- 1Department of Nuclear Medicine/PET Image Center, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Department of Pharmacy, The Second Xiangya Hospital of Central South University, Changsha, China
Objective: To assess the cost effectiveness of radium-223 dichloride for patients with metastatic castration-resistant prostate cancer (mCRPC) in China.
Materials and methods: A Markov model was developed to estimate the long-term health and economic outcomes of radium-223 plus best standard care (BSC) treatment and BSC only for bone mCRPC patients over a lifetime horizon. The patients and interventions were modeled according to the ALSYMPCA trial. Costs were collected from a Chinese health system perspective. Utility values were derived from the published literature. The base-case model results were quality-adjusted life year (QALY), total cost, and incremental cost-utility ratio (ICUR). Uncertainty analyses were performed to assess the robustness of our conclusions.
Results: Compared with the BSC arm, radium-223 achieved an excess 0.344 QALYs with an incremental cost of $29,459, resulting in an ICUR of $85,647 per QALY. The probability of Ra-223 being cost effective for the patients with bone mCRPC was sharply low (<0.5%) at a willingness-to-pay threshold of $38,136/QALY. Uncertainty analyses revealed that the model is robust to all the input parameters.
Conclusion: Radium-223 is unlikely to be cost effective in patients with bone mCRPC at the current WTP threshold, from a Chinese health system perspective. In affluent areas with a high per-capita GDP, radium-223 therapy may be cost effective.
Introduction
Prostate cancer is the most commonly diagnosed cancer and the second leading cause of death among males, with an estimated 1,414,259 new cases and 375,304 fatalities worldwide (Sung et al., 2021; Siegel et al., 2022). The majority of advanced prostate cancers eventually stop responding to the standard androgen deprivation treatment (ADT), which is categorized as castration-resistant prostate cancer (CRPC). Up to 90% of patients with metastatic CRPC (mCRPC) will develop bone metastases (Parker et al., 2013). Clinical practice guidelines from the European Society for Medical Oncology (ESMO), Chinese Society of Clinical Oncology (CSCO), and American Society of Clinical Oncology (ASCO) all recommend the use of abiraterone, enzalutamide, cabazitaxel, and radium-223 dichloride (for men with bone-predominant, symptomatic mCRPC without visceral metastases) in patients with mCRPC who are ineligible for docetaxel (Guidelines of Chinese Society of Clinical Oncology, 2020; Parker et al., 2020; Saylor et al., 2020). According to the ASCO and CSCO guidelines, Sipuleucel-T may be offered to mCRPC with asymptomatic/minimally symptomatic (Guidelines of Chinese Society of Clinical Oncology, 2020; Saylor et al., 2020); and palliative care should be offered to all patients (Guidelines of Chinese Society of Clinical Oncology, 2020; Parker et al., 2020; Saylor et al., 2020). Bisphosphonate or denosumab is recommended as palliative care for patients with bone mCRPC at risk for clinically significant skeletal-related events, and external beam radiotherapy treatment (EBRT) is recommended for palliation of painful, uncomplicated bone metastasis (Guidelines of Chinese Society of Clinical Oncology, 2020; Parker et al., 2020; Saylor et al., 2020). According to the CSCO guidelines, the best standard of care (BSC) includes alleviating pain, nutritional support, psychotherapy, and preventing skeletal-related events (bisphosphonate, EBRT, etc.) (Guidelines of Chinese Society of Clinical Oncology, 2020).
Bone disease and its complication are major causes of death for patients with prostate cancer and have imposed a substantial economic burden and decreased the quality of life (Lange and Vessella, 1998; Roodman, 2004; Hagiwara et al., 2013). Therefore, prevention and delay of the symptomatic skeletal event (SSE) are vital in the management of patients with mCRPC. Denosumab, bisphosphonates, and radioisotopes are among the treatments that can relieve pain and postpone SSE but do not increase survival (Finlay et al., 2005; Lipton, 2010; Fizazi et al., 2011; Sartor et al., 2011). Conversely, available effective therapies including docetaxel, cabazitaxel, abiraterone, and enzalutamide that have been proven to improve mCRPC patients’ survival have not been demonstrated to prevent against SSE(Berthold et al., 2008; de Bono et al., 2010; de Bono et al., 2011; Penson et al., 2016).
Radium-223 dichloride (Ra-223), as a bone-targeted alpha therapy, is a first-in-class radiopharmaceutical that has been shown to have both survival and SSE benefit for CRPC with symptomatic bone metastases (bone mCRPC) (Kluetz et al., 2014), based on the findings of a double-blind, randomized phase 3 ALSYMPCA trial (Parker et al., 2013; Hoskin et al., 2014; Sartor et al., 2014). The ALSYMPCA trial demonstrated that compared with the best standard of care (BSC), Ra-223 significantly prolonged overall survival (median 14.9 months vs. 11.3 months; hazard ratio [HR] 0.70, 95% CI 0.58–0.83) and the time to first SSE (median 15.6 months vs. 9.8 months; HR 0.66, 95% CI 0.52–0.83) for patients with bone mCRPC. Another double-blind randomized phase 3 trial (ERA-223) found that adding Ra-223 to abiraterone plus prednisone or prednisolone did not improve SSE in patients with bone mCRPC, and was associated with a higher risk of bone fractures than adding placebo (Smith et al., 2019). An interim safety analysis of a global, prospective, non-interventional study (REASSURE) indicated that the short-term safety profile of Ra-223 in routine clinical practice was comparable to other clinical studies, irrespective of prior chemotherapy treatment (Dizdarevic et al., 2019).
Due to its superior efficacy, Ra-223 is recommended as category 1 for bone mCRPC in clinical guidelines (Guidelines of Chinese Society of Clinical Oncology, 2020; Nation Comprehensive Cancer Network, 2022), and was approved by the Chinese National Medical Products Administration (NMPA) in 2020. Several studies have evaluated the economic impact of Ra-223 in patients with bone mCRPC in different countries, including Spanish, Ireland, England and Wales, Canada, and Dutch (Tirado Mercier et al., 2018; National Centre for Pharmacoeconomics (NCPE), 2022; National Institute for Health and Care Excellence (NICE), 2022; Canadian Agency for Drugs and Technologies in Health (CADTH), 2016; Peters et al., 2018). However, it is unclear whether Ra-223 is cost effective for Chinese patients with bone mCRPC. Therefore, the aim of this study is to evaluate the cost effectiveness of Ra-223 in the treatment of patients with bone mCRPC from a Chinese health system perspective.
Materials and methods
Methodological design
To meet the aim described above, a cost-utility analysis was carried out to estimate the long-term health and economic outcomes of Ra-223 plus BSC and placebo plus BSC for bone mCRPC patients. A Markov model was developed in TreeAge Pro software (TreeAge Software, Williamstown, MA), and the model simulation outcomes were presented as total costs, quality-adjusted life years (QALYs), and incremental cost-utility ratio (ICUR). All costs and utilities were discounted to 2021 using a 5% annual rate according to China Guidelines for Pharmacoeconomic Evaluations (2020) (Certified Public Accountant, 2020) and an average exchange rate in December 2021 of 1 US dollar (USD) to 6.37 Chinese Yuan (RMB) (The People’s Bank of China, 2022). The willingness-to-pay (WTP) threshold of $38,136/QALY, which equals to three times the per-capita gross domestic product (GDP) of 2021 (National Bureau of Statistics of China, 2022), was used in the cost-utility analysis based on the recommendation of the China Guidelines for Pharmacoeconomic Evaluations.
Patients and intervention
The patients and intervention were modeled according to the ALSYMPCA trial, which verified a superior benefit of Ra-223 plus BSC among mCRPC patients, who previously received treatment of docetaxel or were unsuitable for docetaxel, with two or more bone metastases and no known visceral metastases (Parker et al., 2013). In brief, 921 patients were randomly assigned (2:1) to receive the therapies of Ra-223 (50 kBq/kg, once per 4 weeks, 6 intravenous injections) plus BSC or placebo plus BSC. The BSC treatments were administered to both arms, including local external beam radiation, glucocorticoids, antiandrogens, ketoconazole, estramustine, or diethylstilbestrol.
Model structure
A Markov model was established to estimate the costs and health efficacy of treatment with Ra-223 plus BSC versus BSC alone in patients with bone mCRPC (Figure 1). There are five mutually exclusive health states in the model: progression-free survival (PFS) without SSE, progression (PD) without SSE, PFS with SSE, PD with SSE, and death. In a nutshell, all eligible patients enter the health state “PFS without SSE,” and receive Ra-223 plus BSC treatment (Ra-223 arm) or placebo plus BSC treatment (BSC arm). Patients can move from one state to another or stay in their initial health state at the end of each Markov cycle. The Markov model cycle length was chosen to be 4 weeks, mirroring the Ra-223 therapy cycle.
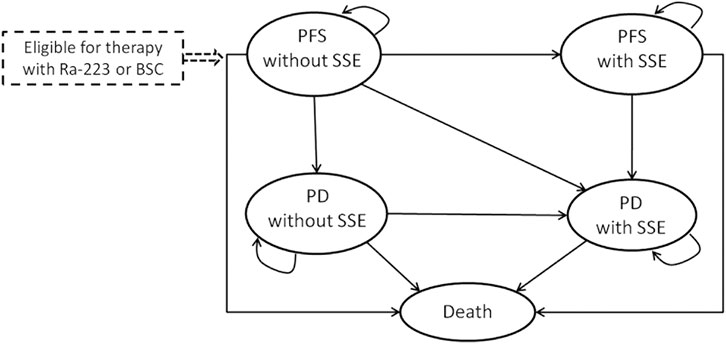
FIGURE 1. Markov model for patients with metastatic castration-resistant prostate cancer. PFS: progression-free survival; SSE: symptomatic skeletal event; Ra-223: radium-223; PD: progression survival; BSC: best standard care.
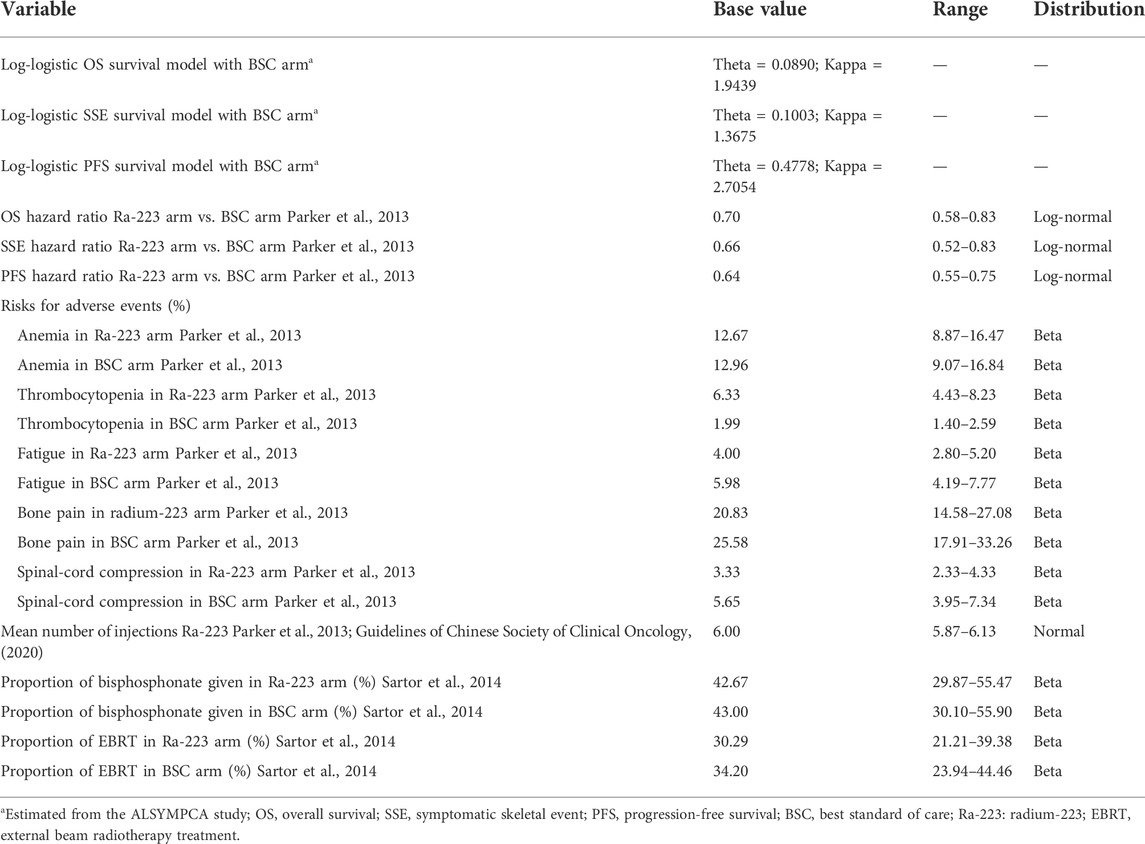
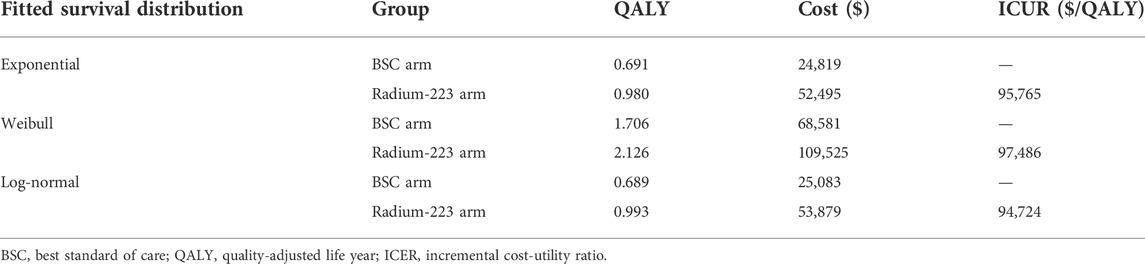
The main variables that determine the Markov transition probabilities for the BSC arm were derived from the ALSYMPCA study (Parker et al., 2013). The standard techniques were used to perform extrapolation of the time to death, the time to SSE, and the time to disease progression (Guyot et al., 2012). The first step was to reconstruct the individual patient-level data (IPD) using published survival curves, the number of patients at risk, and the total events. Second, the four common survival models—exponential, Weibull, log-normal, and log-logistic—were tested using the reconstructed IPD. Finally, the Akaike information criterion, visual inspection, and statistical criteria were used to determine that the log-logistic function should be used for the current economic evaluation. Supplementary Appendix SA1 contains specifics regarding the selection of survival distributions. The hazard ratios (HRs) published in the clinical ALSYMPCA were used to calculate the transition probabilities for the Ra-223 arm. The variables of the log-logistic function and the HRs are displayed in Table 1.
According to the China Guidelines for Pharmacoeconomic Evaluations, the cost-utility analysis of the current model was performed from a Chinese health system perspective using a lifetime horizon and a half-cycle correction.
Inputs used to populate the model
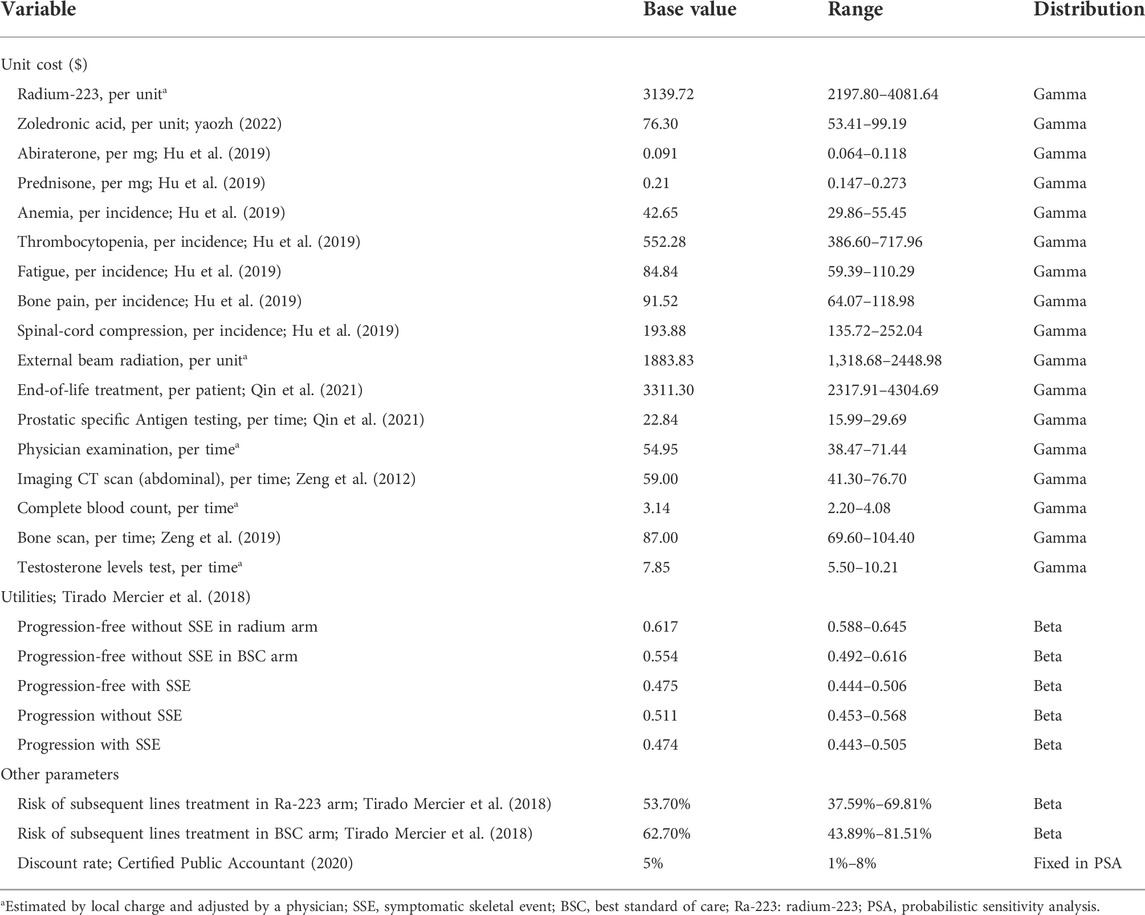
Medical costs of health resources were collected in accordance with the clinical practice of the ALSYMPCA trial. Direct medical costs were calculated in terms of standard procedure for Ra-223 therapy, management of serious adverse events (AEs), BSC (bisphosphonates and local external beam radiation), routine follow-up care (prostatic specific antigen testing, physician examination, imaging CT scan, complete blood count, bone scan, and testosterone levels test), subsequent lines treatment in progression state, and terminal care. Supplementary Appendix SA2 offered information regarding the frequency of health services. The clinical trial’s mean number of Ra-223 injections was used to calculate the drug’s total costs. Only grade 3/4 AEs with a frequency greater than 3% were taken into account to estimate the AEs costs in the current economic evaluation. Risks of bisphosphonate and external beam radiotherapy were multiplied by their unit costs as the BSC cost. Costs of tumor-related orthopedic surgical and pathological fractures were not estimated in our analysis because of their identical incidences and little healthcare spending. Because zoledronic acid was the most commonly used bisphosphonate in the ALSYMPCA trial, we assumed it was available when assessing the cost of bisphosphonate. Routine follow-up care was given according to the Chinese clinical guidelines for the management of prostate cancer (Guidelines of Chinese Society of Clinical Oncology, 2020). All model input parameters estimated or derived from the clinical trial are shown in Table 1.
Based on Chinese experts’ opinion, all patients with progression switched to abiraterone acetate and prednisone as subsequent lines of treatment after 4 weeks of the last injection of Ra-223. The risks of the subsequent lines of treatment in the two arms were obtained from another economic evaluation (Tirado Mercier et al., 2018). Unit costs, shown in Table 2, were derived from published studies (Zeng et al., 2012; Hu et al., 2019; Zeng et al., 2019; Qin et al., 2021), the site of the big data service platform for China’s health industry (yaozh, 2022), or estimated according to local charges and adjusted by a clinical physician from a Chinese health system perspective.
Utility values were obtained from published literature (Tirado Mercier et al., 2018) and displayed in Table 2. The utility values were different between patients treated with Ra-223 and BSC. Once the disease progressed, the same utility values were assumed for both arms.
Uncertainty analyses
We conducted a series of uncertainty analyses to evaluate the robustness of the model results. In one-way sensitivity analysis, model input parameters were varied within their ranges to explore the influence of each individual parameter on the results. The ranges of all parameters were obtained from the relevant derivation or ±30% of the base values. Monte Carlo simulations of 1,000 iterations were performed in a probabilistic sensitivity analysis by setting specific patterns of distribution for each parameter. All ranges and distributions selected for uncertainty analyses were listed in Table 1 and Table 2. Additionally, considering the survival curves used to model the survival curves may impact results greatly, we performed a scenario analysis using different fitted survival curves.
Results
Base-case results
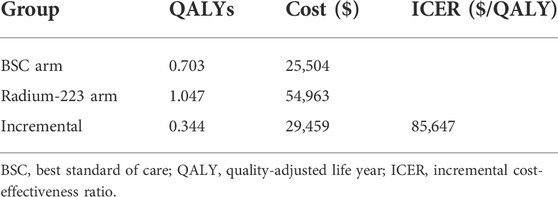
Patients treated with Ra-223 achieved QALYs of 1.047, which was 0.344 more than those receiving BSC treatment. The total cost per patient in the Ra-223 and BSC arms was 54,963 USD and 25,504 USD, respectively. The ICUR for the Ra-223 arm versus the BSC arm was $85,647 per QALY (Table 3).
Sensitivity analyses results
A tornado diagram was used to show how the one-way sensitivity analyses turned out (Figure 2). The ICUR was most affected by the overall survival HR. Other sensitive parameters were risks of subsequent lines of treatment in both groups, the cost of radium-223 and abiraterone, the utility of progression without SSE, and the discount rate. All of the model’s input parameters failed to result in an ICUR below the WTP threshold of $38,136/QALY.
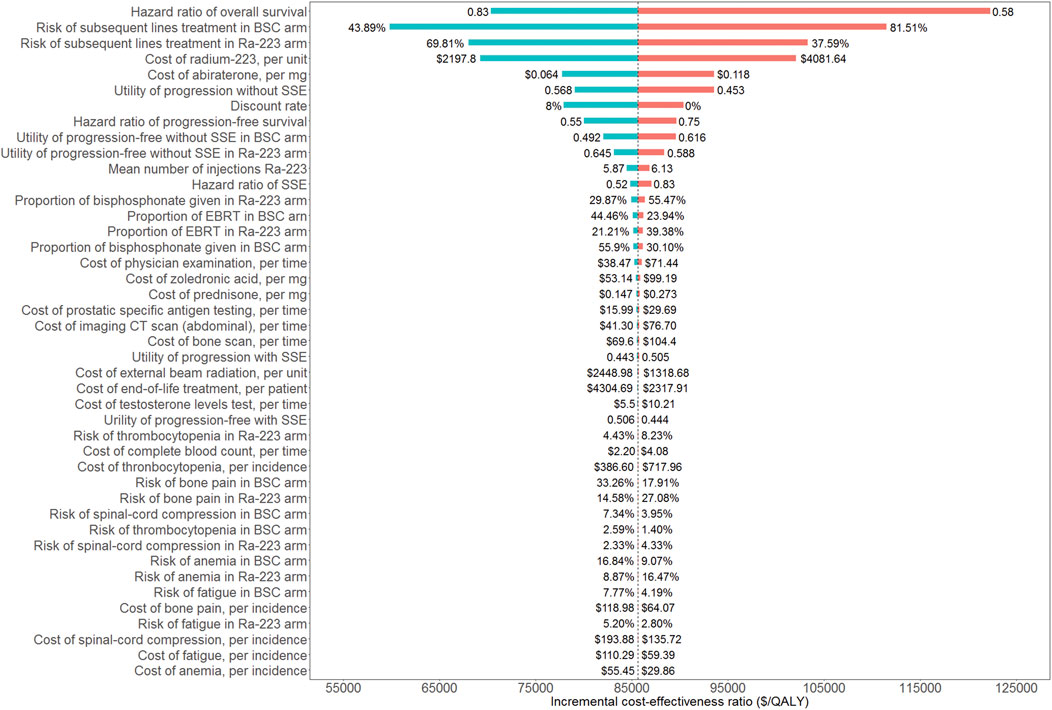
FIGURE 2. Tornado diagram of one-way sensitivity analyses. BSC: best standard care; Ra-223: radium-223; SSE: symptomatic skeletal event; EBRT: external beam radiation treatment.
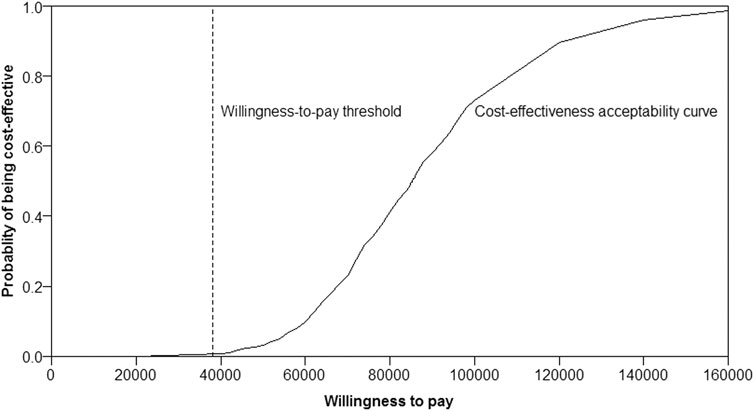
Almost the majority of the dots in the incremental cost-effectiveness scatterplot of 1,000 iterations were above the WTP threshold of $38,136/QALY (Figure 3). If the WTP threshold was more than $85,824/QALY, more than 50% of dots were cost-effective (below the WTP threshold). The probability of Ra-223 being cost-effective for the patients with bone mCRPC was sharply low (<0.5%) at the current WTP threshold ($38,136/QALY), according to the cost-effectiveness acceptability curve (Figure 4).
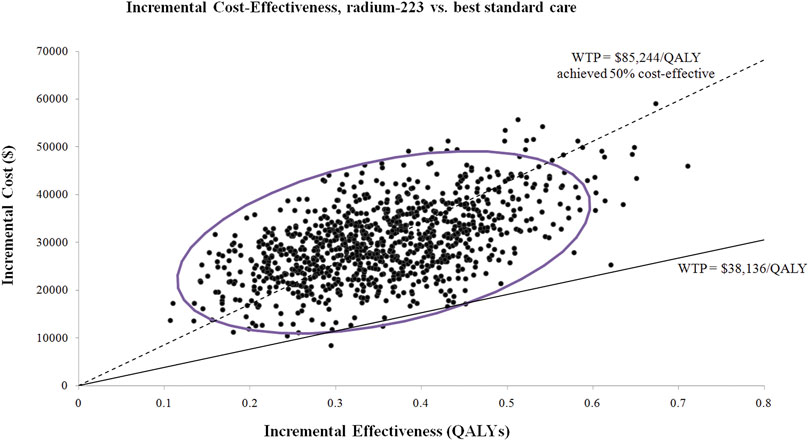
FIGURE 3. Incremental cost-effectiveness scatterplot of 1,000 iterations. WTP: willingness to pay; QALY: quality-adjusted life years.
Scenario analyses using different fitted survival curves were displayed in Table 4. Although different fitted survival distributions impacted the results greatly, all of them did not result in the ICER below the WTP threshold ($38,136/QALY).
Discussion
Ra-223 has been shown to be effective for treating patients with bone mCRPC in the ALSYMPCA trial due to its prolongation of overall survival and the first time to SSE (Parker et al., 2013; Hoskin et al., 2014; Sartor et al., 2014). However, after receiving NMPA approval, it is also necessary to assess its economic impact. In the current study, from a Chinese health system perspective, we evaluated the lifetime horizon costs and effectiveness associated with Ra-223 plus BSC and BSC only for bone mCRPC using a Markov model. The efficacy and safety data were taken from the ALSYMPCA trial, and the cost was collected from the published literature, or estimated according to local charges and adjusted by clinical physicians, on the basis of the clinical practice and the Chinese clinical guidelines. The result of the base-case analysis demonstrated that the ICUR was unfavorable at $85,647 per QALY gained, which was higher than the WTP threshold of $38,136/QALY.
The outcomes of our investigation were robust to all of the input parameters, according to the uncertainty analyses. Although the hazard ratio of the overall survival, risk of subsequent lines of treatment in both groups, and cost of radium-223 impacted the ICUR materially, none of them led to the ICUR lowering the WTP threshold. According to the probabilistic sensitivity analyses, adding Ra-223 to the BSC for patients with bone mCRPC exceeds the current WTP threshold. Only if the WTP threshold is more than $85,824/QALY will the Ra-223 therapy have a greater than 50% chance of being cost-effective. Of note, in the Chinese mainland, there are 31 province-level administrative divisions, in which the per-capita GDP differs significantly. The per-capita GDP ranged from $6,421 (Gansu province) to $28,870 (Beijing city) in 2021 (National Bureau of Statistics of China, 2022). Obviously, the Ra-223 treatment would be a more cost-effective alternative if we used Beijing’s per-capita GDP as the recommended WTP threshold (3 × $28,870 = $86,610) because the probability of Ra-223 being cost-effective is more than 50% when the WTP threshold is higher than $86,610/QALY (Figure 3).
Our research represents, as far as we are aware, the first economic evaluation of Ra-223 from the Chinese health system perspective. We believe that the current findings can offer crucial information to Chinese decision makers, especially the National Healthcare Security Administration and the medical resource payers, as well as other payers of medical resources.
There is another published article that evaluated the cost effectiveness of Ra-223 on the basis of the ALSYMPCA trial, which was performed from the perspective of the Spanish national health system (Tirado Mercier et al., 2018). When compared to BSC treatment alone, Ra-223 in their study achieved an incremental QALYs of 0.35 and a cost of €9,631, and the ICUR was €27,606/QALY. The incremental cost and the ICUR were not comparable between that study and our study because various countries have distinct national characteristics. But our study’s incremental QALYs of 0.344 is comparable to the earlier finding (0.35). An economic evaluation, from the Dutch societal perspective, was conducted to investigate the cost effectiveness of Ra-223 compared with abiraterone, cabazitaxel, and enzalutamide, in patients with mCRPC previously treated with docetaxel (Peters et al., 2018). In that study, compared with cabazitaxel and abiraterone, Ra-223 resulted in €4,465 and €6,092 lower costs and 0.01 and 0.02 higher QALYs, separately, and compared with enzalutamide, Ra-223 achieved slightly lower QALYs (−0.06) and €7,390 lower costs. At the informal WTP threshold of €80,000/QALY, Ra-223 revealed a cost-effective chance with 64%, 61%, and 54%, compared with abiraterone, enzalutamide, and cabazitaxel, respectively. Therefore, they concluded that Ra-223 maybe a less costly strategy compared with abiraterone, cabazitaxel, and enzalutamide.
Some health technology assessment (HTA) reports assessed the cost effectiveness of Ra-223 for patients with mCRPC. The National Centre For Pharmacoeconomics (NCPE) calculated the ICUR of Ra-223 versus BSC with €79,948/QALY (National Centre for Pharmacoeconomics (NCPE), 2022). As such, Ra-223 was deemed not cost effective at a WTP threshold of €45,000/QALY. The NCPE also discussed a company evaluation of Ra-223 versus abiraterone from an indirect comparison analysis and inferred that the probability of cost effectiveness of Ra-223 versus abiraterone was 41% (National Centre for Pharmacoeconomics (NCPE), 2022). The National Institute for Health and Care Excellence (NICE) noted that the ICUR for Ra-223 versus BSC was likely above £50,000/QALY, which is above the normal WTP threshold of £20,000-£20,000 per QALY gained (National Institute for Health and Care Excellence (NICE), 2022). Consequently, the NICE concluded that Ra-223 could not be considered a cost-effective use of national health services (NHS) resources in England and Wales, Ra-223 is recommended only when a negotiated discounted price is reached. In Canada, the Canadian Agency for Drugs and Technologies in Health (CADTH) reviewed the economic evaluation of Ra-223 for patients with bone mCRPC and suggested that Ra-223 was not cost-effective (Canadian Agency for Drugs and Technologies in Health (CADTH), 2016).
Our study has a number of strengths. First, as mentioned above, our study was the first economic evaluation of Ra-223 in China, which can provide Chinese decision makers with crucial data. Second, a large multicentre phase III trial with 921 patients, including Chinese patients, served as the basis of the current study’s model analyses (Parker et al., 2013). The randomized controlled trial is deemed to be the most scientific method and provides the most rigorous evidence for determining the net benefit of a new drug or a therapy procedure. Third, in order to best depict Chinese conditions, the health resources we employed in the model were collected from Chinese published literature or estimated by local charges and adjusted by a physician associated with the clinical practice and the Chinese clinical guidelines. Last but not the least, to test the impact of each input parameter’s certainty on the outcome, the model’s input parameters were varied within the appropriate ranges and defined patterns of distribution. All uncertainty analyses revealed that the results we estimated were robust regardless of how the parameters varied.
There are some limitations in our study to be noted. First off, only grade 3/4 AEs with a percent greater than 3% were taken into account when estimating the AEs costs, which might result in uncertainty about the management costs of AEs. However, the economic evaluation focused on estimating ICUR between the two arms, and the incremental total cost was used to calculate the ICUR. Therefore, the AEs with lower risk would only have a modest impact on the final result. On the other hand, there would be less doubt if patients with grade 1/2 AEs rarely received extra treatment. Last, the findings of the one-way sensitivity analyses showed that the ICUR was not sensitive to the AEs cost and the relative incidence (Figure 2). As with any modeling study, there is an inherent limitation to utilizing the log-logistic function to extrapolate survival curves beyond the time horizon of the clinical trial. The usage of BSC as an additional active treatment is the last limitation. In China, abiraterone ought to be an alternative treatment for those with bone mCRPC. However, currently, there was not yet a clinical trial that directly compared the Ra-223 to abiraterone. We did not use the indirect comparison methods since they should take into account how comparable clinical trials are to one another and would undermine the validity of the current constructed model. We will update our results when suitable clinical data are available. Despite the limitations listed above, this study will nevertheless be helpful to physicians, payers, and decision makers in China as it is the first economic evaluation of Ra-223 from a Chinese perspective.
In conclusion, from the Chinese health system perspective, adding radium-223 to the best standard care is unlikely to be cost effective, in patients with bone metastatic castration-resistant prostate cancer at the current WTP threshold. However, in affluent regions with high per-capita GDP, radium-223 therapy may be cost effective.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
XZ, YW, and XM contributed to the study conception and design. Data collection, analysis, and interpretation were performed by QL and XZ. XZ and QL wrote the first draft of the manuscript. Critical revisions of the manuscript for important intellectual content were performed by all authors. All authors read and approved the final manuscript. YW and XM had final responsibility for the decision to submit for publication.
Funding
This work was funded by the Natural Science Foundation of Hunan Province [grant numbers 2021JJ80080 and 2020JJ4801] and Key Research and Development Program of Hunan Province [grant number 2019SK2252].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1003483/full#supplementary-material
References
Berthold, D. R., Pond, G. R., Soban, F., de Wit, R., Eisenberger, M., and Tannock, I. F. (2008). Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J. Clin. Oncol. 26 (2), 242–245. doi:10.1200/JCO.2007.12.4008
Canadian Agency for Drugs and Technologies in Health (CADTH). Radium-223 for patients with castration resistant prostate cancer with bone metastases: A review of clinical effectiveness, cost-effectiveness and guidelines. CADTH Rapid Response Reports. Ottawa (ON) 2016. Available at: https://www.ncbi.nlm.nih.gov/books/NBK401502/pdf/Bookshelf_NBK401502.pdf [Accessed 13 September, 2022].
Certified Public Accountant. Chinese pharmaceutical association. China guidelines for pharmacoeconomic evaluations (2020). Available at: https://www.cpa.org.cn/index.php?cid=75553&do=info [Accessed 25 July, 2022].
de Bono, J. S., Logothetis, C. J., Molina, A., Fizazi, K., North, S., Chu, L., et al. (2011). Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364 (21), 1995–2005. doi:10.1056/NEJMoa1014618
de Bono, J. S., Oudard, S., Ozguroglu, M., Hansen, S., Machiels, J. P., Kocak, I., et al. (2010). Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 376 (9747), 1147–1154. doi:10.1016/S0140-6736(10)61389-X
Dizdarevic, S., Petersen, P. M., Essler, M., Versari, A., Bourre, J. C., la Fougere, C., et al. (2019). Interim analysis of the REASSURE (Radium-223 alpha emitter agent in non-intervention safety study in mCRPC popUlation for long-teRm evaluation) study: Patient characteristics and safety according to prior use of chemotherapy in routine clinical practice. Eur. J. Nucl. Med. Mol. Imaging 46 (5), 1102–1110. doi:10.1007/s00259-019-4261-y
Finlay, I. G., Mason, M. D., and Shelley, M. (2005). Radioisotopes for the palliation of metastatic bone cancer: A systematic review. Lancet. Oncol. 6 (6), 392–400. doi:10.1016/S1470-2045(05)70206-0
Fizazi, K., Carducci, M., Smith, M., Damiao, R., Brown, J., Karsh, L., et al. (2011). Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 377 (9768), 813–822. doi:10.1016/S0140-6736(10)62344-6
Guidelines of Chinese Society of Clinical Oncology, . Prostate cancer (2020). Available at: https://njguloulib.yuntsg.com/ueditor/jsp/upload/file/20210608/1623115680743018719.pdf. [Accessed 25 July, 2022].
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced secondary analysis of survival data: Reconstructing the data from published kaplan-meier survival curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Hagiwara, M., Delea, T. E., Saville, M. W., and Chung, K. (2013). Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 16 (1), 23–27. doi:10.1038/pcan.2012.42
Hoskin, P., Sartor, O., O'Sullivan, J. M., Johannessen, D. C., Helle, S. I., Logue, J., et al. (2014). Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet. Oncol. 15 (12), 1397–1406. doi:10.1016/s1470-2045(14)70474-7
Hu, X., Qu, S., Yao, X., Li, C., Liu, Y., and Wang, J. (2019). Abiraterone acetate and docetaxel with androgen deprivation therapy in high-volume metastatic hormone-sensitive prostate cancer in China: An indirect treatment comparison and cost analysis. Cost. Eff. Resour. Alloc. 17, 27. doi:10.1186/s12962-019-0193-4
Kluetz, P. G., Pierce, W., Maher, V. E., Zhang, H., Tang, S., Song, P., et al. (2014). Radium Ra 223 dichloride injection: U.S. Food and drug administration drug approval summary. Clin. Cancer Res. 20 (1), 9–14. doi:10.1158/1078-0432.CCR-13-2665
Lange, P. H., and Vessella, R. L. (1998). Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 17 (4), 331–336. doi:10.1023/a:1006106209527
Lipton, A. (2010). Implications of bone metastases and the benefits of bone-targeted therapy. Semin. Oncol. 37 (2), S15–S29. doi:10.1053/j.seminoncol.2010.10.002
Nation Comprehensive Cancer Network. (2022) Data form: Prostate cancer, version 3.2022. Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf [Accessed 25 July, 2022].
National Bureau of Sts atisticof China (2022). National economy continued to recover with major indicators generally improving in august. Available at: http://www.stats.gov.cn/[Accessed 25 July, 2022].
National Centre for Pharmacoeconomics (NCPE) (2022). Cost-effectiveness of radium-223 (Xofigo®) for castration-resistant prostate with symptomatic bone metastases and no known visceral metastases. Available at: https://www.ncpe.ie/wp-content/uploads/2013/12/Xofigo-12122014_final.pdf [Accessed 13 September, 2022].
National Institute for Health and Care Excellence (NICE) (2022). Radium-223 dichloride for treating hormone-relapsed prostate cancer with bone metastases. Available at: https://www.nice.org.uk/guidance/ta412 [Accessed 13 September, 2022].
Parker, C., Castro, E., Fizazi, K., Heidenreich, A., Ost, P., Procopio, G., et al. (2020). Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 31 (9), 1119–1134. doi:10.1016/j.annonc.2020.06.011
Parker, C., Nilsson, S., Heinrich, D., Helle, S. I., O'Sullivan, J. M., Fossa, S. D., et al. (2013). Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369 (3), 213–223. doi:10.1056/NEJMoa1213755
Penson, D. F., Armstrong, A. J., Concepcion, R., Agarwal, N., Olsson, C., Karsh, L., et al. (2016). Enzalutamide versus bicalutamide in castration-resistant prostate cancer: The STRIVE trial. J. Clin. Oncol. 34 (18), 2098–2106. doi:10.1200/JCO.2015.64.9285
Peters, M. L., de Meijer, C., Wyndaele, D., Noordzij, W., Leliveld-Kors, A. M., van den Bosch, J., et al. (2018). Dutch economic value of radium-223 in metastatic castration-resistant prostate cancer. Appl. Health Econ. Health Policy 16 (1), 133–143. doi:10.1007/s40258-017-0350-x
Qin, X., Ye, D., Gu, C., Huang, Y., Gu, W., Dai, B., et al. (2021). Prostate cancer screening using prostate-specific antigen tests in a high-risk population in China: A cost-utility analysis. Curr. Ther. Res. Clin. Exp. 95, 100653. doi:10.1016/j.curtheres.2021.100653
Roodman, G. D. (2004). Mechanisms of bone metastasis. N. Engl. J. Med. 350 (16), 1655–1664. doi:10.1056/NEJMra030831
Sartor, O., Coleman, R., Nilsson, S., Heinrich, D., Helle, S. I., O'Sullivan, J. M., et al. (2014). Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet. Oncol. 15 (7), 738–746. doi:10.1016/S1470-2045(14)70183-4
Sartor, O., Goeckeler, W., and Bruland, O. (2011). Stromal targeted therapy in bone metastatic prostate cancer: Promise delivered. Asian J. Androl. 13 (6), 783–784. doi:10.1038/aja.2011.120
Saylor, P. J., Rumble, R. B., Tagawa, S., Eastham, J. A., Finelli, A., Reddy, P. S., et al. (2020). Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a cancer care ontario guideline. J. Clin. Oncol. 38 (15), 1736–1743. doi:10.1200/JCO.19.03148
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2022. Ca. Cancer J. Clin. 72 (1), 7–33. doi:10.3322/caac.21708
Smith, M., Parker, C., Saad, F., Miller, K., Tombal, B., Ng, Q. S., et al. (2019). Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 20 (3), 408–419. doi:10.1016/S1470-2045(18)30860-X
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
The People’s Bank of China (2022). Exchange rate. Available at: http://www.pbc.gov.cn [Accessed 25 July, 2022].
Tirado Mercier, E., Callejo Velasco, D., Rubio Cabezas, M., Moretones Agut, C., and Granell Villalon, M. (2018). Cost-effectiveness analysis of radium-223 dichloride in metastatic castration-resistant prostate cancer patients without previous chemotherapy treatment in Spain. J. Health Econ. Outcomes Res. 6 (1), 1–14. doi:10.36469/9777
yaozh (2022). The big data service platform for China’s health industry. Available at: https://www.yaozh.com/[Accessed 25 July, 2022].
Zeng, X., Karnon, J., Wang, S., Wu, B., Wan, X., and Peng, L. (2012). The cost of treating advanced non-small cell lung cancer: Estimates from the Chinese experience. PLoS One 7 (10), e48323. doi:10.1371/journal.pone.0048323
Keywords: cost effectiveness, radium-223, metastatic castration-resistant prostate cancer, QALY, China
Citation: Zeng X, Liu Q, Tan C, Wan X, Wang Y and Ma X (2022) Alpha emitter radium-223 in patients with metastatic castration-resistant prostate cancer: A cost-utility analysis. Front. Pharmacol. 13:1003483. doi: 10.3389/fphar.2022.1003483
Received: 26 July 2022; Accepted: 07 October 2022;
Published: 21 October 2022.
Edited by:
Lin Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Yanlei Zhang, Heor of Simcere, ChinaSergio Baldari, University of Messina, Italy
Liang Qu, University of Melbourne, Australia
Copyright © 2022 Zeng, Liu, Tan, Wan, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunhua Wang, d2FuZ3l1bmh1YTA4MDFAY3N1LmVkdS5jbg==; Xiaowei Ma, bWF4aWFvd2VpQGNzdS5lZHUuY24=
†These authors contributed equally to this work
 Xiaohui Zeng
Xiaohui Zeng Qiao Liu
Qiao Liu Chongqing Tan
Chongqing Tan Xiaomin Wan
Xiaomin Wan Yunhua Wang
Yunhua Wang Xiaowei Ma
Xiaowei Ma