- Department of Hematology and Research Laboratory of Hematology, West China Hospital, Sichuan University, Chengdu, China
Background: Selinexor (SEL) is an orally bioavailable, highly-selective, and slowly-reversible small molecule that inhibits Exportin 1. Preclinical studies showed that SEL had synergistic antimyeloma activity with glucocorticoids, proteasome inhibitors (PIs) and immunomodulators. The combination of selinexor and dexamethasone (DEX) has been approved in the United States for patients with penta-refractory multiple myeloma in July 2019. This meta-analysis aimed to investigate the safety and efficacy of selinexor based treatment in Multiple myeloma.
Methods: We systematically searched the Medline (PubMed), Embase, Web of Science, Cochrane Central Register of Controlled Trials Library databases and ClinicalTrials.gov. Outcome measures of efficacy included overall response rate (ORR), clinical benefit rate (CBR), stringent complete response rate (sCR), complete response rate (CR), very good partial response (VGPR), partial response rate (PR), minimal response (MR), rate of stable disease (SDR), rate of progressive disease (PDR) and median progression-free survival (mPFS). Safety was evaluated by the incidences of all grade adverse events and Grade≥3 adverse events. The subgroup analysis was conducted to analyze the difference in different combination treatment regimens (SEL + DEX + PIs vs SEL + DEX).
Results: We included six studies with 477 patients. The pooled ORR, CBR, sCR, CR, VGPR, PR, MR, SDR, and PDR were 43% (18–67%), 55% (32–78%), 5% (−2–13%), 7% (4–11%), 14% (5–24%), 23% (15–31%), 11% (8–14%), 26% (14–38%) and 14% (4–23%), respectively. SEL + DEX + PIs treatment had higher ORR (54 vs 24%, p = 0.01), CBR (66 vs 37%, p = 0.01), sCR (10 vs 2%, p = 0.0008), and VGPR (23 vs 5%, p < 0.00001) compared to SEL + DEX treatment, and lower PDR (4 vs 23%, p < 0.00001) and SDR (17 vs 37%, p = 0.0006). The pooled incidences of any grade and grade≥3 were 45 and 30% in hematological AEs, and in non-hematological AEs were 40 and 30%, respectively. The most common all grade (68%) and grade≥3 (54%) hematological AE were both thrombocytopenia. Fatigue was the most common all grade (62%) and grade≥3 (16%) non-hematological AE. Compared to SEL + DEX treatment, SEL + DEX + PIs treatment had lower incidences of hyponatremia (39 vs 12%, p < 0.00001), nausea (72 vs 52%, p < 0.00001), vomiting (41 vs 23%, p < 0.0001), and weight loss (42 vs 17%, p = 0.03) in all grade AEs. Meanwhile, SEL + DEX + PIs treatment had lower incidences of anemia (36 vs 16%, p = 0.02), fatigue (20 vs 13%, p = 0.04), hyponatremia (22 vs 5%, p < 0.0001) than SEL + DEX treatment in grade≥3 AEs.
Conclusion: Our meta-analysis revealed that selinexor-based regimens could offer reasonable efficacy and tolerable adverse events in patients with multiple myeloma. SEL + DEX + PIs treatments had higher efficacy and lower toxicities than SEL + DEX.
Introduction
Multiple myeloma (MM) is a bone marrow-based malignant plasma-cell disorder that is characterized by production of monoclonal immunoglobulin (M protein), osteolytic bone lesions, hypercalcemia, anemia and associated organ dysfunction (Palumbo and Anderson, 2011). Over the past 2 decades, survival of patients with multiple myeloma has significantly improved as a result of the introduction of autologous stem cell transplantation (ASCT) and several novel classes of drugs, including proteasome inhibitors (PIs) (bortezomib, carfilzomib and ixazomib), immunomodulatory agents (IMiDs) (thalidomide, lenalidomide and pomalidomide), monoclonal antibodies targeting CD38 (daratumumab, isatuximab) and SLAMF7 (elotuzumab), and HDAC inhibitors (panobinostat) (Miguel et al., 2013; Dimopoulos et al., 2015; Lonial et al., 2015; Dimopoulos et al., 2016; San-Miguel et al., 2016; Chim et al., 2018; Goldschmidt et al., 2019; Kumar et al., 2019). However, none of these agents are curative, despite these additions to the MM armamentarium, most patients will relapse and develop refractory disease to all available therapies (Kumar et al., 2019; Kumar et al., 2012; Gandhi et al., 2019). Therefore, it remains a high priority to develop novel, more efficacious and less toxic treatment strategies for patients with relapsed/refractory MM.
Exportin 1 (XPO1), the only known nuclear export protein for most tumor suppressor proteins, cell-cycle regulators, the glucocorticoid receptor, and several eIF4A-bound oncoprotein mRNAs for key oncoproteins, including c-MYC, BCL-2 and Cyclin D (Gaubatz et al., 2001; Culjkovic-Kraljacic et al., 2012; Gravina et al., 2014). XPO1 is overexpressed in multiple myeloma and correlates with increased bone disease and shorter survival (Tiedemann et al., 2012; Tai et al., 2014), a genome-wide RNA interference screen identified XPO1 as an essential gene required for myeloma cell survival and proliferation (Schmidt et al., 2013). Furthermore, elevated levels of XPO1 is associated with the development of resistance to proteasome inhibitors (including bortezomib) (Chanukuppa et al., 2019) and immunomodulatory agents (Bhutani et al., 2017).
Selinexor (SEL) is an orally bioavailable, highly-selective, and slowly-reversible small molecule that binds to the Cys528 residue in the cargo-binding pocket of XPO1 (Gravina et al., 2014). Treatment of cancer cells with selinexor induced nuclear retention of tumor suppressor proteins (TSPs) and blocked the export of eIF4E-bound oncoprotein mRNAs, leading apoptosis, reduced levels of proto-oncoproteins and impaired osteoclastogenesis (Gravina et al., 2014; Tan et al., 2014). Preclinical studies have shown that selective inhibitor of nuclear export compounds showed noticeable anti-myeloma activity largely independent of genotype, as well as synergistic activity with glucocorticoids, proteasome inhibitors, and immunomodulatory drugs (Tai et al., 2014; Turner et al., 2014; Turner et al., 2016a). The combination of selinexor (80 mg, twice per week) and dexamethasone (DEX) has been approved in the United States for patients with penta-refractory multiple myeloma in July 2019 based on the STORM clinical trial. There are several prospective clinical trials having been conducted to investigate the safety and efficacy of selinexor-based treatment in patients with MM. However, a quantitative and comprehensive meta-analysis focus on the safety and efficacy of selinexor-based treatment is still scarce. In this study, we analyzed the comprehensive safety and efficacy of selinexor-based treatments in patients with relapsed or/and refractory MM, and overcome the limitations of individual studies, such as small sample size and lack of statistical power. Besides, we also used subgroup analysis to compare the efficacy and safety of different combination therapies (SEL + DEX + PIs vs SEL + DEX). These findings lead to the evaluation of XPO1 inhibitors for the treatment of MM.
Methods
Study Design, Search Strategy
This systematic review and meta-analysis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We searched the Medline (PubMed), Embase, Web of Science, Cochrane Central Register of Controlled Trials Library databases and ClinicalTrials.gov by a combination of Medical Subject Heading terms and keywords regarding “selinexor” and “multiple myeloma” to identify studies assessing selinexor based treatment in the setting of multiple myeloma. There were no date or language restrictions and the data cut-off for this analysis was March 5, 2021. The detailed search strategy was provided in Supplementary Materials.
Inclusion Criteria and Excluded Criteria
Studies that met the following criterias were included: 1) clinical trials in any phase of selinexor-based therapy for patients with multiple myeloma; 2) full data of the safety and efficacy were available in the articles; 3) the drugs were applied on human; 5) patients’ age was over 18 years.
The studies were excluded if they met the following criterias: 1) the studies were without initial data such as reviews; 2) it was a case report. When more than one study reported the results from the same cohort, only the most recent study was included.
Two authors independently searched, screened, and determined study eligibility, with any disagreement were resolved by discussion.
Data extraction
Two investigators independently reviewed and extracted the following Data: 1) the fundamental characteristics of included studies: the first author name, year of publication, ClinicalTrials.gov number, the phase of the studies, number of participating patients, prior lines of treatment, median age, intervention; 2) the AEs of all grades and grade ≥3 AEs; 3) efficacy outcome such as overall response rate (ORR), clinical benefit rate (CBR), stringent complete response rate (sCR), complete response rate (CR), very good partial response rate (VGPR), partial response rate (PR), minimal response rate (MR), rate of stable disease (SDR), rate of progressive disease (PDR) and median progression-free survival (mPFS). Discrepancies were settled by discussion. For the RCT, we only extracted the information of selinexor-based treatment.
Statistical Analysis
The Revman 5.4 and STATA14 (Stata Corporation) were used in this study. The
Study Qualitative Assessment
We used the Cochrane Collaboration Risk of Bias Tool (Higgins et al., 2011) (Review Manager 5.4) to assess systematic bias of the involved RCT study, which is based on six criteria: sequence generation, allocation concealment, blinding of participants, blinding of outcomes, completeness of the data, and selective outcome reporting. The Methodological Index for Non-randomized Studies (MINORS) was adopted to assess the methodological quality of the inclusive non-RCT studies. MINORS contained 12 items, eight of which were specified for non-comparative studies (Slim et al., 2003). The eight items included: study aims, consecutive patient inclusion criteria, prospective pooling of data, endpoint consistent with the study aim, unbiased evaluation of endpoints, follow-up period, loss to follow-up less than 5%, and prospective calculation of the sample size. The items were scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). Two reviewers independently evaluated the quality of each study, and the discrepancy was solved by consensus.
Results
Study Selection and Characteristics
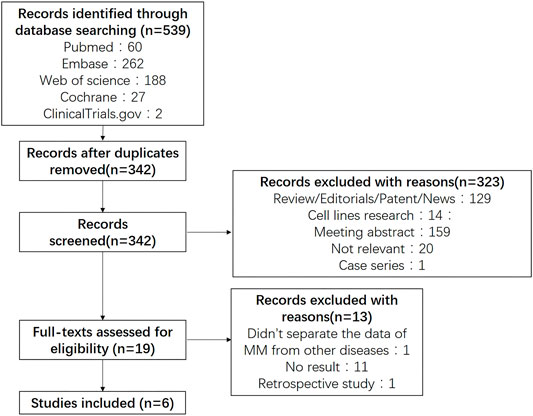
Five hundred and thirty nine potentially relevant studies were identified through searching PubMed, Embase, Web of Science and CENTRAIL. 197 were removed after duplication, 533 were finally excluded due to different reasons. Figure 1 presented the details of our search process. Six studies (26–31) containing 1 phase III trials and 5 phase I/II trials were selected in our meta-analysis.
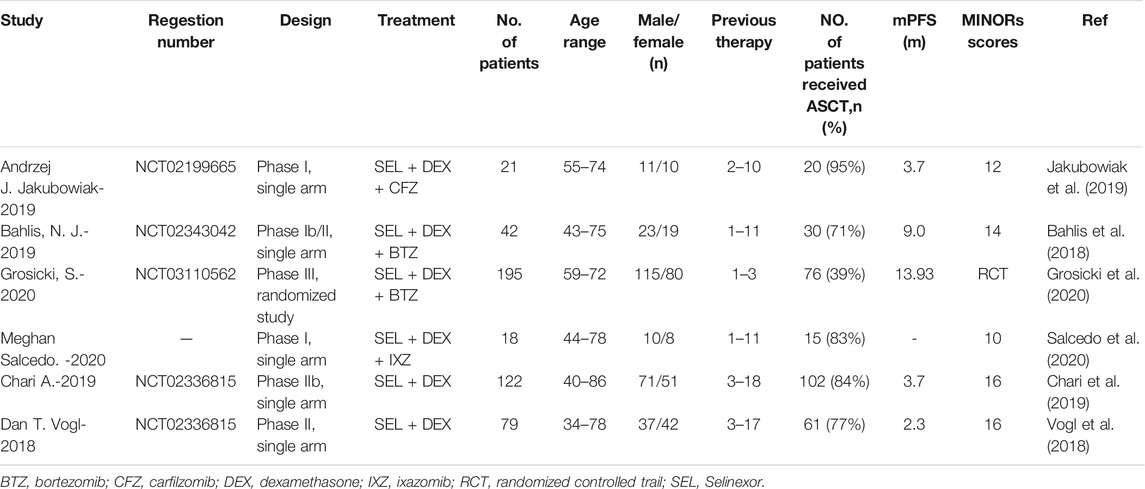
The characteristics of the including articles was listed in Table 1. A total of 477 patients were involved in the six studies with the mean age<70 years, among which 4 studies containing 276 patients using SEL + DEX + PIs treatment (Bahlis et al., 2018; Jakubowiak et al., 2019; Grosicki et al., 2020; Salcedo et al., 2020), two studies containing 201 patients using SEL + DEX treatment (Vogl et al., 2018; Chari et al., 2019). The prior lines of therapy patients received ranged from 1 to 18. The selected studies were published from 2018 to 2020. All studies presented complicate information on AEs and response rate. Five studies with 459 patients reported the median progression-free survival. All studies were open-label clinical trials, five were single-arm, one was RCT.
Assessment of Study Quality
All studies were open-label. The Cochrane Collaboration risk of bias tool was used to assess the methodological quality of the RCT (30), the result of quality evaluation was good. Supplementary Figure S1 shows the risk of bias graph and risk of bias summary of the RCT study. The MINORS scores of other studies ranged from 10 to 16 (Table 1). All studies stated the aim of their study clearly, included of consecutive patients, collected data prospectively and had appropriate endpoints for the aim of the study. Three studies did not report the unbiased assessment of the study endpoint. One study did not report the follow-up period appropriate to the aim of the study. All studies, the patients’ loss to follow-up did not exceed 5%. Two studies did not prospectively calculate the study size. All in all, the overall quality of the selected studies was adequate.
Publication Bias
According to the results of
Efficacy
All study reported the efficacy outcome such as overall response rate (ORR), clinical benefit rate (CBR), stringent complete response rate (sCR), complete response rate (CR), very good partial response (VGPR), partial response rate (PR), minimal response (MR), rate of stable disease (SDR), rate of progressive disease (PDR), and the responses were evaluated using International Myeloma Working Group (IMWG) response criteria.
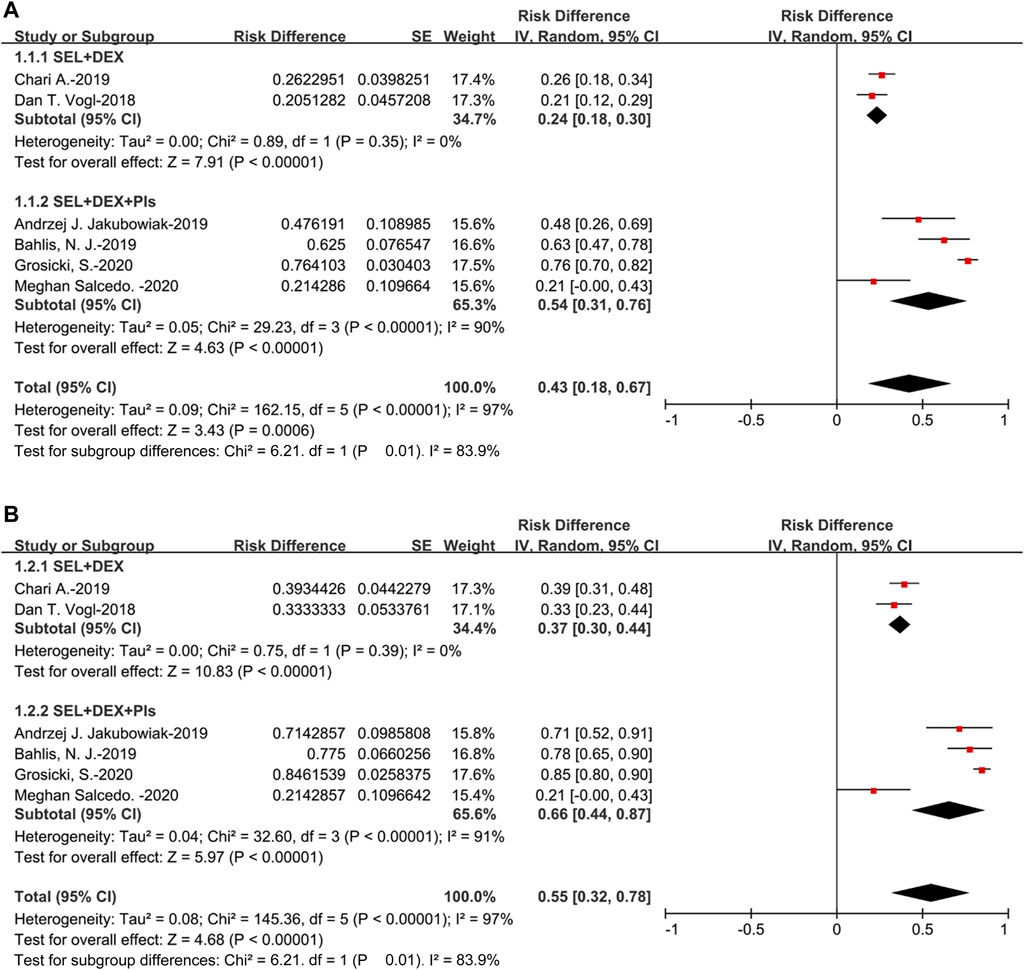
All the studies could be used to evaluate ORR, the pooled ORR was 43% (95%CI: 18–67%), suggested that approximately 43% of the 477 patients displayed PR or better as the best response to selinexor-based regimens. For the SEL + DEX + PIs treatment, the pooled ORR was 54% (95%CI: 31–76%), for the SEL + DEX treatment, the ORR was 24% (95%CI: 18–30%). The results of subanalysis showed that the ORR of SEL + DEX treatment was much lower than SEL + DEX + PIs treatment (24 vs 54%, p = 0.01) (Figure 2).
Additionally, the pooled CBR was 55% (95%CI: 32–78%), suggested that approximately 55% of the 477 patients displayed MR or better as the best response to selinexor-based regimens. For the subanalysis of SEL + DEX + PIs treatment, the pooled CBR was 66% (95%CI: 44–87%), for the SEL + DEX treatment, the CBR was 37% (95%CI: 30–44%). Subgroup analysis of CBR by treatment showed that, in patients using SEL + DEX + PIs treatment, the CBR was higher than in those using SEL + DEX treatment (66 vs 37%, p = 0.01) (Figure 2).
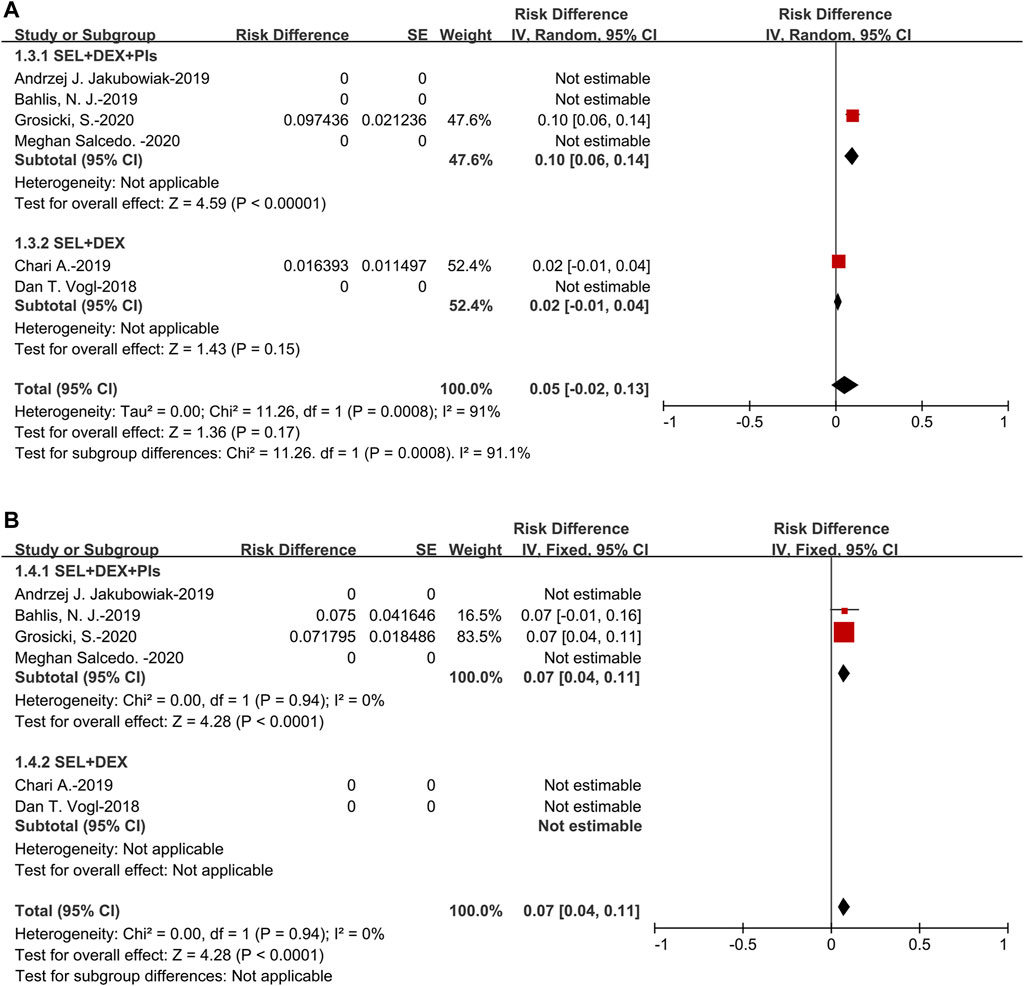
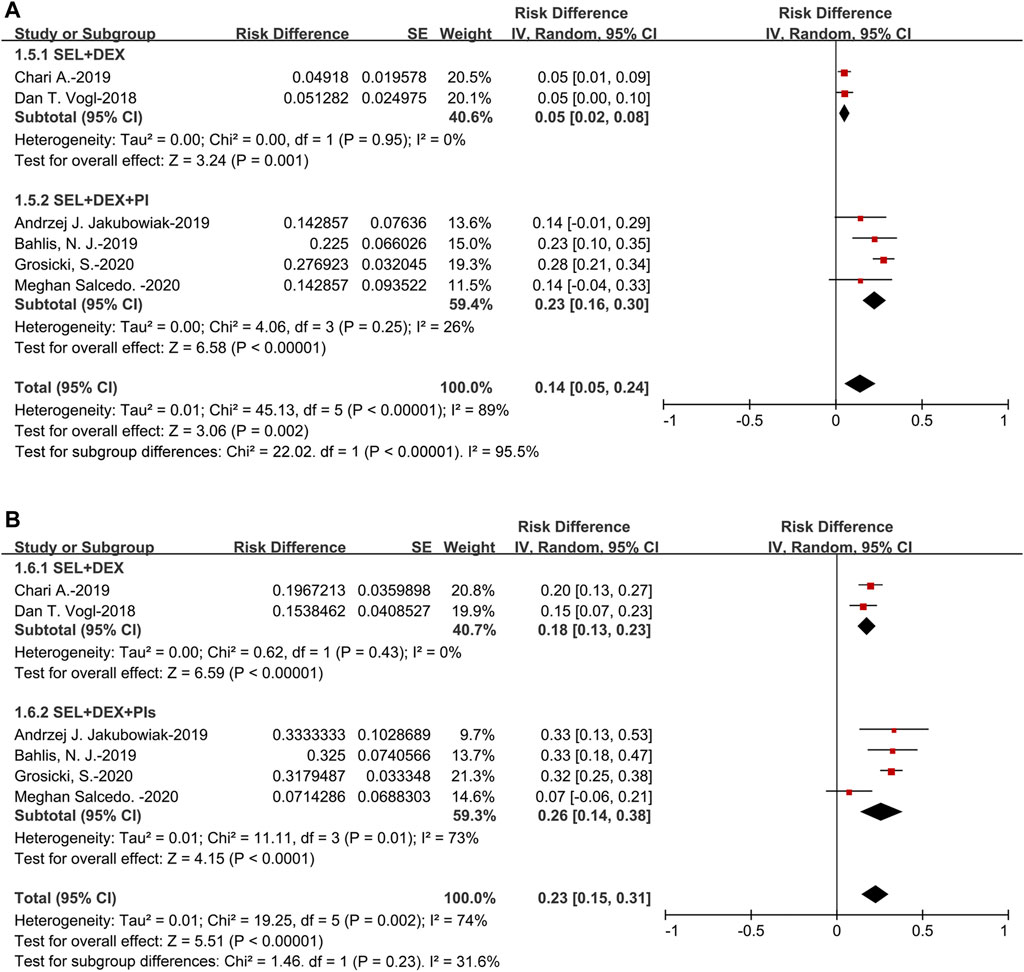
The pooled sCR was 5% (95%CI: 2–13%). For the subanalysis of SEL + DEX + PIs treatment, the pooled sCR was 10% (95%CI: 6–14%), for the SEL + DEX treatment, the sCR was 2% (95%CI: 1–4%). The pooled sCR of patients using SEL + DEX + PIs treatment was higher than in those using SEL + DEX treatment (10 vs 2%, p = 0.0008) (Figure 3).
The pooled CR was 7% (95%CI: 4–11%) (Figure 3). Of the four studies that adopted the SEL + DEX + PIs treatment, two had a CR of 0%, and the other two had CRs of 8% (Bahlis et al., 2018) and 7% (Grosicki et al., 2020), respectively. Both studies that used SEL + DEX treatment (29, 31) had a CR of 0%. Therefore, the subgroup analysis by treatment (SEL + DEX + PIs vs SEL + DEX) was not conducted.
The pooled VGPR was 14% (95%CI: 5–24%). For the subanalysis of SEL + DEX + PIs treatment, the pooled VGPR was 23% (95%CI: 16–30%), for the SEL + DEX treatment, the VGPR was 5% (95%CI: 2–8%). The pooled VGPR of patients using SEL + DEX + PIs treatment was higher than in those using SEL + DEX treatment (23 vs 5%, p < 0.00001) (Figure 4).
The pooled PR was 23% (95%CI: 15–31%). For the subanalysis of SEL + DEX + PIs treatment, the pooled PR was 26% (95%CI: 14–38%), for the SEL + DEX treatment, the PR was 18% (95%CI: 13–23%). The results of subanalysis indicated that no significant difference between the PR of patients using SEL + DEX + PIs treatment and in those using SEL + DEX treatment (26 vs 18%, p = 0.23) (Figure 4).
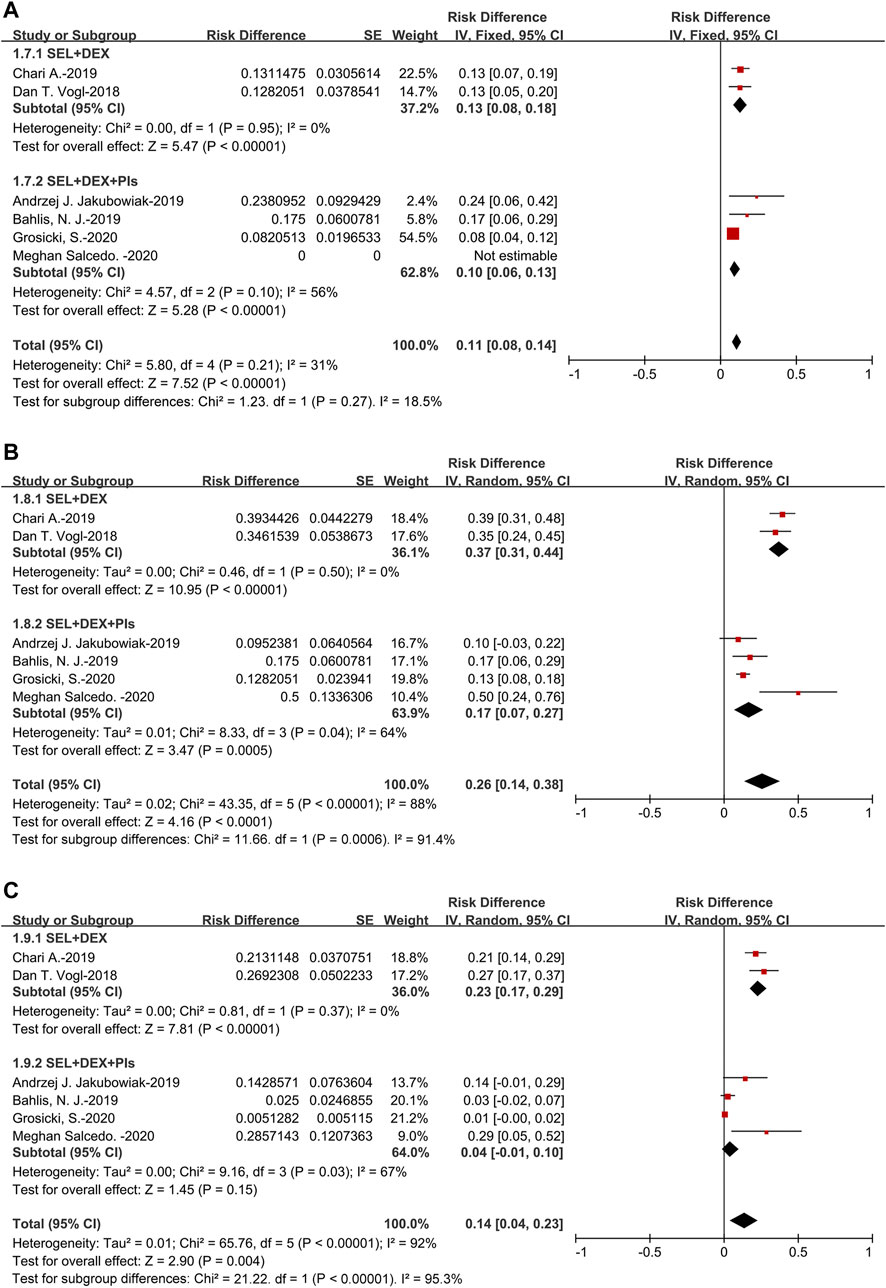
The pooled MR was 11% (95%CI: 8–14%). For the subanalysis of SEL + DEX + PIs treatment, the pooled MR was 10% (95%CI: 6–13%), for the SEL + DEX treatment, the MR was 13% (95%CI: 8–18%). Subgroup analysis of MR suggested that no significant difference existed in the patients using SEL + DEX + PIs treatment and those using SEL + DEX treatment (10 vs 13%, p = 0.27) (Figure 5).
The pooled SDR was 26% (95%CI: 14–38%). For the subanalysis of SEL + DEX + PIs treatment, the pooled SDR was 17% (95%CI: 7–27%), for the SEL + DEX treatment, the SDR was 37% (95%CI: 31–44%). The results of subanalysis indicated that the SDR of patients using SEL + DEX treatment was higher than in those using SEL + DEX + PIs treatment (37 vs 17%, p = 0.0006) (Figure 5).
The pooled PDR was 14% (95%CI: 4–23%). For the subanalysis of SEL + DEX + PIs treatment, the pooled PDR was 4% (95%CI: 1–10%), for the SEL + DEX treatment, the PDR was 23% (95%CI: 17–29%). The results of subanalysis showed that the PDR of patients using SEL + DEX treatment was higher than in those using SEL + DEX + PIs treatment (23 vs 4%, p < 0.00001), which indicated that using SEL + DEX + PIs treatment led to lower disease progression rate than using SEL + DEX treatment (Figure 5). All these results indicated that the efficacy of SEL + DEX + PIs treatment was better than SEL + DEX treatment.
Five studies presented mPFS and the survival curve, with one study (Salcedo et al., 2020) using SEL + DEX + PIs treatment did not present mPFS or the survival curve. For patients using SEL + DEX + PIs treatment, the mPFS in the reported three studies was 3.7, 9.0, and 13.93 months, respectively. For patients using SEL + DEX treatment, the mPFS in the reported two studies was 3.7 and 2.3 months, respectively. It seemed that SEL + DEX + PIs treatment was related to better survival than SEL + DEX treatment. The mPFS recorded in articles on patients were listed in Table1.
Safety
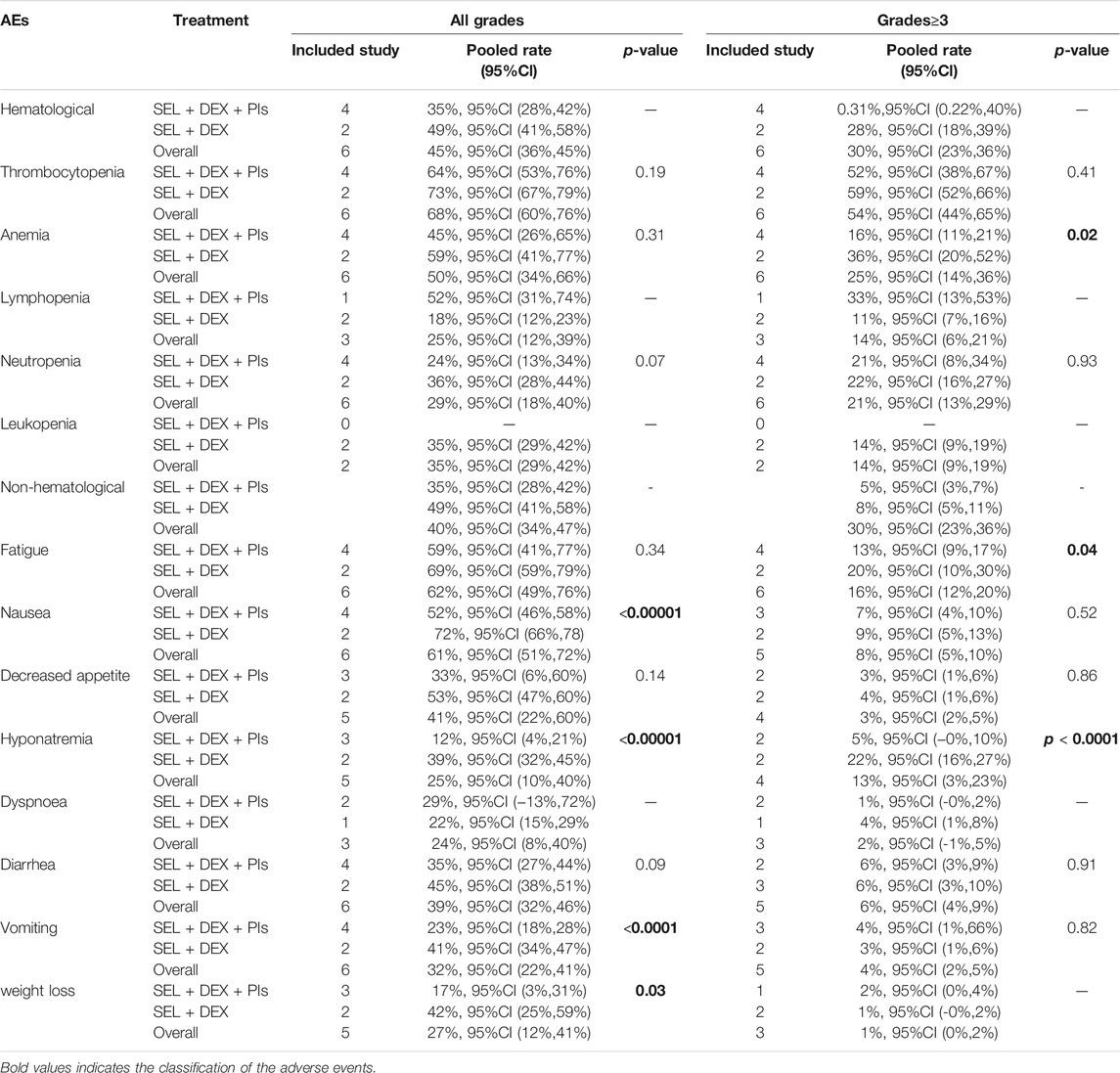
All the involved articles including 477patients reported AEs, so all studies were contributing to the meta-analysis of the all grade AEs rate and the grade≥3 AEs rate. All treatment-emergent AEs in included studies were graded according to the National Cancer Institute-Common Terminology Criteria for Adverse Events. The pooled incidence of all grade hematological AEs was 45%, and the all grade non-hematological AEs was 40%. The most common all grade hematological AE was thrombocytopenia 68%, and the most common all grade non-hematological was fatigue 62%. The pooled incidence of grade≥3 hematological AEs was 30%, and the grade≥3 non-hematological AEs was 30%. The most common grade≥3 hematological AE was thrombocytopenia 54%, and the most common grade≥3 non-hematological was fatigue 16%. Table 2 listed the pooled incidences of treatment-emergent adverse events.
Subgroup analyses of all grade AEs by different treatments suggested that no significant differences occurred in anemia (59 vs 45%, p = 0.31), thrombocytopenia (73 vs 64%, p = 0.19), decreased appetite (53 vs 33%, p = 0.14), diarrhea (45 vs 35%, p = 0.09), fatigue (69 vs 59%, p = 0.34) and neutropenia (36 vs 24%, p = 0.07). Subgroup analyses of all grade AEs by different treatments suggested that compared to SEL + DEX treatment, SEL + DEX + PIs treatment had a lower incidence of hyponatremia (39 vs 12%, p < 0.00001), nausea (72 vs 52%, p < 0.00001), vomiting (41 vs 23%, p < 0.0001) and weight loss (42 vs 17%, p = 0.03).
Subgroup analyses of grade≥3 AEs by different treatments revealed that no statistically significant subgroup differences were found between different treatment in terms of the incidence of decreased appetite (4 vs 3%, p = 0.86), thrombocytopenia (59 vs 52%, p = 0.41), diarrhea (6 vs 6%, p = 0.91), nausea (9 vs 7%, p = 0.52), neutropenia (22 vs 21%, p = 0.93) and vomiting (3 vs 4%, p = 0.82). Subgroup analyses of grade≥3 AEs by different treatments revealed that compared to SEL + DEX treatment, SEL + DEX + PIs treatment had a lower incidence of anemia (36 vs 16%, p = 0.02), fatigue (20 vs 13%, p = 0.04), hyponatremia (22 vs 5%, p < 0.0001).
Disscussion
Multiple myeloma (MM) is a bone marrow-based malignant plasma-cell disorder without curative treatment, most patients will face the risk of relapse and develop refractory disease (Kumar et al., 2012; Gandhi et al., 2019; Kumar et al., 2019). It’s very meaningful to find more efficacious and less toxic treatment strategies for patients with relapsed/refractory multiple myeloma. Selinexor is an orally bioavailable, highly-selective, and slowly-reversible small molecule that binds to the Cys528 residue in the cargo-binding pocket of XPO1(12). We performed this meta-analysis to investigate the efficacy and safety of selinexor based treatments for multiple myeloma patients.
We included six studies, four studies were combined selinexor with dexamethasone and proteasome inhibitors regimens and two studies were combined selinexor with dexamethasone regimens. This review included a total of 276 MM patients treated with SEL + DEX + PIs treatment and 201 patients with SEL + DEX treatment. A random effects model was used when significant heterogeneity was observed in the analysis of efficacy and safety. Galbraith plots provides a visual impression of the amount of heterogeneity and indicates potential sources of heterogeneity. The results showed that one study (Grosicki et al., 2020) contributed mostly to publication bias in the pooled efficacy, and the reason for this phenomenon may be that this study provided the maximum number of enrolled patients. We also performed prespecified exploratory subgroup analyses by different treatments.
Our meta-analysis revealed that selinexor-based regimens could offer reasonable efficacy in patients with multiple myeloma. It worth noting that Selinexor exhibited a higher efficacy against MM resistant to previous therapies. The prior lines of therapy patients received ranged from 1 to 18, including iMiDs (lenalidomide, pomalidomide, thalidomide), PIs (bortezomib, Carfilzomib, Ixazomib), anti-CD38 monoclonal antibody (Daratumumab), Panobinostat, Alkylating Agent, antigen receptor-modified T cell therapy (CART), and ASCT. Patients with RRMM are heterogeneous, despite new treatment regimens improving outcomes, the prognosis remains particularly poor for heavily pretreated and/or multiple treatment-refractory patients (Usmani et al., 2016). Excessive nuclear export is an important factor in both the initiation and progression of cancer and is associated with resistance to chemotherapy (El-Tanani et al., 2016). Data from many preclinical studies confirm that selinexor effectively overcome different drug resistance, including bortezomib (Muz et al., 2017; Chanukuppa et al., 2019), anthracycline (Turner et al., 2013; Turner et al., 2016b), and so on. The results of subanalysis showed that SEL + DEX + PIs treatment could improve ORR, CBR, sCR, and VGPR compared to SEL + DEX treatment, while SEL + DEX treatment was associated with higher PDR (rate of progressive disease) and SDR (rate of stable disease) compared to SEL + DEX + PIs treatment. However, there was no difference in PR and MR between SEL + DEX + PIs treatment and SEL + DEX treatment. The combination of SEL and DEX has been approved by the FDA for patients with penta-refractory multiple myeloma. Due to the novel mechanism of action compared with other agents for MM, combining selinexor with other therapies is regarded as a promising therapeutic strategy. Our analysis revealed that the efficacy of SEL + DEX treatments could be enhanced by combinating with PIs. Furthermore, the pooled ORR (43%) in our analysis of SEL-based treatments was higher than that (39.1%) reported in a meta-analysis of bortezomib-based retreatment in relapsed/refractory myeloma (Knopf et al., 2014), revealing that the efficacy of SEL-based treatments was higher than bortezomib-based retreatment. Although the subgroup analysis of CR was not performed, in the RCT we included (Grosicki et al., 2020), the CR (7%) in the SEL + DEX + BTZ group was higher than the CR (4%) in the BTZ + DEX group. Considering that, selinexor-based regimen would be a good option for patients with R/R MM.
Although the recent approval of therapies, including next-generation PIs and immunomodulatory drugs (IMiDs), monoclonal antibodies (mAbs) and other new drug with novel mechanisms of action, has provided novel treatment options, MM remains an incurable disease, most patients will relapse and develop refractory disease to all available therapies (Kumar et al., 2012; Lonial et al., 2015; Kumar et al., 2019). Agents capable of harnessing the power of cellular immunity have long been sought, however, ASCT has remained the only proven therapy capable of long-term disease eradication through a graft-vs-myeloma effect (Nathwani et al., 2016). However, ASCT have a high treatment and transplant-related mortality rate (Giralt et al., 2015). It’s encouraging that the OS of patients with MM after ASCT has improved over time along with the introduction of new drugs for the treatment of MM (Shimazu et al., 2021). The development of other innovative immunotherapy approaches including monoclonal antibodies, chimeric antigen receptor T-cell therapy, checkpoint blockade, and novel vaccine therapies has led to a new era of immunotherapy in multiple myeloma (Nathwani et al., 2016). In addition to the combination with existing standard-of-care therapies like bortezomib, to explore the option of organically combining cellular immunotherapy with selinexor and transplantation for providing better treatment to multiple myeloma patients. Given the heterogeneity of RRMM, it’s critical to selected treatment plan more properly. Using the predictors of survival in patients such as early platelet engraftment and the administration of a CD34 + HPC count would be a rational choice (Aladağ Karakulak et al., 2020).
In our analysis, the most common AEs in all grade were thrombocytopenia, anemia, fatigue, nausea and decreased appetite. Besides, the most common AEs in grade≥3 were thrombocytopenia, anemia, neutropenia, and fatigue. Notably, hematological toxicities were the most frequent severe AEs. The pooled incidence of thrombocytopenia in all grade was 68%, 95%CI (60%,76%), and 54%, 95%CI (44%,65%) in grade≥3. The subanalysis showed no difference in the incidence of thrombocytopenia between SEL + DEX treatment and SEL + DEX + PIs treatment. Thrombocytopenia occurred in more than half of the patients, which was partly caused by selinexor because of its inhibition of thrombopoietin signaling in the differentiation of stem cells into megakaryocytes (Machlus et al., 2017). As thrombocytopenia was reversible and could be managed with dose interruptions, transfusion of blood products and thrombopoietin-receptor agonists, these patients rarely experienced severe thrombocytopenia (Machlus et al., 2017). Clinicians need to pay attention to the patients’ platelet status and adjust the medication regimen flexibly according to the patient’s platelet reduction. Bortezomib combined with dexamethasone was approved as a standard treatment in the United States for relapsed or refractory multiple myeloma. Peripheral neuropathy is the main dose-limiting toxicity caused by the standard treatment (Delforge et al., 2010; Cavaletti and Jakubowiak, 2010), In our included six studies, only two studies mentioned the incidence of peripheral neuropathy (Bahlis et al., 2018; Grosicki et al., 2020), other studies didn’t mention it because the incidence is too low. Both of the two studies used the selinexor + bortezomib + dexamethasone. However, the only one RCT study suggested that the triplet combination (SEL + DEX + PIs) was well tolerated with much lower rates of peripheral neuropathy compared to bortezomib + dexamethasone treatment in all grade (32 vs 47%) and grade≥3 (5 vs 9%) (Grosicki et al., 2020). Therefore, it seemed that the combination with selinexor was associated with lower rates and severity of bortezomib-induced peripheral neuropathy. The selinexor-based treatments have an improved side effect profile, while the SEL + DEX + PIs treatment has reduced levels of peripheral neuropathy. All patients involved in the meta-analysis received prophylactic use of antiemetics to mitigate gastrointestinal events. However, the all-oral Sid (selinexor, ixazomib, and low-dose dexamethasone) combination therapy for heavily pretreated patients (the patients had a median of five prior lines of therapy, ranged from 1 to 11 lines) with R/R MM resulted in frequent treatment delays and dose reductions owing to thrombocytopenia and gastrointestinal related toxicities, which lead to progression of disease (Salcedo et al., 2020). It suggested that heavily pretreated patients with R/R MM required careful consideration of using the combination therapy.
The subgroup analysis results suggested that SEL + DEX + PIs treatment was more effective and tolerated than SEL + DEX treatment for patients with MM. Preclinical data suggested that combining selinexor with proteasome inhibitors and dexamethasone had a mechanistic rationale (Turner et al., 2016a; Kashyap et al., 2016), as XPO1 is overexpressed in multiple myeloma and correlates with increased bone disease and shorter survival (Tai et al., 2014; Tiedemann et al., 2012), a genome-wide RNA interference screen identified XPO1 as an essential gene required for myeloma cell survival and proliferation (Schmidt et al., 2013). Furthermore, elevated levels of XPO1 is associated with the development of resistance to proteasome inhibitors (including bortezomib) (Chanukuppa et al., 2019) and immunomodulatory agents (Bhutani et al., 2017). Therefore, selinexor could inhibit the XPO1 and improve the resistance to PIs in R/R MM. Considering the relapsed/refractory status of the patient population enrolled in the meta-analysis, the efficacy of patients treated with selinexor-based treatments was satisfactory with tolerable AEs.
There are several inevitable limitations in our analysis. Firstly, most of the involved studies were single-armed studies without double-blinded randomized controlled trials. In addition, the dose of the drugs was different between individual trials, and some AEs were dose dependent. Finally, although the patients were all in advanced stages, the degree of multiple myeloma was dispersive, which might have aroused bias to the final analysis. No survival benefit was confirmed in our study due to the small sample size, the varied length of follow-up time and loss of data in some studies.
In conclusion, our meta-analysis further demonstrated that selinexor-based regimen was a novel and potent treatment option for patients with R/R multiple myeloma. The combination of selinexor with proteasome inhibitors and dexamethasone contributed to improve the efficacy and reduce the incidence of AEs than SEL + DEX treatment. Further studies are needed to explore the feasibility and efficacy of treatment for heavily pretreated patients with R/R MM. Combining XPO1 inhibitors with other effective antimyeloma agents and cellular immunotherapy was expected to be complementary to existing therapies for multiple myeloma, as well as for other neoplasms. Additionally, it is also important to focus on improving patients’ compliance, whether investigating new XPO1 inhibitors or new forms of combination.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
YT and HZ contributed equally to this work. YT and HZ designed the study, and provided the plan, YT and HZ searched the literature, extracted the data, and analyzed the data, YT drafted the initial manuscript and HZ and TN critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by Incubation Program for Clinical Trials (No. 19HXFH030), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thanked all of the participants enrolled in the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.758992/full#supplementary-material
References
Aladağ Karakulak, E., Demiroğlu, H., Büyükaşik, Y., Turgut, M., Aksu, S., Sayinalp, N., et al. (2020). CD34+ Hematopoietic Progenitor Cell Dose as a Predictor of Engraftment and Survival in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation. Turk J. Med. Sci. 50 (8), 1851–1856. doi:10.3906/sag-2001-173
Bahlis, N. J., Sutherland, H., White, D., Sebag, M., Lentzsch, S., Kotb, R., et al. (2018). Selinexor Plus Low-Dose Bortezomib and Dexamethasone for Patients with Relapsed or Refractory Multiple Myeloma. Blood 132 (24), 2546–2554. doi:10.1182/blood-2018-06-858852
Bhutani, M., Zhang, Q., Friend, R., Voorhees, P. M., Druhan, L. J., Barlogie, B., et al. (2017). Investigation of a Gene Signature to Predict Response to Immunomodulatory Derivatives for Patients with Multiple Myeloma: an Exploratory, Retrospective Study Using Microarray Datasets from Prospective Clinical Trials. Lancet Haematol. 4 (9), e443–e51. doi:10.1016/S2352-3026(17)30143-6
Cavaletti, G., and Jakubowiak, A. J. (2010). Peripheral Neuropathy during Bortezomib Treatment of Multiple Myeloma: a Review of Recent Studies. Leuk. Lymphoma 51 (7), 1178–1187. doi:10.3109/10428194.2010.483303
Chanukuppa, V., Paul, D., Taunk, K., Chatterjee, T., Sharma, S., Kumar, S., et al. (2019). XPO1 Is a Critical Player for Bortezomib Resistance in Multiple Myeloma: A Quantitative Proteomic Approach. J. Proteomics 209, 103504. doi:10.1016/j.jprot.2019.103504
Chari, A., Vogl, D. T., Gavriatopoulou, M., Nooka, A. K., Yee, A. J., Huff, C. A., et al. (2019). Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 381 (8), 727–738. doi:10.1056/NEJMoa1903455
Chim, C. S., Kumar, S. K., Orlowski, R. Z., Cook, G., Richardson, P. G., Gertz, M. A., et al. (2018). Management of Relapsed and Refractory Multiple Myeloma: Novel Agents, Antibodies, Immunotherapies and beyond. Leukemia 32 (2), 252–262. doi:10.1038/leu.2017.329
Culjkovic-Kraljacic, B., Baguet, A., Volpon, L., Amri, A., and Borden, K. L. (2012). The Oncogene eIF4E Reprograms the Nuclear Pore Complex to Promote mRNA export and Oncogenic Transformation. Cell Rep 2 (2), 207–215. doi:10.1016/j.celrep.2012.07.007
Delforge, M., Bladé, J., Dimopoulos, M. A., Facon, T., Kropff, M., Ludwig, H., et al. (2010). Treatment-related Peripheral Neuropathy in Multiple Myeloma: the challenge Continues. Lancet Oncol. 11 (11), 1086–1095. doi:10.1016/S1470-2045(10)70068-1
Dimopoulos, M. A., Moreau, P., Palumbo, A., Joshua, D., Pour, L., Hájek, R., et al. (2016). Carfilzomib and Dexamethasone versus Bortezomib and Dexamethasone for Patients with Relapsed or Refractory Multiple Myeloma (ENDEAVOR): a Randomised, Phase 3, Open-Label, Multicentre Study. Lancet Oncol. 17 (1), 27–38. doi:10.1016/S1470-2045(15)00464-7
Dimopoulos, M. A., Richardson, P. G., Moreau, P., and Anderson, K. C. (2015). Current Treatment Landscape for Relapsed And/or Refractory Multiple Myeloma. Nat. Rev. Clin. Oncol. 12 (1), 42–54. doi:10.1038/nrclinonc.2014.200
El-Tanani, M., Dakir, el-H., Raynor, B., and Morgan, R. (2016). Mechanisms of Nuclear Export in Cancer and Resistance to Chemotherapy. Cancers (Basel) 8 (3). doi:10.3390/cancers8030035
Gandhi, U. H., Cornell, R. F., Lakshman, A., Gahvari, Z. J., McGehee, E., Jagosky, M. H., et al. (2019). Outcomes of Patients with Multiple Myeloma Refractory to CD38-Targeted Monoclonal Antibody Therapy. Leukemia 33 (9), 2266–2275. doi:10.1038/s41375-019-0435-7
Gaubatz, S., Lees, J. A., Lindeman, G. J., and Livingston, D. M. (2001). E2F4 Is Exported from the Nucleus in a CRM1-dependent Manner. Mol. Cel Biol 21 (4), 1384–1392. doi:10.1128/MCB.21.4.1384-1392.2001
Giralt, S., Garderet, L., Durie, B., Cook, G., Gahrton, G., Bruno, B., et al. (2015). American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol. Blood Marrow Transpl. 21 (12), 2039–2051. doi:10.1016/j.bbmt.2015.09.016
Goldschmidt, H., Ashcroft, J., Szabo, Z., and Garderet, L. (2019). Navigating the Treatment Landscape in Multiple Myeloma: Which Combinations to Use and when? Ann. Hematol. 98 (1), 1–18. doi:10.1007/s00277-018-3546-8
Gravina, G. L., Senapedis, W., McCauley, D., Baloglu, E., Shacham, S., and Festuccia, C. (2014). Nucleo-cytoplasmic Transport as a Therapeutic Target of Cancer. J. Hematol. Oncol. 7, 85. doi:10.1186/s13045-014-0085-1
Grosicki, S., Simonova, M., Spicka, I., Pour, L., Kriachok, I., Gavriatopoulou, M., et al. (2020). Once-per-week Selinexor, Bortezomib, and Dexamethasone versus Twice-Per-Week Bortezomib and Dexamethasone in Patients with Multiple Myeloma (BOSTON): a Randomised, Open-Label, Phase 3 Trial. Lancet 396 (10262), 1563–1573. doi:10.1016/S0140-6736(20)32292-3
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Jakubowiak, A. J., Jasielec, J. K., Rosenbaum, C. A., Cole, C. E., Chari, A., Mikhael, J., et al. (2019). Phase 1 Study of Selinexor Plus Carfilzomib and Dexamethasone for the Treatment of Relapsed/refractory Multiple Myeloma. Br. J. Haematol. 186 (4), 549–560. doi:10.1111/bjh.15969
Kashyap, T., Argueta, C., Aboukameel, A., Unger, T. J., Klebanov, B., Mohammad, R. M., et al. (2016). Selinexor, a Selective Inhibitor of Nuclear Export (SINE) Compound, Acts through NF-Κb Deactivation and Combines with Proteasome Inhibitors to Synergistically Induce Tumor Cell Death. Oncotarget 7 (48), 78883–78895. doi:10.18632/oncotarget.12428
Knopf, K. B., Duh, M. S., Lafeuille, M. H., Gravel, J., Lefebvre, P., Niculescu, L., et al. (2014). Meta-analysis of the Efficacy and Safety of Bortezomib Re-treatment in Patients with Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 14 (5), 380–388. doi:10.1016/j.clml.2014.03.005
Kumar, S. K., Callander, N. S., Hillengass, J., Liedtke, M., Baljevic, M., Campagnaro, E., et al. (2019). NCCN Guidelines Insights: Multiple Myeloma, Version 1.2020. J. Natl. Compr. Canc Netw. 17 (10), 1154–1165. doi:10.6004/jnccn.2019.0049
Kumar, S. K., Lee, J. H., Lahuerta, J. J., Morgan, G., Richardson, P. G., Crowley, J., et al. (2012). Risk of Progression and Survival in Multiple Myeloma Relapsing after Therapy with IMiDs and Bortezomib: a Multicenter International Myeloma Working Group Study. Leukemia 26 (1), 149–157. doi:10.1038/leu.2011.196
Lonial, S., Dimopoulos, M., Palumbo, A., White, D., Grosicki, S., Spicka, I., et al. (2015). Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 373 (7), 621–631. doi:10.1056/NEJMoa1505654
Machlus, K. R., Wu, S. K., Vijey, P., Soussou, T. S., Liu, Z. J., Shacham, E., et al. (2017). Selinexor-induced Thrombocytopenia Results from Inhibition of Thrombopoietin Signaling in Early Megakaryopoiesis. Blood 130 (9), 1132–1143. doi:10.1182/blood-2016-11-752840
Miguel, J. S., Weisel, K., Moreau, P., Lacy, M., Song, K., Delforge, M., et al. (2013). Pomalidomide Plus Low-Dose Dexamethasone versus High-Dose Dexamethasone Alone for Patients with Relapsed and Refractory Multiple Myeloma (MM-003): a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 14 (11), 1055–1066. doi:10.1016/S1470-2045(13)70380-2
Muz, B., Azab, F., de la Puente, P., Landesman, Y., and Azab, A. K. (2017). Selinexor Overcomes Hypoxia-Induced Drug Resistance in Multiple Myeloma. Transl Oncol. 10 (4), 632–640. doi:10.1016/j.tranon.2017.04.010
Nathwani, N., Larsen, J. T., and Kapoor, P. (2016). Consolidation and Maintenance Therapies for Newly Diagnosed Multiple Myeloma in the Era of Novel Agents. Curr. Hematol. Malig Rep. 11 (2), 127–136. doi:10.1007/s11899-016-0310-9
Palumbo, A., and Anderson, K. (2011). Multiple Myeloma. N. Engl. J. Med. 364 (11), 1046–1060. doi:10.1056/NEJMra1011442
Salcedo, M., Lendvai, N., Mastey, D., Schlossman, J., Hultcrantz, M., Korde, N., et al. (2020). Phase I Study of Selinexor, Ixazomib, and Low-Dose Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 20 (3), 198–200. doi:10.1016/j.clml.2019.12.013
San-Miguel, J. F., Hungria, V. T., Yoon, S. S., Beksac, M., Dimopoulos, M. A., Elghandour, A., et al. (2016). Overall Survival of Patients with Relapsed Multiple Myeloma Treated with Panobinostat or Placebo Plus Bortezomib and Dexamethasone (The PANORAMA 1 Trial): a Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Haematol. 3 (11), e506–e15. doi:10.1016/S2352-3026(16)30147-8
Schmidt, J., Braggio, E., Kortuem, K. M., Egan, J. B., Zhu, Y. X., Xin, C. S., et al. (2013). Genome-wide Studies in Multiple Myeloma Identify XPO1/CRM1 as a Critical Target Validated Using the Selective Nuclear export Inhibitor KPT-276. Leukemia 27 (12), 2357–2365. doi:10.1038/leu.2013.172
Shimazu, Y., Mizuno, S., Fuchida, S-I., Suzuki, K., Tsukada, N., Hanagaishi, A., et al. (2021). Improved Survival of Multiple Myeloma Patients Treated with Autologous Transplantation in the Modern Era of New Medicine. Cancer Sci.. doi:10.1111/cas.15163
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., and Chipponi, J. (2003). Methodological index for Non-randomized Studies (Minors): Development and Validation of a New Instrument. ANZ J. Surg. 73 (9), 712–716. doi:10.1046/j.1445-2197.2003.02748.x
Tai, Y. T., Landesman, Y., Acharya, C., Calle, Y., Zhong, M. Y., Cea, M., et al. (2014). CRM1 Inhibition Induces Tumor Cell Cytotoxicity and Impairs Osteoclastogenesis in Multiple Myeloma: Molecular Mechanisms and Therapeutic Implications. Leukemia 28 (1), 155–165. doi:10.1038/leu.2013.115
Tan, D. S., Bedard, P. L., Kuruvilla, J., Siu, L. L., and Razak, A. R. (2014). Promising SINEs for Embargoing Nuclear-Cytoplasmic export as an Anticancer Strategy. Cancer Discov. 4 (5), 527–537. doi:10.1158/2159-8290.CD-13-1005
Tiedemann, R. E., Zhu, Y. X., Schmidt, J., Shi, C. X., Sereduk, C., Yin, H., et al. (2012). Identification of Molecular Vulnerabilities in Human Multiple Myeloma Cells by RNA Interference Lethality Screening of the Druggable Genome. Cancer Res. 72 (3), 757–768. doi:10.1158/0008-5472.CAN-11-2781
Turner, J. G., Dawson, J., Cubitt, C. L., Baz, R., and Sullivan, D. M. (2014). Inhibition of CRM1-dependent Nuclear export Sensitizes Malignant Cells to Cytotoxic and Targeted Agents. Semin. Cancer Biol. 27, 62–73. doi:10.1016/j.semcancer.2014.03.001
Turner, J. G., Dawson, J., Emmons, M. F., Cubitt, C. L., Kauffman, M., Shacham, S., et al. (2013). CRM1 Inhibition Sensitizes Drug Resistant Human Myeloma Cells to Topoisomerase II and Proteasome Inhibitors Both In Vitro and Ex Vivo. J. Cancer 4 (8), 614–625. doi:10.7150/jca.7080
Turner, J. G., Dawson, J. L., Grant, S., Shain, K. H., Dalton, W. S., Dai, Y., et al. (2016). Treatment of Acquired Drug Resistance in Multiple Myeloma by Combination Therapy with XPO1 and Topoisomerase II Inhibitors. J. Hematol. Oncol. 9 (1), 73. doi:10.1186/s13045-016-0304-z
Turner, J. G., Kashyap, T., Dawson, J. L., Gomez, J., Bauer, A. A., Grant, S., et al. (2016). XPO1 Inhibitor Combination Therapy with Bortezomib or Carfilzomib Induces Nuclear Localization of IκBα and Overcomes Acquired Proteasome Inhibitor Resistance in Human Multiple Myeloma. Oncotarget 7 (48), 78896–78909. doi:10.18632/oncotarget.12969
Usmani, S., Ahmadi, T., Ng, Y., Lam, A., Desai, A., Potluri, R., et al. (2016). Analysis of Real-World Data on Overall Survival in Multiple Myeloma Patients with ≥3 Prior Lines of Therapy Including a Proteasome Inhibitor (PI) and an Immunomodulatory Drug (IMiD), or Double Refractory to a PI and an IMiD. Oncologist 21 (11), 1355–1361. doi:10.1634/theoncologist.2016-0104
Keywords: selinexor, multiple myeloma, XPO1, efficacy, safety, meta-analysis
Citation: Tao Y, Zhou H and Niu T (2021) Safety and Efficacy Analysis of Selinexor-Based Treatment in Multiple Myeloma, a Meta-Analysis Based on Prospective Clinical Trials. Front. Pharmacol. 12:758992. doi: 10.3389/fphar.2021.758992
Received: 15 August 2021; Accepted: 22 November 2021;
Published: 03 December 2021.
Edited by:
J. Luis Espinoza, Kanazawa University, JapanReviewed by:
Yuanbin Song, Sun Yat-sen University Cancer Center, ChinaIbrahim C. Haznedaroglu, Hacettepe University Hospital, Turkey
Copyright © 2021 Tao, Zhou and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Niu, dGluZ25pdUBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yali Tao
Yali Tao Hui Zhou
Hui Zhou Ting Niu
Ting Niu