
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 17 January 2022
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.817885
This article is part of the Research Topic Dietary Supplements, Botanicals and Herbs at The Interface of Food and Medicine View all 37 articles
Barleria terminalis Nees and Calacanthus grandiflorus (Dalzell) Radlk. are endemic medicinal plants of the Western Ghats of India. The aim of the present research work was to investigate phytochemical profile, potent bioactives using RP-HPLC, LC-MS and GC-MS and to evaluate their bioactivities. Acetone was found to be the best extraction medium for separating phytochemicals. Similarly, acetone and methanol extracts exhibited potential antioxidant properties. Ethanol extract of B. terminalis stem showed potent acetylcholinesterase (AChE) (89.10 ± 0.26%) inhibitory activity. Inhibition of α-amylase (36.96 ± 2.96%) activity was observed the best in ethanol extract of B. terminalis leaves and α-glucosidase inhibitory activity (94.33 ± 0.73%) in ethanol extract of C. grandiflorus stem. RP-HPLC analysis confirmed the presence of several phenolic compounds (gallic acid, hydroxybenzoic acid, vanillic acid, chlorogenic acid and coumaric acid) and phenylethanoid glycoside (verbascoside). The highest phenolics content were observed in B. terminalis (GA (4.17 ± 0.002), HBA (3.88 ± 0.001), VA (4.54 ± 0.001), CHLA (0.55 ± 0.004) mg/g DW, respectively). Similarly, LC-MS and GC-MS revealed the presence of phenolics, glycosides, terpenes, steroids, fatty acids, etc. Moreover, positive correlation between studied phytochemicals and antioxidants was observed in principal component analysis. Based on the present investigation, we conclude that B. terminalis and C. grandiflorus can be further explored for their active principles particularly, phenylethanoid glycosides and iridoids and their use in drug industry for pharmaceutical purposes.
Acanthaceae Juss. is a large family comprising about 4,000 species and 220 genera over the globe. It comprises annual, perennial, herbs, shrubs or trees found in tropical, subtropical and a few in temperate regions (Kavitha et al., 2016). Barleria terminalis Nees and Calacanthus grandiflorus (Dalzell) Radlk. are the endemic plants of the Western Ghats of India (Tripp et al., 2013; Patil et al., 2019). The genus Barleria L. contains 285 species found in Asia and Africa, with the majority of its diversity found in tropical eastern and southern Africa (Darbyshire et al., 2019). In India, the genus is represented by 30 species (Patil et al., 2020). Extensive literature survey showed that Barleria is medicinally important genus and locally called as Vajradanti (Banerjee et al., 2012). The plant is widely used in the treatment of human diseases like anemia, toothache, cough, fever, asthma, bronchitis, diabetes, insect bites and inflammations (Banerjee et al., 2012; Sudheer and Praveen, 2021). Aerial parts and leaves of Barleria are known to be a vital source of iridoids (Amoo et al., 2011; Sudheer and Praveen, 2021). The leaves of the plant are known for flavonoids, saponins, sterols, tannins, and terpenoids. Similarly, flowers contain flavonoids and neohesperidoside. In the same way, aerial part contains balarenone, terpenoid, barlerinoside, saponins, flavonoids, phenolics, tannins, steroids, carbohydrates, phytosterols, acetyl-barlerin, β-sitosterol, iridoids and lupulinoside (Ata et al., 2009). This plant contains numerous medicinal properties (Sudheer and Praveen, 2021). On the other hand, there are no reports on the phytochemical or medicinal potential of C. grandiflorus.
So keeping in view the therapeutic potential of Barleria and Calacanthus, leaves and stem extracts of B. terminalis and C. grandiflorus were prepared for the first time utilizing a variety of solvents. The present study was designed to explore the phytochemicals and biological potential of both the species (antioxidant, anti-acetylcholine esterase and antidiabetic) as well as to identify and quantify phenolics and phenylethanoid glycoside (verbascoside) using RP-HPLC. Furthermore, LC-MS and GC-MS were used to screen potent secondary metabolites present in the plants. To the best of our knowledge, this is the first study to look into the potential benefits of the medicinal plants, B. terminalis and C. grandiflorus.
B. terminalis was collected from Lead Botanical Garden, Shivaji University, Kolhapur, Maharashtra (N 16°40.546’, E 074°15.337’). C. grandiflorus were collected from Kumbhavade ghat, Sindhudurg district, Maharashtra (N 16°31.51.9′, E 73°50′01.4′). Both the specimens were deposited in SUK herbarium (Voucher No. SSO 001, SSO 030). Plant material was oven dried for 72 h at 60°C, ground into fine powder, and extraction was done by continuous shaking of finely ground powder (5 g) in respective solvents (30 ml each). Extracts were then centrifuged (5,000 rpm), concentrated and dissolved in 5 ml respective solvent. Prior to analysis, extracts were filtered through 0.2 µm nylon filter (HiMedia, India), used in experiment and stored at 4°C.
TPC was determined as per the method adopted by Patel and Ghane (2021) with some minor modifications. Results were expressed as mg tannic acid equivalent (TAE)/g extract. TFC was quantified according to method described by Sakanaka et al. (2005) and TTC was estimated as per Patel and Ghane (2021) with minor modifications. Catechin was used as standard (mg/ml) and results were expressed in mg catechin equivalent (CE)/g extract.
Total proanthocyanidins were calculated according to the method described by Fawole et al. (2009) with minor modifications. Proanthocyanidins content was expressed as % per g DW and calculated by the following formula:
TIC was estimated according to method described by Levieille and Wilson (2002) with minor modifications. Results were expressed as mg harpagoside equivalent (HE)/g extract.
DPPH radical scavenging activity was determined as per earlier method (Attar and Ghane, 2021). The activity was expressed as mg ascorbic acid equivalent (AAE)/g extract. The FRAP method proposed by Benzie and Strain (1996) was adopted. FRAP values were indicated as mg Fe (II) equivalent/g extract. ABTS radical activity was evaluated according to Patel et al. (2018) with some minor modifications. Activity was expressed as mg trolox equivalent (TE)/g extract. Metal chelating activity was calculated as per Patel and Ghane (2021) and the activity expressed as mg EDTA equivalent (EE)/g extract. Phosphomolybdenum assay was performed according to method described by Prieto et al. (1999). Results were depicted as mg of ascorbic acid equivalent (AAE)/g extract.
AChE inhibitory activity was examined according the method of Ghane et al. (2018) with some minor modifications. Galanthamine hydrobromide was used as standard, and results were expressed in percentage. Inhibition of α-amylase and α-glucosidase was evaluated as per our earlier protocol (Ghane et al., 2018). Acarbose was used as a positive control and activity was evaluated on a percent basis.
Finely ground powder (500 mg) of B. terminalis and C. grandiflorus (leaves and stem) was extracted by using 10 ml methanol in an ultrasound bath. Homogenate was centrifuged, and supernatant collected, condensed and volume adjusted to 1 ml. Prior to analysis, the extract was filtered using 0.2 µm nylon filter (HiMedia, India). Different concentrations of standard solutions (20–100 μg/ml) were prepared and used to plot calibration curve using RP-HPLC.
HPLC apparatus consisted of quaternary pump, autosampler and UV detector (UV 2070) (Jasco, Japan, Model no. LC-2000 plus). Separation was performed using Hiber C18 column (5 μm, 250–4.6 mm). Mobile phase consisted of water: acetonitrile: glacial acetic acid (90:5:5), flow rate was 0.9 ml/min and 20 μl injection volume (Patel and Ghane, 2021). Sample peaks were monitored at 280 nm with 60 min as run time. Phenolic content was determined by comparing with standards and expressed as milligram per gram of dry weight (mg/g DW).
RP-HPLC analysis of verbascoside was carried out using the same instrumentation as specified above. Separation of compound was achieved using methanol and water (90:10) as mobile phase with 1 ml/min flow rate and 20 μl injection volume (Dhakulkar et al., 2005). The peak was monitored at 320 nm with 30 min as run time. Experiments were performed in triplicates for assessing suitability of system and amount of verbascoside expressed as µg/g DW.
LC-MS analysis was done by using HPLC-ESI-MS-NEG-PHENOMENEX in negative ionization mode. System was equipped with binary pump, auto sampler, thermostated column compartment and iFunnel quadrapole time-of-flight spectrometer (Q-TOF). Zorbax eclipse C18 column (4.6 × 250 mm, 5 μm particle size) was used for compounds separation at 25°C temperature. In the present study, 0.1% (v/v) formic acid (A) and acetonitrile (B) was used in gradient elution. Gradient was initiated at 80% A and 20% B to 30% B (after 10 min), followed by 40% B (40 min), 60% B (60 min) and 90% B (80 min) and finally returned to the initial conditions. Solvent system B was injected with flow rate of 0.8 ml/min. Mass spectrometer was operated in range of 100–1,000 m/z. N2 gas was used as a nebulizer. Drying gas flow rate was 8 L/min at 325°C and nebulizer gas at 25 psi with fragmentor voltage 150 V (Patel and Ghane, 2021). For data analysis, mass hunter qualitative analysis software package (Agilent Technologies) was used. Detected compounds were validated on the basis of molecular formula, molecular mass, retention time and m/z ratio. For the authentication of compounds, details were compared with available literature and Metline personal metabolites database.
GC-MS analysis was performed on Model QP 2010 series, Shimadzu, Tokyo, Japan, equipped with AOC-20i auto sampler and RTX-1 fused silica capillary column (30 m length, 0.25 mm id, and 0.25 µm thickness). Helium gas (purity 99.99%) was used as a carrier gas at a flow rate of 1.5 ml/min. The injector temperature was fixed at 280°C and samples were injected through split injection mode. The column oven program was set at 50°C for 2 min, then increased to 28°C with the rate of 10°C/min. Ion source temperature was applied to 230°C and interface temperature was set to 250°C. The mass range from 36 to 800 m/z was scanned at a rate of 3.0 scans/s. The total run time of GC-MS system was 36 min (Patel and Ghane, 2021). Compounds were identified by comparison with authentic spectra obtained from GC-MS library (NIST 11).
All analyses were performed in triplicates and values are represented as average, with standard error. Data obtained from the experiments were subjected to one-way analysis of variance and significant differences between mean values were determined by Duncan’s multiple range test (p ≤ 0.05) using SPSS software ver. 16. Data derived from the studied phytochemicals and antioxidant activities from different extracts were subjected to Principal Component Analysis (PCA) (Minitab software ver. 19).
In phytochemicals such as TPC, TFC, TTC, proanthocyanidins and TIC contents were determined and results are represented in Table 1. Among all the samples, Calacanthus grandiflorus leaves extract showed comparatively higher yield as compared to the stem extracts that ranged from 2.36 to 5.69%.
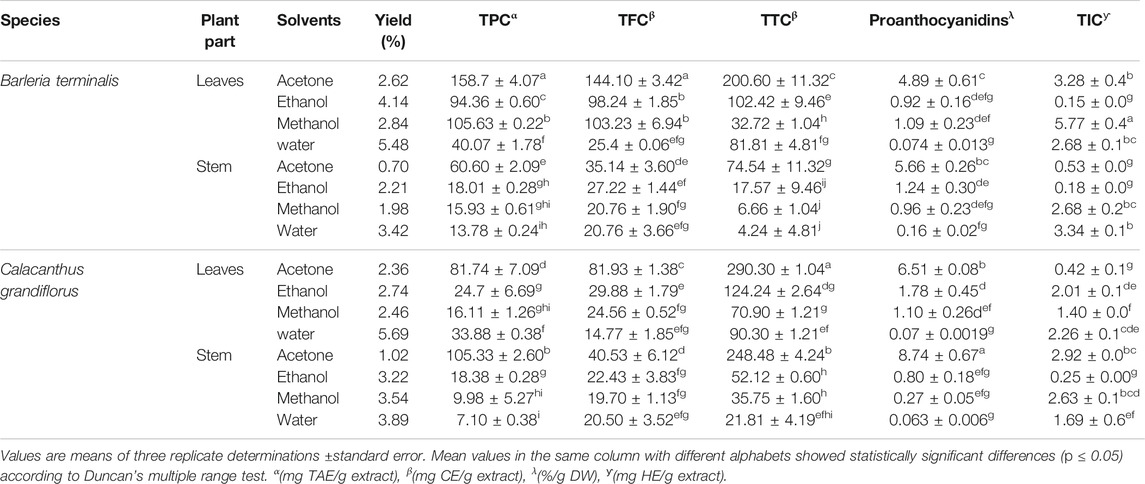
TABLE 1. Extract yield, total phenolics content (TPC), total flavonoids content (TFC), total tannins content (TTC), proanthocyanidins and total iridoids content (TIC) of different solvent extracts of B. terminalis and C. grandiflorus.
In the case of B. terminalis, the highest TPC and TFC content were found in acetone leaves extract (158.76 ± 4.07 mg TAE/g extract and 144.10 ± 3.42 mg CE/g extract, respectively). TTC ranged from 4 to 290 mg CE/g extract where aqueous stem extract possessed the lowest tannin content (4.24 ± 1.21 mg CE/g extract). Similarly, total iridoid content was recorded the highest in methanol extract of leaves (5.77 ± 0.4 mg HE/g extract) and the lowest was found in ethanol extract of stem (0.18 ± 0.0 mg HE/g extract) (Table 1).
In C. grandiflorus, aqueous extract of stem had the least content (7.10 ± 0.38 mg TAE/g extract) of TPC. In the same way, the lowest TFC was exhibited in aqueous leaves extract (14.77 ± 1.85 mg CE/g extract). Acetone extract of leaves revealed promising tannin content (290.30 ± 1.04 mg CE/g extract). Moreover, proanthocyanidins content ranged from 0.06 to 9%/g DW and acetone extract of stem had the highest proanthocyanidins (8.74 ± 0.67%/g DW) whereas water extracts exhibited the least (0.063 ± 0.006%/g DW) content (Table 1).
Antioxidant activities like DPPH, FRAP, ABTS, MCA and PMA from different leaves and stem extracts of both the species are depicted in Figure 1. In B. terminalis, methanol extract of leaves showed promising DPPH scavenging and FRAP activity (182.59 ± 1.57 mg AAE/g extract and 668.24 ± 2.04 mg Fe(II)/g extract, respectively) (Figures 1A,B). Similarly, ABTS activity was noted the highest in the acetone extract of leaves (73.97 ± 0.04 mg TE/g extract) (Figure 1C). Acetone extract of leaves (797.61 ± 8.71 mg AAE/g extract) showed maximum PMA whereas the aqueous extract of stem exhibited the lowest activity (101.5 ± 18.95 mg AAE/g extract) (Figure 1E).
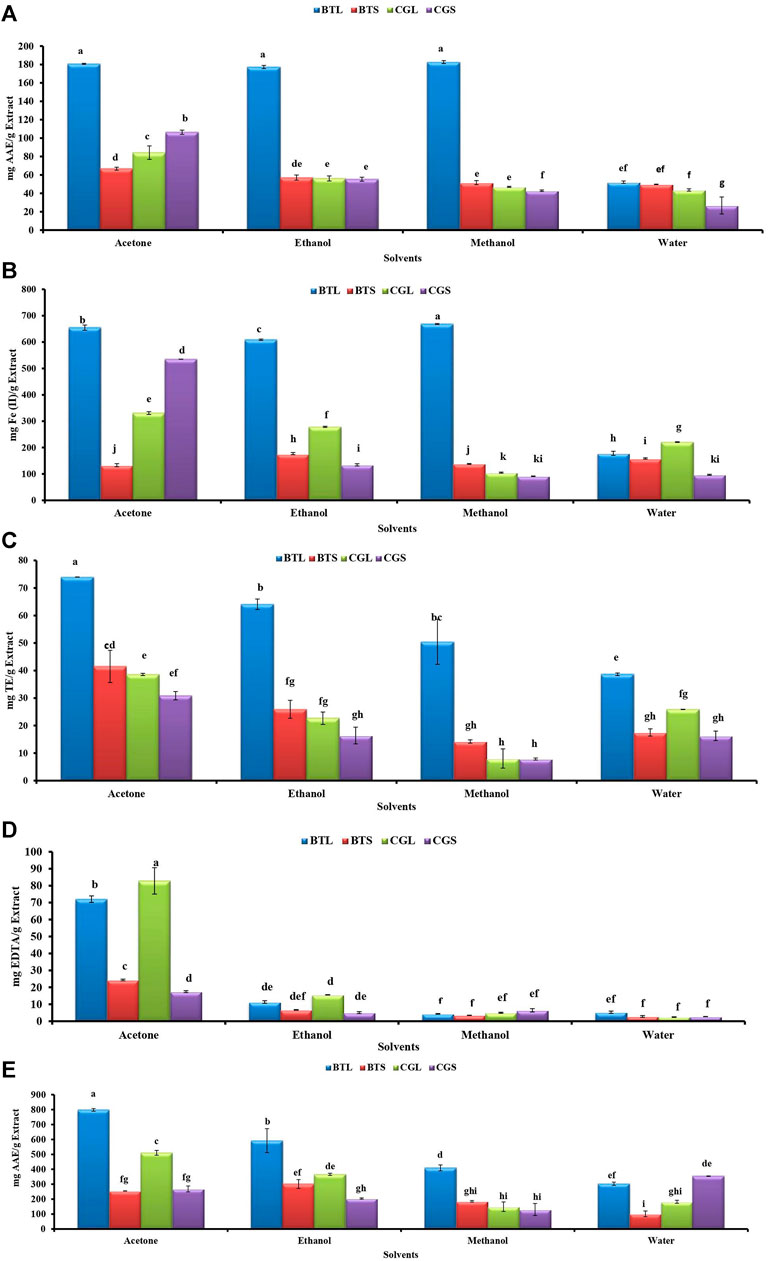
FIGURE 1. Antioxidant activities from the different extracts of B. terminalis and C. grandiflorus (A) DPPH radical scavenging activity, (B) FRAP activity, (C) ABTS + radical scavenging activity, (D) Metal chelating activity, (E) Phosphopmolybdenum reduction activity. Note—All figures indicate different extracts from leaves and stems of BTL—B. terminalis leaves, BTS—B. terminalis stem and CGL—C. grandiflorus leaves and CGS—C. grandiflorus stem. According to DMRT, Bars having different alphabets showed statistically significant differences (p ≤ 0.05).
In C. grandiflorus, DPPH activity was observed the least in the water extract of stem (26.79 ± 9.30 AAE/g extract) (Figure 1A). Methanol extract of stem exhibited the lowest FRAP activity (91.33 ± 0.94 mg Fe (II)/g extract) (Figure 1B). The lowest ABTS activity (7.85 ± 0.37 mg TE/g extract) was registered with methanol extract of stem (Figure 1C). Antioxidant activity in terms of MC was found superior in C. grandiflorus. The acetone extract of leaves exhibited the highest MC activity (82.83 ± 7.79 mg EDTA/g extract) whereas the least activity was observed in the water extracts (2.42 ± 0.14 mg EDTA/g extract) (Figure 1D).
The PCA was performed to understand the relationship between the phytochemicals (TPC, TFC, TTC, proanthocyanidins and TIC) and antioxidant activities (DPPH, FRAP, ABTS, PMA, and MCA) studied from acetone, ethanol, methanol and water extracts of leaves and stem of B. terminalis and C. grandiflorus (Figure 2). From Figures 2A,B, it was confirmed that only acetone along with ABTS, proanthocyanidins, PMA, MCA and TTC occupied positive plane of component 1 in leaves; while in stem with TPC and TTC occupied positive plane of both components. PCA analysis of C. grandiflorus leaves and stem denoted 97 and 93% total variability respectively, out of which component 1 contributed for 87 and 80% variability. Similar to B. terminalis leaves, TPC, TFC, TTC, DPPH, ABTS, FRAP, PMA, MCA and proanthocyanidins enjoyed the positive plane of component 1 and exhibited largest distribution with the coefficients 0.323, 0.331, 0.339, 0.335, 0.288, 0.275, 0.317, 0.336, and 0.331, respectively (Figure 2C). PCA of stem extract of C. grandiflorus showed that all tested parameters enjoyed positive plane of component 1. Among the solvents studied, only acetone and methanol represented positive planes on component 1 and 2, respectively. Variables exhibited the largest distribution with the coefficients 0.353, 0.352, 0.353, 0.339, 0.323, 0.353, 0.061, 0.345, 0.353, and 0.189, respectively (Figure 2D).
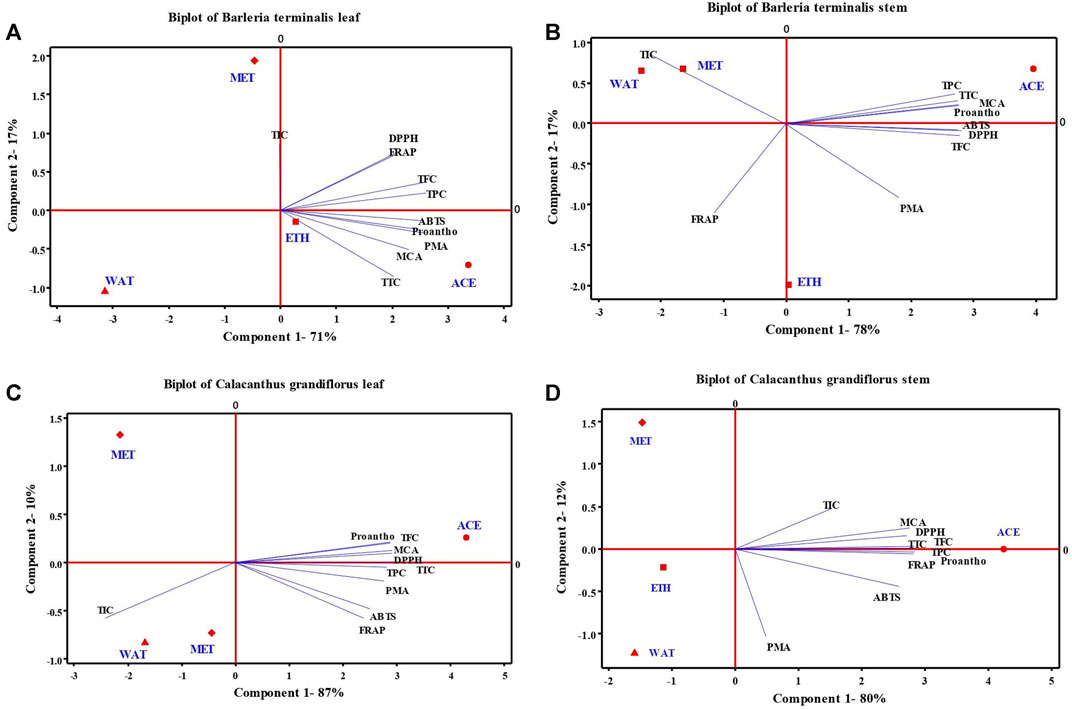
FIGURE 2. Principal component analysis (scores and loading plots, biplot) depends on several phytochemical compounds analyzed in four different leaves and stem extract of B. terminalis (A,B) and C. grandiflorus (C,D) with their antioxidant activities (DPPH, ABTS, FRAP, PMA, and MCA). TPC, total phenolic content; TFC, total flavonoid content; TTC, total tannin content; Proantho, Proanthocyanidins content; TIC, total iridoid content.
Anti-acetylcholinesterase (AChE) and anti-diabetic activities of different solvent extracts of leaves and stem of B. terminalis and C. grandiflorus were performed and the results are depicted in Table 2. Anti-diabetic activity was analyzed by using α-amylase and α-glucosidase inhibition. In B. terminalis, maximum α-amylase inhibition was found in the ethanol extract of leaves (36.96 ± 2.96%) and the least α-glucosidase inhibitory (2.38 ± 2.08%) activity in the aqueous extract of leaves. The ethanol extract of stem showed the highest AChE inhibition (89.10 ± 0.26%) while the aqueous extract the least (9.12 ± 0.26%) (Table 2). Similarly, methanolic extract of leaves of C. grandiflorus exhibited the lowest α-amylase (14.8 ± 0.37%) inhibitory activity whereas the ethanol extract of the stem exhibited the highest α-glucosidase inhibitory (94.33 ± 0.73%) activity (Table 2).
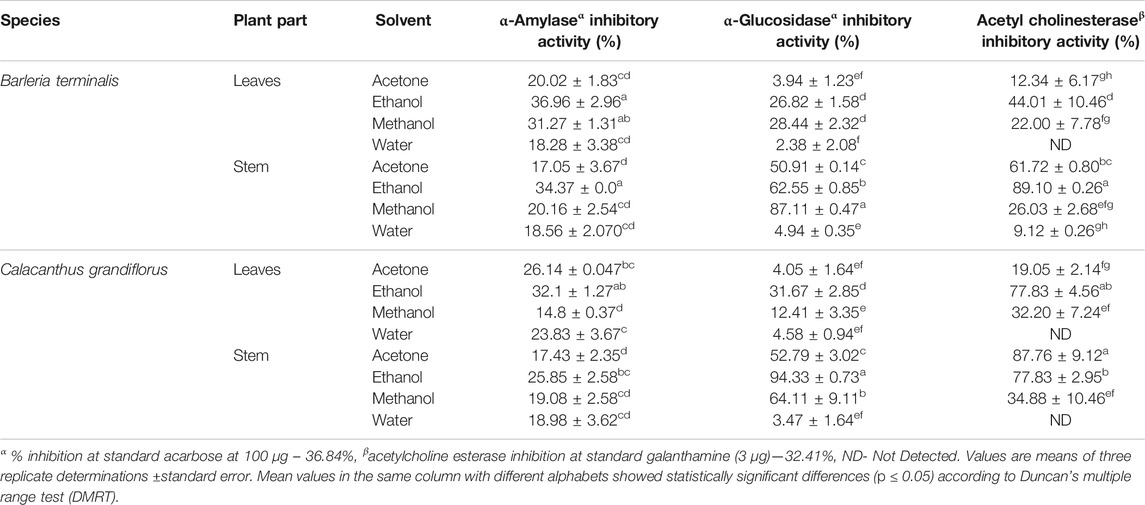
TABLE 2. α-Amylase, α-glucosidase, and acetyl cholinesterase inhibitory activities of leaves and stem extracts of B. terminalis and C. grandiflorus.
Results suggested that phenolics were present in the methanolic leaves and stem extracts of B. terminalis and C. grandiflorus. Five phenolic compounds, viz. gallic acid (GA) (tR 6.0), hydroxybenzoic acid (HBA) (tR 15.71), vanillic acid (VA) (tR 21.62), chlorogenic acid (CHLA) (tR 23.82), and coumaric acid (COA) (tR 44.87) were identified and quantified (Figure 3A; Table 3). The methanolic extract of stem of B. terminalis showed the highest GA content (4.17 ± 0.002 mg/g DW) whereas the lowest was observed in leaves (1.61 ± 0.003 mg/g DW). HBA content (3.88 ± 0.001 mg/g DW) was recorded maximum in leaves (Figure 3B). VA was found maximum in the leaves (4.54 ± 0.001 mg/g DW) and the minimum in the stem (0.45 ± 0.003 mg/g DW). CHLA was detected in very low quantity as compared to other phenolics. In all tested samples, the highest CHLA was recorded in the stem extract (0.55 ± 0.004 mg/g DW) (Table 3).
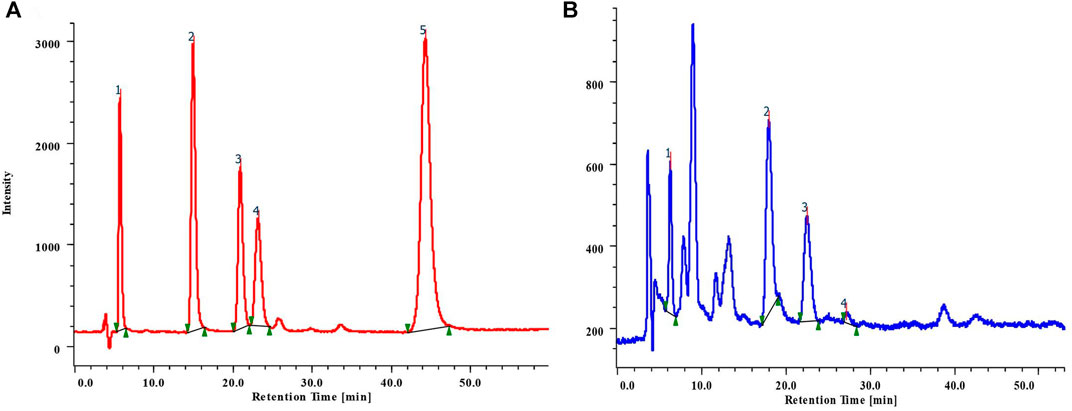
FIGURE 3. (A) RP-HPLC chromatogram of mixture of standard phenolic compounds 1) GA; 2) HBA; 3) VA; 4) CHLA; 5) COA; (B) Phenolics detected from the leaves extract of B. terminalis.
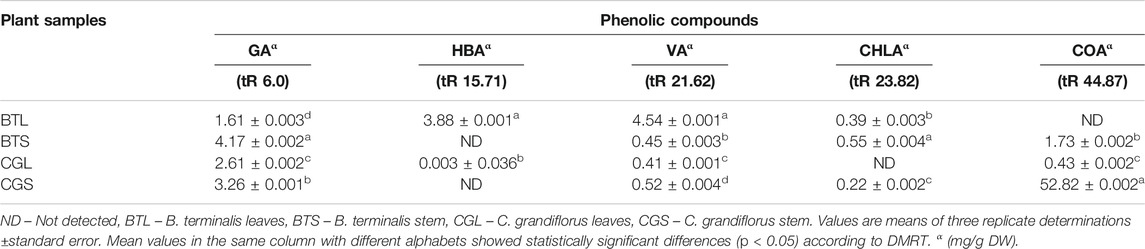
TABLE 3. HPLC analysis of phenolics from the leaves and stem extracts of B. terminalis and C. grandiflorus.
In the case of C. grandiflorus, significant amount of GA content was found in the stem (3.26 ± 0.001 mg/g DW) followed by the leaves (2.61 ± 0.002 mg/g DW) extract. VA was found maximum in the stem (0.52 ± 0.004 mg/g DW) and minimum in the leaves (0.41 ± 0.001 mg/g DW) extract. CHLA was observed to be the lowest in the stem (0.22 ± 0.002 mg/g DW) extract. The highest content of COA was reported in the stem (52.82 ± 0.002 mg/g DW) extract (Table 3).
Verbascoside was detected and quantified from the methanolic extracts of both the species (Figure 4 and Figure 5). All the plant parts showed remarkable quantity of verbascoside, in which the highest content was found in B. terminalis leaves (273.89 ± 0.6 μg/g DW) followed by B. terminalis stem (4.13 ± 0.1 μg/g DW) (Figure 5). Similarly, remarkable quantity of verbascoside was observed in C. grandiflorus stem (2.06 ± 0.1 μg/g DW) and leaves (1.56 ± 0.1 μg/g DW) (Figure 5). Compounds detected in extracts of B. terminalis and C. grandiflorus leaves by LC-MS are shown in Table 4 and Figures 6A,B. Likewise, using GC-MS several bioactive constituents were identified from the methanol extracts of both the species (Table 5). All the compounds from the both species were identified on the basis of chromatogram area, peak, molecular weight and molecular formula.
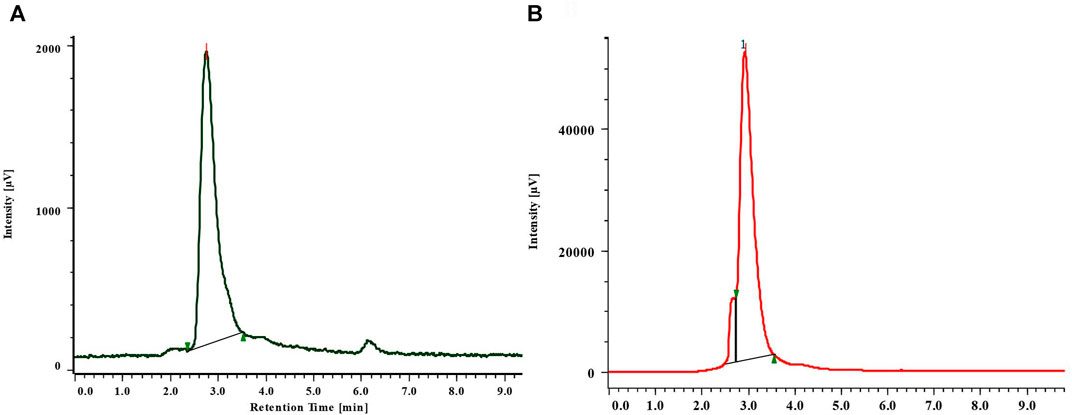
FIGURE 4. RP-HPLC chromatogram of standard (A) and verbascoside from the leaves of B. terminalis (B).
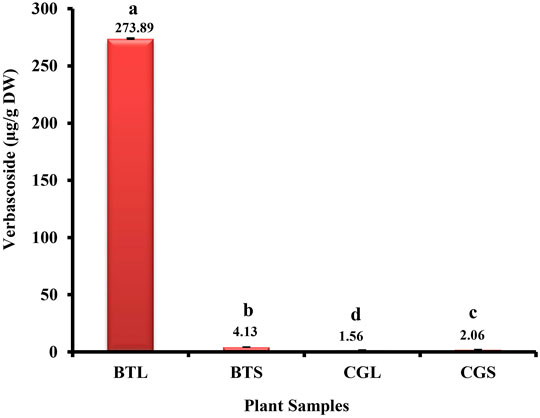
FIGURE 5. HPLC analysis of verbascoside from B. terminalis and C. grandiflorus. BTL—B. terminalis leaves, BTS—B. terminalis stem, CGL—C. grandiflorus leaves, CGS—C. grandiflorus stem.
FIGURE 6. Total ion chromatograms (TICs) of methanol extract of B. terminalis leaves (A) and C. grandiflorus leaves (B).
This investigation reported reliable amount of phenolics, flavonoids, tannins, proanthocyanidins and iriodoids from the leaves and stem of both the species. Our findings support the results of Amoo et al. (2011) who observed significant amount of phenolics, flavonoids, tannins, proanthocyanidins and iridoid content in methanolic extract leaves, stem and root of three Barleria species. Antioxidant potential of leaves and stem of B. terminalis and C. grandiflorus were comparable to the findings of Amoo et al. (2011) and Yadav et al. (2012). Extraction of phytochemicals depends on their solubility in different solvents and hence, the variability (quantitative and qualitative) in phytochemicals could be on account of different solvents used. Also, the extraction is dependent upon the extraction process, the genotype, and many intrinsic and extrinsic factors (Attar and Ghane, 2019).
Amoo et al. (2011) reported that methanol extract of leaves B. greenii and B. albostellata showed promising inhibition of AChE (68 and 22%, respectively). Investigations of α-amylase and α-glucosidase inhibitory activity are the key steps to discover the plants with antidiabetic potential as they play important role in the cure of diabetes (Ghane et al., 2018; Attar and Ghane, 2019; Patel et al., 2020; Taslimi et al., 2020). In the present investigation, correlation among phytochemicals and antioxidant activities from both the leaves and stem extracts of various solvents of both the species was found by PCA. Acetone was found to be the most superior for extracting phytochemicals. Acetone extracts showed higher antioxidant activity than ethanol, methanol and water extracts. This solvent can be used for the extraction of natural antioxidants from both the species.
HPLC analysis confirmed the presence of potent phenolics and phenylethanoid glycoside (verbascoside). Bioactive compounds found in plants also play important role in pharmaceutics and food industry, where some secondary metabolites showed potential biological activities (Nescatelli et al., 2017; Stefanucci et al., 2018; Uysal et al., 2019; Lekhak et al., 2021; Mollica et al., 2021; Patel and Ghane, 2021). Ranade et al. (2016) concluded that among phenolics, GA and CA were found the most common and abundant in leaves and stem of B. prionitis. Verbascoside was firstly isolated from the Mullein plant (Verbascum sinuatum L.). It has potent biological activities such as antioxidant, anti-inflammatory, antineoplastic, wound healing, neuroprotective, etc. (Alipieva et al., 2014). Kanchanapoom et al. (2004) isolated verbascoside from the methanolic extract of entire plant of B. strigosa. Additionally, LC-MS and GC-MS analysis revealed several potent metabolites from the methanolic extract of both species. Studies on phytochemical investigations supported appreciable bioactivities from the studied plants coupled with the presence of bioactive compounds.
The presence of considerable amount of phenolics, flavonoids, tannins, terpenoids, proanthocyanidins and iridoids in B. terminalis and C. grandiflorus led to appreciable antioxidant, antidiabetic, and anti-acetyl cholinesterase potential. Acetone was discovered to be the best solvent for extracting phytochemicals that exhibited potential antioxidant properties in the current study. Ethanol extracts of leaves and stem revealed promising antidiabetic and acetyl cholinesterase inhibitory activities. The presence of phenolics, verbascoside, and other bioactive substances were confirmed using RP-HPLC, LC-MS, and GC-MS. The best source of phenolics and phenylethanoid glycoside was discovered to be B. terminalis leaves. Biological activities and secondary metabolites differ from one organ to the other as well as from one season to the next. As India is home to 30 species of Barleria, it would be worthwhile to undertake extensive research to look into the phytochemical diversity in the genus and identify potential bioactive compounds that might be used to treat diabetes, neurological disorders, and produce new medications. In addition, the pharmaceutical industry needs to pay more attention to the creation of important pharmaceuticals (phenolics, iridoids, and phenylethanoid glycosides) from Barleria.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
SG designed the experiment. SO and SP carried out experiment, SO drafted manuscript and ML, SP, and SG critically reviewed the manuscript. SG participated in statistical analysis and helped to improve the manuscript. All authors contributed to the article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer VAB declared a shared affiliation, with the authors to the handling editor at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Authors are thankful to the Head, Department of Botany, Shivaji University, Kolhapur, India for providing necessary facility. UGC-DRS-SAP and DST-FIST programs are duly acknowledged.
ABTS, (2, 2′-azinobis 3-ethylbenzthiazoline-6-sulfonic acid); AChE, acetylcholinesterase; CHLA, chlorogenic acid; COA, coumaric acid; DPPH, 1,1-diphenyl-2-picrylhydrazyl; DNSA, dinitrosalicylic acid; DTNB, 5, 5-dithiobis-2-nitrobenzoic acid; DW, distilled water; FRAP, ferric reducing antioxidant property; GA, gallic acid; GC-MS, gas chromatography-mass spectrometry; HBA, hydroxybenzoic acid; HR-LC-MS, high-resolution liquid chromatography-mass spectrometry; MCA, metal chelating activity; PMA, phosphomolybdenum activity; PCA, principal component analysis; RP-HPLC, reversed phase high performance liquid chromatography; TIC, total iridoid content; TFC, total flavonoids content; TPC, total phenolic content; TPTZ-HCl, 2,4,6-Tripyridyl-S-triazine- hydrochloric acid; TTC, total tannin content; VA, vanillic acid.
Alipieva, K., Korkina, L., Orhan, I. E., and Georgiev, M. I. (2014). Verbascoside-a Review of its Occurrence, (Bio)synthesis and Pharmacological Significance. Biotechnol. Adv. 32, 1065–1076. doi:10.1016/j.biotechadv.2014.07.001
Amoo, S. O., Ndhlala, A. R., Finnie, J. F., and Van Staden, J. (2011). Antifungal, Acetylcholinesterase Inhibition, Antioxidant and Phytochemical Properties of Three Barleria Species. South Afr. J. Bot. 77, 435–445. doi:10.1016/j.sajb.2010.11.002
Ata, A., Kalhari, K. S., and Samarasekera, R. (2009). Chemical Constituents of Barleria Prionitis and Their Enzyme Inhibitory and Free Radical Scavenging Activities. Phytochemistry Lett. 2, 37–40. doi:10.1016/j.phytol.2008.11.005
Attar, U. A., and Ghane, S. G. (2019). In Vitro antioxidant, Antidiabetic, Antiacetylcholine Esterase, Anticancer Activities and RP-HPLC Analysis of Phenolics from the Wild Bottle Gourd (Lagenaria siceraria (Molina) Standl.). South Afr. J. Bot. 125, 360–370. doi:10.1016/j.sajb.2019.08.004
Attar, U. A., and Ghane, S. G. (2021). Proximate Composition, Ionomics, Phytochemical, Antioxidant, Anti-diabetic and Acetylcholinesterase Inhibitory Activity of Cucumis Species from Western Ghats of India. Indian J. Pharm. Sci. 83 (4), 679–694. doi:10.36468/pharmaceutical-sciences.819
Banerjee, D., Maji, A. K., Mahapatra, S., and Banerji, P. (2012). Barleria prionitis Linn.: A Review of its Traditional Uses, Phytochemistry, Pharmacology and Toxicity. Res. J. Phytochem. 6, 31–41. doi:10.3923/rjphyto.2012.31.41
Benzie, I. F., and Strain, J. J. (1996). The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: the FRAP Assay. Anal. Biochem. 239, 70–76. doi:10.1006/abio.1996.0292
Darbyshire, I., Tripp, E. A., and Chase, F. M. (2019). A Taxonomic Revision of Acanthaceae Tribe Barlerieae in Angola and Namibia. Part 1. Kew Bull. 74 (5), 1–85. doi:10.1007/s12225-018-9791-0
Dhakulkar, S., Ganapathi, T. R., Bhargava, S., and Bapat, V. A. (2005). Induction of Hairy Roots in Gmelina arborea Roxb. And Production of Verbascoside in Hairy Roots. Plant Sci. 169, 812–818. doi:10.1016/j.plantsci.2005.05.014
Fawole, O. A., Ndhlala, A. R., Amoo, S. O., Finnie, J. F., and Van Staden, J. (2009). Anti-inflammatory and Phytochemical Properties of Twelve Medicinal Plants Used for Treating Gastro-Intestinal Ailments in South Africa. J. Ethnopharmacol. 123, 237–243. doi:10.1016/j.jep.2009.03.012
Ghane, S. G., Attar, U. A., Yadav, P. B., and Lekhak, M. M. (2018). Antioxidant, Anti-diabetic, Acetylcholinesterase Inhibitory Potential and Estimation of Alkaloids (Lycorine and Galanthamine) from Crinum Species: An Important Source of Anticancer and Anti-alzheimer Drug. Ind. Crops Prod. 125, 168–177. doi:10.1016/j.indcrop.2018.08.087
Kanchanapoom, T., Noiarsa, P., Ruchirawat, S., Kasai, R., and Otsuka, H. (2004). Phenylethanoid and Iridoid Glycosides from the Thai Medicinal Plant, Barleria strigosa. Chem. Pharm. Bull. 52 (5), 612–614. doi:10.1248/cpb.52.612
Kavitha, K., Sujatha, K., and Manoharan, S. (2016). Antidiabetic Potential of Acanthaceae Family. Int. J. Pharm. Sci. Rev. Res. 36 (1), 30–37.
Lekhak, M. M., Patel, S. B., Otari, S. S., Lekhak, U. M., and Ghane, S. G. (2021). Bioactive Potential and RP-HPLC Detection of Phenolics and Alkaloids (Lycorine and Galanthamine) from Ultrasonic-Assisted Extracts of Crinum Roots. South Afr. J. Bot. doi:10.1016/j.sajb.2021.07.024
Levieille, G., and Wilson, G. (2002). In Vitro propagation and Iridoid Analysis of the Medicinal Species Harpagophytum procumbens and H . zeyheri. Plant Cell Rep. 21, 220–225. doi:10.1007/s00299-002-0520-6
Mollica, A., Scioli, G., Della Valle, A., Cichelli, A., Novellino, E., Bauer, M., et al. (2021). Phenolic Analysis and In Vitro Biological Activity of Red Wine, Pomace and Grape Seeds Oil Derived from Vitis vinifera L. Cv. Montepulciano d’Abruzzo. Antioxidants 10, 2–17. doi:10.3390/antiox10111704
Nescatelli, R., Carradori, S., Marini, F., Caponigro, V., Bucci, R., De Monte, C., et al. (2017). Geographical Characterization by MAE-HPLC and NIR Methodologies and Carbonic Anhydrase Inhibition of Saffron Components. Food Chem. 221, 855–863. doi:10.1016/j.foodchem.2016.11.086
Patel, S. B., and Ghane, S. G. (2021). Phyto-constituents Profiling of Luffa echinata and In Vitro Assessment of Antioxidant, Anti-diabetic, Anticancer and Anti-acetylcholine Esterase Activities. Saudi J. Biol. Sci. 28 (7), 3835–3846. doi:10.1016/j.sjbs.2021.03.050
Patel, S. B., Attar, U. A., and Ghane, S. G. (2018). Antioxidant Potential of Wild Lagenaria siceraria (Molina) Standl. Thai J. Pharm. Sci. 42, 90–96.
Patel, S. B., Attar, U. A., Sakate, D. M., and Ghane, S. G. (2020). Efficient Extraction of Cucurbitacins from Diplocyclos Palmatus (L.) C. Jeffrey: Optimization Using Response Surface Methodology, Extraction Methods and Study of Some Important Bioactivities. Sci. Rep. 10, 2109–2112. doi:10.1038/s41598-020-58924-5
Patil, S. S., Tamboli, A. S., Yadav, S. R., and Lekhak, M. M. (2019). A New Species of Barleria (Acanthaceae), its Morphotaxonomy, Cytogenetics and Phylogenetic Placement. Plant Syst. Evol. 305, 933–947. doi:10.1007/s00606-019-01613-2
Patil, S. S., Ganesan, R., Yadav, S. R., and Lekhak, M. M. (2020). Taxonomy of Barleria morrisiana E. Barnes & C. E. C. Fisch. (Acanthaceae), a Little-Known Species of the Biligiri Ranganathaswamy temple Tiger reserve, Karnataka, India. Curr. Sci. 119 (10), 1611–1612.
Prieto, P., Pineda, M., and Aguilar, M. (1999). Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 269, 337–341. doi:10.1006/abio.1999.4019
Ranade, R., Jain, A., and Joshi, N. (2016). Estimation of Phenolic Compounds by RP-HPLC and Antioxidant Activity in Leaf and Stem Extracts of Barleria prionitis L. Int. J. Pharm. Sci. Res. 7 (6), 2445–2457. doi:10.13040/IJPSR.0975-8232.7(6).2445-57
Sakanaka, S., Tachibana, Y., and Okada, Y. (2005). Preparation and Antioxidant Properties of Extracts of Japanese Persimmon Leaf tea (Kakinoha-Cha). Food Chem. 89, 569–575. doi:10.1016/j.foodchem.2004.03.013
Stefanucci, A., Mollica, A., Macedonio, G., Zengin, G., Ahmed, A. A., and Novellino, E. (2018). Exogenous Opioid Peptides Derived from Food Proteins and Their Possible Uses as Dietary Supplements: A Critical Review. Food Rev. Int. 34 (1), 70–86. doi:10.1080/87559129.2016.1225220
Sudheer, W. N., and Praveen, N. (2021). Phytochemical, Pharmacological and Tissue Culture Studies of Some Important Species of the Genus Barleria L. (Acanthaceae) - a Review. Plant Sci. Today 8 (3), 491–500. doi:10.14719/pst.2021.8.3.1117
Taslimi, P., Köksal, E., Gören, A. C., Bursal, E., Aras, A., Kılıç, Ö., et al. (2020). Anti-alzheimer, Antidiabetic and Antioxidant Potential of Satureja cuneifolia and Analysis of its Phenolic Contents by LC-MS/MS. Arabian J. Chem. 13, 4528–4537. doi:10.1016/j.arabjc.2019.10.002
Tripp, E. A., Daniel, T. F., Fatimah, S., and McDade, L. A. (2013). Phylogenetic Relationships within Ruellieae (Acanthaceae) and a Revised Classification. Int. J. Plant Sci. 174 (1), 97–137. doi:10.1086/668248
Uysal, A., Ozer, O. Y., Zengin, G., Stefanucci, A., Mollica, A., Picot-Allain, C. M. N., et al. (2019). Multifunctional Approaches to Provide Potential Pharmacophores for the Pharmacy Shelf: Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Comput. Biol. Chem. 78, 64–73. doi:10.1016/j.compbiolchem.2018.11.018
Keywords: biological activities, endemic, HPLC, LC-MS, multivariate analysis, phytochemicals
Citation: Otari SS, Patel SB, Lekhak MM and Ghane SG (2022) Phytochemical Studies on Two Unexplored Endemic Medicinal Plants of India, Barleria terminalis and Calacanthus grandiflorus. Front. Pharmacol. 12:817885. doi: 10.3389/fphar.2021.817885
Received: 18 November 2021; Accepted: 27 December 2021;
Published: 17 January 2022.
Edited by:
Alessandra Durazzo, Council for Agricultural Research and Economics, ItalyReviewed by:
Vishwas Ananat Bapat, Shivaji University, IndiaCopyright © 2022 Otari, Patel, Lekhak and Ghane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Savaliram G. Ghane, c2dnLmJvdGFueUB1bmlzaGl2YWppLmFjLmlu, Z2hhbmVyYW1AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.