- 1Department of Biological and Biomedical Sciences, Aga Khan University Hospital, Karachi, Pakistan
- 2Family Medicine, Liaquat National Hospital, Karachi, Pakistan
- 3Department of Pharmaceutics, Faculty of Pharmacy, Hamdard University, Karachi, Pakistan
- 4Department of Bioscience, Salim Habib University, Karachi, Pakistan
- 5Department of Pharmacology and Toxicology, College of Pharmacy, Prince Sattam Bin Abdul Aziz University, Al Kharj, Saudi Arabia
- 6National Institute of Health, Islamabad, Pakistan
- 7Department of Public Health and Nutrition, The University of Haripur, Haripur, Pakistan
Background: Metabolic syndrome (MetS) is a multifactorial disease, whose main stay of prevention and management is life-style modification which is difficult to attain. Combination of herbs have proven more efficacious in multi-targeted diseases, as compared to individual herbs owing to the “effect enhancing and side-effect neutralizing” properties of herbs, which forms the basis of polyherbal therapies This led us to review literature on the efficacy of herbal combinations in MetS.
Methods: Electronic search of literature was conducted by using Cinnahl, Pubmed central, Cochrane and Web of Science, whereas, Google scholar was used as secondary search tool. The key words used were “metabolic syndrome, herbal/poly herbal,” metabolic syndrome, clinical trial” and the timings were limited between 2005–2020.
Results: After filtering and removing duplications by using PRISMA guidelines, search results were limited to 41 studies, out of which 24 studies were evaluated for combinations used in animal models and 15 in clinical trials related to metabolic syndrome. SPICE and SPIDER models were used to assess the clinical trials, whereas, a checklist and a qualitative and a semi-quantitative questionnaire was formulated to report the findings for animal based studies. Taxonomic classification of Poly herbal combinations used in animal and clinical studies was designed.
Conclusion: With this study we have identified the potential polyherbal combinations along with a proposed method to validate animal studies through systematic qualitative and quantitative review. This will help researchers to study various herbal combinations in MetS, in the drug development process and will give a future direction to research on prevention and management of MetS through polyherbal combinations.
Introduction
Non-communicable diseases (NCDs) account for 71% of the deaths worldwide with rising prevalence in lower- and middle-income countries (Huang, 2009; Robinson et al., 2013). NCDs have been ranked as one of the top ten global threats in 2019 by World Health Organization (Khowaja et al., 2007; Robinson et al., 2013). Metabolic syndrome (MetS) is a type of NCD with worldwide prevalence ranging from less than 10% to as much as 84% (Rhee et al., 2010) with the burden being greater in South Asian countries (Sever et al., 2003; Su and Li, 2011).
MetS is characterized by a cluster of three or more features including hyperglycaemia, hypertriglyceridemia, a low level of high-density lipoprotein cholesterol (HDL-C), blood pressure and central obesity (Bodeker and Kronenberg, 2002; Anderson and Taylor, 2012). A person who has at least three out of five of these characteristics is labelled as MetS patient. The following criteria should be met for MetS (AuH, 1998; Anderson and Taylor, 2012): waist circumference more than 35 and 40 inches in women and men, respectively (central obesity); triglycerides (TGs) 150 mg/dl or greater, HDL-C less than 50 and 40 mg/dl in women and men, respectively, blood pressure (BP) of 130/85 mm Hg or higher, fasting blood glucose (FBG) of 100 mg/dl or greater. Besides the above mentioned abnormalities, underlying initiators of MetS are inflammation, oxidative stress and insulin resistance (Ma et al., 2009; Aziz et al., 2013; Amin et al., 2015a). Together these factors pose a three- and five-fold greater risk for cardiovascular disease (CVD) and type II diabetes mellitus (T2DM) respectively (Zimmermann et al., 2007), along with high mortality rate (Gilani and Rahman, 2005).
MetS has multiple aetiologies and therefore no single drug can be effective in reversing this situation. The main stay of prevention and management of individuals at risk is life-style modification. However, those who have high levels of risk factors are the recipients for pharmacological treatment which is aimed towards individual symptoms’ management (AuH, 1998; Devalaraja et al., 2011; Mohamed, 2014). Multiple drugs including drugs to lower the blood glucose level, TGs, and blood pressure (Robinson et al., 2013) may be needed for a long time resulting in drug related complications, low compliance rate and high cost of care (Khowaja et al., 2007; Huang, 2009). Alternately, some researchers suggest to advocate life-style modification as the first line therapy for prevention of a chronic disease, rather than using pharmacological therapies such as metformin in pre-diabetes (Rhee et al., 2010) and statins in mild to moderate dyslipidemia (Sever et al., 2003). Endorsing only life-style modifications is challenging for the physicians especially among high-risk patients such as in obese patients, since compliance to dietary modification, and physical activity is difficult to attain (Samir et al., 2011). Therefore, it is imperative to explore innovative therapies which are cost-effective and acceptable, with fewer adverse effects, in order to reduce the risk of cardiovascular diseases (CVD) through addressing the risk factors.
According to World Health Organization (WHO), up to 80% of the Asian population relies on complementary and alternative/Traditional medicine (CAM/T) for their primary healthcare, possibly because more than 80% of people in developing nations can barely afford basic medical needs (Su and Li, 2011). Interestingly, almost half of the population in the developed world also uses CAM/T therapies (Bodeker and Kronenberg, 2002). Amongst the most common complementary modalities used by individuals with CVD risk factors are natural products (Anderson and Taylor, 2012) that have evidently contributed in the development of modern medicine for cardiovascular disorders (AuH, 1998). MetS requires multiple factors to be addressed simultaneously, therefore polyherbal combinations can offer a safe and more effective therapeutic option. Research has revealed that the multi-component properties of polyherbal combinations make them suitable for treating complex diseases and offer great potential for exhibiting synergistic actions. Evaluation of literature from individual effects of potential polyherbal combinations paves the path for deriving new combinations.
Synergistic therapeutic actions of polyherbal formulations are possible through underlying mechanisms such as regulation of same or different targets in various pathways hence in combination enhance efficacy, regulation of enzymes and transporters to improve oral drug bioavailability, neutralize adverse effects and overcome drug resistance mechanisms. Synergism is observed when multiple chemical constituents are present in single or in combination of herbs (Amin et al., 2015a), which are potential therapeutic options for various disease targets. This forms the basis of polyherbal therapies (Ma et al., 2009; Aziz et al., 2013) and is considered rational and more efficacious in multi-targeted diseases (Zimmermann et al., 2007). The effect-enhancing and side-effect neutralizing properties of polyherbal combinations (Gilani and Rahman, 2005) prompted us to review the literature on the efficacy of polyherbal combinations in metabolic syndrome, the incidence of which is rising globally. This will help researchers to identify various effective polyherbal combinations in MetS, which may help in the drug development process, as well as provide future direction towards research on prevention and cure of a menace like metabolic syndrome. Although synergistic therapeutic interactions of herbal ingredients have been frequently reported, to the best of our knowledge, none of the reports have offered review of polyherbal formulations in MetS. Individual herb reviews related to MetS were limited to functional foods (Mohamed, 2014) and exotic fruits (Devalaraja et al., 2011). Hence, in this review, we present recent literature reporting herb synergisms and efficacy of various polyherbal formulations in MetS. We have identified the herb to be good if it manages to modulate at least 3 out of 5 MetS criteria.
METHODS
Systematic Review Protocol (Search Strategy and Data Sources)
We decided for a qualitative systematic review for which an electronic literature search was carried out to find articles published mainly in the last 15 years (2005–2020).
For this purpose, following databases, and/or search engines were used: Cinnahl, Pubmed central, Cochrane, Web of Science and Scopus. Google scholar was used as secondary search tool.
The key words used were “metabolic syndrome, herbal/polyherbal,” “metabolic syndrome, clinical trial”.
Inclusion Criteria
1. Animal model with MetS that are given more than one herb for treatment.
2. Adults diagnosed with MetS (who qualify for 3 of the 5 MetS parameters: obesity, high blood pressure, hypertriglyceridemia, low HDL, high blood sugar (>100–125 mg/dl).
3. Adults >17 years < 74 years.
Exclusion Criteria
1. Review article.
2. Effect of individual herbs on MetS
3. Effect of interventions through diet, low caloric, mediterranean diet etc., on MetS.
4. Any MetS model used but not for the purpose of assessing effect on MetS, rather individual aspect such as obesity, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis and polycystic ovary syndrome.
Data Analysis and Study Design
All the polyherbal formulations were classified taxonomically and then effect of intervention and evaluation of results were done, based on number of MetS criteria met both in animal and/or humans.
Quality of animal-based studies were assessed by using a qualitative scoring system using 8 questions. Maximum score achieved was 8, with yes = score 1 and no = score 0 with following questions:
1. MetS parameters assessed >3 = score 1; ≤3 = score 0
2. MetS parameters met: 3 out of 5 parameters (good Effect) = 1; <3 out of 5 (not so good) = 0.
3. Dosage of herb provided: Yes = 1; No = 0
4. Components and rationale for dosing: yes = 1; no = 0
5. Animal ethical approval: Yes = 1; No = 0
6. Euthanasia protocol mentioned/followed: Yes = 1; No = 0
7. Model validated for MetS: Yes = 1; No = 0
8. Positive control used: Yes = 1; No = 0
For clinical trial we adopted a mixed model for assessing our articles including SPICE (S = setting; P = population; I = intervention/what; C = comparison/controls E = evaluation/with what result) (Booth, 2006; Cleyle and Booth, 2006) and SPIDER (S = Sample P = phenomenon of interest/intervention I = intervention size, D = design, E = evaluation/outcome R = research type; qualitative, quantitative or mixed type). SPIDER methods had added points for assessing both qualitative and quantitative methods (Cooke et al., 2012). Further aspects of quality of clinical trial were assessed based on following aspects with yes = 1; No = 0 according to an adopted guideline for critical appraisal (Alcántara et al., 2011):
1. The study addresses an appropriate and clearly focused question
2. The assignment of subjects to treatment groups is randomized.
3. An adequate concealment method is used
4. The design keeps subjects and investigators ‘blind’ about treatment allocation.
5. The treatment and control groups are similar at the start of the trial.
6. The only difference between groups is the treatment under investigation.
7. All relevant outcomes are measured in a standard, valid and reliable way
8. What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed?
9. All the subjects are analyzed in the groups to which they were randomly allocated (often referred to as intention to treat analysis)
10. Where the study is carried out at more than one site, results are comparable for all sites.
Besides, following questions were also assessed: concentration of the herb provided or not, quality control of the combination assessed or not and chemical classification done or not.
Results
The selection parameter, applied filters, as well as output of all the searches, are summarised in Figure 1A. In Figure 1B the summary of identified results is presented according to PRISMA guidelines (Page et al., 2019; Maraolo, 2021).
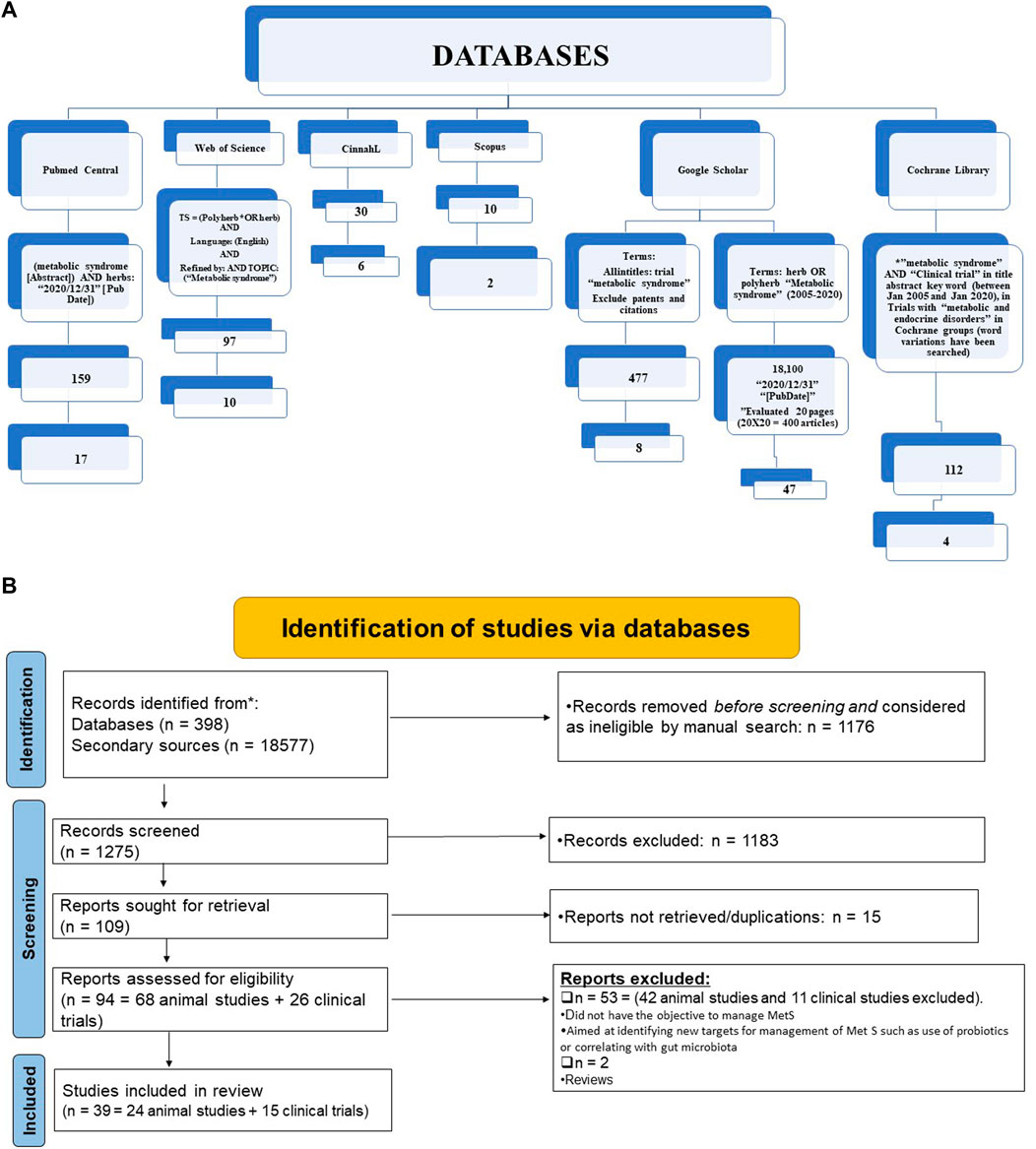
FIGURE 1. Summary of literature search (A) and Analysis of systematic review results according to Prisma guidelines (B).
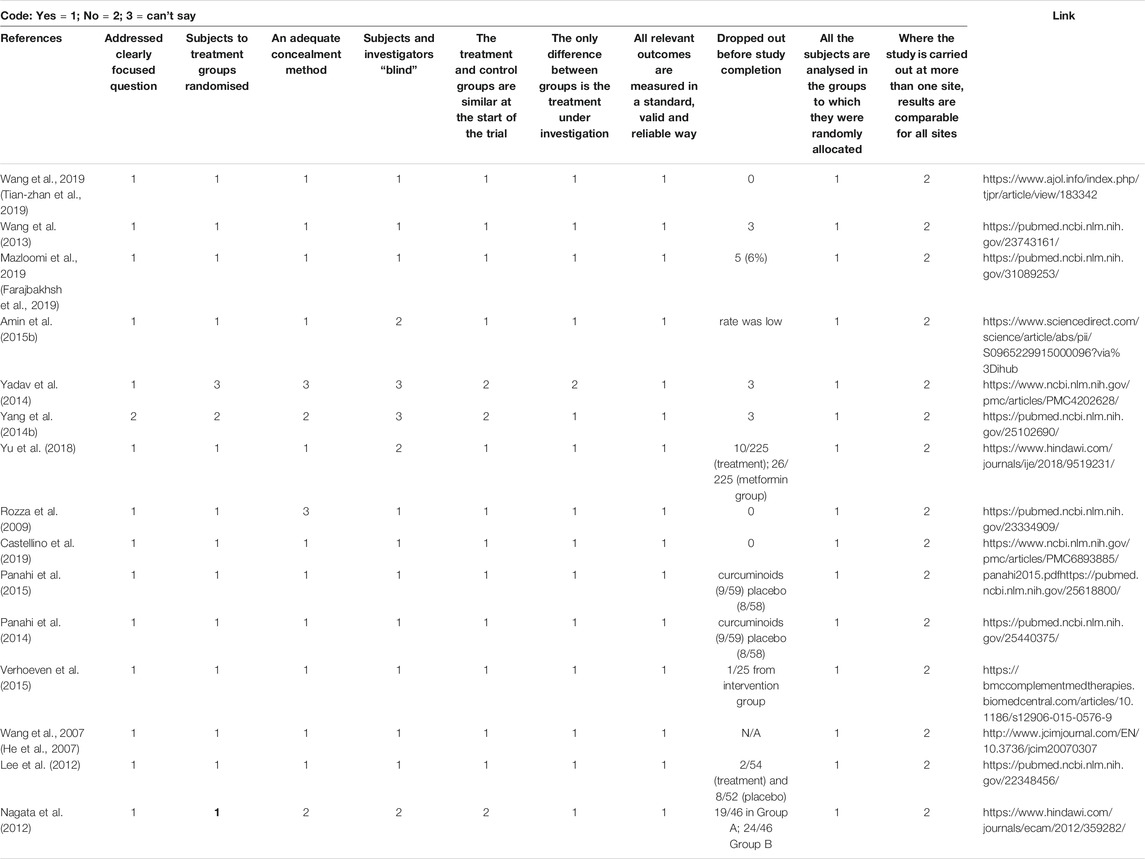
The total reference shortlisted were 109, out of which duplications and or articles which could not be retrieved were removed (n = 15) and number of articles to review were 94. Out of total 94 articles, 26 were divided as clinical trials and remaining 68 articles were either based on animal studies or in-vitro assays. These articles were further shortlisted by reviewing their basic theme and it was identified that some of the articles did not have the objective to manage MetS or were aimed at identifying new targets for management of Met S such as use of probiotics or correlating with gut microbiota (Ni et al., 2018) or the basic target for those studies were to cater different disease, although parameters for MetS were being met. Hence, out of 68 animal studies, filtered animal studies were identified to be 24 which matched our main objective of MetS. The taxonomic classification of polyherbal combinations used both in animal and clinical studies are summarized in Tables 1, 2, respectively. The meta-analysis of animal studies is summarized in Table 3. To further analyze the quality of studies, a semiquantitative scale was used, the details of which are presented separately as Table 4. The maximum score was 8, and references have been aligned from highest score to lowest score.
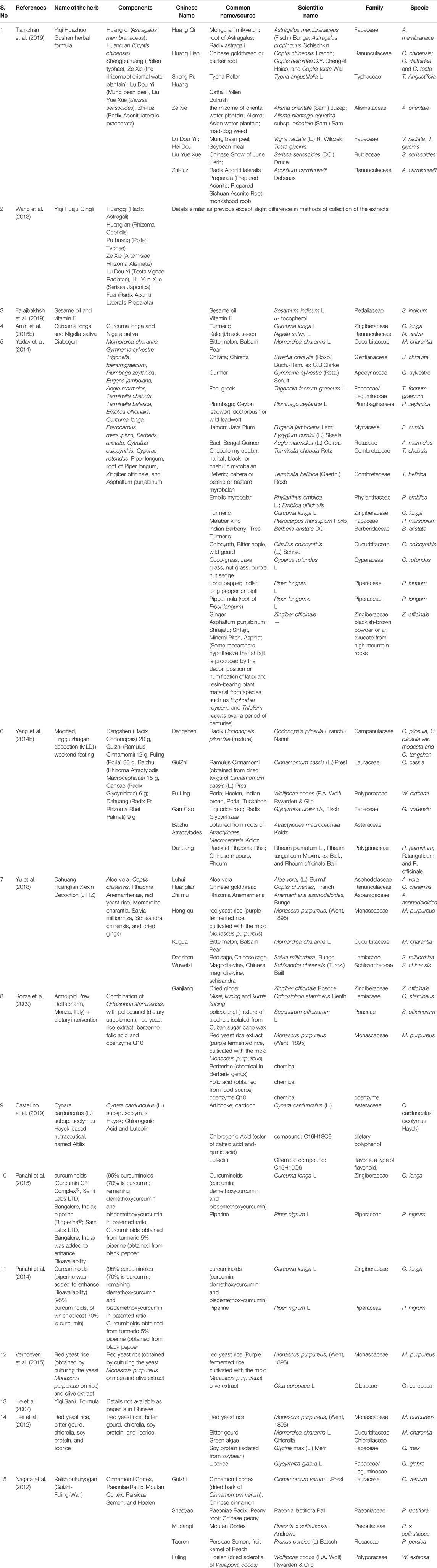
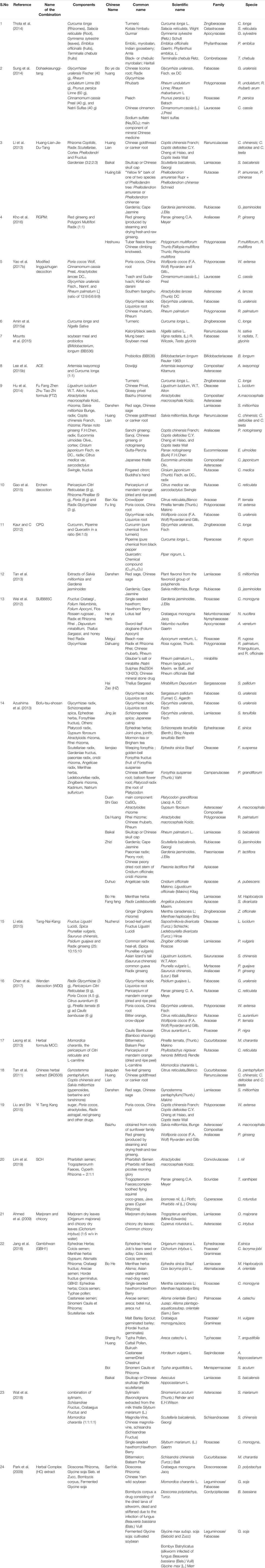
TABLE 2. Taxonomic classification of all the polyherbal combinations used in clinical studies against metabolic syndrome.
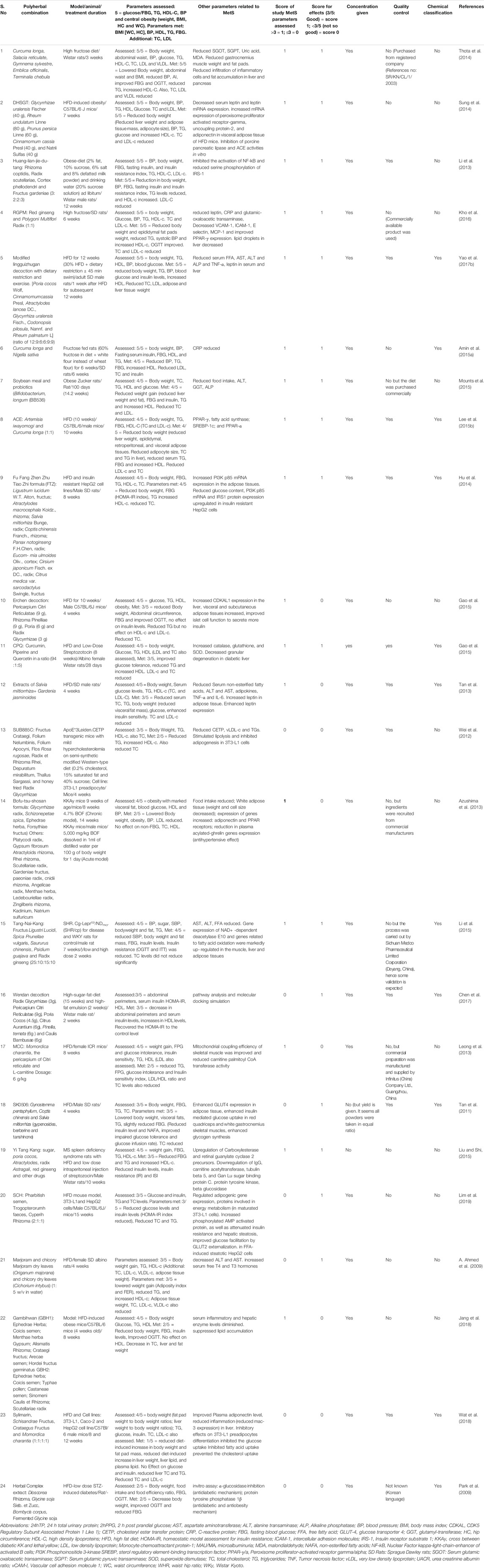
TABLE 3. Summary of metanalysis of Poly herbal combinations used in animal-based models of Metabolic syndrome.
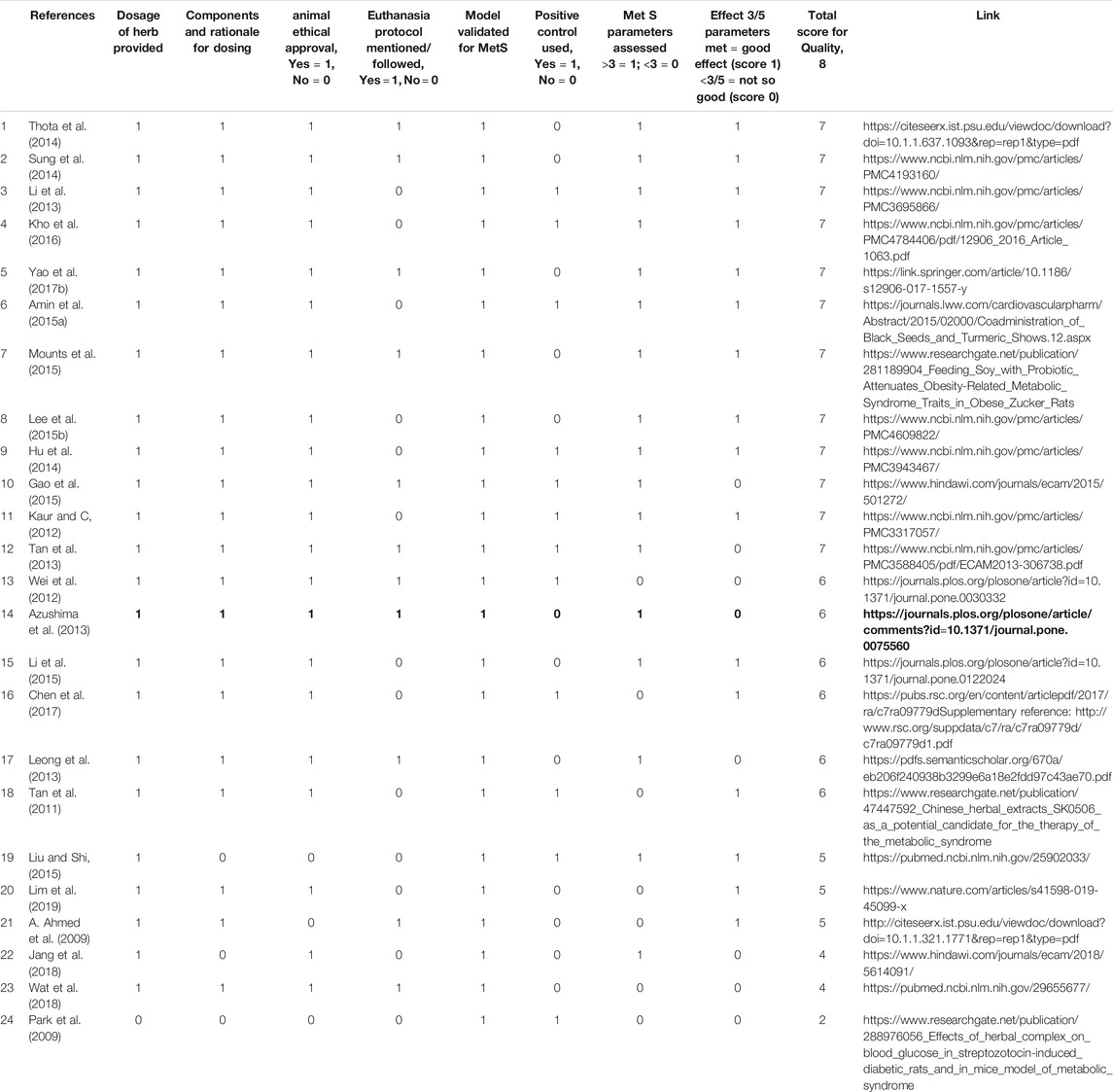
TABLE 4. Qualitative scoring of studies on polyherbal combinations used in animals of Metabolic Syndrome models.
Out of 26 clinical trial articles, 15 articles matched our main objective, and their meta-analysis is presented in Table 5 according to SPIDER model with references. Supplementary Table S1 is attached to shows the analysis by SPICE protocol along with information about other targets met besides the 5 parameters of MetS. Table 6 summarizes the qualitative scoring based on a checklist as mentioned in analysis section along with the online link available for the same. Out of 15 polyherbal combinations that were reviewed three formulations were able to modify 4 MetS parameters clinically. They include Yiqi Huazhuo Gushen herbal formula (Tian-zhan et al., 2019), Yiqi Huaju Qingli Formula (Wang et al., 2013), Sesame oil and vitamin E (Farajbakhsh et al., 2019). Six polyherbal combinations were able to reduce three out of 5 standard MetS parameters. The combinations included, Curcuma longa and Nigella sativa (Amin et al., 2015b), Diabegon (Yadav et al., 2014), modified Lingguizhugan decoction (MLD)+ weekend fasting (Yang et al., 2014a), Dahuang Huanglian Xiexin Decoction (Yu et al., 2018), combination of Nutraceuticals (Rozza et al., 2009) and Altilix supplement containing chlorogenic acid and luteolin (Castellino et al., 2019).
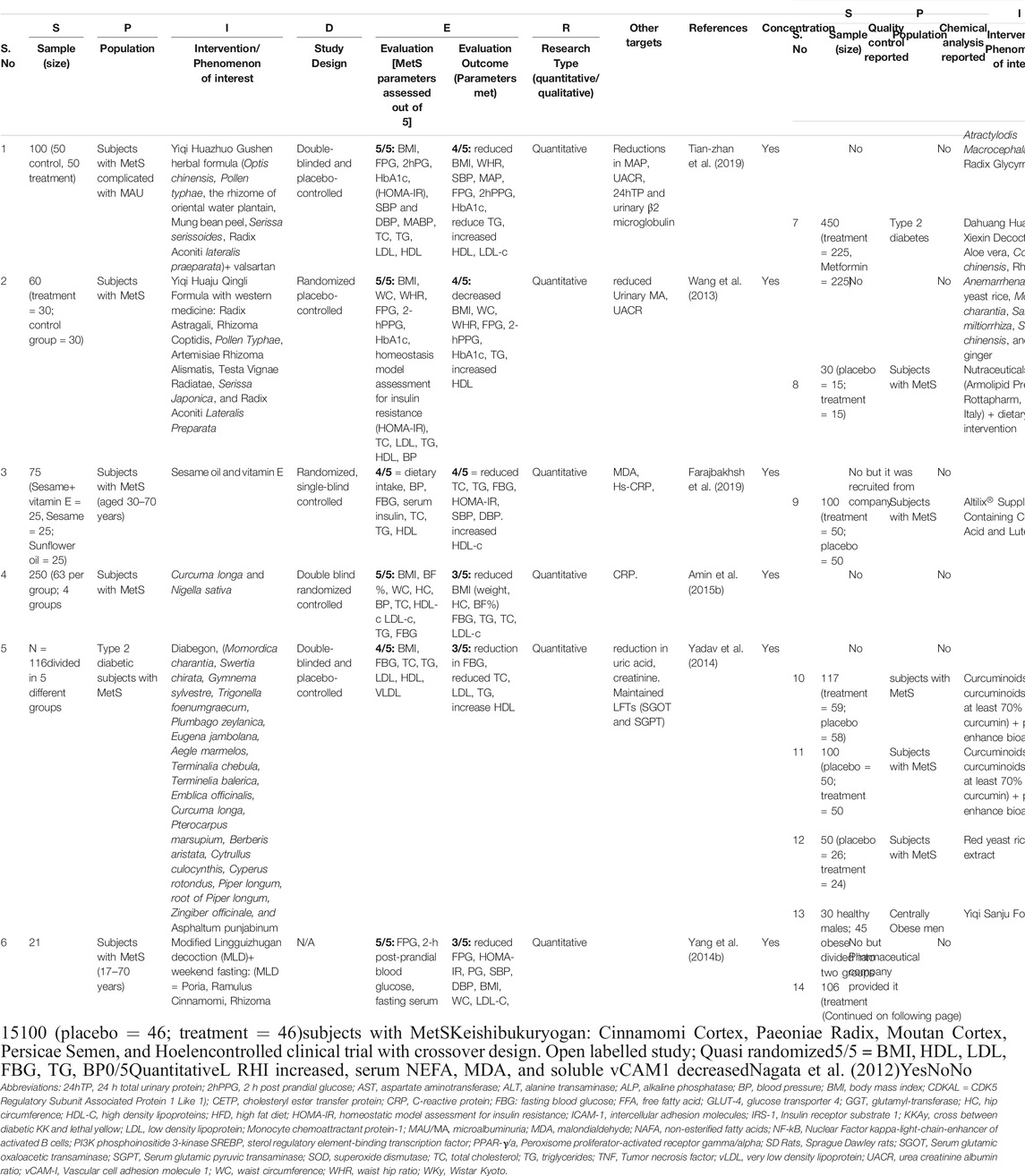
TABLE 5. Summary of meta-analysis of polyherbal combinations used in Clinical studies in patients with MetS according to SPIDER model, concentration, quality control and chemical classifications reports.
Discussion
MetS is a cluster of metabolic abnormalities that appear as a pre-diseased state and predisposes to CVD risk even before overt disease such as diabetes or hypertension develops. Catering those risk factors at this stage could prevent incidence of CVD. Hence, clinicians need to target multiple risk factors simultaneously. As the incidence of MetS is rising, there is a need to identify therapeutic modalities that could address multiple disease targets, offer better compliance, and reduce risk of adverse effects (Reilly and Rader, 2003; Keith et al., 2005). Polyherbal formulations could mutually enhance pharmacological synergy on the targeted disease and often exhibit pharmacological and therapeutic superiority in comparison to isolated single constituents.
The current review focuses on studies published from 2005–2020, reporting the efficacy of polyherbal therapies in MetS. This is attributed to either the action of bioactive ingredients from different herbs on the same molecular target forming a multiple-drug-one-target model (additive effect) and/or the functionally diverse targets but with potentially clinically relevant associations forming a multiple-drug-multiple-target-one-disease (synergistic effect) (Lu et al., 2012; Wang et al., 2012). In the current review, we identified 25 animal based studies in which polyherbal formulations were used in animal models of Mets. We categorised them as good and not very good, based on the modulation of MetS parameters. Studies which were able to modulate 4-5 parameters were considered as very effective, whereas studies that modulated three or less than 3 parameters were marked as not so good. This, however, does not reflect on the quality of review. For the quality of review, we devised an 8-question checklist and marked one point for meeting the criteria and 0 for no meeting the criteria. The overall score was 8.
From the effect point of view, different combinations were identified as very effective in animal based studies. They included combination of Curcuma longa, Salacia reticulate, Gymnema sylvestre, Emblica officinalis, Terminalia chebula (Thota et al., 2014), Glycyrrhizae uralensis Fischer, Rheum undulatum Linne, Prunus persica Linne, Cinnamomum cassia Presl and Natrii Sulfas (Sung et al., 2014), Rhizoma coptidis, Radix scutellariae, Cortex phellodendri and Fructus gardeniae (Li et al., 2013), Red ginseng and Polygoni Multiflori Radix (Kho et al., 2016) and modified lingguizhugan decoction (Yao et al., 2017a). These combinations modulated all the five parameters of MetS including reduction in body weight/obesity, BP, TG, and fasting blood glucose (FBG) and increase in HDL. Additionally, combination of soybean meal and probiotics (Bifidobacterium longum) (Mounts et al., 2015), Fu Fang Zhen Zhu Tiao Zhi formula (Hu et al., 2014), Curcuma Longa and Nigella Sativa (Amin et al., 2015a) and mixed extracts of Artemisia iwayomogi and Curcuma longa (Lee et al., 2015a) improved 4/5 MetS parameters and can be further considered for clinical trials.
These studies however exhibited certain limitations. For example, Lee et al. (2015a), comprehensively studied effect of Artemisia iwayomogi and Curcuma longa extract on metabolic markers along with fine mechanistic details but did nto use positive controls in their study. Similarly, Yao et al. (2017a) did not use positive controls in their study when studying effect of modified Lingguizhugan decoction (MLD) and only selected one dose for intervention. Hence, dose dependent effect couldn’t be assessed. Besides, they did not study the effect mediated by MLD alone and only showed results of MLD with dietary restriction and exercise; additional group of MLD should have been added for confidently claiming the effect of MLD in the study. Amin et al., presented their findings comprehensively about use of combined Curcuma longa and Nigella sativa in MetS models but despite of mention of measuring body weight fortnightly, there were no results about effect on body weight (Amin et al., 2015a).
Some studies showed reduced effect on Met S parameters, but their focus was more on mechanistic details. For instance, study by Gao et al. (2015) on effect of Erchen decoction (ECD) exhibited effect on 3 parameters of MetS including FBG, TG and body weight and abdominal circumference. One of the appreciable aspects of this study is that the researchers reported abdominal circumference and body weight simultaneously. Limited animal studies consider abdominal circumference, which is the actual predictor of MetS. Additionally, molecular mechanisms of ECD on diabetic parameters have been elaborated at genetic level, where expression of CDK5 regulatory subunit associated protein 1 like 1 (CDAK1) has been shown and correlated with improved islet cell function. Since this preparation did not have effect on LDL and HDL, combining it with antidyslipidemic herb, such as Curcuma longa and/or Nigella sativa coupled with low dose of ECD may be a good combination for future studies. Like this, extracts of Salvia miltiorrhiza and Gardenia jasminoides (Tan et al., 2013), showed effect on 3 parameters of Met S, but gave an elaborate mechanism for their antiobesity effect including enhanced leptin expression. Amongst the studies reported in this review, limited studies assessed BP (Thota et al., 2014; Amin et al., 2015a); whereas, most of them did not assess blood pressure in their models, and therefore the studies which have either met 3 or 4 out of 5 parameters of MetS are majorly the ones which did not assess BP in their animal models (Mounts et al., 2015). One of the reasons for this could be that BP monitoring in animals is technically challenging, and assessing it for number of animals, which usually are 40–50 altogether, is highly tedious and time consuming.
The other part of our review focussed on clinical trials in the last 15 years which used polyherbal formulations for the management of MetS. Amongst the combinations reviewed the most effective considered were the ones which met maximum MetS parameters. The maximum parameters modified were 4 out of 5 by 3 combinations including Yiqi Huazhuo Gushen herbal formula (Tian-zhan et al., 2019), Yiqi Huaju Qingli Formula (Wang et al., 2013), and Sesame oil and vitamin E combination (Farajbakhsh et al., 2019). However, these studies were assessed for short period of time ranging from 8 to 12 weeks, which may be helpful in determining the acute effect but not long-term effect and side-effects.
From this perspective a study by Yadav et al. (2014) is worth mentioning who studied the effects of herbal combination “Diabegon” till 1.5 years and monitored the effect on liver and kidney parameters, which showed no toxic effects on these organs. In fact, the combination reduced uric acid and effectively reduced FBG, TG and increased HDL, although BP was not monitored. Another worthy study in this regard was controlled clinical trial which used Keishibukuryogan, a traditional Japanese (Kampo) formula (Nagata et al., 2012) in MetS patients in a cross over design. Although, it did not reduce any MetS parameters, its main outcome was improvement in endothelial function which has a preventive role towards atherosclerosis. Such study designs should be adopted for formulas which have shown promising results in small scale studies.
Some studies design was flawed and therefore the effects could not be validated. For instance, study by Yang et al. (2014a) on MLD along with weekend fasting tested the combination on MetS patients but no comparative control was used. We could not determine whether the effect was due to MLD or weekend fasting. Aims of the study were also not clearly written in the write-up. Similarly, a combination of nutraceuticals with dietary interventions very efficiently reflected the improvement in MetS parameters to an extent that the patients no longer fulfilled the MetS criteria after treatment (10/15) (Rozza et al., 2009). Nevertheless, with such a small sample size, the magnitude of impact could not be extrapolated and needs to be studied further. Some clinical studies assessed only limited parameters of MetS and therefore in terms of effectiveness those combinations are considered as not so good. Nevertheless, that’s not completely true, because the authors did not measure the remaining parameters (He et al., 2007; Panahi et al., 2014; Panahi et al., 2015). Reason for this could be that the main objective of those studies was to explore additional mechanisms of MetS. For instance, Panahi et al., (Panahi et al., 2015) report curcuminoids to reduce 2 out of 5 MetS parameters because they assessed only BP and BMI. Their main finding was anti-inflammatory and antioxidant activities, whereas antidyslipidemic effect was reported in their preceding study (Panahi et al., 2014).
The current review has certain limitations. One of the factors to be considered for future reviews should be to differentiate the polyherbal combinations according to different ethnicities and cultures in which the herb is famously used such as Asian, Chinese and Japanese traditional medicine. The current review can be used by researchers for idnetifying different polyherbal combinations by considering which herbs could simultaneously target many or all risk factors for MetS. For future studies some known anti-obesity and/or antihypertensive herbs shall be considered as an add-on with those polyherbal combinations that predominantly exhibited anti-hyperglycaemic and anti-dyslipidemic effect, to be able to manage multiple MetS parameters simultaneously. This is one of the advantages of such reviews that researchers could identify the missing targets and add herb accordingly for future studies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
AG contributed to conception and along with AP and FA contributed in the design of the study. AP, FA, and AG organized the database and filtered the relevant articles. FA, BF, AS NR, and IH performed the analysis of their respective articles. AP wrote the first draft of the manuscript. FA, BF, AS, NR, and IH wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge Dr. Rizwan Khan, Dean Faculty of Computer Science, Salim Habib University for reflecting ideas about systematic research methodologies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.752926/full#supplementary-material
References
A. Ahmed, L., S. Ramadan, R., and A. Mohamed, R. (2009). Biochemical and Histopathological Studies on the Water Extracts of Marjoram and Chicory Herbs and Their Mixture in Obese Rats. Pakistan J. Nutr. 8, 1581–1587. doi:10.3923/pjn.2009.1581.1587
Alcántara, M., Serra-Aracil, X., Falcó, J., Mora, L., Bombardó, J., and Navarro, S. (2011). Prospective, Controlled, Randomized Study of Intraoperative Colonic Lavage versus Stent Placement in Obstructive Left-Sided Colonic Cancer. World J. Surg. 35 (8), 1904–1910. doi:10.1007/s00268-011-1139-y
Amin, F., Gilani, A. H., Mehmood, M. H., Siddiqui, B. S., and Khatoon, N. (2015). Coadministration of Black Seeds and Turmeric Shows Enhanced Efficacy in Preventing Metabolic Syndrome in Fructose-Fed Rats. J. Cardiovasc. Pharmacol. 65 (2), 176–183. doi:10.1097/FJC.0000000000000179
Amin, F., Islam, N., Anila, N., and Gilani, A. H. (2015). Clinical Efficacy of the Co-administration of Turmeric and Black Seeds (Kalongi) in Metabolic Syndrome - a Double Blind Randomized Controlled Trial - TAK-MetS Trial. Complement. Ther. Med. 23 (2), 165–174. doi:10.1016/j.ctim.2015.01.008
Anderson, J. G., and Taylor, A. G. (2012). Use of Complementary Therapies by Individuals with or at Risk for Cardiovascular Disease: Results of the 2007 National Health Interview Survey. J. Cardiovasc. Nurs. 27 (2), 96–102. doi:10.1097/JCN.0b013e31821888cd
AuH, Gilani. (1998). Novel Developments from Natural Products in Cardiovascular Research. Phytotherapy Research: Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Product. Derivatives 12 (S1), S66–S9.
Aziz, N., Mehmood, M. H., and Gilani, A. H. (2013). Studies on Two Polyherbal Formulations (ZPTO and ZTO) for Comparison of Their Antidyslipidemic, Antihypertensive and Endothelial Modulating Activities. BMC Complement. Altern. Med. 13 (1), 371. doi:10.1186/1472-6882-13-371
Azushima, K., Tamura, K., Wakui, H., Maeda, A., Ohsawa, M., Uneda, K., et al. (2013). Bofu-tsu-shosan, an oriental Herbal Medicine, Exerts a Combinatorial Favorable Metabolic Modulation Including Antihypertensive Effect on a Mouse Model of Human Metabolic Disorders with Visceral Obesity. PLoS ONE 8 (10), e75560. doi:10.1371/journal.pone.0075560
Bodeker, G., and Kronenberg, F. (2002). A Public Health Agenda for Traditional, Complementary, and Alternative Medicine. Am. J. Public Health 92 (10), 1582–1591. doi:10.2105/ajph.92.10.1582
Booth, A. (2006). Clear and Present Questions: Formulating Questions for Evidence Based Practice. Libr. hi tech 24 (3), 355–368. doi:10.1108/07378830610692127
Castellino, G., Nikolic, D., Magán-Fernández, A., Malfa, G. A., Chianetta, R., Patti, A. M., et al. (2019). Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 11 (11). doi:10.3390/nu11112580
Chen, M., Yang, F., Kang, J., Gan, H., Lai, X., and Gao, Y. (2017). Metabolomic Investigation into Molecular Mechanisms of a Clinical Herb Prescription against Metabolic Syndrome by a Systematic Approach. RSC Adv. 7 (87), 55389–55399. doi:10.1039/c7ra09779d
Cleyle, S., and Booth, A. (2006). Clear and Present Questions: Formulating Questions for Evidence Based Practice. Libr. hi tech. 24(3):355–368. doi:10.1108/07378830610692127
Cooke, A. D., Smith, D., and Booth, A. (2012). Beyond PICO: The SPIDER Tool for Qualitative Evidence Synthesis. Qual. Health Res. 22 (10), 1435–1443. doi:10.1177/1049732312452938
Devalaraja, S., Jain, S., and Yadav, H. (2011). Exotic Fruits as Therapeutic Complements for Diabetes, Obesity and Metabolic Syndrome. Food Res. Int. 44 (7), 1856–1865. doi:10.1016/j.foodres.2011.04.008
Farajbakhsh, A., Mazloomi, S. M., Mazidi, M., Rezaie, P., Akbarzadeh, M., Ahmad, S. P., et al. (2019). Sesame Oil and Vitamin E Co-administration May Improve Cardiometabolic Risk Factors in Patients with Metabolic Syndrome: a Randomized Clinical Trial. Eur. J. Clin. Nutr. 73 (10), 1403–1411. doi:10.1038/s41430-019-0438-5
Gao, B. Z., Chen, J. C., Liao, L. H., Xu, J. Q., Lin, X. F., and Ding, S. S. (2015). Erchen Decoction Prevents High-Fat Diet Induced Metabolic Disorders in C57BL/6 Mice. Evid. Based Complement. Alternat Med. 2015, 501272. doi:10.1155/2015/501272
Gilani, A. H., and Rahman, A. U. (2005). Trends in Ethnopharmocology. J. Ethnopharmacol 100 (1-2), 43–49. doi:10.1016/j.jep.2005.06.001
He, C. Y., Wang, W. J., Li, B., Xu, D. S., Chen, W. H., Ying, J., et al. (2007). Clinical Research of Yiqi Sanju Formula in Treating central Obese Men at High Risk of Metabolic Syndrome. Zhong Xi Yi Jie He Xue Bao 5 (3), 263–267. doi:10.3736/jcim20070307
Hu, X., Wang, M., Bei, W., Han, Z., and Guo, J. (2014). The Chinese Herbal Medicine FTZ Attenuates Insulin Resistance via IRS1 and PI3K In Vitro and in Rats with Metabolic Syndrome. J. Transl Med. 12, 47. doi:10.1186/1479-5876-12-47
Huang, P. L. (2009). A Comprehensive Definition for Metabolic Syndrome. Dis. Model. Mech. 2 (5-6), 231–237. doi:10.1242/dmm.001180
Jang, J-W., Lim, D-W., Chang, J-U., and Kim, J-E. (2018). The Combination of Ephedrae Herba and Coicis Semen in Gambihwan Attenuates Obesity and Metabolic Syndrome in High-Fat Diet–Induced Obese Mice. Evidence-Based Complement. Altern. Med. doi:10.1155/2018/5614091
Kaur, G., and C, M. (2012). Amelioration of Obesity, Glucose Intolerance, and Oxidative Stress in High-Fat Diet and Low-Dose Streptozotocin-Induced Diabetic Rats by Combination Consisting of "curcumin with Piperine and Quercetin". ISRN Pharmacol. 2012, 957283. doi:10.5402/2012/957283
Keith, C. T., Borisy, A. A., and Stockwell, B. R. (2005). Multicomponent Therapeutics for Networked Systems. Nat. Rev. Drug Discov. 4 (1), 71–78. doi:10.1038/nrd1609
Kho, M. C., Lee, Y. J., Park, J. H., Cha, J. D., Choi, K. M., Kang, D. G., et al. (2016). Combination with Red Ginseng and Polygoni Multiflori Ameliorates Highfructose Diet Induced Metabolic Syndrome. BMC Complement. Altern. Med. 16, 98. doi:10.1186/s12906-016-1063-7
Khowaja, L. A., Khuwaja, A. K., and Cosgrove, P. (2007). Cost of Diabetes Care in Out-Patient Clinics of Karachi, Pakistan. BMC Health Serv. Res. 7 (1), 189. doi:10.1186/1472-6963-7-189
Lee, I. T., Lee, W. J., Tsai, C. M., Su, I. J., Yen, H. T., and Sheu, W. H. (2012). Combined Extractives of Red Yeast rice, Bitter Gourd, Chlorella, Soy Protein, and Licorice Improve Total Cholesterol, Low-Density Lipoprotein Cholesterol, and Triglyceride in Subjects with Metabolic Syndrome. Nutr. Res. 32 (2), 85–92. doi:10.1016/j.nutres.2011.12.011
Lee, S-J., Han, J-M., Lee, J-S., Son, C-G., Im, H-J., Jo, H-K., et al. (2015). ACE Reduces Metabolic Abnormalities in a High-Fat Diet Mouse Model. Evidence-Based Complement. Altern. Med. 2015. doi:10.1155/2015/352647
Lee, S. J., Han, J. M., Lee, J. S., Son, C. G., Im, H. J., Jo, H. K., et al. (2015). ACE Reduces Metabolic Abnormalities in a High-Fat Diet Mouse Model. Evid Based. Complement. Altern. Med. doi:10.1155/2015/352647
Leong, P. K., Leung, H. Y., Wong, H. S., Chen, J., Ma, C. W., and Yang, Y. (2013). Long-term Treatment with an Herbal Formula MCC Reduces the Weight Gain in High Fat Diet-Induced Obese Mice. Chin. Med. 04 (03), 63–71. doi:10.4236/cm.2013.43010
Li, C. B., Li, X. X., Chen, Y. G., Gao, H. Q., Bu, P. L., Zhang, Y., et al. (2013). Huang-Lian-Jie-Du-Tang Protects Rats from Cardiac Damages Induced by Metabolic Disorder by Improving Inflammation-Mediated Insulin Resistance. PLoS ONE 8 (6), e67530. doi:10.1371/journal.pone.0067530
Li, L., Yoshitomi, H., Wei, Y., Qin, L., Zhou, J., Xu, T., et al. (2015). Tang-Nai-Kang Alleviates Pre-diabetes and Metabolic Disorders and Induces a Gene Expression Switch toward Fatty Acid Oxidation in SHR.Cg-Leprcp/NDmcr Rats. PLoS ONE 10 (4), e0122024. doi:10.1371/journal.pone.0122024
Lim, D. W., Kim, H., Kim, Y. M., Chin, Y. W., Park, W. H., and Kim, J. E. (2019). Drug Repurposing in Alternative Medicine: Herbal Digestive Sochehwan Exerts Multifaceted Effects against Metabolic Syndrome. Sci. Rep. 9, 9055. doi:10.1038/s41598-019-45099-x
Liu, X. X., and Shi, Y. (2015). Intervention Effect of Traditional Chinese Medicine Yi Tang Kang on Metabolic Syndrome of Spleen Deficiency. Asian Pac. J. Trop. Med. 8 (2), 162–168. doi:10.1016/S1995-7645(14)60309-6
Lu, J. J., Pan, W., Hu, Y. J., and Wang, Y. T. (2012). Multi-target Drugs: the Trend of Drug Research and Development. PLoS ONE 7 (6), e40262. doi:10.1371/journal.pone.0040262
Ma, X. H., Zheng, C. J., Han, L. Y., Xie, B., Jia, J., Cao, Z. W., et al. (2009). Synergistic Therapeutic Actions of Herbal Ingredients and Their Mechanisms from Molecular Interaction and Network Perspectives. Drug Discov. Today 14 (11-12), 579–588. doi:10.1016/j.drudis.2009.03.012
Maraolo, A. E. (2021). Una bussola per le revisioni sistematiche: la versione italiana della nuova edizione del PRISMA statement. BMJ 372, n71.
Mohamed, S. (2014). Functional Foods against Metabolic Syndrome (Obesity, Diabetes, Hypertension and Dyslipidemia) and Cardiovasular Disease. Trends Food Sci. Techn. 35 (2), 114–128. doi:10.1016/j.tifs.2013.11.001
Mounts, L., Sunkara, R., Shackelford, L., Ogutu, S., T. Walker, L., and Verghese, M. (2015). Feeding Soy with Probiotic Attenuates Obesity-Related Metabolic Syndrome Traits in Obese Zucker Rats. Fns 06 (09), 780–789. doi:10.4236/fns.2015.69081
Nagata, Y., Goto, H., Hikiami, H., Nogami, T., Fujimoto, M., Shibahara, N., et al. (2012). Effect of Keishibukuryogan on Endothelial Function in Patients with at Least One Component of the Diagnostic Criteria for Metabolic Syndrome: a Controlled Clinical Trial with Crossover Design. Evid. Based Complement. Alternat Med. 2012, 359282. doi:10.1155/2012/359282
Ni, Y., Mu, C., He, X., Zheng, K., Guo, H., and Zhu, W. (2018). Characteristics of Gut Microbiota and its Response to a Chinese Herbal Formula in Elder Patients with Metabolic Syndrome. Drug Discov. Ther. 12 (3), 161–169. doi:10.5582/ddt.2018.01036
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T., Mulrow, C. D., et al. (2019). Research Repository.
Panahi, Y., Hosseini, M. S., Khalili, N., Naimi, E., Majeed, M., and Sahebkar, A. (2015). Antioxidant and Anti-inflammatory Effects of Curcuminoid-Piperine Combination in Subjects with Metabolic Syndrome: a Randomized Controlled Trial and an Updated Meta-Analysis. Clin. Nutr. 34 (6), 1101–1108. doi:10.1016/j.clnu.2014.12.019
Panahi, Y., Khalili, N., Hosseini, M. S., Abbasinazari, M., and Sahebkar, A. (2014). Lipid-modifying Effects of Adjunctive Therapy with Curcuminoids-Piperine Combination in Patients with Metabolic Syndrome: Results of a Randomized Controlled Trial. Complement. Ther. Med. 22 (5), 851–857. doi:10.1016/j.ctim.2014.07.006
Park, H-S., Lee, Y-S., Choi, S-J., Kim, J-K., Lee, Y-L., Kim, H-G., et al. (2009). Effects of Herbal Complex on Blood Glucose in Streptozotocin-Induced Diabetic Rats and in Mice Model of Metabolic Syndrome. Korean J. Pharmacognosy 40 (3), 196–204.
Reilly, M. P., and Rader, D. J. (2003). The Metabolic Syndrome: More Than the Sum of its Parts. Circulation 108 (13), 1546–1551. doi:10.1161/01.CIR.0000088846.10655.E0
Rhee, M. K., Herrick, K., Ziemer, D. C., Vaccarino, V., Weintraub, W. S., Narayan, K. M., et al. (2010). Many Americans Have Pre-diabetes and Should Be Considered for Metformin Therapy. Diabetes care 33 (1), 49–54. doi:10.2337/dc09-0341
Robinson, J. G., Ballantyne, C. M., Hsueh, W. A., Rosen, J. B., Lin, J., Shah, A. K., et al. (2013). Age, Abdominal Obesity, and Baseline High-Sensitivity C-Reactive Protein Are Associated with Low-Density Lipoprotein Cholesterol, Non-high-density Lipoprotein Cholesterol, and Apolipoprotein B Responses to Ezetimibe/simvastatin and Atorvastatin in Patients with Metabolic Syndrome. J. Clin. Lipidol. 7 (4), 292–303. doi:10.1016/j.jacl.2013.03.007
Rozza, F., de Simone, G., Izzo, R., De Luca, N., and Trimarco, B. (2009). Nutraceuticals for Treatment of High Blood Pressure Values in Patients with Metabolic Syndrome. High Blood Press. Cardiovasc. Prev. 16 (4), 177–182. doi:10.2165/11530420-000000000-00000
Samir, N., Mahmud, S., and Khuwaja, A. K. (2011). Prevalence of Physical Inactivity and Barriers to Physical Activity Among Obese Attendants at a Community Health-Care center in Karachi, Pakistan. BMC Res. Notes 4 (1), 174. doi:10.1186/1756-0500-4-174
Sever, P. S., Dahlöf, B., Poulter, N. R., Wedel, H., Beevers, G., Caulfield, M., et al. (2003). Prevention of Coronary and Stroke Events with Atorvastatin in Hypertensive Patients Who Have Average or lower-Than-average Cholesterol Concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a Multicentre Randomised Controlled Trial. Lancet 361 (9364), 1149–1158. doi:10.1016/S0140-6736(03)12948-0
Su, D., and Li, L. (2011). Trends in the Use of Complementary and Alternative Medicine in the United States: 2002-2007. J. Health Care Poor Underserved 22 (1), 296–310. doi:10.1353/hpu.2011.0002
Sung, Y. Y., Kim, D. S., Choi, G., Kim, S. H., and Kim, H. K. (2014). Dohaekseunggi-tang Extract Inhibits Obesity, Hyperlipidemia, and Hypertension in High-Fat Diet-Induced Obese Mice. BMC Complement. Altern. Med. 14 (1), 372. doi:10.1186/1472-6882-14-372
Tan, Y., Kamal, M. A., Wang, Z. Z., Xiao, W., Seale, J. P., and Qu, X. (2011). Chinese Herbal Extracts (SK0506) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Clin. Sci. (Lond) 120 (7), 297–305. doi:10.1042/CS20100441
Tan, Y., Lao, W., Xiao, L., Wang, Z., Xiao, W., Kamal, M. A., et al. (2013). Managing the Combination of Nonalcoholic Fatty Liver Disease and Metabolic Syndrome with Chinese Herbal Extracts in High-Fat-Diet Fed Rats. Evid Based. Complement. Altern. Med. doi:10.1155/2013/306738
Thota, R. N., Paruchuru, D., Naik, R., Metlakunta, A. S., Benarjee, G., and Puchakayala, G. (2014). Effect of Polyherbal Formulation on Metabolic Derangements in Experimental Model of High Fructose Diet Induced Metabolic Syndrome. Int. J. Appl. Biol. Pharm. Techn. 5 (3).
Tian-zhan, W., Qing-ping, H., Bing, W., Wen-jian, W., Xiaodong, F., Yan-ming, H., et al. (2019). Synergistic Effects of Yiqi Huazhuo Gushen Herbal Formula and Valsartan on Metabolic Syndrome Complicated with Microalbuminuria. Trop. J. Pharm. Res. 18 (1), 101–108. doi:10.4314/tjpr.v18i1.15
Verhoeven, V., Van der Auwera, A., Van Gaal, L., Remmen, R., Apers, S., Stalpaert, M., et al. (2015). Can Red Yeast rice and Olive Extract Improve Lipid Profile and Cardiovascular Risk in Metabolic Syndrome?: a Double Blind, Placebo Controlled Randomized Trial. BMC Complement. Altern. Med. 15 (1), 52–58. doi:10.1186/s12906-015-0576-9
Wang, T. Z., Chen, Y., He, Y. M., Fu, X. D., Wang, Y., Xu, Y. Q., et al. (2013). Effects of Chinese Herbal Medicine Yiqi Huaju Qingli Formula in Metabolic Syndrome Patients with Microalbuminuria: a Randomized Placebo-Controlled Trial. J. Integr. Med. 11 (3), 175–183. doi:10.3736/jintegrmed2013032
Wang, Y., Liu, Z., Li, C., Li, D., Ouyang, Y., Yu, J., et al. (2012). Drug Target Prediction Based on the Herbs Components: the Study on the Multitargets Pharmacological Mechanism of Qishenkeli Acting on the Coronary Heart Disease. Evidence-Based Complement. Altern. Med. doi:10.1155/2012/698531
Wat, E., Wang, Y., Chan, K., Law, H. W., Koon, C. M., Lau, K. M., et al. (2018). An In Vitro and In Vivo Study of a 4-herb Formula on the Management of Diet-Induced Metabolic Syndrome. Phytomedicine 42, 112–125. doi:10.1016/j.phymed.2018.03.028
Wei, H., Hu, C., Wang, M., van den Hoek, A. M., Reijmers, T. H., Wopereis, S., et al. (2012). Lipidomics Reveals Multiple Pathway Effects of a Multi-Components Preparation on Lipid Biochemistry in ApoE*3Leiden.CETP Mice. PLoS ONE 7 (1), e30332. doi:10.1371/journal.pone.0030332
Yadav, D., Tiwari, A., Mishra, M., Subramanian, S. S., Baghel, U. S., Mahajan, S., et al. (2014). Anti-hyperglycemic and Anti-hyperlipidemic Potential of a Polyherbal Preparation "Diabegon" in Metabolic Syndrome Subject with Type 2 Diabetes. Afr. J. Tradit Complement. Altern. Med. 11 (2), 249–256. doi:10.4314/ajtcam.v11i2.4
Yang, Y., Li, Q., Chen, S., Ke, B., Huang, Y., and Qin, J. (2014). Effects of Modified Lingguizhugan Decoction Combined with Weekend Fasting on Metabolic Syndrome. J. Tradit Chin. Med. 34 (1), 48–51. doi:10.1016/s0254-6272(14)60053-4
Yang, Y., Li, Q., Chen, S., Ke, B., Huang, Y., and Qin, J. (2014). Effects of Modified Lingguizhugan Decoction Combined with Weekend Fasting on Metabolic Syndrome. J. Tradit Chin. Med. 34 (1), 48–51. doi:10.1016/s0254-6272(14)60053-4
Yao, L., Wei, J., Shi, S., Guo, K., Wang, X., Wang, Q., et al. (2017). Modified Lingguizhugan Decoction Incorporated with Dietary Restriction and Exercise Ameliorates Hyperglycemia, Hyperlipidemia and Hypertension in a Rat Model of the Metabolic Syndrome. BMC Complement. Altern. Med. 17 (1), 132. doi:10.1186/s12906-017-1557-y
Yao, L., Wei, J., Shi, S., Guo, K., Wang, X., Wang, Q., et al. (2017). Modified Lingguizhugan Decoction Incorporated with Dietary Restriction and Exercise Ameliorates Hyperglycemia, Hyperlipidemia and Hypertension in a Rat Model of the Metabolic Syndrome. BMC Complement. Altern. Med. 17, 132. doi:10.1186/s12906-017-1557-y
Yu, X., Xu, L., Zhou, Q., Wu, S., Tian, J., Piao, C., et al. (2018). The Efficacy and Safety of the Chinese Herbal Formula, JTTZ, for the Treatment of Type 2 Diabetes with Obesity and Hyperlipidemia: a Multicenter Randomized, Positive-Controlled, Open-Label Clinical Trial. Int. J. Endocrinol. 2018, 9519231. doi:10.1155/2018/9519231
Keywords: obesity, natural products, clinical trials, animal models, polyherbal
Citation: Palla AH, Amin F, Fatima B, Shafiq A, Rehman NU, Haq Iu and Gilani A-u-H (2021) Systematic Review of Polyherbal Combinations Used in Metabolic Syndrome. Front. Pharmacol. 12:752926. doi: 10.3389/fphar.2021.752926
Received: 03 August 2021; Accepted: 20 September 2021;
Published: 07 October 2021.
Edited by:
Mahendra Rai, Sant Gadge Baba Amravati University, IndiaReviewed by:
Claudio Ferrante, University of Studies G. d’Annunzio Chieti and Pescara, ItalyLuigi Brunetti, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Copyright © 2021 Palla, Amin, Fatima, Shafiq, Rehman, Haq and Gilani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amber Hanif Palla, YW1iZXJwYWxsYUB5YWhvby5jb20=; Anwar-ul-Hassan Gilani, dmNAdW9oLmVkdS5waw==, YW53YXJoZ2lsYW5pQHlhaG9vLmNvbQ==
 Amber Hanif Palla
Amber Hanif Palla Faridah Amin2
Faridah Amin2 Najeeb Ur Rehman
Najeeb Ur Rehman Anwar-ul-Hassan Gilani
Anwar-ul-Hassan Gilani