
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 25 January 2022
Sec. Experimental Pharmacology and Drug Discovery
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.803304
Cancer is a life-threatening disease, contributing approximately 9.4 million deaths worldwide. To address this challenge, scientific researchers have investigated molecules that could act as speed-breakers for cancer. As an abiotic drug delivery system, liposomes can hold both hydrophilic and lipophilic drugs, which promote a controlled release, accumulate in the tumor microenvironment, and achieve elongated half-life with an enhanced safety profile. To further improve the safety and impair the off-target effect, the surface of liposomes could be modified in a way that is easily identified by cancer cells, promotes uptake, and facilitates angiogenesis. Integrins are overexpressed on cancer cells, which upon activation promote downstream cell signaling and eventually activate specific pathways, promoting cell growth, proliferation, and migration. RGD peptides are easily recognized by integrin over expressed cells. Just like a multistage rocket, ligand anchored liposomes can be selectively recognized by target cells, accumulate at the specific site, and finally, release the drug in a specific and desired way. This review highlights the role of integrin in cancer development, so gain more insights into the phenomenon of tumor initiation and survival. Since RGD is recognized by the integrin family, the fate of RGD has been demonstrated after its binding with the acceptor’s family. The role of RGD based liposomes in targeting various cancer cells is also highlighted in the paper.
Since the discovery of liposomes in the 1960s they have contributed to a growth in the drug development industry owing to their unique characteristics such as bio-degradability, biocompatibility, lack of immune system activation, low toxicity, and capability to incorporate both hydrophobic and hydrophilic drugs. Liposomes are nanometric phospholipid bubbles with a lipid bilayer which, after incorporation of lipophilic/hydrophilic drug molecules, prevents the rapid degradation of the drug and also reduces toxicity (Torchilin, 2005; Bulbake et al., 2017). Using the specialized advantages of liposomes, the therapeutic window of potent drug molecules can be expanded by increasing metabolism and absorption, reducing the elimination rate, and promoting the biological half-life (Estanqueiro et al., 2015; Kesharwani et al., 2021).
The FDA has approved several liposome based anti-cancer preparations that have shown promising results even in the last stage of clinical trials. Thus, not only the safety but also the therapeutic profile of anti-cancer agents could be improved. Ever since the development of the first liposomal product, Doxil® (Barenholz, 2012), many more anticancer formulations have been successfully developed, including Depocyt® (Murry and Blaney, 2000), Mepact® (Alphandéry et al., 2015), OnivydeTM (Drummond et al., 2006), Myocet® (Leonard et al., 2009), DaunoXome® (Forssen, 1997), and Marqibo® (Webb et al., 1995). The attributes of liposomes could also be modified depending on the kind of lipids used. In a current study, liposomes composed of porphyrin-phospholipids showed the simultaneous release of cargos upon NIR irradiation (Carter et al., 2014). The composed liposome 1,2-dioleoyl-sn-glycero-3-phospho ethanolamine (DOPE) has shown pH-dependent release, with maximum release at low pH due to the structural transformation of DOPE to hexagonal form, endorsing the destabilization of the bilayer (Soares et al., 2011). Nano-preparation using liposomes also has the potential to deliver poorly water-soluble agents even without adding a surfactant, which can be used to cause toxicity. With dedicated research, many novel liposomes such as pH and thermosensitive, long-circulating, and environmental sensitive liposomes have been developed (Figure 1). Two preparations, using Paclitaxel loaded liposomes (EndoTAG-1, Medigene AG and LEP-ETU, Neopharm, Inc.) reached Phase II clinical trial, which disregarded the use of Cremophore/Kolliphore adjuvant, whose usage causes severe anaphylactic shock. Thus, the maximum tolerated dose of paclitaxel was elevated (Koudelka and Turánek, 2012). A study performed by Petersen et al. showed that 11 published results prolonged the survival time in 11 tumor-bearing mice models treated with doxorubicin liposomal preparation as compared to plain drug. A meta-analysis for screening progression free survival (PFS) and overall survival (OS) using eight clinical studies was performed, comparing the efficacy of anthracycline-based liposomal preparations and conventional anthracycline preparation. No evidence was found claiming PFS and OS in patients. However, the safety profile of liposomal preparation improved. Particularly, the liposomes could not cross the endothelial lining of the blood vessel of the heart, thereby regulating the pronounced cardio-toxicity as compared to the conventional drug (Gh et al., 2016). Such, an early delivery system depended on the passive release process, which ascertains the stimulated release other than the passive release at a specific cancer cell. Due to lack of specificity, liposome’s talent remains vague by not delivering the required amount to the desired area. To overcome such shortcomings, targeted drug delivery is being introduced in the menu of research. Steady release in pH mimicking tumor environment and remote triggering such as using ultrasonic waves and photo-activating substances, modify the drug release profile. The cell selectivity of liposomes by cancer cells could be improved by the surface functionalization of liposomes. This target mediates therapy and could reduce the toxicity, improve the site-specific drug accumulation and internalization along with promoting endosomal escape (Choudhury et al., 2018; Kesharwani et al., 2018).
Incipient tumor cells acquire several biological functions and undergo metabolic changes in the journey of evolution, which permit the cells to become more tumorigenic and eventually invasive (Hu et al., 2015; Kesharwani and Iyer, 2015; Wang et al., 2020). Cancer cells over-express various surface receptors which help them to grow and proliferate (Kesharwani et al.,2011; Kesharwani et al., 2014a; Kesharwani et al.,2014b; Kesharwani et al., 2015a; Kesharwani and Iyer, 2015; Amjad et al., 2017). This accelerates their survival, challenging the chemotherapeutic activity to work against them. Integrins are also among these kinds of receptors, generally and transmembrane receptors have been implicated as playing a prime role in regulating the metabolic process by advocating cellular growth at ground level after their attachment with the ECM. Furthermore, after adhesion with ECM, integrins could regulate traction for invasion and cell migration. To summarize, integrins stimulate the survival, proliferation, and metastasis of tumorigenic cells (Yee et al., 2008; Böger et al., 2014; Duro-Castano et al., 2017). Specifically, they stimulate overexpression of αvβ3 integrin receptor, which has been allied with various types of cancer including glioblastoma, colon, melanoma, breast, pancreatic, prostate ovarian, and cervical cancer. An interesting study by Pierschbacher and Ruoslahti in 1984, described the Arginine-glycine-Aspartate (RGD) cell adhesion sequence, an extremely preserved minimal integrin sequence in fibronectin (Ruoslahti, 1996). This sequence is the smallest that can bind with the αvβ3 integrin receptor overly expressed on the tumor environment. The expression of integrins thus requires the design of agents that can bind with them using RGD as a targeting ligand. Liposomal preparations based on the delivery of chemotherapeutic agents on an integrin over-expressed site could offer multiple ranges of benefits.
This review sheds light on integrin-targeted cancer cells using liposomal preparations functionalized by RGD peptide motifs for attaining the targeted effect. Such a targeted approach is reported as a cell penetration facilitator engulfing theranostic (therapeutic + diagnostic) agents into targeted spots. It focuses attention on the role of integrin in cancer prognosis and also explains its interaction with RGD peptides. This review also highlights the RGD anchored liposomal delivery for various cancers, to compile all available current research.
Integrins are cell surface receptors that are a prominent component of extracellular matrix (ECM), from the generation of 24 transmembrane heterodimers. To date, 18α and 8β subunits have been described on the human genome as having different affinities and specificities towards different ligands (Hynes, 2002; Humphries et al., 2006). For instance, αvβ3 integrin binds an eclectic range of ECM molecules, together with fibrinogen, vitronectin, von Willebrand factor along with proteolyzed forms laminin and collagen, while α5β1 integrin selectively binds fibronectin (van der Flier and Sonnenberg, 2001) The heterodimers of integrin are transported to the Golgi apparatus from the endoplasmic reticulum, which after modification relocated to the cell in an inactive form (De Franceschi et al., 2015). The amino acid terminals of α and β integrins bind together by non-covalent attraction, subsequently giving rise to integrin αβ heterodimers (Seguin et al., 2015).
Integrin directly interacts with the component of the extracellular matrix (ECM) to arbitrate cell adhesion, which is an essential step to carry out several metabolic processes that promote cell growth and viability. Additionally, they directly regulate the invasion and migration of tumor cells after interaction with ECM components, providing a platform for cellular motility and intravasation (Guo and Giancotti, 2004). This property of integrins in tumor cells (relocation and invasion) plays important role in cancer evolution and has recently been reported elsewhere (Aoudjit and Vuori, 2001; SK and DD, 2006). The role of integrins depends on the environmental cues that could either elevate survival or initiate apoptosis (Figure 2). The normal physiology of the human body works to maintain a balance in the functioning of all processes. While on the one hand, the unlighted form of integrin encourages the pro-apoptotic cascade, simultaneously the ligated form of integrin transmits bidirectional survival signals. Such homeostasis upholds the integrity of various cells and organs by averting cell survival. (Askari et al., 2009; Legate et al., 2009; Hamidi and Ivaska, 2018; Cooper and Giancotti, 2019). The activation and affinity of integrin towards ECM increase after recruiting the integrin tail region with adaptor protein talin causes a confirmational change. Moreover, as a consequence of Integrin–ECM interactions at the integrin’s horizon the absorption of focal adhesion is promoted. Ligated integrins in the case of ligated integrins promote the activation of metabolic pathways such as MAPK, PI3K-AKT, augmented nuclear factor-κB (NF-κB), reduced p53 expression, and amplified expression of B-cell lymphoma-2 (BCL-2) and CFLAR gene. The crosstalk between the integrins and the growth factor receptors also preferentially stimulates Raf, mediating cell survival and proliferation, which in turn encourages Ser 338-9 related Raf phosphorylation, inhibiting the risk of cellular death through the intrinsic pathway. While, phosphorylation of Tyr340-1 prevents cell death through the extrinsic pathway (Legate et al., 2006; Landen et al., 2008). The unligated integrins cleaved caspase-8, prompting integrin-mediated death (Stupack et al., 2006). Cell death gets initiated after complete loss of adhesion through a process termed anoikis which proceeds either via intrinsic or extrinsic pathways (De Franceschi et al., 2015).
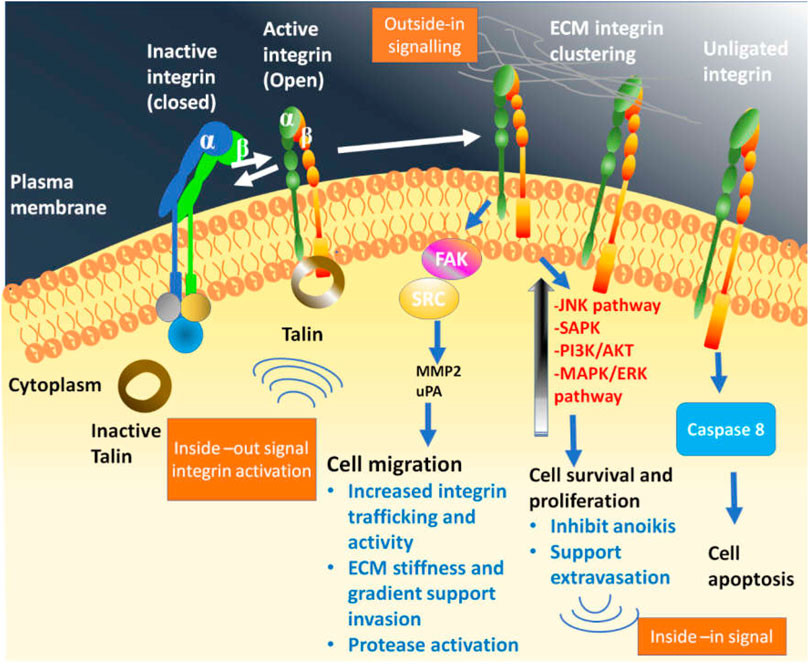
FIGURE 2. Bidirectional signalling by integrin family which is in virtue to confirmational state demonstrate its binding affinity towards extracellular matrix (ECM) and related protein. The bend or closed integrin epitomize the inactive form having low affinity towards ECM, while the straight-extended and upright integrin corresponds to the active form that show high affinity towards ECM, thereby promoting down-stream signalling eliciting cellular response following the ligand attachment. Following the integrin-ligand engagement also called as adhesion, and their clustering on plasma membrane, assembles the multimeric complexes to promote outside-in or downstream signalling. In consequence, phosphorylation of focal adhesion kinase (FAK) initiates, which further activate the steroid receptor coactivator or SRC. In association to this, other metabolic pathway-PI3K/AKT, MAPK/RAS also get activated to produce signal nodes. Talin bind to the β-integrin subunit tail which triggers the open structure. Moreover, integrin dependent signalling compounds and activated integrin in association with receptor tyrosine kinase and ECM encourages the “inside-in” signalling.
The sequence of RGD was the smallest cell adhesion sequence discovered over 3 decades ago. The recognition sites of RGD were also obtained in other ECM proteins (Lawler et al., 1988) such as vitronectin, osteopontin (Horton et al., 1995), bone sialoprotein, fibrinogen, fibronectin, and laminin (Grant et al., 1989), whose receptors were recognized in the integrin family. The recognition by the integrin receptor depends upon the structural conformation of peptide (RGD). Integrin modulated cancer cell surfaces offer a horizon for such peptides to be eventually identified and offer a target binding site (Ali et al., 2017; Wu et al., 2017). A modulation of nanocarriers with RGD binding motif demonstrated high affinity towards cancer cells overexpressing integrin and also offered potential efficiency to conquer tumor progression via inhibition of integrin facilitated functions (Zhang et al., 2011; Ahmad et al., 2021).
The role of RGD is exploited in cellular adhesion, spreading, focal-adhesion, and actin-skeleton formation with integrins that are required for the transmission of signals that regulate the cell cycle and its behavior (Hwang et al., 2007; Bellis, 2011). The binding of RGD-nanoparticle to ECM ligand forces integrin to trigger “outside-in” signaling to enhance the downstream signaling for arresting the cell cycle and promotion. In synergy, the ligand-bounded integrins get internalized into the cells via clathrin-mediated endocytosis for focal adhesion turnover and then transported to late lysosomes or endosomes (Jain et al., 2012; Onodera et al., 2013; Nieberler et al., 2017; Kesharwani et al., 2018).
Few integrins separate from the binding ligands in response to the acidic atmosphere of late endosome/lysosome, and the free integrin returns to the plasma membrane (Ezratty et al., 2009). This framework of recycling integrin towards plasma membrane eventually results in the progression of cancer due to upregulation of neoplastic cells. Such processes (endocytosis or integrin return) promote the binding, internalization, and accumulation of integrin-targeted nanoparticles (Danhier et al., 2012; Dozynkiewicz et al., 2012). As observed in a study by Li et al., modified RGD conjugated nanoparticles facilitate in vivo anti-tumor efficacy (Li et al., 2014). A highly potent αvβ3 ligand, i.e., cyclic RGD-tyrosine-lysine peptide (cRGDyk) modified liposome encapsulating cisplatin elevated therapeutic efficacy against bone metastasis in congruency with pain relief, tumor regression, and improving overall survival improvement (Wang et al., 2014). The selectivity of RGD also depends on its specificity to bind to integrin. For instance, the appointment of integrins β3 is crucial for the cell to cell or cell to matrix interactions, thus connecting a wide network of biological processes (Bialkowska et al., 2000; Arias-Salgado et al., 2003).
In contrast to the αvβ3 integrin, which is generally associated with almost every cancer type, other αIIbβ3 integrins are encountered on the cell surface and perinuclear region of prostate neoplastic cells (Bello et al., 2000; Brüning et al., 2001; Rolli et al., 2003; Müller and Bosserhoff, 2008). However, αvβ3 and various integrin are expressed in normal cells as well but to a low extent. For example, cyclic-RGD peptides or cRGD targeted various integrin including αvβ3 αvβ8, αvβ6, and αvβ5, which were expressed on healthy tissues also, and ultimately produced toxicity in normal cells. Another major issue encountered was that RGD peptide could only recognize αvβ3, but integrin αIIbβ3 was not recognized by the RGD that were present in the perinuclear region of prostate tumor. This accelerated the demand for such modified peptides or specific ligands being taken up by integrins β3 for improving anticancer treatment and minimizing adverse effects. A molecular docking study identified an RGD modified peptide- Arginine-Tryptophan-(D-Arginine)-Asparagine-Arginine named β3Integ that showed high affinity towards integrins β3. In comparison to cRGDyK modified liposomes, β3Integ exhibited 3.3-fold anti-cancer effect. Moreover, no cardiomyocyte toxicity or weight variation change was observed after treatment with β3Integ grafted preparation (Zhang L. et al., 2019). In another approach, glutamic oligopeptides-RGD peptide (GLU6-RGD) derivative modified liposome displayed outstanding in vivo targeting activity in metastatic bone malignancy with high hydroxyapatite (HAP) binding efficiency, improved cytotoxicity and enhanced stability (Zhao et al., 2019). Such dual targeted effects elevate the anti-cancer efficacy of targeted nano molecules.
With many attractive properties, liposomes are a well-recognized nanocarrier in the biomedical industry. However, liposome is not new to the industry. Since the success of Doxil (Doxorubicin loaded liposome) in 1995, liposome made its way from the lab to the clinic (Seynhaeve et al., 2013). With the approval of the FDA, several liposomal preparations were promoted from the bench to bedside proving their efficacy in the last stage of the clinical trial. The significant approach of liposomal therapy is sufficient drug loading, congruous size, and very importantly, steric stabilization, which is necessary for improving circulation time (Allen and Cullis, 2013). Parameters such as controlled release with maximum efficacy of liposomal therapy are essential, which in the case of early liposome is mediated by passive release pattern. Here, due to the leaky vasculature of the tumor, the liposome loaded active anti-cancer drug elicits its action (Andresen et al., 2005; Kesharwani et al., 2015b; Luong et al., 2016; Srivastava et al., 2021), but affects normal cells as well. With insufficient release pattern, SPI-077 liposomes, despite getting internalized in cancer interstitium was not able to meet the efficacy demand (Bandak et al., 1999). Therefore, to meet the demand and provide the clinic with a candidate with a wide therapeutic window, liposomes could be grafted with ligands (Ruoslahti, 2012). By facilitating more uptake by tumor cells, serving in better internalization, and honing the anti-tumor effect, targeted liposomes could follow various pathways to achieve such objectives. Endocytic pathways such as flotillin (<100 nm), caveolae (<80 nm), and clathrin (<300 nm) mediated pathways are employed by liposomes for delivery of ligand mediated selected uptake (He et al., 2010; Bae and Park, 2011). As discussed previously, integrin is over-expressed in numerous cancers, which could be well recognized by RGD peptide. Thus, utilizing the advantage of RGD as ligands and properties of liposomes, multiple researchers have examined its effect on tumor growth. The section below illustrates examples of RGD modified liposomes with efficacy against various metastatic and non-metastatic cancers (Table 1).
Accounting for more than one per 10 new cases, breast cancer ranks top in the most commonly diagnosed cancers among women (Alkabban and Ferguson, 2021). Its therapy involves using drugs that block cancer growth by interfering with respective molecules that hinder proliferation and survival. The cells may overexpress specific receptors, which upon activation promote downstream signaling, and in consequence result in the countenance of certain genes for cancer cell growth, proliferation, migration, survival, angiogenesis, and promotion of other vital cell cycle pathways (Yarden and Sliwkowski, 2001; Murphy and Dickler, 2016). A thorough study of over-expressed receptors could provide a platform for target-based therapy.
Over-expression of integrin in breast cancer cells mediates cell growth after binding with integrin. Hence numerous molecules are being developed towards integrin overexpressed cells, which help with tumor apoptosis and cell death. The liposomes derived from model biological membranes manifest the high potential of gene and drug delivery, molecular imaging, and employ biological cell membranes that mimic the human microenvironment. To achieve extra therapeutic benefits, targeting numerous components such as proteins, antibodies, peptides, and small molecules could reduce the off-target effect of naked nanoparticles (Torchilin, 2005; Ying et al., 2010; Manjappa et al., 2011; Muthu et al., 2011; Wang et al., 2012). The pH-based drug delivery system also earmarks the tumor by releasing cargo in the acidic environment of the tumor (6.5–6.7), while remaining intact at physiological pH. This type of smart, target-based approach guarantees reduced toxicity and elevated therapeutic effect.
To utilize extra-ordinary benefits such as controlled properties and high uptake potential, Chang et al., developed RGD anchored Cholesteryl hemisuccinate (CHEMS) based pH responsive liposome loaded with docetaxel (DTX). CHEMS are protonic lipid having ester derivative at 3-hydroxyl group, utilized in constructing pH responsive drug delivery system. The prepared DTX loaded liposomes (D-LP) and RGD anchored D-LP (RGD-LP) displayed pH dependent size variation. The size of D-LP and RGD-LP increased from 136.4 to 129.4 nm to 961.4 and 685.2 nm with a shift in pH from 7.4 to 4. This is due to the presence CHEMS, which significantly promoted drug release at definite pKa. The targeted preparation showed maximum intensity around the nuclei which indicated selective uptake of ligand bound liposomes in MCF-7 cells bearing mouse model (Chang et al., 2015). Hence, RGD based liposomal therapy could be a promising opportunity for multifunctional drug delivery systems.
In a similar study by another research group, pH sensitive DTX loaded liposomes decorated with PEG linked RGD peptide as targeting ligand have been investigated using a different polymer, Linoleic acid (LA). LA is anionic at physiological pH, however, protonation of unbounded carboxylic acid at the acidic environment (tumor site) alters the confirmational structure, causing destabilization of liposome leading to its collapse or dissociation. As compared with the non-targeted liposome and DTX (Duopafei®), RGD-decorated liposome showed larger AUC, extended mean half-life, and residual time (MRT) along with a slower clearance rate in vivo. The superior performance is ascertained due to the presence of a PEG chain that connected RGD and LA. The linked and unlinked PEG with RGD, swing on the liposomal surface, thereby prolonging the circulation time. The plain liposomes did not exhibit any cytotoxicity in MCF-seven cells, with more than 89% surviving cells, indicating the safety of liposomes in a biological membrane. The IC50 value of RGD grafted liposomes, non-targeted liposome, and Duopafei®) after 72 h was 1.05 ± 0.04, 1.50 ± 0.13, and 1.84 ± 0.10 μg/ml, respectively. Moreover, the smallest mass of tumor was observed in mice treated with RGD bound liposome, which was attributed to good tumor-inhibiting potential due to enhanced uptake by tumor cells. Overall, the preparation represents a new strategy for drug delivery systems with a dual targeting effect to suppress tumor progression (Zuo et al., 2016).
Triple negative breast cancer or TNBC accounts for nearly 20% of all breast carcinoma and is characterized as the most aggressive form of cancer. Currently, for the treatment of TNBC, chemotherapy, radiotherapy, and surgery remain options for the treatment. However, due to meager prognosis and high mortality, the dilemma of treatment should be alleviated urgently (Chang-Qing et al., 2020; Medina et al., 2020). To acquire the best therapeutic response and clinical output, one must understand the normal physiology of the tumor environment. Cancer cells are depleted of oxygen and glucose, which steal nutrients from the normal cells. In contrast to normal cells, tumor cells are depicted by a sharp rise of glucose uptake in an oxygen rich environment, known as the Warburg effect (Gowrishankar et al., 2011). Thus, the Warburg effect, together with proliferation consumes more glucose, making a low-glucose microenvironment in tumor cells.
Breast cancer cells consume fructose 8-10 times higher than the normal cells. Selective from different kinds of hexose transporters, solitary GLUT2 and GLUT5 was capable of carrying fructose (FRU), among which GLUT5 wons the race (Zamora-Leon et al., 1996; BJ et al., 2009; Sheng et al., 2009).
The environment of the tumor along with sufficient information regarding over-expression of receptors have led to the design of targeted drug delivery systems with promising essential candidates against cancer. To improve targetability, Pu and team reported two novel types dual targeting liposomes loaded with Paclitaxel (PTX). One is obtained through the physical mixture of liposome with RGD (targeting αvβ3 integrin over-expressed on TNBC cells) and fructose (to target cells showing Warburg effect) forming RGD-FRU-Lip, another is the covalently linked liposomes that formed Y shaped ligand with RGD and FRU (RGD/FRU/Lip). The results manifested that RGD/FRU/Lip was stable and safe to use, showing hemolysis, which is regarded safe. As compared to free liposome (lip), RGD-lip, FRU-Lip, and RGD-FRU-Lip, RGD/FRU/Lip showed significant cytotoxicity, while free PTX exhibited the least cell viability due to the passive diffusion. Moreover, the upregulation of RGD/FRU/Lip was also significantly enhanced than other preparations, as revealed by confocal microscopy. The result of confocal microscopy showed cytoplasmic localization of liposomes that rarely entered the nucleus facilitated by the release of drugs after phospholipid destruction. The in vivo images 4T1 bearing mice showed maximum targetability 12 h post-treatment with DID-RGD/FRU/Lip. Solely modified liposomes such as DID-RGD-Lip and Did-FRU-Lip also showed comparable results to unmodified liposomes. Even after 24 h, the modified liposomes present in the cancer cells were significantly greater compared to the remaining groups (Figure 3). Thus, researchers studied the environment of the tumor and designed a therapeutic regimen that reduced the undesired effect on normal cells while improving targeting ability (Pu et al., 2019).
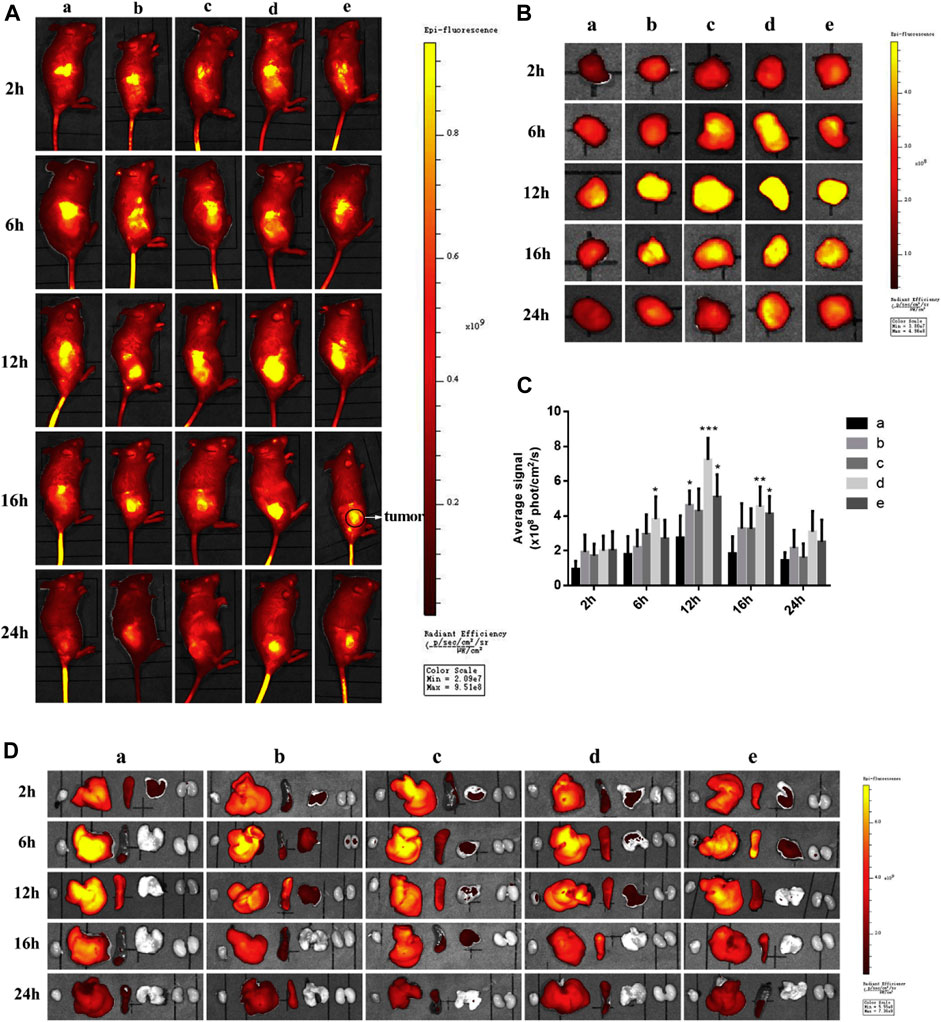
FIGURE 3. In vivo (A) and ex-vivo images (B) 4T1 bearing mice treated with (i) DID-Lip (ii) DID-FRU-Lip (iii) Did-RGD-Lip (iv) Did-RGD/FRU/Lip and (v) Did-RGD-FRU-Lip. Among the modified liposome (iv) showed strongest fluorescent intensity due to targeting effect. (https://doi.org/10.1016/j.ejmech.2019.111720).
For decades the fight against cancer has sought to realize β-catechin as a potential mediator of the Wnt pathway as it plays an important role in homeostasis and expansion of adult tissue via mediating the embryonic and stem cell functions. Particularly in TNBC, this pathway is superiorly activated, contributing towards initiation and metastasis. Thus, to rationalize, Li and team developed Diacedic Norcantharidin (NCTD), an anti-cancer-loaded RGD-coated lipid polymer hybrid (RGD/LPH). They realized that TNBC cells expressed integrin α5 (ITGA5) expression to a far greater extent than normal cells. The targeted formulation showed promising results that enhanced the circulation time and accumulation of NCTD in mice with orthotopic mammary TNBC tumor and lung cancer cells showing metastasis, which offered a platform for a targeted approach in attenuating TNBC tumor. Via downregulating β-catechin, the tumor growth and metastasis also reduced in RGD/LPH treated tumor bearing mice as compared to LPH/NCTD and free NCTD, inflicting the tumor homing effect via binding with integrin (Li et al., 2019). Apart from the delivery of drug based anti-cancer substances, gene therapy also offers a great therapeutic window in cancer therapy. Micro RNA or miRNA are demonstrated in regulating gene expression in cancer and its related conditions (Huang et al., 2013; Mirzazadeh Tekie et al., 2015; Assali et al., 2018). It has been revealed that miR-34a influences cell arrest and apoptosis, however, the major challenge is stability. Nucleases degraded the nucleic acid, thereby reaching the target site in an inactive form. Thus, to deliver miR-34a successfully to the cancer site, Vakhshiteh and team focussed on the ability of liposomes to protect and deliver the genetic agent. To achieve targeted delivery the miR-34a loaded liposomes were PEGylated (to improve the circulation time and protect liposomes from interaction with serum protein) and cRGD to target TNBC and circulating stem cells (CSCs) over-expressing integrin receptors. The targeted liposomal preparation resulted in higher cellular uptake (2X), which indicated miR-34a stability amplification and increased cellular uptake. The preparation also affects the migration of tumor cells by 33% after treatment with targeted liposome than non-targeted liposome, which reduced the cellular migration by 15%. Similarly, invasion of cancerous cells also reduced significantly by 17 and 8%, after being treated with targeted and unligated liposomes. This is due to the higher uptake of cRGD decorated liposomes, which effectively augmented the miR-34a accumulation, thus, substantially curbing invasiveness and malignancy (Vakhshiteh et al., 2020).
Gliomas are a subtype of Central Nervous System (CNS) neoplasm, that originates from the neuroglia such as oligodendrocytes and ependymal cells of the brain. Among all brain cancer, glioma accounts for nearly 30% and approximately 80% among primary malignant brain cancers. However, the aetiology is still unknown (Mishra and Kesharwani, 2016; Reifenberger et al., 2017; Malta et al., 2018; Molinaro et al., 2019; Lin et al., 2020). Today, the standard treatment is surgery followed by chemotherapy and radiotherapy. Limited penetrance of the therapeutic agent and resistance due to blood brain barrier (BBB) and blood brain tumor barrier (BBTB), diminishes the overall 5-years survival rate. Liposomes have gained attention in the therapy of glioma due to their hydrophobic nature, facilitating the diffusion of hydrophilic or lipophilic drugs across the BBTB and BBB (Ruan et al., 2021), and thus accumulating in brain cancer cells. Non-targeted liposomes, however, pose a hurdle as they raise the toxicity by also liberating drugs in normal cells. This explains the value of targeted preparation that avoids off-target drug release (Shi et al., 2008).
In a study by Liu and co-workers, the PTX loaded liposome was modified with cyclic RGD conjugated with another cell penetrating peptide (R8), to obtain R8/cRGD liposomal structure. In contrast to linear RGD, cyclic RGD shows 1,000 times greater targeting ability. On C6 cells, R8-RGD-Liposome and R8-Liposome showed a strong anti-proliferation effect than free PTX due to higher cellular uptake. However, blank liposome exhibited no toxicity, which illustrates its safety. Moreover, R8-RGD-Liposome induced stronger apoptosis (38.30 ± 3.11%) as compared to other preparations. This indicates that R8-RGD augments the cellular accumulation of targeted preparation, releasing more drugs directly to the cytoplasm besides attaining in vitro inhibition. Additionally, PTX-R8-RGD-lip treated mice bearing glioma showed improved efficacy by elevating the survival time to 48 days, which was significantly longer than those treated with free PTX, PTX- R8-lip, and PTX-RGD-lip, showing survival of 32, 36, and 38 days, respectively (Liu et al., 2014). In another interesting study, Docetaxel (DTX) and quantum dot (QD) loaded liposomes coated with d-alpha-tocopheryl polyethylene glycol succinate (TPGS) and RGD were prepared, which shows the ROS scavenging property of TPGS. As compared to non-targeted liposomes (DTX/TPGS/Lip), non-targeted theranostic liposomes (DTX/QDs/TPGS/Lip), plain DTX, and plain QD, DTX/QDs/RGD/TPGS-Lip remarkably reduced ROS levels. The theranostic liposome shadowed integrin-receptor released the therapeutic agent via endocytosis at a specific site that additionally reduced ROS production in the brain. Moreover, the preparation was biocompatible showing no signs of toxicity as evaluated via histological study. Thus, it could be concluded that due to over-expression of integrin, transport of both active moiety and imaging agent, simultaneously, could be potentiated (Singh et al., 2016). A multi targeted approach has also been recently introduced in the treatment of glioma therapy. This could overcome the limitations imposed by BBB and BBTB. To examine enhanced benefits, a study recently reported that two ligands, cRGD, could recognize αvβ3 integrin and small ligand i.e., p-hydroxybenzoic acid (p-HBA), which could target BBB, and which has been connected via appropriate linker forming cRGD-p-HBA, then covalently with Mal-PEG3400-DSPE. The resulting Y shaped complex was then entrapped in the Doxorubicin (DOX) laden liposomes (cRGD-p-HBA-DSPE-DOX-LIP). The in vivo imaging demonstrated maximum fluorescence intensity at 4h, cRGD-p-HBA-DSPE-DOX-LIP was evidenced more significantly in glioma cells than the unmodified and monoligated liposomes, suggesting that the liposomes decorated with Y shaped agents could facilitate in vivo targeted brain delivery. The anti-cancer efficacy of Y-shaped ligand modified liposomes was demonstrated by the U87 glioma-bearing mice model. Credit goes to the synergistic effect mediated by cRGD and p-HBA for transporting targeted nanoparticles across brain barrier pervading, improving drug circulation time, and enhancing targeted delivery towards the brain (Belhadj et al., 2017). However, integrins could be recognized by RGD peptides, which also imposes certain malfunctioning. In a study, it was corroborated that cRGD modified liposomes caused IgG-mediated acute systemic anaphylactic along with anti-cRGD-liposome IgM and IgG production (Wang et al., 2019). The same research group employed a computer-assisted virtual screening method to produce a linear pentapeptide that could recognize the integrin effectively. In contrast to cRGD strengthened liposomes, RW liposomes exhibited better immune-compatibility, enhanced circulation along with vasculogenic mimicry targeting, improved angiogenesis, and therapeutic prowess that could hone the delivery of targeted liposome to integrin expressed cells (Li et al., 2020).
Melanoma is the deadliest form of skin carcinoma. At an early stage, melanoma can be treated successfully with surgery alone with improved survival rates, but after metastasis, survival rates decline significantly. Therefore, the correct initial diagnosis is key for ensuring the best possible prognosis for patients. However, once melanoma turns advanced, surgery no longer remains the pivotal step of treatment (Tsatmali et al., 2002; Viros et al., 2014; Madheswaran et al., 2017; Singh et al., 2020; Kaur and Kesharwani, 2021). Conventional chemotherapy obstructs the way of promising candidates reaching the benchmark, hence TDDS are emerging as the most explored option. Studies have put forth the development of target assisted delivery system of therapeutics with controlled release. In addition, revolutionary sterically stabilized liposomes (SSL) can achieve targeted benefits and increase the circulation time (Immordino et al., 2006). SSL is demonstrated in cancer tissue at higher concentrations due to their enhanced permeability and retention (EPR) effect. For example, in one study SSL with poly (ethylene glycol) (PEG) at the distal end was conjugated with RGD mimetic (considered as RGDm), which due to passive accumulating and enhanced intracellular delivery, improved therapeutic efficacy (Xiong et al., 2005a). In a similar study by the same research group, RGD modified liposomes showed enhanced intracellular delivery of doxorubicin as compared to non-ligated molecules. This could be due to the conjugation of SSL with RGD; however, the drug release pattern was similar to those with SSL-DOX. Thus, it was clinched SSL and RGD conjugate could not elevate the DOX release. Since the negative zeta potential of SSL and RGD-SSL were close enough, the electrostatic between the cell membrane and liposome contributed in part to the increased DOX uptake and release. Moreover, cellular uptake was increased due to RGD binding with integrins present on melanoma cells (Xiong et al., 2005b). Combination therapies are well known when a single drug fails to deliver enough effect. Anti-vascular therapy along with cytotoxic agents could be a blockbuster treatment option. On the one hand, early metastasis and dividing rims of mature cancer cells are sensitive to cytotoxic therapy, while on the other, more mature tumor cells and angiogenic metastasis are sensitive to anti-vascular therapy (Horsman and Siemann, 2006; Pastorino et al., 2006). To validate, Zhang and team loaded anti-vascular agent, namely combretastatin A-4 (also known as CA-4) along with doxorubicin (DOX) in RGD modified liposome, which delayed the subcutaneous tumor growth in mice and was greater than those liposomes laden with individual moiety. Finally, displaying synergistic mutual therapeutics benefit to the dual effect, CA-4 firstly disordered the vascular matrix and thereby accumulated more liposomal DOX inside the cancer site liberating its cytotoxic effect. Hence, combination therapy could be more beneficial and could be effective in cancer therapy, specifically for intractable tumor diseases (Zhang et al., 2010).
Over the last decade, targeted or ligated nanocarriers have been seen in an array of research for specific targeting to procure more output in melanoma therapy (Hunt and Vorburger, 2002). However, little research is concerned with the function of ligand binding on the change of receptors. In an attempt to challenge this issue, stealth liposomes were amended into monomeric RGD (mo-RGD-L), dimeric RGD (di-RGD-L), and a unique dimeric cRGD motif with a linker in-between (P-di-RGD-L). The 3-dimensional models of αvβ3 clustering proposed a receptor interval within ̴ 41 to ̴ 65 Å, whereas the molecular computation confirmed an RGD ligand interval of ̴ 78 Å, 42 Å and 20 Å, for mo-RGD-L, P-di-RGD-L and di-RGD-L, respectively, which confirms P-di-RGD-L is the perfect match between clustered αvβ3 and special dimeric peptide conjugated liposome. In vivo imaging results suggested augmented uptake of P-di-RGD-L as compared to other RGD based preparation, suggesting improved endocytosis and targeting ability of liposomes for tumors, highly expressing αvβ3 integrin (Figure 4) (Guo et al., 2014). However, specific ligands are frequently are not enough to promote the endocytosis or penetrate solid tumor (Liu et al., 2013). Thus, team Shi aimed to modify the paclitaxel loaded liposomes-based preparation by connecting novel c (RGDfK) peptide to the C-terminus of the peptide TH through an ester bond together with TR peptide. The surface plasmon resonance study confirmed excelled binding of TR-liposome at pH 6.5 than RGD-Liposome, TH-Liposome or PEG-liposome. It was established that TR-liposomes got inside the cell through clathrin or caveolin mediated endocytosis, which was not demonstrated by non-modified liposomes. Additionally, the TH-liposomes and RGD-liposomes carrying paclitaxel did not show variable anti-cancer effects in vivo due to the expression of integrin on cancer cells, however, TR-liposomes carrying paclitaxel showed a synergistic anti-cancer effect, which is due more to internalization and higher specificity towards the cancer cells (Shi et al., 2015). Keeping the normal physiology of cancer in mind, one can efficiently deliver a wide network of cancer therapy. In another study, fluorescence imaging of dual targeted liposome carrying galectin-1-specific anginex (AX) and RGD peptide (AX-RGD-LIP) showed 53 ± 6% fluorescence intensity as compared to AX-LIP (43 ± 9%) and RGD-LIP (28 ± 8%). This is due to their targeting efficacy and specificity, which in turn also improved the circulation time (Kluza et al., 2012).
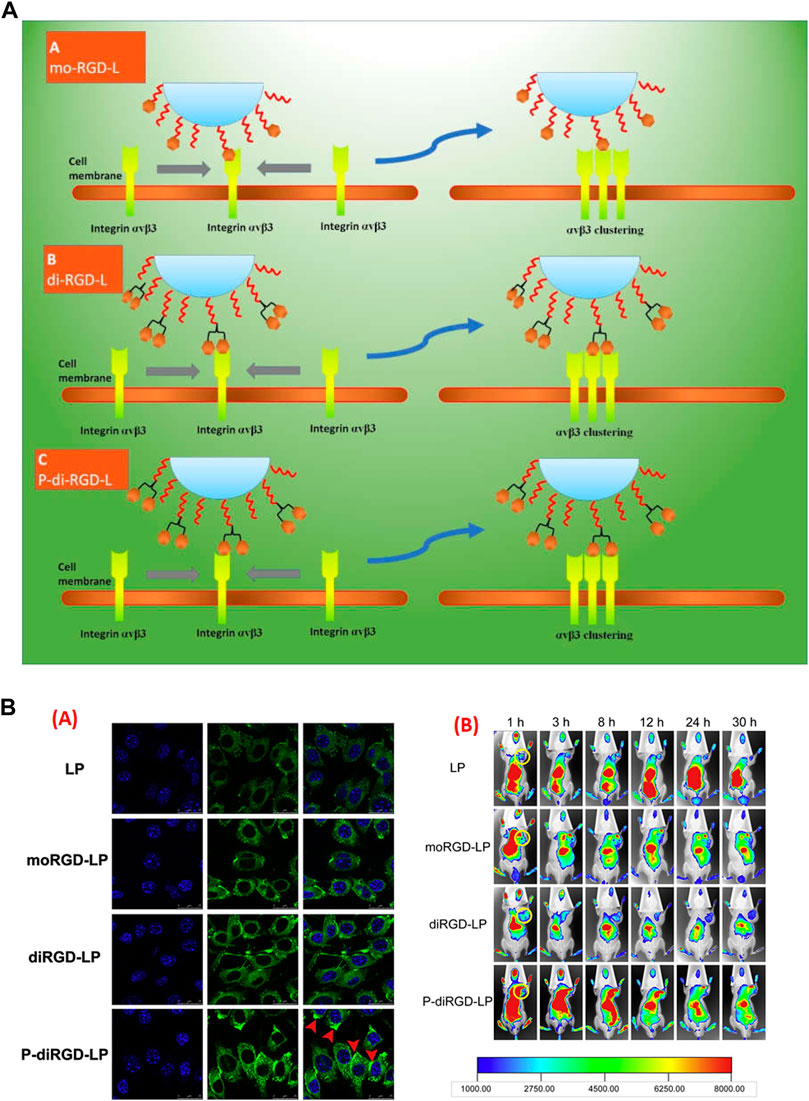
FIGURE 4. (A) Schematic demonstration of different RGD modified nanocarrier towards the adjacent binding site of αvβ3 integrin. In first case, mo-RGD-L molecules, the RGD moiety were too distant to bind with two αvβ3 clustering elements, in second case with di-RGD-L, the two RGD motifs were very near to each other and thus conformationally unrelated to two αvβ3 clustering elements, while P-di-RGD-L were appropriate to bind efficiently to the two integrin cluster elements. (B) (i) Confocal laser microscopic images (CLSM) of melanoma cells (B16) incubated at 37°C, pre-incubated with respected preparations, (ii) In vivo fluorescent images of B16-tumor bearing C57BL/6 mice treated with Liposome, mo-RGD-L, di-RGD-L and P-di-RGD-L, each loaded with DID, confirming P-di-RGD-L exhibited maximum fluorescence at 12 h, which even encountered in following hours as well http://dx.doi.org/10.1016/j.biomaterials.2014.04.031.
With 235,760 new cases and 131,880 deaths worldwide, lung cancer has become the most prevalent cancer in men and the third most prevalent among women. Major risk factors of lung carcinoma are tobacco smoking, workplace exposure to asbestos or arsenic, or environmental exposure to certain pollutants, radon, and harmful gases (Bray et al., 2018; Keikha and Esfahani, 2018; Gorain et al., 2019; Board, 2021). Lung carcinomas are broadly divided into two forms, small cell lung carcinoma, and non-small cell lung cancer. The non-small-cell cancer is further divided into adenocarcinomas, squamous cell cancer, and large-cell lung neoplasm. Adenocarcinoma generally arises in non-smokers, extends towards the periphery, and multiplies in size within 161 days, while squamous cell cancers account for nearly 30% of all lung cancer. It generally originates at the center of the chest, grows slowly, and doubles in size in 88 days. In contrast, small cell lung neoplasm account for nearly 20–25% of lung carcinoma, which tends to originate in central locations, grow swiftly, and double in size in approximately 29 days. The major population diagnosed with small cell lung cancer have metastatic carcinoma (Norouzi and Hardy, 2021). However, therapies such as conventional chemotherapy could reduce the growth of a tumor, but also affects the gastric, cardiac, and neural functions of the body. Meanwhile, nano medicines also present novel ideas that could reduce such toxicity while improving the therapeutic window, but non-targeted preparation does not provide the desired benefit.
To overcome the drawbacks related to passive targeting, ligand-based nano-carriers have been under investigation. αvβ3 integrin is largely expressed on numerous tumors including, lung, breast, colon, pancreatic and hepatic. RGD is a small sequence ligand that shows high affinity towards αvβ3 integrin over-expressing cancer cells. This was proven in a study conducted by Zhang and team, who formulated PEGylated liposome (PEG-LIP) carrying bufalin, a highly toxic and short-lived anti-cancer moiety, finally functionalized with RGD peptide. As compared to plain bufalin (BF), RGD-PEG-BF-LIP significantly inhibited proliferation of A549 (lung cancer) cells showing only 10.11 ± 2.2 viability, which was much greater than PEG-BF-LIP (13.21 ± 3.5%) and pure BF (96.6 ± 3.7%). Strikingly, PEG-BF-LIP also showed significant results because liposomes were modified with DSPE-mPEG (2000) that prolonged the release of BF. The least cell viability by RGD-PEG-BF-LIP was due to the tumor homing effect as RGD peptide was up taken by cancer cells having integrin receptor on the surface (Zhang Y. et al., 2019). Due to the transformations in research, siRNA-based therapy (Butt et al., 2016; Chadar et al., 2021) has also gained significant attention among scientists. Maximum therapeutic benefit could be achieved if such molecules could be made available at the desired site. siRNA, in a specific manner, inhibits the expression of certain genes and proteins that cause tumor growth and metastasis (Kesharwani et al., 2015a; Xiong et al., 2018). However physiological barriers such as nucleases degradation, reticuloendothelial system (RES) uptake, endothelial barrier, and the high rate of elimination are among major hurdles including anionic nature and hydrophilicity, which causes effective protein knockdown and inhibits cellular entry. To overcome such barriers, Khatri et al., prepared the aggregate of siRNA and calcium phosphate through ionic interaction and entrapped it inside the liposomal core. However, due to changes in pH, temperature, and concentration, reproducibility presents another challenge. Hence, a nanocarrier with a well-defined structure could solve this issue. To achieve targeting effect, cRGD peptide was engineered to siRNA entrapped liposomes. The targeted preparation carrying siRNA exhibited 24.2 ± 3.4% gene expression in A549 cells, while bare siRNA unveiled more than 80% gene expression.
The higher the expression of a gene; the poorer its silencing ability. Higher gene expression by naked siRNA can be explained due to low transfection in the cell, while liposomal preparation has higher transfection efficiency, which is due to the structural resemblance between formulation and biological membrane. DOPE in liposomes helped in the amalgamation of cell and endosomal membrane to the liposomes, which deliver the high cytosolic siRNA availability of siRNA with a small expression of the gene. To summarize, liposomes proved an excellent carrier for the delivery of genes, which upon modification, helped in enhancing uptake to promote gene silencing (Khatri et al., 2014).
Liposomes can entrap both hydrophilic and hydrophobic drugs. They are also an important carrier for diagnostic molecules that are required for the detection of cancer growth when extensive techniques such as MRI and X-ray fail due to a lack of inherent sensitivity. In one study, RGD engineered liposomes carrying gadolinium diethylenetriamine penta-acetic acid (GD-DTPA) (RGD-GD-DTPA-LIP) and GD-DTPA-LIP were prepared. Nanoparticles with a high payload of imaging agents along with surface modification with ligand could specifically deliver nanoparticles at the tumor region. As observed by MRI imaging in A449 tumor bearing mice, the intensity within the GD-DTPA treated group reached a maximum after 1 h, which further declined rapidly. The non-targeted liposome also showed maximum intensity after 2 h, however, its surface enhancement rate (SER) also declined and returned to the baseline 6-h post-injection. The maximal SER was observed after targeted therapy after 4 h, which was highest among all groups that also maintained maximum level for 6 h. This shows the efficiency of the ligand that helped in internalization and retention inside the desired cell (Li et al., 2011). Hence, RGD anchored liposomes could not only deliver the chemotherapeutic agent directly to the anticipated site but also make the imaging agent available at cancer cells.
Colon and liver cancer are the deadliest malignancies for both sexes. Environmental associations and genetic factors contribute significantly to their growth. Due to the impact of screening and proper vaccination, the new case rate has fallen in recent years (Gelband et al., 2015; Aljabali et al., 2020; Recio-Boiles and Cagir, 2021). However, much still needs to be done that could hone to hasten the decline. For colon cancer, endoscopic resection, surgical resection, and adjuvant therapy remain the available options for therapy. However, such therapy is available for stage II and above patients, while conventional therapy could be useful up to a certain extent but could not revamp the overall survival (Dawson et al., 2019). Liver cancer is one of the least successfully treated cancers but is among the most readily prevented cancer. The major intervention for liver cancer is the anti-hepatitis vaccine. Currently, there is no standard second-line chemotherapy available for patients who do not respond to sorafenib. Moreover, hepatic or liver carcinoma is considered a chemotherapy-refractory tumor and thus chemotherapy is not given in patients routinely. Among other available drugs, doxorubicin has been studied for advanced hepatic cell carcinoma, but the results remain disappointing (Burroughs et al., 2004; Yeo et al., 2005). Personalized medicine could be a better option for therapy (None et al., 2018) but before researchers directly undertake treatment, they first need to understand the demands of the biological system. Receptor over-expression on tumor cells and their targeting has been the cornerstone of this approach. Among the various vascular markers, anti-vascular targeting of integrins αvβ3, α5β1, and αvβ5 were critically associated with tumor differentiation and its metastasis. Compared to quiescent and pre-existing vessels, a higher expression of integrin in the tumor environment makes them a promising target and target-based research is making headway.
Scientists are not just exploring the ligands, they are also modifying them to improve their targetability. This is demonstrated in a study by Amin and team. They decorated PEGylated liposomes with three peptides individually. The chosen peptides were RGD-d-Tyr-Cys (RGDyC), which is least hydrophilic, RGD-d-Phe-Lys (RGDfK), and RGD-d-Phe-(N-Methyl) Lys [RGDf (N-Met) K] (moderately hydrophilic). The doxorubicin loaded PEGylated liposome with RGDf-(N-Met)-K motif showed 2.46- and 3.93-times higher accumulation in contrast to liposome bearing RGDyC and RGDfK motif. This effect was achieved due to the direct attachment of targeted nanoparticles to vasculature mediated by EPR. The controlled and prolonged release is a prerequisite for any nanoparticle, that opens the door for attachment and effective collision or extravasation. This was the reason RGDfK grafted doxorubicin loaded liposome could not achieve greater accumulation of high tumor level despite high binding ability. The targeted therapy also showed a promising anti-cancer effect in vivo by increasing the survival rate and suppressing the tumor growth in mice (Amin et al., 2013).
In another study, RGD grafted lipid-based nanoparticles carrying both bovine serum albumin (BSA) and chemotherapeutic (PTX) were prepared using calcium carbonate (CaCO3) forming RBPCL. The advantage of CaCO3 in nano-preparation is that it gets destroyed in the acidic microenvironment of the lysosome. This could increase the release of drugs in tumor interstitium. The protein-loaded nanoparticle imitated in vivo circulation in blood, additionally, decomposition of CaCO3 favourably elevated intracellular drug and protein release. Due to the RGD ligand, the uptake of nanoparticle affected the growth of tumor in colon tumor xenograft mice (Peng et al., 2019) (Figure 5). The simple PEGylation of nanoparticle can prolong circulation time by avoiding the interaction of plasma protein and quick removal by reticuloendothelial system (RES). Thus, the carrier can be accumulated at tumor site and is efficiently internalized into the cell. As every coin has two faces, PEGylation seriously affects the interaction of carriers with the cell at their arrival at tumor site (Gullotti and Yeo, 2009). To find a solution, cleavable PEG, through certain linkers on nanocarrier surface, could remove the PEG after it arrives at the tumor cell (Kale and Torchilin, 2007). Considering the “shielding” effect provided by cleavable PEG, a multistage liposomal therapy modified by RGD, cell penetrating peptide [Tat (AYGRKKRRQRRR)] and cleavable PEG has been manufactured by Mei and co-workers. After investigation of the cell trafficking of liposome, it was observed that RGD-TAT modified cleavable PEG labeled liposome (RGD-Tat-C-LIP) was majorly observed in the cytoplasm, however, few were also encountered in lysosomes. This is due to clathrin-mediated endocytosis, which suggests the mitogenic substances ultimately reached the. However, cleavable PEG and Tat co-modified liposome (Tat-C-LIP) were majorly scattered in the cytoplasmic section outside the lysosome which stipulated that both had a capability for lysosomal escape. However, PEG did not shed entirely from the surface of the liposome initially, but, after interaction with the lysosome enzyme, the PEG got detached.
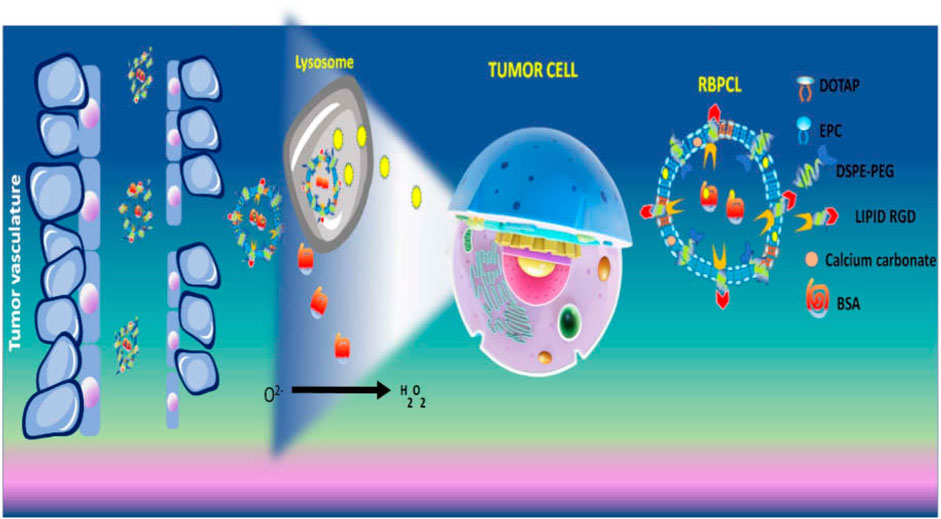
FIGURE 5. Schematic illustration of pH dependent drug release from CaCO3 nanoparticles containing BSA and PTX.
Liposomes were also able to mediate successful lysosomal escape due to the membrane fusion effect of Tat. The dual targeted (Tat and RGD) liposome was strongly internalized in hepatic tumor cells in comparison to a single ligated liposome. The exposed integrin receptor bound to the RGD grafted material and sufficiently internalized the molecule inside the cell (Mei et al., 2014). The biocompatible nature of liposome makes them an important carrier for many anti-cancer drugs that show severe systemic toxicity (Bulbake et al., 2017; Lammers et al., 2008; Park, 2007). Hollow mesoporous silica nanoparticle (MSN) carrying anti-cancer agent-arsenic trioxide was coated with biocompatible liposome by Fei et al., to address the challenges of MSN, such as burst release and low circulation time, poor targetability and severe toxicity. To have a safe delivery system, the nanoparticle was then modified by RGD. In contrast to plain arsenic trioxide, the targeted liposomal encapsulated MSN (RGD-L-MSN) prolonged the AUC and half-life by 2.4 and 1.7 times. Additionally, in H22 tumor grafted mouse model, the nanocarrier amended the anti-cancer potential and targeting ability of the anti-cancer agent (Fei et al., 2017). Very recently, liposomes were prepared with the aim of encapsulating and improving the aqueous solubility of galbanic acid (GA), which is an effective anti-proliferative and anti-angiogenic compound. However, it fails to deliver significant results due to poor bioavailability. To further enhance the targeting effect, the nano-preparation was decorated with RGD peptide (Figure 6). The final formulation was successful at overcoming the solubility issues, due to entrapment of drug in the lipid bilayer. As compared to other preparations, the optimized liposome (size: 100 nm, zeta potential: -22.5, PDI: 0.16 with entrapment efficiency: 77%) showed that slow release is essential for the specific release of the tumor environment. The accumulation of targeted liposomal preparation was more than the non-targeted liposomes in HUVEC cells (colon cancer cells), which was due to tumor homing effect resulting from the interaction of RGD with integrin. The cell viability was less after treatment with RGD modified preparation compared to free galbanic acid. In vivo anti-tumor efficacy was also elevated after treatment with RGD-liposomes bearing galbanic acid (Nik et al., 2021). The outcome of the therapy proved to have strong anti-cancer effects.
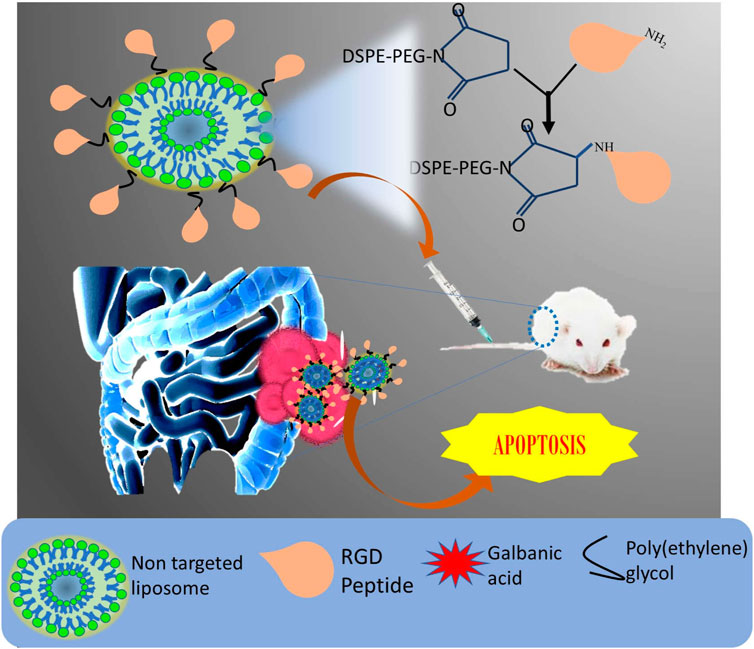
FIGURE 6. Schematic representation of preparation of RGD-decorated liposome loaded with galbanic acid against colon cancer.
Due to their versatile applicability, liposomes represent the most appealing delivery system. From enhancing the solubility of water insoluble drugs to their accumulation at tumor cells, liposomes have beneficial features. A simple modification of liposomes such as PEGylation or using pH or thermosensitive lipid accelerates their clinical demands and safety profile. RGD peptides demonstrate excellent ligand binding ability towards the integrin family and its modification with liposomes provides broad-spectrum therapy. Integrins act as cellular mechanotransducers, which are involved in various cancers such as breast, colon, lung, hepatic, and skin, etc.,
Activation of the integrin mediated bidirectional signaling feeds tumor growth and metastasis. Therefore, targeting integrin in cancer stroma is a crucial avenue for uncovering therapeutic options. In principle, liposomal therapy targets integrin via ligation with RGD peptide perpetuated high uptake, profound release, reduced cell viability, improved cytotoxicity, and elevated survival. Thus, inhibiting the activation of integrins at any stage of cancer could be beneficial for cancer patients. However, RGD engineered liposomes are still in the early stages of development for target mediated treatment. Several challenges such as non-specific binding with serum and recognition by the immune system disable their functions. To date, RGD modified liposomes have not received significant attention in clinical trials. The reason for this could be instability and low drug loading. Moreover, the high rate of cancer heterogeneity poses a major challenge in its utilization.
In general, in vivo cell specificity is somewhat different from in vitro cell targeting as an expression of integrin over endothelial cells. Further knowledge of their behavior in the cellular environment is critical and extensive molecular studies are required before jumping into therapy.
AS: writing—original draft. NA: resources. SM: resources. PK: writing—review and editing.
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia funded this project under Grant Nos. (FP-90-42).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, K., Lee, E. J., Shaikh, S., Kumar, A., Rao, K. M., Park, S. Y., et al. (2021). Targeting Integrins for Cancer Management Using Nanotherapeutic Approaches: Recent Advances and Challenges. Semin. Cancer Biol. 69, 325–336. doi:10.1016/j.semcancer.2019.08.030
Ali, M. R. K., Wu, Y., Tang, Y., Xiao, H., Chen, K., Han, T., et al. (2017). Targeting Cancer Cell Integrins Using Gold Nanorods in Photothermal Therapy Inhibits Migration through Affecting Cytoskeletal Proteins. Proc. Natl. Acad. Sci. U S A. 114, E5655–E5663. doi:10.1073/PNAS.1703151114
Aljabali, A. A. A., Bakshi, H. A., Hakkim, F. L., Haggag, Y. A., Al-Batanyeh, K. M., Al Zoubi, M. S., et al. (2020). Albumin Nano-Encapsulation of Piceatannol Enhances its Anticancer Potential in Colon Cancer via Downregulation of Nuclear P65 and HIF-1α. Cancers (Basel) 12. doi:10.3390/cancers12010113
Alkabban, F. M., and Ferguson, T. (2021). Breast Cancer. Cambridge Handb. Psychol. Heal. Med. Second Ed, 577–580.
Allen, T. M., and Cullis, P. R. (2013). Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 65, 36–48. doi:10.1016/j.addr.2012.09.037
Alphandéry, E., Grand-Dewyse, P., Lefèvre, R., and Mandawala, C. (2015). Cancer Therapy Using Nanoformulated Substances: Scientific, Regulatory and Financial Aspects. Expert Rev. Anticancer Ther. 15, 1233–1255. doi:10.1586/14737140.2015.1086647
Amin, M., Badiee, A., and Jaafari, M. R. (2013). Improvement of Pharmacokinetic and Antitumor Activity of PEGylated Liposomal Doxorubicin by Targeting with N-Methylated Cyclic RGD Peptide in Mice Bearing C-26 colon Carcinomas. Int. J. Pharm. 458, 324–333. doi:10.1016/j.ijpharm.2013.10.018
Amjad, M. W., Kesharwani, P., Mohd Amin, M. C. I., and Iyer, A. K. (2017). Recent Advances in the Design, Development, and Targeting Mechanisms of Polymeric Micelles for Delivery of siRNA in Cancer Therapy. Prog. Polym. Sci. 64, 154–181. doi:10.1016/j.progpolymsci.2016.09.008
Andresen, T. L., Jensen, S. S., and Jørgensen, K. (2005). Advanced Strategies in Liposomal Cancer Therapy: Problems and Prospects of Active and Tumor Specific Drug Release. Prog. Lipid Res. 44, 68–97. doi:10.1016/J.PLIPRES.2004.12.001
Aoudjit, F., and Vuori, K. (2001). Integrin Signaling Inhibits Paclitaxel-Induced Apoptosis in Breast Cancer Cells. Oncogene 20, 4995–5004. doi:10.1038/SJ.ONC.1204554
Arias-Salgado, E. G., Lizano, S., Sarkar, S., Brugge, J. S., Ginsberg, M. H., and Shattil, S. J. (2003). Src Kinase Activation by Direct Interaction with the Integrin Beta Cytoplasmic Domain. Proc. Natl. Acad. Sci. U. S. A. 100, 13298–13302. doi:10.1073/PNAS.2336149100
Askari, J. A., Buckley, P. A., Mould, A. P., and Humphries, M. J. (2009). Linking Integrin Conformation to Function. J. Cel Sci. 122, 165–170. doi:10.1242/JCS.018556
Assali, A., Akhavan, O., Mottaghitalab, F., Adeli, M., Dinarvand, R., Razzazan, S., et al. (2018). Cationic Graphene Oxide Nanoplatform Mediates miR-101 Delivery to Promote Apoptosis by Regulating Autophagy and Stress. Int. J. Nanomedicine 13, 5865–5886. doi:10.2147/IJN.S162647
Bae, Y. H., and Park, K. (2011). Targeted Drug Delivery to Tumors: Myths, Reality and Possibility. J. Control Release 153, 198–205. doi:10.1016/J.JCONREL.2011.06.001
Bandak, S., Goren, D., Horowitz, A., Tzemach, D., and Gabizon, A. (1999). Pharmacological Studies of Cisplatin Encapsulated in Long-Circulating Liposomes in Mouse Tumor Models. Anticancer. Drugs 10, 911–920. doi:10.1097/00001813-199911000-00007
Barenholz, Y. (2012). Doxil®--the First FDA-Approved Nano-Drug: Lessons Learned. J. Control Release 160, 117–134. doi:10.1016/J.JCONREL.2012.03.020
Belhadj, Z., Ying, M., Cao, X., Hu, X., Zhan, C., Wei, X., et al. (2017). Design of Y-Shaped Targeting Material for Liposome-Based Multifunctional Glioblastoma-Targeted Drug Delivery. J. Control Release 255, 132–141. doi:10.1016/J.JCONREL.2017.04.006
Bellis, S. L. (2011). Advantages of RGD Peptides for Directing Cell Association with Biomaterials. Biomaterials 32, 4205–4210. doi:10.1016/J.BIOMATERIALS.2011.02.029
Bello, L., Zhang, J., Nikas, D. C., Strasser, J. F., Villani, R. M., and Cheresh, D. A. (2000). Alpha(v)beta3 and Alpha(v)beta5 Integrin Expression in Meningiomas. Neurosurgery 47, 1185–1195. doi:10.1097/00006123-200011000-00035
Bialkowska, K., Kulkarni, S., Du, X., Goll, D. E., Saido, T. C., and Fox, J. E. (2000). Evidence that Beta3 Integrin-Induced Rac Activation Involves the Calpain-dependent Formation of Integrin Clusters that Are Distinct from the Focal Complexes and Focal Adhesions that Form as Rac and RhoA Become Active. J. Cel Biol. 151, 685–696. doi:10.1083/JCB.151.3.685
Böger, C., Kalthoff, H., Goodman, S. L., Behrens, H. M., and Röcken, C. (2014). Integrins and Their Ligands Are Expressed in Non-small Cell Lung Cancer but Not Correlated with Parameters of Disease Progression. Virchows Arch. 464, 69–78. doi:10.1007/s00428-013-1506-1
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Brüning, A., KöhlerKöhler, T., Quist, S., Wang-Gohrke, S., Moebus, V. J., Kreienberg, R., et al. (2001). Adenoviral Transduction Efficiency of Ovarian Cancer Cells Can Be Limited by Loss of Integrin β3Subunit Expression and Increased by Reconstitution of Integrin αvβ3. Hum. Gene Ther. 12, 391–399. doi:10.1089/10430340150504019
Bulbake, U., Doppalapudi, S., Kommineni, N., and Khan, W. (2017). Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 9, 1–33. doi:10.3390/pharmaceutics9020012
Burroughs, A., Hochhauser, D., and Meyer, T. (2004). Systemic Treatment and Liver Transplantation for Hepatocellular Carcinoma: Two Ends of the Therapeutic Spectrum. Lancet Oncol. 5, 409–418. doi:10.1016/S1470-2045(04)01508-6
Butt, A. M., Amin, M. C., Katas, H., Abdul Murad, N. A., Jamal, R., and Kesharwani, P. (2016). Doxorubicin and siRNA Codelivery via Chitosan-Coated pH-Responsive Mixed Micellar Polyplexes for Enhanced Cancer Therapy in Multidrug-Resistant Tumors. Mol. Pharm. 13, 4179–4190. doi:10.1021/acs.molpharmaceut.6b00776
Carter, K. A., Shao, S., Hoopes, M. I., Luo, D., Ahsan, B., Grigoryants, V. M., et al. (2014). Porphyrin-phospholipid Liposomes Permeabilized by Near-Infrared Light. Nat. Commun. 5 (5), 3546–3556. doi:10.1038/ncomms4546
Chadar, R., AfsanaKesharwani, P., and Kesharwani, P. (2021). Nanotechnology-based siRNA Delivery Strategies for Treatment of Triple Negative Breast Cancer. Int. J. Pharm. 605, 120835. doi:10.1016/J.IJPHARM.2021.120835
Chang, M., Lu, S., Zhang, F., Zuo, T., Guan, Y., Wei, T., et al. (2015). RGD-modified pH-Sensitive Liposomes for Docetaxel Tumor Targeting. Colloids Surf. B Biointerfaces 129, 175–182. doi:10.1016/j.colsurfb.2015.03.046
Chang-Qing, Y., Jie, L., Shi-Qi, Z., Kun, Z., Zi-Qian, G., Ran, X., et al. (2020). Recent Treatment Progress of Triple Negative Breast Cancer. Prog. Biophys. Mol. Biol. 151, 40–53. doi:10.1016/j.pbiomolbio.2019.11.007
Choudhury, H., Pandey, M., Chin, P. X., Phang, Y. L., Cheah, J. Y., Ooi, S. C., et al. (2018). Transferrin Receptors-Targeting Nanocarriers for Efficient Targeted Delivery and Transcytosis of Drugs into the Brain Tumors: a Review of Recent Advancements and Emerging Trends. Drug Deliv. Transl. Res. 8, 1545–1563. doi:10.1007/s13346-018-0552-2
Cooper, J., and Giancotti, F. G. (2019). Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 35, 347–367. doi:10.1016/J.CCELL.2019.01.007
Danhier, F., Pourcelle, V., Marchand-Brynaert, J., Jérôme, C., Feron, O., and Préat, V. (2012). Targeting of Tumor Endothelium by RGD-Grafted PLGA-Nanoparticles. Methods Enzymol. 508, 157–175. doi:10.1016/B978-0-12-391860-4.00008-2
Dawson, H., Kirsch, R., Messenger, D., and Driman, D. (2019). A Review of Current Challenges in Colorectal Cancer Reporting. Arch. Pathol. Lab. Med. 143, 869–882. doi:10.5858/ARPA.2017-0475-RA
De Franceschi, N., Hamidi, H., Alanko, J., Sahgal, P., and Ivaska, J. (2015). Integrin Traffic - the Update. J. Cel Sci. 128, 839–852. doi:10.1242/JCS.161653
Dozynkiewicz, M. A., Jamieson, N. B., MacPherson, I., Grindlay, J., van den Berghe, P. V., and von Thun, A. (2012). Rab25 and CLIC3 Collaborate to Promote Integrin Recycling from Late Endosomes/lysosomes and Drive Cancer Progression. Dev. Cel 22, 131–145. doi:10.1016/J.DEVCEL.2011.11.008
Drummond, D. C., Noble, C. O., Guo, Z., Hong, K., Park, J. W., and Kirpotin, D. B. (2006). Development of a Highly Active Nanoliposomal Irinotecan Using a Novel Intraliposomal Stabilization Strategy. Cancer Res. 66, 3271–3277. doi:10.1158/0008-5472.CAN-05-4007
Duro-Castano, A., Gallon, E., Decker, C., and Vicent, M. J. (2017). Modulating Angiogenesis with Integrin-Targeted Nanomedicines. Adv. Drug Deliv. Rev. 119, 101–119. doi:10.1016/j.addr.2017.05.008
Estanqueiro, M., Amaral, M. H., Conceição, J., and Sousa Lobo, J. M. (2015). Nanotechnological Carriers for Cancer Chemotherapy: The State of the Art. Colloids Surf. B Biointerfaces 126, 631–648. doi:10.1016/j.colsurfb.2014.12.041
Ezratty, E. J., Bertaux, C., Marcantonio, E. E., and Gundersen, G. G. (2009). Clathrin Mediates Integrin Endocytosis for Focal Adhesion Disassembly in Migrating Cells. J. Cel Biol. 187, 733–747. doi:10.1083/JCB.200904054
Fei, W., Zhang, Y., Han, S., Tao, J., Zheng, H., Wei, Y., et al. (2017). RGD Conjugated Liposome-Hollow Silica Hybrid Nanovehicles for Targeted and Controlled Delivery of Arsenic Trioxide against Hepatic Carcinoma. Int. J. Pharm. 519, 250–262. doi:10.1016/j.ijpharm.2017.01.031
Forssen, E. A. (1997). The Design and Development of DaunoXome® for Solid Tumor Targeting In Vivo. Adv. Drug Deliv. Rev. 24, 133–150. doi:10.1016/S0169-409X(96)00453-X
Gelband, H., Chen, C.-J., Chen, W., Franceschi, S., Hall, S. A., London, W. T., et al. (2015). Liver Cancer. Dis. Control Priorities. Cancer Vol. 3, 147–164. doi:10.1596/978-1-4648-0349-9_CH8Third Ed.
Gh, P., Sk, A., Ss, S., and Nm, L.-B. (2016). Meta-analysis of Clinical and Preclinical Studies Comparing the Anticancer Efficacy of Liposomal versus Conventional Non-liposomal Doxorubicin. J. Control Release 232, 255–264. doi:10.1016/J.JCONREL.2016.04.028
Gorain, B., Bhattamishra, S. K., Choudhury, H., Nandi, U., and Pandey, M. (2019). Overexpressed Receptors and Proteins in Lung Cancer. Nanotechnology-Based Target. Drug Deliv. Syst. Lung Cancer 13, 39–75. doi:10.1016/B978-0-12-815720-6.00003-4
Gowrishankar, G., Zitzmann-Kolbe, S., Junutula, A., Reeves, R., Levi, J., Srinivasan, A., et al. (2011). GLUT 5 Is Not Over-expressed in Breast Cancer Cells and Patient Breast Cancer Tissues. PLoS One 6, e26902. doi:10.1371/JOURNAL.PONE.0026902
Grant, D. S., Tashiro, K-I., Segui-Real, B., Yamada, Y., Martin, G. R., and Kleinman, H. K. (1989). Two Different Laminin Domains Mediate the Differentiation of Human Endothelial Cells into Capillary-like Structures In Vitro. Cell 58, 933–943. doi:10.1016/0092-8674(89)90945-8
Gullotti, E., and Yeo, Y. (2009). Extracellularly Activated Nanocarriers: a New Paradigm of Tumor Targeted Drug Delivery. Mol. Pharm. 6, 1041–1051. doi:10.1021/MP900090Z
Guo, W., and Giancotti, F. G. (2004). Integrin Signalling during Tumour Progression. Nat. Rev. Mol. Cel Biol. 5, 816–826. doi:10.1038/NRM1490
Guo, Z., He, B., Jin, H., Zhang, Haoran., Dai, W., Zhang, L., et al. (2014). Targeting Efficiency of RGD-Modified Nanocarriers with Different Ligand Intervals in Response to Integrin αvβ3 Clustering. Biomaterials 35, 6106–6117. doi:10.1016/j.biomaterials.2014.04.031
Hamidi, H., and Ivaska, J. (20182018). Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Rev. Cancer 189 18, 533–548. doi:10.1038/s41568-018-0038-z
He, C., Hu, Y., Yin, L., Tang, C., and Yin, C. (2010). Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 31, 3657–3666. doi:10.1016/J.BIOMATERIALS.2010.01.065
Horsman, M. R., and Siemann, D. W. (2006). Pathophysiologic Effects of Vascular-Targeting Agents and the Implications for Combination with Conventional Therapies. Cancer Res. 66, 11520–11539. doi:10.1158/0008-5472.CAN-06-2848
Horton, M. A., Nesbit, M. A., and Helfrich, M. H. (1995). Interaction of Osteopontin with Osteoclast Integrins. Ann. N. Y. Acad. Sci. 760, 190–200. doi:10.1111/J.1749-6632.1995.TB44630.X
Hu, G., Zhang, H., Zhang, L., Ruan, S., He, Q., and Gao, H. (2015). Integrin-mediated Active Tumor Targeting and Tumor Microenvironment Response Dendrimer-Gelatin Nanoparticles for Drug Delivery and Tumor Treatment. Int. J. Pharm. 496, 1057–1068. doi:10.1016/j.ijpharm.2015.11.025
Huang, T., Alvarez, A., Hu, B., and Cheng, S.-Y. (2013). Noncoding RNAs in Cancer and Cancer Stem Cells. Chinj. Cancer 32, 582. doi:10.5732/CJC.013.10170
Humphries, J. D., Byron, A., and Humphries, M. J. (2006). Integrin Ligands at a Glance. J. Cel Sci. 119, 3901–3903. doi:10.1242/JCS.03098
Hunt, K. K., and Vorburger, S. A. (2002). Tech.Sight. Gene Therapy. Hurdles and Hopes for Cancer Treatment. Science 297, 415–416. (80-. ). doi:10.1126/SCIENCE.297.5580.415
Hwang, D. S., Sim, S. B., and Cha, H. J. (2007). Cell Adhesion Biomaterial Based on Mussel Adhesive Protein Fused with RGD Peptide. Biomaterials 28, 4039–4046. doi:10.1016/J.BIOMATERIALS.2007.05.028
Hynes, R. O. (2002). Integrins: Bidirectional, Allosteric Signaling Machines. Cell 110, 673–687. doi:10.1016/S0092-8674(02)00971-6
Immordino, M. L., Dosio, F., and Cattel, L. (2006). Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomedicine 1, 297.
Jain, K., Kesharwani, P., Gupta, U., and Jain, N. K. (2012). A Review of Glycosylated Carriers for Drug Delivery. Biomaterials 33, 4166–4186. doi:10.1016/J.BIOMATERIALS.2012.02.033
Kale, A. A., and Torchilin, V. P. (2007). Enhanced Transfection of Tumor Cells In Vivo Using “Smart” pH-Sensitive TAT-Modified Pegylated Liposomes. J. Drug Target. 15, 538. doi:10.1080/10611860701498203
Kaur, H., and Kesharwani, P. (2021). Advanced Nanomedicine Approaches Applied for Treatment of Skin Carcinoma. J. Control Release 337, 589–611. doi:10.1016/J.JCONREL.2021.08.003
Keikha, M., and Esfahani, B. N. (2018). The Relationship between Tuberculosis and Lung Cancer. Adv. Biomed. Res. 7, 58. doi:10.4103/ABR.ABR_182_17
Kesharwani, P., Banerjee, S., Gupta, U., Mohd Amin, M. C. I., Padhye, S., Sarkar, F. H., et al. (2015a). PAMAM Dendrimers as Promising Nanocarriers for RNAi Therapeutics. Mater. Today 18, 565–572. doi:10.1016/j.mattod.2015.06.003
Kesharwani, P., Gothwal, A., Iyer, A. K., Jain, K., Chourasia, M. K., and Gupta, U. (2018). Dendrimer Nanohybrid Carrier Systems: an Expanding Horizon for Targeted Drug and Gene Delivery. Drug Discov. Today 23, 300–314. doi:10.1016/j.drudis.2017.06.009
Kesharwani, P., and Iyer, A. K. (2015). Recent Advances in Dendrimer-Based Nanovectors for Tumor-Targeted Drug and Gene Delivery. Drug DiscovToday 20, 536–547. doi:10.1016/j.drudis.2014.12.012
Kesharwani, P., Jain, K., and Jain, N. K. (2014a). Dendrimer as Nanocarrier for Drug Delivery. Prog. Polym. Sci. 39, 268–307. doi:10.1016/j.progpolymsci.2013.07.005
Kesharwani, P., Md, S., Alhakamy, N. A., Hosny, K. M., and Haque, A. (2021). QbD Enabled Azacitidine Loaded Liposomal Nanoformulation and its In Vitro Evaluation. Polymers (Basel) 13, 250. doi:10.3390/polym13020250
Kesharwani, P., Tekade, R. K., Gajbhiye, V., Jain, K., and Jain, N. K. (2011). Cancer Targeting Potential of Some Ligand-Anchored Poly(propylene Imine) Dendrimers: A Comparison. Nanomedicine Nanotechnology, Biol. Med. 7, 295–304. doi:10.1016/j.nano.2010.10.010
Kesharwani, P., Tekade, R. K., and Jain, N. K. (2015b). Dendrimer Generational Nomenclature: the Need to Harmonize. Drug Discov. Today. doi:10.1016/j.drudis.2014.12.015
Kesharwani, P., Tekade, R. K., and Jain, N. K. (2014b). Generation Dependent Cancer Targeting Potential of Poly(propyleneimine) Dendrimer. Biomaterials 35, 5539–5548. doi:10.1016/j.biomaterials.2014.03.064
Khatri, N., Baradia, D., Vhora, I., Rathi, M., and Misra, A. (2014). CRGD Grafted Liposomes Containing Inorganic Nano-Precipitate Complexed siRNA for Intracellular Delivery in Cancer Cells. J. Control Release 182, 45–57. doi:10.1016/j.jconrel.2014.03.003
Kluza, E., Jacobs, I., Hectors, S. J. C. G., Mayo, K. H., Griffioen, A. W., Strijkers, G. J., et al. (2012). Dual-targeting of α Vβ 3 and Galectin-1 Improves the Specificity of Paramagnetic/fluorescent Liposomes to Tumor Endothelium In Vivo. J. Control Release 158, 207–214. doi:10.1016/j.jconrel.2011.10.032
Koudelka, S., and Turánek, J. (2012). Liposomal Paclitaxel Formulations. J. Control Release 163, 322–334. doi:10.1016/J.JCONREL.2012.09.006
Lammers, T., Hennink, W. E., and Storm, G. (2008). Tumour-targeted Nanomedicines: Principles and Practice. Br. J. Cancer. doi:10.1038/sj.bjc.6604483
Landen, C. N., Kim, T. J., Lin, Y. G., Merritt, W. M., Kamat, A. A., Han, L. Y., et al. (2008). Tumor-selective Response to Antibody-Mediated Targeting of αvβ3 Integrin in Ovarian Cancer. Neoplasia 10, 1259–1267. doi:10.1593/neo.08740
Lawler, J., Weinstein, R., and Hynes, R. O. (1988). Cell Attachment to Thrombospondin: the Role of ARG-GLY-ASP, Calcium, and Integrin Receptors. J. Cel Biol. 107, 2351. doi:10.1083/JCB.107.6.2351
Legate, K. R., Montañez, E., Kudlacek, O., and Fässler, R. (2006). ILK, PINCH and Parvin: the tIPP of Integrin Signalling. Nat. Rev. Mol. Cel Biol. 7, 20–31. doi:10.1038/NRM1789
Legate, K. R., Wickström, S. A., and Fässler, R. (2009). Genetic and Cell Biological Analysis of Integrin Outside-In Signaling. Genes Dev. 23, 397–418. doi:10.1101/GAD.1758709
Leonard, R. C. F., Williams, S., Tulpule, A., Levine, A. M., and Oliveros, S. (2009). Improving the Therapeutic index of Anthracycline Chemotherapy: Focus on Liposomal Doxorubicin (MyocetTM). The Breast 18, 218–224. doi:10.1016/J.BREAST.2009.05.004
Li, J., Chai, Z., Lu, J., Xie, C., Ran, D., Wang, S., et al. (2020). Ɑ V β 3 -targeted Liposomal Drug Delivery System with Attenuated Immunogenicity Enabled by Linear Pentapeptide for Glioma Therapy. J. Control Release 322, 542–554. doi:10.1016/j.jconrel.2020.04.009
Li, W., Su, B., Meng, S., Ju, L., Yan, L., Ding, Y., et al. (2011). RGD-targeted Paramagnetic Liposomes for Early Detection of Tumor: In Vitro and In Vivo Studies. Eur. J. Radiol. 80, 598–606. doi:10.1016/j.ejrad.2011.01.051
Li, Y., Xiao, Y., Lin, H. P., Reichel, D., Bae, Y., Lee, E. Y., et al. (2019). In Vivo β-catenin Attenuation by the Integrin α5-targeting Nano-Delivery Strategy Suppresses Triple Negative Breast Cancer Stemness and Metastasis. Biomaterials 188, 160–172. doi:10.1016/j.biomaterials.2018.10.019
Li, Y., Zheng, X., Sun, Y., Ren, Z., Li, X., and Cui, G. (2014). RGD-fatty Alcohol-Modified Docetaxel Liposomes Improve Tumor Selectivity In Vivo. Int. J. Pharm. 468, 133–141. doi:10.1016/j.ijpharm.2014.04.001
Lin, S., Xu, H., Zhang, A., Ni, Y., Xu, Y., Meng, T., et al. (2020). Prognosis Analysis and Validation of m6A Signature and Tumor Immune Microenvironment in Glioma. Front. Oncol. 0, 1916. doi:10.3389/FONC.2020.541401
Liu, Y., Ji, M., Wong, M. K., Joo, K. I., and Wang, P. (20132013). Enhanced Therapeutic Efficacy of iRGD-Conjugated Crosslinked Multilayer Liposomes for Drug Delivery. Biomed. Res. Int. doi:10.1155/2013/378380
Liu, Y., Ran, R., Chen, J., Kuang, Q., Tang, J., Mei, L., et al. (2014). Biomaterials Paclitaxel Loaded Liposomes Decorated with a Multifunctional Tandem Peptide for Glioma Targeting. Biomaterials 35, 4835–4847. doi:10.1016/j.biomaterials.2014.02.031
Luong, D., Kesharwani, P., Deshmukh, R., Mohd Amin, M. C. I., Gupta, U., Greish, K., et al. (2016). PEGylated PAMAM Dendrimers: Enhancing Efficacy and Mitigating Toxicity for Effective Anticancer Drug and Gene Delivery. Acta Biomater. 43, 14–29. doi:10.1016/J.ACTBIO.2016.07.015
Madheswaran, T., Baskaran, R., Yoo, B. K., and Kesharwani, P. (2017). In Vitro and In Vivo Skin Distribution of 5α-Reductase Inhibitors Loaded into Liquid Crystalline Nanoparticles. J. Pharm. Sci. 106, 3385–3394. doi:10.1016/j.xphs.2017.06.016
Malta, T. M., Souza, C. F., Sabedot, T. S., Silva, T. C., Mosella, M. S., Kalkanis, S. N., et al. (2018). Glioma CpG Island Methylator Phenotype (G-CIMP): Biological and Clinical Implications. Neuro. Oncol. 20, 608–620. doi:10.1093/NEUONC/NOX183
Manjappa, A. S., Chaudhari, K. R., Venkataraju, M. P., Dantuluri, P., Sidda, C., et al. (2011). Antibody Derivatization and Conjugation Strategies: Application in Preparation of Stealth Immunoliposome to Target Chemotherapeutics to Tumor. J. Controlled Release 150, 2–22. doi:10.1016/J.JCONREL.2010.11.002
Medina, M. A., Oza, G., Sharma, A., Arriaga, L. G., Hernández, J. M. H., Rotello, V. M., et al. (2020). Triple-negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int. J. Environ. Res. Public Health. doi:10.3390/ijerph17062078
Mei, L., Fu, L., Shi, K., Zhang, Q., Liu, Y., Tang, J., et al. (2014). Increased Tumor Targeted Delivery Using a Multistage Liposome System Functionalized with RGD, TAT and Cleavable PEG. Int. J. Pharm. 468, 26–38. doi:10.1016/j.ijpharm.2014.04.008
Mirzazadeh Tekie, F. S., Atyabi, F., Soleimani, M., Arefian, E., Atashi, A., Kiani, M., et al. (2015). Chitosan Polyplex Nanoparticle Vector for miR-145 Expression in MCF-7: Optimization by Design of experiment. Int. J. Biol. Macromol. 81, 828–837. doi:10.1016/J.IJBIOMAC.2015.09.014
Mishra, V., and Kesharwani, P. (2016). Dendrimer Technologies for Brain Tumor. Drug Discov. Today 21. doi:10.1016/j.drudis.2016.02.006
Molinaro, A. M., Taylor, J. W., Wiencke, J. K., and Wrensch, M. R. (2019). Genetic and Molecular Epidemiology of Adult Diffuse Glioma. Nat. Rev. Neurol. 15, 405–417. doi:10.1038/S41582-019-0220-2
Müller, D. W., and Bosserhoff, A.-K. (2008). Integrin β3 Expression Is Regulated by Let-7a miRNA in Malignant Melanoma. Oncogene 27, 6698–6706. doi:10.1038/ONC.2008.282
Murphy, C. G., and Dickler, M. N. (2016). Endocrine Resistance in Hormone-Responsive Breast Cancer: Mechanisms and Therapeutic Strategies. Endocr. Relat. Cancer 23, R337–R352. doi:10.1530/ERC-16-0121
Murry, D. J., and Blaney, S. M. (2000). Clinical Pharmacology of Encapsulated Sustained-Release Cytarabine. Ann. Pharmacother. 34, 1173–1178. doi:10.1345/APH.19347
Muthu, M. S., Kulkarni, S. A., Xiong, J., and Feng, S. S. (2011). Vitamin E TPGS Coated Liposomes Enhanced Cellular Uptake and Cytotoxicity of Docetaxel in Brain Cancer Cells. Int. J. Pharm. 421, 332–340. doi:10.1016/J.IJPHARM.2011.09.045
Nieberler, M., Reuning, U., Reichart, F., Notni, J., Wester, H. J., Schwaiger, M., et al. (2017). Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers (Basel) 9 (9), 116. doi:10.3390/cancers9090116
Nik, M. E., Jaafari, M. R., Mashreghi, M., Nikoofal-Sahlabadi, S., Amin, M., Sadeghnia, H. R., et al. (2021). The Effect of RGD-Targeted and Non-targeted Liposomal Galbanic Acid on the Therapeutic Efficacy of Pegylated Liposomal Doxorubicin: From Liposomal Preparation to In-Vivo Studies. Int. J. Pharm. 604, 120710. doi:10.1016/j.ijpharm.2021.120710
None, A., Jain, V., Haider, N., and Jain, K. (2018). 3D Printing in Personalized Drug Delivery. Curr. Pharm. Des. 24, 5062–5071. doi:10.2174/1381612825666190215122208
Norouzi, M., and Hardy, P. (2021). Clinical Applications of Nanomedicines in Lung Cancer Treatment. Acta Biomater. 121, 134–142. doi:10.1016/j.actbio.2020.12.009
Onodera, Y., Nam, J. M., and Sabe, H. (2013). Intracellular Trafficking of Integrins in Cancer Cells. Pharmacol. Ther. 140, 1–9. doi:10.1016/J.PHARMTHERA.2013.05.007
Park, K. (2007). Nanotechnology: What it Can Do for Drug Delivery. J. Control Release. doi:10.1016/j.jconrel.2007.05.003
Pastorino, F., Brignole, C., Di Paolo, D., Nico, B., Pezzolo, A., Marimpietri, D., et al. (2006). Targeting Liposomal Chemotherapy via Both Tumor Cell-specific and Tumor Vasculature-specific Ligands Potentiates Therapeutic Efficacy. Cancer Res. 66, 10073–10082. doi:10.1158/0008-5472.CAN-06-2117
Peng, J. Q., Fumoto, S., Suga, T., Miyamoto, H., Kuroda, N., Kawakami, S., et al. (2019). Targeted Co-delivery of Protein and Drug to a Tumor In Vivo by Sophisticated RGD-Modified Lipid-Calcium Carbonate Nanoparticles. J. Control Release 302, 42–53. doi:10.1016/j.jconrel.2019.03.021
Pu, Y., Zhang, H., Peng, Y., Fu, Q., Yue, Q., Zhao, Y., et al. (2019). Dual-targeting Liposomes with Active Recognition of GLUT5 and αvβ3 for Triple-Negative Breast Cancer. Eur. J. Med. Chem. 183. doi:10.1016/j.ejmech.2019.111720
Reifenberger, G., Wirsching, H-G., Knobbe-Thomsen, C. B., and Weller, M. (2017). Advances in the Molecular Genetics of Gliomas- Implications for Classification and Therapy. Nat. Rev. Clin. Oncol. 14, 434–452. doi:10.1038/NRCLINONC.2016.204
Rolli, M., Fransvea, E., Pilch, J., Saven, A., and Felding-Habermann, B. (2003). Activated Integrin αvβ3 Cooperates with Metalloproteinase MMP-9 in Regulating Migration of Metastatic Breast Cancer Cells. Proc. Natl. Acad. Sci. U. S. A. 100, 9482. doi:10.1073/PNAS.1633689100
Ruan, S., Zhou, Y., Jiang, X., and Gao, H. (2021). Rethinking Critid Procedure of Brain Targeting Drug Delivery: Circulation, Blood Brain Barrier Recognition, Intracellular Transport, Diseased Cell Targeting, Internalization, and Drug Release. Adv. Sci. 8, 2004025. doi:10.1002/ADVS.202004025
Ruoslahti, E. (2012). Peptides as Targeting Elements and Tissue Penetration Devices for Nanoparticles. Adv. Mater. 24, 3747–3756. doi:10.1002/ADMA.201200454
Ruoslahti, E. (1996). Rgd and Other Recognition Sequences for Integrins. Annu. Rev. Cel Dev. Biol. 11, 697–715. doi:10.1146/annurev.cellbio.12.1.697
Seguin, L., Desgrosellier, J. S., Weis, S. M., and Cheresh, D. A. (2015). Integrins and Cancer: Regulators of Cancer Stemness, Metastasis, and Drug Resistance. Trends Cel Biol 25, 234–240. doi:10.1016/J.TCB.2014.12.006
Seynhaeve, A. L. B., Dicheva, B. M., Hoving, S., Koning, G. A., and ten Hagen, T. L. M. (2013). Intact Doxil Is Taken up Intracellularly and Released Doxorubicin Sequesters in the Lysosome: Evaluated by In Vitro/In Vivo Live Cell Imaging. J. Control Release 172, 330–340. doi:10.1016/j.jconrel.2013.08.034
Sheng, H., Niu, B., and Sun, H. (2009). Metabolic Targeting of Cancers: From Molecular Mechanisms to Therapeutic Strategies. Curr. Med. Chem. 16, 1561–1587. doi:10.2174/092986709788186255
Shi, J., Wang, L., Kim, Y. S., Zhai, S., Liu, Z., Chen, X., et al. (2008). Improving Tumor Uptake and Excretion Kinetics of 99mTc-Labeled Cyclic Arginine-Glycine-Aspartic (RGD) Dimers with Triglycine Linkers. J. Med. Chem. 51, 7980–7990. doi:10.1021/jm801134k
Shi, K., Li, J., Cao, Z., Yang, P., Qiu, Y., Yang, B., et al. (2015). A pH-Responsive Cell-Penetrating Peptide-Modified Liposomes with Active Recognizing of Integrin αvβ3 for the Treatment of Melanoma. J. Control Release 217, 138–150. doi:10.1016/j.jconrel.2015.09.009
Singh, R. P., Sharma, G., Kumari, L., Koch, B., Singh, S., Bharti, S., et al. (2016). RGD-TPGS Decorated Theranostic Liposomes for Brain Targeted Delivery. Colloids Surf. B Biointerfaces. doi:10.1016/j.colsurfb.2016.07.058
Singh, S., Numan, A., Agrawal, N., Tambuwala, M. M., Singh, V., and Kesharwani, P. (2020). Role of Immune Checkpoint Inhibitors in the Revolutionization of Advanced Melanoma Care. Int. Immunopharmacol. doi:10.1016/j.intimp.2020.106417
Sk, M., and Dd, S. (2006). Integrin-regulated FAK-Src Signaling in normal and Cancer Cells. Curr. Opin. Cel Biol. 18, 516–523. doi:10.1016/J.CEB.2006.08.011
Soares, D. C. F., De Oliveira, M. C., De Barros, A. L. B., Cardoso, V. N., and Ramaldes, G. A. (2011). Liposomes Radiolabeled with 159Gd: In Vitro Antitumoral Activity, Biodistribution Study and Scintigraphic Image in Ehrlich Tumor Bearing Mice. Eur. J. Pharm. Sci. 43, 290–296. doi:10.1016/J.EJPS.2011.05.006
Srivastava, S., Mahor, A., Singh, G., Bansal, K., Singh, P. P., Gupta, R., et al. (2021). Formulation Development, In Vitro and In Vivo Evaluation of Topical Hydrogel Formulation of Econazole Nitrate-Loaded β-cyclodextrin Nanosponges. J. Pharm. Sci. doi:10.1016/J.XPHS.2021.07.008
Stupack, D. G., Teitz, T., Potter, M. D., Mikolon, D., Houghton, P. J., Kidd, V. J., et al. (2006). Potentiation of Neuroblastoma Metastasis by Loss of Caspase-8. Nature 439, 95–99. doi:10.1038/NATURE04323
Torchilin, V. P. (2005). Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. doi:10.1038/nrd1632
Trayner, B. J., Grant, T. N., West, F. G., and Cheeseman, C. I. (2009). Synthesis and Characterization of 6-Deoxy-6-Fluoro-D-Fructose as a Potential Compound for Imaging Breast Cancer with PET. Bioorg. Med. Chem. 17, 5488–5495. doi:10.1016/J.BMC.2009.06.034
Tsatmali, M., Ancans, J., and Thody, A. J. (2002). Melanocyte Function and its Control by Melanocortin Peptides. J. Histochem. Cytochem. 50, 125–133. doi:10.1177/002215540205000201
Vakhshiteh, F., Khabazian, E., Atyabi, F., Ostad, S. N., Madjd, Z., and Dinarvand, R. (2020). Peptide-conjugated Liposomes for Targeted miR-34a Delivery to Suppress Breast Cancer and Cancer Stem-like Population. J. Drug Deliv. Sci. Technol. 57, 101687. doi:10.1016/j.jddst.2020.101687
van der Flier, A., and Sonnenberg, A. (2001). Function and Interactions of Integrins. Cell Tissue Res 305, 285–298. doi:10.1007/S004410100417
Viros, A., Sanchez-Laorden, B., Pedersen, M., Furney, S. J., Rae, J., Hogan, K., et al. (2014). Ultraviolet Radiation Accelerates BRAF-Driven Melanomagenesis by Targeting TP53. Nature 511, 478–482. doi:10.1038/nature13298
Wang, F., Chen, L., Zhang, R., Chen, Z., and Zhu, L. (2014). RGD Peptide Conjugated Liposomal Drug Delivery System for Enhance Therapeutic Efficacy in Treating Bone Metastasis from Prostate Cancer. J. Control Release 196, 222–233. doi:10.1016/j.jconrel.2014.10.012
Wang, X., Wang, H., Jiang, K., Zhang, Y., Zhan, C., Ying, M., et al. (2019). Liposomes with Cyclic RGD Peptide Motif Triggers Acute Immune Response in Mice. J. Control Release 293, 201–214. doi:10.1016/J.JCONREL.2018.12.003
Wang, Y., Hu, W., Ding, B., Chen, D., and Cheng, L. (2020). cRGD Mediated Redox and pH Dual Responsive Poly(amidoamine) Dendrimer-Poly(ethylene Glycol) Conjugates for Efficiently Intracellular Antitumor Drug Delivery. Colloids Surf. B Biointerfaces 194, 111195. doi:10.1016/j.colsurfb.2020.111195
Wang, Z., Yu, Y., Dai, W., Lu, J., Cui, J., Wu, H., et al. (2012). The Use of a Tumor Metastasis Targeting Peptide to Deliver Doxorubicin-Containing Liposomes to Highly Metastatic Cancer. Biomaterials 33, 8451–8460. doi:10.1016/J.BIOMATERIALS.2012.08.031
Webb, M. S., Harasym, T. O., Masin, D., Bally, M. B., and Mayer, L. D. (1995). Sphingomyelin-cholesterol Liposomes Significantly Enhance the Pharmacokinetic and Therapeutic Properties of Vincristine in Murine and Human Tumour Models. Br. J. Cancer 72, 896–904. doi:10.1038/BJC.1995.430
Wu, P.-H., Onodera, Y., Ichikawa, Y., Rankin, E. B., Giaccia, A. J., Watanabe, Y., et al. (2017). Targeting Integrins with RGD-Conjugated Gold Nanoparticles in Radiotherapy Decreases the Invasive Activity of Breast Cancer Cells. Int. J. Nanomedicine 12, 5069–5085. doi:10.2147/IJN.S137833
Xiong, X. B., Huang, Y., Lu, W. L., Zhang, X., Zhang, H., Nagai, T., et al. (2005a). Enhanced Intracellular Delivery and Improved Antitumor Efficacy of Doxorubicin by Sterically Stabilized Liposomes Modified with a Synthetic RGD Mimetic. J. Control Release 107, 262–275. doi:10.1016/j.jconrel.2005.03.030
Xiong, X. B., Huang, Y., Lu, W. L., Zhang, X., Zhang, H., Nagai, T., et al. (2005b). Intracellular Delivery of Doxorubicin with RGD-Modified Sterically Stabilized Liposomes for an Improved Antitumor Efficacy: In Vitro and In Vivo. J. Pharm. Sci. 94, 1782–1793. doi:10.1002/jps.20397
Xiong, Z., Shen, M., and Shi, X. (2018). Dendrimer-based Strategies for Cancer Therapy: Recent Advances and Future Perspectives. Sci. China Mater. 61, 1387–1403. doi:10.1007/s40843-018-9271-4
Yarden, Y., and Sliwkowski, M. X. (2001). Untangling the ErbB Signalling Network. Nat. Rev. Mol. Cel Biol. 2, 127–137. doi:10.1038/35052073
Yee, K. L., Weaver, V. M., and Hammer, D. A. (2008). Integrin-mediated Signalling through the MAP-Kinase Pathway. IET Syst. Biol. 2, 8–15. doi:10.1049/iet-syb:20060058
Yeo, W., Mok, T. S., Zee, B., Leung, T. W., Lai, P. B., Lau, W. Y., et al. (2005). A Randomized Phase III Study of Doxorubicin versus Cisplatin/interferon Alpha-2b/doxorubicin/fluorouracil (PIAF) Combination Chemotherapy for Unresectable Hepatocellular Carcinoma. J. Natl. Cancer Inst. 97, 1532–1538. doi:10.1093/JNCI/DJI315
Ying, X., Wen, H., Lu, W. L., Du, J., Guo, J., Tian, W., et al. (2010). Dual-targeting Daunorubicin Liposomes Improve the Therapeutic Efficacy of Brain Glioma in Animals. J. Control Release 141, 183–192. doi:10.1016/J.JCONREL.2009.09.020
Zamora-Leon, S. P., Golde, D. W., Concha, I. I., Rivas, C. I., Delgado-Lopez, F., Baselga, J., et al. (1996). Expression of the Fructose Transporter GLUT5 in Human Breast Cancer. Proc. Natl. Acad. Sci. U. S. A. 93, 1847–1852. doi:10.1073/PNAS.93.5.1847
Zhang, L., Shan, X., Meng, X., Gu, T., Lu, Q., Zhang, J., et al. (2019). The First Integrins β3-mediated Cellular and Nuclear Targeting Therapeutics for Prostate Cancer. Biomaterials 223, 119471. doi:10.1016/j.biomaterials.2019.119471
Zhang, L., Zhu, S., Qian, L., Pei, Y., Qiu, Y., and Jiang, Y. (2011). RGD-modified PEG-PAMAM-DOX Conjugates: In Vitro and In Vivo Studies for Glioma. Eur. J. Pharm. Biopharm. 79, 232–240. doi:10.1016/j.ejpb.2011.03.025
Zhang, Y. fei., Wang, J. cheng., Bian, D. yan., Zhang, X., and Zhang, Q. (2010). Targeted Delivery of RGD-Modified Liposomes Encapsulating Both Combretastatin A-4 and Doxorubicin for Tumor Therapy: In Vitro and In Vivo Studies. Eur. J. Pharm. Biopharm. 74, 467–473. doi:10.1016/j.ejpb.2010.01.002
Zhang, Y., Tian, Z., Zhao, X., Li, N., Garamus, V. M., Yin, P., et al. (2019). Dual-modified Bufalin Loaded Liposomes for Enhanced Tumor Targeting. Colloids Surf. A Physicochem. Eng. Asp. 571, 72–79. doi:10.1016/j.colsurfa.2019.03.060
Zhao, Z., Zhao, Y., Xie, C., Chen, C., Lin, D., Wang, S., et al. (2019). Dual-active Targeting Liposomes Drug Delivery System for Bone Metastatic Breast Cancer: Synthesis and Biological Evaluation. Chem. Phys. Lipids 223, 104785. doi:10.1016/j.chemphyslip.2019.104785
Keywords: liposome, cancer, targeted therapy, integrin, RGD peptide, toxicity, nanomedicine
Citation: Sheikh A, Alhakamy NA, Md S and Kesharwani P (2022) Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 12:803304. doi: 10.3389/fphar.2021.803304
Received: 17 November 2021; Accepted: 22 December 2021;
Published: 25 January 2022.
Edited by:
Wei Tao, Harvard Medical School, United StatesReviewed by:
Phei Er Saw, Sun Yat-sen Memorial Hospital, ChinaCopyright © 2022 Sheikh, Alhakamy, Md and Kesharwani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prashant Kesharwani, cHJhc2hhbnRkb3BzQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.