
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 13 December 2021
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.786596
This article is part of the Research TopicInsights in Gastrointestinal and Hepatic Pharmacology: 2021View all 30 articles
 Chayanis Kositamongkol1†
Chayanis Kositamongkol1† Sukrit Kanchanasurakit2,3,4,5,6†
Sukrit Kanchanasurakit2,3,4,5,6† Chiraphong Auttamalang3
Chiraphong Auttamalang3 Nutkamon Inchai3
Nutkamon Inchai3 Thanatchaporn Kabkaew3
Thanatchaporn Kabkaew3 Sarunporn Kitpark3
Sarunporn Kitpark3 Nathorn Chaiyakunapruk7†
Nathorn Chaiyakunapruk7† Acharaporn Duangjai8†
Acharaporn Duangjai8† Surasak Saokaew3,4,5,6,9,10*†
Surasak Saokaew3,4,5,6,9,10*† Pochamana Phisalprapa1*†
Pochamana Phisalprapa1*†Background: The effects of coffee consumption on hepatic outcomes are controversial. This study investigated the associations between coffee consumption and the incidence of non-alcoholic fatty liver disease (NAFLD) in the general population and the reduction of liver fibrosis among patients with NAFLD.
Methods: The study consisted of two parts: an umbrella review and a systematic review and meta-analysis (SRMA). The searches for each part were performed separately using PubMed, EMBASE, Cochrane, Scopus, and CINAHL databases. All articles published up to September 2021 were reviewed. To be eligible, studies for the umbrella review were required to report outcomes that compared the risks of NAFLD in the general population and/or liver fibrosis in patients with NAFLD who did and did not drink coffee. Our SRMA included primary studies reporting the effects of coffee consumption on NAFLD-related outcomes. The outcomes were pooled using a random-effects model and reported in both qualitative and quantitative terms (pooled risk ratio, odds ratio, and weighted mean difference).
Results: We identified four published SRMAs during the umbrella review. Most studies showed that individuals in the general population who regularly drank coffee were significantly associated with a lower NAFLD incidence than those who did not. Our SRMA included nine studies on the effects of coffee consumption on NAFLD incidence. Pooled data from 147,875 subjects showed that coffee consumption was not associated with a lower NAFLD incidence in the general population. The between-study heterogeneity was high (I2, 72–85%). Interestingly, among patients with NAFLD (5 studies; n = 3,752), coffee consumption was significantly associated with a reduction in liver fibrosis (odds ratio, 0.67; 95% CI, 0.55 to 0.80; I2, 3%). There were no differences in the coffee consumption of the general population and of those with NAFLD (4 studies; n = 19,482) or by patients with no/mild liver fibrosis and those with significant fibrosis (4 studies; n = 3,331).
Conclusions: There are contrasting results on the effects of coffee on NAFLD prevention in the general population. Benefits of coffee consumption on liver fibrosis were seen among patients with NAFLD.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021226607, identifier CRD42021226607
Non-alcoholic fatty liver disease (NAFLD) is a global chronic health problem. Meta-analyses have reported a prevalence ranging between 25 and 32% (Younossi et al., 2016; Park et al., 2021). The incidence of NAFLD has tended to increase over time due to behavioral changes leading to unhealthy lifestyles in the global population (European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity, 2016; Li et al., 2019; Calabrò et al., 2020). The latest meta-analyses showed that the incidence of NAFLD was approximately 42.8–50.9 per 1,000 person-years (Li et al., 2019; Park et al., 2021). Based on current evidence, NAFLD can lead to many serious complications. They include hepatic-related events (such as liver cirrhosis, end-stage liver disease, and hepatocellular carcinoma) and non-hepatic-related events (for example, cardiovascular and chronic kidney diseases) (European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity, 2016; Chalasani et al., 2018; Eslam et al., 2020; Park et al., 2021). The burdens of these diseases and their sequelae are problematic in both developed and developing countries. Based on the results of modeling studies, NAFLD, non-alcoholic steatohepatitis (NASH), and liver fibrosis are projected to create very large clinical and economic burdens (Younossi et al., 2019; Tampi et al., 2020; Ito et al., 2021; Park et al., 2021; Phisalprapa et al., 2021). Slowing the incidence rates and preventing the sequelae of NAFLD are important solutions to reduce the burdens.
Behavioral modification, weight reduction, and drug therapies are hoped to be effective treatments for NAFLD. Despite the fact that there is no approved pharmacological treatment for NAFLD and non-biopsy-proven patients (European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity, 2016; Chalasani et al., 2018; Leoni et al., 2018), behavioral changes that lead to weight reduction, fat- and carbohydrate-diet adjustment, exercise, proper sleep, and coffee consumption have caught the attention of researchers (European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity; Chalasani et al., 2018; Leoni et al., 2018; Calabrò et al., 2020). Numerous studies have shown that coffee consumption has many positive effects on the liver, such as reducing the risk of fatty liver disease, lowering the severity of hepatic steatosis, and slowing the progression of fibrosis (Molloy et al., 2012; Zelber-Sagi et al., 2015). Recent meta-analyses and narrative reviews have reported many favorable clinical effects in people who regularly consume coffee or caffeine-containing products compared with those who do not (Calabrò et al., 2020)—for example, coffee consumption could be a protective factor against NAFLD and liver fibrosis (Marventano et al., 2016; Shen et al., 2016; Wijarnpreecha et al., 2017; Chen et al., 2019; Calabrò et al., 2020). Additionally, coffee extract improved the lipid profile and body mass index of patients with NAFLD (Hosseinabadi et al., 2020). Various mechanisms of NAFLD and liver fibrosis have been proposed, one of which is associated with transforming growth factor-beta (TGF-β) and oxidative stress (Tarantino et al., 2019). The hypotheses of the positive effects of coffee on the liver are that caffeine may help reduce TGF-β in liver cells and inhibit hepatic stellate cell activity (Shim et al., 2013). Additionally, non-caffeine substances, such as cafestol, kahweol, and chlorogenic acid, are antioxidants that may help reduce triglyceride and cholesterol deposition in hepatocytes and prevent cancer. Moreover, phenolic substances, including ferulic acid, have been introduced for the treatment of cardiovascular diseases and diabetes (Calabrò et al., 2020).
However, several studies have reported non-significant benefits of coffee on clinical and laboratory outcomes related to NAFLD (Calabrò et al., 2020). The effects of coffee consumption on the risk of NAFLD in the general population and liver fibrosis in patients with NAFLD are controversial. Therefore, we conducted this study using a reliable methodology—an umbrella review—to investigate the summarized effects of coffee consumption found by previous systematic reviews and meta-analyses. Additionally, we conducted a new systematic review and meta-analysis (SRMA) that included recently published primary studies to obtain up-to-date results with more power from a larger sample size.
This study consisted of two parts: an umbrella review and a SRMA. Separate searches were carried out to obtain articles for each part of the study. PubMed, EMBASE, Cochrane, Scopus, and CINAHL databases were searched. There was no language restriction in our inclusion criteria for eligible studies. The searches were performed on September 9, 2021. This research project was conducted and reported according to the statement of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (Liberati et al., 2009; Page et al., 2021). The study was registered with PROSPERO (registration number, CRD42021226607).
The search terms for the umbrella review were (“coffee consumption” OR “caffeine consumption”) AND (“NAFLD” OR “non-alcoholic fatty liver disease”) AND (“systematic review” OR “meta-analysis”). The eligibility criteria were as follows:
1. A systematic review with/without meta-analysis
2. A meta-analysis that reported any pooled results of risk ratios (RRs) or odds ratios (ORs) by comparing exposures (did and did not drink coffee) with outcomes (incidence of NAFLD and/or liver fibrosis)
3. A meta-analysis that reported the mean differences (MDs) in the coffee consumption of the two groups of subjects with different hepatic outcomes
4. Research on NAFLD and liver fibrosis that had been diagnosed by reliable methods, such as elevated liver enzymes, ultrasonography, and/or liver biopsy
Publications that met the eligibility criteria were included in the umbrella review.
The search terms for the SRMA were (“coffee consumption” OR “caffeine consumption”) AND (“NAFLD” OR “non-alcoholic fatty liver disease” OR “NASH” OR “non-alcoholic steatohepatitis” OR “fatty liver”). The eligibility criteria were as follows:
1. A case–control, cohort, or cross-sectional design study
2. A study that compared liver outcomes (NAFLD incidence and/or liver fibrosis) of two groups of participants who did and did not drink coffee and reported the results as adjusted RRs or ORs with 95% confidence intervals (95% CI)
3. A study that reported the MDs with 95% CI in the coffee consumption of the two groups of subjects with different hepatic outcomes
4. Research on NAFLD and liver fibrosis that had been diagnosed using reliable methods, such as elevated liver enzymes, ultrasonography, and/or liver biopsy
Primary studies that met the eligibility criteria were included in the SRMA.
In the umbrella review, we extracted information from the included systematic reviews and meta-analyses following the Joanna Briggs Institute data extraction guide (Aromataris et al., 2015). The methodological quality of a meta-analysis was evaluated using A Measurement Tool to Assess Systematic Reviews (AMSTAR) 2 (Shea et al., 2017). If two or more studies were published in the same 24-month period for the same category of exposure and same outcomes, we selected the one with the highest AMSTAR 2 score for further analysis.
The quality of the primary studies in the SRMA was evaluated according to the Newcastle–Ottawa Quality Assessment Scale (NOS). Specifically, for the quality assessment of cross-sectional studies, the modified NOS described by Herzog et al. (2013) was used.
In the umbrella review, we comprehensively summarized the findings of previously published systematic reviews and meta-analyses. However, we did not review the full texts of the primary study articles examined by each meta-analysis.
For the SRMA, we used the DerSimonian and Laird random-effects model. The outcomes of interest were as follows:
1. An association between coffee consumption (1 to 2 and >2 cups/day versus <1 cup/day) and the incidence of NAFLD
2. An association between coffee consumption and reduction in liver fibrosis among patients with NAFLD
3. The difference in the consumption of coffee by the general population and by patients with NAFLD
4. The difference in coffee consumption of patients with NAFLD with no/mild and significant stages of liver fibrosis
The results are reported as pooled RRs and weighted mean differences (WMDs) along with 95% CIs. The I2 statistic was calculated to determine the between-study heterogeneity. Publication bias was evaluated using Begg’s and Egger’s tests and by a visual investigation of funnel plots for potential asymmetry. All analyses were performed using Stata 14.1 (Stata Corp, College Station, TX, United States). A p-value of less than 0.05 was considered statistically significant.
A total of 1,266 studies were retrieved using our search keywords. After the exclusion of 33 duplicate studies, the titles and abstracts of the remaining 1,233 studies were screened. This resulted in the elimination of 1,226 more studies (1,195 were not related and 31 used other study designs). Seven studies were available for full-length article review. Three of these were excluded because they did not report outcomes or have a study population within the scope of interest. The four studies (Shen et al., 2016; Wijarnpreecha et al., 2017; Chen et al., 2019; Hayat et al., 2021) that fully met the eligibility criteria were included in the umbrella review (Figure 1A). Their summarized quantitative and qualitative findings are presented in Figures 2, 3, respectively. They were considered high-quality reviews based on AMSTAR 2 criteria (Table 1). We did not test them for publication bias as the number of studies was too small.
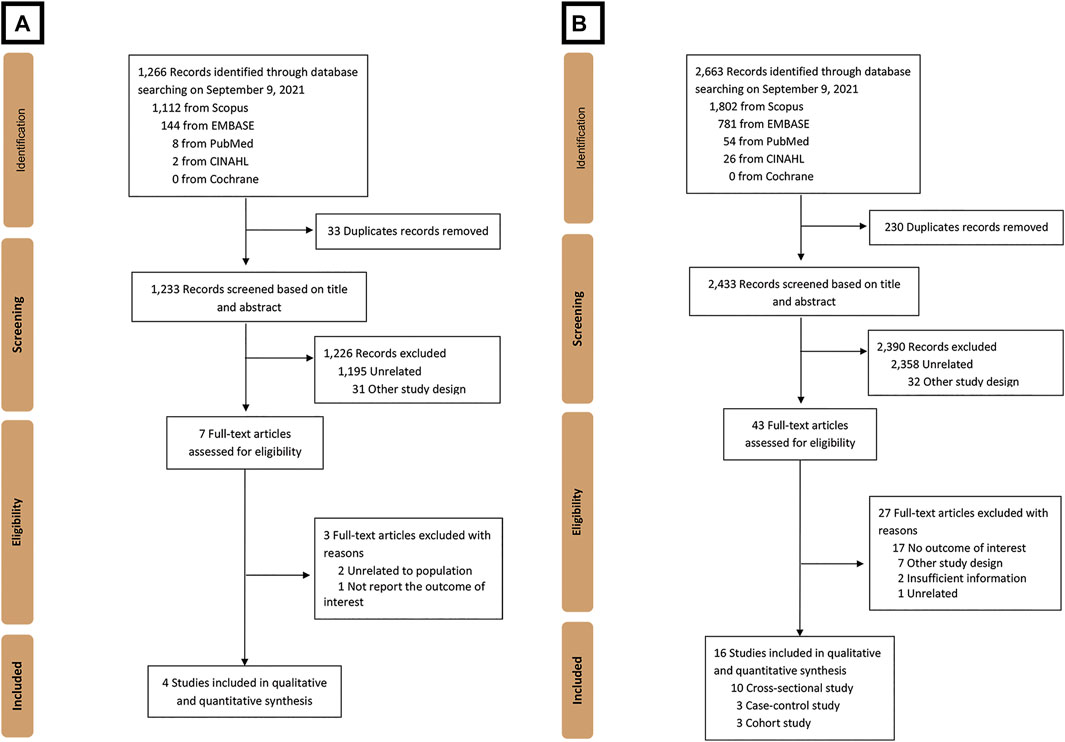
FIGURE 1. Flow diagram of the study identification, inclusion, and exclusion of (A) umbrella review and (B) systematic review and meta-analysis.
Quantitative analyses from three studies by Wijarnpreecha et al. (2017), Chen et al. (2019), and Hayat et al. (2021) provided significant association outcomes between coffee consumption and incidence of NAFLD, with pooled RRs of 0.71 (95% CI, 0.60–0.85), 0.94 (95% CI, 0.92–0.97), and 0.77 (95% CI, 0.60–0.98), respectively. In contrast, the study by Shen et al. (2016) reported a non-significant outcome for daily coffee consumption, with an MD of 23.06 mg/day (95% CI, −8.67–54.80). The results are illustrated in Figure 2. The qualitative outcomes included the perspective of doctors that coffee consumption provided a beneficial protective effect on NAFLD among the general population (Figure 3).
Quantitative outcomes from three studies by Wijarnpreecha et al. (2017), Shen et al. (2016), and Hayat et al. (2021) indicated that there was a significant association between coffee consumption and liver fibrosis in patients with NAFLD, with a pooled RR of 0.70 (95% CI, 0.60–0.82), MD of −91.35 mg/day (95% CI, −139.42–−43.27), and RR of 0.68 (95% CI, 0.58–0.79; Figure 2). The qualitative outcomes included the perspective of doctors that coffee was beneficial to the fibrosis outcomes of patients with NAFLD (Figure 3).
A total of 2,663 studies were retrieved using our search keywords. After excluding duplicate records, the titles and abstracts of 2,433 studies were screened. Of these, 2,390 were excluded due to irrelevance, leaving 43 for full-length article review. Twenty-seven of these were subsequently excluded because they did not meet our eligibility criteria. Eventually, 16 studies (n = 171,096) were available for data analysis (Figure 1B) (Ruhl and Everhart, 2005; Catalano et al., 2010; Funatsu et al., 2011; Anty et al., 2012; Birerdinc et al., 2012; Gutiérrez-Grobe et al., 2012; Molloy et al., 2012; Bambha et al., 2014; Graeter et al., 2015; Imatoh et al., 2015; Zelber-Sagi et al., 2015; Alferink et al., 2017; Setiawan et al., 2017; Soleimani et al., 2019; Chung et al., 2020; Mikolasevic et al., 2021). The characteristics of the included studies were extracted (Table 2). Quality evaluations using NOS indicated that all were high-quality studies (Figure 4).
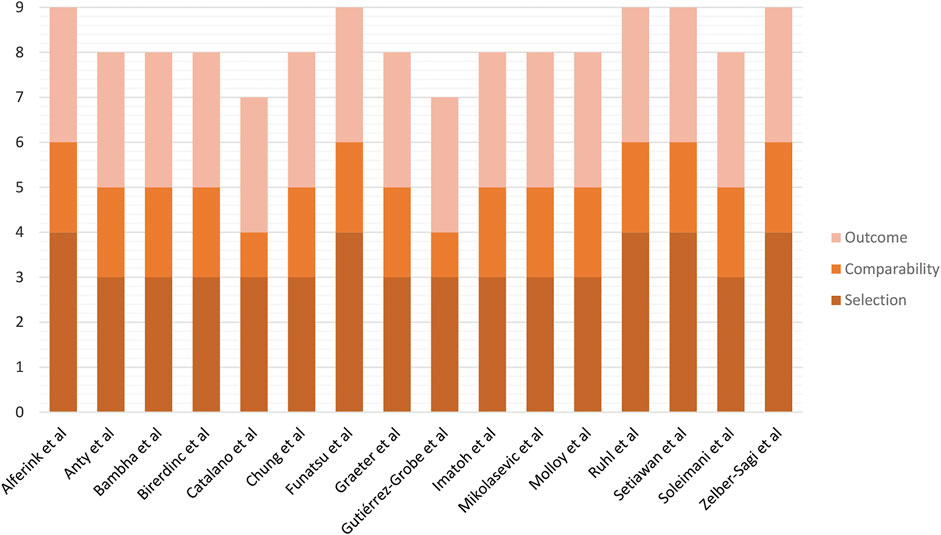
FIGURE 4. Risk of bias assessment of cohort studies included in the meta-analysis using the Newcastle–Ottawa Scale.
The pooled RR of nine observational studies (n = 147,875) did not show a significant difference in the incidence of NAFLD of the population who drank one or more cups of coffee per day and <1 cup/day versus those who did not (Ruhl and Everhart, 2005; Catalano et al., 2010; Bambha et al., 2014; Graeter et al., 2015; Imatoh et al., 2015; Zelber-Sagi et al., 2015; Setiawan et al., 2017; Chung et al., 2020; Mikolasevic et al., 2021). The analyses revealed that neither the consumption of one to two cups of coffee/day nor the consumption of even more than two cups/day was associated with a reduction in the incidence of NAFLD in the general population compared with the population who drank less than one cup of coffee per day (RR, 1.00; 95% CI, 0.93–1.07; I2, 72% and RR, 0.91; 95% CI, 0.80–1.03; I2, 85%, respectively; Figure 5).
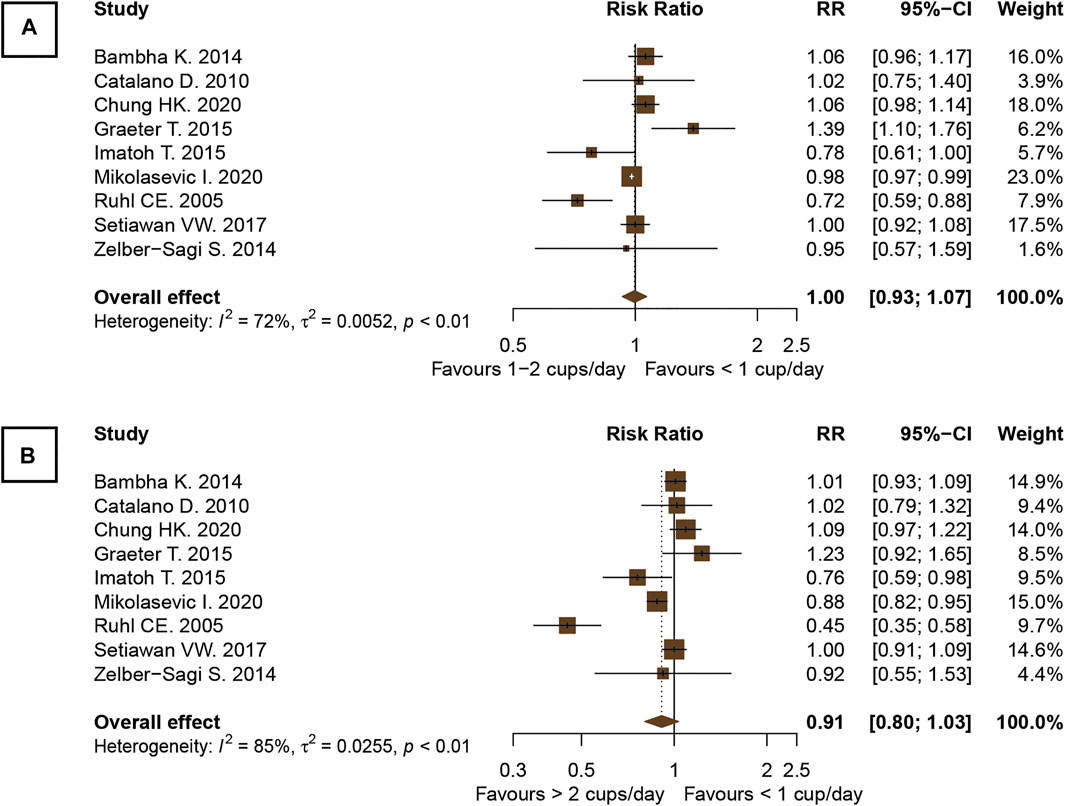
FIGURE 5. Forest plots of the association of coffee consumption and the incidence of NAFLD in the general population: (A) 1 to 2 cups of coffee per day versus <1 cup per day and (B) >2 cups per day versus <1 cup per day.
A total of five studies (n = 2,948) were included in this outcome analysis (Anty et al., 2012; Bambha et al., 2014; Zelber-Sagi et al., 2015; Alferink et al., 2017; Soleimani et al., 2019). The results showed that coffee consumption was significantly associated with a reduction in hepatic fibrosis in patients with NAFLD (OR, 0.67; 95% CI, 0.55–0.80; I2, 3%; Figure 6).
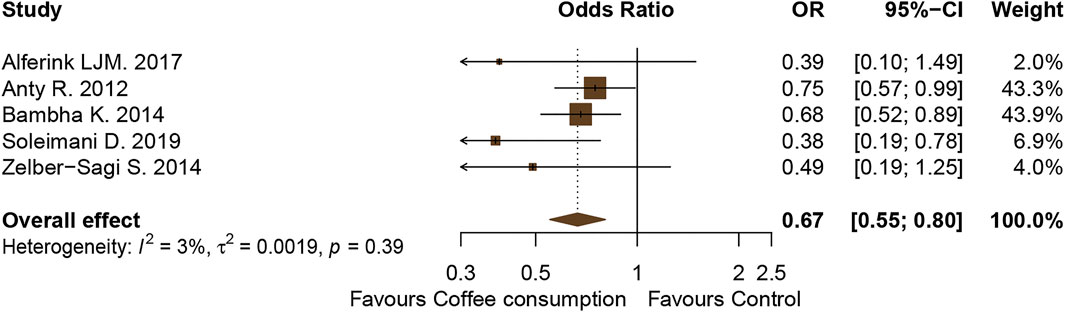
FIGURE 6. Forest plot of the association of coffee consumption and the liver fibrosis outcomes of patients with NAFLD who drank coffee versus those who did not drink coffee (control).
Four observational studies explored the association between coffee consumption by the general population and by patients with NAFLD (Catalano et al., 2010; Funatsu et al., 2011; Birerdinc et al., 2012; Gutiérrez-Grobe et al., 2012). There was a total of 19,482 individuals (general population, 17,322; NAFLD patients, 2,160). The pooled result showed that the general population did not consume more coffee than people with NAFLD (WMD, −17.11 mg/day; 95% CI, −51.41–17.20; I2, 85%), as illustrated in Supplementary Figure S1.
Four observational studies investigated the difference in the coffee consumption of NAFLD patients with no/mild and significant stages of liver fibrosis (Anty et al., 2012; Molloy et al., 2012; Bambha et al., 2014; Alferink et al., 2017). Of the 3,331 patients, 2,912 had no/mild fibrosis, while 419 had significant fibrosis. The pooled result showed that there was no difference in the coffee consumption of the two groups of patients with different stages of fibrosis (WMD, −33.78 mg/day; 95% CI, −90.26–22.71; I2, 81%). The comparison is illustrated in Supplementary Figure S2.
There was no evidence of publication bias among the included studies, as illustrated in Figures 7, 8. For articles comparing the association of coffee consumption of one to two cups/day and of <1 cup/day with the incidence of NAFLD in the general population, the p-values were 0.677 for Begg’s test and 0.747 for Egger’s test. Regarding the association of coffee consumption of >2 cups/day and of <1 cup/day with the incidence of NAFLD in the general population, the p-value of Begg’s and Egger’s tests was 0.835 and 0.533, respectively. Furthermore, there was no publication bias among the studies investigating the association of coffee consumption with liver fibrosis in patients with NAFLD. The p-value for Begg’s and Egger’s tests was 0.327 and 0.060, respectively.
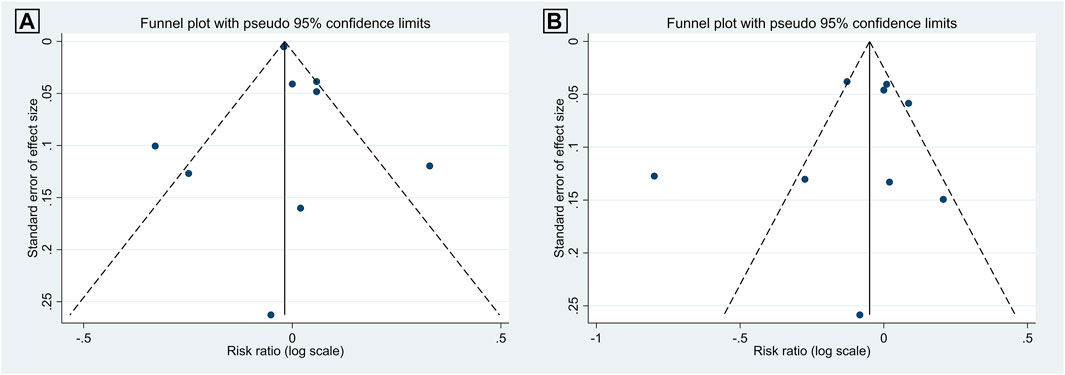
FIGURE 7. Funnel plots of the studies investigating the effects of coffee consumption on the incidence of NAFLD in the general population: (A) 1 to 2 cups/day versus <1 cup/day and (B) two cups/day versus <1 cup/day.
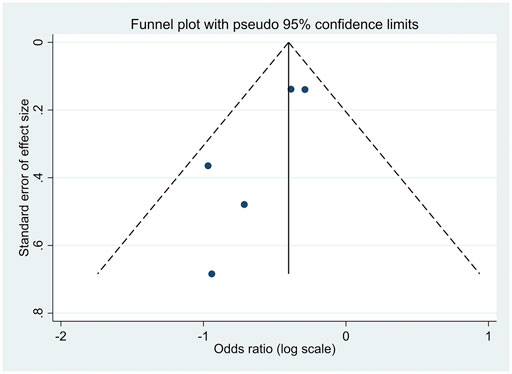
FIGURE 8. Funnel plot of the studies investigating the effects of coffee consumption and liver fibrosis in patients with NAFLD.
Both the qualitative and quantitative outcomes of the umbrella review showed that most of the previous systematic reviews and meta-analyses reported an association between coffee consumption and a reduction in the incidence of NAFLD in the general population. Moreover, the results showed that, from the point of view of doctors, coffee consumption had the beneficial effect of reducing the degree of liver fibrosis among patients with NAFLD.
Many studies have reported the hepatoprotective effect of coffee. This may be the result of several caffeine and non-caffeine substances (Shen et al., 2016; Wijarnpreecha et al., 2017; Chen et al., 2019; Hayat et al., 2021). Caffeine was shown to reduce oxidative stress and liver inflammation in an in vitro study. Moreover, its mechanisms of action showed signs of anti-fibrotic properties in hepatocytes (Gressner et al., 2008). Preclinical research found that other non-caffeine compounds also showed antioxidative characteristics that could reduce inflammatory reactions (Furtado et al., 2012). In addition to anti-inflammatory effects, antioxidants also have a significant role in reducing the accumulation of lipids in liver cells (Calabrò et al., 2020). The outcomes of the included systematic reviews and meta-analyses were particularly evident in their clinical aspects. They established that consuming coffee provided the benefits of reducing both the risk of NAFLD in the general population and the severity of fibrosis in patients with NAFLD (Shen et al., 2016; Wijarnpreecha et al., 2017; Chen et al., 2019; Hayat et al., 2021).
However, in the present SRMA, which included a much larger number of samples, we found that coffee consumption was not associated with a lower incidence of NAFLD in the general population. This non-significant outcome may be due to many reasons—for example, there were heterogeneities between the primary studies in terms of their methodologies, the differences in the types and amounts of coffee consumed, and the various tools used to investigate the basis of coffee consumption in the subjects. Thus, we were unable to confirm the protective effect of coffee against NAFLD in the general population. Even individuals who consumed more than two cups of coffee daily did not show a lower risk of NAFLD than those who drank less than one cup per day. However, among patients with NAFLD, coffee consumption was significantly associated with a reduction in hepatic fibrosis. On the other hand, the pooled results of four studies (Anty et al., 2012; Molloy et al., 2012; Bambha et al., 2014; Alferink et al., 2017) with a total of 3,331 patients with NAFLD indicated that there was no difference between the coffee intake of patients with no/mild liver fibrosis and those with significant liver fibrosis. Thus, more research is needed on the coffee intake required to reduce the severity of liver fibrosis in patients with NAFLD.
The benefits of coffee for patients with NAFLD can be explained by the underlying mechanism of NAFLD. Since coffee is composed of many substances, those that offer any effect that intervenes in the etiology of NAFLD should result in favorable clinical outcomes. As mentioned above, both caffeine and non-caffeine substances were preliminarily proven in preclinical studies to be able to block pathways associated with oxidative stress and TGF-β. These pathways happen to be one of the mechanisms behind the incidence of NAFLD and liver fibrosis (Tarantino et al., 2019). Still, various factors could alter clinical results in the real world. The inconsistent findings of our study were not unexpected due to the number of undetermined confounders and variations among the included clinical studies.
The strengths of our study were that we not only evaluated and summarized previously published SRMAs on the topics mentioned above but also rigorously performed a new SRMA to determine the effects of coffee consumption. The umbrella review and SRMA were conducted following standard guidelines. The searches were performed using various reliable databases and were assessed without language restriction. The SRMAs in the umbrella review were considered high-quality reviews according to AMSTAR 2 criteria. As far as we are concerned, our SRMA encompasses the most up-to-date evidence available and measures the effects of coffee consumption using the largest sample size currently available. Despite conflicting results on the protective effects of NAFLD in the general population, the benefits of coffee consumption on liver fibrosis were identified in patients with NAFLD. The results of this study may encourage researchers to develop well-designed studies to confirm the hepatoprotective effects of coffee, which should be useful in clinical practice.
This study had several limitations. First, it consisted of observational studies that could only show an association—not a causal relationship—between coffee consumption and liver outcomes. Second, the methodologies of the included studies were quite heterogeneous. Remarkable heterogeneity among the studies was also shown through the I2 statistic. Therefore, this current investigation adopted a random-effects model to pool the outcomes for the meta-analysis. Several of the included studies diagnosed NAFLD based on ultrasonography without confirmation by liver biopsy. Furthermore, the measurement of coffee intake in the primary articles was derived using a questionnaire at a single time point. This may not have provided an accurate assessment of the general coffee intake. The studies also used various means to measure coffee consumption, and some might have been prone to recall bias. Third, relevant information on coffee consumption, which may have influenced the outcomes of the present work, was not provided in the primary articles. This included details of the type of coffee, the components of the coffee, the brewing method, the amount of coffee per cup, and the drinking time. Thus, we were unable to analyze alterations in results when these factors changed. As an example, adding milk or sugar to coffee may increase fat accumulation in the liver, resulting in more unfavorable outcomes. Fourth, confounders of the included population were not used to adjust the outcomes in our analyses. The confounders were, for example, comorbidities, smoking, alcohol intake, physical activity, diet, and socioeconomic status.
In conclusion, our umbrella review and SRMA showed contrasting results on the benefits of coffee consumption on liver outcomes among the general population. Whether the consumption of coffee can be considered a preventive measure against NAFLD requires further investigation with a more rigid methodology. However, the results of both the umbrella review and the SRMA showed that the benefits of coffee consumption on liver fibrosis were significant for patients with NAFLD.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding authors.
This systematic review and meta-analysis was exempt from ethics approval because data were collected and synthesized from previous studies. The authors followed applicable EQUATOR Network (https://www.equator-network.org) guidelines during the conduct of the project.
CK, SuK, CA, NI, TK, SaK, NC, SS, and PP contributed to study concept and design. CK, SuK, CA, NI, TK, SaK, and SS contributed to acquisition of data. CK, SuK, CA, NI, TK, SaK, AD, and SS contributed to statistical analysis and interpretation of data. CK, SuK, CA, NI, TK, SaK, AD, SS, and PP contributed to the drafting of the manuscript. CK, SuK, NC, AD, SS, and PP contributed to the critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN) (grant number: FF65-UoE005), School of Pharmaceutical Sciences, University of Phayao. The funding source had no role in the study design, collection, analysis, and interpretation of data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
We thank the University of Phayao for its financial support of this research project. We are also grateful to Miss Pinyapat Ariyakunaphan for coordinating the project. Finally, we also acknowledge the careful and professional English language editing of this paper by David Park.
The supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.786596/full#supplementary-material
AMSTAR, A MeaSurement Tool to Assess systematic Reviews; BMI, body mass index; CI, confidence interval; JBI, Joanna Briggs Institute; MD, mean difference; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NOS, Newcastle–Ottawa quality assessment scale; OR, odds ratio; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RR, risk ratio; SRMA, systematic review and meta-analysis; WMD, weighted mean difference.
Alferink, L. J. M., Fittipaldi, J., Kiefte-de Jong, J. C., Taimr, P., Hansen, B. E., Metselaar, H. J., et al. (2017). Coffee and Herbal tea Consumption Is Associated with Lower Liver Stiffness in the General Population: The Rotterdam Study. J. Hepatol. 67 (2), 339–348. doi:10.1016/j.jhep.2017.03.013
Anty, R., Marjoux, S., Iannelli, A., Patouraux, S., Schneck, A. S., Bonnafous, S., et al. (2012). Regular Coffee but Not Espresso Drinking Is Protective against Fibrosis in a Cohort Mainly Composed of Morbidly Obese European Women With NAFLD Undergoing Bariatric Surgery. J. Hepatol. 57 (5), 1090–1096. doi:10.1016/j.jhep.2012.07.014
Aromataris, E., Fernandez, R., Godfrey, C. M., Holly, C., Khalil, H., and Tungpunkom, P. (2015). Summarizing Systematic Reviews: Methodological Development, Conduct and Reporting of an Umbrella Review Approach. Int. J. Evid. Based Healthc. 13 (3), 132–140. doi:10.1097/XEB.0000000000000055
Bambha, K., Wilson, L. A., Unalp, A., Loomba, R., Neuschwander-Tetri, B. A., Brunt, E. M., et al. (2014). Coffee Consumption in NAFLD Patients with Lower Insulin Resistance Is Associated with Lower Risk of Severe Fibrosis. Liver Int. 34 (8), 1250–1258. doi:10.1111/liv.12379
Birerdinc, A., Stepanova, M., Pawloski, L., and Younossi, Z. M. (2012). Caffeine Is Protective in Patients with Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 35 (1), 76–82. doi:10.1111/j.1365-2036.2011.04916.x
Calabrò, A., Procopio, A. C., Primerano, F., Larussa, T., Luzza, F., Di Renzo, L., et al. (2020). Beneficial Effects of Coffee in Non-Alcoholic Fatty Liver Disease: A Narrative Review. Hepatoma Res., 2020. doi:10.20517/2394-5079.2020.63
Catalano, D., Martines, G. F., Tonzuso, A., Pirri, C., Trovato, F. M., and Trovato, G. M. (2010). Protective Role of Coffee in Non-Alcoholic Fatty Liver Disease (NAFLD). Dig. Dis. Sci. 55 (11), 3200–3206. doi:10.1007/s10620-010-1143-3
Chalasani, N., Younossi, Z., Lavine, J. E., Charlton, M., Cusi, K., Rinella, M., et al. (2018). The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 67 (1), 328–357. doi:10.1002/hep.29367
Chen, Y. P., Lu, F. B., Hu, Y. B., Xu, L. M., Zheng, M. H., and Hu, E. D. (2019). A Systematic Review and a Dose-Response Meta-Analysis of Coffee Dose and Nonalcoholic Fatty Liver Disease. Clin. Nutr. 38 (6), 2552–2557. doi:10.1016/j.clnu.2018.11.030
Chung, H. K., Nam, J. S., Lee, M. Y., Kim, Y. B., Won, Y. S., Song, W. J., et al. (2020). The Increased Amount of Coffee Consumption Lowers the Incidence of Fatty Liver Disease in Korean Men. Nutr. Metab. Cardiovasc. Dis. 30 (10), 1653–1661. doi:10.1016/j.numecd.2020.05.026
Eslam, M., Sanyal, A. J., George, J., and International Consensus, P. (2020). MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 158 (7), 1999–e1. doi:10.1053/j.gastro.2019.11.312
European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity(2016). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-alcoholic Fatty Liver Disease. J. Hepatol. 64 (6), 1388–1402.
Funatsu, K., Yamashita, T., and Nakamura, H. (2011). Coffee Consumption Is Associated with a Lower Incidence of Fatty Liver in Middle-Aged Men. J. Health Sci. 57 (5), 406–413. doi:10.1248/jhs.57.406
Furtado, K. S., Prado, M. G., Aguiar E Silva, M. A., Dias, M. C., Rivelli, D. P., Rodrigues, M. A., et al. (2012). Coffee and Caffeine Protect Against Liver Injury Induced by Thioacetamide in Male Wistar Rats. Basic Clin. Pharmacol. Toxicol. 111 (5), 339–347. doi:10.1111/j.1742-7843.2012.00903.x
Graeter, T., Niedermayer, P. C., Mason, R. A., Oeztuerk, S., Haenle, M. M., Koenig, W., et al. (2015). Coffee Consumption and NAFLD: a Community Based Study on 1223 Subjects. BMC Res. Notes 8, 640. doi:10.1186/s13104-015-1645-3
Gressner, O. A., Lahme, B., Rehbein, K., Siluschek, M., Weiskirchen, R., and Gressner, A. M. (2008). Pharmacological Application of Caffeine Inhibits TGF-Beta-Stimulated Connective Tissue Growth Factor Expression in Hepatocytes via PPARgamma and SMAD2/3-Dependent Pathways. J. Hepatol. 49 (5), 758–767. doi:10.1016/j.jhep.2008.03.029
Gutiérrez-Grobe, Y., Chávez-Tapia, N., Sánchez-Valle, V., Gavilanes-Espinar, J. G., Ponciano-Rodríguez, G., Uribe, M., et al. (2012). High Coffee Intake Is Associated with Lower Grade Nonalcoholic Fatty Liver Disease: The Role of Peripheral Antioxidant Activity. Ann. Hepatol. 11 (3), 350–355. doi:10.1016/s1665-2681(19)30931-7
Hayat, U., Siddiqui, A. A., Okut, H., Afroz, S., Tasleem, S., and Haris, A. (2021). The Effect of Coffee Consumption on the Non-Alcoholic Fatty Liver Disease and Liver Fibrosis: A Meta-Analysis of 11 Epidemiological Studies. Ann. Hepatol. 20, 100254. doi:10.1016/j.aohep.2020.08.071
Herzog, R., Álvarez-Pasquin, M. J., Díaz, C., Del Barrio, J. L., Estrada, J. M, and Gil, Á. (2013). Are Healthcare Workers’ Intentions to Vaccinate Related to Their Knowledge, Beliefs and Attitudes? A Systematic Review. BMC Public Health 13, 154. doi:10.1186/1471-2458-13-154
Hosseinabadi, S., Rafraf, M., Asghari, S., Asghari-Jafarabadi, M., and Vojouhi, S. (2020). Effect of Green Coffee Extract Supplementation on Serum Adiponectin Concentration and Lipid Profile in Patients with Non-alcoholic Fatty Liver Disease: A Randomized, Controlled Trial. Complement. Ther. Med. 49, 102290. doi:10.1016/j.ctim.2019.102290
Imatoh, T., Kamimura, S., and Miyazaki, M. (2015). Coffee but Not Geen tea Consumption Is Associated with Prevalence and Severity of Hepatic Steatosis: The Impact on Leptin Level. Eur. J. Clin. Nutr. 69 (9), 1023–1027. doi:10.1038/ejcn.2015.23
Ito, T., Ishigami, M., Zou, B., Tanaka, T., Takahashi, H., Kurosaki, M., et al. (2021). The Epidemiology of NAFLD and Lean NAFLD in Japan: A Meta-Analysis With Individual and Forecasting Analysis, 1995–2040. Hepatol. Int. 15 (2), 366–379. doi:10.1007/s12072-021-10143-4
Leoni, S., Tovoli, F., Napoli, L., Serio, I., Ferri, S., and Bolondi, L. (2018). Current Guidelines for the Management of Non-alcoholic Fatty Liver Disease: A Systematic Review with Comparative Analysis. World J. Gastroenterol. 24 (30), 3361–3373. doi:10.3748/wjg.v24.i30.3361
Li, J., Zou, B., Yeo, Y. H., Feng, Y., Xie, X., Lee, D. H., et al. (2019). Prevalence, Incidence, and Outcome of Non-Alcoholic Fatty Liver Disease in Asia, 1999–2019: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 4 (5), 389–398. doi:10.1016/S2468-1253(19)30039-1
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Marventano, S., Salomone, F., Godos, J., Pluchinotta, F., Del Rio, D., Mistretta, A., et al. (2016). Coffee and Tea Consumption in Relation with Non-Alcoholic Fatty Liver and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. 35 (6), 1269–1281. doi:10.1016/j.clnu.2016.03.012
Mikolasevic, I., Domislovic, V., Filipec Kanizaj, T., Radic-Kristo, D., Krznaric, Z., Milovanovic, T., et al. (2021). Relationship Between Coffee Consumption, Sleep Duration and Smoking Status With Elastographic Parameters of Liver Steatosis and Fibrosis; Controlled Attenuation Parameter and Liver Stiffness Measurements. Int. J. Clin. Pract. 75 (3), e13770. doi:10.1111/ijcp.13770
Molloy, J. W., Calcagno, C. J., Williams, C. D., Jones, F. J., Torres, D. M., and Harrison, S. A. (2012). Association of Coffee and Caffeine Consumption With Fatty Liver Disease, Nonalcoholic Steatohepatitis, and Degree of Hepatic Fibrosis. Hepatology 55 (2), 429–436. doi:10.1002/hep.24731
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Park, J., Lee, E. Y., Li, J., Jun, M. J., Yoon, E., Ahn, S. B., et al. (2021). NASH/Liver Fibrosis Prevalence and Incidence of Non-Liver Comorbidities Among People with NAFLD and Incidence of NAFLD by Metabolic Comorbidites: Lessons From South Korea. Dig. Dis.
Phisalprapa, P., Prasitwarachot, R., Kositamongkol, C., Hengswat, P., Srivanichakorn, W., Washirasaksiri, C., et al. (2021). Economic Burden of Non-Alcoholic Steatohepatitis With Significant Fibrosis in Thailand. BMC Gastroenterol. 21 (1), 135. doi:10.1186/s12876-021-01720-w
Ruhl, C. E., and Everhart, J. E. (2005). Coffee and Caffeine Consumption Reduce the Risk of Elevated Serum Alanine Aminotransferase Activity in the United States. Gastroenterology 128 (1), 24–32. doi:10.1053/j.gastro.2004.09.075
Setiawan, V. W., Porcel, J., Wei, P., Stram, D. O., Noureddin, N., Lu, S. C., et al. (2017). Coffee Drinking and Alcoholic and Nonalcoholic Fatty Liver Diseases and Viral Hepatitis in the Multiethnic Cohort. Clin. Gastroenterol. Hepatol. 15 (8), 1305–1307. doi:10.1016/j.cgh.2017.02.038
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews that Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 358, j4008. doi:10.1136/bmj.j4008
Shen, H., Rodriguez, A. C., Shiani, A., Lipka, S., Shahzad, G., Kumar, A., et al. (2016). Association Between Caffeine Consumption and Nonalcoholic Fatty Liver Disease: A Systemic Review and Meta-Analysis. Therap Adv. Gastroenterol. 9 (1), 113–120. doi:10.1177/1756283X15593700
Shim, S. G., Jun, D. W., Kim, E. K., Saeed, W. K., Lee, K. N., Lee, H. L., et al. (2013). Caffeine Attenuates Liver Fibrosis via Defective Adhesion of Hepatic Stellate Cells in Cirrhotic Model. J. Gastroenterol. Hepatol. 28 (12), 1877–1884. doi:10.1111/jgh.12317
Soleimani, D., Ranjbar, G., Rezvani, R., Goshayeshi, L., Razmpour, F., and Nematy, M. (2019). Dietary Patterns in Relation to Hepatic Fibrosis Among Patients With Nonalcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 12, 315–324. doi:10.2147/DMSO.S198744
Tampi, R. P., Wong, V. W., Wong, G. L., Shu, S. S., Chan, H. L., Fung, J., et al. (2020). Modelling the Economic and Clinical burden of Non-Alcoholic Steatohepatitis in East Asia: Data From Hong Kong. Hepatol. Res. 50 (9), 1024–1031. doi:10.1111/hepr.13535
Tarantino, G., Citro, V., and Capone, D. (2019). Nonalcoholic Fatty Liver Disease: A Challenge from Mechanisms to Therapy. J. Clin. Med. 9 (1). doi:10.3390/jcm9010015
Wijarnpreecha, K., Thongprayoon, C., and Ungprasert, P. (2017). Coffee Consumption and Risk of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 29 (2), e8–e12. doi:10.1097/MEG.0000000000000776
Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., and Wymer, M. (2016). Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 64 (1), 73–84. doi:10.1002/hep.28431
Younossi, Z. M., Tampi, R., Priyadarshini, M., Nader, F., Younossi, I. M., and Racila, A. (2019). Burden of Illness and Economic Model for Patients With Nonalcoholic Steatohepatitis in the United States. Hepatology 69 (2), 564–572. doi:10.1002/hep.30254
Keywords: coffee, non-alcoholic fatty liver disease, liver fibrosis, umbrella review, meta-analysis
Citation: Kositamongkol C, Kanchanasurakit S, Auttamalang C, Inchai N, Kabkaew T, Kitpark S, Chaiyakunapruk N, Duangjai A, Saokaew S and Phisalprapa P (2021) Coffee Consumption and Non-alcoholic Fatty Liver Disease: An Umbrella Review and a Systematic Review and Meta-analysis. Front. Pharmacol. 12:786596. doi: 10.3389/fphar.2021.786596
Received: 30 September 2021; Accepted: 11 November 2021;
Published: 13 December 2021.
Edited by:
Ralf Weiskirchen, RWTH Aachen University, GermanyReviewed by:
Giovanni Tarantino, University of Naples Federico II, ItalyCopyright © 2021 Kositamongkol, Kanchanasurakit, Auttamalang, Inchai, Kabkaew, Kitpark, Chaiyakunapruk, Duangjai, Saokaew and Phisalprapa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Surasak Saokaew, c3VyYXNhay5zYUB1cC5hYy50aA==; Pochamana Phisalprapa, Y29jb19hMTA1QGhvdG1haWwuY29t
†ORCID: Chayanis Kositamongkol, orcid.org/0000–0001–7182–0733; Sukrit Kanchanasurakit, orcid.org/0000-0002-1268-2665; Nathorn Chaiyakunapruk, orcid.org/0000-0003-4572-8794; Acharaporn Duangjai, orcid.org/0000-0002-5153-8738; Surasak Saokaew, orcid.org/0000-0002-1382-0660; Pochamana Phisalprapa, orcid.org/0000-0003-1995-4405
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.