
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 02 November 2021
Sec. Neuropharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.770329
This article is part of the Research Topic Current Trends in Medicinal Plant Research and Neurodegenerative Disorders View all 16 articles
Background: The incidence of cerebral ischemia disease leading cause of death in human population worldwide. Treatment of cerebral ischemia remains a clinical challenge for researchers and mechanisms of cerebral ischemia remain unknown. During the cerebral ischemia, inflammatory reaction and oxidative stress plays an important role. The current investigation scrutinized the neuroprotective and anti-inflammatory role of pterostilbene against cerebral ischemia in middle cerebral artery occlusion (MCAO) rodent model and explore the underlying mechanism.
Methods: The rats were divided into following groups viz., normal, sham, MCAO and MCAO + pterostilbene (25 mg/kg) group, respectively. The groups received the oral administration of pterostilbene for 30 days followed by MCAO induction. The neurological score, brain water content, infarct volume and Evan blue leakage were estimated. Hepatic, renal, heart, inflammatory cytokines and inflammatory mediators were estimated.
Results: Pterostilbene treatment significantly (p < 0.001) improved the body weight and suppressed the glucose level and brain weight. Pterostilbene significantly (p < 0.001) reduced the hepatic, renal and heart parameters. Pterostilbene significantly (p < 0.001) decreased the level of glutathione (GSH), glutathione peroxidase (GPx), superoxide dismutase (SOD) and decreased the level of malonaldehyde (MDA), 8-Hydroxy-2′-deoxyguanosine (8-OHdG). Pterostilbene significantly (p < 0.001) inflammatory cytokines and inflammatory parameters such as cyclooxygenase-2 (COX-2), inducible nitric oxidase synthase (iNOS) and prostaglandin (PGE2). Pterostilbene significantly (p < 0.001) down-regulated the level of metalloproteinases (MMP) such as MMP-2 and MMP-9. Pterostilbene suppressed the cellular swelling, cellular disintegration, macrophage infiltration, monocyte infiltration and polymorphonuclear leucocyte degranulation in the brain.
Conclusion: In conclusion, Pterostilbene exhibited the neuroprotective effect against cerebral ischemia in rats via anti-inflammatory mechanism.
Stroke is a complicated disease that causes the number of death and disability whole over the world, and it is accompanied by cognitive dysfunctions (Vakili et al., 2014; Wang K. et al., 2020). According to the World Health Organization (WHO), over 15 million people are affected by stroke (Alva-Díaz et al., 2020). Ischemia stroke is the most frequent type of stroke, accounting for 87 percent of all instances. It is caused by thromboembolic blockage of cerebral arteries, which causes an ischemic cascade and tissue injury (Özön and Cüce, 2020; Tuo et al., 2021). Cerebral ischemia disease is the major neurological disease worldwide and incidence of cerebral ischemia rapidly increases last few decades (Yuan et al., 2020). The treatment of cerebral ischemia still challenge to the researcher (Wang et al., 2018; Leung et al., 2020). But few research suggest that neurovascular units involve in the disease (Pan et al., 2018) and the researcher targeting the neurovascular unit (NVU) for the treatment of overall disease, which exhibited the protective against the neuronal damage, neurons and blood brain barrier (BBB) such as extracellular matrix, vascular endothelial cells, microglia and astrocytes (Crofts et al., 2020; Lake et al., 2017).
During the ischemic cascade, oxygen and energy deprivation start the production of reactive oxygen species (ROS), followed through inflammatory reaction, deposition of intracellular calcium and glutamate excitotoxicity (Michalski et al., 2017; Tuo et al., 2021). Ischemic tissue reperfusion enhances the neuroinflammatory reaction and production of ROS. Various studies have shown that oxidative stress is linked to neuronal cell death in ischemic lesions (Patel et al., 2020; Yan et al., 2020). ROS such as nitric oxide (NO), hydrogen peroxide (H2O2), superoxide anion (O2) and hydroxyl free radicals destroys the cellular structures includes nucleic acid, proteins, lipids, redox sensitive enzymes, membrane receptors and channels, which eventually induces the neuronal injury in the ischemic lesions (Jin et al., 2016; Liang et al., 2019; Leung et al., 2020). Furthermore, the increased proportion of ROS leakage from the mitochondrial cytochrome C, which further recruits caspases, worsening neuronal death after ischemia and reperfusion injury (Tang et al., 2016; Han et al., 2018; Zhang et al., 2020). Due to increase the level of ROS leading the cell death, inflammatory reaction and neural dysfunction after the reperfusion (Zhang et al., 2018; Leung et al., 2020). Following cerebral ischemia, an inflammatory response plays a key role in the development of secondary brain injury. Inflammatory mediators include COX-2, nuclear factor kappa B (NF-κB) and MMPs play a significant role in the expansion of cerebral ischemic (Li et al., 2017; Zhang et al., 2018). During the cerebral ischemia injury, the level of above discuss enzymes boosted to considerable level in the brain area. In the treatment of cerebral ischemia, antioxidants and anti-inflammatory agents are helpful (Liang et al., 2020).
Extracellular Matrix (ECM) degrading enzymes such as MMP-2 and MMP-9, are most directly linked to BBB degradation (Traystman, 2003; Ma et al., 2020). The inhibition of ECM degrading enzymes considerably suppresses the cerebral infarct volume and cerebral edema induced via ischemia and suppress the BBB injury (Turner and Sharp, 2016). Previous study showed that the reperfusion is the protective in suppressing the ischemic brain injury and also showed the beneficial effect for recovering the various reversible injury (Gresoiu and Christou, 2020; Yu et al., 2020). Though, few research suggest that the blood reperfusion for ischemic tissue could causes the dysfunction and injury in some cases (Gresoiu and Christou, 2020). Furthermore, consideration is always taken of how to inhibit reperfusion injury during the treatment of ischemic stroke (Vakili et al., 2014). During the ischemic reperfusion stop or abrupt the blood supply in the brain area which resultant shortage the oxygen and glucose level in the brain that leading to the anaerobic pathway of glycolytic cycle. The glycolytic cycle generate the high H+ ion, lactic acid and reversal to the mitochondrial matrix (Liang et al., 2019; Wang et al., 2020a; Zhao et al., 2020). All the cascade suppressed the cytoplasmic pH and increase the formation of free radicals. Due to continuous production of free radicals, its finally induces the oxidative stress and start the inflammatory reaction (Li et al., 2017; Zhang et al., 2018).
More than hundred traditional Chinese medicine (TCM) patents have been filed in China in the last few years for the treatment of ischemic stroke, including therapies for ischemia reperfusion injury (Gupta et al., 2010; Peng et al., 2019; Singh et al., 2020). Dietary phytochemicals getting more popularity due to its wide range of benefits includes regulation of immune system, anti-oxidation and suppression of inflammation. Resveratrol (polyphenol flavonoids) isolated from the skin of red grape and suggested the protective effect against various inflammatory reaction. Pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene) is a stilbene, that is naturally occurring methoxylated analog of Revesterol, commonly found in blueberry (Acharya and Ghaskadbi, 2013; Shi et al., 2019). Pterostilbene shows the higher bioactivity and bioavailability than resveratrol due to presence of two extra methyl group in the structure (Supplementary Figure S1). The structure of pterostilbene showed the removal of two hydroxy group with two methoxy group, which increase the bioactivity and oral bioavailability and demonstrate the prolong metabolism (Wu et al., 2017; Yang et al., 2017; Shi et al., 2019). Pterostilbene having the bioavailability (85%) as compared to the bioavailability of revesterol (20%) in animal model. Additionally, clinical studies showed that the pterostilbene may be a potent anti-inflammatory drug against various diseases (Acharya and Ghaskadbi, 2013; Shi et al., 2019; Zhang et al., 2021). In this experimental study, we try to explore the neuroprotective and anti-inflammatory effect of pterostilbene against the MCAO rat model.
Swiss Wistar rats (weight 200 ± 20 g, sex-both, aged 8–10 weeks) were used in this study. The whole experimental study was carried out using the institutional animal ethical committee guidelines. All the rats were kept in the standard laboratory condition 20 ± 5°C temperature, 12 h dark and 12 h light cycle and 65% relative humidity. All the rats were received the standard pellet (rat chow) and water ab libitum. The rats were kept 7 days for acclimatization for adopting the laboratory condition.
Briefly, the rats were anesthetized using the 50 mg/kg intraperitoneal injection of ketamine hydrochloride and 5 mg/kg xylazine and monitored accordingly. The common carotid artery was isolated and the branches of external carotid artery (right) were carefully removed (Pan et al., 2018). To obstruct the source of MCAO, a nylon monofilament suture (4–0) with a silicon coated tip was pushed to the internal carotid artery (Yuan et al., 2020). The same operation was performed on the normal group (sham group), but no suture was put. After cerebral ischemia (2 h), the suture was removed for reperfusion (24 h). The rats were divided into different groups such as Group I: control; Group II: Sham; Group III: MCAO and Group IV: MCAO + pterostilbene (35 mg/kg), respectively.
Neurological dysfunction were assessed after 2 h of MCAO and then every day for the next 5 days (Shamim and Khan, 2019). The behavioural impairments and post chemical motor were examined using a 4-point neuro score (Yuan et al., 2020). The grade for neurological impairments was as follows:
Symptoms are absent in grade 0.
Grade 1: bending of the forelimbs
Grade 2: reduced lateral push (and forelimb flexion) resistance without circling
Grade 3: circle and the same conduct as grade 2.
The rats were euthanized at the end of the protocol so that blood (plasma/serum) and organs (liver and brain) could be collected and kept at 80°C. All the blood and tissue samples kept for the histopathological evaluation and biochemical estimation.
For the determination of BBB permeability, the all-group rats were received the Evans blue (2%) in the tail vein before the sacrifice (2 h). after that removed the brain tissue and weighted and placed into the 1 ml dimethylformamide and incubated for next 24 h at 60°C. Afterthat centrifuge at 1 g rpm for 10 min to separate the supernatant (Pan et al., 2018). The supernatant collected and take absorbance at 620 nm wavelength using the spectrophotometer.
For the determination of cerebral edema, the brain water content was estimated using the dry weight method of previous reported method with minor modification (Yang et al., 2020). Briefly, the brain tissue was successfully removed after the reperfusion (24 h) and weight immediately and baked in the oven for 24 h at 120°C and again weight.
The brain water content was estimated using the following formula
Turbid Metric Immunoassay model was used for the estimation of serum C-reactive protein (CRP) using the kit (Conformidad Europea, Spain).
Enzymatic colorimetric GOD-POD kit was used for the estimation of blood glucose (Global, United Kingdom). Lactate dehydrogenase (LDH) assay kit was used for the determination of LDH activity (Institute of Biological Engineering of Nanjing Jiancheng, Nanjing, China) following the manufacture protocol.
For the estimation of lipid parameters such as high-density lipoprotein cholesterol (HDL-c), triglyceride (TG) and cholesterol (TC) was estimated using the enzymatic kit kit (Institute of Biological Engineering of Nanjing Jiancheng, Nanjing, China). The low-density lipoprotein cholesterol (LDL-c) and very low-density lipoprotein cholesterol (VLDL-c) were determined using the Friedewald’s formula (Mendes de Cordova et al., 2018).
Berthelot colorimetric model was used for the estimation of urea concentration in the serum. Jaffe’s method was used for the determination of creatinine using the enzymatic kit (Biogene Diagnostics, United States). Blood urea nitrogen (BUN) and uric acid was estimated using the previous reported method with minor modification (Pandey et al., 2018; Kumar et al., 2021).
The hepatic parameters include alkaline phosphatase (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using the enzymatic kit (Institute of Biological Engineering of Nanjing Jiancheng, Nanjing, China).
The antioxidant parameters include SOD, GSH, GPx, MDA and CAT were estimated using the previous reported method with minor modification (Bhatt et al., 2017, 2018).
MMP-2 and MMP-9 were estimated using the gelatinase assay kit (Chemicon International, Inc. Temecula, CA, United States) following the manufacture protocol.
Pro-inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-4 (IL-4), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1 (IL-10) and interleukin-1 (IL-1) were estimated using the Enzyme-Linked Immunosorbent Assay Kits (ELISA) (Institute of Biological Engineering of Nanjing Jiancheng, Nanjing, China) following the manufacture protocol.
Inflammatory mediators such as NF-κB, i-NOS, NO, COX-2 and PGE2 using the Enzyme-Linked Immunosorbent Assay Kits (ELISA) (Institute of Biological Engineering of Nanjing Jiancheng, Nanjing, China) following the manufacture protocol.
The current study’s findings were given as a mean standard error mean (S.E.M.). The statistical analysis was performed using Graphpad Prism 7. The difference between the intergroups was determined using a post hoc t test. The significance level was set at p < 0.05.
During cerebral ischemia, it increases the neurological score. A neurological score of 0 was observed in the normal and sham group rats. The MCAO group rats showed an enhanced neurological score, which started from day 0 and reached its maximum on day 1 and remained at the higher end of the protocol (day 5). Pterostilbene treated rats significantly (p < 0.001) suppressed the neurological score. Pterostilbene treated rats exhibited a reduced neurological score at different time intervals (day 0, 1, and 5) as compared to the MCAO group (Figure 1A).
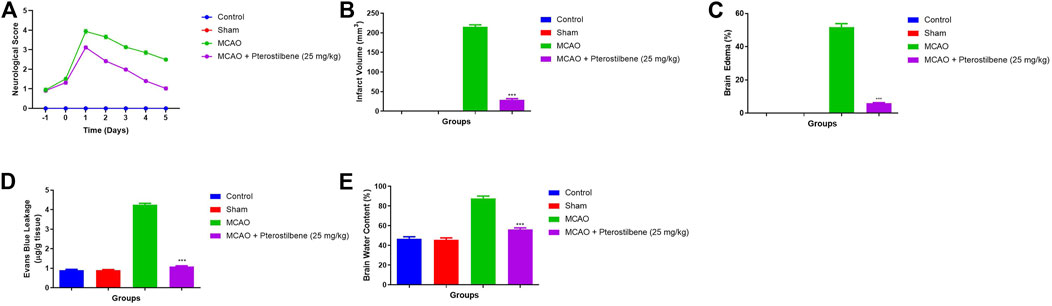
FIGURE 1. showed the neurological parameter and score of among experimental groups. (A) neurological score, (B) infarct volume, (C) brain edema, (D) evans blue leakage and (E) brain water content. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
Infarct volume (Figure 1B), brain edema (Figure 1C), Evans blue leakage (Figure 1D) and brain water content (Figure 1E) were higher observed in the MCAO group rats. Pterostilbene treated rats exhibited the suppressed level of infarct volume, brain edema, Evans blue leakage and brain water content.
The body weight was slightly reduced in the sham control and MCAO group rats. MCAO group rats receiving pterostilbene significantly (p < 0.001) improved their body weight (Figure 2A).
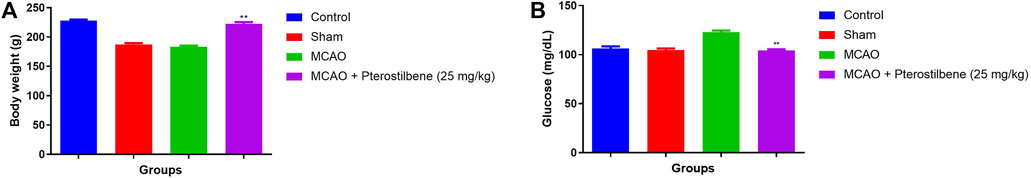
FIGURE 2. showed the body weight and glucose level of among experimental groups. (A) body weight and (B) blood glucose level. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
The blood glucose remained constant in the normal and sham control group rats. The blood glucose level slightly decreased in the MCAO group. Pterostilbene treated group rats improved the blood glucose level (Figure 2B).
The brain weight slightly enhanced in the sham group rats as compared to the normal rats. MCAO group rats exhibited the increased brain weight as compared to all experimental group. MCAO group rats treated with the pterostilbene treated group rats demonstrated the reduced brain weight (Figure 3).
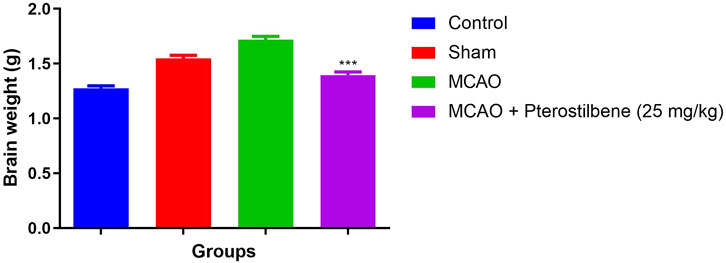
FIGURE 3. showed the brain weight of among experimental groups. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
The level of LDH (Figure 4A) and CRP (Figure 4B) were estimated in the different groups. The level of LDH (691 ± 12.45 U/L) and CRP (10.89 ± 1.32 mg/dl) slightly enhanced in the MCAO group and pterostilbene treated rats significantly (p < 0.05) reduced the level of LDH (687 ± 11.34 U/L) and CRP (9.91 ± 1.93 mg/dl).
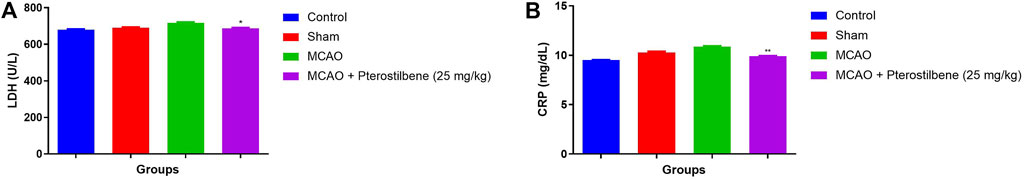
FIGURE 4. showed the LDH and CRP level of among experimental groups. (A) LDH and (B) CRP. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
MCAO group rats displayed the boosted level of ALT (25.45 ± 2.38 U/L), AST (40.73 ± 4.93 U/L) and ALP (51.61 ± 2.83 U/L) as compared to normal and sham group rats. After the pterostilbene treatment reduced the level of hepatic parameters ALT (23.04 ± 2.93 U/L), AST (31.59 ± 3.82 U/L) and ALP (42.82 ± 3.89 U/L) (Figure 5).
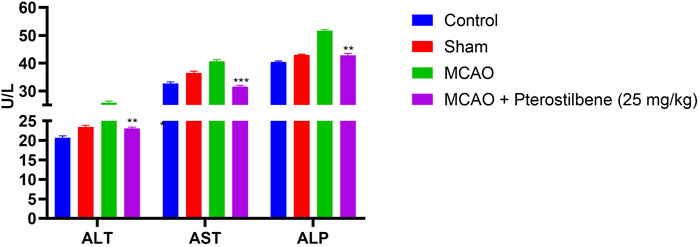
FIGURE 5. showed the hepatic parameters of among experimental groups. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
The levels of Uric acid (4.67 ± 0.83 mg/dl) (Figure 6A), creatinine (0.56 ± 0.03 mg/dl) (Figure 6B), BUN (22.51 ± 1.34 mg/dl) (Figure 6C) and urea (49.46 ± 3.94 mg/dl) (Figure 6D) were observed in different experimental group. MCAO group rats demonstrated the increased level of uric acid (2.62 ± 0.67 mg/dl), creatinine (0.35 ± 0.04 mg/dl), BUN (16.54 ± 2.32 mg/dl) and urea (33.05 ± 2.93 mg/dl), which was significantly (p < 0.001) diminished after the pterostilbene treatment.
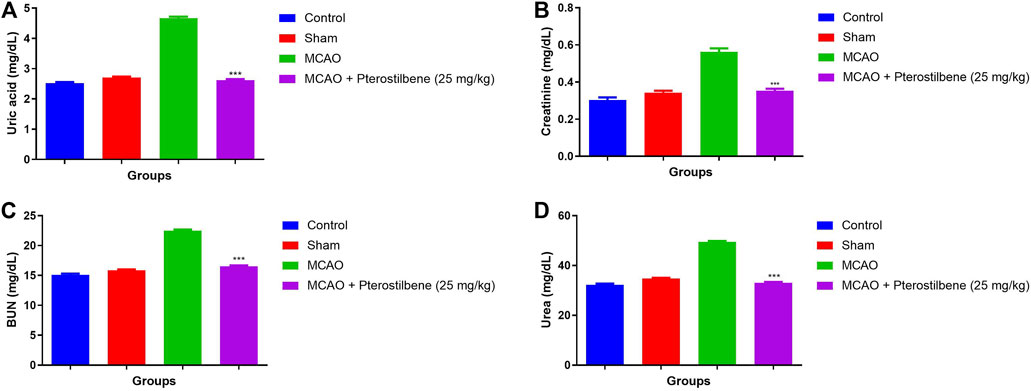
FIGURE 6. showed the renal parameters of among experimental groups. (A) uric acid, (B) creatinine, (C) BUN and (D) urea. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
The NO level was considerably boosted in the MCAO group rats as compared to normal and sham control. MCAO group rats received the NO significantly (p < 0.001) repressed the level (Figure 7).
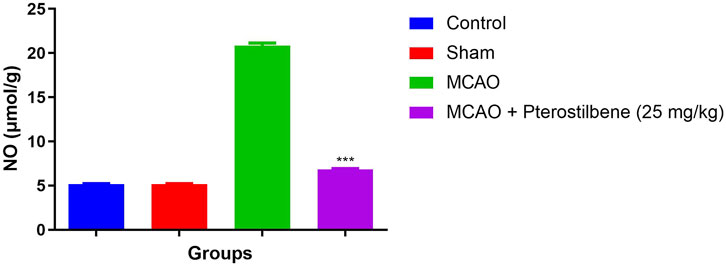
FIGURE 7. showed the level of nitric oxide of among experimental groups. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
Figure 8 exhibited the lipid parameters in experimental groups. The level of lipid parameters such as TG, LDL, TC, VLDL boosted and HDL level reduced in the MCAO group. Pterostilbene treated rats exhibited the reduced level of TG, LDL, TC, VLDL and enhanced level of HDL.
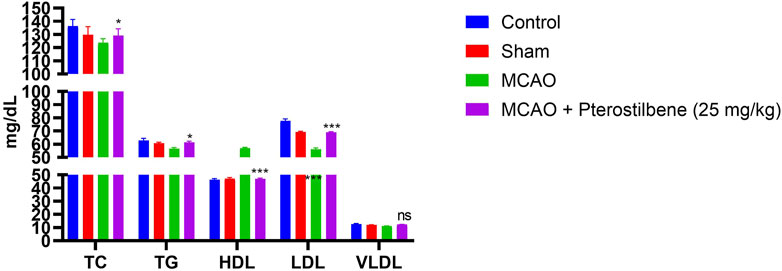
FIGURE 8. showed the lipid parameters of among experimental groups. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
Figure 9 exhibited the antioxidant parameters of different group rats. MCAO group rats exhibited the decreased level of SOD (55.03 ± 5.32 U/mg protein) (Figure 9A), CAT (5.57 ± 0.83 U/mg protein) (Figure 9B), GPx (7.11 ± 0.93 U/mg protein) (Figure 9C), GSH (0.47 ± 0.04 U/mg protein) (Figure 9D) and enhanced level of MDA (263.4 ± 10.34 U/mg protein) (Figure 9E), 8OdhG OdhG (0.97 ± 0.09 μg/mg protein) (Figure 9F) as compared to normal and sham group rats. MCAO group rats treated with pterostilbene significantly (p < 0.001) improved the level of SOD (102.34 ± 7.54 U/mg protein), CAT (28.85 ± 1.33 U/mg protein), GPx (33.73 ± 4.34 U/mg protein), GSH (1.34 ± 0.45 U/mg protein) and suppressed the level of MDA (89.45 ± 3.45 U/mg protein), 8-OdhG (0.28 ± 0.05 μg/mg protein).
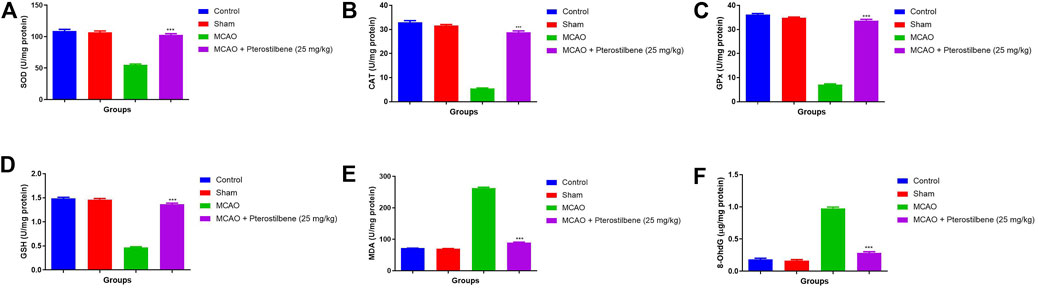
FIGURE 9. showed the antioxidant parameters of among experimental groups. (A) SOD, (B) CAT, (C) GPx, (D) GSH, (E) MDA and (F) 8-OhdG. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
The level of inflammatory cytokines boosted during the cerebral ischemia due to expansion of inflammatory disease. MCAO group rats exhibited the increased level of TNF-α (41.34 ± 1.57 pg/mg) (Figure 10A), IL-1β (14.37 ± 1.23 pg/mg) (Figure 10B), IL-6 (41.45 ± 2.34 pg/mg) (Figure 10C) and reduced level of (9.89 ± 1.34 pg/mg) IL-10 (Figure 10D). MCAO group rats received the pterostilbene treatment significantly diminished the level of TNF-α (10.34 ± 0.89 pg/mg) (Figure 10A), IL-1β (5.23 ± 0.83 pg/mg) (Figure 10B), IL-6 (17.04 ± 2.93 pg/mg) (Figure 10C) and improved the level of IL-10 (28.34 ± 1.83 pg/mg) (Figure 10D).
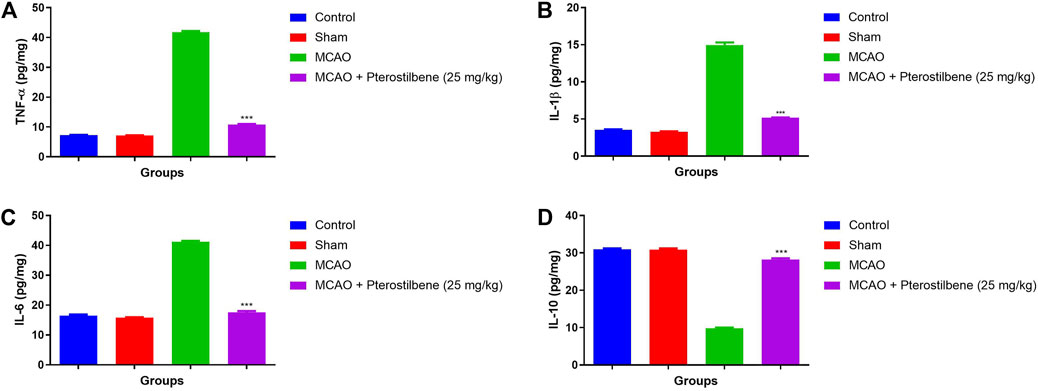
FIGURE 10. showed the inflammatory cytokines of among experimental groups. (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-10. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
Figures 11A–C showed the level of inflammatory parameters in different experimental group. MCAO group rats showed the improved level of COX-2 (37.34 ± 2.34 pg/mg) (Figure 11A), PGE2 (41.93 ± 3.23 pg/mg) (Figure 11B) and iNOS (36.54 ± 2.93 pg/mg) (Figure 11C) and pterostilbene treatment reduced the inflammatory parameters such as COX-2 (12.34 ± 0.45 pg/mg), PGE2 (10.02 ± 0.93 pg/mg) and iNOS (10.34 ± 1.83 pg/mg).
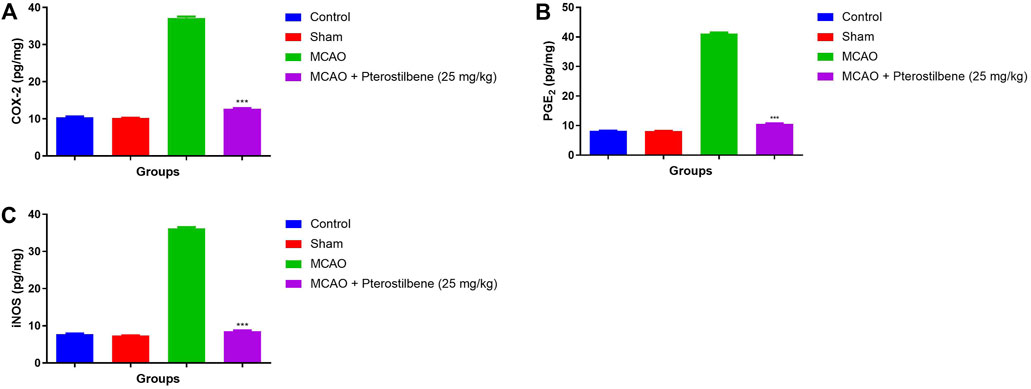
FIGURE 11. showed the inflammatory mediators of among experimental groups. (A) COX-2, (B) PGE2 and (C) iNOS. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
MCAO group showed the increased level of MMP-2 (1.13 ± 0.37 pg/mg) (Figure 12A) and MMP-9 (1.08 ± 0.89 pg/mg) (Figure 12B) as compared to different group. MCAO group treated with the pterostilbene significantly (p < 0.001) suppressed the level of MMP-2 (0.28 ± 0.08 pg/mg) and MMP-9 (0.56 ± 0.04 pg/mg).
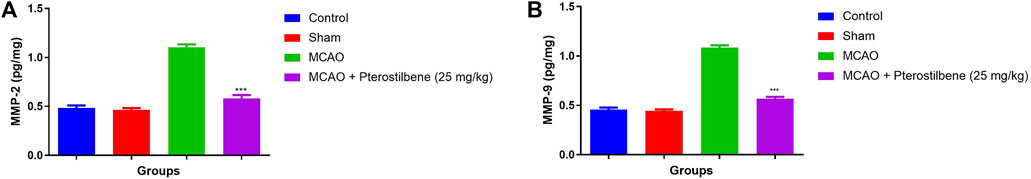
FIGURE 12. showed the MMP level of among experimental groups. (A) MMP-2 and (B) MMP-9. Values are expressed as mean ± SEM. The t-test was used for the significant difference between groups: *p < 0.05, **p < 0.01, ***p < 0.005. NS, non-significant.
Table 1 demonstrated the effect of pterostilbene on the brain tissue histopathology. MCAO group rats demonstrated the development of cellular swelling, cellular disintegration, macrophage infiltration, monocyte infiltration and polymorphonuclear leucocyte degranulation. Pterostilbene treatment suppressed the cellular swelling, cellular disintegration, macrophage infiltration, monocyte infiltration and polymorphonuclear leucocyte degranulation.
Stroke and its related disorder cause the disability and death whole over the world. The loss of cerebral blood flow during an ischemic stroke causes metabolic, biochemical and hemodynamic alteration in the damaged brain area to shut down, resulting in brain injury and reduced or lost brain function (Wang et al., 2020b; Yuan et al., 2021b). The neuronal necrosis is aggravated by reperfusion after an ischemic insult. Although the cause of cerebral ischemic stroke is complicated, some pathogenic mechanisms have shown that increasing oxidative stress and reducing endogenous antioxidant enzymes, can restore the most harmful effects of the disease (Bu et al., 2019; Shi et al., 2021). Some studies showed that oxidate stress and inflammatory reactions play a significant role to induces the serve toxicity in the biological macromolecules, which further leading the injury in the cell and tissue (Wang et al., 2020b; Shi et al., 2021). MCAO is a well-established animal model for ischemic stroke-related brain injury in order to study about the stroke phenomena and to assess the efficacy of possible neuroprotection therapies. Furthermore, neutralizing the oxidative stress, inflammatory reaction, enhanced antioxidant intake would be a beneficial therapy for the treatment of ischemic disease (Pan et al., 2018). Herb and its phytoconstituent having long history to treat the oxidative stress and inflammatory disease. Previous studies showed that the plant and its phytoconstituent having the potent anti-inflammatory, antitumor, antidiabetic and antioxidant activity (Wang et al., 2020b; Tian et al., 2020; Shi et al., 2021). The neuroprotective effect of pterostilbene against MCAO-induced cerebral ischemia reperfusion was studied in this experimental study.
During the cerebral ischemia, start the production of oxygen free radicals (OFR) via enzymatic and non-enzymatic systems and later on they can attack on the polyunsaturated fatty acid (PUFA), which are commonly found in the brain cells and vascular endothelial cells (Bu et al., 2019; Yuan et al., 2021b; Shi et al., 2021). Free radical starts the lipid peroxidation reaction which further start the production of various products such as hydroxy, aldehyde (MDA), keto, inner peroxy or carbonyl radicals. Some reported suggest that oxidant and free radicals play a crucial role in the expansion of edema and disruption of BBB after the cerebral ischemia (Chen et al., 2013; Bu et al., 2019). Drugs that exhibited the neuroprotective effect via targeting the production of free radicals to treat the disease. MDA (an indicator of lipid peroxidation, LPO) used to estimation of LPO degree and indirectly shows the cell injury in the brain (Stegner et al., 2019; Wang et al., 2020b). According to the results, pterostilbene showed the neuroprotective effect via decreasing the brain lesions and edema may be related to its effect in restriction the free radical production and improved the antioxidant effect. GSH neutralize the hydroperoxides and other toxic free radicals in the brain tissue (Wang et al., 2020b; Yuan et al., 2021b). GSH is endogenous antioxidant enzymes against the oxidative stress. SOD averts formation of hydroxyl radical through catalyzing the super radicals into the H2O2. CAT detoxifies the H2O2 into the water and molecular oxygen. In the brain tissue, CAT is the 2nd line antioxidant that protect brain from oxidative injury. During the cerebral ischemia and oxidation of free radical, the activity of SOD reduced in the brain tissue and serum (Si et al., 2009; Zhang et al., 2019). Large amount of SOD in consumed for neutralize or scavenge the free radicals. Due to continues uses of the SOD to neutralize or scavenge the free radicals, the activity of SOD reduces. However, the activity of SOD indirectly reflects the level of scavenged free radicals (Si et al., 2009; Li et al., 2020). Our result clearly showed that pterostilbene may contribute to suppressing the excessive production of free radical and enhance the SOD activity in the serum and brain. Pterostilbene treated group exhibited the reduction in the MDA content and enhancement of SOD content, but the result not significant (He et al., 2019; Chen et al., 2020). On the basis of result, we can say that Pterostilbene altered the cerebral ischemia via scavenging pathway of LPO, not antioxidant effects.
Stroke is a primary cause of acquired impairment in adults and one of the top causes of death worldwide, with a complicated pathological process (He et al., 2019; Chen et al., 2020). Recently, the researcher targeting the neurovascular unit for the treatment of stroke. Neurovascular unit is made up to several neurons, glial cells (microglia and astrocyte), extracellular matrix (ECM), endothelial cells (vascular cells) and BBB (Pan et al., 2018). The BBB is the most important component of the neurovascular unit, and MMPs have long been thought to play a role in the breakdown of the BBB following a stroke. During the early stage of stroke, MMPs induce the various neruo vascular dysfunction includes leakage of BBB and neuronal death. Previous report showed that MMP-9 is unusually expressed in brains suffering from cerebral ischemia injury and its enhance the brain injury and breakdown of BBB (Li et al., 2017). The patients suffer from the ischemic stroke exhibit the enhance level of MMP (MMP-2 and MMP-9) in the circulation (Gu et al., 2013; Li et al., 2017). The increase level of MMP-9 in the circulation related with the poorer prognosis. The reduction of MMP-9 in the circulation and gene considerably decreased the bleeding complications and infarct size (Li et al., 2017). Our result showed that pterostilbene may protect the BBB permeability via suppressing of MMP-9. Studies that used MMP inhibitors before a stroke, showed similar benefits, which matched our findings.
The recruitment of systemic macrophages and endogenous microglia indicates an early inflammatory response in cerebral ischemia injury. Inflammatory cells interact with the cerebral stimulus through production of pleiotropic mediators such as prostanoids, cytokines and chemokines (Chen et al., 2020; Li et al., 2020). The inflammatory or immune response following a stroke includes changes in numerous pro and anti-inflammatory cytokines and chemokines in the brain tissue. Previous reports suggest that targeting the inflammatory cytokines is beneficial for the treatment of cerebral ischemia (Li et al., 2020). Inflammatory reaction initiates the tissue lesions such as accumulation of free radicals as well targeting the toxic enzyme of brain tissue. Report suggest that various inflammatory mediators interact with each other and enhance the brain injury. Increases the level of inflammatory cytokines in the brain and begins the breakdown of BBB during cerebral ischemia (Gong et al., 2014; Li et al., 2020).
Report suggests that the TNF-α is activated during the brain ischemia. TNF-α generated through monocytes and macrophages and its over generated due to activation of inflammation related cells during the cerebral ischemia, that boost the local inflammation reaction in the brain tissue (Chen et al., 2020; Li et al., 2020). The level of cytokines increased as a result of leukocyte leakage into the circulation, causing an influx of neutrophils, macrophages, and microglia to enter the ischemic area, causing neuronal cell death and injury. TNF-α induces the cerebral endothelial cells injury and also enhance the BBB permeability, thus contributing to the formation of brain edema (Gong et al., 2014; Li et al., 2020). Moreover, the antiedematous effect of pterostilbene due to reduction the synthesis of TNF-α and also provide the protection of the BBB against disruption. Other inflammatory cytokines such as IL-6 and IL-1β and NF-κB activation play an important role in the pathogenesis of brain edema (He et al., 2017; Hou et al., 2018; Li et al., 2020). Recently report suggest that pterostilbene considerably suppressed the inflammatory cytokines and activated the NF-κB in the endothelial cells and lipopolysaccharide induced lung injury (Liu et al., 2016). All these results suggest that the preventive effect of pterostilbene, at least in part, from suppression the cytokines production and subsequently their signaling pathways. More molecular research needs to elucidate these possibilities.
During cerebral ischemic injury, enhance the level of iNOS due to development of injury cause by inflammatory cytokines and mediators (Li et al., 2017; Zhang et al., 2020). During the normal process, NO generated from the oxidation of L-arginine and further catalysed through synthesis of nitric oxide. The iNOS level boosted in the cerebral ischemic group, confirm the expansion of inflammation and pterostilbene considerably suppressed the iNOS level and confirm the reduction in the inflammatory reaction. Inflammatory mediators and cytokines increased the level of immunocyte such as neutrophils and macrophages (Gong et al., 2014; Li et al., 2017; Zhang et al., 2020). The boosted level of iNOS, PGE2 and COX-2, observed after the cerebral ischemia, which further expand the brain injury via inducing the damage in the neuronal cells (Gong et al., 2014; Li et al., 2021).
Prostaglandin is formed when arachidonic acid (AA) is metabolised, and it has been shown to have both a pro and anti-inflammatory effect in mammalian systems, as well as other physiological activities (Liang et al., 2020; Yuan and Zhang, 2021). COX is thought to have a role in brain homeostasis, such as blood flow regulation. Due to COX’s important involvement in vasodilation, rodents lacking the enzyme were more prone to stroke. COX is divided into two isomers (COX-1 and COX-2), which have similar catalytic actions but differing physiological functions (Liang et al., 2020). COX-2 is an inducible COX that catalyses the first committed step in prostaglandin production from arachidonic acid (Liang et al., 2020; Yuan et al., 2021a). COX-2 levels and PG production increase in endothelial cells, neurons, and glial cells in the brain tissue in response to diverse stimuli such as inflammatory mediators, excitatory synaptic activity, hypoxia, and growth factors. Previous report suggest that the COX-2 enzymatic activity involved in the stroke animal model that leads to the enhanced stroke damage and neuronal death (Wei et al., 2016; Liang et al., 2020). The use of pharmacological or genetic techniques to suppress COX-2-dependent PG production reduces infarct volume. As a result, a major effort is ongoing to investigate how to minimise the PG receptor pathways that cause COX-2-mediated cerebral damage after stroke. COX-2 levels are higher in infarcted human brains, according to clinical and prior studies, and it is found in both glial and neuronal cells. Previous research suggests that the enhanced level of COX-2 infiltrating the vascular cells, neutrophils and neurons in the peri-infarct zone (Abd El-Aal et al., 2013; Yu et al., 2015; Wei et al., 2016). It is well documented that COX-2 play an important role to providing the protection of brain tissue against cerebral ischemic injury during the brain injury in the rodents. The higher level of COX-2 rodent having higher infarcts volume after experimental stroke. In animal models, selective pharmacologic suppression of COX-2 activity has shown to be a viable therapeutic target for stroke. Prostanoids (prostaglandin E2 and prostaglandin D2) formed through the COX pathway. (Liang et al., 2020; Yuan et al., 2021a). The level of PGE2 have been boosted in the brain after the cerebral ischemia (Liang et al., 2020). Following doubts regarding the safety of COX-2 inhibitors in 2004, most research on the role of the cyclooxygenase system in stroke focused on the PGE2 and PGD2 receptors (Ahmad and Graham, 2010). The actions of PGE2 receptors are triggered by four G-protein coupled receptors, which are the mediators of stroke injury (Liang et al., 2020; Yuan and Zhang, 2021). The level of PGE2 rose during cerebral ischemia due to the production of brain damage. Our control group rats showed a similar finding, with pterostilbene administration significantly suppressing COX-2 and PGE2, indicating an anti-inflammatory effect (Duan et al., 2016; Sotomayor-Sobrino et al., 2019). NF-κB is another significant transcription factors which activates after the reperfusion of cerebral ischemia (Simão et al., 2012; Yuan et al., 2021a). It is well known that oxidative stress/ROS activates the NF-κB signaling pathway that are involved in the various inflammatory reaction initiate after the cerebral ischemia. NF-κB play a crucial role in implementation of various inflammatory reactions that induces the brain injury during the cerebral ischemia (Abd El-Aal et al., 2013; Wei et al., 2016). NF-κB triggered the various parameters such as inflammatory cytokines and inflammatory mediators that are involved in the brain injury during the cerebral ischemia injury (Liang et al., 2020). Targeting the NF-κB is the novel approach to treat the cerebral ischemia. In this study, pterostilbene considerably suppressed the level of COX-2, PGE2 and NF-κB, suggesting the anti-inflammatory potential against cerebral ischemia.
In conclusion, the current investigation showed that pterostilbene has beneficial and protective effect against cerebral ischemia reperfusion injury. Pterostilbene significantly reduced the brain edema, infarct volume and neurological score. Pterostilbene maintain the liver enzymes, renal and lipid parameters at a baseline level. Pterostilbene considerably altered the level of antioxidant enzymes in the brain tissue and reduces the oxidative stress. Pterostilbene significantly suppressed the level of inflammatory cytokines, inflammatory mediators and increased the level of anti-inflammatory cytokines. These findings show that pterostilbene may be an effective treatment for cerebral ischemic stroke. It also emphasises pterostilbene’s clinical applications in the treatment of cerebral ischemia reperfusion.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by All the experimental study was approved from the Gansu Provincial Hospital and performed as per the guidelines of the Institute.
WY design and performed the experimental study. DR, XF, JH, DW, TL, and DZ. interpret the biochemical data. All the authors equally contributed in proof reading.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.770329/full#supplementary-material
MCAO, Middle cerebral artery occlusion; GSH, Glutathione; GPx, Glutathione peroxidase; SOD, Superoxide dismutase; MDA, Malonaldehyde; 8-OHdG, 8-Hydroxy-2′-deoxyguanosine; COX-2, Cyclooxygenase-2; iNOS Inducible nitric oxidase synthase; PGE2, Prostaglandin; MMP, Metalloproteinases; NVU, Neurovascular unit; BBB, Blood brain barrier; ECM, Extracellular Matrix; WHO, World Health Organization; ROS, Reactive oxygen species; NO, Nitric oxide; H2O2, Hydrogen peroxide; O2, Superoxide anion; NF-Κb, Nuclear factor kappa B; Tcm, Traditional Chinese medicine; CRP, C-reactive protein; LDH, Lactate dehydrogenase; HDL-c, High-density lipoprotein cholesterol; TG, Triglyceride; TC, Total cholesterol; LDL-c, Low-density lipoprotein cholesterol; VLDL-c, Very low-density lipoprotein cholesterol; BUN, Blood urea nitrogen; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; IL-1β, Interleukin-1β; IL-4, Interleukin-4; TNF-α, Tumor necrosis factor-α; IL-6, Interleukin-6; IL-10, Interleukin-1; IL-1, Interleukin-1; ELISA, Enzyme-Linked Immunosorbent Assay Kits; SEM, Standard error mean; PUFA, Polyunsaturated fatty acid; LPO, Lipid peroxidation.
Abd El-Aal, S. A., El-Sawalhi, M. M., Seif-El-Nasr, M., and Kenawy, S. A. (2013). Effect of Celecoxib and L-NAME on Global Ischemia-Reperfusion Injury in the Rat Hippocampus. Drug Chem. Toxicol. 36, 385–395. doi:10.3109/01480545.2012.749270
Acharya, J. D., and Ghaskadbi, S. S. (2013). Protective Effect of Pterostilbene against Free Radical Mediated Oxidative Damage. BMC Complement. Altern. Med. 13(1), 238. doi:10.1186/1472-6882-13-238
Ahmad, M., and Graham, S. H. (2010). Inflammation after Stroke: Mechanisms and Therapeutic Approaches. Transl. Stroke Res. 1, 74–84. doi:10.1007/s12975-010-0023-7
Alva-Díaz, C., Huerta-Rosario, A., Pacheco-Barrios, K., Molina, R. A., Navarro-Flores, A., Aguirre-Quispe, W., et al. (2020). Neurological Diseases in Peru: a Systematic Analysis of the Global burden Disease Study. Arq. Neuropsiquiatr. 78, 282–289. doi:10.1590/0004-282x20200018
Bhatt, P. C., Verma, A., Al-Abbasi, F. A., Anwar, F., Kumar, V., and Panda, B. P. (2017). Development of Surface-Engineered PLGA Nanoparticulate-Delivery System of Tet1-Conjugated Nattokinase Enzyme for Inhibition of Aβ40 Plaques in Alzheimer's Disease. Int. J. Nanomed. 12, 8749–8768. doi:10.2147/IJN.S144545
Bhatt, P. C., Pathak, S., Kumar, V., and Panda, B. P. (2018). Attenuation of Neurobehavioral and Neurochemical Abnormalities in Animal Model of Cognitive Deficits of Alzheimer's Disease by Fermented Soybean Nanonutraceutical. Inflammopharmacol 26, 105–118. doi:10.1007/s10787-017-0381-9
Bu, J., Shi, S., Wang, H.-Q., Niu, X.-S., Zhao, Z.-F., et al. (2019). Acacetin Protects against Cerebral Ischemia-Reperfusion Injury via the NLRP3 Signaling Pathway. Neural Regen. Res. 14, 605. doi:10.4103/1673-5374.247465
Chen, X.-m., Chen, H.-s., Xu, M.-j., and Shen, J.-g. (2013). Targeting Reactive Nitrogen Species: A Promising Therapeutic Strategy for Cerebral Ischemia-Reperfusion Injury. Acta Pharmacol. Sin. 34, 67–77. doi:10.1038/aps.2012.82
Chen, X., Yao, Z., Peng, X., Wu, L., Wu, H., Ou, Y., et al. (2020). Eupafolin Alleviates Cerebral Ischemia/reperfusion Injury in Rats via Blocking the TLR4/NF-κB Signaling Pathway. Mol. Med. Rep. 22, 5135–5144. doi:10.3892/mmr.2020.11637
Crofts, A., Kelly, M. E., and Gibson, K. L. (2020). Imaging Functional Recovery Following Ischemic Stroke: Clinical and Preclinical fMRI Studies. J. Neuroimaging 30 (1), 5–14. doi:10.1111/jon.12668
Duan, X., Wen, Z., Shen, H., Shen, M., and Chen, G. (2016). Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell Longev. 2016, 1–17. doi:10.1155/2016/1203285
Gong, G., Xiang, L., Yuan, L., Hu, L., Wu, W., Cai, L., et al. (2014). Protective Effect of Glycyrrhizin, a Direct HMGB1 Inhibitor, on Focal Cerebral Ischemia/reperfusion-Induced Inflammation, Oxidative Stress, and Apoptosis in Rats. PLoS One 9, e89450. doi:10.1371/journal.pone.0089450
Gresoiu, M., and Christou, S. (2020). Hypoxic Ischaemic Brain Injury. Anaesth. Intensive Care Med. 21, 298–304. doi:10.1016/j.mpaic.2020.03.009
Gu, J.-H., Ge, J.-B., Li, M., Xu, H.-D., Wu, F., and Qin, Z.-H. (2013). Poloxamer 188 Protects Neurons against Ischemia/Reperfusion Injury through Preserving Integrity of Cell Membranes and Blood Brain Barrier. PLoS One 8, e61641. doi:10.1371/journal.pone.0061641
Gupta, Y. K., Briyal, S., and Gulati, A. (2010). Therapeutic Potential of Herbal Drugs in Cerebral Ischemia. Indian J. Physiol. Pharmacol. 54 (2), 99–122. doi:10.25259/IJPP_90_2010
Han, X. R., Wen, X., Wang, Y. J., Wang, S., Shen, M., Zhang, Z. F., et al. (2018). Retracted : Protective Effects of microRNA‐431 against Cerebral Ischemia‐reperfusion Injury in Rats by Targeting the Rho/Rho‐kinase Signaling Pathway. J. Cel. Physiol. 233, 5895–5907. doi:10.1002/jcp.26394
He, Q., Li, Z., Wang, Y., Hou, Y., Li, L., and Zhao, J. (2017). Resveratrol Alleviates Cerebral Ischemia/reperfusion Injury in Rats by Inhibiting NLRP3 Inflammasome Activation through Sirt1-dependent Autophagy Induction. Int. Immunopharmacol. 50, 208–215. doi:10.1016/j.intimp.2017.06.029
He, J., Li, H., Li, G., and Yang, L. (2019). Hyperoside Protects against Cerebral Ischemia-Reperfusion Injury by Alleviating Oxidative Stress, Inflammation and Apoptosis in Rats. Biotechnol. Biotechnol. Equip. 33, 798–806. doi:10.1080/13102818.2019.1620633
Hou, Y., Wang, Y., He, Q., Li, L., Xie, H., Zhao, Y., et al. (2018). Nrf2 Inhibits NLRP3 Inflammasome Activation through Regulating Trx1/TXNIP Complex in Cerebral Ischemia Reperfusion Injury. Behav. Brain Res. 336, 32–39. doi:10.1016/j.bbr.2017.06.027
Jin, F. M., Zhang, Z. X., Wang, Y., Zhao, H. R., Yang, Y. Y., Huang, X., et al. (2016). Protective Effect of Ento-I Plastic against Cerebral Ischemia-Reperfusion Injury in Rats 12, 947. J. Int. Pharm. Res. doi:10.13220/j.cnki.jipr.2016.03.019
Kumar, V., Sachan, R., Rahman, M., Rub, R. A., Patel, D. K., Sharma, K., et al. (2021). Chemopreventive Effects of Melastoma malabathricum L. Extract in Mammary Tumor Model via Inhibition of Oxidative Stress and Inflammatory Cytokines. Biomed. Pharmacother. 137, 111298. doi:10.1016/j.biopha.2021.111298
Lake, E. M. R., Bazzigaluppi, P., Mester, J., Thomason, L. A. M., Janik, R., Brown, M., et al. (2017). Neurovascular Unit Remodelling in the Subacute Stage of Stroke Recovery. Neuroimage 146, 869–882. doi:10.1016/j.neuroimage.2016.09.016
Leung, S. W., Lai, J. H., Wu, J. C.-C., Tsai, Y.-R., Chen, Y.-H., Kang, S.-J., et al. (2020). Neuroprotective Effects of Emodin against Ischemia/Reperfusion Injury through Activating Erk-1/2 Signaling Pathway. Ijms 21, 2899. doi:10.3390/ijms21082899
Li, W., Suwanwela, N. C., and Patumraj, S. (2017). Curcumin Prevents Reperfusion Injury Following Ischemic Stroke in Rats via Inhibition of NF-κB, ICAM-1, MMP-9 and Caspase-3 Expression. Mol. Med. Rep. 16, 4710–4720. doi:10.3892/mmr.2017.7205
Li, L., Sun, L., Qiu, Y., Zhu, W., Hu, K., and Mao, J. (2020). Protective Effect of Stachydrine against Cerebral Ischemia-Reperfusion Injury by Reducing Inflammation and Apoptosis through P65 and JAK2/STAT3 Signaling Pathway. Front. Pharmacol. 11, 64. doi:10.3389/fphar.2020.00064
Li, F., Xu, Y., Li, X., Wang, X., Yang, Z., Li, W., et al. (2021). Triblock Copolymer Nanomicelles Loaded with Curcumin Attenuates Inflammation via Inhibiting the NF-Κb Pathway in the Rat Model of Cerebral Ischemia. Ijn 16, 3173–3183. doi:10.2147/IJN.S300379
Liang, W., Lin, C., Yuan, L., Chen, L., Guo, P., Li, P., et al. (2019). Preactivation of Notch1 in Remote Ischemic Preconditioning Reduces Cerebral Ischemia-Reperfusion Injury through Crosstalk with the NF-Κb Pathway. J. Neuroinflamm. 16. doi:10.1186/s12974-019-1570-9
Liang, S., Chen, Z., Li, H., Cang, Z., Yin, K., Wu, M., et al. (2020). Neuroprotective Effect of Umbelliferone against Cerebral ischemia/Reperfusion Induced Neurological Deficits: In-Vivo and In-Silico Studies. J. Biomol. Struct. Dyn. 0, 1–11. doi:10.1080/07391102.2020.1780153
Liu, J., Fan, C., Yu, L., Yang, Y., Jiang, S., Ma, Z., et al. (2016). Pterostilbene Exerts an Anti-inflammatory Effect via Regulating Endoplasmic Reticulum Stress in Endothelial Cells. Cytokine 77, 88–97. doi:10.1016/j.cyto.2015.11.006
Ma, R., Xie, Q., Li, Y., Chen, Z., Ren, M., Chen, H., et al. (2020). Animal Models of Cerebral Ischemia: A Review. Biomed. Pharmacother. 131, 110686. doi:10.1016/j.biopha.2020.110686
Mendes de Cordova, C. M., de Santa Helena, E. T., Galgowski, C., Figueira, V. H., Setter, G. B., Markus, M. R. P., et al. (2018). Evaluation of a New Equation for LDL-C Estimation and Prediction of Death by Cardiovascular Related Events in a German Population-Based Study Cohort. Scand. J. Clin. Lab. Invest. 78, 187–196. doi:10.1080/00365513.2018.1432070
Michalski, D., Hofmann, S., Pitsch, R., Grosche, J., and Härtig, W. (2017). Neurovascular Specifications in the Alzheimer-like Brain of Mice Affected by Focal Cerebral Ischemia: Implications for Future Therapies. Jad 59, 655–674. doi:10.3233/JAD-170185
Özön, A. Ö., and Cüce, F. (2020). Clinical and Radiological Evaluation of Epilepsy after Ischemic Cerebrovascular Disease. Gulhane Med. J., 62(2):126–130. doi:10.4274/gulhane.galenos.2019.998
Pan, Z., Cui, M., Dai, G., Yuan, T., Li, Y., Ji, T., et al. (2018). Protective Effect of Anthocyanin on Neurovascular Unit in Cerebral Ischemia/Reperfusion Injury in Rats. Front. Neurosci. 12. doi:10.3389/fnins.2018.00947
Pandey, P., Bhatt, P. C., Rahman, M., Patel, D. K., Anwar, F., Al-Abbasi, F., et al. (2018). Preclinical Renal Chemo-Protective Potential of Prunus Amygdalus Batsch Seed Coat via Alteration of Multiple Molecular Pathways. Arch. Physiol. Biochem. 124, 88–96. doi:10.1080/13813455.2017.1364773
Patel, P., Barve, K., and Bhatt, L. K. (2020). Narirutin-rich Fraction from Grape Fruit Peel Protects against Transient Cerebral Ischemia Reperfusion Injury in Rats. Nutr. Neurosci. 2020, 1–10. doi:10.1080/1028415X.2020.1821518
Peng, T., Jiang, Y., Farhan, M., Lazarovici, P., Chen, L., and Zheng, W. (2019). Anti-Inflammatory Effects of Traditional Chinese Medicines on Preclinical In Vivo Models of Brain Ischemia-Reperfusion-Injury: Prospects for Neuroprotective Drug Discovery and Therapy. Front. Pharmacol. 10, 204. doi:10.3389/fphar.2019.00204
Shamim, M., and Khan, N. I. (2019). Neuroprotective Effect ofPanax Ginsengextract against Cerebral Ischemia-Reperfusion-Injury-Induced Oxidative Stress in Middle Cerebral Artery Occlusion Models. Facets 4, 52–68. doi:10.1139/facets-2018-0025
Shi, L., Liu, Q., Tang, J.-h., Wen, J.-j., and Li, C. (2019). Protective Effects of Pterostilbene on Ulcerative Colitis in Rats via Suppressing NF-Κb Pathway and Activating PPAR-γ. Eur. J. Inflamm. 17, 205873921984015. doi:10.1177/2058739219840152
Shi, C. X., Ding, Y. B., Yu, F., Jin, J., Li, T., Ma, J. H., et al. (2021). Effects of Sevoflurane post-conditioning in Cerebral Ischemia-Reperfusion Injury via TLR4/NF-Κb Pathway in Rats. Eur. Rev. Med. Pharmacol. Sci. 22(6):1770–1775. doi:10.26355/eurrev_201803_14595
Si, Y. l., Zhang, J. y., and Yan, G. T. (2009). Protective Effect of Leptin against Cerebral Ischemia/reperfusion Injury in Mice. Nan Fang Yi Ke Da Xue Xue Bao 29 (4), 598–601. doi:10.27382/IJPP_1-_2009
Simão, F., Matté, A., Pagnussat, A. S., Netto, C. A., and Salbego, C. G. (2012). Resveratrol Preconditioning Modulates Inflammatory Response in the Rat hippocampus Following Global Cerebral Ischemia. Neurochem. Int. 61, 659–665. doi:10.1016/j.neuint.2012.06.009
Singh, S., Shafi, S., Shrivastav, A., Khan, S., Ahmad, T., and Khan, N. A. (2020). A Review on Herbal Plants Used in Brain Ischemic and Reperfusion Injury. J. Drug Deliv. Ther. 10, 376–379. doi:10.22270/jddt.v10i5.4415
Sotomayor-Sobrino, M. A., Ochoa-Aguilar, A., Méndez-Cuesta, L. A., and Gómez-Acevedo, C. (2019). Interacciones Neuroinmunológicas en el ictus. Neurología 34, 326–335. doi:10.1016/j.nrl.2016.08.003
Stegner, D., Klaus, V., and Nieswandt, B. (2019). Platelets as Modulators of Cerebral Ischemia/Reperfusion Injury. Front. Immunol. 10, 2505. doi:10.3389/fimmu.2019.02505
Tang, X.-J., Yang, M.-H., Cao, G., Lu, J.-T., Luo, J., Dai, L.-J., et al. (2016). Protective Effect of microRNA-138 against Cerebral Ischemia/reperfusion Injury in Rats. Exp. Ther. Med. 11, 1045–1050. doi:10.3892/etm.2016.3021
Tian, F., Liu, R., Fan, C., Sun, Y., Huang, X., Nie, Z., et al. (2020). Effects of Thymoquinone on Small-Molecule Metabolites in a Rat Model of Cerebral Ischemia Reperfusion Injury Assessed Using Maldi-Msi. Metabolites 10, 27. doi:10.3390/metabo10010027
Traystman, R. J. (2003). Animal Models of Focal and Global Cerebral Ischemia. ILAR J. 44, 85–95. doi:10.1093/ilar.44.2.85
Tuo, Q. z., Zhang, S. T., and Lei, P. (2021). Mechanisms of Neuronal Cell Death in Ischemic Stroke and Their Therapeutic Implications. Med. Res. Rev. doi:10.1002/med.21817
Turner, R. J., and Sharp, F. R. (2016). Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cel. Neurosci. 10, 56. doi:10.3389/fncel.2016.00056
Vakili, A., Einali, M. R., and Bandegi, A. R. (2014). Protective Effect of Crocin against Cerebral Ischemia in a Dose-dependent Manner in a Rat Model of Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 23, 106–113. doi:10.1016/j.jstrokecerebrovasdis.2012.10.008
Wang, Y., Ren, Q., Zhang, X., Lu, H., and Chen, J. (2018). Neuroprotective Mechanisms of Calycosin against Focal Cerebral Ischemia and Reperfusion Injury in Rats. Cell. Physiol. Biochem. 45, 537–546. doi:10.1159/000487031
Wang, K., Ru, J., Zhang, H., Chen, J., Lin, X., Lin, Z., et al. (2020a). Melatonin Enhances the Therapeutic Effect of Plasma Exosomes against Cerebral Ischemia-Induced Pyroptosis through the TLR4/NF-Κb Pathway. Front. Neurosci. 14, 848. doi:10.3389/fnins.2020.00848
Wang, Y., Xiao, G., He, S., Liu, X., Zhu, L., Yang, X., et al. (2020b). Protection against Acute Cerebral Ischemia/reperfusion Injury by QiShenYiQi via Neuroinflammatory Network Mobilization. Biomed. Pharmacother. 125, 109945. doi:10.1016/j.biopha.2020.109945
Wei, J., Sun, C. L., Liu, C., and Zhang, Q. M. (2016). Cerebrovascular Protective Effect of Combination of Tetradrine and Atorvastatin against Cerebral Ischemia-Reperfusion Injury in Rats via Inhibition of Inflammatory Mediators. Int. J. Clin. Exp. Med. 9 (11), 22807–22813.
Wu, M., Lu, S., Zhong, J., Huang, K., and Zhang, S. (2017). Protective Effects of Pterostilbene against Myocardial Ischemia/Reperfusion Injury in Rats. Inflammation 40, 578–588. doi:10.1007/s10753-016-0504-2
Yan, M., Li, M., Gu, S., Sun, Z., Ma, T., and Ma, X. (2020). Ginkgo Biloba Extract Protects Diabetic Rats against Cerebral Ischemia-Reperfusion Injury by Suppressing Oxidative Stress and Upregulating the Expression of Glutamate Transporter 1. Mol. Med. Rep. 21, 1809–1818. doi:10.3892/mmr.2020.10990
Yang, Y., Fan, C., Wang, B., Ma, Z., Wang, D., Gong, B., et al. (2017). Pterostilbene Attenuates High Glucose-Induced Oxidative Injury in Hippocampal Neuronal Cells by Activating Nuclear Factor Erythroid 2-related Factor 2. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1863, 827–837. doi:10.1016/j.bbadis.2017.01.005
Yang, T., Feng, C., Wang, D., Qu, Y., Yang, Y., Wang, Y., et al. (2020). Neuroprotective and Anti-inflammatory Effect of Tangeretin against Cerebral Ischemia-Reperfusion Injury in Rats. Inflammation 43, 2332–2343. doi:10.1007/s10753-020-01303-z
Yu, H., Wu, M., Zhao, P., Huang, Y., Wang, W., and Yin, W. (2015). Neuroprotective Effects of Viral Overexpression of microRNA-22 in Rat and Cell Models of Cerebral Ischemia-Reperfusion Injury. J. Cel. Biochem. 116, 233–241. doi:10.1002/jcb.24960
Yu, L., Su, X., Li, S., Zhao, F., Mu, D., and Qu, Y. (2020). Microglia and Their Promising Role in Ischemic Brain Injuries: An Update. Front. Cel. Neurosci. 14, 211. doi:10.3389/fncel.2020.00211
Yuan, S., and Zhang, T. (2021). Boeravinone B Protects Brain against Cerebral Ichemia Reperfusion Injury in Rats: Possible Role of Anti-inflammatory and Antioxidant. J. Oleo Sci. 70, 927–936. doi:10.5650/jos.ess21037
Yuan, Y., Men, W., Shan, X., Zhai, H., Qiao, X., Geng, L., et al. (2020). Baicalein Exerts Neuroprotective Effect against Ischaemic/Reperfusion Injury via Alteration of NF-kB and LOX and AMPK/Nrf2 Pathway. Inflammopharmacology 28, 1327–1341. doi:10.1007/s10787-020-00714-6
Yuan, H., Yang, Q., Yang, B., Xu, H., Nasif, O., Muruganantham, S., et al. (2021a). Phyllanthin Averts Oxidative Stress and Neuroinflammation in Cerebral Ischemic-Reperfusion Injury through Modulation of the NF-Κb and AMPK/Nrf2 Pathways. J. Environ. Pathol. Toxicol. Oncol. 40, 85–97. doi:10.1615/JEnvironPatholToxicolOncol.2020036307
Yuan, Q., Yuan, Y., Zheng, Y., Sheng, R., Liu, L., Xie, F., et al. (2021b). Anti-cerebral Ischemia Reperfusion Injury of Polysaccharides: A Review of the Mechanisms. Biomed. Pharmacother. 137, 111303. doi:10.1016/j.biopha.2021.111303
Zhang, W., Song, J. K., Zhang, X., Zhou, Q. M., He, G. R., Xu, X. N., et al. (2018). Salvianolic Acid A Attenuates Ischemia Reperfusion Induced Rat Brain Damage by Protecting the Blood Brain Barrier through MMP-9 Inhibition and Anti-inflammation. Chin. J. Nat. Med. 16(3):184–193. doi:10.1016/S1875-5364(18)30046-3
Zhang, W. F., Jin, Y. C., Li, X. M., Yang, Z., Wang, D., and Cui, J. J. (2019). Protective Effects of Leptin against Cerebral Ischemia/reperfusion Injury. Exp. Ther. Med. 17, 3282–3290. (Review). doi:10.3892/etm.2019.7377
Zhang, C., Chen, S., Zhang, Z., Xu, H., Zhang, W., Xu, D., et al. (2020). Asiaticoside Alleviates Cerebral Ischemia-Reperfusion Injury via NOD2/Mitogen-Activated Protein Kinase (MAPK)/Nuclear Factor Kappa B (NF-κB) Signaling Pathway. Med. Sci. Monit. 26, e920325. doi:10.12659/MSM.920325
Zhang, Y., Han, Z., Jiang, A., Wu, D., Li, S., Liu, Z., et al. (2021). Protective Effects of Pterostilbene on Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting NF-Κb and Activating Nrf2/HO-1 Signaling Pathways. Front. Pharmacol. 11, 591836. doi:10.3389/fphar.2020.591836
Keywords: cerebral ischemia reperfusion, pterostilbene, inflammation, oxidative stress, COX-2
Citation: Yan W, Ren D, Feng X, Huang J, Wang D, Li T and Zhang D (2021) Neuroprotective and Anti-Inflammatory Effect of Pterostilbene Against Cerebral Ischemia/Reperfusion Injury via Suppression of COX-2. Front. Pharmacol. 12:770329. doi: 10.3389/fphar.2021.770329
Received: 03 September 2021; Accepted: 13 October 2021;
Published: 02 November 2021.
Edited by:
Muhammad Ayaz, University of Malakand, PakistanReviewed by:
Muhammad Ikram, Gyeongsang National University, South KoreaCopyright © 2021 Yan, Ren, Feng, Huang, Wang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjun Yan, eWFud2VuanVuMDcwMEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.