
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 24 November 2022
Sec. Pharmacoepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1003370
This article is part of the Research Topic Optimizing Medicines for Healthy Ageing View all 6 articles
Objective: Findings among studies evaluating the effect of statin use and OA development in a 2020 meta-analysis of data from 11 observational studies of statin use and osteoarthritis (OA) revealed controversial results. We aimed to determine the associations between statin use and OA-related outcomes in an updated meta-analysis.
Methods: The protocol was registered with PROSPERO (CRD42020163983). A systematic literature retrieval was performed in the online databases, including PubMed, Cochrane Library, Embase, Web of Science, and Scopus, from inception to 1 June 2022, for clinical studies that compared the effects of statin users vs. nonusers on OA-related outcomes risks. Systematic reviews and meta-analyses were performed to estimate the correlations between statin use and OA-related outcomes. Tendency analysis was also used to estimate dose-response effects. The risk of bias was evaluated with the Newcastle–Ottawa scale.
Results: We included 23 studies involving more than 6,000,000 participants. Statin use was associated with increased OA risk (OR 1.099 [95%CI 1.002–1.206, p = 0.045]). Higher statin doses had higher OA risk (simvastatin equivalent daily of >40 mg). OA and related surgery risks were significantly reduced in statin users using antihypertensive drugs (AHDs). No significant differences were seen in other outcomes.
Conclusion: This meta-analysis inferred that statin use might be associated with increased OA development, especially at higher doses. The present study highlights the importance of recognizing potential OA risk in the population with long-term and/or high-dose statin use, especially in older populations. In addition, AHDs are associated with lower OA risk and fewer surgeries in hypertensive statin users. Due to limitations of heterogeneity and confounders, more rigorous studies are needed to define the correlations between statin use and OA-related outcomes.
Osteoarthritis (OA) is the most common degenerative joint disease, and the major cause of joint pain and disability. OA is increasingly recognized as worldwide health concern due to low quality of life and huge social and economic burden (Hunter and Bierma-Zeinstra, 2019; Katz Jeffrey et al., 2021). OA pathogenesis is complex and remains largely unclear. In the late stage of OA, surgeries are effective but have a limited long-term prognosis and prosthesis life (Evans et al., 2019). Therefore, early pharmacological intervention should be considered, but challenges remain due to limited analgesic outcomes and the occurrence of adverse events (Gregori et al., 2018). Metabolic factors like dyslipidemia were confirmed as being significantly associated with OA (Mobasheri et al., 2017; Choi et al., 2019; Song et al., 2021). The proinflammatory effects of lipids, adipokine-linked proinflammatory cytokines and pathways have been reported to be associated with OA pathogeneses (Gkretsi et al., 2011; Neumann et al., 2016). Therefore, lipid-related metabolisms and pathways could be attractive targets for OA management.
Statins are currently the most effective drugs used as lipid-lowering agents. In clinical practice, statins are commonly prescribed in cardiovascular diseases and dyslipidemia treatments, especially in older populations with multiple geriatric and metabolic diseases. In addition, statins have been shown to function as agents of anti-inflammation, offering cartilage protection (Ridker et al., 2005; Gkretsi et al., 2011). Hence, there is a growing interest in statins as potential disease-modifying agents for OA (Baker et al., 2011; Gkretsi et al., 2011). However, statin use is also reported to be linked to adverse musculoskeletal and metabolic effects, but this conclusion remains controversial (Mansi et al., 2013; Thompson et al., 2016).
A 2020 meta-analysis looking at the association between statin use and OA development and progression provided controversial results due to multiple limitations (Wang et al., 2020). Meanwhile, multiple OA-related outcomes, such as OA surgical risks, remain to be investigated. It is unclear if statin use is associated with OA-related outcomes, although some studies have reported inspiring outcomes (Clockaerts et al., 2012; Valdes et al., 2014; Michaëlsson et al., 2017; Haj-Mirzaian et al., 2019; Veronese et al., 2019). Therefore, the purpose of this study was to conduct a systematic review and meta-analysis to estimate the effects of statin use on OA-related outcomes.
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Liberati et al., 2009) and guidance from the Cochrane Collaboration (Higgins et al., 2019), and the protocol was registered in with PROSPERO (CRD42020163983). The PRISMA checklists and amendments from the primary version were available in Supplementary Data. A systematic online search was conducted using electronic databases, including PubMed, Cochrane Library, Embase, Web of Science, and Scopus, without language limitations, from inception to 1 June 2022. The online retrieval was performed with the terms: “osteoarthritis OR degenerative joint disease” and “statin.” The detailed search strategy is provided in Supplementary Data. The first search was performed on 1 January 2020 and updated on 1 June 2022.
Study selection was based on the PICOS statement. Population/patient: participants using statins with controls from the same population without statin medication prior to identification; intervention: statin use; comparison: statin users vs. nonusers on OA-related outcomes; outcome: data concerning OA-related outcomes, including statin-related OA risk; OA-related surgery; duration and dosage of statin use and OA risk; study design: clinical randomized/case-control/cohort studies. Non-clinical studies, studies with potential bias in participant populations, or studies without available data or conclusion (including reviews, protocols, etc.) were excluded. This part of the study was independently performed by two investigators in duplicate and double-checked by a third investigator. Discordant judgments were addressed by open discussion with the research team and resolved by consensus.
Two investigators independently performed the data extraction and quality assessments. From each included study, we extracted information such as demographic data, conclusions and clinical significance. If multiple studies were analyzed with potential overlapping participants, we retained studies with the most detailed methods, the most participants and the most adjusted confounding factors. If multiple non-overlapping data were found, the data were allowed and regarded as independent.
The risk of bias of included studies was assessed with the Newcastle–Ottawa scale (NOS) (Wells et al., 2000), which evaluates studies with >7 stars or those with greater than median stars to be of high quality with low risk for bias.
Data of statin users vs. nonusers on OA-related outcomes risks were calculated, including data concerning OA-related outcomes, including statin-related OA risk; OA-related surgery; duration and dosage of statin use and OA risk. OA diagnoses were defined by radiologic (Kellgren-Lawrence grade ≥2) or clinical evidence, and endpoint events were defined as OA-related outcome events. Propensity score-matched data were preferred for the entire analysis, then data of the largest number of confounders adjusted, followed by raw data. Ratios and eligible data were used. Hazard ratios (HRs), odds ratios (ORs) and relative ratios (RRs) in this study were used, deemed as homogenous and analyzed together.
When combination or transformation of study estimation values were needed, in the situations like multiple co-existing estimations obtained from the same reference control group, the method developed by Hamling’s study was used when participant numbers at every level were available (Hamling et al., 2008). Otherwise the method presented in Gao’s study was used (Gao et al., 2018). If transformations were not applicable, estimations using the most participants, the longest durations, or the highest doses were used. When two estimates were compared, and relative OR value and 95% CI were to be used, the method presented in Altman and Bland (2003) was used.
Meta-analyses were performed using the meta package for R software (version 3.6.3) (Balduzzi et al., 2019). Heterogeneity was identified by applying the DerSimonian–Laird method of the Q-test and was quantified using I2 values. Pooled data had low heterogeneity if p was greater than 0.1 and I2 was less than 50%. In this case, a fixed-effects model was used; otherwise, a random-effects model was used (Egger et al., 1997; Higgins et al., 2003). Statistical analyses were two-sided, and a p-value less than 0.05 was considered significant.
Sensitivity analysis were performed to reduce heterogeneity and detect additional potential correlations. Subgroup analyses were performed in the overall cohorts where significant heterogeneity was detected and were based on extracted data. Egger’s linear regression and Begg’s rank correlation tests were performed to evaluate potential publication bias for outcomes with more than 10 included studies (Begg and Mazumdar, 1994; Sterne et al., 2000; Mathur Maya and VanderWeele Tyler, 2020).
For data deemed ineligible for meta-analyses, we listed and re-analyzed all data without statistical syntheses; however, the strengths of this evidence were considered to be inferior to those of the meta-analyses.
The systematic literature search originally retrieved 1574 unique citations. A total of 23 studies (20 cohort studies and three case-control studies) met the eligibility criteria and were included in this study (Figure 1) (Beattie et al., 2005; Chodick et al., 2010; Chaganti et al., 2012; Clockaerts et al., 2012; Kadam et al., 2013; Mansi et al., 2013; Riddle et al., 2013; Cemeroglu et al., 2014; Valdes et al., 2014; Frey et al., 2017; Garcia-Gil et al., 2017; Michaëlsson et al., 2017; Roy et al., 2017; Burkard et al., 2018; Cheng et al., 2018; Eymard et al., 2018; Haj-Mirzaian et al., 2019; Jonsson et al., 2019; Veronese et al., 2019; Cook et al., 2020; Sarmanova et al., 2020; Milena et al., 2021; Perry et al., 2021). Most of these studies were from famous research cohorts or databases, like the Clinical Practice Research Datalink (CPRD) and the Osteoarthritis Initiative (OAI) comprising more than 6,000,000 participants. Detailed characteristics of the included studies and participants are shown in Table 1. Due to the very large amount of basic data, we listed the data sources and potentially duplicate data in Supplementary Table S1. Assessments of methodologic quality according to NOS are summarized in Supplementary Table S2, and most studies scored above 6 of 9.
Based on the extracted data and transformed data, statistical analyses were performed in several cohorts, including OA incident risk (risk cohort), OA-related surgery risk (surgery cohort), duration and dosage of statin use and OA incident risk (duration/dosage cohort), OA progression risk (progression risk) and antihypertension drug and statin co-use and OA incident/surgery risk (AHD cohort). The cohort outcomes and evidence analysis of non-meta-analyzed studies are displayed in Tables 2, 3.
Of the 23 included studies, 12 had estimated statin use and OA risk in hazard risk, odds ratio, and relative risk (HR/OR/RR) data and raw numbers. Statin use was detected as being associated with slightly higher OA risk in overall adjusted estimations (OR 1.099 [95% CI 1.002–1.206, p = 0.045]). Similar outcomes were seen in the raw data (OR 1.247 [95% CI 0.988–1.573, p = 0.063]) (Figures 2A,B). After non-meta-analyzed evidence was re-analyzed, no consistent conclusion was obtained about statin use and OA risk, but statin use may increase knee pain and function loss (Table 3).
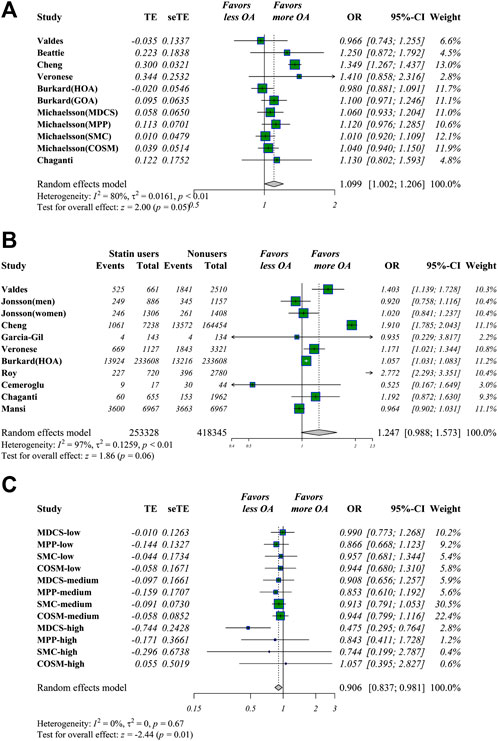
FIGURE 2. Meta-analysis of estimations of statin use and OA risk. Meta-analysis of overall adjusted estimations (A) and raw data (B) of statin users vs. nonusers for OA risk. Statin use was linked to significantly higher OA risk in adjusted estimations in adjusted OR values and raw data (C) Meta-analysis of potential impacts of antihypertensive drugs (AHDs) on hypertensive statin users. AHD use may be linked to decreased OA risk in hypertensive statin users.
For estimations concerning statin use and OA-related surgery, five candidate studies were selected where an OA-related surgery (total joint arthroplasty/revision/osteotomy) was defined as the endpoint event. Statin use was linked to a higher number of OA-related surgery in raw data (OR 1.216 [95% CI 1.190–1.242, p < 0.001]) but not in adjusted data.
Six studies examined OA progression. The relevant data were reported and analyzed in two ways: the follow-up endpoint vs. baseline (OR 1.034 [95% CI 0.798–1.341, p = 0.800]) and adjusted estimations (OR 0.867 [95% CI 0.557–1.349, p = 0.527]). No significant outcomes were detected in either analysis, indicating that statin use was not associated with OA progression or radiologic progression (Perry et al., 2021) (Table 3).
The links between statin use duration and OA risks were analyzed in nine studies. Significantly higher risks were only detected in cohorts of “former statin use” (OR 1.169 [95% CI 1.022–1.338, p = 0.023]), but the definition was not clear. To further estimate the continuous tendency of duration–risk correlations, Pearson’s correlation coefficients and fitting curves were calculated and visualized with the ggplot2 and ggpubr packages (Supplementary Figures S1A,B). Correlation coefficients of two measurements of tendency did not indicate a significant trend (R = −0.063, p = 0.89; R = 0.53, p = 0.28). Comparisons of the estimations were made every two adjacent durations, and meta-analyses of these ratios were performed. No significant outcomes were found, indicated that when other factors were consistent, statin use duration was not associated with OA risk.
The non-meta-analyzed data of two duration cohorts in Chodick et al. (2010) revealed that higher persistent statin use could decrease OA risk in the >1-year follow-up cohort when correlation coefficients were calculated and a significant negative correlation was observed (R = −0.91, p = 0.034). This negative correlation was not observed in the >5-year cohort (R = 0.51, p = 0.38). However, a higher risk of OA was seen in the >5-year cohort (OR 1.065 [95%CI 1.016–1.116, p < 0.001]), and meta-analyzed estimations in the same persistence of >5-year vs. >1-year was significantly increased (OR 1.204 [95% CI 1.143–1.270, p < 0.001]) (Table 3). No other significant outcome or tendency was found.
Dose analysis was tested in five studies. Based on the provided data and statin dose equivalent conversions (Weng et al., 2010), two subgroups were identified: 1) Statin users vs. nonusers, and 2) antihypertensive drugs (AHDs) with statin use vs. AHDs without statin use. For better visual and qualitative presentations regarding dose estimation correlations, the subgroups of these two groups were set as nonusers, low-dose (simvastatin daily <20 mg), low-medium (<40 mg), medium (40 mg), medium-high (40–80 mg), and high-dose (>80 mg). Medium-high (OR 1.079 [95% CI 1.012–1.151, p = 0.021]) and high doses (OR 1.383 [95% CI 1.054–1.815, p = 0.019]) of statin use were significantly associated with higher OA risks. A sudden increase in ORs was seen in the high-dose subgroup compared with the other dose groups, suggesting that a dose threshold could exist beyond which higher doses are associated with significantly higher OA risk. When 20 mg/d simvastatin equivalent was set as a threshold, overall >20 mg/d vs. <20 mg/d of statin use calculated by meta-analyzing the estimations of a higher dose vs. adjacent lower one, higher statin use was associated with increased OA risk (OR 1.108 [95% CI 1.004–1.222, p = 0.041]).
For the tendency analysis of dose-risk estimations from the low-dose to high-dose, positive trends that approached significance were detected (p = 0.8, R = 0.057) (Supplementary Figure S1C). However, based on the evidence obtained from meta-analyses of each adjacent higher dose vs. lower, higher statin doses slightly increased OA risk in the overall cohort (OR 1.039 [95% CI 1.000–1.080, p = 0.049]).
Cochran–Armitage tests for trends were performed using the DescTools package for non-meta-analyzed studies where binomial case and control numbers of every dose level were available. Two studies (Kadam et al., 2013; Cheng et al., 2018) indicated that OA risk was detected as being significantly reduced with higher statin dose, as the dose-HR correlation fell when the nonuser group was removed. Note, however, the nonuser group was not set as the reference in Cheng et al. (2018). If the nonuser group was transformed as the reference, statin use may significantly increase OA risk, regardless of dose level (Table 3). The evidence above indicates that, when other factors are consistent, higher statin doses may be linked to increased OA risk.
Only Michaëlsson et al. (2017) analyzed the association of AHDs and OA-related outcomes of statin users. The effect of AHDs was only analyzed in sensitivity analyses without an explanation of why it was listed as a potential confounder factor. In this four-center study, we set two groups as group-1 and group-2 in available data. Group 1 included the estimations of participants with both AHD and statin use vs. AHD users only; meanwhile, group 2 was defined as overall statin users vs. nonusers, regardless of AHD use. Using the method of Altman Altman and Bland (2003), we calculated the estimations of group 1 vs. group 2 by corresponding study cohort, then these calculated estimations were meta-analyzed and defined as EST-1 (data from Table 2 of Michaëlsson et al., 2017). EST-2 was defined as group 1 vs. group 2 above, and estimations of every dose were used (data from Table 4 of Michaëlsson et al., 2017). In these comparisons, AHDs were considered a potential intervention. AHDs were associated with significantly decreased OA risk and OA-related surgeries in both EST-1 (OA risk (OR 0.901 [95% CI 0.825–0.984, p = 0.020]), surgery (OR 0.912 [95% CI 0.833–0.999, p = 0.048])) and EST-2 (OA risk (OR 0.906 [95% CI 0.837–0.981, p = 0.015]), surgery (OR 0.882 [95% CI 0.783–0.994, p = 0.039])), but these outcomes may be only available for hypertensive statin users (Figure 2C). These outcomes may be explained as follows: because comparisons involved whether to administer AHDs, the risks of OA and related surgeries were lower in the AHD group because of significant blood pressure control compared with those in the overall group, as hypertension was determined to be linked to a higher likelihood of OA development. Because group 2 included both hypertensive participants with and without AHD use, the impact of AHDs in decreasing OA-related outcomes may be inferred as more significant when the blood pressure is well-controlled in hypertensive statin users than when it is not well controlled. However, the existence of interactions between AHDs and statins remains unclear and needs further investigation.
All cohorts were subjected to heterogeneity testing, but significant heterogeneity was only detected in the raw data of the risk analyses. Subgroup analyses were only conducted based on both adjusted estimations and raw data of the OA-risk analyses, and outcomes were preferred when obtained from multiple studies. Statin use was detected as being significantly increased in a Caucasian population, in cohort study design, in longer follow-up durations and in small-joint OA like hand OA, suggesting that these situations of statin use need further consideration. No additional significant outcomes were detected, and the outcomes of the subgroup analysis are listed in Supplementary Table S3.
Sensitivity analyses were performed with no eliminations that could have been caused by multiple and heterogeneous methodologies. Meta-regression was thus performed for raw data analysis. The overall variables accounted for all heterogeneity (97.40%), and among those variables, race, lesion, follow-up periods and NOS scores accounted for 54.45%, 38.75%, 43.41% and 18.06%, respectively. Publication bias tests were only conducted in risk analysis, and bias was not found in Egger’s or Begg’s tests for any cohort.
Beyond using statins as first-line medications to treat atherosclerotic cardiovascular events and dyslipidemia, the anti-inflammatory and cartilage-protective effects have been reported by numerous studies. Our meta-analysis comprehensively investigates whether statin use leads to OA-related outcomes, and suggest that statin use may be associated with OA-related outcomes, especially at higher doses. In addition, AHDs significantly decrease OA and related surgeries risks in hypertensive statin users.
In the previous meta-analysis (Wang et al., 2020), methodologic confusion led to controversial conclusions regarding statin use and OA development. First, the retrieval and use of the literature did not follow PRISMA guidelines. For instance, a study reported as a letter by Valdes et al. (2014) was included, but letter type was excluded during their literature retrieval. More eligible studies were included in our study compared with that study. Secondly, inappropriate data uses and transformations were also present in that study; the estimation transformation method was not appropriate and had no basis for some estimations with the same baseline reference, and some data regarding other OA types were ignored. Lastly, duplicate data were potentially used. Thus, previous meta-analysis was deemed controversial, and we attempted to overcome these issues in this meta-analysis.
The overall outcomes indicated that statin use may slightly increase the risk of OA, which was inconsistent with the previous meta-analysis. Statins have been reported to protect cartilage, and views are presented. First, cholesterol and fat were shown to be positively correlated with OA severity (Ali et al., 2016). Statins decrease circulating and intra-chondrocyte cholesterol and fats, downregulate and inhibit adipo-related inflammatory cytokines, and upregulate cartilage-protective factors. Second, it has been reported that vascular pathologies, including arteriosclerosis, could contribute to OA (Liu et al., 2019). The anti-arteriosclerosis function of statins could, therefore, be an attractive therapeutic target for OA. Third, it is believed that metabolic syndrome (MetS) is closely correlated with OA, and statins are known to reduce inflammatory cytokines in MetS and related diseases (Tabrizi et al., 2019). Fourth, statins have the effect of preventing inflammatory cell infiltration (Akasaki et al., 2009), as they are anti-oxidative and anti-inflammatory, thus alleviating pain and cartilage degeneration, blocking the further progression of symptoms.
As statins are widely and long-termly used in older people, their potential contributions to musculoskeletal disorders deserve considerable attention. Some studies reported that statin use was linked to musculoskeletal conditions, including arthropathies (Mansi et al., 2013). Statins have also been reported linked to commonly occurring myopathies with statin-associated muscle symptoms (SAMS) (Bouitbir et al., 2020). Thus, statin-related adverse effects, such aspotential weakening of musculoskeletal mechanical properties (Eliasson et al., 2019) and some metabolic interferences like diabetes mellitus (DM) may lead to OA. Based on this evidence and our outcomes, we hypothesized that statins increase OA-related outcome risks through musculoskeletal and metabolic interference pathways. Musculoskeletal disorders and associated symptoms induced by statins could lead to greater mechanical loads and reduced physical activities. However, whether statin use leads to decreased muscle and tendon function has remained controversial. In the subgroup analysis, more significant likelihood was found in hand OA than large-joint OA, and flexible joints were more likely to be interfered with by statins, which inferred that apart from mechanical loads, extensive musculoskeletal metabolic disturbance may exist due to statin use. Another potential mechanism is that a high percentage of older people with multiple comorbidities are included. They are more likely to develop MetS and sarcopenia, and it is possible that beneficial effects of statins are limited. Thus, detailed comorbidities of participants’ baseline conditions are needed to confirm or reject the presence of MetS or sacropenia. However, it is still important to highlight that attention should be paid to potential OA risks in long-term statin users, especially older people.
For statin use and OA-related outcomes, people taking higher statin doses are more likely to develop OA. High-dose statin use may increase SAMS development, and simvastatin >40 mg/d equivalents was associated with higher osteoporosis and diabetes risks (Swerdlow et al., 2015; Leutner et al., 2019). The statin use durations were detected as not associated with higher OA risk, but short-term statin use was reported to lead to musculoskeletal diseases, including OA, in subgroup analysis (Mansi et al., 2016). AHDs were detected as associated with lower OA and related surgeries risks in statin users, but this may not indicate that there must be interactions between AHDs and statins. Michaëlsson et al. (2017) took AHDs as the basis for the sensitivity analysis in their study, but the reason was not mentioned. AHDs were analyzed due to considerable data available for researching potential correlations. Hypertension was reported to be closely linked to OA, but the beneficial effects of AHDs in decreasing OA development risks and potential interactions with statins were inconclusive. Thus, these outcomes require additional studies for confirmation.
This study was performed following recommendations for rigorous meta-analyses. A major strength was the inclusion of large sample size comprising multiple population-based studies performed under rigorous research conditions or from databases based on credible records. This process provided an advantage by eliminating biases. Therefore, we feel that our meta-analysis outcomes are robust and valuable for further investigations, especially in patients with MetS. In our study, multiple statin use analyses were performed using major parameters, such as duration, doses, and multiple OA-related outcomes. We also used tendency analyses for dynamic estimations.
Although several challenges were encountered and resolved, there were also several significant limitations. First, as a limitation of drug-related meta-analysis of multiple retrospective large-sample studies, it is premature to arrive at an absolute causality. Heterogeneous baseline conditions, and confounding factors and methodologies were present, and heterogeneity could not be completely eliminated. Therefore, the conclusion was limited and challenged by inconsistent quality and multiple confounders in the included studies, let alone the lack of a rigorous causal relationship. Second, some important definitions of statin use were lacking or controversial. For example, statin prescriptions did not necessarily qualify as use or even regular daily use, and no consistent definition of statin use was present. However, as OA was a long-term progressive degenerative disease, logically and theoretically, it may be inferred that OA progress, incident and surgery risk are significantly elevated with long-duration and/or high-dose use when statins were deemed associated with OA. Multi-dimensional analysis was only performed in one included study that used total statin dosage taken within a certain duration (Cheng et al., 2018). Statin use duration and dose cannot be investigated simultaneously with the present available data. Third, the included studies should have had more detailed data, like dyslipidemia conditions, body mass index (BMI) and work intensity of participants, and data needed for transformation. Especially subgroup analysis of various types of statins could not be conducted due to lack of detailed information about the types of statins taken by the patients. In addition, the limited number of studies resulted in limited power for estimations in some analyses. We hope to eliminate heterogeneity and potential bias by including studies with confounding factors adjusted and with large number of participants. We hope that future rigorous studies could help to analyze each parameter of statin use and OA-related outcomes in more detail.
In this meta-analysis based on multiple population-based studies, the statistical outcomes inferred that statin use might be associated with increased OA development risk, especially at higher doses. These findings do not support the argument that statins alleviate OA risks and highlighted the importance of recognizing potential OA risk in the population with long-term and/or high-dose statin use, especially in an older population. In addition, AHDs reduced OA incident risk and OA-related surgeries in hypertensive statin users. However, because the methodologies and parameters of the included studies were various and heterogenous, more rigorous, multi-dimension studies with greater numbers of participants are needed to confirm our conclusions.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
ZZ and XL developed the idea for the present study; ZZ and XM conducted the search strategy and literature screening, which was checked by CD, and discrepancies were settled with discussions among the research group; ZZ and CD performed data extraction and methodologic quality assessments, and discrepancies were settled with discussions among the research group and resolved by consensus. At least two authors accessed and verified the data. ZZ and XM performed data analysis and underlying data verification again. ZZ and CD performed the statistical analysis; FZ, QW and XL critically checked the methods and results. ZZ wrote the draft manuscript; comprehensive revisions were made by all authors. XL (the corresponding author) attests that all authors who meet the authorship criteria are listed. All authors confirm that they have full access to all the data in the study and approve and accept responsibility for submitting the present version of the manuscript for publication.
This study was funded by the Joint Program of Key Research and Development of Liaoning Province of China (No. 2020JH2/10300138) and the Natural Science Foundation of Liaoning Province (No. 20180530044).
We want to acknowledge Shengjing Hospital, the doctors and students, and all of the participants who contributed to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1003370/full#supplementary-material
Supplementary Figure S1 | Correlation analysis of statin use duration and dosage and OA risk. (A,B) display two kinds of duration descriptions of statin use. The horizontal coordinate indicates statin use duration, and the ordinate coordinate indicates the OR values and 95% CIs of statin users vs. nonusers. Statin use dose-OA risk correlation analysis of (C) overall statin users and (D) statin users with antihypertensive drug use. The horizontal coordinate here indicates statin use dosage. No significant correlations and tendencies were detected.
Akasaki, Y., Matsuda, S., Nakayama, K., Fukagawa, S., Miura, H., and Iwamoto, Y. (2009). Mevastatin reduces cartilage degradation in rabbit experimental osteoarthritis through inhibition of synovial inflammation. Osteoarthr. Cartil. 17 (2), 235–243. doi:10.1016/j.joca.2008.06.012
Ali, S. A., Al-Jazrawe, M., Ma, H., Whetstone, H., Poon, R., Farr, S., et al. (2016). Regulation of cholesterol homeostasis by hedgehog signaling in osteoarthritic cartilage. Arthritis Rheumatol. 68 (1), 127–137. doi:10.1002/art.39337
Altman, D. G., and Bland, J. M. (2003). Interaction revisited: The difference between two estimates. BMJ 326 (7382), 219. doi:10.1136/bmj.326.7382.219
Baker, J. F., Walsh, P., and Mulhall, K. J. (2011). Statins: A potential role in the management of osteoarthritis? Jt. Bone Spine 78 (1), 31–34. doi:10.1016/j.jbspin.2010.02.035
Balduzzi, S., Rücker, G., and Schwarzer, G. (2019). How to perform a meta-analysis with R: A practical tutorial. Evid. Based. Ment. Health 22 (4), 153–160. doi:10.1136/ebmental-2019-300117
Beattie, M. S., Lane, N. E., Hung, Y. Y., and Nevitt, M. C. (2005). Association of statin use and development and progression of hip osteoarthritis in elderly women. J. Rheumatol. 32 (1), 106–110. doi:10.1300/J094v13n02_11
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50 (4), 1088–1101. doi:10.2307/2533446
Bouitbir, J., Sanvee, G. M., Panajatovic, M. V., Singh, F., and Krahenbuhl, S. (2020). Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacol. Res. 154, 104201. doi:10.1016/j.phrs.2019.03.010
Burkard, T., Hügle, T., Layton, J. B., Glynn, R. J., Bloechliger, M., Frey, N., et al. (2018). Risk of incident osteoarthritis of the hand in statin initiators: A sequential cohort study. Arthritis Care Res. Hob. 70 (12), 1795–1805. doi:10.1002/acr.23616
Cemeroglu, O., Aydın, H. I., Yasar, Z. S., Bozduman, F., Saglam, M., Selcoki, Y., et al. (2014). Hand and heart, hand in hand: Is radiological hand osteoarthritis associated with atherosclerosis? Int. J. Rheum. Dis. 17 (3), 299–303. doi:10.1111/1756-185x.12251
Chaganti, R. K., Tolstykh, I., Lane, N. E., McCullough, C., Javaid, M., Driban, J., et al. (2012). The association of statin use and incident radiographic knee osteoarthritis. Osteoarthr. Cartil. 20 (1), S154. doi:10.1016/j.joca.2012.02.227
Cheng, Y. Y., Kao, C. L., Lin, S. Y., Chang, S. T., Wei, T. S., Chang, S. N., et al. (2018). Effect of an increased dosage of statins on spinal degenerative joint disease: A retrospective cohort study. BMJ Open 8 (2), e017442. doi:10.1136/bmjopen-2017-017442
Chodick, G., Amital, H., Shalem, Y., Kokia, E., Heymann, A. D., Porath, A., et al. (2010). Persistence with statins and onset of rheumatoid arthritis: A population-based cohort study. PLoS Med. 7 (9), e1000336. doi:10.1371/journal.pmed.1000336
Choi, W. S., Lee, G., Song, W. H., Koh, J. T., Yang, J., Kwak, J. S., et al. (2019). The CH25H-CYP7B1-RORα axis of cholesterol metabolism regulates osteoarthritis. Nature 566 (7743), 254–258. doi:10.1038/s41586-019-0920-1
Clockaerts, S., Van Osch, G. J., Bastiaansen-Jenniskens, Y. M., Verhaar, J. A. N., Van GlabbeekF., , Van Meurs, J. B., et al. (2012). Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann. Rheum. Dis. 71 (5), 642–647. doi:10.1136/annrheumdis-2011-200092
Cook, M. J., Sorial, A. K., Lunt, M., Board, T. N., and O'Neill, T. W. (2020). Effect of timing and duration of statin exposure on risk of hip or knee revision arthroplasty: A population-based cohort study. J. Rheumatol. 47 (3), 441–448. doi:10.3899/jrheum.180574
Egger, M., Smith, G. D., and Phillips, A. N. (1997). Meta-analysis: Principles and procedures. BMJ 315 (7121), 1533–1537. doi:10.1136/bmj.315.7121.1533
Eliasson, P., Dietrich-Zagonel, F., Lundin, A. C., Aspenberg, P., Wolk, A., and Michaelsson, K. (2019). Statin treatment increases the clinical risk of tendinopathy through matrix metalloproteinase release - a cohort study design combined with an experimental study. Sci. Rep. 9 (1), 17958. doi:10.1038/s41598-019-53238-7
Evans, J. T., Walker, R. W., Evans, J. P., Blom, A. W., Sayers, A., and Whitehouse, M. R. (2019). How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 393 (10172), 655–663. doi:10.1016/S0140-6736(18)32531-5
Eymard, F., Parsons, C., Edwards, M. H., Petit-Dop, F., Reginster, J. Y., Bruyere, O., et al. (2018). Statin use and knee osteoarthritis progression: Results from a post-hoc analysis of the SEKOIA trial. Jt. Bone Spine 85 (5), 609–614. doi:10.1016/j.jbspin.2017.09.014
Frey, N., Hügle, T., Jick, S. S., Meier, C. R., and Spoendlin, J. (2017). Hyperlipidaemia and incident osteoarthritis of the hand: A population-based case-control study. Osteoarthr. Cartil. 25 (7), 1040–1045. doi:10.1016/j.joca.2017.01.014
Gao, S. Y., Wu, Q. J., Sun, C., Zhang, T. N., Shen, Z. Q., Liu, C. X., et al. (2018). Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: A systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med. 16 (1), 205. doi:10.1186/s12916-018-1193-5
Garcia-Gil, M., Reyes, C., Ramos, R., Sanchez-Santos, M. T., Prieto-Alhambra, D., Spector, T. D., et al. (2017). Serum lipid levels and risk of hand osteoarthritis: The chingford prospective cohort study. Sci. Rep. 7 (1), 3147. doi:10.1038/s41598-017-03317-4
Gkretsi, V., Simopoulou, T., and Tsezou, A. (2011). Lipid metabolism and osteoarthritis: Lessons from atherosclerosis. Prog. Lipid Res. 50 (2), 133–140. doi:10.1016/j.plipres.2010.11.001
Gregori, D., Giacovelli, G., Minto, C., Barbetta, B., Gualtieri, F., Azzolina, D., et al. (2018). Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: A systematic review and meta-analysis. JAMA 320 (24), 2564–2579. doi:10.1001/jama.2018.19319
Haj-Mirzaian, A., Bahram, M., Ali, G., Conaghan, P. G., Lima, J. A. C., Blaha, M. J., et al. (2019). Statin use and knee osteoarthritis outcome measures according to the presence of heberden nodes: Results from the osteoarthritis initiative. Radiology 293, 396–404. doi:10.1148/radiol.2019190557
Hamling, J., Lee, P., Weitkunat, R., and Ambuhl, M. (2008). Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat. Med. 27 (7), 954–970. doi:10.1002/sim.3013
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P. T., Thomas, J., and Chandler, J., (2019). Cochrane handbook for systematic reviews of interventions version 6.0 (updated july 2019). London, United Kingdom: John Wiley & Sons.
Hunter, D. J., and Bierma-Zeinstra, S. (2019). Osteoarthritis. Lancet 393 (10182), 1745–1759. doi:10.1016/S0140-6736(19)30417-9
Jonsson, H., Fisher, D. E., Eiriksdottir, G., Aspelund, T., Klein, R., Gudnason, V., et al. (2019). Hand and knee osteoarthritis are associated with reduced diameters in retinal vessels: The AGES-reykjavik study. Rheumatol. Int. 39 (4), 669–677. doi:10.1007/s00296-019-04243-6
Kadam, U. T., Blagojevic, M., and Belcher, J. (2013). Statin use and clinical osteoarthritis in the general population: A longitudinal study. J. Gen. Intern. Med. 28 (7), 943–949. doi:10.1007/s11606-013-2382-8
Katz Jeffrey, N., Arant Kaetlyn, R., and Loeser Richard, F. (2021). Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA 325, 568–578. doi:10.1001/jama.2020.22171
Leutner, M., Matzhold, C., Bellach, L., Deischinger, C., Harreiter, J., Thurner, S., et al. (2019). Diagnosis of osteoporosis in statin-treated patients is dose-dependent. Ann. Rheum. Dis. 78 (12), 1706–1711. doi:10.1136/annrheumdis-2019-215714
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Liu, W., Balu, N., Canton, G., Hippe, D. S., Watase, H., Waterton, J. C., et al. (2019). Understanding atherosclerosis through an osteoarthritis data set. Arterioscler. Thromb. Vasc. Biol. 39 (6), 1018–1025. doi:10.1161/ATVBAHA.119.312513
Mansi, I., Frei, C. R., Pugh, M. J., Makris, U., and Mortensen, E. M. (2013). Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern. Med. 173 (14), 1–10. doi:10.1001/jamainternmed.2013.6184
Mansi, I. A., English, J., Zhang, S., Mortensen, E. M., and Halm, E. A. (2016). Long-term outcomes of short-term statin use in healthy adults: A retrospective cohort study. Drug Saf. 39 (6), 543–559. doi:10.1007/s40264-016-0412-2
Mathur Maya, B., and VanderWeele Tyler, J. (2020). Sensitivity analysis for unmeasured confounding in meta-analyses. J. Am. Stat. Assoc. 115, 163–172. doi:10.1080/01621459.2018.1529598
Michaëlsson, K., Lohmander, L. S., Turkiewicz, A., Wolk, A., and EnglundM., (2017). Association between statin use and consultation or surgery for osteoarthritis of the hip or knee: A pooled analysis of four cohort studies. Osteoarthr. Cartil. 25 (11), 1804–1813. doi:10.1016/j.joca.2017.07.013
Milena, S., Harmer, A. R., Agaliotis, M., Nairn, L., Bridgett, L., March, L., et al. (2021). Clinical risk factors associated with radiographic osteoarthritis progression among people with knee pain: A longitudinal study. Arthritis Res. Ther. 23, 160. doi:10.21203/rs.3.rs-67862/v1
Mobasheri, A., Rayman, M. P., Gualillo, O., Sellam, J., van der Kraan, P., and Fearon, U. (2017). The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 13 (5), 302–311. doi:10.1038/nrrheum.2017.50
Neumann, E., Junker, S., Schett, G., Frommer, K., and Muller-Ladner, U. (2016). Adipokines in bone disease. Nat. Rev. Rheumatol. 12 (5), 296–302. doi:10.1038/nrrheum.2016.49
Perry, T. A., Wang, X., Nevitt, M., Abdelshaheed, C., Arden, N., and Hunter, D. J. (2021). Association between current medication use and progression of radiographic knee osteoarthritis: Data from the osteoarthritis initiative. Rheumatol. Oxf. Engl. 60, 4624–4632. doi:10.1093/rheumatology/keab059
Riddle, D. L., Moxley, G., and Dumenci, L. (2013). Associations between statin use and changes in pain, function and structural progression: A longitudinal study of persons with knee osteoarthritis. Ann. Rheum. Dis. 72 (2), 196–203. doi:10.1136/annrheumdis-2012-202159
Ridker, P. M., Cannon, C. P., Morrow, D., Rifai, N., Rose, L. M., McCabe, C. H., et al. (2005). C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352 (1), 20–28. doi:10.1056/NEJMoa042378
Roy, S., Weinstock, J. L., Ishino, A. S., Benites, J. F., Pop, S. R., Perez, C. D., et al. (2017). Association of cognitive impairment in patients on 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors. J. Clin. Med. Res. 9 (7), 638–649. doi:10.14740/jocmr3066w
Sarmanova, A., Doherty, M., Kuo, C., Wei, J., Abhishek, A., Mallen, C., et al. (2020). Statin use and risk of joint replacement due to osteoarthritis and rheumatoid arthritis: A propensity-score matched longitudinal cohort study. Rheumatol. Oxf. 59 (10), 2898–2907. doi:10.1093/rheumatology/keaa044
Song, Y., Liu, J., Zhao, K., and Gao, L. (2021). Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 33, 1911–1925. doi:10.1016/j.cmet.2021.09.001
Sterne, J. A., Gavaghan, D., and Egger, M. (2000). Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 53 (11), 1119–1129. doi:10.1016/S0895-4356(00)00242-0
Swerdlow, D. I., Preiss, D., Kuchenbaecker, K. B., Holmes, M. V., Engmann, J. E. L., Shah, T., et al. (2015). HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: Evidence from genetic analysis and randomised trials. Lancet 385 (9965), 351–361. doi:10.1016/S0140-6736(14)61183-1
Tabrizi, R., Tamtaji, O. R., Mirhosseini, N., Lankarani, K. B., Akbari, M., Dadgostar, E., et al. (2019). The effects of statin use on inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 141, 85–103. doi:10.1016/j.phrs.2018.12.010
Thompson, P. D., Panza, G., Zaleski, A., and Taylor, B. (2016). Statin-associated side effects. J. Am. Coll. Cardiol. 67 (20), 2395–2410. doi:10.1016/j.jacc.2016.02.071
Valdes, A. M., Zhang, W., Muir, K., Maciewicz, R. A., Doherty, S., and DohertyM., (2014). Use of statins is associated with a lower prevalence of generalised osteoarthritis. Ann. Rheum. Dis. 73 (5), 943–945. doi:10.1136/annrheumdis-2013-204382
Veronese, N., Koyanagi, A., Stubbs, B., Cooper, C., Guglielmi, G., Rizzoli, R., et al. (2019). Statin use and knee osteoarthritis outcomes: A longitudinal cohort study. Arthritis Care Res. Hob. 71 (8), 1052–1058. doi:10.1002/acr.23735
Wang, J., Dong, J., Yang, J., Wang, Y., and Liu, J. (2020). Association between statin use and incidence or progression of osteoarthritis: meta-analysis of observational studies. Osteoarthr. Cartil. 28 (9), 1170–1179. doi:10.1016/j.joca.2020.04.007
Wells, G. A., Shea, B., and O’Connell, D., The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2000, Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed March 1, 2022).
Keywords: statin, osteoarthritis, meta-analysis, risk, antihypertensive drugs
Citation: Zhang Z, Deng C, Ma X, Wu Q, Zhou F and Liu X (2022) The association between statin use and osteoarthritis-related outcomes: An updated systematic review and meta-analysis. Front. Pharmacol. 13:1003370. doi: 10.3389/fphar.2022.1003370
Received: 26 July 2022; Accepted: 25 October 2022;
Published: 24 November 2022.
Edited by:
Amy Page, University of Western Australia, AustraliaReviewed by:
Joshua Lee, Victoria University, Australia, AustraliaCopyright © 2022 Zhang, Deng, Ma, Wu, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueyong Liu, bGl1c2poQHNqLWhvc3BpdGFsLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.