
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 24 November 2021
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.779606
This article is part of the Research Topic Fibrosis in the Respiratory and Digestive Systems View all 50 articles
Long non-coding RNAs (lncRNAs) can potentially regulate all aspects of cellular activity including differentiation and development, metabolism, proliferation, apoptosis, and activation, and benefited from advances in transcriptomic and genomic research techniques and database management technologies, its functions and mechanisms in physiological and pathological states have been widely reported. Liver fibrosis is typically characterized by a reversible wound healing response, often accompanied by an excessive accumulation of extracellular matrix. In recent years, a range of lncRNAs have been investigated and found to be involved in several cellular-level regulatory processes as competing endogenous RNAs (ceRNAs) that play an important role in the development of liver fibrosis. A variety of lncRNAs have also been shown to contribute to the altered cell cycle, proliferation profile associated with the accelerated development of liver fibrosis. This review aims to discuss the functions and mechanisms of lncRNAs in the development and regression of liver fibrosis, to explore the major lncRNAs involved in the signaling pathways regulating liver fibrosis, to elucidate the mechanisms mediated by lncRNA dysregulation and to provide new diagnostic and therapeutic strategies for liver fibrosis.
As a globally important public health problem, liver fibrosis is typically characterized by a reversible wound healing response and an accompanying imbalance between increased synthesis and deposition and decreased degradation of extracellular matrix (ECM), resulting in programmed overaccumulation of ECM components (Nudelman et al., 1998; Aydin and Akcali, 2018). Numerous epidemiological studies have revealed the etiological role of various chronic liver diseases and associated liver injury-healing reactions in liver fibrosis, such as hepatitis (non-alcoholic steatohepatitis (NASH), hepatitis B and C and so on) and biliary obstruction, which are closely associated with its progression (Parola and Pinzani, 2019). Mechanistic studies at the cellular level suggest that hepatic stellate cells (HSCs) located in the Disse space between hepatic sinusoidal endothelial cells and hepatic epithelial cells and maintaining a close interaction with both are the main sites for the production of ECM components (Geerts, 2001; Khomich et al., 2019), and furthermore, numerous studies have revealed that their intracellular lipid droplets, which are specific organelles for hepatic retinoic acid storage (Blaner et al., 2009; Elpek, 2014), could lead to liver disease disorders through efflux, depletion, and loss. undesirable progression (Yin et al., 2013; Ray, 2014; Krizhanovsky et al., 2008). Thus, studies on the activation mechanisms of hematopoietic stem cells are of great concern in proposing new therapies against hepatic fibrosis and in improving the original strategies (Figure 1).
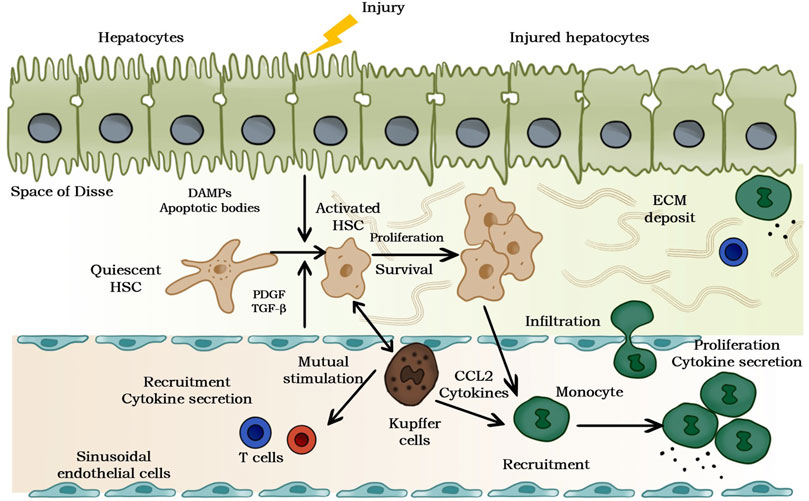
FIGURE 1. Pathogenesis of liver fibrosis. The release of damage-related patterns (DAMPs). and apoptotic bodies can be induced by chronic hepatocyte injury, which activates hematopoietic stem cells and recruits immune cells. Moreover, the complex multidirectional interaction between activated hematopoietic stem cells and Kupffer cells and innate immune cells promotes transformation and differentiation into proliferation and ECM to generate myofibroblasts.
In recent years, numerous non-coding RNAs (ncRNAs) molecules have been identified benefiting from the application of RNA microarrays and next-generation transcriptome sequencing technologies, enabling humans to deepen their understanding of the pathophysiology of multiple diseases from a new perspective (Consortium et al., 2007). ncRNAs are well known for not encoding proteins at the RNA level but can perform as key regulators of multiple regulatory gene expression as well as cellular signaling pathways (Heo et al., 2019). NcRNAs are categorized according to their relative size into two types: small or short non-coding RNAs (miRNAs) of less than 200 nucleotides (nt) and long non-coding RNAs (lncRNAs) of greater than 200 nucleotides (Riaz and Li, 2019). The most prominently researched endogenous small ncRNAs, known as miRNAs, mainly regulate the post-transcriptional levels of target genes by binding to the 3′ untranslated region (3′ UTR) of mRNAs, thus playing an important role in regulating the cell growth cycle as well as the expression of specific cell differentiation and cell death-related genes, lipid metabolism, and inflammatory responses. miRNAs have shown association with various liver diseases including liver fibrosis (Zhang CY. et al., 2016; Lan et al., 2018; Zhao et al., 2019).
As a novel ncRNAs, lncRNAs are predominantly transcribed by RNA polymerase II and exhibit multiple functions at the molecular level (Figure 2) (Ma et al., 2016b), the lncRNAs are classified according to their relative position on the chromosome to the coding gene as: 1. antisense lncRNAs, 2. intronic lncRNAs, 3. divergent lncRNAs, 4. intergenic lncRNAs, 5. promoter upstream lncRNAs, 6. promter-associated lncRNAs, 7. transcription start site-associated lncRNAs. LncRNAs regulate the expression of different genes based on their different cellular locations in multiple molecular mechanistic pathways including chromatin modification, transcriptional regulation, and post-transcriptional regulation (Zhang et al., 2014; Kopp and Mendell, 2018).
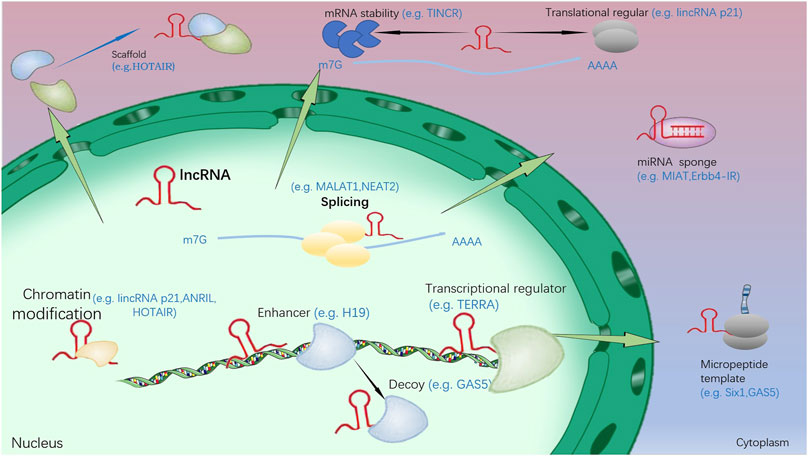
FIGURE 2. The function and regulation mechanism of lncRNA. (1): In the nucleus, lncRNA could inhibit and/or activate gene expression by transferring chromatin modifiers and various transcriptional regulators into DNA. In addition, target gene activation could be further enhanced by lncRNA. They can also induce proteins to move away from specific DNA locations and pass as molecular decoys. (2): In the cytoplasm, lncRNA could bring two or more proteins into a complex by acting as a scaffold. In addition, they could regulate other transcripts or proteins by acting as sponges and protein templates, or regulating mRNA degradation and translation.
Tsix inhibits Xist transcription by recruiting CTCF to the Xist promoter region, while JPx inhibits CTCF transcriptional repression of Xist by binding CTCC (CCCTC-binding factor); Khps1a participates in the T-DMR (tissue-dependent) region demethylation of Sphk1 by an unknown mechanism; Dum cis-recruits DNMT1, DNMT3A, DNMT3B, and etc. to the promoter region of the neighboring gene Dppa2. differentially methylated region) of Sphk1; Dum cis-recruits DNMT1, DNMT3A and DNMT3B to the promoter region of the neighboring gene Dppa2 and causes silencing of methylation in this region, thereby suppressing Dppa2 expression and leading to differentiation of skeletal muscle myogenic cells into myoblasts (Guttman et al., 2011; Bian et al., 2019; He et al., 2020).
Xist (X chromosome inactivation specific transcript) and RepA (transcript of the adenine repeat region at the 5′ end of the Xist gene) synergistically wrap the X chromosome and recruit PRC2 to establish H3K27me3 to cause X chromosome inactivation; Bxd (Bithoraxoid) binds to ubx-TRE (Ubx cis-regulatory trithorax response elements) and recruits ASH1 to activate Ubx transcription; HOTAIR regulates the expression of the HoxD gene cluster in trans by interacting with two histone modification complexes: catalytic PRC2 complex established by H3K27me3, and the LSD1-CoREST-REST complex catalyzing H3K4me2/3 erasure (lysine-specific demethylase1-RE1-Silencing Transcription factor corepressor 1-RE1 -Silencing Transcription factor complex); HOTTIP (HoxA transcript at the distal tip) recruits MLL (Mixed lineage) via WDR5 (WD repeat-containing protein 5) leukemia) to the 5′ region of the HoxA gene cluster, which catalyzes the establishment of H3K4me3 and activates the expression of genes such as Hoxa11 and Hoxa13 in cis; Mira can form a DNA/RNA heterodimer with its locus and recruit MLL1, a member of the TrxG complex, which catalyzes the establishment of H3K4me3 and activates adjacent genes Hoxa6 and Hoxa7 expression, leading to differentiation of mES to the germline; Evf2 acts as a coactivator of DLX2 at high concentrations, enhancing Dlx5/6 enhancer activity and activating Dlx5 and Dlx6 transcription, while at low concentrations it can cis-suppress Dlx6 transcription through its Dlx6 antisense property, by recruiting MECP2, and thus HDAC Dlx5; asOct4-pg5 can recruit histone methyltransferases such as EZH2 and G9a to bind to the promoter regions of Oct4 and Oct4-pg5, establishing repressive chromatin modifications such as H3K27me3 and H3K9me3, which in turn repress transcription of Oct4 and Oct4-pg5, and when as Oct4-pg5 is combined with PURA (purine rich element binding protein A) and NCL (nucleolin), the ability to recruit EZH2 and G9a is lost and the repressive function is lost; the nascent ANRIL (antisense noncoding RNA in the INK4 locus) binds to CBX7 and ANRIL binds to CBX7 and promotes heterochromatin formation, while the formed ANRIL-CBX7 complex unbinds CBX7 to H3K27me3, leaving transcriptional repression in a dynamic state of flux. ANRIL recruits PRC2, allowing the INK4β/INK4α/ARF gene cluster to establish repressive modifications such as H3K27me3. ANRIL binds to SUZ12 and cis-represses INK4β transcription; DBE-T cis-recruits Ash1L to the 4q35 region, catalyzing the establishment of activating chromatin modifications such as H3K36me2, which activates gene transcription in the 4q35 region, ultimately leading to FSHD (facioscapulohumeral muscular dystrophy) disease (Guttman et al., 2011; Kopp and Mendell, 2018; Riaz and Li, 2019).
Xite and DXPas34 regulate Tsix expression in cis with enhancer activity; transcription of SER3 gene biosynthesis-associated 3-phosphoglycerate dehydrogenase in the biosynthesis of serine is regulated by lncRNA SRG1 (SER3 regulatory gene 1); Pwr1 interferes with the transcription of Icr1; Icr1 interferes with the transcription of Fol11; DHFRtinc interferes with the transcription of DHFR; Airn interferes with the transcription of Igf2r; Gas5 inhibits the binding of activated GR to target genes (Zhang et al., 2014; Ma et al., 2016b).
Malat1 regulates variable shear of Cat1 pre-mRNA; Zeb2-anti is involved in variable shear of Zed2; PTENpg1 asRNA β promotes PTENpg1 exit from the nucleus; Neat1 promotes retention of mRNA with IRAlus structure in the 3′ UTR region within paraspeckles; BACE1-AS increases BACE1 stability; MDRL as ceRNA promotes pri-miR484 maturation (Bian et al., 2019; He et al., 2020).
It has been reported that lncRNAs are usually involved in the progression of human-related diseases by such ways as being deregulated (Guttman et al., 2011; He et al., 2020), and some studies have shown that lncRNAs are involved in the key process of liver fibrosis by acting as regulators of HSC activation (Wang et al., 2014; Bian et al., 2019). Even though we can observe an impressive amount of literature suggesting an important role of lncRNAs in the liver fibrosis process, it is undeniable that the detailed mechanisms of lncRNAs in liver fibrosis remain unclear until now. In this review, we aim to provide a review of the latest developments in lncRNAs research, elaborate on the interactions between lncRNAs and miRNAs, and further evaluate the potential of lncRNAs as new therapeutic targets in liver fibrosis.
The distribution of lncRNAs in liver fibrosis has been detected by the latest high-throughput methods such as next-generation sequencing and microarrays (Zheng et al., 2015; Xiong et al., 2016), and the pleiotropic nature of lncRNAs has been demonstrated in the activation and apoptosis of HSCs and the progression of multiple liver fibrosis by interacting with molecules such as miRNAs, specific structural domains, and proteins to regulate key genes in liver fibrosis, thus exerting their potential (Bu et al., 2020). In this paper, we review the role of lncRNAs in liver fibrosis and their potential mechanisms in the development of liver fibrosis. Table 1 provides a summary of the expression patterns, functional roles, and regulatory mechanisms of lncRNAs.
Panzitt et al. identified for the first time the highly up-regulated hepatocellular carcinoma LncRNA (HULC) of approximately 500 nucleotides containing two exons located on chromosome 6p24.3 as the most highly expressed lncRNA in human hepatocellular carcinoma, whose transcribed RNA does not have a considerable open reading frame nor does it produce any protein (Panzitt et al., 2007). The HULC promoter and its first exon are in a long terminal repeat sequence (LTR) retrotransposon-like sequence (Kapusta et al., 2013). The upregulation trend of HULC can be observed in all accessible studies on hepatocellular carcinoma (HCC) (Chen et al., 2017; Xin et al., 2018; Ghafouri-Fard et al., 2020b). Several literatures have reported that HULC is upregulated in cancer and is regulated as an oncogene lncRNA in tumorigenesis and progression (Kitagawa et al., 2013; Parasramka et al., 2016). Considering its high expression in HCC cells, previous studies have also shown the potential of HULC as a novel antitumor therapeutic agent (Klec et al., 2019). cAMP response element binding protein (CREB) is usually bound to and activated by the target promoter (Mayr and Montminy, 2001; Kong et al., 2016), and Wang et al. demonstrated the presence of CREB binding sites in the HULC promoter region and the ability to further activate the HULC promoter (Wang et al., 2010), which can affect the expression of HULC at the transcriptional level (Shen X. et al., 2019). Shen et al. revealed the role of lncRNA HULC in the progression of liver fibrosis in rats with nonalcoholic fatty liver disease (NFALD) and that inhibition of HULC suppressed steatosis. The degree of hepatic steatosis, inflammation, hepatocyte red lipid vesicles and apoptosis were also significantly reduced with the knockdown of HULC gene. Inhibition of HULC significantly reduced liver fibrosis scores and liver fibrosis indices (HA, LN, PC III, and IV-C) (Shen X. et al., 2019). Inhibition of HULC improved liver fibrosis and reduced hepatocyte apoptosis in NAFLD rats. In general, the above findings not only provide valuable candidate molecular markers for liver fibrosis and indicators of advanced liver fibrosis but also provide new insights into the role of lncRNA in the biology of cancer.
Nuclear paraspeckle assembly transcript 1 (NEAT1) was characterized as an unusual RNA polymerase II transcript that lacks introns and accompanied by non-canonical processing of the non-polyadenylated 3′-end by RNase P (Ding et al., 2019). NEAT1 was found to be upregulated in gastric adenocarcinoma and human laryngeal squamous cell carcinoma (Ma et al., 2016a; Wang et al., 2016), which suggested that it promotes tumor development by promoting cell proliferation and survival as well as inhibiting apoptosis (Choudhry et al., 2015). A similar situation has been observed in hepatocellular carcinoma (Guo et al., 2015). Yu et al. examined NEAT1 expression in cCl4-induced mice. qRT-PCR analysis showed increased expression of NEAT1 in CCl4-treated livers compared to control livers, and a significant increase in NEAT1 expression in HSCs was also observed during different weeks of CCl4 treatment (Yu et al., 2017b), Huang et al. screened the aberrantly expressed microRNAs in the CCl4-induced mouse liver fibrosis model by analyzing the GSE77271 microRNA microarray based on the Agilent-046065 mouse miRNA V19.0 platform. Neat1 simultaneously targeted miR-148a-3p and miR-22-3p, and showed the most significant increase in liver fibrosis mice that displayed the most marked increase in expression upregulation, and its expression in the CCl4 group exceeded 2-fold that of the control group (Huang et al., 2021), and inhibition of NEAT1 was observed to reverse isotropic liver fibrosis with concomitant reduction in α-SMA and type I collagen content, which was further confirmed by NEAT1 knockdown assays and NEAT1 overexpression assays (Yu et al., 2017b). A similar situation was confirmed in the alcoholic steatohepatitis (ASH) assay by Ye et al. (Ye et al., 2020). Inhibition of NEAT1 suppressed ethanol-stimulated elevated lipid metabolism and inhibited inflammatory responses in AML-12 cells. More importantly, inhibition of NEAT1 upregulated ethanol-induced hepatic function in ASH mice and inhibited lipid, inflammatory responses, hepatocyte apoptosis, and hepatic fibrosis, demonstrating that knockdown of NEAT1 inhibited hepatic fibrosis in ASH mice and thus slowed down the development of ASH (Ye et al., 2020). Related mechanistic studies suggest that Kruppel-like factor 6 (KLF6), as an important pro-fibrotic gene, is involved in the regulation of liver fibrosis by NEAT1 (Yu et al., 2017b), and that NEAT1 overexpression induces KLF6 mRNA and protein expression. However, it is of interest that KLF6 knockdown experiments showed NEAT1-induced proliferation of HSC, while KLF6 siRNA blocked NEAT1-induced α- SMA and type I collagen production, suggesting that NEAT1 could mediate HSC activation through KLF6 (Yu et al., 2017b). Huang et al. suggested that NEAT1 knockdown could inhibit the process of liver fibrosis and HSCs activation by regulating the expression of a cellular adhesion element 3 (Cyth3) associated with allosteric insulin signaling in mammals (Jux et al., 2019; Huang et al., 2021). And Ye et al. further identified that downregulation of NEAT1 could limit the inflammatory response and liver fibrosis in ASH mice by reducing suppressor of cytokine signaling 2 (SOCS2) (Ye et al., 2020), which is a feedback inhibitor of the growth hormone/insulin-like growth factor axis (Monti-Rocha et al., 2018).
It was first reported by Chaudhry in 2013 that a new full-length 2,176 bp oncogenic lncRNA, known as lncRNA small nucleolar rna host gene 7 (SNHG7), expressed in lymphoblastoid cell lines TK6 and WTK1 (Chaudhry, 2013), which is located on chromosome 9q34.3. Recent studies have shown a significant increase in its expression in tumor cells of digestive system, breast, and prostate (Wu F. et al., 2020; Wu X. et al., 2020), and further studies have demonstrated that SNHG7 is widely involved in the proliferation, invasion and migration of various tumor cells (Xia et al., 2020), including its regulation in the progression of HCC and liver fibrosis (Cui et al., 2017). Just as Xie et al. found increased expression of SNHG7 in primary HSC mice as well liver fibrosis, suggesting its regulation of HSC activation (Xie et al., 2021), and SNHG7 knockdown experiments showed decreased expression levels of α-SMA and Col. I (Xie et al., 2021), similarly SNHG7 inhibition was associated with reduced survival and proliferation rates in liver fibrosis mice. Current studies have identified several types of high confidence indicators of autophagy, such as the cytoplasmic form of LC3, a key protein in autophagosome formation (LC3-I), the active membrane-bound form of LC3 (LC3-II), and Beclin1 (Wirawan et al., 2012; Alirezaei et al., 2015; Dodson et al., 2017; Feng et al., 2017). Xie et al. revealed that knockdown of SNHG7 could reduce the decrease the expression level of Beclin1, LC3-II and LC3-I ratio, demonstrating the inhibitory effect of SNHG7 knockdown on HSC autophagy (Xie et al., 2021). DNMT3A induces a DNMT-regulated DNA ab initio methylation process, and DNA methylation/hydroxy methylation, a key step in HSC activation and liver fibrosis development, can be inhibited by activation of DNMT3A expression in HSCs (Garzon et al., 2009; Page et al., 2016). Several recent mechanistic studies suggest that SNHG7 knockdown is significantly associated with low expression levels of DNMT3A. These results confirm the relationship between SNHG7 and DNMT3A, which are novel regulators of HSC activation, autophagy, and proliferation in liver fibrosis (Xie et al., 2021). Yu et al. identified a positive correlation between SNHG7 levels and type I collagen mRNA levels in patients with cirrhosis (Yu et al., 2017b). In addition, SNHG7 showed a significant association in regulating activated HSCs proliferation and the cell cycle associated with increased G0/G1 phase cells and decreased S phase cells. SNHG7 knockdown experiments performed in activated HSCs inhibited type I collagen expression (Yu et al., 2017b), as well as collagen deposition and hydroxyproline due to carbon tetrachloride were similarly blocked by silencing of SNHG7 in vivo, suggesting that inhibition of liver fibrosis can be mediated by downregulation of SNHG7 (Yu et al., 2017b). Furthermore, Yu et al. demonstrated at the mechanistic level the role of SNHG7 in regulating the expression level of irregular fragment polarity protein 2 (DVL2) (Yu et al., 2017b; Nielsen et al., 2019), which was positively correlated with DVL2, the deletion of which also blocked its effect on HSCs activation (Yu et al., 2017b). In conclusion, all these data suggest that SNHG7 is an impressive possible therapeutic target and a potential diagnostic marker for liver fibrosis.
LncRNA H19 is expressed only by the maternal allele 11p15.5, which can encode 2.3 kb RNA and is transcribed by RNA polymerase II (Gabory et al., 2006), splicing and polyadenylation (Ghafouri-Fard et al., 2020a). It is exported from the nucleus to the cytoplasm, adjacent to the insulin-like growth factor 2 (IGF2) gene, and they are expressed from the maternal and paternal genetic chromosomes, respectively (Raveh et al., 2015; Wang J. et al., 2020). H19 RNA molecules have now been observed to be present in the cytoplasm at much higher levels than in the nucleus. H19 plays an essential role in biological processes such as apoptosis, angiogenesis, inflammation and cell death through regulatory RNA or ribosomal regulators (Yoshimura et al., 2018). This includes the regulation of proliferation, invasion, and metastasis processes in a variety of tumors of the digestive system (Zhou et al., 2017; Wei LQ. et al., 2019). Multiple complex mechanisms have been demonstrated in different cancers (Zhang D. M. et al., 2017; Zhou et al., 2017). Of interest is the upregulation of the level of intracellular transcripts (Zhu et al., 2019) and extracellular exosomes (Li X. et al., 2018), known as lncRNA-H19, observed in activated HSCs, which is thought to be associated with HSCs-activated metabolic processes like lipid (Liu et al., 2018) and cholesterol metabolism (Xiao et al., 2019). Previous research concluded that the level of fibrosis in the liver was positively correlated with the level of H19, and that H19 knockdown attenuated Bcl-2-induced liver injury (Zhang Y. et al., 2016), while conversely H19 overexpression significantly exacerbated the process of HSCs and EMT activation in hepatocytes (Zhu et al., 2019). Song et al. demonstrated the overexpression of H19 in bile duct ligation (BDL)-induced liver fibrosis with abnormal liver function parameters (Song et al., 2017), and identified a new downstream target gene of ZEB1, called EpCAM (Song et al., 2017), which promotes cholestatic liver fibrosis by interacting with the ZEB1 protein to prevent its binding to the EpCAM promoter and thus the inhibitory effect of ZEB1 (Song et al., 2017). Liu et al. reported that cholangiocyte-derived exosomal H19 promotes cholestatic liver injury in Mdr2−/− mice and promotes HSCs transdifferentiation and activation, along with upregulation of fibrotic gene expression in HSCs-derived fibroblasts (Liu et al., 2019). Wang et al. discovered that H19 can promote RARα and RXRβ mRNA and protein synthesis (Wang ZM. et al., 2020), and its reduced expression reversed the extent of HSCs activation induced due to increased retinoic acid signaling. Meanwhile, it should be mentioned that H19 knockdown-mediated HSCs inactivation was inhibited by the activation of retinoic acid signaling. Furthermore, they demonstrated that H19 enhancement was positively associated with a synergistic increase in retinoic acid metabolism during HSCs activation (Wang ZM. et al., 2020). More significantly, they confirmed that inhibition of ethanol dehydrogenase III (ADH3) completely abolished the effect of H19-mediated retinoic acid signaling, and that dihydroartemisinin (DHA), a natural inhibitor of H19, reduced both H19 and ADH3 expression and thus inhibited HSC activation (Wang ZM. et al., 2020). Taken together, these results reveal some of the molecular mechanisms underlying the increase in retinoic acid signaling during HSCs activation and suggest that the lncRNA-H19/ADH3 pathway is a potential target for the treatment of liver fibrosis. Similarly, H19 expression levels were increased in CCl4-induced fibrotic liver thereby activating HSCs (Wang Z. et al., 2020). Further studies revealed the role of hypoxia-inducible factor-1α (HIF-1α) in promoting H19 expression by binding to the H19 promoter at two hypoxia response element (HRE) sites located at 492–499 and 515–522 bp (Wang Z. et al., 2020). H19 knockdown experiments also resulted in significant inhibition of HSC activation and attenuated liver fibrosis, suggesting that lncRNAH19 may be a potential target for antifibrotic therapeutic approaches. Moreover, the H19 silencing assay reduced the degree of lipid oxidation and the H19 knockdown assay restored the levels of lipid droplets, triglycerides, cholesteryl esters and retinyl esters in HSCs without changes in lipid uptake and synthesis (Wang Z. et al., 2020). In conclusion, as described above, the results highlight the role of H19 in the proliferation, activation and metabolism of lipid droplets in HSCs and reveal its feasibility as a new molecular target to attenuate liver fibrosis.
Ji et al. characterized a metastasis-associated lung adenocarcinoma transcript (MALAT1) transcribed by RNA polymerase II located on human chromosome 11q13 and mouse chromosome 19qA (Zhang et al., 2012; Wilusz, 2016), which is widely known for its properties in predicting early NSCLC metastasis and survival (Ji et al., 2003), the major transcript of MALAT1 is approximately mid-8 kb in humans and 6.7 kb in mice (Wilusz et al., 2008). MALAT1-associated small cytoplasmic RNA (mascRNA) is a larger fragment of approximately 6.7 kb and a smaller fragment of 61 nucleotides produced by the action of ribonuclease P and ribonuclease Z on MALAT1 (Brown et al., 2014), while the larger fragment or mature transcript is highly stable due to a unique triple-helix structure at the 3′ end that protects it from nucleic acid exonucleases (Zhang et al., 2012). The highly conserved and widespread expression of MALAT1 in mammalian tissues and cancers implies its functional importance; MALAT-1 dysregulation in a variety of cancers has been extensively studied. In most cases, it functions as a promoting role in the development of different types of tumors (Kim et al., 2018; Feng et al., 2019). MALAT1 upregulation is closely associated with the development of cancers such as lung (Wei S. et al., 2019), glioblastoma (Voce et al., 2019), esophageal squamous cell carcinoma (Chen M. et al., 2018), renal cell carcinoma (Zhang H. et al., 2019), colorectal cancer (Zhang H. et al., 2019; Xie et al., 2019), osteosarcoma (Chen Y. et al., 2018), multiple myeloma (Amodio et al., 2018), gastric cancer (Zhang YF. et al., 2020), gallbladder cancer (Lin et al., 2019), and other cancers (Tian and Xu, 2015), as well as other clinicopathological features including tumor location, tumor size, differentiation and tumor stage (Goyal et al., 2021). Numerous studies have shown that as a biomarker for tumor diagnosis and prognosis, the abnormal expression of MALAT1 in tumor tissues and/or body fluids is highly indicative (Leti et al., 2017; Peng et al., 2018). In a physical and functional interaction study with the liver fibrosis process, Yu et al. found that MALAT1 expression was significantly upregulated in fibrotic liver tissues and simultaneously activated HSCs (Yu et al., 2015a), while silencing MALAT1 suppressed the mRNA levels of α-SMA and Col.I and downregulated the protein levels of α-SMA and collagen type I in HSC respectively (Yu et al., 2015a). Sirius red staining of collagen in mouse liver tissue resulted in the observation that mice transduced by silencing MALAT1 showed a 54% downregulation of collagen accumulation compared to CCl4-treated mice (Yu et al., 2015a), reflecting the role of MALAT1 in accelerating the progression of liver fibrosis in vivo. Dai et al. used arsenite treatment of L-02 cells as well as co-culture of LX-2 cells and found that MALAT1 expression levels increased as well as co-culture promoting activation of LX-2 cells (Dai et al., 2019). They further discovered that MALAT1 levels were increased in exosomes of arsenite-treated L-02 cells and LX-2 cells exposed to exosomes from arsenite-treated L-02 cells (Dai et al., 2019), and these exosomes also promoted LX-2 cell activation; blocking MALAT1 expression simultaneously inhibited these changes, thus suggesting a mechanism by which MALAT1 induces LX-2 cell activation via exosomes. Silent information regulator 1 (SIRT1), as a member of the mammalian sirtuin family of proteins (SIRT1-SIRT7) (Dai et al., 2019), is homologous to the yeast Sir2 protein, and SIRT1 is involved in a variety of biological processes and exhibits multiple physiological functions through the deacetylation of many non-histone proteins (Houtkooper et al., 2012). Wu et al. verified the important role of SIRT1 in hepatic stellate cell activation and reversal and its overexpression counteracting TGF-β1-induced LX-2 cell activation (Wu et al., 2015), suggesting its potential as an alternative for the treatment of liver fibrosis. Further studies found that the evolutionarily highly conserved MALAT1 has a strong tendency to interact with SIRT1 (discriminatory power 100%) (Wu et al., 2015), which was verified in CCL4-treated mice and LX-2 cells exposed to TGF-β1, considering that the expression level of MALAT1 mRNA was significantly upregulated and accompanied by negative changes in SIRT1 protein (Wu et al., 2015). MALAT1 silencing assay yielded results that eliminated the TGF-β1-induced upregulation of myofibroblast markers and the downregulation of SIRT1 protein. These phenomena suggest a role for MALAT1 in mediating the expression as well as the function of SIRT1 in regulating liver fibrosis. In conclusion, these findings highlight the role of MALAT1 in liver fibrosis and suggest a mechanism for fibrosis development (Wu et al., 2015). However, future efforts should be devoted to elucidating other regulatory mechanisms and clinical implications of MALAT1 in liver fibrosis.
HOXA transcript at the distal tip (HOTTIP) is a functionally characterized lncRNA (Li et al., 2016). Wang et al. demonstrated that the HOTTIP gene is located at chromosomal locus 7p15.2 and encodes a 4665 bp transcript (Wang et al., 2011). Its function is to directly interact with the Trithorax protein WDR5 and induce open DNA chromatin conformation, target the WDR5/MLL complex and drive histone H3 lysine 4 trimethylation for transcriptional regulation of the 50-terminal HOXA locus gene (Wang et al., 2011). This suggests that HOTTIP is not only involved in developmental processes but also enhances the effect of this lncRNA as a cancer-associated lncRNA considering its role as a signaling transmitter from higher-order chromosome conformation to chromatin coding (Wang et al., 2011). Overall survival (OS), distant metastasis (DM), lymph node metastasis (LNM), and tumor staging of human tumors have been extensively studied and determined to be closely associated with HOTTIP expression, suggesting that HOTTIP expression may influence the prognosis and metastasis of several human cancers (Broerse and Crassini, 1984; Quagliata et al., 2014). The most representative case is the high HOTTIP expression in human HCC specimens (Quagliata et al., 2014) and its close correlation with clinical progression and disease outcome (Tsang et al., 2015). It is worth mentioning that HOTTIP has long been shown to be dysregulated in the early stages of hepatocellular carcinoma formation, and recent studies suggest a positive correlation between its expression and liver fibrosis progression (Yang et al., 2019; He et al., 2020). Zheng et al. verified the specific expression status of HOTTIP in liver fibrotic tissue and primary quiescent HSC (Zheng et al., 2019), and their qRT-PCR results showed a 22.6-fold increase in HOTTIP expression on day 10 compared to day 2 increased 22.6-fold, similar to the results from the group of oil-treated mice compared to the group of CCl4-treated mice suggesting a significant upregulation of HOTTIP expression in HSCs however this phenomenon was not observed in hepatocytes (Zheng et al., 2019). Furthermore, the mRNA and protein levels of α-SMA and Col. I was also found to be reduced by hot-end silencing but Edu incorporation assay demonstrated that hot-end downregulation inhibited the proliferation of activated HSCs. The above results suggest that HOTTIP downregulation could inhibit HSCs activation and proliferation. Related mechanistic studies suggest that HOTTIP is a target of miR-150 and is also recruited to Ago2-associated miRNPs (Zheng et al., 2019), possibly acting through miR-150 association. In addition, bioinformatics analysis and luciferase analysis of a series of experiments also confirmed the role of serum response factor (SRF) as a target of miR-150. They further demonstrated that the inhibition of HSCs activation was caused by an increase in SRF mRNA expression due to HOTTIP overexpression (Zheng et al., 2019). Li et al. revealed that HOTTIP expression was significantly upregulated in fibrotic and cirrhotic liver samples, with the highest in cirrhotic samples (Li Z. et al., 2018), and this was also found in liver fibrotic tissue, primary HSC and activated LX-2 cells. Inhibition of HOTTIP at the mRNA and protein levels was effective in reducing the expression of α- SMA and Col. I. They found that downregulation of HOTTIP attenuated CCl4-induced liver fibrosis in mice. In contrast, the relative survival of HSC in LX-2 cells and the mRNA and protein levels of α-SMA and Col. I were significantly reduced by HOTTIP knockdown (Li Z. et al., 2018). Li et al. proposed a possible mechanism to promote HSC activation, i.e., negative regulation of HOTTIP mediated by miR-148a, considering that TGFBR1 and TGFBR2 were identified as miR-148a novel targets in HSCs. TGFBR1 and TGFBR2 levels were increased by high levels of HOTTIP, which led to the progression of liver fibrosis (Li Z. et al., 2018). These results highlight the potential of the HOTTIP/miR-148a/TGFBR1/TGFBR2 axis as a potential marker and target in patients with liver fibrosis. In conclusion, HOTTIP promotes HSCs cell proliferation and activation suggesting its possible role as a fibrogenic gene in liver fibrosis and plays a key role as a prognostic marker and novel therapeutic target. However, these still need to be investigated further.
The 7,598 nucleotide lncRNA sequence localized on chromosome 22q12.2, also known as taurine upregulated gene 1 (TUG1), was initially identified in a genomic screen of taurine-treated mouse retinal cells (Young et al., 2005; Zhang et al., 2013). Functional studies in mice further demonstrated that knockdown of TUG1 inhibits retinal developmental processes (Khalil et al., 2009). Khalil et al. demonstrated by whole genome RNA immunoprecipitation analysis that approximately 20% of lncRNAs (including TUG1) with methyltransferase activity promote demethylation and trimethylation of lysine residue 27 of histone 3 (H3K27me3) in the target gene and inhibit its expression by binding to polyclonal repressor complex 2 (PRC2), which inhibits its expression (van Kruijsbergen et al., 2015). Besides other PRC2-associated lncRNAs involved in tumorigenesis and progression, TUG1 regulates the biological behavior and molecular mechanisms of different cancer cells, including cell proliferation, invasion, apoptosis, differentiation, migration, drug resistance, radiation resistance, angiogenesis, mitochondrial bioenergetics, epithelial-mesenchymal transition (EMT), and regulation of blood-tumor barrier permeability among other different cancer cell (Niland et al., 2012; Katsushima et al., 2016; Cai et al., 2017; Chiu et al., 2018). TUG1 is closely associated with the mediation of radio resistance and angiogenesis in hepatoblastoma (Dong et al., 2016). TUG1 has also been extensively studied in liver diseases such as cirrhosis and liver fibrosis. Zhang et al. demonstrated that TUG1 is highly expressed in liver sinusoidal endothelial cells (LSEC), and the results of TUG1 knockdown experiments revealed inhibition of the extent of expression of autophagy and EMT-related genes (Zhang R. et al., 2020). In contrast, knockdown of TUG1 eliminated the most significant increase in autophagy-related genes in LPS-treated LSEC under starvation. The increase in ATG5 expression while inhibition of ATG5 attenuated autophagy and EMT (Zhang R. et al., 2020). Han et al. demonstrated that TUG1 was overexpressed in liver samples from patients with CCl4 and BDL-induced liver fibrosis in vivo as well as cirrhosis and activated HSCs while promoting a degree of expression of SMA, Col1a1, Mmp2/9/10, and Timp1. The possibility that TUG1 accelerates the progression of liver fibrosis by promoting the expression of these pro-fibrotic genes through downregulation of miR-29b is mechanistically argued (Han et al., 2018). Collectively, these studies revealed the mechanisms of TUG1 play a crucial role in liver fibrosis, suggesting its ability to monitor human liver fibrosis and its potential to be a candidate biomarker for new therapeutic strategies.
LincRNA-p21 (long intergenic non-coding RNA p21) localized at human chromosome 6p21.2 situated approximately 15 kb upstream of the cell cycle regulatory gene p21/Cdkn1a and approximately 3.0 kb in length has been described as an inducer of p53-dependent apoptosis in mouse embryonic fibroblasts (Huarte et al., 2010). lincRNA-p21 is available in two types both containing an exon and an Alu isoforms with a reverse repeat element (Yoon et al., 2012a; Yoon et al., 2012b; Wilusz and Wilusz, 2012). Coordinates the degree of autoregulation and expression of its target transcripts by interacting with RNA-binding proteins, miRNA, and mRNA targets (Yoon et al., 2012a). As a transcriptional target of p53 it is involved in the p53 pathway, downregulating many p53 target genes and triggering the apoptotic process by physically interacting with the p53 repressor complex (Fatica and Bozzoni, 2014). lincRNA-p21 has also been reported to regulate gene expression by directing protein binding partners in chromatin localization and thus directly binding to target mRNAs to act as a translational repressor and thus by activating p21 in cis participate in the regulation of the G1/S checkpoint (Dimitrova et al., 2014). It is also noteworthy that it can feedback regulate p53 activity by regulating the interaction of p53, p300, and MDM2 (Wu et al., 2014; Tang et al., 2015), thus participating in different tumorigenesis including hepatocellular carcinoma (Jia et al., 2016). In terms of tumor invasion lincRNA-p21 overexpression can be inhibited by Notch pathway (Wang et al., 2017). Besides it plays a key regulatory role in DNA damage response, apoptosis, and cell proliferation among other different processes (Ozgur et al., 2013). Zheng et al. observed in animal experiments that lincRNA-p21 expression was downregulated in liver fibrosis (Zheng et al., 2015). lincRNA-p21 was negatively correlated with disease progression and HSCs activation status, while in vitro and in vivo distribution inhibited HSCs activation and reduced liver fibrosis progression. Notably the reversibility of the inhibitory effect of lincRNA-p21 was confirmed by the removal of lincRNA-p21 leading to classical morphological changes associated with HSCs activation. lincRNA-p21 was found by Zhang et al. to inhibit the cell cycle and proliferation of primary HSCs by enhancing p21 (Zheng et al., 2015), while Tu et al. found a significant increase in hepatocyte lincRNA-p21 expression during hepatic fibrosis (Tu et al., 2017). These suggest that lincRNA-p21 contributes to a positive role in hepatocyte apoptosis and inhibition of hepatocyte growth in fibrotic livers. Knockdown of hepatocyte lincRNA-p21 attenuated CCl4-induced hepatocyte apoptosis thereby reducing CCl4-induced inflammatory cell infiltration and secretion levels of pro-inflammatory and pro-fibrotic cytokines in the fibrotic liver. Mechanistic studies have shown that inhibition of miR-30 impairs the effect of lincRNA-p21 in the development of liver fibrosis (Tu et al., 2017). lincRNA-p21/miR-30 axis has been highlighted as a potential marker and target for patients with liver fibrosis. Yang et al. found that lincRNA-p21 overexpression promotes hepatocyte apoptosis, but its results can be blocked by thymosin β4 (Tβ4) blocked, and additionally Tβ4 reversed lincRNA-p21- induced cleavage of caspase-3 and caspase-9 levels (Tu et al., 2017). LincRNA-p21 overexpression increases the levels of fibrosis-associated proteins (type I collagen, α- SMA, and TIMP-1) and induces hydroxyproline and ALT production leading to pathological damage of liver tissue and progression of fibrosis. The potential utility of lincRNA-p21 in predicting cirrhosis is supported by the results of downregulation of serum lincRNA-p21 levels in cirrhotic patients (Yang L. et al., 2020). Yu et al. reported a decrease in serum lincRNA-p21 levels in patients with chronic hepatitis B that negatively correlated with the stage of liver fibrosis, thus revealing its diagnostic value (Yu et al., 2017c). There was also a negative correlation between serum lincRNA-p21 levels and markers of liver fibrosis (including α-SMA and Col. I) but not in markers of viral replication, liver inflammatory activity and liver function. The primary HSC culture results suggested that the deletion of lincRNA-p21 expression was associated with promoter methylation, and these conditions implied the potential of serum lincRNA-p21 as a potential biomarker of liver fibrosis in patients with chronic hepatitis B/cirrhosis. Promoter methylation may be involved in the downregulation of lincRNA-p21 in liver fibrosis (Yu et al., 2017c). Collectively, these findings demonstrate the ability of lincRNA-p21 to act as a mediator of HSCs activation and proliferation, suggesting its potential as a new therapeutic target for liver fibrosis.
The maternally expressed gene 3 (MEG3), located within the human chromosome 14q32.3 DLK1-MEG3 locus (Wylie et al., 2000), is 35 kb in size consisting of 10 exons (Zhou et al., 2012) and encodes an approximately 1.6 kb long non-coding RNA as a contained 10 exons (Zhang et al., 2010). Selectively spliced transcripts of Gtl2 (gene trap site 2 (Gtl2) is the mouse homolog of human MEG3) extend to contain intron-encoded C/D box SNORNAs and miRNAs, suggesting that Gtl2 may function as a host gene for these small RNAs (Cavaille et al., 2002; Lin et al., 2003; Tierling et al., 2006). MEG3 can be observed in unimprinted embryonic cells to silence genes involved in neurogenesis by regulating the chromatin targeting of multicomb proteins and plays an important role in neuronal development (Mercer et al., 2008; Kaneko et al., 2014; Mondal et al., 2015). Recent studies have suggested that MEG3 may act as a tumor suppressor considering the extent to which its loss of expression in several cancers is associated with inhibition of cell proliferation (Ghafouri-Fard and Taheri, 2019). Yu et al. showed that the process of liver fibrosis is accompanied by a decrease in MEG3 in vivo and in vitro and that restoration of MEG3 expression inhibits liver fibrosis while reducing α-SMA and type I collagen production (Yu et al., 2018). MEG3 overexpression inhibits HSC activation through EMT and is associated with E-calcium activation. The Hedgehog (Hh) pathway is one of the pathways involved in HSC activation by MEG3 as an EMT process. Smoothing (SMO) plays an important role in the Hh pathway. Bioinformatics analysis, RNA immunoprecipitation and deletion mapping results suggest that the interaction between MEG3 and SMO is involved in EMT repression caused by MEG3 overexpression (Yu et al., 2018). Gene expression in the DLK1-MEG3 region is controlled by two differentially methylated regions (DMRs) consisting of multiple methylated CpG sites located approximately 13 kb upstream of the MEG3 transcription start site intergenic DMR (IG-DMR) and overlaps with a 1.5-kb upstream promoter in the post-fertilization-derived secondary (MEG3-DMR) (Murphy et al., 2003), indicating the important role that DNA methylation plays in silencing the MGE3 gene (Anwar et al., 2012). The most widely studied epigenetic modification, DNA methylation and its relevance to the pathogenesis of liver fibrosis have been well established experimentally (Benetatos et al., 2008; Li et al., 2010), and previous studies have suggested a role for DNA methylation in the deletion of MEG3 expression in tumors (Zhao et al., 2005; Benetatos et al., 2008). He et al. revealed that MEG3 levels were significantly reduced in CCl4-induced liver fibrosis in mice and humans, while MSP was significantly reduced in CCl4-treated mouse liver tissue and human liver fibrosis tissue and TGF-β1-treated LX-2 cells where MEG3 promoter methylation was observed (He et al., 2014). The effect of 5-azadC to block MEG3 methylation could be achieved by the methylation inhibitor 5-azadC significantly eliminating TGF-β1-induced aberrant MEG3 hypermethylation and restoring MEG3 in TGF-β1-treated LX-2 cells thereby inhibiting HSC activation and proliferation expression illustration (He et al., 2014). The inhibition of activation and the degree of proliferation of LX-2 cells and the reversal of methylation of the MEG3 promoter were both closely associated with the deletion of DNMT1 thereby restoring MEG3 expression. While 5-azadC treatment or knockdown of DNMT1 downregulated mRNA and protein production of α-SMA and Col. I in TGF-β1-treated LX-2 cells, overexpression of MEG3 was detected in TGF-β1-treated LX-2 cells (He et al., 2014), which significantly activated p53 protein levels and induced a Bax/Bcl-2 ratio accompanied by a significant increase in cytoplasmic cytochrome c significantly increased. These suggest that the p53-dependent mitochondrial apoptotic pathway is partially involved in the MEG3-induced apoptosis process (He et al., 2014). In conclusion, these findings demonstrate that MEG3 may play an important role in stellate cell activation and liver fibrosis progression and presents as a new potential treatment target for liver fibrosis.
Situated at 1q25 and composed of 12 exons, GAS5 was originally identified from a subtractive cDNA library, named according to the increased level of expression found in mammalian cells at growth arrest (Sun et al., 2017). Its exons are selectively spliced to produce two possible mature lncRNAs: GAS5a and GAS5b (Li J. et al., 2018) and 11 introns responsible for encoding 10 cassettes of C/D small nucleolar RNA (snoRNA) (Ni et al., 2019). Sequence similarity to the hormone receptor element of the glucocorticoid receptor (GR) in terms of function inhibits the effect of GR on its target gene expression (Zhong et al., 2020). Considering other regions of sequence similarity suggests a role for this lncRNA in regulating the function of other hormones such as androgen, progesterone, and salt corticosteroid receptors (Dong P. et al., 2019; Yang X. et al., 2020). Additionally, plasma GAS5 is involved during diabetes and coronary heart disease. Yu et al. showed that GAS5 could directly bind to miR-222 in mouse, rat and human fibrotic liver samples as well as in activated HSC but its overexpression inhibited the activation of primary HSC in vitro while attenuating collagen accumulation levels in fibrotic liver tissues in vivo, but this was not observed in response to GAS5 is predominantly localized in the cytoplasm (Yu et al., 2015b) accompanied by a higher copy number than miR-222 and is noted to increase p27 protein levels by binding to miR-222, thereby acting as a suppressor in HSC activation and proliferation (Yu et al., 2015b). Han et al. revealed that GAS5 expression was strongly correlated with liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD) (Han et al., 2020), and plasma GAS5 expression was significantly higher in patients with advanced stages than in non-advanced stages (Han et al., 2020). The progression of fibrosis was linearly correlated with plasma GAS5 expression, which also suggests the potential of plasma GAS5 as a noninvasive marker of liver fibrosis in patients with NAFLD (Dong Z. et al., 2019). Dong et al. investigated CCl4-induced in vivo assays in model rats and TGF-β1-induced in vitro assays in HSC and found that miR-23a expression was significantly increased while compared with miR-23a Compared with the NC group (Dong Z. et al., 2019), miR-23a inhibitor did not affect the expression levels of E-calmodulin, α-SMA and type I collagen in normal rats while up-regulating the expression levels of E-calmodulin and down-regulating the expression levels of α-SMA and type I collagen in model rats, suggesting that miR-23a plays a critical regulatory role in the development of liver fibrosis (Dong Z. et al., 2019). Further co-transfection revealed that the relative luciferase activity of pGL3-GAS5-wt was inhibited by miR-23a mimics while the luciferase activity of miR-23a NC and pGL3- GAS5-mut was unchanged (Dong Z. et al., 2019). RNA pull-down analysis suggested that approximately 5% of GAS5 bound to miR-23a compared to 100% of GAS5 in total RNA, and these results suggest that miR-23a could pull down GAS5 in liver tissue and HSC. lncRNA GAS5 silencing resulted in increased expression levels of miR-23a while addition of exogenous miR-23a resulted in downregulation of lncRNA GAS5 expression levels, this evidence suggested the ability of lncRNA GAS5 to bind directly to miR-23a. Thus, the ability of lncRNA GAS5 to act as a sponge platform for miR-23a and competitively reduce the expression level of miR-23a to inhibit liver fibrosis can be confirmed. Additionally, it is essential to mention the fact that TCM has been selected as an alternative therapy for liver fibrosis in view of the ineffectiveness and frequent occurrence of adverse side effects of synthetic drugs currently used to treat liver diseases, including liver fibrosis (Lam et al., 2016). Dahuang Zhezhuo Pill (DHZCP) as a typical Chinese medicine can inhibit the proliferation of vascular smooth muscle cells or further development of liver fibrosis in vivo by inhibiting the MAPK pathway (Zhang et al., 2009; Cai et al., 2010). Gong et al. identified that the proliferation of HSC was significantly inhibited after overexpression of GAS5 and DHZCP reversed the relative mRNA expression of GAS5, which suggest that DHZCP can mitigate liver fibrosis by enhancing the GAS5 expression (Gong et al., 2018).
Competitive endogenous RNAs (ceRNAs) act as reciprocal regulators of transcripts at the post-transcriptional level through competing shared miRNAs (Salmena et al., 2011; Qi et al., 2015). ceRNA hypothesis suggests that it provides a pathway to predict the non-coding function of any non-featured RNA transcript by identifying putative miRNA binding sites and linking the function of protein-coding mRNAs to that of e.g., miRNA, lncRNA, and MiRNAs negatively regulate gene expression at the post-transcriptional level by direct base pairing with target sites within the untranslated region of messenger RNAs (Franco-Zorrilla et al., 2007; Thomson and Dinger, 2016; Braga et al., 2020), considering that more than 60% of human protein-coding genes are under the selective pressure of MiRNAs and that any transcript containing miRNA response elements could theoretically function as ceRNAs the ability to function (Salmena et al., 2011; Li et al., 2014; Yang X. et al., 2020), which may typify a wide range of post-transcriptional forms of regulation of gene expression in physiology and pathology (Karreth et al., 2011; An et al., 2017; Wang L. X. et al., 2019). Many lncRNAs may have poor results on their effectiveness as ceRNAs under steady-state conditions due to low abundance and/or nuclear localization. Thousands of lncRNAs have cell type, tissue type, developmental stage, and disease-specific expression patterns and localization suggesting that in some cases individual lncRNAs may be effective natural miRNA sponges (Guttman and Rinn, 2012; Tay et al., 2014) (Figure 3). Preliminary experimental evidence has been given for ceRNA crosstalk results between the tumor suppressor gene PTEN and the pseudogene PTENP1 (Tay et al., 2011), and recent studies have focused on the ability of lncRNAs to act as ceRNAs to regulate miRNA concentrations and biological functions in hepatic fibrosis. using CCl4-induced mice (Zhang et al., 2018; He et al., 2020; Mahpour and Mullen, 2021). Zhu et al. explored that overexpression of H19 significantly exacerbated hepatocyte HSC and EMT activation (Zhu et al., 2019). Dual luciferase reporter analysis mechanistically revealed that miR-148a significantly inhibited the luciferase activity of pmirGLO-H19-WT and deregulated this inhibition by targeted mutation of the binding site. miR-148a inhibitor rescued H19 levels in LX-2 cells but miR-148a mimicked the down-regulated H19 levels in L-02 cells. Overexpression of H19 did not affect miR-148a levels in fibrotic livers but miR-148a could inhibit HSC and EMT activation by targeting ubiquitin-specific protease 4 (USP4) (Zhu et al., 2019). They demonstrated that the maintenance of USP4 levels could be mediated by H19 as ceRNA spongy miR-148a hair, and this evidence suggests that H19 may be a promising target for the treatment of liver fibrosis through the novel H19/miR-148a/USP4 axis that can promote liver fibrosis in HSC and hepatocytes (Zhu et al., 2019). Yu et al. found that liver fibrosis tissue and activation reduced levels of lincRNA-p21 expression in HSC, and overexpression of lincRNA-p21 played a key role in the inhibition of its activation by inducing a significant reduction in HSC expression of α-SMA and Col (Yu et al., 2016). Noticeably, these effects were blocked if in the absence of lincRNA-p21-induced PTEN enhancement, and these circumstances demonstrate the fact that lincRNAp21 inhibits liver fibrosis through PTEN. Further studies showed that miR-181b mimics inhibited the effect of lincRNA-p21 on PTEN expression and HSC activation. Combined with the above data lincRNA-p21 enhances PTEN expression levels by competitively binding miR-181b (Yu et al., 2016). Thus, these results reveal a novel lincRNA-p21-miR-181b-PTEN signaling cascade in liver fibrosis and its potential to suggest lincRNA-p21 as a molecular target for anti-fibrotic therapy. Yu et al. confirmed that lincRNA-p21 inhibits miR-17-5p levels, with this phenomenon missing in lincRNA-p21-miR-17-5p binding site could block miR-17-5p expression in the inhibition assay (Yu et al., 2017a). The function of miR-29b in mediating the downregulation of extracellular matrix genes involved in the TGF-β and NF-κB signaling pathways in HSC has long been reported (Roderburg et al., 2011). Han et al. suggested that TUG1 promotes the expression of these pro-fibrotic genes through the downregulation of miR-29b and thus plays a ceRNA role in accelerating the progression of liver fibrosis (Han et al., 2018). Xie et al. demonstrated that SNHG7 as a ceRNA can also bind to miR-29b in HSC and inhibit the expression level of miR-29b, which may affect the expression of DNMT3A (a downstream target gene of miR-29b) thus regulating the activation, autophagy, and proliferation of HSC (Xie et al., 2021). The downregulation of miR-378a-3p, a target of SNHG7, which is co-localized with SNHG7 in the cytoplasm, could block SNHG7 deletion and thus alleviate the outcome of HSC activation. Analogously, SNHG7-induced HSC activation was almost confirmed to be blocked by irregular fragment polarity protein 2 (DVL2) knockdown of the target site of miR-378a-3p (Yu et al., 2019). These discoveries suggest that lncRNA SNHG7 may interact with different miRNAs to play a critical role in the development of liver fibrosis. Zhou et al. investigated sperm-mediated primary HSC and found that lncRNA Gm5091 overexpressed and knocked downplayed important roles in negatively regulating cell migration, ROS content, IL-1β secretion and HSC activation, respectively (Zhou et al., 2018). lncRNA Gm5091 exhibited direct binding to miR-27b, miR-23b, and miR-24 and inhibited miR-27b, miR-23b, and miR-24 expression. All these suggest the potential of lncRNA Gm5091 to function as ceRNA and thus attenuate liver fibrosis through spongy miR-27b/23b/24 (Zhou et al., 2018). lncRNA ATB containing a common binding site for miR-200a was found to be upregulated in fibrotic liver tissue and simultaneously involved in LX-2 cell activation by Fu et al. in the same field. Knockdown experiments of lncRNA ATB upregulated endogenous miR-200a while downregulating β-catenin expression while suppressing the activation state of LX-2 cells (Fu et al., 2017). Significant increase in lncRNA NEAT1 expression in vitro and in vivo, as well as the inhibitory effect of its deletion on liver fibrosis were observed (Yu et al., 2017b). lncRNA NEAT1 and miR-122 interacted directly in that lncRNA NEAT1 could regulate KLF6 expression in liver fibrosis by competitively binding to miR-122, thereby accelerating HSC activation and increased cell proliferation and collagen activation (Yu et al., 2017b). The lncRNA NEAT1, which is upregulated in NAFLD progression, binds to miR-506, and GLI3, and regulates GLI3 expression levels as well as fibrosis, inflammatory response and lipid metabolism in NAFLD by secreting miR-506 and miR-506/GLI3 axis, respectively (Jin et al., 2019). lncRNA NEAT1 was found to be elevated in ash by Ye et al. was elevated in ash and acted as a ceRNA sponge for miR-129-5p′s ability to suppress SOCS2 expression. It is also important to note that inhibition of lncRNA NEAT1 inhibits the development of liver fibrosis and ASH by elevating miR-129-5p and inhibiting SOCS2 (Ye et al., 2020). These findings clarify that lncRNA NEAT1 may contribute to the development of liver fibrosis and provide new insights into the pathogenesis and potential therapeutic strategies for liver fibrosis. In conclusion these results suggest that lncRNA-miRNA interactions regulate target genes and play a role in liver fibrosis, and these evidence will provide the basis for a better understanding of this interaction to develop a new liver fibrosis treatment strategy (Table 2).
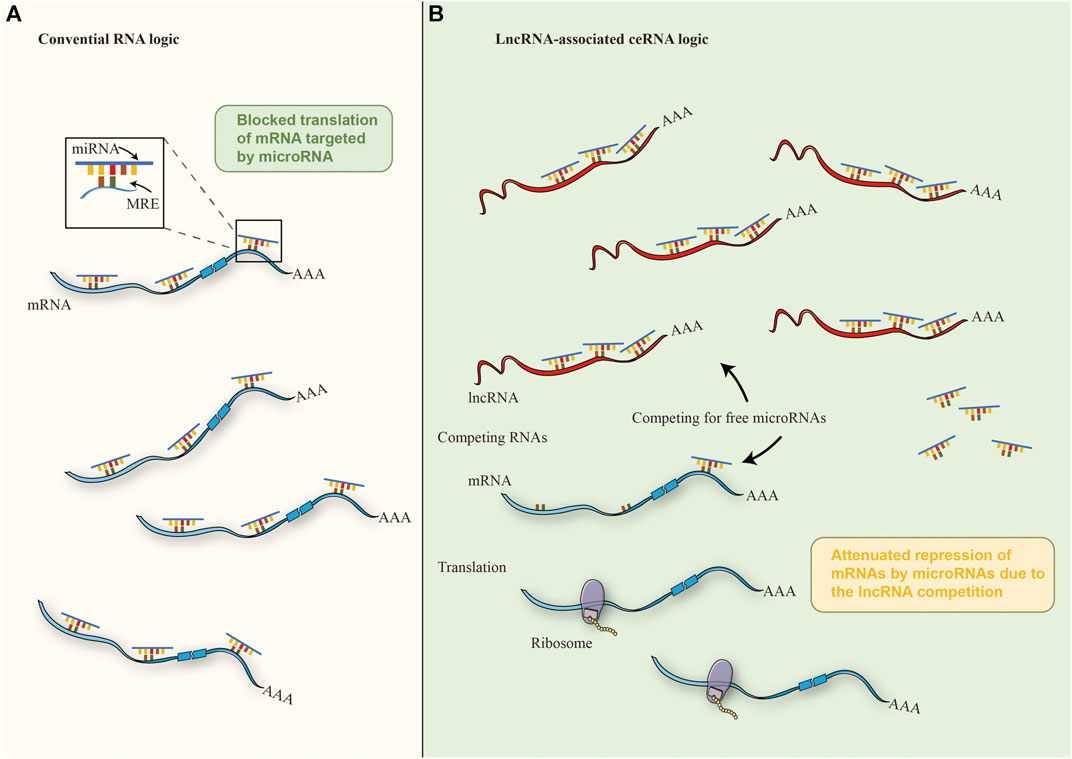
FIGURE 3. The mechanism of ceRNA. (A) In the cytoplasm, miRNAs could regulate 3′- UTR of mRNAs through base pairing with partial complementarity in the conventional crosstalk of RNA transcripts, thus inhibiting mRNAs. (B) Under the ceRNA mechanism of cancer cells, miRNAs are isolated from each other by abnormally expressed lncrna and MREs, thus reducing the interaction between miRNA and mRNA, thereby weakening the inhibition of downstream mRNA.
Deep understanding of the disease is essential to improve patient survival and to identify effective biomarkers for the development of liver fibrosis. How to detect liver fibrosis early in disease progression and develop effective therapies is critical in reducing the risk of cirrhosis, subsequent decompensation or liver cancer and reducing cancer mortality (Chang et al., 2015; Tacke and Trautwein, 2015; Aydin and Akcali, 2018). We already know that multiple signaling pathways are involved in the pathogenesis of liver fibrosis (Yang et al., 2014; Roehlen et al., 2020; Zhu et al., 2021). We therefore summarize some of the regulatory mechanisms associated with hepatic fibrosis development and progression (Figure 4).
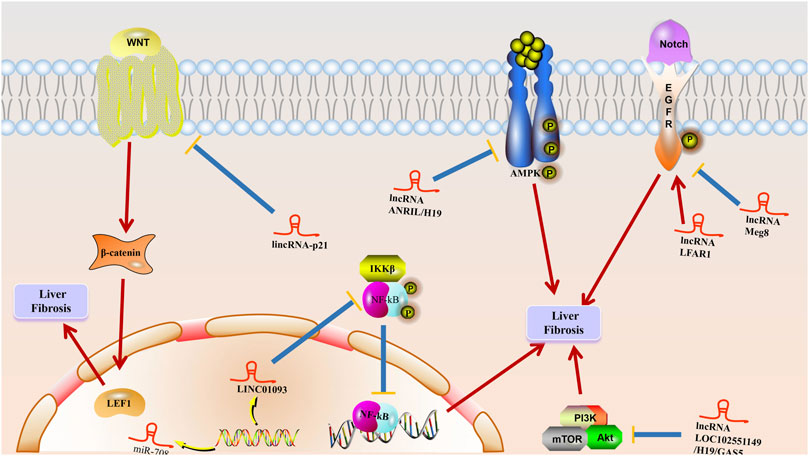
FIGURE 4. The mechanism of lncRNA to liver fibrosis. Multiple stimuli such as chronic hepatitis B (CHB) damage hepatocytes to initiate wound healing responses, and LncRNAs play a role in promoting activation and apoptosis of hepatic stellate cells and inducing epithelial-mesenchymal transition (EMT) at multiple stages, leading to excessive accumulation of extracellular matrix (ECM) proteins in hepatocytes, resulting in liver fibrosis generation and progression.
The importance of lncRNAs in mediating various signaling pathways has been recently highlighted in the direction of liver fibrosis onset and progression as well (Peng et al., 2018; Yang et al., 2019; Ganguly and Chakrabarti, 2021). The Notch signaling pathway, which induces developmental interactions and is a major player in liver biology and pathophysiology (Kovall et al., 2017; Nowell and Radtke, 2017; Meurette and Mehlen, 2018), is thought to be involved in cell proliferation, survival, apoptosis and differentiation events at various stages of development thereby controlling events such as organogenesis and morphogenesis (Zhang K. et al., 2019; Chen T. et al., 2020), as well as being significantly associated with HSC activation and HCs EMT in liver fibrosis (Zhang K. et al., 2019). Chen et al. found that lncRNA Meg8, through the Notch pathway inhibited hepatic stellate cell activation and EMT in hepatocytes while its silencing assay exhibited a significant promotion of Notch2, Notch3 and Hes1 expression levels in primary HSC and LX-2 cells (Chen T. et al., 2020). lncRNAs Notch2, Notch3, and Hes1 expression could also be inhibited by knocking down lncRNAs in primary HCs and AML-12 cells Meg8 was significantly increased. Increased mRNA and protein levels of type I collagen and α-SMA were observed in LX-2 cells transfected with lncRNA Meg8 siRNA, while knockdown lncRNA Meg8 experiments showed that overexpression of type I collagen and α-SMA was eliminated by RO4929097, evidence suggesting that this signal may be involved in mediating the function of lncRNA Meg8 (Chen T. et al., 2020). The regulatory role of lncRNAs in liver fibrosis via the Notch signaling pathway was recently reported, and protein and mRNA levels of Notch signaling-related molecules and target genes Notch2, Notch3, and Hes1 were reduced in HSCs with lncRNA LFAR1 downregulation and increased in HSCs with lncRNA LFAR1 overexpression (Zhang K. et al., 2017). CCl4-and BDL-treated mice showed significantly increased expression of Notch2, Notch3, Hes1, and Hey2 compared to lenti NC infection. Lentivirus-mediated knockdown of lncRNA LFAR1 resulted in decreased expression of Notch2, Notch 3, Hes1, and Hey2 while suppressing CCl4-and BDL-induced upregulation of these genes, and this evidence suggests that lncRNA LFAR1 promotes processes such as liver fibrosis and HSC activation through activation of the Notch signaling pathway as well as acting as a Notch signaling pathway provides new insights to elucidate the molecular mechanisms of liver fibrosis (Zhang K. et al., 2017).
The Wnt/β-catenin signaling pathway, which is highly conserved among species and controls a variety of biological processes during animal development and life cycle (Zhou and Liu, 2015; Fu et al., 2018; Zuo et al., 2019), is essential in the regulation of EMT and can recur during the onset and progression of various diseases (Sebio et al., 2014; Schunk et al., 2021). Salvianolic acid B (Sal B), one of the water-soluble components extracted from Salvia miltiorrhiza, plays an important role in the treatment and inhibition of activated HSC, and increases the expression of lincRNA-p21 (Yu et al., 2017a). Sal-B increased the expression of P-β-catenin and decreased the cytoplasmic and nuclear expression levels of β-catenin thus significantly reducing the pathway activity while this phenomenon could be restored by lincRNA-p21 knockdown. The deletion of lincRNA-p21 is involved in the inhibition of Sal-B-induced P-β-cateni and the restoration of reduced β-linked proteins in the cytoplasm and nucleus, suggesting that Sal-B may inhibit Wnt/β-linked protein pathway processes through lincRNA-p21 (Yu et al., 2017a). In conclusion, the Wnt/β-linked protein pathway inhibited by lincRNA-p21 is involved in the effect of Sal B on HSC activation and thus inhibits HSC activation and provides new evidence for the role of Wnt/β-linked protein signaling inhibited by lincRNA-p21 in the progression of liver fibrosis disease. proliferation, survival, differentiation, and invasion (Lee JJ. et al., 2015; Alzahrani, 2019; Corti et al., 2019).
lncRNAs silencing experiments can reduce the phosphorylation levels of ERK, Akt, and mTOR, while PI3K/AKT/mTOR signaling has also been reported to be closely associated with HSC proliferation, activation, and ECM synthesis, which is also significantly inhibited by pharmacological and genetic approaches through inhibition of PI3K signaling (Khemlina et al., 2017; Kong et al., 2020; Jung et al., 2021). Huang et al. revealed that increased expression of H19 could be inhibited by LY294002. These results suggest a role for lncRNA H19 in HSC activation as a downstream site regulated by the PI3K/AKT/mTOR pathway (Huang et al., 2019). Beyond this the pathway of lncRNA H19 promoting HSC activation through autophagy must be highlighted. It has also been reported that lncRNA H19 significantly decreased the expression of p-AKT and p-mTOR, and this effect was further enhanced by LY294002 and rapamycin (Huang et al., 2019). This suggests that lncRNA H19 can be involved in the PI3K/AKT/mTOR-promoted autophagy-activated HSC pathway 30735452. Dong et al. reported that silencing of lncRNA GAS5 increased the expression levels of p-PI3K, p-Akt, and p-mTOR thus revealing that activation of PI3K/Akt/mTOR signaling pathway in liver fibrosis can be mediated by the lncRNA GAS5 (Dong Z. et al., 2019). LOC102551149 knockdown assay promoted the expression of p-PI3K, p-Akt, and p-mTOR in activated HSC, while the overexpression of LOC102551149 in activated HSC 30735452 suppressed the expression levels of p-PI3K, p-Akt, and p-mTOR (Dong Z. et al., 2019). This evidence imply that lncRNAs can reduce the activation response of HSC by inhibiting the activation of PI3K/AKT/mTOR signaling pathway in liver fibrosis (Karin et al., 2002; De Simone et al., 2015).
The NF-κB signaling pathway, an important transcription factor for many inflammatory mediators and cytokines, remains a dormant molecule in the cytoplasm by binding tightly to IκB inhibitor proteins (Inoue et al., 1992; Yang et al., 2012), and phosphorylation of IκB by IκB kinase (IKK) upon stimulation separates IκB from NF-κB leading to translocation and activation of NF-κB, a process reported to be involved in the formation and progression of liver fibrosis (Luedde and Schwabe, 2011; Wang T. et al., 2019; Zhang K. et al., 2020; Zhao et al., 2020). Shi et al. found that LINC01093 31450097 knockdown assay confirmed the promotion of NF-κB p65 nuclear translocation and elevated levels of NF-kB p65 in the cytoplasm (Shi et al., 2019). On the contrary, overexpression of LINC01093 is involved in the inhibition of nuclear translocation of NF-κB p65 leading to an increase in the nuclear level of NF-κB p65 and a decrease in NF-κB p65 at the cytoplasmic level, and this evidence suggests that overexpression of LINC01093 could be involved in inhibiting hepatocyte apoptosis and attenuating the process of liver fibrosis by suppressing the NF-κB signaling pathway (Shi et al., 2019).
The AMP-activated protein kinase (AMPK) signaling pathway, which plays an important role in regulating cellular energy homeostasis, could respond to changes in intracellular adenine nucleotide levels and is involved in the process of HSC activation (Shackelford and Shaw, 2009; Mihaylova and Shaw, 2011; Zhao et al., 2017). Yang et al. determined that the proliferation rate of HSC transfected with LncRNA- ANRIL siRNA was significantly higher than that of NC and vector-identified AMPK as a key gene in LncRNA-ANRIL-mediated HSC activation (Zhang T. et al., 2019; Kim MH. et al., 2020). Overexpression of LncRNA-ANRIL suppressed the level of phosphorylated AMPK in activated HSC while LncRNA-ANRIL-siRNA increased the level of phosphorylated AMPK in activated HSC, this evidence suggest that LncRNA-ANRIL deletion can trigger HSC activation through AMPK pathway (Yang JJ. et al., 2020). Wang et al. revealed that lncRNA- H19 regulates lipid droplet metabolism by mechanisms that rely on the AMPKα pathway acting as a sensor for maintaining energy homeostasis (Wang Z. et al., 2020). The upregulated lncRNA-H19 initiates the catabolic pathway by binding to AMPKα to maintain the necessary energy supply. In addition to acting as a scaffold between AMPKα and LKB1, lncRNA-H19 links AMPKα and LKB1 and plays a facilitating role in the phosphorylation of AMPKα by LKB1 (Wang Z. et al., 2020). lncRNA-H19/AMPKα pathway is thought to be involved in HSC activation-induced lipid droplet disappearance in liver fibrosis given that lncRNA-H19 can be observed to induce HSC -formation of the AMPKα/LKB1 complex in LX2 cells and its potential as a novel target for liver fibrosis treatment (Wang Z. et al., 2020).
In conclusion, these findings highlight the possibility that there may be new therapeutic targets and biomarkers for liver fibrosis in the future from lncRNAs, Figure 4 schematically demonstrates the potential mechanism of lncRNA on liver fibrosis.
As a serious infectious disease caused by hepatitis B virus (HBV) infection, hepatitis B currently infects 350–400 million people worldwide (McMahon, 2009). Patients with chronic hepatitis B (CHB) are characterized by progressive liver fibrosis and inflammation (Guo et al., 2021) as the main pathological manifestations representing the ultimate common pathway for almost all types of CLD (Cai et al., 2020; Roehlen et al., 2020). However, it must be emphasized that liver fibrosis characterized by excessive accumulation of extracellular matrix (ECM) proteins also represents a manifestation of the liver’s trauma healing response to various types of liver injury (e.g., HBV infection) (Lee Y. A. et al., 2015; Tacke and Trautwein, 2015). The application of liver biopsy as the gold standard for assessing the presence and staging of liver fibrosis is often limited by its invasive nature, possible complications, and potential sampling errors. Consequently, there is a need for effective early detection studies of liver fibrosis to control and treat the patient’s liver fibrosis progression (Cadranel et al., 2000; Bravo et al., 2001; Rockey et al., 2009). lncRNAs are frequently deregulated in a variety of human diseases as well as in many important biological processes thereby generating abnormal lncRNAs involved in the development of various diseases 29330108 (Peng et al., 2017; Ma et al., 2018). It must be emphasized that lncRNAs are stable in the circulatory system and readily detectable in serum due to their inability to be degraded by nucleases (Faghihi et al., 2008; Gupta et al., 2010), a property that makes them highly diagnostic in different diseases including liver fibrosis (Zhang K. et al., 2017; Liu et al., 2019). They further demonstrated that reduced serum lincRNA-p21 levels in chronic hepatitis B patients correlated with fibrosis stage (Yu et al., 2017c). Subject operating characteristic curve (ROC) analysis suggested that serum lincRNA-p21 could differentiate chronic hepatitis B patients with liver fibrosis from healthy controls, specifically the area under the ROC curve (AUC) was 0.854 [0.805–0.894], with a sensitivity and specificity of 100 and 70%, respectively, at a critical value of 3.65. A sensitivity of 100% and specificity of 70% accompanied by an AUC of 0.760 (0.682–0.826) in differentiating chronic hepatitis B patients with low fibrosis scores from healthy controls; a sensitivity of 100% and specificity of 73.3% accompanied by an AUC of 0.856 in differentiating chronic hepatitis B patients with moderate fibrosis scores from healthy controls (0.801–0.901); 100% sensitivity and 77.5% specificity accompanied by an AUC of 0.935 (0.882–0.969) were observed in differentiating patients with chronic hepatitis B with high fibrosis score versus healthy controls (Yu et al., 2017c). Furthermore, the levels of lincRNA-p21 could be distinguished in chronic hepatitis B patients with different fibrosis scores, specifically: 70.9% sensitivity and 92.3% specificity (AUC 0.875, 0.800–0.930) for moderate fibrosis score and mild fibrosis score; 81.4% sensitivity and 96.1% specificity (AUC 0.954, 0.859–0.993) for high fibrosis score and low fibrosis score; and Yu et al. showed that serum lincRNA-p21 levels were associated with liver Fibrosis markers including α-SMA and Col1A1 were negatively correlated but markers of viral replication, liver inflammatory activity and liver function showed no correlation (Yu et al., 2017c). lncRNA SNHG7 was also found to be correlated with liver fibrosis progression by Yu et al. (Yu et al., 2019) and ROC curve analysis showed an area under the ROC curve (AUC) of 0.955 (95% confidence interval [CI], 0.868–0.990), where it is noteworthy that at a critical value of 1.0, its sensitivity is 90% and specificity is 100%, suggesting its potential as a potential diagnostic biomarker for liver fibrosis. lncRNA SNHG7 is higher in the cytoplasm of human LX-2 cells as well as primary HSC than in the nucleus (Yu et al., 2019), and this evidence indicates that the expression of lincRNA-p21 and lncRNA SNHG7 plays a key role in the progression of liver fibrosis and its potential as a potential biomarker of liver fibrosis. Han et al. experimentally confirmed that plasma lncRNA GAS5 was significantly elevated in patients with advanced fibrosis compared to patients without progressive fibrosis, but this did not show any statistical difference in tissues, but lncRNA GAS5 tissue expression was positively correlated with the stage of fibrosis prior to the development of cirrhosis as well as significantly downregulating lncRNA in plasma of NAFLD patients with cirrhosis GAS5 expression (Han et al., 2020). However, significant differences in tissue levels of lncRNA GAS5 were not shown in patients with advanced fibrosis and cirrhosis, a phenomenon that emphasizes the accuracy of the association between plasma levels and fibrosis stage. The significance of serum lncRNA GAS5 in the diagnosis of liver fibrosis was proposed by Gou et al. through the detection of abnormalities in lncRNA GAS5 in the serum of patients with chronic hepatitis B, and although the significance of serum lncRNA GAS5 in the age and gender distribution subgroups were not statistically significant (Han et al., 2020). The results of qRT PCR analysis suggested lower serum lncRNA GAS5 levels in CHB patients, and the results of ROC curve analysis showed that serum lncRNA GAS5 could effectively differentiate between CHB liver fibrosis patients and healthy controls (AUC of 0.993, 0.972–0.992). Altogether, circulating elevated lncRNA GAS5 levels correlated with the progression of liver fibrosis prior to the development of cirrhosis can be used to serve as a valid non-invasive marker in patients with NAFLD and CHB with liver fibrosis (Han et al., 2020). Chen et al. contributed significantly to the promotion of lncRNA MEG3 as a serum bi-diagnostic marker for chronic hepatitis B and to improve early diagnosis and treatment outcomes (Chen et al., 2019). qRT PCR data showed a significant decrease in serum lncRNA MEG3 levels in patients with chronic hepatitis B. lncRNA MEG3 expression was negatively correlated with the degree of liver fibrosis (AUC of 0.8844 and the critical value was 5.112) in the low-level fibrosis group versus the control group (AUC and critical value were 0.5237 and 2.988, respectively) (Chen et al., 2019), in the moderate fibrosis group and the control group (AUC and critical value were 0.7085 and 3.812, respectively), and in the high fibrosis group and the control group (AUC and critical value were 0.9395 and 4.689, respectively). Finally, they focused on the possibility of lncRNA MEG3 levels as a differentiating marker in chronic hepatitis B types with different degrees of liver fibrosis (Chen et al., 2019). The AUC and critical values were found to be 0.8281 and 3.963 for the low and intermediate level fibrosis groups, respectively. 0.8857 and 4.818 for the high and low levels fibrosis groups, respectively, and conversely, 0.7861 and 5.312 for the high and intermediate level fibrosis groups, respectively. The results show the important diagnostic value of serum lncRNA MEG3 in patients with chronic hepatitis B combined with liver fibrosis. Yu et al. also concluded that lncRNA MEG3 was negatively correlated with the transcript level of α- SMA and positively correlated with E-calmodulin mRNA expression. Moreover, the increase in fibrosis score was accompanied by a gradual increase in liver MEG3ΔCt value, which indicated that MEG3 expression was negatively correlated with fibrosis score (Yu et al., 2018). In conclusion, all the above results demonstrate that lncRNA MEG3 is a biomarker in the detection and prognosis of liver fibrosis.
Given the current delayed diagnosis and relapse as the biggest barriers to liver fibrosis treatment, ideal biomarkers are of great importance for clinical efforts such as improving early diagnosis rates. These results suggest a potential role of lncRNAs in the diagnosis and prognosis of liver fibrosis. Nevertheless, we must realize that the exact molecular mechanism of the role of lncRNAs in liver fibrosis is still unclear therefore the functional role of lncRNAs in liver fibrosis still needs further exploration and validation including clinical applications.
lncRNAs have been receiving increasing attention along with the rapid development of the field of molecular biology, and breakthroughs in new high-throughput sequencing technologies such as RNA-Seq, microarrays and deep sequencing have provided the basis for expanding our understanding of complex transcriptomic networks and enabling us to identify the dysregulated expression of various lncRNAs in liver fibrosis. Our review details the role of lncRNAs as important regulators in the development of liver fibrosis and the relationship between aberrant lncRNA expression and HSC activation (Yang et al., 2019; De Vincentis et al., 2020). In addition to this, given the increasing number of studies providing data on lncRNAs measured between normal and liver fibrotic tissues, it not only suggests that lncRNAs may be involved in the progression of liver fibrosis but also provides a solid theoretical basis for lncRNAs to become biomarkers for the clinical diagnosis of liver fibrosis (Jiang and Zhang, 2017; Unfried and Fortes, 2020). However, we still need to clarify the regulatory network of lncRNAs in liver fibrosis and the underlying molecular mechanisms are still complex and still inconclusive (Kim YA. et al., 2020; Ganguly and Chakrabarti, 2021). Therefore, the next work should focus on screening effective lncRNAs for the diagnosis and treatment of liver fibrosis and actively promote the development of effective lncRNAs that can be applied in the clinical setting.
On the other hand, a series of lipid bilayer membrane-bound organelles that are released by cells into the environment, which we call “EVs” (Xu et al., 2016), vary in size and could be released from almost all cells under appropriate physiological and pathological conditions (Walker et al., 2019; Mo et al., 2021). One of the hot topics of research is their cargo-carrying function given that their cargo can partially reflect the cellular properties of their origin, exosomes carry significantly different types of RNAs compared to parental cells (Abels and Breakefield, 2016; van Niel et al., 2018). Most importantly, ncRNAs can be shipped in a way that avoids the fate of unprotected ncRNAs that are readily degraded by RNA enzymes in the blood and can furthermore maintain their integrity and activity in circulation (Shen M. et al., 2019; Mori et al., 2019; Hu et al., 2020). Liu et al. found that hepatic lncRNA H19 expression levels correlated with serum exosomal lncRNA H19 levels and severity of liver fibrosis in a mouse model of cholestatic liver injury and in human patients with primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC). In contrast, exogenous lncRNA H19 promotes liver fibrosis and enhances the activation and proliferation of HSC (Liu et al., 2019). Our review highlights the status of exogenous lncRNA H19 as a potential diagnostic marker and therapeutic target in the development of cholestatic liver fibrosis, and the potential of targeting these intercellular signaling mechanisms and mediators to increase sensitivity and improve response to conventional therapeutic agents used to treat liver fibrosis and to complement exogenous lncRNAs strategies in liver prevention, diagnosis and treatment. Finally, we must emphasize that gene editing is a technique for targeted modification of DNA nucleotide sequences characterized by the precise severing of targeted DNA fragments and insertion of new gene fragments (Jinek et al., 2012), and that CRISPR/Cas9, which has been successfully applied to the disruption of protein-coding24 sequences in various organisms (Sharma et al., 2021), is a very powerful gene editing tool, and that it also plays a key role in the progression and development of liver fibrosis (Barrangou et al., 2007; Strotskaya et al., 2017). For example, RSPO4-CRISPR applied in a rat model of liver fibrosis showed excellent performance in reducing liver injury and restoring the gut microbiota (Yu et al., 2021). HSC reprogramming via exon-mediated CRISPR/dCas9-VP64 delivery (Luo et al., 2021) has also been reported. Among them it is important to note that lncRNAs have been successfully edited/regulated by the CRISPR/Cas9 system therefore no transgene needs to be introduced (Konermann et al., 2015; Chen B. et al., 2020). Due to its specificity, efficiency, simplicity, and versatility CRISPR/Cas9 has achieved many encouraging successes as a powerful genome engineering tool for the treatment of many diseases including cancer (Goyal et al., 2017; Esposito et al., 2019). For example, the fact that CRISPR/Cas9’s specifically designed GRNA targeting suppresses the upregulated lncRNA UCA1 (uroepithelial carcinoma associated 1) in bladder cancer once again highlights the potential of the CRISPR/Cas9 system for regulating the expression of lncRNAs and for further use as a therapeutic approach in clinical cancer treatment (Yang et al., 2018; Shen M. et al., 2019). Therefore, it is reasonable to assume that CRISPR/Cas9 can regulate the expression of lncRNAs and thus achieve the treatment of liver fibrosis through relevant molecular mechanisms. Importantly, our review provides a summary of the stages by which this budding and maturing technology can be used in the future for drug discovery, cancer therapy, and treatment of other genetic diseases previously considered incurable.
Research on the involvement of lncRNAs in regulating the development of liver fibrosis are increasing year by year, and the results of in vivo and ex vivo experiments confirm the significant effect of overexpression and knockdown of lncRNAs in reducing or enhancing the extent of liver fibrosis, suggesting that lncRNAs are promising as new targets for liver fibrosis treatment. The expression of lncRNAs may be a suitable candidate in the issue of potential markers for the diagnosis and prognosis of liver fibrosis. lncRNAs regulate the proliferation, activation and apoptosis of HSC involved in the process of liver fibrosis. The regulation of lncRNAs expression mediated by miRNAs and the inverse regulation of miRNAs expression by lncRNAs are demonstrated. miRNAs are involved in the regulation of lncRNAs expression through sequence-specific binding between them. Multiple molecular mechanisms regulated by lncRNAs including NF-κB signaling pathway are involved in the pathological process of liver fibrosis, while examples of successful implementation of strategies applying regulation of lncRNA expression in preclinical models can already be observed. On the one hand, we can optimistically anticipate the promising clinical applications of therapeutic strategies based on the regulation of lncRNA expression, but on the other hand, we must realize that their safety and reliability still depend on the advancement of knowledge and sophisticated technologies.
ZW, XY, SG, and FY drafted the manuscript. CL revised the manuscript. ZC, RC, and XX reviewed and modified the manuscript. All authors agreed on the final version.
This study was supported by the grants from the Inflammation and Immune Mediated Diseases Laboratory of Anhui Province (IMMDL202010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AMPK, AMP-activated protein kinase; ncRNAs, non-coding RNAs; lncRNAs, Long non-coding RNAs; ceRNAs, competing endogenous RNAs; miRNAs, micro RNAs; ECM, extracellular matrix; NASH, non-alcoholic steatohepatitis; HSCs, hepatic stellate cells; Xist X, chromosome inactivation specific transcript; HULC, hepatocellular carcinoma; NEAT1, Nuclear paraspeckle assembly transcript 1; SNHG7, small nucleolar RNA host gene 7; MALAT1, metastasis-associated lung adenocarcinoma transcript; HOTTIP, HOXA transcript at the distal tip; TUG1, taurine upregulated gene 1; MEG3, maternally expressed gene 3.
Abels, E. R., and Breakefield, X. O. (2016). Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol 36 (3), 301–312. doi:10.1007/s10571-016-0366-z
Alirezaei, M., Flynn, C. T., Wood, M. R., Harkins, S., and Whitton, J. L. (2015). Coxsackievirus Can Exploit LC3 in Both Autophagy-dependent and -independent Manners In Vivo. Autophagy 11 (8), 1389–1407. doi:10.1080/15548627.2015.1063769
Alzahrani, A. S. (2019). PI3K/Akt/mTOR Inhibitors in Cancer: At the Bench and Bedside. Semin. Cancer Biol. 59, 125–132. doi:10.1016/j.semcancer.2019.07.009
Amodio, N., Stamato, M. A., Juli, G., Morelli, E., Fulciniti, M., Manzoni, M., et al. (2018). Drugging the lncRNA MALAT1 via LNA gapmeR ASO Inhibits Gene Expression of Proteasome Subunits and Triggers Anti-multiple Myeloma Activity. Leukemia 32 (9), 1948–1957. doi:10.1038/s41375-018-0067-3
An, Y., Furber, K. L., and Ji, S. (2017). Pseudogenes Regulate Parental Gene Expression via ceRNA Network. J. Cel Mol Med 21 (1), 185–192. doi:10.1111/jcmm.12952
Anwar, S. L., Krech, T., Hasemeier, B., Schipper, E., Schweitzer, N., Vogel, A., et al. (2012). Loss of Imprinting and Allelic Switching at the DLK1-MEG3 Locus in Human Hepatocellular Carcinoma. PLoS One 7 (11), e49462. doi:10.1371/journal.pone.0049462
Aydin, M. M., and Akcali, K. C. (2018). Liver Fibrosis. Turk J. Gastroenterol. 29 (1), 14–21. doi:10.5152/tjg.2018.17330
Barrangou, R., Fremaux, C., Deveau, H., Richards, M., Boyaval, P., Moineau, S., et al. (2007). CRISPR Provides Acquired Resistance against Viruses in Prokaryotes. Science 315 (5819), 1709–1712. doi:10.1126/science.1138140
Benetatos, L., Dasoula, A., Hatzimichael, E., Georgiou, I., Syrrou, M., and Bourantas, K. L. (2008). Promoter Hypermethylation of the MEG3 (DLK1/MEG3) Imprinted Gene in Multiple Myeloma. Clin. Lymphoma Myeloma 8 (3), 171–175. doi:10.3816/CLM.2008.n.021
Bian, E. B., Xiong, Z. G., and Li, J. (2019). New Advances of lncRNAs in Liver Fibrosis, with Specific Focus on lncRNA-miRNA Interactions. J. Cel Physiol 234 (3), 2194–2203. doi:10.1002/jcp.27069
Blaner, W. S., O'Byrne, S. M., Wongsiriroj, N., Kluwe, J., D'Ambrosio, D. M., Jiang, H., et al. (2009). Hepatic Stellate Cell Lipid Droplets: a Specialized Lipid Droplet for Retinoid Storage. Biochim. Biophys. Acta 1791 (6), 467–473. doi:10.1016/j.bbalip.2008.11.001
Braga, E. A., Fridman, M. V., Moscovtsev, A. A., Filippova, E. A., Dmitriev, A. A., and Kushlinskii, N. E. (2020). LncRNAs in Ovarian Cancer Progression, Metastasis, and Main Pathways: ceRNA and Alternative Mechanisms. Int. J. Mol. Sci. 21 (22). doi:10.3390/ijms21228855
Bravo, A. A., Sheth, S. G., and Chopra, S. (2001). Liver Biopsy. N. Engl. J. Med. 344 (7), 495–500. doi:10.1056/NEJM200102153440706
Broerse, J., and Crassini, B. (1984). Investigations of Perception and Imagery Using CAEs: the Role of Experimental Design and Psychophysical Method. Percept Psychophys 35 (2), 155–164. doi:10.3758/bf03203895
Brown, J. A., Bulkley, D., Wang, J., Valenstein, M. L., Yario, T. A., Steitz, T. A., et al. (2014). Structural Insights into the Stabilization of MALAT1 Noncoding RNA by a Bipartite Triple helix. Nat. Struct. Mol. Biol. 21 (7), 633–640. doi:10.1038/nsmb.2844
Bu, F. T., Wang, A., Zhu, Y., You, H. M., Zhang, Y. F., Meng, X. M., et al. (2020). LncRNA NEAT1: Shedding Light on Mechanisms and Opportunities in Liver Diseases. Liver Int. 40 (11), 2612–2626. doi:10.1111/liv.14629
Cadranel, J. F., Rufat, P., and Degos, F. (2000). Practices of Liver Biopsy in France: Results of a Prospective Nationwide Survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32 (3), 477–481. doi:10.1053/jhep.2000.16602
Cai, B., Dongiovanni, P., Corey, K. E., Wang, X., Shmarakov, I. O., Zheng, Z., et al. (2020). Macrophage MerTK Promotes Liver Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab 31 (2), 406–e7. e407. doi:10.1016/j.cmet.2019.11.013
Cai, H., Liu, X., Zheng, J., Xue, Y., Ma, J., Li, Z., et al. (2017). Long Non-coding RNA Taurine Upregulated 1 Enhances Tumor-Induced Angiogenesis through Inhibiting microRNA-299 in Human Glioblastoma. Oncogene 36 (3), 318–331. doi:10.1038/onc.2016.212
Cai, H. B., Sun, X. G., Liu, Z. F., Liu, Y. W., Tang, J., Liu, Q., et al. (2010). Effects of Dahuangzhechong Pills on Cytokines and Mitogen Activated Protein Kinase Activation in Rats with Hepatic Fibrosis. J. Ethnopharmacol 132 (1), 157–164. doi:10.1016/j.jep.2010.08.019
Cavaillé, J., Seitz, H., Paulsen, M., Ferguson-Smith, A. C., and Bachellerie, J. P. (2002). Identification of Tandemly-Repeated C/D snoRNA Genes at the Imprinted Human 14q32 Domain Reminiscent of Those at the Prader-Willi/Angelman Syndrome Region. Hum. Mol. Genet. 11 (13), 1527–1538. doi:10.1093/hmg/11.13.1527
Chang, J., Lan, T., Li, C., Ji, X., Zheng, L., Gou, H., et al. (2015). Activation of Slit2-Robo1 Signaling Promotes Liver Fibrosis. J. Hepatol. 63 (6), 1413–1420. doi:10.1016/j.jhep.2015.07.033
Chaudhry, M. A. (2013). Expression Pattern of Small Nucleolar RNA Host Genes and Long Non-coding RNA in X-Rays-Treated Lymphoblastoid Cells. Int. J. Mol. Sci. 14 (5), 9099–9110. doi:10.3390/ijms14059099
Chen, B., Deng, S., Ge, T., Ye, M., Yu, J., Lin, S., et al. (2020a). Live Cell Imaging and Proteomic Profiling of Endogenous NEAT1 lncRNA by CRISPR/Cas9-mediated Knock-In. Protein Cell 11 (9), 641–660. doi:10.1007/s13238-020-00706-w
Chen, M., Xia, Z., Chen, C., Hu, W., and Yuan, Y. (2018a). LncRNA MALAT1 Promotes Epithelial-To-Mesenchymal Transition of Esophageal Cancer through Ezh2-Notch1 Signaling Pathway. Anticancer Drugs 29 (8), 767–773. doi:10.1097/CAD.0000000000000645
Chen, M. J., Wang, X. G., Sun, Z. X., and Liu, X. C. (2019). Diagnostic Value of LncRNA-MEG3 as a Serum Biomarker in Patients with Hepatitis B Complicated with Liver Fibrosis. Eur. Rev. Med. Pharmacol. Sci. 23 (10), 4360–4367. doi:10.26355/eurrev_201905_17943
Chen, T., Lin, H., Chen, X., Li, G., Zhao, Y., Zheng, L., et al. (2020b). LncRNA Meg8 Suppresses Activation of Hepatic Stellate Cells and Epithelial-Mesenchymal Transition of Hepatocytes via the Notch Pathway. Biochem. Biophys. Res. Commun. 521 (4), 921–927. doi:10.1016/j.bbrc.2019.11.015
Chen, X., Lun, L., Hou, H., Tian, R., Zhang, H., and Zhang, Y. (2017). The Value of lncRNA HULC as a Prognostic Factor for Survival of Cancer Outcome: A Meta-Analysis. Cell Physiol Biochem 41 (4), 1424–1434. doi:10.1159/000468005
Chen, Y., Huang, W., Sun, W., Zheng, B., Wang, C., Luo, Z., et al. (2018b). LncRNA MALAT1 Promotes Cancer Metastasis in Osteosarcoma via Activation of the PI3K-Akt Signaling Pathway. Cel Physiol Biochem 51 (3), 1313–1326. doi:10.1159/000495550
Chiu, H. S., Somvanshi, S., Patel, E., Chen, T. W., Singh, V. P., Zorman, B., et al. (2018). Pan-Cancer Analysis of lncRNA Regulation Supports Their Targeting of Cancer Genes in Each Tumor Context. Cell Rep 23 (1), 297–e12. e212. doi:10.1016/j.celrep.2018.03.064
Choudhry, H., Albukhari, A., Morotti, M., Haider, S., Moralli, D., Smythies, J., et al. (2015). Tumor Hypoxia Induces Nuclear Paraspeckle Formation through HIF-2α Dependent Transcriptional Activation of NEAT1 Leading to Cancer Cell Survival. Oncogene 34 (34), 4482–4490. doi:10.1038/onc.2014.378
Consortium, E. P., Birney, E., Stamatoyannopoulos, J. A., Dutta, A., Guigó, R., Gingeras, T. R., et al. (2007). Identification and Analysis of Functional Elements in 1% of the Human Genome by the ENCODE Pilot Project. Nature 447 (7146), 799–816. doi:10.1038/nature05874
Corti, F., Nichetti, F., Raimondi, A., Niger, M., Prinzi, N., Torchio, M., et al. (2019). Targeting the PI3K/AKT/mTOR Pathway in Biliary Tract Cancers: A Review of Current Evidences and Future Perspectives. Cancer Treat. Rev. 72, 45–55. doi:10.1016/j.ctrv.2018.11.001
Cui, H., Zhang, Y., Zhang, Q., Chen, W., Zhao, H., and Liang, J. (2017). A Comprehensive Genome-wide Analysis of Long Noncoding RNA Expression Profile in Hepatocellular Carcinoma. Cancer Med. 6 (12), 2932–2941. doi:10.1002/cam4.1180
Dai, X., Chen, C., Xue, J., Xiao, T., Mostofa, G., Wang, D., et al. (2019). Exosomal MALAT1 Derived from Hepatic Cells Is Involved in the Activation of Hepatic Stellate Cells via miRNA-26b in Fibrosis Induced by Arsenite. Toxicol. Lett. 316, 73–84. doi:10.1016/j.toxlet.2019.09.008
De Simone, V., Franzè, E., Ronchetti, G., Colantoni, A., Fantini, M. C., Di Fusco, D., et al. (2015). Th17-type Cytokines, IL-6 and TNF-α Synergistically Activate STAT3 and NF-kB to Promote Colorectal Cancer Cell Growth. Oncogene 34 (27), 3493–3503. doi:10.1038/onc.2014.286
De Vincentis, A., Rahmani, Z., Muley, M., Vespasiani-Gentilucci, U., Ruggiero, S., Zamani, P., et al. (2020). Long Noncoding RNAs in Nonalcoholic Fatty Liver Disease and Liver Fibrosis: State-Of-The-Art and Perspectives in Diagnosis and Treatment. Drug Discov. Today 25 (7), 1277–1286. doi:10.1016/j.drudis.2020.05.009
Dimitrova, N., Zamudio, J. R., Jong, R. M., Soukup, D., Resnick, R., Sarma, K., et al. (2014). LincRNA-p21 Activates P21 in Cis to Promote Polycomb Target Gene Expression and to Enforce the G1/S Checkpoint. Mol. Cel 54 (5), 777–790. doi:10.1016/j.molcel.2014.04.025
Ding, F., Lai, J., Gao, Y., Wang, G., Shang, J., Zhang, D., et al. (2019). NEAT1/miR-23a-3p/KLF3: a Novel Regulatory axis in Melanoma Cancer Progression. Cancer Cel Int 19, 217. doi:10.1186/s12935-019-0927-6
Dodson, M., Wani, W. Y., Redmann, M., Benavides, G. A., Johnson, M. S., Ouyang, X., et al. (2017). Regulation of Autophagy, Mitochondrial Dynamics, and Cellular Bioenergetics by 4-hydroxynonenal in Primary Neurons. Autophagy 13 (11), 1828–1840. doi:10.1080/15548627.2017.1356948
Dong, P., Xiong, Y., Yue, J., J B Hanley, S., Kobayashi, N., Todo, Y., et al. (2019a). Exploring lncRNA-Mediated Regulatory Networks in Endometrial Cancer Cells and the Tumor Microenvironment: Advances and Challenges. Cancers (Basel) 11 (2), 234. doi:10.3390/cancers11020234
Dong, R., Liu, G. B., Liu, B. H., Chen, G., Li, K., Zheng, S., et al. (2016). Targeting Long Non-coding RNA-TUG1 Inhibits Tumor Growth and Angiogenesis in Hepatoblastoma. Cell Death Dis 7 (6), e2278. doi:10.1038/cddis.2016.143
Dong, Z., Li, S., Wang, X., Si, L., Ma, R., Bao, L., et al. (2019b). lncRNA GAS5 Restrains CCl4-Induced Hepatic Fibrosis by Targeting miR-23a through the PTEN/PI3K/Akt Signaling Pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 316 (4), G539–G550. doi:10.1152/ajpgi.00249.2018
Elpek, G. Ö. (2014). Cellular and Molecular Mechanisms in the Pathogenesis of Liver Fibrosis: An Update. World J. Gastroenterol. 20 (23), 7260–7276. doi:10.3748/wjg.v20.i23.7260
Esposito, R., Bosch, N., Lanzós, A., Polidori, T., Pulido-Quetglas, C., and Johnson, R. (2019). Hacking the Cancer Genome: Profiling Therapeutically Actionable Long Non-coding RNAs Using CRISPR-Cas9 Screening. Cancer Cell 35 (4), 545–557. doi:10.1016/j.ccell.2019.01.019
Faghihi, M. A., Modarresi, F., Khalil, A. M., Wood, D. E., Sahagan, B. G., Morgan, T. E., et al. (2008). Expression of a Noncoding RNA Is Elevated in Alzheimer's Disease and Drives Rapid Feed-Forward Regulation of Beta-Secretase. Nat. Med. 14 (7), 723–730. doi:10.1038/nm1784
Fatica, A., and Bozzoni, I. (2014). Long Non-coding RNAs: New Players in Cell Differentiation and Development. Nat. Rev. Genet. 15 (1), 7–21. doi:10.1038/nrg3606
Feng, C., Zhao, Y., Li, Y., Zhang, T., Ma, Y., and Liu, Y. (2019). LncRNA MALAT1 Promotes Lung Cancer Proliferation and Gefitinib Resistance by Acting as a miR-200a Sponge. Arch. Bronconeumol 55 (12), 627–633. doi:10.1016/j.arbres.2019.03.026
Feng, L., Zhang, J., Zhu, N., Ding, Q., Zhang, X., Yu, J., et al. (2017). Ubiquitin Ligase SYVN1/HRD1 Facilitates Degradation of the SERPINA1 Z Variant/α-1-Antitrypsin Z Variant via SQSTM1/p62-dependent Selective Autophagy. Autophagy 13 (4), 686–702. doi:10.1080/15548627.2017.1280207
Franco-Zorrilla, J. M., Valli, A., Todesco, M., Mateos, I., Puga, M. I., Rubio-Somoza, I., et al. (2007). Target Mimicry Provides a New Mechanism for Regulation of microRNA Activity. Nat. Genet. 39 (8), 1033–1037. doi:10.1038/ng2079
Fu, N., Zhao, S. X., Kong, L. B., Du, J. H., Ren, W. G., Han, F., et al. (2017). LncRNA-ATB/microRNA-200a/β-catenin Regulatory axis Involved in the Progression of HCV-Related Hepatic Fibrosis. Gene 618, 1–7. doi:10.1016/j.gene.2017.03.008
Fu, X., Zhu, X., Qin, F., Zhang, Y., Lin, J., Ding, Y., et al. (2018). Linc00210 Drives Wnt/β-Catenin Signaling Activation and Liver Tumor Progression through CTNNBIP1-dependent Manner. Mol. Cancer 17 (1), 73. doi:10.1186/s12943-018-0783-3
Gabory, A., Ripoche, M. A., Yoshimizu, T., and Dandolo, L. (2006). The H19 Gene: Regulation and Function of a Non-coding RNA. Cytogenet. Genome Res. 113 (1-4), 188–193. doi:10.1159/000090831
Ganguly, N., and Chakrabarti, S. (2021). Role of Long Non-coding RNAs and R-elated E-pigenetic M-echanisms in L-iver F-ibrosis (Review). Int. J. Mol. Med. 47 (3). doi:10.3892/ijmm.2021.4856
Garzon, R., Liu, S., Fabbri, M., Liu, Z., Heaphy, C. E. A., Callegari, E., et al. (2009). MicroRNA-29b Induces Global DNA Hypomethylation and Tumor Suppressor Gene Reexpression in Acute Myeloid Leukemia by Targeting Directly DNMT3A and 3B and Indirectly DNMT1. Blood 113 (25), 6411–6418. doi:10.1182/blood-2008-07-170589
Geerts, A. (2001). History, Heterogeneity, Developmental Biology, and Functions of Quiescent Hepatic Stellate Cells. Semin. Liver Dis. 21 (3), 311–335. doi:10.1055/s-2001-17550
Ghafouri-Fard, S., Esmaeili, M., Taheri, M., and Samsami, M. (2020b). Highly Upregulated in Liver Cancer (HULC): An Update on its Role in Carcinogenesis. J. Cel Physiol 235 (12), 9071–9079. doi:10.1002/jcp.29765
Ghafouri-Fard, S., and Taheri, M. (2019). Maternally Expressed Gene 3 (MEG3): A Tumor Suppressor Long Non Coding RNA. Biomed. Pharmacother. 118, 109129. doi:10.1016/j.biopha.2019.109129
Ghafouri-Fard, S., Esmaeili, M., and Taheri, M. (2020a). H19 lncRNA: Roles in Tumorigenesis. Biomed. Pharmacother. 123, 109774. doi:10.1016/j.biopha.2019.109774
Gong, Z., Deng, C., Xiao, H., Peng, Y., Hu, G., Xiang, T., et al. (2018). Effect of Dahuang Zhechong Pills on Long Non-coding RNA Growth Arrest Specific 5 in Rat Models of Hepatic Fibrosis. J. Tradit Chin. Med. 38 (2), 190–196. doi:10.1016/j.jtcm.2018.04.007
Goyal, A., Myacheva, K., Gross, M., Klingenberg, M., Duran Arqué, B., and Diederichs, S. (2017). Challenges of CRISPR/Cas9 Applications for Long Non-coding RNA Genes. Nucleic Acids Res. 45 (3), e12. doi:10.1093/nar/gkw883
Goyal, B., Yadav, S. R. M., Awasthee, N., Gupta, S., Kunnumakkara, A. B., and Gupta, S. C. (2021). Diagnostic, Prognostic, and Therapeutic Significance of Long Non-coding RNA MALAT1 in Cancer. Biochim. Biophys. Acta Rev. Cancer 1875 (2), 188502. doi:10.1016/j.bbcan.2021.188502
Guo, S., Chen, W., Luo, Y., Ren, F., Zhong, T., Rong, M., et al. (2015). Clinical Implication of Long Non-coding RNA NEAT1 Expression in Hepatocellular Carcinoma Patients. Int. J. Clin. Exp. Pathol. 8 (5), 5395–5402.
Guo, Y., Li, C., Zhang, R., Zhan, Y., Yu, J., Tu, J., et al. (2021). Epigenetically-regulated Serum GAS5 as a Potential Biomarker for Patients with Chronic Hepatitis B Virus Infection. Cbm 32, 137–146. doi:10.3233/CBM-203169
Gupta, R. A., Shah, N., Wang, K. C., Kim, J., Horlings, H. M., Wong, D. J., et al. (2010). Long Non-coding RNA HOTAIR Reprograms Chromatin State to Promote Cancer Metastasis. Nature 464 (7291), 1071–1076. doi:10.1038/nature08975
Guttman, M., Donaghey, J., Carey, B. W., Garber, M., Grenier, J. K., Munson, G., et al. (2011). lincRNAs Act in the Circuitry Controlling Pluripotency and Differentiation. Nature 477 (7364), 295–300. doi:10.1038/nature10398
Guttman, M., and Rinn, J. L. (2012). Modular Regulatory Principles of Large Non-coding RNAs. Nature 482 (7385), 339–346. doi:10.1038/nature10887
Han, M. H., Lee, J. H., Kim, G., Lee, E., Lee, Y. R., Jang, S. Y., et al. (2020). Expression of the Long Noncoding RNA GAS5 Correlates with Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Genes (Basel) 11 (5), 545. doi:10.3390/genes11050545
Han, X., Hong, Y., and Zhang, K. (2018). TUG1 Is Involved in Liver Fibrosis and Activation of HSCs by Regulating miR-29b. Biochem. Biophys. Res. Commun. 503 (3), 1394–1400. doi:10.1016/j.bbrc.2018.07.054
He, Y., Wu, Y. T., Huang, C., Meng, X. M., Ma, T. T., Wu, B. M., et al. (2014). Inhibitory Effects of Long Noncoding RNA MEG3 on Hepatic Stellate Cells Activation and Liver Fibrogenesis. Biochim. Biophys. Acta 1842 (11), 2204–2215. doi:10.1016/j.bbadis.2014.08.015
He, Z., Yang, D., Fan, X., Zhang, M., Li, Y., Gu, X., et al. (2020). The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Int. J. Mol. Sci. 21 (4), 1482. doi:10.3390/ijms21041482
Heo, M. J., Yun, J., and Kim, S. G. (2019). Role of Non-coding RNAs in Liver Disease Progression to Hepatocellular Carcinoma. Arch. Pharm. Res. 42 (1), 48–62. doi:10.1007/s12272-018-01104-x
Houtkooper, R. H., Pirinen, E., and Auwerx, J. (2012). Sirtuins as Regulators of Metabolism and Healthspan. Nat. Rev. Mol. Cel Biol 13 (4), 225–238. doi:10.1038/nrm3293
Hu, W., Liu, C., Bi, Z. Y., Zhou, Q., Zhang, H., Li, L. L., et al. (2020). Comprehensive Landscape of Extracellular Vesicle-Derived RNAs in Cancer Initiation, Progression, Metastasis and Cancer Immunology. Mol. Cancer 19 (1), 102. doi:10.1186/s12943-020-01199-1
Huang, T. J., Ren, J. J., Zhang, Q. Q., Kong, Y. Y., Zhang, H. Y., Guo, X. H., et al. (2019). IGFBPrP1 Accelerates Autophagy and Activation of Hepatic Stellate Cells via Mutual Regulation between H19 and PI3K/AKT/mTOR Pathway. Biomed. Pharmacother. 116, 109034. doi:10.1016/j.biopha.2019.109034
Huang, W., Huang, F., Zhang, R., and Luo, H. (2021). LncRNA Neat1 Expedites the Progression of Liver Fibrosis in Mice through Targeting miR-148a-3p and miR-22-3p to Upregulate Cyth3. Cell Cycle 20 (5-6), 490–507. doi:10.1080/15384101.2021.1875665
Huarte, M., Guttman, M., Feldser, D., Garber, M., Koziol, M. J., Kenzelmann-Broz, D., et al. (2010). A Large Intergenic Noncoding RNA Induced by P53 Mediates Global Gene Repression in the P53 Response. Cell 142 (3), 409–419. doi:10.1016/j.cell.2010.06.040
Inoue, J., Kerr, L. D., Kakizuka, A., and Verma, I. M. (1992). I Kappa B Gamma, a 70 Kd Protein Identical to the C-Terminal Half of P110 NF-Kappa B: a New Member of the I Kappa B Family. Cell 68 (6), 1109–1120. doi:10.1016/0092-8674(92)90082-n
Ji, P., Diederichs, S., Wang, W., Böing, S., Metzger, R., Schneider, P. M., et al. (2003). MALAT-1, a Novel Noncoding RNA, and Thymosin Beta4 Predict Metastasis and Survival in Early-Stage Non-small Cell Lung Cancer. Oncogene 22 (39), 8031–8041. doi:10.1038/sj.onc.1206928
Jia, M., Jiang, L., Wang, Y. D., Huang, J. Z., Yu, M., and Xue, H. Z. (2016). lincRNA-p21 Inhibits Invasion and Metastasis of Hepatocellular Carcinoma through Notch Signaling-Induced Epithelial-Mesenchymal Transition. Hepatol. Res. 46 (11), 1137–1144. doi:10.1111/hepr.12659
Jiang, X., and Zhang, F. (2017). Long Noncoding RNA: a New Contributor and Potential Therapeutic Target in Fibrosis. Epigenomics 9 (9), 1233–1241. doi:10.2217/epi-2017-0020
Jin, S. S., Lin, X. F., Zheng, J. Z., Wang, Q., and Guan, H. Q. (2019). lncRNA NEAT1 Regulates Fibrosis and Inflammatory Response Induced by Nonalcoholic Fatty Liver by Regulating miR-506/GLI3. Eur. Cytokine Netw. 30 (3), 98–106. doi:10.1684/ecn.2019.0432
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., and Charpentier, E. (2012). A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 337 (6096), 816–821. doi:10.1126/science.1225829
Jung, K., Kim, M., So, J., Lee, S. H., Ko, S., and Shin, D. (2021). Farnesoid X Receptor Activation Impairs Liver Progenitor Cell-Mediated Liver Regeneration via the PTEN-Pi3k-AKT-mTOR Axis in Zebrafish. Hepatology 74 (1), 397–410. doi:10.1002/hep.31679
Jux, B., Gosejacob, D., Tolksdorf, F., Mandel, C., Rieck, M., Namislo, A., et al. (2019). Cytohesin-3 Is Required for Full Insulin Receptor Signaling and Controls Body Weight via Lipid Excretion. Sci. Rep. 9 (1), 3442. doi:10.1038/s41598-019-40231-3
Kaneko, S., Bonasio, R., Saldaña-Meyer, R., Yoshida, T., Son, J., Nishino, K., et al. (2014). Interactions between JARID2 and Noncoding RNAs Regulate PRC2 Recruitment to Chromatin. Mol. Cel 53 (2), 290–300. doi:10.1016/j.molcel.2013.11.012
Kapusta, A., Kronenberg, Z., Lynch, V. J., Zhuo, X., Ramsay, L., Bourque, G., et al. (2013). Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs. Plos Genet. 9 (4), e1003470. doi:10.1371/journal.pgen.1003470
Karin, M., Cao, Y., Greten, F. R., and Li, Z. W. (2002). NF-kappaB in Cancer: from Innocent Bystander to Major Culprit. Nat. Rev. Cancer 2 (4), 301–310. doi:10.1038/nrc780
Karreth, F. A., Tay, Y., Perna, D., Ala, U., Tan, S. M., Rust, A. G., et al. (2011). In Vivo identification of Tumor- Suppressive PTEN ceRNAs in an Oncogenic BRAF-Induced Mouse Model of Melanoma. Cell 147 (2), 382–395. doi:10.1016/j.cell.2011.09.032
Katsushima, K., Natsume, A., Ohka, F., Shinjo, K., Hatanaka, A., Ichimura, N., et al. (2016). Targeting the Notch-Regulated Non-coding RNA TUG1 for Glioma Treatment. Nat. Commun. 7, 13616. doi:10.1038/ncomms13616
Khalil, A. M., Guttman, M., Huarte, M., Garber, M., Raj, A., Rivea Morales, D., et al. (2009). Many Human Large Intergenic Noncoding RNAs Associate with Chromatin-Modifying Complexes and Affect Gene Expression. Proc. Natl. Acad. Sci. U S A. 106 (28), 11667–11672. doi:10.1073/pnas.0904715106
Khemlina, G., Ikeda, S., and Kurzrock, R. (2017). The Biology of Hepatocellular Carcinoma: Implications for Genomic and Immune Therapies. Mol. Cancer 16 (1), 149. doi:10.1186/s12943-017-0712-x
Khomich, O., Ivanov, A. V., and Bartosch, B. (2019). Metabolic Hallmarks of Hepatic Stellate Cells in Liver Fibrosis. Cells 9 (1). doi:10.3390/cells9010024
Kim, J., Piao, H. L., Kim, B. J., Yao, F., Han, Z., Wang, Y., et al. (2018). Long Noncoding RNA MALAT1 Suppresses Breast Cancer Metastasis. Nat. Genet. 50 (12), 1705–1715. doi:10.1038/s41588-018-0252-3
Kim, M. H., Seong, J. B., Huh, J. W., Bae, Y. C., Lee, H. S., and Lee, D. S. (2020a). Peroxiredoxin 5 Ameliorates Obesity-Induced Non-alcoholic Fatty Liver Disease through the Regulation of Oxidative Stress and AMP-Activated Protein Kinase Signaling. Redox Biol. 28, 101315. doi:10.1016/j.redox.2019.101315
Kim, Y. A., Park, K. K., and Lee, S. J. (2020b). LncRNAs Act as a Link between Chronic Liver Disease and Hepatocellular Carcinoma. Int. J. Mol. Sci. 21 (8), 2883. doi:10.3390/ijms21082883
Kitagawa, M., Kitagawa, K., Kotake, Y., Niida, H., and Ohhata, T. (2013). Cell Cycle Regulation by Long Non-coding RNAs. Cell Mol Life Sci 70 (24), 4785–4794. doi:10.1007/s00018-013-1423-0
Klec, C., Gutschner, T., Panzitt, K., and Pichler, M. (2019). Involvement of Long Non-coding RNA HULC (Highly Up-Regulated in Liver Cancer) in Pathogenesis and Implications for Therapeutic Intervention. Expert Opin. Ther. Targets 23 (3), 177–186. doi:10.1080/14728222.2019.1570499
Konermann, S., Brigham, M. D., Trevino, A. E., Joung, J., Abudayyeh, O. O., Barcena, C., et al. (2015). Genome-scale Transcriptional Activation by an Engineered CRISPR-Cas9 Complex. Nature 517 (7536), 583–588. doi:10.1038/nature14136
Kong, D., Zhang, Z., Chen, L., Huang, W., Zhang, F., Wang, L., et al. (2020). Curcumin Blunts Epithelial-Mesenchymal Transition of Hepatocytes to Alleviate Hepatic Fibrosis through Regulating Oxidative Stress and Autophagy. Redox Biol. 36, 101600. doi:10.1016/j.redox.2020.101600
Kong, X., Qian, X., Duan, L., Liu, H., Zhu, Y., and Qi, J. (2016). microRNA-372 Suppresses Migration and Invasion by Targeting P65 in Human Prostate Cancer Cells. DNA Cel Biol 35 (12), 828–835. doi:10.1089/dna.2015.3186
Kopp, F., and Mendell, J. T. (2018). Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 172 (3), 393–407. doi:10.1016/j.cell.2018.01.011
Kovall, R. A., Gebelein, B., Sprinzak, D., and Kopan, R. (2017). The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev. Cel 41 (3), 228–241. doi:10.1016/j.devcel.2017.04.001
Krizhanovsky, V., Yon, M., Dickins, R. A., Hearn, S., Simon, J., Miething, C., et al. (2008). Senescence of Activated Stellate Cells Limits Liver Fibrosis. Cell 134 (4), 657–667. doi:10.1016/j.cell.2008.06.049
Lam, P., Cheung, F., Tan, H. Y., Wang, N., Yuen, M. F., and Feng, Y. (2016). Hepatoprotective Effects of Chinese Medicinal Herbs: A Focus on Anti-inflammatory and Anti-oxidative Activities. Int. J. Mol. Sci. 17 (4), 465. doi:10.3390/ijms17040465
Lan, T., Li, C., Yang, G., Sun, Y., Zhuang, L., Ou, Y., et al. (2018). Sphingosine Kinase 1 Promotes Liver Fibrosis by Preventing miR-19b-3p-Mediated Inhibition of CCR2. Hepatology 68 (3), 1070–1086. doi:10.1002/hep.29885
Lee, J. J., Loh, K., and Yap, Y. S. (2015a). PI3K/Akt/mTOR Inhibitors in Breast Cancer. Cancer Biol. Med. 12 (4), 342–354. doi:10.7497/j.issn.2095-3941.2015.0089
Lee, Y. A., Wallace, M. C., and Friedman, S. L. (2015b). Pathobiology of Liver Fibrosis: a Translational success story. Gut 64 (5), 830–841. doi:10.1136/gutjnl-2014-306842
Leti, F., Legendre, C., Still, C. D., Chu, X., Petrick, A., Gerhard, G. S., et al. (2017). Altered Expression of MALAT1 lncRNA in Nonalcoholic Steatohepatitis Fibrosis Regulates CXCL5 in Hepatic Stellate Cells. Transl Res. 190, 25–e21. doi:10.1016/j.trsl.2017.09.001
Li, J., Yang, C., Li, Y., Chen, A., Li, L., and You, Z. (2018a). LncRNA GAS5 Suppresses Ovarian Cancer by Inducing Inflammasome Formation. Biosci. Rep. 38 (2). doi:10.1042/BSR20171150
Li, J. H., Liu, S., Zhou, H., Qu, L. H., and Yang, J. H. (2014). starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and Protein-RNA Interaction Networks from Large-Scale CLIP-Seq Data. Nucleic Acids Res. 42, D92–D97. Database issue. doi:10.1093/nar/gkt1248
Li, T., Mo, X., Fu, L., Xiao, B., and Guo, J. (2016). Molecular Mechanisms of Long Noncoding RNAs on Gastric Cancer. Oncotarget 7 (8), 8601–8612. doi:10.18632/oncotarget.6926
Li, X., Liu, R., Huang, Z., Gurley, E. C., Wang, X., Wang, J., et al. (2018b). Cholangiocyte-derived Exosomal Long Noncoding RNA H19 Promotes Cholestatic Liver Injury in Mouse and Humans. Hepatology 68 (2), 599–615. doi:10.1002/hep.29838
Li, Y., Zhu, J., Tian, G., Li, N., Li, Q., Ye, M., et al. (2010). The DNA Methylome of Human Peripheral Blood Mononuclear Cells. Plos Biol. 8 (11), e1000533. doi:10.1371/journal.pbio.1000533
Li, Z., Wang, J., Zeng, Q., Hu, C., Zhang, J., Wang, H., et al. (2018c). Long Noncoding RNA HOTTIP Promotes Mouse Hepatic Stellate Cell Activation via Downregulating miR-148a. Cel Physiol Biochem 51 (6), 2814–2828. doi:10.1159/000496012
Lin, N., Yao, Z., Xu, M., Chen, J., Lu, Y., Yuan, L., et al. (2019). Long Noncoding RNA MALAT1 Potentiates Growth and Inhibits Senescence by Antagonizing ABI3BP in Gallbladder Cancer Cells. J. Exp. Clin. Cancer Res. 38 (1), 244. doi:10.1186/s13046-019-1237-5
Lin, S. P., Youngson, N., Takada, S., Seitz, H., Reik, W., Paulsen, M., et al. (2003). Asymmetric Regulation of Imprinting on the Maternal and Paternal Chromosomes at the Dlk1-Gtl2 Imprinted Cluster on Mouse Chromosome 12. Nat. Genet. 35 (1), 97–102. doi:10.1038/ng1233
Liu, C., Yang, Z., Wu, J., Zhang, L., Lee, S., Shin, D. J., et al. (2018). Long Noncoding RNA H19 Interacts with Polypyrimidine Tract-Binding Protein 1 to Reprogram Hepatic Lipid Homeostasis. Hepatology 67 (5), 1768–1783. doi:10.1002/hep.29654
Liu, R., Li, X., Zhu, W., Wang, Y., Zhao, D., Wang, X., et al. (2019). Cholangiocyte-Derived Exosomal Long Noncoding RNA H19 Promotes Hepatic Stellate Cell Activation and Cholestatic Liver Fibrosis. Hepatology 70 (4), 1317–1335. doi:10.1002/hep.30662
Luedde, T., and Schwabe, R. F. (2011). NF-kappaB in the Liver-Llinking Injury, Fibrosis, and Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 8 (2), 108–118. doi:10.1038/nrgastro.2010.213
Luo, N., Li, J., Chen, Y., Xu, Y., Wei, Y., Lu, J., et al. (2021). Hepatic Stellate Cell Reprogramming via Exosome-Mediated CRISPR/dCas9-VP64 Delivery. Drug Deliv. 28 (1), 10–18. doi:10.1080/10717544.2020.1850917
Ma, Y., Liu, L., Yan, F., Wei, W., Deng, J., and Sun, J. (2016a). Enhanced Expression of Long Non-coding RNA NEAT1 Is Associated with the Progression of Gastric Adenocarcinomas. World J. Surg. Oncol. 14 (1), 41. doi:10.1186/s12957-016-0799-3
Ma, Y., Yang, Y., Wang, F., Moyer, M. P., Wei, Q., Zhang, P., et al. (2016b). Long Non-coding RNA CCAL Regulates Colorectal Cancer Progression by Activating Wnt/β-Catenin Signalling Pathway via Suppression of Activator Protein 2α. Gut 65 (9), 1494–1504. doi:10.1136/gutjnl-2014-308392
Ma, Y., Zhang, J., Wen, L., and Lin, A. (2018). Membrane-lipid Associated lncRNA: A New Regulator in Cancer Signaling. Cancer Lett. 419, 27–29. doi:10.1016/j.canlet.2018.01.008
Mahpour, A., and Mullen, A. C. (2021). Our Emerging Understanding of the Roles of Long Non-coding RNAs in normal Liver Function, Disease, and Malignancy. JHEP Rep. 3 (1), 100177. doi:10.1016/j.jhepr.2020.100177
Mayr, B., and Montminy, M. (2001). Transcriptional Regulation by the Phosphorylation-dependent Factor CREB. Nat. Rev. Mol. Cel Biol 2 (8), 599–609. doi:10.1038/35085068
McMahon, B. J. (2009). The Natural History of Chronic Hepatitis B Virus Infection. Hepatology 49 (5 Suppl. l), S45–S55. doi:10.1002/hep.22898
Mercer, T. R., Dinger, M. E., Sunkin, S. M., Mehler, M. F., and Mattick, J. S. (2008). Specific Expression of Long Noncoding RNAs in the Mouse Brain. Proc. Natl. Acad. Sci. U S A. 105 (2), 716–721. doi:10.1073/pnas.0706729105
Meurette, O., and Mehlen, P. (2018). Notch Signaling in the Tumor Microenvironment. Cancer Cell 34 (4), 536–548. doi:10.1016/j.ccell.2018.07.009
Mihaylova, M. M., and Shaw, R. J. (2011). The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cel Biol 13 (9), 1016–1023. doi:10.1038/ncb2329
Mo, Z., Cheong, J. Y. A., Xiang, L., Le, M. T. N., Grimson, A., and Zhang, D. X. (2021). Extracellular Vesicle-Associated Organotropic Metastasis. Cell Prolif 54 (1), e12948. doi:10.1111/cpr.12948
Mondal, T., Subhash, S., Vaid, R., Enroth, S., Uday, S., Reinius, B., et al. (2015). MEG3 Long Noncoding RNA Regulates the TGF-β Pathway Genes through Formation of RNA-DNA Triplex Structures. Nat. Commun. 6, 7743. doi:10.1038/ncomms8743
Monti-Rocha, R., Cramer, A., Gaio Leite, P., Antunes, M. M., Pereira, R. V. S., Barroso, A., et al. (2018). SOCS2 Is Critical for the Balancing of Immune Response and Oxidate Stress Protecting against Acetaminophen-Induced Acute Liver Injury. Front. Immunol. 9, 3134. doi:10.3389/fimmu.2018.03134
Mori, M. A., Ludwig, R. G., Garcia-Martin, R., Brandão, B. B., and Kahn, C. R. (2019). Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cel Metab 30 (4), 656–673. doi:10.1016/j.cmet.2019.07.011
Murphy, S. K., Wylie, A. A., Coveler, K. J., Cotter, P. D., Papenhausen, P. R., Sutton, V. R., et al. (2003). Epigenetic Detection of Human Chromosome 14 Uniparental Disomy. Hum. Mutat. 22 (1), 92–97. doi:10.1002/humu.10237
Ni, W., Yao, S., Zhou, Y., Liu, Y., Huang, P., Zhou, A., et al. (2019). Long Noncoding RNA GAS5 Inhibits Progression of Colorectal Cancer by Interacting with and Triggering YAP Phosphorylation and Degradation and Is Negatively Regulated by the m6A Reader YTHDF3. Mol. Cancer 18 (1), 143. doi:10.1186/s12943-019-1079-y
Nielsen, C. P., Jernigan, K. K., Diggins, N. L., Webb, D. J., and MacGurn, J. A. (2019). USP9X Deubiquitylates DVL2 to Regulate WNT Pathway Specification. Cel Rep 28 (4), 1074–e5. e1075. doi:10.1016/j.celrep.2019.06.083
Niland, C. N., Merry, C. R., and Khalil, A. M. (2012). Emerging Roles for Long Non-coding RNAs in Cancer and Neurological Disorders. Front. Genet. 3, 25. doi:10.3389/fgene.2012.00025
Nowell, C. S., and Radtke, F. (2017). Notch as a Tumour Suppressor. Nat. Rev. Cancer 17 (3), 145–159. doi:10.1038/nrc.2016.145
Nudelman, R., Ardon, O., Hadar, Y., Chen, Y., Libman, J., and Shanzer, A. (1998). Modular Fluorescent-Labeled Siderophore Analogues. J. Med. Chem. 41 (10), 1671–1678. doi:10.1021/jm970581b
Özgür, E., Mert, U., Isin, M., Okutan, M., Dalay, N., and Gezer, U. (2013). Differential Expression of Long Non-coding RNAs during Genotoxic Stress-Induced Apoptosis in HeLa and MCF-7 Cells. Clin. Exp. Med. 13 (2), 119–126. doi:10.1007/s10238-012-0181-x
Page, A., Paoli, P., Moran Salvador, E., White, S., French, J., and Mann, J. (2016). Hepatic Stellate Cell Transdifferentiation Involves Genome-wide Remodeling of the DNA Methylation Landscape. J. Hepatol. 64 (3), 661–673. doi:10.1016/j.jhep.2015.11.024
Panzitt, K., Tschernatsch, M. M., Guelly, C., Moustafa, T., Stradner, M., Strohmaier, H. M., et al. (2007). Characterization of HULC, a Novel Gene with Striking Up-Regulation in Hepatocellular Carcinoma, as Noncoding RNA. Gastroenterology 132 (1), 330–342. doi:10.1053/j.gastro.2006.08.026
Parasramka, M. A., Maji, S., Matsuda, A., Yan, I. K., and Patel, T. (2016). Long Non-coding RNAs as Novel Targets for Therapy in Hepatocellular Carcinoma. Pharmacol. Ther. 161, 67–78. doi:10.1016/j.pharmthera.2016.03.004
Parola, M., and Pinzani, M. (2019). Liver Fibrosis: Pathophysiology, Pathogenetic Targets and Clinical Issues. Mol. Aspects Med. 65, 37–55. doi:10.1016/j.mam.2018.09.002
Peng, H., Wan, L. Y., Liang, J. J., Zhang, Y. Q., Ai, W. B., and Wu, J. F. (2018). The Roles of lncRNA in Hepatic Fibrosis. Cell Biosci 8, 63. doi:10.1186/s13578-018-0259-6
Peng, W. X., Koirala, P., and Mo, Y. Y. (2017). LncRNA-mediated Regulation of Cell Signaling in Cancer. Oncogene 36 (41), 5661–5667. doi:10.1038/onc.2017.184
Qi, X., Zhang, D. H., Wu, N., Xiao, J. H., Wang, X., and Ma, W. (2015). ceRNA in Cancer: Possible Functions and Clinical Implications. J. Med. Genet. 52 (10), 710–718. doi:10.1136/jmedgenet-2015-103334
Quagliata, L., Matter, M. S., Piscuoglio, S., Arabi, L., Ruiz, C., Procino, A., et al. (2014). Long Noncoding RNA HOTTIP/HOXA13 Expression Is Associated with Disease Progression and Predicts Outcome in Hepatocellular Carcinoma Patients. Hepatology 59 (3), 911–923. doi:10.1002/hep.26740
Raveh, E., Matouk, I. J., Gilon, M., and Hochberg, A. (2015). The H19 Long Non-coding RNA in Cancer Initiation, Progression and Metastasis - a Proposed Unifying Theory. Mol. Cancer 14, 184. doi:10.1186/s12943-015-0458-2
Ray, K. (2014). Liver: Hepatic Stellate Cells Hold the Key to Liver Fibrosis. Nat. Rev. Gastroenterol. Hepatol. 11 (2), 74. doi:10.1038/nrgastro.2013.244
Riaz, F., and Li, D. (2019). Non-coding RNA Associated Competitive Endogenous RNA Regulatory Network: Novel Therapeutic Approach in Liver Fibrosis. Curr. Gene Ther. 19 (5), 305–317. doi:10.2174/1566523219666191107113046
Rockey, D. C., Caldwell, S. H., Goodman, Z. D., Nelson, R. C., and Smith, A. D.American Association for the Study of Liver Diseases (2009). Liver Biopsy. Hepatology 49 (3), 1017–1044. doi:10.1002/hep.22742
Roderburg, C., Urban, G. W., Bettermann, K., Vucur, M., Zimmermann, H., Schmidt, S., et al. (2011). Micro-RNA Profiling Reveals a Role for miR-29 in Human and Murine Liver Fibrosis. Hepatology 53 (1), 209–218. doi:10.1002/hep.23922
Roehlen, N., Crouchet, E., and Baumert, T. F. (2020). Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 9 (4), 875. doi:10.3390/cells9040875
Salmena, L., Poliseno, L., Tay, Y., Kats, L., and Pandolfi, P. P. (2011). A ceRNA Hypothesis: the Rosetta Stone of a Hidden RNA Language? Cell 146 (3), 353–358. doi:10.1016/j.cell.2011.07.014
Schunk, S. J., Floege, J., Fliser, D., and Speer, T. (2021). WNT-β-catenin Signalling - a Versatile Player in Kidney Injury and Repair. Nat. Rev. Nephrol. 17 (3), 172–184. doi:10.1038/s41581-020-00343-w
Sebio, A., Kahn, M., and Lenz, H. J. (2014). The Potential of Targeting Wnt/β-Catenin in colon Cancer. Expert Opin. Ther. Targets 18 (6), 611–615. doi:10.1517/14728222.2014.906580
Shackelford, D. B., and Shaw, R. J. (2009). The LKB1-AMPK Pathway: Metabolism and Growth Control in Tumour Suppression. Nat. Rev. Cancer 9 (8), 563–575. doi:10.1038/nrc2676
Sharma, G., Sharma, A. R., Bhattacharya, M., Lee, S. S., and Chakraborty, C. (2021). CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases. Mol. Ther. 29 (2), 571–586. doi:10.1016/j.ymthe.2020.09.028
Shen, M., Dong, C., Ruan, X., Yan, W., Cao, M., Pizzo, D., et al. (2019a). Chemotherapy-Induced Extracellular Vesicle MiRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer Res. 79 (14), 3608–3621. doi:10.1158/0008-5472.CAN-18-4055
Shen, X., Guo, H., Xu, J., and Wang, J. (2019b). Inhibition of lncRNA HULC Improves Hepatic Fibrosis and Hepatocyte Apoptosis by Inhibiting the MAPK Signaling Pathway in Rats with Nonalcoholic Fatty Liver Disease. J. Cel Physiol 234 (10), 18169–18179. doi:10.1002/jcp.28450
Shi, X., Jiang, X., Yuan, B., Liu, T., Tang, Y., Che, Y., et al. (2019). LINC01093 Upregulation Protects against Alcoholic Hepatitis through Inhibition of NF-Κb Signaling Pathway. Mol. Ther. Nucleic Acids 17, 791–803. doi:10.1016/j.omtn.2019.06.018
Song, Y., Liu, C., Liu, X., Trottier, J., Beaudoin, M., Zhang, L., et al. (2017). H19 Promotes Cholestatic Liver Fibrosis by Preventing ZEB1-Mediated Inhibition of Epithelial Cell Adhesion Molecule. Hepatology 66 (4), 1183–1196. doi:10.1002/hep.29209
Strotskaya, A., Savitskaya, E., Metlitskaya, A., Morozova, N., Datsenko, K. A., Semenova, E., et al. (2017). The Action of Escherichia coli CRISPR-Cas System on Lytic Bacteriophages with Different Lifestyles and Development Strategies. Nucleic Acids Res. 45 (4), 1946–1957. doi:10.1093/nar/gkx042
Sun, D., Yu, Z., Fang, X., Liu, M., Pu, Y., Shao, Q., et al. (2017). LncRNA GAS5 Inhibits Microglial M2 Polarization and Exacerbates Demyelination. EMBO Rep. 18 (10), 1801–1816. doi:10.15252/embr.201643668
Tacke, F., and Trautwein, C. (2015). Mechanisms of Liver Fibrosis Resolution. J. Hepatol. 63 (4), 1038–1039. doi:10.1016/j.jhep.2015.03.039
Tang, S. S., Zheng, B. Y., and Xiong, X. D. (2015). LincRNA-p21: Implications in Human Diseases. Int. J. Mol. Sci. 16 (8), 18732–18740. doi:10.3390/ijms160818732
Tay, Y., Kats, L., Salmena, L., Weiss, D., Tan, S. M., Ala, U., et al. (2011). Coding-independent Regulation of the Tumor Suppressor PTEN by Competing Endogenous mRNAs. Cell 147 (2), 344–357. doi:10.1016/j.cell.2011.09.029
Tay, Y., Rinn, J., and Pandolfi, P. P. (2014). The Multilayered Complexity of ceRNA Crosstalk and Competition. Nature 505 (7483), 344–352. doi:10.1038/nature12986
Thomson, D. W., and Dinger, M. E. (2016). Endogenous microRNA Sponges: Evidence and Controversy. Nat. Rev. Genet. 17 (5), 272–283. doi:10.1038/nrg.2016.20
Tian, X., and Xu, G. (2015). Clinical Value of lncRNA MALAT1 as a Prognostic Marker in Human Cancer: Systematic Review and Meta-Analysis. BMJ Open 5 (9), e008653. doi:10.1136/bmjopen-2015-008653
Tierling, S., Dalbert, S., Schoppenhorst, S., Tsai, C. E., Oliger, S., Ferguson-Smith, A. C., et al. (2006). High-resolution Map and Imprinting Analysis of the Gtl2-Dnchc1 Domain on Mouse Chromosome 12. Genomics 87 (2), 225–235. doi:10.1016/j.ygeno.2005.09.018
Tsang, F. H., Au, S. L., Wei, L., Fan, D. N., Lee, J. M., Wong, C. C., et al. (2015). Long Non-coding RNA HOTTIP Is Frequently Up-Regulated in Hepatocellular Carcinoma and Is Targeted by Tumour Suppressive miR-125b. Liver Int. 35 (5), 1597–1606. doi:10.1111/liv.12746
Tu, X., Zhang, Y., Zheng, X., Deng, J., Li, H., Kang, Z., et al. (2017). TGF-β-induced Hepatocyte lincRNA-P21 Contributes to Liver Fibrosis in Mice. Sci. Rep. 7 (1), 2957. doi:10.1038/s41598-017-03175-0
Unfried, J. P., and Fortes, P. (2020). LncRNAs in HCV Infection and HCV-Related Liver Disease. Int. J. Mol. Sci. 21 (6). doi:10.3390/ijms21062255
van Kruijsbergen, I., Hontelez, S., and Veenstra, G. J. (2015). Recruiting Polycomb to Chromatin. Int. J. Biochem. Cel Biol 67, 177–187. doi:10.1016/j.biocel.2015.05.006
van Niel, G., D'Angelo, G., and Raposo, G. (2018). Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cel Biol 19 (4), 213–228. doi:10.1038/nrm.2017.125
Voce, D. J., Bernal, G. M., Wu, L., Crawley, C. D., Zhang, W., Mansour, N. M., et al. (2019). Temozolomide Treatment Induces lncRNA MALAT1 in an NF-Κb and P53 Codependent Manner in Glioblastoma. Cancer Res. 79 (10), 2536–2548. doi:10.1158/0008-5472.CAN-18-2170
Walker, S., Busatto, S., Pham, A., Tian, M., Suh, A., Carson, K., et al. (2019). Extracellular Vesicle-Based Drug Delivery Systems for Cancer Treatment. Theranostics 9 (26), 8001–8017. doi:10.7150/thno.37097
Wang, F., Yuan, J. H., Wang, S. B., Yang, F., Yuan, S. X., Ye, C., et al. (2014). Oncofetal Long Noncoding RNA PVT1 Promotes Proliferation and Stem Cell-like Property of Hepatocellular Carcinoma Cells by Stabilizing NOP2. Hepatology 60 (4), 1278–1290. doi:10.1002/hep.27239
Wang, J., Liu, X., Wu, H., Ni, P., Gu, Z., Qiao, Y., et al. (2010). CREB Up-Regulates Long Non-coding RNA, HULC Expression through Interaction with microRNA-372 in Liver Cancer. Nucleic Acids Res. 38 (16), 5366–5383. doi:10.1093/nar/gkq285
Wang, J., Sun, J., and Yang, F. (2020a). The Role of Long Non-coding RNA H19 in Breast Cancer. Oncol. Lett. 19 (1), 7–16. doi:10.3892/ol.2019.11093
Wang, K. C., Yang, Y. W., Liu, B., Sanyal, A., Corces-Zimmerman, R., Chen, Y., et al. (2011). A Long Noncoding RNA Maintains Active Chromatin to Coordinate Homeotic Gene Expression. Nature 472 (7341), 120–124. doi:10.1038/nature09819
Wang, L. X., Wan, C., Dong, Z. B., Wang, B. H., Liu, H. Y., and Li, Y. (2019a). Integrative Analysis of Long Noncoding RNA (lncRNA), microRNA (miRNA) and mRNA Expression and Construction of a Competing Endogenous RNA (ceRNA) Network in Metastatic Melanoma. Med. Sci. Monit. 25, 2896–2907. doi:10.12659/MSM.913881
Wang, P., Wu, T., Zhou, H., Jin, Q., He, G., Yu, H., et al. (2016). Long Noncoding RNA NEAT1 Promotes Laryngeal Squamous Cell Cancer through Regulating miR-107/CDK6 Pathway. J. Exp. Clin. Cancer Res. 35, 22. doi:10.1186/s13046-016-0297-z
Wang, T., Fu, X., Jin, T., Zhang, L., Liu, B., Wu, Y., et al. (2019b). Aspirin Targets P4HA2 through Inhibiting NF-Κb and LMCD1-AS1/let-7g to Inhibit Tumour Growth and Collagen Deposition in Hepatocellular Carcinoma. EBioMedicine 45, 168–180. doi:10.1016/j.ebiom.2019.06.048
Wang, X., Ruan, Y., Wang, X., Zhao, W., Jiang, Q., Jiang, C., et al. (2017). Long Intragenic Non-coding RNA lincRNA-P21 Suppresses Development of Human Prostate Cancer. Cel Prolif 50 (2). doi:10.1111/cpr.12318
Wang, Z., Yang, X., Kai, J., Wang, F., Wang, Z., Shao, J., et al. (2020b). HIF-1α-upregulated lncRNA-H19 Regulates Lipid Droplet Metabolism through the AMPKα Pathway in Hepatic Stellate Cells. Life Sci. 255, 117818. doi:10.1016/j.lfs.2020.117818
Wang, Z. M., Xia, S. W., Zhang, T., Wang, Z. Y., Yang, X., Kai, J., et al. (2020c). LncRNA-H19 Induces Hepatic Stellate Cell Activation via Upregulating Alcohol Dehydrogenase III-Mediated Retinoic Acid Signals. Int. Immunopharmacol 84, 106470. doi:10.1016/j.intimp.2020.106470
Wei, L. Q., Li, L., Lu, C., Liu, J., Chen, Y., and Wu, H. (2019a). Involvement of H19/miR-326 axis in Hepatocellular Carcinoma Development through Modulating TWIST1. J. Cel Physiol 234 (4), 5153–5162. doi:10.1002/jcp.27319
Wei, S., Wang, K., Huang, X., Zhao, Z., and Zhao, Z. (2019b). LncRNA MALAT1 Contributes to Non-small Cell Lung Cancer Progression via Modulating miR-200a-3p/programmed Death-Ligand 1 axis. Int. J. Immunopathol Pharmacol. 33, 2058738419859699. doi:10.1177/2058738419859699
Wilusz, C. J., and Wilusz, J. (2012). HuR and Translation-Tthe Missing Linc(RNA). Mol. Cel 47 (4), 495–496. doi:10.1016/j.molcel.2012.08.005
Wilusz, J. E., Freier, S. M., and Spector, D. L. (2008). 3' End Processing of a Long Nuclear-Retained Noncoding RNA Yields a tRNA-like Cytoplasmic RNA. Cell 135 (5), 919–932. doi:10.1016/j.cell.2008.10.012
Wilusz, J. E. (2016). Long Noncoding RNAs: Re-writing Dogmas of RNA Processing and Stability. Biochim. Biophys. Acta 1859 (1), 128–138. doi:10.1016/j.bbagrm.2015.06.003
Wirawan, E., Lippens, S., Vanden Berghe, T., Romagnoli, A., Fimia, G. M., Piacentini, M., et al. (2012). Beclin1: a Role in Membrane Dynamics and beyond. Autophagy 8 (1), 6–17. doi:10.4161/auto.8.1.16645
Wu, F., Sui, Y., Wang, Y., Xu, T., Fan, L., and Zhu, H. (2020a). Long Noncoding RNA SNHG7, a Molecular Sponge for microRNA-485, Promotes the Aggressive Behavior of Cervical Cancer by Regulating PAK4. Onco Targets Ther. 13, 685–699. doi:10.2147/OTT.S232542
Wu, G., Cai, J., Han, Y., Chen, J., Huang, Z. P., Chen, C., et al. (2014). LincRNA-p21 Regulates Neointima Formation, Vascular Smooth Muscle Cell Proliferation, Apoptosis, and Atherosclerosis by Enhancing P53 Activity. Circulation 130 (17), 1452–1465. doi:10.1161/CIRCULATIONAHA.114.011675
Wu, X., Yuan, Y., Ma, R., Xu, B., and Zhang, R. (2020b). lncRNA SNHG7 Affects Malignant Tumor Behaviors through Downregulation of EZH2 in Uveal Melanoma Cell Lines. Oncol. Lett. 19 (2), 1505–1515. doi:10.3892/ol.2019.11240
Wu, Y., Liu, X., Zhou, Q., Huang, C., Meng, X., Xu, F., et al. (2015). Silent Information Regulator 1 (SIRT1) Ameliorates Liver Fibrosis via Promoting Activated Stellate Cell Apoptosis and Reversion. Toxicol. Appl. Pharmacol. 289 (2), 163–176. doi:10.1016/j.taap.2015.09.028
Wylie, A. A., Murphy, S. K., Orton, T. C., and Jirtle, R. L. (2000). Novel Imprinted DLK1/GTL2 Domain on Human Chromosome 14 Contains Motifs that Mimic Those Implicated in IGF2/H19 Regulation. Genome Res. 10 (11), 1711–1718. doi:10.1101/gr.161600
Xia, Q., Li, J., Yang, Z., Zhang, D., Tian, J., and Gu, B. (2020). Long Non-coding RNA Small Nucleolar RNA Host Gene 7 Expression Level in Prostate Cancer Tissues Predicts the Prognosis of Patients with Prostate Cancer. Medicine (Baltimore) 99 (7), e18993. doi:10.1097/MD.0000000000018993
Xiao, Y., Liu, R., Li, X., Gurley, E. C., Hylemon, P. B., Lu, Y., et al. (2019). Long Noncoding RNA H19 Contributes to Cholangiocyte Proliferation and Cholestatic Liver Fibrosis in Biliary Atresia. Hepatology 70 (5), 1658–1673. doi:10.1002/hep.30698
Xie, J. J., Li, W. H., Li, X., Ye, W., and Shao, C. F. (2019). LncRNA MALAT1 Promotes Colorectal Cancer Development by Sponging miR-363-3p to Regulate EZH2 Expression. J. Biol. Regul. Homeost Agents 33 (2), 331–343.
Xie, Z., Wu, Y., Liu, S., Lai, Y., and Tang, S. (2021). LncRNA-SNHG7/miR-29b/DNMT3A axis Affects Activation, Autophagy and Proliferation of Hepatic Stellate Cells in Liver Fibrosis. Clin. Res. Hepatol. Gastroenterol. 45 (2), 101469. doi:10.1016/j.clinre.2020.05.017
Xin, X., Wu, M., Meng, Q., Wang, C., Lu, Y., Yang, Y., et al. (2018). Long Noncoding RNA HULC Accelerates Liver Cancer by Inhibiting PTEN via Autophagy Cooperation to miR15a. Mol. Cancer 17 (1), 94. doi:10.1186/s12943-018-0843-8
Xiong, D., Sheng, Y., Ding, S., Chen, J., Tan, X., Zeng, T., et al. (2016). LINC00052 Regulates the Expression of NTRK3 by miR-128 and miR-485-3p to Strengthen HCC Cells Invasion and Migration. Oncotarget 7 (30), 47593–47608. doi:10.18632/oncotarget.10250
Xu, R., Greening, D. W., Zhu, H. J., Takahashi, N., and Simpson, R. J. (2016). Extracellular Vesicle Isolation and Characterization: toward Clinical Application. J. Clin. Invest. 126 (4), 1152–1162. doi:10.1172/JCI81129
Yang, J., Kantrow, S., Sai, J., Hawkins, O. E., Boothby, M., Ayers, G. D., et al. (2012). INK4a/ARF [corrected] Inactivation with Activation of the NF-κB/IL-6 Pathway Is Sufficient to Drive the Development and Growth of Angiosarcoma. Cancer Res. 72 (18), 4682–4695. doi:10.1158/0008-5472.CAN-12-0440
Yang, J., Meng, X., Pan, J., Jiang, N., Zhou, C., Wu, Z., et al. (2018). CRISPR/Cas9-mediated Noncoding RNA Editing in Human Cancers. RNA Biol. 15 (1), 35–43. doi:10.1080/15476286.2017.1391443
Yang, J. J., Tao, H., and Li, J. (2014). Hedgehog Signaling Pathway as Key Player in Liver Fibrosis: New Insights and Perspectives. Expert Opin. Ther. Targets 18 (9), 1011–1021. doi:10.1517/14728222.2014.927443
Yang, J. J., Yang, Y., Zhang, C., Li, J., and Yang, Y. (2020a). Epigenetic Silencing of LncRNA ANRIL Enhances Liver Fibrosis and HSC Activation through Activating AMPK Pathway. J. Cel Mol Med 24 (4), 2677–2687. doi:10.1111/jcmm.14987
Yang, L., Fu, W. L., Zhu, Y., and Wang, X. G. (2020b). Tβ4 Suppresses lincRNA-P21-Mediated Hepatic Apoptosis and Fibrosis by Inhibiting PI3K-AKT-NF-Κb Pathway. Gene 758, 144946. doi:10.1016/j.gene.2020.144946
Yang, X., Xie, Z., Lei, X., and Gan, R. (2020c). Long Non-coding RNA GAS5 in Human Cancer. Oncol. Lett. 20 (3), 2587–2594. doi:10.3892/ol.2020.11809
Yang, Z., Jiang, S., Shang, J., Jiang, Y., Dai, Y., Xu, B., et al. (2019). LncRNA: Shedding Light on Mechanisms and Opportunities in Fibrosis and Aging. Ageing Res. Rev. 52, 17–31. doi:10.1016/j.arr.2019.04.001
Ye, J., Lin, Y., Yu, Y., and Sun, D. (2020). LncRNA NEAT1/microRNA-129-5p/SOCS2 axis Regulates Liver Fibrosis in Alcoholic Steatohepatitis. J. Transl Med. 18 (1), 445. doi:10.1186/s12967-020-02577-5
Yin, C., Evason, K. J., Asahina, K., and Stainier, D. Y. (2013). Hepatic Stellate Cells in Liver Development, Regeneration, and Cancer. J. Clin. Invest. 123 (5), 1902–1910. doi:10.1172/JCI66369
Yoon, J. H., Abdelmohsen, K., Srikantan, S., Yang, X., Martindale, J. L., De, S., et al. (2012a). LincRNA-p21 Suppresses Target mRNA Translation. Mol. Cel 47 (4), 648–655. doi:10.1016/j.molcel.2012.06.027
Yoon, J. H., Srikantan, S., and Gorospe, M. (2012b). MS2-TRAP (MS2-tagged RNA Affinity Purification): Tagging RNA to Identify Associated miRNAs. Methods 58 (2), 81–87. doi:10.1016/j.ymeth.2012.07.004
Yoshimura, H., Matsuda, Y., Yamamoto, M., Kamiya, S., and Ishiwata, T. (2018). Expression and Role of Long Non-coding RNA H19 in Carcinogenesis. Front. Biosci. (Landmark Ed. 23, 614–625. doi:10.2741/4608
Young, T. L., Matsuda, T., and Cepko, C. L. (2005). The Noncoding RNA Taurine Upregulated Gene 1 Is Required for Differentiation of the Murine Retina. Curr. Biol. 15 (6), 501–512. doi:10.1016/j.cub.2005.02.027
Yu, F., Dong, P., Mao, Y., Zhao, B., Huang, Z., and Zheng, J. (2019). Loss of lncRNA-SNHG7 Promotes the Suppression of Hepatic Stellate Cell Activation via miR-378a-3p and DVL2. Mol. Ther. Nucleic Acids 17, 235–244. doi:10.1016/j.omtn.2019.05.026
Yu, F., Geng, W., Dong, P., Huang, Z., and Zheng, J. (2018). LncRNA-MEG3 Inhibits Activation of Hepatic Stellate Cells through SMO Protein and miR-212. Cel Death Dis 9 (10), 1014. doi:10.1038/s41419-018-1068-x
Yu, F., Guo, Y., Chen, B., Shi, L., Dong, P., Zhou, M., et al. (2017a). LincRNA-p21 Inhibits the Wnt/β-Catenin Pathway in Activated Hepatic Stellate Cells via Sponging MicroRNA-17-5p. Cel Physiol Biochem 41 (5), 1970–1980. doi:10.1159/000472410
Yu, F., Jiang, Z., Chen, B., Dong, P., and Zheng, J. (2017b). NEAT1 Accelerates the Progression of Liver Fibrosis via Regulation of microRNA-122 and Kruppel-like Factor 6. J. Mol. Med. (Berl) 95 (11), 1191–1202. doi:10.1007/s00109-017-1586-5
Yu, F., Lu, Z., Cai, J., Huang, K., Chen, B., Li, G., et al. (2015a). MALAT1 Functions as a Competing Endogenous RNA to Mediate Rac1 Expression by Sequestering miR-101b in Liver Fibrosis. Cell Cycle 14 (24), 3885–3896. doi:10.1080/15384101.2015.1120917
Yu, F., Lu, Z., Chen, B., Dong, P., and Zheng, J. (2016). Identification of a Novel lincRNA-P21-miR-181b-PTEN Signaling Cascade in Liver Fibrosis. Mediators Inflamm. 2016, 9856538. doi:10.1155/2016/9856538
Yu, F., Zheng, J., Mao, Y., Dong, P., Lu, Z., Li, G., et al. (2015b). Long Non-coding RNA Growth Arrest-specific Transcript 5 (GAS5) Inhibits Liver Fibrogenesis through a Mechanism of Competing Endogenous RNA. J. Biol. Chem. 290 (47), 28286–28298. doi:10.1074/jbc.M115.683813
Yu, F., Zhou, G., Huang, K., Fan, X., Li, G., Chen, B., et al. (2017c). Serum lincRNA-P21 as a Potential Biomarker of Liver Fibrosis in Chronic Hepatitis B Patients. J. Viral Hepat. 24 (7), 580–588. doi:10.1111/jvh.12680
Yu, L., Wang, L., Wu, X., and Yi, H. (2021). RSPO4-CRISPR Alleviates Liver Injury and Restores Gut Microbiota in a Rat Model of Liver Fibrosis. Commun. Biol. 4 (1), 230. doi:10.1038/s42003-021-01747-5
Zhang, B., Arun, G., Mao, Y. S., Lazar, Z., Hung, G., Bhattacharjee, G., et al. (2012). The lncRNA Malat1 Is Dispensable for Mouse Development but its Transcription Plays a Cis-Regulatory Role in the Adult. Cel Rep 2 (1), 111–123. doi:10.1016/j.celrep.2012.06.003
Zhang, C. Y., Yuan, W. G., He, P., Lei, J. H., and Wang, C. X. (2016a). Liver Fibrosis and Hepatic Stellate Cells: Etiology, Pathological Hallmarks and Therapeutic Targets. World J. Gastroenterol. 22 (48), 10512–10522. doi:10.3748/wjg.v22.i48.10512
Zhang, D. M., Lin, Z. Y., Yang, Z. H., Wang, Y. Y., Wan, D., Zhong, J. L., et al. (2017a). IncRNA H19 Promotes Tongue Squamous Cell Carcinoma Progression through Beta-catenin/GSK3beta/EMT Signaling via Association with EZH2. Am. J. Transl Res. 9 (7), 3474–3486.
Zhang, H., Li, W., Gu, W., Yan, Y., Yao, X., and Zheng, J. (2019a). MALAT1 Accelerates the Development and Progression of Renal Cell Carcinoma by Decreasing the Expression of miR-203 and Promoting the Expression of BIRC5. Cel Prolif 52 (5), e12640. doi:10.1111/cpr.12640
Zhang, K., Han, X., Zhang, Z., Zheng, L., Hu, Z., Yao, Q., et al. (2017b). The Liver-Enriched Lnc-LFAR1 Promotes Liver Fibrosis by Activating TGFβ and Notch Pathways. Nat. Commun. 8 (1), 144. doi:10.1038/s41467-017-00204-4
Zhang, K., Shi, Z., Zhang, M., Dong, X., Zheng, L., Li, G., et al. (2020a). Silencing lncRNA Lfar1 Alleviates the Classical Activation and Pyoptosis of Macrophage in Hepatic Fibrosis. Cel Death Dis 11 (2), 132. doi:10.1038/s41419-020-2323-5
Zhang, K., Shi, Z. M., Chang, Y. N., Hu, Z. M., Qi, H. X., and Hong, W. (2014). The Ways of Action of Long Non-coding RNAs in Cytoplasm and Nucleus. Gene 547 (1), 1–9. doi:10.1016/j.gene.2014.06.043
Zhang, K., Zhang, M., Yao, Q., Han, X., Zhao, Y., Zheng, L., et al. (2019b). The Hepatocyte-Specifically Expressed Lnc-HSER Alleviates Hepatic Fibrosis by Inhibiting Hepatocyte Apoptosis and Epithelial-Mesenchymal Transition. Theranostics 9 (25), 7566–7582. doi:10.7150/thno.36942
Zhang, Q., Geng, P. L., Yin, P., Wang, X. L., Jia, J. P., and Yao, J. (2013). Down-regulation of Long Non-coding RNA TUG1 Inhibits Osteosarcoma Cell Proliferation and Promotes Apoptosis. Asian Pac. J. Cancer Prev. 14 (4), 2311–2315. doi:10.7314/apjcp.2013.14.4.2311
Zhang, R., Huang, X. Q., Jiang, Y. Y., Li, N., Wang, J., and Chen, S. Y. (2020b). LncRNA TUG1 Regulates Autophagy-Mediated Endothelial-Mesenchymal Transition of Liver Sinusoidal Endothelial Cells by Sponging miR-142-3p. Am. J. Transl Res. 12 (3), 758–772.
Zhang, T., Hu, J., Wang, X., Zhao, X., Li, Z., Niu, J., et al. (2019c). MicroRNA-378 Promotes Hepatic Inflammation and Fibrosis via Modulation of the NF-Κb-Tnfα Pathway. J. Hepatol. 70 (1), 87–96. doi:10.1016/j.jhep.2018.08.026
Zhang, X., Rice, K., Wang, Y., Chen, W., Zhong, Y., Nakayama, Y., et al. (2010). Maternally Expressed Gene 3 (MEG3) Noncoding Ribonucleic Acid: Isoform Structure, Expression, and Functions. Endocrinology 151 (3), 939–947. doi:10.1210/en.2009-0657
Zhang, Y., Liu, C., Barbier, O., Smalling, R., Tsuchiya, H., Lee, S., et al. (2016b). Bcl2 Is a Critical Regulator of Bile Acid Homeostasis by Dictating Shp and lncRNA H19 Function. Sci. Rep. 6, 20559. doi:10.1038/srep20559
Zhang, Y. F., Li, C. S., Zhou, Y., and Lu, X. H. (2020c). Propofol facilitates cisplatin sensitivity via lncRNA MALAT1/miR-30e/ATG5 axis through suppressing autophagy in gastric cancer. Life Sci. 244, 117280. doi:10.1016/j.lfs.2020.117280
Zhang, Y. H., Liu, J. T., Wen, B. Y., and Liu, N. (2009). Mechanisms of Inhibiting Proliferation of Vascular Smooth Muscle Cells by Serum of Rats Treated with Dahuang Zhechong Pill. J. Ethnopharmacol 124 (1), 125–129. doi:10.1016/j.jep.2009.04.012
Zhang, Z., Qian, W., Wang, S., Ji, D., Wang, Q., Li, J., et al. (2018). Analysis of lncRNA-Associated ceRNA Network Reveals Potential lncRNA Biomarkers in Human Colon Adenocarcinoma. Cel Physiol Biochem 49 (5), 1778–1791. doi:10.1159/000493623
Zhao, J., Dahle, D., Zhou, Y., Zhang, X., and Klibanski, A. (2005). Hypermethylation of the Promoter Region Is Associated with the Loss of MEG3 Gene Expression in Human Pituitary Tumors. J. Clin. Endocrinol. Metab. 90 (4), 2179–2186. doi:10.1210/jc.2004-1848
Zhao, J., Han, M., Zhou, L., Liang, P., Wang, Y., Feng, S., et al. (2020). TAF and TDF Attenuate Liver Fibrosis through NS5ATP9, TGFβ1/Smad3, and NF-Κb/nlrp3 Inflammasome Signaling Pathways. Hepatol. Int. 14 (1), 145–160. doi:10.1007/s12072-019-09997-6
Zhao, Y., Hu, X., Liu, Y., Dong, S., Wen, Z., He, W., et al. (2017). ROS Signaling under Metabolic Stress: Cross-Talk between AMPK and AKT Pathway. Mol. Cancer 16 (1), 79. doi:10.1186/s12943-017-0648-1
Zhao, Z., Lin, C. Y., and Cheng, K. (2019). siRNA- and miRNA-Based Therapeutics for Liver Fibrosis. Transl Res. 214, 17–29. doi:10.1016/j.trsl.2019.07.007
Zheng, J., Dong, P., Mao, Y., Chen, S., Wu, X., Li, G., et al. (2015). lincRNA-p21 Inhibits Hepatic Stellate Cell Activation and Liver Fibrogenesis via P21. FEBS J. 282 (24), 4810–4821. doi:10.1111/febs.13544
Zheng, J., Mao, Y., Dong, P., Huang, Z., and Yu, F. (2019). Long Noncoding RNA HOTTIP Mediates SRF Expression through Sponging miR-150 in Hepatic Stellate Cells. J. Cel Mol Med 23 (2), 1572–1580. doi:10.1111/jcmm.14068
Zhong, Q., Wang, Z., Liao, X., Wu, R., and Guo, X. (2020). LncRNA GAS5/miR-4465 axis R-egulates the M-alignant P-otential of N-asopharyngeal C-arcinoma by T-argeting COX2. Cell Cycle 19 (22), 3004–3017. doi:10.1080/15384101.2020.1816280
Zhou, B., Yuan, W., and Li, X. (2018). LncRNA Gm5091 Alleviates Alcoholic Hepatic Fibrosis by Sponging miR-27b/23b/24 in Mice. Cell Biol Int 42 (10), 1330–1339. doi:10.1002/cbin.11021
Zhou, L., and Liu, Y. (2015). Wnt/β-catenin Signalling and Podocyte Dysfunction in Proteinuric Kidney Disease. Nat. Rev. Nephrol. 11 (9), 535–545. doi:10.1038/nrneph.2015.88
Zhou, W., Ye, X. L., Xu, J., Cao, M. G., Fang, Z. Y., Li, L. Y., et al. (2017). The lncRNA H19 Mediates Breast Cancer Cell Plasticity during EMT and MET Plasticity by Differentially Sponging miR-200b/c and Let-7b. Sci. Signal. 10 (483), eaak9557. doi:10.1126/scisignal.aak9557
Zhou, Y., Zhang, X., and Klibanski, A. (2012). MEG3 Noncoding RNA: a Tumor Suppressor. J. Mol. Endocrinol. 48 (3), R45–R53. doi:10.1530/JME-12-0008
Zhu, C., Tabas, I., Schwabe, R. F., and Pajvani, U. B. (2021). Maladaptive Regeneration - the Reawakening of Developmental Pathways in NASH and Fibrosis. Nat. Rev. Gastroenterol. Hepatol. 18 (2), 131–142. doi:10.1038/s41575-020-00365-6
Zhu, J., Luo, Z., Pan, Y., Zheng, W., Li, W., Zhang, Z., et al. (2019). H19/miR-148a/USP4 axis Facilitates Liver Fibrosis by Enhancing TGF-β Signaling in Both Hepatic Stellate Cells and Hepatocytes. J. Cel Physiol 234 (6), 9698–9710. doi:10.1002/jcp.27656
Zuo, X.-x., Yang, Y., Zhang, Y., Zhang, Z.-g., Wang, X.-f., and Shi, Y.-g. (2019). Platelets Promote Breast Cancer Cell MCF-7 Metastasis by Direct Interaction: Surface Integrin α2β1-contacting-mediated Activation of Wnt-β-Catenin Pathway. Cell Commun Signal 17 (1), 142. doi:10.1186/s12964-019-0464-x
Keywords: lncRNAs, liver fibrosis, ceRNAs, HSCs, therapeutic strategies
Citation: Wang Z, Yang X, Gui S, Yang F, Cao Z, Cheng R, Xia X and Li C (2021) The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Front. Pharmacol. 12:779606. doi: 10.3389/fphar.2021.779606
Received: 19 September 2021; Accepted: 02 November 2021;
Published: 24 November 2021.
Edited by:
Jian Gao, Shanghai Children’s Medical Center, ChinaReviewed by:
Cheng Chen, Hefei Institutes of Physical Science (CAS), ChinaCopyright © 2021 Wang, Yang, Gui, Yang, Cao, Cheng, Xia and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanying Li, bGN5MDU2NTAwQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.