
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 20 December 2021
Sec. Pharmacoepidemiology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.766125
 Xiang Zhou1†
Xiang Zhou1† Xiaofei Ye2†
Xiaofei Ye2† Xiaojing Guo2†
Xiaojing Guo2† Dongxu Liu1†
Dongxu Liu1† Jinfang Xu2
Jinfang Xu2 Fangyuan Hu2
Fangyuan Hu2 Yinghong Zhai1
Yinghong Zhai1 Yongqing Gao1
Yongqing Gao1 Xiao Xu1
Xiao Xu1 Ziwei Dong1
Ziwei Dong1 Jia He1,2*
Jia He1,2*Background: Sodium-glucose co-transporter-2 inhibitors (SGLT2is) are widely used in clinical practice for their demonstrated cardiorenal benefits, but multiple adverse events (AEs) have been reported. We aimed to describe the distribution of SGLT2i-related AEs in different systems and identify important medical event (IME) signals for SGLT2i.
Methods: Data from the first quarter (Q1) of 2013–2021 Q2 in FAERS were selected to conduct disproportionality analysis. The definition of AEs and IMEs relied on the system organ classes (SOCs) and preferred terms (PTs) by the Medical Dictionary for Regulatory Activities (MedDRA-version 24.0). Two signal indicators, the reported odds ratio (ROR) and information component (IC), were used to estimate the association between SGLT2is and IMEs.
Results: A total of 57,818 records related to SGLT2i, with 22,537 SGLT2i-IME pairs. Most SGLT2i-related IMEs occurred in monotherapy (N = 21,408, 94.99%). Significant signals emerged at the following SOCs: “metabolism and nutrition disorders” (N = 9,103; IC025 = 4.26), “renal and urinary disorders” (3886; 1.20), “infections and infestations” (3457; 0.85). The common strong signals were observed in diabetic ketoacidosis, ketoacidosis, euglycaemic diabetic ketoacidosis and Fournier’s gangrene. Unexpected safety signals such as cellulitis, osteomyelitis, cerebral infarction and nephrolithiasis were detected.
Conclusion: Our pharmacovigilance analysis showed that a high frequency was reported for IMEs triggered by SGLT2i monotherapy. Different SGLT2is caused different types and the association strengths of IMEs, while they also shared some specific PTs. Most of the results are generally consistent with previous studies, and more pharmacoepidemiological studies are needed to validate for unexpected AEs. Based on risk-benefit considerations, clinicians should be well informed about important medical events that may be aggravated by SGLT2is.
Sodium-glucose co-transporter-2 inhibitors (SGLT2is) is a relatively new class of oral hypoglycemic agents indicated for adults with type 2 diabetes, which has been proven to be beneficial in reducing glycosylated hemoglobin, lowering blood pressure, losing weight, and improving blood lipids (Taylor et al., 2019; Sridhar et al., 2020). They have also been reported to be superior to placebo treatments in preventing cardiovascular events, improving end-stage renal disease, and reducing mortality (Bonora et al., 2021; Cahn et al., 2021; Ciardullo et al., 2021). In patients with type 2 diabetes who have atherosclerotic cardiovascular disease or established kidney disease or heart failure, SGLT2is is recommended by the American Diabetes Association (ADA) as the priority choice after metformin (American Diabetes, 2021). Currently, the FDA has approved four gliflozin congeners, named dapagliflozin, canagliflozin, empagliflozin, and ertugliflozin. Ipragliflozin, luseogliflozin, and tofogliflozin were launched in Japan. Remogliflozin has only been approved in India (Kuchay et al., 2021).
However, with their widespread use, AEs related to them have been reported (Puckrin et al., 2018; Ueda et al., 2018; Douros et al., 2020; Rampersad et al., 2020; Caparrotta et al., 2021). The common AEs include diabetic ketoacidosis (DKA), acute kidney injury (AKI), pyelonephritis, capacity depletion, genital fungi, and urinary tract infection (Blau et al., 2017; Fadini et al., 2017; Ueda et al., 2018; Caparrotta et al., 2021; Tuttle et al., 2021). They can be explained by the inherent mechanism of action of the drug (SGLT2is lower blood sugar levels by increasing urinary glucose excretion) or by the fact that the applicable users are generally prone to them. Moreover, the FDA issued warnings that canagliflozin is associated with an increased risk for lower-limb amputation and SGLT2is are associated with Fournier’s gangrene (FG). Canagliflozin may indirectly disrupt the homeostasis of calcium and phosphorus, contributing to weight loss, hypotension, loss of bone mineral density, and increased falls (Fadini and Avogaro, 2017; Zhou et al., 2019; Jackson and Moseley, 2020). FG is an extremely rare but life-threatening bacterial infection, which is also known as perineal necrotizing fasciitis. Although diabetes is a risk factor for developing FG, this infection remains rare in patients with it (Bersoff-Matcha et al., 2019). This number only includes reports submitted to the FDA, so there may be other cases that we are not aware of.
SGLT2is may trigger serious AEs, which may remain undetected. Therefore, a comprehensive analysis of important medical events (IMEs) is necessary to uncover the signals of all SGLT2is-IMEs pairs. This study aimed to analyze IMEs associated with SGLT2is in all SOCs by mining the large pharmacovigilance database (FAERS). We presented more descriptive information on patient characterizations as Supplementary Data to previous studies and mapped signal profiles to visually represent the correlation between SGLT2is and IMEs, to more quickly identify potential safety issues and provide recommendations for clinical use.
The FAERS database is a spontaneous reporting system (SRS) collecting global reports of AEs to support the FDA’s post-marketing surveillance for drugs and biotherapy products. It enables signal detection and quantification of the association between drugs and reporter AEs (Min et al., 2018; Zhai et al., 2019). Data is aggregated quarterly and contains AE reports, medication error reports, and product quality complaints. In this study, we extracted all reports of SGLT2i from FAERS between 2013Q1 (first FDA approval) and 2021Q2 (the latest). Considering the existence of duplicate records in FAERS, we performed the deduplication process. According to the FDA recommendation (Hu et al., 2020), duplicate records are removed by selecting the latest FDA_DT when the CASEID and FDA_DT are the same.
Canagliflozin, empagliflozin, dapagliflozin, ertugliflozin, ipragliflozin, tofogliflozin, luseogliflozin, remogliflozin, and their combination with other drugs all included. Monotherapy here was defined as a specific SGLT2i used alone, which means that this specific SGLT2i is the “primary suspect” in the ROLE_COD field of the DRUG file and without other antidiabetic drugs in the same report as “secondary suspect,” “concomitant” or “interacting.” Combination therapy/polytherapy was defined as concurrent administration of SGLT2is and other antidiabetic agents, which implies that in the same report if the specific SGLT2i is the “primary suspect,” the other antidiabetic agent is the “secondary suspect,” “concomitant” or “interacting,” and vice versa. Since FAERS does not standardize drug names, both brand names and generic names were used to identify records of target drugs. The index of all drug names was shown in Supplementary Table S1. All AEs were coded as preferred terms (PTs) according to MedDRA (version 24.0). The primary system organ classes (SOCs) corresponding to these PTs were also listed, and SOCs were equivalent to the system classification in other medical terms. Subsequently, we used the Important Medical Events (IMEs) list to filter out those associated with SGLT2is and mapped a signal profile of SGLT2is-induced IMEs. The list of IMEs is developed by European Medicines Agency (EMA) to help prioritize the review reports of suspected adverse drug reactions (ADRs) in the framework of routine pharmacovigilance activities and is used to identify reports of suspected ADRs which deserve attention. The full IME list is openly available on the website of https://www.ema.europa.eu/documents/other/important-medical-event-terms-list-version-meddra-version-240_en.xlsx. Severe outcomes included life-threatening events or those causing hospitalization, disability, or death. Unexpected AE was defined as any significant adverse event that was not mentioned in the FDA drug prescribing information.
Disproportionality analysis was performed in our study to indicate the presence of signals of potential increased risk of drug-related AE. In quantitative signal detection, a disproportionately high frequency of drug-event pairs in the database compared to expected may represent a significant signal (van Puijenbroek et al., 2002; Montastruc et al., 2011). We used two disproportional signal detection methods based on the frequency and Bayesian theories - the proportional report odds ratio (ROR) and Bayesian confidence propagation neural network of information components (IC) - to verify the stability of the detected signals. Considering that a spurious association can occur when events with very low expected frequency, statistical shrinkage transformation was performed to obtain conservative results (Noren et al., 2013; Zhai et al., 2019). The shrinkage transformation of ROR and IC is calculated as follows:
All the measures of disproportionality are based on the same principles of calculation using the 2 × 2 table (Supplementary Table S2). Nobserved = a, which is is the observed number of records of interested drug-AE pairs, Nexpected is the expected number of records of interested drug-AE pairs, Ndrug is the total number of records of the target drug, Nevent is the total number of records of target AEs, and Ntotal is the total number of records in the whole database.
The 95% confidence intervals for ROR (ROR025) and IC (IC025) were calculated to describe the association strength of SGLT2is and IMEs. A significant signal was defined as ROR025 greater than 1 with at least three cases or IC025 above zero (van Puijenbroek et al., 2002). All analyses were performed SAS version 9.4 (SAS Institute Inc., Cary, NC, United States).
From 2013q1 to 2021q2, a total of 11,822,884 AE reports were submitted to the FDA, with 57,818 related to the use of SGLT2i, of which 22,537 (38.98% to all SGLT2i) reported events were IMEs. The distribution of gliflozin congeners and the clinical characteristics of the patients are described in Tables 1,2. In general, the distribution of each indicator was similar in SGLT2i-related AEs and IMEs. For SGLT2i-related IMEs, monotherapy (94.99%) accounted for a much larger proportion than combination therapy, with canagliflozin constituting nearly half (48.26%). The vast majority of reports cited SGLT2i as the primary suspect drug (94.55%). Most reported cases were male (48.60%). The median age was 58 (interquartile range [IQR] 49–67, and nearly half (47.53%) of the cases were under 65 years old. The median weight of the available data was 84 kg (IQR 70–100 kg). There is an increasing trend overall in reporting years, with peaks in 2015 and 2018. This may be due to the concern raised by the FDA issuing some black box warnings about SGLT2is at these times. Hospitalization and other serious medical events were the most frequently reported outcomes. During the study period (nearly 9 years), 2,464 patients developed severe outcome events (except hospitalization) reported in IME. It implied that SGLT2is may have potentially dangerous attributes.
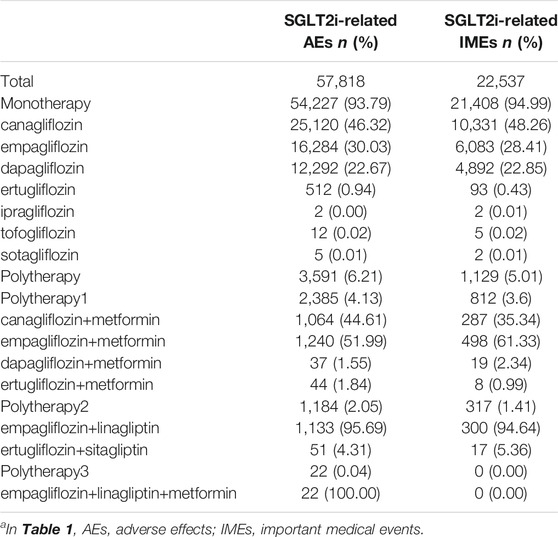
TABLE 1. Distribution of SGLT2i across therapiesa.
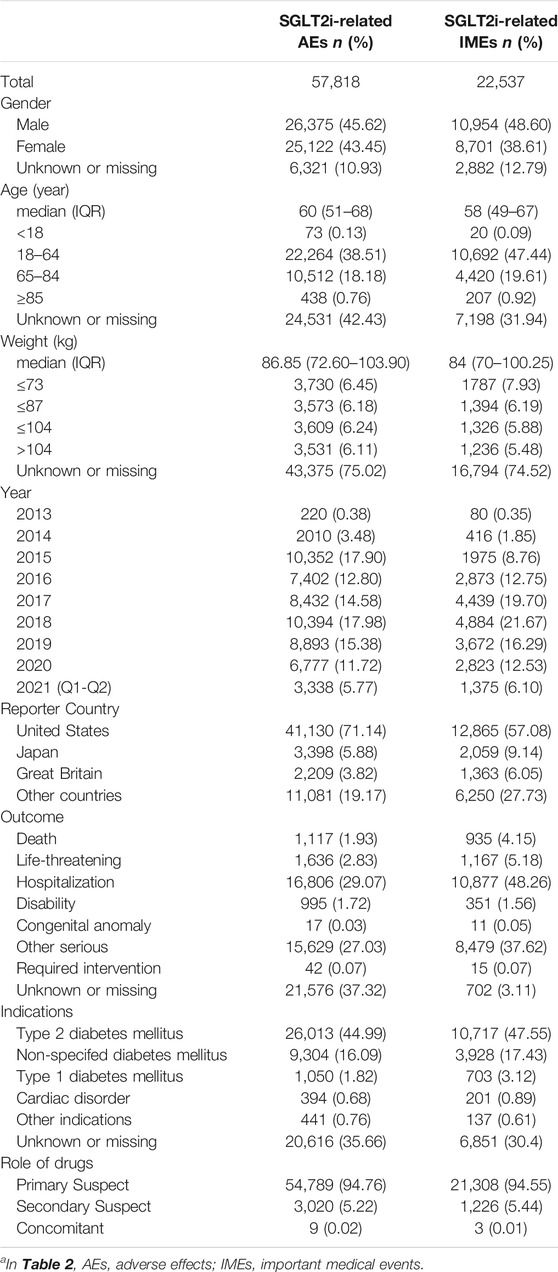
TABLE 2. Characteristics of patients with SGLT2i-related AEs/IMEsa.
Considering SGLT2i as a class, the signal strengths of its associated AEs or IMEs at different SOCs are presented in Table 3. Significant signal overlap emerged in three SOCs: “infections and infestations” (IC025 of AEs = 1.67; IC025 of IMEs = 0.85), “metabolism and nutrition disorders” (3.33; 4.26), “renal and urinary disorders” (1.94; 1.20). In addition, SGLT2i-induced AEs also showed significance in “investigations,” “reproductive system and breast disorders” and “surgical and medical procedures.” Among them, “Reproductive system and breast disorders” had the lowest number of reports but relatively strong association (N = 911, IC025 = 0.88).
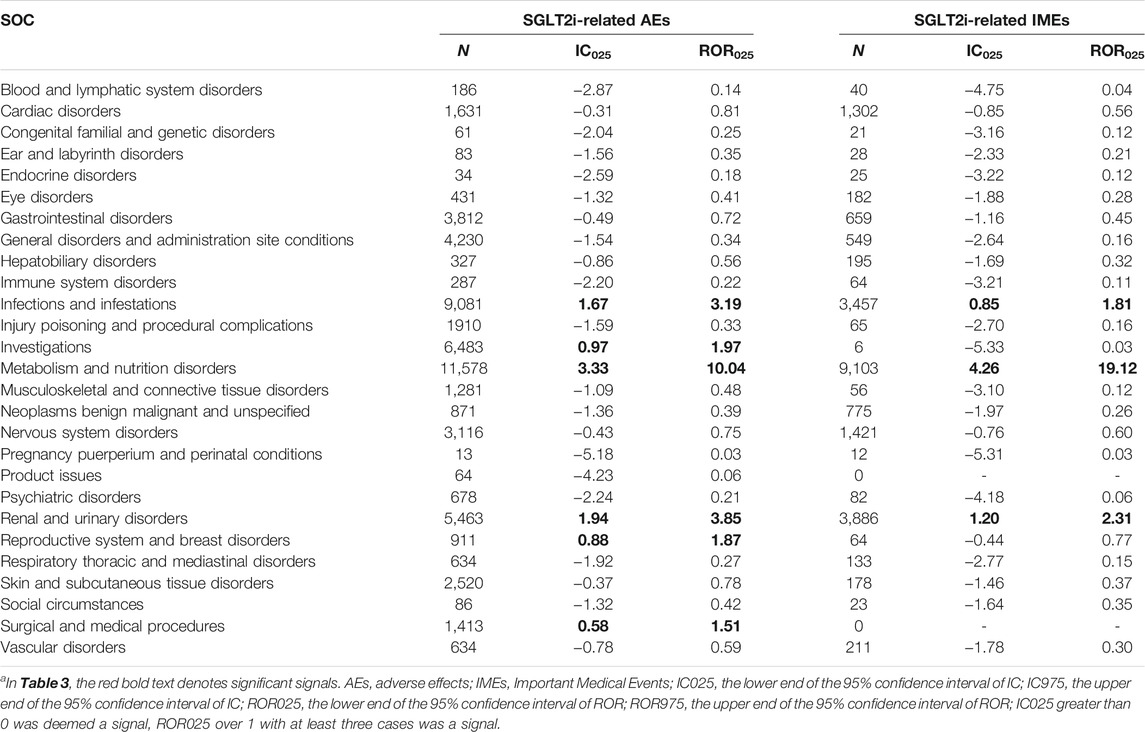
TABLE 3. Signal strength of SGLT2i and AEs/IMEs at the System Organ Class (SOC) levela.
We further analyzed the different drugs in the monotherapy with each specific IME to explore whether there was an association between them. Since the number of drug-specific IME combinations for ipragliflozin, tofogliflozin, and sotagliflozin was less than 3, these three drugs were not included in this analysis. The top 50 (116 in total) significant signals ranked by frequency are listed in Figure 1. We used the IC025 value as an indicator and defined a strong signal when the IC025 value was not lower than 4. A full list of IMEs for SGLT2i can be accessed in the Supplementary Figure S1 and Supplementary Table S3. The distribution of significant signals in Figure 1 and Supplementary Figure S1 is approximately the same. The PTs in them were sorted in descending order within the corresponding SOCs.
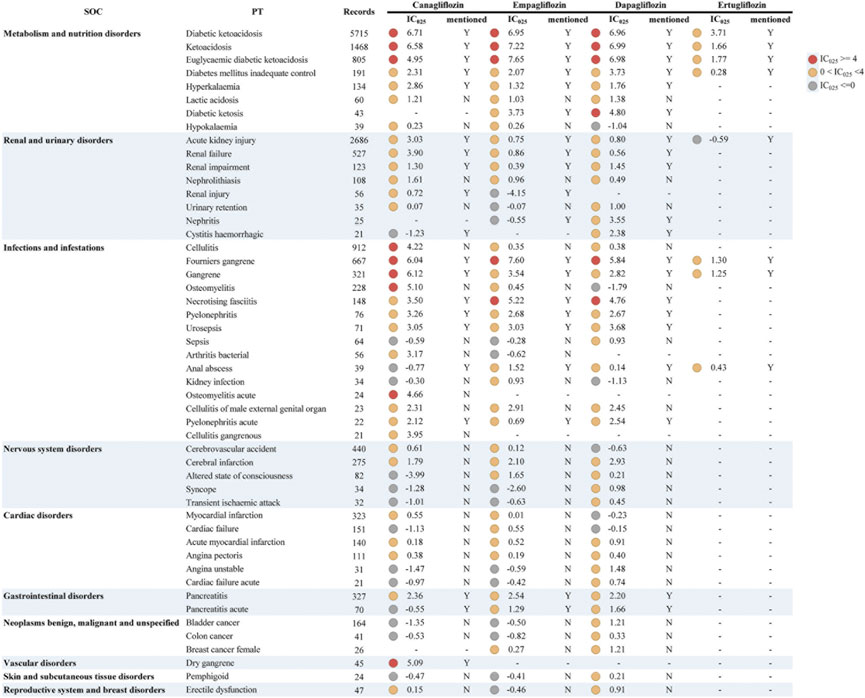
FIGURE 1. Signal profiles of top 50 IMEs induced by SGLT2i monotherapy-based on all other drugs as “non-case”* *In Figure 1, IMEs, Important Medical Events; SOC, System Organ Class; PT, Preferred Term; IC, information component; IC025, the lower limit of the 95% confidence interval of IC. IC025 greater than 0 was deemed a signal. IC025 no less than 4 was deemed a strong signal.
For the complete signal spectrum, SGLT2i-involved IMEs were mainly distributed in “infections and infestations” (n = 40), accordingly, the most signals appeared. Although relatively few IMEs were monitored in “metabolism and nutrition disorders,” the strong signal distribution was the most intensive. This result was consistent with the strength of the association between IMEs and specific SOCs in Table 3. From the specific drug perspective, dapagliflozin presented the broadest spectrum with a total of 69 potential signals detected, ranging from ventricular tachycardia (IC025 = 0.06) to ketoacidosis (IC025 = 6.99). In addition to the currently known AEs (diabetic ketoacidosis, Fournier’s gangrene, etc.), there remained 46 IMEs not previously mentioned in the prescribing information for dapagliflozin, of which stronger signals were found for epididymitis, cerebral infarction, and brain stem infarction. For canagliflozin, a total of 64 significant signals were observed, with IC025 values ranging from 0.02 (bladder transitional cell carcinoma) to 6.71 (diabetic ketoacidosis). Strong signals emerged in ketoacidosis (unspecified type), gangrene (unspecified type), osteomyelitis, and cellulitis. The latter two were not observed in the instructions, and acute osteomyelitis and dry gangrene were unique strong signals for canagliflozin. There were 61 PTs significantly associated with empagliflozin. Of these, euglycaemic diabetic ketoacidosis (euDKA), Fournier’s gangrene (FG), ketoacidosis, diabetic ketoacidosis (DKA), necrotising fasciitis showed a robust correlation (corresponding IC025 = 7.65, 7.60, 7.22, 6.95, 5.22) and the first four PTs are shared with canagliflozin and dapagliflozin. However, the drug with the fewest PTs was ertugliflozin, with only eight signals detected and all overlapping with other drugs. Notably, DKA, ketoacidosis, euDKA, FG, gangrene, and “diabetes mellitus inadequate control” were the six PTs common to these four SGLT2is, the first four of which showed reported with high frequency and marked strong intensity.
We also used other antidiabetic drugs as “non-cases” to adjust for the background risk of such events in diabetic patients, taking into account the effect of the underlying disease (Figure 2 and Supplementary Table S4). The results indicated a general decrease in IC025 values and no strong signal. Similar to all drugs as non-cases, the signals were mostly observed in “infections and infestations.” Likewise, dapagliflozin also had the widest signal spectrum (n = 38), ranging from pyelonephritis (IC025 = 0.0005) to nephritis (1.82). The strong signal with dapagliflozin found in Figure 1 also appears in Figure 2, but the signal strength is substantially reduced. Nephritis, diabetic ketosis, cystitis haemorrhagic, and cerebral infarction with relatively strong signals (corresponding IC025 = 1.82, 1.60, 1.50, 1.24). This was followed by canagliflozin (n = 27), where cellulitis, osteomyelitis, and gangrene had the top three signal strengths (1.78, 1.76, 1.54). Notably, the signal of Fournier’s gangrene and euglycaemic diabetic ketoacidosis vanished in canagliflozin and ertugliflozin, while stronger signals were consistently shown in empagliflozin. Cellulitis, osteomyelitis, cerebral infarction, and nephrolithiasis, showed unexpected signals in both analyses.
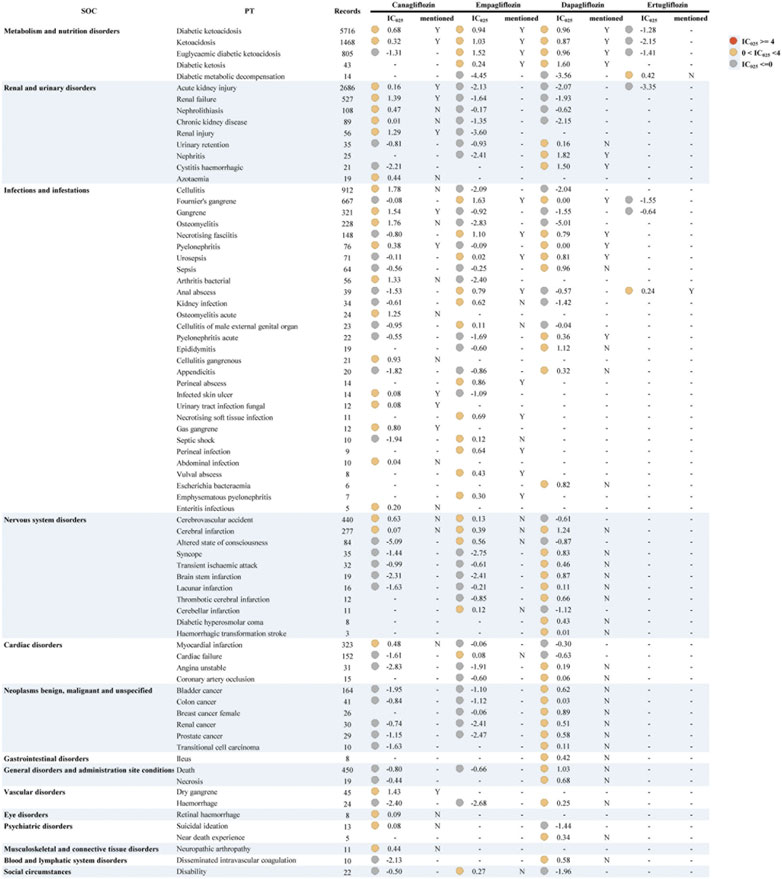
FIGURE 2. Signal profiles of all IMEs induced by SGLT2i monotherapy-based on other antidiabetic drugs as “non-case”* *In Figure 2, IMEs, Important Medical Events; SOC, System Organ Class; PT, Preferred Term; IC, information component; IC025, the lower limit of the 95% confidence interval of IC. IC025 greater than 0 was deemed a signal. IC025 no less than 4 was deemed a strong signal.
We presented common significant signals for serious outcomes in monotherapy (Table 4). DKA, ketoacidosis, euDKA, FG, and gangrene were frequently reported. Further analysis indicated that DKA and ketoacidosis seemed to be more strongly associated with canagliflozin, empagliflozin, and dapagliflozin (Supplementary Figure S2). Slightly lower frequency and association of canagliflozin with euDKA compared to empagliflozin and dapagliflozin. A similar scenario occurs for the combination of dapagliflozin with FG. Age below 65 years or men seems to be more likely to suffer from FG (Supplementary Figure S3). Notably, empagliflozin alone or combined metformin\linagliptin was highly associated with developing these four SGLT2i-related IMEs.
In this study, the potential adverse signals of SGLT2i were mined based on the FAERS database using ROR and IC methods. The two methods are based on different statistical ideas (ROR belongs to the frequency method and IC is based on the Bayesian method) which can reflect the target drug-AE association in a mutually validated, rapid, and quantitative way. In addition to analyzing all SGLT2i-induced AEs like previous studies (Raschi et al., 2017; Ueda et al., 2018; Caparrotta et al., 2021), we focused on the IMEs associated with SGLT2i, mapping an important potential signal spectrum for monotherapy in a visual manner. Our findings are as follows.
According to our analysis, DKA, ketoacidosis, euDKA, FG, gangrene, and “inadequate diabetic control” were the six common IMEs after receiving the four FDA-approved SGLT2is. Further analysis demonstrated that the first five AEs were more likely to occur with SGLT2i compared to other hypoglycemic agents. They were all illustrated in the instructions, and the first four PTs appeared as highly significant signals in all four drugs. These AEs have been proven in previous studies or clinical trials (Ueda et al., 2018; Scheen, 2019; Toyama et al., 2019; Hu et al., 2020). EuDKA is a rare complication of diabetes, with normal or slightly increased blood glucose levels. This often masks the symptoms of DKA and easily leads to a missed diagnosis. The FDA states that regardless of current blood glucose levels (even if blood glucose levels are below 250 mg/dl), physicians should discontinue the drug and handle it as soon as the patient develops signs and symptoms of ketoacidosis. In post-marketing surveillance of diabetic patients receiving SGLT2 inhibitors (Bersoff-Matcha et al., 2019; Fadini et al., 2019; Hu et al., 2020), reports of necrotizing fasciitis of the perineum (Fournier’s gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, were identified. Cases have been reported in both women and men, with men being more susceptible (Kuchinka et al., 2019; Wang et al., 2020), and we found the same results. In addition, we found a strong association between empagliflozin and FG occurrence whether based on all other drugs or other antiglycemic agents. The same results emerged in a study based on FAERS exploring the association of FG with SGLT2i (Hu et al., 2020).
A total of 11 PTs exist that are not mentioned in the SGLT2i instructions, mainly related to cardio-cerebral diseases and infectious diseases (Cellulitis). For example, “Acute myocardial infarction,” “Angina pectoris,” “Coronary artery stenosis,” and “Cerebral infarction” were identified as significant signals. Notably, compared to other antiglycemic medications, the frequency of cellulitis, osteomyelitis, cerebral infarction, and nephrolithiasis was higher in SGLT2is users. Although SGLT2i has been confirmed to have cardiovascular protective effects, a few studies noted that some patients developed some of these AEs shortly after receiving treatment, and most of these cases occurred in Japan. (Ito et al., 2016; Goda et al., 2018). In contrast, a meta-analysis provided evidence that SGLT2is had no significant effect on ischemic events caused by atherosclerotic coronary artery disease in patients with type 2 diabetes (Ye et al., 2021). Whether SGLT2is contribute to these AEs is controversial and without evidence from large pharmacoepidemiological studies or clinical trials. However, what we do know is that SGLT2i treatment can lead to volume depletion or even dehydration due to excessive diuresis. And dehydration may lead to thromboembolism (e.g., cerebral infarction). Therefore, patients should be encouraged to drink appropriate amounts of water regularly. Meanwhile, cerebral infarction and myocardial infarction are also rare and serious complications of DKA (Yaroglu Kazanci et al., 2015).
In both SGLT2i-induced AEs and IMEs, we obtained signals of infectious, metabolic, and urinary/renal events, which was generally consistent with the safety data obtained from RCTs (Scheen, 2019; American Diabetes, 2021). It also demonstrated the credible predictive ability of SRS in pharmacovigilance. Thus, before initiating therapy, prescribers should be aware of patients with concomitant diseases of the above systems and keep them under surveillance to avoid deterioration. In addition, an SRS-based study (Raschi et al., 2017) monitored the significance of “skin and subcutaneous tissue disorders,” which was not highlighted in our results.
Our study has several limitations. Firstly, FAERS has inherent flaws as an SRS, such as underreporting, duplicate records, uneven information quality, lack of controls, and inability to calculate incidence rates. Although we performed manual correction and de-duplication, there may be records of target drugs that were not included. Secondly, although the use of disproportionality analysis in pharmacovigilance is well established, a limitation of such methods is the lack of a gold standard for assessing the validity and magnitude of suspected safety issues (Almenoff et al., 2007). We defined graded thresholds of signal intensity that allow clinicians to understand suspicious events more intuitively and quickly. Moreover, the significant signals monitored only represent potential associations rather than causality. Finally, we only analyzed the single and combined usage of SGLT2i, without comparing other hypoglycemic agents. This will be considered as our sequential study. Nevertheless, FAERS has provided us with a huge amount of drug safety data for free. We have systematically mined and identified potential IME signals associated with SGLT2is, as well as provided a signaling profile of SGLT2i-induced IMEs, which could provide valuable evidence for further research and clinical practice in this area.
With the widespread clinical use of SGLT2i, concerns about safety issues have arisen. This study comprehensively explored the potential IME signals of SGLT2i based on all other drugs and other antidiabetic drugs as non-cases. The type and magnitude of IMEs varied among different SGLT2is. Except for cellulitis, osteomyelitis, and some cardiac/cerebral events, most results were consistent with previous studies. Our results can only indicate that these IMEs are overreported in SGLT2i, while proof of causality requires additional evidence, such as pharmacoepidemiological studies, pharmacokinetic and pharmacodynamics plausibility, or from pharmacological properties and pathophysiology.
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Conception or design of the work: XZ, XY, and JH; Acquisition, analysis, or interpretation of data: XZ, FH, and YZ; Management and checking of all data: XG, JX, YG, DL, XX, and ZD; Drafting the article: XZ. All authors critically reviewed the manuscript and interpreted the results. The final manuscript was read, checked, and approved by all authors.
(National Nature Science Foundation of China (No. 82073671)); (the Leading Talents of Public Health in Shanghai (No. GWV-10.2-XD22)); (the Shanghai Municipal Commission of Health and Family Planning Fund for Excellent Young Scholars (No. 2018YQ47)); (the Nature Science Foundation of Shanghai (No.18ZR1449500)); (the National Thirteen Five Year Plan Major Special Project (No. 2017ZX09304016)); (the Excellent Young Scholars of Public Health in Shanghai (No. GWV-10.2-YQ33)); (Military Key Discipline Construction Project (Health Service–Naval Health Service Organization and Command) (No. 03)) the National Key R&D Program of China (No. 2017YFC0908005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.766125/full#supplementary-material
Almenoff, J. S., Pattishall, E. N., Gibbs, T. G., DuMouchel, W., Evans, S. J., and Yuen, N. (2007). Novel Statistical Tools for Monitoring the Safety of Marketed Drugs. Clin. Pharmacol. Ther. 82 (2), 157–166. doi:10.1038/sj.clpt.6100258
American Diabetes, A. (2021). 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care 44 (Suppl. 1), S111–S124. doi:10.2337/dc21-S009
Bersoff-Matcha, S. J., Chamberlain, C., Cao, C., Kortepeter, C., and Chong, W. H. (2019). Fournier Gangrene Associated with Sodium-Glucose Cotransporter-2 Inhibitors: A Review of Spontaneous Postmarketing Cases. Ann. Intern. Med. 170 (11), 764–769. doi:10.7326/M19-0085
Blau, J. E., Tella, S. H., Taylor, S. I., and Rother, K. I. (2017). Ketoacidosis Associated with SGLT2 Inhibitor Treatment: Analysis of FAERS Data. Diabetes Metab. Res. Rev. 33 (8), e2924. doi:10.1002/dmrr.2924
Bonora, B. M., Raschi, E., Avogaro, A., and Fadini, G. P. (2021). SGLT-2 Inhibitors and Atrial Fibrillation in the Food and Drug Administration Adverse Event Reporting System. Cardiovasc. Diabetol. 20 (1), 39. doi:10.1186/s12933-021-01243-4
Cahn, A., Raz, I., Leiter, L. A., Mosenzon, O., Murphy, S. A., Goodrich, E. L., et al. (2021). Cardiovascular, Renal, and Metabolic Outcomes of Dapagliflozin versus Placebo in a Primary Cardiovascular Prevention Cohort: Analyses from DECLARE-TIMI 58. Dia Care 44, 1159–1167. doi:10.2337/dc20-2492
Caparrotta, T. M., Greenhalgh, A. M., Osinski, K., Gifford, R. M., Moser, S., Wild, S. H., et al. (2021). Sodium-Glucose Co-transporter 2 Inhibitors (SGLT2i) Exposure and Outcomes in Type 2 Diabetes: A Systematic Review of Population-Based Observational Studies. Diabetes Ther. 12 (4), 991–1028. doi:10.1007/s13300-021-01004-2
Ciardullo, S., Trevisan, R., and Perseghin, G. (2021). Sodium-glucose Transporter 2 Inhibitors for Renal and Cardiovascular Protection in US Adults with Type 2 Diabetes: Impact of the 2020 KDIGO Clinical Practice Guidelines. Pharmacol. Res. 166, 105530. doi:10.1016/j.phrs.2021.105530
Douros, A., Lix, L. M., Fralick, M., Dell'Aniello, S., Shah, B. R., Ronksley, P. E., et al. (2020). Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis : A Multicenter Cohort Study. Ann. Intern. Med. 173 (6), 417–425. doi:10.7326/M20-0289
Fadini, G. P., and Avogaro, A. (2017). SGLT2 Inhibitors and Amputations in the US FDA Adverse Event Reporting System. Lancet Diabetes Endocrinol. 5 (9), 680–681. doi:10.1016/s2213-8587(17)30257-7
Fadini, G. P., Bonora, B. M., and Avogaro, A. (2017). SGLT2 Inhibitors and Diabetic Ketoacidosis: Data from the FDA Adverse Event Reporting System. Diabetologia 60 (8), 1385–1389. doi:10.1007/s00125-017-4301-8
Fadini, G. P., Sarangdhar, M., De Ponti, F., Avogaro, A., and Raschi, E. (2019). Pharmacovigilance Assessment of the Association Between Fournier's Gangrene and Other Severe Genital Adverse Events with SGLT-2 Inhibitors. BMJ Open Diabetes Res. Care 7 (1), e000725. doi:10.1136/bmjdrc-2019-000725
Goda, M., Yamakura, T., Sasaki, K., Tajima, T., and Ueno, M. (2018). Safety and Efficacy of Canagliflozin in Elderly Patients with Type 2 Diabetes Mellitus: A 1-year Post-marketing Surveillance in Japan. Curr. Med. Res. Opin. 34 (2), 319–327. doi:10.1080/03007995.2017.1392293
Hu, Y., Bai, Z., Tang, Y., Liu, R., Zhao, B., Gong, J., et al. (2020). Fournier Gangrene Associated with Sodium-Glucose Cotransporter-2 Inhibitors: A Pharmacovigilance Study with Data from the U.S. FDA Adverse Event Reporting System. J. Diabetes Res. 2020, 3695101. doi:10.1155/2020/3695101
Ito, H., Shinozaki, M., Nishio, S., and Abe, M. (2016). SGLT2 Inhibitors in the Pipeline for the Treatment of Diabetes Mellitus in Japan. Expert Opin. Pharmacother. 17 (15), 2073–2084. doi:10.1080/14656566.2016.1232395
Jackson, K., and Moseley, K. F. (2020). Diabetes and Bone Fragility: SGLT2 Inhibitor Use in the Context of Renal and Cardiovascular Benefits. Curr. Osteoporos. Rep. 18 (5), 439–448. doi:10.1007/s11914-020-00609-z
Kuchay, M. S., Farooqui, K. J., Mishra, S. K., and Mithal, A. (2021). Glucose Lowering Efficacy and Pleiotropic Effects of Sodium-Glucose Cotransporter 2 Inhibitors. Adv. Exp. Med. Biol. 1307, 213–230. doi:10.1007/5584_2020_479
Kuchinka, J., Matykiewicz, J., Wawrzycka, I., Kot, M., Karcz, W., and Głuszek, S. (2019). Fournier's Gangrene - Challenge for Surgeon. Pol. Przegl Chir 92 (5), 1–5. doi:10.5604/01.3001.0013.5894
Min, J., Osborne, V., Kowalski, A., and Prosperi, M. (2018). Reported Adverse Events with Painkillers: Data Mining of the US Food and Drug Administration Adverse Events Reporting System. Drug Saf. 41 (3), 313–320. doi:10.1007/s40264-017-0611-5
Montastruc, J. L., Sommet, A., Bagheri, H., and Lapeyre-Mestre, M. (2011). Benefits and Strengths of the Disproportionality Analysis for Identification of Adverse Drug Reactions in a Pharmacovigilance Database. Br. J. Clin. Pharmacol. 72 (6), 905–908. doi:10.1111/j.1365-2125.2011.04037.x
Norén, G. N., Hopstadius, J., and Bate, A. (2013). Shrinkage Observed-To-Expected Ratios for Robust and Transparent Large-Scale Pattern Discovery. Stat. Methods Med. Res. 22 (1), 57–69. doi:10.1177/0962280211403604
Puckrin, R., Saltiel, M. P., Reynier, P., Azoulay, L., Yu, O. H. Y., and Filion, K. B. (2018). SGLT-2 Inhibitors and the Risk of Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Acta Diabetol. 55 (5), 503–514. doi:10.1007/s00592-018-1116-0
Rampersad, C., Kraut, E., Whitlock, R. H., Komenda, P., Woo, V., Rigatto, C., et al. (2020). Acute Kidney Injury Events in Patients with Type 2 Diabetes Using SGLT2 Inhibitors Versus Other Glucose-Lowering Drugs: A Retrospective Cohort Study. Am. J. Kidney Dis. 76 (4), 471–479.e1. doi:10.1053/j.ajkd.2020.03.019
Raschi, E., Parisotto, M., Forcesi, E., La Placa, M., Marchesini, G., De Ponti, F., et al. (2017). Adverse Events with Sodium-Glucose Co-transporter-2 Inhibitors: A Global Analysis of International Spontaneous Reporting Systems. Nutr. Metab. Cardiovasc. Dis. 27 (12), 1098–1107. doi:10.1016/j.numecd.2017.10.008
Scheen, A. J. (2019). An Update on the Safety of SGLT2 Inhibitors. Expert Opin. Drug Saf. 18 (4), 295–311. doi:10.1080/14740338.2019.1602116
Sridhar, V. S., Rahman, H. U., and Cherney, D. Z. I. (2020). What Have We Learned About Renal protection from the Cardiovascular Outcome Trials and Observational Analyses with SGLT2 Inhibitors? Diabetes Obes. Metab. 22 Suppl 1, 55–68. doi:10.1111/dom.13965
Taylor, S. I., Blau, J. E., Rother, K. I., and Beitelshees, A. L. (2019). SGLT2 Inhibitors as Adjunctive Therapy for Type 1 Diabetes: Balancing Benefits and Risks. Lancet Diabetes Endocrinol. 7 (12), 949–958. doi:10.1016/s2213-8587(19)30154-8
Toyama, T., Neuen, B. L., Jun, M., Ohkuma, T., Neal, B., Jardine, M. J., et al. (2019). Effect of SGLT2 Inhibitors on Cardiovascular, Renal and Safety Outcomes in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 21 (5), 1237–1250. doi:10.1111/dom.13648
Tuttle, K. R., Brosius, F. C., Cavender, M. A., Fioretto, P., Fowler, K. J., Heerspink, H. J. L., et al. (2021). SGLT2 Inhibition for CKD and Cardiovascular Disease in Type 2 Diabetes: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Am. J. Kidney Dis. 77 (1), 94–109. doi:10.1053/j.ajkd.2020.08.003
Ueda, P., Svanström, H., Melbye, M., Eliasson, B., Svensson, A. M., Franzén, S., et al. (2018). Sodium Glucose Cotransporter 2 Inhibitors and Risk of Serious Adverse Events: Nationwide Register Based Cohort Study. BMJ 363, k4365. doi:10.1136/bmj.k4365
van Puijenbroek, E. P., Bate, A., Leufkens, H. G., Lindquist, M., Orre, R., and Egberts, A. C. (2002). A Comparison of Measures of Disproportionality for Signal Detection in Spontaneous Reporting Systems for Adverse Drug Reactions. Pharmacoepidemiol. Drug Saf. 11 (1), 3–10. doi:10.1002/pds.668
Wang, T., Patel, S. M., Hickman, A., Liu, X., Jones, P. L., Gantz, I., et al. (2020). SGLT2 Inhibitors and the Risk of Hospitalization for Fournier's Gangrene: A Nested Case-Control Study. Diabetes Ther. 11 (3), 711–723. doi:10.1007/s13300-020-00771-8
Yaroglu Kazanci, S., Yesilbas, O., Ersoy, M., Kihtir, H. S., Yildirim, H. M., and Sevketoglu, E. (2015). Cerebral Infarction and Femoral Venous Thrombosis Detected in a Patient with Diabetic Ketoacidosis and Heterozygous Factor V Leiden G1691A and PAI-1 4G/5G Mutations. J. Pediatr. Endocrinol. Metab. 28 (9-10), 1183–1186. doi:10.1515/jpem-2015-0056
Ye, G., Wang, S., and Peng, D. (2021). Effects of SGLT2 Inhibitor on Ischemic Events Stemming from Atherosclerotic Coronary Diseases: A Systematic Review and Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials. J. Cardiovasc. Pharmacol. 77, 787–795. doi:10.1097/FJC.0000000000001018
Zhai, Y., Ye, X., Hu, F., Xu, J., Guo, X., Zhuang, Y., et al. (2019). Endocrine Toxicity of Immune Checkpoint Inhibitors: A Real-World Study Leveraging US Food and Drug Administration Adverse Events Reporting System. J. Immunother. Cancer 7 (1), 286. doi:10.1186/s40425-019-0754-2
Keywords: SGLT2 inhibitor, important medical events, adverse event, FAERS, pharmacovigilance, disproportionality, diabetes
Citation: Zhou X, Ye X, Guo X, Liu D, Xu J, Hu F, Zhai Y, Gao Y, Xu X, Dong Z and He J (2021) Safety of SGLT2 Inhibitors: A Pharmacovigilance Study from 2013 to 2021 Based on FAERS. Front. Pharmacol. 12:766125. doi: 10.3389/fphar.2021.766125
Received: 28 August 2021; Accepted: 22 November 2021;
Published: 20 December 2021.
Edited by:
Emanuel Raschi, University of Bologna, ItalyReviewed by:
Charles Khouri, Centre Hospitalier Universitaire de Grenoble, FranceCopyright © 2021 Zhou, Ye, Guo, Liu, Xu, Hu, Zhai, Gao, Xu, Dong and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia He, aGVqaWE2M0B5ZWFoLm5ldA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.