Corrigendum: The Protective Effects of Lipid-Lowering Agents on Cardiovascular Disease and Mortality in Maintenance Dialysis Patients: Propensity Score Analysis of a Population-Based Cohort Study
- 1Division of Nephrology, Department of Internal Medicine, Shin-Kong Wu Ho-Su Memorial Hospital, Taipei City, Taiwan
- 2Department of Medicine, School of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
- 3Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, New Taipei City, Taiwan
- 4AI Development Center, Fu Jen Catholic University, New Taipei City, Taiwan
- 5Division of Nephrology, Department of Internal Medicine, Hsin-Jen Hospital, New Taipei City, Taiwan
Lipid-lowering agents display limited benefits on cardiovascular diseases and mortality in patients undergoing dialysis. Therefore, they are not routinely recommended for dialysis patients. The aim of this study was to assess the effects of lipid-lowering agents on clinical outcomes in dialysis patients on the basis of real-world evidence. This research used Taiwan’s National Health Insurance Research Database to identify dialysis patients from January 2009 to December 2015; patients were then categorized into a case group treated with lipid-lowering agents (n = 3,933) and a control group without lipid-lowering agents (n = 24,267). Patients were matched by age, sex, and comorbidities in a 1:1 ratio. This study used the Cox regression model to estimate the hazard ratios (HRs) for mortality and major adverse cardiovascular events (MACEs) for events recorded until December 2017. During a mean follow-up period of approximately 3.1 years, 1726 [43.9%, incidence 0.123/person-year (PY)] deaths and 598 (15.2%, incidence 0.047/PY) MACEs occurred in the case group and 2031 (51.6%, incidence 0.153/PY) deaths and 649 (16.5% incidence 0.055/PY) MACEs occurred in the control group. In the multivariable analysis of the Cox regression model, lipid-lowering agent users showed a significantly lower risk of death [HR: 0.75; 95% confidence interval (CI): 0.70–0.80] and MACEs (HR: 0.88; 95% CI: 0.78–0.98) than lipid-lowering agent non-users. Moreover, the survival benefit of lipid-lowering agents was significant across most subgroups. Dialysis patients treated with lipid-lowering agents display a 25 and 12% reduction in their risk of mortality and MACEs, respectively. Therefore, lipid-lowering agents might be considered when treating dialysis patients with hyperlipidemia.
Introduction
Patients with chronic kidney disease (CKD) are at a high risk of morbidity and mortality owing to cardiovascular disease (CVD) (Jankowski et al., 2021); the risk of CVD starts from an early CKD stage and increases along with a decline in the estimated glomerular filtration rate (Yu et al., 2021). While entering into dialysis-dependent end-stage renal disease (ESRD), the risk of CVD significantly increases compared with that in the general population, accounting for >50% of the mortality (Chronic Kidney Disease Prognosis Consortium et al., 2010; Sharma and Sarnak, 2017; Johansen et al., 2021).
Dyslipidemia is a traditional risk factor contributing to CVD, and its correction has been considered the mainstay of treatment in high-risk groups (Lee et al., 2017; Hedayatnia et al., 2020). The first large-scale study investigating the effects of lipid-lowering agents was the Scandinavian Simvastatin Survival Study (4S), which demonstrated that this statin reduced cardiovascular events and mortality in patients with coronary artery disease (Pedersen et al., 1994). Recently, the benefit of statin therapy in CVD is well studied, and it is recommended for the primary prevention of CVD in those at a high risk of atherosclerosis (Ziaeian and Fonarow, 2017; Arnett et al., 2019; Byrne et al., 2019). Furthermore, some studies have suggested that achieving a very low level of low-density lipoprotein cholesterol (LDL-C) (<30 mg/dl) using high-intensity statin was safe and beneficial for the very high–risk population (Nicholls et al., 2016; Giugliano et al., 2017a; Giugliano et al., 2017b). However, the mechanisms and impacts of dyslipidemia remain unclear in patients with ESRD undergoing dialysis. The most widely accepted hypothesis is the carbamylation of LDL-C in the uremia stage (Kraus and Kraus, 2001; Ok et al., 2005). Carbamylated LDL has been shown to be a potent atherogenic factor in vitro and in vivo. Through endothelial cell damage, the degree of vascular smooth muscle cell proliferation and endothelial cell apoptosis was proportional to the degree of LDL carbamylation and subsequently induced accelerated atherosclerosis (Ok et al., 2005; Apostolov et al., 2010).
Because dyslipidemia is prevalent in patients with ESRD undergoing dialysis (Qunibi, 2015), two large randomized controlled trials have been conducted to investigate its correction by using lipid-lowering agents in merely dialysis patients. The Die Deutsche Diabetes Dialyze Studie (4D study) and A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events (AURORA) showed no benefit of statin use in dialysis patients (Wanner et al., 2005; Fellström et al., 2009). Moreover, the Study of Heart and Renal Protection (SHARP) including both non–dialysis-dependent CKD and dialysis patients showed that statin therapy did not significantly reduce the major adverse cardiovascular events (MACEs) in the subgroup of dialysis patients (hemodialysis and peritoneal dialysis) (Baigent et al., 2011). Therefore, lipid-lowering agents have not been routinely recommended for dialysis patients with hyperlipidemia owing to the aforementioned results in the current guideline (Mach et al., 2020).
Conversely, some previous observational studies have reported that lipid-lowering therapy is associated with reduced mortality in dialysis patients (Seliger et al., 2002; Mason et al., 2005; Jung et al., 2020). A recent study using a population-based nationwide dataset in Korea showed that statins combined with ezetimibe significantly lowered the all-cause mortality in adult patients undergoing maintenance hemodialysis (Jung et al., 2020), and the finding of which suggested that the application of lipid-lowering agents in the dialysis population might be re-evaluated. Moreover, hypertriglyceridemia is commonly observed in dialysis patients, but the therapeutic benefit of using the fenofibrate to lower triglyceride levels is unclear in this group (Hung et al., 2009; Mikolasevic et al., 2017). However, some studies had reported that the use of fenofibrate can provide cardiovascular and mortality benefits in non-dialysis CKD patients (Ting et al., 2012; Yen et al., 2021). Accordingly, we conducted this study to investigate whether the risk of MACEs and all-cause mortality could be reduced by lipid-lowering agents (statin and fenofibrate) in dialysis patients, including hemodialysis and peritoneal dialysis, on the basis of a real-world database.
Materials and Methods
Data Sources and Research Samples
In this retrospective study, the source of information was the National Health Insurance Research Database (NHIRD). The NHIRD was established in 1996, including coverage for nearly 99% of beneficiaries who were certified citizens in Taiwan since 1998, and it provides diverse medical information, with inpatient and outpatient demographics, clinical records, diagnosis, procedure codes, and medical expenses (Hsieh et al., 2019). The diagnosis code was based on the International Classification of Diseases (9th and 10th) Clinical Modification (ICD-9-CM; ICD-10-CM) codes. The benefits of using NHIRD include the access to long-term follow-up data.
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Fu Jen Catholic University (IRB approval number: No. C104016). All claim records were anonymized before analysis, and the requirement of written informed consent was waived by the Institutional Review Board of Fu Jen Catholic University.
Study Population and Exclusion Criteria
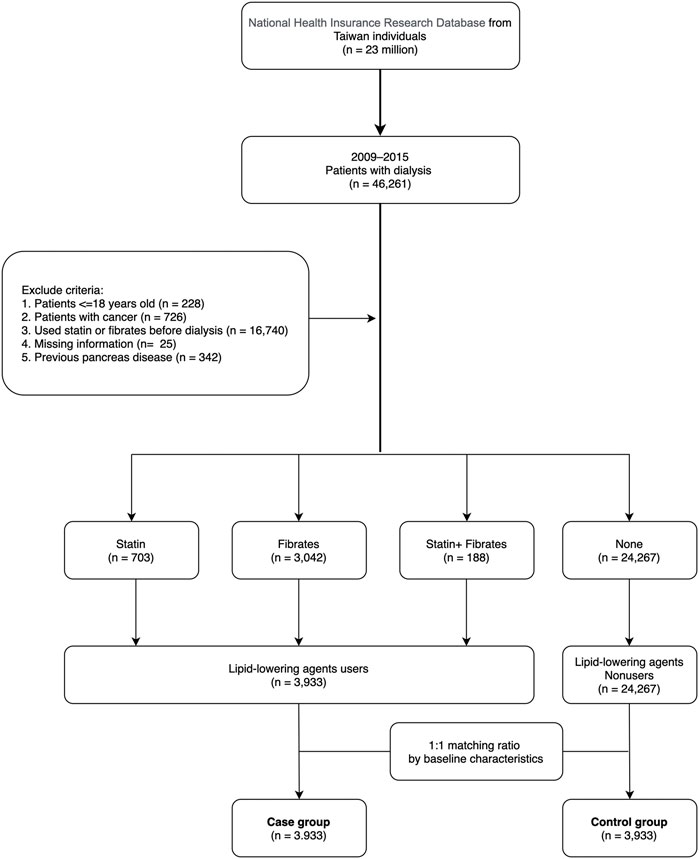
The data for this study were collected between 1 January 2009 and 31 December 2015. Patients undergoing peritoneal dialysis or hemodialysis for more than 3 months were included (n = 46,261). The codes of dialysis procedures are shown in Supplementary Table S1. Exclusion criteria were age less than 18 years (n = 228), missing information (n = 25), cancer diagnosis (n = 726), use of statin or fibrates before entering the dialysis-dependent CKD stage (n = 16,740), and having multiple pancreatic diseases (acute pancreatitis, complications pancreas transplantation, pancreas damage of head, chronic inflammatory pancreatitis, and defect of pancreas, n = 342) before the index date. A total of 28,200 patients were categorized into case and control groups, and cases using lipid-lowering agents (including statin and fibrate for 2 months) (n = 3,933) were matched with controls (n = 24,267) who did not use lipid-lowering agents within 6 months after entering the dialysis-dependent CKD stage. To reduce bias in our research, we used a 1:1 ratio propensity score matching to the baseline information including sex, age, baseline comorbidities, Charlson comorbidity index score (CCIS) (Charlson et al., 1987), and hospital area (Figure 1).
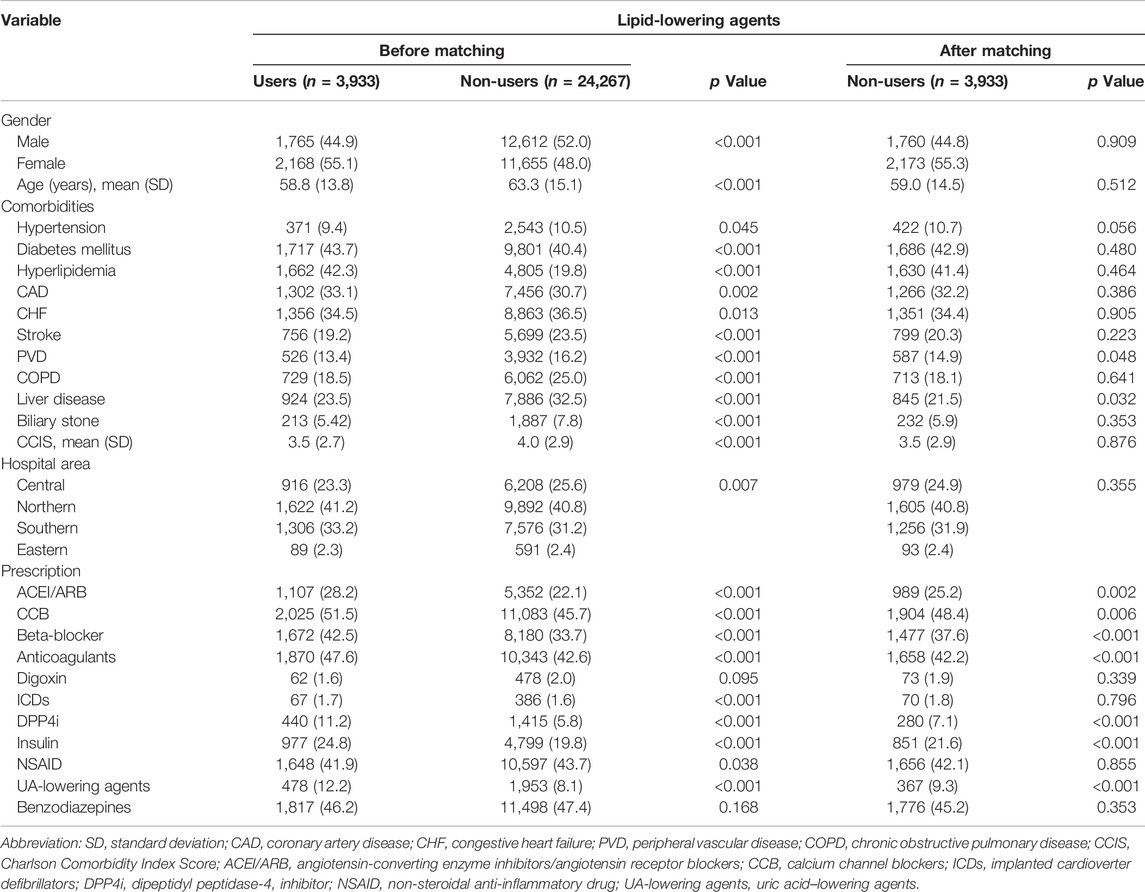
Baseline comorbidities, namely, hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, chronic heart failure, stroke, peripheral artery occlusion disease, chronic obstructive pulmonary disease, liver disease, and biliary stone, were defined as diseases when they had at least three outpatient diagnoses or one inpatient diagnosis within 1 year before the index date (Supplementary Table S1). The usage of other drugs, namely, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB), calcium channel blockers (CCB), beta-blockers, anticoagulants, digoxin, dipeptidyl peptidase-4 inhibitor (DDP4i), insulin, nonsteroidal anti-inflammatory drugs (NSAIDs), uric acid–lowering agents, and benzodiazepines was considered when the patients used the drug for at least 2 months within 6 months before the index date. The prescription of implanted cardioverter defibrillators (ICDs) was also collected before the index date (Supplementary Table S2).
Clinical Outcomes
The index date for users was the date after using lipid-lowering agents for 2 months and that for non-users was the date after entering dialysis for 6 months. Patients were followed up until the occurrence of clinical events including all-cause death, ischemic stroke, and MACEs (the composite cardiovascular events: myocardial infarction, cerebrovascular disease, heart failure, and arrhythmia), or the end of the research (2017-12-31), whichever occurred first. The date of death was collected by linking to the database of the National Register of Deaths. Stroke and MACEs were mainly diagnosed according to the ICD code (Supplementary Table S1).
Statistical Analysis
Continuous data are expressed as mean ± standard deviation and categorical data as proportions (%). Categorical variables were analyzed using chi-squared tests. Continuous variables were analyzed using paired t-tests to validate demographic characteristics, including sex and age, and baseline comorbidities between case and control groups. The probability of propensity score was defined by covariates and estimated by using a logistic or probit regression model. There are two important points in propensity matching. First, the researchers have to make sure the matching ratio between the explored and unexplored groups. Second, researchers must determine which baseline variables were the confounding factors and eliminate them (Austin, 2008; Austin and Cafri, 2020). This research used propensity score matching with a 1:1 ratio and used baseline variables to eliminate the discrepancy between lipid-lowering agent users and non-users (Austin, 2011). An intention-to-treatment basis was adopted according to the patients’ initial lipid-lowering agent use status without consideration of the subsequent regimen change.
Event-free survival curves were created using the Kaplan–Meier method and tested with the log-rank test. Moreover, the Cox proportional hazard model was adopted to estimate the hazard ratio (HR) with 95% confidence intervals (CI) of clinical outcomes as a function of lipid-lowering agent use. The assumption of proportionality was not violated by testing for an interaction between time and the variables. We also performed a subgroup analysis stratified by comorbidities. All statistical analyses and figures were created using 9.4 version of SAS (SAS Institute, Cary, NC, United States) software. For all tests, two-tailed p-values of <0.05 were considered statistically significant.
Results
Patient Characteristics
We enrolled 28,200 dialysis patients in the present study (Figure 1). Among them, 3,933 patients (13.9%) used lipid-lowering agents for at least 2 months after entering dialysis. The mean age of lipid-lowering agent users (case group) was 58.8 years, of whom 44.9% were men, 9.4% had hypertension, 43.7% had diabetes mellitus, 33.1% had coronary artery disease, and 19.2% had a stroke history. Moreover, 41.2% of patients were from northern Taiwan and 33.2% were from southern Taiwan (Table 1). Before matching, lipid-lowering agent users had lesser comorbidities than lipid-lowering agent non-users. However, the baseline demographic data and comorbidities between lipid-lowering agent users and non-users showed no significant difference after matching. For baseline medical prescriptions after matching, lipid-lowering agent users had a higher prescription rate in ACEI/ARB, CCB, beta-blockers, anticoagulants, DPP4i, insulin, and uric acid–lowering agents than in lipid-lowering agent non-users after matching, as shown in Table 1.
In the group of lipid-lowering agents users, the numbers of patients who did not use lipid-lowering agents after the index day were 157 (4%) within 1 year and 297 (7.5%) within 2 years. However, the non-users of lipid-lowering agents were kept free from the lipid-lowering agents for 1 and 2 years after the index day.
Benefit of Using Lipid-Lowering Agents in Dialysis Patients
The total follow-up summation is 86,627 person-year (PY) during the study period (Table 2). A total of 16,866 patients (59.8%) died, 4,512 patients (16%) had a new-onset MACE, and 954 patients (3.4%) experienced the ischemic stroke. Before matching, the lipid-lowering agent users had a better incidence rate than non-users in mortality (0.123/PY vs 0.208/PY) and MACEs (0.47/PY vs 0.60/PY) but not in ischemic stroke (0.01 vs 0.01%). Moreover, the Kaplan–Meier event-free curves for all-cause mortality (Figure 2A) and MACEs (Figure 2B) among lipid-lowering agents users compared with non-users were both significant (p < 0.05) after matching. This finding indicated that the use of lipid-lowering agents was associated with a lower risk of mortality and MACEs.
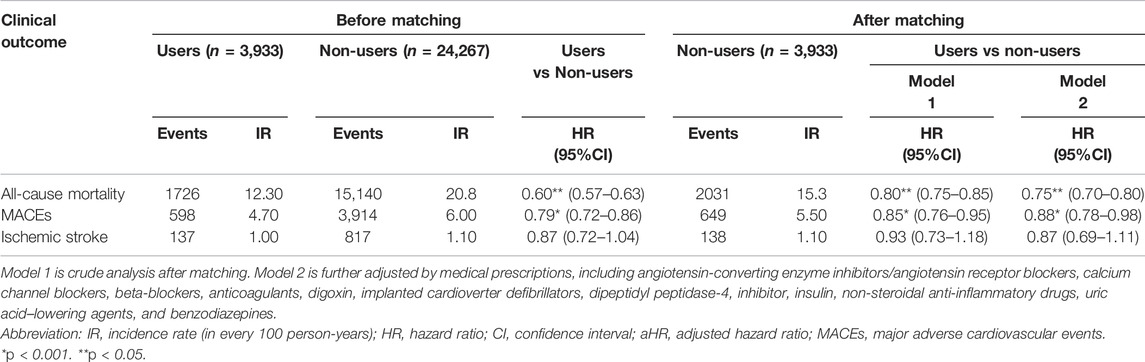
TABLE 2. Risk of clinical outcomes in patients with dialysis comparing lipid-lowering agents users vs. non-users.
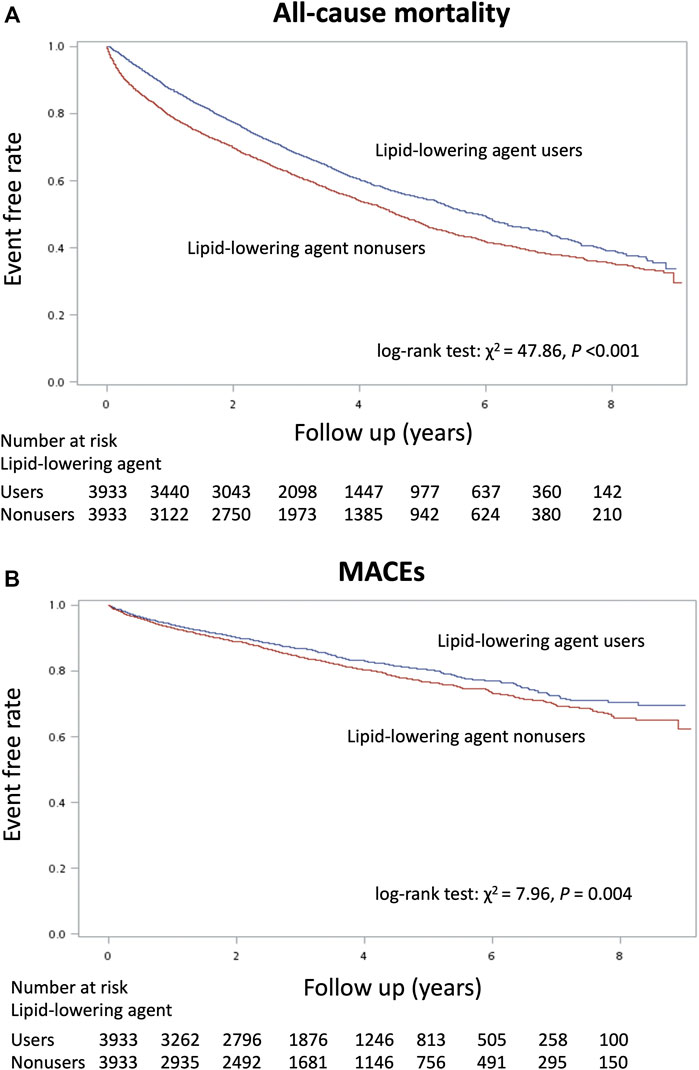
FIGURE 2. Kaplan–Meier cumulative event-free plots of (A) mortality and (B) major adverse cardiovascular events (MACEs) in the study population according to whether the lipid-lowering agents were used or not.
As shown in Table 2, we found that treatment with lipid-lowering agents in dialysis patients significantly decreased their risk of mortality (HR, 0.60; 95% CI, 0.57–0.63) and MACEs (HR, 0.79; 95% CI, 0.72–0.86) before matching, and the beneficial effects of lipid-lowering agents were maintained after propensity score matching [HR: 0.80 (0.75–0.85); 0.85 (0.76–0.95), respectively]. After further adjusting the medical prescriptions, such significance was also kept [HR: 0.75 (0.70–0.80) for mortality; 0.88 (0.78–0.98) for MACEs]. The risk of ischemic stroke event had no significant difference between the matching groups [HR: 0.93 (0.73–1.18) in the crude model; 0.87 (0.69–1.11) in the multivariable adjusting model].
In Table 3, it shows the events of composites of MACEs between lipid-lowering agents users and non-users during the observation period, of which myocardial infarction had a significant difference between groups (3.5 vs 2.4%, p = 0.003).

TABLE 3. Components of MACEs between patients with dialysis comparing lipid-lowering agents users vs. non-users.
Subgroup Analysis
We conducted a series of stratified analyses to test the reliability of our results (Figure 3). The decreased HRs of mortality among dialysis patients in favor of lipid-lowering agents were consistent across all patient subgroups. Patients of young age, those with diabetes, and those without a history of stroke and congestive heart failure showed a significantly lower risk of MACEs.
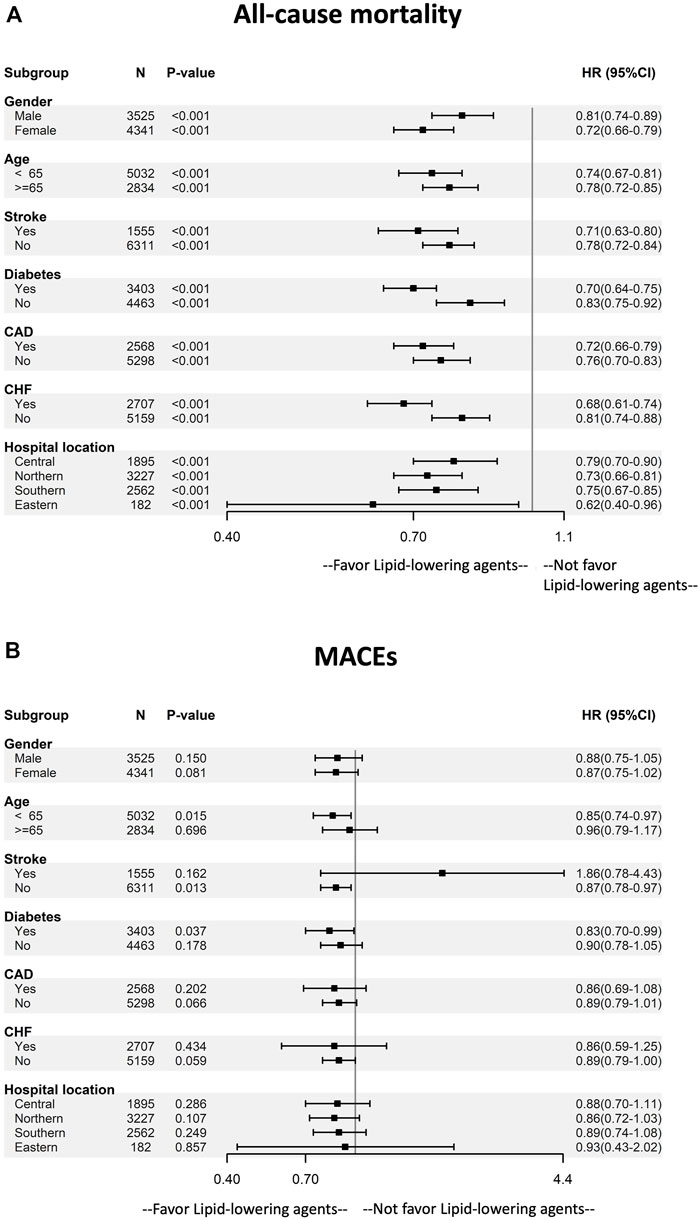
FIGURE 3. Subgroup analysis of the effect of lipid-lowering agents use on (A) mortality and (B) MACEs by baseline characteristics. The multivariable adjusting model was the same as model 2 in Table 2. Abbreviation: CAD, coronary artery disease; CHF, congestive heart disease; MACEs, major adverse cardiovascular events.
Discussion
We have provided real-world evidence on the effects of lipid-lowering agents in reducing cardiovascular events and mortality in dialysis patients using an NHIRD dataset. This finding is crucial to disclose that dyslipidemia correction still plays a role in the improvement of CVD and mortality in dialysis patients, which belong to the high-risk CVD group. Our study extends the current knowledge about the use of lipid-lowering agents in the dialysis population who were eligible to use lipid-lowering agents which may be associated with the reduced mortality and MACEs, and the risk reduction of MACEs may be attributed to the prevention of myocardial infarction. Therefore, we suggested that lipid-lowering agents (stain or fenofibrate) may be applied to dialysis patients with dyslipidemia.
There has been a longstanding debate regarding the use of lipid-lowering agents in chronic dialysis patients. However, this question became unequivocal since the outcomes of the 4D, AURORA, and SHARP trials revealed negative results in the dialysis population without overt CAD (Wanner et al., 2005; Fellström et al., 2009; Baigent et al., 2011). In the 4D study, diabetes patients undergoing chronic hemodialysis were randomly assigned to receive a statin (atorvastatin) or a placebo. In the statin-treated group, LDL cholesterol levels significantly reduced from baseline since the first 4 weeks and were maintained for 5 years. This reduction in LDL cholesterol did not offer benefit in reducing MACEs compared with placebo, suggesting that it was too late to use statin in the dialysis population (Wanner et al., 2005). Regarding the AURORA study conducted in hemodialysis patients with or without diabetes, similar results were noted between the statin (rosuvastatin) and placebo groups (Fellström et al., 2009). Moreover, the SHARP study showed that after an average follow-up period of 4.9 years, a significant reduction in major atherosclerotic events was observed in the recruited participants. However, this positive finding was not significant in the subgroup based on dialysis at enrollment (Baigent et al., 2011). Moreover, a meta-analysis combined these three studies and showed that statin use had a significant benefit on nonfatal atherosclerotic CV events [odds ratio (OR), 0.89, 95% CI, 0.8–0.99] but not on coronary events (OR, 0.97; 95% CI, 0.88–1.06), ischemic stroke (OR, 1.29, 95% CI, 0.72–2.31), and all-cause mortality (OR, 0.88; 95% CI, 0.74–1.04) (Green et al., 2013).
Because the effects of lipid-lowering agents in dialysis patients were not as expected for the general population in these large randomized controlled trials (RCTs), there are several possible explanations for these negative results. The first one is the complexity of CVD in patients undergoing dialysis. Apart from traditional risk factors, several types of pathogenesis contribute to atherosclerosis and the decline in heart function. The so-called non-traditional factors include mineral and bone metabolism disorder, oxidative stress, and anemia (Yao et al., 2004). Uremic toxin, p-cresol, and indoxyl sulfate have been linked with atherosclerosis and vascular calcification (Barreto et al., 2009; Opdebeeck et al., 2020). In addition, the increased level of fibroblast growth factor 23 as an indicator of renal function decline has shown its deleterious effects on cardiomyocytes (Gutiérrez et al., 2008). These factors render the association of a direct causal relationship between LDL cholesterol and atheroma less relevant (Mihaylova et al., 2012; Fulcher et al., 2015). In addition, there are aspects of CVD in ESRD, such as arrhythmia and heart failure, that affect non-atheromatous pathogenesis (Methven et al., 2017; Saran et al., 2017). Intervention in only one dimension of lipid control will not solve these issues as noted in these large RCT studies.
Despite the negative results from RCT trials, some studies found that ESRD groups undergoing dialysis could benefit from lipid-lowering agents. In the post hoc analysis of the 4D study, statin still lowered the risk of cardiovascular events when the LDL level was >145 mg/dl (März et al., 2011). This suggested that in dialysis patients with a severe degree of dyslipidemia, aggressive lipid-lowering therapy can also reduce cardiovascular mortality in a long-term observational period. Moreover, statin treatment in dialysis patients after acute myocardial infarction improves overall mortality (Chung et al., 2017). Statin treatment significantly decreases overall mortality in ESRD patients with acute myocardial infarction compared with the non-statin group. This is more prominent in the cardiac shock patient subgroup. These results are compatible with those from other studies, supporting a measurable benefit from statins in ESRD patients (Chung et al., 2017).
In our study using Taiwan’s NHIRD, chronic dialysis patients using lipid-lowering agents had a better outcome in MACEs, especially the composite of myocardial infarction, and all-cause mortality, suggesting that such agents may be helpful in this population. However, the reasons for the discordance between RCTs and the observational study findings about the use of lipid-lowering agents in dialysis patients are still undetermined. We proposed some possible explanations. In our previous single-center study cohort, we observed an inverted U-curve association between serum indoxyl sulfate levels and cardiovascular events in chronic hemodialysis patients (Tsai et al., 2021). The reported data indicate that although indoxyl sulfate, a recognized uremic toxin, may contribute to atherosclerosis and cardiovascular pathogenesis in vivo and in vitro, its higher levels led to a better cardiovascular outcome. In our observation, the underlying nutritional status may explain this reverse epidemiology (Zha and Qian, 2017; Hanna et al., 2020). Regarding the lipid-lowering issue, only well-nourished patients in chronic dialysis would be suitable to receive such agents. This may be a reasonable explanation for this finding in our population-based cohort study. Also, the other explanation is that RCTs have a good enteral validity but a relatively low external validity and generalization due to their highly selective population and tightly control setting. Indeed, in real-world practice, patients have higher heterogenicity than those in RCTs. Therefore, a discrepancy exists between RCTs and the studies using real-word data (Kim et al., 2018).
Our study had several strengths. First, we used data from a nationwide database, meaning that the study’s results can be generalized. Second, the sample size and observation time were adequate to obtain sufficient inferences. Despite its strengths, our study had several potential limitations. First, NHIRD, an original claim database for reimbursement, does not offer clinical information such as biochemical data, inflammatory burden, blood pressure, and body characteristics (weight, height, waist circumference, and body fat percentage) which might have an impact on the development of MACEs (Sardu et al., 2019; Sardu et al., 2021a; Sardu et al., 2021b). However, our study’s large number size with the method of propensity score matching can alleviate this bias. Second, we cannot determine whether the study patients showed regular drug compliance because exposure to lipid-lowering agents was based on prescription information only. Third, this was not a RCT; thus, the unbalanced baseline between the two groups was a major concern, which might induce a bias of confounding by indication (Kyriacou and Lewis, 2016). However, we used the propensity score matching to reduce this bias as the method of propensity score matching is well developed to balance the underlying difference between groups. Also, we further adjusted the medications and ICDs use, the devices could significantly ameliorate clinical cardiovascular outcomes (Sardu et al., 2017), and the significant results in our study were kept.
In conclusion, although lipid-lowering agents are not recommended for routine use in dialysis patients with hyperlipidemia according to the clinical guideline, our results demonstrated significant benefits of the use of lipid-lowering agents on clinical outcomes, including MACEs, and all-cause death, in such a population.
Data Availability Statement
The datasets presented in this article are not readily available because all claim records were anonymized before analysis. Thus, Fu Jen Catholic University Ethics Institutional Review Board was exempted from a full ethical review, and the requirement to obtain informed consent was waived. Requests to access the datasets should be directed to https://dep.mohw.gov.tw/dos/np-1714-113.html.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Y-WF, MC, and M-HT conceived and designed the experiments. MC, H-HL, and M-HT performed the experiments. Y-CH and MC analyzed the data. Contributed reagents/materials/analysis tools. All authors wrote the manuscript. M-HT and Y-WF approved the manuscript. M-HT and MC contributed equally to this work.
Funding
Shin Kong Wu Ho-Su Memorial Hospital sponsored this study (109-SKH-FJU-05).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.804000/full#supplementary-material
References
Apostolov, E. O., Ray, D., Savenka, A. V., Shah, S. V., and Basnakian, A. G. (2010). Chronic Uremia Stimulates LDL Carbamylation and Atherosclerosis. J. Am. Soc. Nephrol. 21 (11), 1852–1857. doi:10.1681/asn.2010040365
Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., et al. (2019). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 74 (11), 1376–1414. doi:10.1016/j.jacc.2019.03.009
Austin, P. C. (2008). A Critical Appraisal of Propensity-Score Matching in the Medical Literature between 1996 and 2003. Stat. Med. 27 (12), 2037–2049. doi:10.1002/sim.3150
Austin, P. C. (2011). An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav. Res. 46 (3), 399–424. doi:10.1080/00273171.2011.568786
Austin, P. C., and Cafri, G. (2020). Variance Estimation when Using Propensity-Score Matching with Replacement with Survival or Time-To-Event Outcomes. Stat. Med. 39 (11), 1623–1640. doi:10.1002/sim.8502
Baigent, C., Landray, M. J., Reith, C., Emberson, J., Wheeler, D. C., Tomson, C., et al. (2011). The Effects of Lowering LDL Cholesterol with Simvastatin Plus Ezetimibe in Patients with Chronic Kidney Disease (Study of Heart and Renal Protection): a Randomised Placebo-Controlled Trial. Lancet 377 (9784), 2181–2192. doi:10.1016/S0140-6736(11)60739-3
Barreto, F. C., Barreto, D. V., Liabeuf, S., Meert, N., Glorieux, G., Temmar, M., et al. (2009). Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 4 (10), 1551–1558. doi:10.2215/cjn.03980609
Byrne, P., Cullinan, J., Smith, A., and Smith, S. M. (2019). Statins for the Primary Prevention of Cardiovascular Disease: an Overview of Systematic Reviews. BMJ Open 9 (4), e023085. doi:10.1136/bmjopen-2018-023085
Charlson, M. E., Pompei, P., Ales, K. L., and MacKenzie, C. R. (1987). A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 40 (5), 373–383. doi:10.1016/0021-9681(87)90171-8
Chronic Kidney Disease Prognosis Consortium, , Matsushita, K., van der Velde, M., Astor, B. C., Woodward, M., Levey, A. S., et al. (2010). Association of Estimated Glomerular Filtration Rate and Albuminuria with All-Cause and Cardiovascular Mortality in General Population Cohorts: a Collaborative Meta-Analysis. Lancet 375 (9731), 2073–2081. doi:10.1016/S0140-6736(10)60674-5
Chung, C. M., Lin, M. S., Chang, C. H., Cheng, H. W., Chang, S. T., Wang, P. C., et al. (2017). Moderate to High Intensity Statin in Dialysis Patients after Acute Myocardial Infarction: A National Cohort Study in Asia. Atherosclerosis 267, 158–166. doi:10.1016/j.atherosclerosis.2017.09.018
Fellström, B. C., Jardine, A. G., Schmieder, R. E., Holdaas, H., Bannister, K., Beutler, J., et al. (2009). Rosuvastatin and Cardiovascular Events in Patients Undergoing Hemodialysis. N. Engl. J. Med. 360 (14), 1395–1407. doi:10.1056/NEJMoa0810177
Fulcher, J., Fulcher, J., O'Connell, R., Voysey, M., Emberson, J., Blackwell, L., et al. (2015). Efficacy and Safety of LDL-Lowering Therapy Among Men and Women: Meta-Analysis of Individual Data from 174,000 Participants in 27 Randomised Trials. Lancet 385 (9976), 1397–1405. doi:10.1016/s0140-6736(14)61368-4
Giugliano, R. P., Pedersen, T. R., Park, J. G., De Ferrari, G. M., Gaciong, Z. A., Ceska, R., et al. (2017a). Clinical Efficacy and Safety of Achieving Very Low LDL-Cholesterol Concentrations with the PCSK9 Inhibitor Evolocumab: a Prespecified Secondary Analysis of the FOURIER Trial. Lancet 390 (10106), 1962–1971. doi:10.1016/S0140-6736(17)32290-0
Giugliano, R. P., Wiviott, S. D., Blazing, M. A., De Ferrari, G. M., Park, J. G., Murphy, S. A., et al. (2017b). Long-term Safety and Efficacy of Achieving Very Low Levels of Low-Density Lipoprotein Cholesterol : A Prespecified Analysis of the IMPROVE-IT Trial. JAMA Cardiol. 2 (5), 547–555. doi:10.1001/jamacardio.2017.0083
Green, D., Ritchie, J. P., and Kalra, P. A. (2013). Meta-analysis of Lipid-Lowering Therapy in Maintenance Dialysis Patients. Nephron Clin. Pract. 124 (3-4), 209–217. doi:10.1159/000357676
Gutiérrez, O. M., Mannstadt, M., Isakova, T., Rauh-Hain, J. A., Tamez, H., Shah, A., et al. (2008). Fibroblast Growth Factor 23 and Mortality Among Patients Undergoing Hemodialysis. N. Engl. J. Med. 359 (6), 584–592. doi:10.1056/NEJMoa0706130
Hanna, R. M., Ghobry, L., Wassef, O., Rhee, C. M., and Kalantar-Zadeh, K. (2020). A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 49 (1-2), 202–211. doi:10.1159/000504240
Hedayatnia, M., Asadi, Z., Zare-Feyzabadi, R., Yaghooti-Khorasani, M., Ghazizadeh, H., Ghaffarian-Zirak, R., et al. (2020). Dyslipidemia and Cardiovascular Disease Risk Among the MASHAD Study Population. Lipids Health Dis. 19 (1), 42. doi:10.1186/s12944-020-01204-y
Hsieh, C. Y., Su, C. C., Shao, S. C., Sung, S. F., Lin, S. J., Kao Yang, Y. H., et al. (2019). Taiwan's National Health Insurance Research Database: Past and Future. Clin. Epidemiol. 11, 349–358. doi:10.2147/CLEP.S196293
Hung, S. Y., Liou, H. H., Ger, L. P., Chen, L. K., Liu, M. C., Chung, H. M., et al. (2009). Clustering of Unconventional Cardiovascular Risk Factors Among Taiwanese Hemodialysis Patients. Am. J. Nephrol. 30 (3), 222–231. doi:10.1159/000218105
Jankowski, J., Floege, J., Fliser, D., Böhm, M. N., and Marx, N. (2021). Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 143 (11), 1157–1172. doi:10.1161/CIRCULATIONAHA.120.050686
Johansen, K. L., Chertow, G. M., Foley, R. N., Gilbertson, D. T., Herzog, C. A., Ishani, A., et al. (2021). US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 77 (4 Suppl. 1), A7–A8. doi:10.1053/j.ajkd.2021.01.002
Jung, J., Bae, G. H., Kang, M., Kim, S. W., and Lee, D. H. (2020). Statins and All-Cause Mortality in Patients Undergoing Hemodialysis. J. Am. Heart Assoc. 9 (5), e014840. doi:10.1161/JAHA.119.014840
Kim, H. S., Lee, S., and Kim, J. H. (2018). Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J. Korean Med. Sci. 33 (34), e213. doi:10.3346/jkms.2018.33.e213
Kraus, L. M., and Kraus, A. P. (2001). Carbamoylation of Amino Acids and Proteins in Uremia. Kidney Int. Suppl. 78, S102–S107. doi:10.1046/j.1523-1755.2001.59780102.x
Kyriacou, D. N., and Lewis, R. J. (2016). Confounding by Indication in Clinical Research. JAMA 316 (17), 1818–1819. doi:10.1001/jama.2016.16435
Lee, J. S., Chang, P. Y., Zhang, Y., Kizer, J. R., Best, L. G., and Howard, B. V. (2017). Triglyceride and HDL-C Dyslipidemia and Risks of Coronary Heart Disease and Ischemic Stroke by Glycemic Dysregulation Status: The Strong Heart Study. Diabetes Care 40 (4), 529–537. doi:10.2337/dc16-1958
Mach, F., Baigent, C., Catapano, A. L., Koskinas, K. C., Casula, M., Badimon, L., et al. (2020). 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 41 (1), 111–188. doi:10.1093/eurheartj/ehz455
März, W., Genser, B., Drechsler, C., Krane, V., Grammer, T. B., Ritz, E., et al. (2011). Atorvastatin and Low-Density Lipoprotein Cholesterol in Type 2 Diabetes Mellitus Patients on Hemodialysis. Clin. J. Am. Soc. Nephrol. 6 (6), 1316–1325. doi:10.2215/cjn.09121010
Mason, N. A., Bailie, G. R., Satayathum, S., Bragg-Gresham, J. L., Akiba, T., Akizawa, T., et al. (2005). HMG-coenzyme a Reductase Inhibitor Use Is Associated with Mortality Reduction in Hemodialysis Patients. Am. J. Kidney Dis. 45 (1), 119–126. doi:10.1053/j.ajkd.2004.09.025
Methven, S., Steenkamp, R., and Fraser, S. (2017). UK Renal Registry 19th Annual Report: Chapter 5 Survival and Causes of Death in UK Adult Patients on Renal Replacement Therapy in 2015: National and Centre-specific Analyses. Nephron 137 (Suppl. 1), 117–150. doi:10.1159/000481367
Mihaylova, B., Mihaylova, B., Emberson, J., Blackwell, L., Keech, A., Simes, J., et al. (2012). The Effects of Lowering LDL Cholesterol with Statin Therapy in People at Low Risk of Vascular Disease: Meta-Analysis of Individual Data from 27 Randomised Trials. Lancet 380 (9841), 581–590. doi:10.1016/s0140-6736(12)60367-5
Mikolasevic, I., Žutelija, M., Mavrinac, V., and Orlic, L. (2017). Dyslipidemia in Patients with Chronic Kidney Disease: Etiology and Management. Int. J. Nephrol. Renovasc Dis. 10, 35–45. doi:10.2147/IJNRD.S101808
Nicholls, S. J., Puri, R., Anderson, T., Ballantyne, C. M., Cho, L., Kastelein, J. J., et al. (2016). Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 316 (22), 2373–2384. doi:10.1001/jama.2016.16951
Ok, E., Basnakian, A. G., Apostolov, E. O., Barri, Y. M., and Shah, S. V. (2005). Carbamylated Low-Density Lipoprotein Induces Death of Endothelial Cells: a Link to Atherosclerosis in Patients with Kidney Disease. Kidney Int. 68 (1), 173–178. doi:10.1111/j.1523-1755.2005.00391.x
Opdebeeck, B., D'Haese, P. C., and Verhulst, A. (2020). Molecular and Cellular Mechanisms that Induce Arterial Calcification by Indoxyl Sulfate and P-Cresyl Sulfate. Toxins (Basel) 12 (1), 58. doi:10.3390/toxins12010058
Pedersen, T. R., Kjekshus, J., Berg, K., Haghfelt, T., Fargeman, O., Thorgeirsson, G., et al. (1994). Randomised Trial of Cholesterol Lowering in 4444 Patients with Coronary Heart Disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344 (8934), 1383–1389.
Qunibi, W. Y. (2015). Dyslipidemia in Dialysis Patients. Semin. Dial. 28 (4), 345–353. doi:10.1111/sdi.12375
Saran, R., Robinson, B., Abbott, K. C., Agodoa, L. Y., Albertus, P., Ayanian, J., et al. (2017). US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 69 (3 Suppl. 1), A7–A8. doi:10.1053/j.ajkd.2016.12.004
Sardu, C., Barbieri, M., Santamaria, M., Giordano, V., Sacra, C., Paolisso, P., et al. (2017). Multipolar Pacing by Cardiac Resynchronization Therapy with a Defibrillators Treatment in Type 2 Diabetes Mellitus Failing Heart Patients: Impact on Responders Rate, and Clinical Outcomes. Cardiovasc. Diabetol. 16 (1), 75. doi:10.1186/s12933-017-0554-2
Sardu, C., D'Onofrio, N., Torella, M., Portoghese, M., Loreni, F., Mureddu, S., et al. (2019). Pericoronary Fat Inflammation and Major Adverse Cardiac Events (MACE) in Prediabetic Patients with Acute Myocardial Infarction: Effects of Metformin. Cardiovasc. Diabetol. 18 (1), 126. doi:10.1186/s12933-019-0931-0
Sardu, C., D’Onofrio, N., Torella, M., Portoghese, M., Mureddu, S., Loreni, F., et al. (2021a). Metformin Therapy Effects on the Expression of Sodium-Glucose Cotransporter 2, Leptin, and SIRT6 Levels in Pericoronary Fat Excised from Pre-diabetic Patients with Acute Myocardial Infarction. Biomedicines 9 (8), 904. doi:10.3390/biomedicines9080904
Sardu, C., Gatta, G., Pieretti, G., Viola, L., Sacra, C., Di Grezia, G., et al. (2021b). Pre-Menopausal Breast Fat Density Might Predict MACE during 10 Years of Follow-Up. JACC: Cardiovasc. Imaging 14 (2), 426–438. doi:10.1016/j.jcmg.2020.08.028
Seliger, S. L., Weiss, N. S., Gillen, D. L., Kestenbaum, B., Ball, A., Sherrard, D. J., et al. (2002). HMG-CoA Reductase Inhibitors Are Associated with Reduced Mortality in ESRD Patients. Kidney Int. 61 (1), 297–304. doi:10.1046/j.1523-1755.2002.00109.x
Sharma, S., and Sarnak, M. J. (2017). Epidemiology: The Global burden of Reduced GFR: ESRD, CVD and Mortality. Nat. Rev. Nephrol. 13 (8), 447–448. doi:10.1038/nrneph.2017.84
Ting, R. D., Keech, A. C., Drury, P. L., Donoghoe, M. W., Hedley, J., Jenkins, A. J., et al. (2012). Benefits and Safety of Long-Term Fenofibrate Therapy in People with Type 2 Diabetes and Renal Impairment: the FIELD Study. Diabetes Care 35 (2), 218–225. doi:10.2337/dc11-1109
Tsai, M.-H., Chang, C.-H., Liou, H.-H., and Fang, Y.-W. (2021). Inverted U-Curve Association between Serum Indoxyl Sulfate Levels and Cardiovascular Events in Patients on Chronic Hemodialysis. J. Clin. Med. 10 (4), 744. doi:10.3390/jcm10040744
Wanner, C., Krane, V., März, W., Olschewski, M., Mann, J. F., Ruf, G., et al. (2005). Atorvastatin in Patients with Type 2 Diabetes Mellitus Undergoing Hemodialysis. N. Engl. J. Med. 353 (3), 238–248. doi:10.1056/NEJMoa043545
Yao, Q., Pecoits-Filho, R., Lindholm, B., and Stenvinkel, P. (2004). Traditional and Non-traditional Risk Factors as Contributors to Atherosclerotic Cardiovascular Disease in End-Stage Renal Disease. Scand. J. Urol. Nephrol. 38 (5), 405–416. doi:10.1080/00365590410031715
Yen, C. L., Fan, P. C., Lin, M. S., Lee, C. C., Tu, K. H., Chen, C. Y., et al. (2021). Fenofibrate Delays the Need for Dialysis and Reduces Cardiovascular Risk Among Patients with Advanced CKD. J. Clin. Endocrinol. Metab. 106 (6), 1594–1605. doi:10.1210/clinem/dgab137
Yu, Y., Dong, Z., Li, Y., Zhang, J., Yin, S., Gao, X., et al. (2021). The Cardiovascular and Cerebrovascular Health in North China from 2006 to 2011: Results from the KaiLuan Study. Front. Cardiovasc. Med. 8, 683416. doi:10.3389/fcvm.2021.683416
Zha, Y., and Qian, Q. (2017). Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients 9 (3), 208. doi:10.3390/nu9030208
Keywords: dialysis, National Health Insurance Research Database, lipid-lowering agents, mortality, major adverse cardiovascular events
Citation: Tsai M-H, Chen M, Huang Y-C, Liou H-H and Fang Y-W (2022) The Protective Effects of Lipid-Lowering Agents on Cardiovascular Disease and Mortality in Maintenance Dialysis Patients: Propensity Score Analysis of a Population-Based Cohort Study. Front. Pharmacol. 12:804000. doi: 10.3389/fphar.2021.804000
Received: 28 October 2021; Accepted: 31 December 2021;
Published: 28 January 2022.
Edited by:
Norberto Perico, Mario Negri Pharmacological Research Institute (IRCCS), ItalyReviewed by:
Jia-Feng Chang, En Chu Kong Hospital, TaiwanCelestino Sardu, University of Campania Luigi Vanvitelli, Italy
Copyright © 2022 Tsai, Chen, Huang, Liou and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Wei Fang, m005916@gmail.com
†These authors have contributed equally to this work and share first authorship
 Ming-Hsien Tsai
Ming-Hsien Tsai Mingchih Chen
Mingchih Chen Yen-Chun Huang
Yen-Chun Huang Hung-Hsiang Liou5
Hung-Hsiang Liou5