- 1Department of Pharmacy, China-Japan Union Hospital of Jilin University, Jilin University, Changchun, China
- 2Center of Reproductive Medicine and Center of Prenatal Diagnosis, The First Hospital, Jilin University, Changchun, China
- 3Department of Neurosurgery, China-Japan Union Hospital of Jilin University, Jilin University, Changchun, China
Sepsis is a syndrome with high mortality, which seriously threatens human health. During the pandemic of coronavirus disease 2019 (COVID-19), some severe and critically ill COVID-19 patients with multiple organ dysfunction developed characteristics typical of sepsis and met the diagnostic criteria for sepsis. Timely detection of cytokine storm and appropriate regulation of inflammatory response may be significant in the prevention and treatment of sepsis. This study evaluated the efficacy and safety of specific interleukin (IL)-1 inhibitors, specific IL-6 inhibitors, and GM-CSF blockades in the treatment of COVID-19 (at the edge of sepsis) patients through systematic review and meta-analysis. Methodology: A literature search was conducted on PubMed, EMBASE, Clinical Key, Cochrane Library, CNKI, and Wanfang Database using proper keywords such as “SARS-CoV-2,” “Corona Virus Disease 2019,” “COVID-19,” “anakinra,” “tocilizumab,” “siltuximab,” “sarilumab,” “mavrilimumab,” “lenzilumab,” and related words for publications released until August 22, 2021. Other available resources were also used to identify relevant articles. The present systematic review was performed based on PRISMA protocol. Results: Based on the inclusion and exclusion criteria, 43 articles were included in the final review. The meta-analysis results showed that tocilizumab could reduce the mortality of patients with COVID-19 (at the edge of sepsis) [randomized controlled trials, RCTs: odds ratio (OR) 0.71, 95%CI: 0.52–0.97, low-certainty evidence; non-RCTs: risk ratio (RR) 0.68, 95%CI: 0.55–0.84, very low-certainty evidence) as was anakinra (non-RCTs: RR 0.47, 95%CI: 0.34–0.66, very low-certainty evidence). Sarilumab might reduce the mortality of patients with COVID-19 (at the edge of sepsis), but there was no statistical significance (OR 0.65, 95%CI: 0.36–1.2, low-certainty evidence). For safety outcomes, whether tocilizumab had an impact on serious adverse events (SAEs) was very uncertain (RCTs: OR 0.87, 95%CI: 0.38–2.0, low-certainty evidence; non-RCTs 1.18, 95%CI: 0.83–1.68, very low-certainty evidence) as was on secondary infections (RCTs: OR 0.71, 95%CI: 0.06–8.75, low-certainty evidence; non-RCTs: RR 1.15, 95%CI: 0.89–1.49, very low-certainty evidence). Conclusions: This systematic review showed that tocilizumab, sarilumab, and anakinra could reduce the mortality of people with COVID-19 (at the edge of sepsis), and tocilizumab did not significantly affect SAEs and secondary infections. The current evidence of the studies on patients treated with siltuximab, mavrilimumab, and lenzilumab is insufficient. In order to establish evidence with stronger quality, high-quality studies are needed.
Systematic Review Registration: PROSPERO (https://www.crd.york.ac.uk/prospero/), identifier CRD42020226545
1 Introduction
Sepsis is a life-threatening organ dysfunction syndrome caused by host response imbalance due to an infection or infectious factors. The mortality and treatment expenditure of sepsis are relatively high, and there is no specific drug so far. An article published in The Lancet in 2020 pointed out that the number of sepsis patients worldwide reached 48.9 million in 2017, among which 11 million patients died, accounting for one-fifth of the global death toll (Rudd et al., 2020).
During the pandemic of coronavirus disease 2019 (COVID-19), patients with severe and critically ill COVID-19 may develop circulation disorders and severe lung damage. Some patients with multiple organ dysfunction, such as that of the liver and kidney, showed typical characteristics of sepsis and meet the diagnostic criteria for sepsis (Li et al., 2020). According to Sepsis-3, sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection. The organ dysfunction can be represented by an increase in the Sequential (Sepsis-Related) Organ Failure Assessment (SOFA) score of 2 points or more, which is associated with an in-hospital mortality greater than 10% (Singer et al., 2016). Recent studies have shown that patients with severe and critical diseases may experience immune hyperactivity with increased levels of interleukin (IL)-1, IL-6, granulocyte–monocyte colony-stimulating factor (GM-CSF), interferon-γ-inducible protein 10 (IP-10), tumor necrosis factor-α (TNF-α), and other several inflammatory cytokines and were associated with adverse clinical outcomes (Huang et al., 2020; Qin et al., 2020; Coomes and Haghbayan, 2020; Lucas et al., 2020). Therefore, inhibition of proinflammatory cytokines may be a potential therapeutic strategy in COVID-19 (at the edge of sepsis) patients. This study was the first to screen COVID-19 patients with sepsis or at the edge of sepsis through the SOFA score and systematically reviewed the efficacy and safety of anti-cytokine therapy, such as specific IL-1, IL-6 inhibitors, and anti-GM-CSF in COVID-19 patients with organ dysfunction (SOFA ≥2). This paper could help sepsis treatment strategy researchers to grasp the current status of anti-cytokine therapy for COVID-19 patients (at the edge of sepsis) and provide a new perspective for clinical treatment.
2 Methodology
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (Supplementary Material S1) (Moher et al., 2009) and registered with the National Institute for Health Research international prospective register of systematic reviews (PROSPERO registration number: CRD42020226545) (Wang et al., 2020).
2.1 Search Strategy and Selection Criteria
Electronic searches were carried out in PubMed, EMBASE, Clinical Key, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang Database. The search terms that we used were “SARS-CoV-2,” “corona virus disease 2019,” “COVID-19,” “anakinra,” “tocilizumab,” “siltuximab,” “sarilumab,” “mavrilimumab,” and “lenzilumab” and relevant keywords for publications released until August 22, 2021. The search strategies are available as supplementary data (Supplementary Material S1). Other available resources were also used to identify relevant articles. The language will be limited to Chinese and English. Eligible articles were identified for inclusion by screening the titles, abstracts, and full text. Other relevant studies were manually screened by investigators from the reference list of included studies for further analysis. There was no date limit. Two independent reviewers (YW and KZ) carried out the search in a standardized process, followed with identifying eligible records through the examination of each title, abstract, and full text. Disagreements were resolved by consensus, and unresolved conflicts were decided by a third reviewer (QY).
The studies were selected based on the following inclusion criteria: (1) The patients were diagnosed with SARS-CoV-2 infection and their SOFA score (include mean value, median, and absolute value) ≥2 or, according to the SOFA scoring tool, a certain system index (including mean, median, and absolute value) should be within the range corresponding to the system score ≥2—for example, PaO2/FiO2 ratio (P/F) (including absolute value, mean value, or median value) was less than 300 mmHg (Singer et al., 2016). A SpO2/FiO2 ratio (S/F) of 315 corresponded with a P/F ratio of 300 mmHg [S/F = 64 + 0.84*(P/F)] (Rice et al., 2007). In this review, we defined such COVID-19 patients to be at the edge of sepsis. (2) The intervention of interest was a specific IL-1 inhibitor (anakinra), specific IL-6 inhibitors (tocilizumab, siltuximab, and sarilumab), GM-CSF blockades (mavrilimumab and lenzilumab) with or without standard of care (or treatment), and glucocorticoids. Comparator treatments included placebo, standard of care (or treatment), glucocorticoids, or no intervention; studies with no comparator group were also included. (3) Randomized clinical trials (RCTs), cohort studies, case–control studies, case series, case reports, clinical guidelines, protocols for clinical trials, and any other gray literatures will be included. The studies will not be limited in terms of country. The exclusion criteria were as follows: (1) The patients were not diagnosed as COVID-19; (2) The SOFA score (absolute value, mean value, or median value) of the patients was less than 2 or did not reach 2 on any of the system indicators; (3) Data on SOFA score or certain indicators in the SOFA scoring tool for the patients studied were not available in the study text, additional materials, or any other relevant resources; and (4) Studies without an available full text or whose data were incomplete or unavailable, posters, commentaries, letters, opinion articles, and in vitro studies were excluded. The defined primary outcome was all-cause mortality at 28–30 days. The safety outcomes included serious adverse events (SAEs) and (serious) secondary infection. Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 4.0 (National Institutes of Health, 2017).
2.2 Data Extraction and Quality (Risk of Bias) Assessment
Two independent reviewers (YW and KZ) extracted data from the eligible studies, and a third one (QY) validated it. The following information will be extracted: year of publication, authors, country, study type, sample size, participant demographics, time of administration, intervention characteristics (name of agent, dose, and route), concomitant medications, survival outcome, treatment-related adverse events, and conclusions of the authors.
The included studies were assessed in terms of potential bias by two reviewers (RD and RL) independently. The third researcher (XL) was consulted for resolving any difference of opinion. The Quality Assessment for Case Series of the National Institute for Health and Care Excellence will be used to evaluate the quality of the case reports (series). The total score is 8 points, in which a score of 4–8 is high quality, and a score less than 4 is low quality. The methodological quality for cohort and case–control studies was assessed based on the Newcastle–Ottawa Scale (NOS) (NOS, 2020). The total score is 9 points, in which scores of 0–3, 4–6, and 7–9 are respectively considered as low, moderate, and high quality. The methodological quality of RCTs was assessed based on the “Risk of Bias” 2.0 tool (Sterne et al., 2019). Each checklist item was judged as “low,” “moderate,” “serious,” and “critical,” The quality of evidence was assessed by using the “Grading of Recommendations Assessment Development and Evaluation (GRADE)” tool (Granholm et al., 2019). The quality of evidence of each outcome is classified as “high,” “moderate,” “low,” or “very low”.
2.3 Data Synthesis and Analysis
The Review Manager version 5.4.1 software was used for analyses. One reviewer (YW) would have to enter the data into the software, and another reviewer (M.L) would have to check the data for accuracy. For dichotomous outcomes, the number of events and total number of participants in the two groups were recorded. The different types of studies were analyzed separately, such as non-RCTs (cohorts and case–control studies) and RCTs. The risk ratio (RR) and odds ratio (OR) with 95% confidence intervals (CIs) were respectively assessed for non-RCTs and RCTs. Fixed-effects model was used if the result of the Q test was not significant (p > 0.1) and I2 <50%. Chi-square test, with a significance level at p ≤ 0.1, was used to assess the heterogeneity of treatment effects between trials. The I2 statistic was used to quantify possible heterogeneity (75–100% considerable heterogeneity). We would explore potential causes through sensitivity and subgroup analyses if heterogeneity had been above 80%. We would not have conducted a meta-analysis if we had not found a reason for heterogeneity. If we could not perform a meta-analysis, we had planned to comment on the results from all studies.
3 Results
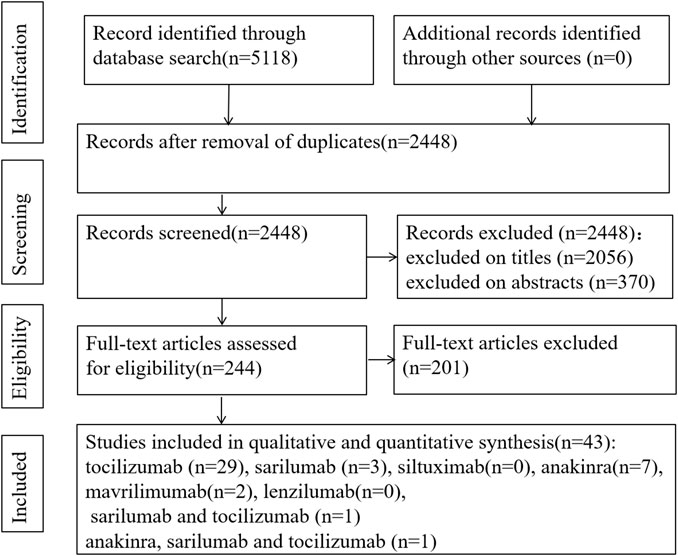
3.1 Search Results
Because of insufficient evidence available from RCTs, we also included cohort studies, case–control studies, and case reports (series). The search of the electronic databases on Aug 22, 2021 yielded a total of 5,118 studies. Following the elimination of duplicates and screening of titles and abstracts, we evaluated 244 articles in full text. Among these, we found 43 eligible articles (5 RCTs, 16 cohort studies, 2 case–control studies, and 20 case reports) (Figure 1) (Salvarani et al., 2021; REMAP-CAP Investigators et al., 2021; Canziani et al., 2020; Menzella et al., 2020; Fisher et al., 2021; Campochiaro et al., 2020; Vazquez Guillamet et al., 2021; Rajendram et al., 2021;Huang et al., 2021; Galván-Román et al., 2021; Saffo et al., 2021; Somers et al., 2021; Brosnahan et al., 2021; Corominas et al., 2021; Cavalli et al., 2021; Abe et al., 2021; Patel et al., 2020; Mady et al., 2020; Bernardo et al., 2020; Morillas et al., 2020; Cascella et al., 2020; Al-Kaf et al., 2021; Eroglu et al., 2021; Kataoka et al., 2021; Kishaba et al., 2021; Leelayuwatanakul et al., 2021; McKenzie et al., 2021; Nourié et al., 2021; Ladna et al., 2021; Senegaglia et al., 2021; Thammathiwat et al., 2021; Lescure et al., 2021; Della-Torre et al., 2020; Gremese et al., 2020; Kyriazopoulou et al., 2021a; Bozzi et al., 2021; Franzetti et al., 2021; Kyriazopoulou et al., 2021b; Erden et al., 2021; Filocamo et al., 2020; Franzetti et al., 2020; Cremer et al., 2021; De Luca et al., 2020). All studies were published in peer-reviewed journals.
In the process of full-text review, there were four articles for which we failed to obtain the full texts. The four studies were related to tocilizumab. Two studies did not report the efficacy and safety of tocilizumab (Garg et al., 2020; Kashin et al., 2020). The other two studies were case reports, in which one patient developed tuberculosis reactivation during treatment and the other patient had a secondary infection. The authors of the two case reports suggested that patients might be at a high risk for secondary infection after receiving tocilizumab or tocilizumab combined with glucocorticoid. They suggested that clinicians should use tocilizumab with caution and screen for latent tuberculosis before medication (Mazankova et al., 2020; Moideen et al., 2020).
3.2 Risk of Bias Assessment
The risk of bias of the RCTs was low to moderate, respectively. The results are shown in Supplementary Figure S1 (Supplementary Material S2, Appendix p1). Some studies reported only one outcome, and we assessed the risk of bias for the results—for instance, bias in the measurement of outcomes was not available for safety for the study of Brosnahan et al. (2021) because they did not report it. For mortality outcomes, the methodological quality of 16 cohorts was moderate to high, and those of 2 case–control studies were moderate. For safety outcomes, the methodological quality of 14 cohorts was low to high, and those of 2 case–control studies were low to moderate (NOS assessment results are shown in Supplementary Material S2, Appendix p2–40). The methodological quality evaluation results of the included case reports (series) showed that the quality was low to moderate (the results of quality are shown in Supplementary Material S2, Appendix, p41–42).
3.3 Characteristics of Patients
The 43 studies included were identified and critically evaluated, which included a total of 4,951 patients with confirmed SARS-CoV-2 infection, of whom 2,243 received mechanical ventilation. Only 11 studies reported the SOFA score of enrolled patients, of which 4 studies reported SOFA scores greater than or equal to 6 (tocilizumab), 3 studies reported scores between 4 and 5 (tocilizumab), and 4 studies reported scores between 2 and 3 (3 for anakinra, 1 for mavrilimumab). The remaining 32 articles reported the respiratory status (including P/F or S/F) and platelet of patients, of which 5 studies included patients with P/F less than or equal to 100 mmHg and of which 13 studies reported patients with P/F between 100 and 200 mmHg.
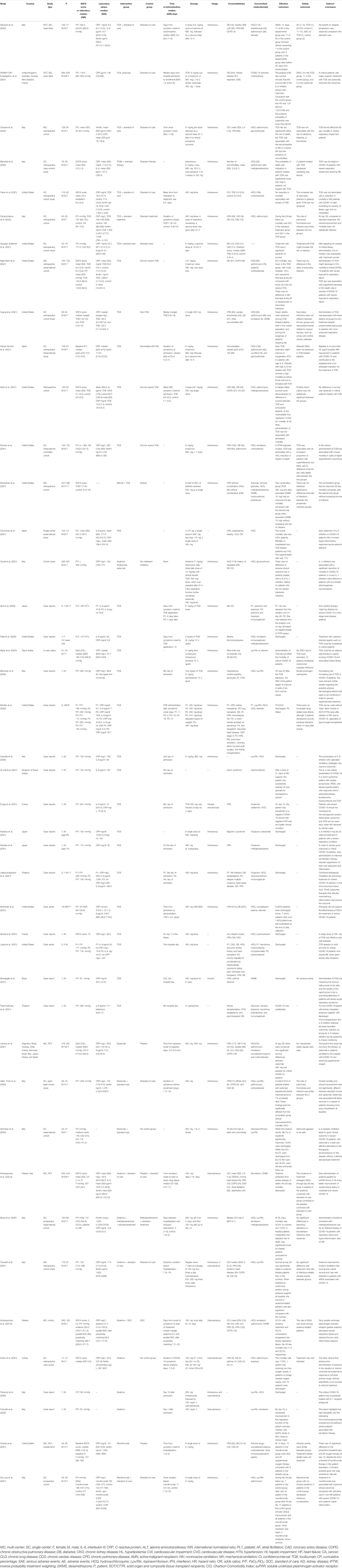
Most patients received standard of care (or standard of treatment) based on local treatment guidelines. However, the medication regimens of the standard of care were different, mainly including antiviral drugs, antibiotics, glucocorticoids, and other symptomatic drugs. Anti-cytokine agents were mainly used by intravenous injection and, in a few studies, by subcutaneous administration. In addition, there is still no consensus on the dosage of anti-cytokine agents for such patients until now. In the included articles, the dosage of most patients was as follows: tocilizumab, 8 mg/kg/dose and up to a maximum of 800 mg; sarilumab, 400 mg/dose with 1 to 2 doses; anakinra 100 mg/dose 1–4 times a day; and mavrilimumab 6 mg/kg/dose. The characteristics of the included studies are presented in Table 1.
3.4 Results of the Meta-analysis
We cannot conduct a quantitative analysis of anakinra, sarilumab, and mavrilimumab for some outcomes, owing to differences in outcomes reported, study design, and limited study numbers. Especially for mavrilimumab, only one RCT and one cohort met the inclusion criteria. If we could not perform a meta-analysis, we commented on the results from all included studies.
3.4.1 Mortality Outcome (All-Cause Mortality at Days 28–30)
Tocilizumab
Among the 14 controlled studies, one RCT and 6 cohorts neither reported a difference for mortality at days 28–30 between the tocilizumab and control groups. Compared to the control group, the results of RCTs showed that the use of tocilizumab for patients with COVID-19 (at the edge of sepsis) might decrease the mortality rate (OR 0.71, 95%CI: 0.52–0.97, I2 = 0%), and there was a significant difference between the two groups (Figure 2A). The non-RCTs showed a similar result (RR 0.68, 95%CI: 0.55–0.84, I2 = 45%), and there was statistical significance (Figure 2B).
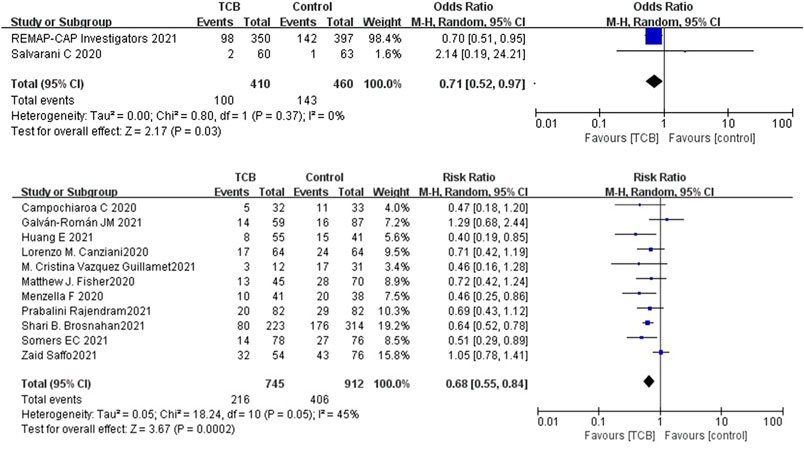
FIGURE 2. (A) Results from randomized controlled trials (RCTs): the mortality outcome of tocilizumab for COVID-19 (at the edge of sepsis).
Sarilumab
For sarilumab, of the studies that met the inclusion criteria, only two RCTs (one of the RCTs studied tocilizumab and sarilumab) and two non-RCTs provided data on mortality outcome. Among the two non-RCTs, one cohort did not set up a control group. Compared to the control group, the results of RCTs showed that the use of sarilumab for patients with COVID-19 (at the edge of sepsis) might reduce the mortality rate (OR 0.65, 95%CI: 0.36–1.2, I2 = 8%), but there was no significant difference between the two groups (Figure 3). However, due to the lack of research, data synthesis for outcomes of non-RCTs was not conducted.
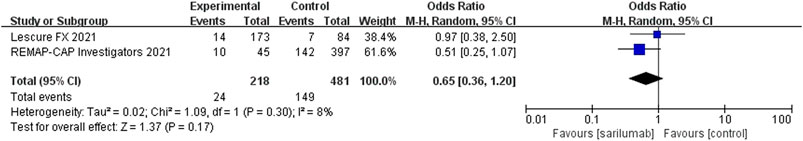
FIGURE 3. Results from randomized controlled trials: the mortality outcome of sarilumab for COVID-19 (at the edge of sepsis).
Anakinra
For anakinra, of the studies that met the inclusion criteria, 1 RCT and 4 non-RCTs provided data on mortality outcome. Due to the insufficiency of RCTs, we only quantitatively synthesized the results of non-RCTs. Compared to the control group, the results of non-RCTs showed that the use of anakinra for patients with COVID-19 (at the edge of sepsis) might reduce the mortality rate (RR 0.47, 95%CI: 0.34–0.66, I2 = 0%), and there was statistical significance (Figure 4).
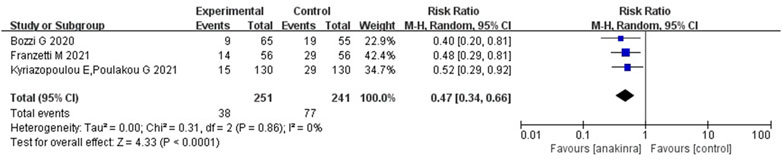
FIGURE 4. Results from non- randomized controlled trials: the mortality outcome of anakinra for COVID-19 (at the edge of sepsis).
Mavrilimumab
The only RCT, published in 2021, explored outcomes in 21 patients who received mavrilimumab and 19 patients who received placebo. The median (IQR) baseline SOFA score of enrolled patients was 2 (2 to 3). The study reported no significant association with the proportion of patients alive and off oxygen therapy at day 14. The other cohort, published in 2020, explored outcomes in 12 patients who received mavrilimumab and 26 patients who received standard of care. The median (IQR) P/F ratio of the mavrilimumab and control group was 196 (167–215) and 217 (138–258) mmHg, respectively. The study reported that mavrilimumab was associated with a reduced mortality rate and improved clinical outcomes. The benefits of mavrilimumab therapy for those patients remained uncertain, given the insufficient controlled studies and the small sample size.
3.4.2 Safety Outcomes
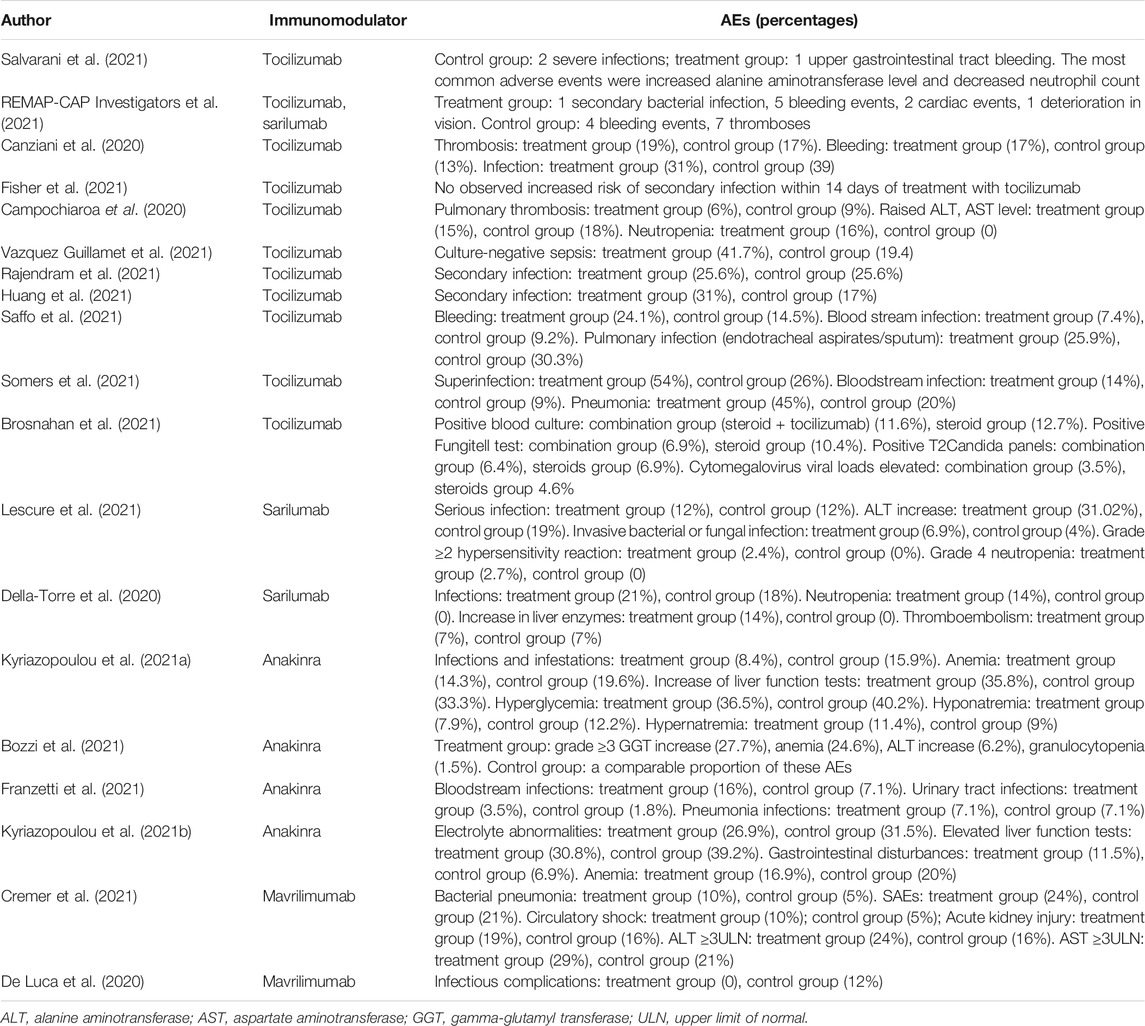
Treatment-related adverse events (TRAEs) were reported in the majority of research and typically included neutropenia, secondary infections, increase in liver enzymes, and thromboembolism (Table 2). Due to the insufficient studies of safety outcome, we only conducted a quantitative synthesis for tocilizumab.
Tocilizumab
Both 2 RCTs reported SAEs and secondary infections; 4 of 11 non-RCTs reported SAEs and 10 reported secondary infections. Tocilizumab was associated with less SAEs (OR 0.87, 95%CI: 0.38–2.00, I2 = 0%) and lower rates of secondary infections (OR 0.71, 95%CI: 0.06–8.75, I2 = 42%) compared with the control groups, which both did not reach significance in RCTs (Figures 5A,B). For non-RCTs, tocilizumab was associated with slightly more SAEs (RR 1.18, 95%CI: 0.83–1.68, I2 = 0) and secondary infections (RR 1.15, 95%CI: 0.89–1.49, I2 = 49%) compared with the control arm, but there was no statistical significance (Figures 6A,B).
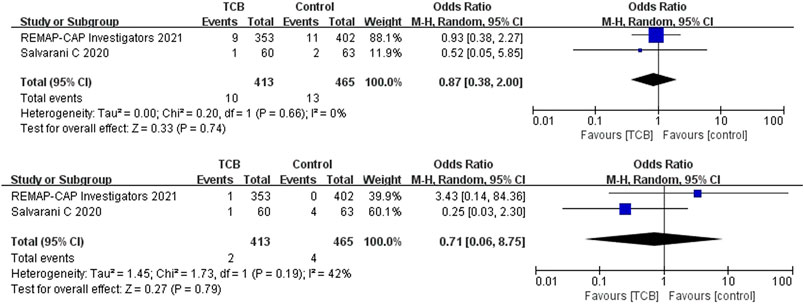
FIGURE 5. (A) Results from randomized controlled trials (RCTs): the serious adverse events (SAEs) of tocilizumab for COVID-19 (at the edge of sepsis).
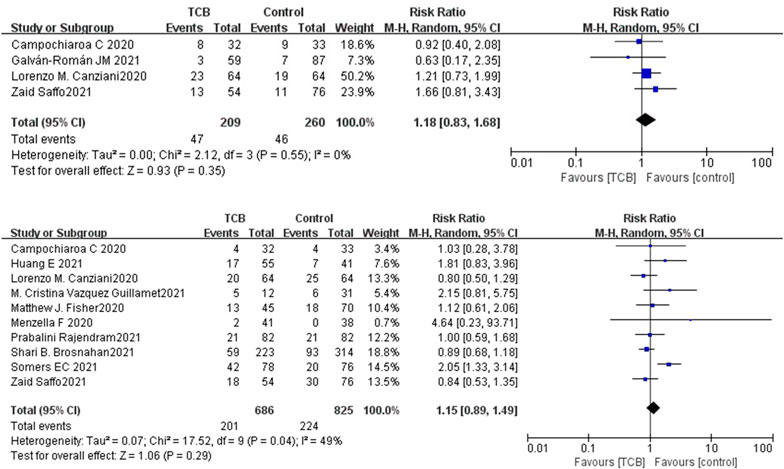
FIGURE 6. (A) Results from non-randomized controlled trials (non-RCTs): the serious adverse events of tocilizumab for COVID-19 (at the edge of sepsis).
Other Anti-cytokine Agents
The included RCTs reported that the incidence of treatment-emergent SAEs through day 28 was higher in the placebo and standard-of-care group (21.2%) compared to the anakinra and standard-of-care group (16.5%). The non-serious TRAEs were similar in both treatment groups (Kyriazopoulou et al., 2021a). Only two cohorts reported secondary infection outcomes, and none reported SAEs. Both Franzetti M et al. and Bozzi G et al. reported that the rate of adverse events related to infection (or bloodstream infections) was similar between groups—for example, 26.8% occurred in the anakinra group and 16.1% in the control group (Bozzi et al., 2021; Franzetti et al., 2021). Among these infectious events, 9/56 developed bloodstream infections in the anakinra group and 4/56 in the control group (Franzetti et al., 2021). Meanwhile, they all suggested that special attention should be paid to possible infective reactivations or bacterial sepsis due to anakinra. In studies with a comparator arm exploring outcomes from patients who received mavrilimumab or sarilumab, the frequency of TRAEs was similar in both treatment and comparator groups.
3.5 Quality of Evidence
For mortality outcomes, the quality of evidence of tocilizumab for COVID-19 (at the edge of sepsis) was of low and very low quality for RCTs and non-RCTs, respectively. Meanwhile, the quality of evidence of sarilumab and anakinra for COVID-19 (at the edge of sepsis) was of low and very low quality, respectively. As for the SAEs and secondary infections of tocilizumab for COVID-19 (at the edge of sepsis), the quality of evidence was all low for RCTs and very low for non-RCTs, respectively. The results are shown in Supplementary Table S8 (Supplementary Material S2, Appendix p43–45).
4 Discussion
In terms of etiology, sepsis can be classified as bacterial sepsis, fungal sepsis, and viral sepsis based on different pathogens. Sepsis patients with a SOFA score of 2 or more in a general hospital population with presumed infection had an increased risk of death by 2–25 times compared to patients with a SOFA score of less than 2 (Singer et al., 2016; Seymour et al., 2016). The population included in this study was COVID-19 patients with SOFA score ≥2, who were already in the state of sepsis or were about to deteriorate into sepsis, and these patients urgently needed appropriate, safe, and effective treatment. In this study, we evaluated the efficacy and safety of tocilizumab, sarilumab, siltuximab, anakinra, mavrilimumab, and lenzilumab to provide relevant clinical evidence and research ideas for treatment.
4.1 Anti-cytokine Therapy
The local inflammatory response caused by an infection can promote the replacement of damaged tissues by new tissues and play a role in weakening the damage that has occurred, but when excessive inflammation occurs, it may cause systemic inflammatory response syndrome (SIRS) and lead to sepsis. Therefore, timely detection of cytokine storms and proper regulation of inflammatory response may be of great significance to the prevention of sepsis. The “Expert Consensus on Early Prevention and Blocking of Sepsis in China” recommended that when infected patients experience significant increases in cytokines or inflammatory imbalances, the inflammation should be adjusted as soon as possible using glucocorticoids, nonsteroidal anti-inflammatory drugs, traditional Chinese medicine preparations, antibodies targeting inflammatory mediators, etc. (Emergency medicine branch of CPAM et al., 2020). Many studies showed that the factors mainly involved in SIRS and compensatory anti-inflammatory response syndrome include TNF-α, IL-1, IL-6, etc. The Expert Consensus suggested that, for patients with high-risk sepsis infection, cytokine monitoring should be carried out regularly (2–4-h repetition) to find suspected sepsis patients in time. At present, the cytokine commonly detected in hospitals is IL-6. As a cytokine, IL-6 mainly stimulates the proliferation and differentiation of cells involved in immune response and plays an important role in the anti-infection immune response (Emergency Medicine Branch of CPAM et al., 2020).
IL-6 inhibitors include tocilizumab, sarilumab and siltuximab. Tocilizumab and sarilumab were approved for rheumatoid arthritis, and siltuximab was approved for Castleman’s disease. The IL-1 receptor antagonist (anakinra) is a cornerstone treatment for hyperinflammatory conditions such as Still’s disease. Some studies showed that cytokine-directed agents such as IL-6 and IL-1 inhibitors might be effective in the treatment of cytokine storm syndromes, including macrophage activation syndrome and cytokine release syndrome (La Rosée et al., 2019). The GM-CSF blockade included mavrilimumab and lenzilumab, which is designed to prevent and treat cytokine storm (De Luca et al., 2020; Aroldi et al., 2019). This systematic review identified and summarized RCTs, non-RCTs, and case reports (series) to evaluate the effect and safety of tocilizumab, sarilumab, siltuximab, anakinra, mavrilimumab, and lenzilumab. The meta-analysis results showed that tocilizumab might reduce the mortality of patients with COVID-19 (at the edge of sepsis) (RCTs: OR: 0.71, 95%CI: 0.52–0.97, low-certainty evidence; non-RCTs: RR: 0.68, 95%CI: 0.55–0.84, very low-certainty evidence) as was anakinra (non-RCTs: RR: 0.47, 95%CI: 0.34–0.66, very low-certainty evidence). Sarilumab might reduce the mortality of patients with COVID-19 (at the edge of sepsis), but there was no statistical significance (OR: 0.65, 95%CI: 0.36–1.2, low-certainty evidence). For safety outcomes, whether tocilizumab had an impact on SAEs was very uncertain (RCTs: OR: 0.87, 95%CI: 0.38–2.0, low-certainty evidence; non-RCTs: OR: 1.18, 95%CI: 0.83–1.68, very low-certainty evidence) as was on secondary infections (RCTs: OR: 0.71, 95%CI: 0.06–8.75, low-certainty evidence; non-RCTs: RR: 1.15, 95%CI: 0.89–1.49, very low-certainty evidence).
4.2 Special Population
At present, there are still few large-scale randomized controlled prospective studies on COVID-19 (at the edge of sepsis). The experiences of case or case series still have a certain reference significance for clinical treatment, especially for the individualized treatment of special populations, such as critically ill children, immunocompromised individuals, and elderly patients with a variety of chronic diseases. Patel PA et al. reported a case of severe pediatric COVID-19 presenting with respiratory failure and severe thrombocytopenia. On day 7, because of continued fever and elevated inflammatory markers, remdesivir and tocilizumab were given. On the next day, she had significant clinical improvement, so the treatment with cytokine-directed agents may be considered in critically ill patients (Patel et al., 2020).
Patients with impaired immune function are more at risk in case of adverse outcomes. Leelayuwatanaku N et al. reported two patients (P/F < 300 mmHg) with human immunodeficiency virus (HIV) infection and multiple myeloma relapse, respectively. After tocilizumab, hemoperfusion, and immunoglobulin comprehensive treatment, their P/F levels increased significantly, and they survived to discharge (Leelayuwatanaku et al., 2021). In addition, Kataoka H et al. reported an 85-year-old patient with Sjögren’s syndrome, whose P/F decreased to 100 mmHg. After receiving a single dose of tocilizumab, the symptoms improved. This patient represents a supplementary case confirming the safety and efficacy of tocilizumab for elderly COVID-19 patients with autoimmune diseases. It is also suggested that combination therapy may be a promising treatment for severe COVID-19 in immunocompromised hosts (Kataoka et al., 2021).
The experience of COVID-19 patients with solid organ and composite tissue transplantation has not been reported in detail before. Morillas JA et al. reported 5 patients with COVID-19 (P/F < 300 mmHg) who received kidney transplantation, lung transplantation, face transplantation, and liver transplantation, respectively. These patients also had chronic diseases, such as heart diseases, bladder cancer, rheumatic heart disease, etc. Their C-reactive protein (CRP) levels decreased significantly within a few days after the application of tocilizumab. The findings showed that tocilizumab could be used without major direct toxicity in solid organ and composite tissue transfer recipients early after initiation of mechanical exploitation due to COVID-19, regardless of the type of organ transferred. However, the authors suggested that the diagnosis and side effects need to be further studied (Morillas et al., 2020). Ladna M et al. and Thammathiwa T et al. shared the treatment experiences of transplant patients, respectively. One patient who received a kidney and heart transplant in February 2020 had a relatively poor clinical condition with a P/F level of 117 mmHg. On day1, he was given a dose of 400 mg of tocilizumab, broad-spectrum antibiotics, and hydroxychloroquine, and transplant immunosuppression with tacrolimus was continued. After 11 days of treatment, he was discharged without supplemental oxygen requirement (Ladna et al., 2021; Thammathiwat et al., 2021).
In addition to tocilizumab treatment cases, there are few case reports on IL-1 receptor antagonist. Filocamo G et al. and Franzetti M et al. in Italy treated two severe patients (P/F <200 mmHg) with IL-1 receptor antagonist anakinra. These studies suggested that, in the cytokine storm occurring during severe COVID-19 pneumonia, the high tolerability, short half-life, and immunomodulatory profile of anakinra may be useful. IL-1 inhibition may represent a safe and promising strategy to reduce inflammation, thus preventing multi-organ dysfunction (Filocamo et al., 2020; Franzetti et al., 2020).
4.3 Limitation
First, the lack of RCTs limited our analyses. Some included studies were case reports or series and had no proper control groups. Meanwhile, some articles of which the full texts or data were not accessible and those in languages other than Chinese and English were excluded from the analysis. This might have led to overlooking some critical findings or observations. In addition, in this study, the SOFA score or related indicators of some patients included in the study were median or mean, so not all patients were septic patients, but the results of this population also reflected a trend problem because some patients might be or would be in a state of sepsis. Thirdly, we found that most patients use antiviral drugs, glucocorticoids, immunoglobulins, plasma, broad-spectrum antibiotics, and other drugs at the same time. We cannot rule out the impact of these drugs on the disease.
5 Conclusion
The results of this systematic review showed that tocilizumab, sarilumab, and anakinra might reduce the mortality of people with COVID-19 (at the edge of sepsis), and tocilizumab did not significantly affect SAEs and secondary infections. However, given the limited clinical researches and low-quality evidence, this conclusion needs more clinical evidence to be verified. In addition, so far, there is still no unified opinion on the timing, dosage, usage, and applicable population of these drugs all over the world, which also adds to the uncertainty of the conclusion of this study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
YW and KZ contributed equally to this work. YW, KZ, and QY conceived and designed the study protocol. YW and KZ executed the search strategy and screening and performed data extraction. RL, RD, and XL performed risk of bias assessments, and YW and RL assessed the quality of evidence. YW, ML, and RD analyzed or interpreted the data. YW and KZ drafted the manuscript. QY contributed to writing—review and editing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Supplementary Material
The supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.804250/full#supplementary-material
References
Abe, T., Izumo, T., Ueda, A., Hayashi, M., and Ishibashi, Y. (2021). Successful Treatment of Two Japanese ESRD Cases With Severe COVID-19 Pneumonia. CEN Case Rep. 10 (1), 42–45. doi:10.1007/s13730-020-00512-7
Al-Kaf, F. A., Al Garni, T. A., Al-Harbi, N., Sandokji, H., and Samargandy, S. (2021). Cardiac Tamponade, Sever Hypothyroidism and Acute Respiratory Distress Syndrome (ARDS) With COVID-19 Infection. J. Saudi Heart Assoc. 33 (1), 71–76. doi:10.37616/2212-5043.1235
Aroldi, A., Chiarle, R., and Gambacorti-Passerini, C. (2021). Clinical Benefit of Lenzilumab in Cases of Coronavirus Disease 2019. Mayo Clin. Proc. 96 (3), 817. doi:10.1016/j.mayocp.2020.12.030
Bernardo, L., Del Sesto, S., Giordano, L., Benincaso, A. R., Biondi, P., Goj, V., et al. (2020). Severe Prolonged Neutropenia Following Administration of Tocilizumab in a Patient Affected by COVID-19: a Case Report and Brief Review of the Literature. Drugs Ther. Perspect. 14, 1–5. doi:10.1007/s40267-020-00777-z
Bozzi, G., Mangioni, D., Minoia, F., Aliberti, S., Grasselli, G., Barbetta, L., et al. (2021). Anakinra Combined With Methylprednisolone in Patients With Severe COVID-19 Pneumonia and Hyperinflammation: An Observational Cohort Study. J. Allergy Clin. Immunol. 147 (2), 561–e4. e4. doi:10.1016/j.jaci.2020.11.006
Brosnahan, S. B., Chen, X. J. C., Chung, J., Altshuler, D., Islam, S., Thomas, S. V., et al. (2021). Low-Dose Tocilizumab With High-Dose Corticosteroids in Patients Hospitalized for COVID-19 Hypoxic Respiratory Failure Improves Mortality Without Increased Infection Risk. Ann. Pharmacother. 28, 10600280211028882. doi:10.1177/10600280211028882
Campochiaro, C., Della-Torre, E., Cavalli, G., De Luca, G., Ripa, M., Boffini, N., et al. (2020). Efficacy and Safety of Tocilizumab in Severe COVID-19 Patients: a Single-centre Retrospective Cohort Study. Eur. J. Intern. Med. 76, 43–49. doi:10.1016/j.ejim.2020.05.021
Canziani, L. M., Trovati, S., Brunetta, E., Testa, A., De Santis, M., Bombardieri, E., et al. (2020). Interleukin-6 Receptor Blocking with Intravenous Tocilizumab in COVID-19 Severe Acute Respiratory Distress Syndrome: A Retrospective Case-Control Survival Analysis of 128 Patients. J. Autoimmun. 114, 102511. doi:10.1016/j.jaut.2020.102511
Cascella, M., Mauro, I., De Blasio, E., Crispo, A., Del Gaudio, A., Bimonte, S., et al. (2020). Rapid and Impressive Response to a Combined Treatment With Single-Dose Tocilizumab and NIV in a Patient With COVID-19 Pneumonia/ARDS. Medicina (Kaunas). 56 (8), 377. doi:10.3390/medicina56080377
Cavalli, G., Larcher, A., Tomelleri, A., Campochiaro, C., Della-Torre, E., De Luca, G., et al. (2021). Interleukin-1 and Interleukin-6 Inhibition Compared With Standard Management in Patients With COVID-19 and Hyperinflammation: a Cohort Study. Lancet Rheumatol. 3 (4), e253–e261. doi:10.1016/S2665-9913(21)00012-6
Coomes, E. A., and Haghbayan, H. (2020). Interleukin-6 in Covid-19: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 30 (6), 1–9. doi:10.1002/rmv.2141
Corominas, H., Castellví, I., Pomar, V., Antonijoan, R., Mur, I., Matas, L., et al. (2021). Effectiveness and Safety of Intravenous Tocilizumab to Treat COVID-19-Associated Hyperinflammatory Syndrome: Covizumab-6 Observational Cohort. Clin. Immunol. 223, 108631. doi:10.1016/j.clim.2020.108631
Cremer, P. C., Abbate, A., Hudock, K., McWilliams, C., Mehta, J., Chang, S. Y., et al. (2021). Mavrilimumab in Patients With Severe COVID-19 Pneumonia and Systemic Hyperinflammation (MASH-COVID): an Investigator Initiated, Multicentre, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Rheumatol. 3 (6), e410–e418. doi:10.1016/S2665-9913(21)00070-9
De Luca, G., Cavalli, G., Campochiaro, C., Della-Torre, E., Angelillo, P., Tomelleri, A., et al. (2020). GM-CSF Blockade With Mavrilimumab in Severe COVID-19 Pneumonia and Systemic Hyperinflammation: a Single-Centre, Prospective Cohort Study. Lancet Rheumatol. 2 (8), e465–e473. doi:10.1016/S2665-9913(20)30170-3
Della-Torre, E., Campochiaro, C., Cavalli, G., De Luca, G., Napolitano, A., La Marca, S., et al. (2020). Interleukin-6 Blockade With Sarilumab in Severe COVID-19 Pneumonia With Systemic Hyperinflammation: an Open-Label Cohort Study. Ann. Rheum. Dis. 79 (10), 1277–1285. doi:10.1136/annrheumdis-2020-218122
Emergency medicine branch of CPAM (2020). Emergency Medicine Branch of Chinese Medical Association, Emergency Physicians branch of Chinese Medical Doctor Association, Professional Committee of Emergency Medicine of Chinese People's Liberation Army. Expert Consensus on Early Prevention and Blocking of Sepsis in China. Cli Emer J. (China). 21 (7), 517–529.
Erden, A., Ozdemir, B., Karakas, O., Mutlu, N. M., Izdes, S., Kalem, A. K., et al. (2021). Evaluation of 17 Patients With COVID-19 Pneumonia Treated With Anakinra According to HScore, SOFA, MuLBSTA, and Brescia-COVID Respiratory Severity Scale (BCRSS) Scoring Systems. J. Med. Virol. 93 (3), 1532–1537. doi:10.1002/jmv.26473
Eroglu, A., Kartal, S., and Saral, O. B. (2021). Helmet Mask and Tocilizumab for a Patient With Hemophagocytic Lymphohistiocytosis Syndrome and COVID-19: a Case Report. Braz. J. Anesthesiol. 71 (1), 79–83. doi:10.1016/j.bjane.2020.10.009
Filocamo, G., Mangioni, D., Tagliabue, P., Aliberti, S., Costantino, G., Minoia, F., et al. (2020). Use of Anakinra in Severe COVID-19: A Case Report. Int. J. Infect. Dis. 96, 607–609. doi:10.1016/j.ijid.2020.05.026
Fisher, M. J., Marcos Raymundo, L. A., Monteforte, M., Taub, E. M., and Go, R. (2021). Tocilizumab in the Treatment of Critical COVID-19 Pneumonia: A Retrospective Cohort Study of Mechanically Ventilated Patients. Int. J. Infect. Dis. 103, 536–539. doi:10.1016/j.ijid.2020.12.021
Franzetti, M., Forastieri, A., Borsa, N., Pandolfo, A., Molteni, C., Borghesi, L., et al. (2021). IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective, Observational Study. J. Immunol. 206 (7), 1569–1575. doi:10.4049/jimmunol.2001126
Franzetti, M., Pozzetti, U., Carugati, M., Pandolfo, A., Molteni, C., Faccioli, P., et al. (2020). Interleukin-1 Receptor Antagonist Anakinra in Association With Remdesivir in Severe COVID-19: A Case Report. Int. J. Infect. Dis. 97, 215–218. doi:10.1016/j.ijid.2020.05.050
Galván-Román, J. M., Rodríguez-García, S. C., Roy-Vallejo, E., Marcos-Jiménez, A., Sánchez-Alonso, S., Fernández-Díaz, C., et al. (2021). IL-6 Serum Levels Predict Severity and Response to Tocilizumab in COVID-19: An Observational Study. J. Allergy Clin. Immunol. 147 (1), 72–80. e8. doi:10.1016/j.jaci.2020.09.018
Garg, N., and Lee, Y. I. (2020). Reactivation TB With Severe COVID-19. Chest. 158, A777. doi:10.1016/j.chest.2020.08.724
Granholm, A., Alhazzani, W., and Møller, M. H. (2019). Use of the GRADE Approach in Systematic Reviews and Guidelines. Br. J. Anaesth. 123 (5), 554–559. doi:10.1016/j.bja.2019.08.015
Gremese, E., Cingolani, A., Bosello, S. L., Alivernini, S., Tolusso, B., Perniola, S., et al. (2020). Sarilumab Use in Severe SARS-CoV-2 Pneumonia. EClinicalMedicine. 27, 100553. doi:10.1016/j.eclinm.2020.100553
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet. 395 (10223), 497–506. doi:10.1016/S0140-6736(20)30183-5
Huang, E., Isonaka, S., Yang, H., Salce, E., Rosales, E., and Jordan, S. C. (2021). Tocilizumab Treatment in Critically Ill Patients With COVID-19: A Retrospective Observational Study. Int. J. Infect. Dis. 105, 245–251. doi:10.1016/j.ijid.2021.02.057
Kashin, M., Gorski, J., and Minkin, R. (2020). Lung Abscesses in A Patient Treated With Tocilizumab for Covid-19 Pneumonia Complicated by Severe Hypoxemic Respiratory Failure. Chest. 158 (4), A2568. doi:10.1016/j.chest.2020.09.165
Kataoka, H., Kodama, F., Tomita, T., Kondo, M., Nagasaka, A., Nishikawa, S., et al. (2021). Immediate Amelioration of Severe Respiratory Distress in Sjögren's Syndrome With COVID-19 Treated With a Single Dose of Off-Label Tocilizumab. Intern. Med. 60 (4), 639–643. doi:10.2169/internalmedicine.6010-20
Kishaba, T., Maeda, A., Fukuoka, S., Imai, T., Takakura, S., Yokoyama, S., et al. (2021). A Case Report of Super Responder of Critical COVID-19 Pneumonia. J. Med. Invest. 68 (1.2), 192–195. doi:10.2152/jmi.68.192
Kyriazopoulou, E., Panagopoulos, P., Metallidis, S., Dalekos, G. N., Poulakou, G., Gatselis, N., et al. (2021a). An Open Label Trial of Anakinra to Prevent Respiratory Failure in COVID-19. Elife. 10, e66125. doi:10.7554/eLife.66125
Kyriazopoulou, E., Poulakou, G., Milionis, H., Metallidis, S., Adamis, G., Tsiakos, K., et al. (2021b). Early Treatment of COVID-19 with Anakinra Guided by Soluble Urokinase Plasminogen Receptor Plasma Levels: a Double-Blind, Randomized Controlled Phase 3 Trial. Nat. Med. 27 (10), 1752–1760. doi:10.1038/s41591-021-01499-z
La Rosée, P., Horne, A., Hines, M., von Bahr Greenwood, T., Machowicz, R., Berliner, N., et al. (2019). Recommendations for the Management of Hemophagocytic Lymphohistiocytosis in Adults. Blood. 133 (23), 2465–2477. doi:10.1182/blood.2018894618
Ladna, M., Villanueva, F. L., Maharrey, P. B., and Lascano, J. (2021). Post-Transplant Patients With COVID-19 Associated Acute Respiratory Distress Syndrome, a Role for Tociluzumab: A Case Series. Respir. Med. Case Rep. 32, 101319. doi:10.1016/j.rmcr.2020.101319
Leelayuwatanakul, N., Kongpolprom, N., Sriprasart, T., Phoophiboon, V., Thanthitaweewat, V., Thawanaphong, S., et al. (2021). Multimodality Treatment in Immunocompromised Patients With Severe COVID-19: the Role of IL-6 Inhibitor, Intravenous Immunoglobulin, and Haemoperfusion. Respirol Case Rep. 9 (4), e0733. doi:10.1002/rcr2.733
Lescure, F. X., Honda, H., Fowler, R. A., Lazar, J. S., Shi, G., Wung, P., et al. (2021). Sarilumab COVID-19 Global Study Group. Sarilumab in Patients Admitted to Hospital With Severe or Critical COVID-19: a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 9 (5), 522–532. doi:10.1016/S2213-2600(21)00099-0
Li, H., Liu, L., Zhang, D., Xu, J., Dai, H., Tang, N., et al. (2020). SARS-CoV-2 and Viral Sepsis: Observations and Hypotheses. Lancet. 395 (10235), 1517–1520. doi:10.1016/S0140-6736(20)30920-X
Lucas, C., Wong, P., Klein, J., Castro, T. B. R., Silva, J., Sundaram, M., et al. (2020). Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature. 584 (7821), 463–469. doi:10.1038/s41586-020-2588-y
Mady, A., Aletreby, W., Abdulrahman, B., Lhmdi, M., Noor, A. M., Alqahtani, S. A., et al. (2020). Tocilizumab in the Treatment of Rapidly Evolving COVID-19 Pneumonia and Multifaceted Critical Illness: A Retrospective Case Series. Ann. Med. Surg. (Lond). 60, 417–424. doi:10.1016/j.amsu.2020.10.061
Mazankova, L. N., Osmanov, I. M., Samitova, E. R., Malakhov, A. B., Koroid, V. V., Nedostoev, A. A., et al. (2020). A Teenager With a Severe Form of COVID-19. Rossiyskiy Vestnik Perinatologii i Pediatrii. 65 (5), 58–65. doi:10.21508/1027-4065-2020-65-5-58-65
McKenzie, M. G., Lee, Y. M., Mathew, J., Anderson, M., Vo, A. T., Akinyele, S., et al. (2021). Tocilizumab for the Critically Ill With Severe COVID-19: A Community Hospital Case Series. J. Pharm. Pract. 19, 8971900211002353. doi:10.1177/08971900211002353
Menzella, F., Fontana, M., Salvarani, C., Massari, M., Ruggiero, P., Scelfo, C., et al. (2020). Efficacy of Tocilizumab in Patients With COVID-19 ARDS Undergoing Noninvasive Ventilation. Crit. Care. 24 (1), 589. doi:10.1186/s13054-020-03306-6
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Plos Med. 6 (7), e1000097. doi:10.1371/journal.pmed.1000097
Moideen, S., Maheshwari, V., Syam Prakash, K. R., Rajagopal, S., and Sherief, S. H. (2020). Different Treatment Approaches, Clinical Outcome, Effectiveness of the Drug towards Covid 19 With 5 Different Cases: A Case Series. Int. J. Pharm. Sci. Rev. Res. 65 (1), 98–103. doi:10.47583/ijpsrr.2020.v65i01.014
Morillas, J. A., Marco Canosa, F., Srinivas, P., Asadi, T., Calabrese, C., Rajendram, P., et al. (2020). Tocilizumab Therapy in 5 Solid and Composite Tissue Transplant Recipients With Early ARDS Due to SARS-CoV-2. Am. J. Transpl. 20 (11), 3191–3197. doi:10.1111/ajt.16080
National Institutes of Health(NIH) (2017). Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 (Accessed October 17, 2021).
NOS (2020). Abbreviations.com. STANDS4 LLC. Available at: https://www.abbreviations.com/term/1418908 (Accessed October 17, 2021).
Nourié, N., Chamaa, M. A., Mouawad, S., Kotait, M. M., Finianos, S., Azar, H., et al. (2021). Effective Treatment With Tocilizumab in a COVID-19 Patient on Maintenance Hemodialysis: A Case Report. CEN Case Rep. 10 (3), 364–369. doi:10.1007/s13730-021-00577-y
Patel, P. A., Chandrakasan, S., Mickells, G. E., Yildirim, I., Kao, C. M., and Bennett, C. M. (2020). Severe Pediatric COVID-19 Presenting With Respiratory Failure and Severe Thrombocytopenia. Pediatrics. 146 (1), e20201437. doi:10.1542/peds.2020-1437
Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., et al. (2020). Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 71 (15), 762–768. doi:10.1093/cid/ciaa248
Rajendram, P., Sacha, G. L., Mehkri, O., Wang, X., Han, X., Vachharajani, V., et al. (2021). Tocilizumab in Coronavirus Disease 2019-Related Critical Illness: A Propensity Matched Analysis. Crit. Care Explor. 3 (1), e0327. doi:10.1097/CCE.0000000000000327
REMAP-CAP Investigators Gordon, A. C., Mouncey, P. R., Al-Beidh, F., Rowan, K. M., and Nichol, A. D. (2021). Interleukin-6 Receptor Antagonists in Critically Ill Patients With Covid-19. N. Engl. J. Med. 384 (16), 1491–1502. doi:10.1056/NEJMoa2100433
Rice, T. W., Wheeler, A. P., Bernard, G. R., Hayden, D. L., Schoenfeld, D. A., Ware, L. B., et al. (2007). Comparison of the SpO2/FIO2 Ratio and the PaO2/FIO2 Ratio in Patients With Acute Lung Injury or ARDS. Chest. 132 (2), 410–417. doi:10.1378/chest.07-0617
Rudd, K. E., Johnson, S. C., Agesa, K. M., Shackelford, K. A., Tsoi, D., Kievlan, D. R., et al. (2020). Global, Regional, and National Sepsis Incidence and Mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet. 395 (10219), 200–211. doi:10.1016/S0140-6736(19)32989-7
Saffo, Z., Guo, W., Springer, K., Maksimowicz-McKinnon, K., Kak, V., McKinnon, J. E., et al. (2021). The Role of Tocilizumab Therapy in Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2. J. Osteopath Med. 121 (8), 705–714. doi:10.1515/jom-2020-0292
Salvarani, C., Dolci, G., Massari, M., Merlo, D. F., Cavuto, S., Savoldi, L., et al. (2021). Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 181 (1), 24–31. doi:10.1001/jamainternmed.2020.6615
Senegaglia, A. C., Rebelatto, C. L. K., Franck, C. L., Lima, J. S., Boldrini-Leite, L. M., Daga, D. R., et al. (2021). Combined Use of Tocilizumab and Mesenchymal Stromal Cells in the Treatment of Severe Covid-19: Case Report. Cell Transpl. 30, 9636897211021008. doi:10.1177/09636897211021008
Seymour, C. W., Liu, V. X., Iwashyna, T. J., Brunkhorst, F. M., Rea, T. D., Scherag, A., et al. (2016). Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 315 (8), 762–774. doi:10.1001/jama.2016.0288
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 315 (8), 801–810. doi:10.1001/jama.2016.0287
Somers, E. C., Eschenauer, G. A., Troost, J. P., Golob, J. L., Gandhi, T. N., Wang, L., et al. (2021). Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19. Clin. Infect. Dis. 73 (2), e445–e454. doi:10.1093/cid/ciaa954
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 366, l4898. doi:10.1136/bmj.l4898
Thammathiwat, T., Tungsanga, S., Tiankanon, K., Torvorapanit, P., Chumpangern, W., Udomkarnjananun, S., et al. (2021). A Case of Successful Treatment of Severe COVID-19 Pneumonia With Favipiravir and Tocilizumab in post-kidney Transplant Recipient. Transpl. Infect. Dis. 23 (1), e13388. doi:10.1111/tid.13388
Vazquez Guillamet, M. C., Kulkarni, H. S., Montes, K., Samant, M., Shaikh, P. A., Betthauser, K., et al. (2021). Interleukin-6 Trajectory and Secondary Infections in Mechanically Ventilated Patients With Coronavirus Disease 2019 Acute Respiratory Distress Syndrome Treated With Interleukin-6 Receptor Blocker. Crit. Care Explor. 3 (2), e0343. doi:10.1097/CCE.0000000000000343
Wang, Y., Dai, R., Lv, X., Qian, Y., and Zhu, K. (2020). Specific Interleukin-1 Inhibitors, Specific Interleukin-6 Inhibitors and Anti-GM-CSF for COVID-19 (At the Edge of Sepsis): a Living System Review. PROSPERO 2020 CRD42020226545 Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020226545 (Accessed October 17, 2021).
Keywords: specific interleukin-1 inhibitors, specific interleukin-6 inhibitors, GM-CSF blockades, coronavirus disease 2019 (COVID-19), SARS-CoV-2, sepsis
Citation: Wang Y, Zhu K, Dai R, Li R, Li M, Lv X and Yu Q (2022) Specific Interleukin-1 Inhibitors, Specific Interleukin-6 Inhibitors, and GM-CSF Blockades for COVID-19 (at the Edge of Sepsis): A Systematic Review. Front. Pharmacol. 12:804250. doi: 10.3389/fphar.2021.804250
Received: 29 October 2021; Accepted: 23 November 2021;
Published: 21 January 2022.
Edited by:
Yan Kang, Sichuan University, ChinaReviewed by:
Wared Nour-Eldine, Qatar Biomedical Research Institute, QatarPasquale Pagliano, University of Salerno, Italy
Copyright © 2022 Wang, Zhu, Dai, Li, Li, Lv and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Yu, eXVxaWFuQGpsdS5lZHUuY24=
†These authors have contributed equally to this work.
 Ying Wang
Ying Wang Kun Zhu
Kun Zhu Rulin Dai2
Rulin Dai2 Miao Li
Miao Li