
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol., 20 December 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.809467
This article is part of the Research TopicThe Effect of Anti-Cancer Drug Therapies in the Treatment of Lung CancerView all 29 articles
 Yadong Wang1,2†
Yadong Wang1,2† Tiange Wang2,3†
Tiange Wang2,3† Jianchao Xue1,2†
Jianchao Xue1,2† Ziqi Jia1,4
Ziqi Jia1,4 Xinyu Liu1,4
Xinyu Liu1,4 Bowen Li1,2
Bowen Li1,2 Ji Li5
Ji Li5 Xiaoguang Li6
Xiaoguang Li6 Weiwei Wang1
Weiwei Wang1 Zhongxing Bing1
Zhongxing Bing1 Lei Cao1
Lei Cao1 Zhili Cao1
Zhili Cao1 Naixin Liang1*
Naixin Liang1*Tumour lysis syndrome (TLS) represents a group of fatal metabolic derangements resulting from the rapid breakdown of tumour cells. TLS typically occurs soon after the administration of chemotherapy in haematologic malignancies but is rarely observed in solid tumours. Here, we report a case of brigatinib-induced TLS after treatment with sequential anaplastic lymphoma kinase (ALK) inhibitors in a patient with advanced ALK-rearranged lung adenocarcinoma. The patient was treated sequentially with crizotinib, alectinib, and ensartinib. High-throughput molecular profiling after disease progression indicated that brigatinib may overcome ALK resistance mutations, so the patient was administered brigatinib as the fourth-line treatment. After 22 days of therapy, he developed oliguria, fever, and progressive dyspnoea. Clinical manifestations and laboratory findings met the diagnostic criteria for TLS. The significant decrease in the abundance of ALK mutations in plasma indicated a therapeutic response at the molecular level. Consequently, the diagnosis of brigatinib-induced TLS was established. To the best of our knowledge, this is the first case of TLS induced by sequential targeted therapy in non-small cell lung cancer. With the extensive application of sequential therapy with more potent next-generation targeted therapeutic drugs, special attention should be given to this rare but severe complication.
Tumour lysis syndrome (TLS) is a life-threatening oncology emergency. It is induced by a massive release of intracellular contents into the circulation when tumour cells breakdown spontaneously or after the initiation of chemotherapy (Howard et al., 2011). TLS mostly develops in the context of haematologic malignancies and is relatively rare in solid tumours (Cairo et al., 2010). Only a few cases of TLS have been reported in non-small cell lung cancer (NSCLC), most of which are induced by chemotherapy (Myint et al., 2015).
With the development of molecular diagnoses and targeted therapies, small molecular tyrosine kinase inhibitors (TKIs) have become the cornerstone of treatment for patients with targetable mutations. The most common therapeutic targets include epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) genomic alterations in NSCLC (Recondo et al., 2018). Over the past years, EGFR-TKIs and ALK-TKIs have continuously evolved from the first generation to the third generation (Mok et al., 2009; Solomon et al., 2014; Ramalingam et al., 2020; Shaw et al., 2020). In patients with sensitizing mutations who experienced progression after first-line targeted therapy, sequential treatment with more potent next-generation inhibitors is recommended to overcome drug resistance and improve patient outcomes (Mok et al., 2017; Shaw et al., 2019; Nishio et al., 2021). However, the usage of more potent next-generation inhibitors may increase the risk of rapid breakdown of tumour cells and the incidence of TLS. In this study, we describe a patient with metastatic ALK-rearranged NSCLC who received multiple prior ALK inhibitors during his treatment course and subsequently died from brigatinib-induced TLS.
A 39-year-old nonsmoking male presented to our hospital with chest pain in November 2017 (Figure 1A). The patient had no significant medical or family history of malignancy. Chest computed tomography (CT) demonstrated a 26-mm solid nodule in the hilum of the right lung (Figure 1B). Positron-emission tomography/computed tomography (PET/CT) revealed multiple right pleural and bilateral supraclavicular lymph node metastases without distant metastasis. The patient was diagnosed with lung adenocarcinoma (pT4N3M1a, stage IVA) following a tissue biopsy via video-assisted thoracoscopic surgery (VATS). Next-generation sequencing (NGS) with a 168-gene sequencing panel (Burning Rock, Guangzhou, China) revealed echinoderm microtubule-associated protein-like 4 (EML4)-ALK rearrangement and TP53 mutation (Figure 2A). The patient was administered crizotinib (250 mg twice daily) as the first-line treatment and achieved a partial response according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). After effective treatment for 11 months, he was admitted to our hospital again for dyspnoea. CT showed a large right pleural effusion. Thoracentesis was performed to collect pleural effusion for circulating tumour DNA (ctDNA) analysis. NGS identified two emerging mutations in the ALK kinase domain, F1174L and G1269A, indicating the onset of resistance to crizotinib.
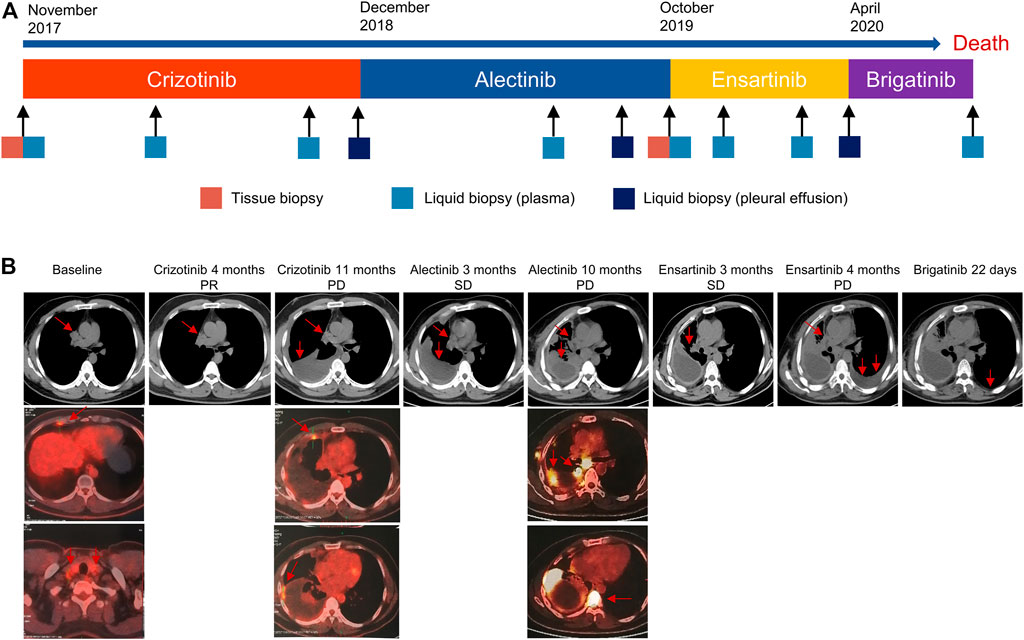
FIGURE 1. The Clinical course of the disease, treatment history, and response evaluation. (A) Timeline of treatment and molecular profiling based on tissue and liquid biopsies. (B) Duration of disease response evaluated by CT and PET/CT. CT, computed tomography; PET/CT, positron-emission tomography/computed tomography.
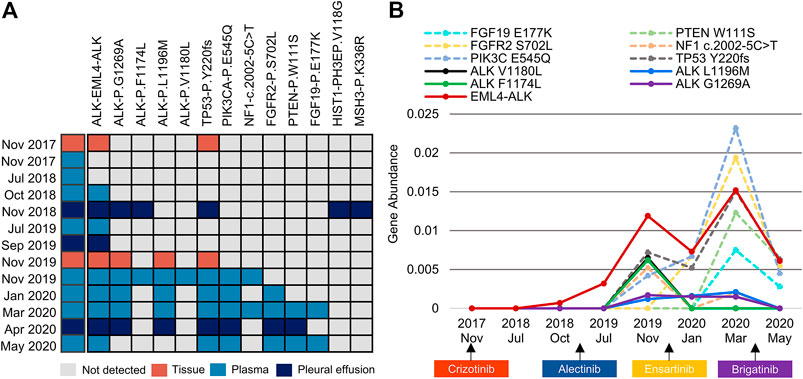
FIGURE 2. An overview of somatic mutation profiles within tissue and liquid biopsies using the next-generation sequencing technique. (A) Each row represents one individual biopsy sample, and each column represents one somatic genetic alteration. (B) Dynamic changes in the gene abundance of plasma ctDNA. Solid lines represent ALK mutations, and dashed lines represent concomitant mutations. ctDNA, circulating tumour DNA.
Crizotinib was discontinued, and he was administered alectinib (600 mg twice daily) as the second-line treatment. Stable disease (SD) was maintained for 10 months, during which only EML4-ALK was detectable in the plasma and pleural effusion. The patient gradually developed low back pain. PET/CT demonstrated bone metastasis and systemic progression. VATS biopsy was performed again, and NGS of metastatic tissues identified ALK L1196M and G1269A mutations in addition to EML4-ALK rearrangement and TP53 mutation. Meanwhile, plasma ctDNA revealed the other two ALK F1174L and V1180L mutations. Considering disease progression and drug accessibility, he was switched to ensartinib (225 mg once daily) as the third-line treatment. Subsequent CT confirmed encapsulated pleural effusion after 3 months of therapy, and he had SD that lasted for 4 months. Although the ALK F1174L and V1180L mutations were subsequently not detectable in plasma, the mutational spectrum became significantly complicated with the emergence of various concomitant mutations.
In April 2020, the patient experienced progressive left pleural effusion and started receiving brigatinib (90 mg once daily and then 180 mg once daily at 1 week) as the fourth-line treatment. Symptoms or laboratory abnormalities associated with TLS were not observed before initiation of brigatinib except for slightly elevated lactate dehydrogenase (Table 1). On Day 6, he reported mild relief from dyspnoea, and brigatinib was continued. However, the patient presented to the emergency department with oliguria, fever, and progressive dyspnoea after 22 days of therapy. The electrocardiogram revealed atrial fibrillation. Laboratory tests revealed the following results: hyperuricaemia (956 μmol/L), hyperkalaemia (6.27 mmol/L), hyperphosphatemia (2.65 mmol/L), hypocalcaemia (1.93 mmol/L), elevated urea nitrogen (36.4 mmol/L), creatinine (320.2 μmol/L), and lactate dehydrogenase (1,477 U/L). The estimated glomerular filtration rate (eGFR) was 19.5 ml/min/1.73 m2. Dynamic monitoring of plasma ctDNA revealed a significant decrease in the abundance of EML4-ALK rearrangement from 1.52 to 0.61%. The other resistance-associated ALK point mutations disappeared (Figure 2B).
Based on clinical manifestations and laboratory findings, the diagnosis of brigatinib-induced TLS was established. The patient immediately received intubation and mechanical ventilation for progressive respiratory failure. He was subsequently treated with vigorous volume expansion, torasemide, sodium polystyrene sulfonate, calcium gluconate, and ceftazidime. Despite these aggressive medical interventions, his clinical and biochemical parameters did not improve, and continuous renal replacement therapy was required. The patient and his family refused emergent haemodialysis or further interventions due to the dismal prognosis. He was transitioned to comfort care and passed away within 24 h.
TLS is a potentially lethal complication in the therapy of tumours with a mortality rate of up to 35% in solid tumours (Baeksgaard and Sørensen, 2003). TLS occurs frequently in patients with haematologic malignancies after the initiation of cytotoxic chemotherapy, such as acute lymphoblastic leukaemia and high-grade non-Hodgkin’s lymphoma (Cairo and Bishop, 2004). An increasing number of TLS cases have been reported in patients with solid tumours that were previously rarely associated with this complication (Mirrakhimov et al., 2014). Moreover, with the advent of more potent targeted agents, TLS induced by targeted therapy has been reported in several solid tumours, such as melanoma and colorectal, liver, kidney, and breast cancers (Krishnan et al., 2008; Huang and Yang, 2009; Michels et al., 2010; Handy et al., 2021; Tachibana et al., 2021). To the best of our knowledge, this is the first case of TLS induced by targeted therapy in NSCLC.
The pathophysiological mechanisms of TLS are mainly due to the rapid lysis of tumour cells with a massive release of intracellular contents, such as potassium, phosphorus, and nucleic acids that are ultimately metabolized to uric acids (Howard et al., 2011). These chemical substances are mainly excreted through the kidney, which exceeds the compensatory capacity of the kidney and leads to metabolic disorders and electrolyte disturbances. The most commonly employed criteria of TLS diagnosis were developed by Cairo and Bishop in 2004 (Cairo and Bishop, 2004). In their classification system, TLS is classified as laboratory TLS or clinical TLS. Laboratory TLS requires at least two of the following laboratory abnormalities within 3 days before or up to 7 days after the initiation of therapy: hyperuricaemia, hyperkalaemia, hyperphosphatemia, and hypocalcaemia. Clinical TLS is defined as the presence of laboratory TLS and at least one of the following clinical complications: renal insufficiency, seizures, and cardiac arrhythmias or sudden death (Cairo and Bishop, 2004). In our case, uric acid, potassium, and phosphate levels met the criteria for laboratory TLS, and the creatine level, atrial fibrillation, and death met the criteria for clinical TLS.
Brigatinib is a potent next-generation ALK inhibitor with demonstrated activity against ALK rearrangement and multiple mutations in the ALK kinase domain that confer resistance (Kim et al., 2017; Camidge et al., 2018; Camidge et al., 2020; Nishio et al., 2021; Stinchcombe et al., 2021). In our case, the patient was treated with crizotinib as the first-line treatment and subsequently switched to alectinib due to disease progression. Stable disease was maintained for 10 months, after which the patient gradually developed bone metastasis and systemic progression. NGS identified acquired ALK resistance mutations, including F1174L, G1269A, L1196M, and V1180L. At that time, lorlatinib was not approved in China, and the patient declined platinum-doublet chemotherapy. Yang et al. reported that ensartinib could exhibit clinical activity in patients with acquired resistance mutations to some other second-generation ALK TKIs (Yang et al., 2020; Yang et al., 2021). Therefore, the patient was administered ensartinib as the third-line treatment and subsequently experienced progressive left pleural effusion. The phase 2 J-ALTA trial confirmed the antitumour activity of brigatinib in patients with ALK-rearranged NSCLC refractory to alectinib or other ALK-TKIs (Nishio et al., 2021). Previous literature reported that brigatinib may overcome acquired ALK resistance mutations in this patient, so the patient chose brigatinib as the fourth-line treatment (Gainor et al., 2016; Zhang et al., 2016; Recondo et al., 2018; Gristina et al., 2020).
During the patient’s treatment course, repeat molecular profiling based on tissue or liquid biopsies played an important role in monitoring the evolution of resistance and tailoring individualized treatment after disease progression (Horn et al., 2019). In recent years, liquid biopsy has gradually become an alternative or complementary approach to tissue biopsy for diagnostic, predictive, and prognostic purposes. Compared with traditional tissue biopsy, liquid biopsy has notable advantages, such as its minimally invasive nature, ability to reflect tumour heterogeneity, and capacity to be repeatedly performed to realize dynamic monitoring of the disease (Russo et al., 2021). Liquid biopsy is particularly suitable for patients for whom sufficient tumour tissues cannot be obtained for molecular profiling. However, liquid biopsy exhibits various limitations due to both biological and technological issues. Foremost among them is the risk of a false-negative result, which might affect subsequent clinical decisions and outcomes. In addition, some other limitations, such as discordance with tissue specimens, remain to be further addressed by technical advances and the establishment of standard processes. In this patient, dynamic monitoring of plasma ctDNA revealed a significant decrease in the abundance of EML4-ALK rearrangement and the disappearance of other resistance-associated ALK point mutations. It is difficult to directly evaluate the effect of brigatinib because the patient only received it for less than 1 month. However, the significant decrease or clearance of ALK mutations after treatment with brigatinib indicated a therapeutic response at the molecular level (Wang et al., 2018). Therefore, the diagnosis of brigatinib-induced TLS was established after carefully excluding other possible contributing factors.
The existing Cairo-Bishop criteria were mainly based on the clinical features and treatment paradigms of haematologic malignancies. Thus, it is less suited for solid tumours and new treatment modalities, such as targeted therapy and immunotherapy. The Cairo-Bishop criteria have several limitations and need to be further improved. First, receipt of chemotherapy is the basic foundation for the diagnosis of TLS. However, TLS can develop spontaneously or after the initiation of surgery, radiotherapy, targeted therapy, and immunotherapy in solid tumours (Noh et al., 2008; Shenoy, 2009; Shin et al., 2019; Sugimoto et al., 2020; Tachibana et al., 2021). With the emergence of more potent therapeutic modalities and multiple combined therapies, especially in advanced tumours, predisposing factors of TLS should not just be limited to chemotherapy. Second, the time of occurrence of TLS in the Cairo-Bishop criteria may need to be redefined. Most conventional chemotherapy agents kill tumour cells through cytotoxic effects with rapid onset of action. Therefore, chemotherapy-induced TLS was diagnosed relatively early. Nevertheless, the mechanism of chemotherapy is considerably different from that of targeted therapy and immunotherapy. Targeted therapy acts specifically on certain signalling pathways and precisely kills tumour cells (Recondo et al., 2018). The principle of immunotherapy is to activate the patient’s own immune system to attack malignant tumour cells (Alsaab et al., 2017). Consequently, these therapeutic agents may have a slower onset of efficacy and relatively moderate response at the beginning of therapy compared with chemotherapy. Nicholaou et al. reported a case of sunitinib-induced TLS in a patient with kidney renal clear cell carcinoma after 14 days of treatment (Nicholaou et al., 2007). Huang et al. reported a case of sorafenib-induced TLS after 30 days of treatment in an advanced hepatocellular carcinoma patient (Huang and Yang, 2009). In our case, the patient developed TLS after 22 days of brigatinib treatment. These results indicate that the Cairo-Bishop criteria may potentially exclude some patients who develop TLS over a 7-days period after initiating treatment. Therefore, the Cairo-Bishop criteria need to be dynamically updated to adapt the features of novel treatment strategies.
Given the seriousness and urgency of the TLS, early prevention and early recognition are vital for reducing mortality. Major risk factors for TLS include but are not limited to a large tumour burden, tumours with a high proliferative rate, highly effective antitumour treatment, liver metastases, and baseline renal insufficiency (Amiri, 2015). According to these risk factors, patients can be divided into low-risk, intermediate-risk, and high-risk groups, which is of central importance to enable stratified management. Prevention measures include adequate hydration and prophylactic administration of rasburicase in high-risk patients, adequate hydration plus allopurinol or rasburicase for intermediate-risk patients, and careful monitoring for low-risk patients (Coiffier et al., 2008; Cairo et al., 2010). Additionally, repeat dynamic detection of laboratory and clinical indicators before and during treatment is recommended. In our case, the patient was at an advanced stage with multiple metastases and had been sequentially treated with crizotinib, alectinib, and ensartinib before initiation of brigatinib. Therefore, multiline TKI therapy and a high tumour load may be associated with rapid lysis of tumour cells and the occurrence of TLS when the patient was treated with a more potent next-generation TKI. Actually, with the extensive application of sequential therapy with potent next-generation TKIs, such as osimertinib and lorlatinib, this risk factor should attract sufficient attention from clinicians (Ramalingam et al., 2020; Shaw et al., 2020). In addition, patients with NSCLC typically receive oral small molecular TKIs at home instead of at the hospital. Thus, these patients require careful observation and appropriate laboratory tests in the initial period of targeted therapy.
As soon as symptoms or laboratory abnormalities associated with TLS are observed, multidisciplinary diagnostic assessment and strict monitoring are necessary. Accurate and timely diagnosis of TLS is a prerequisite for taking effective measures to prevent the disease from further worsening and reduce overall mortality. Once a definite diagnosis of TLS has been established, antineoplastic treatment that may lead to TLS should be discontinued. Frequent monitoring examinations involving continuous electrocardiogram, repeat blood biochemistry parameters and urine analysis should be performed. The patient should be monitored and treated in the intensive care unit with an experienced multidisciplinary team according to the standard guidelines for TLS (Jones et al., 2015).
Another noteworthy concern is whether molecular targeted drugs could be restarted after recovery from TLS. In clinical practice, the decision must be made regarding the potential clinical benefit versus the relative risk of the re-emergence of TLS. Several studies reported that readministration of molecular targeted drugs at a reduced dose could confer a survival benefit with no TLS recurrence (Joshita et al., 2010; Michels et al., 2010; Shimizu et al., 2021). Therefore, resumption of prior treatment may be attempted with careful monitoring if the targeted drug is irreplaceable or significantly more effective than the alternatives.
In summary, our case indicated that TLS can be induced at a relatively late timing by sequential targeted therapy in NSCLC. Given the seriousness of TLS and the extensive use of sequential targeted therapy, clinicians should maintain a high clinical suspicion for TLS and take appropriate measures to reduce mortality.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
NL conceived the study and reviewed the article. YW wrote the original draft and prepared the figures. YW, TW, JX, ZJ, XnL, and BL collected and analyzed the clinical data. All authors contributed to the article and approved the final manuscript.
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2020-I2M-C&T-A-003), CSCO-MSD fund (Y-MSD2020-0270), Beijing Health Promotion Association (BJHPA-FW-XHKT-2020040400344), Ministry of Science and Technology of the People’s Republic of China, Special Data Service for Oncology, The National Population and Health Scientific Data Sharing Platform (NCMI-ABD02-201809; NCMI-YF02N-201906) and Wu Jieping Medical Foundation Precision Treatment for Thoracic and Abdominal Cancer Fund (320.6750.19092-43).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the patient’s family for providing written informed consent for publication of this case report.
Alsaab, H. O., Sau, S., Alzhrani, R., Tatiparti, K., Bhise, K., Kashaw, S. K., et al. (2017). PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 8, 561. doi:10.3389/fphar.2017.00561
Amiri, F. S. (2015). Concurrent Acute Spontaneous Tumor Lysis Syndrome Complicated with Multiple Organ Failure in a Patient with Pre-existing Undiagnosed Lung Cancer. CEN Case Rep. 4 (2), 233–237. doi:10.1007/s13730-015-0175-0
Baeksgaard, L., and Sørensen, J. B. (2003). Acute Tumor Lysis Syndrome in Solid Tumors-Aa Case Report and Review of the Literature. Cancer Chemother. Pharmacol. 51 (3), 187–192. doi:10.1007/s00280-002-0556-x
Cairo, M. S., and Bishop, M. (2004). Tumour Lysis Syndrome: New Therapeutic Strategies and Classification. Br. J. Haematol. 127 (1), 3–11. doi:10.1111/j.1365-2141.2004.05094.x
Cairo, M. S., Coiffier, B., Reiter, A., and Younes, A. (2010). Recommendations for the Evaluation of Risk and Prophylaxis of Tumour Lysis Syndrome (TLS) in Adults and Children with Malignant Diseases: An Expert TLS Panel Consensus. Br. J. Haematol. 149 (4), 578–586. doi:10.1111/j.1365-2141.2010.08143.x
Camidge, D. R., Kim, H. R., Ahn, M. J., Yang, J. C., Han, J. Y., Lee, J. S., et al. (2018). Brigatinib Versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 379 (21), 2027–2039. doi:10.1056/NEJMoa1810171
Camidge, D. R., Kim, H. R., Ahn, M. J., Yang, J. C. H., Han, J. Y., Hochmair, M. J., et al. (2020). Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J. Clin. Oncol. 38 (31), 3592–3603. doi:10.1200/jco.20.00505
Coiffier, B., Altman, A., Pui, C. H., Younes, A., and Cairo, M. S. (2008). Guidelines for the Management of Pediatric and Adult Tumor Lysis Syndrome: An Evidence-Based Review. J. Clin. Oncol. 26 (16), 2767–2778. doi:10.1200/jco.2007.15.0177
Gainor, J. F., Dardaei, L., Yoda, S., Friboulet, L., Leshchiner, I., Katayama, R., et al. (2016). Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 6 (10), 1118–1133. doi:10.1158/2159-8290.Cd-16-0596
Gristina, V., La Mantia, M., Iacono, F., Galvano, A., Russo, A., and Bazan, V. (2020). The Emerging Therapeutic Landscape of ALK Inhibitors in Non-Small Cell Lung Cancer. Pharmaceuticals (Basel) 13 (12), 474. doi:10.3390/ph13120474
Handy, C., Wesolowski, R., Gillespie, M., Lause, M., Sardesai, S., Williams, N., et al. (2021). Tumor Lysis Syndrome in a Patient with Metastatic Breast Cancer Treated with Alpelisib. Breast Cancer (Auckl) 15, 117822342110374. doi:10.1177/11782234211037421
Horn, L., Whisenant, J. G., Wakelee, H., Reckamp, K. L., Qiao, H., Leal, T. A., et al. (2019). Monitoring Therapeutic Response and Resistance: Analysis of Circulating Tumor DNA in Patients With ALK+ Lung Cancer. J. Thorac. Oncol. 14 (11), 1901–1911. doi:10.1016/j.jtho.2019.08.003
Howard, S. C., Jones, D. P., and Pui, C. H. (2011). The Tumor Lysis Syndrome. N. Engl. J. Med. 364 (19), 1844–1854. doi:10.1056/NEJMra0904569
Huang, W. S., and Yang, C. H. (2009). Sorafenib Induced Tumor Lysis Syndrome in an Advanced Hepatocellular Carcinoma Patient. World J. Gastroenterol. 15 (35), 4464–4466. doi:10.3748/wjg.15.4464
Jones, G. L., Will, A., Jackson, G. H., Webb, N. J., and Rule, S. (2015). Guidelines for the Management of Tumour Lysis Syndrome in Adults and Children with Haematological Malignancies on Behalf of the British Committee for Standards in Haematology. Br. J. Haematol. 169 (5), 661–671. doi:10.1111/bjh.13403
Joshita, S., Yoshizawa, K., Sano, K., Kobayashi, S., Sekiguchi, T., Morita, S., et al. (2010). A Patient with Advanced Hepatocellular Carcinoma Treated with Sorafenib Tosylate Showed Massive Tumor Lysis with Avoidance of Tumor Lysis Syndrome. Intern. Med. 49 (11), 991–994. doi:10.2169/internalmedicine.49.3153
Kim, D. W., Tiseo, M., Ahn, M. J., Reckamp, K. L., Hansen, K. H., Kim, S. W., et al. (2017). Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 35 (22), 2490–2498. doi:10.1200/jco.2016.71.5904
Krishnan, G., D'Silva, K., and Al-Janadi, A. (2008). Cetuximab-Related Tumor Lysis Syndrome in Metastatic colon Carcinoma. J. Clin. Oncol. 26 (14), 2406–2408. doi:10.1200/jco.2007.14.7603
Michels, J., Lassau, N., Gross-Goupil, M., Massard, C., Mejean, A., and Escudier, B. (2010). Sunitinib Inducing Tumor Lysis Syndrome in a Patient Treated for Renal Carcinoma. Invest. New Drugs 28 (5), 690–693. doi:10.1007/s10637-009-9275-z
Mirrakhimov, A. E., Ali, A. M., Khan, M., and Barbaryan, A. (2014). Tumor Lysis Syndrome in Solid Tumors: An up to Date Review of the Literature. Rare Tumors 6 (2), 5389. doi:10.4081/rt.2014.5389
Mok, T. S., Wu, Y-L., Ahn, M-J., Garassino, M. C., Kim, H. R., Ramalingam, S. S., et al. (2017). Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 376 (7), 629–640. doi:10.1056/NEJMoa1612674
Mok, T. S., Wu, Y. L., Thongprasert, S., Yang, C. H., Chu, D. T., Saijo, N., et al. (2009). Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 361 (10), 947–957. doi:10.1056/NEJMoa0810699
Myint, Z. W., Verla-Tebit, E., Cho, B. B., Goodner, S. A., Stelow, E. B., Weiss, G. R., et al. (2015). Tumor Lysis Syndrome in a Patient with Metastatic Non-small Cell Lung Cancer: Case Report and Literature Review. Cancer Treat. Commun. 4, 10–14. doi:10.1016/j.ctrc.2015.03.002
Nicholaou, T., Wong, R., and Davis, I. D. (2007). Tumour Lysis Syndrome in a Patient with Renal-Cell Carcinoma Treated with Sunitinib Malate. Lancet 369 (9577), 1923–1924. doi:10.1016/s0140-6736(07)60903-9
Nishio, M., Yoshida, T., Kumagai, T., Hida, T., Toyozawa, R., Shimokawaji, T., et al. (2021). Brigatinib in Japanese Patients With ALK-Positive NSCLC Previously Treated With Alectinib and Other Tyrosine Kinase Inhibitors: Outcomes of the Phase 2 J-ALTA Trial. J. Thorac. Oncol. 16 (3), 452–463. doi:10.1016/j.jtho.2020.11.004
Noh, G. Y., Choe, D. H., Kim, C. H., and Lee, J. C. (2008). Fatal Tumor Lysis Syndrome during Radiotherapy for Non-small-cell Lung Cancer. J. Clin. Oncol. 26 (36), 6005–6006. doi:10.1200/jco.2008.19.4308
Ramalingam, S. S., Vansteenkiste, J., Planchard, D., Cho, B. C., Gray, J. E., Ohe, Y., et al. (2020). Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 382 (1), 41–50. doi:10.1056/NEJMoa1913662
Recondo, G., Facchinetti, F., Olaussen, K. A., Besse, B., and Friboulet, L. (2018). Making the First Move in EGFR-Driven or ALK-Driven NSCLC: First-Generation or Next-Generation TKI? Nat. Rev. Clin. Oncol. 15 (11), 694–708. doi:10.1038/s41571-018-0081-4
Russo, A., Incorvaia, L., Del Re, M., Malapelle, U., Capoluongo, E., Gristina, V., et al. (2021). The Molecular Profiling of Solid Tumors by Liquid Biopsy: A Position Paper of the AIOM-SIAPEC-IAP-SIBioC-SIC-SIF Italian Scientific Societies. ESMO Open 6 (3), 100164. doi:10.1016/j.esmoop.2021.100164
Shaw, A. T., Bauer, T. M., de Marinis, F., Felip, E., Goto, Y., Liu, G., et al. (2020). First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 383 (21), 2018–2029. doi:10.1056/NEJMoa2027187
Shaw, A. T., Solomon, B. J., Besse, B., Bauer, T. M., Lin, C. C., Soo, R. A., et al. (2019). ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-small-Cell Lung Cancer. J. Clin. Oncol. 37 (16), 1370–1379. doi:10.1200/jco.18.02236
Shenoy, C. (2009). Acute Spontaneous Tumor Lysis Syndrome in a Patient with Squamous Cell Carcinoma of the Lung. QJM 102 (1), 71–73. doi:10.1093/qjmed/hcn129
Shimizu, Y., Sunagozaka, H., Yamagata, K., Hirai, H., Miura, M., Yonemoto, Y., et al. (2021). Lenvatinib-Induced Tumor Lysis Syndrome in a Patient with Advanced Hepatocellular Carcinoma: A Case Report. Clin. J. Gastroenterol. 14 (2), 645–649. doi:10.1007/s12328-020-01306-1
Shin, T. H., Inagaki, E., Ganta, T., Hartshorn, K., Litle, V. R., and Suzuki, K. (2019). Tumor Lysis Syndrome After Bilobectomy for Typical Carcinoid Tumor of the Lung. Ann. Thorac. Surg. 107 (3), e199–e201. doi:10.1016/j.athoracsur.2018.06.089
Solomon, B. J., Mok, T., Kim, D. W., Wu, Y. L., Nakagawa, K., Mekhail, T., et al. (2014). First-Line Crizotinib Versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 371 (23), 2167–2177. doi:10.1056/NEJMoa1408440
Stinchcombe, T. E., Doebele, R. C., Wang, X., Gerber, D. E., Horn, L., and Camidge, D. R. (2021). Preliminary Clinical and Molecular Analysis Results From a Single-Arm Phase 2 Trial of Brigatinib in Patients with Disease Progression After Next-Generation ALK Tyrosine Kinase Inhibitors in Advanced ALK+ NSCLC. J. Thorac. Oncol. 16 (1), 156–161. doi:10.1016/j.jtho.2020.09.018
Sugimoto, S., Terashima, T., Yamashita, T., Iida, N., Kitahara, M., Hodo, Y., et al. (2020). Tumor Lysis Syndrome in a Patient with Metastatic Melanoma Treated with Nivolumab. Clin. J. Gastroenterol. 13 (5), 935–939. doi:10.1007/s12328-020-01164-x
Tachibana, K., Ohe, S., Tanaka, M., Maniwa, T., and Isei, T. (2021). Tumor Lysis Syndrome Induced by BRAF/MEK Double Blockade in a Patient with Metastatic Melanoma: A First Case Report. J. Dermatol. 48 (7), e324–e326. doi:10.1111/1346-8138.15894
Wang, Z., Cheng, Y., An, T., Gao, H., Wang, K., Zhou, Q., et al. (2018). Detection of EGFR Mutations in Plasma Circulating Tumour DNA as a Selection Criterion for First-Line Gefitinib Treatment in Patients with Advanced Lung Adenocarcinoma (BENEFIT): a Phase 2, Single-Arm, Multicentre Clinical Trial. Lancet Respir. Med. 6 (9), 681–690. doi:10.1016/s2213-2600(18)30264-9
Yang, Y., Huang, J., Wang, T., Zhou, J., Zheng, J., Feng, J., et al. (2021). Decoding the Evolutionary Response to Ensartinib in Patients With ALK-Positive NSCLC by Dynamic Circulating Tumor DNA Sequencing. J. Thorac. Oncol. 16 (5), 827–839. doi:10.1016/j.jtho.2021.01.1615
Yang, Y., Zhou, J., Zhou, J., Feng, J., Zhuang, W., Chen, J., et al. (2020). Efficacy, Safety, and Biomarker Analysis of Ensartinib in Crizotinib-Resistant, ALK-Positive Non-Small-Cell Lung Cancer: A Multicentre, Phase 2 Trial. Lancet Respir. Med. 8 (1), 45–53. doi:10.1016/s2213-2600(19)30252-8
Zhang, S., Anjum, R., Squillace, R., Nadworny, S., Zhou, T., Keats, J., et al. (2016). The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin. Cancer Res. 22 (22), 5527–5538. doi:10.1158/1078-0432.Ccr-16-0569
Keywords: tumour lysis syndrome, non-small cell lung cancer, targeted therapy, brigatinib, acute kidney injury, case report
Citation: Wang Y, Wang T, Xue J, Jia Z, Liu X, Li B, Li J, Li X, Wang W, Bing Z, Cao L, Cao Z and Liang N (2021) Fatal Tumour Lysis Syndrome Induced by Brigatinib in a Lung Adenocarcinoma Patient Treated With Sequential ALK Inhibitors: A Case Report. Front. Pharmacol. 12:809467. doi: 10.3389/fphar.2021.809467
Received: 05 November 2021; Accepted: 01 December 2021;
Published: 20 December 2021.
Edited by:
Pasquale Pisapia, University of Naples Federico II, ItalyReviewed by:
Valerio Gristina, University of Palermo, ItalyCopyright © 2021 Wang, Wang, Xue, Jia, Liu, Li, Li, Li, Wang, Bing, Cao, Cao and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naixin Liang, cHVtY2huZWxzb25AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.