
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 21 January 2022
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.784281
This article is part of the Research Topic Plant Extracts and Natural Products as Protective Agents Against Toxins View all 4 articles
Pesticides are used in large quantities infrequently, resulting in environmental damage and health issues. The goal of the current study was to explore the ameliorating effect of Ocimum basilicum (Basil) leaves essential oil versus the harmful effects of β-cyfluthrin in rat liver. Male Wistar rats were classified at random into four groups; negative control (corn oil), basil leaves essential oil (BEO, 3 ml/kg), β-cyfluthrin (positive control) (β-Cyf; 15 mg/kg BW, 1/25 LD50), and BEO plus β-Cyf, respectively. The rats were given their doses orally every day for a month. Results revealed that BEO yielded 6.32 mg/g with 33 identified components, representing 97% of the total oil. BEO implicated a considerable level of total phenolic contents, DPPH radical scavenging capacity, ABTS activity, and FRAP. The treatment of β-Cyf dramatically elevated lipid peroxidation (TBARS and H2O2) (LPO), protein oxidation (PC, AOPP, and HYP), and considerably reduced enzymatic (SOD, CAT, GPx, GR, and GST) and non-enzymatic (GSH) antioxidants. After β-Cyf treatment, hematological parameters, body and liver weights, enzyme activity (AST, ALT, ALP, and LDH), as well as protein, albumin, globulin, and total bilirubin levels were all considerably affected. Furthermore, β-Cyf increased the expression of pro-inflammatory genes (TNF-α, IL-6) as well as DNA damage and cell cycle arrest in the G0/G1 phase and decreased the number of cells in S and G2/M phase of liver cells. Moreover, rats given BEO then intoxicated with β-Cyf showed substantial changes in the majority of the parameters tested. Finally, BEO was shown to have high antioxidant efficacy in combating β-Cyf toxicity because of its high phenolic content.
Pyrethroids insecticides, synthetic derivatives of pyrethrins, are utilized worldwide because of their high toxicity to insects and minimal efficiency in mammals (Das et al., 2017; Tang et al., 2018). β-cyfluthrin (β-Cyf), a photostable type II pyrethroid insecticide, is widely utilized in agriculture and household applications (Wang et al., 2020). Workers may be exhibited to β-Cyf as a consequence of occupational exposure; pest control operations and contaminated food and water (Gorell et al., 1998). The mechanism of action was discovered to be the interaction of β-Cyf with sodium ion-gated channels, which leads to membrane depolarization and loss of electrical excitability in the nervous system. Another mechanism is linked to the disruption in calcium concentration in neurons through the inhibition of calcium transporter enzymes (Wolansky et al., 2006). Furthermore, different data revealed that pyrethroids caused oxidative stress and generated reactive oxygen species (ROS) during their metabolism in mammals (Jebur et al., 2014), leading to increased lipid peroxidation (Afolabi et al., 2019).
The botanical medicinal products with abundant phenolic components are gaining popularity because of their potential to sweep free radicals (Gülçin et al., 2007). Basil; the common name of Ocimum basilicum L.; is a herbaceous and aromatic plant from the Lamiaceae Martinov family, Genus: Ocimum L. and is cultivated all over the world (Shirazi et al., 2014). Basil is regarded as one of the most effective botanical medications for treating a variety of ailments (Patel et al., 2018).
Antioxidant, anti-aging, anti-cancer, antiviral, antibacterial, anti-genotoxic, and anti-inflammatory properties are among its many benefits (Teofilović et al., 2021). Also, basil has been demonstrated to have a variety of pharmacological effects in many disorders, including hepatic fibrosis (Alomar and Al-Attar, 2019), diabetes (Widjaja and Rusdiana, 2019), asthma (Eftekhar et al., 2019), anemia (Zangeneh et al., 2019), and brain damage (Singh et al., 2018). Furthermore, simultaneous therapy with O. basilicum protects against oxidative stress caused by Mercury chloride and cadmium (Sakr and Nooh, 2013; Sunday et al., 2018), as well as deltamethrin. (Sakr and Al-Amoudi, 2012). Therefore, the goal of this investigation was to assess the protective effects of O. basilicum essential oil against hepatotoxicity induced by β-cyfluthrin in rats.
β-cyfluthrin (Purity >99%) was acquired from Nanjing Panfeng Chem Ltd. (Nanjing, Jiangsu, China). The rest of the reagents were analytical reagent grade. Fresh basil leaves (Ocimum basilicum L. (Lamiaceae) were obtained from the Faculty of Science, where they were recognized and authenticated as Basil; the common name of Ocimum basilicum L.; Lamiaceae Martinov family, Genus: Ocimum L. by a plant taxonomy specialist at the Department of Botany, Faculty of Science, Alexandria University, Alexandria, Egypt.
Basil dry leaves (100 g) were crushed and hydrodistilated with sterile water (1 L) for 3 h using a Clevenger-type device. The essential oil was extracted and dried over anhydrous sodium sulfate before being kept at 4°C for further use.
A Thermo Scientific GC/MS version (5) 2009 system with TG-5MS column (30mX0.32mmID) was used to identify the chemical elements of the extracted essential oil. At a flow rate of 1 ml/min, helium was used as the carrier gas. Five μl essential oil was diluted to 1 ml with dichloromethane, then 2 μl was injected on splitless mode for 1 min, followed by a 1:10 split flow. GC oven temperature was held at 45°C for 2 min then was programmed from 45°C to 165°C at 4°C/min; from 165°C to 280°C at 15°C/min after which was kept constant at 280°C for 10 min. At 250°C, both the interface and injection temperatures were set. The ionization voltage was 70 eV with a mass range between 40–800 m/z (Adams, 2006). The components of the essential oil were identified using mass fragmentation patterns, which were compared to the mass spectral database (version 2) of the National Institute of Standards and Technology (NIST), and their relative percentages were estimated using GC peak regions.
The total phenolic contents in BEO were determined spectrophotometrically using Folin-Ciocalteau method (Kang et al., 2010). The absorbance was measured at 765 nm against blank. The calibration curve was produced using pyrocatechol as a standard. The total phenolic content of the extract was measured in milligrams of pyrocatechol equivalents (PE) per Gram of extract.
The reducing ability of antioxidants toward the DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) radical was estimated to determine the free radical scavenging activity of the sample extract (Kang et al., 2011). Using butylated hydroxytoluene (BHT) as a positive control, the DPPH radical scavenging activity of each sample was measured at 515 nm and computed as follows: percent inhibition = [(AB-AA)/AB] x 100, where AB and AA are the absorbance values of the control and test samples, respectively. The IC50 value is used to represent the radical-scavenging activity of DPPH.
With slight adjustments, the antioxidant capacity was calculated using the approach reported by Re et al. (1999). A spectrophotometer was used to measure the absorbance at 734 nm. The percent inhibition of ABTS•+ was calculated for each test sample using the formula: (percent inhibition) = [100*(Ac–As/Ac)], where Ac represents the control sample absorbance and As represents the test sample absorbance. The amount of oil required to scavenge 50% of the ABTS radicals (IC50) was estimated. Trolox was utilized as a control substance.
As a measure of “antioxidant power,” a ferric reducing ability (FRAP) assay (Benzie and Strain, 1996) was used to estimate the total antioxidant capacity of the sample. The change in absorbance at 593 nm is measured in this experiment.
Twenty-eight male Wistar rats (150 ± 10 g) were given by the Faculty of Medicine, Alexandria University, Alexandria, Egypt. The local Research Ethical Committee accepted the experimental design, which followed the National Institutes of Health’s guidelines (NIH). The rats were kept in groups and fed rat chow and water ad libitum while being kept at 21–24°C and 40–60% relative humidity with 12-h light-dark cycles. Animals were randomly divided into four groups (n = 7/group) after 2 weeks of acclimatization. The first group was given corn oil (negative control), the second group received basil essential oil (BEO; 3 ml/kg), the third group (positive control) was treated with β-cyfluthrin (β-Cyf; 15 mg/kg/day; 1/25 LD50), and the fourth group-administered BEO an hour before β-Cyf intoxication. Dosages of β-Cyf (Singh et al., 2009) and BEO (Farghali et al., 2014) were given orally for 1 month daily. After the end of the treatment period, rats were anesthetized using isoflurane, euthanized and blood and livers were collected for further analysis. The liver was cut into three pieces: the first was held at 20°C (for biochemical assays), the second was kept at 80°C (for RNA extraction), and the third was used right away (for comet assay and flow cytometry).
The body weights were recorded at the start of the treatment period (starting body weight) and at the end of the treatment period (final body weight). The livers were dissected and weighed after the associated tissues were cut away. In addition, organ relative weight was reported as g/100 g of body weight.
Blood samples were drawn through cardiac puncture and allowed to clot for 30 min at 25°C before being centrifuged for 15 min at 3000 g. Serum was taken and kept at −80°C until it was utilized to determine liver function indicators. Other blood samples were placed in EDTA tubes and analyzed using an automated analyzer for the identification of the complete blood count (CBC) (Sysmex kx-21n Automated Hematology Analyzer; JAPAN CARE CO., LTD.).
After rats’ dissection, the liver of each rat was taken and homogenized in sodium-potassium phosphate buffer (0.01 mol/L, pH 7.4). The homogenates were centrifuged for 20 min at 10,000 g (4°C), and the supernatants were used in various assays.
Thiobarbituric acid-reactive substances (TBARS) and hydrogen peroxide (H2O2) were measured in liver homogenate according to the methods of Ohkawa et al. (1979) and Velikova et al. (2000), respectively. The concentrations of lipid hydroperoxides (LOOH) in liver homogenates were assessed using Nourooz-Zadeh et al. (1994) method. The oxidative protein damage was measured spectrophotometrically in the tissue by evaluating the advanced oxidized protein products (AOPP) (Witko-Sarsat et al., 1996) and protein carbonyl (PC) (Reznick and Packer, 1994). The levels of hydroxyproline (HYP) were assessed using the method of Bergman and Loxley (1963).
The content of reduced glutathione (GSH) in liver homogenates was determined using the method of Ellman (1959) after the reaction with 5,5′- dithiobis-(2-nitrobenzoic acid). The activities of glutathione peroxidase (GPx; EC 1.11.1.9) and glutathione reductase (GR; EC 1.6.4.2) were evaluated according to Hafeman et al. (1974) while superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), and glutathione S-transferase (GST; EC 2.5.1.18) were carried out using the methods of Misra and Fridovich (1972), Aebi (1984), and Habig et al. (1974), respectively.
The activities of liver aminotransferases (AST; EC 2.6.1.1 and ALT; EC 2.6.1.2), alkaline phosphatase (ALP; EC 3.1.3.1), and lactate dehydrogenase (LDH; EC 1.1.1.27) activities, as well as total protein, albumin, and total bilirubin, were measured using commercially available kits from Biodiagnostic Company, Egypt.
Real-time PCR was used to assess the relative expression of inflammation-related genes (TNF-α, IL-6) in the liver (qPCR). Total RNA was obtained using a commercial kit (Gene JET RNA Purification Kit), as directed by the manufacturer (Thermo Scientific, #K0731, United States). After evaluation of RNA level by Nanodrop (Quawell, Q3000, United States), cDNA was produced by reverse transcription using a commercial kit (RevertAid H Minus Reverse Transcriptase) as described in the manufacturer’s instruction (Thermo Scientific, # EP0451, United States). The qPCR was performed using StepOnePlus real-time PCR system (Applied Biosystem, United States) and a mixture of cDNA, 2X Maxima SYBR Green Master Mix (Thermo Scientific, #K0221, United States), and gene-specific primers (Table 1). The β-actin gene was utilized as a reference (internal control) to calculate the fold change in target genes. The relative gene expression was calculated using the 2–ΔΔCt method The threshold cycle numbers (Ct) of the target gene were normalized to that of reference (ref.) gene, in both the test groups and the control group by using the following equations:
ΔCt test = Ct (target in test groups)—Ct (ref in test groups)
ΔCt calibrator = Ct (target in control)—Ct (ref in control)
The ΔCt of the test genes were normalized to the ΔCt of the calibrator:
ΔΔCt = ΔCt (Test)—ΔCt (Calibrator)
Fold change of relative gene expression was calculated as follow:
Fold Change = 2–ΔΔCt
Alkaline single-cell gel electrophoresis was used to measure DNA damage parameters in liver tissues including tail length, percentage of degraded DNA, and tail moment (comet assay). The DNA fragment movement patterns of 100 cells for each dose level were investigated using GelRed stain (red fluorescence) and a 40× objective on a fluorescent microscope with an excitation filter of 420–490 nm (issue 510 nm). Komet 5 image analysis software was used to accomplish the imaging (Kinetic Imaging, Liverpool, United Kingdom).
Flow cytometry was used to determine the influence of various treatments on the number of cells in each phase of the cell cycle, and it was carried out as previously described by Abdelwahab et al. (2019) with minor changes. Collagenase enzyme (0.1 percent, Invitrogen, United States) was used to lyse freshly dissected liver tissues, which were subsequently mesh filtered (0.7 mm nylon) and centrifuged (4,000 rpm/5 min). The cells were fixed and stained with propidium iodide before being examined with an Attune flow cytometer (Applied Bio-system, United States). Each cell cycle phase’s number of cells was counted and expressed as a percentage of the total number of cells.
Results from all groups were represented as means with standard errors (SEM) and analyzed with SPSS software (version 22, IBM Co., Armonk, NY). ANOVA and Tukey’s post-hoc test were used to compare between groups. The sample size (7 rats/group) used with Cohen’s d of two achieved power of 92.91% via Two- Sample t-test using Effect Size (PASS program version 15). p-values ≤ 0.05 were deemed significant.
The overall content of the basil essential oil (yield = 6.32 mg/g) contained 33 compounds, accounting for 97.0% of the total oil (Figure 1; Table 2), including monoterpenes, sesquiterpenes compounds, and phenylpropanoid derivatives. Estragole was the main component (28.6%). Linalool, the second most significant molecule, had a peak area of 21.7%. Other notable chemicals included (E)-methyl cinnamate (14.3%), α-cadinol (7.1%), eugenol (5.9%), 1,8-cineole (4.0%), methyl eugenol (3.1%), and α-bergamotene (2.2%).
In the present study, O. basilicum leaves essential oil showed relatively high phenolic content with 40.9 mg PE/g. In comparison to the standard, BEO has a comparatively strong DPPH radical scavenging capacity, with an IC50 value of 10.79 mg/ml. BHT acid had a strong IC50 value of 6.80 mg/ml as a control. According to the findings, the BEO appears to have high antiradical activity, which could be ascribed to its high phenolic content, which traps the cation radical ABTS•+ by supplying H+. BEO had an IC50 (ABTS) of 0.69 ± 0.020 mg/ml, while Trolox had an IC50 (ABTS) of 3.35 ± 0.003. Furthermore, the FRAP assay is quick and easy to carry out, and the reaction is repeatable and linearly proportional to the antioxidant content present. It is calculated by comparing the total amount of antioxidants to the sample’s reducing capability. BEO has high reducing power (FRAP) equal (IC50 = 1.378 g/L), but less than the reducing power of butylated hydroxytoluene (BHT) (IC50 = 0.908 g/L) (Table 3).
During the research period, no mortality occurred while burrowing, salivation, tremors, writhing, and convulsions, are only observed as a few clinical indications of poisoning. In rats exposed to β-Cyf, there was a substantial decrease in body weights and an increase in relative liver weights when compared to control rats. In comparison to the β-Cyf exposed group, rats supplemented with BEO then treated with β-Cyf showed considerable alleviation in these parameters. BEO did not generate any substantial changes in rats when given alone (Table 4).
Results of the hematological parameters in rats treated with β-Cyf showed a significant drop in hemoglobin (Hb) concentration, red blood cells (RBC), hematocrit (Ht), and thrombocyte values and an increase in white blood cells (WBC) when compared to the control group. Administration with basil alone showed minor changes in the hematological parameters compared to the control group while administration of BEO before β-Cyf intoxication showed significant restoration in these parameters near the normal level in comparison to the β-Cyf group (Table 5).
To examine the effects of β-Cyf administration on lipid peroxidation, TBARs, H2O2, and lipid hydroperoxide (LOOH) levels were determined. Significant induction in TBARs, H2O2, and LOOH concentrations after β-Cyf administration was detected as compared to the control group while rats who received BEO plus β-Cyf displayed a considerable decline in TBARS, H2O2, and LOOH concentrations as compared to the β-Cyf treated group. In addition, protein carbonyl (PC) and advanced oxidized protein products (AOPP) markers of protein oxidation were markedly increased in the liver of the β-Cyf intoxicated group. The increase in PC and AOPP levels in the liver was reduced by BEO administration. Hepatic hydroxyproline (HYP) levels were analyzed to evaluate hepatic collagen production after β-Cyf administration. The current data showed a significant increase in hepatic HYP levels in β-Cyf -treated rats in comparison to the control group. However, high levels of HYP levels dropped due to BEO administration before β-Cyf treatment. The liver of rats supplemented with BEO alone showed significant changes in the levels of most products of lipid peroxidation and protein oxidation as compared to the control group (Figure 2).
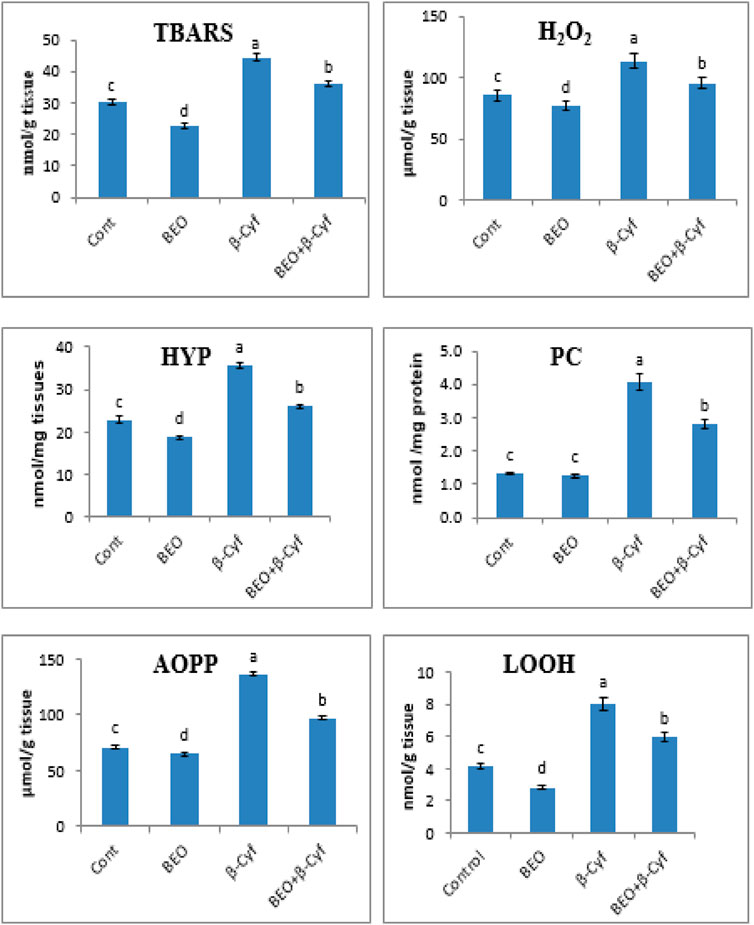
FIGURE 2. Lipid peroxidation and protein oxidation in the liver of male rats given BEO, β-Cyf, and BEO plus β–Cyf. Values are expressed as mean ± SE of seven rats per group. Columns with different letters are significantly different at p < 0.05. Groups are compared as follows: BEO and β–Cyf are compared to negative control while BEO + β-Cyf group are compared to the positive control group, β-Cyf. Abbreviations: TBARS, thiobarbituric acid reactive substances, H2O2; hydrogen peroxide, HYP; hepatic hydroxyproline, LOOH; lipid hydroperoxide, PC; protein carbonyl, AOPP; advanced oxidized protein product.
Antioxidant enzyme activity (SOD, CAT, GPx, GR, and GST) and GSH levels are decreased significantly in liver homogenates of β-Cyf treated animals as compared to control. Otherwise, a significant induction in the same parameters was detected in the animals that received BEO plus β-Cyf as compared to the β-Cyf treated group. The use of BEO alone resulted in significant improvement in these indices (Figure 3).
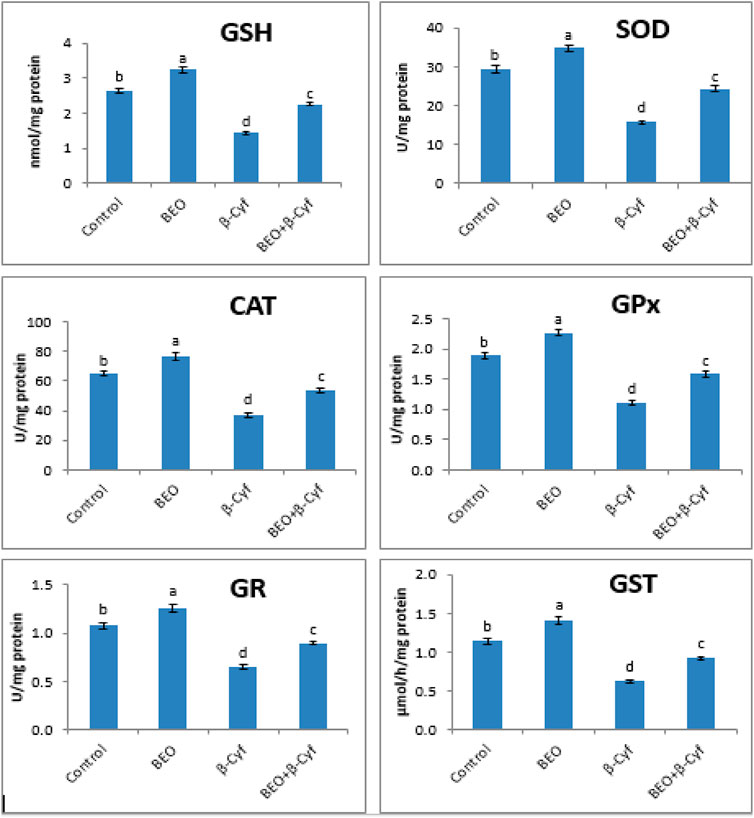
FIGURE 3. Reduced glutathione (GSH) content and activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione S-transferase (GST) in the liver of male rats treated with BEO, β-Cyf, and BEO plus β–Cyf. Values are expressed as mean ± SE of seven rats per group. Columns with different letters are significantly different at p < 0.05. Groups are compared as follows: BEO and β–Cyf groups are compared to the negative control group while BEO + β-Cyf group is compared to the positive control group, β-Cyf.
Hepatic enzymes as AST, ALT, ALP, and LDH are concerned as biomarkers of hepatotoxicity. Rats administered with β-Cyf exhibited alterations in AST, ALT, and ALP activities as well as protein content, total protein, albumin, globulin, and total bilirubin in both rat liver homogenate and serum as compared to the reference group (p < 0.05) while LDH activity is elevated significantly. Rats treated with BEO plus β-Cyf displayed significant modulation in enzyme activities and biochemical parameters as compared with the β-Cyf -treated group. Administration of BEO alone induced significant changes in some of these parameters (Table 6).
In the liver tissue of rats treated with β-Cyf, qPCR results demonstrated significant increases in mRNA levels of pro-inflammatory cytokines TNF-α and IL-6 when compared to the control group. Animals pretreated with BEO then intoxicated with β-Cyf showed significant downregulation in the studied genes (Figure 4).

FIGURE 4. Relative expression of IL-6 and TNF-α genes in rats’ liver tissues of different groups. Data are presented as fold change (mean) ± SEM (n = 7/group). Different letters show significant differences at p < 0.05. Groups are compared as follows: BEO and β–Cyf groups are compared to the negative control group while BEO + β-Cyf group is compared to the positive control group, β-Cyf.
The DNA damage in the liver of rats in different groups was assessed using a comet test. In comparison to control (G1) and other treated groups, administration of β-Cyf (G3) resulted in a substantial increase in DNA damage (p < 0.05), as evidenced by an increase in tail length and tail moment. Because BEO was given before β-Cyf intoxication, the induction of DNA injury was reduced (G4). The control group (G1) and the BEO group (G2), on the other hand, showed no difference in DNA damage (tail length) (Figure 5).
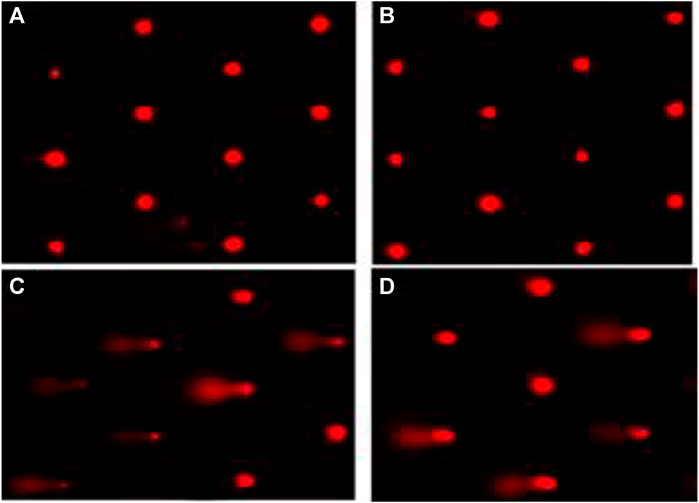
FIGURE 5. Liver DNA damage detected by the comet assay. (A): cont. (B): BEO; (C): β-Cyf and (D): BEO plus β–Cyf. Groups are compared as follows: BEO and β–Cyf groups are compared to the negative control group while BEO + β-Cyf group is compared to the positive control group, β-Cyf.
The cells in the G0/G1 phase were arrested after treatment with β-Cyf, as evidenced by a considerable (p < 0.05) increase in the number of cells in this phase compared to the reference group. However, the β-Cyf treatment significantly reduced the number of cells in the S phase and G2/M phase (p < 0.05). Further, the number of cells in each cell cycle was recovered to a level comparable to the control group after treatment with BEO (Figure 6).
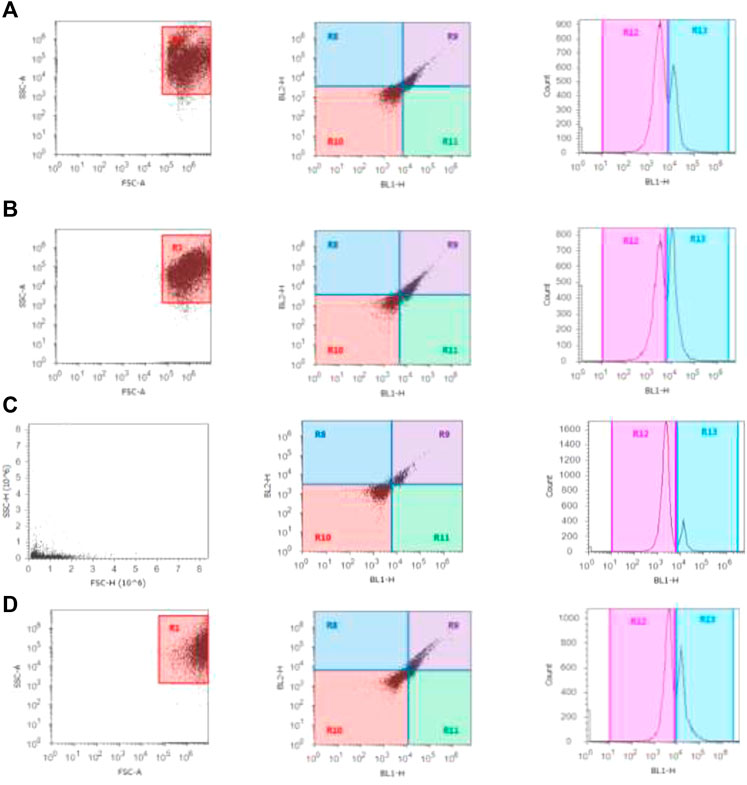
FIGURE 6. Cell cycle scatter and histograms for different groups (A–D). The forward scatter (FS) and side scatter (SS) were measured to identify single cells. The X-axis in the histogram depicts propidium iodide fluorescence as a function of DNA content, while Y-axis reflects cells count in the 3 cell cycle phases (G0/G1, S, and G2/M).
The use of pyrethroids in large quantities has increased the risks to the environment and human health. Several studies have shown that oxidative stress has a role in pyrethroid toxicity in vivo and in vitro (Prasanthi et al., 2005; Jebur et al., 2014). The toxic effect of β-Cyf on the gastrointestinal tract, which results in a decrease in food consumption and nutritional absorption, could explain the observed drop in rat body weight (Mohafrash et al., 2017). Furthermore, the enhanced cell division as an adaptive strategy of the liver to improve its function could be connected to the increase in relative liver weight in β-Cyf treated rats (Rifat et al., 2012). Also, the observed drop in RBCs count and Hb concentration could be attributable to excessive erythrocyte collapse and/or β-Cyf toxic effect on bone marrow (Verma et al., 2013). Furthermore, leukocytosis can be thought of as the body’s response to a strange material entering the body (Saxena and Tomar, 2003). So, the alteration in hematological parameters could be due to the direct harmful effect of β-Cyf.
Pyrethroids caused cellular oxidative damage and the buildup of peroxidation products, which are commonly utilized as oxidative stress markers (El-Demerdash, 2011; Mossa et al., 2013). In the present investigation, β-Cyf exposure caused oxidative stress, as demonstrated by significant induction in lipid peroxidation and protein oxidation products, such as TBARs, H2O2, PC, LOOH, and AOPP. Jebur et al. (2014) suggested that the increased levels of free radicals and weakened antioxidant defenses indicated that β-Cyf had the potential to cause cellular damage by reducing membrane mobility. Furthermore, because Type II pyrethroids are hydrophobic esters with a cyano group, β-Cyf toxicity is most likely caused by the release of unstable metabolites called cyanohydrins, which result in the creation of free radicals (El-Demerdash, 2007). Additionally, pyrethroid-induced oxidative toxicity is likely due to their lipophilicity, which allows them to easily percolate through cellular membranes (Nasuti et al., 2003). Liver fibrosis is characterized by the buildup of collagen in the liver, which is an unusual feature (Bissel et al., 1990). The observed increase in HYP; the main characteristic ingredient in collagen; reflects the amount of collagen and conveys the extent of fibrosis induced by β-Cyf (Hanauske-Abel, 1996).
Glutathione is the most important antioxidant in the antioxidant defense system, as it keeps cells’ redox balance in check and protects them from free radicals. GSH aids in ROS elimination through its action as a non-enzymatic antiradical and as a substrate for certain enzymes such as GPx (Tsukamoto et al., 2002). Many authors have indicated that the absence of sulfhydryl groups puts cells at oxidative stress risk (Bizzozero et al., 2006; Rosa et al., 2007). Higher ROS production, as demonstrated by increased LPO, could explain the detected drop in GSH level as well as GPx and GR activities in β-Cyf treated rats. Enzymatic antioxidants such as SOD and CAT are also thought to be the first line of defense against the damaging effects of ROS generated by pyrethroids on biological macromolecules (El-Demerdash, 2007). Due to its change by xenobiotics, GST, a phase II enzyme, acts as a biomarker; also, it binds and removes metabolites produced by phase I enzymes of the cytochrome P450 monooxygenase system. Further, the observed decrease in GST activity caused by β-Cyf could be due to GSH depletion via a detoxifying mechanism (Ranjbar et al., 2005). In addition, numerous authors have observed that pyrethroid exposure impairs enzymatic antioxidants (Jebur et al., 2014; Syed et al., 2016), implying that the antioxidant defense system is unable to cope with the flood of ROS created by β-Cyf exposure. This occurs as a result of enzyme depletion or inhibition during the breakdown of free radicals, or as a result of direct inhibition of enzymes by β-Cyf.
The observed changes in hepatic enzymes activity (ALT, AST, ALP, and LDH) in β-Cyf treated rats could be attributable to LPO, which disrupts the integrity of cellular membranes, allowing cytoplasmic enzymes to leak into the circulation following hepatocellular injury (Gokcimen et al., 2007). In parallel, several authors concluded that pyrethroids exposure caused changes in enzymes activity since they have the potency to induce hepatic injury and physiological impairments (El-Demerdash, 2007; Jebur et al., 2014; Verma et al., 2016). Protein is also one of the most sensitive cell components to free radical damage. Excessive protein loss via nephrosis, or induction of proteolytic enzymes activity or degradation, were the main causes of the observed drop in protein content (Chatterjea and Shinde, 2002). In addition, a change in normal liver function could be linked to the significant drop in total protein content and albumin in β-Cyf treated rats (Bhushan et al., 2013). Furthermore, an increase in total bilirubin may be caused by decreased liver output or occlusion of the bile ducts as a result of liver cell failure (Sanjiv, 2002).
In the current study, TNF-α and IL-6 gene expressions were significantly elevated in the β-Cyf treated rats. Similarly, increased expression of the interleukin-6 receptor (IL-6R) and tumor necrosis factor receptor superfamily (TNFRSF10A) genes, as well as inflammation, have been related to β-Cyf (Abdou et al., 2017). Furthermore, pyrethroid-produced free radicals are thought to play a role in the generation of proinflammatory cytokines like IL-12, INF-γ, and TNF-α (Hussein and Ahmed, 2016). ROS are also potent activators of the NF-ĸB transcription factor, which is involved in innate immunity and inflammation. TNF-α, IL-6, and other inflammation-related genes are all upregulated when NF-ĸB is activated (Tabrez et al., 2014). Furthermore, previous studies showed that the increase of IL-6 and TNF-α, associated with up-regulation of NF-kappaβ, IL-1β, IL-10, P53, and pro-apoptotic gene Bax (Ahmed et al., 2017; Nna et al., 2018). Hepatic protein depletion elucidates disruption to membrane integrity, which in turn indicates the admission of a toxicant into the cell. Both the toxicant and its metabolic intermediates can cause a range of problems within the cell, including the release of numerous hydrolytic enzymes such as nucleases and proteases making cells more susceptible to chromosomal damage and cell cycle disruption (Singh and Saxena, 2002). Also, the decline in hepatic protein may be affecting genetics by modifying the cell cycle, resulting in hepatic cell proliferation (Omotuyi et al., 2006). Furthermore, various authors have documented that pesticides can damage DNA in hepatocytes, lymphocytes, and other cells in the body, which is consistent with the increase in tail length and DNA percent of rat liver intoxicated with β-Cyf (Cortes-Gutierrez et al., 2011; Hussain et al., 2011). The harmful influence of β-Cyf on rat liver DNA is due to its buildup in liver tissue, which leads to an increase in free radical generation, oxidative stress, and apoptosis (Hussein and Ahmed, 2016).
Oral BEO administration was able to reduce LPO and increase antioxidant status because of its wide range of phenolic components and natural products (Kwon et al., 2019). The increase in radical scavenging activity of BEO in this study was most likely due to its significant component estragole. The most prominent component in Egyptian basil leaves essential oil, according to Chenni et al. (2016), is linalool, and the chemicals observed in this study were equivalent to those found by Falowo et al. (2019). The differences in total phenolic compounds (TPC) between our study and others can be attributed to the different environmental circumstances (Ahmed et al., 2019). According to the findings, BEO has a high reducing scavenging activity and a high DPPH radical scavenging ability, indicating that the oxygenated phenolic monoterpene and a mixture of mono and sesquiterpene hydrocarbons are the most likely culprits (Pripdeevech et al., 2010). According to Ofem et al. (2012) and Zangeneh et al. (2019), BEO improved hematological parameter disorders, and this effect may be attributed to the proportion of iron present in basil (Nworgu et al., 2013), which can stimulate the production of RBC and promote blood Hb level to recover their deficiency induced by β-Cyf.
LPO, alterations in enzymatic and non-enzymatic antioxidants, and liver biomarkers induced by β-Cyf were all mitigated by BEO supplementation. This is due to basil’s high concentration of phenolic components as phenolic acids and flavonoids, which have redox characteristics and can scavenge free radicals (Ahmed et al., 2019). Basil is beneficial in the treatment of hepatic fibrosis, with a significant decrease in hydroxyproline deposition in hepatocytes confirming the alleviation of fibrotic changes in the liver and promoting liver regeneration in fibrotic rats (Parola and Robino, 2001). Basil’s antifibrotic properties are related to its flavonoids which have high antioxidant capacity. In addition, Basil extract decreased the gene expression of fibrosis-related extracellular matrix components and hindered the conversion of hepatic stellate cells to myofibroblasts (Salmah et al., 2005). Also, oral administration of BEO basil extracts reduced inflammation in rats exposed to β-Cyf. These findings are in line with several findings that reported that basil possesses anti-inflammatory properties via blocking inflammatory mediator receptors and cell migration to stimulation sites (Aye et al., 2019; Takeuchi et al., 2020).
In general, according to our findings, β-Cyf exposure is linked to increased oxidative stress and the production of reactive oxygen species (ROS), which interact with macromolecules in liver tissue and cause lipid peroxidation, protein oxidation, fibrosis, hematotoxicity, depletion in enzymatic and non-enzymatic antioxidants, increased pro-inflammatory cytokines, DNA damage, and arrested cells in the G0/G1 phase. So, the administration of basil leaves essential oil as a phytochemical drug, has been documented to have a plethora of phenolic constituents that may explain its strong free radical scavenging effect leading to the modulation of the observed β-Cyf toxicity verifying our hypothesis which is BEO could ameliorate the toxic effect of β-Cyf due to its high antiradical and antioxidant activity.
β-cyfluthrin induced oxidative stress in rat liver tissue, leading to disturbances in antioxidant status and liver function biomarkers. Pre-treatment with BEO improved most of the enzymatic activities lost due to β-Cyf toxicity. BEO displayed great DPPH radical scavenging capacity and powerful antioxidant activity because of its phenolic components that act as strong free radicals’ scavengers. Furthermore, BEO exhibits anti-inflammatory and antioxidant capabilities that can alleviate β-Cyf-induced hepatotoxicity in rats by inhibiting oxidative stress, DNA damage, inflammation, and cell cycle arrest. So, basil is a low-cost botanical drug that is widely available and has been shown to have a high antioxidant capacity.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This manuscript includes animal experiments, and the animal handling, housing, and care, as well as the experimental protocol, were approved by the Animal Care and Ethics Review Committee at the Faculty of Medicine, Alexandria University, Alexandria, Egypt.
AJ: practical work, data analysis, writing–original draft. RE-S: practical work, data generation, manuscript revision. FE-D: conceptualization, methodology, manuscript preparation, and publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors gratefully acknowledge the Institute of Graduate Studies and Research, Alexandria University, where the research work was done.
Abdelwahab, M. A., El-Barbary, A. A., El-Said, K. S., Betiha, M., Elkholy, H. M., Chiellini, E., et al. (2019). Functionalization of Poly(3-Hydroxybutyrate) with Different Thiol Compounds Inhibits MDM2-P53 Interactions in MCF7 Cells. J. Appl. Polym. Sci. 136, 46924. doi:10.1002/app.46924
Abdou, H. M., Mohamed, N. A., Awad, D., and El-Qazaz, I. (2017). β-Cyfluthrin – Induced Impairment in Neurotransmitters, Testicular Function and Gene Expressions of Male Rats: The Protective Effect of Omega-3. IJPSR 8 (7), 2819–2831.
Adams, R. P. (2006). Identification of Essential Oil Components by Gas Chromatography and Mass Spectrometry. 4th edition. Carol Stream: Allured Pub. Corp.
Aebi, H. (1984). Catalase In Vitro. Methods Enzymol. 105, 121–126. doi:10.1016/s0076-6879(84)05016-3
Afolabi, O. K., Aderibigbe, F. A., Folarin, D. T., Arinola, A., and Wusu, A. D. (2019). Oxidative Stress and Inflammation Following Sub-lethal Oral Exposure of Cypermethrin in Rats: Mitigating Potential of Epicatechin. Heliyon 5, e02274. doi:10.1016/j.heliyon.2019.e02274
Ahmed, A. F., Attia, F. A. K., Liu, Z., Li, C., Wei, J., and Kang, W. (2019). Antioxidant Activity and Total Phenolic Content of Essential Oils and Extracts of Sweet Basil (Ocimum Basilicum L.) Plants. Food Sci. Hum. Wellness 8, 299–305. doi:10.1016/j.fshw.2019.07.004
Ahmed, R., Tanvir, E. M., Hossen, M. S., Afroz, R., Ahmmed, I., Rumpa, N. E., et al. (2017). Antioxidant Properties and Cardioprotective Mechanism of Malaysian Propolis in Rats. Evid. Based Complement. Alternat. Med. 2017, 5370545. doi:10.1155/2017/5370545
Alomar, M. Y., and Al-Attar, A. M. (2019). Effect of Basil Leaves Extract on Liver Fibrosis Induced by Thioacetamide in Male Rats. Int. J. Pharmacol. 15, 478–485. doi:10.3923/ijp.2019.478.485
Aye, A., Jeon, Y.-D., Lee, J.-H., Bang, K.-S., and Jin, J.-S. (2019). Anti-inflammatory Activity of Ethanol Extract of Leaf and Leaf Callus of Basil (Ocimum Basilicum L.) on RAW 264.7 Macrophage Cells. Orient Pharm. Exp. Med. 19, 217–226. doi:10.1007/s13596-019-00372-2
Benzie, I. F., and Strain, J. J. (1996). The Ferric Reducing Ability of Plasma (FRAP) as a Measure of "antioxidant Power": the FRAP Assay. Anal. Biochem. 239, 70–76. doi:10.1006/abio.1996.0292
Bergman, I., and Loxley, R. (1963). Two Improved and Simplified Methods for the Spectrophotometric Determination of Hydroxyproline. Anal. Chem. 35, 1961–1965. doi:10.1021/ac60205a053
Bhushan, B., Saxena, P. N., and Saxena, N. (2013). Biochemical and Histological Changes in Rat Liver Caused by Cypermethrin and Beta-Cyfluthrin. Arh Hig Rada Toksikol 64, 57–67. doi:10.2478/10004-1254-64-2013-2184
Bissell, D. M., Friedman, S. L., Maher, J. J., and Roll, F. J. (1990). Connective Tissue Biology and Hepatic Fibrosis: Report of a Conference. Hepatology 11, 488–498. doi:10.1002/hep.1840110322
Bizzozero, O. A., Ziegler, J. L., De Jesus, G., and Bolognani, F. (2006). Acute Depletion of Reduced Glutathione Causes Extensive Carbonylation of Rat Brain Proteins. J. Neurosci. Res. 83, 656–667. doi:10.1002/jnr.20771
Chatterjea, M. N., and Shinde, R. (2002). Textbook of Medical Biochemistry. 5th ed. Jaypee BrothersNew Delhi: Medical Publishers Ltd., 317.
Chenni, M., El Abed, D., Rakotomanomana, N., Fernandez, X., and Chemat, F. (2016). Comparative Study of Essential Oils Extracted from Egyptian Basil Leaves (Ocimum Basilicum L.) Using Hydro-Distillation and Solvent-free Microwave Extraction. Molecules 21, E113. doi:10.3390/molecules21010113
Cortés-Gutiérrez, E. I., Dávila-Rodríguez, M. I., Fernández, J. L., López-Fernández, C., Gosálbez, A., and Gosálvez, J. (2011). New Application of the Comet Assay: Chromosome-Ccomet Assay. J. Histochem. Cytochem. 59, 655–660. doi:10.1369/0022155411410884
Das, T., Ghosh, R., Ghosh, R., Paramanik, A., Pradhan, A., Dey, S. K., et al. 2017). Dose-Dependent Hematological, Hepatic and Gonadal Toxicity of Cypermethrin in Wistar Rats. Toxicol. Forensic Med. Open J. 2: 74–83. doi:10.17140/TFMOJ-2-122
Eftekhar, N., Moghimi, A., Mohammadian Roshan, N., Saadat, S., and Boskabady, M. H. (2019). Immunomodulatory and Anti-inflammatory Effects of Hydro-Ethanolic Extract of Ocimum Basilicum Leaves and its Effect on Lung Pathological Changes in an Ovalbumin-Induced Rat Model of Asthma. BMC Complement. Altern. Med. 19, 349. doi:10.1186/s12906-019-2765-4
El-Demerdash, F. M. (2007). Lambda-cyhalothrin-induced Changes in Oxidative Stress Biomarkers in Rabbit Erythrocytes and Alleviation Effect of Some Antioxidants. Toxicol. Vitro 21, 392–397. doi:10.1016/j.tiv.2006.09.019
El-Demerdash, F. M. (2011). Oxidative Stress and Hepatotoxicity Induced by Synthetic Pyrethroids-Organophosphate Insecticides Mixture in Rat. J. Environ. Sci. Health C Environ. Carcinog Ecotoxicol Rev. 29, 145–158. doi:10.1080/10590501.2011.577679
Ellman, G. L. (1959). Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 82, 70–77. doi:10.1016/0003-9861(59)90090-6
Falowo, A. B., Mukumbo, F. E., Idamokoro, E. M., Afolayan, A. J., and Muchenje, V. (2019). Phytochemical Constituents and Antioxidant Activity of Sweet Basil (Ocimum Basilicum L.) Essential Oil on Ground Beef from Boran and Nguni Cattle. Int. J. Food Sci. 2019, 1–8. doi:10.1155/2019/2628747
Farghali, H. M. A., Ghozy, S. F. A. E., and El-Mehiry, H. F. (2014). Study the Effect of Basil Oil as Herbal Treatment of Acetylsalicylate Induced Gastric Ulcer in Experimental Rat Model. Glob. Veterinaria 12, 431–448.
Gokcimen, A., Gulle, K., Demirin, H., Bayram, D., Kocak, A., and Altuntas, I. (2007). Effects of Diazinon at Different Doses on Rat Liver and Pancreas Tissues. Pestic. Biochem. Physiol. 87, 103–108. doi:10.1016/j.pestbp.2006.06.011
Gorell, J. M., Johnson, C. C., Rybicki, B. A., Peterson, E. L., and Richardson, R. J. (1998). The Risk of Parkinson's Disease with Exposure to Pesticides, Farming, Well Water, and Rural Living. Neurology 50, 1346–1350. doi:10.1212/wnl.50.5.1346
Gülçin, I., Elmastaş, M., and Aboul-Enein, H. Y. (2007). Determination of Antioxidant and Radical Scavenging Activity of Basil (Ocimum Basilicum L. Family Lamiaceae) Assayed by Different Methodologies. Phytother Res. 21, 354–361. doi:10.1002/ptr.2069
Habig, W. H., Pabst, M. J., and Jakoby, W. B. (1974). Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 249, 7130–7139. doi:10.1016/s0021-9258(19)42083-8
Hafeman, D. G., Sunde, R. A., and Hoekstra, W. G. (1974). Effect of Dietary Selenium on Erythrocyte and Liver Glutathione Peroxidase in the Rat. J. Nutr. 104, 580–587. doi:10.1093/jn/104.5.580
Hanauske-Abel, H. M. (1996). “Fibrosis: Representative Molecular Elements, a Basic Concept and Emerging Targets for Suppressive Treatment,” in Hepatology: A Textbook of Liver Disease. Editors D. Zakim, and T. D. Boyer (Philadelphia: W.B. Saunders Company), 465–506.
Hussain, R., Mahmood, F., Khan, M. Z., Khan, F., and Muhammad, F. (2011). Pathological and Genotoxic Effects of Atrazine in Male Japanese Quail (Coturnix japonica). Ecotoxicology, 20: 1–8. doi:10.1007/s10646-010-0515-y
Hussein, M. M., and Ahmed, M. M. (2016). The Th1/Th2 Paradigm in Lambda Cyhalothrin-Induced Spleen Toxicity: The Role of Thymoquinone. Environ. Toxicol. Pharmacol. 41, 14–21. doi:10.1016/j.etap.2015.11.008
Jebur, A. B., Nasr, H. M., and El-Demerdash, F. M. (2014). Selenium Modulates β-cyfluthrin-induced Liver Oxidative Toxicity in Rats. Environ. Toxicol. 29, 1323–1329. doi:10.1002/tox.21863
Kang, W.-Y., Li, C.-F., and Liu, Y.-X. (2010). Antioxidant Phenolic Compounds and Flavonoids of Mitragyna Rotundifolia (Roxb.) Kuntze In Vitro. Med. Chem. Res. 19, 1222–1232. doi:10.1007/s00044-009-9265-x
Kang, W.-Y., Song, Y.-L., and Zhang, L. (2011). α-Glucosidase Inhibitory and Antioxidant Properties and Antidiabetic Activity of Hypericum Ascyron L. Med. Chem. Res. 20, 809–816. doi:10.1007/s00044-010-9391-5
Kwon, D. Y., Li, X., Kim, J. K., and Park, S. U. (2019). Molecular Cloning and Characterization of Rosmarinic Acid Biosynthetic Genes and Rosmarinic Acid Accumulation in Ocimum Basilicum L. Saudi J. Biol. Sci. 26, 469–472. doi:10.1016/j.sjbs.2017.03.010
Misra, H. P., and Fridovich, I. (1972). The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 247, 3170–3175. doi:10.1016/s0021-9258(19)45228-9
Mohafrash, S. M. M., Abdel-Hamid, H. F., and Mossa, A. H. (2017). Adverse Effects of Sixty Days Sub-chronic Exposure to β -cyfluthrin on Male Rats. J. Environ. Sci. Technol. 10, 1–12.
Mossa, A.-T. H., Refaie, A. A., Ramadan, A., and Bouajila, J. (2013).Amelioration of Prallethrin Induced Oxidative Stress and Hepatotoxicity in Rat by the Administration of Origanum Majorana Essential Oil, Biomed. Res. Int. 2013, 859085. doi:10.1155/2013/859085
Nasuti, C., Cantalamessa, F., Falcioni, G., and Gabbianelli, R. (2003). Different Effects of Type I and Type II Pyrethroids on Erythrocyte Plasma Membrane Properties and Enzymatic Activity in Rats. Toxicology 191, 233–244. doi:10.1016/s0300-483x(03)00207-5
Nna, V. U., Abu Bakar, A. B., Md Lazin, M. R. M. L., and Mohamed, M. (2018). Antioxidant, Anti-inflammatory and Synergistic Anti-hyperglycemic Effects of Malaysian Propolis and Metformin in Streptozotocin-Induced Diabetic Rats. Food Chem. Toxicol. 120, 305–320. doi:10.1016/j.fct.2018.07.028
Nourooz-Zadeh, J., Tajaddini-Sarmadi, J., and Wolff, S. P. (1994). Measurement of Plasma Hydroperoxide Concentrations by the Ferrous Oxidation-Xylenol orange Assay in Conjunction with Triphenylphosphine. Anal. Biochem. 220, 403–409. doi:10.1006/abio.1994.1357
Nworgu, F., Yekini, B., and Oduola, O. (2013). Effects of Basil Leaf (Ocimum Gratissimum) Supplement on Some Blood Parameters of Growing Pullets. Int. J. Agric. 3, 480–488. doi:10.9734/ajea/2013/2947
Ofem, O., Ani, E., and Eno, A. (2012). Effect of Aqueous Leaves Extract of Ocimum Gratissimum on Hematological Parameters in Rats. Int. J. Appl. Basic Med. Res. 2, 38–42. doi:10.4103/2229-516X.96807
Ohkawa, H., Ohishi, N., and Yagi, K. (1979). Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 95, 351–358. doi:10.1016/0003-2697(79)90738-3
Omotuyi, I. O., Oluyemi, K. A., Omofoma, C. O., Josiah, S. J., Adesany, O. A., and Saalu, L. C. (2006). Cyfluthrin Induced Hepatotoxicity in Rats. Afr. J. Biotechnol. 5 (20), 1909–1912.
Parola, M., and Robino, G. (2001). Oxidative Stress-Related Molecules and Liver Fibrosis. J. Hepatol. 35, 297–306. doi:10.1016/s0168-8278(01)00142-8
Patel, M., Gadhvi, H., Patel, S., Mankad, A., Pandya, H., and Rawal, R. (2018). Holy Basil: Holy Herb to Multimodal Medicine for Human Health. Pharma Inn J. 7, 418–423.
Prasanthi, K., Muralidhara, M., and Rajini, P. S. (2005). Morphological and Biochemical Perturbations in Rat Erythrocytes Following In Vitro Exposure to Fenvalerate and its Metabolite. Toxicol. Vitro 19, 449–456. doi:10.1016/j.tiv.2004.12.003
Pripdeevech, P., Chumpolsri, W., Suttiarporn, P., and Wongpornchai, S. (2010). The Chemical Composition and Antioxidant Activities of Basil from Thailand Using Retention Indices and Comprehensive Two-Dimensional Gas Chromatography. Jscs 75, 1503–1513. doi:10.2298/jsc100203125p
Ranjbar, A., Solhi, H., Mashayekhi, F. J., Susanabdi, A., Rezaie, A., and Abdollahi, M. (2005). Oxidative Stress in Acute Human Poisoning with Organophosphorus Insecticides; a Case Control Study. Environ. Toxicol. Pharmacol. 20, 88–91. doi:10.1016/j.etap.2004.10.007
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 26 (9–10), 1231–1237. doi:10.1016/s0891-5849(98)00315-3
Reznick, A. Z., and Packer, L. (1994). Oxidative Damage to Proteins: Spectrophotometric Method for Carbonyl Assay. Methods Enzymol. 233, 357–363. doi:10.1016/s0076-6879(94)33041-7
Rifat, F., Sharma, M., Srivastava, P., and Sisodia, R. (2012). Effects of Cyfluthrin on Biochemical and Histopathological Alterations in Liver of Swiss Albino Mice. Int. J. Scient. Res. Rev. 1, 1–12.
Rosa, R. M., Hoch, N. C., Furtado, G. V., Saffi, J., and Henriques, J. A. (2007). DNA Damage in Tissues and Organs of Mice Treated with Diphenyl Diselenide. Mutat. Res. 633, 35–45. doi:10.1016/j.mrgentox.2007.05.006
Sakr, S. A., and Nooh, H. Z. (2013). Effect of Ocimum Basilicum Extract on Cadmium-Induced Testicular Histomorphometric and Immunohistochemical Alterations in Albino Rats. Anat. Cel Biol 46, 122–130. doi:10.5115/acb.2013.46.2.122
Sakr, S. A., and Al-Amoudi, W. M. (2012). Effect of Leave Extract of Ocimum Basilicum on Deltamethrin Induced Nephrotoxicity and Oxidative Stress in Albino Rats. J. App. Pharm. Sci. 2, 22–27. doi:10.7324/japs.2012.2507
Salmah, I., Mahmood, A. A., and Sidik, K. (2005). Synergistic Effects of Alcoholic Extract of Sweet Basil (Ocimum Basilicum L.) Leaves and Honey on Cutaneous Wound Healing in Rats. Int. J. Mol. Med. Adv. Sci. 1, 220–224.
Sanjiv, C. (2002). The Liver Book: A Comprehensive Guide to Diagnosis, Treatment and Recovery. New York: Atria Jimcafe Company.
Saxena, P. N., and Tomar, V. (2003). Assessment of Comparative Hemotoxicity of Cybil and Fenvalerate in Rattus norvegicus. Bull. Environ. Contam. Toxicol. 70, 839–846. doi:10.1007/s00128-003-0058-5
Shirazi, M. T., Gholami, H., Kavoosi, G., Rowshan, V., and Tafsiry, A. (2014). Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Tagetes minuta and Ocimum Basilicum Essential Oils. Food Sci. Nutr. 2, 146–155. doi:10.1002/fsn3.85
Singh, A. K., Saxena, P. N., and Sharma, H. N. (2009). Stress Induced by Beta-Cyfluthrin, a Type-2 Pyrethroid, on Brain Biochemistry of Albino Rat (Rattus norvegicus). Biol. Med. 1, 74–86.
Singh, V., Krishan, P., and Shri, R. (2018). Improvement of Memory and Neurological Deficit with Ocimum Basilicum L. Extract after Ischemia Reperfusion Induced Cerebral Injury in Mice. Metab. Brain Dis. 33, 1111–1120. doi:10.1007/s11011-018-0215-5
Singh, V. K., and Saxena, P. N. (2002). Genotoxic Potential of Cypermethrin in Mammalian Haemopoietic System. Him J. Env. Zool. 16, 195–202.
Sunday, A., Adelakun, S. A., Olusegun, D., Omotoso, D. O., Grace, T., and Akingbade, G. T. (2018). Therapeutics Potential of Ocimum Basilicum Following Mercury Chloride-Induced Hepatotoxicity in Rats (Rattus norvegicus). Braz. J. Biol. Sci. 5, 725–737. doi:10.21472/bjbs.051110
Syed, F., Chandravanshi, L. P., Khanna, V. K., and Soni, I. (2016). Beta-cyfluthrin Induced Neurobehavioral Impairments in Adult Rats. Chem. Biol. Interact 243, 19–28. doi:10.1016/j.cbi.2015.11.015
Tabrez, S., Priyadarshini, M., Priyamvada, S., Khan, M. S., Na, A., and Zaidi, S. K. (2014). Gene-environment Interactions in Heavy Metal and Pesticide Carcinogenesis. Mutat. Res. Genet. Toxicol. Environ. Mutagen 760, 1–9. doi:10.1016/j.mrgentox.2013.11.002
Takeuchi, H., Takahashi-Muto, C., Nagase, M., Kassai, M., Tanaka-Yachi, R., and Kiyose, C. (2020). Anti-inflammatory Effects of Extracts of Sweet Basil (Ocimum Basilicum L.) on a Co-culture of 3T3-L1 Adipocytes and RAW264. 7 Macrophages. J. Oleo Sci. 69, 487–493. doi:10.5650/jos.ess19321
Tang, W., Wang, D., Wang, J., Wu, Z., Li, L., Huang, M., et al. (2018). Pyrethroid Pesticide Residues in the Global Environment: an Overview. Chemosphere 191, 990–1007. doi:10.1016/j.chemosphere.2017.10.115
Teofilović, B., Grujić-Letić, N., Karadžić, M., Kovačević, S., Podunavac-Kuzmanović, S., Gligorić, E., et al. (2021). Analysis of Functional Ingredients and Composition of Ocimum Basilicum, South. Afri J. Bot. 141, 227–234. doi:10.1016/j.sajb.2021.04.035
Tsukamoto, M., Tampo, Y., Sawada, M., and Yonaha, M. (2002). Paraquat Induced Oxidative Stress and Dysfunction of the Glutathione Redox Cycle in Pulmonary Microvascular Endothelial Cells. Toxicol. Appl. Pharmacol. 178: 82–87. doi:10.1006/taap.2001.9325
Velikova, V., Yordanov, I., and Edreva, A. (2000). Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants. Plant Sci. 151, 59–66. doi:10.1016/s0168-9452(99)00197-1
Verma, R., Awasthi, K. K., Rajawat, N. K., Soni, I., and John, P. J. (2016). Curcumin Modulates Oxidative Stress and Genotoxicity Induced by a Type II Fluorinated Pyrethroid, Beta-Cyfluthrin. Food Chem. Toxicol. 97, 168–176. doi:10.1016/j.fct.2016.09.014
Verma, R., Awasthi, K. K., Soni, I., and John, P. J. (2013). Evaluation of Cytogenetic Effects of -cyfluthrin in Swiss Albino Mice. Int. J. Curr. Microbiol. Appl. Sci. 6, 30–40.
Wang, Z., Chen, L., Zhang, L., Zhang, W., Deng, Y., Liu, R., et al. (2020). Thermal Effects on Tissue Distribution, Liver Biotransformation, Metabolism and Toxic Responses in Mongolia Racerunner (Eremias Argus) after Oral Administration of Beta-Cyfluthrin. Environ. Res. 185, 109393. doi:10.1016/j.envres.2020.109393
Widjaja, S. S., Rusdiana, M. S., and Savira, M. (2019). Glucose Lowering Effect of Basil Leaves in Diabetic Rats. Open Access Maced J. Med. Sci. 7, 1415–1417. doi:10.3889/oamjms.2019.293
Witko-Sarsat, V., Friedlander, M., Capeillère-Blandin, C., Nguyen-Khoa, T., Nguyen, A. T., Zingraff, J., et al. (1996). Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 49, 1304–1313. doi:10.1038/ki.1996.186
Wolansky, M. J., Gennings, C., and Crofton, K. M. (2006). Relative Potencies for Acute Effects of Pyrethroids on Motor Function in Rats. Toxicol. Sci. 89, 271–277. doi:10.1093/toxsci/kfj020
Keywords: Ocimum basilicum leaves extract, β-cyfluthrin, oxidative stress, enzymes, rat liver, molecular changes
Citation: Jebur AB, El-Sayed RA and El-Demerdash FM (2022) Ocimum basilicum Essential Oil Modulates Hematotoxicity, Oxidative Stress, DNA Damage, and Cell Cycle Arrest Induced by β-cyfluthrin in Rat Liver. Front. Pharmacol. 12:784281. doi: 10.3389/fphar.2021.784281
Received: 27 September 2021; Accepted: 29 December 2021;
Published: 21 January 2022.
Edited by:
Mariano Bizzarri, Sapienza University of Rome, ItalyReviewed by:
Sadaf Jahan, Majmaah University, Saudi ArabiaCopyright © 2022 Jebur, El-Sayed and El-Demerdash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatma M. El-Demerdash, ZWxkZW1lcmRhc2hmQHlhaG9vLmNvbQ==, ZWxkZW1lcmRhc2hmQGFsZXh1LmVkdS5lZw==, b3JjaWQub3JnLzAwMDAtMDAwMS01NjI0LTkxNDU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.