
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 15 September 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1001363
This article is part of the Research TopicExperimental Models of Epilepsy and Related Comorbidities, Volume IIView all 7 articles
 Xianhao Huo1,2,3†
Xianhao Huo1,2,3† Xingguo Xu1†
Xingguo Xu1† Mei Li2,3
Mei Li2,3 Lifei Xiao1,2,3
Lifei Xiao1,2,3 Yangyang Wang2,3,4
Yangyang Wang2,3,4 Wenchao Li2,3,4
Wenchao Li2,3,4 Chaofan Wang1,2,3
Chaofan Wang1,2,3 Tao Sun1,2*
Tao Sun1,2*Purpose: To explore the effectiveness of different anti-seizure medications in preventing early and late post-traumatic epilepsy (PTE). The efficacy, treatment-related side-effects, and mortality of the different treatments were compared using a ranking model to identify the optimal treatment.
Methods: A comprehensive literature search was performed using Pubmed, Medline, Embase, and Cochrane library databases. All relevant published articles up to 10 March 2022 were evaluated. The quality of the extracted data was assessed using either the Cochrane risk of bias tool or the Newcastle-Ottawa scale. The primary outcome measures were early or late post-traumatic seizures. The secondary outcome measures were mortality, treatment-related adverse effects, length of hospital stay, and length of stay within the intensive care unit (ICU).
Results: A total of seven randomized controlled trials and 18 non-randomized controlled trials were included in this network meta-analysis. The trials included six interventions: Phenytoin (PHT)+phenobarbital (PB), levetiracetam (LEV), PHT, PHT-LEV, lacosamide (LCM), and valproate (VPA). All interventions except VPA significantly reduced the rate of early PTE in TBI patients compared with the placebo. Seven studies reported the impact of four treatments (PHT + PB, LEV, PHT, VPA) on late seizures and showed a significant reduction in the incidence of late seizures in patients with TBI compared with placebo. The impact of PHT, LEV, and VPA on mortality was reported in nine studies. PHT had no impact on mortality, but patients treated with both LEV and VPA had higher mortality than those treated with placebo. The treatment-related adverse effects of LEV, PHT, and LCM were reported in five studies. LEV and PHT had higher treatment-related adverse effects incidence than placebo, while LCM had no effect on treatment related-adverse effects.
Conclusion: LEV and PHT prevented early and late PTE. PHT also reduced the mortality rate in patients with TBI. Both LEV and PHT had higher treatment-related adverse effects compared with placebo. However, LEV had a slightly lower incidence of treatment-related adverse effects when compared with PHT. Compared with PHT, LEV did not reduce the length of hospital stay but shortened the length of ICU stays. Therefore, based on the findings of this meta-analysis, we speculate that LEV is the best treatment option for TBI patients. However, further high-quality randomized controlled trials are required to confirm these findings.
Traumatic brain injury (TBI) is mainly caused by direct or indirect external forces on the head. More than 50 million people worldwide suffer from TBI each year. Common causes of TBI include car accidents, injuries from falls, and heavy blows to the head (Winkler et al., 2016; Vella et al., 2017). These injuries can result in various disabilities, including neurological deficits, memory loss, and other negative results, making TBI a chronic health condition and a global healthcare burden (Yuan and Wang, 2020).
Post-traumatic epilepsy (PTE) is a recognized complication of TBI. Depending on the location and severity of the bleeding, PTE can occur immediately within 24 h after trauma, early within the first 7 days following trauma, and late after 7 days following trauma (Turnbull et al., 2016; Chartrain et al., 2017). Over the past 30 years, the cumulative incidence of PTE was 2% for mild brain injury, 4% for moderate brain injury, and 15% for severe brain injury (Saletti et al., 2019; Liou et al., 2020). PTE following TBI may further exacerbate the effects of TBI on memory and cognition, damage the cerebrovascular system or blood-brain barrier, and lead to depression or post-traumatic stress disorder (Castriotta et al., 2007; Luo et al., 2014; Muccigrosso et al., 2016; Vos et al., 2018). PTE also makes treating the primary injury more difficult and increases the costs associated with treatment, imposing a serious economic and life burden on the patients’ families and society. Therefore, the prevention of PTE is an important clinical goal in the treatment of TBI.
Prophylactic treatment with anti-seizure medications (ASMs) is increasingly being used to reduce the risk of developing PTE following TBI. The main ASMs used in clinical practice include phenytoin sodium (PHT) (Harris et al., 2020), sodium valproate (VPA) (Ma et al., 2010), phenobarbital (PB) (Servit and Musil, 1981), lamotrigine (LAM) (Kaufman, 2011), levetiracetam (LEV) (Khan et al., 2016), oxcarbazepine (OCBZ) (Degrauw et al., 2018), topiramate (TPM) (Courchia et al., 2018), and carbamazepine (CBZ) (Kirmani et al., 2016). Among these, PHT, OCBZ and CBZ have the common notion of the mechanism, which was reduced high-frequency repetitive discharges of action potentials by enhancing sodium channel inactivation. VPA has multiple mechnisms of action, including GABA potentiation, blocking of T-type calcium channels, and blocking of sodium channels. The main mechanism of PB in preventing seizures is through binding the γ-aminobutyric acid (GABA)-A receptor, prolong the opening of the associated chloride channel. The mechanism of LAM is blocked sodium channels, and reduces Ca2+-mediated transmitter release. The main mechanism of LEV is binding to the synaptic vesicle protein SV2A; inhibits high-voltage-activated Ca2+ channels; reverses the negative allosteric effect of GABA/Gly receptor antagonists. TPM has multiple mechanisms of blocking Na+ channels, increasing γ-aminobutyric acid-mediated inhibition and blocking glutamate-mediated neural excitation, affecting Cl− membrane operation and Ca2+ channel blockade. Based on the different mechanisms of the different ASMs and the limitation of the available evidence, the use of ASMs for the prevention of epileptic seizures after TBI is still controversial. A propensity score analysis conducted by Liou et al. (Liou et al., 2020). Revealed that ASMs were ineffective in preventing seizures after TBI, and the benefit of routine prophylactic ASMs treatment in TBI patients needs to be reassessed (Saletti et al., 2019). Studies have shown that VPA was associated with higher mortality in patients with TBI (Temkin et al., 1999), while carbamazepine and PHT were associated with severe adverse effects. As a result, there is a need to identify the optimal ASM therapy to prevent epileptic seizures in TBI patients. ASMs such as LEV and PHT are increasingly being used in clinical practice due to the many favorable features of these drugs. The Brain Trauma Foundation (BTF) guidelines have acknowledged the potential role of PHT and LEV in the management of early PTE but did not provide any clinical recommendations on using these drugs due to insufficient evidence (Carney et al., 2017). As a result, the fourth edition of the BTF guidelines recommends using preventive PHT in the first week following TBI. Still, it does not provide any recommendations for the use of ASMs as a prophylactic treatment for late epileptic seizures (Carney et al., 2017).
In recent years, several studies have been published evaluating the use of ASMs following TBI. However, the current evidence is based on low-quality studies with small sample size. This highlights the need for a meta-analysis to evaluate the current evidence and provide a less biased and more accurate estimation of the clinical problem (Guyatt et al., 1995; Lee, 2018). A network meta-analysis would be ideal in this case since it can be used to compare multiple interventions indirectly by setting a common control group for analysis.
Therefore this study aimed to perform a ranked network meta-analysis to evaluate the effectiveness of ASMs in preventing early or late seizures in TBI patients. In addition, the mortality rate and treatment-related adverse effects of the various therapies were also evaluated.
The systematic review and network meta-analysis were performed according to the checklist of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) extension statement for network meta-analysis.
The inclusion and exclusion criteria for this study were based on the PICOS strategy (P: patient/population, I: intervention, C: comparison/control, O: outcome, S: study design). The population criteria of the included literature were patients above the age of 14 years who suffered a TBI(including the presence of subarachnoid hemorrhage, epidural hematoma, subdural hematoma, parenchymal hemorrhage, diffuse axonal injury, and depressed skull fracture confirmed by CT scan), with a time to injury shorter than 24 h. The interventions involved the use of ASMs such as phenytoin, valproate, PB, LAM, LEV, oxcarbazepine, topiramate, and carbamazepine. The outcome indicators were early seizures, late seizures, mortality, and adverse effect. The meta-analysis included randomized controlled trials, prospective cohort, retrospective, or observational studies.
Studies that evaluated the use of ASMs in patients with non-TBI (e.g., cerebral infarction, brain tumor, etc.), spontaneous cerebral hemorrhage, and a history of seizures or trauma were excluded. In addition, studies that included patients treated with ASMs prior to injury or between injury, pregnant or lactating women, and patients with a history of food and drug allergies, severe cardiac, hepatic, or renal dysfunction, chronic alcohol or drug abuse, and severe psychiatric disorders were also excluded. Furthermore, case reports, single-arm studies, literature reviews, letters to the editor, related trials on children, animal experiments, and studies that did not report the outcome indicators were also excluded.
The primary outcome measures were early or late post-traumatic seizures. The secondary outcome measures were mortality, treatment-related side effects, and length of hospital and intensive care unit (ICU) stay.
A comprehensive literature search of studies published up to 10 March 2022, was conducted using Pubmed, Cochrane Library, Embase, and Medline. The keywords used for the search were “brain injury”, “head injury”, “brain hemorrhage”, “parenchymal hemorrhage”, “intracranial hemorrhage”, “subarachnoid hemorrhage”, “epidural hemorrhage”, “anti-epileptic”, “anticonvulsant”, “antiseizure”, “Phenytoin”, “Valproate”, “Phenobarbital”, “Lamotrigine”, “Levetiracetam”, “Oxcarbazepine”, “Topiramate”, and “Carbamazepine”. The references of relevant published systematic reviews were searched manually to identify additional literature. The World Health Organization (WHO) clinical trial registry was also searched manually to identify ongoing and completed unpublished clinical trials evaluating the use of ASMs in TBI patients.
Two professionally trained researchers independently screened all retrieved literature separately. The quality of the included randomized controlled trials was assessed using the Cochrane risk of bias tool. This tool assesses the quality of clinical trials based on six aspects; randomization, allocation concealment, blind application, data completeness, selective reporting, and other biases. The included prospective and retrospective non-randomized controlled trials were evaluated using the Newcastle-Ottawa Scale (NOS). The NOS tool assesses the quality of studies from three aspects: selectivity, comparability, and outcomes. Any disagreements encountered during the screening for relevant articles, quality assessment, and data analysis were resolved through consultations. A third researcher was consulted whenever the two researchers failed to reach an agreement. The quality of the evidence was finally assessed using the Grading of Recommendations Assessment and Development and Evaluation (GRADE) framework.
The author, publication year, country, study type, age, and intervention measures were extracted from each article. In addition, the total number of patients, details of drug treatment, the incidence of PTE, treatment-related adverse effects, and the mortality for each treatment group were also extracted. For studies with missing data, the author of the published research was contacted to obtain additional information.
Heterogeneity testing was carried out for all the included articles. The fixed-effect model was used for non-heterogenous studies with a p > 0.1 and I2 < 20%. For all other studies, the random-effects model was adopted. The surface under the cumulative ranking curve (SUCRA) was used to rank the treatment effects. In addition, the node-splitting method was used to conduct a consistency test to determine whether the direct and indirect evidence could be combined. The statistical analyses were performed by Revman Software (Version 5.3; The Cochrane Collaboration) and Stata (version16.0; Corporation, College Station, TX). A 2-tailed p-value below 0.05 was considered statistically significant for all statistical tests.
A total of 16,497 articles were initially retrieved, of which 13021were duplicated and were therefore excluded from the meta-analysis. Another 225 papers were excluded as the research purpose and/or the literature type were not in line with the aims of this meta-analysis. An additional 53 articles were excluded as these did not meet the eligibility criteria of this meta-analysis. Finally, 25 articles with a total sample size of 6,466 cases were included in the network meta-analysis, including seven randomized controlled trials (Young et al., 1983a; Young et al., 1983b; Temkin et al., 1990; Temkin et al., 1999; Szaflarski et al., 2010; Khan et al., 2016; Younus et al., 2018), four prospective studies (Jones et al., 2008; Inaba et al., 2013; Gabriel and Rowe, 2014; Khor et al., 2018), 13 retrospective studies (Wohns and Wyler, 1979; Servit and Musil, 1981; Ma et al., 2010; Debenham et al., 2011; Caballero et al., 2013; Kruer et al., 2013; Bhullar et al., 2014; Javed et al., 2016; Zangbar et al., 2016; Hazama et al., 2018; Kwon et al., 2019; Harris et al., 2020; Nguyen et al., 2021), and one non-randomized trial (Klein et al., 2012) as shown in Figure 1. The characteristics of the study design, interventions, and sample size for each treatment group are summarized in (Tables 1, 2). The studies evaluated six interventions, including PHT + PB, LEV, PHT, PHT-LEV, LCM, and valproate.
According to the Cochrane risk of bias tool, the seven randomized controlled trials included in this meta-analysis used correct random assignment methods, had complete outcome data information and were not selectively reported. The study by Temkin et al. (1990) was not blinded at trial implementation and outcome assessment, while the study by Szaflarski et al. (2010) was not blinded at outcome assessment. We could not determine whether the blinding method in the studies by Khan et al. (2016) and Younus et al. (2018) was correctly implemented during the intervention and evaluation of the outcome measures. Since it was not possible to determine the level of bias in the included randomized controlled studies, the quality of the studies was classified as moderate (Supplementary Figure S1). The total NOS score of the included 18 non-randomized trials was above 5, indicating high quality within these studies (Supplementary Table S1).
Twenty-two studies reported the rate of early seizures in patients with TBI after treatment with different ASMs. The results of the subgroup analysis are available in Supplementary Figure S2A. Since the subgroups had significant heterogeneity (I2 > 20%, p < 0.1), the random-effects model was adopted. Patients treated with PHT had a significantly lower rate of early seizures when compared with those treated with placebo (p = 0.007). However, the rate of early seizures did not differ significantly between PHT and other ASMs, including LEV, VPA, PHT-LEV, and LCM (All p > 0.05). In addition, VPA and LEV had no significant impact on the rate of early seizures compared with the placebo (p = 0.31, 0.54, respectively).
Seven studies reported the incidence of late seizures in patients with TBI after treatment with different ASMs. These were analyzed in subgroups as shown in Supplementary Figure S2B. Since the heterogeneity between subgroups (I2 > 20%, p < 0.1) was significant, the random-effects model was adopted. PHT + PB significantly reduced the rate of late seizures compared to a placebo (p < 0.05). However, the incidence of late seizures following PHT treatment did not differ significantly from that of LEV, VPA, and a placebo (all p > 0.05).
Nine studies reported mortality (the cause is TBI) in patients with TBI after treatment with different ASMs. The results of the subgroup analysis are summarized in Supplementary Figure S2C. Since there is no significant difference in heterogeneity between subgroups (I2 < 20%, p > 0.1), the fixed-effect model was adopted. PHT had no significant impact on mortality compared with the placebo, VPA, and LEV (all p > 0.05). The mortality between patients treated with LEV and the placebo did not vary significantly (p = 0.56).
The adverse effects of these drugs mainly were fever, cardiovascular, hematologic and dermatologic abnormalities (like bradycardia, myocardial infarction, atrial fibrillation, neutropenia, thrombocytopenia, rash, skin itchiness, etc), abnormal liver and kidney function (like elevated liver enzymes, acute kidney injury, diabetes insipidus, etc), and so on. In this network meta-analysis, five studies reported treatment-related adverse effects in TBI patients after treatment with different ASMs. Because of the details of treatment-related adverse effects reported by these studies were not completely consistent, this study only analyzed the overall incidence of treatment-related adverse effects. The results of the subgroup analysis of these studies are summarized in Supplementary Figure S2D. Since the subgroups had significant heterogeneity (I2 > 20%, p < 0.1), the random-effects model was adopted. PTH did not increase the incidence of treatment-related adverse effects compared with placebo and LEV (p = 0.23 and p = 0.24), but LCM significantly reduced the incidence of treatment-related adverse effects compared with the PHT (p < 0.05).
The length of hospital stay was reported in eight studies, while the length of ICU stay was reported in five studies. The results of the subgroup analysis are summarized in Supplementary Figures S3A, 3B). Since the subgroups had significant heterogeneity (I2 > 20%, p < 0.1), the random-effects model was adopted. LEV did not reduce the length of hospital stay (p = 0.37) but significantly reduced the length of ICU stay (p = 0.04) compared to PHT. However, PHT significantly prolonged the length of hospital stay compared to the placebo (p < 0.05).
The credibility of the evidence for early seizures, late seizures, mortality, and the treatment-related adverse effects was very low in the non-randomized controlled trials due to the use of an observational study design and the indirectness of several comparisons (Tables 3). Therefore further high-quality research is needed to provide more evidence.
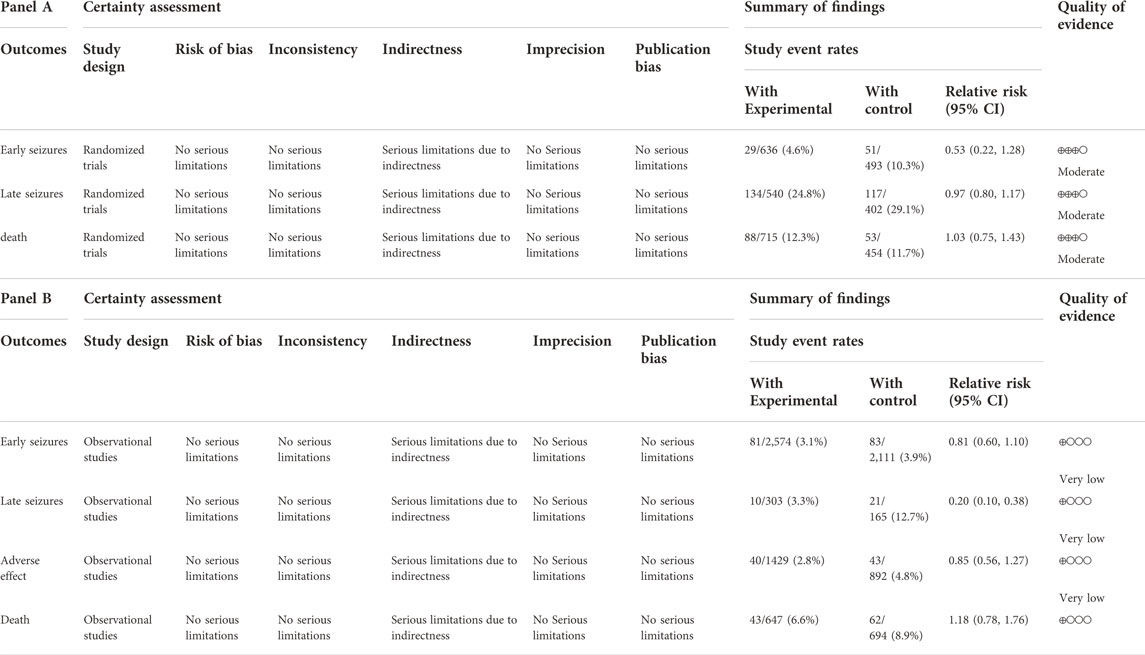
TABLE 3. (Panel A) GRADE assessment of the quality of evidence based on the RCT. (Panel B) GRADE assessment of the quality of evidence based on the observational study.
Figure 2 illustrates a diagram of the network meta-analysis for the different interventions. A direct line between two interventions indicates evidence of direct comparison, while no line indicates no evidence of direct comparison. The size of the dots represents the different sample sizes, and the thickness of the lines represents the number of studies. The PHT, LEV, and placebo treatment groups had the largest sample sizes and the largest number of direct or indirect entries in the comparative trials.
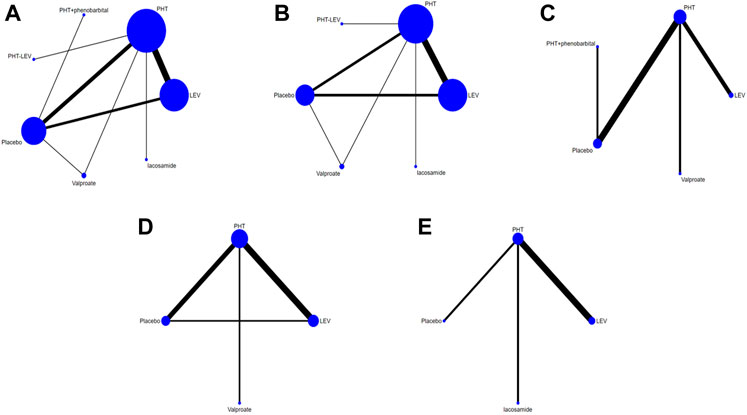
FIGURE 2. Network Chart; (A) The network chart of total seizures, (B). The network chart of early seizures, (C). The network chart of late seizures, (D). The network chart of death, (E). The network chart of the adverse effect.
Since both direct and indirect evidence was present in this study, consistency testing was performed before integrating the results. No evidence of inconsistency was found in this network model as the differences were no statistically significant (p > 0.05). Therefore the included direct and indirect evidence was combined.
Figure 3A shows the network meta-analysis sequence diagram for PTE occurrence after treatment with different ASMs. In this figure the larger area under the curve, this ASM has a lower incidence of PTE. The PTE reduction efficacy of six different ASMs, including PHT + PB, LEV, PHT, PHT-LEV, LCM, and VPA, were compared with a placebo. All treatments except VPA significantly reduced the incidence of PTE in TBI patients. PHT + PB had the highest impact in reducing PTE, followed by LEV, PHT, PHT-LEV, LCM, placebo, and VPA.
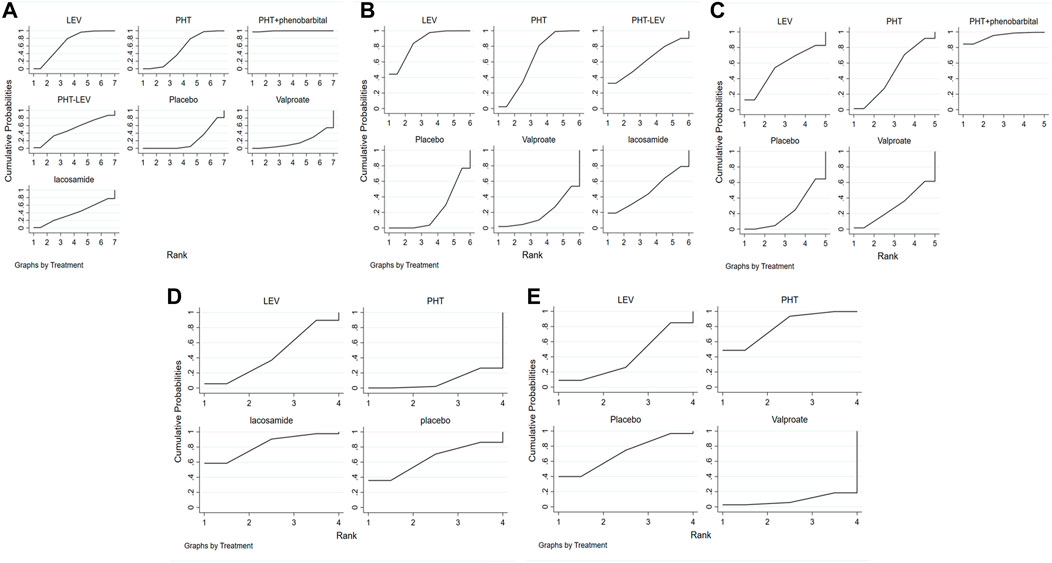
FIGURE 3. The Rank Chart; (A) The rank chart of total seizures, (B). The rank chart of early seizures, (C). The rank chart of late seizures, (D). The rank chart of the adverse effect, (E). The rank chart of death.
The occurrence of early seizures following treatment with five ASMs (LEV, PHT, PHT-LEV, LCM, VPA) was evaluated in 22 studies. Figure 3B shows the network meta-analysis sequence diagram for the rate of early seizures after treatment with different ASMs. In this figure the larger area under the curve, this ASM has a lower rate of early seizures. All treatments except for VPA significantly reduced the rate of early seizures compared to placebo in patients with TBI. LEV had the highest efficacy, followed by PHT, PHT-LEV, LCM, placebo, and VPA. Late seizures were reported in seven studies. Figure 3C shows the network meta-analysis sequence diagram for the rate of late seizures after treatment with different ASMs. In this figure the larger area under the curve, this ASM has a lower rate of late seizures. These studies evaluated four ASMs, including PHT + PB, LEV, PHT, and VPA. All the four regimens significantly reduced the rate of late seizures in patients with TBI compared with placebo. PHT + PB had the highest efficacy in reducing the onset of late seizures, followed by LEV, PHT, VPA.
The overall treatment-related adverse effects were reported in five studies for three different treatment regimes (PHT, LEV, and LCM). This analysis was used to compare the treatment-related adverse effects between different regimes. Figure 3D shows that the larger area under the curve, this ASM has a lower rate of treatment-related adverse effects. All treatment regimes except LCM had significantly higher treatment-related adverse effects when compared with a placebo. PHT had the highest rate of treatment-related adverse effects, followed by LEV, placebo, and LCM. Patient mortality for three treatment regimes (PHT, LEV, and VPA) was reported in nine studies. Figure 3E shows the network meta-analysis sequence diagram for the rate of mortality after treatment with different ASMs. In this figure the larger area under the curve, this ASM has a lower rate of mortality. Therefore, this figure showed that the mortality in patients treated with LEV and VPA was significantly higher than that in patients treated with the placebo, but the mortality in patients treated with PHT was lower that in patients treated with the placebo.
The treatment of PTE in patients with TBI is still controversial. PHT was the historical gold standard for the prevention of PTE, as revealed in a landmark randomized, double-blind, placebo-controlled trial conducted by Kruer et al. (2013). However, although PHT reduced the incidence of early seizures in TBI patients compared with the placebo, it did not reduce the incidence of late seizures or mortality. Conversely, a retrospective study conducted in 2013 by Bhullar et al. (2014) showed that PHT treatment is not effective in reducing early PTE.
For more than a decade, experts in neuroscience have explored the efficacy and safety of various ASMs in the prevention of PTE after TBI. However, the reported efficacy of these treatments in preventing PTE is still inconclusive. As a result, the current treatment for PTE is based on the recommendations of BTF guidelines, clinical experience, and the clinician’s subjective opinions. As a result, there is a need to identify the optimal ASMs treatment for TBI patients.
In this study, we for the first time conducted a comprehensive search of clinical studies related to all drug regimens for preventing seizures after TBI and used the principle of indirect comparison of the Network meta-analysis. By establishing a common control between all the ASMs, to compare the efficacy and safety of different ASMs.
In the beginning, we performed a traditional meta-analysis to compare the onset of early and late seizures and mortality in subgroups of patients treated with different ASMs. Consistent with the trial of Temkin et al. (1999), our findings showed that PHT could reduce the incidence of early seizures but not the incidence of late seizures and mortality in patients with TBI compared with placebo. Therefore, we hypothesized that PHT might be an effective ASM for the prevention of early PTE. Our analysis found no significant differences between PHT and LEV in preventing early and late seizures, treatment-related adverse effects, and mortality in patients with TBI. In the conventional meta-analysis by Zafar et al. (2012), PHT and LEV were found to be equally effective in preventing early and late seizures. However, the studies in the meta-analysis (Zafar et al., 2012) also included patients with brain injury caused by surgery, tumors, and spontaneous brain hemorrhage. Furthermore, Zafar et al. (2012) did not analyze the adverse effects and mortality associated with the two treatments. In a systematic evaluation published in 2016 (Xu et al., 2016), LEV and PHT were also found to be equally effective in preventing seizures in patients with brain injury. Nevertheless, in this study, LEV had a better safety profile than PHT. However, it is important to note that Xu et al. (2016). Included patients with brain injury caused by a spontaneous brain hemorrhage, while our study only included patients with brain injury caused by trauma. Therefore, we believe that the efficacy and safety of LEV and PHT in preventing seizures in patients with TBI still need to be further explored. In addition, we also found that VPA, PHT + LEV, and LCM did not reduce early seizures in patients with TBI compared with PHT. Conversely, VPA and PHT + LEV did not reduce late seizures in patients with TBI, but PHT combined with PB reduced late seizures in patients with TBI. The reliability of these conclusions requires further investigation as these regimens involved only one study with a small sample size and insufficient strength of evidence.
Subsequently, based on the available evidence and the results of the traditional meta-analysis, a network meta-analysis was performed to compare the direct and indirect outcomes of the six treatment regimens (PHT + PB, LEV, PHT, PHT-LEV, LCM, and VPA). This network meta-analysis included 25 studies with a total sample size of 6,466. The SUCRA ranking was used to compare the treatment efficacy, adverse effects, and mortality of all six regimens.
Twenty-two studies evaluated the impact of five treatment regimens, namely LEV, PHT, PHT-LEV, LCM, and VPA, in reducing the incidence of early PTE. All treatment regimes except VPA could significantly reduce the incidence of early PTE compared with placebo. In terms of late seizures, seven studies revealed that PHT + PB, LEV, PHT, and VPA could reduce the late PTE rate in TBI patients compared with placebo.
However, the current network meta-analysis included only one study on PHT combined with PB. The study had a very small sample size, so the role of this regimen in reducing the incidence of seizures after TBI still needs to be further explored.
In the nine studies evaluating patient mortality, LEV and VPA had higher patient mortality than placebo. However, there was no significant difference in the mortality between PHT and the placebo. In the ranking analysis for the five studies that evaluated treatment-related adverse effects, both LEV and PHT had higher treatment-related adverse effects than the placebo. Although PHT and LEV did not reduce the length of hospital stay, they significantly reduced the length of ICU stay.
This network meta-analysis has some limitations that have to be acknowledged. Studies evaluating PHT and LEV were based on very large-scale, high-quality randomized controlled trials. However, studies evaluating the remaining four treatment options involved a small number of studies. Therefore, the relevant ranking of these four treatment options is unreliable and still requires further pooled analysis. This network meta-analysis contains 18 non-randomized controlled trials with a low GRADE rating which may reduce the strength of evidence for the conclusions of this study. The number of studies reporting the incidence of late seizures, mortality, and treatment-related adverse effects was small and mostly involved an observational research design with a small sample size. Therefore the conclusions in this part of the study are less convincing and need to be revalidated by high-quality, large-sample randomized controlled trials. In addition, the overall incidence of treatment-related adverse effects was not explored in most of the six treatment regimens. As a result, we could not perform a clusterank analysis to evaluate the effectiveness and safety of the different treatments and the impact of adverse effects on treatment efficacy.
LEV and PHT prevented early and late PTE in patients with TBI. PHT also reduced the mortality rate in patients with TBI. Both LEV and PHT had higher treatment-related adverse effects compared with placebo. However, LEV had a slightly lower incidence of treatment-related adverse effects when compared with PHT. Compared with PHT, LEV did not reduce the length of hospital stay but shortened the length of ICU stays. Therefore, based on the findings of this meta-analysis, we speculate that LEV is the best treatment option for TBI patients. However, further high-quality randomized controlled trials are required to confirm these findings.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
TS performed the study subject and design, interpretation of data and manuscript drafting. XH and XX contributed to the data extraction, statistical analysis and manuscript revising. ML contributed to the data extraction and interpretation of data. LX and YW performed statistical analysis. WL and CW performed quality assessment.
This work was supported by Key Research and Development Program of Ningxia (2016B207).
We would like to thank TopEdit (www.topeditsci.com) for the English language editing of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1001363/full#supplementary-material
PHT, phenytoin; PB, phenobarbital; LEV, levetiracetam; LCM, lacosamide; VPA, valproate; LAM, lamotrigine; OCBZ, oxcarbazepine; CBZ, carbamazepine; TPM, topiramate; TBI, traumatic brain injury; PTE, post-traumatic epilepsy; AEDs, antiepileptic drugs; ICU, intensive care unit; BTF, Brain Trauma Foundation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; WHO, World Health Organization; NOS, Newcastle-Ottawa Scale; GRADE, Grading of Recommendations Assessment and Development and Evaluation; SUCRA, surface under the cumulative ranking curve.
Bhullar, I. S., Johnson, D., Paul, J. P., Kerwin, A. J., Tepas, J. R., and Frykberg, E. R. (2014). More harm than good: Antiseizure prophylaxis after traumatic brain injury does not decrease seizure rates but may inhibit functional recovery. J. Trauma Acute Care Surg. 76 (1), 54–60. discussion 60-1. doi:10.1097/TA.0b013e3182aafd15 | |
Caballero, G. C., Hughes, D. W., Maxwell, P. R., Green, K., Gamboa, C. D., and Barthol, C. A. (2013). Retrospective analysis of levetiracetam compared to phenytoin for seizure prophylaxis in adults with traumatic brain injury. Hosp. Pharm. 48 (9), 757–761. doi:10.1310/hpj4809-757 | |
Carney, N., Totten, A. M., O'Reilly, C., Ullman, J. S., Hawryluk, G. W., Bell, M. J., et al. (2017). Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 80 (1), 6–15. doi:10.1227/NEU.0000000000001432 | |
Castriotta, R. J., Wilde, M. C., Lai, J. M., Atanasov, S., Masel, B. E., and Kuna, S. T. (2007). Prevalence and consequences of sleep disorders in traumatic brain injury. J. Clin. Sleep. Med. 3 (4), 349–356. doi:10.5664/jcsm.26855 | |
Chartrain, A. G., Yaeger, K., Feng, R., Themistocleous, M. S., Dangayach, N. S., Margetis, K., et al. (2017). Antiepileptics for post-traumatic seizure prophylaxis after traumatic brain injury. Curr. Pharm. Des. 23 (42), 6428–6441. doi:10.2174/1381612823666171031100139 | |
Courchia, B., Kurtom, W., Pensirikul, A., Del-Moral, T., and Buch, M. (2018). Topiramate for seizures in preterm infants and the development of necrotizing enterocolitis. Pediatrics 142 (1), e20173971. doi:10.1542/peds.2017-3971 | |
Debenham, S., Sabit, B., Saluja, R. S., Lamoureux, J., Bajsarowicz, P., Maleki, M., et al. (2011). A critical look at phenytoin use for early post-traumatic seizure prophylaxis. Can. J. Neurol. Sci. 38 (6), 896–901. doi:10.1017/s031716710001249x | |
Degrauw, X., Thurman, D., Xu, L., Kancherla, V., and Degrauw, T. (2018). Epidemiology of traumatic brain injury-associated epilepsy and early use of anti-epilepsy drugs: An analysis of insurance claims data, 2004-2014. Epilepsy Res. 146, 41–49. doi:10.1016/j.eplepsyres.2018.07.012 | |
Gabriel, W. M., and Rowe, A. S. (2014). Long-term comparison of GOS-E scores in patients treated with phenytoin or levetiracetam for posttraumatic seizure prophylaxis after traumatic brain injury. Ann. Pharmacother. 48 (11), 1440–1444. doi:10.1177/1060028014549013 | |
Guyatt, G. H., Sackett, D. L., Sinclair, J. C., Hayward, R., Cook, D. J., and Cook, R. J. (1995). Users' guides to the medical literature. IX. A method for grading health care recommendations. Evidence-Based Medicine Working Group. JAMA 274 (22), 1800–1804. doi:10.1001/jama.274.22.1800 | |
Harris, L., Hateley, S., Tsang, K. T., Wilson, M., and Seemungal, B. M. (2020). Impact of anti-epileptic drug choice on discharge in acute traumatic brain injury patients. J. Neurol. 267 (6), 1774–1779. doi:10.1007/s00415-020-09769-5 | |
Hazama, A., Ziechmann, R., Arul, M., Krishnamurthy, S., Galgano, M., and Chin, L. S. (2018). The effect of keppra prophylaxis on the incidence of early onset, post-traumatic brain injury seizures. Cureus 10 (5), e2674. doi:10.7759/cureus.2674 | |
Inaba, K., Menaker, J., Branco, B. C., Gooch, J., Okoye, O. T., Herrold, J., et al. (2013). A prospective multicenter comparison of levetiracetam versus phenytoin for early posttraumatic seizure prophylaxis. J. Trauma Acute Care Surg. 74 (3), 766–771. discussion 771-3. doi:10.1097/TA.0b013e3182826e84 | |
Javed, G., Waqas, M., Qadeer, M., Bari, E., and Murtaza, G. (2016). Use of levetiracetam in prophylaxis of early post-traumatic seizures. Turk. Neurosurg. 26 (5), 732–735. doi:10.5137/1019-5149.JTN.8245-13.1 | |
Jones, K. E., Puccio, A. M., Harshman, K. J., Falcione, B., Benedict, N., Jankowitz, B. T., et al. (2008). Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurg. Focus. 25 (4), E3. doi:10.3171/FOC.2008.25.10.E3 | |
Kaufman, K. R. (2011). Antiepileptic drugs in the treatment of psychiatric disorders. Epilepsy Behav. 21 (1), 1–11. doi:10.1016/j.yebeh.2011.03.011 | |
Khan, S. A., Bhatti, S. N., Khan, A. A., Khan, A. E., Muhammad, G., Gul, N., et al. (2016). Comparison of efficacy of phenytoin and levetiracetam for prevention of early post traumatic seizures. J. Ayub Med. Coll. Abbottabad. 28 (3), 455–460. |
Khor, D., Wu, J., Hong, Q., Benjamin, E., Xiao, S., Inaba, K., et al. (2018). Early seizure prophylaxis in traumatic brain injuries revisited: A prospective observational study. World J. Surg. 42 (6), 1727–1732. doi:10.1007/s00268-017-4373-0 | |
Kirmani, B. F., Robinson, D. M., Fonkem, E., Graf, K., and Huang, J. H. (2016). Role of anticonvulsants in the management of posttraumatic epilepsy. Front. Neurol. 7, 32. doi:10.3389/fneur.2016.00032 | |
Klein, P., Herr, D., Pearl, P. L., Natale, J., Levine, Z., Nogay, C., et al. (2012). Results of phase 2 safety and feasibility study of treatment with levetiracetam for prevention of posttraumatic epilepsy. Arch. Neurol. 69 (10), 1290–1295. doi:10.1001/archneurol.2012.445 | |
Kruer, R. M., Harris, L. H., Goodwin, H., Kornbluth, J., Thomas, K. P., Slater, L. A., et al. (2013). Changing trends in the use of seizure prophylaxis after traumatic brain injury: A shift from phenytoin to levetiracetam. J. Crit. Care. 28 (5), e9–13. doi:10.1016/j.jcrc.2012.11.020 | |
Kwon, S. J., Barletta, J. F., Hall, S. T., Mangram, A. J., Dzandu, J. K., Abdulhamid, M., et al. (2019). Lacosamide versus phenytoin for the prevention of early post traumatic seizures. J. Crit. Care. 50, 50–53. doi:10.1016/j.jcrc.2018.11.010 | |
Lee, Y. H. (2018). An overview of meta-analysis for clinicians. Korean J. Intern. Med. 33 (2), 277–283. doi:10.3904/kjim.2016.195 | |
Liou, J., Chang, Y., Lee, H., Wu, M., Hou, Y., and Liou, W. (2020). Preventing epilepsy after traumatic brain injury: A propensity score analysis. J. Chin. Med. Assoc. 83 (10), 950–955. doi:10.1097/JCMA.0000000000000414 | |
Luo, J., Nguyen, A., Villeda, S., Zhang, H., Ding, Z., Lindsey, D., et al. (2014). Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front. Neurol. 5, 12. doi:10.3389/fneur.2014.00012 | |
Ma, C. Y., Xue, Y. J., Li, M., Zhang, Y., and Li, G. Z. (2010). Sodium valproate for prevention of early posttraumatic seizures. Chin. J. Traumatol. 13 (5), 293–296. |
Muccigrosso, M. M., Ford, J., Benner, B., Moussa, D., Burnsides, C., Fenn, A. M., et al. (2016). Cognitive deficits develop 1month after diffuse brain injury and are exaggerated by microglia-associated reactivity to peripheral immune challenge. Brain Behav. Immun. 54, 95–109. doi:10.1016/j.bbi.2016.01.009 | |
Nguyen, J. V., Yaw, T., and Gratton, H. (2021). Incidence of neurobehavioral side effects associated with levetiracetam compared to phenytoin in traumatic brain injury patients. Brain Inj. 35 (8), 902–906. doi:10.1080/02699052.2021.1927184 | |
Saletti, P. G., Ali, I., Casillas-Espinosa, P. M., Semple, B. D., Lisgaras, C. P., Moshe, S. L., et al. (2019). In search of antiepileptogenic treatments for post-traumatic epilepsy. Neurobiol. Dis. 123, 86–99. doi:10.1016/j.nbd.2018.06.017 | |
Servit, Z., and Musil, F. (1981). Prophylactic treatment of posttraumatic epilepsy: Results of a long-term follow-up in czechoslovakia. Epilepsia 22 (3), 315–320. doi:10.1111/j.1528-1157.1981.tb04115.x | |
Szaflarski, J. P., Sangha, K. S., Lindsell, C. J., and Shutter, L. A. (2010). Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit. Care. 12 (2), 165–172. doi:10.1007/s12028-009-9304-y | |
Temkin, N. R., Dikmen, S. S., Anderson, G. D., Wilensky, A. J., Holmes, M. D., Cohen, W., et al. (1999). Valproate therapy for prevention of posttraumatic seizures: A randomized trial. J. Neurosurg. 91 (4), 593–600. doi:10.3171/jns.1999.91.4.0593 | |
Temkin, N. R., Dikmen, S. S., Wilensky, A. J., Keihm, J., Chabal, S., and Winn, H. R. (1990). A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N. Engl. J. Med. 323 (8), 497–502. doi:10.1056/NEJM199008233230801 | |
Turnbull, D., Singatullina, N., and Reilly, C. (2016). A systematic appraisal of neurosurgical seizure prophylaxis: Guidance for critical care management. J. Neurosurg. Anesthesiol. 28 (3), 233–249. doi:10.1097/ANA.0000000000000206 | |
Vella, M. A., Crandall, M. L., and Patel, M. B. (2017). Acute management of traumatic brain injury. Surg. Clin. North Am. 97 (5), 1015–1030. doi:10.1016/j.suc.2017.06.003 | |
Vos, B. C., Nieuwenhuijsen, K., and Sluiter, J. K. (2018). Consequences of traumatic brain injury in professional American football players: A systematic review of the literature. Clin. J. Sport Med. 28 (2), 91–99. doi:10.1097/JSM.0000000000000432 | |
Winkler, E. A., Minter, D., Yue, J. K., and Manley, G. T. (2016). Cerebral edema in traumatic brain injury: Pathophysiology and prospective therapeutic targets. Neurosurg. Clin. N. Am. 27 (4), 473–488. doi:10.1016/j.nec.2016.05.008 | |
Wohns, R. N., and Wyler, A. R. (1979). Prophylactic phenytoin in severe head injuries. J. Neurosurg. 51 (4), 507–509. doi:10.3171/jns.1979.51.4.0507 | |
Xu, J. C., Shen, J., Shao, W. Z., Tang, L. J., Sun, Y. Z., Zhai, X. F., et al. (2016). The safety and efficacy of levetiracetam versus phenytoin for seizure prophylaxis after traumatic brain injury: A systematic review and meta-analysis. Brain Inj. 30 (9), 1054–1061. doi:10.3109/02699052.2016.1170882 | |
Young, B., Rapp, R. P., Norton, J. A., Haack, D., Tibbs, P. A., and Bean, J. R. (1983b). Failure of prophylactically administered phenytoin to prevent early posttraumatic seizures. J. Neurosurg. 58 (2), 231–235. doi:10.3171/jns.1983.58.2.0231 | |
Young, B., Rapp, R. P., Norton, J. A., Haack, D., Tibbs, P. A., and Bean, J. R. (1983a). Failure of prophylactically administered phenytoin to prevent late posttraumatic seizures. J. Neurosurg. 58 (2), 236–241. doi:10.3171/jns.1983.58.2.0236 | |
Younus, S. M., Basar, S., Gauri, S. A., Khan, A. A., Imran, M., Abubakar, S., et al. (2018). Comparison of phenytoin versus levetiracetam in early seizure prophylaxis after traumatic brain injury, at a tertiary care hospital in karachi, Pakistan. Asian J. Neurosurg. 13 (4), 1096–1100. doi:10.4103/ajns.AJNS_125_17 | |
Yuan, W. H., and Wang, S. J. (2020). Posttraumatic epilepsy after traumatic brain injury and prophylactic administration of antiepileptic drugs. J. Chin. Med. Assoc. 83 (10), 885–886. doi:10.1097/JCMA.0000000000000395 | |
Zafar, S. N., Khan, A. A., Ghauri, A. A., and Shamim, M. S. (2012). Phenytoin versus Leviteracetam for seizure prophylaxis after brain injury - a meta analysis. BMC Neurol. 12, 30. doi:10.1186/1471-2377-12-30 | |
Keywords: traumatic brain injury, post-traumatic epilepsy, antiepileptic drugs, PHT, LEV
Citation: Huo X, Xu X, Li M, Xiao L, Wang Y, Li W, Wang C and Sun T (2022) Effectiveness of antiseizure medications therapy in preventing seizures in brain injury patients: A network meta-analysis. Front. Pharmacol. 13:1001363. doi: 10.3389/fphar.2022.1001363
Received: 23 July 2022; Accepted: 30 August 2022;
Published: 15 September 2022.
Edited by:
Rita Citraro, University Magna Graecia of Catanzaro, ItalyReviewed by:
Ke-Yang Chen, Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, ChinaCopyright © 2022 Huo, Xu, Li, Xiao, Wang, Li, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Sun, c3VudGFvX254bXVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.