- 1Department of Pharmacology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
- 2Department of Pharmacotherapy, College of Pharmacy, University of Utah, Salt Lake City, UT, United States
- 3Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
- 4Department of Pediatrics, Khon Kaen Hospital, Khon Kaen, Thailand
- 5Department of Pediatrics, Udon Thani Hospital, Udon Thani, Thailand
- 6Division of Pharmacogenomics and Personalized Medicine, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 7Laboratory for Pharmacogenomics, Somdech Phra Debaratana Medical Center (SDMC), Ramathibodi Hospital, Bangkok, Thailand
Backgound: The high incidence of thiopurine-induced myelosuppression in Asians is known to be attributable to genetic variation in thiopurine metabolism. A quantitative synthesis to summarize the genetic association with thiopurine-induced myelosuppression in Asians was therefore conducted.
Methods: A Literature search was performed from January 2016 to May 2021 in the following databases: PubMed, Web of Science, and Embase and addition search included the studies from Zhang et al. Two reviewers independently extracted the following data: the author’s name, year of publication, ethnicity, drugs, diseases, genetic polymorphisms, onset, type of myelosuppression and results of Hardy-Weinberg equilibrium. The Newcastle-Ottawa Scale was used to assess the quality of the studies. The pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated to evaluate the associations of NUDT15 and the risk of thiopurine-induced myelosuppression stratified by onset and type of myelosuppressive. Subgroup analysis by NUDT15 genetic polymorphisms was performed.
Results: A total of 30 studies was included in this meta-analysis. The overall OR for the relationship between NUDT15 genetic polymorphisms and thiopurine-induced early onset of leukopenia and neutropenia in Asian populations were 11.43 (95% CI 7.11–18.35) and 16.35 (95% CI 10.20–26.22). Among NUDT15 polymorphisms, NUDT15*3 showed a significantly increased risk of early leukopenia (OR 15.31; 95% CI 9.65–24.27) and early neutropenia (OR 15.85; 95% CI 8.80–28.53). A significantly higher thiopurine-induced early neutropenic risk was also found for NUDT15*2 (OR 37.51; 95% CI 1.99–708.69). Whereas, NUDT15*5 and NUDT15*6 variants showed a lower risk of leukopenia.
Conclusion: This study suggests that NUDT15*3 and NUDT15*2 are important genetic markers of thiopurine-induced early onset of myelotoxicity in Asians, therefore, early detection of these variants before initiating thiopurine therapy is necessary.
Introduction
Thiopurines, 6-mercaptopurine (6-MP) and azathioprine (AZA), are purine analogs that are closely related in their structures (Coulthard and Hogarth, 2005). 6-MP is widely used in the treatment of acute lymphoblastic leukemia (ALL) as part of a combination regimen at the maintenance phase and used as an immunosuppressive agent for maintaining the remission of the disease. It is also prescribed off-label for the treatment of inflammatory bowel disease (IBD) (Dean, 2012). AZA is commonly used in management of autoimmune disorders e.g. Crohn’s disease, rheumatoid arthritis, and systemic lupus erythematosus (SLE) (Zaza et al., 2010), whereas thiopurine drugs have been shown to be effective in maintaining disease remission; however, almost 30–40% of patients discontinue therapy due to adverse effects, particularly myelosuppression (Lee et al., 2015), in which its incidence is higher in Asian populations than in Caucasian populations (Kakuta et al., 2018b). Thiopurine-induced myelosuppression often causes infectious complications and some patients require therapy interruption leading to suboptimal treatment and unfavorable outcomes (Relling et al., 1999; Hindorf et al., 2006). These adverse effects are known to be caused by individual differences in thiopurine metabolism, which is affected by the genetic variations.
6-MP is metabolized into inactive 6- methyl mercaptopurine (6-MMP) by Thiopurine S-methyltransferase (TPMT) (Lennard, 1992). It is well known that TPMT polymorphisms result in TPMT deficiency thereby increasing concentration of 6-thioguanine nucleotide (6-TGN) levels related to myelosuppression (Weinshilboum and Sladek, 1980). The frequencies of TPMT polymorphisms are ethnic differences which are higher in Caucasians than in the Asian population, which may explain the high incidence of thiopurine-induced myelosuppression in Asians (Collie-Duguid et al., 1999). Recently, several lines of evidence reported that the nucleoside diphosphate–linked moiety X-type motif 15 (NUDT15) was strongly associated with thiopurine-induced myelosuppression, specifically in Asian populations (Yang et al., 2014; Yang et al., 2015; Moriyama et al., 2016). The distribution of NUDT15 genetic polymorphism was reported to be the most common in East Asians, but rare in Caucasians (Yang et al., 2014; Yang et al., 2015; Moriyama et al., 2016). NUDT15 enzyme dephosphorylates 6-TGN then prevent the incorporation into DNA or RNA (Moriyama et al., 2016) therefore decreasing of the enzymatic activity which observed in NUDT15 variants, particularly NUDT15*3 was leading to thiopurine-induced myelosuppression (Moriyama et al., 2016).
Although the association between the NUDT15*3 variant and thiopurine-induced myelotoxicity is well recognized, there is still controversy about the increased risk of thiopurine hematotoxicity in patients who carry other variants of this gene, particularly NUDT15*2, NUDT15*5, NUDT15*6 which exist in high allele frequencies in Asian populations (Yang et al., 2014; Yang et al., 2015; Moriyama et al., 2016; Kim et al., 2017a). A quantitative synthesis of the existing genetic association studies to summarize the magnitude of the genetic association of all common variants of NUDT15 on thiopurine-induced toxicity was therefore conducted in order to ensure proper treatment and minimize the risk of thiopurine-induced myelosuppression in the Asian populations.
Methods
Data Sources and Search Strategy
A literature -search was conducted using PubMed, Web of Science, and Embase. Searches were performed using keywords and synonyms for NUDT15 and thiopurines and relevant terms for myelosuppression. MeSH terms in PubMed were used when available. There was no language or study design restriction, but only human studies were included. In the present meta-analysis, an updated search included the studies from Zhang et al., the previously systematic review and meta-analysis (Zhang et al., 2018), and additional studies published between January 1, 2016 and May 14, 2021.
Study Selection
Two reviewers (KK and WT) independently assessed abstracts and titles retrieved from the comprehensive searches for study inclusion. Articles from the updated search and from Zhang et al. were included if they met the inclusion criteria, as follows: 1) the study population was of patients treated with thiopurine drugs: 6-MP, AZA or 6-TG 2) Studied the association between genetic polymorphisms of NUDT15 and thiopurine-induced myelosuppression in an Asian population 3) the outcomes of interest included myelosuppression (anemia, neutropenia, leukopenia, and thrombocytopenia) 4) the study provided sufficient information to calculate the genetic association with thiopurine-induced myelosuppression. Exclusion criteria are 1) studies not relevant to pharmacogenetic of NUDT15 and TPMT and thiopurine-induced toxicity in Asian; 2) not clinical study; 3) review study, systematic review, meta-analysis, letters, editorials, opinion, commentaries, case report; 4) no full text available; 5) not report odds ratio or no sufficient information for calculate odd ratio or data not related to dose reduction. Any disagreements were discussed until consensus between the two reviewers could be reached.
Data Extraction and Quality Assessment
Data extraction was performed by two independent reviewers. Any disagreement was discussed and the data checked again to arrive at an agreement. The following data were extracted from each study: the first author’s last name, year of publication, ethnicity, drugs used, disease type, genetic polymorphisms, onset and type of myelosuppression. The Hardy-Weinberg equilibrium (HWE) was tested to check if the included individuals were in equilibrium for the frequencies of genotypes (Salanti et al., 2005; Mayo, 2008). Equilibrium implies that the included individuals were likely representative of the population (Smits et al., 2005; Thakkinstian et al., 2005). The quality of the selected studies was evaluated using the Newcastle–Ottawa Scale (NOS) (Wells et al., 2014). This scale is an 8-item instrument, categorized into the following three domains: selection of participants, comparability between groups, and the assessment of exposures and outcomes. A system of stars was used to provide quality ratings for studies.
Statistical Analysis
The pooled odd ratio (OR) and 95% CI were calculated to determine the association between genetic polymorphisms and the risk of thiopurine-induced myelosuppression. All analyses were performed with the method by DerSimonian and Laird (DerSimonian and Laird, 1986) using a random-effects model. Subgroup analysis was performed based on NUDT15 variants. Statistical heterogeneity was assessed via the Q statistic and I2 tests (Higgins and Thompson, 2002). p ≤ 0.10 indicated heterogeneity between studies. I2 values of 25 and 50% denoted low heterogeneity and moderate heterogeneity, across studies (Higgins et al., 2003). The Funnel plot, Begg test, and Egger test were used to evaluate small study effect (Begg and Berlin, 1989). All analyses were performed in STATA version 13.0 (StataCorp, College Station, Texas, USA).
Results
Study Selection
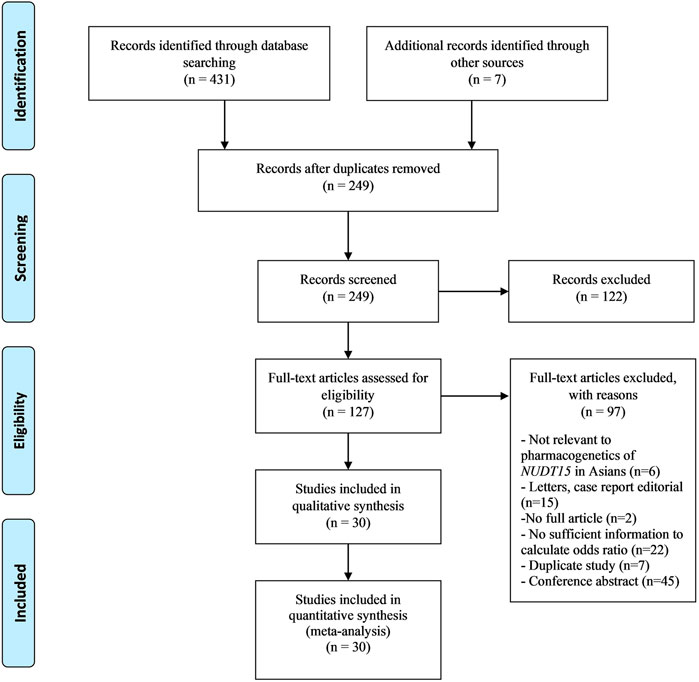
A total of 431 studies were identified from an updated literature search while an additional seven studies from a previous systematic meta-analysis by Zhang et al. (2018) were reviewed. Of the original 431 articles, 30 studies were included in the meta-analysis. No additional articles were identified via a review of the bibliographies of the included studies. (Figure 1).
Study Characteristics
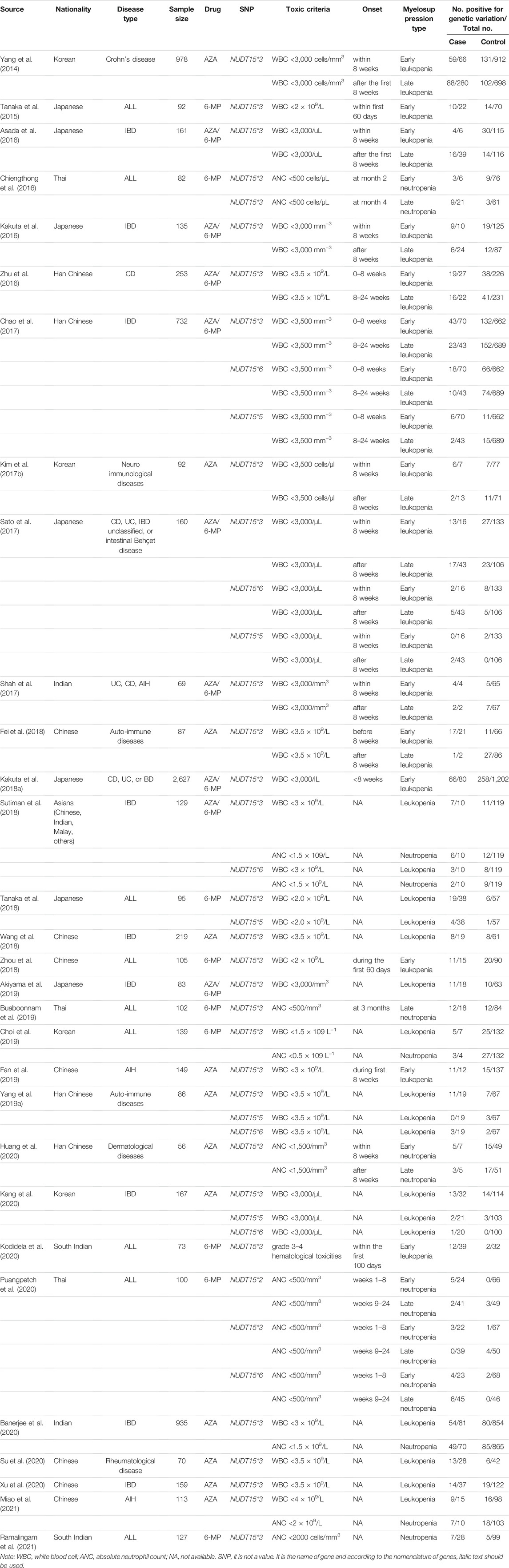
Characteristics of 30 studies are described in Table 1. All studies were conducted among Asians populations. There were 1,167 cases with leukopenia and 240 cases with neutropenia. Among these, 14 studies were conducted in IBD (Yang et al., 2014; Asada et al., 2016; Kakuta et al., 2016; Zhu et al., 2016; Chao et al., 2017; Sato et al., 2017; Shah et al., 2017; Kakuta et al., 2018a; Sutiman et al., 2018; Wang et al., 2018; Akiyama et al., 2019; Banerjee et al., 2020; Kang et al., 2020; Xu et al., 2020), nine studies in ALL (Tanaka et al., 2015; Chiengthong et al., 2016; Tanaka et al., 2018; Zhou et al., 2018; Buaboonnam et al., 2019; Choi et al., 2019; Kodidela et al., 2020; Puangpetch et al., 2020; Ramalingam et al., 2021) and seven studies in other autoimmune diseases (autoimmune hepatitis, dermatological diseases and rheumatological diseases) (Kim et al., 2017b; Fei et al., 2018; Yang et al., 2019a; Fan et al., 2019; Huang et al., 2020; Su et al., 2020; Miao et al., 2021).
Myelosuppression was categorized based on the onset and characteristics of blood cell type, including of leukopenia and neutropenia. The onset of myelosuppression within 8 weeks was defined as early whereas the onset after 8 weeks was defined as late. For those studies without a clear description of onset of myelosuppression, the onset was classified based on duration of the study. Among studies conducted in ALL patients, the 6-MP dose was 40–75 mg/m2/d, while the studies conducted in IBD or autoimmune diseases was AZA 0.5–3 mg/kg/d. The distribution of observed alleles and expected alleles of each genetic variation were consistent with HWE, except for three studies (Tanaka et al., 2015; Kakuta et al., 2018a; Tanaka et al., 2018).
Quality Assessment
The methodological quality of all studies is summarized as a mean Newcastle-Ottawa Scale score of 8 (range, 7–9; maximum score, 9, see Supplementary Table S2).
Quantitative Synthesis
Association between genetic polymorphisms involved in thiopurine metabolism and risk of myelosuppression.
NUDT15*3 Polymorphisms (rs116855232)
In thirty studies with NUDT15*3, there were 476 cases with early leukopenia from 15 studies, 691 cases with late leukopenia from 19 studies. Of these, 338 cases (71%) with early onset and 281 cases (40.67%) with late onset carried the NUDT15*3 variant. The higher risk for development of early leukopenia was found to be significantly associated with NUDT15*3 carriers (OR 15.31; 95% CI 9.65–24.27, I2 = 63.6%) (Figure 2) compared to those patients with late onset (OR 4.9; 95% CI 3.56–6.74, I2 = 47.4) (Supplementary Figure S1). In addition, there were 105 cases with early neutropenia. Of these, 60 cases (57.14%) carried NUDT15*3. The study also found a strong relationship between NUDT15*3 and risk of early neutropenia in studied patients (OR 15.85; 95% CI 8.8–28.53, I2 7.4%, p = 0.356) (Figure 3).

FIGURE 2. The forest plots for the association of NUDT15 variants with thiopurine-induced early leukopenia. Note: Width of the box indicates the precision of the estimates; diamond, the overall summary estimate for the analysis (width of the diamond represents the 95% CI).
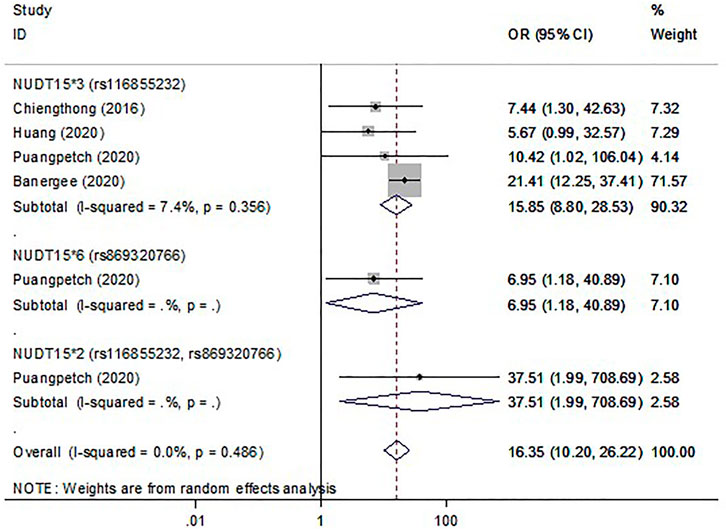
FIGURE 3. The forest plots for the association of NUDT15 variants with thiopurine-induced early neutropenia. Note: Width of the box indicates the precision of the estimates; diamond, the overall summary estimate for the analysis (width of the diamond represents the 95% CI).
NUDT15*5 (rs186364861), NUDT15*6 (rs869320766) and NUDT15*2 (rs116855232, rs869320766)
Six studies were included to determine the association of NUDT15*5 and NUDT15*6 with the risk of myelosuppression. Overall, with those who carried either NUDT15*5 or NUDT15*6, 15.12% (26 of 172) cases had early leukopenia whereas 10.70% (32 of 299) cases had late leukopenia. For neutropenia, 17.39% (4 of 23) cases with early onset and 14.55% (8 of 55) cases with late onset carried NUDT15*6. The OR for early and late leukopenia was about 3.01–4.9 for NUDT15*5 and NUDT15*6 variants (Figure 2, Supplementary Figure S1). Only one study showed a significant 6.95-fold higher risk for NUDT15*6 variant carriers to develop early neutropenia (OR 6.95; 95% CI 1.18–40.89) (Figure 3). There was one study reporting the significant association between NUDT15*2 and early neutropenia in ALL patients (OR 37.51; 95% CI 1.99–708.69) (Figure 3) but not for late neutropenia (Supplementary Figure S2).
Small Study Effect
Begg’s and Egger’s test were performed to assess for small study effect. The Egger’s test and Begg’s test were not significant for all analysis (p > 0.05, see Supplementary Table S1), except for late neutropenia. However, no asymmetry was found in the funnel plot indicating a lack of evidence for a small study effect (see Supplementary Figures S3–6).
Discussion
Recently, a number of studies suggested that NUDT15*3 was a novel predictor of thiopurine-induced myelosuppression in Asians (Yang et al., 2014; Yang et al., 2015; Moriyama et al., 2016). To date, more than 20 variants of the NUDT15 gene have been reported (Yang et al., 2019b). The common variant alleles are NUDT15*2 (rs869320766; c.36_37insGGAGTC and rs116855232; c.415C > T), NUDT15*3 (rs116855232; c.415C > T), NUDT15*5 (rs186364861; c.52G > A), and NUDT15*6 (rs869320766; c.36_37insGGAGTC) (Moriyama et al., 2016) in which NUDT13*3 is the most prevalent in Asian populations (Yang et al., 2014; Yang et al., 2015; Moriyama et al., 2016; Kim et al., 2017a; Khaeso et al., 2021). This current study’s results indicate that the overall OR for the relationship between NUDT15 genetic polymorphisms and thiopurine-induced early onset of leukopenia and neutropenia were 11.43 (95%CI 7.11–18.35) and 16.35 (95% CI 10.20–26.22). The higher risk was noted in patients who carried NUDT15*3 more than any other variant with an OR of 15.31 for early leukopenia and 15.85 for early neutropenia. In addition, an almost 38-fold increase of risk of early neutropenia was also found for NUDT15*2 carrier patients.
NUDT15*3 and NUDT15*2 showed a 100% loss of enzyme activity (Moriyama et al., 2016). The recent Clinical Implementation Consortium (CPIC) Guidelines for Thiopurine classified an individual carrying one normal function allele with NUDT15*2 or NUDT15*3 allele as an intermediate metabolizer whereas an individual carrying these two no function alleles were poor metabolizers (Relling and Schwab, 2019). It has been reported that patients with NUDT15*1/*2 which contain both rs869320766 and rs116855232 had a similar degree of 6-MP intolerance as the NUDT15*1/*3 (which contained a single rs116855232 SNP) (Moriyama et al., 2016). A similar result was reported in that the NUDT15*2 variant showed an approximate 38-fold higher risk of early neutropenia in ALL patients who were treated with 6-MP (Puangpetch et al., 2020). This suggested that these two variants of NUDT15 proteins may have exhibited similar enzymatic activity (Moriyama et al., 2016).
Unlike NUDT15*3 and NUDT15*2, an in vitro study reported that NUDT15*5 and NUDT15*6 showed a loss of enzyme activity of about 50–60%, however, in vivo activities of these enzymes were not quite clear (Moriyama et al., 2016). Previous studies showed controversy about the increased risk of thiopurine hematotoxicity in patients who carried these variant alleles, particularly NUDT15*6 (Sato et al., 2017; Sutiman et al., 2018; Tanaka et al., 2018). The previous meta-analysis has reported a lower diagnostic accuracy for NUDT15*6 and NUDT15*5 compared to NUDT15*3 (Cargnin et al., 2018), consistent with the current results that revealed a lower risk of OR in patients who carried NUDT15*5 or NUDT15*6 with the risk of myelosuppression compared to NUDT15*3.
The results from this meta-analysis showed that the lower number of OR was found in late onset of myelosuppression and that these may be because thiopurine toxicity often occurs in the first few months of the maintenance phase. The reason may be due to the fact that practically, the thiopurine dose was gradually adjusted to the tolerated dose which resulted in no myelosuppressive effect. Therefore NUDT15*3 polymorphism had the increased risk of thiopurine-induced leukopenia and neutropenia in particular, as early as the first 2 months of the maintenance phase of treatment.
One limitation that deserves discussion is a significant heterogeneity observed amongst studies evaluating the association between with NUDT15*3 carriers and early leukopenia. We could not explore the cause of this heterogeneity. Despite the fact that we used a random-effects model to pool the results across studies, the results of the meta-analysis for early leukopenia should be interpreted with caution.
Conclusion
In summary, this study found a strong relationship between NUDT15*3 and NUDT15*2 variants and thiopurine-induced early onset myelosuppression. The rs116855232 SNP which exists in both of these variants appears to be a key SNP for thiopurine-induced hematoxtoxicity. The genotyping of the rs116855232 which has a high prevalence in Asian populations should be considered prior prescribing thiopurine drugs in order to predict the risk of early myelosuppression.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
KK, SU, NC, and WT study design. KK and SU search strategy and literature search. KK and WT data extraction. KK, SU, and NC data analysis, assessment of study quality and risk of bias. All authors contributed to manuscript drafting and final approval of the manuscript.
Funding
This work was supported by grants from the Health System Research Institute (under Genomics Thailand Strategic Fund, HSRI 64–082), Faculty of Medicine, Khon Kaen University, Thailand (grant number IN64111) and the scholarship support from the Graduate School, Khon Kaen University through the Research Fund for Supporting Lecturers to Admit High Potential Student to Study and Research on His Expert Program Year 2019 (grant number 621H219).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge Prof. James A. Will, University of Wisconsin-Madison, for critical review and editing the manuscript via Publication Clinic KKU, Thailand.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.784712/full#supplementary-material
References
Akiyama, S., Matsuoka, K., Fukuda, K., Hamada, S., Shimizu, M., Nanki, K., et al. (2019). Long-term Effect of NUDT15 R139C on Hematologic Indices in Inflammatory Bowel Disease Patients Treated with Thiopurine. J. Gastroenterol. Hepatol. 34, 1751–1757. doi:10.1111/jgh.14693
Asada, A., Nishida, A., Shioya, M., Imaeda, H., Inatomi, O., Bamba, S., et al. (2016). NUDT15 R139C-Related Thiopurine Leukocytopenia Is Mediated by 6-thioguanine Nucleotide-independent Mechanism in Japanese Patients with Inflammatory Bowel Disease. J. Gastroenterol. 51, 22–29. doi:10.1007/s00535-015-1142-4
Banerjee, R., Ravikanth, V. V., Pal, P., Bale, G., Avanthi, U. S., Goren, I., et al. (2020). NUDT15 C415T Variant Compared with TPMT Genotyping in Predicting Azathioprine-Induced Leucopenia: Prospective Analysis of 1014 Inflammatory Bowel Disease Patients in India. Aliment. Pharmacol. Ther. 52, 1683–1694. doi:10.1111/apt.16137
Begg, C. B., and Berlin, J. A. (1989). Publication Bias and Dissemination of Clinical Research. J. Natl. Cancer Inst. 81, 107–115. doi:10.1093/jnci/81.2.107
Buaboonnam, J., Sripatanatadasakul, P., Treesucon, A., Glomglao, W., Siraprapapat, P., Narkbunnam, N., et al. (2019). Effect of NUDT15 on Incidence of Neutropenia in Children with Acute Lymphoblastic Leukemia. Pediatr. Int. 61, 754–758. doi:10.1111/ped.13905
Cargnin, S., Genazzani, A. A., Canonico, P. L., and Terrazzino, S. (2018). Diagnostic Accuracy of NUDT15 Gene Variants for Thiopurine-Induced Leukopenia: a Systematic Review and Meta-Analysis. Pharmacol. Res. 135, 102–111. doi:10.1016/j.phrs.2018.07.021
Chao, K., Wang, X., Cao, Q., Qian, J., Wu, K., Zhu, X., et al. (2017). Combined Detection of NUDT15 Variants Could Highly Predict Thiopurine-Induced Leukopenia in Chinese Patients with Inflammatory Bowel Disease: a Multicenter Analysis. Inflamm. Bowel Dis. 23, 1592–1599. doi:10.1097/MIB.0000000000001148
Chiengthong, K., Ittiwut, C., Muensri, S., Sophonphan, J., Sosothikul, D., Seksan, P., et al. (2016). NUDT15 c.415C>T Increases Risk of 6-mercaptopurine Induced Myelosuppression during Maintenance Therapy in Children with Acute Lymphoblastic Leukemia. Haematologica 101, e24–6. doi:10.3324/haematol.2015.134775
Choi, R., Sohn, I., Kim, M. J., Woo, H. I., Lee, J. W., Ma, Y., et al. (2019). Pathway Genes and Metabolites in Thiopurine Therapy in Korean Children with Acute Lymphoblastic Leukaemia. Br. J. Clin. Pharmacol. 85, 1585–1597. doi:10.1111/bcp.13943
Collie-Duguid, E. S., Pritchard, S. C., Powrie, R. H., Sludden, J., Collier, D. A., Li, T., et al. (1999). The Frequency and Distribution of Thiopurine Methyltransferase Alleles in Caucasian and Asian Populations. Pharmacogenetics 9, 37–42. doi:10.1097/00008571-199902000-00006
Coulthard, S., and Hogarth, L. (2005). The Thiopurines: an Update. Invest. New Drugs 23, 523–532. doi:10.1007/s10637-005-4020-8
Dean, L. (2012). “Mercaptopurine Therapy and TPMT Genotype,” in Medical Genetics Summaries. Editors V. M. Pratt, H. L. McLeod, W. S. Rubinstein, S. A. Scott, L. C. Dean, B. L. Kattmanet al. (Bethesda (MD).
DerSimonian, R., and Laird, N. (1986). Meta-analysis in Clinical Trials. Control. Clin. Trials 7, 177–188. doi:10.1016/0197-2456(86)90046-2
Fan, X., Yin, D., Men, R., Xu, H., and Yang, L. (2019). NUDT15 Polymorphism Confer Increased Susceptibility to Thiopurine-Induced Leukopenia in Patients with Autoimmune Hepatitis and Related Cirrhosis. Front. Pharmacol. 10, 346. doi:10.3389/fphar.2019.00346
Fei, X., Shu, Q., Zhu, H., Hua, B., Wang, S., Guo, L., et al. (2018). NUDT15 R139C Variants Increase the Risk of Azathioprine-Induced Leukopenia in Chinese Autoimmune Patients. Front. Pharmacol. 9, 460. doi:10.3389/fphar.2018.00460
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327, 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P., and Thompson, S. G. (2002). Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 21, 1539–1558. doi:10.1002/sim.1186
Hindorf, U., Lindqvist, M., Hildebrand, H., Fagerberg, U., and Almer, S. (2006). Adverse Events Leading to Modification of Therapy in a Large Cohort of Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 24, 331–342. doi:10.1111/j.1365-2036.2006.02977.x
Huang, P. W., Tseng, Y. H., and Tsai, T. F. (2020). Predictive Value of NUDT15 Variants on Neutropenia Among Han Chinese Patients with Dermatologic Diseases: A Single-center Observational Study. Dermatol. Ther. (Heidelb) 10, 263–271. doi:10.1007/s13555-020-00360-4
Kakuta, Y., Kawai, Y., Okamoto, D., Takagawa, T., Ikeya, K., Sakuraba, H., et al. (2018a). NUDT15 Codon 139 Is the Best Pharmacogenetic Marker for Predicting Thiopurine-Induced Severe Adverse Events in Japanese Patients with Inflammatory Bowel Disease: a Multicenter Study. J. Gastroenterol. 53, 1065–1078. doi:10.1007/s00535-018-1486-7
Kakuta, Y., Kinouchi, Y., and Shimosegawa, T. (2018b). Pharmacogenetics of Thiopurines for Inflammatory Bowel Disease in East Asia: Prospects for Clinical Application of NUDT15 Genotyping. J. Gastroenterol. 53, 172–180. doi:10.1007/s00535-017-1416-0
Kakuta, Y., Naito, T., Onodera, M., Kuroha, M., Kimura, T., Shiga, H., et al. (2016). NUDT15 R139C Causes Thiopurine-Induced Early Severe Hair Loss and Leukopenia in Japanese Patients with IBD. Pharmacogenomics J. 16, 280–285. doi:10.1038/tpj.2015.43
Kang, B., Kim, T. J., Choi, J., Baek, S. Y., Ahn, S., Choi, R., et al. (2020). Adjustment of Azathioprine Dose Should Be Based on a Lower 6-TGN Target Level to Avoid Leucopenia in NUDT15 Intermediate Metabolisers. Aliment. Pharmacol. Ther. 52, 459–470. doi:10.1111/apt.15810
Khaeso, K., Nakkam, N., Komwilaisak, P., Wongmast, P., Chainansamit, S. O., Dornsena, A., et al. (2021). Genetic Polymorphisms of Drug-Metabolizing Enzymes Involved in 6-Mercaptopurine-Induced Myelosuppression in Thai Pediatric Acute Lymphoblastic Leukemia Patients. J. Pediatr. Genet. 10, 29–34. doi:10.1055/s-0040-1715818
Kim, H. T., Choi, R., Won, H. H., Choe, Y. H., Kang, B., Lee, K., et al. (2017a). NUDT15 Genotype Distributions in the Korean Population. Pharmacogenet Genomics 27, 197–200. doi:10.1097/FPC.0000000000000274
Kim, S. Y., Shin, J. H., Park, J. S., Kang, S. Y., Nam, T. S., Kim, J. K., et al. (2017b). NUDT15 p.R139C Variant Is Common and Strongly Associated with Azathioprine-Induced Early Leukopenia and Severe Alopecia in Korean Patients with Various Neurological Diseases. J. Neurol. Sci. 378, 64–68. doi:10.1016/j.jns.2017.04.041
Kodidela, S., Dorababu, P., Thakkar, D. N., Dubashi, B., Sundaram, R., Muralidharan, N., et al. (2020). Association of NUDT15*3 and FPGS 2572C>T Variants with the Risk of Early Hematologic Toxicity during 6-MP and Low-Dose Methotrexate-Based Maintenance Therapy in Indian Patients with Acute Lymphoblastic Leukemia. Genes (Basel) 11. doi:10.3390/genes11060594
Lee, K. M., Kim, Y. S., Seo, G. S., Kim, T. O., and Yang, S. K.IBD Study Group of the Korean Association for the Study of Intestinal Diseases (2015). Use of Thiopurines in Inflammatory Bowel Disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 13, 193–207. doi:10.5217/ir.2015.13.3.193
Lennard, L. (1992). The Clinical Pharmacology of 6-mercaptopurine. Eur. J. Clin. Pharmacol. 43, 329–339. doi:10.1007/BF02220605
Mayo, O. (2008). A century of Hardy-Weinberg Equilibrium. Twin Res. Hum. Genet. 11, 249–256. doi:10.1375/twin.11.3.249
Miao, Q., Yan, L., Zhou, Y., Li, Y., Zou, Y., Wang, L., et al. (2021). Association of Genetic Variants in TPMT, ITPA, and NUDT15 with Azathioprine-Induced Myelosuppression in Southwest china Patients with Autoimmune Hepatitis. Sci. Rep. 11, 7984. doi:10.1038/s41598-021-87095-0
Moriyama, T., Nishii, R., Perez-Andreu, V., Yang, W., Klussmann, F. A., Zhao, X., et al. (2016). NUDT15 Polymorphisms Alter Thiopurine Metabolism and Hematopoietic Toxicity. Nat. Genet. 48, 367–373. doi:10.1038/ng.3508
Puangpetch, A., Tiyasirichokchai, R., Pakakasama, S., Wiwattanakul, S., Anurathapan, U., Hongeng, S., et al. (2020). NUDT15 Genetic Variants Are Related to Thiopurine-Induced Neutropenia in Thai Children with Acute Lymphoblastic Leukemia. Pharmacogenomics 21, 403–410. doi:10.2217/pgs-2019-0177
Ramalingam, R., Kaur, H., Scott, J. X., Sneha, L. M., Arun Kumar, G. P., Srinivasan, A., et al. (2021). Pharmacogenetic Evaluation of 6-Mercaptopurine-Mediated Toxicity in Pediatric Acute Lymphoblastic Leukemia Patients from a South Indian Population. Pharmacogenomics 22, 401–411. doi:10.2217/pgs-2020-0193
Relling, M. V., Hancock, M. L., Boyett, J. M., Pui, C. H., and Evans, W. E. (1999). Prognostic Importance of 6-mercaptopurine Dose Intensity in Acute Lymphoblastic Leukemia. Blood 93, 2817–2823. doi:10.1182/blood.v93.9.2817.409k04_2817_2823
Relling, M. V., Schwab, M., Whirl-Carrillo, M., Suarez-Kurtz, G., Pui, C. H., Stein, C. M., et al. (2019). Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 105, 1095–1105. doi:10.1002/cpt.1304
Salanti, G., Sanderson, S., and Higgins, J. P. (2005). Obstacles and Opportunities in Meta-Analysis of Genetic Association Studies. Genet. Med. 7, 13–20. doi:10.1097/01.gim.0000151839.12032.1a
Sato, T., Takagawa, T., Kakuta, Y., Nishio, A., Kawai, M., Kamikozuru, K., et al. (2017). NUDT15, FTO, and RUNX1 Genetic Variants and Thiopurine Intolerance Among Japanese Patients with Inflammatory Bowel Diseases. Intest Res. 15, 328–337. doi:10.5217/ir.2017.15.3.328
Shah, S. A., Paradkar, M., Desai, D., and Ashavaid, T. F. (2017). Nucleoside Diphosphate-Linked Moiety X-type Motif 15 C415T Variant as a Predictor for Thiopurine-Induced Toxicity in Indian Patients. J. Gastroenterol. Hepatol. 32, 620–624. doi:10.1111/jgh.13494
Smits, K. M., Schouten, J. S., Smits, L. J., Stelma, F. F., Nelemans, P., and Prins, M. H. (2005). A Review on the Design and Reporting of Studies on Drug-Gene Interaction. J. Clin. Epidemiol. 58, 651–654. doi:10.1016/j.jclinepi.2005.01.001
Su, S. S., Lin, Y. F., and Zhou, H. (2020). Association of Thiopurine S-Methyltransferase and NUDT15 Polymorphisms with Azathioprine-Induced Myelotoxicity in Chinese Patients with Rheumatological Disease. Chin. Med. J. (Engl) 133, 1002–1004. doi:10.1097/CM9.0000000000000756
Sutiman, N., Chen, S., Ling, K. L., Chuah, S. W., Leong, W. F., Nadiger, V., et al. (2018). Predictive Role of NUDT15 Variants on Thiopurine-Induced Myelotoxicity in Asian Inflammatory Bowel Disease Patients. Pharmacogenomics 19, 31–43. doi:10.2217/pgs-2017-0147
Tanaka, Y., Kato, M., Hasegawa, D., Urayama, K. Y., Nakadate, H., Kondoh, K., et al. (2015). Susceptibility to 6-MP Toxicity Conferred by a NUDT15 Variant in Japanese Children with Acute Lymphoblastic Leukaemia. Br. J. Haematol. 171, 109–115. doi:10.1111/bjh.13518
Tanaka, Y., Nakadate, H., Kondoh, K., Nakamura, K., Koh, K., and Manabe, A. (2018). Interaction between NUDT15 and ABCC4 Variants Enhances Intolerability of 6-mercaptopurine in Japanese Patients with Childhood Acute Lymphoblastic Leukemia. Pharmacogenomics J. 18, 275–280. doi:10.1038/tpj.2017.12
Thakkinstian, A., McElduff, P., D'Este, C., Duffy, D., and Attia, J. (2005). A Method for Meta-Analysis of Molecular Association Studies. Stat. Med. 24, 1291–1306. doi:10.1002/sim.2010
Wang, H. H., He, Y., Wang, H. X., Liao, C. L., Peng, Y., Tao, L. J., et al. (2018). Comparison of TPMT and NUDT15 Polymorphisms in Chinese Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 24, 941–948. doi:10.3748/wjg.v24.i8.941
Weinshilboum, R. M., and Sladek, S. L. (1980). Mercaptopurine Pharmacogenetics: Monogenic Inheritance of Erythrocyte Thiopurine Methyltransferase Activity. Am. J. Hum. Genet. 32, 651–662.
Wells, G., Shea, B., O'Connell, D., Peterson, J., Welch, V., Losos, M., et al. (2014). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses [Online]. Ottawa Hospital Research Institutewebsite. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed March 22, 2021).
Xu, Y., Qiao, Y. Q., Li, H. Y., Zhou, M., Cai, C. W., Shen, J., et al. (2020). NUDT15 Genotyping during Azathioprine Treatment in Patients with Inflammatory Bowel Disease: Implications for a Dose-Optimization Strategy. Gastroenterol. Rep. (Oxf) 8, 437–444. doi:10.1093/gastro/goaa021
Yang, J., Wang, P., Qin, Z., Jia, M., Zhang, C., Tian, X., et al. (2019a). NUDT15 and TPMT Genetic Polymorphisms Are Related to Azathioprine Intolerance in Chinese Patients with Rheumatic Diseases. Genet. Test. Mol. Biomarkers 23, 751–757. doi:10.1089/gtmb.2018.0313
Yang, J. J., Landier, W., Yang, W., Liu, C., Hageman, L., Cheng, C., et al. (2015). Inherited NUDT15 Variant Is a Genetic Determinant of Mercaptopurine Intolerance in Children with Acute Lymphoblastic Leukemia. J. Clin. Oncol. 33, 1235–1242. doi:10.1200/JCO.2014.59.4671
Yang, J. J., Whirl-Carrillo, M., Scott, S. A., Turner, A. J., Schwab, M., Tanaka, Y., et al. (2019b). Pharmacogene Variation Consortium Gene Introduction: NUDT15. Clin. Pharmacol. Ther. 105, 1091–1094. doi:10.1002/cpt.1268
Yang, S. K., Hong, M., Baek, J., Choi, H., Zhao, W., Jung, Y., et al. (2014). A Common Missense Variant in NUDT15 Confers Susceptibility to Thiopurine-Induced Leukopenia. Nat. Genet. 46, 1017–1020. doi:10.1038/ng.3060
Zaza, G., Cheok, M., Krynetskaia, N., Thorn, C., Stocco, G., Hebert, J. M., et al. (2010). Thiopurine Pathway. Pharmacogenet Genomics 20, 573–574. doi:10.1097/FPC.0b013e328334338f
Zhang, A. L., Yang, J., Wang, H., Lu, J. L., Tang, S., and Zhang, X. J. (2018). Association of NUDT15 c.415C>T Allele and Thiopurine-Induced Leukocytopenia in Asians: a Systematic Review and Meta-Analysis. Ir J. Med. Sci. 187, 145–153. doi:10.1007/s11845-017-1608-x
Zhou, H., Li, L., Yang, P., Yang, L., Zheng, J. E., Zhou, Y., et al. (2018). Optimal Predictor for 6-mercaptopurine Intolerance in Chinese Children with Acute Lymphoblastic Leukemia: NUDT15, TPMT, or ITPA Genetic Variants. BMC Cancer 18, 516. doi:10.1186/s12885-018-4398-2
Zhu, X., Wang, X. D., Chao, K., Zhi, M., Zheng, H., Ruan, H. L., et al. (2016). NUDT15 Polymorphisms Are Better Than Thiopurine S-Methyltransferase as Predictor of Risk for Thiopurine-Induced Leukopenia in Chinese Patients with Crohn's Disease. Aliment. Pharmacol. Ther. 44, 967–975. doi:10.1111/apt.13796
Keywords: nucleoside diphosphate–linked moiety X-type motif 15 (NUDT15), thiopurine drugs, hematotoxicity, genetic polymorphism, precision medicine, Meta-analysis, Systematic review
Citation: Khaeso K, Udayachalerm S, Komvilaisak P, Chainansamit S-o, Suwannaying K, Laoaroon N, Kuwatjanakul P, Nakkam N, Sukasem C, Puangpetch A, Tassaneeyakul W and Chaiyakunapruk N (2021) Meta-Analysis of NUDT15 Genetic Polymorphism on Thiopurine-Induced Myelosuppression in Asian Populations. Front. Pharmacol. 12:784712. doi: 10.3389/fphar.2021.784712
Received: 28 September 2021; Accepted: 16 November 2021;
Published: 02 December 2021.
Edited by:
Giuseppe Toffoli, Aviano Oncology Reference Center (IRCCS), ItalyReviewed by:
Swarup A. Shah, P. D. Hinduja Hospital and Medical Research Centre, IndiaSonja Pavlovic, University of Belgrade, Serbia
Copyright © 2021 Khaeso, Udayachalerm, Komvilaisak, Chainansamit, Suwannaying, Laoaroon, Kuwatjanakul, Nakkam, Sukasem, Puangpetch, Tassaneeyakul and Chaiyakunapruk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wichittra Tassaneeyakul, d2ljaGl0dHJhLnRhc3NhbmVleWFrdWxAZ21haWwuY29t; Nathorn Chaiyakunapruk, bmF0aG9ybi5jaGFpeWFrdW5hcHJ1a0B1dGFoLmVkdQ==
 Kanyarat Khaeso
Kanyarat Khaeso Sariya Udayachalerm2
Sariya Udayachalerm2 Nontaya Nakkam
Nontaya Nakkam Chonlaphat Sukasem
Chonlaphat Sukasem Nathorn Chaiyakunapruk
Nathorn Chaiyakunapruk