
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 18 November 2021
Sec. Drugs Outcomes Research and Policies
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.756582
Background and Objective: Over the past few years, mirabegron has been increasingly used as a therapeutic option for neurogenic lower urinary tract dysfunction. Here, we carried out a meta-analysis to investigate the efficacy and safety of mirabegron for the treatment of neurogenic lower urinary tract dysfunction.
Methods: We used a range of databases to retrieve randomized controlled trials (RCTs) relating to mirabegron in patients with neurogenic lower urinary tract dysfunction: PubMed, Embase, and Cochrane Library; our strategy conformed to the PICOS (populations, interventions, comparators, outcomes, and study designs) strategy.
Results: Our analyses involved four RCTs involving 245 patients. We found that mirabegron treatment resulted in a significant improvement in bladder compliance [mean difference (MD) = 19.53, 95% confidence interval (CI): 14.19 to 24.87, P < 0.00001], urinary incontinence episodes (MD = −0.78, 95% CI: −0.89 to −0.67, P < 0.00001) and Incontinence Quality of Life (I-QOL) (MD = 8.02, 95% CI: 3.20 to 12.84, P = 0.001). Significant differences were detected in terms of Patient Perception of Bladder Condition (PPBC) (MD = −0.54, 95% CI: −1.46 to 0.39, P = 0.26) and urinary urgency episodes (MD = −0.72, 95% CI: −3.1 to 1.66, P = 0.55). With regard to safety, there were no significant differences between mirabegron and control groups in terms of the incidence of drug-related adverse events [odds ratio (OR): 0.83, 95% CI: 0.43 to 1.59, P = 0.57], arrhythmias (OR: 1.27, 95% CI: 0.37 to 4.38, P = 0.70), hypertension (OR: 0.70, 95% CI: 0.13 to 3.82, P = 0.68), or post-voiding residual volume (MD: 1.62, 95% CI: −9.00 to 12.24, P = 0.77).
Conclusion: Mirabegron is an efficacious and safe treatment for patients with neurogenic lower urinary tract dysfunction.
Patients suffering from spinal cord injury (SCI) and neurological disorders (e.g., multiple sclerosis (MS) and Parkinson’s disease) often present with neurogenic lower urinary tract dysfunction (NLUTD) (Stöhrer et al., 2009; Harris and Lemack, 2016). The typical clinical symptoms of NLUTD usually manifest as dysuria, urgency, urinary incontinence, and impaired bladder emptying. Patients with severe NLUTD can develop renal failure and complicated urinary tract infections and may even die. At present, anticholinergic (antimuscarinic) drugs are recommended as the first-line treatment for NLUTD. Although some studies have reported that anticholinergic (antimuscarinic) medications can effectively improve urodynamic parameters in patients with NLUTD (Madhuvrata et al., 2012; Sugiyama et al., 2017), these medicines are associated with side effects (e.g., dry mouth and constipation) that limit their use in the long term (Averbeck and Madersbacher, 2011; Manack et al., 2011; Wagg et al., 2012). Therefore, there is a clear need to develop novel, effective, and safe therapeutic modalities for NLUTD.
Mirabegron, a β3-adrenoceptor agonist, is commonly applied to treat idiopathic overactive bladder in the clinic and works by stimulating β3-adrenergic receptors to induce detrusor relaxation (Kashyap and Tyagi, 2013). Compared with anticholinergic (antimuscarinic) drugs, mirabegron has similar levels of efficacy but with superior safety (Maman et al., 2014; Chapple et al., 2017). More recently, mirabegron has been gradually applied for the treatment of NLUTD. However, few evidence-based studies have been conducted on the feasibility of using mirabegron as a treatment for NLUTD. In view of their superior safety profile, mirabegron is expected to become a new option for the treatment of NLUTD.
In this systematic review and meta-analysis, we assessed the efficacy and safety of mirabegron for the treatment of NLUTD to provide a feasible reference for clinical medication. Our study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.
Three of the authors identified randomized controlled trials (RCTs) relating to the impact of mirabegron in the treatment of NLUTD from the PubMed, Embase, and Cochrane Library databases, in accordance with the PICOS (populations, interventions, comparators, outcomes, and study designs) strategy; the search strategy is summarized in Table 1. Our database searches included the following search terms: NLUTD, SCI, neurological disorders (MS and Parkinson’s disease), mirabegron, and RCTs. Our analysis was registered with PROSPERO (Reference: CRD42021256235). References from the included articles were also reviewed by the three authors to identify additional relevant articles.
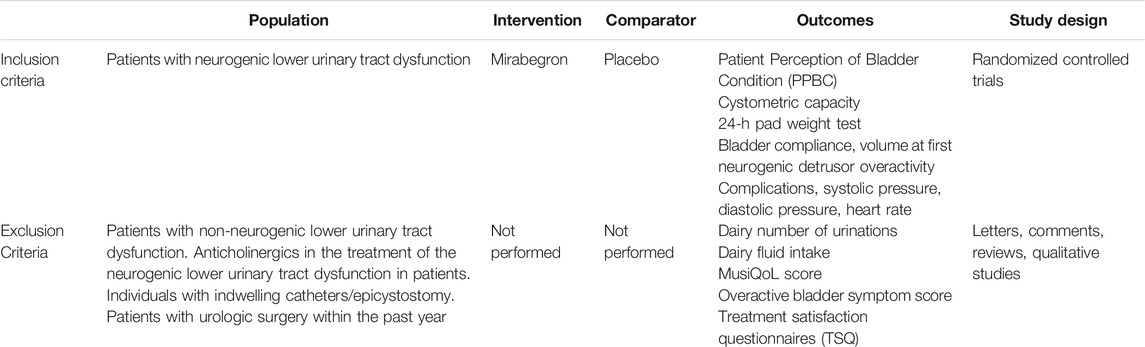
TABLE 1. Search strategy according to populations, interventions, comparators, outcomes, and study designs (PICOS).
To be included in our study, the RCTs needed to satisfy the following criteria: 1) the study analyzed the effect of mirabegron on NLUTD, 2) full-text content was available, and 3) the study provided complete and precise data (including the sample size of participants and the results of each indicator). There were stricter inclusion and exclusion criteria for RCTs, compared with other prospective and retrospective studies.
The quality of the selected RCTs was assessed by applying the Jadad scale (Alejandro, 1998). In addition, the assessment method included patient allocation, the concealment of allocation, blinding methodology, and the number of patients who were lost to follow-up. In accordance with the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions V.5.1.0 (DerSimonian and Laird, 1986), we classified the quality of each study as follows: 1) the study achieved all quality criteria with a low-risk of bias, 2) the study achieved most quality criteria with a moderate risk of bias, and 3) the study achieved few quality criteria with a high risk of bias. All authors achieved good levels of agreement when applying this classification.
We extracted a range of valuable information from each of the RCTs: 1) the name of the first author; 2) the study type; 3) the sample size of each group; 4) the treatment modality; 5) the dosage and time of treatment; and 6) the study outcome, including bladder compliance, Incontinence-Quality of Life (I-QOL), urinary incontinence episodes, urinary urgency episodes, Patient Perception of Bladder Condition (PPBC), the incidence of drug-related adverse events, arrhythmias, hypertension, and post-voiding residual volume.
We performed statistical analysis using Review Manager software (RevMan, version 5.3.0, Cochrane Collaboration) (Higgins and Green, 2008). Differences in bladder compliance; the mean score for the I-QOL and PPBC; and the incidence of drug-related adverse events, arrhythmias, hypertension, and post-voiding residual volume were used to investigate the efficacy of mirabegron for the treatment of NLUTD. Continuous data were evaluated by mean difference (MD) and dichotomous data are expressed by odds ratios (ORs) with 95% confidence intervals (CIs) (DerSimonian and Laird, 2015). When the p value was greater than 0.05, the study was regarded as being homogenous. A fixed-effects model was applied to homogenous studies. In contrast, a random-effects model was applicable to heterogeneous studies. We used the I2 statistic to test for inconsistency. A p value <0.05 was considered to indicate statistical significance.
After applying the inclusion/exclusion criteria, a total of 286 articles were identified from the databases. First, we screened the titles and abstracts; this led to the removal of 249 articles. When considering the remaining 19 articles, we excluded 14 articles because useful data were missing. One article was eliminated due to duplication. Finally, our analyses involved four high-quality RCTs (Zachariou et al., 2017; Krhut et al., 2018; Welk et al., 2018; Cho et al., 2021). Figure 1 shows a flowchart that presents the selection process. Study features and patient characteristics are given in Table 2.
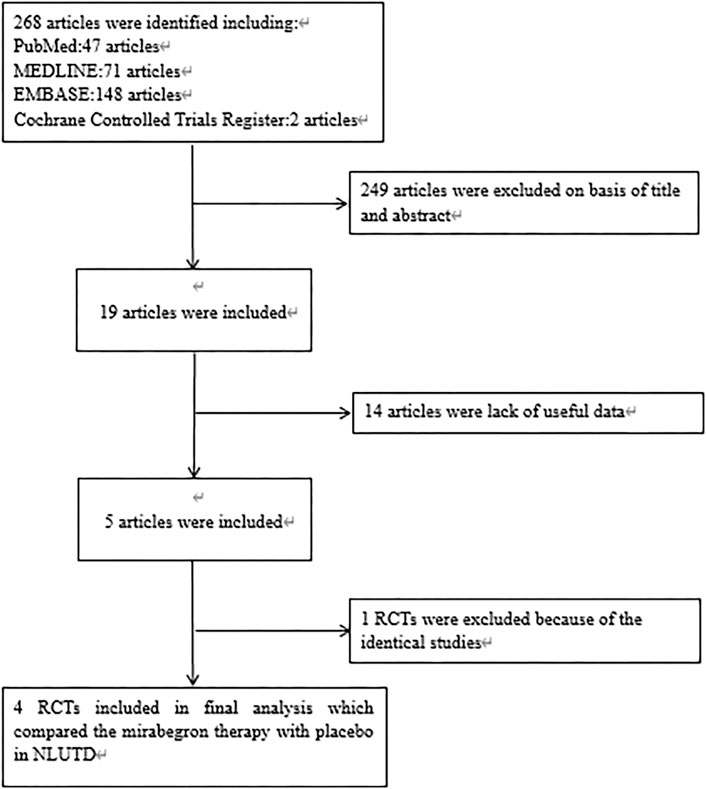
FIGURE 1. Flowchart of the study selection process. RCT, randomized controlled trials; NLUTD, neurogenic lower urinary tract dysfunction.
The included studies were all RCTs; three of these were randomized, double-blind, and placebo-controlled trials (Krhut et al., 2018; Welk et al., 2018; Cho et al., 2021). The quality grade of three of the included RCTs (Krhut et al., 2018; Welk et al., 2018; Cho et al., 2021) was rated as A; one RCT (Zachariou et al., 2017) was rated as B. One study failed to complete follow-up (Cho et al., 2021), and four patients were lost to follow up. Further details relating to study quality are given in Table 3.
Three RCTs analyzed the differences in PPBC across the 352 patients (the mirabegron group consisted of 106 patients, whereas the placebo group consisted of 109 patients) (Figure 2A). Because of p < 0.05, we performed a random-effects model; this showed a MD of –0.54 (95% CI: 1.46 to 0.39, I2 = 94%, Chi-squared value = 32.17, p = 0.26). Our analysis indicated that the effect of mirabegron on PPBC was similar to that of the placebo.
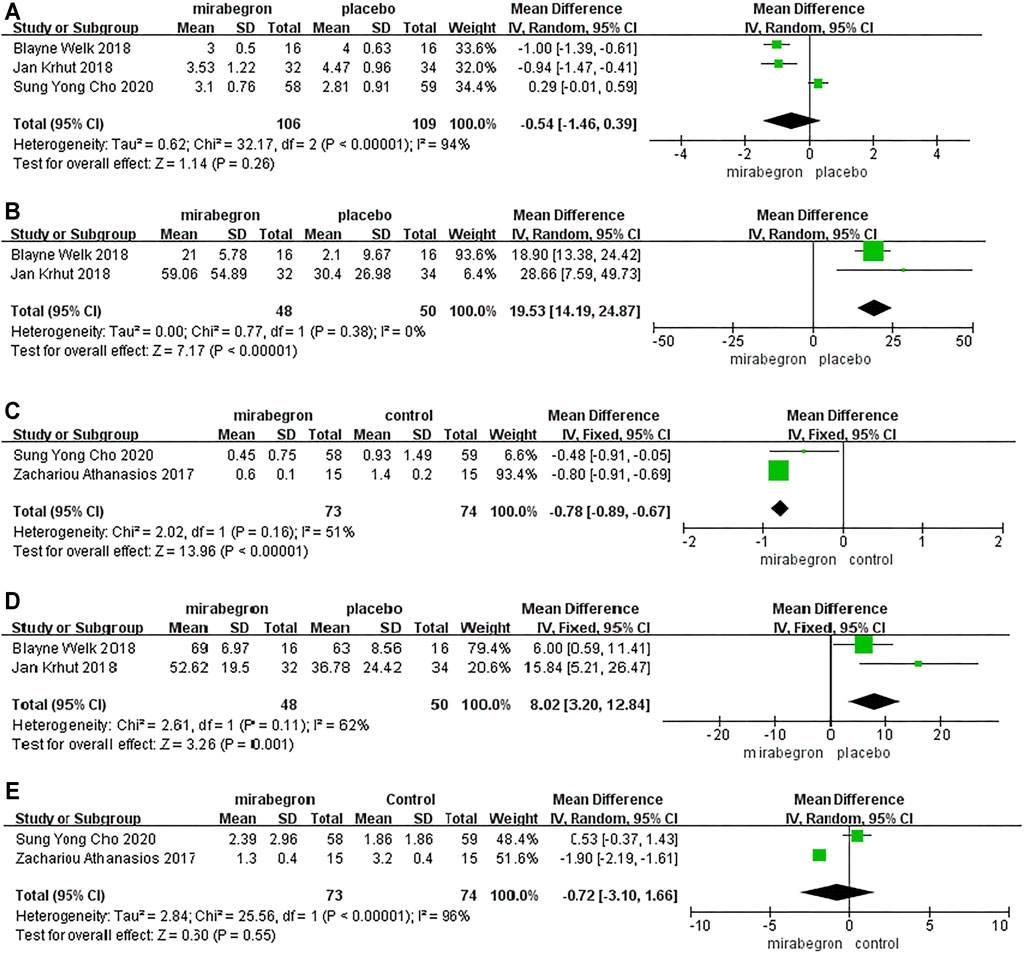
FIGURE 2. Forest plots showing changes in (A) patient perception of bladder condition (PPBC), (B) bladder compliance (C) urinary incontinence episodes, (D) Incontinence Quality of Life (I-QOL), and (E) urinary urgency episodes.
Two RCTs reported differences in the bladder compliance of 98 patients (48 in the mirabegron group and 50 in the placebo group) (Figure 2B). A random-effects model showed that patients experienced significantly improved bladder compliance following treatment with mirabegron (MD = 19.53; 95% CI: 14.19 to 24.87, p ≤ 0.00001).
Two RCTs reported differences in the urinary incontinence episodes of 147 patients (73 in the mirabegron group and 73 in the control group) (Figure 2C). A fixed-effects model indicated that mirabegron significantly improved urinary incontinence episodes in patients with NLUTD (MD = −0.78, 95% CI: −0.89 to −0.67, p < 0.00001).
Two RCTs reported differences in the bladder compliance of 98 patients (48 in the mirabegron group and 50 in the placebo group). Pooled results from a fixed-effects model showed that a statistically significant improvement was recorded in the mirabegron group in terms of the I-QOL scores (MD = 8.02, 95% CI: 3.20 to 12.84, p = 0.001) (Figure 2D).
Two RCTs reported differences in the urinary urgency episodes of 147 patients (73 in the mirabegron group and 74 in the control group). Pooled results from a random-effects model suggested that the mirabegron group did not differ significantly from that of the control group with regard to improving urinary urgency episodes (MD = −0.72, 95% CI: −3.1 to 1.66, p = 0.55) (Figure 2E).
Because of p > 0.05, we performed a fixed-effects model to compare the occurrence of drug-related adverse events between the two groups from three RCTs (Figure 3A). The model indicated that the OR was 0.83, the 95% CI was 0.43 to 1.59, the I2 was 0%, and the Chi-squared value was 1.45 (p = 0.57), thus indicating that there was no significant difference between the two groups with regard to the occurrence of drug-related adverse events.
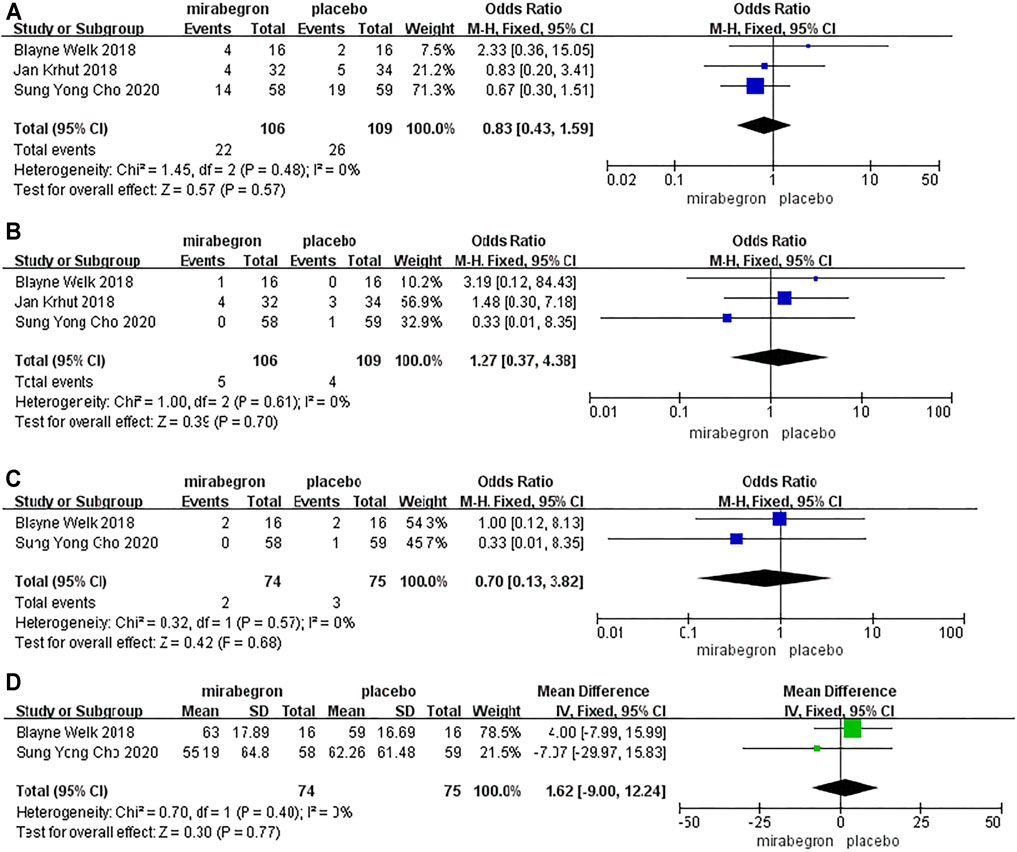
FIGURE 3. Forest plots showing changes in (A) adverse events, (B) heart rate, (C) blood pressure, and (D) post-voiding residual volume.
Because of p > 0.05, we performed a fixed-effects model to analyze the incidence of abnormal heart rate between the two groups from three RCTs (106 patients received mirabegron, whereas 109 patients received placebo treatment) (Figure 3B). The model indicated that the OR was 1.27, the 95% CI was 0.37 to 4.38, the I2 was 0%, and the Chi-squared value was 1.00 (p = 0.70), thus indicating that the mirabegron and placebo groups were similar in terms of the incidence of abnormal heart rate.
Two RCTs, including 149 patients (74 in the mirabegron group and 75 in the placebo group), evaluated the risk of abnormal blood pressure (Figure 3C). We utilized a fixed-effects model to analyze these data as p > 0.05. The model indicated that the OR was 0.70, the 95% CI was 0.13 to 3.82, the I2 was 0%, and the Chi-squared value was 0.32 (p = 0.68), thus indicating that there were no significant differences between the two groups with regard to abnormal blood pressure.
Two RCTs, including 149 patients (74 received mirabegron treatment and 75 received placebo treatment), analyzed post-voiding residual volume (Figure 3D). We used a fixed-effects model to analyze these data, as p > 0.05. There was no significant difference between the two groups with regard to post-voiding residual volume (MD = −1.62; 95% CI: −9.00 to 12.24, p = 0.77).
Previous epidemiological surveys have shown that the prevalence of SCI in Europe was 0.298%, whereas that of MS was 0.11% (Kingwell et al., 2013; Lee et al., 2014). Developing countries have also been shown to be associated with a high prevalence (2.189%) of cerebrovascular accidents (Przydacz et al., 2017). Studies have also shown that 57%–83% of stroke patients will develop lower urinary tract symptoms just 1 month after cerebrovascular accident (Besiroglu et al., 2015). NLUTD arises from any alteration of the normal neural control mechanisms and can be the consequence of a number of nervous system diseases: SCI, MS, and Parkinson’s. The lower urinary tract is made up of the bladder and the urethra and implements its biological function via the storage and voiding of urine. Any neurological lesions or injuries that occur in this complex pathway may contribute to NLUTD. Of the numerous complications of NLUTD, renal failure is the leading cause of mortality (Groen et al., 2016). In addition, lower urinary tract symptoms can exert a serious negative impact on the quality of life. Until now, the management of NLUTD has remained as a major challenge facing the field of urology.
For many years, anticholinergic (antimuscarinic) drugs have been the most frequently used treatment for NLUTD in the clinical setting (Siddiqui et al., 2010; Madhuvrata et al., 2012). Although these drugs are effective in the improvement of cystometric capacity and bladder compliance (Madhuvrata et al., 2012), they are also associated with a high incidence of adverse drug reactions that often leads to treatment discontinuation (Nicholas et al., 2015). Therefore, it is essential that we identify alternative drugs that are both safe and effective.
Mirabegron is a β3-adrenergic agonist that is expected to become an efficient alternative to antimuscarinic agents due to its promising efficacy on the overactive bladder (Chapple et al., 2014). β-adrenoceptors can be divided into three subtypes: β1, β2, and β3 (Bylund et al., 1994). β3-receptors are predominately expressed in the heart, gastrointestinal tract, brain, prostate, and bladder detrusor (Ursino et al., 2009). However, because the β-adrenoceptors are widely expressed in the cardiovascular system, β-receptor agonists tend to induce adverse cardiovascular reactions. Because of its high selectivity toward β3-adrenoreceptors, mirabegron is rarely associated with complications (Korstanje et al., 2017). Previous studies have indicated that β3-adrenoceptor agonists exhibit the potential to cause human ureter relaxation (Matsumoto et al., 2013). The mechanisms of action by which mirabegron differs from anticholinergic drugs relate to the relaxation of the detrusor muscles in the storage phase; these effects occur via the stimulation of β3-adrenoreceptors. Previous studies have indicated that mirabegron was superior to antimuscarinic drugs in terms of cardiovascular complications (Rosa et al., 2018).
In the recent years, the role of mirabegron on NLUTD has attracted increasing levels of attention. For example, Beauval et al. proved that mirabegron treatment significantly improved micturition frequency and non-voiding contractions in a rat model of SCI (Beauval et al., 2015). In another study, Chen et al. reported the beneficial effects of mirabegron on the lower urinary tract symptoms of patients suffering from Parkinson’s disease and stroke (Chen and Kuo, 2019). Another retrospective chart review by Wöllner et al. concluded that mirabegron treatment has several advantages for patients with neurogenic detrusor overactivity (Wöllner and Pannek, 2016). Furthermore, Karakus et al. demonstrated that mirabegron is an effective and safe option for erectile function in men with an overactive bladder and erectile dysfunction (Karakus et al., 2021). Mullen et al. showed that mirabegron was effective in improving the urinary symptoms of patients with both overactive bladder and benign prostatic hyperplasia (Mullen and Kaplan, 2021).
We evaluated the treatment outcome of patients with several tools: the PPBC, I-QOL, and the Treatment Satisfaction-Visual Analog Scale (TS-VAS). PPBC is an evaluation form developed by the European Medical Evaluation Association to assess the global urinary incontinence problem and aims to report a subject’s subjective sensation of problems relating to the lower urinary tract (Coyne et al., 2006). The I-QoL, as a simple clinical investigation method, was originally designed to investigate the quality of life of women suffering from stress urinary incontinence (Patrick et al., 1999). The TS-VAS is used to record the subjective satisfaction of a patient with regard to their treatment. The results of these analyses were rated from 0 (none) to 100 (completely).
In our meta-analysis, we included four RCTs involving 245 patients who suffered from NLUTD. We assessed both the efficacy and safety of mirabegron for the treatment of NLUTD. The pooled results highlighted the significant superiority of mirabegron in terms of improving bladder compliance, urinary incontinence episodes, and I-QOL scores than placebo. In terms of PPBC and urinary urgency episodes, mirabegron therapy does not appear to differ from that of the placebo group. In a previous study, Krhut et al. found that mirabegron was superior to the placebo in terms of improving volume at the first detrusor contraction; it also improved TS-VAS and reduced urine leakage over 24 h (Krhut et al., 2018). In one previous RCT, the neurogenic bladder symptom score of a mirabegron group was significantly higher than the placebo group (Welk et al., 2018). With regard to safety, we found no significant difference between the mirabegron group and the placebo group in terms of the incidence of drug-related adverse events, arrhythmias, hypertension, and post-voiding residual volume. With this meta-analysis, we concluded that mirabegron can significantly improve the symptoms of NLUTD and has a superior clinical safety profile when compared with a placebo. These findings provide the basis for the continued use of mirabegron as an effective therapeutic strategy for the NLUTD.
Our meta-analysis has several strengths. First, the studies that we analyzed were all RCT; this means that the risk of bias was low. Second, to the best of our knowledge, very few previous reports have attempted to investigate the efficacy and safety of mirabegron for the treatment of NLUTD. Our study provides a strong support for the clinical use of mirabegron in NLUTD. However, there are also some limitations that need to be considered. First, the number of studies included in this analysis was inadequate and could have resulted in publication bias. To address this, our future research will focus on the most recent RCTs. Second, this study was not able to evaluate the long-term effects of mirabegron. As a result, our findings need to be confirmed by performing more high-quality RCTs.
Our study indicated that mirabegron was effective in relieving NLUTD symptoms and exhibited a favorable safety profile.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
JW and YC designed the research, interpreted the data, and revised the paper. DZ, FS, HY, XB, and DW performed the data extraction and carried out the meta-analysis. DZ drafted the paper. All of the authors approved the submitted and final versions.
This work was supported by grants from the National Nature Science Foundation of China (nos. 81,870,525 and 81,572,835) and Taishan Scholars Program of Shandong Province (no. tsqn201909199).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Averbeck, M. A., and Madersbacher, H. (2011). Constipation and LUTS - How Do They Affect Each Other? Int. Braz. J. Urol. 37 (1), 16-28. doi:10.1590/s1677-55382011000100003
Beauval, J. B., Guilloteau, V., Cappellini, M., Westfall, T. D., Rischmann, P., Palea, S., et al. (2015). Comparison of the Effects of β3 -adrenoceptor Agonism on Urinary Bladder Function in Conscious, Anesthetized, and Spinal Cord Injured Rats. Neurourol Urodyn 34 (6), 578-585. doi:10.1002/nau.22629
Besiroglu, H., Otunctemur, A., and Ozbek, E. (2015). The Relationship between Metabolic Syndrome, its Components, and Erectile Dysfunction: a Systematic Review and a Meta-Analysis of Observational Studies. J. Sex. Med. 12 (6), 1309-1318. doi:10.1111/jsm.12885
Bylund, D. B., Eikenberg, D. C., Hieble, J. P., Langer, S. Z., Lefkowitz, R. J., Minneman, K. P., et al. (1994). International Union of Pharmacology Nomenclature of Adrenoceptors. Pharmacol. Rev. 46 (2), 121-136.
Chapple, C. R., Cardozo, L., Nitti, V. W., Siddiqui, E., and Michel, M. C. (2014). Mirabegron in Overactive Bladder: A Review of Efficacy, Safety, and Tolerability. Neurourol Urodyn 33 (1), 17-30. doi:10.1002/nau.22505
Chapple, C. R., Nazir, J., Hakimi, Z., Bowditch, S., Fatoye, F., Guelfucci, F., et al. (2017). Persistence and Adherence with Mirabegron Versus Antimuscarinic Agents in Patients with Overactive Bladder: A Retrospective Observational Study in UK Clinical Practice. Eur. Urol. 72 (3), 389-399. doi:10.1016/j.eururo.2017.01.037
Chen, S. F., and Kuo, H. C. (2019). Therapeutic Efficacy of Low-Dose (25 mg) Mirabegron Therapy for Patients with Mild to Moderate Overactive Bladder Symptoms Due to central Nervous System Diseases. Low Urin Tract Symptoms 11 (2), O53-O58. doi:10.1111/luts.12215
Cho, S. Y., Jeong, S. J., Lee, S., Kim, J., Lee, S. H., Choo, M. S., et al. (2021). Mirabegron for Treatment of Overactive Bladder Symptoms in Patients with Parkinson's Disease: A Double-Blind, Randomized Placebo-Controlled Trial (Parkinson's Disease Overactive Bladder Mirabegron, PaDoMi Study). Neurourol Urodyn 40 (1), 286–294. doi:10.1002/nau.24552
Coyne, K. S., Matza, L. S., Kopp, Z., and Abrams, P. (2006). The Validation of the Patient Perception of Bladder Condition (PPBC): A Single-Item Global Measure for Patients with Overactive Bladder. Eur. Urol. 49 (6), 1079-1086. doi:10.1016/j.eururo.2006.01.007
DerSimonian, R., and Laird, N. (1986). Meta-Analysis in Clinical Trials. Control. Clin. T Rials 7, 177–188. doi:10.1016/0197-2456(86)90046-2
DerSimonian, R., and Laird, N. (2015). Meta-Analysis in Clinical Trials Revisited. Contemp. Clin. Trials 45, 139–145. doi:10.1016/j.cct.2015.09.002
Groen, J., Pannek, J., Castro Diaz, D., Del Popolo, G., Gross, T., Hamid, R., et al. (2016). Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur. Urol. 69 (2), 324-333. doi:10.1016/j.eururo.2015.07.071
Harris, C. J., and Lemack, G. E. (2016). Neurourologic Dysfunction: Evaluation, Surveillance and Therapy. Curr. Opin. Urol. 26 (4), 290-294. doi:10.1097/MOU.0000000000000290
J. P. Higgins, and S. Green (Editors) (2008). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, United Kingdom: The Cochrane Collaboration. Version 5.3.0. Available at: www.cochranehandbook.org. doi:10.1002/9780470712184.ch2
Karakus, S., Musicki, B., and Burnett, A. L. (2021). Mirabegron Improves Erectile Function in Men with Overactive Bladder and Erectile Dysfunction: A 12-week Pilot Study. Int. J. Impot Res. 32 (3). doi:10.1038/s41443-021-00455-2
Kashyap, M., and Tyagi, P. (2013). The Pharmacokinetic Evaluation of Mirabegron as an Overactive Bladder Therapy Option. Expert Opin. Drug Metab. Toxicol. 9 (5), 617-627. doi:10.1517/17425255.2013.786700
Kingwell, E., Marriott, J. J., Jetté, N., Pringsheim, T., Makhani, N., Morrow, S. A., et al. (2013). Incidence and Prevalence of Multiple Sclerosis in Europe: A Systematic Review. BMC Neurol. 13 (1), 128. doi:10.1186/1471-2377-13-128
Korstanje, C., Suzuki, M., Yuno, K., Sato, S., Ukai, M., Schneidkraut, M. J., et al. (2017). Translational Science Approach for Assessment of Cardiovascular Effects and Proarrhythmogenic Potential of the Beta-3 Adrenergic Agonist Mirabegron. J. Pharmacol. Toxicol. Methods 87, 74-81. doi:10.1016/j.vascn.2017.04.008
Krhut, J., Borovička, V., Bílková, K., Sýkora, R., Míka, D., Mokriš, J., et al. (2018). Efficacy and Safety of Mirabegron for the Treatment of Neurogenic Detrusor Overactivity-Prospective, Randomized, Double-Blind, Placebo-Controlled Study. Neurourol Urodyn 37 (7), 2226-2233. doi:10.1002/nau.23566
Lee, B. B., Cripps, R. A., Fitzharris, M., and Wing, P. C. (2014). The Global Map for Traumatic Spinal Cord Injury Epidemiology: Update 2011, Global Incidence Rate. Spinal Cord 52 (2), 110-116. doi:10.1038/sc.2012.158
Madhuvrata, P., Singh, M., Hasafa, Z., and Abdel-Fattah, M. (2012). Anticholinergic Drugs for Adult Neurogenic Detrusor Overactivity: A Systematic Review and Meta-Analysis. Eur. Urol. 62 (5), 816-830. doi:10.1016/j.eururo.2012.02.036
Maman, K., Aballea, S., Nazir, J., Desroziers, K., Neine, M. E., Siddiqui, E., et al. (2014). Comparative Efficacy and Safety of Medical Treatments for the Management of Overactive Bladder: A Systematic Literature Review and Mixed Treatment Comparison. Eur. Urol. 65 (4), 755-765. doi:10.1016/j.eururo.2013.11.010
Manack, A., Motsko, S. P., Haag-Molkenteller, C., Dmochowski, R. R., Goehring, E. L., Nguyen-Khoa, B. A., et al. (2011). Epidemiology and Healthcare Utilization of Neurogenic Bladder Patients in a US Claims Database. Neurourol Urodyn 30 (3), 395-401. doi:10.1002/nau.21003
Matsumoto, R., Otsuka, A., Suzuki, T., Shinbo, H., Mizuno, T., Kurita, Y., et al. (2013). Expression and Functional Role of β3 -Adrenoceptors in the Human Ureter. Int. J. Urol. 20 (10), 1364-1370. doi:10.1111/iju.12093
Mullen, G. R., and Kaplan, S. A. (2021). Efficacy and Safety of Mirabegron in Men with Overactive Bladder Symptoms and Benign Prostatic Hyperplasia. Curr. Urol. Rep. 22 (1), 5. doi:10.1007/s11934-020-01017-7
Nicholas, R. S., Friede, T., Hollis, S., and Young, C. A. (2015). Withdrawn: Anticholinergics for Urinary Symptoms in Multiple Sclerosis. Cochrane Database Syst. Rev. 6, CD004193. doi:10.1002/14651858.CD004193.pub3
Patrick, D. L., Martin, M. L., Bushnell, D. M., Yalcin, I., Wagner, T. H., and Buesching, D. P. (1999). Quality of Life of Women with Urinary Incontinence: Further Development of the Incontinence Quality of Life Instrument (I-QOL). Urology 53 (1), 71-76. doi:10.1016/s0090-4295(98)00454-3
Przydacz, M., Denys, P., and Corcos, J. (2017). What Do We Know about Neurogenic Bladder Prevalence and Management in Developing Countries and Emerging Regions of the World? Ann. Phys. Rehabil. Med. 60 (5), 341-346. doi:10.1016/j.rehab.2017.02.008
Rosa, G. M., Baccino, D., Valbusa, A., Scala, C., Barra, F., Brunelli, C., et al. (2018). Cardiovascular Effects of Antimuscarinic Agents and Beta3-Adrenergic Receptor Agonist for the Treatment of Overactive Bladder. Expert Opin. Drug Saf. 17 (5), 487-497. doi:10.1080/14740338.2018.1453496
Siddiqui, N. Y., Wu, J. M., and Amundsen, C. L. (2010). Efficacy and Adverse Events of Sacral Nerve Stimulation for Overactive Bladder: A Systematic Review. Neurourol Urodyn 29 (S1), S18–S23. doi:10.1002/nau.20786
Stöhrer, M., Blok, B., Castro-Diaz, D., Chartier-Kastler, E., Del Popolo, G., Kramer, G., et al. (2009). EAU Guidelines on Neurogenic Lower Urinary Tract Dysfunction. Eur. Urol. 56 (1), 81-88. doi:10.1016/j.eururo.2009.04.028
Sugiyama, H., Uemura, O., Mori, T., Okisio, N., Unai, K., and Liu, M. (2017). Effect of Imidafenacin on the Urodynamic Parameters of Patients with Indwelling Bladder Catheters Due to Spinal Cord Injury. Spinal Cord 55 (2), 187-191. doi:10.1038/sc.2016.168
Ursino, M. G., Vasina, V., Raschi, E., Crema, F., and De Ponti, F. (2009). The Beta3-Adrenoceptor as a Therapeutic Target: Current Perspectives. Pharmacol. Res. 59 (4), 221-234. doi:10.1016/j.phrs.2009.01.002
Wagg, A., Compion, G., Fahey, A., and Siddiqui, E. (2012). Persistence with Prescribed Antimuscarinic Therapy for Overactive Bladder: A UK Experience. BJU Int. 110 (11), 1767-1774. doi:10.1111/j.1464-410X.2012.11023.x
Welk, B., Hickling, D., McKibbon, M., Radomski, S., and Ethans, K. (2018). A Pilot Randomized-Controlled Trial of the Urodynamic Efficacy of Mirabegron for Patients with Neurogenic Lower Urinary Tract Dysfunction. Neurourol Urodyn 37 (8), 2810-2817. doi:10.1002/nau.23774
Wöllner, J., and Pannek, J. (2016). Initial Experience with the Treatment of Neurogenic Detrusor Overactivity with a New β-3 Agonist (Mirabegron) in Patients with Spinal Cord Injury. Spinal Cord 54 (1), 78-82. doi:10.1038/sc.2015.195
Keywords: meta-analysis, mirabegron, neurogenic lower urinary tract dysfunction, RCT, randomized controlled trial, systematic review
Citation: Zhang D, Sun F, Yao H, Bao X, Wang D, Cui Y and Wu J (2021) The Efficacy and Safety of Mirabegron for the Treatment of Neurogenic Lower Urinary Tract Dysfunction: A Systematic Review and Meta-analysis. Front. Pharmacol. 12:756582. doi: 10.3389/fphar.2021.756582
Received: 10 August 2021; Accepted: 05 October 2021;
Published: 18 November 2021.
Edited by:
Jean Paul Deslypere, Aesculape CRO, BelgiumReviewed by:
Philip Van Kerrebroeck, Maastricht University, NetherlandsCopyright © 2021 Zhang, Sun, Yao, Bao, Wang, Cui and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jitao Wu, d2p0dXJvbG9neUAxNjMuY29t; Yuanshan Cui, OTc4OTQ2NzAwQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.