
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 23 November 2021
Sec. Integrative and Regenerative Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.772678
This article is part of the Research Topic Recent Trends in Pharmacological Treatment of Musculoskeletal Disorders View all 21 articles
Osteoarthritis (OA) is a degenerative joint disease in the musculoskeletal system with a relatively high incidence and disability rate in the elderly. It is characterized by the degradation of articular cartilage, inflammation of the synovial membrane, and abnormal structure in the periarticular and subchondral bones. Although progress has been made in uncovering the molecular mechanism, the etiology of OA is still complicated and unclear. Nevertheless, there is no treatment method that can effectively prevent or reverse the deterioration of cartilage and bone structure. In recent years, in the field of pharmacology, research focus has shifted to disease prevention and early treatment rather than disease modification in OA. Biologic agents become more and more attractive as their direct or indirect intervention effects on the initiation or development of OA. In this review, we will discuss a wide spectrum of biologic agents ranging from DNA, noncoding RNA, exosome, platelet-rich plasma (PRP), to protein. We searched for key words such as OA, DNA, gene, RNA, exosome, PRP, protein, and so on. From the pharmacological aspect, stem cell therapy is a very special technique, which is not included in this review. The literatures ranging from January 2016 to August 2021 were included and summarized. In this review, we aim to help readers have a complete and precise understanding of the current pharmacological research progress in the intervention of OA from the biological aspect and provide an indication for the future translational studies.
Osteoarthritis (OA) is a degenerative chronic joint disease mainly affects the elderly, causing pain and loss of movement function. The trends of an aging population worldwide and increasing obesity are likely to make OA a leading cause of disability in the elderly (Hunter et al., 2020). Although many risk factors such as abnormal joint biomechanics, bone-mass index, joint injury, and genetic variations have been identified in the causation of OA, the etiology of OA is still poorly understood. In a traditional point of view, cartilage degradation was purely caused by mechanical imbalance (Francisco et al., 2018). Currently, increasing evidence shows that OA is a complex condition, in which the whole joints, including cartilage, subchondral bone, and synovium probably, are all involved in the pathogenesis (Goldring and Goldring, 2016), among which degradation of cartilage caused by matrix proteases plays a pivotal role (Pérez-García et al., 2019). In general, OA is a disease resulting from an imbalance between catabolic and anabolic events. In recent years, biologic agents become more and more attractive as they either target specific catabolic events, such as inflammation or matrix degradation, or promote anabolic events, such as anti-inflammation or chondrogenesis. In this review, we provide an update of the current treatment strategies and recent research progress in the pharmacological intervention of OA from the biology aspect (Figure 1).
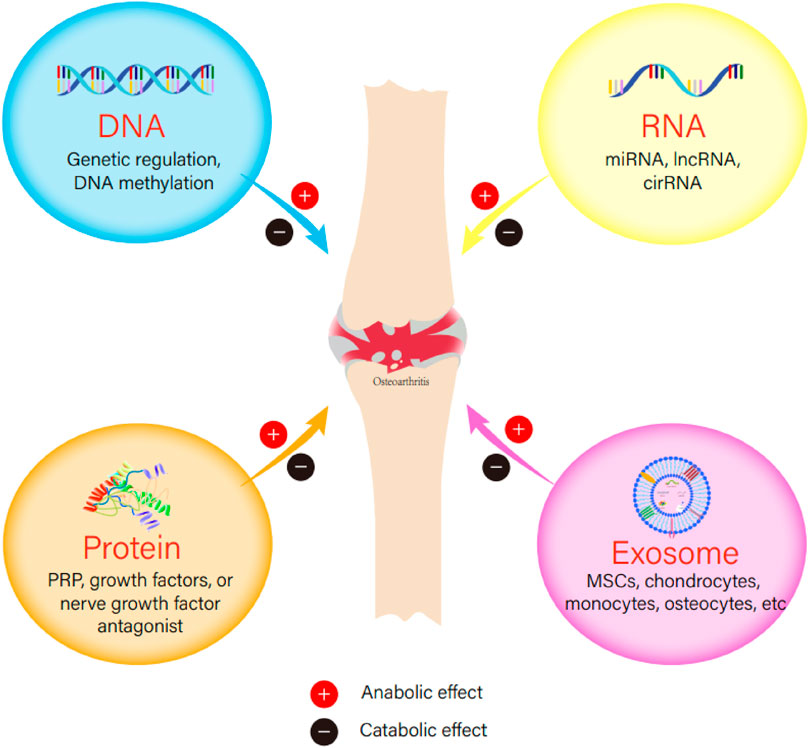
FIGURE 1. The anabolic or catabolic effect of DNA, RNA, protein, or exosome in the initiation or development of osteoarthritis.
We searched PubMed for combination of the following indexed subject headings [MeSH]: osteoarthritis, DNA, noncoding RNAs, exosomes, platelet-rich plasma, and proteins.
Clinical management for OA patients depends on their development stages of the disease. As the pathogenesis of OA is complicated, there is still no specific intervention for the treatment of OA. The primary goal for OA management is to alleviate pain and stiffness and maintain the joint function (Hermann et al., 2018). The treatment strategies for OA can be divided into three categories: nonpharmacological interventions, pharmacological interventions, and surgical interventions. Current consensus guidelines recommend the use of combination of nonpharmacological interventions, pharmacological interventions, and surgical intervention where necessary. The majority of individuals with OA can be managed successfully with a combination of nonpharmacological interventions and pharmacological interventions. However, surgical approaches should be considered at the late stages to repair the cartilage lesions or even replace the joint to regain the function.
Lifestyle modification and physical therapy are the two main nonpharmacological interventions. Body weight control in obese patients improves the symptoms and reduces the risk of symptomatic OA will develop. Exercise strengthens the muscle around the joints and maintain the stability. Physical therapy, such as pulsed electromagnetic fields (Yang et al., 2018a), extracorporeal shock wave therapy (ESWT) (Yu et al., 2017), acupuncture (Tu et al., 2021), and so on, improves the mobility and relieves the symptoms. Chondroitin sulfate and glucosamine have been used as dietary supplements.
Nonpharmacological interventions could be insufficient for many patients who develop symptomatic OA. Pharmaceutical agents, especially acetaminophen and nonsteroidal anti-inflammatory drugs, play a key role in symptom control. Other agents such as duloxetine (Weng et al., 2020), opioids, intra-articular steroid (Wijn et al., 2020), and viscosupplement injections are also approved for OA management. These drugs may effectively relieve the pain. However, many safety concerns have been raised regarding their side effects.
Surgical interventions are inevitable for many patients. Joint reservation surgeries, such as high tibial osteotomy and joint distraction, have shown symptomatic improvement (van der Woude et al., 2017). However, evidence for the long-term effectiveness is still to be confirmed. Unicompartmental knee arthroplasty (Murray and Parkinson, 2018), total knee arthroplasty (TKA) (Gademan et al., 2016), and total hip arthroplasty are widely accepted by the patients with end-stage OA.
DNA (Minchin and Lodge, 2019) is a double-stranded and long-chain polymer composed of four deoxynucleotides. DNA fragments with genetic information are called genes. At present, many genes are reported to be related to the occurrence and development of OA by increasing susceptibility, enhancing cartilaginous matrix degradation, preventing cartilage from repair, increasing the expression of inflammatory factors, or promoting fibroblast transformation. First, the susceptibility genes of OA mainly include ASPN (Wang et al., 2018), ADIPOQ (Shang et al., 2019), AKNA (Zhao et al., 2020a), DPEP1 (Zhang et al., 2021a), rs1065080 (Lu et al., 2019a), TLR7 (Wang et al., 2020a), RTP4 (Wang et al., 2020a), CRIP1 (Wang et al., 2020a), ZNF688 (Wang et al., 2020a), TOP1 (Wang et al., 2020a), EIF1AY (Wang et al., 2020a), RAB2A (Wang et al., 2020a), ZNF281 (Wang et al., 2020a), UIMC1 (Wang et al., 2020a), and PRKACB (Zhao, 2021). Second, the genes that promote the degradation of cartilage mainly include ADAMTS5 (Jiang et al., 2021), ADAM12 (Lv et al., 2017), JUN (Rhee et al., 2017), PTGS2 (Zhou et al., 2019a), MMP1 (Zhou et al., 2019a), MMP3 (Zhou et al., 2019a), MMP13 (Zhou et al., 2019a), AGT (Wang et al., 2020b), and rs2830585 (Zhou et al., 2019b). Third, several genes such as BMP3 (He et al., 2018), rs1799750 (Geng et al., 2018), and CHI3L1 (Song et al., 2021) show an inhibitory effect on cartilage repair. Fourth, the genes that regulate the expression of inflammatory cytokines in chondrocytes mainly include renin (Wu et al., 2019a), ACE (Wu et al., 2019a), Ang II (Wu et al., 2019a), AT1R (Wu et al., 2019a), AT2R (Wu et al., 2019a), ATF3 (Iezaki et al., 2016), PTGS2 (Lin et al., 2018; Wang et al., 2020a), CCL20 (Lin et al., 2018), CHI3L1 (Lin et al., 2018), LIF (Lin et al., 2018), CXCL8 (Lin et al., 2018), and CXCL12 (Lin et al., 2018). Last but not least, COL6A3/ACTG1 (Li et al., 2020a) and fibronectin1 (FN1) (Wu et al., 2020a) were found involved in fibroblast transformation. Although many catabolic genes have been found, there are very limited key anabolic genes that can promote the proliferation or differentiation of chondrocytes or encode key anchoring collagen molecules and the corresponding genes including GDF5 (Sun et al., 2021), Gas7 (Zhong et al., 2020), PRELP (Li et al., 2019a), TGF-β (Tao et al., 2016), SOX9 (Tao et al., 2016), and COL9A1 (Durand et al., 2020).
Genetic modification of joints has been achieved in preclinical models by ex vivo and in vivo strategies using a variety of vectors (Evans et al., 2018). Delivering genes from the body to the joints through direct intra-articular injection is a feasible way to speed up treatment. However, many vectors are inflammatory, immunogenic, or unsafe or provide only short-term transgene expression after successfully transferring cells into joint tissues. In order to solve this problem, an ideal delivery vector in vivo has been discovered; it is the adeno-associated virus (AAV), which is safer, more effective, and less immunogenic than other vectors (Evans et al., 2018). In addition, AAV also prolongs the expression time of transgenes in joints. When injected into the joint, the recombinant AAV will transduce synovial lining cells and chondrocytes at the thickness of the articular cartilage (Watson Levings et al., 2018). Besides the genetic regulation, epigenetic regulations, such as DNA methylation, may be also involved in OA pathology. Hypermethylation leads to a decrease in the expression of COL9A1, destroys the integrity of cartilage, and promotes the development of OA (Miranda-Duarte, 2018). SOX9 is a key transcription factor for cartilage formation in chondrocytes. The DNA methylation of SOX9 gene promoter in chondrocytes of patients with OA increases. This increase in methylation reduces the binding affinity of transcription factors, thereby reducing the expression of SOX9 in OA chondrocytes (He et al., 2020a). The DNA methyltransferases could be the potential targets to the treatment of OA in the future.
As mentioned, many studies in OA have focused on the epigenetic regulation of its pathogenesis and potential targets for therapy, specifically noncoding RNA (ncRNA). Human genome is estimated to contain ∼2% protein-coding RNA, whereas a vast majority of the genome comprises ncRNA. These ncRNAs, such as microRNA (miRNA), long noncoding RNA (lncRNA), and circular RNA (circRNA), are involved in the pathological development of OA, which can be used as diagnostic and therapeutic markers for OA progression and prognosis. Recent preclinical evidence shows that many ncRNAs can directly affect the expression of key genes involved in OA, which have great translational potential in OA treatment (Duan et al., 2020). Future research on elucidating the role of ncRNAs will also help in better understanding the etiology of OA. In particular, research and development of therapeutic targets for OA provide important clues (Cong et al., 2017). However, studies also report that many ncRNAs are considered the critical elements in cancer development (He et al., 2021a). Sufficient preclinical safety inspections should be performed before clinical use (Xie et al., 2020a).
Among those ncRNAs, miRNAs are most popular in recent years, with approximately 22 nucleotides, functioning in RNA silencing and posttranscriptional regulation of gene expression. Many studies have reported that several miRNAs could play an important role in regulating bone and cartilage homeostasis (Shen et al., 2019) (Table 1), through regulating the signaling pathways involved in extracellular matrix (ECM) degradation, apoptosis or hypotrophy of chondrocytes, or synovial inflammation.
lncRNAs are another type of ncRNAs that are longer than 200 nucleotides (Zhang et al., 2021b). LncRNA–RNA interaction controls mRNA translation and degradation, or as silent miRNA sponges. They are also regarded as important regulators of cartilage development (Table 2). The anti-OA mechanism of lncRNA may be achieved by competitively binding miRNA, reducing the binding of miRNA and downstream genes, and increasing the transcription and expression of downstream genes (Wu et al., 2019b).
CircRNA is a covalently closed circRNA molecule that contains exon sequences and is spliced at the canonical splicing site (Tam et al., 2019), functioning as miRNA sponges or competing endogenous RNAs that naturally sequester and competitively inhibit miRNA activity. CircRNAs also emerge as a new player in the development of OA through mechanisms such as interfering chondrocyte proliferation and apoptosis, regulating ECM degradation, and inflammation (Yang et al., 2020) (Table 3).
The protein currently used in clinical practice is mainly platelet-rich plasma (PRP) (Szwedowski et al., 2021). PRP is an autologous plasma preparation rich in platelets whose plasma concentration is higher than the normal concentration in whole blood. The basic principle of therapeutic potential of high-concentration platelets is based on their ability to provide superphysiological amounts of essential growth factors to provide regenerative stimulation that can promote tissue repair. PRP preparations need to be activated before use (Gentile et al., 2020). Intra-articular injections of PRP may be an effective alternative treatment to pain killers for knee OA (Rajan et al., 2020). It significantly promoted the proliferation of chondrocytes, decreased apoptosis, and increased autophagy by regulating the markers including FOXO1, FOXO3, and HIF-1 in osteoarthritic chondrocytes (Moussa et al., 2017). The concentration of white blood cells during the leukocyte-rich PRP (LR-PRP) preparation will affect its efficacy (Yaşar Şirin et al., 2017). It is reported that compared with the LR-PRP, the leukocyte-poor PRP (LP-PRP) has an effect on improving the proliferation of chondrocytes. The lubricating property of hyaluronic acid (HA) facilitates the movement of joints. And a combination of HA and PRP (HA–PRP) (Zhao et al., 2020b) could exert a beneficial synergistic effect for OA treatment. However, up until now, the preparation method and the components of PRP have still not been standardized, making the efficacy of PRP therapy to be inconclusive.
In addition to PRP, the proteins currently studied include nerve growth factor antibody (Grässel and Muschter, 2020) or its antagonists (Denk et al., 2017), fibroblast growth factor (FGF) (Xie et al., 2020b), insulin-like growth factor–binding proteins (IGFBP) (Tanaka et al., 2021), growth and differentiation factor 5 (Kania et al., 2020), Wnt16 (Tong et al., 2019), low-density lipoprotein receptor–related protein 5 (Wu et al., 2017a), neuropeptide Y (NPY) (Kang et al., 2020), and so on. Among the proteins, fasinumab (Dakin et al., 2020), tanezumab (Berenbaum et al., 2020), sprifermin (Eckstein et al., 2020), teriparatide (Apostu et al., 2019), and so on, have shown various effects on the management of OA in clinical trials. Nerve factor antibodies and their antagonists, fasinumab and tanezumab, can improve pain, and the antagonists have the most significant effect. Tanezumab can easily lead to rapidly progressive OA. FGF, GDF5, Wnt16, NPY, sprifermin, and teriparatide are related to cartilage repair. IGFBP is related to cartilage matrix synthesis. The binding of low-density lipoprotein receptor–related protein and sclerostin can inhibit the degradation of normal chondrocytes, but it does not seem to have such an effect in OA. The specific reason is not clear.
Recently, histone modifications have been recognized as another important epigenetic regulation in OA-related genes. LSD1 KDM4B, KDM6A, KDM6B, EZH2, and DOT1L were reported to be the major epigenetic regulators in OA onset and progression through their methyltransferases and demethylase activities by binding to the OA-related gene (e.g., Runx2, Nfat1, and Sox9) promoters or by interplaying with OA-associated signaling transduction pathways (Sacks et al., 2018). Modified histone domains have thus become epigenetic signatures, which will either mark for gene activation or gene repression. The role of methyltransferases and demethylase in epigenetic regulations also indicate they could be potential targets for the management of OA.
Exosomes are small, single-membrane, secreted organelles with a diameter of approximately 30–200 nm. They have the same topological structure as cells and are rich in selected proteins, lipids, nucleic acids, and glycoconjugates (Pegtel and Gould, 2019). Exosomes mainly mediate cell–cell communication through direct membrane fusion or protein–protein interaction (Wu et al., 2020b). The source of exosomes comes in many forms (Ni et al., 2020a), including peripheral blood (Chang et al., 2018), synovial fluid (Gao et al., 2020), mesenchymal stem cells (Tofiño-Vian et al., 2018), embryonic stem cells (Wang et al., 2017a), vascular endothelial cells (Yang et al., 2021a), dental pulp stem cells (Lin et al., 2021), monocytes (Bai et al., 2020), amniotic fluid stem cells (Beretti et al., 2018), chondrogenic progenitor cells (Toh et al., 2017), chondrocytes (Zheng et al., 2019), PRP (Liu et al., 2019a), osteocytes (Lyu et al., 2020) (Table 4), and so on. Exosomes with different origins may have different functions. Exosomes in the joint microenvironment are involved in the development of OA. Most therapeutic exosomes may have an anabolic effect by promoting expression of chondrogenic markers or cartilage ECM or exert an effect by inhibiting inflammation, hypertrophy, or apoptosis of chondrocytes (Zhou et al., 2020) showing great potential for OA therapy.
Sustained-release drug delivery systems have been developed by a combination of exosomes and tissue engineering strategies, showing great promising results in recent research by delivering targeted drug or nucleic acids for regenerative medicine (Akbari et al., 2020). However, because of complexity in the components and rare understanding of their functions, exosomes remain challenges for clinical applications (Zhou et al., 2020).
In order to analyze the research trends in the field of OA treatment using the biologic agent in recent years, we have reviewed relevant literature on DNA, RNA, protein, and exosome in the past 5 years on PubMed and also subdivided RNA into circRNA, lncRNA, and miRNA. We present a graphic (Figure 2) and the corresponding table (Supplementary Table S1) to show the literature trend in the past 5 years from January 2016 to August 2021. From the results, we can see that the number of articles of each type of biological agent has increased throughout the past 5 years. Among the four types of biologic agents, the most abundant research on proteins was found, followed by RNA, then DNA, and finally exosomes. Within RNA, miRNA has been studied most intensively, followed by lncRNA, and finally circRNA. This result shows that the research on proteins and RNA is relatively mature, but DNA and exosomes are new highlights in recent years. Within RNA, there are relatively many studies on miRNA and relatively fewer studies on lncRNA and circRNA. Therefore, DNA, exosomes, lncRNA, and circRNA may all become new research hotspots.
DNA, RNA, and protein described in this article have shown various regulatory effects on the pathological process of OA. Some of those are expected to become targets in terms of diagnosis and treatment of OA. In general, the effects of biologic agents are divided into two aspects: catabolic or anabolic effect by deteriorating or preventing OA occurrence or development. The catabolic effect is mainly to recruit inflammatory cells, inhibit chondrocyte proliferation, accelerate matrix degradation, or induce cell apoptosis. In opposite, the anabolic effect is mainly to reduce the production of catabolic enzymes, promote the proliferation of chondrocytes, inhibit chondrocyte apoptosis, promote the expression of ECM, or inhibit the expression of inflammatory factors. The main pathways involved in OA treatment are NF-κB, Notch, Wnt/β-catenin, TGF-β, Erk, p38 MAPK, JAK2/STAT3, and so on. At present, most researches on biologic agents are in vitro experiments or animal model experiments. There are still many obstacles to overcome for the biologics agents: (1) safety concern is the first to be considered when applying viral vectors to deliver plasmids, ncRNAs, which may bind to multiple targets; and exosomes and proteins, which may result in immunoresponse and disease transmission; (2) efficacy of most of the biologic agents in OA therapy is various and still yet to be verified; (3) heterogeneity of disease may also affect the therapeutic outcomes. With the advancement of molecular biotechnology in future research, translation research should be considered to address the limitations before clinical trials.
We have reviewed a wide spectrum of biologic agents in OA therapy, including DNA, RNA, protein, and exosomes, which provide an insight in finding potential therapeutic targets. Although significant progress has been made in this field, translational research is needed to further address the safety concerns, various efficacies, and heterogenetic of OA.
JD, ZZ, ZS, and HC did literature retrieval and prepared the draft, JD, JH, and YN made the first revision of the manuscript, HZ and BW finalized the manuscript.
This project was funded by Natural Science Foundation of Guangdong Province Science and Technology Department (2020A1515010003 and 2019A1515110724), Natural Science Foundation of China (NSFC, 81874000), Medical Research Fund of Guangdong Province (A2020151), “Peaking Plan” for the construction of high-level hospital at Affiliated Hospital of Guangdong Medical University (20501DFY20190168), Discipline construction project of Guangdong Medical University (4SG21002G), and also supported by the Science and Technology Project of Zhanjiang city (2020A01025 and 2019A01029).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.772678/full#supplementary-material
CircRNA, circular RNA; FGF, fibroblast growth factor; HA–PRP, hyaluronic acid–platelet-rich plasma; IGFBP, insulin-like growth factor–binding proteins; lncRNA, long noncoding RNA; LP-PRP, leukocyte-poor–platelet-rich plasma; LR-PRP, leukocyte-rich–platelet-rich plasma; miRNA, microRNA; OA, osteoarthritis; PRP, platelet-rich plasma; TGF-β, transforming growth factor β.
Ai, D., and Yu, F. (2019). LncRNA DNM3OS Promotes Proliferation and Inhibits Apoptosis through Modulating IGF1 Expression by Sponging MiR-126 in CHON-001 Cells. Diagn. Pathol. 14, 106. doi:10.1186/s13000-019-0877-2
Akbari, A., Jabbari, N., Sharifi, R., Ahmadi, M., Vahhabi, A., Seyedzadeh, S. J., et al. (2020). Free and Hydrogel Encapsulated Exosome-Based Therapies in Regenerative Medicine. Life Sci. 249, 117447. doi:10.1016/j.lfs.2020.117447
Apostu, D., Lucaciu, O., Mester, A., Oltean-Dan, D., Baciut, M., Baciut, G., et al. (2019). Systemic Drugs with Impact on Osteoarthritis. Drug Metab. Rev. 51, 498–523. doi:10.1080/03602532.2019.1687511
Bai, J., Zhang, Y., Zheng, X., Huang, M., Cheng, W., Shan, H., et al. (2020). LncRNA MM2P-Induced, Exosome-Mediated Transfer of Sox9 from Monocyte-Derived Cells Modulates Primary Chondrocytes. Cell Death Dis 11, 763. doi:10.1038/s41419-020-02945-5
Berenbaum, F., Blanco, F. J., Guermazi, A., Miki, K., Yamabe, T., Viktrup, L., et al. (2020). Subcutaneous Tanezumab for Osteoarthritis of the Hip or Knee: Efficacy and Safety Results from a 24-week Randomised Phase III Study with a 24-week Follow-Up Period. Ann. Rheum. Dis. 79, 800–810. doi:10.1136/annrheumdis-2019-216296
Beretti, F., Zavatti, M., Casciaro, F., Comitini, G., Franchi, F., Barbieri, V., et al. (2018). Amniotic Fluid Stem Cell Exosomes: Therapeutic Perspective. Biofactors 44, 158–167. doi:10.1002/biof.1407
Cao, L., Wang, Y., Wang, Q., and Huang, J. (2018). LncRNA FOXD2-AS1 Regulates Chondrocyte Proliferation in Osteoarthritis by Acting as a Sponge of miR-206 to Modulate CCND1 Expression. Biomed. Pharmacother. 106, 1220–1226. doi:10.1016/j.biopha.2018.07.048
Çelik, E., Bayram, C., and Denkbaş, E. B. (2019). Chondrogenesis of Human Mesenchymal Stem Cells by microRNA Loaded Triple Polysaccharide Nanoparticle System. Mater. Sci. Eng. C Mater. Biol. Appl. 102, 756–763. doi:10.1016/j.msec.2019.05.006
Chang, Y. H., Wu, K. C., Harn, H. J., Lin, S. Z., and Ding, D. C. (2018). Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cel Transpl. 27, 349–363. doi:10.1177/0963689717723636
Cheleschi, S., Tenti, S., Mondanelli, N., Corallo, C., Barbarino, M., Giannotti, S., et al. (2019). MicroRNA-34a and MicroRNA-181a Mediate Visfatin-Induced Apoptosis and Oxidative Stress via NF-Κb Pathway in Human Osteoarthritic Chondrocytes. Cells 8. doi:10.3390/cells8080874
Chen, C., Yin, P., Hu, S., Sun, X., and Li, B. (2020). Circular RNA-9119 Protects IL-1β-treated Chondrocytes from Apoptosis in an Osteoarthritis Cell Model by Intercepting the microRNA-26a/PTEN axis. Life Sci. 256, 117924. doi:10.1016/j.lfs.2020.117924
Chen, G., Liu, T., Yu, B., Wang, B., and Peng, Q. (2020). CircRNA-UBE2G1 Regulates LPS-Induced Osteoarthritis through miR-373/HIF-1a axis. Cell Cycle 19, 1696–1705. doi:10.1080/15384101.2020.1772545
Chen, K., Zhu, H., Zheng, M. Q., and Dong, Q. R. (2019). LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage, 1947603519855759.
Chu, P., Wang, Q., Wang, Z., and Gao, C. (2019). Long Non-coding RNA Highly Up-Regulated in Liver Cancer Protects Tumor Necrosis Factor-Alpha-Induced Inflammatory Injury by Down-Regulation of microRNA-101 in ATDC5 Cells. Int. Immunopharmacol 72, 148–158. doi:10.1016/j.intimp.2019.04.004
Cong, L., Zhu, Y., and Tu, G. (2017). A Bioinformatic Analysis of microRNAs Role in Osteoarthritis. Osteoarthritis Cartilage 25, 1362–1371. doi:10.1016/j.joca.2017.03.012
D'Adamo, S., Cetrullo, S., Guidotti, S., Borzì, R. M., and Flamigni, F. (2017). Hydroxytyrosol Modulates the Levels of microRNA-9 and its Target Sirtuin-1 Thereby Counteracting Oxidative Stress-Induced Chondrocyte Death. Osteoarthritis Cartilage 25, 600–610. doi:10.1016/j.joca.2016.11.014
Dakin, P., Kivitz, A. J., Gimbel, J. S., Skrepnik, N., DiMartino, S. J., Emeremni, C. A., et al. (2020). Efficacy and Safety of Fasinumab in Patients with Chronic Low Back Pain: a Phase II/III Randomised Clinical Trial. Ann. Rheum. Dis. 80, 509–517. doi:10.1136/annrheumdis-2020-217259
Denk, F., Bennett, D. L., and McMahon, S. B. (2017). Nerve Growth Factor and Pain Mechanisms. Annu. Rev. Neurosci. 40, 307–325. doi:10.1146/annurev-neuro-072116-031121
Duan, L., Liang, Y., Xu, X., Wang, J., Li, X., Sun, D., et al. (2020). Noncoding RNAs in Subchondral Bone Osteoclast Function and Their Therapeutic Potential for Osteoarthritis. Arthritis Res. Ther. 22, 279. doi:10.1186/s13075-020-02374-x
Durand, A. L., Dufour, A., Aubert-Foucher, E., Oger-Desfeux, C., Pasdeloup, M., Lustig, S., et al. (2020). The Lysine Specific Demethylase-1 Negatively Regulates the COL9A1 Gene in Human Articular Chondrocytes. Int. J. Mol. Sci. 21. doi:10.3390/ijms21176322
Eckstein, F., Kraines, J. L., Aydemir, A., Wirth, W., Maschek, S., and Hochberg, M. C. (2020). Intra-articular Sprifermin Reduces Cartilage Loss in Addition to Increasing Cartilage Gain Independent of Location in the Femorotibial Joint: post-hoc Analysis of a Randomised, Placebo-Controlled Phase II Clinical Trial. Ann. Rheum. Dis. 79, 525–528. doi:10.1136/annrheumdis-2019-216453
Evans, C. H., Ghivizzani, S. C., and Robbins, P. D. (2018). Gene Delivery to Joints by Intra-articular Injection. Hum. Gene Ther. 29, 2–14. doi:10.1089/hum.2017.181
Francisco, V., Pérez, T., Pino, J., López, V., Franco, E., Alonso, A., et al. (2018). Biomechanics, Obesity, and Osteoarthritis. The Role of Adipokines: When the Levee Breaks. J. Orthop. Res. 36, 594–604. doi:10.1002/jor.23788
Fu, Q., Zhu, J., Wang, B., Wu, J., Li, H., Han, Y., et al. (2021). LINC02288 Promotes Chondrocyte Apoptosis and Inflammation through miR-374a-3p Targeting RTN3. J. Gene Med. 23, e3314. doi:10.1002/jgm.3314
Gademan, M. G., Hofstede, S. N., Vliet Vlieland, T. P., Nelissen, R. G., and Marang-van de Mheen, P. J. (2016). Indication Criteria for Total Hip or Knee Arthroplasty in Osteoarthritis: a State-Of-The-Science Overview. BMC Musculoskelet. Disord. 17, 463. doi:10.1186/s12891-016-1325-z
Gao, F., Peng, C., Zheng, C., Zhang, S., and Wu, M. (2019). miRNA-101 Promotes Chondrogenic Differentiation in Rat Bone Marrow Mesenchymal Stem Cells. Exp. Ther. Med. 17, 175–180. doi:10.3892/etm.2018.6959
Gao, K., Zhu, W., Li, H., Ma, D., Liu, W., Yu, W., et al. (2020). Association between Cytokines and Exosomes in Synovial Fluid of Individuals with Knee Osteoarthritis. Mod. Rheumatol. 30, 758–764. doi:10.1080/14397595.2019.1651445
Geng, R., Xu, Y., Hu, W., and Zhao, H. (2018). The Association between MMP-1 Gene Rs1799750 Polymorphism and Knee Osteoarthritis Risk. Biosci. Rep. 38. doi:10.1042/BSR20181257
Gentile, P., Calabrese, C., De Angelis, B., Dionisi, L., Pizzicannella, J., Kothari, A., et al. (2020). Impact of the Different Preparation Methods to Obtain Autologous Non-activated Platelet-Riched Plasma (A-PRP) and Activated Platelet-Riched Plasma (AA-PRP) in Plastic Surgery: Wound Healing and Hair Regrowth Evaluation. Int. J. Mol. Sci. 21. doi:10.3390/ijms21020431
Ghafouri-Fard, S., Abak, A., Tavakkoli Avval, S., Rahmani, S., Shoorei, H., Taheri, M., et al. (2021). Contribution of miRNAs and lncRNAs in Osteogenesis and Related Disorders. Biomed. Pharmacother. 142, 111942. doi:10.1016/j.biopha.2021.111942
Goldring, S. R., and Goldring, M. B. (2016). Changes in the Osteochondral Unit during Osteoarthritis: Structure, Function and Cartilage-Bone Crosstalk. Nat. Rev. Rheumatol. 12, 632–644. doi:10.1038/nrrheum.2016.148
Grässel, S., and Muschter, D. (2020). Recent Advances in the Treatment of Osteoarthritis. F1000Res 9. doi:10.12688/f1000research.22115.1
Guillén, M. I., Compañ, A., and Alcaraz, M. J. (2021). Evaluation of Extracellular Vesicles from Adipose Tissue-Derived Mesenchymal Stem Cells in Primary Human Chondrocytes from Patients with Osteoarthritis. Methods Mol. Biol. 2269, 221–231. doi:10.1007/978-1-0716-1225-5_15
Han, W., and Liu, J. (2018). LncRNA-p21 Inhibited the Proliferation of Osteosarcoma Cells via the miR-130b/PTEN/AKT Signaling Pathway. Biomed. Pharmacother. 97, 911–918. doi:10.1016/j.biopha.2017.11.014
He, B., and Jiang, D. (2020). HOTAIR-induced Apoptosis Is Mediated by Sponging miR-130a-3p to Repress Chondrocyte Autophagy in Knee Osteoarthritis. Cell Biol Int 44, 524–535. doi:10.1002/cbin.11253
He, C. P., Jiang, X. C., Chen, C., Zhang, H. B., Cao, W. D., Wu, Q., et al. (2021). The Function of lncRNAs in the Pathogenesis of Osteoarthritis. Bone Jt. Res 10, 122–133. doi:10.1302/2046-3758.102.BJR-2020-0228.R1
He, J., Cao, W., Azeem, I., and Shao, Z. (2020). Epigenetics of Osteoarthritis: Histones and TGF-Β1. Clin. Chim. Acta 510, 593–598. doi:10.1016/j.cca.2020.08.011
He, J., Su, X., and Xie, W. (2020). MiR-582-3p Alleviates Osteoarthritis Progression by Targeting YAP1. Mol. Immunol. 128, 258–267. doi:10.1016/j.molimm.2020.10.022
He, X., Gao, K., Lu, S., and Wu, R. (2021). LncRNA HOTTIP Leads to Osteoarthritis Progression via Regulating miR-663a/Fyn-Related Kinase axis. BMC Musculoskelet. Disord. 22, 67. doi:10.1186/s12891-020-03861-7
He, Y., Yao, W., Zhang, M., Zhang, Y., Zhang, D., Jiang, Z., et al. (2018). Changes in Osteogenic Gene Expression in Hypertrophic Chondrocytes Induced by SIN-1. Exp. Ther. Med. 16, 609–618. doi:10.3892/etm.2018.6261
Hermann, W., Lambova, S., and Muller-Ladner, U. (2018). Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 14, 108–116. doi:10.2174/1573397113666170829155149
Hu, G., Zhao, X., Wang, C., Geng, Y., Zhao, J., Xu, J., et al. (2017). MicroRNA-145 Attenuates TNF-α-Driven Cartilage Matrix Degradation in Osteoarthritis via Direct Suppression of MKK4. Cel Death Dis 8, e3140. doi:10.1038/cddis.2017.522
Hu, J., Wang, Z., Shan, Y., Pan, Y., Ma, J., and Jia, L. (2018). Long Non-coding RNA HOTAIR Promotes Osteoarthritis Progression via miR-17-5p/FUT2/β-Catenin axis. Cel Death Dis 9, 711. doi:10.1038/s41419-018-0746-z
Hu, Y., Li, S., and Zou, Y. (2019). Knockdown of LncRNA H19 Relieves LPS-Induced Damage by Modulating miR-130a in Osteoarthritis. Yonsei Med. J. 60, 381–388. doi:10.3349/ymj.2019.60.4.381
Huang, B., Yu, H., Li, Y., Zhang, W., and Liu, X. (2019). Upregulation of Long Noncoding TNFSF10 Contributes to Osteoarthritis Progression through the miR-376-3p/FGFR1 axis. J. Cel Biochem 120, 19610–19620. doi:10.1002/jcb.29267
Huang, J., Liu, L., Yang, J., Ding, J., and Xu, X. (2019). lncRNA DILC Is Downregulated in Osteoarthritis and Regulates IL-6 Expression in Chondrocytes. J. Cel Biochem 120, 16019–16024. doi:10.1002/jcb.28880
Huang, J., Zhao, L., Fan, Y., Liao, L., Ma, P. X., Xiao, G., et al. (2019). The microRNAs miR-204 and miR-211 Maintain Joint Homeostasis and Protect against Osteoarthritis Progression. Nat. Commun. 10, 2876. doi:10.1038/s41467-019-10753-5
Huang, T., Wang, J., Zhou, Y., Zhao, Y., Hang, D., and Cao, Y. (2019). LncRNA CASC2 Is Up-Regulated in Osteoarthritis and Participates in the Regulation of IL-17 Expression and Chondrocyte Proliferation and Apoptosis. Biosci. Rep. 39. doi:10.1042/BSR20182454
Hunter, D. J., March, L., and Chew, M. (2020). Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet 396, 1711–1712. doi:10.1016/S0140-6736(20)32230-3
Iezaki, T., Ozaki, K., Fukasawa, K., Inoue, M., Kitajima, S., Muneta, T., et al. (2016). ATF3 Deficiency in Chondrocytes Alleviates Osteoarthritis Development. J. Pathol. 239, 426–437. doi:10.1002/path.4739
Ji, M. L., Jiang, H., Wu, F., Geng, R., Ya, L. K., Lin, Y. C., et al. (2020). Precise Targeting of miR-141/200c Cluster in Chondrocytes Attenuates Osteoarthritis Development. Ann. Rheum. Dis. 80 (3), 356–366. doi:10.1136/annrheumdis-2020-218469
Ji, Q., Qi, D., Xu, X., Xu, Y., Goodman, S. B., Kang, L., et al. (2018). Cryptotanshinone Protects Cartilage against Developing Osteoarthritis through the miR-106a-5p/GLIS3 Axis. Mol. Ther. Nucleic Acids 11, 170–179. doi:10.1016/j.omtn.2018.02.001
Ji, Q., Qiao, X., Liu, Y., Wang, D., and Yan, J. (2020). Silencing of Long-chain N-on-coding RNA GAS5 in O-steoarthritic C-hondrocytes I-s M-ediated by T-argeting the miR-34a/Bcl-2 axis. Mol. Med. Rep. 21, 1310–1319. doi:10.3892/mmr.2019.10900
Jiang, L., Lin, J., Zhao, S., Wu, J., Jin, Y., Yu, L., et al. (2021). ADAMTS5 in Osteoarthritis: Biological Functions, Regulatory Network, and Potential Targeting Therapies. Front. Mol. Biosci. 8, 703110. doi:10.3389/fmolb.2021.703110
Jiang, M., Liu, J., Luo, T., Chen, Q., Lu, M., and Meng, D. (2019). LncRNA PACER Is Down-Regulated in Osteoarthritis and Regulates Chondrocyte Apoptosis and lncRNA HOTAIR Expression. Biosci. Rep. 39. doi:10.1042/BSR20190404
Kang, X., Qian, Z., Liu, J., Feng, D., Li, H., Zhang, Z., et al. (2020). Neuropeptide Y Acts Directly on Cartilage Homeostasis and Exacerbates Progression of Osteoarthritis through NPY2R. J. Bone Miner Res. 35, 1375–1384. doi:10.1002/jbmr.3991
Kania, K., Colella, F., Riemen, A. H. K., Wang, H., Howard, K. A., Aigner, T., et al. (2020). Regulation of Gdf5 Expression in Joint Remodelling, Repair and Osteoarthritis. Sci. Rep. 10, 157. doi:10.1038/s41598-019-57011-8
Kim, Y. G., Choi, J., and Kim, K. (2020). Mesenchymal Stem Cell-Derived Exosomes for Effective Cartilage Tissue Repair and Treatment of Osteoarthritis. Biotechnol. J. 15, e2000082. doi:10.1002/biot.202000082
Komori, T. (2016). Glucocorticoid Signaling and Bone Biology. Horm. Metab. Res. 48, 755–763. doi:10.1055/s-0042-110571
Lei, J., Fu, Y., Zhuang, Y., Zhang, K., and Lu, D. (2019). LncRNA SNHG1 Alleviates IL-1β-induced Osteoarthritis by Inhibiting miR-16-5p-Mediated P38 MAPK and NF-Κb Signaling Pathways. Biosci. Rep. 39. doi:10.1042/BSR20191523
Li, B. F., Zhang, Y., Xiao, J., Wang, F., Li, M., Guo, X. Z., et al. (2017). Hsa_circ_0045714 Regulates Chondrocyte Proliferation, Apoptosis and Extracellular Matrix Synthesis by Promoting the Expression of miR-193b Target Gene IGF1R. Hum. Cel 30, 311–318. doi:10.1007/s13577-017-0177-7
Li, C., Luo, J., Xu, X., Zhou, Z., Ying, S., Liao, X., et al. (2020). Single Cell Sequencing Revealed the Underlying Pathogenesis of the Development of Osteoarthritis. Gene 757, 144939. doi:10.1016/j.gene.2020.144939
Li, C., Pan, S., Song, Y., Li, Y., and Qu, J. (2019). Silence of lncRNA MIAT Protects ATDC5 Cells against Lipopolysaccharides challenge via Up-Regulating miR-132. Artif. Cell Nanomed Biotechnol 47, 2521–2527. doi:10.1080/21691401.2019.1626410
Li, H., Yang, H. H., Sun, Z. G., Tang, H. B., and Min, J. K. (2019). Whole-transcriptome Sequencing of Knee Joint Cartilage from Osteoarthritis Patients. Bone Jt. Res 8, 290–303. doi:10.1302/2046-3758.87.BJR-2018-0297.R1
Li, L., Lv, G., Wang, B., and Kuang, L. (2018). The Role of lncRNA XIST/miR-211 axis in Modulating the Proliferation and Apoptosis of Osteoarthritis Chondrocytes through CXCR4 and MAPK Signaling. Biochem. Biophys. Res. Commun. 503, 2555–2562. doi:10.1016/j.bbrc.2018.07.015
Li, L., Zhang, L., Zhang, Y., Jiang, D., Xu, W., Zhao, H., et al. (2019). Inhibition of Long Non-coding RNA CTD-2574D22.4 Alleviates LPS-Induced Apoptosis and Inflammatory Injury of Chondrocytes. Curr. Pharm. Des. 25, 2969–2974. doi:10.2174/1381612825666190801141801
Li, Q., Zhang, Z., Guo, S., Tang, G., Lu, W., and Qi, X. (2019). LncRNA ANCR Is Positively Correlated with Transforming Growth Factor-Β1 in Patients with Osteoarthritis. J. Cel Biochem 120, 14226–14232. doi:10.1002/jcb.28881
Li, X., Huang, T. L., Zhang, G. D., Jiang, J. T., and Guo, P. Y. (2019). LncRNA ANRIL Impacts the Progress of Osteoarthritis via Regulating Proliferation and Apoptosis of Osteoarthritis Synoviocytes. Eur. Rev. Med. Pharmacol. Sci. 23, 9729–9737. doi:10.26355/eurrev_201911_19535
Li, X., Ren, W., Xiao, Z. Y., Wu, L. F., Wang, H., and Guo, P. Y. (2018). GACAT3 Promoted Proliferation of Osteoarthritis Synoviocytes by IL-6/STAT3 Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 22, 5114–5120. doi:10.26355/eurrev_201808_15705
Li, X., Yu, M., Chen, L., Sun, T., Wang, H., Zhao, L., et al. (2019). LncRNA PMS2L2 Protects ATDC5 Chondrocytes against Lipopolysaccharide-Induced Inflammatory Injury by Sponging miR-203. Life Sci. 217, 283–292. doi:10.1016/j.lfs.2018.12.020
Li, X. F., Wang, Z. Q., Li, L. Y., Zhao, G. Q., and Yu, S. N. (2018). Downregulation of the Long Noncoding RNA MBNL1-AS1 Protects Sevoflurane-Pretreated Mice against Ischemia-Reperfusion Injury by Targeting KCNMA1. Exp. Mol. Med. 50, 115–116. doi:10.1038/s12276-018-0133-y
Li, Y., Li, S., Luo, Y., Liu, Y., and Yu, N. (2017). LncRNA PVT1 Regulates Chondrocyte Apoptosis in Osteoarthritis by Acting as a Sponge for miR-488-3p. DNA Cel Biol 36, 571–580. doi:10.1089/dna.2017.3678
Li, Y., Li, Z., Li, C., Zeng, Y., and Liu, Y. (2019). Long Noncoding RNA TM1P3 Is Involved in Osteoarthritis by Mediating Chondrocyte Extracellular Matrix Degradation. J. Cel Biochem 120, 12702–12712. doi:10.1002/jcb.28539
Li, Y. F., Li, S. H., Liu, Y., and Luo, Y. T. (2017). Long Noncoding Rna Cir Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis by Acting as a Sponge for Mir-27b. Cell Physiol Biochem 43, 602–610. doi:10.1159/000480532
Li, Z., Cheng, J., and Liu, J. (2020). Baicalin Protects Human OA Chondrocytes against IL-1β-Induced Apoptosis and ECM Degradation by Activating Autophagy via MiR-766-3p/AIFM1 Axis. Drug Des. Devel Ther. 14, 2645–2655. doi:10.2147/DDDT.S255823
Li, Z., Yuan, B., Pei, Z., Zhang, K., Ding, Z., Zhu, S., et al. (2019). Circ_0136474 and MMP-13 Suppressed Cell Proliferation by Competitive Binding to miR-127-5p in Osteoarthritis. J. Cel Mol Med 23, 6554–6564. Circ_0136474. doi:10.1111/jcmm.14400
Liao, H. X., Zhang, Z. H., Chen, H. L., Huang, Y. M., Liu, Z. L., and Huang, J. (2021). CircHYBID Regulates Hyaluronan Metabolism in Chondrocytes via Hsa-miR-29b-3p/TGF-Β1 axis. Mol. Med. 27, 56. doi:10.1186/s10020-021-00319-x
Lin, J., Wu, G., Zhao, Z., Huang, Y., Chen, J., Fu, C., et al. (2018). Bioinformatics Analysis to Identify Key Genes and Pathways Influencing Synovial Inflammation in Osteoarthritis. Mol. Med. Rep. 18, 5594–5602. doi:10.3892/mmr.2018.9575
Lin, S. S., Yuan, L. J., Niu, C. C., Tu, Y. K., Yang, C. Y., and Ueng, S. W. N. (2019). Hyperbaric Oxygen Inhibits the HMGB1/RAGE Signaling Pathway by Upregulating Mir-107 Expression in Human Osteoarthritic Chondrocytes. Osteoarthritis Cartilage 27, 1372–1381. doi:10.1016/j.joca.2019.05.011
Lin, T., Wu, N., Wang, L., Zhang, R., Pan, R., and Chen, Y-F. (2021). Inhibition of Chondrocyte Apoptosis in a Rat Model of Osteoarthritis by Exosomes Derived from miR-140-5p-Overexpressing Human Dental Pulp Stem Cells. Int. J. Mol. Med. 47. doi:10.3892/ijmm.2020.4840
Liu, F., Liu, X., Yang, Y., Sun, Z., Deng, S., Jiang, Z., et al. (2020). NEAT1/miR-193a-3p/SOX5 axis Regulates Cartilage Matrix Degradation in Human Osteoarthritis. Cel Biol Int 44, 947–957. doi:10.1002/cbin.11291
Liu, G., Wang, Y., Zhang, M., and Zhang, Q. (2019). Long Non-coding RNA THRIL Promotes LPS-Induced Inflammatory Injury by Down-Regulating microRNA-125b in ATDC5 Cells. Int. Immunopharmacol 66, 354–361. doi:10.1016/j.intimp.2018.11.038
Liu, J., Yu, Q., Ye, Y., Yan, Y., and Chen, X. (2018). Abnormal Expression of miR-4784 in Chondrocytes of Osteoarthritis and Associations with Chondrocyte Hyperplasia. Exp. Ther. Med. 16, 4690–4694. doi:10.3892/etm.2018.6739
Liu, Q., Hu, X., Zhang, X., Dai, L., Duan, X., Zhou, C., et al. (2016). The TMSB4 Pseudogene LncRNA Functions as a Competing Endogenous RNA to Promote Cartilage Degradation in Human Osteoarthritis. Mol. Ther. 24, 1726–1733. doi:10.1038/mt.2016.151
Liu, X., Liu, L., Zhang, H., Shao, Y., Chen, Z., Feng, X., et al. (2019). MiR-146b Accelerates Osteoarthritis Progression by Targeting Alpha-2-Macroglobulin. Aging (Albany NY) 11, 6014–6028. doi:10.18632/aging.102160
Liu, X., Wang, L., Ma, C., Wang, G., Zhang, Y., and Sun, S. (2019). Exosomes Derived from Platelet-Rich Plasma Present a Novel Potential in Alleviating Knee Osteoarthritis by Promoting Proliferation and Inhibiting Apoptosis of Chondrocyte via Wnt/β-Catenin Signaling Pathway. J. Orthop. Surg. Res. 14, 470. doi:10.1186/s13018-019-1529-7
Liu, Y., Li, Q., Gao, Z., Lei, F., and Gao, X. (2021). Circ-SPG11 Knockdown Hampers IL-1β-induced Osteoarthritis Progression via Targeting miR-337-3p/ADAMTS5. J. Orthop. Surg. Res. 16, 392. doi:10.1186/s13018-021-02526-y
Lu, C., Li, Z., Hu, S., Cai, Y., and Peng, K. (2019). LncRNA PART-1 Targets TGFBR2/Smad3 to Regulate Cell Viability and Apoptosis of Chondrocytes via Acting as miR-590-3p Sponge in Osteoarthritis. J. Cel Mol Med 23, 8196–8205. doi:10.1111/jcmm.14690
Lu, C., Shu, J., Han, Y., Ren, X. Y., Xu, K., Fan, H., et al. (2019). The Polymorphism of SMAD3 Rs1065080 Is Associated with Increased Risk for Knee Osteoarthritis. Mol. Biol. Rep. 46, 4501–4505. doi:10.1007/s11033-019-04905-5
Lu, J., Ji, M. L., Zhang, X. J., Shi, P. L., Wu, H., Wang, C., et al. (2017). MicroRNA-218-5p as a Potential Target for the Treatment of Human Osteoarthritis. Mol. Ther. 25, 2676–2688. doi:10.1016/j.ymthe.2017.08.009
Lu, X., Li, Y., Chen, H., Pan, Y., Lin, R., and Chen, S. (2021). miR-335-5P Contributes to Human Osteoarthritis by Targeting HBP1. Exp. Ther. Med. 21, 109. doi:10.3892/etm.2020.9541
Lv, Z. T., Liang, S., Huang, X. J., Cheng, P., Zhu, W. T., and Chen, A. M. (2017). Association between ADAM12 Single-Nucleotide Polymorphisms and Knee Osteoarthritis: A Meta-Analysis. Biomed. Res. Int. 2017, 5398181. doi:10.1155/2017/5398181
Lyu, H., Xiao, Y., Guo, Q., Huang, Y., and Luo, X. (2020). The Role of Bone-Derived Exosomes in Regulating Skeletal Metabolism and Extraosseous Diseases. Front Cel Dev Biol 8, 89. doi:10.3389/fcell.2020.00089
Ma, H. R., Mu, W. B., Zhang, K. Y., Zhou, H. K., Jiang, R. D., and Cao, L. (2020). CircVCAN Regulates the Proliferation and Apoptosis of Osteoarthritis Chondrocyte through NF-Κb Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 24, 6517–6525. doi:10.26355/eurrev_202006_21635
Minchin, S., and Lodge, J. (2019). Understanding Biochemistry: Structure and Function of Nucleic Acids. Essays Biochem. 63, 433–456. doi:10.1042/EBC20180038
Miranda-Duarte, A. (2018). DNA Methylation in Osteoarthritis: Current Status and Therapeutic Implications. Open Rheumatol. J. 12, 37–49. doi:10.2174/1874312901812010037
Moussa, M., Lajeunesse, D., Hilal, G., El Atat, O., Haykal, G., Serhal, R., et al. (2017). Platelet Riched Plasma (PRP) Induces Chondroprotection via Increasing Autophagy, Anti-inflammatory Markers, and Decreasing Apoptosis in Human Osteoarthritic Cartilage. Exp. Cel Res 352, 146–156. doi:10.1016/j.yexcr.2017.02.012
Murray, D. W., and Parkinson, R. W. (2018). Usage of Unicompartmental Knee Arthroplasty. Bone Jt. J 100-b, 432–435. doi:10.1302/0301-620X.100B4.BJJ-2017-0716.R1
Ni, J. L., Dang, X. Q., and Shi, Z. B. (2020). CircPSM3 Inhibits the Proliferation and Differentiation of OA Chondrocytes by Targeting miRNA-296-5p. Eur. Rev. Med. Pharmacol. Sci. 24, 3467–3475. doi:10.26355/eurrev_202004_20805
Ni, Z., Zhou, S., Li, S., Kuang, L., Chen, H., Luo, X., et al. (2020). Exosomes: Roles and Therapeutic Potential in Osteoarthritis. Bone Res. 8, 25. doi:10.1038/s41413-020-0100-9
Park, S., Lee, M., Chun, C. H., and Jin, E. J. (2019). The lncRNA, Nespas, Is Associated with Osteoarthritis Progression and Serves as a Potential New Prognostic Biomarker. Cartilage 10, 148–156. doi:10.1177/1947603517725566
Pegtel, D. M., and Gould, S. J. (2019). Exosomes. Annu. Rev. Biochem. 88, 487–514. doi:10.1146/annurev-biochem-013118-111902
Pérez-García, S., Carrión, M., Gutiérrez-Cañas, I., Villanueva-Romero, R., Castro, D., Martínez, C., et al. (2019). Profile of Matrix-Remodeling Proteinases in Osteoarthritis: Impact of Fibronectin. Cells 9, 40. doi:10.3390/cells9010040
Qi, K., Lin, R., Xue, C., Liu, T., Wang, Y., Zhang, Y., et al. (2019). Long Non-coding RNA (LncRNA) CAIF Is Downregulated in Osteoarthritis and Inhibits LPS-Induced Interleukin 6 (IL-6) Upregulation by Downregulation of MiR-1246. Med. Sci. Monit. 25, 8019–8024. doi:10.12659/MSM.917135
Qiu, M., Liu, D., and Fu, Q. (2021). MiR-129-5p Shuttled by Human Synovial Mesenchymal Stem Cell-Derived Exosomes Relieves IL-1β Induced Osteoarthritis via Targeting HMGB1. Life Sci. 269, 118987. doi:10.1016/j.lfs.2020.118987
Rajan, P. V., Ng, M. K., Klika, A., Kamath, A. F., Muschler, G. F., Higuera, C. A., et al. (2020). The Cost-Effectiveness of Platelet-Rich Plasma Injections for Knee Osteoarthritis: A Markov Decision Analysis. J. Bone Jt. Surg Am 102, e104. doi:10.2106/JBJS.19.01446
Rhee, J., Park, S. H., Kim, S. K., Kim, J. H., Ha, C. W., Chun, C. H., et al. (2017). Inhibition of BATF/JUN Transcriptional Activity Protects against Osteoarthritic Cartilage Destruction. Ann. Rheum. Dis. 76, 427–434. doi:10.1136/annrheumdis-2015-208953
Sacks, D., Sacks, D., Baxter, B., Campbell, B. C. V., Carpenter, J. S., Cognard, C., et al. (2018). Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. AJNR Am. J. Neuroradiol 39, E61–E632. doi:10.3174/ajnr.A5638
Sekar, D. (2021). Implications of microRNA 21 and its Involvement in the Treatment of Different Type of Arthritis. Mol. Cel Biochem 476, 941–947. doi:10.1007/s11010-020-03960-y
Shang, H., Hao, Y., Hu, W., Hu, X., and Jin, Q. (2019). Association between ADIPOQ Gene Variants and Knee Osteoarthritis in a Chinese Population. Biosci. Rep. 39. doi:10.1042/BSR20182104
Shen, S., Wu, Y., Chen, J., Xie, Z., Huang, K., Wang, G., et al. (2019). CircSERPINE2 Protects against Osteoarthritis by Targeting miR-1271 and ETS-Related Gene. Ann. Rheum. Dis. 78, 826–836. doi:10.1136/annrheumdis-2018-214786
Si, H. B., Zeng, Y., Liu, S. Y., Zhou, Z. K., Chen, Y. N., Cheng, J. Q., et al. (2017). Intra-articular Injection of microRNA-140 (miRNA-140) Alleviates Osteoarthritis (OA) Progression by Modulating Extracellular Matrix (ECM) Homeostasis in Rats. Osteoarthritis Cartilage 25, 1698–1707. doi:10.1016/j.joca.2017.06.002
Song, Y., Hao, D., Jiang, H., Huang, M., Du, Q., Lin, Y., et al. (2021). Nrf2 Regulates CHI3L1 to Suppress Inflammation and Improve Post-Traumatic Osteoarthritis. Jir Vol. 14, 4079–4088. doi:10.2147/jir.s310831
Sun, K., Guo, J., Yao, X., Guo, Z., and Guo, F. (2021). Growth Differentiation Factor 5 in Cartilage and Osteoarthritis: A Possible Therapeutic Candidate. Cell Prolif 54, e12998. doi:10.1111/cpr.12998
Szwedowski, D., Szczepanek, J., Paczesny, Ł., Zabrzyński, J., Gagat, M., Mobasheri, A., et al. (2021). The Effect of Platelet-Rich Plasma on the Intra-articular Microenvironment in Knee Osteoarthritis. Int. J. Mol. Sci. 22. doi:10.3390/ijms22115492
Tam, C., Wong, J. H., Tsui, S. K. W., Zuo, T., Chan, T. F., and Ng, T. B. (2019). LncRNAs with miRNAs in Regulation of Gastric, Liver, and Colorectal Cancers: Updates in Recent Years. Appl. Microbiol. Biotechnol. 103, 4649–4677. doi:10.1007/s00253-019-09837-5
Tanaka, N., Tsuno, H., Ohashi, S., Iwasawa, M., Furukawa, H., Kato, T., et al. (2021). The Attenuation of Insulin-like Growth Factor Signaling May Be Responsible for Relative Reduction in Matrix Synthesis in Degenerated Areas of Osteoarthritic Cartilage. BMC Musculoskelet. Disord. 22, 231. doi:10.1186/s12891-021-04096-w
Tang, L. P., Ding, J. B., Liu, Z. H., and Zhou, G. J. (2018). LncRNA TUG1 Promotes Osteoarthritis-Induced Degradation of Chondrocyte Extracellular Matrix via miR-195/MMP-13 axis. Eur. Rev. Med. Pharmacol. Sci. 22, 8574–8581. doi:10.26355/eurrev_201812_16620
Tao, K., Rey-Rico, A., Frisch, J., Venkatesan, J. K., Schmitt, G., Madry, H., et al. (2016). rAAV-Mediated Combined Gene Transfer and Overexpression of TGF-β and SOX9 Remodels Human Osteoarthritic Articular Cartilage. J. Orthop. Res. 34, 2181–2190. doi:10.1002/jor.23228
Tofiño-Vian, M., Guillén, M. I., Pérez Del Caz, M. D., Silvestre, A., and Alcaraz, M. J. (2018). Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cel Physiol Biochem 47, 11–25. doi:10.1159/000489739
Toh, W. S., Lai, R. C., Hui, J. H. P., and Lim, S. K. (2017). MSC Exosome as a Cell-free MSC Therapy for Cartilage Regeneration: Implications for Osteoarthritis Treatment. Semin. Cel Dev Biol 67, 56–64. doi:10.1016/j.semcdb.2016.11.008
Tong, W., Zeng, Y., Chow, D. H. K., Yeung, W., Xu, J., Deng, Y., et al. (2019). Wnt16 Attenuates Osteoarthritis Progression through a PCP/JNK-mTORC1-PTHrP cascade. Ann. Rheum. Dis. 78, 551–561. doi:10.1136/annrheumdis-2018-214200
Tu, J. F., Yang, J. W., Shi, G. X., Yu, Z. S., Li, J. L., Lin, L. L., et al. (2021). Efficacy of Intensive Acupuncture versus Sham Acupuncture in Knee Osteoarthritis: A Randomized Controlled Trial. Arthritis Rheumatol. 73, 448–458. doi:10.1002/art.41584
van der Woude, J. A. D., Wiegant, K., van Heerwaarden, R. J., Spruijt, S., van Roermund, P. M., Custers, R. J. H., et al. (2017). Knee Joint Distraction Compared with High Tibial Osteotomy: a Randomized Controlled Trial. Knee Surg. Sports Traumatol. Arthrosc. 25, 876–886. doi:10.1007/s00167-016-4131-0
Wang, H., Zhang, H., Sun, Q., Yang, J., Zeng, C., Ding, C., et al. (2019). Chondrocyte mTORC1 Activation Stimulates miR-483-5p via HDAC4 in Osteoarthritis Progression. J. Cel Physiol 234, 2730–2740. doi:10.1002/jcp.27088
Wang, H., Zhang, X., Wu, W., Zhang, M., Sam, N. B., and Niu, L. (2018). Association between the Aspartic Acid D-Repeat Polymorphisms and Osteoarthritis Susceptibility: An Updated Systematic Review and Meta-Analyses. Medicine (Baltimore) 97, e13163. doi:10.1097/MD.0000000000013163
Wang, J., Chen, L., Jin, S., Lin, J., Zheng, H., Zhang, H., et al. (2017). Altered Expression of microRNA-98 in IL-1β-induced Cartilage Degradation and its Role in Chondrocyte Apoptosis. Mol. Med. Rep. 16, 3208–3216. doi:10.3892/mmr.2017.7028
Wang, P., Dong, R., Wang, B., Lou, Z., Ying, J., Xia, C., et al. (2019). Genome-wide microRNA Screening Reveals miR-582-5p as a Mesenchymal Stem Cell-specific microRNA in Subchondral Bone of the Human Knee Joint. J. Cel Physiol 234, 21877–21888. doi:10.1002/jcp.28751
Wang, W., Han, X., Zhao, T., Zhang, X., Qu, P., and Zhao, H. (2020). AGT, Targeted by miR-149-5p, Promotes IL-6-induced Inflammatory Responses of Chondrocytes in Osteoarthritis via Activating JAK2/STAT3 Pathway. Clin. Exp. Rheumatol. 38, 1088–1095.
Wang, W. T., Huang, Z. P., Sui, S., Liu, J. H., Yu, D. M., and Wang, W. B. (2020). microRNA-1236 Promotes Chondrocyte Apoptosis in Osteoarthritis via Direct Suppression of PIK3R3. Life Sci. 253, 117694. doi:10.1016/j.lfs.2020.117694
Wang, X., Yu, Y., Huang, Y., Zhu, M., Chen, R., Liao, Z., et al. (2020). Identification of Potential Diagnostic Gene Biomarkers in Patients with Osteoarthritis. Sci. Rep. 10, 13591. doi:10.1038/s41598-020-70596-9
Wang, X. B., Zhao, F-C., Yi, L-H., Tang, J-L., Zhu, Z-Y., Pang, Y., et al. (2019). MicroRNA-21-5p as a Novel Therapeutic Target for Osteoarthritis. Rheumatology 58, 1485–1497. doi:10.1093/rheumatology/kez102
Wang, Y., Cao, L., Wang, Q., Huang, J., and Xu, S. (2019). LncRNA FOXD2-AS1 Induces Chondrocyte Proliferation through Sponging miR-27a-3p in Osteoarthritis. Artif. Cell Nanomed Biotechnol 47, 1241–1247. doi:10.1080/21691401.2019.1596940
Wang, Y., Wu, C., Yang, Y., Ren, Z., Lammi, M. J., and Guo, X. (2019). Preliminary Exploration of Hsa_circ_0032131 Levels in Peripheral Blood as a Potential Diagnostic Biomarker of Osteoarthritis. Genet. Test. Mol. Biomarkers 23, 717–721. doi:10.1089/gtmb.2019.0036
Wang, Y., Wu, C., Zhang, Y., Yang, Y., Ren, Z., Lammi, M. J., et al. (2020). Screening for Differentially Expressed circRNA between Kashin-Beck Disease and Osteoarthritis Patients Based on circRNA Chips. Clin. Chim. Acta 501, 92–101. doi:10.1016/j.cca.2019.10.026
Wang, Y., Yu, D., Liu, Z., Zhou, F., Dai, J., Wu, B., et al. (2017). Exosomes from Embryonic Mesenchymal Stem Cells Alleviate Osteoarthritis through Balancing Synthesis and Degradation of Cartilage Extracellular Matrix. Stem Cel Res Ther 8, 189. doi:10.1186/s13287-017-0632-0
Watson Levings, R. S., Broome, T. A., Smith, A. D., Rice, B. L., Gibbs, E. P., Myara, D. A., et al. (2018). Gene Therapy for Osteoarthritis: Pharmacokinetics of Intra-articular Self-Complementary Adeno-Associated Virus Interleukin-1 Receptor Antagonist Delivery in an Equine Model. Hum. Gene Ther. Clin. Dev. 29, 90–100. doi:10.1089/humc.2017.142
Wei, W., He, S., Wang, Z., Dong, J., Xiang, D., Li, Y., et al. (2019). LINC01534 Promotes the Aberrant Metabolic Dysfunction and Inflammation in IL-1β-Simulated Osteoarthritic Chondrocytes by Targeting miR-140-5p. Cartilage, 1947603519888787.
Wen, X., Li, H., Sun, H., Zeng, A., Lin, R., Zhao, J., et al. (2020). MiR-455-3p Reduces Apoptosis and Alleviates Degeneration of Chondrocyte through Regulating PI3K/AKT Pathway. Life Sci. 253, 117718. doi:10.1016/j.lfs.2020.117718
Weng, C., Xu, J., Wang, Q., Lu, W., and Liu, Z. (2020). Efficacy and Safety of Duloxetine in Osteoarthritis or Chronic Low Back Pain: a Systematic Review and Meta-Analysis. Osteoarthritis Cartilage 28, 721–734. doi:10.1016/j.joca.2020.03.001
Wijn, S. R. W., Rovers, M. M., van Tienen, T. G., and Hannink, G. (2020). Intra-articular Corticosteroid Injections Increase the Risk of Requiring Knee Arthroplasty. Bone Jt. J 102-b, 586–592. doi:10.1302/0301-620X.102B5.BJJ-2019-1376.R1
Woods, S., Barter, M. J., Elliott, H. R., McGillivray, C. M., Birch, M. A., Clark, I. M., et al. (2019). miR-324-5p Is up Regulated in End-Stage Osteoarthritis and Regulates Indian Hedgehog Signalling by Differing Mechanisms in Human and Mouse. Matrix Biol. 77, 87–100. doi:10.1016/j.matbio.2018.08.009
Wu, J., Ma, L., Wu, L., and Jin, Q. (2017). Wnt-β-catenin Signaling Pathway Inhibition by Sclerostin May Protect against Degradation in Healthy but Not Osteoarthritic Cartilage. Mol. Med. Rep. 15, 2423–2432. doi:10.3892/mmr.2017.6278
Wu, X., Wang, Y., Xiao, Y., Crawford, R., Mao, X., and Prasadam, I. (2020). Extracellular Vesicles: Potential Role in Osteoarthritis Regenerative Medicine. J. Orthop. Translat 21, 73–80. doi:10.1016/j.jot.2019.10.012
Wu, Y., Hong, Z., Xu, W., Chen, J., Wang, Q., Chen, J., et al. (2021). Circular RNA circPDE4D Protects against Osteoarthritis by Binding to miR-103a-3p and Regulating FGF18. Mol. Ther. 29, 308–323. doi:10.1016/j.ymthe.2020.09.002
Wu, Y., Lu, X., Li, M., Zeng, J., Zeng, J., Shen, B., et al. (2019). Renin-angiotensin System in Osteoarthritis: A New Potential Therapy. Int. Immunopharmacol 75, 105796. doi:10.1016/j.intimp.2019.105796
Wu, Y., Lu, X., Shen, B., and Zeng, Y. (2019). The Therapeutic Potential and Role of miRNA, lncRNA, and circRNA in Osteoarthritis. Curr. Gene Ther. 19, 255–263. doi:10.2174/1566523219666190716092203
Wu, Y., Zhang, Y., Zhang, Y., and Wang, J. J. (2017). CircRNA Hsa_circ_0005105 Upregulates NAMPT Expression and Promotes Chondrocyte Extracellular Matrix Degradation by Sponging miR-26a. Cel Biol Int 41, 1283–1289. doi:10.1002/cbin.10761
Wu, Z., Shou, L., Wang, J., and Xu, X. (2020). Identification of the Key Gene and Pathways Associated with Osteoarthritis via Single-Cell RNA Sequencing on Synovial Fibroblasts. Medicine (Baltimore) 99, e21707. doi:10.1097/MD.0000000000021707
Xiao, J., Wang, R., Zhou, W., Cai, X., and Ye, Z. (2021). Circular RNA CSNK1G1 Promotes the Progression of Osteoarthritis by Targeting the miR-4428/FUT2 axis. Int. J. Mol. Med. 47, 232–242. doi:10.3892/ijmm.2020.4772
Xiao, Y., Bao, Y., Tang, L., and Wang, L. (2019). LncRNA MIR4435-2HG Is Downregulated in Osteoarthritis and Regulates Chondrocyte Cell Proliferation and Apoptosis. J. Orthop. Surg. Res. 14, 247. doi:10.1186/s13018-019-1278-7
Xiao, Y., Yan, X., Yang, Y., and Ma, X. (2019). Downregulation of Long Noncoding RNA HOTAIRM1 Variant 1 Contributes to Osteoarthritis via Regulating miR-125b/BMPR2 axis and Activating JNK/MAPK/ERK Pathway. Biomed. Pharmacother. 109, 1569–1577. doi:10.1016/j.biopha.2018.10.181
Xie, F., Liu, Y. L., Chen, X. Y., Li, Q., Zhong, J., Dai, B. Y., et al. (2020). Role of MicroRNA, LncRNA, and Exosomes in the Progression of Osteoarthritis: A Review of Recent Literature. Orthop. Surg. 12, 708–716. doi:10.1111/os.12690
Xie, Y., Zinkle, A., Chen, L., and Mohammadi, M. (2020). Fibroblast Growth Factor Signalling in Osteoarthritis and Cartilage Repair. Nat. Rev. Rheumatol. 16, 547–564. doi:10.1038/s41584-020-0469-2
Xu, J., and Xu, Y. (2017). The lncRNA MEG3 Downregulation Leads to Osteoarthritis Progression via miR-16/SMAD7 axis. Cell Biosci 7, 69. doi:10.1186/s13578-017-0195-x
Xu, W., Gao, P., Zhang, Y., Piao, L., and Dong, D. (2019). microRNA-138 Induces Cell Survival and Reduces WNT/β-catenin Signaling of Osteoarthritis Chondrocytes through NEK2. IUBMB Life 71, 1355–1366. doi:10.1002/iub.2050
Xue, H., Tu, Y., Ma, T., Wen, T., Yang, T., Xue, L., et al. (2019). miR-93-5p Attenuates IL-1β-induced Chondrocyte Apoptosis and Cartilage Degradation in Osteoarthritis Partially by Targeting TCF4. Bone 123, 129–136. doi:10.1016/j.bone.2019.03.035
Xue, H., Yu, P., Wang, W. Z., Niu, Y. Y., and Li, X. (2020). The Reduced lncRNA NKILA Inhibited Proliferation and Promoted Apoptosis of Chondrocytes via miR-145/sp1/nf-Κb Signaling in Human Osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 24, 535–548. doi:10.26355/eurrev_202001_20030
Yang, D. W., Zhang, X., Qian, G. B., Jiang, M. J., Wang, P., and Wang, K. Z. (2019). Downregulation of Long Noncoding RNA LOC101928134 Inhibits the Synovial Hyperplasia and Cartilage Destruction of Osteoarthritis Rats through the Activation of the Janus Kinase/signal Transducers and Activators of Transcription Signaling Pathway by Upregulating IFNA1. J. Cel Physiol 234, 10523–10534. doi:10.1002/jcp.27730
Yang, M., Yan, X., Yuan, F. Z., Ye, J., Du, M. Z., Mao, Z. M., et al. (2021). MicroRNA-210-3p Promotes Chondrogenic Differentiation and Inhibits Adipogenic Differentiation Correlated with HIF-3α Signalling in Bone Marrow Mesenchymal Stem Cells. Biomed. Res. Int. 2021, 6699910. doi:10.1155/2021/6699910
Yang, Q., Li, X., Zhou, Y., Fu, W., Wang, J., and Wei, Q. (2019). A LINC00341-Mediated Regulatory Pathway Supports Chondrocyte Survival and May Prevent Osteoarthritis Progression. J. Cel Biochem 120, 10812–10820. doi:10.1002/jcb.28372
Yang, R. Z., Zheng, H. L., Xu, W. N., Zheng, X. F., Li, B., Jiang, L. S., et al. (2021). Vascular Endothelial Cell-Secreted Exosomes Facilitate Osteoarthritis Pathogenesis by Promoting Chondrocyte Apoptosis. Aging (Albany NY) 13, 4647–4662. doi:10.18632/aging.202506
Yang, X., He, H., Gao, Q., and He, C. (2018). Pulsed Electromagnetic Field Improves Subchondral Bone Microstructure in Knee Osteoarthritis Rats through a Wnt/β-Catenin Signaling-Associated Mechanism. Bioelectromagnetics 39, 89–97. doi:10.1002/bem.22106
Yang, Y., Wang, Y., Jia, H., Li, B., Xing, D., and Li, J. J. (2020). MicroRNA-1 Modulates Chondrocyte Phenotype by Regulating FZD7 of Wnt/β-Catenin Signaling Pathway. Cartilage. [Epub ahead of print]. doi:10.1177/1947603520973255
Yang, Z., Tang, Y., Lu, H., Shi, B., Ye, Y., Xu, G., et al. (2018). Long Non-coding RNA Reprogramming (lncRNA-ROR) Regulates Cell Apoptosis and Autophagy in Chondrocytes. J. Cel Biochem 119, 8432–8440. doi:10.1002/jcb.27057
Yaşar Şirin, D., Yılmaz, İ., İsyar, M., Öznam, K., and Mahiroğulları, M. (2017). Does Leukocyte-Poor or Leukocyte-Rich Platelet-Rich Plasma Applied with Biopolymers Have Superiority to Conventional Platelet-Rich Plasma Applications on Chondrocyte Proliferation?. Eklem Hastalik Cerrahisi 28, 142–151. doi:10.5606/ehc.2017.55186
Ye, D., Jian, W., Feng, J., and Liao, X. (2018). Role of Long Noncoding RNA ZFAS1 in Proliferation, Apoptosis and Migration of Chondrocytes in Osteoarthritis. Biomed. Pharmacother. 104, 825–831. doi:10.1016/j.biopha.2018.04.124
Ying, H., Wang, Y., Gao, Z., and Zhang, Q. (2019). Long Non-coding RNA Activated by Transforming Growth Factor Beta Alleviates Lipopolysaccharide-Induced Inflammatory Injury via Regulating microRNA-223 in ATDC5 Cells. Int. Immunopharmacol 69, 313–320. doi:10.1016/j.intimp.2019.01.056
Yoshiko, Y., and Minamizaki, T. (2020). Emerging Roles of microRNAs as Extracellular Vesicle Cargo Secreted from Osteoblasts. J. Oral Biosci. 62, 228–234. doi:10.1016/j.job.2020.05.006
Yu, C., Shi, D., Li, Z., Wan, G., and Shi, X. (2019). Long Noncoding RNA CHRF Exacerbates IL-6-induced Inflammatory Damages by Downregulating microRNA-146a in ATDC5 Cells. J. Cel Physiol 234, 21851–21859. doi:10.1002/jcp.28749
Yu, F., Xie, C., Sun, J., Feng, H., and Huang, X. (2018). Circular RNA Expression Profiles in Synovial Fluid: a Promising New Class of Diagnostic Biomarkers for Osteoarthritis. Int. J. Clin. Exp. Pathol. 11, 1338–1346.
Yu, L., Liu, S., Zhao, Z., Xia, L., Zhang, H., Lou, J., et al. (2017). Extracorporeal Shock Wave Rebuilt Subchondral Bone In Vivo and Activated Wnt5a/Ca2+ Signaling In Vitro. Biomed. Res. Int. 2017, 1404650. doi:10.1155/2017/1404650
Zhai, X., Meng, R., Li, H., Li, J., Jing, L., Qin, L., et al. (2017). miR-181a Modulates Chondrocyte Apoptosis by Targeting Glycerol-3-Phosphate Dehydrogenase 1-Like Protein (GPD1L) in Osteoarthritis. Med. Sci. Monit. 23, 1224–1231. doi:10.12659/msm.899228
Zhang, G., Wu, Y., Xu, D., and Yan, X. (2016). Long Noncoding RNA UFC1 Promotes Proliferation of Chondrocyte in Osteoarthritis by Acting as a Sponge for miR-34a. DNA Cel Biol 35, 691–695. doi:10.1089/dna.2016.3397
Zhang, L., Zhang, P., Sun, X., Zhou, L., and Zhao, J. (2018). Long Non-coding RNA DANCR Regulates Proliferation and Apoptosis of Chondrocytes in Osteoarthritis via miR-216a-5p-JAK2-STAT3 axis. Biosci. Rep. 38. doi:10.1042/BSR20181228
Zhang, W., Cheng, P., Hu, W., Yin, W., Guo, F., Chen, A., et al. (2018). Inhibition of microRNA-384-5p Alleviates Osteoarthritis through its Effects on Inhibiting Apoptosis of Cartilage Cells via the NF-Κb Signaling Pathway by Targeting SOX9. Cancer Gene Ther. 25, 326–338. doi:10.1038/s41417-018-0029-y
Zhang, W., Hu, C., Zhang, C., Luo, C., Zhong, B., and Yu, X. (2021). MiRNA-132 Regulates the Development of Osteoarthritis in Correlation with the Modulation of PTEN/PI3K/AKT Signaling. BMC Geriatr. 21, 175. doi:10.1186/s12877-021-02046-8
Zhang, W., Zhang, C., Hu, C., Luo, C., Zhong, B., and Yu, X. (2020). Circular RNA-CDR1as Acts as the Sponge of microRNA-641 to Promote Osteoarthritis Progression. J. Inflamm. (Lond) 17, 8. doi:10.1186/s12950-020-0234-y
Zhang, W., Qi, L., Chen, R., He, J., Liu, Z., Wang, W., et al. (2021). Circular RNAs in Osteoarthritis: Indispensable Regulators and Novel Strategies in Clinical Implications. Arthritis Res. Ther. 23, 23. doi:10.1186/s13075-021-02420-2
Zhang, Y., Wang, F., Chen, G., He, R., and Yang, L. (2019). LncRNA MALAT1 Promotes Osteoarthritis by Modulating miR-150-5p/AKT3 axis. Cel Biosci 9, 54. doi:10.1186/s13578-019-0302-2
Zhang, Z., Mei, Y., Feng, M., Wang, C., Yang, P., and Tian, R. (2021). The Relationship between Common Variants in the DPEP1 Gene and the Susceptibility and Clinical Severity of Osteoarthritis. Int. J. Rheum. Dis. 24 (9), 1192–1199. doi:10.1111/1756-185x.14182
Zhao, C. (2021). Identifying the Hub Gene and Immune Infiltration of Osteoarthritis by Bioinformatical Methods. Clin. Rheumatol. 40, 1027–1037. doi:10.1007/s10067-020-05311-0
Zhao, J., Huang, H., Liang, G., Zeng, L. F., Yang, W., and Liu, J. (2020). Effects and Safety of the Combination of Platelet-Riched Plasma (PRP) and Hyaluronic Acid (HA) in the Treatment of Knee Osteoarthritis: a Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 21, 224. doi:10.1186/s12891-020-03262-w
Zhao, T., Ma, C., Xie, B., Zhao, B., Wang, W., and Liu, J. (2020). Evaluation of Common Variants in the AKNA Gene and Susceptibility to Knee Osteoarthritis Among the Han Chinese. Genet. Test. Mol. Biomarkers 24, 425–430. doi:10.1089/gtmb.2020.0014
Zhao, X., Li, H., and Wang, L. (2019). MicroRNA-107 Regulates Autophagy and Apoptosis of Osteoarthritis Chondrocytes by Targeting TRAF3. Int. Immunopharmacol 71, 181–187. doi:10.1016/j.intimp.2019.03.005
Zhao, X., Meng, F., Hu, S., Yang, Z., Huang, H., Pang, R., et al. (2020). The Synovium Attenuates Cartilage Degeneration in KOA through Activation of the Smad2/3-Runx1 Cascade and Chondrogenesis-Related miRNAs. Mol. Ther. Nucleic Acids 22, 832–845. doi:10.1016/j.omtn.2020.10.004
Zheng, L., Wang, Y., Qiu, P., Xia, C., Fang, Y., Mei, S., et al. (2019). Primary Chondrocyte Exosomes Mediate Osteoarthritis Progression by Regulating Mitochondrion and Immune Reactivity. Nanomedicine (Lond) 14, 3193–3212. doi:10.2217/nnm-2018-0498
Zheng, X., Zhao, F. C., Pang, Y., Li, D. Y., Yao, S. C., Sun, S. S., et al. (2017). Downregulation of miR-221-3p Contributes to IL-1β-induced Cartilage Degradation by Directly Targeting the SDF1/CXCR4 Signaling Pathway. J. Mol. Med. (Berl) 95, 615–627. doi:10.1007/s00109-017-1516-6
Zhong, G., Long, H., Ma, S., Shunhan, Y., Li, J., and Yao, J. (2019). miRNA-335-5p Relieves Chondrocyte Inflammation by Activating Autophagy in Osteoarthritis. Life Sci. 226, 164–172. doi:10.1016/j.lfs.2019.03.071
Zhong, W., Li, X., Pathak, J. L., Chen, L., Cao, W., Zhu, M., et al. (2020). Dicalcium Silicate Microparticles Modulate the Differential Expression of circRNAs and mRNAs in BMSCs and Promote Osteogenesis via Circ_1983-miR-6931-Gas7 Interaction. Biomater. Sci. 8, 3664–3677. doi:10.1039/d0bm00459f
Zhou, J., Li, C., Yu, A., Jie, S., Du, X., Liu, T., et al. (2019). Bioinformatics Analysis of Differentially Expressed Genes Involved in Human Developmental Chondrogenesis. Medicine (Baltimore) 98, e16240. doi:10.1097/MD.0000000000016240
Zhou, Q. F., Cai, Y. Z., and Lin, X. J. (2020). The Dual Character of Exosomes in Osteoarthritis: Antagonists and Therapeutic Agents. Acta Biomater. 105, 15–25. doi:10.1016/j.actbio.2020.01.040
Zhou, X., Jiang, L., Fan, G., Yang, H., Wu, L., Huang, Y., et al. (2019). Role of the ciRS-7/miR-7 axis in the Regulation of Proliferation, Apoptosis and Inflammation of Chondrocytes Induced by IL-1β. Int. Immunopharmacol 71, 233–240. doi:10.1016/j.intimp.2019.03.037
Zhou, X., Jiang, L., Zhang, Y., Zhang, J., Zhou, D., Wu, L., et al. (2019). Genetic Variation of Aggrecanase-2 (ADAMTS5) in Susceptibility to Osteoarthritis. Braz. J. Med. Biol. Res. 52, e8109. doi:10.1590/1414-431X20188109
Zhou, Z. B., Du, D., Huang, G. X., Chen, A., and Zhu, L. (2018). Circular RNA Atp9b, a Competing Endogenous RNA, Regulates the Progression of Osteoarthritis by Targeting miR-138-5p. Gene 646, 203–209. doi:10.1016/j.gene.2017.12.064
Zhou, Z. B., Huang, G. X., Fu, Q., Han, B., Lu, J. J., Chen, A. M., et al. (2019). circRNA.33186 Contributes to the Pathogenesis of Osteoarthritis by Sponging miR-127-5p. Mol. Ther. 27, 531–541. doi:10.1016/j.ymthe.2019.01.006
Zhu, H., Hu, Y., Wang, C., Zhang, X., and He, D. (2020). CircGCN1L1 Promotes Synoviocyte Proliferation and Chondrocyte Apoptosis by Targeting miR-330-3p and TNF-α in TMJ Osteoarthritis. Cel Death Dis 11, 284. doi:10.1038/s41419-020-2447-7
Zhu, J. K., He, T. D., Wei, Z. X., and Wang, Y. M. (2018). LncRNA FAS-AS1 Promotes the Degradation of Extracellular Matrix of Cartilage in Osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 22, 2966–2972. doi:10.26355/eurrev_201805_15051
Keywords: osteoarthritis, DNA, RNA, exosomes, platelet-rich plasma, protein, gene
Citation: Deng J, Zong Z, Su Z, Chen H, Huang J, Niu Y, Zhong H and Wei B (2021) Recent Advances in Pharmacological Intervention of Osteoarthritis: A Biological Aspect. Front. Pharmacol. 12:772678. doi: 10.3389/fphar.2021.772678
Received: 08 September 2021; Accepted: 04 October 2021;
Published: 23 November 2021.
Edited by:
Liangliang Xu, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Wenxiang Cheng, Shenzhen Institutes of Advanced Technology, ChinaCopyright © 2021 Deng, Zong, Su, Chen, Huang, Niu, Zhong and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Zhong, emhoNzIxOEBxcS5jb20=; Bo Wei, d2VianhtY0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.