
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 16 December 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.795381
This article is part of the Research Topic Chemosensitizing Effect of Natural Products against Cancers: Applications in Enhancing Chemotherapy and Immunotherapy View all 14 articles
Mutation of the BRAF proto-oncogene is found in approximately 10% of colorectal cancers (CRC), with much of the mutation conferred by a V600E mutation. Unlike other CRC subtypes, BRAF-mutant CRC have had relatively limited response to conventional therapies and overall poor survival. We present the case of a 75-year-old man with severe nonischemic cardiomyopathy on a LifeVest who was found to have a transverse colonic mass with widespread hepatic metastatic disease and was subsequently found to have BRAFV600E-mutant CRC (MSI High/dMMR). After a failed therapy with FOLFOX and pembrolizumab, the patient was started on a regimen of vemurafenib, irinotecan, and cetuximab (VIC) based on the SWOG 1406 trial which had shown improved progression-free survival and response rate for the treatment of BRAFV600E-mutant metastatic CRC. After 40 cycles of VIC, the patient attained complete response and is in remission off chemotherapy with significant improvement. This case highlights the effectiveness of the triple-regimen of vemurafenib, irinotecan, and cetuximab as a treatment option for BRAFV600E-mutant CRC, which is a treatment regimen based on the SWOG 1406 trial, and also demonstrates the synergistic role of BRAFV600E inhibitors and EGFR inhibitors in the treatment of BRAFV600E-mutant CRC.
Colorectal cancer (CRC) is one of the leading causes of death in the world. In the United States alone, it represents the third most common cancer mortality with up to 53,200 deaths in 2020 (Biller and Schrag, 2021).
Up to a third of patients initially present with stage IV metastatic disease at the time of diagnosis, requiring systemic therapy for chance of cure (Zacharakis et al., 2010). 5-Fluorouracil was the first chemotherapy which proved effective against CRC, and new therapeutic agents and regimens have since been adopted (Duschinsky et al., 1957; Heidelberger et al., 1957; Biller and Schrag, 2021).
The mitogen-activated protein kinases (MAPK) is a chain of kinases and proteins that relay extracellular signals via a biochemical cascade to influence essential intracellular processes such as growth, differentiation, migration, and apoptosis (Dhillon et al., 2007). A dysregulation of this signal cascade can therefore lead to uncontrolled growth and proliferation, leading to tumorigenesis (Ascierto et al., 2012). BRAF is one of the protein kinases that is frequently mutated, perhaps due to fewer mutations necessary for its constitutive activation (Michaloglou et al., 2008). Mutation of the BRAF proto-oncogene is found in approximately 10% of colorectal cancers (CRC), with much of the mutation conferred by a V600E mutation (Kopetz et al., 2019). BRAF-mutant CRCs pose a special challenge for oncologists due to their relatively limited response to conventional therapies and overall poor survival (Kopetz et al., 2019).
Greater understanding of the pathogenesis of CRC has paved the way to numerous therapies, ranging from chemotherapy to targeted therapies. Currently, irinotecan combined with cetuximab is one of the regimens approved for treatment of metastatic CRC (Kopetz et al., 2017; Kopetz et al., 2021). On April 8, 2020, the Food and Drug Administration (FDA) approved encorafenib in combination with cetuximab for the treatment of patients with metastatic CRC with BRAFV600E mutation after a positive Phase III trial (Kopetz et al., 2019). In addition, an older randomized clinical trial called SWOG 1406 showed that the addition of vemurafenib to the irinotecan/cetuximab regimen showed improved progression-free survival and response rate for the treatment of BRAFV600E-mutant metastatic CRC (Kopetz et al., 2017; Kopetz et al., 2021). Here we present a case highlighting the effectiveness of the triple-combination therapy of vemurafenib, irinotecan, and cetuximab in the treatment of metastatic BRAFV600E-mutant CRC. This regimen, based on the SWOG 1406 trial, demonstrates a potential new option for treating metastatic BRAFV600E-mutant CRC. We also explore other potential treatment options currently under study, which reveal the synergistic role of an EGFR inhibitor and a BRAFV600E inhibitor.
We present the case of a 75-year-old man with severe nonischemic cardiomyopathy on LifeVest who initially presented on December 2017 with progressive abdominal pain for 2 months. The patient was initially suspected as having a gastric ulcer by his primary care physician and was therefore referred to a gastroenterologist for endoscopic evaluation. Upper endoscopy however was only positive for a hiatal hernia. The patient’s abdominal pain continued to worsen, and he developed additional symptoms nausea, decreased appetite, fatigue, constipation. The patient visited the emergency room given the progress nature of his symptoms. CT imaging showed a circumferential narrowing of the mid-transverse colon with widespread hepatic metastatic disease. Subsequent colonoscopy showed a fungating, infiltrating, and ulcerated partially obstructing mass in the transverse colon with subsequent biopsy revealing poorly differentiated invasive adenocarcinoma on January 2018. Molecular testing was positive for BRAF-V600E mutation. Mismatch repair protein analysis was notable for loss of expression of MLH1 and PMS2. Microsatellite instability analysis was MSI-High, with instability of microsatellite markers NR-21, BAT-26, BAT-25, NR-24, and MONO-27.
The patient was started on palliative FOLFOX and was then started on bevacizumab during the third cycle of FOLFOX. This regimen was chosen due to initial concerns for GI perforation and overall poor performance status. CT chest/abdomen/pelvis was obtained every 3 months to assess therapy response. However, subsequent imaging showed progression of disease despite four cycles of FOLFOX and two cycles of bevacizumab. The treatment regimen was switched to pembrolizumab, but disease progression was still seen despite three cycles of pembrolizumab.
In May 2018 the patient was started on a triple-combination therapy consisting of vemurafenib, irinotecan, and cetuximab (VIC), which was based on the SWOG 1406 clinical trial (Kopetz et al., 2017). This decision was made based on his prior therapy failures as well as the promising results of the SWOG 1406 clinical trial (Kopetz et al., 2017). At this point in time, the regimen of encorafenib and cetuximab had not yet been FDA approved (Kopetz et al., 2019). Extensive discussion was held with the patient and family regarding therapy strategy and side-effect profile, and ultimately they agreed to the experimental therapy with continued goal of palliative therapy. The dosing and frequency was modeled after that of the SWOG 1406 clinical trial (Kopetz et al., 2017; Kopetz et al., 2021). The patient received IV cetuximab at a dose of 500 mg/m2 (800 mg for our patient) followed by IV irinotecan at 180 mg/m2 (288 mg for our patient) on days 1 and 15, with courses repeated every 28 days (Kopetz et al., 2017; Kopetz et al., 2021). The patient also received PO vemurafenib 960 mg twice daily on days 1 through 28, with a single cycle consisting of 28 days. After five cycles of VIC therapy, the patient’s metastatic lesions of the liver had significantly decreased in size. CT imaging was continually obtained every 3 months, and each additional imaging showed either stable disease or disease regression. After 40 cycles of VIC, the patient attained complete response and is in remission off chemotherapy with significant improvement in his weight, performance status, and quality of life (Figure 1, Figure 2). As of today October 2021 the patient has remained disease-free off of chemotherapy for 9 months without obvious disease recurrence, but continued surveillance CT imaging will still be performed to monitor for recurrence.
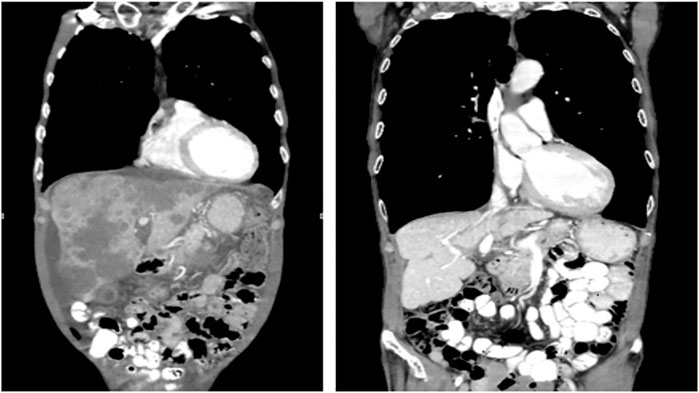
FIGURE 1. Extensive hepatic metastasis present before initiation of VIC (left). In remission after 40 cycles of VIC (right).
Of note, before the patient was started on VIC, the patient had been followed by a cardiologist for his nonischemic cardiomyopathy with a severely depressed LVEF 20–24%. The patient was not a candidate for an automatic implantable cardioverter defibrillator (AICD) and therefore had been on a LifeVest. Given the overall poor outlook from his metastatic disease, the patient had decided to discontinue his LifeVest for improved quality of life at the risk of a sudden cardiac death. However, after attaining complete response to VIC, the patient was able to undergo implantation of a cardiac resynchronization therapy with defibrillation (CRT-D) device with significant improvement to his functional status. The patient and his family have been very happy with the care they received in the hospital and the clinic.
Vemurafenib is a selective BRAFV600Einhibitor that had been approved by the FDA in 2011 for treatment of BRAFV600E-mutant metastatic melanoma, though it had limited efficacy when used as monotherapy against CRC (Korphaisarn and Kopetz, 2016). Irinotecan, a topoisomerase I inhibitor, was approved for treatment of metastatic CRC refractory to 5-FU in 1996 and is now a key drug for treatment of metastatic CRC given the survival advantage of irinotecan-based regimen (Fujita et al., 2015). Cetuximab is a monoclonal antibody against the epidermal growth factor receptor (EGFR) inhibitor. A 2004 clinical trial showed clinically-significant activity of cetuximab when given alone or in combination with irinotecan for irinotecan-refractory metastatic CRC, with greater ORR and PFS for the combination therapy as opposed to the monotherapy (Cunningham et al., 2004).
For our patient, we have chosen the triple therapy of VIC (vemurafenib + irinotecan + cetuximab) because at the time of therapy initiation (2018) the doublet regimen of encorafenib and cetuximab, based on the positive phase III trial in 2019 (8), had not yet been FDA approved. Our patient was started on VIC in 2018, and the preliminary results of the randomized phase II SWOG 1406 clinical trial published in 2017 had shown clinically-significant benefit of adding vemurafenib to the combined irinotecan/cetuximab therapy (Kopetz et al., 2017). The SWOG 1406 trial was comprised of 106 participants who had previously received 1 or 2 prior regimens except for anti-EGFR agents (Kopetz et al., 2017; Kopetz et al., 2021). Compared to the doublet irinotecan/cetuximab therapy, the triple therapy showed improved median PFS (4.4 vs 2 months) and improved response rate (17 vs 4%) (Kopetz et al., 2017; Kopetz et al., 2021). The primary conclusion of the study was that inhibition of both EGFR and BRAF combined with irinotecan was effective for the treatment of BRAFV600E-mutant CRC (Kopetz et al., 2017; Kopetz et al., 2021). Our patient’s PFS (36 months) has far exceeded what was shown in the study. The reason for our patient’s superior clinical response is unclear, but we suspect this was due to a distinct molecular and immunohistochemical characteristic of our patient’s cancer that was more responsive to the VIC regimen. A significant challenge was the patient’s cardiac comorbidity, namely his cardiomyopathy with an LVEF 20–24%. In view of this condition, the patient required close monitoring of his QTc (by EKG before each cycle) and volume status while receiving his chemotherapy. The overall poor prognosis had made him ineligible for an ICD, and in fact the patient’s LifeVest was discontinued during the initial stages of chemotherapy to improve his quality of life. This is no longer the case after having achieved remission, and now the patient was able to have an CRT-D device implanted.
A major strength to this case is that this case was not directly affiliated with the SWOG clinical study. We believe this allowed for a non-biased approach to our treatment regimen and assessment of the treatment response. The limitation of this case report, on the other hand, is that this is a report regarding a single patient. However, not many publications experimenting with the VIC regimen are found in the literature aside from the primary SWOG trial. One similar finding is a 2020 case report demonstrating complete response of metastatic BRAFV600E-mutant CRC to a regimen of fluorouracil + VIC (Wang et al., 2019). The 44 year-old patient was refractory to a regimen of capecitabine + oxaliplatin and had developed metastatic disease (Wang et al., 2019). The patient’s first cycle was irinotecan + 5-FU with subsequent cycles composed of a quad-therapy of 5-FU + VIC, and after 10 total cycles the patient had attained complete recession of metastases (Wang et al., 2019). The general conclusion of this case was similar to that of our case and the SWOG trial, aside from the concomitant usage of 5-FU along with the VIC.
The BEACON CRC phase III trial showed effectiveness of the triple regimen of encorafenib + cetuximab + binimetinib compared to the control (cetuximab + irinotecan or cetuximab + FOLFIRI) for treatment-refractory metastatic CRC (Kopetz et al., 2019). There was a clinically significant increase in median overall survival of patients who had received the triple therapy (9.0 months) compared to the control group (5.4 months) (Kopetz et al., 2019). It is worth noting that the trial had also explored the efficacy of a doublet therapy (encorafenib + cetuximab) which had a median overall survival of 8.4 months (Kopetz et al., 2019). This doublet therapy of encorafenib + cetuximab is now FDA approved for treatment of metastatic BRAFV600E-mutant CRC. Of note, encorafenib is a BRAFV600E inhibitor just like vemurafenib which gives further gives credence to the effectiveness of utilizing both EGFR and BRAF inhibition as in like the SWOG trial.
Currently, there is research analyzing the effectiveness of the triple regimen (encorafenib + cetuximab + binimetinib) for metastatic BRAFV600E-mutant CRC (Grothey et al., 2020). The ANCHOR CRC trial is currently in phase II, and so far it has shown effectiveness of the afore-mentioned triple therapy as a first-line metastatic treatment, with an overall response rate of 50 and 85% of patients with decreased tumor size so far (Grothey et al., 2020). This is in contrast to the BEACON CRC trial which had enrolled patients with treatment-refractory metastatic CRC (Kopetz et al., 2019).
A recent 2018 study of 142 patients analyzed the treatment efficacy of dabrafenib + panitumumab + trametinib (D + P + T) (Corcoran et al., 2018). Dabrafenib is a BRAFV600E inhibitor and panitumumab is an inhibitor of EGFR, just like vemurafenib and cetuximab are, respectively (Addeo et al., 2010; Abraham and Stenger, 2014). This study also added trametinib, a MEK inhibitor which had been FDA approved for use in BRAFV600E/V600K mutated melanoma (Hoffner and Benchich, 2018). The median PFS for the D + P + T, D + P, and T + P arms were 4.2, 2.6, and 3.5 months respectively (Corcoran et al., 2018). The overall response rate was 21, 10, and 0% respectively (Corcoran et al., 2018). The median overall survival was 9.1 months, 13.2, and 8.2 respectively (Corcoran et al., 2018). In conclusion, the usage of the triple regimen of MEK + BRAFV600E + EGFR inhibitors had greater overall response rate and PFS, while the dual-regimen of BRAFV600E + EGFR inhibitors had the greater median overall survival.
Overall, our case highlights the effectiveness of the triple-regimen of vemurafenib, irinotecan, and cetuximab as a treatment option for BRAFV600E-mutant CRC, a treatment regimen based on the SWOG 1406 trial. Our case demonstrates a patient with serious cardiac comorbidity who was able to tolerate the cancer regimen and attain complete response. The current treatment trend for this metastatic BRAFV600E-mutant CRC at this time appears to revolve around the usage of both a BRAF inhibitor as well as an EGFR mutation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
CS and EA reviewed the literature; CS, EA and AM contributed to the design of the manuscript and manuscript drafting, and revision of the manuscript for intellectual content; CS imaged analysis and interpretation; AM was responsible for the critical revision of the manuscript for intellectual content; and all authors issued final approval for the version to be submitted.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The assistance provided by Eva Zsigmond as scientific editing was greatly appreciated.
Abraham, J., and Stenger, M. (2014). Dabrafenib in Advanced Melanoma with BRAF V600E Mutation. J. Community Support. Oncol. 12 (2), 48–49. doi:10.12788/jcso.0014
Addeo, R., Caraglia, M., Cerbone, D., Frega, N., Cimmino, G., Abbruzzese, A., et al. (2010). Panitumumab: a New Frontier of Target Therapy for the Treatment of Metastatic Colorectal Cancer. Expert Rev. Anticancer Ther. 10 (4), 499–505. doi:10.1586/era.10.28
Ascierto, P. A., Kirkwood, J. M., Grob, J. J., Simeone, E., Grimaldi, A. M., Maio, M., et al. (2012). The Role of BRAF V600 Mutation in Melanoma. J. Transl Med. 10, 85. doi:10.1186/1479-5876-10-85
Biller, L. H., and Schrag, D. (2021). Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 325 (7), 669–685. doi:10.1001/jama.2021.0106
Corcoran, R. B., André, T., Atreya, C. E., Schellens, J. H. M., Yoshino, T., Bendell, J. C., et al. (2018). Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer. Cancer Discov. 8 (4), 428–443. doi:10.1158/2159-8290.CD-17-1226
Cunningham, D., Humblet, Y., Siena, S., Khayat, D., Bleiberg, H., Santoro, A., et al. (2004). Cetuximab Monotherapy and Cetuximab Plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 351 (4), 337–345. doi:10.1056/NEJMoa033025
Dhillon, A. S., Hagan, S., Rath, O., and Kolch, W. (2007). MAP Kinase Signalling Pathways in Cancer. Oncogene 26 (22), 3279–3290. doi:10.1038/sj.onc.1210421
Duschinsky, R., Pleven, E., and Heidelberger, C. (1957). The Synthesis of 5-fluoropyrimidines. J. Am. Chem. Soc. 79, 4559–4560. doi:10.1021/ja01573a087
Fujita, K., Kubota, Y., Ishida, H., and Sasaki, Y. (2015). Irinotecan, a Key Chemotherapeutic Drug for Metastatic Colorectal Cancer. World J. Gastroenterol. 21 (43), 12234–12248. doi:10.3748/wjg.v21.i43.12234
Grothey, A., Tabernero, J., Taieb, J., Yaeger, R., Yoshino, T., Maiello, E., et al. (2020). LBA-5 ANCHOR CRC: a Single-Arm, Phase 2 Study of Encorafenib, Binimetinib Plus Cetuximab in Previously Untreated BRAF V600E-Mutant Metastatic Colorectal Cancer. Ann. Oncol. 31, S242–S243. doi:10.1016/j.annonc.2020.04.080
Heidelberger, C., Chaudhuri, N. K., Danneberg, P., Mooren, D., Griesbach, L., Duschinsky, R., et al. (1957). Fluorinated Pyrimidines, a New Class of Tumour-Inhibitory Compounds. Nature 179 (4561), 663–666. doi:10.1038/179663a0
Hoffner, B., and Benchich, K. (2018). Trametinib: A Targeted Therapy in Metastatic Melanoma. J. Adv. Pract. Oncol. 9 (7), 741–745. doi:10.6004/jadpro.2018.9.7.5
Kopetz, S., Grothey, A., Yaeger, R., Van Cutsem, E., Desai, J., Yoshino, T., et al. (2019). Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 381 (17), 1632–1643. doi:10.1056/NEJMoa1908075
Kopetz, S., Guthrie, K. A., Morris, V. K., Lenz, H. J., Magliocco, A. M., Maru, D., et al. (2021). Randomized Trial of Irinotecan and Cetuximab with or without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406). J. Clin. Oncol. 39 (4), 285–294. doi:10.1200/JCO.20.01994
Kopetz, S., McDonough, S. L., Morris, V. K., Lenz, H-J., Magliocco, A. M., Atreya, C. E., et al. (2017). Randomized Trial of Irinotecan and Cetuximab with or without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG 1406). J. Clin. Oncol. 35 (4), 520. doi:10.1200/jco.2017.35.4_suppl.520
Korphaisarn, K., and Kopetz, S. (2016). BRAF-directed Therapy in Metastatic Colorectal Cancer. Cancer J. 22 (3), 175–178. doi:10.1097/PPO.0000000000000189
Michaloglou, C., Vredeveld, L. C., Mooi, W. J., and Peeper, D. S. (2008). BRAF(E600) in Benign and Malignant Human Tumours. Oncogene 27 (7), 877–895. doi:10.1038/sj.onc.1210704
Wang, Z., Dai, W. P., and Zang, Y. S. (2019). Complete Response with Fluorouracil and Irinotecan with a BRAFV600E and EGFR Inhibitor in BRAF-Mutated Metastatic Colorectal Cancer: a Case Report. Onco Targets Ther. 12, 443–447. doi:10.2147/OTT.S180845
Keywords: BRAF, CRC, Vemurafenib, Irinotecan, Cetuximab, Metastasis, SWOG, Immunotherapy, Chemotherapy
Citation: Cho SM, Esmail A and Abdelrahim M (2021) Triple-Regimen of Vemurafenib, Irinotecan, and Cetuximab for the Treatment of BRAFV600E-Mutant CRC: A Case Report and Review. Front. Pharmacol. 12:795381. doi: 10.3389/fphar.2021.795381
Received: 15 October 2021; Accepted: 05 November 2021;
Published: 16 December 2021.
Edited by:
Devesh Tewari, Lovely Professional University, IndiaReviewed by:
Lars Petter Jordheim, Université de Lyon, FranceCopyright © 2021 Cho, Esmail and Abdelrahim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maen Abdelrahim, bWFiZGVscmFoaW1AaG91c3Rvbm1ldGhvZGlzdC5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.