- 1Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 2Department of Clinical Laboratory, Affiliated Hospital of Jining Medical University, Jining, China
Background: TNF-α has been reported to be closely associated with autoimmune inflammatory diseases. This study aims to investigate the role of TNF-α -308(rs1800629) G/A gene polymorphisms as well as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in predicting the efficacy and safety of TNF inhibitors (TNFi) in patients with ankylosing spondylitis (AS), rheumatoid arthritis (RA), and psoriasis arthritis (PsA).
Methods: A total of 515 subjects (181 AS, 144 RA, 48 PsA, 10 hyperbilirubinemia, 10 hyperlipidemia and 122 healthy control) were recruited in this study. The accuracy of RT-PCR methods for identifying individual TNF-α -308 genotypes was assessed using sequencing as the gold standard. Baseline NLR and PLR of patients with AS, RA and PsA and healthy controls (HC) were calculated and compared. Meanwhile, differences between responders and non-responders to TNFi treatment as well as between individuals with and without adverse effects (AE) among responders were compared.
Results: The RT-PCR method is stable and reliable for TNF-α -308G/A gene polymorphism analysis, independent of sample status. The GG genotype was overwhelmingly represented, with relatively few GA genotype, whilst the AA genotype was not detected in this study. There was no observed association between TNF-α-308G/A polymorphism and susceptibility in AS, RA or PsA patients. Patients with AS, RA, and PsA had a higher NLR, compared to the HC group. Apart from PsA patients, AS and RA patients had a higher PLR, compared to the HC group. NLR was positively correlated with PLR. Furthermore, a lack of response was more frequently observed in AS and RA patients that carrying the GA genotype than the GG genotype. AS and RA patients with AE had higher NLR and PLR, compared with the non-AE group.
Conclusion: Our study preliminarily shown that combining TNF-α -308G/A polymorphisms with NLR and PLR can predict the responsiveness and safety of anti-TNF therapy in patients with AS or RA.
Introduction
Ankylosing spondylitis (AS), rheumatoid arthritis (RA) and psoriasis arthritis (PsA) are common autoimmune-mediated inflammatory diseases. The primary clinical feature of AS is the involvement of peripheral joints, which eventually leads to ossification of entheses and loss of joint mobility (Sode et al., 2018). According to a preliminary survey, the prevalence of AS in China is approximately 0.3% (Shen et al., 2013). AS can affect the quality of life, mortality and work capacity of patients, also imposes a heavy burden on society (Boonen and van der Linden, 2006). Simultaneously, RA is characterized by persistent synovitis, systemic inflammation, and autoantibodies. Individuals with RA have increased mortality due to cardiovascular disease and infection (Scott et al., 2010). PsA is an immune-mediated, genetic disease manifesting in the joints. Patients with psoriasis were at an increased risk of developing serious health problems, such as psoriatic arthritis, metabolic syndrome and cardiovascular disorders (Boehncke and Schön, 2015). Notably, it is difficult to achieve stable disease in PsA patients with concomitant other diseases. It has been reported that PsA patients with hepatic steatosis (HS) and carotid plaques (CPs) were at increased risk of relapse and failure to achieve minimal disease activity (MDA) after anti-TNF-α treatment (Di Minno et al., 2012). AS, RA, and PsA caused serious threats to human health, while both genetic and environmental factors play an important role in their pathogenesis.
Tumor necrosis factor alpha (TNF-α) is a cytokine that produced by macrophages or monocytes in acute inflammation which involved in a range of cellular signaling events (Idriss and Naismith, 2000). Serum levels of TNF-α have been reported to be significantly higher in patients with AS, RA, and PsA compared to the healthy individuals (Gratacós et al., 1994; Kyriakou et al., 2014). This implies that TNF-α may have an essential role in the development of autoimmune inflammatory diseases. On the other hand, single nucleotide gene polymorphisms (SNPs) of the TNF-α promoter are gradually being of interest. The G-to-A transition at position -308 (rs1800629) has been the most extensively investigated. However, the association of SNP at the TNF-α -308G/A with autoimmune diseases such as AS, RA, and PsA has not been fully elucidated.
In recent years, there is growing evidence of the value of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in assessing disease activity and prognosis in systemic inflammatory diseases (Qin et al., 2016; Wu et al., 2016; Gasparyan et al., 2019). Besides, elevated NLR and PLR are considered as biomarkers of poor prognosis in patients with cancer (Hirahara et al., 2019; Ni et al., 2020). Thus, NLR and PLR are inexpensive and easily accessible indicators that have a wide range of clinical applications. Unfortunately, studies of NLR and PLR in evaluating the efficacy and safety of anti-TNF-α therapy in patients with AS, RA, and PsA are still lacking.
Resistance to conventional drugs has become a major challenge in the treatment of autoimmune diseases. Based on the hypothesis that excessive TNF-α accumulation may cause deleterious effects, a variety of treatments targeting TNF-α (such as Infliximab, Etanercept, Certolizumab pegol, Adalimumab) have been introduced in autoimmune diseases and have shown favorable performance (Chatzantoni and Mouzaki, 2006). However, 40–60% of patients did not respond to TNF-α inhibitors (TNFi) while they were exposed to the risk of serious adverse effects, including serious infections and even oncogenesis (Strand et al., 2007). Biomarkers based on individual differences are desirable to evaluate the effectiveness of anti-TNF-α therapy. Several studies have shown that TNF-α -308G/A can be an excellent predictor of the responsiveness to TNF-α inhibitors (Maxwell et al., 2008; Netz et al., 2017). Similarly, NLR and PLR have been preliminarily studied in predicting the responsiveness and drug persistence of anti-TNF-α agents in patients with RA (Lee et al., 2019). Specifically, their predictive power in AS, RA, and PsA needs to be further investigated.
In this study, we focused on the utility of TNF-α -308G/A polymorphisms, NLR, and PLR as potential biomarkers in AS, RA, and PsA, as well as the role of TNF-α -308G/A polymorphisms in combination with NLR and PLR in predicting the efficacy and safety of anti-TNF-α therapy.
Materials and Methods
Subjects
A total of 515 subjects (181 AS, 144 RA, 48 PsA, 10 hyperbilirubinemia, 10 hyperlipidemia and 122 healthy control) were recruited from Peking Union Medical College Hospital (PUMCH), Chinese Academy of Medical Sciences between July 2019 and August 2021 in this study. Of these patients, 181 patients were diagnosed with AS according to the Modified New York criteria by the American College of Rheumatology (van der Linden et al., 1984). 144 patients were defined as RA according to the American Rheumatism Association 1987 revised criteria (Arnett et al., 1988) and 48 patients were defined as PsA fulfilling classification criteria for psoriatic arthritis (Taylor et al., 2006). Hyperbilirubinemia (HB) and hyperlipidemia (HLP) were diagnosed by combining clinical symptoms and laboratory results (Sullivan and Rockey, 2017; Stewart et al., 2020). Data including demographic information, medical history, laboratory results and drug consumption information were collected through the hospital information system (HIS) and laboratory information system (LIS) of PUMCH. The eligibility criteria of patient-recruitment were: (1) confirmed diagnosis of RA, AS, PsA, HB, or HLP; (2) complete demographic data, medical history can be obtained; (3) EDTA anticoagulated whole blood samples with acceptable quality were available. The exclusion criteria adopted in the research were as follows: (1) presence of other autoimmune diseases, malignancies, acute infections, hematological diseases, immunodeficiency diseases and other serious conditions; (2) exposure to biologic therapy due to other diseases prior to enrollment; (3) patients have been misdiagnosed several times and have received several inappropriate treatments before being properly diagnosed; (4) an unwillingness or inability to cooperate. In addition, the healthy individuals selected as control group had no family history of autoimmunity or chronic inflammatory disease and no abnormal laboratory results. Duplicate individuals were removed using a unique identification code while the whole blood collected was kept with EDTA from individuals for subsequent experiments. The NLR is calculated dividing the absolute count of neutrophils by the absolute count of lymphocytes, and the PLR is calculated dividing the platelet count by the absolute count of lymphocytes. The patient’s responsiveness to TNF-α inhibitors was evaluated by the clinicians based on a combination of the patient’s joint or spinal involvement, patient description and laboratory testing. For AS, patients were defined as responders if their Ankylosing Spondylitis Disease Activity Score (ASDAS) decreased by ≥ 1.1 units compared to baseline score, otherwise they were defined as non-responders (Machado et al., 2011). Disease Activity Score using 28 joint counts (DAS28) was used to assess the responsiveness of patients with RA. Patients with DAS28 reduction >1.2 or DAS28 < 5.1 and a DAS28 reduction between 0.6 and 1.2 were considered responders, otherwise they were considered non-responders (van Riel and Renskers, 2016). For PsA, the treatment response was evaluated by the Disease Activity Index for Psoriatic Arthritis (DAPSA). Patients were regarded as responders when their DAPSA score improved by 50% or more relative to baseline levels, otherwise they were regarded as non-responders (Schoels et al., 2016).
Ethics Statement
This study was conducted in accordance with the recommendations of the PUMCH. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of PUMCH (No. HS2019035).
Genotyping
Genomic DNA was extracted from whole peripheral blood via DNA extraction kits (Tianlong Technology Co. LTD, Xi’an, China). Genotyping for TNF-α -308 (rs1800629) was performed using TNF-α -308G/A gene polymorphisms RT-PCR detection kit (Wuhan HealthCare Biotechnology Co., Ltd., Wuhan, China). Genotypes were interpreted with the criteria of Supplementary Table S1. Meanwhile, genotyping was achieved by TaqMan chemistry using the Applied Biosystems real-time Prism 3730XL Sequence Detection System (ABI Inc. CA, United States) according to the Applied Biosystem protocol.
Statistical Analysis
All the data were analyzed by Excel 2019 (Microsoft Inc., United States), and SPSS 20.0 (SPSS Inc., Chicago, IL, United States) software. The concordance between genotyping methods was analyzed by using kappa test. Correlation tests were performed by using the Spearman’s correlation analysis. Differences between groups were assessed using the Wilcoxon or Chi-square test. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by using logistic regression analysis. The Hardy-Weinberg equilibrium (HWE) was evaluated by online website (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). p < 0.05 was considered statistically significant.
Results
Methodological Assessment of Genotype Identification
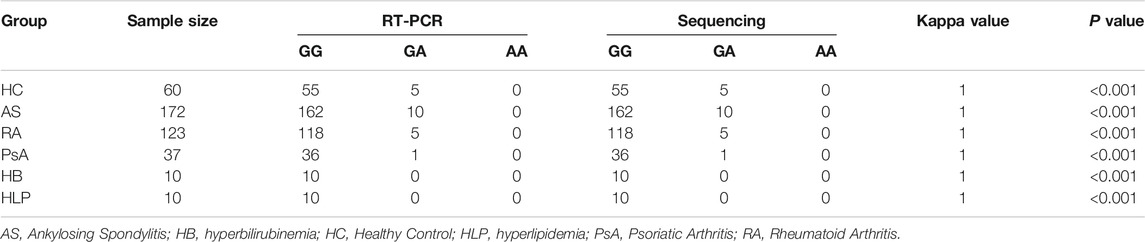
In this study, the TNF-α -308G/A polymorphisms detection kit (RT-PCR) was used to identify the genotype of each subject. To ensure the reliability of genotype identification for each participant, a subset of subjects (60 HC, 172 AS, 123 RA, 37 PsA, 10 HB and 10 HLP) were selected to assess the accuracy of the RT-PCR method, using the sequencing method as the gold standard. As depicted in Table 1; Figure 1, there was an excellent agreement between the two methods in the HC, AS, RA and PsA populations. Notably, favorable results were also achieved with the RT-PCR method in patients with HB and HP, indicating that RT-PCR method is accurate and reliable, independent of bilirubin and lipid interference in the samples.
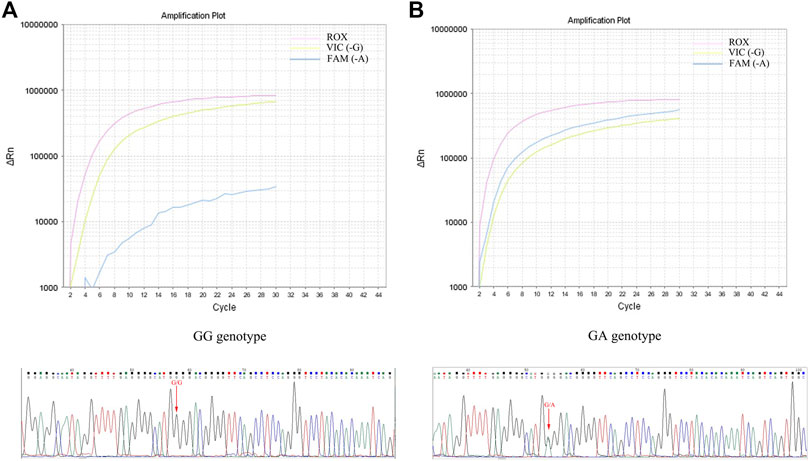
FIGURE 1. Determination of TNF-α -308G/A genotype using RT-PCR and sequencing. (A): GG genotype amplification curve and sequencing peaks map; (B): GA genotype amplification curve and sequencing peaks map.
Genotype Distributions of TNF-α -308G/A Gene Polymorphisms in the RA, AS, PsA Patients and HC
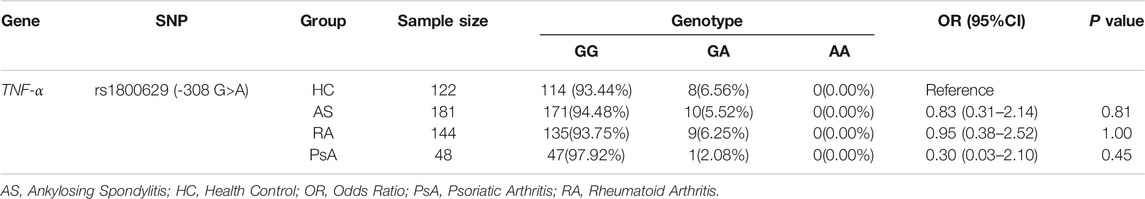
A total of 181 patients with AS, 144 with RA, 48 with PsA and 122 HC were included in the study to examine the polymorphisms of TNF-α -308G/A. The clinical characteristics and baseline laboratory data of all participants were summarized in Table 2. All samples were genotyped by RT-PCR method. The distribution and frequency of TNF-α -308G/A genotypes were shown in Table 3. The genotypes distribution agreed with the Hardy-Weinberg equilibrium (p = 0.52). Overall, the GG genotype was overwhelmingly represented, with relatively few GA genotype, whilst the AA genotype was not detected in this study. Furthermore, logistic regression analyses revealed that TNF-α -308G/A polymorphisms were not associate with susceptibility of AS, RA, or PsA (p > 0.05).
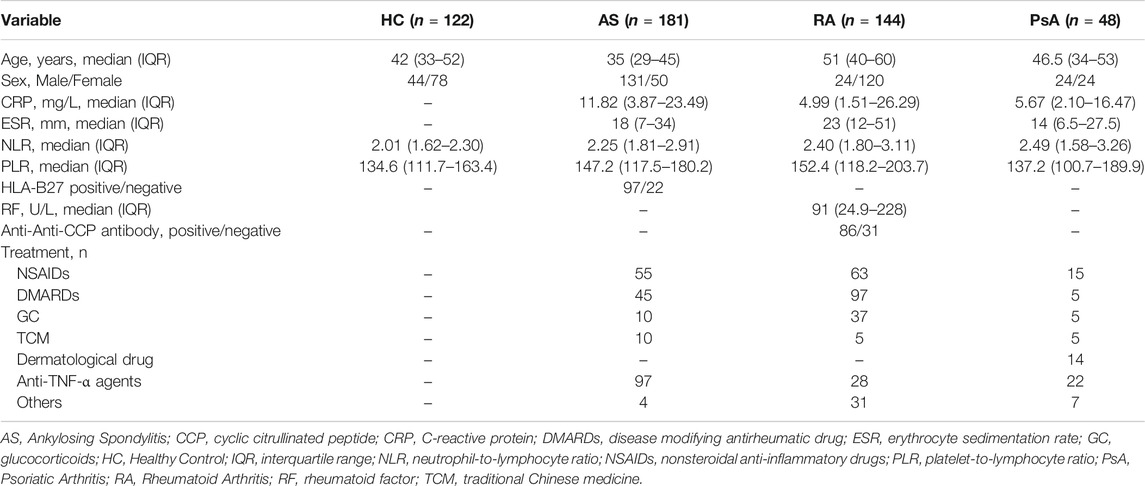
TABLE 2. Clinical characteristics and baseline laboratory data in HC and patients with AS, RA and PsA.
NLR and PLR in the RA, AS, PsA Patients and HC
To explore the potential value of NLR and PLR as biomarkers, baseline NLR and PLR of AS, RA, PsA patients and HC were calculated. As shown in Figure 2A, patients with AS, RA and PsA had a higher NLR, compared to the HC group. Apart from PsA patients, AS and RA patients had a higher PLR, compared to the HC group. (Figure 2B). We further compared the differences of NLR and PLR among individuals with different genotypes and showed that only in the RA group, patients with the GA genotype had a higher NLR than the GG genotype. (Figures 2C,D). It was intriguing to note that NLR was positively correlated with PLR in the AS (r = 0.53, p < 0.001), RA (r = 0.64, p < 0.001), PsA (r = 0.75, p < 0.001) and HC groups (r = 0.48, p < 0.001). (Figures 2E–H). Moreover, we observed a positive correlation among NLR, PLR, CRP and ESR in the AS and RA groups. (Supplementary Figure S1).
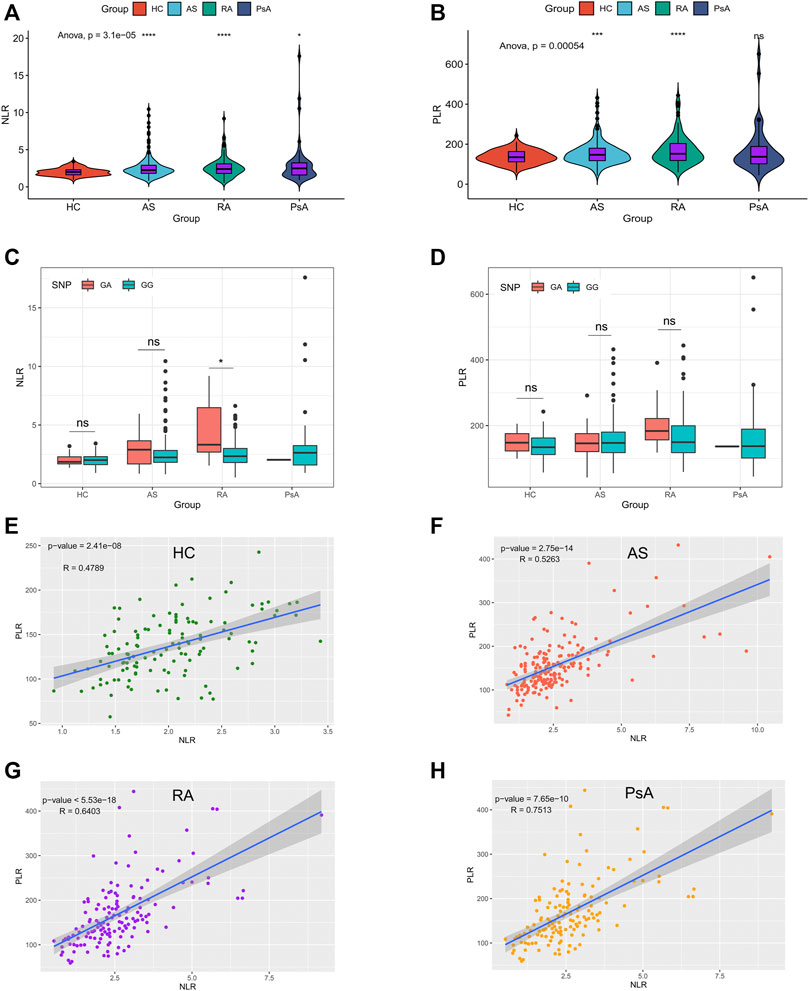
FIGURE 2. NLR and PLR in the RA, AS, PsA patients and HC. (A–B): baseline levels of NLR and PLR in subjects in the AS, RA, PsA, and HC groups, ****p < 0.0001, ***p < 0.001, *p < 0.05; (C–D): baseline levels of NLR and PLR in subjects with different genotypes in AS, RA, PsA, and HC groups, *p < 0.05, ns, no significance; (E–H): The correlation between PLR and NLR in HC, AS, RA, and PsA group.
TNF-α -308G/A Polymorphisms Combined With Baseline NLR and PLR Predict Responsiveness and Safety of TNF-α Inhibitors
In this study, we collected information on medication administration for all patients via the HIS system. As illustrated in Table 2, non-steroidal anti-inflammatory drugs (NSAIDs) and disease modifying antirheumatic drug (DMARDs) were often the first choice of drugs for the treatment of auto-immune inflammatory diseases. Unfortunately, we have observed that a significant proportion of patients do not respond to these traditional medicines. The introduction of TNFi brought a ray of hope for these patients. Anti-TNF-α therapies are now widely used in the clinic, however, there are still some patients who are not responsive to TNFi and under enormous financial pressure as well as being exposed to the risk of adverse effects (AE).
In this study, the AS, RA and PsA groups had 97, 28 and 22 patients who received TNFi such as etanercept or adalimumab. (Table 4). Most patients showed satisfactory efficacy with TNFi, but a few non-responders remained. The differences in clinical features between responders and non-responders in patients with AS and RA have been shown in Table 5. We found a lack of response was more frequently observed in AS and RA patients carrying the GA genotype than the GG genotype (p < 0.05). For RA patients, non-responders had higher level of NLR and PLR compared to responders, whereas for AS patients this difference was not observed. Therefore, TNF-α -308G/A polymorphisms may be used as a predictor of responsiveness to TNFi.

TABLE 4. Categories and therapeutic effectiveness of TNF-α inhibitors in patients with AS, RA and PsA.
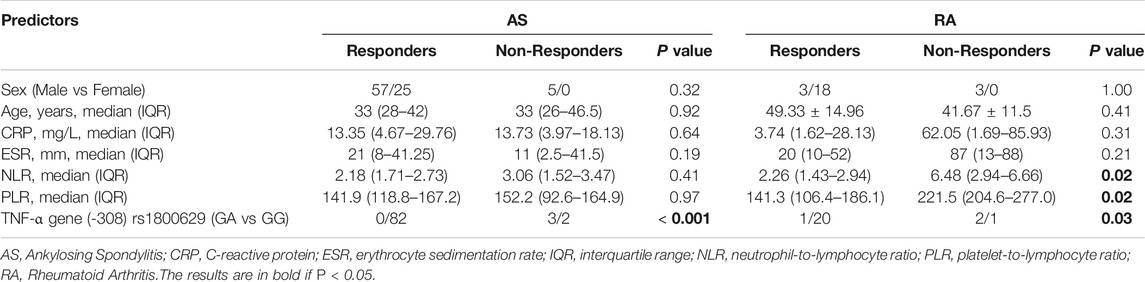
TABLE 5. Differences in clinical features between responders and non-responders in patients with AS and RA.
Remarkably, we also observed that most patients carrying the GG genotype showed a superior response to TNFi, but there were still some patients suffering from AE, such as somatic pains, iritis, or tuberculosis, which led them to switch to another TNFi or even abandon anti-TNF-α treatment. Meanwhile, patients carrying the GG genotype have a wide range of values for NLR and PLR. On this basis, we intended to explore whether NLR and PLR could further identify patients exposed to the risk of AE. As shown in Figure 3, AS and RA patients with AE had higher NLR and PLR, compared with the non-AE group. However, for PsA patients, there was no significant difference in NLR and PLR between the AE and non-AE groups. In addition, we also found that during the process of anti-TNF-α treatment, the change trend in NLR, PLR over time was consistent with the change in disease activity (Figure 4). Overall, combining TNF-α -308G/A polymorphisms with NLR and PLR can predict the responsiveness and safety of anti-TNF therapy in patients with AS or RA.
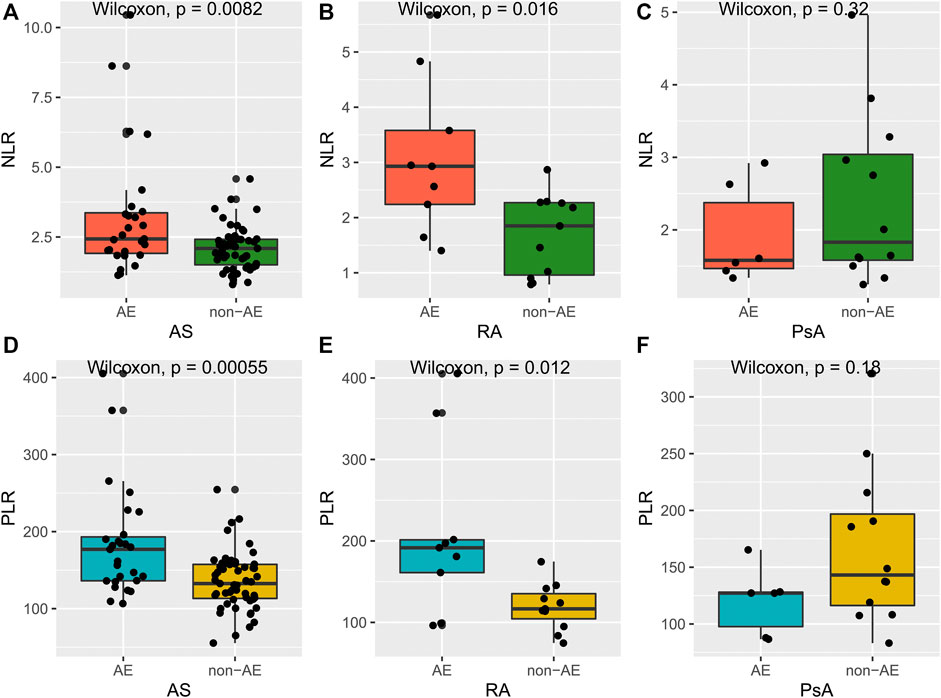
FIGURE 3. Differences of NLR and PLR between AE responders and non-AE responders. (A–C): differences of NLR between AE responders and non-AE responders in AS, RA, and PsA group; (D–F): differences of PLR between AE responders and non-AE responders in AS, RA, and PsA group.
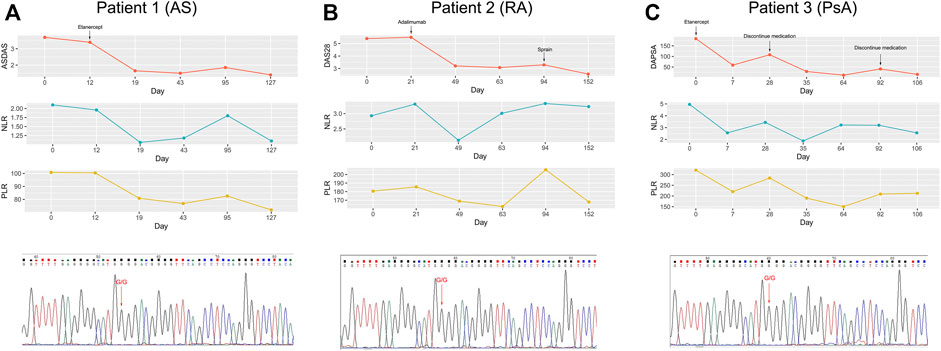
FIGURE 4. Correlation of changes in disease activity with changes in PLR and NLR over the course of TNFi treatment. (A): patient 1 with AS carrying the GG genotype; (B): patient 2 with RA carrying the GG genotype; (C): patient 3 with PsA carrying the GG genotype.
Discussion
TNF-α was initially identified as a factor that can induce tumor cell necrosis, but was latterly found to be involved in the pathogenic process of autoimmune diseases as an important pro-inflammatory factor (Jang et al., 2021). The accumulation of TNF-α can ultimately contribute to the development of chronic inflammation and tissue destruction. It has been reported that TNF-α promoter -308A allele in Crohn’s disease increased TNF production promoting inflammatory activity and were associated with worse responsiveness to anti-TNF-α therapy (González et al., 2003; Netz et al., 2017). On this basis, we speculated that TNF-α -308A may also have a similar role in autoimmune diseases such as AS, RA, and PsA.
Various methods have been reported to determine the genotype of TNF-α -308G/A, such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Sun et al., 2013), restriction fragment length polymorphism (RFLP) (Manolova et al., 2014), and high resolution melting (HRM) (Li et al., 2014). However, the accuracy of these methods as genotyping has rarely been verified. In this study, to identify each subject’s genotype accurately, we selected a subset of the population was genotyped using RT-PCR with the sequencing method as the gold standard. The results showed an extremely high level of agreement between the two methods. Besides, the genotype analysis of patients with HB and HLP was not interfered by bilirubin and lipids. Therefore, the RT-PCR method is stable and reliable for TNF-α -308G/A gene polymorphism analysis, independent of sample status.
Interestingly, the GG genotype was overwhelming (over 90%) in both disease groups and healthy controls, the GA genotype was relatively rare (2.08–6.56%), and the AA genotype was not detected. The frequency of genotype distribution in our study was analogous to two other studies from China (Sun et al., 2013; Hu et al., 2018). In contrast to our study, the frequency of the GA genotype was slightly higher in the Bulgarian and Danish populations, accounting for about 20% of the population (Manolova et al., 2014; Sode et al., 2018). In the UK population, the frequency of the GA genotype even reaches 32% (Maxwell et al., 2008). This discrepancy may be due to racial differences and suggested that the A allele in the TNF-α -308 gene is relatively scarce in the Chinese population.
The association between TNF-α -308 G/A and AS has been well studied but still controversial. Sode et al. and Hu et al. have reported that heterozygous variant genotypes of TNF-α -308G/A were associated with reduced risk of AS (Hu et al., 2018; Sode et al., 2018). Nevertheless, in the reports of Fraile et al. and Sun et al., no significant differences in the genotype and allele frequencies of TNF-α -308G/A (rs1800629) polymorphism were observed between AS patients and healthy individuals (Fraile et al., 1998; Sun et al., 2013). Our findings were analogous to the latter, with no explicit evidence of an association between AS and TNF-α -308G/A being observed. A meta-analysis based on 35 case-control studies showed that rs1800629 polymorphism significantly increased the risk of AS in Caucasians and decreased the risk of AS in mixed populations (Gao et al., 2021). Therefore, it is conceivable that race and sample size are crucial factors contributing to the conclusion and that future large-scale multicenter studies are needed to elucidate the role of TNF-α -308G/A polymorphisms in AS.
Our study also indicated that TNF-α -308G/A polymorphisms were not associated with susceptibility to RA. Li et al. reported that the rs1800629 A allele of TNF-α was negatively associated with RA in the Chinese population (Li et al., 2014). But several studies have concluded that TNF-α -308G/A polymorphisms were not associated with RA susceptibility (Sun et al., 2013; Manolova et al., 2014; Cadena-Sandoval et al., 2018). A meta-analysis of 19 studies involving 2584 patients with RA and 3254 controls revealed that the TNF-α rs1800629 G/A polymorphism may represent a significant risk factor for RA in Latin Americans but not in the European, Arab, or Asian populations (Song et al., 2014). It was consistent with our research.
For PsA, our study demonstrated that individuals with the GA genotype appear to have a reduced risk of PsA, but the difference was not statistically significant (OR = 0.30, 95%CI 0.03–2.10, p = 0.45). A previous meta-analysis consisting of 2253 psoriasis patients and 1947 controls highlighted that TNF-α -308G/A polymorphism was significantly associated with decreased risk of psoriasis (GA versus GG: OR 0.67, 95%CI 0.57–0.78, p < 0.001) (Zhuang et al., 2013). Perhaps the small sample size could explain the failure to observe significant differences in our results. Therefore, in the future, studies with larger samples containing different stages of PsA need to be performed to draw a reliable conclusion.
Generally, the neutrophil count in the blood increases as the inflammatory disease progresses, while the lymphocyte count decreases. There is growing evidence that platelets are activated and play vital roles in the inflammatory response (Thomas and Storey, 2015). Thus, NLR and PLR are regarded as robust indicators that reflect the progression of inflammatory diseases. Our study showed that patients with AS and RA had higher NLR and PLR compared with HC group, which is in accordance with previous reports (Erre et al., 2019; Zeb et al., 2019). Notably, NLR was elevated in PsA patients compared with HC group, while PLR was not. Surprisingly, our results were perfectly concordant with the study by Kim et al. (Kim et al., 2016). Mean platelet volume (MPV) has been reported to significantly increase in patients with PsA as a marker of platelet activation (Canpolat et al., 2010). Thus, both the number and volume of platelets may expand over the course of PsA whereas PLR solely considers the number of platelets. Furthermore, when we stratified the different disease groups and healthy controls by genotype, there were no significant differences regarding NLR and PLR between individuals carrying the GG genotype and the GA genotype. It implied that there was no obvious correlation between TNF-α -308G/A polymorphisms and NLR or PLR. Nevertheless, NLR was positively correlated with PLR in AS, RA, PsA patients and in the HC group, revealing a well-defined agreement between the NLR and PLR. When considering the association between NLR, PLR and other laboratory indicators, such as CRP or ESR, higher correlations were observed in patients with AS and RA. These correlations have also been confirmed in previous publications (Kim et al., 2016; Qin et al., 2016), suggesting NLR and PLR as new inexpensive and available biomarkers that can effectively reflect disease activity.
TNFi can be of significant benefit to patients with AS, especially in cases when conventional treatment has failed, but non-response to its application also raised a concern. On the other hand, TNFi is enormously expensive and carries the risk of adverse effects, thus there is an urgent need for a program to guide the use of the drug based on individual variation. Our survey data showed that 97 patients with AS, 28 patients with RA, and 22 patients with PsA were given treatment with TNFi and most of them exhibited a favorable outcome, while just ten were non-responders. Despite the small size of the non-response cases, the fact that six of the ten cases were of the GA genotype implied that the GA genotype may be a predictor of treatment failure. Our study agreed with the findings of Ma et al., in their study, the GG genotype was more frequent in good responders to TNF-α inhibitors in patients with AS (Ma et al., 2017). Certainly, given the rarity of the A allele in the Chinese population, continuous studies with larger populations are desirable to further clarify the relationship between TNF-α -308G/A polymorphisms and responsiveness to TNF-α inhibitors.
The association between TNF-α -308G/A polymorphism and responsiveness of TNFi in patients with RA was as well controversial. A meta-analysis conducted by Lee et al. asserted that RA patients carrying the A allele had a worse response to TNF-α inhibitors than those carrying the G allele, but they denied this in a consequent updated meta-analysis (Lee et al., 2006; Lee et al., 2010). Therefore, more and more rigorous studies will be needed in the future to reach a sufficiently scientific conclusion. Song et al. have reported that PsA individuals carrying the TNF-α -308 G allele shown better responsiveness to TNFi than those with A alleles in Caucasians (Song et al., 2015). Unfortunately, similar studies in Chinese populations are conspicuously rare. In our report, although the number of cases was limited, 18 of the 19 patients carrying the GG genotype responded to TNFi whilst one patient carrying the GA genotype did not respond. It was also an implicit association of TNF-α -308G/A polymorphisms with responsiveness to TNFi in Chinese PsA patients, which of course needs further verification subsequently.
Another concern about TNFi is its adverse effects. We have observed that some responders suffered from AE that forced them to change to another TNFi or to give up biological treatments. This situation imposed a heavy financial burden on them and taken a psychological toll on them. Lee et al. reported that in patients with RA, higher baseline NLR predicted poorer persistence of TNFi, including lack of efficacy and adverse events (Lee et al., 2019). Since NLR and PLR are taken in a wide range of values in responders, we speculated that NLR and PLR may be biomarkers for identifying patients at high risk of AE. We analyzed data on NLR and PLR in responders carrying the GG genotype and showed that in patients with AS and RA, NLR and PLR were higher in the AE group than in the non-AE group. This implied that responders exposed to AE may have a greater inflammatory burden that impaired the persistence of TNFi. In contrast, this difference was not observed in PsA patients. We considered two reasons might help explain it, one being that our study included fewer patients with PsA and the other being that because PsA has both psoriasis and arthritis symptoms, diagnosis is complicated and various treatments may have led to inflammatory storms in the body before a definitive diagnosis was obtained. Finally, we analyzed follow-up data on NLR and PLR during TNFi treatment and the results revealed a visible correlation between disease activity and NLR or PLR. Hence, NLR and PLR may be reliable biomarkers for the follow-up of patients undergoing TNFi treatment.
However, there were some limitations in our study. Firstly, our sample size was limited, and larger sample sizes would be required in future studies. Secondly, the A allele was remarkably scarce in our study in the Chinese population, but this was consistent with the relevant published studies across other cohorts. Thirdly, in this study we only examined the TNF-α -308G/A polymorphism; other SNPs of TNF-α should be included in future studies.
Conclusion
Our study preliminarily shown that TNF-α -308G/A polymorphisms may be used as a potential biological marker to evaluate the responsiveness to TNFi in AS or RA patients. Higher baseline NLR and PLR could identify responders at higher risk of AE. Overall, combining TNF-α -308G/A polymorphisms with NLR and PLR can predict the responsiveness and safety of anti-TNF therapy in patients with AS or RA.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of PUMCH (No. HS2019035). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ZW, YD contributed to conception and design; HZ and RZ collected samples and medical record data; LK and YD performed the experiments; ZG rechecked the data and revised the manuscript; FS helped for the data statistical analysis; ZW, LK and YD performed the data statistical analysis and wrote the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
Funding
This work was supported by the Beijing Key Clinical Specialty for Laboratory Medicine Excellent Project (No. ZK201000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.811719/full#supplementary-material
References
Arnett, F. C., Edworthy, S. M., Bloch, D. A., McShane, D. J., Fries, J. F., Cooper, N. S., et al. (1988). The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum. 31 (3), 315–324. doi:10.1002/art.1780310302
Boehncke, W. H., and Schön, M. P. (2015). Psoriasis. Lancet 386 (9997), 983–994. doi:10.1016/s0140-6736(14)61909-7
Boonen, A., and van der Linden, S. M. (2006). The burden of Ankylosing Spondylitis. J. Rheumatol. Suppl. 78, 4–11.
Cadena-Sandoval, D., Alemán-Ávila, I., Barbosa-Cobos, R. E., Becerril-Mendoza, L. T., Fragoso, J. M., and Ramírez-Bello, J. (2018). Tumor Necrosis Factor (TNF) and TNFR1 Polymorphisms Are Not Risk Factors for Rheumatoid Arthritis in a Mexican Population. Mol. Biol. Rep. 45 (3), 227–232. doi:10.1007/s11033-018-4155-2
Canpolat, F., Akpinar, H., and Eskioğlu, F. (2010). Mean Platelet Volume in Psoriasis and Psoriatic Arthritis. Clin. Rheumatol. 29 (3), 325–328. doi:10.1007/s10067-009-1323-8
Chatzantoni, K., and Mouzaki, A. (2006). Anti-TNF-alpha Antibody Therapies in Autoimmune Diseases. Curr. Top. Med. Chem. 6 (16), 1707–1714. doi:10.2174/156802606778194217
Di Minno, M. N., Peluso, R., Iervolino, S., Lupoli, R., Russolillo, A., Tarantino, G., et al. (2012). Hepatic Steatosis, Carotid Plaques and Achieving MDA in Psoriatic Arthritis Patients Starting TNF-α Blockers Treatment: a Prospective Study. Arthritis Res. Ther. 14 (5), R211. doi:10.1186/ar4049
Erre, G. L., Paliogiannis, P., Castagna, F., Mangoni, A. A., Carru, C., Passiu, G., et al. (2019). Meta-analysis of Neutrophil-To-Lymphocyte and Platelet-To-Lymphocyte Ratio in Rheumatoid Arthritis. Eur. J. Clin. Invest. 49 (1), e13037. doi:10.1111/eci.13037
Fraile, A., Nieto, A., Beraún, Y., Vinasco, J., Matarán, L., and Martín, J. (1998). Tumor Necrosis Factor Gene Polymorphisms in Ankylosing Spondylitis. Tissue Antigens 51 (4 Pt 1), 386–390. doi:10.1111/j.1399-0039.1998.tb02978.x
Gao, S., Liang, W., Xu, T., Xun, C., Cao, R., Deng, Q., et al. (2021). Associations of Tumor Necrosis Factor Alpha Gene Polymorphisms and Ankylosing Spondylitis Susceptibility: A Meta-Analysis Based on 35 Case-Control Studies. Immunological Invest. doi:10.1080/08820139.2021.1882485
Gasparyan, A. Y., Ayvazyan, L., Mukanova, U., Yessirkepov, M., and Kitas, G. D. (2019). The Platelet-To-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 39 (4), 345–357. doi:10.3343/alm.2019.39.4.345
González, S., Rodrigo, L., Martínez-Borra, J., López-Vázquez, A., Fuentes, D., Niño, P., et al. (2003). TNF-alpha -308A Promoter Polymorphism Is Associated with Enhanced TNF-Alpha Production and Inflammatory Activity in Crohn's Patients with Fistulizing Disease. Am. J. Gastroenterol. 98 (5), 1101–1106. doi:10.1111/j.1572-0241.2003.07416.x
Gratacós, J., Collado, A., Filella, X., Sanmartí, R., Cañete, J., Llena, J., et al. (1994). Serum Cytokines (IL-6, TNF-Alpha, IL-1 Beta and IFN-Gamma) in Ankylosing Spondylitis: a Close Correlation between Serum IL-6 and Disease Activity and Severity. Br. J. Rheumatol. 33 (10), 927–931. doi:10.1093/rheumatology/33.10.927
Hirahara, T., Arigami, T., Yanagita, S., Matsushita, D., Uchikado, Y., Kita, Y., et al. (2019). Combined Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Predicts Chemotherapy Response and Prognosis in Patients with Advanced Gastric Cancer. BMC Cancer 19 (1), 672. doi:10.1186/s12885-019-5903-y
Hu, N., Cui, Y., Yang, Q., Wang, L., Yang, X., and Xu, H. (2018). Association of Polymorphisms in TNF and GRN Genes with Ankylosing Spondylitis in a Chinese Han Population. Rheumatol. Int. 38 (3), 481–487. doi:10.1007/s00296-017-3899-7
Idriss, H. T., and Naismith, J. H. (2000). TNF Alpha and the TNF Receptor Superfamily: Structure-Function Relationship(s). Microsc. Res. Tech. 50 (3), 184–195. doi:10.1002/1097-0029(20000801)50:3<184:Aid-jemt2>3.0.Co;2-h
Jang, D. I., Lee, A. H., Shin, H. Y., Song, H. R., Park, J. H., Kang, T. B., et al. (2021). The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 22 (5), 2719. doi:10.3390/ijms22052719
Kim, D. S., Shin, D., Lee, M. S., Kim, H. J., Kim, D. Y., Kim, S. M., et al. (2016). Assessments of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Korean Patients with Psoriasis Vulgaris and Psoriatic Arthritis. J. Dermatol. 43 (3), 305–310. doi:10.1111/1346-8138.13061
Kyriakou, A., Patsatsi, A., Vyzantiadis, T. A., and Sotiriadis, D. (2014). Serum Levels of TNF-α, IL-12/23p40, and IL-17 in Plaque Psoriasis and Their Correlation with Disease Severity. J. Immunol. Res. 2014, 467541. doi:10.1155/2014/467541
Lee, H. N., Kim, Y. K., Kim, G. T., Ahn, E., So, M. W., Sohn, D. H., et al. (2019). Neutrophil-to-lymphocyte and Platelet-To-Lymphocyte Ratio as Predictors of 12-week Treatment Response and Drug Persistence of Anti-tumor Necrosis Factor-α Agents in Patients with Rheumatoid Arthritis: a Retrospective Chart Review Analysis. Rheumatol. Int. 39 (5), 859–868. doi:10.1007/s00296-019-04276-x
Lee, Y. H., Ji, J. D., Bae, S. C., and Song, G. G. (2010). Associations between Tumor Necrosis Factor-Alpha (TNF-Alpha) -308 and -238 G/A Polymorphisms and Shared Epitope Status and Responsiveness to TNF-Alpha Blockers in Rheumatoid Arthritis: a Metaanalysis Update. J. Rheumatol. 37 (4), 740–746. doi:10.3899/jrheum.090707
Lee, Y. H., Rho, Y. H., Choi, S. J., Ji, J. D., and Song, G. G. (2006). Association of TNF-Alpha -308 G/A Polymorphism with Responsiveness to TNF-Alpha-Blockers in Rheumatoid Arthritis: a Meta-Analysis. Rheumatol. Int. 27 (2), 157–161. doi:10.1007/s00296-006-0175-7
Li, F., Gao, J., Sokolove, J., Xu, J., Zheng, J., Zhu, K., et al. (2014). Polymorphisms in the TNF-α, TNFR1 Gene and Risk of Rheumatoid Arthritis in Chinese Han Population. Int. J. Immunogenet. 41 (6), 499–502. doi:10.1111/iji.12148
Ma, H. J., Yin, Q. F., Wu, Y., and Guo, M. H. (2017). TNF-α-308 Polymorphism Determines Clinical Manifestations and Therapeutic Response of Ankylosing Spondylitis in Han Chinese. Med. Clin. (Barc) 149 (12), 517–522. doi:10.1016/j.medcli.2017.04.023
Machado, P., Landewé, R., Lie, E., Kvien, T. K., Braun, J., Baker, D., et al. (2011). Ankylosing Spondylitis Disease Activity Score (ASDAS): Defining Cut-Off Values for Disease Activity States and Improvement Scores. Ann. Rheum. Dis. 70 (1), 47–53. doi:10.1136/ard.2010.138594
Manolova, I., Ivanova, M., Stoilov, R., Rashkov, R., and Stanilova, S. (2014). Association of Single Nucleotide Polymorphism at Position -308 of the Tumor Necrosis Factor-Alpha Gene with Ankylosing Spondylitis and Rheumatoid Arthritis. Biotechnol. Biotechnol. Equip 28 (6), 1108–1114. doi:10.1080/13102818.2014.972147
Maxwell, J. R., Potter, C., Hyrich, K. L., Barton, A., Barton, A., Worthington, J., et al. (2008). Association of the Tumour Necrosis Factor-308 Variant with Differential Response to Anti-TNF Agents in the Treatment of Rheumatoid Arthritis. Hum. Mol. Genet. 17 (22), 3532–3538. doi:10.1093/hmg/ddn245
Netz, U., Carter, J. V., Eichenberger, M. R., Dryden, G. W., Pan, J., Rai, S. N., et al. (2017). Genetic Polymorphisms Predict Response to Anti-tumor Necrosis Factor Treatment in Crohn's Disease. World J. Gastroenterol. 23 (27), 4958–4967. doi:10.3748/wjg.v23.i27.4958
Ni, L., Tao, J., Xu, J., Yuan, X., Long, Y., Yu, N., et al. (2020). Prognostic Values of Pretreatment Neutrophil-To-Lymphocyte and Platelet-To-Lymphocyte Ratios in Endometrial Cancer: a Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 301 (1), 251–261. doi:10.1007/s00404-019-05372-w
Qin, B., Ma, N., Tang, Q., Wei, T., Yang, M., Fu, H., et al. (2016). Neutrophil to Lymphocyte Ratio (NLR) and Platelet to Lymphocyte Ratio (PLR) Were Useful Markers in Assessment of Inflammatory Response and Disease Activity in SLE Patients. Mod. Rheumatol. 26 (3), 372–376. doi:10.3109/14397595.2015.1091136
Schoels, M. M., Aletaha, D., Alasti, F., and Smolen, J. S. (2016). Disease Activity in Psoriatic Arthritis (PsA): Defining Remission and Treatment success Using the DAPSA Score. Ann. Rheum. Dis. 75 (5), 811–818. doi:10.1136/annrheumdis-2015-207507
Scott, D. L., Wolfe, F., and Huizinga, T. W. (2010). Rheumatoid Arthritis. Lancet 376 (9746), 1094–1108. doi:10.1016/s0140-6736(10)60826-4
Shen, B., Shi, Z. J., Lv, H. S., Xu, J. Z., Yan, S. G., Zhang, K., et al. (2013). Consensus of Chinese Orthopaedic Experts on Diagnosis and Treatment of Ankylosing Spondylitis. Orthop. Surg. 5 (1), 1–5. doi:10.1111/os.12016
Sode, J., Bank, S., Vogel, U., Andersen, P. S., Sørensen, S. B., Bojesen, A. B., et al. (2018). Genetically Determined High Activities of the TNF-Alpha, IL23/IL17, and NFkB Pathways Were Associated with Increased Risk of Ankylosing Spondylitis. BMC Med. Genet. 19 (1), 165. doi:10.1186/s12881-018-0680-z
Song, G. G., Bae, S. C., Kim, J. H., and Lee, Y. H. (2014). Association between TNF-α Promoter -308 A/G Polymorphism and Rheumatoid Arthritis: a Meta-Analysis. Rheumatol. Int. 34 (4), 465–471. doi:10.1007/s00296-013-2919-5
Song, G. G., Seo, Y. H., Kim, J. H., Choi, S. J., Ji, J. D., and Lee, Y. H. (2015). Association between TNF-α (-308 A/G, -238 A/G, -857 C/T) Polymorphisms and Responsiveness to TNF-α Blockers in Spondyloarthropathy, Psoriasis and Crohn's Disease: a Meta-Analysis. Pharmacogenomics 16 (12), 1427–1437. doi:10.2217/pgs.15.90
Stewart, J., McCallin, T., Martinez, J., Chacko, S., and Yusuf, S. (2020). Hyperlipidemia. Pediatr. Rev. 41 (8), 393–402. doi:10.1542/pir.2019-0053
Strand, V., Kimberly, R., and Isaacs, J. D. (2007). Biologic Therapies in Rheumatology: Lessons Learned, Future Directions. Nat. Rev. Drug Discov. 6 (1), 75–92. doi:10.1038/nrd2196
Sullivan, J. I., and Rockey, D. C. (2017). Diagnosis and Evaluation of Hyperbilirubinemia. Curr. Opin. Gastroenterol. 33 (3), 164–170. doi:10.1097/mog.0000000000000354
Sun, R., Huang, Y., Zhang, H., and Liu, R. (2013). MMP-2, TNF-α and NLRP1 Polymorphisms in Chinese Patients with Ankylosing Spondylitis and Rheumatoid Arthritis. Mol. Biol. Rep. 40 (11), 6303–6308. doi:10.1007/s11033-013-2743-8
Taylor, W., Gladman, D., Helliwell, P., Marchesoni, A., Mease, P., and Mielants, H. (2006). Classification Criteria for Psoriatic Arthritis: Development of New Criteria from a Large International Study. Arthritis Rheum. 54 (8), 2665–2673. doi:10.1002/art.21972
Thomas, M. R., and Storey, R. F. (2015). The Role of Platelets in Inflammation. Thromb. Haemost. 114 (3), 449–458. doi:10.1160/th14-12-1067
van der Linden, S., Valkenburg, H. A., and Cats, A. (1984). Evaluation of Diagnostic Criteria for Ankylosing Spondylitis. A Proposal for Modification of the New York Criteria. Arthritis Rheum. 27 (4), 361–368. doi:10.1002/art.1780270401
van Riel, P. L., and Renskers, L. (2016). The Disease Activity Score (DAS) and the Disease Activity Score Using 28 Joint Counts (DAS28) in the Management of Rheumatoid Arthritis. Clin. Exp. Rheumatol. 34 (5 Suppl. 101), S40–s44.
Wu, Y., Chen, Y., Yang, X., Chen, L., and Yang, Y. (2016). Neutrophil-to-lymphocyte Ratio (NLR) and Platelet-To-Lymphocyte Ratio (PLR) Were Associated with Disease Activity in Patients with Systemic Lupus Erythematosus. Int. Immunopharmacol 36, 94–99. doi:10.1016/j.intimp.2016.04.006
Zeb, A., Khurshid, S., Bano, S., Rasheed, U., Zammurrad, S., Khan, M. S., et al. (2019). The Role of the Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio as Markers of Disease Activity in Ankylosing Spondylitis. Cureus 11 (10), e6025. doi:10.7759/cureus.6025
Keywords: Ankylosing spondylitis, rheumatoid arthritis, psoriasis arthritis, tumor necrosis factor alpha, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio
Citation: Wang Z, Kong L, Zhang H, Sun F, Guo Z, Zhang R and Dou Y (2022) Tumor Necrosis Factor Alpha -308G/A Gene Polymorphisms Combined with Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratio Predicts the Efficacy and Safety of Anti-TNF-α Therapy in Patients with Ankylosing Spondylitis, Rheumatoid Arthritis, and Psoriasis Arthritis. Front. Pharmacol. 12:811719. doi: 10.3389/fphar.2021.811719
Received: 09 November 2021; Accepted: 30 December 2021;
Published: 21 January 2022.
Edited by:
Zuben E. Sauna, United States Food and Drug Administration, United StatesReviewed by:
Giovanni Tarantino, University of Naples Federico II, ItalyYuan-Hao Wu, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
Copyright © 2022 Wang, Kong, Zhang, Sun, Guo, Zhang and Dou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaling Dou, cHVtY2hkeWw2N0AxNjMuY29t, ZG91eWxAcHVtY2guY24=
†These authors have contributed equally to this work and share first authorship
 Ziran Wang
Ziran Wang Lingjun Kong1†
Lingjun Kong1† Han Zhang
Han Zhang Yaling Dou
Yaling Dou