
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 13 January 2022
Sec. Inflammation Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.814953
This article is part of the Research Topic Insights in Inflammation Pharmacology: 2022 View all 8 articles
Background: The pathogenesis of chronic rhinosinusitis with nasal polyps (CRSwNP) and mechanisms underlying different responses to systemic glucocorticoids (GC) remain unclear. The major aim of this study was to explore the transcriptomic and oxidative lipidomic signatures and the effects of GC in patients with different clinical responses.
Methods: Nasal polyp biopsies were obtained before and after 14-day oral GC treatment from 16 patients with CRSwNP, and normal nasal mucosa specimens were collected from 12 control subjects. RNA sequencing and oxidative lipidomics were performed, and differential gene expression analysis was conducted in the Responder and Non-responder groups at baseline and after treatment.
Results: In the Responder group, GC significantly improved clinical symptoms and reduced tissue eosinophil infiltration. Meanwhile, GC led to a pronounced transcriptomic reversion with robust suppression of inflammatory responses and abnormal metabolism of extracellular matrix, as well as restoration of cilia function. However, non-responders were mainly characterized by epithelial hyperplasia and keratinization, with much less transcriptomic improvement after GC treatment. Higher expression of type 2 inflammatory molecules (CCL13, IGHE, CCL18, CCL23, CCR3, and CLC) with lower levels of LACRT, PPDPFL, DES, C6, MUC5B, and SCGB3A1 were related to a stronger clinical response to GC. Besides decreased prostaglandins and increased leukotrienes, increased dysregulation in other oxylipid mediators derived from polyunsaturated fatty acids was determined in nasal polyps, which was ameliorated by GC treatment.
Conclusion: Systemic GC exert anti-inflammatory effects, improve tissue remodeling, restore cilia function, and ameliorate dysregulation of oxylipid mediator pathway in CRSwNP. GC-responders exhibited different transcriptomic signatures from non-responders.
Chronic rhinosinusitis with nasal polyp (CRSwNP) is a common chronic inflammatory disorder leading to nasal obstruction, rhinorrhea, loss of smell, and facial pain for over 12 weeks (Fokkens et al., 2020). CRSwNP is a heterogeneous condition with different phenotypes and endotypes. Most cases in western countries are characterized by T-helper (Th) 2 biased inflammation and tissue eosinophilia; however, Chinese patients present with mixed Th1/Th2/Th17 responses and tissue neutrophilia (Zhang et al., 2008; Cao et al., 2009; Wang et al., 2016). Thus, elucidating the poorly understood pathogenesis of CRSwNP may help develop new potential therapeutic targets.
Oxylipins formed from polyunsaturated fatty acids (PUFAs) via lipoxygenase (LOX) and cyclooxygenase (COX) pathways play key roles in apoptosis, tissue repair, blood vessel permeability, inflammation, and immune activity (Gabbs et al., 2015). Prostaglandin (PG) and leukotrienes (LT) are the most widely investigated eicosanoids in allergic diseases and CRSwNP (Peebles, 2019; Miyata et al., 2020a). Dysregulation in the arachidonic acid (AA) metabolism pathway, which is characterized by upregulation of the proinflammatory leukotriene pathway and downregulation of the anti-inflammatory prostaglandin E2 (PGE2) pathway, has been detected in nasal polyps, especially in eosinophilic cases and patients with aspirin-exacerbated respiratory diseases (AERD) (Pérez-Novo et al., 2005; Wu et al., 2016; Kowalski et al., 2019). Multi-omics analysis has revealed that LTD4 production in nasal polyp-derived eosinophils is selectively enhanced (Miyata et al., 2019). Other oxylipids derived from PUFAs also play an important role in inflammatory processes (Marion-Letellier et al., 2015). For example, AA-derived lipoxins (LXA4 and LXB4), docosahexaenoic acid (DHA)-derived protectins (PD1 and PDX), resolvin (Rv) D series and maresins (Mar-1 and Mar-2), and eicosapentaenoic acid (EPA)-derived resolvin E series are generally termed specialized pro-resolving mediators (SPMs) that inhibit neutrophil chemotaxis, enhance efferocytosis, and promote resolution of inflammation (Buckley et al., 2014; Miyata et al., 2020b). However, a detailed and extensive quantification of oxylipid mediators in nasal polyps is lacking.
Glucocorticoids (GC) mainly exert their anti-inflammatory effects by binding to the GC receptor (GR) (Lee, 2015). The interactions between GRα and the transcription factor (activator protein-1 and NF-κB) repress the transcription of proinflammatory genes. GC also activate anti-inflammatory genes through interaction with GC-responsive elements (Hox et al., 2020). A short course of systemic GC is widely used in patients with CRSwNP, which could alleviate symptoms and reduce polyp size (Fokkens et al., 2020). However, a proportion of patients are GC-insensitive, and their response to GC varies in distinct endotypes of CRSwNP (Wen et al., 2012; Milara et al., 2015; Milara et al., 2017; Wu et al., 2019). To date, the mechanisms underlying the pharmacological effects and the resistance to oral GC in CRSwNP patients are still not entirely clear. Considering the adverse effects of systemic GC, it is necessary to identify better biomarkers for predicting clinical response. Therefore, in this study, we assessed the transcriptomic and oxidative lipidomic signatures of nasal polyps before and after systemic GC treatment. These results demonstrated that, in addition to anti-inflammatory effects, systemic GC could improve tissue remodeling, restore cilia function, and ameliorate dysregulation of oxylipid mediator pathway in CRSwNP. We also detected different transcriptomic signatures in GC-responders and non-responders and identified a pool of promising candidate biomarkers predicting a better clinical response.
This prospective study was approved by the ethics committee of Peking Union Medical College Hospital (JS-1989). All patients and participants provided written informed consents before enrollment. Sixteen patients with CRSwNP and 12 control subjects were enrolled from Peking Union Medical College Hospital during April 2019 to August 2020. CRSwNP was diagnosed according to the European position paper on rhinosinusitis and nasal polyps 2012 guidelines (Fokkens et al., 2012). Exclusion criteria included allergic fungal rhinosinusitis, AERD, cystic fibrosis, use of oral GC within 6 months, use of nasal GC or antibiotics within 4 weeks, and comorbid conditions where systemic GC were contraindicated. Oral GC (methylprednisone 0.4 mg/kg/d) were given to eligible patients for 14 days before their scheduled operations. Use of antihistamines or topical GC was stopped during this period. Fresh polyp tissues were biopsied before and after treatment. The control group comprised subjects undergoing septoplasty for anatomic variation, transnasal endoscopic removal of nonfunctional pituitary adenoma or transnasal endoscopic repair of spontaneous cerebrospinal fluid leak. Normal nasal mucosa specimens were collected from controls during surgery.
Nasal symptoms (nasal obstruction, rhinorrhea, loss of smell, and facial pain) were assessed using a scale of 0–3 (0 = none, 1 = mild and occasionally present, 2 = moderate and frequently present, 3 = severe and continuously present) and the Total Nasal Symptom Score (TNSS) was calculated as the sum of the four individual symptom scores. Nasal polyp size was assessed through nasal endoscopy using the Nasal Polyp Size Score (NPSS) system (Supplementary Table S1) (Hong et al., 2018). NPSS and TNSS were assessed at baseline and after 14-days treatment. Patients were divided into two subgroups (Supplementary Figure S1): Responder group (change in NPSS >1 point) and Non-responder group (change in NPSS ≤ 1 point) (Milara et al., 2015; Milara et al., 2017; Hong et al., 2018; Wu et al., 2019). Details are described in the Methods of Supplementary Material.
RNA sequencing was performed on the Illumina Novaseq 6,000 platform, and details are given in the Methods of Supplementary Materials. Fragments per kilo-base of exon per million fragments mapped (FPKM) of each gene was calculated based on the length and reads count mapped to this gene. Differential expression analysis was performed using DESeq2 R package. The resulting p-value was adjusted using Benjamini and Honchberg’s approach for controlling the false discovery rate. Differentially expressed genes (DEGs) were defined as genes with absolute Log2FoldChange (Log2FC) > 1 and adjusted P (Padj) < 0.05. The raw sequence data have been submitted to the Genome Sequence Archive for Human in National Genomics Data Center, China National Center for Bioinformation at http://bigd.big.ac.cn/gsa-human, with the accession number HRA000808. Gene Ontology (GO) enrichment analysis of DEGs was conducted by the clusterProfiler R package and the enriched GO terms were visualized with R package GOplot (Walter et al., 2015).
Paraffin sections of nasal tissues were stained with hematoxylin and eosin (H&E, for evaluating infiltrating eosinophils) and immunohistochemical staining (for evaluation of FOXJ1+ ciliated cells and P63+ basal cells). The numbers of infiltrating eosinophils in nasal polyp tissues were counted as described in a previous study (Zhu et al., 2020). The IHC results were evaluated through a modified semiquantitative system (Supplementary Figure S2) (Li et al., 2011). Details are described in the Methods of Supplementary Material.
The contents of oxylipids derived from AA, DHA, EPA, linoleic acid (LA), α-linolenic acid (ALA), γ-linolenic acid (GLA), and dihomo-γ-linolenic acid (DGLA) in the tissue specimens were measured with the AB Sciex QTRAP6500 LC-MS/MS platform. Details are described in the Methods of Supplementary Material.
Data analysis was conducted using GraphPad Prism (version 7.0, GraphPad Software) and SPSS software (version 23.0, IBM Corporation). Wilcoxon matched-pairs signed rank test was used to compare the two groups of paired data. Mann–Whitney U test was used for the comparison of unpaired data (except for age, which was compared with an unpaired t-test). Categorical data were compared using a Fisher’ exact test. p < 0.05 was considered statistically significant.
Sixteen patients with CRSwNP were divided into two groups based on the change of NPSS: Responder (R, n = 11) and Non-responder groups (N, n = 5). The clinical data before and after treatment are shown in Table 1. The change of TNSS score was greater in the Responder group than in the Non-responder group. At baseline, blood and tissue eosinophil counts were significantly higher in the Responder group than in the Non-responder group. After treatment, blood eosinophil and basophil counts were reduced in the two groups, meanwhile, tissue eosinophil count decreased significantly in the Responder group (see H&E images in Supplementary Figure S3).
We preformed RNA Sequencing on paired polyp biopsies (pre- and post-treatment) from 14 patients (nine responders and five non-responders) and nasal mucosa from five control subjects. The global expression patterns of different samples were evaluated using principal component analysis (PCA, Supplementary Figure S4A). The separation between R_Pre and Control was larger than that between N_Pre and Control. Compared with the control, DEGs in all CRSwNP, Responder and Non-responder groups at baseline are shown in Supplementary Figures S5A, C, E and the significantly enriched GO terms are shown in Figures 1A–C. We identified 3,533 DEGs (Supplementary Figure S5A, 1487 upregulated and 2046 downregulated) in all CRSwNP subjects compared with controls. The most significantly enriched and upregulated (Z-score > 0) GO terms were leukocyte migration/chemotaxis and extracellular matrix organization, whereas the most significantly enriched and downregulated (Z-score < 0) GO terms were related to cilia (Figure 1A and Supplementary Table S2). To better show the differences in cilia-related GO terms between nasal polyps and controls, all the involved genes and enriched terms are shown using GOChord plot in Supplementary Figure S5B.
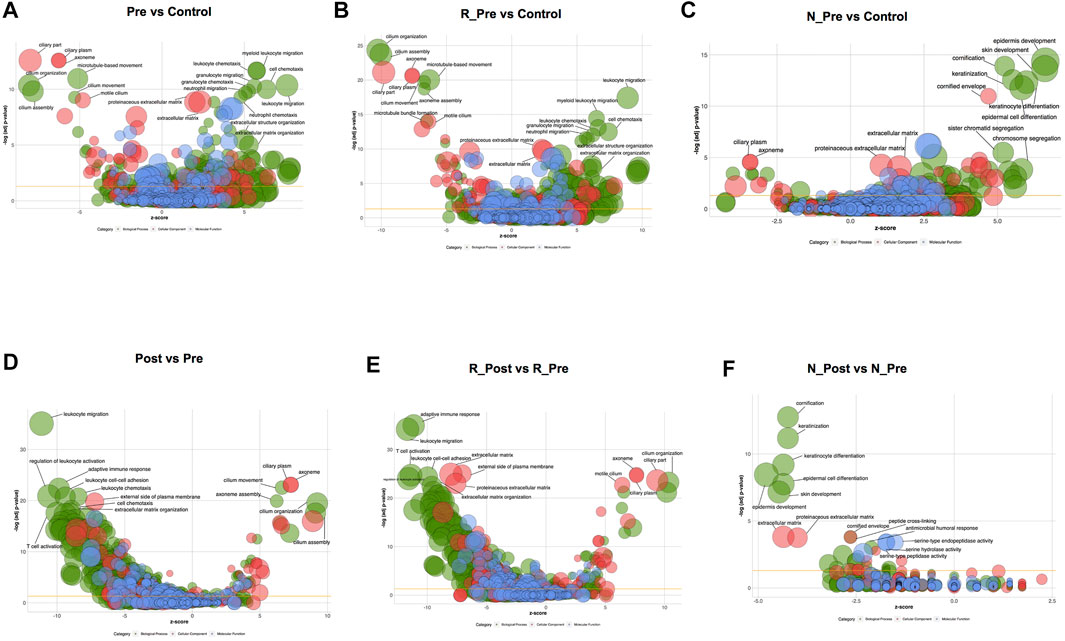
FIGURE 1. Gene Ontology (GO) terms enriched in CRSwNP at baseline compared with the Control in all patients (A), Responders (B), and Non-responders (C), see Supplementary Tables S2–S4 for more details. GO terms enriched in nasal polyps after oral glucocorticoids treatment compared to baseline in all patients (D), Responders (E), and Non-responders (F), see Supplementary Tables S8–S10 for more details. The area of bubble presents the number of genes in each term (Biological Process terms in green; Cellular Component terms in red; Molecular Function terms in blue) with -Log adjusted p-value the Y-axis, with z-score the X-axis (upregulated, z-score > 0; downregulated, z-score < 0). Abbreviation: Pre, all CRSwNP_Pre-treatment; Post, all CRSwNP_Post-treatment; R_Pre, Responder_Pre-treatment; N_Pre, Non-responder_Pre-treatment; R_Post, Responder_Post-treatment; N_Post, Non-responder_Post-treatment.
A larger number of DEGs (Supplementary Figure S5C, 2006 upregulated and 2817 downregulated) were identified when comparing the Responder group with the control. Leukocyte migration/chemotaxis and extracellular matrix organization were the most significantly enriched and upregulated (Z-score > 0) GO terms (Figure 1B and Supplementary Table S3). The expression levels of multiple chemotactic factors and receptors for Th2 cells and eosinophils (CCL8, CCL11, CCL13, CCL17, CCL18, CCL22, CCL24, CCL26, CCR3, CCR4, and CCR8), monocytes (CCL2, CCL3, CCL7, and CCR2), and neutrophils (CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8, CXCR1, and CXCR2) were significantly higher in the Responder group (Figure 2A, Supplementary Figure S6A and Supplementary Table S5). The expression levels of type 2 cytokines (IL5, IL13, and IL13RA2), and proinflammatory cytokines (IL6, IL6R, TNF, and TNFRSF1B) were significantly higher in the Responder group than in the Non-responder group and controls, indicating the augmented inflammatory responses. The expression levels of ECM components, enzymes and inhibitors in the metabolism of ECM, as well as cytokines regulating the metabolic process were significantly elevated in the Responder group at baseline (Figure 2B, Supplementary Figure S6B and Supplementary Table S6). The dysregulation of coagulation system also plays a role in the pathogenesis of tissue remodeling in nasal polyps (Takabayashi et al., 2013a; Takabayashi et al., 2013b; Kim et al., 2015). We detected increased activity of coagulation factor XIII-A (F13A1), decreased expression of fibrinolytic genes (plasminogen, PLG and tissue type plasminogen activator, PLAT), and increased expression of inhibitors of fibrinolysis (serpin family E member 1, SERPINE1 and serpin family B member 2, SERPINB2) in the Responder group, which could explain the fibrin accumulation in nasal polyps (Figure 2B).
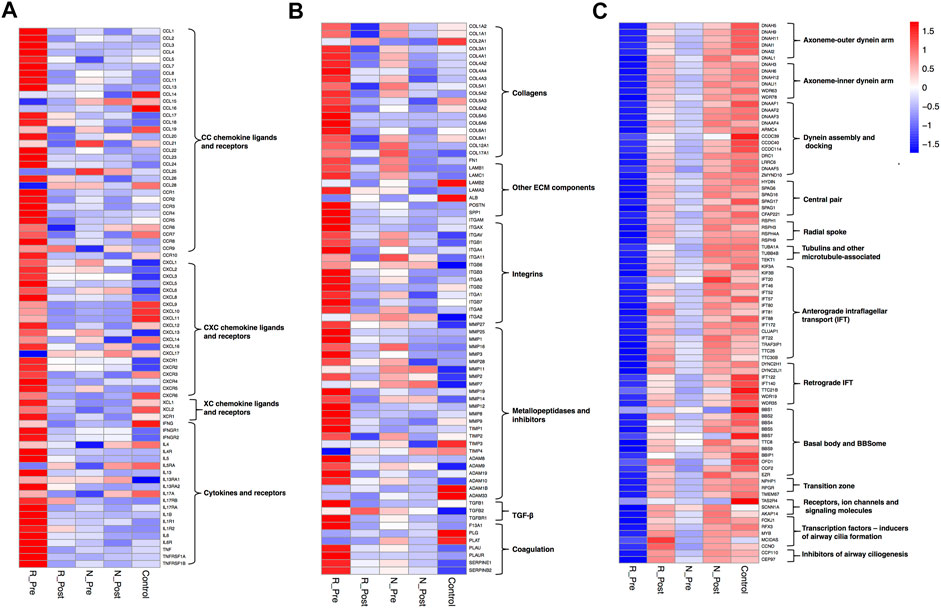
FIGURE 2. Heatmaps depicting the mean mRNA expression values of multiple chemokines, cytokines and corresponding receptors (A), genes involved in extracellular matrix metabolism (B), and genes related to cilia (C) in R_Pre (n = 9), R_Post (n = 9), N_Pre (n = 5), N_Post (n = 5), and Control (n = 5). See Supplementary Figure S6 and Supplementary Tables S5–S7 for more details.
GO terms related to cilia were the most significantly enriched and downregulated (Z-score < 0) in the Responder group compared with the control (Figure 1B). The significantly enriched cilia-related GO terms involved 264 genes, 79.5% of which were downregulated (Supplementary Figure S5D). The expression levels of key genes encoding components of axoneme (outer and inner dynein arm), central pairs, radial spoke and tubulin, others involved in dynein assembly and docking, intraflagellar transport, and regulators of ciliogenesis process (Callejas-Díaz et al., 2020), were impaired (Figure 2C, Supplementary Figure S6C and Supplementary Table S7).
A total of 1,480 DEGs (Supplementary Figure S5E, 692 upregulated and 788 downregulated) were identified in the Non-responder group compared with the control, and the significantly upregulated GO terms (Z-score > 0) were epidermis development, keratinization, chromosome segregation, and extracellular matrix (Figure 1C and Supplementary Table S4). The upregulated keratinization markers (keratins, KRT16, KRT78, KRT6C, KRT24, KRT6A, KRT14, KRT6B, KRT10, and KRT31; small proline rich proteins, SPRR2E, LCE3D, SPRR1B, SPRR3, SPRR2A, SPRR1A, SPRR2D, and SPRR2F; cornifelin, CNFN) indicated distinctive squamous metaplasia in the Non-responder group (see H&E images in Supplementary Figure S3). Furthermore, upregulation of genes related to chromosome segregation (KNL1, BUB1B, BUB1, TOP2A, PTTG1, TTK, MKI67, etc.) indicated epithelial hyperplasia in the Non-responder group. The expression of multiple cytokines and corresponding receptors was comparable with that in the control (Figure 2A), and less DEGs related to the metabolism of ECM were detected in the Non-responder group than in the Responder group (Figure 2B).
GO terms related to cilia were also the most significantly enriched and downregulated (Z-score < 0) in the Non-responder group compared with the control (Figure 1C). We observed that the significantly enriched cilia-related GO terms involved 59 genes, 64.4% of these were downregulated (Supplementary Figure S5F). The decrease in the expression of cilia-related genes was smaller in the Non-responder group than that in the Responder group (Figure 2C).
Therefore, enhanced inflammatory responses, severely abnormal metabolism of ECM, and cilia dysfunction were the prominent transcriptomic signatures of nasal polyps in the Responder group. However, nasal polyps of the non-responders were characterized by epithelial hyperplasia and keratinization.
The Post (Post-treatment) group was an intermediary phenotype between Pre (Pre-treatment) and Control on the PCA plot, and a smaller separation was seen between N_Post and N_Pre than between R_Post and R_Pre (Supplementary Figure S4A). To elucidate the molecular mechanisms underlying the therapeutic effects of systemic GC, transcriptomic changes were identified using pairwise comparison. There were 1,439 upregulated and 1853 downregulated genes when comparing Post to Pre in all CRSwNP subjects (Supplementary Figure S7A). The most significantly enriched and downregulated (Z-score < 0) GO terms were leukocyte migration/chemotaxis, adaptive immune response, regulation of leukocyte activation, and extracellular matrix organization, whereas the most significantly enriched and upregulated (Z-score > 0) GO terms were related to cilia (Figure 1D, Supplementary Figure S7B and Supplementary Table S8).
Consistent with the clinically therapeutic effects, there were more DEGs caused by GC treatment in the Responder group (Supplementary Figure S7C, 2027 upregulated and 2,169 downregulated). Furthermore, the expression of 1,110 (55.3%) of the 2006 overexpressed genes decreased significantly and that of 1,192 (42.3%) of the 2,817 underexpressed genes increased significantly after treatment (Supplementary Figure S4B). The most significantly enriched and downregulated (Z-score < 0) GO terms were adaptive immune response, leukocyte migration, T cell activation, and extracellular matrix organization (Figure 1E and Supplementary Table S9). The expression of multiple type 2 cytokines and chemokines, and proinflammatory cytokines was suppressed by systemic GC (Figure 2A). Moreover, there was a strong improvement in the dysregulated expression of ECM metabolism-related genes (Figure 2B). The abnormal expression of coagulation factors involved in tissue remodeling of nasal polyps was also significantly corrected using GC, with a significant increase in PLG and a decrease in F13A, SERPINE1, and SERPINB2.
GO terms related to cilia were the most significantly enriched and upregulated (Z-score > 0) (Figure 1E). The most significantly enriched cilia-related GO terms involved 228 DEGs (85.5% upregulated, Supplementary Figure S7D). Genes involved multiple aspects of cilia were significantly increased after GC treatment (Figure 2C). Semiquantitative assessment of FOXJ1 protein immunostaining confirmed the RNA-sequencing data. The median score of FOXJ1 was decreased in the Responder group at baseline than in the control (p = 0.003), and this was significantly upregulated after GC treatment (p = 0.020, Figure 3A).
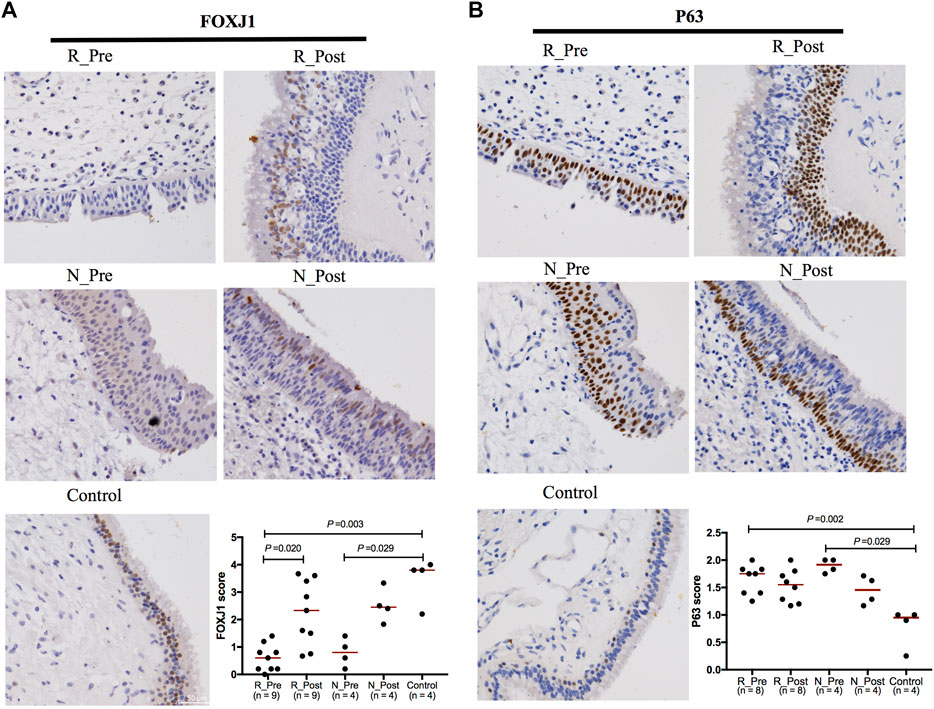
FIGURE 3. Expression of FOXJ1 and P63 determined by immunohistochemistry. Representative immunostaining images and analysis of the semiquantitative scores for the ciliated cell marker FOXJ1 (A) and basal cell marker P63 (B). Wilcoxon matched-pairs signed rank test was used to compare two groups of paired data. Mann-Whitney U test was used for the comparison of unpaired data. The median values of the semiquantitative scores were indicated by horizontal red lines.
Only 211 genes were significantly modulated by GC in the Non-responder group (57 upregulated and 154 downregulated, Supplementary Figure S7E). Furthermore, the expression of 54 (7.8%) of the 692 overexpressed genes in non-responders decreased significantly, and that of 7 (0.8%) of the 788 underexpressed genes increased significantly after treatment (Supplementary Figure S4C). Nonetheless, the most significantly enriched and downregulated (Z-score < 0) GO terms were cornification, keratinization, epidermis development, and extracellular matrix (Figure 1F and Supplementary Table S10). Significant reduction in the expression of keratinization-associated genes, including IVL, KRT14, KRT13, KRT16, KRT6C, KRT6A, KRT6B, SPRR1B, SPRR2E, SPRR2D, and SPRR2A, was observed after GC treatment. Several genes involved in the metabolism of ECM were also significantly modulated by GC (Figure 2B).
Although the upregulation of cilia-related genes in the Non-responder group was not as significant as that in the Responder group (Figure 2C), GO terms related to cilia were enriched in the upregulated DEGs in N_Post versus N_Pre (Supplementary Figure S7F). As confirmed with IHC, the median score of FOXJ1 was also decreased in the Non-responder group at baseline (p = 0.029), but the upregulation after GC treatment was not statistically significant (p > 0.05, Figure 3A).
To elucidate changes of other epithelial subsets in nasal polyps, we depicted a heatmap comparing the expression of marker genes of basal, glandular, and goblet/secretory cells used in a single-cell RNA-sequencing study of CRS (Supplementary Figure S8 and Supplementary Table S11) (Ordovas-Montanes et al., 2018). As basal cell hyperplasia is a hallmark of nasal polyps, we detected increased expression of basal cell marker in nasal polyps compared with the control (although only the Padj value of S100A2 was less than 0.05); however, the suppression caused by GC was not significant both in the Responder and Non-responder groups. Comparable results were obtained from IHC: the median score of basal cell marker P63 was significantly higher both in the Responder and Non-responder groups at baseline than in the control (p = 0.002 and 0.029, respectively); however, the downregulation after GC treatment was not significant (p > 0.05, Figure 3B). The expression of glandular cell marker genes (LTF, TCN1, LYZ, SLPI, PIP, BPIFB1, and BPIFA1) was decreased in the Responder group at baseline compared with the control, and this was upregulated by GC treatment. The decrease of goblet/secretory cell markers (MUC5B, SCGB1A1, and SCGB3A1) was also partially reversed after GC treatment in the Responder group. However, in the Non-responder group, there was a slight tendency for the expression of glandular cell markers to decrease after GC treatment, with no significant increase in the expression of secretory cell markers.
Therefore, systemic GC exert robust suppression in the inflammatory responses and abnormal ECM metabolism, and restore cilia function in the Responder group. However, GC mainly inhibited keratinization with slight improvement of abnormal ECM metabolism and cilia dysfunction in the Non-responder group.
To identify the candidate biomarkers predicting a favorable clinical response to oral GC, we compared the transcriptome of responders with non-responders at baseline. There were 800 upregulated and 208 downregulated genes in the Responder group compared with the Non-responder group (Supplementary Figure S9A), and the top upregulated and downregulated protein-coding genes are shown in Table 2. Higher expression of type 2 inflammatory molecules (CCL13, IGHE, CCL18, CCL23, CCR3, CLC, CCL24, and CCL26) with lower levels of LACRT, PPDPFL, DES, C6, MUC5B, and SCGB3A1 were related to a stronger clinical response to systemic GC. Charcot–Leyden crystal (CLC), serum amyloid A (SAA), IL-25, and the ratio of 11β-hydroxysteroid dehydrogenase 1/11β-hydroxysteroid dehydrogenase 2 (11β-HSD1/11β-HSD2) have been identified as biomarkers predicting GC response in patients with CRSwNP (Hong et al., 2018; Lu et al., 2018; Jiang et al., 2019; Wu et al., 2019). Compared with the Non-responder group, in the Responder group, the expression of CLC, 11β-HSD1 and the ratio of 11β-HSD1/11β-HSD2 were significantly increased; however, the transcription level of IL-25 was decreased and no significant difference was found in the expression of SAA1 (Supplementary Figure S9B).
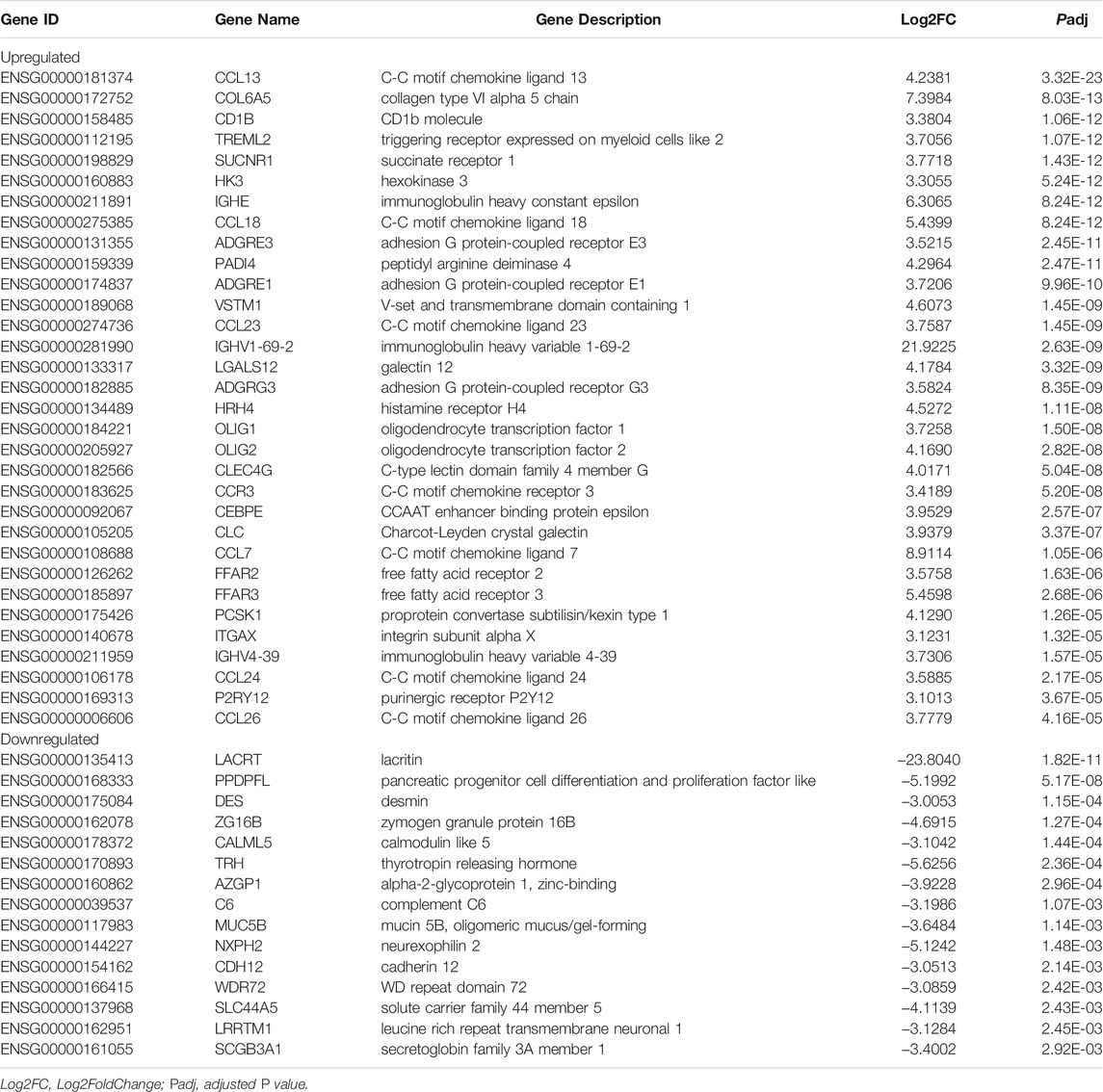
TABLE 2. Top upregulated and downregulated protein-coding genes in the Responder group compared to the Non-responder group at baseline.
Unsaturated fatty acid biosynthetic process was one of the significantly enriched GO terms when comparing R_Post with R_Pre (Padj = 0.016, Z-score = −3.742). We further quantified the oxylipid mediator profiles derived from PUFAs in paired polyp biopsies from 16 patients (11 responders and 5 non-responders) and nasal mucosa tissues from 12 control subjects. Similar to the transcriptome data, the Post group was an intermediary phenotype between the Pre and Control on the PCA plot (Figure 4A). A total of 71 oxylipid mediators derived from AA, DHA, EPA, LA, ALA, GLA, and DGLA were measured and 67 were detected (Figure 4B and Supplementary Table S12).
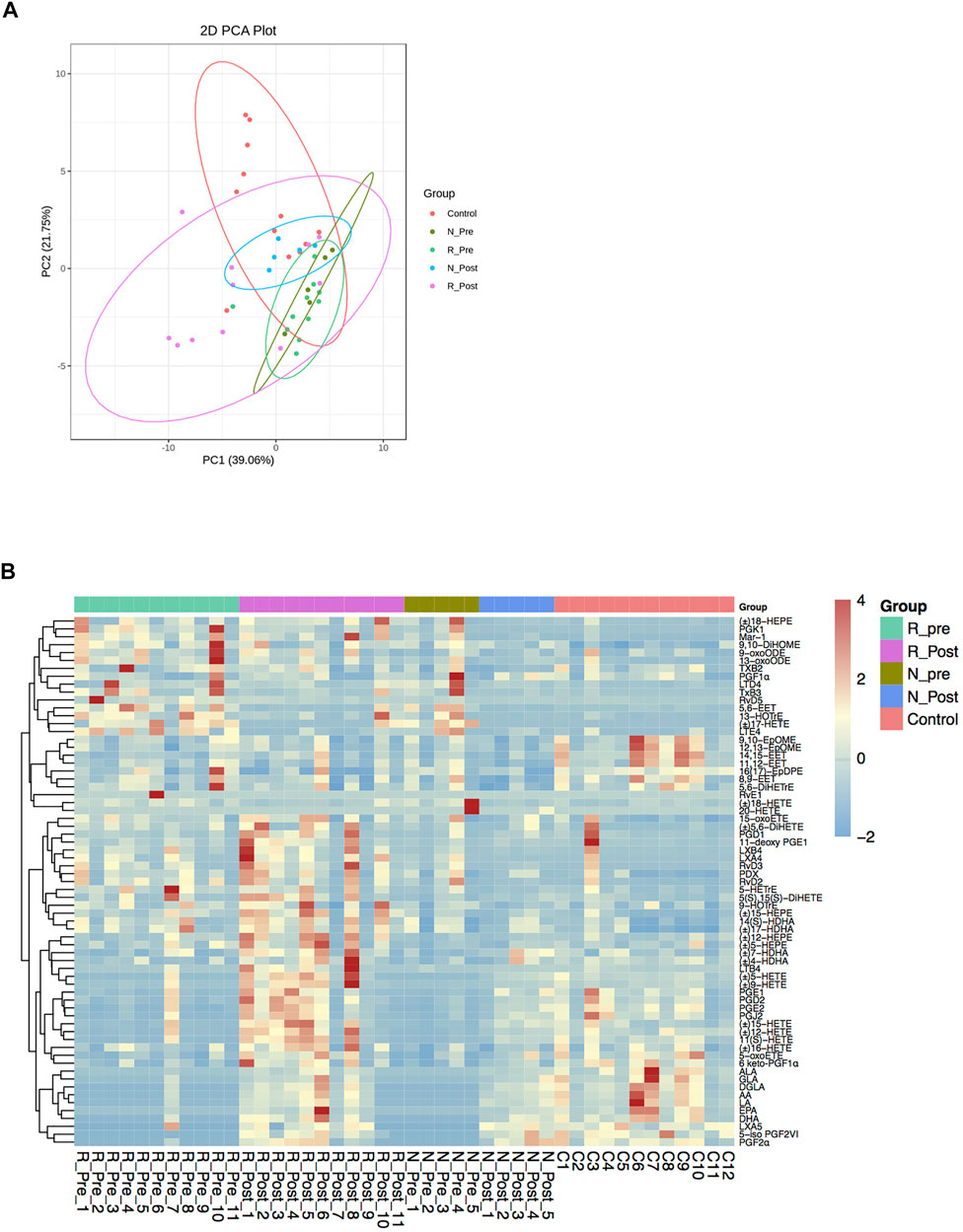
FIGURE 4. Profiles of oxylipid mediators. Principal component analysis (PCA) of oxidative lipidomics (A) and heatmap of the oxylipid mediators (B) in R_Pre (n = 11), R_Post (n = 11), N_Pre (n = 5), N_Post (n = 5), and Control (n = 12) groups. Abbreviation: AA, arachidonic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; DiHOME, dihydroxy-octadecenoic acid; EET, epoxy-eicosatrienoic acids; EPA, eicosapentaenoic acid; EpDPE, epoxy-docosapentaenoic acid; EpOME, epoxy-octadecenoic acid; GLA, γ-linolenic acid; HDHA, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETE, hydroxy-eicosatetraenoic acid; HOTrE, hydroxy-octadecatrienoic acid; LA, linoleic acid; LT, leukotriene; LX, lipoxin; Mar, maresin; oxoETE, oxo-eicosatetraenoic acid; oxoODE, oxo-octadecadienoic acid; PD, Protectin; PG, prostaglandin; Rv, resolvins; TX, thromboxane.
Treatment with GC significantly modulated the expression of multiple enzyme (ALOX5, ALOX5AP, PTGS1, PTGS2, GGT5, DPEP2, and DPEP3) and receptor genes (CYSLTR2, PTGDR2, PTGER3, and PTGER4) involved in the PUFA metabolism and signaling pathway in the Responder group (Figures 5A–C). The levels of AA, ALA, DHA, EPA, LA, GLA, and DGLA were all reduced in nasal polyps at baseline, with a significant increase after GC treatment (Figure 5D). The downregulated oxylipids produced from AA through the LOX pathway, including 5-HETE, 12-HETE, 15-HETE and 5-oxoETE, were increased after GC treatment (Figure 5E). The level of the downstream product of 15-lipoxygenase, 15-oxoETE, was higher in the Responder group at baseline than in the control, with no significant change after treatment. The levels of prostaglandins (PGD2, PGE2, and PGJ2) formed from AA via the COX pathway were decreased in nasal polyps at baseline, which were increased after treatment. GC also reversed the increase of LTD4 and LTE4 in nasal polyps. In contrast, LTB4 was increased after treatment (Figure 5F). Furthermore, multiple SPMs (PDX, Mar-1, RvD2, RvD5, and LXA4) derived from AA and DHA were increased in nasal polyps at baseline compared with the control, among which RvD5 was significantly decreased after GC treatment in the Responder group (Figure 5G).
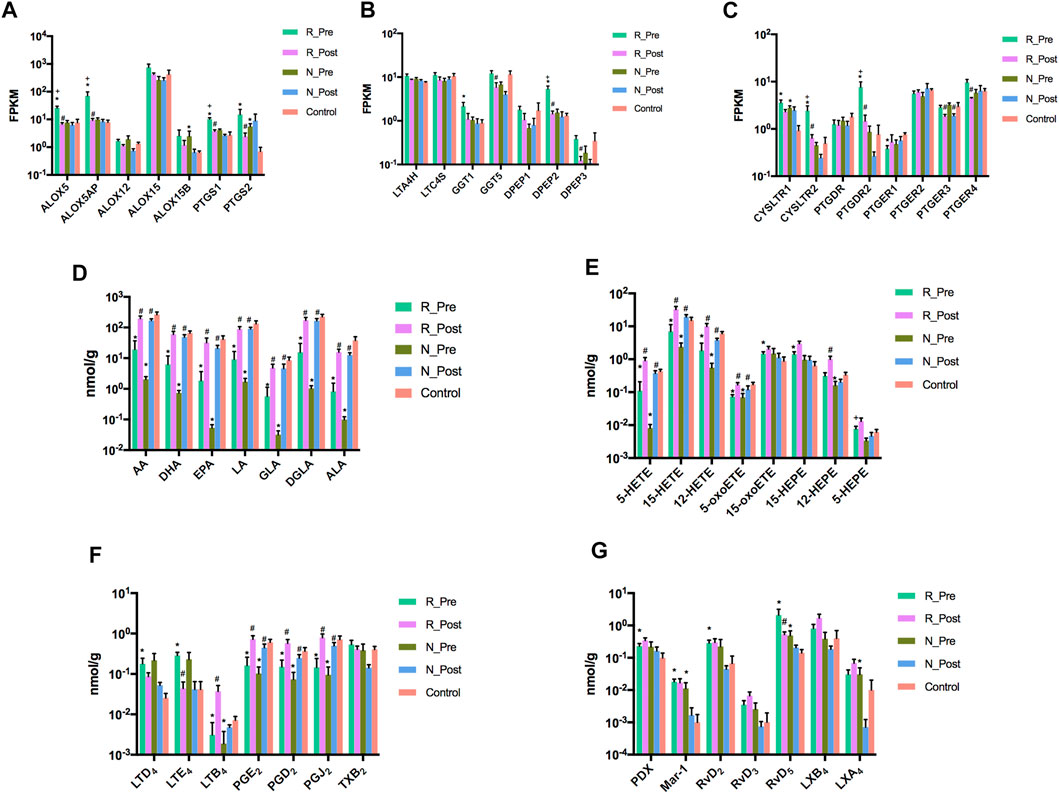
FIGURE 5. Glucocorticoids ameliorate the dysregulation of lipid oxidative metabolism and signaling pathway in nasal polyps. The mRNA expression levels of lipoxygenases and cyclooxygenases (A), other enzymes responsible for the synthesis of cysteinyl leukotrienes (B) and receptors for cysteinyl leukotrienes and prostaglandins (C) in R_Pre (n = 9), R_Post (n = 9), N_Pre (n = 5), N_Post (n = 5), and Control (n = 5) groups. Amounts of various fatty acids (D), oxylipids derived from AA and EPA through lipoxygenase pathway (E), cysteinyl leukotrienes, prostaglandins and thromboxane (F), and specialized pro-resolving mediators (G) in R_Pre (n = 11), R_Post (n = 11), N_Pre (n = 5), N_Post (n = 5), and Control (n = 12) groups. *p < 0.05 compared to control, #p < 0.05 compared to baseline, +p < 0.05 compared to N_Pre. For comparison of the amounts of oxylipid mediators, Wilcoxon matched-pairs signed rank test and Mann-Whitney U test were used to compare two groups of paired and unpaired data. All data were expressed as mean ± standard error of the mean (SEM).
When comparing the oxylipid profiles in the Responder and Non-responder groups at baseline, we found that the separation between the two groups on the PCA plot was smaller than the separation of transcriptome patterns (Figure 4A). Compared with the Non-responder group, in the Responder group, only a significant increase of EPA-derived 5-HEPE was detected (Figure 5E and Supplementary Table S12).
Considering the transcriptomic and lipidomic data, there was a severe disturbance in the lipid oxidative metabolism process in CRSwNP, which could be ameliorated by GC treatment.
A short course of systemic GC is widely used in CRSwNP, and not all patients respond equally. Therefore, it is important to understand the mechanisms underlying the effects of systemic GC and to find potential biomarkers predicting treatment response. In this study, we performed transcriptome sequencing and LC-MS/MS based oxidative lipidomics followed by comparison of nasal polyps and healthy nasal mucosa, with a pairwise comparison of polyp tissues before and after GC treatment. Similar to the results of a previous transcriptome study (Peng et al., 2019), cilia dysfunction, inflammatory responses, and abnormal metabolism of ECM were the gene signatures of the Responder group at baseline. Specifically, most responders were Th2 response dominated (IL-5 high), with increased Th1/Th17 reactions (IFN-γ/IL-17 high) in a small proportion of responders. However, a distinct transcriptomic profile was observed in the Non-responder group, characterized by epithelial hyperplasia and keratinization. As GC mainly exert therapeutic effects by relieving inflammatory responses, nasal polyps originating from epithelial hyperplasia with mild inflammation respond poorly to the treatment. A previous study reported that a large group of CRSwNP patients were key cytokine (IL-5/IL-17/IFN-γ)-negative in China (Ba et al., 2011). Our data showed that almost all non-responders were key cytokine-negative. Meanwhile, we provided a pool of promising candidate biomarkers, including higher expression of type 2 inflammatory molecules (CCL13, IGHE, CCL18, CCL23, CCR3, and CLC) and lower levels of LACRT, PPDPFL, DES, C6, MUC5B, and SCGB3A1, predicting a better response to systemic GC.
Studies have shown the anti-inflammatory effects of GC in nasal polyposis with the downregulation of multiple chemokines and cytokines (Jahnsen et al., 1999; Woodworth et al., 2004; Bolger et al., 2007). Furthermore, GC could change the remodeling patterns of nasal polyp with significant improvements in a variety of remodeling markers (Wang et al., 2015; Zhang et al., 2019). A recent study of exosomal proteomic arrays revealed that 16 (89%) of the 18 highly underexpressed proteins in CRSwNP were upregulated after systemic GC treatment; however, only 22 (42%) of the 53 overexpressed proteins were downregulated (Workman et al., 2020). We identified much less DEGs at baseline and lower transcriptomic response to GC treatment in the Non-responder group than in the Responder group. Along with the decrease of infiltrating eosinophil count, genes associated with inflammatory responses and ECM metabolism were significantly suppressed by GC treatment in the Responder group. The coagulation system dysregulation also plays a role in the tissue remodeling of nasal polyps with reduction of PLAT and upregulation of F13A1 (Kim et al., 2015). We found that a short course of systemic GC decreased the expression of coagulation factor (F13A1) and inhibitors of fibrinolysis (SERPINE1 and SERPINB2), and increased the expression of fibrinolytic genes (PLG and PLAT) in the Responder group; therefore, GC could alleviate fibrin deposition in nasal polyps. Suppression of SERPINB2 by GC in asthmatics has also been reported using microarrays (Woodruff et al., 2007).
A pseudostratified columnar respiratory epithelium in the upper airways, containing ciliated, goblet/secretory, and basal cells, provides barrier defense, innate immune, and tissue repair function (Bankova and Barrett, 2020). Ciliated and goblet cells are involved in mucociliary clearance, whereas basal cells are multipotent stem-like epithelial progenitors. The potential role of epithelial cells in initiating and perpetuating the inflammation condition in CRS has been highlighted (Bankova and Barrett, 2020). The epithelium in nasal polyps is usually characterized by loss of ciliated cells, cilia dysfunction, and basal cell hyperplasia (Gudis et al., 2012; Li et al., 2014; Ordovas-Montanes et al., 2018). In vitro studies have also shown that cilia function and ciliogenesis are impaired significantly in the air–liquid interface culture of nasal polyp epithelial cells (Lai et al., 2011; Li et al., 2014; Callejas-Díaz et al., 2020). DNA microarray of matched nasal polyp tissues revealed that oral steroids promote epithelial repair (Li et al., 2009). In a small group of asthmatics, oral steroids were found to have a positive effect on ciliogenesis (Heino et al., 1988). Consistent with the results of these studies, we found that cilia-related genes and GO terms were significantly downregulated in CRSwNP, particularly in the Responder group. Cilia-related gene expression was significantly improved after GC treatment, with that of ciliated cell marker FOXJ1 confirmed by IHC. No significant change was noted in the expression of MUC5AC; however, the expression of MUC5B, SCGB1A1, and SCGB3A1 was lower at baseline compared with that in the controls and was elevated by GC in the Responder group. In a study of murine models, MUC5B (instead of MUC5AC) was found to be essential for mucociliary clearance (Roy et al., 2014). Therefore, it is assumed that mucociliary clearance is improved by systemic GC. The anti-inflammatory gene SCGB1A1 (uteroglobin) was also found to be upregulated in nasal polyps after local treatment with GC (Benson et al., 2004). As the majority of glandular cell markers are antimicrobial peptides and proteins (AMPs), the decrease of the expression of these genes in responders indicates impaired innate host defense function and reduced number of submucosal glands, which is consistent with the previous studies (Seshadri et al., 2012; Wei et al., 2014; Huang et al., 2021). GC may enhance the innate immune response by upregulating the expression of AMPs, which might be attributed to the increase of the number of submucosal glands.
Multiple genes encoding key enzymes and receptors in the unsaturated fatty acid metabolism and signaling pathway were found to be upregulated in the Responder group at baseline, and their expression decreased significantly after GC treatment. Upregulation of the proinflammatory leukotriene pathway and downregulation of anti-inflammatory PGE2 pathway play a key role in the pathogenesis of CRSwNP (Pérez-Novo et al., 2005; Wu et al., 2016; Du et al., 2017). Cysteinyl leukotriene receptor antagonists have been used in the treatment of asthma, allergic rhinitis, and CRSwNP (Du et al., 2017; Fokkens et al., 2020). Eosinophils derived from nasal polyps produce a higher amount of LTD4 and lower amount of prostaglandins than eosinophils isolated from peripheral blood (Miyata et al., 2019). Previously, Negri et al. (Negri et al., 2008) showed that GC were inhibitors of cysteinyl leukotriene metabolism and signaling pathway in immune cells. Similarly, we found that the expression of enzymes (ALOX5, ALOX5AP, GGT1, and DPEP2) responsible for the production of cysteinyl leukotrienes and their receptors (CYSLTR1, CYSLTR2), as well as the amounts of cysteinyl leukotrienes (LTD4 and LTE4), were increased in the Responder group at baseline, which was reversed by GC. The expression of ALOX15 is higher in eosinophilic nasal polyps (Imoto et al., 2020), and a missense variant in ALOX15 protects against nasal polyps (Kristjansson et al., 2019). 15-oxoETE, a downstream product of the 15-lipoxygenase pathway, is elevated in nasal polyps, especially in patients with AERD (Stevens et al., 2021). In this study, we detected that ALOX15 and 15-oxoETE were upregulated in the Responder group at baseline. Although decreased LXA4 and PD1 were detected in asthma (Miyata et al., 2020b), LXA4, RvD2, and PDX were increased in nasal polyps (Pérez-Novo et al., 2005; Vickery et al., 2021). In addition, maresins were found to be upregulated in nasal secretions from CRSwNP patients when compared with healthy controls and subjects with upper respiratory tract infection (Beegun et al., 2021). In this study, we found that multiple SPMs were increased in nasal polyps, which could be explained by an excessive response to chronic inflammation, and dysfunction or resistance of SPMs. AA-derived SPMs could induce resolution of inflammatory response and tissue remodeling in asthma, although more stable and safer analogs should be developed for clinical use (Insuela et al., 2020). The anti-inflammatory properties of omega-3 PUFAs are well documented, indicating their therapeutic potential in chronic inflammatory diseases (Yates et al., 2014; Calder, 2017). The anti-allergic effects of omega-3 PUFA-derived metabolites have been detected in food and airway allergy models in mice (Kunisawa et al., 2015; Mochimaru et al., 2018; Sawane et al., 2019). Although the lack of consistency in clinical studies makes dietary PUFA supplementation less universally effective in the treatment of asthma and other allergic diseases, maybe these compounds are effective in patients of select phenotypes (Wendell et al., 2014; Venter et al., 2019). Therefore, investigation into the dysregulated PUFA metabolism in CRSwNP could offer novel therapeutic targets that warrant further investigation.
However, unlike the considerable discrepancy in the transcriptome expression patterns of PUFA metabolism-related genes between the Responder and Non-responder groups, the oxylipid profiles were similar in the two groups. This could be explained by post-transcriptional regulation, as well as the complex and unclear metabolic process. As the contents of most oxylipid mediators are extremely low in nasal tissues and the half-life of these mediators is short, the detection accuracy of the lipidomic methodologies and measurement time could also influence the quantification.
This study has some strengths, but also several limitations. To the best of our knowledge, this is the first report on the multi-omic signatures of GC-responders and non-responders with comprehensive examination of changes caused by a short course of systemic GC. Though the relatively small sample size in the current study may decrease the statistical power, we do provide a pool of promising candidate biomarker genes predicting GC responses, but this needs screening and validation in a larger multicenter cohort. We performed bulk RNA sequencing using the whole tissue samples, which lacks the specific expression data from different cell types compared to single-cell RNA sequencing. The variable background of the control subjects is also a limitation of this study.
In conclusion, our results demonstrate that GC-responders and non-responders possess different transcriptomic signatures. Systemic GC exert anti-inflammatory effects, improve tissue remodeling and restore cilia function. Besides the decreased prostaglandins and increased leukotrienes, a more profound dysregulation of other oxylipid mediators derived from polyunsaturated fatty acids is determined in nasal polyps, which is ameliorated by GC treatment. We also provide a broad set of candidate biomarker genes leading to a better strategy for systemic GC use, however, this needs further screening and validation in a larger cohort.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Conception and design: WL, JZ, and ZZ; performing experiments, data acquisition, analysis, and interpretation: ZZ, WW,YZ, XW, LW, and JH; drafting the manuscript: ZZ, WW; revising the manuscript: WL, JZ, XW, YZ, LW, and JH; final approval of the manuscript: all authors.
This work was supported by the National Natural Science Foundation of China (82071027 and 82071791); the Natural Science Foundation of Beijing (7202162); the Nonprofit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019XK320034); the CAMS Initiative for Innovative Medicine (2021-I2M-1-005, 2021-I2M-1-035 and 2021-I2M-1-053), and CAMS Central Public Welfare Scientific Research Institute Basal Research Expenses (2018PT32004 and 2018PT31052).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.814953/full#supplementary-material
Ba, L., Zhang, N., Meng, J., Zhang, J., Lin, P., Zhou, P., et al. (2011). The Association between Bacterial Colonization and Inflammatory Pattern in Chinese Chronic Rhinosinusitis Patients with Nasal Polyps. Allergy 66 (10), 1296–1303. doi:10.1111/j.1398-9995.2011.02637.x
Bankova, L. G., and Barrett, N. A. (2020). Epithelial Cell Function and Remodeling in Nasal Polyposis. Ann. Allergy Asthma Immunol. 124 (4), 333–341. doi:10.1016/j.anai.2020.01.018
Beegun, I., Koenis, D. S., Alusi, G., and Dalli, J. (2021). Dysregulated Maresin Concentrations in Plasma and Nasal Secretions from Patients with Chronic Rhinosinusitis. Front. Immunol. 12, 733019. doi:10.3389/fimmu.2021.733019
Benson, M., Carlsson, L., Adner, M., Jernås, M., Rudemo, M., Sjögren, A., et al. (2004). Gene Profiling Reveals Increased Expression of Uteroglobin and Other Anti-inflammatory Genes in Glucocorticoid-Treated Nasal Polyps. J. Allergy Clin. Immunol. 113 (6), 1137–1143. doi:10.1016/j.jaci.2004.02.028
Bolger, W. E., Joshi, A. S., Spear, S., Nelson, M., and Govindaraj, K. (2007). Gene Expression Analysis in Sinonasal Polyposis before and after Oral Corticosteroids: a Preliminary Investigation. Otolaryngol. Head Neck Surg. 137 (1), 27–33. doi:10.1016/j.otohns.2007.01.023
Buckley, C. D., Gilroy, D. W., and Serhan, C. N. (2014). Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 40 (3), 315–327. doi:10.1016/j.immuni.2014.02.009
Calder, P. C. (2017). Omega-3 Fatty Acids and Inflammatory Processes: from Molecules to Man. Biochem. Soc. Trans. 45 (5), 1105–1115. doi:10.1042/BST20160474
Callejas-Díaz, B., Fernandez, G., Fuentes, M., Martínez-Antón, A., Alobid, I., Roca-Ferrer, J., et al. (2020). Integrated mRNA and microRNA Transcriptome Profiling during Differentiation of Human Nasal Polyp Epithelium Reveals an Altered Ciliogenesis. Allergy 75 (10), 2548–2561. doi:10.1111/all.14307
Cao, P. P., Li, H. B., Wang, B. F., Wang, S. B., You, X. J., Cui, Y. H., et al. (2009). Distinct Immunopathologic Characteristics of Various Types of Chronic Rhinosinusitis in Adult Chinese. J. Allergy Clin. Immunol. 124 (3), 478–484. e1-2. doi:10.1016/j.jaci.2009.05.017
Du, J., Ba, L., Zhou, J., Yu, L., Liu, R., Zhang, J., et al. (2017). The Role of Cysteinyl Leukotrienes and Their Receptors in Refractory Nasal Polyps. Prostaglandins Leukot. Essent. Fatty Acids 126, 39–48. doi:10.1016/j.plefa.2017.09.009
Fokkens, W. J., Lund, V. J., Hopkins, C., Hellings, P. W., Kern, R., Reitsma, S., et al. (2020). European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 58 (29), 1–464. doi:10.4193/Rhin20.600
Fokkens, W. J., Lund, V. J., Mullol, J., Bachert, C., Alobid, I., Baroody, F., et al. (2012). European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 23, 31–298.
Gabbs, M., Leng, S., Devassy, J. G., Monirujjaman, M., and Aukema, H. M. (2015). Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 6 (5), 513–540. doi:10.3945/an.114.007732
Gudis, D., Zhao, K. Q., and Cohen, N. A. (2012). Acquired Cilia Dysfunction in Chronic Rhinosinusitis. Am. J. Rhinol Allergy 26 (1), 1–6. doi:10.2500/ajra.2012.26.3716
Heino, M., Karjalainen, J., Ylikoski, J., Laitinen, A., and Laitinen, L. A. (1988). Bronchial Ciliogenesis and Oral Steroid Treatment in Patients with Asthma. Br. J. Dis. Chest 82 (2), 175–178. doi:10.1016/0007-0971(88)90040-x
Hong, H., Chen, F., Sun, Y., Yang, Q., Gao, W., Cao, Y., et al. (2018). Nasal IL-25 Predicts the Response to Oral Corticosteroids in Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 141 (5), 1890–1892. doi:10.1016/j.jaci.2017.10.050
Hox, V., Lourijsen, E., Jordens, A., Aasbjerg, K., Agache, I., Alobid, I., et al. (2020). Benefits and Harm of Systemic Steroids for Short- and Long-Term Use in Rhinitis and Rhinosinusitis: an EAACI Position Paper. Clin. Transl Allergy 10, 1. doi:10.1186/s13601-019-0303-6
Huang, Y., Wang, M., Hong, Y., Bu, X., Luan, G., Wang, Y., et al. (2021). Reduced Expression of Antimicrobial Protein Secretory Leukoprotease Inhibitor and Clusterin in Chronic Rhinosinusitis with Nasal Polyps. J. Immunol. Res. 2021, 1057186. doi:10.1155/2021/1057186
Imoto, Y., Takabayashi, T., Sakashita, M., Kato, Y., Yoshida, K., Kidoguchi, M., et al. (2020). Enhanced 15-Lipoxygenase 1 Production Is Related to Periostin Expression and Eosinophil Recruitment in Eosinophilic Chronic Rhinosinusitis. Biomolecules 10 (11), 1. doi:10.3390/biom10111568
Insuela, D. B. R., Ferrero, M. R., Coutinho, D. S., Martins, M. A., and Carvalho, V. F. (2020). Could Arachidonic Acid-Derived Pro-resolving Mediators Be a New Therapeutic Strategy for Asthma Therapy? Front. Immunol. 11, 580598. doi:10.3389/fimmu.2020.580598
Jahnsen, F. L., Haye, R., Gran, E., Brandtzaeg, P., and Johansen, F. E. (1999). Glucocorticosteroids Inhibit mRNA Expression for Eotaxin, Eotaxin-2, and Monocyte-Chemotactic Protein-4 in Human Airway Inflammation with Eosinophilia. J. Immunol. 163 (3), 1545–1551.
Jiang, L., Zhou, M., Deng, J., Sun, Y., Zuo, K., Zheng, R., et al. (2019). The Ratio of 11β-Hydroxysteroid Dehydrogenase 1/11β-Hydroxysteroid Dehydrogenase 2 Predicts Glucocorticoid Response in Nasal Polyps. Eur. Arch. Otorhinolaryngol. 276 (1), 131–137. doi:10.1007/s00405-018-5201-3
Kim, D. Y., Cho, S. H., Takabayashi, T., and Schleimer, R. P. (2015). Chronic Rhinosinusitis and the Coagulation System. Allergy Asthma Immunol. Res. 7 (5), 421–430. doi:10.4168/aair.2015.7.5.421
Kowalski, M. L., Agache, I., Bavbek, S., Bakirtas, A., Blanca, M., Bochenek, G., et al. (2019). Diagnosis and Management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI Position Paper. Allergy 74 (1), 28–39. doi:10.1111/all.13599
Kristjansson, R. P., Benonisdottir, S., Davidsson, O. B., Oddsson, A., Tragante, V., Sigurdsson, J. K., et al. (2019). A Loss-Of-Function Variant in ALOX15 Protects against Nasal Polyps and Chronic Rhinosinusitis. Nat. Genet. 51 (2), 267–276. doi:10.1038/s41588-018-0314-6
Kunisawa, J., Arita, M., Hayasaka, T., Harada, T., Iwamoto, R., Nagasawa, R., et al. (2015). Dietary ω3 Fatty Acid Exerts Anti-allergic Effect through the Conversion to 17,18-epoxyeicosatetraenoic Acid in the Gut. Sci. Rep. 5, 9750. doi:10.1038/srep09750
Lai, Y., Chen, B., Shi, J., Palmer, J. N., Kennedy, D. W., and Cohen, N. A. (2011). Inflammation-mediated Upregulation of Centrosomal Protein 110, a Negative Modulator of Ciliogenesis, in Patients with Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 128 (6), 1207–e1. doi:10.1016/j.jaci.2011.09.001
Lee, S. H. (2015). Mechanisms of Glucocorticoid Action in Chronic Rhinosinusitis. Allergy Asthma Immunol. Res. 7 (6), 534–537. doi:10.4168/aair.2015.7.6.534
Li, C. W., Cheung, W., Lin, Z. B., Li, T. Y., Lim, J. T., and Wang, D. Y. (2009). Oral Steroids Enhance Epithelial Repair in Nasal Polyposis via Upregulation of the AP-1 Gene Network. Thorax 64 (4), 306–312. doi:10.1136/thx.2008.106096
Li, C. W., Shi, L., Zhang, K. K., Li, T. Y., Lin, Z. B., Lim, M. K., et al. (2011). Role of P63/p73 in Epithelial Remodeling and Their Response to Steroid Treatment in Nasal Polyposis. J. Allergy Clin. Immunol. 127 (3), 765–772 e12. doi:10.1016/j.jaci.2010.12.011
Li, Y. Y., Li, C. W., Chao, S. S., Yu, F. G., Yu, X. M., Liu, J., et al. (2014). Impairment of Cilia Architecture and Ciliogenesis in Hyperplastic Nasal Epithelium from Nasal Polyps. J. Allergy Clin. Immunol. 134 (6), 1282–1292. doi:10.1016/j.jaci.2014.07.038
Lu, H., Lin, X. S., Yao, D. M., Zhuang, Y. Y., Wen, G. F., Shi, J., et al. (2018). Increased Serum Amyloid A in Nasal Polyps Is Associated with Systemic Corticosteroid Insensitivity in Patients with Chronic Rhinosinusitis with Nasal Polyps: a Pilot Study. Eur. Arch. Otorhinolaryngol. 275 (2), 401–408. doi:10.1007/s00405-017-4809-z
Marion-Letellier, R., Savoye, G., and Ghosh, S. (2015). Polyunsaturated Fatty Acids and Inflammation. IUBMB Life 67 (9), 659–667. doi:10.1002/iub.1428
Milara, J., Morell, A., Ballester, B., Armengot, M., Morcillo, E., and Cortijo, J. (2017). MUC4 Impairs the Anti-inflammatory Effects of Corticosteroids in Patients with Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 139 (3), 855–e13. e3. doi:10.1016/j.jaci.2016.06.064
Milara, J., Peiró, T., Armengot, M., Frias, S., Morell, A., Serrano, A., et al. (2015). Mucin 1 Downregulation Associates with Corticosteroid Resistance in Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 135 (2), 470–476. doi:10.1016/j.jaci.2014.07.011
Miyata, J., Fukunaga, K., Kawashima, Y., Ohara, O., and Arita, M. (2020). Cysteinyl Leukotriene Metabolism of Human Eosinophils in Allergic Disease. Allergol. Int. 69 (1), 28–34. doi:10.1016/j.alit.2019.06.002
Miyata, J., Fukunaga, K., Kawashima, Y., Ohara, O., Kawana, A., Asano, K., et al. (2020). Dysregulated Metabolism of Polyunsaturated Fatty Acids in Eosinophilic Allergic Diseases. Prostaglandins Other Lipid Mediat 150, 106477. doi:10.1016/j.prostaglandins.2020.106477
Miyata, J., Fukunaga, K., Kawashima, Y., Watanabe, T., Saitoh, A., Hirosaki, T., et al. (2019). Dysregulated Fatty Acid Metabolism in Nasal Polyp-Derived Eosinophils from Patients with Chronic Rhinosinusitis. Allergy 74 (6), 1113–1124. doi:10.1111/all.13726
Mochimaru, T., Fukunaga, K., Miyata, J., Matsusaka, M., Masaki, K., Kabata, H., et al. (2018). 12-OH-17,18-Epoxyeicosatetraenoic Acid Alleviates Eosinophilic Airway Inflammation in Murine Lungs. Allergy 73 (2), 369–378. doi:10.1111/all.13297
Negri, J., Early, S. B., Steinke, J. W., and Borish, L. (2008). Corticosteroids as Inhibitors of Cysteinyl Leukotriene Metabolic and Signaling Pathways. J. Allergy Clin. Immunol. 121 (5), 1232–1237. doi:10.1016/j.jaci.2008.02.007
Ordovas-Montanes, J., Dwyer, D. F., Nyquist, S. K., Buchheit, K. M., Vukovic, M., Deb, C., et al. (2018). Allergic Inflammatory Memory in Human Respiratory Epithelial Progenitor Cells. Nature 560 (7720), 649–654. doi:10.1038/s41586-018-0449-8
Peebles, R. S. (2019). Prostaglandins in Asthma and Allergic Diseases. Pharmacol. Ther. 193, 1–19. doi:10.1016/j.pharmthera.2018.08.001
Peng, Y., Zi, X. X., Tian, T. F., Lee, B., Lum, J., Tang, S. A., et al. (2019). Whole-transcriptome Sequencing Reveals Heightened Inflammation and Defective Host Defence Responses in Chronic Rhinosinusitis with Nasal Polyps. Eur. Respir. J. 54 (5). doi:10.1183/13993003.00732-2019
Pérez-Novo, C. A., Watelet, J. B., Claeys, C., Van Cauwenberge, P., and Bachert, C. (2005). Prostaglandin, Leukotriene, and Lipoxin Balance in Chronic Rhinosinusitis with and without Nasal Polyposis. J. Allergy Clin. Immunol. 115 (6), 1189–1196. doi:10.1016/j.jaci.2005.02.029
Roy, M. G., Livraghi-Butrico, A., Fletcher, A. A., McElwee, M. M., Evans, S. E., Boerner, R. M., et al. (2014). Muc5b Is Required for Airway Defence. Nature 505 (7483), 412–416. doi:10.1038/nature12807
Sawane, K., Nagatake, T., Hosomi, K., Hirata, S. I., Adachi, J., Abe, Y., et al. (2019). Dietary Omega-3 Fatty Acid Dampens Allergic Rhinitis via Eosinophilic Production of the Anti-allergic Lipid Mediator 15-Hydroxyeicosapentaenoic Acid in Mice. Nutrients 11 (12), 1. doi:10.3390/nu11122868
Seshadri, S., Lin, D. C., Rosati, M., Carter, R. G., Norton, J. E., Suh, L., et al. (2012). Reduced Expression of Antimicrobial PLUNC Proteins in Nasal Polyp Tissues of Patients with Chronic Rhinosinusitis. Allergy 67 (7), 920–928. doi:10.1111/j.1398-9995.2012.02848.x
Stevens, W. W., Staudacher, A. G., Hulse, K. E., Carter, R. G., Winter, D. R., Abdala-Valencia, H., et al. (2021). Activation of the 15-lipoxygenase Pathway in Aspirin-Exacerbated Respiratory Disease. J. Allergy Clin. Immunol. 147 (2), 600–612. doi:10.1016/j.jaci.2020.04.031
Takabayashi, T., Kato, A., Peters, A. T., Hulse, K. E., Suh, L. A., Carter, R., et al. (2013). Excessive Fibrin Deposition in Nasal Polyps Caused by Fibrinolytic Impairment through Reduction of Tissue Plasminogen Activator Expression. Am. J. Respir. Crit. Care Med. 187 (1), 49–57. doi:10.1164/rccm.201207-1292OC
Takabayashi, T., Kato, A., Peters, A. T., Hulse, K. E., Suh, L. A., Carter, R., et al. (2013). Increased Expression of Factor XIII-A in Patients with Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 132 (3), 584–e4. e4. doi:10.1016/j.jaci.2013.02.003
Venter, C., Meyer, R. W., Nwaru, B. I., Roduit, C., Untersmayr, E., Adel-Patient, K., et al. (2019). EAACI Position Paper: Influence of Dietary Fatty Acids on Asthma, Food Allergy, and Atopic Dermatitis. Allergy 74 (8), 1429–1444. doi:10.1111/all.13764
Vickery, T. W., Armstrong, M., Kofonow, J. M., Robertson, C. E., Kroehl, M. E., Reisdorph, N. A., et al. (2021). Altered Tissue Specialized Pro-resolving Mediators in Chronic Rhinosinusitis. Prostaglandins Leukot. Essent. Fatty Acids 164, 102218. doi:10.1016/j.plefa.2020.102218
Walter, W., Sánchez-Cabo, F., and Ricote, M. (2015). GOplot: an R Package for Visually Combining Expression Data with Functional Analysis. Bioinformatics 31 (17), 2912–2914. doi:10.1093/bioinformatics/btv300
Wang, C., Lou, H., Wang, X., Wang, Y., Fan, E., Li, Y., et al. (2015). Effect of Budesonide Transnasal Nebulization in Patients with Eosinophilic Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 135 (4), 922–e6. doi:10.1016/j.jaci.2014.10.018
Wang, X., Zhang, N., Bo, M., Holtappels, G., Zheng, M., Lou, H., et al. (2016). Diversity of TH Cytokine Profiles in Patients with Chronic Rhinosinusitis: A Multicenter Study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 138 (5), 1344–1353. doi:10.1016/j.jaci.2016.05.041
Wei, Y., Xia, W., Ye, X., Fan, Y., Shi, J., Wen, W., et al. (2014). The Antimicrobial Protein Short Palate, Lung, and Nasal Epithelium Clone 1 (SPLUNC1) Is Differentially Modulated in Eosinophilic and Noneosinophilic Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 133 (2), 420–428. doi:10.1016/j.jaci.2013.09.052
Wen, W., Liu, W., Zhang, L., Bai, J., Fan, Y., Xia, W., et al. (2012). Increased Neutrophilia in Nasal Polyps Reduces the Response to Oral Corticosteroid Therapy. J. Allergy Clin. Immunol. 129 (6), 1522. doi:10.1016/j.jaci.2012.01.079
Wendell, S. G., Baffi, C., and Holguin, F. (2014). Fatty Acids, Inflammation, and Asthma. J. Allergy Clin. Immunol. 133 (5), 1255–1264. doi:10.1016/j.jaci.2013.12.1087
Woodruff, P. G., Boushey, H. A., Dolganov, G. M., Barker, C. S., Yang, Y. H., Donnelly, S., et al. (2007). Genome-wide Profiling Identifies Epithelial Cell Genes Associated with Asthma and with Treatment Response to Corticosteroids. Proc. Natl. Acad. Sci. U S A. 104 (40), 15858–15863. doi:10.1073/pnas.0707413104
Woodworth, B. A., Joseph, K., Kaplan, A. P., and Schlosser, R. J. (2004). Alterations in Eotaxin, Monocyte Chemoattractant Protein-4, Interleukin-5, and Interleukin-13 after Systemic Steroid Treatment for Nasal Polyps. Otolaryngol. Head Neck Surg. 131 (5), 585–589. doi:10.1016/j.otohns.2004.05.028
Workman, A. D., Miyake, M. M., Nocera, A. L., Mueller, S. K., Finn, K., Otu, H. H., et al. (2020). Unexpected Effects of Systemic Steroids on the CRSwNP Proteome: Is Protein Upregulation More Important Than Inhibition? Int. Forum Allergy Rhinol 10 (3), 334–342. doi:10.1002/alr.22497
Wu, D., Yan, B., Wang, Y., Zhang, L., and Wang, C. (2019). Charcot-Leyden crystal Concentration in Nasal Secretions Predicts Clinical Response to Glucocorticoids in Patients with Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 144 (1), 345. doi:10.1016/j.jaci.2019.03.029
Wu, X., Hong, H., Zuo, K., Han, M., Li, J., Wen, W., et al. (2016). Expression of Leukotriene and its Receptors in Eosinophilic Chronic Rhinosinusitis with Nasal Polyps. Int. Forum Allergy Rhinol 6 (1), 75–81. doi:10.1002/alr.21625
Yates, C. M., Calder, P. C., and Ed Rainger, G. (2014). Pharmacology and Therapeutics of omega-3 Polyunsaturated Fatty Acids in Chronic Inflammatory Disease. Pharmacol. Ther. 141 (3), 272–282. doi:10.1016/j.pharmthera.2013.10.010
Zhang, N., Van Zele, T., Perez-Novo, C., Van Bruaene, N., Holtappels, G., DeRuyck, N., et al. (2008). Different Types of T-Effector Cells Orchestrate Mucosal Inflammation in Chronic Sinus Disease. J. Allergy Clin. Immunol. 122 (5), 961–968. doi:10.1016/j.jaci.2008.07.008
Zhang, Y., Lou, H., Wang, Y., Li, Y., Zhang, L., and Wang, C. (2019). Comparison of Corticosteroids by 3 Approaches to the Treatment of Chronic Rhinosinusitis with Nasal Polyps. Allergy Asthma Immunol. Res. 11 (4), 482–497. doi:10.4168/aair.2019.11.4.482
Keywords: chronic rhinosinusitis with nasal polyps, cilia, glucocorticoids, oxylipid mediator, transcriptomic sequencing
Citation: Zhu Z, Wang W, Zha Y, Wang X, Wang L, Han J, Zhang J and Lv W (2022) Transcriptomic and Lipidomic Profiles in Nasal Polyps of Glucocorticoid Responders and Non-Responders: Before and After Treatment. Front. Pharmacol. 12:814953. doi: 10.3389/fphar.2021.814953
Received: 23 November 2021; Accepted: 23 December 2021;
Published: 13 January 2022.
Edited by:
Emanuela Ricciotti, University of Pennsylvania, United StatesCopyright © 2022 Zhu, Wang, Zha, Wang, Wang, Han, Zhang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianmin Zhang, anpoYW5nNDJAMTYzLmNvbQ==; Wei Lv, bGlsaTIwMDIwNjE1QHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.