
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 03 November 2021
Sec. Inflammation Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.775521
 Qingqing Shao1
Qingqing Shao1 Fan Wu1
Fan Wu1 Tong Liu1
Tong Liu1 Wenjia Wang1
Wenjia Wang1 Tianli Liu1
Tianli Liu1 Ximing Jin1
Ximing Jin1 Lijun Xu1
Lijun Xu1 Yonggui Ma2
Yonggui Ma2 Guangying Huang1
Guangying Huang1 Zhuo Chen1,3*
Zhuo Chen1,3*Objectives: Genital herpes (GH) is a common sexually transmitted disease mainly caused by herpes simplex virus 2 (HSV-2). JieZe-1 (JZ-1) is an in-hospital prescription that has been used in Tongji Hospital for many years to treat various lower female genital tract infectious diseases. Our previous study showed that JZ-1 can protect against HSV-2 infection in vitro by inducing autophagy. However, whether JZ-1 can protect against HSV-2 infection in vivo, and the underlying mechanisms involved still remain unclear. Therefore, this study was designed to address above questions.
Methods: 8-week-old female balb/c mice were injected intravaginally with HSV-2 to establish GH model. The symptom score, body weight, and histological examination were recorded to assess the animal model of HSV-2 infected and the therapeutic effect of JZ-1. Inflammatory response was determined by detecting inflammatory cells infiltration and local cytokines levels. After then, under autophagy inhibitor chloroquine application, we measured the levels of cell apoptosis and autophagy and investigated the relationship between enhanced autophagy and cell apoptosis. Next, the classic PI3K/Akt/mTOR axis was examined, and in vitro experiment was carried out for further verification.
Results: Our results showed that JZ-1 administration significantly reduces symptom score, increases weight gain and alleviates histological damage in HSV-2 infection-induced GH in balb/c mice. JZ-1 administration obviously ameliorates inflammatory responses with reduced T-lymphocytes, T helper cells, macrophages and neutrophils infiltration, and local IL-1β, IL-6, TNF-α and CCL2 levels. HSV-2 infection leads to massive cell apoptosis, which was also restored by JZ-1. Meanwhile, we found that HSV-2 infection blocks autophagic flux in vivo and JZ-1 administration induces autophagy. After chloroquine application, it was observed that the inhibition of autophagy is strongly associated with increased cell apoptosis, whereas the promotion of autophagy remarkedly decreases apoptosis. These results suggested that JZ-1 inhibits cell apoptosis in GH by inducing autophagy, which was further supported in later in vitro experiment. Additionally, PI3K/Akt/mTOR signaling pathway was also downregulated by JZ-1 administration.
Conclusion: Our data demonstrated that JZ-1 can alleviate HSV-2 infection-induced GH in balb/c mice by inhibiting cell apoptosis via inducing autophagy, and the underlying mechanisms may be associated with the inhibition of PI3K/Akt/mTOR pathway.
GH is a common sexually transmitted disease mainly caused by HSV-2, which brings a huge concern on personal and public health worldwide owing to its highly contagious. The prevalence of HSV-2 infection is strongly associated with increasing sexual activity, especially after adulthood (Fleming et al., 1997). It was estimated that the HSV-2 serum infection rate is as high as 20–80% in different populations of the world (Paz-Bailey et al., 2007). As the complete cure of HSV-2 infection is challenging, it will always lead to recurrent genital ulcers during the patients’ lifespan. Moreover, the mucosal destruction caused by genital ulcers provides easy access for the acquisition of human immunodeficiency virus (HIV), which increases the risk of sexually acquired HIV associated with HSV-2 infection (Corey et al., 2004). In addition, HSV-2 infection during pregnancy can lead to neonatal herpes as a result of vertical transmission (Kim and Lee, 2020). Currently, the main treatments for HSV-2 infection are nucleoside antiviral drugs, such as acyclovir, penciclovir and famciclovir. These antiviral drugs act by interfering with viral DNA polymerase, thereby inhibiting the replication of the viral genome (Kukhanova et al., 2014). However, these drugs are not very effective for skin lesion as they only shorten the recovery time of the lesions in 1–2 days in most cases, and the problem of drug resistance is also becoming increasingly prominent (Alvarez et al., 2020). Therefore, there is urgent need to explore new drugs that are more effective and long-lasting.
During HSV-2 infection, virus-driven cell death largely contributes to the development of clinical symptoms, like blister, ulcer and inflammation. Among which, autophagy and apoptosis were the most well-studied forms of cell death in virus infection. Autophagy is regarded as a cell homeostasis process that is maintained by fusion of autophagosomes with lysosomes to form autolysosomes. Increasing evidence revealed that viruses and intracellular bacteria can block autophagic flux in host cells to promote the progression of infection. Conversely, the activation of autophagy could be an effective approach to limit HSV-2 infection (Cavignac and Esclatine, 2010). Apoptosis is another kind of programmed cell death, which is characterized by cell shrinkage, formation of apoptotic bodies and nuclear fragmentation (Wyllie, 1997). It was found that apoptosis can be triggered by two distinct signaling pathways, the intrinsic and extrinsic pathways. The intrinsic apoptotic pathway is caused by a variety of intracellular stress conditions, including cytokine deprivation, DNA damage, oxidative stress, cytoplasmic Ca2+ overload and endoplasmic reticulum stress. And the extrinsic apoptotic pathway is elicited by extracellular stress stimulation that is sensed and triggered through activation of death receptors of the tumor necrosis factor (TNF) family, including TNF receptor 1 (TNF-R1), Fas and TNF-related apoptosis-inducing ligand (TRAIL) receptors (Galluzzi et al., 2012a; Zhou et al., 2017). Both pathways will converge on the activation of the cysteine-specific aspartic protease 3 (caspase-3) enzyme, causing proteolysis and ultimately leading to cell death (Galluzzi et al., 2012b). Consistent with autophagy, apoptosis is also closely related to virus infection. A number of studies have demonstrated that virus-mediated host cell apoptosis plays a crucial role in the pathogenesis of herpesviruses-related diseases, including HSV-2, varicella-zoster virus (VZV), murine cytomegalovirus (MCMV) and Epstein–Barr virus (EBV) (Yedowitz and Blaho, 2005; Stefanidou et al., 2013; Zhou et al., 2017). Autophagy usually precedes apoptosis in cells. In general, autophagy is a strategy for cells to adapt and respond to stress. Apoptosis program is activated when the stress exceeds the critical duration or intensity threshold acceptable to the cell (Marino et al., 2014). Therefore, promoting autophagy has an inhibitory role on cell apoptosis during virus infection, which has been confirmed by various investigations (Pei et al., 2016; Peng et al., 2016; Lu et al., 2020).
Traditional Chinese medicine (TCM) has a long history in the treatment of GH, and it was shown to exert good clinical effects both in short-term and long-term treatments with few side effects (Wang, 2008). In the theory of TCM, GH belongs to “sore of vulvae” and “leukorrheal disease”. The pathogenesis of this disease is summarized into three aspects: dampness, poison and deficiency. Dampness and heat are the main characteristics in the acute stage of GH, while in the recurrence and non-episodic stage, dampness, heat and deficiency exist simultaneously. Our research object JZ-1 is derived from the modification of Yihuang Tang, which was first described in Fu Qingzhu Nvke in the Qing Dynasty as a treatment for leukorrheal diseases. JZ-1 is composed of Phellodendron chinense Schneid. (Phellodendri Chinensis Cortex), Ginkgo biloba L. (Ginkgo Semen), Solanum nigrum L. (Solanum Nigrum), Taraxacum mongolicum Hand.-Mazz (Taraxaci Herba), Thlaspi arvense Linn. (Herba Patriniae), Dictamnus dasycarpus Turcz. (Dictamni Cortex), Smilax glabra Roxb. (Smilacis Glabrae Rhizoma), Paeonia suffruticosa Andr. (Moutan Cortex), Mentha haplocalyx Briq. (Menthae Haplocalycis Herba) and Borneolum Syntheticum. As an in-hospital preparation, JZ-1 has been used in Tongji Hospital for many years to treat various lower female genital tract infectious diseases including GH, and it has been proven effective. However, the underlying mechanisms of JZ-1 on GH are not fully elucidated.
Our previous study found that HSV-2 infection inhibited autophagy in vitro, and the antiviral effect of JZ-1 can be achieved by inducing autophagic flux (Shao et al., 2020). However, whether JZ-1 can protect against HSV-2 infection by inducing autophagic flux in vivo, and what is the relationship between enhanced autophagy and cell apoptosis under JZ-1 treatment. Given that, this work was designed to address above questions. In this study, we performed in vivo experiments to explore whether JZ-1 can promote autophagy in HSV-2 infected balb/c mice model and evaluated the potential connection between JZ-1-enhanced autophagy and cell apoptosis in vivo and in vitro by applying an autophagy flux inhibitor.
JZ-1 consists of 10 traditional Chinese medicines, and the details of the herbal ingredients are shown in Table 1. All herbs were purchased from Hubei Shengdetang Chinese Herbal Pieces Co., Ltd. (Qianjiang, China). In brief, Phellodendri Chinensis Cortex was extracted twice using 70% ethanol. Except for Borneolum Syntheticum, the rest of herbs were boiled for 3 h, and then concentrated to 1.10∼1.20 g/ml gel under 60°C. After that, the above two extracts were combined, and then Borneolum Syntheticum was added to it. Finally, the solution was mixed and cooled to room temperature to obtain JZ-1 gel. For in vitro experiments, the process of JZ-1 preparation followed the same procedure but was not made to gel form. The quality control for JZ-1 has been evaluated by high-performance liquid chromatography (HPLC) fingerprinting in our recent publication (Duan et al., 2020).
8-week-old female balb/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. and housed in Animal Biosafety Level 2 of Tongji Hospital under conditions of 12 h dark/light cycle, 60 ± 5% relative humidity and 20 ± 2°C environmental temperature. The animal experiment was reviewed and approved by the Animal Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of science and technology. After 1-week acclimation, balb/c mice were injected intramuscularly with progesterone (Zhejiang Xianju Pharmaceutical Co., Ltd.) daily (150 mg/kg) for 5 days, and then HSV-2 (2×104∼2 × 106 PFU/ml) was injected into the vagina of mice. Firstly, we explored the appropriate timepoint for drug intervention, thus eight timepoints (D1, D3, D5, D7, D9, D12 and D14) were set up after HSV-2 infection. The most typical timepoint was chosen for subsequent experiments by examining severity of HSV-2 infection.
Then the follow-up study was conducted based on the selected timepoint, and the following groups were included: normal group (blank gel), model group (HSV-2 + blank gel), JL group (HSV-2+0.5 mg/ml JZ-1 gel), JM group (HSV-2+1.5 mg/ml JZ-1 gel), JH group (HSV-2+2.5 mg/ml JZ-1 gel), acyclovir group (HSV-2+ acyclovir gel), chloroquine group (CQ intraperitoneal injection), chloroquine + model group (HSV-2+chloroquine), JZ-1+chloroquine + model group (HSV-2+CQ+2.5 mg/ml JZ-1 gel). Briefly, from day 1 on, mice in each group were injected with gel into the vagina twice a day until the end of the experiment. On day 6, all mice except mice of the normal group and chloroquine group were anesthetized, and then HSV-2 was injected into the vagina of mice. Chloroquine was injected intraperitoneally at a dose of 60 mg/kg every 3 days from the day before the model was established. On the end of the experiment, the mice were anaesthetized with 1% pentobarbital. After collecting blood sample, the mice were euthanized with CO2. After HSV-2 injection, the symptom score was determined daily according to Table 2.
Chloroquine was purchased from Sigma-Aldrich, and bafilomycin A1 (Baf A1) was from Selleck. The following antibodies in immunoblotting and immunohistochemistry were used: anti-LC3B, anti-cleaved caspase-3, anti-Bcl-2, anti-p-Akt, anti-Akt, anti-p-PI3K, anti-PI3K, anti-mTOR, anti-p-mTOR and anti-gD were obtained from Cell Signaling Technology (Beverly, MA, United States). The antibodies for CD3 and MPO were from ABclonal Technology (Wuhan, China). Bax antibody was purchased from Proteintech (Wuhan, China). CD68 antibody was provided by Abcam (Cambridge, MA). CD4 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody for β-actin was purchased from Servicebio Co.,Ltd. (Wuhan, China).
Fresh vulva tissues were fixed with 4% paraformaldehyde or placed instantly in liquid nitrogen and then transferred into −80°C refrigerator. Paraffin-embedded slides were used for hematoxylin-eosin (H&E) staining according to the standard protocol. The vulva histological images were captured by an Olympus BX51 system (Olympus, Japan).
Paraffin-embedded vulva slides were dewaxed by dimethylbenzene, polarized with descending concentrations of alcohol (75, 95, 100%, 5 min each), and rinsed with deionized water. After that, antigen retrieval was performed with a microwave. Endogenous peroxidase activity was blocked by incubating slides in 3% H2O2 at room temperature for 30 min. The 10% normal goat serum was used to block the slides for 1 h. Primary antibodies (anti-CD3, anti-CD4, anti-MPO, anti-CD68, anti-LC3B and anti-cleaved caspase-3) were applied overnight at 4°C followed by incubation of HRP-conjugated secondary antibody for 60 min at room temperature. The slides were visualized by DAB and counterstained with hematoxylin. Images were taken by an Olympus BX51 system and analyzed by ImageJ software (National Institutes of Health, United States).
Total RNA was extracted from the vulva tissue of the mice with TRIzol reagent (YEASEN, China) and reverse transcribed into cDNA using the Hieff First Strand cDNA Synthesis Super Mix (YEASEN, China) according to the manufacturer’s protocol. qRT-PCR was performed on a LightCycler®96 system (Roche Diagnostics, Mannheim, Germany) using Hieff qPCR SYBR Green Master Mix (YEASEN, China). The results were analyzed by the 2−ΔΔCT method with β-actin as an internal control. The sequences of the primers used in this study are listed in Table 3.
Paraffin-embedded vulva slides were routinely dewaxed and dehydrated, and then, the slides were immersed in 3% H2O2 methanol solution for 30 min at room temperature to terminate the peroxidase activity. Subsequently, the proteinase K working solution was supplemented and the slides were incubated at 37°C for 30 min. The slides were then immersed in 0.1% TrionX-100 and 0.1% sodium citrate solution and subjected to recovery at room temperature for 5 min. Then the TUNEL reaction mixture and the subsequent staining were proceeded according to the provided TUNEL kit instructions.
The fresh vulva tissues were collected and fixed overnight in cold glutaraldehyde fixative (2.5% in 0.1 mol/L cacodylate buffer, pH 7.4) and post-fixed in osmium tetroxide. After dehydration with a series of graded ethanol concentrations, the samples were rinsed with propylene oxide and impregnated with epoxy resins. Electron micrographs were taken by the transmission electron microscope.
The human immortalized vaginal epithelial cell line (VK2/E6E7) was purchased from the American Type Culture Collection, and the Vero cell line was obtained from the Chinese Type Culture Collection. The VK2/E6E7 cells were cultured in Cn-TPR medium (CELLnTEC, Switzerland), and the Vero cells were cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum. The cells were grown at 37°C in a 5% CO2 atmosphere. According to our previous study, Baf A1 (100 nM) was cotreated with HSV-2 in the absence or presence of 5 mg/ml JZ-1 for the experiments for 24 h.
Total protein was extracted from the vulva tissue of the mice or VK2/E6E7 cells with standard protocols, and the protein concentrations were quantified by the bicinchoninic acid (BCA) protein assay kit. Protein samples (40 μg/lane) were separated by SDS-PAGE (120 v, 90 min) and transferred to nitrocellulose (NC) membranes or polyvinylidene difluoride (PVDF) membranes. After blocking with 5% BSA powder with ultrapure water for 1 h, the membranes were incubated overnight with primary antibodies (anti-LC3B, anti-cleaved caspase-3, anti-Bcl-2, anti-bax, anti-p-Akt, anti-Akt, anti-p-PI3K, anti-PI3K, anti-mTOR, anti-gD and anti-β-actin) at 4°C. After washing three times with TBST for 10 min each, the membranes were incubated with secondary antibodies for 1 h at room temperature, washed another three times, and then visualized by an Odyssey Infrared Imaging system (LI-COR Biosciences, United States). The band densities were quantified by ImageJ (National Institutes of Health, United States).
All data are presented as means ± SEM. Statistical analyses were performed followed this rule: Firstly, the normality of data is tested by Shapiro-Wilk test. Secondly, data that fits the normal distribution are tested for homogeneity of variance via one-way ANOVA. Finally, Tukey’s multiple comparisons test can be performed to compare multiple groups only if there is no significant variance inhomogeneity between groups. Mann-Whitney U test should be used as an alternative post hoc test when the data show non-normality or variance inhomogeneity. Statistics were analyzed using the GraphPad Prism 8 software, and p < 0.05 was considered as statistically significant.
To determine the appropriate timepoint for the degree of genital herpes caused by HSV-2 infection in balb/c mice, we set 8 timepoints after HSV-2 infection (D1, D3, D5, D7, D9, D12 and D14) (Figure 1A). The results showed that on the 9th day after vaginal administration of HSV-2, the mice had the highest vulvar symptom score and the most significant weight loss (Figures 1B,C). The results of H&E staining also showed that the structure was disordered and the degree of inflammatory cell infiltration was the heaviest on the 9th day (Figure 1D). In addition, the expression level of vulvar virus protein gD was the highest on the 9th day (Figures 1E–G). Therefore, the 9th day after vaginal administration of HSV-2 was selected as the timepoint for the follow-up experiments.
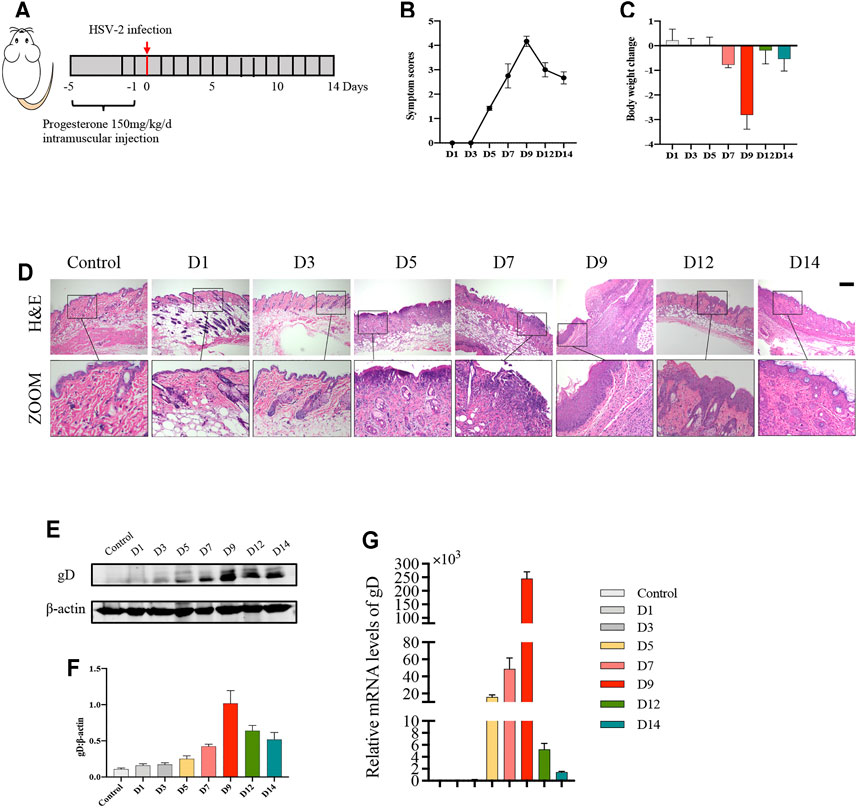
FIGURE 1. Balb/c mice have the most severe symptoms on the 9th day after HSV-2 infection. (A) The protocol of timepoint exploring for animal experiment. (B) Symptom scores of different groups in each timepoint. (C) Body weight change of different groups in each timepoint (compared with body weight of mice before treatment. (D) Pictures of the vulva of balb/c mice. (E) Representative western blots for gD protein expressions in vulva. (F) The quantification of gD western blots. (G) The mRNA levels of gD. All data are presented as means ± SEM. p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***); (n = 6).
In order to clarify the anti-HSV-2 effect of JZ-1, three doses of JZ-1 were used in HSV-2 infected balb/c mice, and acyclovir was chosen as the positive control drug (Figure 2A). Compared to the model group, JH administration exerted a significant symptom-improving and weight-regaining effect similar to acyclovir, and JM is less effective in improving symptoms and weight than JH. While JL administration had a trend of symptom-improving and weight-regaining although it was not statistically significant (Figures 2B–F). The results of H&E staining showed that the vulva structure of the mice in model group was disordered, the epidermis was hyperplasia, and there were a large number of inflammatory cell infiltration, which was largely restored by JZ-1 administration, especially JH administration (Figure 2G).
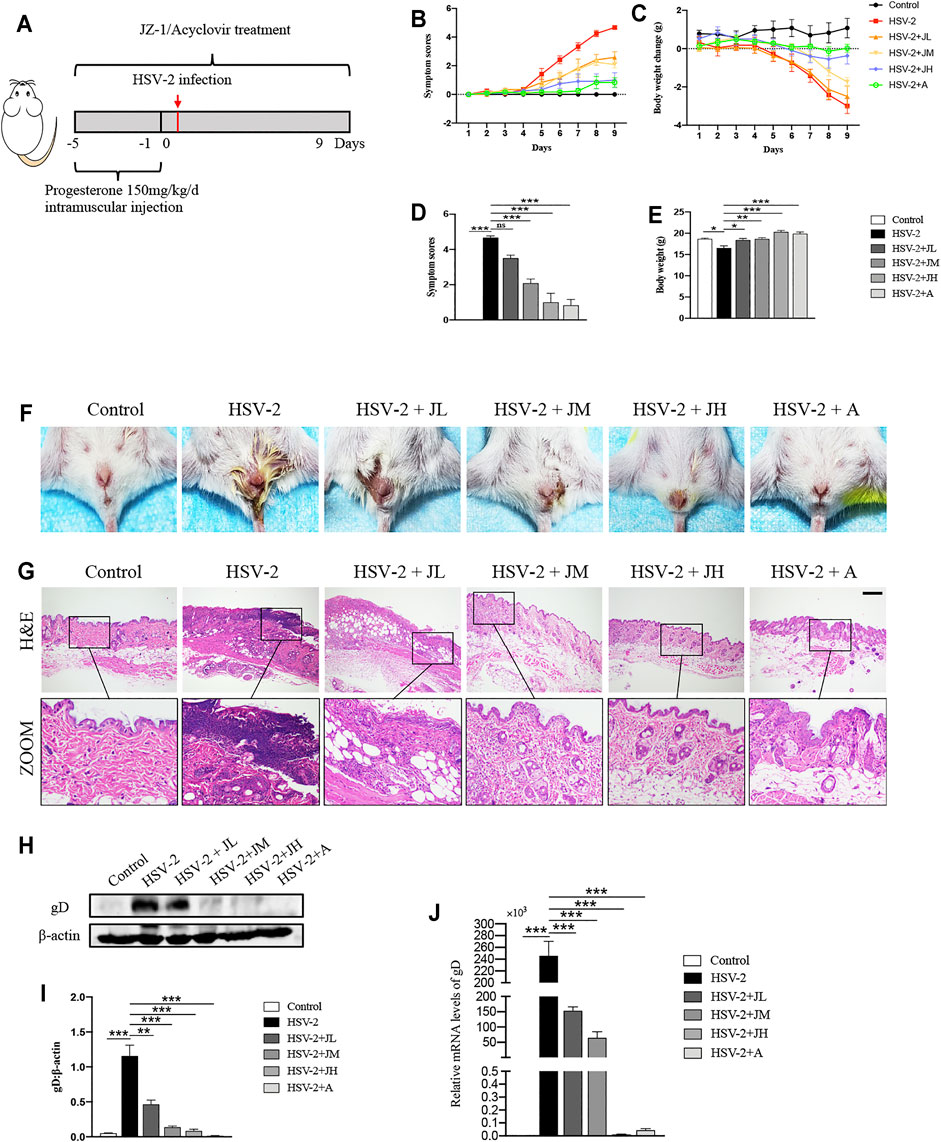
FIGURE 2. JZ-1 administration significantly alleviates HSV-2-induced GH in balb/c mice. (A) The protocol of animal experiment. (B) Symptom scores of different groups over time. (C) Body weight change of different groups over time (compared with body weight of mice before treatment. (D) Symptom scores were recorded at the ending of experiment. (E) Body weight was recorded at the ending of experiment. (F) Pictures of the vulva of balb/c mice. (G) Representative liver H&E staining of different groups. Scale bar, 200 μm. (H) Representative western blots for gD protein expressions in vulva. (I) The quantification of gD western blots. (J) The mRNA levels of gD in vulva. All data are presented as means ± SEM. p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***); (n = 6).
HSV-2 glycoprotein D (gD) is a receptor binding protein required by most alpha herpes viruses including HSV-2 and thus plays a vital role in the spread of the virus (Connolly et al., 2021). The trend of the expression levels of gD was also consistent with the histological results (Figures 2H–J). Collectively, these data suggested that JZ-1 administration significantly alleviates HSV-2-induced GH. Due to JH showed the best therapeutic effects, hence, we choose JH group as the representative JZ-1 intervention group in later mechanism investigations.
To investigate the effects of JZ-1 on inflammatory response in GH, we detected the levels of inflammatory cell infiltration and pro-inflammatory cytokines expression. As shown in Figures 3A–D, the results of IHC staining showed that, compared with control group, T-lymphocytes (CD3-positive), T helper cells (CD4-positive), macrophages (CD68-positive) and neutrophils (MPO-positive) in model group were significantly increased. Compared with model group, JZ-1 significantly decreased the inflammatory cell infiltration. Also, we examined the mRNA levels of pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α and chemokines CCL2, using qRT-PCR assays. Consistent with the results of IHC staining, the mRNA levels of pro-inflammatory cytokines increased obviously in the HSV-2 infected balb/c mice, whereas those increased pro-inflammatory cytokines were largely restored by JH administration (Figures 3E–H). Altogether, these results indicated that JZ-1 administration inhibits inflammatory response in HSV-2-induced GH.
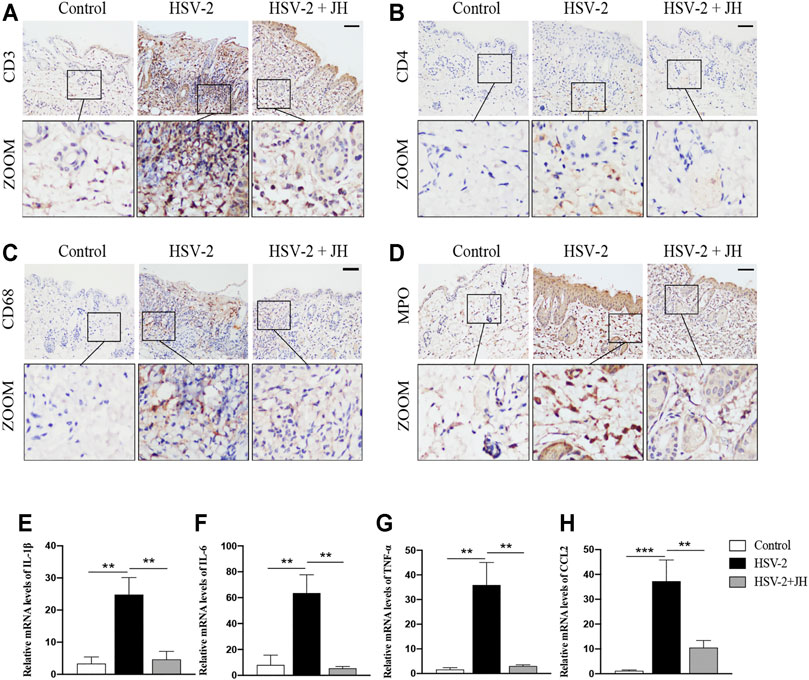
FIGURE 3. JZ-1 administration inhibits inflammatory response in HSV-2-induced GH. (A–D) Representative immunohistochemistry staining for CD3, CD4, CD68 and MPO in vulva of different groups. Scale bar, 100 μm. (E–H) The mRNA levels of IL-1β, IL-6, TNF-α and CCL2 in vulva of different groups. All data are presented as means ± SEM. p < 0.05 (*); (n = 6).
It has been proved that cell apoptosis plays a key role in the development and progression of herpesviruses-related diseases. To determine whether JZ-1 could affect cell apoptosis in HSV-2-induced GH, TUNEL staining was applied to observe apoptotic cells. TUNEL staining results revealed that cell apoptosis of model group was remarkedly increased compared to that of control group (green area), and JZ-1 administration suppressed excessive cell apoptosis compared to model group (Figure 4A). Meanwhile, cell apoptosis was also detected by apoptotic maker cleaved caspase-3 IHC staining, and the results were similar to TUNEL staining (Figure 4B). Next, we tested the protein expression of cleaved caspase-3, Bcl-2 (a well-known anti-apoptotic protein) and Bax (a well-known pro-apoptotic protein) by western blot. As expected, compared to model group, JH treatment brought a significant reduction in cleaved caspase-3 and Bax expressions, and increased the expression of the anti-apoptotic protein Bcl-2 (Figures 4C,D). Therefore, our findings demonstrated that JZ-1 administration suppresses cell apoptosis in HSV-2-induced GH.
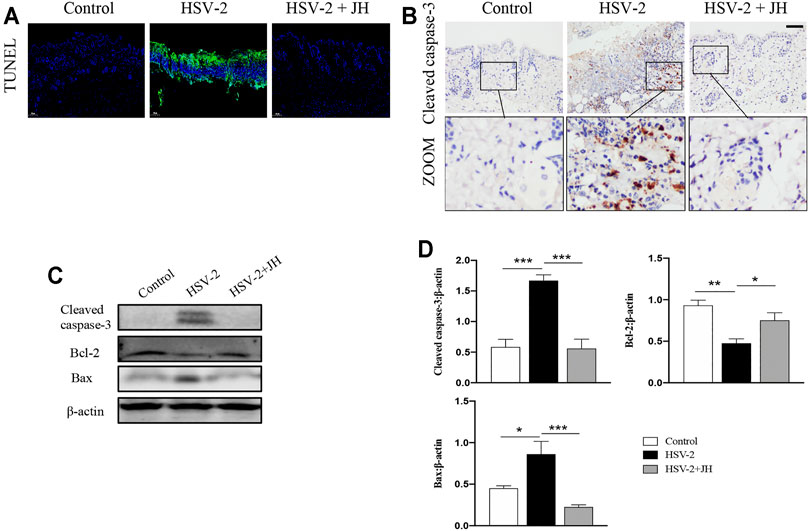
FIGURE 4. JZ-1 administration suppresses cell apoptosis in HSV-2-induced GH. (A) Representative TUNEL staining in vulva. (B) Representative immunohistochemistry staining for cleaved caspase-3 in vulva. (C) Representative western blots for cleaved caspase-3 and Bax in vulva. (D) The quantification of cleaved caspase-3 and Bax western blots. All data are presented as means ± SEM. p < 0.01 (**), and p < 0.001 (***); (n = 6).
Our previous work has confirmed that HSV-2 infection can inhibit autophagy flux in vitro, but it is unclear whether HSV-2 infection also blocks autophagic flux in vivo. Microtubule-associated protein 1 LC3B is an indicator of autophagic flux. LC3 is initially synthesized in an unprocessed form, proLC3, which is proteolytically processed into the cytosolic LC3B–I isoform, and is finally modified into the PE-conjugated form, LC3-II, when autophagy is induced. In addition to LC3, SQSTM1/p62 can also be used as protein marker, the SQSTM1 protein serves as a link between LC3 and ubiquitinated substrates. And Atg5 mediate phagophore expansion and autophagosome formation during autophagy induced (Klionsky et al., 2016). Usually, the expression level of LC3B-II is positively correlated with autophagy. However, the accumulation of LC3B-II also occurs when autophagy flux is blocked. Therefore, to elucidate the true effect of HSV-2 infection on autophagic flux in vivo, we applied the autophagy flux inhibitor chloroquine (CQ) in later investigations (Figure 5A). Inhibiting autophagy in advance by CQ allows us to intuitively observe the true influence of upstream factor on autophagic flux.
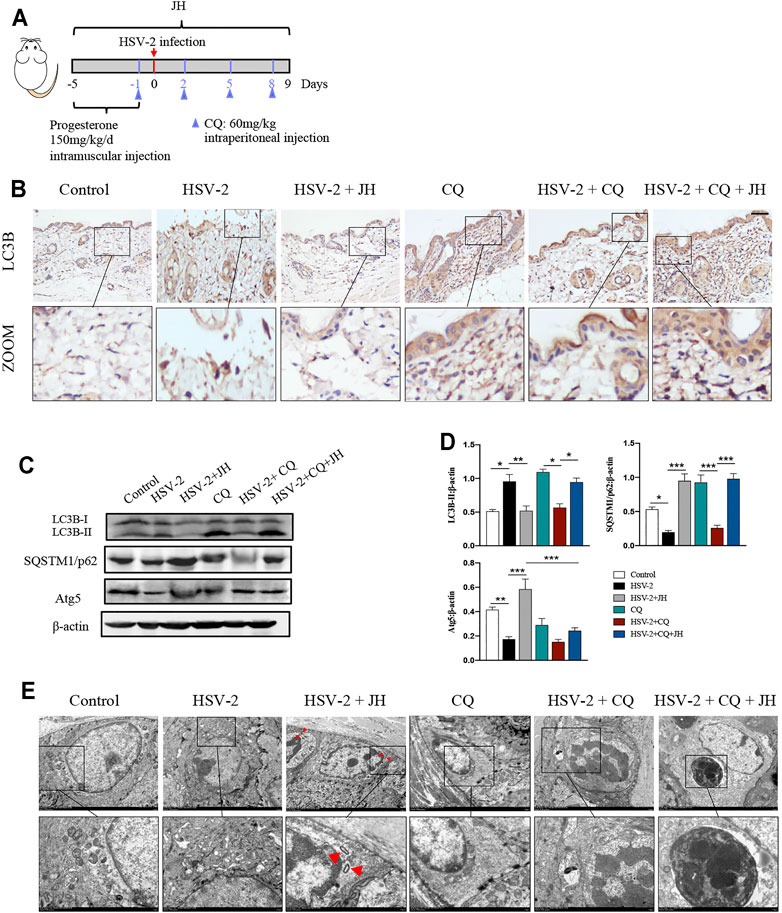
FIGURE 5. HSV-2 infection leads to a blockage of autophagic flux in vivo and JZ-1 administration induces autophagy in HSV-2-induced GH. (A) The protocol of administration of CQ in animal experiment. (B) Representative immunohistochemistry staining for LC3B in vulva of different groups. (C) Representative western blot for LC3B-II, SQSTM1/p62 and Atg5 protein expressions in vulva. (D) The quantification of LC3B-II, SQSTM1/p62 and Atg5 western blot. (E) TEM of different groups in vulva (the red arrow indicates the autophagy structure). All data are presented as means ± SEM. p < 0.01 (**), and p < 0.001 (***); (n = 6).
As shown in Figures 5B–D, the results showed that the expression of LC3B in model group was higher and the expression of SQSTM1/p62 decreased than those in control group, while JH treatment restored their expression. If only these three groups are set, we may draw a wrong conclusion that HSV-2 infection will increase autophagy, while JZ-1 will inhibit autophagy in vivo. Actually, after the application of CQ, we can learn the true influence of HSV-2 infection and JZ-1 on autophagic flux in vivo. Compared with CQ group, it was observed that the expression of LC3B and SQSTM1/p62 in HSV-2+CQ group were both reduced, and compared with HSV-2+CQ group, JH administration obviously increased LC3B and SQSTM1/p62 expression. In addition, the expression of Atg5 decreased in model group compared with control group, while JH treatment increased its expression significantly (Figures 5B–D). Consistently, the TEM results found that JH group showed more autophagy structures than HSV-2 group (Figure 5E). Altogether, these results revealed that HSV-2 infection blocks autophagic flux in vivo, while JZ-1 administration induces autophagy in GH.
According to above results, we have verified that HSV-2 infection can induce cell apoptosis and block autophagy flux in vivo, while JH can suppress cell apoptosis and induce autophagy flux. Since promoting autophagy has an inhibitory role on cell apoptosis during virus infection, thus we wonder whether the anti-apoptotic effect of JZ-1 is mediated by its induction of autophagy. Compared with CQ group, HSV-2+CQ group showed massive apoptotic cells. Meanwhile, it was also found that HSV-2+CQ group displayed more apoptotic cells than HSV-2 group (Figures 6A,B). These results suggested that the inhibition of autophagy is strongly associated with increased cell apoptosis, which was consistent with previous studies. As expected, the apoptosis level in HSV-2+CQ + JH group was significantly increased and the autophagy level was obviously decreased compared to HSV-2+JH group (Figures 6A,B and Figures 5B–D). These results were also supported by further western blots and qRT-PCR assay (Figures 6C–E). Taken together, our data demonstrated that the anti-apoptotic effect of JZ-1 can be mediated by its induction of autophagy.
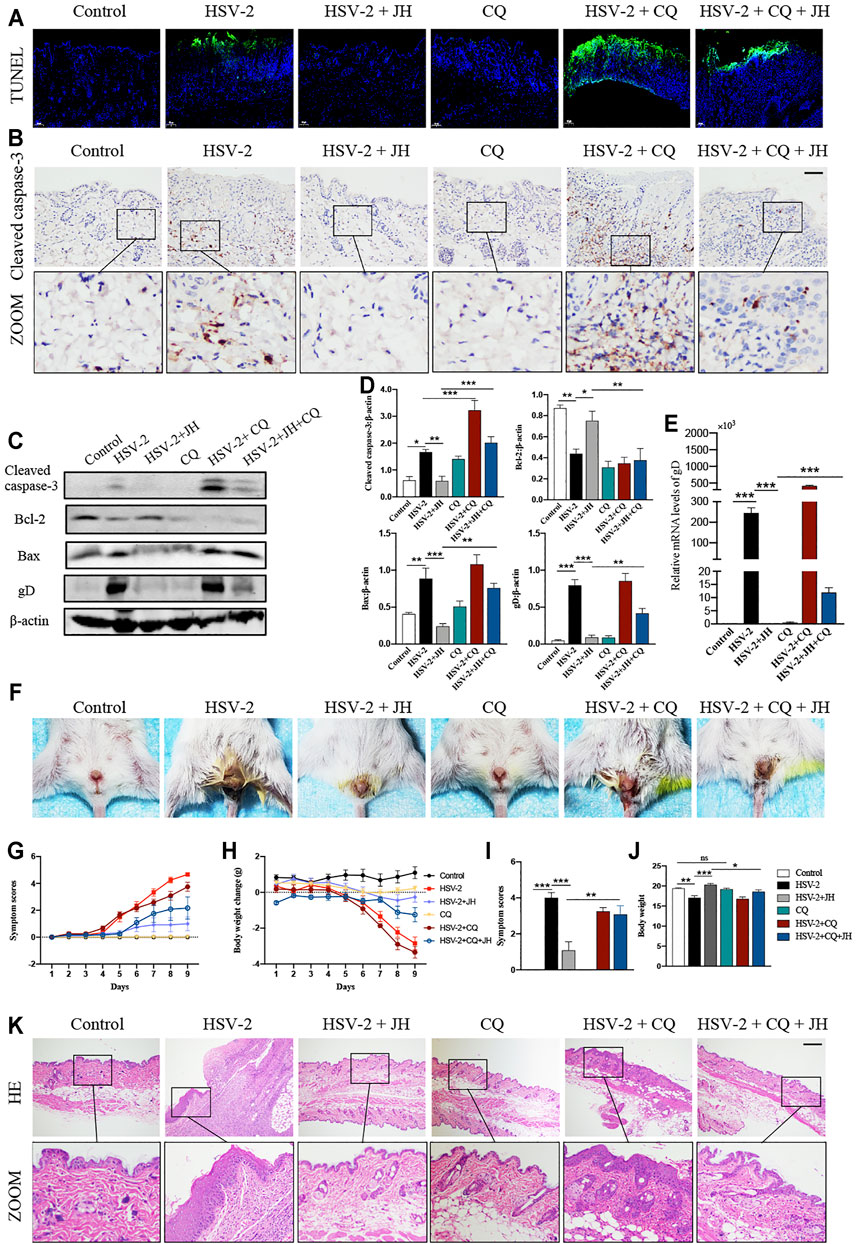
FIGURE 6. The anti-apoptotic effect of JZ-1 is mediated by its induction of autophagy. (A) Representative TUNEL staining in vulva of different groups. (B) Representative immunohistochemistry staining for cleaved caspase-3 in vulva of different groups. (C) Representative western blots for cleaved caspase-3, Bax and gD in vulva of different groups. (D) The quantification of cleaved caspase-3, Bax and gD western blots. (E) The mRNA levels of gD in vulva of different groups. (F) Pictures of the vulva of balb/c mice. (G) Symptom scores of different groups. (H) Body weight of different groups. (I–J) Statistical analysis of symptom scores ND Body weight. (K) Representative liver H&E staining of different groups. All data are presented as means ± SEM. p < 0.01 (**), and p < 0.001 (***); (n = 6).
Besides, it was observed that the degree of vulva ulceration in the mice of HSV-2+CQ and HSV-2 + CQ + JH group is also heavier than that of HSV-2 group and HSV-2 + JH group, respectively (Figure 6F), the symptom score is higher, and the weight loss is more significant (Figures 6G–J). The result of H&E staining showed the same trend (Figure 6K). This phenomenon is attributed to that the treatment of CQ inhibits the beneficial effects of autophagy and exacerbates cell apoptosis in GH.
The mammalian kinase target of rapamycin (mTOR) is the main regulator of autophagy, which is regulated by classic PI3K/Akt signaling pathway (Manning and Cantley, 2007; Heras-Sandoval et al., 2014). In our previous study, we have shown that JZ-1 can induce autophagy in vitro via inhibiting PI3K/Akt/mTOR signaling axis to protect against HSV-2 infection. Therefore, we also detected this pathway in this in vivo study. The results showed that the phosphorylation level of PI3K, Akt, and mTOR in HSV-2 group were significantly increased compared to the control group, and JH administration restored the phosphorylation level of these proteins (Figures 7A,B). These results indicated that, similar to in vitro study, JZ-1 induces autophagy in HSV-2-induced GH and its underlying mechanisms may be associated with the inhibition of PI3K/Akt/mTOR pathway.
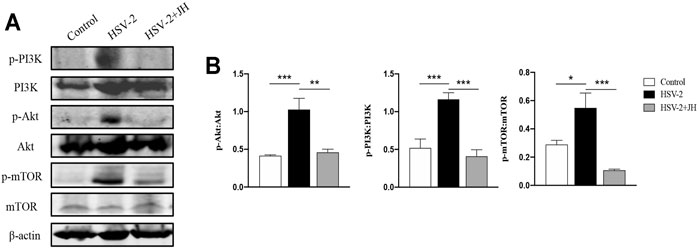
FIGURE 7. JZ-1 inhibits PI3K/Akt/mTOR pathway in HSV-2-induced GH. (A) Representative western blots for p-PI3K, PI3K, p-Akt, Akt, p-mTOR, and mTOR in vulva of different groups. (B) The quantification of p-PI3K, PI3K, p-Akt, Akt, p-mTOR, and mTOR western blots. All data are presented as means ± SEM. p < 0.01 (**), and p < 0.001 (***); (n = 6).
As a supplement, we further verified the above mechanism on VK2/E6E7 cells. In the in vitro study, we applied bafilomycin A1 (Baf A1) as the inhibitor of autophagy flux. As shown in Figures 8A,B, the expression levels of cleaved caspase-3 and Bax significantly increased both in HSV-2 and HSV-2+Baf A1 groups, and JZ-1 administration obviously reduced the expression of above proteins and induced autophagy process. Meanwhile, the cell apoptosis level in HSV-2+Baf A1 and HSV-2+Baf A1+JZ-1 group were significantly higher than those in HSV-2 and HSV-2+JZ-1 group, respectively. These results demonstrated that JZ-1 can exert its anti-apoptotic effect by inducing autophagy in VK2/E6E7 cells, and the inhibition of autophagy impairs its anti-apoptotic effect.
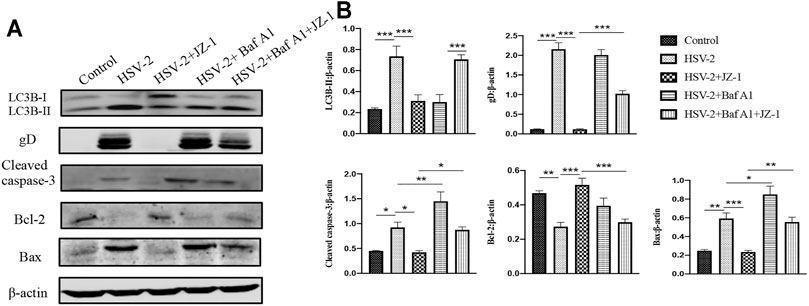
FIGURE 8. JZ-1 can exert its anti-apoptotic effect by inducing autophagy in VK2/E6E7 cells. (A) Representative western blots for LC3B-II, gD, cleaved caspase-3, and Bax in VK2/E6E7 cells of different groups. (B) The quantification of LC3B-II, gD, cleaved caspase-3, and Bax western blots. All data are presented as means ± SEM. p < 0.01 (**), and p < 0.001 (***); (n = 6).
In recent years, although the prevalence of genital herpes caused by HSV-1 has increased (Looker et al., 2015), but compared with HSV-2, HSV-1 is much less likely cause recurrent genital infections (Engelberg et al., 2003). HSV-2 infection still accounts for the vast majority of global genital herpes diseases, about 95% (Looker et al., 2020). The typical symptoms of genital herpes are frequent, painful, recurrent genital lesions, and are accompanied by many social and psychological distress (Gupta et al., 2007). In addition to the characteristics of recurrence, the reasons why it is hard to control HSV-2 infection may include: 1) GH caused by HSV-2 infection is transmitted by sexual activities, and the preventive strategies are not strictly adopted worldwide. 2) Current drug treatments are not satisfactory, and some people are resistant to the nucleoside analogues. 3) There is no breakthrough in vaccine research. 4) Additionally, the research on the mechanism of HSV-2 infection is also lacking at present.
The therapeutic effects of TCM on infectious diseases has been verified in multiple public health incidents. Chinese medicine has played a critical role in treating whether the severe acute respiratory syndrome (SARS) in 2003 or the coronavirus disease 2019 (COVID-19). Similarly, traditional Chinese medicine and its active ingredients have been proven to have significant anti-herpes simplex virus effects (Li et al., 2018; Shen et al., 2019; Feng et al., 2020). JZ-1 is an in-hospital preparation that has been used clinically for many years. It has a good effect on common female lower genital tract infections such as vaginitis and cervicitis. Clinical and experimental studies have shown that JZ-1 can significantly improve the symptoms of vaginal congestion, cervical erosion, abnormal vaginal discharge, vulvar itching and frequent urination caused by U. urealyticum infection (Hong et al., 2007), and can prevent C. albicans and T. vaginalis infections in vivo and in vitro (Chen et al., 2009a; Chen et al., 2009b; Chen et al., 2009c). Our previous in vitro experiments have also confirmed that the Chinese medicine JZ-1 has a significant anti-HSV-2 infection effect (Shao et al., 2020). However, it is unclear whether JZ-1 can protect against HSV-2 infection in vivo, and the underlying mechanisms for JZ-1 also needs to be clarified. In this present study, we confirmed the in vivo anti-HSV-2 infection effect of JZ-1, and revealed that JZ-1 exerts the anti-HSV-2 infection effect by inhibiting cell apoptosis via inducing autophagy.
Apoptosis and autophagy are the most common and widely studied types of programmed cell death. It is well-known that there is a complex functional relationship between apoptosis and autophagy. Generally, autophagy constitutes a stress adaptation that avoids cell death (inhibit apoptosis), whereas under certain circumstances, it constitutes an alternative cell-death pathway (promote apoptosis). In this study, we found that HSV-2 infection induced cell apoptosis, and JZ-1 can exhibit the anti-viral effect by inhibiting apoptosis both in vivo and in vitro. From our previous study, we learned that HSV-2 infection could inhibit the autophagy flux in vitro, which is conducive to the virus evading cell phagocytosis. Hence, we planned to investigate the effects of JZ-1 on autophagy flux in vivo. We found that in many studies, it is unscientific that only use the changes of the key autophagy protein LC3B to draw a conclusion about autophagy flux changes. According to the latest edition of the autophagy monitoring guidelines (Klionsky et al., 2016), a variety of methods for monitoring autophagy flux are recommended, but it is clearly stated that the conclusion that autophagy flux is induced or inhibited cannot be drawn based on a single method. In previous in vitro experiments, we chose autophagy flux inhibitor bafilomycin A1 to verify the true effect of HSV-2 on autophagy flux. Similarly, in this study, we used CQ, another autophagy flux inhibitor, to explore the true effect of HSV-2 infection on autophagy flux in balb/c mice. Autophagosomes need to be fused with lysosomes to form autolysosomes to perform their functions after forming, and autophagy flux would be blocked if autophagosomes cannot fuse with lysosomes, which means that autophagy is inhibited. The role of CQ is to hinder the fusion of autophagosomes and lysosomes (Mauthe et al., 2018). In this study, the expression level of LC3B-II increases in vulva of HSV-2 infected balb/c mice, which is consistent with the performance of autophagy being induced under normal circumstances, but we cannot conclude that HSV-2 induces autophagy only based on the changes of LC3B-II. According to our results, after the application of CQ, compared with CQ group, the expression of LC3B-II in HSV-2+CQ group did not further increase, but decreased; and the expression of LC3B-II in HSV-2+CQ + JH group significantly increased compared to HSV-2+CQ group, which indicated that HSV-2 infection blocked autophagy flux, and JH administration induced autophagy flux. Therefore, at least during HSV-2 infection, we demonstrated that promoting autophagy inhibits cell apoptosis. Additionally, we suggested that in the study of autophagy, relevant compounds and various methods should be used rationally, and conclusions should be made cautiously to ensure the authenticity and scientificity.
Next, we explored the underlying mechanism that regulates autophagy under JZ-1 treatment. The PI3K/Akt/mTOR axis is well-recognized as an important signaling pathway for regulating autophagy, which has been confirmed in our previous in vitro experiments. PI3K/Akt/mTOR signal pathway consisting of two parts: phosphatidylinositol 3-kinase (PI3K) and its downstream molecule serine/threonine protein kinase B (PKB; also known as Akt) (Xu et al., 2020), which plays a vital role in many cellular processes essential for homeostasis, including the cell cycle, cell survival, cell growth and proliferation, inflammation, metabolism, and apoptosis (Sun et al., 2020). In this study, we detected the phosphorylation levels of PI3K, Akt and mTOR, and found that HSV-2 infection induces the activation of this pathway, while JH inhibits this pathway. These results indicated that the promotive effect of JZ-1 on autophagy may be mediated by the PI3K/Akt/mTOR signaling pathway.
In conclusion, this study explored the potential effects and underlying mechanisms of JZ-1 on HSV-2 infection-induced genital herpes in balb/c mice. Our data demonstrated that JZ-1 can alleviate HSV-2 infection-induced GH in balb/c mice by inhibiting cell apoptosis via inducing autophagy, and the underlying mechanisms may be associated with the inhibition of PI3K/Akt/mTOR pathway (Figure 9).
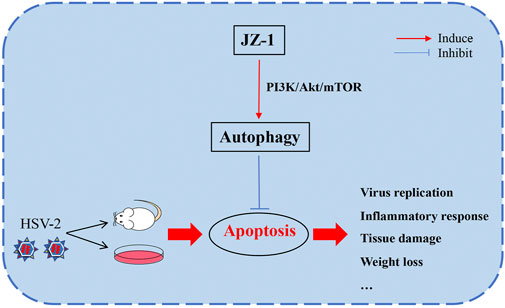
FIGURE 9. JZ-1 alleviates HSV-2 infection-induced genital herpes in balb/c mice by inhibiting cell apoptosis via inducing autophagy. In the pathogenesis of GH, HSV-2 infection leads to massive cell apoptosis, which largely promote the progression of disease. JZ-1 administration can alleviate HSV-2 infection-induced GH in balb/c mice by inhibiting cell apoptosis via inducing autophagy, and the underlying mechanisms may be associated with the inhibition of PI3K/Akt/mTOR pathway.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Animal Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (HUST).
ZC conceived the idea and organized implementation. QS performed main experiments and analyzed data. FW complete the investigation, methodology, data planning, and verification, TL and QS conducted the animal experiments. QS and ZC were responsible for manuscript preparation and revision. YM and LX prepared the Chinese medicine formula JZ-1. WW, TL, and XJ assisted the experiment. GH guided the subject.
This work was supported by the National Natural Science Foundation of China (No. 81874483).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
JZ-1, JieZe-1; JH, high-dose JZ-1 gel; GH, genital herpes; HSV-2, herpes simplex virus type-2; TEM, transmission electron microscope; HIV, human immunodeficiency virus; TUNEL, terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling; Baf A1, Bafilomycin A1; CQ, chloroquine; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; IL-6, interleukin 6; IFN, interferon; TNF, tumor necrosis factor; LC3, microtubule-associated protein 1 light chain 3(MAP1LC3/LC3); Atg, autophagy-related; gD, glycoprotein D; H&E, hematoxylin-eosin; TCM, traditional Chinese medicine.
Alvarez, D. M., Castillo, E., Duarte, L. F., Arriagada, J., Corrales, N., Farias, M. A., et al. (2020). Current Antivirals and Novel Botanical Molecules Interfering with Herpes Simplex Virus Infection. Front. Microbiol. 11, 139. doi:10.3389/fmicb.2020.00139
Cavignac, Y., and Esclatine, A. (2010). Herpesviruses and Autophagy: Catch Me if You Can. Viruses 2 (1), 314–333. doi:10.3390/v2010314
Chen, Z., Kong, X., Wang, R., Ma, Y., Xu, L., Lu, F., et al. (2009a). Investigation on Prevention Effects of Jieze NO.2 on candida Albicans Vaginitis. Matern. Child Health Care China 24 (06), 788–790.
Chen, Z., Wang, R., Ma, Y., Kong, X., and Lu, F. (2009b). Effect of Jieze2 on Trichomonas Vaginalis In Vitro. Matern. Child Health Care China 24 (03), 344–346.
Chen, Z., Wang, R., Ma, Y., Kong, X., Lu, F., and Huang, G. (2009c). The Effect of Jieze NO.2 Jel on Trichomonas Vaginitis in Rats. Matern. Child Health Care China 24 (05), 644–646.
Connolly, S. A., Jardetzky, T. S., and Longnecker, R. (2021). The Structural Basis of Herpesvirus Entry. Nat. Rev. Microbiol. 19 (2), 110–121. doi:10.1038/s41579-020-00448-w
Corey, L., Wald, A., Celum, C. L., and Quinn, T. C. (2004). The Effects of Herpes Simplex Virus-2 on HIV-1 Acquisition and Transmission: A Review of Two Overlapping Epidemics. Jaids-Journal of Acquired Immune Deficiency Syndromes 35 (5), 435–445. doi:10.1097/00126334-200404150-00001
Duan, Q., Liu, T., Yuan, P., Huang, C., Shao, Q., Xu, L., et al. (2020). Antiviral Effect of Chinese Herbal Prescription JieZe-1 on Adhesion and Penetration of VK2/E6E7 with Herpes Simplex Viruses Type 2. J. Ethnopharmacol 249, 112405. doi:10.1016/j.jep.2019.112405
Engelberg, R., Carrell, D., Krantz, E., Corey, L., and Wald, A. (2003). Natural History of Genital Herpes Simplex Virus Type 1 Infection. Sex. Transm. Dis. 30 (2), 174–177. doi:10.1097/00007435-200302000-00015
Feng, X., Cao, S., Qiu, F., and Zhang, B. (2020). Traditional Application and Modern Pharmacological Research of Artemisia Annua L. Pharmacol. Ther. 216, 107650. doi:10.1016/j.pharmthera.2020.107650
Fleming, D. T., McQuillan, G. M., Johnson, R. E., Nahmias, A. J., Aral, S. O., Lee, F. K., et al. (1997). Herpes Simplex Virus Type 2 in the United States, 1976 TO 1994. New Engl. J. Med. 337 (16), 1105–1111. doi:10.1056/Nejm199710163371601
Galluzzi, L., Kepp, O., and Kroemer, G. (2012a). Mitochondria: Master Regulators of Danger Signalling. Nat. Rev. Mol. Cell Biol 13 (12), 780–788. doi:10.1038/nrm3479
Galluzzi, L., Vitale, I., Abrams, J. M., Alnemri, E. S., Baehrecke, E. H., Blagosklonny, M. V., et al. (2012b). Molecular Definitions of Cell Death Subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19 (1), 107–120. doi:10.1038/cdd.2011.96
Gupta, R., Warren, T., and Wald, A. (2007). Genital Herpes. Lancet 370 (9605), 2127–2137. doi:10.1016/S0140-6736(07)61908-4
Heras-Sandoval, D., Perez-Rojas, J. M., Hernandez-Damian, J., and Pedraza-Chaverri, J. (2014). The Role of PI3K/AKT/mTOR Pathway in the Modulation of Autophagy and the Clearance of Protein Aggregates in Neurodegeneration. Cell Signal. 26 (12), 2694–2701. doi:10.1016/j.cellsig.2014.08.019
Hong, W., Zhuo, C., Ping, X., and Lijun, X. (2007). Clinical Research of Jieze No.1 on Cervicitis Caused by Ureaplasma Urealyticum and Effect on Ureaplasma Urealyticum In Vitro. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong 2007 (01), 87–90. doi:10.1007/s11655-008-0088-2
Kim, H. C., and Lee, H. K. (2020). Vaccines against Genital Herpes: Where Are We? Vaccines (Basel) 8 (3). doi:10.3390/vaccines8030420
Klionsky, D. J., Abdelmohsen, K., Abe, A., Abedin, M. J., Abeliovich, H., Acevedo Arozena, A., et al. (2016). Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (3rd Edition). Autophagy 12 (1), 1–222. doi:10.1080/15548627.2015.1100356
Kukhanova, M. K., Korovina, A. N., and Kochetkov, S. N. (2014). Human Herpes Simplex Virus: Life Cycle and Development of Inhibitors. Biochemistry-Moscow 79 (13), 1635–1652. doi:10.1134/S0006297914130124
Li, W., Wang, X. H., Luo, Z., Liu, L. F., Yan, C., Yan, C. Y., et al. (2018). Traditional Chinese Medicine as a Potential Source for HSV-1 Therapy by Acting on Virus or the Susceptibility of Host. Int. J. Mol. Sci. 19 (10). doi:10.3390/ijms19103266
Looker, K. J., Johnston, C., Welton, N. J., James, C., Vickerman, P., Turner, K. M. E., et al. (2020). The Global and Regional burden of Genital Ulcer Disease Due to Herpes Simplex Virus: a Natural History Modelling Study. BMJ Glob. Health 5 (3), e001875. doi:10.1136/bmjgh-2019-001875
Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., Gottlieb, S. L., et al. (2015). Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One 10 (10), e0140765. doi:10.1371/journal.pone.0140765
Lu, Z. Y., Cheng, M. H., Yu, C. Y., Lin, Y. S., Yeh, T. M., Chen, C. L., et al. (2020). Dengue Nonstructural Protein 1 Maintains Autophagy through Retarding Caspase-Mediated Cleavage of Beclin-1. Int. J. Mol. Sci. 21 (24). doi:10.3390/ijms21249702
Manning, B. D., and Cantley, L. C. (2007). AKT/PKB Signaling: Navigating Downstream. Cell 129 (7), 1261–1274. doi:10.1016/j.cell.2007.06.009
Marino, G., Niso-Santano, M., Baehrecke, E. H., and Kroemer, G. (2014). Self-consumption: the Interplay of Autophagy and Apoptosis. Nat. Rev. Mol. Cell Biol 15 (2), 81–94. doi:10.1038/nrm3735
Mauthe, M., Orhon, I., Rocchi, C., Zhou, X., Luhr, M., Hijlkema, K. J., et al. (2018). Chloroquine Inhibits Autophagic Flux by Decreasing Autophagosome-Lysosome Fusion. Autophagy 14 (8), 1435–1455. doi:10.1080/15548627.2018.1474314
Paz-Bailey, G., Ramaswamy, M., Hawkes, S. J., and Geretti, A. M. (2007). Herpes Simplex Virus Type 2: Epidemiology and Management Options in Developing Countries. Sex. Transm. Infections 83 (1), 16–22. doi:10.1136/sti.2006.020966
Pei, J., Deng, J., Ye, Z., Wang, J., Gou, H., Liu, W., et al. (2016). Absence of Autophagy Promotes Apoptosis by Modulating the ROS-dependent RLR Signaling Pathway in Classical Swine Fever Virus-Infected Cells. Autophagy 12 (10), 1738–1758. doi:10.1080/15548627.2016.1196318
Peng, J., Zhu, S., Hu, L., Ye, P., Wang, Y., Tian, Q., et al. (2016). Wild-type Rabies Virus Induces Autophagy in Human and Mouse Neuroblastoma Cell Lines. Autophagy 12 (10), 1704–1720. doi:10.1080/15548627.2016.1196315
Shao, Q., Liu, T., Wang, W., Duan, Q., Liu, T., Xu, L., et al. (2020). The Chinese Herbal Prescription JZ-1 Induces Autophagy to Protect against Herpes Simplex Virus-2 in Human Vaginal Epithelial Cells by Inhibiting the PI3K/Akt/mTOR Pathway. J. Ethnopharmacol 254, 112611. doi:10.1016/j.jep.2020.112611
Shen, M. X., Ma, N., Li, M. K., Liu, Y. Y., Chen, T., Wei, F., et al. (2019). Antiviral Properties of R. Tanguticum Nanoparticles on Herpes Simplex Virus Type I In Vitro and In Vivo. Front. Pharmacol. 10, 959. doi:10.3389/fphar.2019.00959
Stefanidou, M., Ramos, I., Mas Casullo, V., Trepanier, J. B., Rosenbaum, S., Fernandez-Sesma, A., et al. (2013). Herpes Simplex Virus 2 (HSV-2) Prevents Dendritic Cell Maturation, Induces Apoptosis, and Triggers Release of Proinflammatory Cytokines: Potential Links to HSV-HIV Synergy. J. Virol. 87 (3), 1443–1453. doi:10.1128/JVI.01302-12
Sun, K., Luo, J., Guo, J., Yao, X., Jing, X., and Guo, F. (2020). The PI3K/AKT/mTOR Signaling Pathway in Osteoarthritis: a Narrative Review. Osteoarthritis Cartilage 28 (4), 400–409. doi:10.1016/j.joca.2020.02.027
Wang, G. (2008). A Systematic Review of Traditional Chinese Medicine for the Treatment of Genital Herpes Which Could clear Away Heat-Toxin ,eliminate Dampness, Tonify Qi and Strengthen the Body Resistance. Chengdu, China: Chengdu University of Traditional Chinese Medicine.
Wyllie, A. H. (1997). Apoptosis: an Overview. Br. Med. Bull. 53 (3), 451–465. doi:10.1093/oxfordjournals.bmb.a011623
Xu, F., Na, L., Li, Y., and Chen, L. (2020). Roles of the PI3K/AKT/mTOR Signalling Pathways in Neurodegenerative Diseases and Tumours. Cell Biosci 10, 54. doi:10.1186/s13578-020-00416-0
Yedowitz, J. C., and Blaho, J. A. (2005). Herpes Simplex Virus 2 Modulates Apoptosis and Stimulates NF-Kappa B Nuclear Translocation during Infection in Human Epithelial HEp-2 Cells. Virology 342 (2), 297–310. doi:10.1016/j.virol.2005.07.036
Keywords: JZ-1, HSV-2 infection, genital herpes, apoptosis, autophagy, PI3K/Akt/mTOR pathway
Citation: Shao Q, Wu F, Liu T, Wang W, Liu T, Jin X, Xu L, Ma Y, Huang G and Chen Z (2021) JieZe-1 Alleviates HSV-2 Infection-Induced Genital Herpes in Balb/c Mice by Inhibiting Cell Apoptosis via Inducing Autophagy. Front. Pharmacol. 12:775521. doi: 10.3389/fphar.2021.775521
Received: 14 September 2021; Accepted: 22 October 2021;
Published: 03 November 2021.
Edited by:
Gerard Bannenberg, Global Organization for EPA and DHA Omega-3s (GOED), United StatesReviewed by:
Alon Schneider Hait, Aarhus University, DenmarkCopyright © 2021 Shao, Wu, Liu, Wang, Liu, Jin, Xu, Ma, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuo Chen, Y2hlbnpAdGpoLnRqbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.