- 1Department of Pharmacy, Peking University Third Hospital, Beijing, China
- 2School of Basic Medical Sciences and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China
- 3Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Sciences, Peking University Health Science Center, Beijing, China
- 4College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, United States
- 5Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
Background and Aim: As one of the second-generation epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors, afatinib brings survival benefits to patients with common and rare EGFR mutations. This study aimed to compare the effectiveness and safety of 30 and 40 mg of afatinib in patients with non–small cell lung cancer (NSCLC) using qualitative and quantitative analysis methods so as to provide reference for clinical medication.
Methods: The PubMed, Embase, ClinicalTrials.gov, Cochrane Library, China National Knowledge Infrastructure, and WanFang databases were thoroughly searched from inception to February 26, 2021. Two researchers independently screened the literature, extracted data, and evaluated the quality. RevMan and Stata 15.0 were used for meta-analysis.
Results: Twelve cohort studies including 1290 patients for final analysis were selected; of which, 1129 patients were analyzed to measure the effectiveness outcomes and 470 patients were analyzed for safety outcomes. In patients with non-brain metastasis, the progression-free survival of the first- or second-line treatment with reduced-dose afatinib was equivalent to the conventional dose. In terms of safety, the reduced dose could significantly lower the incidence of severe diarrhea and severe rash, but not the total incidence of diarrhea, rash, and all levels of paronychia.
Conclusions: The incidence of common serious adverse reactions was significantly lower with 30 mg of afatinib than with 40 mg of afatinib in patients with NSCLC. The effectiveness appeared to be similar to that in patients with non-brain metastasis. This study provides a reference for clinical dose reduction of afatinib.
Systematic Review Registration: [PROSPERO], identifier [CRD42021238043]
Background
According to the International Agency for Research on Cancer’s (IARC) 2020 cancer report, lung cancer is currently the most common cause of cancer deaths (GLOBOCAN, 2020). Non–small cell lung cancer (NSCLC) accounts for about 85% of all lung cancers, and the 5-year survival rate for advanced patients was only 23% (Miller et al., 2019). In recent years, the drug treatment for NSCLC has developed from chemotherapeutic drugs to targeted drugs, wherein epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are targeted drugs that target EGFR for anticancer treatment. The EGFR-TKI therapy has shown significant survival benefits in patients with EGFR-mutant NSCLC. It has become the first-line treatment recommended by the guidelines of the National Comprehensive Cancer Network, the European Society for Medical Oncology, and the Chinese Society of Clinical Oncology (NCCN, 2021; ESMO, 2020; CSCO, 2020).
As a second-generation EGFR-TKI, afatinib can bind to the kinase domains of multiple subtypes in the EGFR family and inhibit the autophosphorylation of tyrosine kinases. Afatinib treatment has better progression-free survival (PFS) than the first-generation drugs and in patients with exon19 deletion mutation, which approximately accounts for 45% of EGFR mutations and showed better benefits (Yang et al., 2015; Park et al., 2016). However, the incidence of afatinib-related adverse reactions has been high and more serious because of the pan-target characteristics of afatinib. The most common adverse reactions are diarrhea, rash, and paronychia, which have a negative impact on the quality of life in patients. Afatinib can improve disease-related symptoms, such as chest pain and dyspnea, of patients with lung cancer compared with the placebo group. However, it increases diarrhea and loss of appetite at the same time, according to the patient-reported outcome analysis of clinical registration research LUX-Lung 1 (Hirsh et al., 2013).
According to the label, the recommended initial dose of afatinib is 40 mg, which can be reduced by 10 mg if not tolerated (Afatinib label, 2019). In clinical trials, the proportion of patients with dose reduction is 28–53% (Sequist et al., 2013; Wu et al., 2014), and more than half of the patients undergo dose modification due to adverse reactions in real-world studies (Kim et al., 2017; Cheema et al., 2019). It is an important clinical issue, but whether dose reduction can reduce the incidence of adverse reactions and achieve similar clinical effectiveness simultaneously remains elusive.
Some observational studies reported the effectiveness and safety of afatinib dose reduction, but no systematic review or meta-analysis integrated the existing study results. Therefore, this study aimed to compare the effectiveness and safety of 30 and 40 mg of afatinib in patients with NSCLC using qualitative and quantitative analyses to provide reference for clinical medication.
Methods
This systematic review and meta-analysis was performed and reported according to the meta-analysis of observational studies in epidemiology (Stroup et al., 2000) and the preferred reporting items for systematic reviews and meta-analyses checklists (Liberati et al., 2009). The review protocol was registered in the International Prospective Register of Systematic Reviews and the registration number is CRD42021238043.
Search Strategy and Eligibility Criteria
The search terms were related to “afatinib” and “non–small cell lung cancer.” The PubMed, Cochrane Library, EMBASE, ClinicalTrials.gov, China National Knowledge Infrastructure, and WanFang databases were searched to identify relevant publications in English and Chinese from inception to February 2021. The search syntax is provided in Supplementary Table SA.
The inclusion criteria were as follows: comparison of the effectiveness and safety of afatinib dose reduction in patients with NSCLC, age 18 years and above, using afatinib alone or in combination with other non–EGFR-TKI drugs. The exclusion criteria were as follows: doses were mixed when evaluating the effectiveness and safety of afatinib 30 and 40 mg, non-English or Chinese studies, no availability of full-text articles, letters, abstracts, meeting proceedings, and case reports. Two reviewers (WANG and DU) independently screened titles and abstracts for the eligibility of identified studies and then independently reviewed full-text articles. Disagreements were resolved by referring to a third reviewer (CHEN).
Data Extraction
Two reviewers (WANG and DU) extracted data independently, using a predefined data extraction file. The following baseline characteristics were extracted from the included studies: first author, year of publication, study design, country in which the study was performed, study period, number of included patients, patient baseline characteristics and drug regimen of the intervention (30 mg of afatinib) and comparator (40 mg of afatinib) groups, and the effectiveness and safety outcomes of the intervention and comparator groups, such as the objective response rate (ORR), disease control rate (DCR), PFS, and incidence of common adverse events of all grades.
Quality Assessment
Two reviewers independently assessed the methodological quality of included studies using modified Newcastle–Ottawa Scales (Ga Wells et al., 2021). Disagreements were resolved by consensus.
Primary and Secondary Outcomes
The primary outcome of effectiveness was PFS. The secondary outcomes included the ORR and DCR. The ORR was defined as the sum of the proportions of complete response and partial response, and the DCR was defined as the sum of the proportions of complete response, partial response, and stable disease; evaluations were based on the Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 (Eisenhauer et al., 2009). The primary outcome of safety is the incidence of adverse events of all grades (diarrhea, rash, and paronychia). The incidence and severity of all adverse events were documented according to the common terminology criteria for the same.
Statistical Analysis
We used Stata 15.0 for statistical analysis of PFS and used Review Manager (Revman, version 5.3) for other statistical analyses. The dichotomous outcome was reported as the risk ratio (RR), the survival outcome was reported as the median survival ratio (MSR), and the data were analyzed using a random-effect meta-analysis based on the Mantel-Haenszel and DerSimonian—Laird model accompanying 95% confidence intervals (CIs). Statistical heterogeneity between studies was assessed using I2 and χ2 (test level α = 0.1) statistics. In the case of statistical heterogeneity, the sources of heterogeneity were either explored through the subgroup and sensitivity analyses or only descriptive analysis was performed. Publication bias was assessed using Egger’s test when at least 10 studies were included.
Results
Search
The search strategy yielded 5260 citations. After removing duplicate citations, 4428 unique titles and abstracts were screened and 55 full-text articles were assessed for eligibility. Twelve cohort studies were eligible for inclusion, which comprised eight prospective studies and four retrospective studies (Arrieta et al., 2015; Moran et al., 2017; Yang et al., 2017; Lim et al., 2018; Ninomiya et al., 2018; Tan et al., 2018; Tanaka et al., 2018; Halmos et al., 2019; Tamura et al., 2019; Wang et al., 2019; Wei et al., 2019; Ko et al., 2021) (see Figure 1).
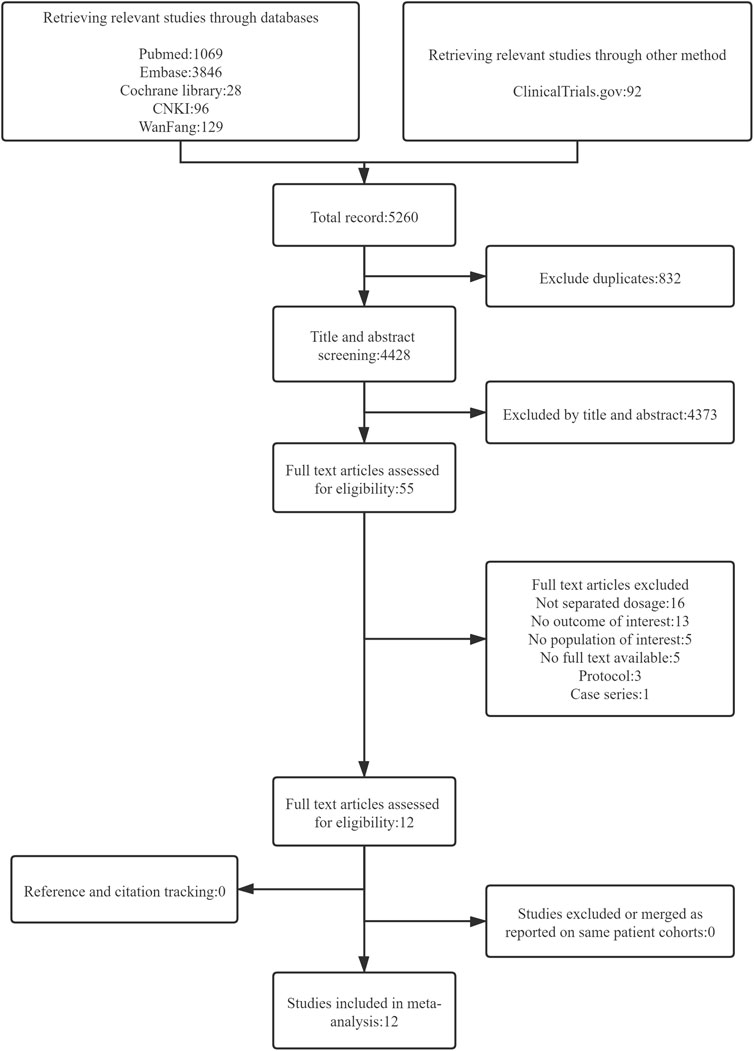
FIGURE 1. Flow diagram representing search and selection of studies comparing 30 versus 40 mg of afatinib treatment in patients with EGFR-mutant NSCLC.
Baseline Study Characteristics and Quality Assessment
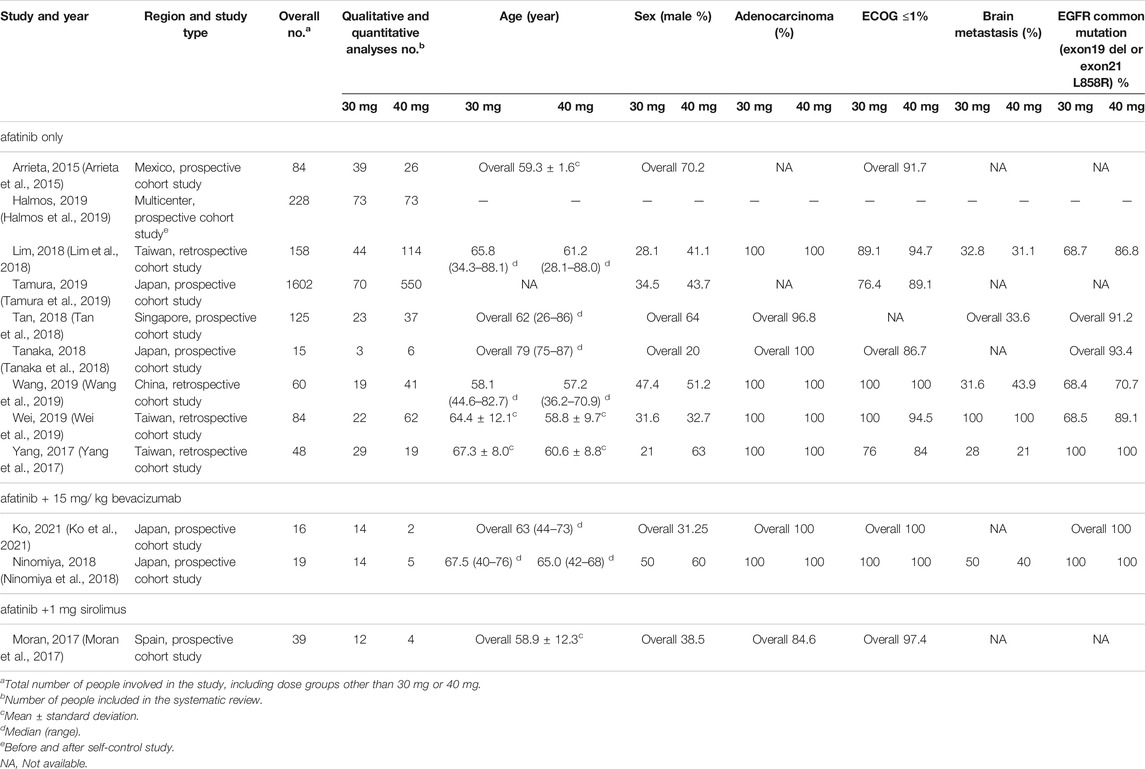
Table 1 shows the baseline characteristics of included studies. In total, 12 studies, including 1290 patients, were selected for qualitative and quantitative analyses, with 362 patients in the 30-mg afatinib group and 928 in the 40-mg afatinib group. Moreover, 1129 patients were analyzed to measure effectiveness outcomes and 470 patients were analyzed to measure safety outcomes. The dosage regimen included afatinib administration only in nine studies and afatinib administration with concomitant medication in three studies. Most studies (9 studies, 75%) were launched in Asia, including 1 in Mexico, 1 in Spain, and 1 globally across 13 countries. The overall quality of the included studies was good. Among the 12 cohort studies included, 3 had a risk of bias in the assessment of prognostic factors and 2 did not match or were adjusted for a few plausible prognostic variables. Supplementary Table SB presents details of the risk-of-bias assessment for observational studies.
Effectiveness Outcome Measures
Progression-free Survival
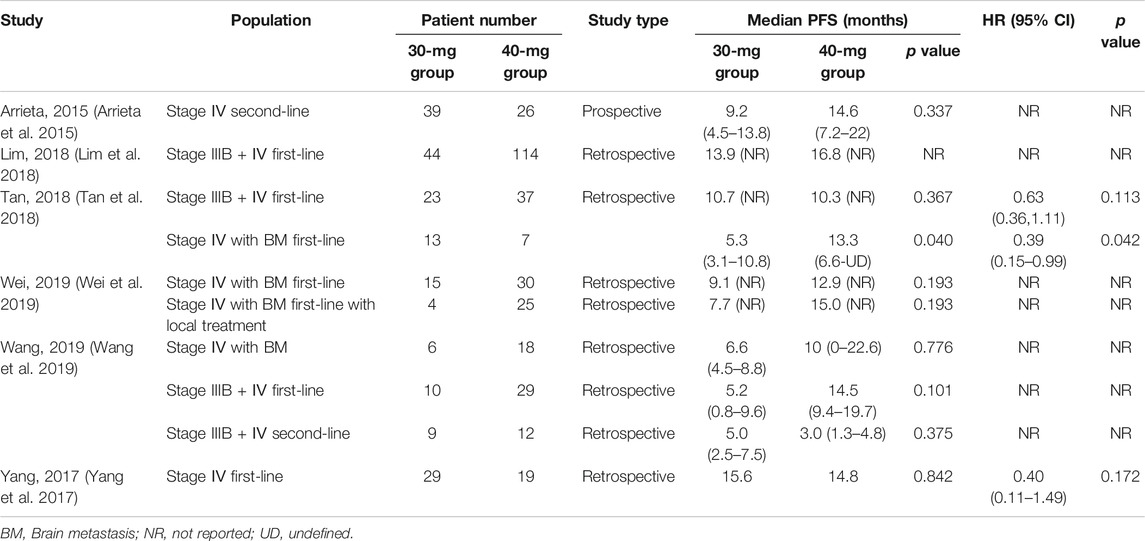
PFS was reported in 6 studies involving 509 patients (Arrieta et al., 2015; Yang et al., 2017; Lim et al., 2018; Tan et al., 2018; Wang et al., 2019; Wei et al., 2019); median PFS data were presented as the MSR, and meta-analysis was performed in Stata 15 using the metan command. Significant heterogeneity was found between studies (I2 = 85.4%). After applying various subgroup analyses, the heterogeneity was still high (I2 = 70.1–89.1%). Therefore, only descriptive analysis was performed on the effectiveness outcome, as shown in Supplementary Figures S1-6.
Six studies used log-rank analysis to compare the median survival of patients using reduced and routine doses of afatinib, all with p-value > 0.05. Kim et al. (Kim et al., 2019) drew the Kaplan–Meier curve of 165 patients with the first-line use of afatinib and found that reducing the dose to 30 mg did not affect the PFS of the patients. However, Tan et al. (Tan et al., 2018) showed that in using afatinib as a first-line treatment in patients with stage IV NSCLC with brain metastases, 40 mg afatinib demonstrated better PFS than 30 mg afatinib (median PFS, 30 vs. 40 mg, 5.3 vs. 13.3 months, p = 0.04). Furthermore, the results were stable after being adjusted by the Cox regression model [hazard ratio (HR), 0.39 (0.15–0.99), p = 0.042]. However, Wei et al. (Wei et al., 2019) found no significant difference in PFS between the 30- and 40-mg afatinib groups in the same population (median PFS 30 vs. 40 mg, 9.1 vs. 12.9 months, p = 0.193). Table 2 shows the results of the six studies.
ORR and DCR
The ORR and DCR were reported in four studies with 871 patients (Yang et al., 2017; Lim et al., 2018; Tamura et al., 2019; Wei et al., 2019), and all of them used afatinib as the first-line treatment. The heterogeneity between the studies was acceptable. No statistically significant difference in the ORR [RR = 0.93 (0.81, 1.06)] and DCR [RR = 1.01 (0.96, 1.05)] was found between the 40- and 30-mg afatinib groups (see Figure 2).
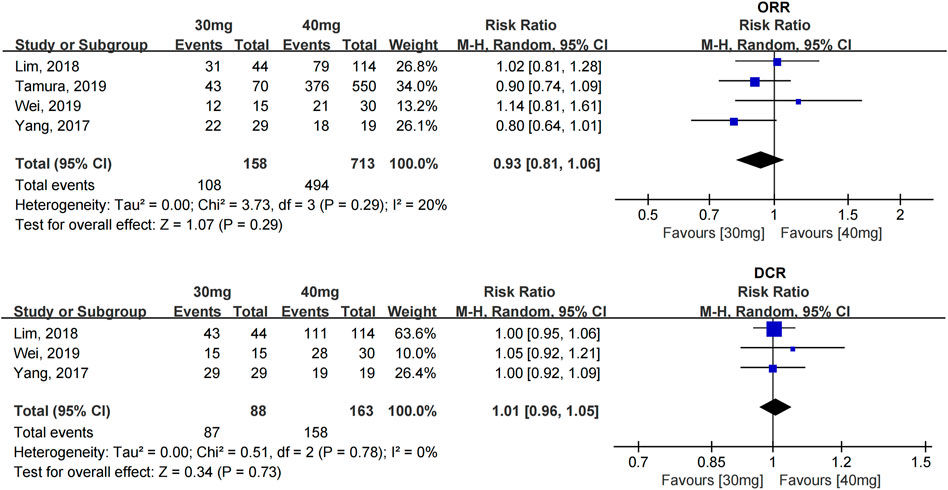
FIGURE 2. Forest plot of the ORR and DCR in the meta-analysis of patients treated with 30 and 40 mg afatinib.
Safety Outcome Measures
Diarrhea
The incidence of diarrhea was reported in seven studies with 454 patients (Yang et al., 2017; Lim et al., 2018; Ninomiya et al., 2018; Tanaka et al., 2018; Halmos et al., 2019; Wang et al., 2019; Ko et al., 2021), and no significant difference was found in the incidence of diarrhea between patients taking 30 and 40 mg afatinib [RR = 0.75 (0.52, 1.08), p = 0.35]. A subgroup analysis showed that when afatinib was used alone or combined with 15 mg/kg bevacizumab, no significant difference in the incidence of diarrhea was found among patients with a reduced dose compared with the conventional dose of afatinib. [RR = 0.75 (0.52, 1.08), p = 0.35], [RR = 0.91 (0.68, 1.21), p = 0.53].
The incidence of severe diarrhea (≥grade 3) was reported in seven studies with 312 patients (Yang et al., 2017; Lim et al., 2018; Ninomiya et al., 2018; Tanaka et al., 2018; Halmos et al., 2019; Wang et al., 2019; Ko et al., 2021); no heterogeneity was found between these studies. Dose modification of afatinib to 30 mg significantly reduced the incidence of severe diarrhea [RR = 0.22 (0.10, 0.49), p = 0.0002] (see Figure 3).
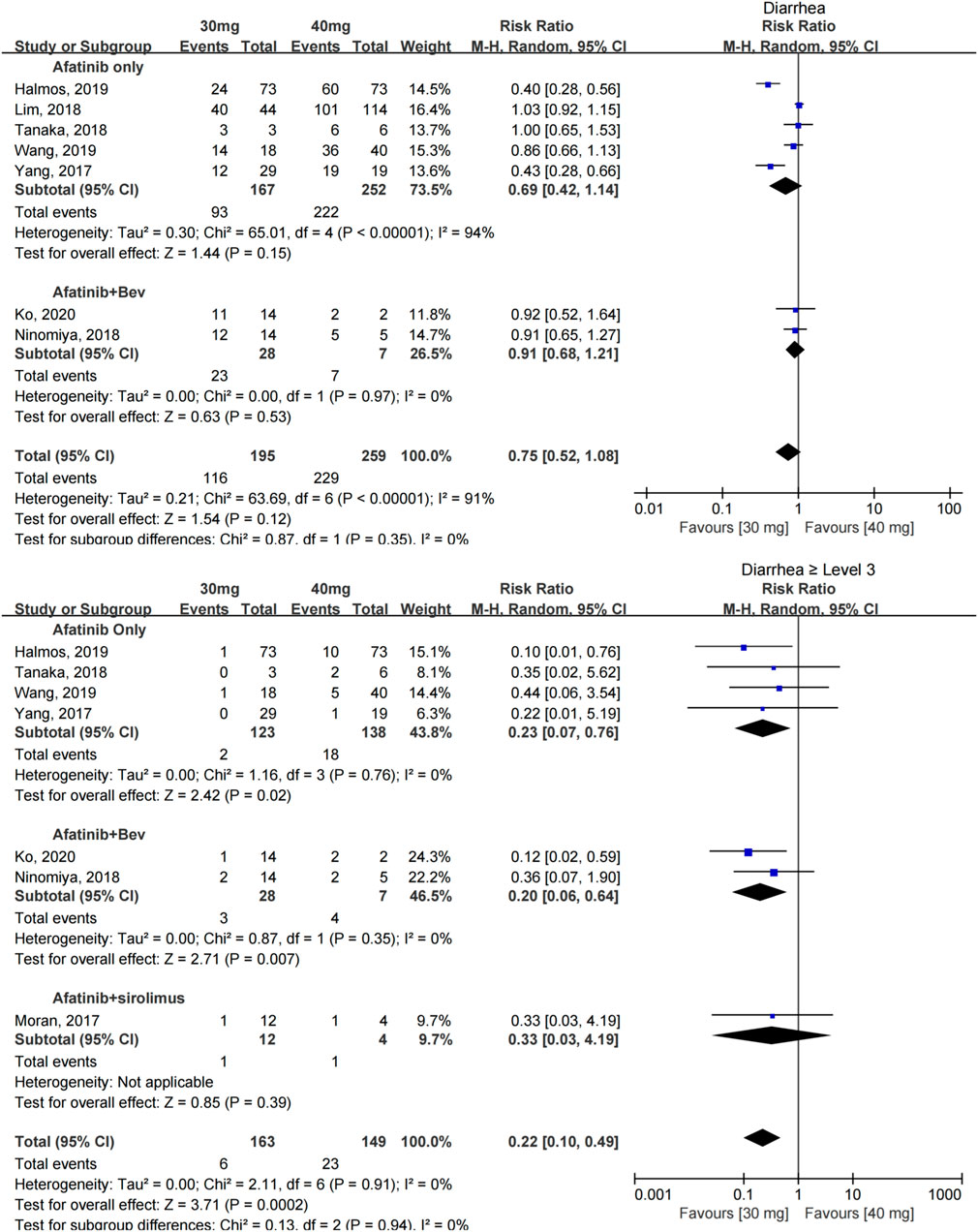
FIGURE 3. Forest plot of the incidence of diarrhea and ≥grade 3 diarrhea in the meta-analysis of patients treated with 30 and 40 mg of afatinib.
Rash
The incidence of rash was reported in seven studies with 454 patients (Yang et al., 2017; Lim et al., 2018; Ninomiya et al., 2018; Tanaka et al., 2018; Halmos et al., 2019; Wang et al., 2019; Ko et al., 2021); 30 mg of afatinib did not show a reduction in the incidence of rash in patients [RR = 0.84 (0.67, 1.06), p = 0.15], and the subgroup analysis showed no significant difference whether afatinib was used alone [RR = 0.80 (0.60, 1.06), p = 0.12] or in combination with other drugs [RR = 1.03 (0.71, 1.49), p = 0.87].
The incidence of severe rash (≥grade 3) was reported in 6 studies with 280 patients (Yang et al., 2017; Ninomiya et al., 2018; Tanaka et al., 2018; Halmos et al., 2019; Wang et al., 2019; Ko et al., 2021), and little heterogeneity was observed. Patients taking 30 mg afatinib had a lower risk of developing severe rash [RR = 0.28 (0.10, 0.78), p = 0.02] (see Figure 4).

FIGURE 4. Forest plot of the incidence of rash and ≥grade 3 rash in the meta-analysis of patients treated with 30 and 40 mg of afatinib.
Paronychia
The incidence of paronychia was reported in 6 studies with 308 patients (Yang et al., 2017; Lim et al., 2018; Ninomiya et al., 2018; Tanaka et al., 2018; Wang et al., 2019; Ko et al., 2021), and the incidence of paronychia in the two groups was similar [RR = 0.89 (0.67, 1.20), p = 0.45].
The incidence of severe paronychia (≥grade 3) was reported in five studies with 141 patients (Yang et al., 2017; Ninomiya et al., 2018; Tanaka et al., 2018; Wang et al., 2019; Ko et al., 2021), and no statistical difference was found in the incidence of severe paronychia [RR = 0.28 (0.07, 1.10), p = 0.07] (see Figure 5).
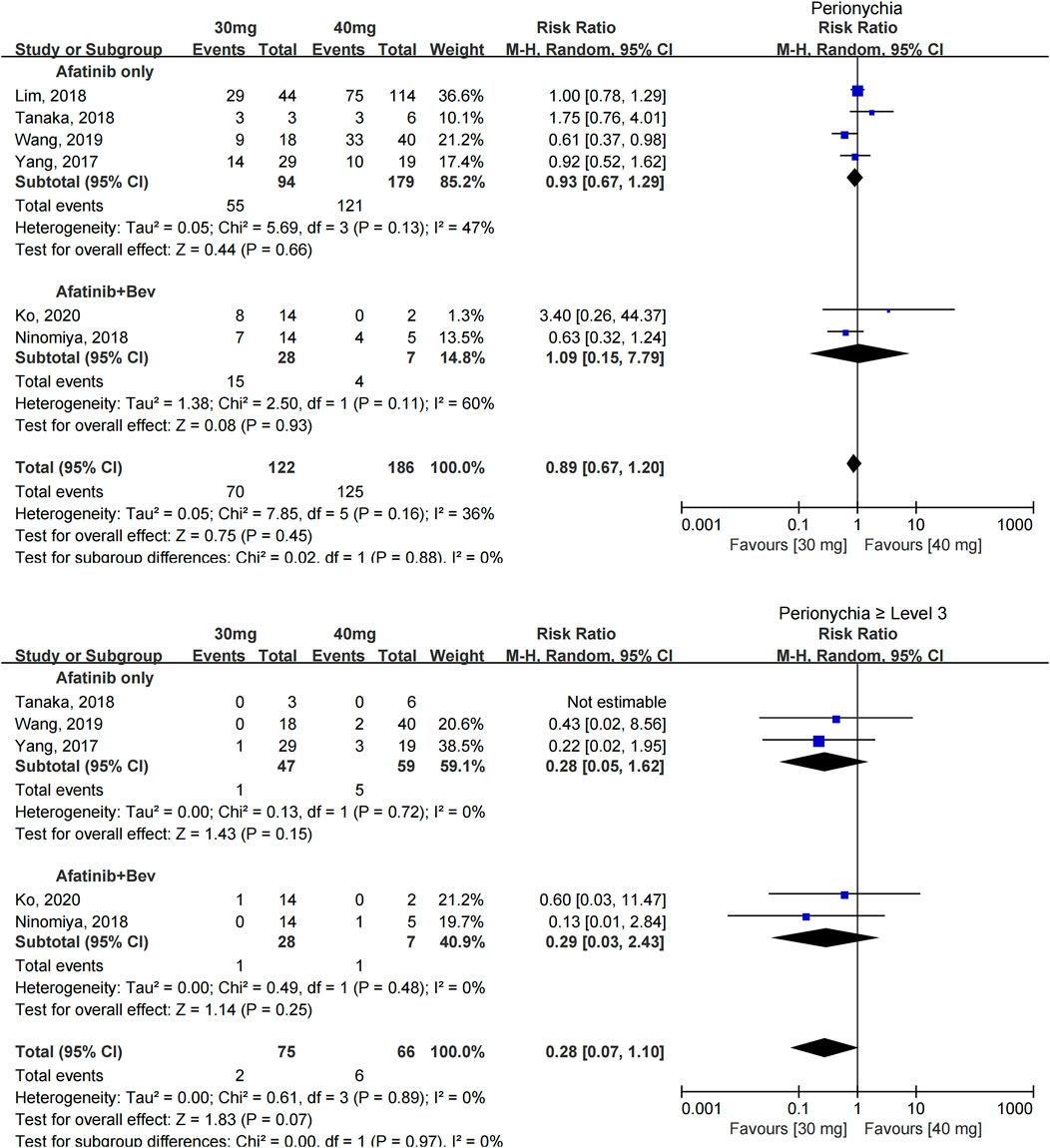
FIGURE 5. Forest plot of the incidence of paronychia and ≥grade 3 paronychia in the meta-analysis of patients treated with 30 and 40 mg afatinib.
Sensitivity Analysis
For different outcome measures, sensitivity analysis was performed by excluding individual studies one by one. The results showed that meta-analysis results did not change in direction after excluding any study, indicating that these meta-analysis results were relatively stable.
Discussion
The present systematic review and meta-analysis was the first study to compare the effectiveness and safety of patients with NSCLC using 30 and 40 mg afatinib. The results showed that in patients with advanced NSCLC without brain metastases, the PFS appeared to be equivalent between the 30- and 40-mg afatinib groups, irrespective of whether afatinib was used as a first-line or second-line treatment; however, the data were limited. No statistical difference in the ORR and DCR was found between the two groups of patients who used afatinib as the first-line treatment. In terms of safety, whether afatinib was used alone or in combination with other drugs, reduced-dose afatinib could significantly reduce the incidence of severe diarrhea and rash; however, the results did not indicate that dose reduction could reduce the incidence of paronychia at all levels.
Afatinib was approved by the U.S. Food and Drug Administration in 2013. As the second-generation EGFR-TKI, afatinib not only showed a better survival benefit for patients with common EGFR mutations (exon 19del and exon 21 L858R) but was also effective for patients with rare mutations (Banno et al., 2018; Chang et al., 2021). However, the incidence of adverse reactions was also significantly higher than that of the first- and third-generation drugs. The risk of diarrhea was the highest among the first- and third-generation drugs (RR = 38.88, p < 0.001) (Yin et al., 2021). Afatinib-related adverse reactions not only reduced the quality of life in patients but also increased their financial burden due to the cost of management (Villa et al., 2016; Subramanian et al., 2019). The median occurrence time of adverse reactions in patients using afatinib was mainly within 1 month from the initial medication (Cheema et al., 2017). Therefore, clinically, some doctors consider reducing the dose empirically at the first administration, while no definite evidence exists for clinical dose reduction currently. Of the 12 studies, 6 reported initial dose reduction and others included dose escalation trials (5 clinical phase I or II) and self-control analyses before and after the trial (1 study). After analyzing the patient characteristics of the included studies, the population characteristics of the 30-mg afatinib group were as follows. Gender: Six studies presented the baseline characteristics of patients with different doses, which revealed that more female patients took reduced doses than male patients. Especially in the study by Yang (Yang et al., 2017), the proportion of women taking 30 mg afatinib was twice that of those taking 40 mg afatinib (79 vs. 37%) (Miller et al., 2019). Weight: Only two studies reported the baseline information on body surface area (BSA) and weight; hence, the data are not summarized in Table 1. Patients who received reduced doses had significantly lower BSA (1.5 ± 0.2 vs. 1.7 ± 0.1, p = 0.0055) and smaller body weight (weight ≥ 60 kg, 19.1 vs. 33.4%). Age: Five studies presented the age of patients in different dose groups, revealing that patients in the 30-mg afatinib group were older. In the study of Lim et al. (Lim et al., 2018) and Yang et al. (Yang et al., 2017), the age of the 30-mg afatinib group was statistically significantly higher than that of the 40-mg afatinib group [65.8 (34.3–88.1) vs. 61.2 (28.1–88.0), p < 0.05; 67.3 ± 8.0 vs. 60.6 ± 8.8, p < 0.05]. The aforementioned population characteristics of reduced doses were basically consistent with the results of afatinib population pharmacokinetic studies (Freiwald et al., 2014), which indicated that women, less weight, low creatinine clearance, and high total protein levels tended to associate with greater drug exposure, and the bioavailability of afatinib in patients with a poor physical status increased.
Therefore, the theoretical basis for reduction in afatinib could be explained by the dose-exposure–response relationship. The steady-state plasma trough concentration after administering 30 mg afatinib was significantly lower than that of patients taking 40 mg afatinib daily (p = 0.02) (Nakao et al., 2019). The steady-state trough blood concentration of patients with serious adverse reactions requiring dose reduction or withdrawal could be twice that of other patients (Chiba, 2016). The blood concentration positively correlated with the severity of diarrhea in the early phase (r = 0.498, p < 0.05) (Hayashi et al., 2019). However, no afatinib exposure–response study was available to explore the relationship between blood concentration and effectiveness. In the phase I trial of afatinib, a high-dose intermittent administration of 55 mg afatinib daily (3 weeks for medication/1 week for rest) achieved a Cmax about four times that of 40 mg afatinib daily, but no definite ORR exists (Marshall et al., 2013).
This study had several limitations. First, the number of relevant original studies was limited, further subgroup analysis based on population characteristics could not be performed, and the number of studies for each outcome was not enough to assess publication bias. Second, few included studies adjusted the outcome results for multivariate analysis, which might affect the accuracy of the final analysis results. Third, for the analysis of PFS, quantitative analysis could not be performed due to the obvious heterogeneity among the relevant studies.
Conclusion
This study was the first systematic review and meta-analysis evaluating the effectiveness and safety outcomes of dose reduction of afatinib used in patients with NSCLC. The results showed that reduced-dose afatinib could significantly reduce the incidence of common serious adverse events. The effectiveness appeared to be comparable between the regular- and reduced-dose group, although the evidence was inadequate and of low quality. Further studies are needed to identify the appropriate population for initial dose reduction to provide more individualized and precise treatment to the patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author Contributions
ZWA: Conceptualization, methodology, formal analysis, data curation, writing–original draft, and writing–reviewing and editing; XD: Methodology, formal analysis, and data curation; KC: Methodology, writing–reviewing and editing; SL: Data curation; ZY: Writing–reviewing and editing; ZWU: Writing–reviewing and editing; LY: Funding acquisition; DC: Writing–reviewing and editing; WL: Conceptualization, funding acquisition, project administration, supervision, and writing–reviewing and editing.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82104294) and the 13th Five-Year Plan National Science and Technology Major Project (Nos. 2017ZX09304012-007 and 2017ZX09304012-006, Ministry of Science of Technology of China).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.781084/full#supplementary-material
References
Afatinib label, Washiongton (USA), (2019). Updated 2019/10/11. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/201292s015lbl.pdf.
Arrieta, O., De la Torre-Vallejo, M., López-Macías, D., Orta, D., Turcott, J., Macedo-Pérez, E. O., et al. (2015). Nutritional Status, Body Surface, and Low Lean Body Mass/Body Mass Index Are Related to Dose Reduction and Severe Gastrointestinal Toxicity Induced by Afatinib in Patients with Non-small Cell Lung Cancer. Oncologist 20 (8), 967–974. doi:10.1634/theoncologist.2015-0058
Banno, E., Togashi, Y., Nakamura, Y., Chiba, M., Kobayashi, Y., Hayashi, H., et al. (2018). Sensitivities to Various EGFR-TKIs of Uncommon EGFR Mutations L861Q and S768I: What Is the Optimal EGFR-TKI. Cancer Sci. 107 (8), 1134–1140. doi:10.1111/cas.12980
Chang, G. C., Lam, D. C., Tsai, C. M., Chen, Y. M., Shih, J. Y., Aggarwal, S., et al. (2021). Experience from Asian Centers in a Named-Patient-Use Program for Afatinib in Patients with Advanced Non-small-cell Lung Cancer Who Had Progressed Following Prior Therapies, Including Patients with Uncommon EGFR Mutations. Int. J. Clin. Oncol. 26 (5), 841–850. doi:10.1007/s10147-021-01869-0
Cheema, P. K., Thawer, A., Leake, J., Cheng, S. Y., Khanna, S., and Charles Victor, J. (2019). Multi-disciplinary Proactive Follow-Up Algorithm for Patients with Advanced NSCLC Receiving Afatinib. Support Care Cancer 27 (3), 1029–1039. doi:10.1007/s00520-018-4392-x
Cheema, P. K., Thawer, A., Leake, J., Cheng, S. Y., Khanna, S., and Victor, J. C. (2017). Pharmacist Led Proactive Follow-Up Algorithm for Advanced EGFR Positive NSCLC Patients on Afatinib. Ann. Oncol. 28, iii40. doi:10.1093/annonc/mdx091.031
Chiba, R. (2016). The Relationship between Plasma Afatinib Concentration and the Severity of Adverse Events. Respirology 21, 79.
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R., et al. (2009). New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 45 (2), 228–247. Oxford, England : 1990. doi:10.1016/j.ejca.2008.10.026
ESMO. Clinical Practice Living Guidelines – Metastatic Non-small-cell Lung Cancer (2020). Available at: https://www.esmo.org/guidelines/lung-and-chest-tumours.
Freiwald, M., Schmid, U., Fleury, A., Wind, S., Stopfer, P., and Staab, A. (2014). Population Pharmacokinetics of Afatinib, an Irreversible ErbB Family Blocker, in Patients with Various Solid Tumors. Cancer Chemother. Pharmacol. 73 (4), 759–770. doi:10.1007/s00280-014-2403-2
Ga Wells, B. S., O'Connell, D., Peterson, J., Welch, V., Losos, M., and Tugwell, P.,(2021). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
GLOBOCAN 2020 (2021). New Global Cancer Data. Lyon: International Agency for Research on Cancer. Available at: https://www.iarc.fr/faq/latest-global-cancer-data-2020-qa.
Halmos, B., Tan, E. H., Soo, R. A., Cadranel, J., Lee, M. K., Foucher, P., et al. (2019). Impact of Afatinib Dose Modification on Safety and Effectiveness in Patients with EGFR Mutation-Positive Advanced NSCLC: Results from a Global Real-World Study (RealGiDo). Lung Cancer 127, 103–111. doi:10.1016/j.lungcan.2018.10.028
Hayashi, H., Iihara, H., Hirose, C., Fukuda, Y., Kitahora, M., Kaito, D., et al. (2019). Effects of Pharmacokinetics-Related Genetic Polymorphisms on the Side Effect Profile of Afatinib in Patients with Non-small Cell Lung Cancer. Lung Cancer 134, 1–6. doi:10.1016/j.lungcan.2019.05.013
Hirsh, V., Cadranel, J., Cong, X. J., Fairclough, D., Finnern, H. W., Lorence, R. M., et al. (2013). Symptom and Quality of Life Benefit of Afatinib in Advanced Non-small-cell Lung Cancer Patients Previously Treated with Erlotinib or Gefitinib: Results of a Randomized Phase IIb/III Trial (LUX-Lung 1). J. Thorac. Oncol. 8 (2), 229–237. doi:10.1097/JTO.0b013e3182773fce
Kim, Y., Lee, S. H., Ahn, J. S., Ahn, M. J., Park, K., and Sun, J. M. (2019). Efficacy and Safety of Afatinib for EGFR-Mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib. Cancer Res. Treat. 51 (2), 502–509. doi:10.4143/crt.2018.117
Kim, Y., Sun, J., Park, K., Park, S. E., Lee, S., Ahn, M., et al. (2017). P3.01-023 First-Line Afatinib for Non-small Cell Lung Cancer in Real World Practice. J. Thorac. Oncol. 12 (11), S2209. doi:10.1016/j.jtho.2017.09.1464
Ko, R., Shukuya, T., Imamura, C. K., Tokito, T., Shimada, N., Koyama, R., et al. (2021). Phase I Study of Afatinib Plus Bevacizumab in Patients with Advanced Non-squamous Non-small Cell Lung Cancer Harboring EGFR Mutations. Transl. Lung Cancer Res. 10 (1), 183–192. doi:10.21037/tlcr-20-824
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Lim, C. K., Wei, Y. F., Tsai, M. S., Chen, K. Y., Shih, J. Y., and Yu, C. J. (2018). Treatment Effectiveness and Tolerability of Afatinib at Different Doses in Patients with EGFR-Mutated Lung Adenocarcinoma: How Low Can We Go. Eur. J. Cancer 103, 32–40. Oxford, England : 1990. doi:10.1016/j.ejca.2018.07.128
Marshall, J., Hwang, J., Eskens, F. A., Burger, H., Malik, S., Uttenreuther-Fischer, M., et al. (2013). A Phase I, Open-Label, Dose Escalation Study of Afatinib, in a 3-Week-On/1-Week-Off Schedule in Patients with Advanced Solid Tumors. Invest. New Drugs 31 (2), 399–408. doi:10.1007/s10637-012-9890-y
Miller, K. D., Nogueira, L., Mariotto, A. B., Rowland, J. H., Yabroff, K. R., Alfano, C. M., et al. (2019). Cancer Treatment and Survivorship Statistics, 2019. CA Cancer J. Clin. 69 (5), 363–385. doi:10.3322/caac.21565
Moran, T., Palmero, R., Provencio, M., Insa, A., Majem, M., Reguart, N., et al. (2017). A Phase Ib Trial of Continuous Once-Daily Oral Afatinib Plus Sirolimus in Patients with Epidermal Growth Factor Receptor Mutation-Positive Non-small Cell Lung Cancer And/or Disease Progression Following Prior Erlotinib or Gefitinib. Lung Cancer 108, 154–160. doi:10.1016/j.lungcan.2017.03.009
Nakao, K., Kobuchi, S., Marutani, S., Iwazaki, A., Tamiya, A., Isa, S., et al. (2019). Population Pharmacokinetics of Afatinib and Exposure-Safety Relationships in Japanese Patients with EGFR Mutation-Positive Non-small Cell Lung Cancer. Sci. Rep. 9 (1), 18202. doi:10.1038/s41598-019-54804-9
NCCN. Non-Small Cell Lung Cancer Clinical Practice Guidelines (Version 6) 2021 Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450.
Ninomiya, T., Nogami, N., Kozuki, T., Harada, D., Kubo, T., Ohashi, K., et al. (2018). A Phase I Trial of Afatinib and Bevacizumab in Chemo-Naïve Patients with Advanced Non-small-cell Lung Cancer Harboring EGFR Mutations: Okayama Lung Cancer Study Group Trial 1404. Lung Cancer 115, 103–108. doi:10.1016/j.lungcan.2017.11.025
Park, K., Tan, E. H., O'Byrne, K., Zhang, L., Boyer, M., Mok, T., et al. (2016). Afatinib versus Gefitinib as First-Line Treatment of Patients with EGFR Mutation-Positive Non-small-cell Lung Cancer (LUX-Lung 7): a Phase 2B, Open-Label, Randomised Controlled Trial. Lancet Oncol. 17 (5), 577–589. doi:10.1016/S1470-2045(16)30033-X
Sequist, L. V., Yang, J. C., Yamamoto, N., O'Byrne, K., Hirsh, V., Mok, T., et al. (2013). Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 31 (27), 3327–3334. doi:10.1200/JCO.2012.44.2806
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of Observational Studies in Epidemiology: a Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 283 (15), 2008–2012. doi:10.1001/jama.283.15.2008
Subramanian, J., Fernandes, A. W., Laliberté, F., Pavilack, M., DerSarkissian, M., and Duh, M. S. (2019). The Rate of Occurrence, Healthcare Resource Use and Costs of Adverse Events Among Metastatic Non-small Cell Lung Cancer Patients Treated with First- and Second-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Lung Cancer 138, 131–138. doi:10.1016/j.lungcan.2019.07.021
Tamura, K., Nukiwa, T., Gemma, A., Yamamoto, N., Mizushima, M., Ochai, K., et al. (2019). Real-world Treatment of over 1600 Japanese Patients with EGFR Mutation-Positive Non-small Cell Lung Cancer with Daily Afatinib. Int. J. Clin. Oncol. 24 (8), 917–926. doi:10.1007/s10147-019-01439-5
Tan, W. L., Ng, Q. S., Lim, C., Tan, E. H., Toh, C. K., Ang, M. K., et al. (2018). Influence of Afatinib Dose on Outcomes of Advanced EGFR-Mutant NSCLC Patients with Brain Metastases. BMC cancer 18 (1), 1198. doi:10.1186/s12885-018-5110-2
Tanaka, H., Taima, K., Tanaka, Y., Itoga, M., Ishioka, Y., Nakagawa, H., et al. (2018). A Phase I Study of Afatinib for Patients Aged 75 or Older with Advanced Non-small Cell Lung Cancer Harboring EGFR Mutations. Med. Oncol. 35 (3), 34. doi:10.1007/s12032-018-1098-3
Villa, S., Brioschi, E., and Ravasio, R. (2016). Analysis of the Costs Associated with the Management of Adverse Events Compared through TKIs in the Treatment of Patients with Non-small Cells Lung Cancer EGFR Mutated. Value in Health 19 (7), A723–A724. doi:10.1016/j.jval.2016.09.2159
Wang, S., Xing, P., Yang, K., Hao, X., Ma, D., Mu, Y., et al. (2019). Efficacy and Safety of Afatinib in a Chinese Population with Advanced Lung Adenocarcinoma with Sensitive EGFR Mutations. Thorac. Cancer 10 (6), 1461–1468. doi:10.1111/1759-7714.13095
Wei, Y. F., Lim, C. K., Tsai, M. S., Huang, M. S., and Chen, K. Y. (2019). Intracranial Responses to Afatinib at Different Doses in Patients with EGFR-Mutated Non-small-cell Lung Carcinoma and Brain Metastases. Clin. Lung Cancer 20 (3), e274–e283. doi:10.1016/j.cllc.2019.02.009
Wu, Y. L., Zhou, C., Hu, C. P., Feng, J., Lu, S., Huang, Y., et al. (2014). Afatinib versus Cisplatin Plus Gemcitabine for First-Line Treatment of Asian Patients with Advanced Non-small-cell Lung Cancer Harbouring EGFR Mutations (LUX-Lung 6): an Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 15 (2), 213–222. doi:10.1016/S1470-2045(13)70604-1
Yang, C. J., Tsai, M. J., Hung, J. Y., Lee, M. H., Tsai, Y. M., Tsai, Y. C., et al. (2017). The Clinical Efficacy of Afatinib 30 Mg Daily as Starting Dose May Not Be Inferior to Afatinib 40 Mg Daily in Patients with Stage IV Lung Adenocarcinoma Harboring Exon 19 or Exon 21 Mutations. BMC Pharmacol. Toxicol. 18 (1), 82. doi:10.1186/s40360-017-0190-1
Yang, J. C., Wu, Y. L., Schuler, M., Sebastian, M., Popat, S., Yamamoto, N., et al. (2015). Afatinib versus Cisplatin-Based Chemotherapy for EGFR Mutation-Positive Lung Adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of Overall Survival Data from Two Randomised, Phase 3 Trials. Lancet Oncol. 16 (2), 141–151. doi:10.1016/S1470-2045(14)71173-8
Keywords: afatinib, non–small cell lung cancer, dose reduction, effectiveness, safety, meta-analysis
Citation: Wang Z, Du X, Chen K, Li S, Yu Z, Wu Z, Yang L, Chen D and Liu W (2021) Impact of Dose Reduction of Afatinib Used in Patients With Non–Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:781084. doi: 10.3389/fphar.2021.781084
Received: 22 September 2021; Accepted: 25 October 2021;
Published: 29 November 2021.
Edited by:
Pasquale Pisapia, University of Naples Federico II, ItalyReviewed by:
Vincenzo L’Imperio, University of Milano-Bicocca, ItalyElham Sajjadi, University of Milan, Italy
Copyright © 2021 Wang, Du, Chen, Li, Yu, Wu, Yang, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dingding Chen, Y2hkZEBjcHUuZWR1LmNu; Wei Liu, YW5kdGhlbjAwMjNAMTYzLmNvbQ==
 Ziyu Wang
Ziyu Wang Xin Du
Xin Du Ken Chen4
Ken Chen4 Li Yang
Li Yang Wei Liu
Wei Liu