. (2006). Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis 185 (2), 320–326. doi:10.1016/j.atherosclerosis.2005.06.001
Polikandrioti, M., Koutelekos, I., Vasilopoulos, G., Gerogianni, G., Gourni, M., Zyga, S., et al. (2018). Anxiety and depression in patients with permanent atrial fibrillation: Prevalence and associated factors. Cardiol. Res. Pract. 2018, 7408129. doi:10.1155/2018/7408129
Rasul, A., Millimouno, F. M., Ali Eltayb, W., Ali, M., Li, J., and Li, X. (2013). Pinocembrin: a novel natural compound with versatile pharmacological and biological activities. Biomed. Res. Int. 2013, 379850. doi:10.1155/2013/379850
Severino, P., Mariani, M. V., Maraone, A., Piro, A., Ceccacci, A., Tarsitani, L., et al. (2019). Triggers for atrial fibrillation: The role of anxiety. Cardiol. Res. Pract. 2019, 1208505. doi:10.1155/2019/1208505
Shimoni, Y., Hunt, D., Chuang, M., Chen, K. Y., Kargacin, G., and Severson, D. L. (2005). Modulation of potassium currents by angiotensin and oxidative stress in cardiac cells from the diabetic rat. J. Physiol. 567 (1), 177–190. doi:10.1113/jphysiol.2005.090639
Shu, L., Xie, J., Yang, M., Li, Z., Li, Z., Liao, D., et al. (2018). A review of emotion recognition using physiological signals. Sensors (Basel, Switz. 18 (7), E2074. doi:10.3390/s18072074
Sovari, A. A., Rutledge, C. A., Jeong, E. M., Dolmatova, E., Arasu, D., Liu, H., et al. (2013). Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circ. Arrhythm. Electrophysiol. 6 (3), 623–631. doi:10.1161/CIRCEP.112.976787
Sovari, A. A. (2016). Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol. Res. Pract. 2016, 9656078. doi:10.1155/2016/9656078
Thompson, T. S., Barksdale, D. J., Mounsey, J. P., Pursell, I., and Gehi, A. K. (2013). The effect of anxiety and depression on symptoms attributed to atrial fibrillation. Pacing Clin. Electrophysiol. 37 (4), 439–446. doi:10.1111/pace.12292
Tse, G., Li, K. H. C., Cheung, C. K. Y., Letsas, K. P., Bhardwaj, A., Sawant, A. C., et al. (2021). Higher dispersion measures of conduction and repolarization in type 1 compared to non-type 1 brugada syndrome patients: An electrocardiographic study from a single center. Front. Cardiovasc. Med. 8, 132. doi:10.3389/fcvm.2018.00132
Tully, P. J., Bennetts, J. S., Baker, R. A., McGavigan, A. D., Turnbull, D. A., and Winefield, H. R. (2011). Anxiety, depression, and stress as risk factors for atrial fibrillation after cardiac surgery. Heart Lung 40 (1), 4–11. doi:10.1016/j.hrtlng.2009.12.010
Wang, W., Zheng, L., Xu, L., Tu, J., and Gu, X. (2020). Pinocembrin mitigates depressive-like behaviors induced by chronic unpredictable mild stress through ameliorating neuroinflammation and apoptosis. Mol. Med. 26 (1), 53. doi:10.1186/s10020-020-00179-x
Yang, T., Yang, P., Roden, D. M., and Darbar, D. (2010). Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart rhythm. 7 (9), 1246–1252. doi:10.1016/j.hrthm.2010.05.032
Ye, T., Zhang, C., Wu, G., Wan, W., Liang, J., Liu, X., et al. (2019). Pinocembrin attenuates autonomic dysfunction and atrial fibrillation susceptibility via inhibition of the NF-κB/TNF-α pathway in a rat model of myocardial infarction. Int. Immunopharmacol. 77, 105926. doi:10.1016/j.intimp.2019.105926
Ye, T., Zhang, C., Wu, G., Wan, W., Guo, Y., Fo, Y., et al. (2020). Pinocembrin decreases ventricular fibrillation susceptibility in a rat model of depression. Front. Pharmacol. 11, 547966. doi:10.3389/fphar.2020.547966
Zhu, W., Wu, R. D., Lv, Y. G., Liu, Y. M., Huang, H., and Xu, J. Q. (2020). BRD4 blockage alleviates pathological cardiac hypertrophy through the suppression of fibrosis and inflammation via reducing ROS generation. Biomed. Pharmacother. 121, 109368. doi:10.1016/j.biopha.2019.109368
Keywords: pinocembrin, anxiety disorder, atrial fibrillation, electrophysiological remodeling, oxidative stress
Citation: Ran Q, Zhang C, Wan W, Ye T, Zou Y, Liu Z, Yu Y, Zhang J, Shen B and Yang B (2022) Pinocembrin ameliorates atrial fibrillation susceptibility in rats with anxiety disorder induced by empty bottle stimulation. Front. Pharmacol. 13:1004888. doi: 10.3389/fphar.2022.1004888
Received: 27 July 2022; Accepted: 11 October 2022;
Published: 20 October 2022.
Edited by:
Keliang Xie, Tianjin Medical University, ChinaReviewed by:
Li Ma, Shanghai Children’s Hospital, ChinaCopyright © 2022 Ran, Zhang, Wan, Ye, Zou, Liu, Yu, Zhang, Shen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Shen, c2hlbmJvd2hkeEAxMjYuY29t; Bo Yang, eXliYjExMkB3aHUuZWR1LmNu
†These authors have contributed equally to the work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.