- 1Department of Nephrology, Chinese PLA General Hospital, Chinese PLA Institute of Nephrology, State Key Laboratory of Kidney Diseases, National Clinical Research Center for Kidney Diseases, Beijing Key Laboratory of Kidney Diseases, Beijing, China
- 2Department of Nephrology, Peking University Shenzhen Hospital, Shenzhen, China
The seven members of the insulin-like growth factor (IGF) binding protein family (IGFBPs) were initially considered to be the regulatory proteins of IGFs in the blood circulation, mainly as the subsequent reserve for bidirectional regulation of IGF function during environmental changes. However, in recent years, IGFBPs has been found to have many functions independent of IGFs. The role of IGFBPs in regulating transcription, inducing cell migration and apoptosis is closely related to the occurrence and development of kidney disease. IGFBP-1, IGFBP-3, IGFBP-4 are closely associated with diabetes and diabetic nephropathy. IGFBP-3, IGFBP-4, IGFBP-5, IGFBP-6 are involved in different kidney disease such as diabetes, FSGS and CKD physiological process as apoptosis proteins, IGFBP-7 has been used in clinical practice as a biomarker for early diagnosis and prognosis of AKI. This review focuses on the differential expression and pathogenesis of IGFBPs in kidney disease.
Introduction
Insulin-like growth factors (IGFs), including IGF-I and IGF-II, are members of the insulin superfamily of growth promoting peptides, and are one of the most abundant and common growth factor polypeptides. IGFs have seven exclusive high-affinity IGF binding proteins (IGFBPs) in vivo. IGFBPs exists in blood circulation, extracellular tissue fluid and intracellular tissues, and they can regulate the half-life of IGF in blood circulation, the distribution of IGF in tissues and its binding to cell receptors (Baxter, 2014). It is precisely because of the presence of these seven IGFBPs that complicates the biological utilization and signal transduction of IGF.
IGFBPs is a class of secreted proteins that is able to interact with many ligands other than IGFs, and most of these interactions are believed to be independent of the IGF-IGFR signaling pathway, so these functions of IGFBPs are independent of IGFs/IGFR (Holbourn et al., 2008). At present, IGFBPs research is mainly focused on the tumor field. Multitudes of preclinical studies have shown that IGFBPs can inhibit the growth of tumors, but some studies believe that IGFBPs also exist as an oncogene. In addition, there is evidence that some IGFBPs may be potential biomarkers that can be used to evaluate tumor prognosis or therapeutic resistance.
In recent years, the role of IGFBPs in kidney disease has been taking serious gradually. Studies showed that the growth binding protein family are associated with the development of kidney, primary renal diseases such as mesangial proliferation of IgA nephropathy (IgAN), secondary kidney disease such as diabetic nephropathy (DN), and chronic kidney disease (CKD). Similar to tumors, researchers are also keen to find early diagnosis and prognostic biomarkers for these various kidney diseases. Currently, IGFBP-7 has been used as an early diagnosis and prognostic marker for acute renal insufficiency (AKI). Other IGFBPs are also identified as biomarkers in different kidney diseases (Table 1). This review will review the basic research of the application of IGFBP family in kidney disease, and summarize the research status of IGFBPs in kidney disease, so as to provide some reference for new research.
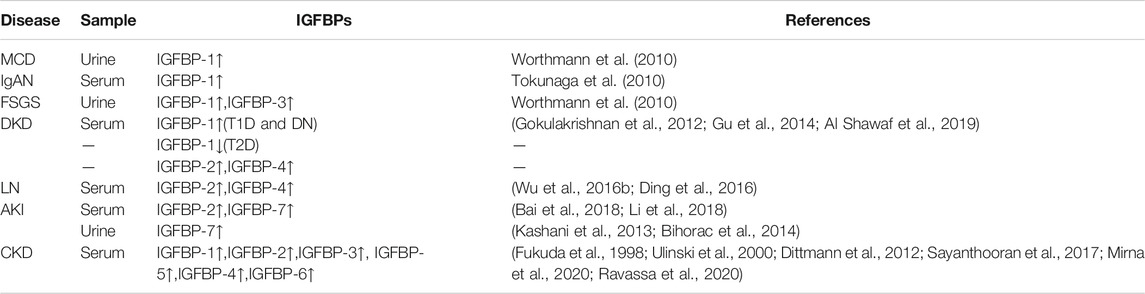
TABLE 1. IGFBPs expression of kidney disease in serum and urine. More and more studies have been conducted on the expression of IGFBPs in renal diseases, mainly focusing on CKD and AKI. All IGFBPs expressions were elevated in the serum of CKD patients, and IGFBP-7 has been a representative marker of AKI, but studies of IGFBPs on primary renal disease still rare.
The Basic Molecular Biology of the IGFBP Family
According to evolution tracking, the homology of IGFBP family genes expanded in the two basal vertebrate tetraploidization (2R) and recombined in the genome of early prochordate animals (Daza et al., 2011). Based on the strict definition of structure and function, there are six recognized IGFBP family proteins named IGFBP-1∼IGFBP-6. Although there is still debate about whether IGFBP-7 should be classified as IGFBPs or defined as IGFBP-related protein (IGFBP-rPs) due to its weaker affinity for IGF-I and IGF-II compared with IGFBP-1∼IGFBP-6, the name “IGFBP-7” is still widely used in the research.
The precursory IGFBP sequence generally has 240–328 amino acid residues before the cleavage of the signal peptide (Daza et al., 2011). Common post-translational modifications of IGFBP family proteins include n-terminal glycosylation, phosphorylation of serine/threonine, and partial proteolysis. The main domains of IGFBPs mainly include IGF binding domains at the amino terminal, heparin binding domains, insulin binding domains and the acid-labile subunit (ALS) in the central region, as well as RGD integrin-binding domains at the carboxyl terminal and nuclear localization domains (Firth and Baxter, 2002). Therefore, IGFBP family has a wide range of molecular functions. In addition to binding to IGF, IGFBP can also bind to cell membrane receptors, integrin family, and play a role directly in the nucleus. IGFBP-3, IGFBP-5 and IGFBP-6 enter the nucleus by binding to the nuclear transporter importin-β through NLS, the same nuclear localization domain at the c-terminal (Schedlich et al., 2000). There is no c-terminal NLS subunit in IGFBP-2, but a structure domain similar to NLS is in the center of IGFBP-2, which is suspected to mediate the nucleation of IGFBP-2 after the combination of importin-β (Azar et al., 2013). However, there is no research showing that the remaining IGFBP are able to import to the nucleus.
IGFBP-1
IGFBP-1 is the first IGF-binding protein with the molecular weight of 27.9 KDa. IGFBP-1 is high expressed in the female reproductive system and liver, but is low expressed in the kidney. In kidney, IGFBP-1 is mainly expressed in the glomerulus. IGFBP-1 is associated with body and kidney development. All of the whole weight, kidney weight and nephron of IGFBP-1 transgenic mice reduce slightly (Rajkumar et al., 1995). IGFBP-1 plays an important role in diabetes and diabetic nephropathy. IGFBP-1 is associated closely with obesity and insulin resistance (Maddux et al., 2006; Rajwani et al., 2012; Bernardo et al., 2015; Zaghlool et al., 2021). The diagnosis, treatment and prognosis of type 1 and type 2 diabetes mellitus are very different, and the mechanisms of diabetic kidney disease (DKD) are still unclear, the treatment of DKD is a difficulty in clinic. Type 1 diabetes (T1D) and DN had increased circulating IGFBP-1 level and decreased DNA methylation levels of the IGFBP-1 gene. Whereas, low serum IGFBP-1 levels and increased DNA methylation levels in the IGFBP-1 gene were associated with the risk of type 2 diabetes (T2D) (Gu et al., 2014), implies that IGFBP-1 is involved in different types of diabetes by different mechanisms. Glomerulus IGFBP-1 is reduced in early type 2 DKD and controlled by PI3K–FoxO1 activity in podocytes (Lay et al., 2021), thereby to play a role in DKD. In this way, IGFBP-1 may be a promising candidate for diagnoses and therapeutic development in the field of DM and DKD.
In addition, researchers explored the role of IGFBP-1 in other kidney disease. IGFBP-1 expression is elevated in IgA nephropathy, FSGS, acute kidney injury (AKI) and CKD. And it is correlated with estimated glomerular filtration rate (eGFR), cell proliferation (Gleeson et al., 2001), glomerular sclerosis (Doublier et al., 2000; Worthmann et al., 2010), erythropoietin induced stress state (Yamashita et al., 2016) and interstitial fibrosis (Tokunaga et al., 2010). But these mechanism needs to be tested further.
IGFBP-2
IGFBP-2 mRNA was high expressed in the liver and pancreas, less expressed in the kidney. In kidney, IGFBP-2 is mainly expressed in the mesangial cells. The molecular weight of IGFBP-2 is about 34.8 KDa. Studies of IGFBP-2 focused in secondary nephritis caused by autoimmune disease. Clinical studies found that serum IGFBP-2 is increased in lupus nephritis (LN), but there is controversy in whether IGFBP-2 is related to renal function. In some studies, serum IGFBP-2 level is correlated positively with serum creatinine and can be used as a marker of to reflect the activity and chronic degree of nephritis (Wu et al., 2016a; Ding et al., 2016). But in another study detected by cytokine antibody array, IGFBP-2 is highly related to the activity of SLE and LN, but no significant association with reduced renal function (Yan et al., 2020). This difference in results may due to different race, sample size and detection methods, thus we need more studies to get verification. In basic studies, the increased expression of IGFBP-2 in the outer cortical glomerulus may be associated with glomerular sclerosis and renal loss in lupus nephritis (Mohammed et al., 2003), and it may inhibit the mesangial proliferation induced by IGF-1 and enhance the extracellular matrix deposition (Wolf et al., 2000).
Except LN, IGFBP-2 in the blood can be used as an early diagnostic marker for AKI, and its sensitivity is higher than creatinine, urea nitrogen and cystatin C (Li et al., 2018), and this might be induced by hypoxia (Minchenko et al., 2015). In studies related to chronic kidney disease, circulating IGFBP-2 increased in CKD patients with different conditions, including experimental uremia, CKD caused by heart failure, and children with CKD (Powell et al., 1996; Tönshoff et al., 1997; Mahesh and Kaskel, 2008; Narayanan et al., 2012; Mirna et al., 2020; Ravassa et al., 2020). What’s more, clinical studies have also tracked the renal function level and plasma IGFBP-2 concentration of more than 400 patients with diabetic nephropathy over an 8-year period, suggesting that IGFBP-2 is a biomarker to predict longitudinal deterioration of renal function in patients with type 2 diabetes (Narayanan et al., 2012).
IGFBP-3
IGFBP-3 is highly expressed in female reproductive system and less expressed in kidney. The molecular weight of IGFBP-3 is 31.6 KDa. IGFBP-3 is the most important IGFBPs in the blood, combining 75–90% IGF-I in circulation (Oh, 2012). Compared with other IGFBPs, IGFBP-3 is involved in a wider range of kidney diseases.
In primary nephropathy, IGFBP-3 is involved in the development of IgA nephropathy and podocytosis. IgA nephropathy is an important type of mesangial proliferative nephropathy. IGFBP-3 was found to be up-regulated in the kidney of experimental IgA nephropathy (Tokunaga et al., 2010). Further research found that in mesangial cells, the expression and release of IGFBP-3 were regulated by IGF and TGF-β. IGFBP-3, TGF-β and IGF form feedback regulation in the glomerulus locally (Grellier et al., 1996). Micropathological nephropathy (MCD) and focal segmentsclerosing nephritis (FSGS) are podocyte diseases. There are few positive podocyte marker (PDX) cells in MCD and more positive cells in FSGS (Worthmann et al., 2010), suggesting that the glomerular podocyte shedding of FSGS is serious, and apoptosis is the important cause of podocyte shedding. Consistent with the excretion rate of PDX cells, the urine excretion rate and the expression in plasma of IGFBP-3 in active FSGS model mice and FSGS patients were significantly up-regulated (Srivastava et al., 2019), IGFBP-3 can be used as a noninvasive biomarker for diagnosis and prognosis of FSGS. In podocytosis, rodent studies have shown that IGFBP-3 regulate the TGF-β/BMP-7 signaling pathway of podocytes and induce apoptosis of podocytes (Peters et al., 2006), what’s more, TGF-β induce up-regulation of IGFBP-3 mRNA expression in human podocytes (Worthmann et al., 2010). In addition, some tumor studies have confirmed that IGFBP-3 promote cell apoptosis through the P53 pathway induced by TGF-β or TNF-α (Besset et al., 1996; Williams et al., 2000). These studies remind us that there might be a positive feedback pathway between TGF-β and IGFBP-3, suggesting a role for IGFBP-3 as a general mediator of programmed death.
The pro-apoptotic effect of IGFBP-3 is also reflected in diabetic nephropathy. IGFBP-3 play an in-depth role in diabetes via apoptosis. Clinical studies showed that IGFBP-3 is down-regulated in Type 2 Diabetes patients with renal disfunction, and predict future renal decline in people with type 2 diabetes combined with apoA4 and CD5L (Peters et al., 2017; Peters et al., 2019). In terms of mechanism, IGFBP-3 mediates high glucose-induced apoptosis by blocking Akt phosphorylation at threonine 308 (pThr308) in mesangial cell and increasing oxidative stress in proximal tubular epithelial Cells (Vasylyeva et al., 2005; Yoo et al., 2011).
Except these, a study including a large population based of 4,028 men and women aged 20–81 years with adjusting for age, waist circumference and type 2 diabetes mellitus showed that IGFBP-3 increases in the blood circulation of in CKD and is negatively correlated with eGFR (Dittmann et al., 2012), However, whether IGFBP-3 is involved in the pathogenesis of CKD or merely serves as a biomarker to indicate the presence of CKD remains to be further studied.
IGFBP-4
IGFBP-4 is highly expressed in the female reproductive system and the liver, and a little less in kidney. IGFBP-4 has the molecular weight of 27.9 KDa. Like other IGFBPs, IGFBP-4 plays an important role in diabetes mellitus and diabetic nephropathy. People with DN have a significant increase in their plasma IGFBP-1 and IGFBP-4 (Al Shawaf et al., 2019), more importantly, IGFBP-4 fragments (including N- and C-terminal fragments (NT-IGFBP-4 and CT-IGFBP-4)) are related to cardiovascular mortality in type 1 diabetes patients no matter with or without nephropathy (Hjortebjerg et al., 2015). Cardiovascular events are endpoint of death in the vast majority of patients with DN, in this way, IGFBP-4 is potential to serve as a predictive marker for DN patients without affected by their own kidney disease.
Among other kidney diseases, IGFBP-4 was found high expressed in the serum of CKD patients, and this is correlated with the kidney failure degree, the reduced osteogenesis during osteodystrophia (Van Doorn et al., 2001), and growth retardation in children with CKD (Ulinski et al., 2000). Other studies have found that the serum concentration of IGFBP-4 is closely associated with the chronic index of lupus nephritis and the estimated glomerular filtration rate (eGFR), and can be used as a marker for lupus nephritis (Wu et al., 2016b).
IGFBP-5
IGFBP-5 is highly expressed in the female reproductive system, and the expression of the kidney is medium. The molecular weight of IGFBP-5 is 30.6 KDa. Previous studies showed that IGFBP-5 is highest expressed in mesangial cells (Matsell et al., 1994), thus most of the early studies are concentrated on mesangial cells. The heparin domain of IGFBP-5 mediate the migration of the mesangial cells by combining cdc42 in the high glucose environment (Abrass et al., 1997; Berfield et al., 2000), and IGFBP-5 increases in the glomerular hypertrophy of early diabetes (Schaeffer et al., 2010). But recent studies of single-cell sequencing and our exploration showed that IGFBP-5 is highly expressed in the renal interstitial, which is the highest in kidney vascular endothelial cell and closely related to CKD (Karaiskos et al., 2018; Park et al., 2018). Studies of IGFBP-5 in renal diseases are rare and non-systematic, however, its role in tumor migration, proliferation (Dong et al., 2020) and tissue fibrosis (Nguyen et al., 2018) suggests that IGFBP-5 might be a potential maker which can not be ignored in kidney disease.
IGFBP-6
IGFBP-6 is the smallest IGFBP with the molecular weight of 25.3 KDa mRNA and protein expression level of IGFBP-6 is high in the kidney. Interestingly, IGFBP-6 can be generated by cleavage of IGFBP-2 by protease in canine renal tubular epithelial cells (MDCK) (Shalamanova et al., 2001). IGFBP-6 was infrequently studied in the kidney, while mostly in proteomic studies of CKD. The abundance of IGFBP-6 in plasma of adults and children with CKD or ERSD were all significantly up-regulated (Jarkovská et al., 2005; Christensson et al., 2018), Consistent with these, the monitoring of plasma IGFBP-6 before and after kidney transplantation and at the time of rejection showed that IGFBP-6 indicate the status of renal function in patients with chronic renal insufficiency (Fukuda et al., 1998), level of IGFBP-6 increases significantly by 8–25 times with the decrease of renal function (Jehle et al., 2000). Meanwhile, the urine abundance of IGFBP-6 gradually increased with kidney developments (CharltonNorwood et al., 2012; Wang and Li, 2015), and is associated with developmental retardation in children with CKD (Powell et al., 1997), suggesting that it was related to the kidney development process. However, these studies did not refer to the mechanism of IGFBP-6 involvement. But IGFBP-6 has been widely studied in tumor and nervous system as a pro-apoptotic protein (Wang et al., 2017; Qiu et al., 2018). Since cell senescence and apoptosis are also important in development and chronic kidney disease, we speculated that IGFBP-6 might be involved in development and fibrosis by regulating apoptosis of renal cells, but more basic research evidence is needed.
IGFBP-7
IGFBP-7 is high expressed in liver, kidney, bone and muscle, and the expression level is higher in renal tubules. The molecular weight of IGFBP-7 is 29.1 kda. Whether IGFBP-7 should be classified into the IGFBP family is still controversial currently. Because of the weak binding forces between IGFBP-7 and IGF, some studies suggest that they should be classified as IGFBP related proteins (IGFBP-rps). But IGFBP-7 plays a quite important role in kidney, and the main use of IGFBP-7 is the early predictive and prognostic marker for AKI(Bai et al., 2018; Cho et al., 2019). The diagnostic performance of TIMP-2 and IGFBP-7 as biomarkers of AKI was first described in Sapphire study (Kashani et al., 2013). This observational study including 744 patients across 20 North American and 15 European centers led to subsequent clinical study boom on IGFBP-7 and AKI induced by various causes. A year later, the Topaz study prospectively validated the urinary [TIMP-2]•[IGFBP-7] test’s ability (at the 0.3 cutoff level) to identify critically ill patients at high risk for developing moderate to severe AKI within 12 h with the high sensitivity of 92% [95% confidence interval (CI), 85–98%] (Bihorac et al., 2014). Based on Sapphire and Topaz study, the urine compound of TIMP-2 and IGFBP-7 became the first US Food and Drug Administration (FDA)-approved biomarker for risk assessment of AKI in ICU patients in 2014 (Us Food and Drug Administ, 2014). In terms of pathogenic mechanism, TIMP-2 and IGFBP-7 have been confirmed to participate in cell apoptosis by p53, p21, p27 and ERK1/2 signaling as G1 cell-cycle arrest maker during the early phases of cell injury (Kashani et al., 2013; Wang et al., 2018).
Summary and Outlook
The mRNA expression of IGFBP family is generally low in the kidney, and so far there is less research about IGFBPs in kidney disease, thus the location of most IGFBP in the kidney is not clear. The IGFBP family has a variety of functions, including control the development of the kidney by interacting with IGF, and regulating the biological process of cell proliferation, apoptosis and differentiation independent of IGF, thus participating in the development of IgA nephropathy, podocyte disease, lupus nephritis and diabetic nephropathy. IGFBP-1, IGFBP-3, IGFBP-4 are closely associated with diabetes and diabetic nephropathy. IGFBP-3, IGFBP-4, IGFBP-5, IGFBP-6 are involved in different kidney disease such as diabetes, FSGS and CKD physiological process as apoptosis proteins, IGFBP-7 has been used in clinical practice as a biomarker for early diagnosis and prognosis of AKI. Although the current studies on the mechanism of IGFBPs in kidney disease are still few and unsystematic. We describe the possible pathogenesis of IGFBPs in renal disease in Figure 1 based on current studies. The existing studies suggest that the role of IGFBPs in kidney disease should not be ignored. We believe that future studies will reveal more important functions of IGFBP family.
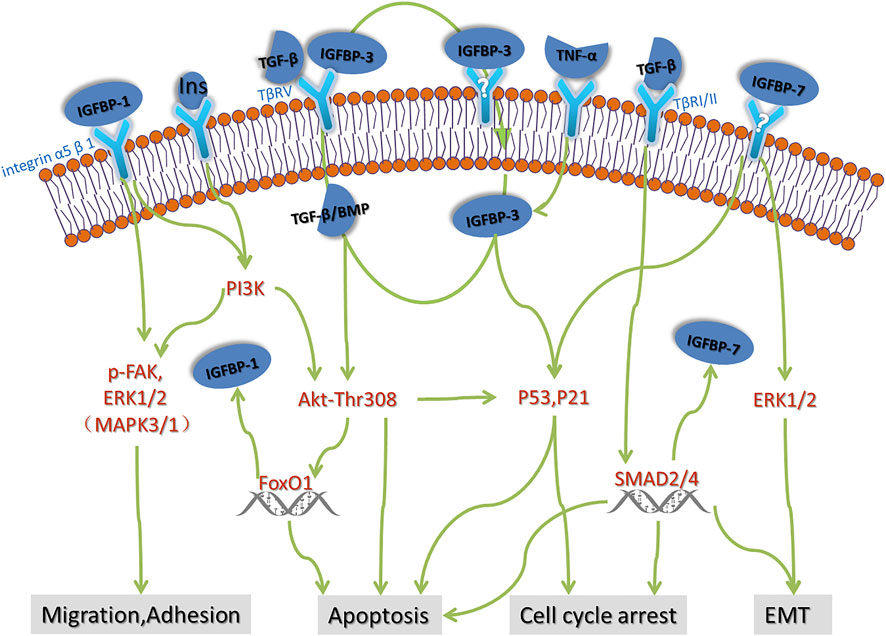
FIGURE 1. Possible pathogenesis of IGFBPs in renal disease. There are few studies on the mechanism of action of IGFBPs in renal disease. Based on the advances in oncology and rheumatology, we speculate that some of the possible mechanisms of action of IGFBPs in renal disease including cell migration, adhesion, apoptosis, cell cycle arrest and EMT, which need to be further verified by strict basic experiments.
Author Contributions
SW and KC: Literature retrieval, sorting and article writing. DW and QH: topic design and guidance.
Funding
This work was supported by the Fostering Fund of National Key Research and Development Project (2018YFE0126600), Chinese PLA General Hospital for the National Distinguished Young Scholar Science Fund (2019- JQPY-002), the National Natural Science Foundation of China (Nos. 81870491 and 82070741).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrass, C. K., Berfield, A. K., and Andress, D. L. (1997). Heparin Binding Domain of Insulin-like Growth Factor Binding Protein-5 Stimulates Mesangial Cell Migration. Am. J. Physiol. 273 (6), F899–F906. doi:10.1152/ajprenal.1997.273.6.F899
Al Shawaf, E., Abu-Farha, M., Devarajan, S., Alsairafi, Z., Al-Khairi, I., Cherian, P., et al. (2019). ANGPTL4: A Predictive Marker for Diabetic Nephropathy. J. Diabetes Res. 2019, 4943191. doi:10.1155/2019/4943191
Azar, W. J., Zivkovic, S., Werther, G. A., and Russo, V. C. (2013). IGFBP-2 Nuclear Translocation Is Mediated by a Functional NLS Sequence and is Essential for its Pro-Tumorigenic Actions in Cancer Cells. Oncogene 33 (5), 578–588. doi:10.1038/onc.2012.630
Bai, Z., Fang, F., Xu, Z., Lu, C., Wang, X., Chen, J., et al. (2018). Serum and Urine FGF23 and IGFBP-7 for the Prediction of Acute Kidney Injury in Critically Ill Children. BMC Pediatr. 18 (1), 192. doi:10.1186/s12887-018-1175-y
Baxter, R. C. (2014). IGF Binding Proteins in Cancer: Mechanistic and Clinical Insights. Nat. Rev. Cancer 14 (5), 329–341. doi:10.1038/nrc3720
Berfield, A. K., Andress, D. L., and Abrass, C. K. (2000). IGFBP-5(201-218) Stimulates Cdc42GAP Aggregation and Filopodia Formationin Migrating Mesangial Cells. Kidney Int. 57 (5), 1991–2003. doi:10.1046/j.1523-1755.2000.00049.x
Bernardo, A. P., Oliveira, J. C., Santos, O., Carvalho, M. J., Cabrita, A., and Rodrigues, A. (2015). Insulin Resistance in Nondiabetic Peritoneal Dialysis Patients: Associations with Body Composition, Peritoneal Transport, and Peritoneal Glucose Absorption. Clin. J. Am. Soc. Nephrol. 10 (12), 2205–2212. doi:10.2215/CJN.03170315
Besset, V., Le Magueresse-Battistoni, B., Collette, J., and Benahmed, M. (1996). Tumor Necrosis Factor Alpha Stimulates Insulin-like Growth Factor Binding Protein 3 Expression in Cultured Porcine Sertoli Cells. Endocrinology 137 (1), 296–303. doi:10.1210/endo.137.1.8536626
Bihorac, A., Chawla, L. S., Shaw, A. D., Al-Khafaji, A., Davison, D. L., Demuth, G. E., et al. (2014). Validation of Cell-Cycle Arrest Biomarkers for Acute Kidney Injury Using Clinical Adjudication. Am. J. Respir. Crit. Care Med. 189 (8), 932–939. doi:10.1164/rccm.201401-0077OC
Charlton, J. R., Norwood, V. F., Kiley, S. C., Gurka, M. J., and Chevalier, R. L. (2012). Evolution of the Urinary Proteome During Human Renal Development and Maturation: Variations with Gestational and Postnatal Age. Pediatr. Res. 72 (2), 179–185. doi:10.1038/pr.2012.63
Cho, W. Y., Lim, S. Y., Yang, J. H., Oh, S. W., Kim, M.-G., and Jo, S.-K. (2019). Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-like Growth Factor-Binding Protein 7 as Biomarkers of Patients with Established Acute Kidney Injury. Korean J. Intern. Med. 35 (3), 662-671. doi:10.3904/kjim.2018.266
Christensson, A., Ash, J. A., Delisle, R. K., Gaspar, F. W., Ostroff, R., Grubb, A., et al. (2018). The Impact of the Glomerular Filtration Rate on the Human Plasma Proteome. Proteomics Clin. Appl. 12 (3), e1700067. doi:10.1002/prca.201700067
Daza, D. O., Sundström, G., Bergqvist, C. A., Duan, C., and Larhammar, D. (2011). Evolution of the Insulin-like Growth Factor Binding Protein (IGFBP) Family. Endocrinology 152 (6), 2278–2289. doi:10.1210/en.2011-0047
Ding, H., Kharboutli, M., Saxena, R., and Wu, T. (2016). Insulin-like Growth Factor Binding Protein-2 as a Novel Biomarker for Disease Activity and Renal Pathology Changes in Lupus Nephritis. Clin. Exp. Immunol. 184 (1), 11–18. doi:10.1111/cei.12743
Dittmann, K., Wallaschofski, H., Rettig, R., Stracke, S., Endlich, K., Völzke, H., et al. (2012). Association Between Serum Insulin-like Growth Factor I or IGF-Binding Protein 3 and Estimated Glomerular Filtration Rate: Results of a Population-Based Sample. BMC Nephrol. 13, 169. doi:10.1186/1471-2369-13-169
Dong, C., Zhang, J., Fang, S., and Liu, F. (2020). IGFBP5 Increases Cell Invasion and Inhibits Cell Proliferation by EMT and Akt Signaling Pathway in Glioblastoma Multiforme Cells. Cell Div 15, 4. doi:10.1186/s13008-020-00061-6
Doublier, S., Seurin, D., Fouqueray, B., Verpont, M. C., Callard, P., Striker, L. J., et al. (2000). Glomerulosclerosis in Mice Transgenic for Human Insulin-like Growth Factor-Binding Protein-1. Kidney Int. 57 (6), 2299–2307. doi:10.1046/j.1523-1755.2000.00090.x
Firth, S. M., and Baxter, R. C. (2002). Cellular Actions of the Insulin-like Growth Factor Binding Proteins. Endocr. Rev. 23 (6), 824–854. doi:10.1210/er.2001-0033
Fukuda, I., Hizuka, N., Okubo, Y., Takano, K., Asakawa-Yasumoto, K., Shizume, K., et al. (1998). Changes in Serum Insulin-like Growth Factor Binding Protein-2, -3, and -6 Levels in Patients with Chronic Renal Failure Following Renal Transplantation. Growth Horm. IGF Res. 8 (6), 481–486. doi:10.1016/s1096-6374(98)80301-8
Gleeson, L. M., Chakraborty, C., Mckinnon, T., and Lala, P. K. (2001). Insulin-like Growth Factor-Binding Protein 1 Stimulates Human Trophoblast Migration by Signaling through Alpha 5 Beta 1 Integrin via Mitogen-Activated Protein Kinase Pathway. J. Clin. Endocrinol. Metab. 86 (6), 2484–2493. doi:10.1210/jcem.86.6.7532
Gokulakrishnan, K., Velmurugan, K., Ganesan, S., and Mohan, V. (2012). Circulating Levels of Insulin-like Growth Factor Binding Protein-1 in Relation to Insulin Resistance, Type 2 Diabetes Mellitus, and Metabolic Syndrome (Chennai Urban Rural Epidemiology Study 118). Metabolism 61 (1), 43–46. doi:10.1016/j.metabol.2011.05.014
Grellier, P., Sabbah, M., Fouqueray, B., Woodruff, K., Yee, D., Abboud, H. E., et al. (1996). Characterization of Insulin-like Growth Factor Binding Proteins and Regulation of IGFBP3 in Human Mesangial Cells. Kidney Int. 49 (4), 1071–1078. doi:10.1038/ki.1996.156
Gu, T., Falhammar, H., Gu, H. F., and Brismar, K. (2014). Epigenetic Analyses of the Insulin-like Growth Factor Binding Protein 1 Gene in Type 1 Diabetes and Diabetic Nephropathy. Clin. Epigenetics 6 (1), 10. doi:10.1186/1868-7083-6-10
Hjortebjerg, R., Tarnow, L., Jorsal, A., Parving, H. H., Rossing, P., Bjerre, M., et al. (2015). IGFBP-4 Fragments as Markers of Cardiovascular Mortality in Type 1 Diabetes Patients with and without Nephropathy. J. Clin. Endocrinol. Metab. 100 (8), 3032–3040. doi:10.1210/jc.2015-2196
Holbourn, K. P., Acharya, K. R., and Perbal, B. (2008). The CCN Family of Proteins: Structure-Function Relationships. Trends Biochem. Sci. 33 (10), 461–473. doi:10.1016/j.tibs.2008.07.006
Jarkovská, Z., Rosická, M., Krsek, M., Sulková, S., Haluzík, M., Justová, V., et al. (2005). Plasma Ghrelin Levels in Patients with End-Stage Renal Disease. Physiol. Res. 54 (4), 403–408.
Jehle, P. M., Ostertag, A., Schulten, K., Schulz, W., Jehle, D. R., Stracke, S., et al. (2000). Insulin-like Growth Factor System Components in Hyperparathyroidism and Renal Osteodystrophy. Kidney Int. 57 (2), 423–436. doi:10.1046/j.1523-1755.2000.00862.x
Karaiskos, N., Rahmatollahi, M., Boltengagen, A., Liu, H., Hoehne, M., Rinschen, M., et al. (2018). A Single-Cell Transcriptome Atlas of the Mouse Glomerulus. J. Am. Soc. Nephrol. 29 (8), 2060–2068. doi:10.1681/ASN.2018030238
Kashani, K., Al-Khafaji, A., Ardiles, T., Artigas, A., Bagshaw, S. M., Bell, M., et al. (2013). Discovery and Validation of Cell Cycle Arrest Biomarkers in Human Acute Kidney Injury. Crit. Care 17 (1), R25. doi:10.1186/cc12503
Lay, A. C., Hale, L. J., Stowell-Connolly, H., Pope, R. J. P., Nair, V., Ju, W., et al. (2021). IGFBP-1 Expression Is Reduced in Human Type 2 Diabetic Glomeruli and Modulates β1-integrin/FAK Signalling in Human Podocytes. Diabetologia 64 (7), 1690–1702. doi:10.1007/s00125-021-05427-1
Li, H. L., Yan, Z., Ke, Z. P., Tian, X. F., Zhong, L. L., Lin, Y. T., et al. (2018). IGFBP2 Is a Potential Biomarker in Acute Kidney Injury (AKI) and Resveratrol-Loaded Nanoparticles Prevent AKI. Oncotarget 9 (93), 36551–36560. doi:10.18632/oncotarget.25663
Maddux, B. A., Chan, A., De Filippis, E. A., Mandarino, L. J., and Goldfine, I. D. (2006). IGF-binding Protein-1 Levels are Related to Insulin-Mediated Glucose Disposal and are a Potential Serum Marker of Insulin Resistance. Diabetes Care 29 (7), 1535–1537. doi:10.2337/dc05-1367
Mahesh, S., and Kaskel, F. (2008). Growth Hormone Axis in Chronic Kidney Disease. Pediatr. Nephrol. 23 (1), 41–48. doi:10.1007/s00467-007-0527-x
Matsell, D. G., Delhanty, P. J., Stepaniuk, O., Goodyear, C., and Han, V. K. (1994). Expression of Insulin-like Growth Factor and Binding Protein Genes during Nephrogenesis. Kidney Int. 46 (4), 1031–1042. doi:10.1038/ki.1994.364
Minchenko, D. O., Kharkova, Ap., Karbovskyi, Ll., and Minchenko, O. H. (2015). Expression of Insulin-like Growth Factor Binding Protein Genes and its Hypoxic Regulation in U87 Glioma Cells Depends on ERN1 Mediated Signaling Pathway of Endoplasmic Reticulum Stress. Endocr. Regul. 49 (2), 73–83. doi:10.4149/endo_2015_02_73
Mirna, M., Topf, A., Wernly, B., Rezar, R., Paar, V., Jung, C., et al. (2020). Novel Biomarkers in Patients with Chronic Kidney Disease: An Analysis of Patients Enrolled in the GCKD-Study. J. Clin. Med. 9 (3), 886. doi:10.3390/jcm9030886
Mohammed, J. A., Mok, A. Y., Parbtani, A., and Matsell, D. G. (2003). Increased Expression of Insulin-like Growth Factors in Progressive Glomerulonephritis of the MRL/lpr Mouse. Lupus 12 (8), 584–590. doi:10.1191/0961203303lu422oa
Narayanan, R. P., Fu, B., Heald, A. H., Siddals, K. W., Oliver, R. L., Hudson, J. E., et al. (2012). IGFBP2 is a Biomarker for Predicting Longitudinal Deterioration in Renal Function in Type 2 Diabetes. Endocr. Connect 1 (2), 95–102. doi:10.1530/EC-12-0053
Nguyen, X. X., Muhammad, L., Nietert, P. J., and Feghali-Bostwick, C. (2018). IGFBP-5 Promotes Fibrosis via Increasing Its Own Expression and that of Other Pro-fibrotic Mediators. Front Endocrinol. (Lausanne) 9, 601. doi:10.3389/fendo.2018.00601
Oh, Y. (2012). The Insulin-like Growth Factor System in Chronic Kidney Disease: Pathophysiology and Therapeutic Opportunities. Kidney Res. Clin. Pract. 31 (1), 26–37. doi:10.1016/j.krcp.2011.12.005
Park, J., Shrestha, R., Qiu, C., Kondo, A., Huang, S., Werth, M., et al. (2018). Single-cell Transcriptomics of the Mouse Kidney Reveals Potential Cellular Targets of Kidney Disease. Science 360 (6390), 758–763. doi:10.1126/science.aar2131
Peters, I., Tossidou, I., Achenbach, J., Woroniecki, R., Mengel, M., Park, J. K., et al. (2006). IGF-binding Protein-3 Modulates TGF-beta/BMP-Signaling in Glomerular Podocytes. J. Am. Soc. Nephrol. 17 (6), 1644–1656. doi:10.1681/ASN.2005111209
Peters, K. E., Davis, W. A., Ito, J., Bringans, S. D., Lipscombe, R. J., and Davis, T. M. E. (2019). Validation of a Protein Biomarker Test for Predicting Renal Decline in Type 2 Diabetes: The Fremantle Diabetes Study Phase II. J. Diabetes Complications 33 (12), 107406. doi:10.1016/j.jdiacomp.2019.07.003
Peters, K. E., Davis, W. A., Ito, J., Winfield, K., Stoll, T., Bringans, S. D., et al. (2017). Identification of Novel Circulating Biomarkers Predicting Rapid Decline in Renal Function in Type 2 Diabetes: The Fremantle Diabetes Study Phase II. Diabetes Care 40 (11), 1548–1555. doi:10.2337/dc17-0911
Powell, D. R., Liu, F., Baker, B. K., Hintz, R. L., Durham, S. K., Brewer, E. D., et al. (1997). Insulin-like Growth Factor-Binding Protein-6 Levels Are Elevated in Serum of Children with Chronic Renal Failure: a Report of the Southwest Pediatric Nephrology Study Group. J. Clin. Endocrinol. Metab. 82 (9), 2978–2984. doi:10.1210/jcem.82.9.4215
Powell, D. R., Liu, F., Baker, B. K., Lee, P. D., and Hintz, R. L. (1996). Insulin-like Growth Factor Binding Proteins as Growth Inhibitors in Children with Chronic Renal Failure. Pediatr. Nephrol. 10 (3), 343–347. doi:10.1007/BF00866778
Qiu, F., Gao, W., and Wang, B. (2018). Correlation of IGFBP-6 Expression with Apoptosis and Migration of Colorectal Carcinoma Cells. Cancer Biomark 21 (4), 893–898. doi:10.3233/CBM-170947
Rajkumar, K., Barron, D., Lewitt, M. S., and Murphy, L. J. (1995). Growth Retardation and Hyperglycemia in Insulin-like Growth Factor Binding Protein-1 Transgenic Mice. Endocrinology 136 (9), 4029–4034. doi:10.1210/endo.136.9.7544274
Rajwani, A., Ezzat, V., Smith, J., Yuldasheva, N. Y., Duncan, E. R., Gage, M., et al. (2012). Increasing Circulating IGFBP1 Levels Improves Insulin Sensitivity, Promotes Nitric Oxide Production, Lowers Blood Pressure, and Protects against Atherosclerosis. Diabetes 61 (4), 915–924. doi:10.2337/db11-0963
Ravassa, S., Beaumont, J., Cediel, G., Lupón, J., López, B., Querejeta, R., et al. (2020). Cardiorenal Interaction and Heart Failure Outcomes. A Role for Insulin-like Growth Factor Binding Protein 2 ? Revista Española de Cardiología(English Edition) 73 (10), 835–843. doi:10.1016/j.rec.2019.10.012
Sayanthooran, S., Magana-Arachchi, D. N., Gunerathne, L., and Abeysekera, T. (2017). Potential Diagnostic Biomarkers for Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka: a Pilot Study. BMC Nephrol. 18 (1), 31. doi:10.1186/s12882-017-0440-x
Schaeffer, V., Hansen, K. M., Morris, D. R., and Abrass, C. K. (2010). Reductions in Laminin Beta2 mRNA Translation Are Responsible for Impaired IGFBP-5-Mediated Mesangial Cell Migration in the Presence of High Glucose. Am. J. Physiol. Ren. Physiol 298 (2), F314–F322. doi:10.1152/ajprenal.00483.2009
Schedlich, L. J., Le Page, S. L., Firth, S. M., Briggs, L. J., Jans, D. A., and Baxter, R. C. (2000). Nuclear Import of Insulin-like Growth Factor-Binding Protein-3 and -5 Is Mediated by the Importin Beta Subunit. J. Biol. Chem. 275 (31), 23462–23470. doi:10.1074/jbc.M002208200
Shalamanova, L., Kübler, B., Scharf, J. G., and Braulke, T. (2001). MDCK Cells Secrete Neutral Proteases Cleaving Insulin-like Growth Factor-Binding Protein-2 to -6. Am. J. Physiol. Endocrinol. Metab. 281 (6), E1221–E1229. doi:10.1152/ajpendo.2001.281.6.E1221
Srivastava, P., Solanki, A. K., Arif, E., Wolf, B. J., Janech, M. G., Budisavljevic, M. N., et al. (2019). Development of a Novel Cell-Based Assay to Diagnose Recurrent Focal Segmental Glomerulosclerosis Patients. Kidney Int. 95 (3), 708–716. doi:10.1016/j.kint.2018.10.030
Tokunaga, K., Uto, H., Takami, Y., Mera, K., Nishida, C., Yoshimine, Y., et al. (2010). Insulin-like Growth Factor Binding Protein-1 Levels Are Increased in Patients with IgA Nephropathy. Biochem. Biophys. Res. Commun. 399 (2), 144–149. doi:10.1016/j.bbrc.2010.07.032
Tönshoff, B., Powell, D. R., Zhao, D., Durham, S. K., Coleman, M. E., Domené, H. M., et al. (1997). Decreased hepatic insulin-like growth factor (IGF)-I and increased IGF binding protein-1 and -2 gene expression in experimental uremia. Endocrinology 138 (3), 938–946.
Ulinski, T., Mohan, S., Kiepe, D., Blum, W. F., Wingen, A. M., Mehls, O., et al. (2000). Serum Insulin-like Growth Factor Binding Protein (IGFBP)-4 and IGFBP-5 in Children with Chronic Renal Failure: Relationship to Growth and Glomerular Filtration Rate. The European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. Pediatr. Nephrol. 14 (7), 589–597. doi:10.1007/s004670000361
Us Food and Drug Administration. Letter to Astute Medical. Updated on Sep2014. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf13/den130031.pdf.
Van Doorn, J., Cornelissen, Aj., and Van Buul-Offers, S. C. (2001). Plasma Levels of Insulin-like Growth Factor Binding Protein-4 (IGFBP-4) under normal and Pathological Conditions. Clin. Endocrinol. (Oxf) 54 (5), 655–664. doi:10.1046/j.1365-2265.2001.01248.x
Vasylyeva, Tl., Chen, X., and Ferry, Rj. (2005). Insulin-like Growth Factor Binding Protein-3 Mediates Cytokine-Induced Mesangial Cell Apoptosis. Growth Horm. IGF Res. 15 (3), 207–214. doi:10.1016/j.ghir.2005.02.008
Wang, S., Liu, Y., Wu, C., Zhao, W., Zhang, J., Bao, G., et al. (2017). The Expression of IGFBP6 after Spinal Cord Injury: Implications for Neuronal Apoptosis. Neurochem. Res. 42 (2), 455–467. doi:10.1007/s11064-016-2092-9
Wang, X., Ma, T., Wan, X., Meng, Y., Zhao, Z., Bian, J., et al. (2018). IGFBP7 Regulates Sepsis-Induced Acute Kidney Injury through ERK1/2 Signaling. J. Cell Biochem.. doi:10.1002/jcb.28035
Wang, Z., and Li, M. (2015). Evolution of the Urinary Proteome during Human Renal Development and Maturation. Adv. Exp. Med. Biol. 845, 95–101. doi:10.1007/978-94-017-9523-4_10
Williams, A. C., Collard, T. J., Perks, C. M., Newcomb, P., Moorghen, M., Holly, J. M., et al. (2000). Increased P53-dependent Apoptosis by the Insulin-like Growth Factor Binding Protein IGFBP-3 in Human Colonic Adenoma-Derived Cells. Cancer Res. 60 (1), 22–27.
Wolf, E., Lahm, H., Wu, M., Wanke, R., and Hoeflich, A. (2000). Effects of IGFBP-2 Overexpression in vitro and in vivo. Pediatr. Nephrol. 14 (7), 572–578. doi:10.1007/s004670000362
Worthmann, K., Peters, I., Kümpers, P., Saleem, M., Becker, J. U., Agustian, P. A., et al. (2010). Urinary Excretion of IGFBP-1 and -3 Correlates with Disease Activity and Differentiates Focal Segmental Glomerulosclerosis and Minimal Change Disease. Growth Factors 28 (2), 129–138. doi:10.3109/08977190903512594
Wu, T., Ding, H., Han, J., Arriens, C., Wei, C., Han, W., et al. (2016). Antibody-Array-Based Proteomic Screening of Serum Markers in Systemic Lupus Erythematosus: A Discovery Study. J. Proteome Res. 15 (7), 2102–2114. doi:10.1021/acs.jproteome.5b00905
Wu, T., Xie, C., Han, J., Ye, Y., Singh, S., Zhou, J., et al. (2016). Insulin-Like Growth Factor Binding Protein-4 as a Marker of Chronic Lupus Nephritis. PLoS One 11 (3), e0151491. doi:10.1371/journal.pone.0151491
Yamashita, T., Noiri, E., Hamasaki, Y., Matsubara, T., Ishii, T., Yahagi, N., et al. (2016). Erythropoietin Concentration in Acute Kidney Injury Is Associated with Insulin-like Growth Factor-Binding Protein-1. Nephrology (Carlton) 21 (8), 693–699. doi:10.1111/nep.12656
Yan, C., Yu, L., Zhang, X. L., Shang, J. J., Ren, J., Fan, J., et al. (2020). Cytokine Profiling in Chinese SLE Patients: Correlations with Renal Dysfunction. J. Immunol. Res. 2020, 8146502. doi:10.1155/2020/8146502
Yoo, E -G., Lee, W. J., Kim, J. H., Chae, H -W., Hyun, S. E., Kim, D. H., et al. (2011). Insulin-Like Growth Factor-Binding Protein-3 Mediates High Glucose-Induced Apoptosis by Increasing Oxidative Stress in Proximal Tubular Epithelial Cells. Endocrinology 152 (8), 3135–3142. doi:10.1210/en.2010-1122
Keywords: IGFBPs, kidney disease, function, mechanism, biomarker
Citation: Wang S, Chi K, Wu D and Hong Q (2021) Insulin-Like Growth Factor Binding Proteins in Kidney Disease. Front. Pharmacol. 12:807119. doi: 10.3389/fphar.2021.807119
Received: 01 November 2021; Accepted: 08 December 2021;
Published: 22 December 2021.
Edited by:
Zhiyong Guo, Second Military Medical University, ChinaReviewed by:
Prasanna K. Santhekadur, JSS Academy of Higher Education and Research, IndiaJianlou Niu, Wenzhou Medical University, China
Copyright © 2021 Wang, Chi, Wu and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Hong, aG9uZ3F1YW5AMzAxaG9zcGl0YWwuY29tLmNu
 Shuqiang Wang
Shuqiang Wang Kun Chi
Kun Chi Di Wu1
Di Wu1 Quan Hong
Quan Hong