- 1Evidence Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
- 2Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province, Lanzhou, China
- 3Department of Pharmacy, Peking University Third Hospital, Beijing, China
- 4Institute for Drug Evaluation, Peking University Health Science Center, Beijing, China
- 5Department of Pediatrics, Peking University Third Hospital, Beijing, China
Background: Dosing strategies of β-lactams and vancomycin should be optimized according to pharmacokinetic/pharmacodynamic principles. However, there is no available data indicating the implementation of extended infusion (EI) or continuous infusion (CI) administration in the management of neonatal sepsis.
Methods: A nationwide cross-sectional survey was conducted and the pediatricians from 31 provinces in China were enrolled. A multidisciplinary team created the questionnaire, which had three sections and a total of 21 questions with open- and closed-ended responses. The survey was then conducted using an internet platform in an anonymous way. The data was eventually gathered, compiled, and examined. To identify the risk factors associated with the implementation of EI/CI, logistic regression was carried out.
Results: A total of 1501 respondents answered the questionnaires. The implementation of EI/CI of β-lactams and vancomycin were only available to one-third of the respondents, and the prolonged strategy was primarily supported by guidelines (71.25%) and advice from medical specialists (55.18%). A significant fraction (72.94%–94.71%) lacked a strong understanding of the infusions’ stability. Additionally, it was discovered that more frequent MDT discussions about antibiotic use and the appropriate time pediatricians worked in the neonatal ward were associated with an increase in the use of the EI/CI strategy.
Conclusion: The EI/CI strategy in neonatal sepsis was not well recognized in China, and it is necessary to establish a solid MDT team with regularly collaborates. In the near future, guidelines regarding prolonged infusion management in neonatal sepsis should be developed.
Introduction
The management of neonatal and pediatric sepsis remains a significant and challenging public burdensome health issue on a worldwide scale (Ruth et al., 2014; Weiss et al., 2015; Shane et al., 2017). Globally pooled data revealed that the case fatality rate of pediatric sepsis was approximately 31.7% in developing countries and 19.3% in developed countries (Tan et al., 2019), and the mortality of neonatal sepsis ranged from 11% to 19% (Fleischmann-Struzek et al., 2018). Nevertheless, current guidelines and opinions recommended that dosing strategies of antibiotics should be optimized according to pharmacokinetic/pharmacodynamic (PK/PD) principles (Rhodes et al., 2017; Weiss et al., 2020) and encouraged to provide individualized antimicrobial dosing approaches for special populations whenever possible (Hartman et al., 2020; Tu et al., 2021; Cao et al., 2022) given the scarcity of antibiotic agents in the pediatric population and the rise in antibiotic resistance.
In neonatal sepsis, the choice of antibiotic is complex and should be based on clinical syndrome, underlying disease, drug intolerances, and local pathogen susceptibility (Plunkett and Tong, 2015). β-lactams and vancomycin are two of the most frequently utilized antimicrobials due to their broad range of activity and broad therapeutic index (Plunkett and Tong, 2015; Phe et al., 2020; Burgunder et al., 2022). β-lactams, the cornerstone of the empirical antibacterial therapy in neonatal sepsis owing to their rare toxicity with only a few exceptions (Muller et al., 2018), mainly include three categories: penicillins (e.g., amoxicillin, ampicillin, and oxacillin, et al.), cephalosporins (e.g., cefazolin, cefuroxime, and ceftriaxone et al.) and carbapenems (e.g., meropenem, imipenem-cistatin, and ertapenem, et al.). Vancomycin, one of the most frequently prescribed glycopeptides in neonatal infections, is recommended to cover vascular catheter-associated coagulase-negative staphylococci and Meticillin resistant Staphylococcus aureus(Jacqz-Aigrain et al., 2015; Minotti et al., 2021).
Generally, PK refers to the study of concentration changes of a drug over a given time period, and PD describes the relationship between PK exposure and pharmacological effect (Abdul-Aziz et al., 2015). As time-dependent antibiotics, extended infusion (EI) or continuous infusion (CI) administration of β-lactams could theoretically lead to improved PK/PD profiles when the concentration remains above the minimum inhibitory concentration (MIC) of the causative pathogen (fT > MIC) for a longer duration (Rizk et al., 2017; Veiga and Paiva, 2018). Vancomycin could also theoretically better meet PD targets when provided as prolonged administration (DiMondi and Rafferty, 2013; Gwee et al., 2014; Alonso-Moreno et al., 2021). Although EI/CI administration for critically ill patients has been suggested by clinical practice guidelines (Bretonnière et al., 2015; Egi et al., 2021; Evans et al., 2021), there lacks convincing evidence demonstrating its superiority compared with a short-term infusion or bolus dosing in children (Zhou et al., 2021), and pediatricians seem to be not knowledgeable of it or to apply it frequently in clinical practice. There are currently no data available demonstrating the practical use of EI/CI approaches in the management of neonatal sepsis in China.
This study is a subset of a national survey titled “Current status of drug optimization for the application of β-lactams antibiotics and vancomycin in the treatment of neonatal sepsis in China” (OBTAINS study). We aimed to conduct a cross-sectional survey of pediatricians in China to characterize the current implementation of EI/CI-lactams or vancomycin in neonatal sepsis and discover its potential factors.
Methods
Study design and participants
A nationwide cross-sectional survey was conducted to assess the clinical administration of EI/CI of β-lactams or vancomycin in neonatal sepsis in China using an online questionnaire. The target population was pediatricians who had experience in neonatal sepsis management, regardless of their pediatric secondary specialty in both private and public hospitals in China. A convenient sampling approach was applied to enroll participants throughout 31 Chinese provinces from April 22 to June 3, 2022. The participants received invitations to answer the questions through the link to the questionnaire platform via social media (WeChat group) or E-mail. Weekly reminders were sent as needed. Participation was anonymous and unpaid.
The study was performed in accordance with the Declaration of Helsinki and approved by the Peking University Third Hospital Medical Science Research Ethics Committee (M2022245).
Questionnaire development
The questionnaire, which is a component of the OBTAINS study, consists of three sections, comprising a total of 21 questions with open- and closed-ended answers. The purpose of first section (7 questions) aimed to collect the demographic information of the respondents. Their clinical practice experience in neonatal wards was investigated in the second section (4 questions). Next, if the respondents had experience in EI/CI of β-lactams (penicillins: benzylpenicillin, amoxicillin, ampicillin, oxacillin, cloxacillin, amoxicillin-clavulanate, ampicillin-sulbactam, and piperacillin-tazobactam; cephalosporins: cefazolin, cefuroxime, ceftriaxone, cefotaxime, ceftizoxime, ceftazidime, ceftazidime-avibactam, cefoperazone-sulbactam, cefepime, and ceftaroline); latamoxef; and carbapenems: meropenem, imipenem-cistatin, biapenem, and ertapenem) and vancomycin in the management of neonatal sepsis, eight multiple-choice questions and two single-choice questions were required to be further answered. This section mainly reflected their experience with specific drugs with EI/CI administration, the method of obtaining this optimization method, the timing and reasons, the infusion time, the knowledge of antibiotic stability (amoxicillin, cloxacillin, ampicillin-sulbactam, piperacillin-tazobactam, cefotaxime, ceftazidime, cefepime, meropenem, imipenem-cistatin, and vancomycin), the loading dose, and the attitudes towards EI/CI administration based on the clinical observation. Surveys would skip to the end if respondents selected “no experience in EI/CI administration”.
Following a comprehensive literature review (Zhou et al., 2021), a multidisciplinary team of pediatricians, pharmacists, infectious disease specialists, and epidemiologists developed the questionnaire. The appearance validity of the questionnaire was then evaluated through an initial pilot test with eight specialists, including four pediatricians, two pharmacists, one epidemiologist, and one infectious diseases specialist. They were invited to provide feedbacks and suggestions on the understandability and logicality of the questions, the rationality of response time, and the validity of the survey platform before it was available on the Questionnaire Star platform (https://www.wjx.cn/). The English version of the questionnaire is available in Supplementary Table S1.
Data collection and statistical analyses
The data were collected, cleaned and standardized by pairs of investigators (Z.P.X. and C.Y.C.) via Microsoft Excel 2019. Frequencies and proportions for categorical data were used primarily to summarize and describe participants’ demographic characteristics and their current clinical practice of EI/CI administration. A multivariable logistic regression model was applied to explore which demographic characteristics or clinical practice experience were associated with the implementation of EI/CI administration using the enter method for covariate selection. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated quantify the associations. All statistical tests were two-sided, and differences with p < 0.05 were considered statistically significant. The analyses were performed using IBM® SPSS®, Statistics 27.0, and Tableau (2022.1 version) was used to generate the figures.
Results
Respondent characteristics
A total of 1,501 pediatricians from 31 provincial administrative regions of China responded to the survey. The demographic characteristics of the participants are shown in Table 1, while Figure 1 and Figure 2 illustrate the geographic distribution of the responses.
Clinical experience of neonatal sepsis management
The neonatal ward was where the most of the responders (54.56%, 819/1501) spent 10–12 months of the year at work. The most frequent ways of obtaining the experience of neonatal sepsis antibiotic management included pediatric team experience (69.22%, 1039/1501), classes from neonatal or pediatric specialists (79.21%, 1189/1501), and guidelines, consensus or medical textbooks (67.95%, 1020/1501). A total of 44.57% (669/1501) and 33.84% (508/1501) of the respondents gained experience via consulting clinical pharmacists and anti-infection specialists, respectively.
Unfortunately, 36.64% (550/1501) of the participants stated that their neonatal antibiotic management did not involve a multidisciplinary therapy (MDT) team. A total of 27.78% (417/1501) conducted MDTs by inviting formal medical consultations, whereas 16.59% (249/1501) addressed the antibiotic-related questions only through private communication with anti-infection specialists or pharmacists. Additionally, 31.78% (477/1501) of the pediatricians reported having no MDT discussion, whereas 41.71% (626/1501) said that MDT discussion was carried out depended on the necessity. Only 13.19% (198/1501) of the respondents had regular MDT discussions on varying frequencies.
Extended infusion or continuous infusion of β-lactams and vancomycin implementation
Overall, only 31.51% (473/1501) of respondents reported that they had experience with EI/CI of β-lactams and vancomycin in neonatal sepsis. More than half indicated that they carried out EI/CI administration when the patients had a strong possibility of serious infections with multiple risk factors (55.81%, 264/473) or the blood cultures revealed that they were intermediate or resistant to β-lactams or vancomycin (53.07%, 251/473). Furthermore, 27.06% (128/1501) reported that they frequently used EI/CI therapy, and 40.17% (190/1501) claimed that the patients might benefit from EI/CI administration when initial antibiotics were ineffective.
The majority of respondents stated that they acquired the EI/CI approach via clinical practice guidelines (71.25%, 337/473) or lectures given by anti-infection specialists (55.18%, 261/473). Less than half of the participants indicated that they learned about the EI/CI approach via clinical pharmacists’ lectures (47.36%, 224/473), academic conferences (47.15%, 223/473) or their department guidance (45.45%, 215/473). Regarding infusion time, most of the respondents had experience with EI administration for 2–3 h (76.74% for β-lactams, 363/473; 81.61% for vancomycin, 386/473), whereas very few had experiences with CI administration (3.38% for β-lactams, 16/473; 8; 5.07% for vancomycin, 24/473) (Table 2).
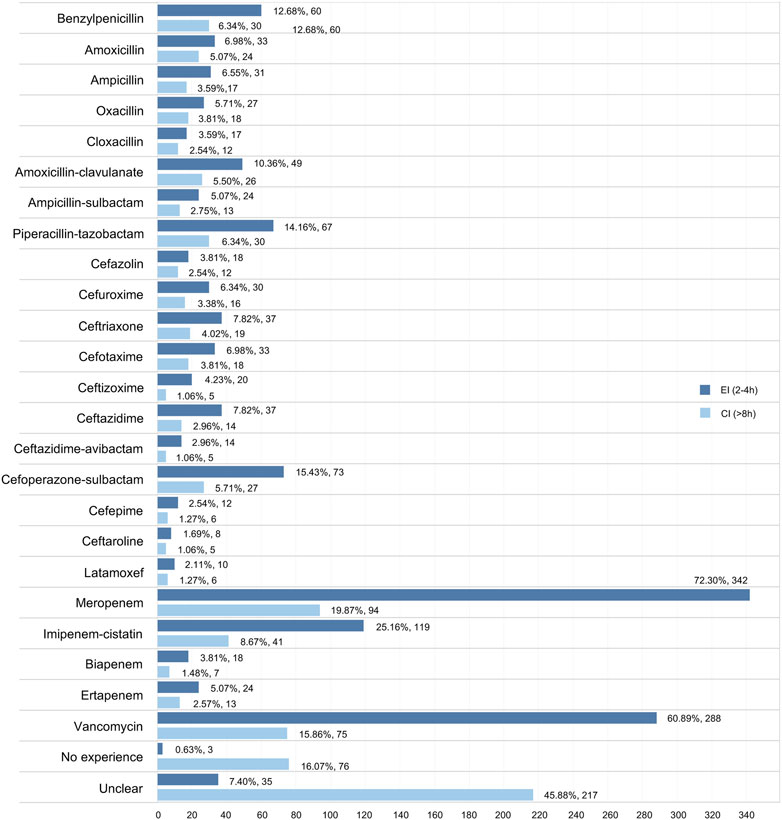
We investigated the experience of EI/CI administration in neonatal sepsis in 24 antibiotics, including 23 types of β-lactams and vancomycin. Overall, the EI (2–4 h) approach was conducted more frequently than the CI (>8 h) approach in all drugs of interest, while meropenem (25.16% in EI; 19.87% in CI) and vancomycin (60.89% in EI; 15.86% in EI) were the two drugs most likely to undergo EI/CI administration. Furthermore, piperacillin-tazobactam (14.16%) was the most commonly reported drug in the penicillin category with the EI/CI method, whereas cefoperazone-sulbactam (15.43%) was the most widely reported drug in the cephalosporin category (Figure 3).
In terms of loading dose of β-lactams or vancomycin, nearly half of the respondents (50.95%, 241/473) reported they would conduct a loading dose depending on the condition and severity of infection, whereas 17.12% (81/473) administered loading dose on a regular basis. The attitude towards EI/CI administration in neonatal physicians was investigated in this questionnaire, and approximately one-third (29.81%, 141/473) of the participants were convinced that they had achieved better clinical improvements. However, 32.35% (153/473) indicated they could not draw definite conclusions about the efficacy due to their limited experience, and 16.70% (79/473) reported there was inconsistency of efficacy based on previous cases. Only 4.65% (79/473) believed there was no clinical improvement in clinical efficacy following EI/CI administration (Table 3).
Respondents’ knowledge about antibiotic stability
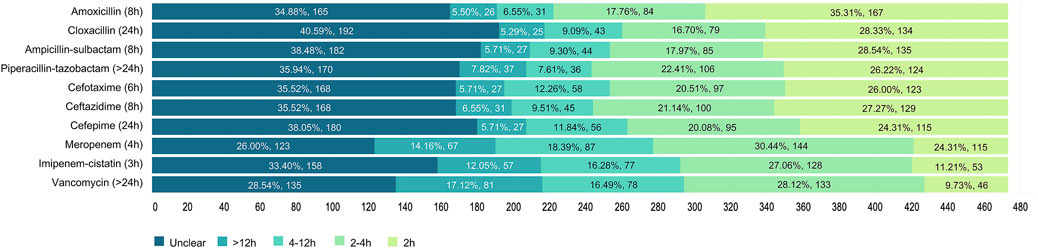
It was unknown how long intravenous β-lactams and vancomycin would remain stable. The most correctly recognized antibiotics in terms of infusion stability, according to published stability studies (Longuet et al., 2016; Loeuille et al., 2022), were imipenem-cistatin (27.06%), meropenem (18.39%), vancomycin (17.12%) and cefotaxime (12.26%), whereas less than 10% of the respondents could clearly identify the right stable time. Nearly 40% (34.88%–40.59%), 30% (26.00%–33.40%) and 28.54% of the participants stated that they were unclear about the stability of infusions in cephalosporins, carbapenems and vancomycin, respectively (Figure 4).
Factors influencing extended infusion or continuous infusion implementation
The findings of the logistic regression indicated that the respondents with a master (p = 0.008) or Doctor degree (p = 0.011) and those who worked in a higher hospital grade (p = 0.004) or neonatal intensive care unit (p = 0.002) were more likely to administrate EI/CI. Additionally, individuals who spent 4–6 months (p = 0.003) in the neonatal department or worked in a well-established MDT team for neonatal sepsis (p = 0.023) with daily (p = 0.047), 2–3 times weekly (p = 0.044) or once weekly (p = 0.032) discussion were significantly linked to greater rates of EI/CI implementation (Supplementary Table S2).
Discussion
To the best of our knowledge, this large nationwide survey represents the first cross-sectional study to investigate the implementation of EI/CI of β-lactams and vancomycin in neonatal sepsis in China. The findings could contribute to identify pediatricians’ current knowledge, clinical practice and attitude regarding this antimicrobial optimization strategy.
Generally, the findings of this survey were well represented by 1501 pediatric physicians in mainland China according to their demographic characteristics and clinical experience. It is worth mentioning that only approximately one-third of the respondents had access to EI/CI of β-lactams (most frequently meropenem, imipenem-cistatin, cefoperazone-sulbactam and piperacillin-tazobactam) and vancomycin for 2–3 h in most cases, with selective administration of a loading dose in critically infected neonates. Notably, the prolonged strategy was primarily supported by guidelines and consultations from medical specialists (such as anti-infection specialists, clinical pharmacists), but the attitude and clinical outcomes were of considerable uncertainty and wide variability among the respondents. A large proportion of respondents did not clearly know or provide accurate answers to the stability of nine kinds of β-lactams and vancomycin in our study. Due to unawareness of or uncertainty over the stability of infusion, pediatricians may consequently decide to abandon the EI/CI strategy.
Furthermore, the multivariate analysis findings demonstrated several potential factors, including the education level, the hospital grade, and the department or ward pediatricians worked in, that could be connected to the implementation of the EI/CI strategy in neonatal sepsis. Additionally, we discovered that an increase in the use of the EI/CI approach was linked to the appropriate time pediatricians worked in the neonatal department/ward, the well-developed MDT team for neonatal sepsis, and more frequent MDT discussion on antibiotic use. Several published studies reported that MDT intervention could benefit anti-infection management in the NICU (Hamza et al., 2022). Neonatologists are encouraged to develop and implement antibiotic utilization strategies in collaboration with surgeons (surgical prophylaxis, the management of surgical infections) (Robinson et al., 2020), infectious disease specialists (MIC and drug resistance), pharmacists (PK/PD theories, toxicities, interactions, and drug information), nurses (EI/CI care), and other NICU providers (Katz et al., 2021). Moreover, it is imperative to note that autonomous learning about antimicrobial stewardship in neonatal sepsis through lectures, guidelines and consultations was not substantially correlated with a higher proportion of EI/CI use in our analysis. Consequently, future quality improvement studies or antimicrobial stewardship practices should deliberately arise awareness, optimize processes, and utilize multidisciplinary resources for neonatologists (Li et al., 2022).
Previously, research on EI/CI strategies administered in intensive care units for adults demonstrated that even though optimized administration of β-lactams and vancomycin were universally acknowledged by intensive care specialists, limited fraction of them actually implemented these principles in clinical practice owing to the lack of knowledge and difficulties in accessing information (Dulhunty et al., 2011; Buyle et al., 2013; Tabah et al., 2015; Charmillon et al., 2016; Liebchen et al., 2020). However, only a few studies have focused on pediatric populations with severe infections and optimized antimicrobial dosing strategies (Knoderer et al., 2014). To our knowledge, few large sample-sized cross-sectional surveys have been carried out in China. It is of irreplaceability to further disseminate the knowledge about the PK/PD theory of prolonged strategy in time-dependent antibiotics, current best clinical evidence on efficacy and safety and implementation experience of EI/CI approaches (Tabah et al., 2015).
The studies indicated that the majority of neonatologists could recognize that antibacterial agents’ PK parameters (such as volume of distribution and drug clearance) are significantly altered in critically infected children (Hartman et al., 2020). However, the optimal antibiotic optimization in accordance with PK/PD theory remained multifaceted and poses particular challenges to neonatal pediatricians (Tängdén et al., 2017; Phe et al., 2020). The potential barriers to its implementation might be mainly attributed to the lack of pharmacological knowledge, the absence of an MDT team, and the lack of confidence in clinical practice owing to limited efficacy and safety evidence in pediatrics. Recent published systematic reviews have demonstrated that EI/CI had generally improved clinical outcomes and safety profiles compared with intermittent infusion strategy, with a greater probability of achieving the target (Costenaro et al., 2020; Alonso-Moreno et al., 2021; Zhou et al., 2021). It is undeniable that the EI/CI strategy has emerged as a promising option (De Waele et al., 2014), even though PK of antibiotics may be affected in critically ill neonates undergoing significant physiological alterations and the appropriateness of EI/CI administration remains further PK/PD or clinical evaluation data (Wang et al., 2020; Saito et al., 2021; Yonwises et al., 2021). A clinical practice guideline describing the methods and procedures for using EI/CI of β-lactams and vancomycin in neonatal sepsis management is consequently urgently required based on our study findings.
To further overcome these obstacles, specific implementation strategies should be developed accordingly (Correa et al., 2020). First, guidelines, research or clinical experience need to be disseminated to department administrators, pediatricians and patients’ families (Boland et al., 2019a; Boland et al., 2019b). Second, it is necessary to establish an MDT team regarding antibacterial agents, with at least anti-infection specialists, clinical pharmacists and microbiologists on board (Whalen et al., 2021). Third, the department’s policies, guidelines, or a trustable clinical decision support system may aid pediatricians in standardizing their EI/CI strategies and boosting their self-assurance (Euteneuer et al., 2019; Persad et al., 2021; Daphtary and Baloglu, 2022).
Nonetheless, there are several limitations in this study. Owing to resource constraints, we used a convenience sampling method to ensure that a specific number of results were available for individual province from China rather than conducting a strict stratified random sample across the nation. The questionnaire results might therefore be assumed representative. Additionally, the questions in the survey concerning clinical experience only reflect the impressions and attitudes towards the respondents other than the actual daily clinical practice. Finally, as a cross-sectional study, its causal association should be determined cautiously. The non-significant results in certain domains do not indicate a lack of association but may be due to limited sample size.
Conclusion
In conclusion, this national survey demonstrated that the EI/CI strategy in neonatal sepsis was only recognized by one-third of Chinese neonatologists. To improve the implementation of antibacterial optimization, a solid MDT team should be developed with regularly collaborates. Guidelines regarding prolonged infusion management in neonatal sepsis would be beneficial for clinical practice and should be developed in the near future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Peking University Third Hospital Medical Science Research Ethics Committee (M2022245).
Author contributions
Conceptualization and design: PZ, XT, KY; Ethic approval: GC and PZ; Methodology: PZ, YC, KY; Acquisition, analysis, or interpretation of data: PZ, YC; Resources: PZ, XT; Visualization: PZ; Drafting of the manuscript: PZ; Supervision: SZ, XT, KY, YX; Revision of the manuscript: KY, XT; All authors have approved the final version of this manuscript.
Funding
This study was financially supported by Beijing Natural Science Foundation (S160004).
Acknowledgments
We thank Zhenhuan Wang from the First Hospital of Tsinghua University and Yingqiu Ying and Ling Liu from Peking University Third Hospital for evaluating the initial questionnaire. We thank all respondents who participated in this survey.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.995528/full#supplementary-material
References
Abdul-Aziz, M. H., Lipman, J., Mouton, J. W., Hope, W. W., and Roberts, J. A. (2015). Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: Optimizing efficacy and reducing resistance development. Semin. Respir. Crit. Care Med. 36 (1), 136–153. doi:10.1055/s-0034-1398490
Alonso-Moreno, M., Mejías-Trueba, M., Herrera-Hidalgo, L., Goycochea-Valdivia, W. A., and Gil-Navarro, M. V. (2021). Efficacy and safety of continuous infusion of vancomycin in children: A systematic review. Antibiot. (Basel) 10 (8), 912. doi:10.3390/antibiotics10080912
Boland, L., Graham, I. D., Légaré, F., Lewis, K., Jull, J., Shephard, A., et al. (2019a). Barriers and facilitators of pediatric shared decision-making: A systematic review. Implement. Sci. 14 (1), 7. doi:10.1186/s13012-018-0851-5
Boland, L., Lawson, M. L., Graham, I. D., Légaré, F., Dorrance, K., Shephard, A., et al. (2019b). Post-training shared decision making barriers and facilitators for pediatric healthcare providers: A mixed-methods study. Acad. Pediatr. 19 (1), 118–129. doi:10.1016/j.acap.2018.05.010
Bretonnière, C., Leone, M., Milési, C., Allaouchiche, B., Armand-Lefevre, L., Baldesi, O., et al. (2015). Strategies to reduce curative antibiotic therapy in intensive care units (adult and paediatric). Intensive Care Med. 41 (7), 1181–1196. doi:10.1007/s00134-015-3853-7
Burgunder, L., Heyrend, C., Olson, J., Stidham, C., Lane, R. D., Workman, J. K., et al. (2022). Medication and fluid management of pediatric sepsis and septic shock. Paediatr. Drugs 24 (3), 193–205. doi:10.1007/s40272-022-00497-z
Buyle, F. M., Decruyenaere, J., De Waele, J., Tulkens, P. M., Van Audenrode, T., Depuydt, P., et al. (2013). A survey of beta-lactam antibiotics and vancomycin dosing strategies in intensive care units and general wards in Belgian hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 32 (6), 763–768. doi:10.1007/s10096-012-1803-7
Cao, G., Zhou, P., Zhang, H., Sun, B., Tong, X., and Xing, Y. (2022). Extended infusion of meropenem in neonatal sepsis: A historical cohort study. Antibiot. (Basel) 11 (3), 341. doi:10.3390/antibiotics11030341
Charmillon, A., Novy, E., Agrinier, N., Leone, M., Kimmoun, A., Levy, B., et al. (2016). The ANTIBIOPERF study: A nationwide cross-sectional survey about practices for β-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin. Microbiol. Infect. 22 (7), 625–631. doi:10.1016/j.cmi.2016.04.019
Correa, V. C., Lugo-Agudelo, L. H., Aguirre-Acevedo, D. C., Contreras, J. A. P., Borrero, A. M. P., Patiño-Lugo, D. F., et al. (2020). Individual, health system, and contextual barriers and facilitators for the implementation of clinical practice guidelines: A systematic metareview. Health Res. Policy Syst. 18 (1), 74. doi:10.1186/s12961-020-00588-8
Costenaro, P., Minotti, C., Cuppini, E., Barbieri, E., Giaquinto, C., and Donà, D. (2020). Optimizing antibiotic treatment strategies for neonates and children: Does implementing extended or prolonged infusion provide any advantage? Antibiot. (Basel) 9 (6), E329. doi:10.3390/antibiotics9060329
Daphtary, K., and Baloglu, O. (2022). Clinical informatics and quality improvement in the pediatric intensive care unit. Pediatr. Clin. North Am. 69 (3), 573–586. doi:10.1016/j.pcl.2022.01.014
De Waele, J. J., Lipman, J., Akova, M., Bassetti, M., Dimopoulos, G., Kaukonen, M., et al. (2014). Risk factors for target non-attainment during empirical treatment with β-lactam antibiotics in critically ill patients. Intensive Care Med. 40 (9), 1340–1351. doi:10.1007/s00134-014-3403-8
DiMondi, V. P., and Rafferty, K. (2013). Review of continuous-infusion vancomycin. Ann. Pharmacother. 47 (2), 219–227. doi:10.1345/aph.1R420
Dulhunty, J. M., Paterson, D., Webb, S. A., and Lipman, J. (2011). Antimicrobial utilisation in 37 Australian and New Zealand intensive care units. Anaesth. Intensive Care 39 (2), 231–237. doi:10.1177/0310057x1103900212
Egi, M., Ogura, H., Yatabe, T., Atagi, K., Inoue, S., Iba, T., et al. (2021). The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-sscg 2020). Acute Med. Surg. 8 (1), e659. doi:10.1002/ams2.659
Euteneuer, J. C., Kamatkar, S., Fukuda, T., Vinks, A. A., and Akinbi, H. T. (2019). Suggestions for model-informed precision dosing to optimize neonatal drug therapy. J. Clin. Pharmacol. 59 (2), 168–176. doi:10.1002/jcph.1315
Evans, L., Rhodes, A., Alhazzani, W., Antonelli, M., Coopersmith, C. M., French, C., et al. (2021). Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47 (11), 1181–1247. doi:10.1007/s00134-021-06506-y
Fleischmann-Struzek, C., Goldfarb, D. M., Schlattmann, P., Schlapbach, L. J., Reinhart, K., and Kissoon, N. (2018). The global burden of paediatric and neonatal sepsis: A systematic review. Lancet. Respir. Med. 6 (3), 223–230. doi:10.1016/s2213-2600(18)30063-8
Gwee, A., Cranswick, N., Metz, D., Coghlan, B., Daley, A. J., Bryant, P. A., et al. (2014). Neonatal vancomycin continuous infusion: Still a confusion? Pediatr. Infect. Dis. J. 33 (6), 600–605. doi:10.1097/inf.0000000000000243
Hamza, W. S., Hamed, E. A. M., Alfadhli, M. A., and Ramadan, M. A. (2022). A multidisciplinary intervention to reduce central line-associated bloodstream infection in pediatrics and neonatal intensive care units. Pediatr. Neonatol. 63 (1), 71–77. doi:10.1016/j.pedneo.2021.08.010
Hartman, S. J. F., Brüggemann, R. J., Orriëns, L., Dia, N., Schreuder, M. F., and de Wildt, S. N. (2020). Pharmacokinetics and target attainment of antibiotics in critically ill children: A systematic review of current literature. Clin. Pharmacokinet. 59 (2), 173–205. doi:10.1007/s40262-019-00813-w
Jacqz-Aigrain, E., Leroux, S., Zhao, W., van den Anker, J. N., and Sharland, M. (2015). How to use vancomycin optimally in neonates: Remaining questions. Expert Rev. Clin. Pharmacol. 8 (5), 635–648. doi:10.1586/17512433.2015.1060124
Katz, S., Banerjee, R., and Schwenk, H. (2021). Antibiotic stewardship for the neonatologist and perinatologist. Clin. Perinatol. 48 (2), 379–391. doi:10.1016/j.clp.2021.03.009
Knoderer, C. A., Nichols, K. R., and Cox, E. G. (2014). Optimized antimicrobial dosing strategies: A survey of pediatric hospitals. Paediatr. Drugs 16 (6), 523–529. doi:10.1007/s40272-014-0093-1
Li, X., Li, Y., Guo, K., Chen, N., Cui, X., Bai, Z., et al. (2022). Evidence based social science in China paper 3: The quality of social science RCTs published from 2000-2020. J. Clin. Epidemiol. 141, 64–73. doi:10.1016/j.jclinepi.2021.09.014
Liebchen, U., Paal, M., Scharf, C., Schroeder, I., Grabein, B., Zander, J., et al. (2020). The ONTAI study - a survey on antimicrobial dosing and the practice of therapeutic drug monitoring in German intensive care units. J. Crit. Care 60, 260–266. doi:10.1016/j.jcrc.2020.08.027
Loeuille, G., D'Huart, E., Vigneron, J., Nisse, Y. E., Beiler, B., Polo, C., et al. (2022). Stability studies of 16 antibiotics for continuous infusion in intensive care units and for performing outpatient parenteral antimicrobial therapy. Antibiot. (Basel) 11 (4), 458. doi:10.3390/antibiotics11040458
Longuet, P., Lecapitaine, A. L., Cassard, B., Batista, R., Gauzit, R., Lesprit, P., et al. (2016). Preparing and administering injectable antibiotics: How to avoid playing God. Med. Mal. Infect. 46 (5), 242–268. doi:10.1016/j.medmal.2016.01.010
Minotti, C., Barbieri, E., Giaquinto, C., and Donà, D. (2021). Vancomycin use in children and neonates across three decades: A bibliometric analysis of the top-cited articles. Pathogens 10 (10), 1343. doi:10.3390/pathogens10101343
Muller, A. E., Huttner, B., and Huttner, A. (2018). Therapeutic drug monitoring of beta-lactams and other antibiotics in the intensive care unit: Which agents, which patients and which infections? Drugs 78 (4), 439–451. doi:10.1007/s40265-018-0880-z
Persad, E., Jost, K., Honoré, A., Forsberg, D., Coste, K., Olsson, H., et al. (2021). Neonatal sepsis prediction through clinical decision support algorithms: A systematic review. Acta Paediatr. 110 (12), 3201–3226. doi:10.1111/apa.16083
Phe, K., Heil, E. L., and Tam, V. H. (2020). Optimizing pharmacokinetics-pharmacodynamics of antimicrobial management in patients with sepsis: A review. J. Infect. Dis. 222 (2), S132–s141. doi:10.1093/infdis/jiaa118
Rhodes, A., Evans, L. E., Alhazzani, W., Levy, M. M., Antonelli, M., Ferrer, R., et al. (2017). Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 45 (3), 486–552. doi:10.1097/ccm.0000000000002255
Rizk, N. A., Kanafani, Z. A., Tabaja, H. Z., and Kanj, S. S. (2017). Extended infusion of beta-lactam antibiotics: Optimizing therapy in critically-ill patients in the era of antimicrobial resistance. Expert Rev. anti. Infect. Ther. 15 (7), 645–652. doi:10.1080/14787210.2017.1348894
Robinson, E. D., Volles, D. F., Kramme, K., Mathers, A. J., and Sawyer, R. G. (2020). Collaborative antimicrobial stewardship for surgeons. Infect. Dis. Clin. North Am. 34 (1), 97–108. doi:10.1016/j.idc.2019.11.002
Ruth, A., McCracken, C. E., Fortenberry, J. D., Hall, M., Simon, H. K., and Hebbar, K. B. (2014). Pediatric severe sepsis: Current trends and outcomes from the pediatric health information systems database. Pediatr. Crit. Care Med. 15 (9), 828–838. doi:10.1097/pcc.0000000000000254
Saito, J., Shoji, K., Oho, Y., Kato, H., Matsumoto, S., Aoki, S., et al. (2021). Population pharmacokinetics and pharmacodynamics of meropenem in critically ill pediatric patients. Antimicrob. Agents Chemother. 65 (2), e01909-e01920. doi:10.1128/aac.01909-20
Shane, A. L., Sánchez, P. J., and Stoll, B. J. (2017). Neonatal sepsis. Lancet 390 (10104), 1770–1780. doi:10.1016/s0140-6736(17)31002-4
Tabah, A., De Waele, J., Lipman, J., Zahar, J. R., Cotta, M. O., Barton, G., et al. (2015). The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 70 (9), 2671–2677. doi:10.1093/jac/dkv165
Tan, B., Wong, J. J., Sultana, R., Koh, J., Jit, M., Mok, Y. H., et al. (2019). Global case-fatality rates in pediatric severe sepsis and septic shock: A systematic review and meta-analysis. JAMA Pediatr. 173 (4), 352–362. doi:10.1001/jamapediatrics.2018.4839
Tängdén, T., Ramos Martín, V., Felton, T. W., Nielsen, E. I., Marchand, S., Brüggemann, R. J., et al. (2017). The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 43 (7), 1021–1032. doi:10.1007/s00134-017-4780-6
Tu, Q., Cotta, M., Raman, S., Graham, N., Schlapbach, L., and Roberts, J. A. (2021). Individualized precision dosing approaches to optimize antimicrobial therapy in pediatric populations. Expert Rev. Clin. Pharmacol. 14 (11), 1383–1399. doi:10.1080/17512433.2021.1961578
Veiga, R. P., and Paiva, J. A. (2018). Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 22 (1), 233. doi:10.1186/s13054-018-2155-1
Wang, Z. M., Chen, X. Y., Bi, J., Wang, M. Y., Xu, B. P., Tang, B. H., et al. (2020). Reappraisal of the optimal dose of meropenem in critically ill infants and children: A developmental pharmacokinetic-pharmacodynamic analysis. Antimicrob. Agents Chemother. 64 (8), e00760–e20. doi:10.1128/aac.00760-20
Weiss, S. L., Fitzgerald, J. C., Pappachan, J., Wheeler, D., Jaramillo-Bustamante, J. C., Salloo, A., et al. (2015). Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med. 191 (10), 1147–1157. doi:10.1164/rccm.201412-2323OC
Weiss, S. L., Peters, M. J., Alhazzani, W., Agus, M. S. D., Flori, H. R., Inwald, D. P., et al. (2020). Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 46 (1), 10–67. doi:10.1007/s00134-019-05878-6
Whalen, E., Ely, E., and Brown, A. (2021). The role of a multidisciplinary team in a pediatric pulmonary hypertension center. Pediatr. Pulmonol. 56 (3), 630–635. doi:10.1002/ppul.24761
Yonwises, W., Wacharachaisurapol, N., Anugulruengkitt, S., Maimongkol, P., and Treyaprasert, W. (2021). Population pharmacokinetics of meropenem in critically ill infant patients. Int. J. Infect. Dis. 111, 58–64. doi:10.1016/j.ijid.2021.08.031
Keywords: extended infusion, continuous infusion, β-lactams, vancomycin, neonatal sepsis, cross-sectional survey
Citation: Zhou P, Cheng Y, Cao G, Xing Y, Zhai S, Tong X and Yang K (2022) The OBTAINS study: A nationwide cross-sectional survey on the implementation of extended or continuous infusion of β-lactams and vancomycin among neonatal sepsis patients in China. Front. Pharmacol. 13:1001924. doi: 10.3389/fphar.2022.1001924
Received: 24 July 2022; Accepted: 23 September 2022;
Published: 10 October 2022.
Edited by:
Elena Y. Enioutina, The University of Utah, United StatesReviewed by:
Jeffrey Lipman, The University of Queensland, AustraliaYogan Khatri, Cayman Chemical, United States
Copyright © 2022 Zhou, Cheng, Cao, Xing, Zhai, Tong and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kehu Yang, eWFuZ2toLWVibUBsenUuZWR1LmNu; Xiaomei Tong, dG9uZ3hpYW9tZWlAYmptdS5lZHUuY24=
 Pengxiang Zhou
Pengxiang Zhou Yinchu Cheng
Yinchu Cheng Guangna Cao5
Guangna Cao5 Suodi Zhai
Suodi Zhai Kehu Yang
Kehu Yang