
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 04 October 2021
Sec. Neuropharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.750397
This article is part of the Research Topic Stimulant Use and Addictive Disorder View all 22 articles
 Shiqiu Meng1†
Shiqiu Meng1† Wei Yan2†
Wei Yan2† Xiaoxing Liu2†
Xiaoxing Liu2† Yimiao Gong2
Yimiao Gong2 Shanshan Tian2
Shanshan Tian2 Ping Wu1
Ping Wu1 Yan Sun1
Yan Sun1 Jie Shi1
Jie Shi1 Lin Lu1,2
Lin Lu1,2 Kai Yuan2*
Kai Yuan2* Yanxue Xue1,3*
Yanxue Xue1,3*Social factors strongly contribute to drug use and relapse, and epidemiological studies have found that members of peer groups influence each other to use drugs. However, previous animal models mostly failed to incorporate social factors and demonstrate the effects of social partners on drug addiction and relapse. In the present study, we investigated the transfer of relapse to cocaine seeking between drug-addicted partners in rats. Male Sprague–Dawley rats were pair-housed and subjected to training and extinction of cocaine self-administration and conditioned place preference (CPP). 24 h after extinction test, the targeted rats interacted with a cocaine-primed (relapsed) partner or stranger, or saline-injected (unrelapsed) partner for 30 min, after which the targeted rats were tested for drug seeking behavior. We found that social interaction with a relapsed partner increased drug seeking behavior in cocaine self-administration and CPP models in rats, while social interaction with an unrelapsed partner or relapsed stranger had no effect on cocaine seeking. Moreover, the effect of social interaction on cocaine seeking could last for at least 1 day. Our findings demonstrate a facilitation effect of relapsed social partners on drug relapse in rats and provide a novel animal model for social transfer of drug relapse.
Drug addiction is a chronic recurrent disease bringing heavy burden to individuals, families, and society (Volkow and Boyle, 2018), and is characterized by high rates of relapse even after treatment (Sinha, 2011). Social and environmental factors are acknowledged as determinants of drug use and relapse, and among various factors, social partners may be critical (Bahr et al., 2005; Simons-Morton and Chen, 2006). Epidemiological investigations have demonstrated that people easily become addicts if their friends are addicted to drugs (Walden et al., 2004; Bahr et al., 2005; Simons-Morton and Chen, 2006). Some clinical research indicates that social interaction with alcohol-addicted partners not only accelerated acquisition of addiction, but also enhanced alcohol tolerance (Caudill and Marlatt, 1975; Lied and Marlatt, 1979; Caudill and Kong, 2001; Larsen et al., 2010; Kirkpatrick and de Wit, 2013), demonstrating that social interaction with addicted partners may play a facilitation effect on addiction. Having drug-using friends has also been found to increase risks of heroin relapse in patients under methadone maintenance treatment (Li et al., 2012). On the contrary, joining recovery communities like Alcoholics Anonymous (Kelly et al., 2012; Frings et al., 2021) and interacting with non-addicted peers to change their social network (Bathish et al., 2017) promotes recovery from addiction. However, due to ethical reasons, human studies on impact of social partners on illicit drug addiction are limited.
Animal models are critical to reveal the neural mechanisms of drug addiction and help clinicians to develop potential treatments, whereas only a few studies have incorporated social factors and demonstrated the effects of social partners on drug addiction. For example, Smith et al. (2015) have found that self-administration behavior of rats is promoted when they are reared with a partner and both trained with cocaine self-administration, while the addictive behavior decreases when the partners have no access to cocaine. Compared with peers who did not experience cocaine self-administration training, rats showed more preference to partners with whom they were trained for self-administration together (Smith and Pitts, 2014), especially those who took similar drugs (Smith et al., 2015). Similar results were also found in the conditioned place preference (CPP) model (Larsen et al., 2010; Koordeman et al., 2011). The above findings suggest that social interaction with addicted partners affects the acquisition and maintenance of addiction. However, the impact of social partners on addiction relapse needs further study.
Numerous studies have demonstrated that after abstinence or extinction, drug craving can still be triggered once the animals are exposed to drug or drug-related cues (e.g., sound and light) (Davachi, 2006; Brandon et al., 2007; Goldfarb and Sinha, 2018). Thus, we speculate that drug craving may also be prompted by social interaction with relapsed partners. In the present study, we established an animal model of social transfer of relapse and investigated the effect of social interaction on cocaine seeking behavior after extinction.
Experiment 1Effects of social interaction with relapsed partner. Rats were pair-housed and subjected to 10 days cocaine self-administration training, followed by extinction. 24 h after extinction test, one of the two rats (relapsed partner) was primed with cocaine (10 mg/kg, i.p.), and subjected to 1 h reinstatement test. Next, the relapsed partner was put back to their homecage, and interacted with the other rat (targeted rat) for 30 min. Then the targeted rat was immediately subjected to 1 h reinstatement test.
Experiment 2Effects of social interaction with unrelapsed partner. Rats were pair-housed and subjected to cocaine self-administration training and extinction. 24 h after extinction test, one of the two rats (unrelapsed partner) was injected with saline (1 ml/kg, i.p.), and subjected to reinstatement test. Next, the unrelapsed partner was put back to their homecage, and interacted with the other rat (targeted rat) for 30 min. Then the targeted rat was subjected to reinstatement test immediately.
Experiment 3Effects of social interaction with relapsed partner for different time. Rats were pair-housed and subjected to cocaine self-administration training and extinction. 24 h after extinction test, one of the two rats (relapsed partner) was primed with cocaine (10 mg/kg, i.p.), and subjected to reinstatement test. Next, the other rat (targeted rat) interacted with the relapsed partner in their homecage for 0, 10, 30, or 60 min, followed by reinstatement test immediately.
Experiment 4The maintenance of facilitation effect after 30 min social interaction with the relapsed partner. Rats were pair-housed and subjected to cocaine self-administration training and extinction. 24 h after extinction test, one of the two rats (relapsed partner) was primed with cocaine (10 mg/kg, i.p.), and subjected to reinstatement test. Next, the other rat (targeted rat) interacted with the relapsed partner in their homecage for 30 min, and subjected to reinstatement test immediately (0 min), or 1–7 days later.
Experiment 5Effects of social interaction with relapsed stranger. Rats were pair-housed and subjected to cocaine self-administration training and extinction. 24 h after extinction test, a rat kept in another cage (relapsed stranger) was injected with a single dose of cocaine (10 mg/kg, i.p.) and subjected to reinstatement test. Then the relapsed stranger was put into the homecage of the targeted rat (its partner was removed from the cage) for 30 min social interaction, and the targeted rat was subjected to reinstatement test immediately.
Experiment 6Effects of social interaction on relapse to cocaine-induced CPP. Rats were pair-housed and subjected to the baseline test. After CPP training and extinction, the rats were subjected to the extinction test. 24 h later, the targeted rats interacted with relapsed partners, unrelapsed partners, or relapsed strangers for 30 min in homecages, and then were subjected to reinstatement test immediately.
We used adult male Sprague–Dawley (SD) rats (280–300 g upon arrival) purchased from Vital River Laboratories. The rats were pair-housed in an animal facility with appropriate temperature (22 ± 2°C) and humidity (50 ± 10%), as well as freely accessible water and food. The lighting time was controlled, under a 12 h light/dark circle. All behavioral experiments were performed under the dark circle and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the Biomedical Ethics Committee for Animal Use and Protection of Peking University.
Rats were under anesthetic through pentobarbital sodium (20 mg/ml, 60 mg/kg, i.p.) and received catheters insertion into the right jugular vein with the tip reaching the opening of right atrium. After the surgery, rats were recovered for 7 days with penicillin administration (resolved in 0.2% heparin sodium) every day, preventing infection and cannula blocking.
Cocaine-HCl was purchased from the Qinghai Pharmaceutical Factory and resolved in 0.9% saline (5 mg/ml).
The training procedure was based on our previous studies (Xue et al., 2012; Luo et al., 2015). The chambers (AniLab Software and Instruments) were equipped with two nosepoke holes 9 cm above the underside, one was active-nosepoke hole and the other was inactive-nosepoke hole. The two rats in each cage were trained at the same time in different chambers for fixed ratio 1 (FR1) cocaine self-administration training for 10 days during three 1 h sessions per day with 5 min intervals. Every session started from the illumination of a house light. Poking to the active-nosepoke hole led to a cocaine infusion (0.75 mg/kg), accompanied with a 5 s tone-light cue, while poking to the inactive-nosepoke hole did not result in cocaine infusion or tone-light cue. There was a 40 s time-out phase between each infusion, after which the house light would turn on again. The number and time of active nosepokes, inactive nosepokes, and infusions were recorded. To prevent rats administrating overdose of cocaine, the number of infusions was limited to 20 times in each session. After training, the rats were returned to their homecages.
The two rats in each cage were subjected to extinction at the same time in different chambers. During the extinction sessions, the conditions were the same as those during the self-administration training. But there was no cocaine infusion after rats poked the active-nosepoke hole. At the end of extinction every day, they were put back in their homecages. The extinction was performed until the number of active-nosepokes decreased to below 20% of the mean nosepokes during the last 3 days of self-administration training for at least two consecutive days. 24 h later, the rats were subjected to test for drug seeking (extinction test).
The procedure was based on our previous studies (Xue et al., 2012). Three-chamber apparatuses were used, and time the rats spent in each chamber was recorded. The two rats in each cage were trained at the same time in different apparatuses. For the baseline test (day 1), there were no partitions among the chambers. Rats were put into the middle chamber and allowed to move freely for 15 min. Rats that presented a preference for one of the boxes (resistance time >540 s) were ruled out. Then the rats were trained for cocaine-induced CPP for 8 days. Rats received intraperitoneal cocaine (10 mg/kg, day 2/4/6/8) or saline (1 ml/kg, day 3/5/7/9) injections alternatively and were confined to the conditioning chambers for 45 min after injection. The rats were returned to their homecages after training every day. 24 h after the last-day training (day 10), drug seeking test (training test) was performed.
The two rats in each cage were subjected to extinction at the same time in different chambers. The conditions for extinction were the same as training except that no injections were given. At the end of extinction every day, they were put back in their homecages. After 8 days of extinction, rats were subjected to drug seeking test (extinction test).
For cocaine priming or saline injection, the rats were intraperitoneally injected with 10 mg/kg cocaine or 1 ml/kg saline, and then they were delivered to drug seeking test (reinstatement test).
To investigate the effect of social interaction with a relapsed partner, one rat in each cage was intraperitoneally injected with cocaine (10 mg/kg), and tested for drug seeking. Next, it was put back to its homecage as a relapsed partner, and interacted with the other rat (targeted rat) for 0, 10, 30, or 60 min.
To test the effect of social interaction with an unrelapsed partner, saline (1 ml/kg) was intraperitoneally injected to one rat in each cage, and then drug seeking was tested. Then it was put back to its homecage as an unrelapsed partner, and interacted with the other rat (targeted rat) for 30 min.
To investigate the effect of social interaction with a relapsed stranger, one rat in each cage was intraperitoneally injected with cocaine (10 mg/kg) and tested for drug seeking. Then it was put back to another cage as a relapsed stranger, and interacted with the rat (targeted rat) in this cage for 30 min. The partner of the targeted rat was removed from their homecage in advance.
After social interaction, the targeted rats were subjected to drug seeking test (reinstatement test).
The conditions during the drug seeking tests in the self-administration model were the same as those during the extinction sessions, and the tests lasted for 1 h.
The conditions during the drug seeking tests in the CPP model were the same as those during the baseline test, and the tests lasted for 15 min. The time spent in the cocaine-paired chamber minus the time spent in the saline-paired chamber was calculated as the CPP score.
All of the statistical analyses were performed using SPSS 20.0 software (SPSS, Chicago, IL, United States). The data were expressed as mean ± SEM, and analyzed by repeated measures analysis of variance (ANOVA) with appropriate within-group factors for each experiment (see Results), followed by least significant difference (LSD) post hoc tests in Experiment 6. Values of p < 0.05 were considered statistically significant.
First, we investigated the effect of social interaction on cocaine seeking behavior in the self-administration model. We first explored whether social interaction with a relapsed partner would induce relapse (Figures 1A–C). The repeated measures ANOVA with the within-subjects factors (extinction test and reinstatement test) showed that cocaine injection increased the number of active-nosepokes (F1,10 = 6.670, p < 0.05), and had no effect on the number of inactive-nosepokes (p > 0.05) in the reinstatement test, indicating that cocaine priming induced reinstatement of drug seeking (Figure 1D). In the targeted rats group, the repeated measures ANOVA of nosepokes with the within-subjects factors (extinction test and reinstatement test) revealed that the number of active-nosepokes was increased after social interaction with relapsed partner (F1,10 = 17.862, p < 0.01), and inactive-nosepokes had no change (p > 0.05) in the reinstatement test, suggesting that social interaction with relapsed partner resulted in relapse of cocaine seeking (Figure 1E).
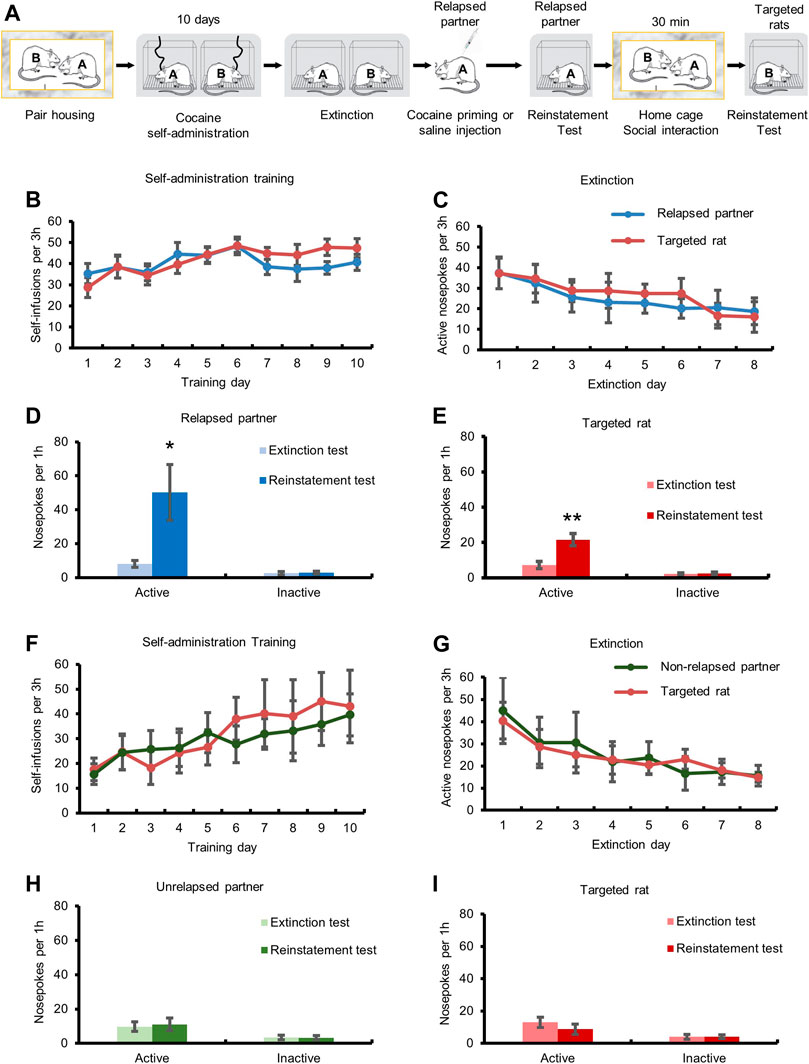
FIGURE 1. Social interaction with relapsed partner induced relapse to cocaine seeking behavior. (A) Experimental timeline. (B,F) Targeted rats and partners acquired cocaine self-administration training. (C,G) Drug-seeking behavior of targeted rats and partners was extinguished. (D) Cocaine injection triggered drug seeking behavior. (E) Drug-seeking behavior of targeted rats was increased after social interaction with relapsed partner (Data are shown as mean ± SEM. **p < 0.01, compared with extinction test. n = 11). (H) Saline injection had no effect on drug seeking behavior. (I) Drug-seeking behavior of targeted rats had no change after social interaction with unrelapsed partner (Data are shown as mean ± SEM. n = 7).
We also assessed the effect of social interaction with an unrelapsed partner on cocaine seeking (Figures 1A,F,G). The repeated measures ANOVA showed that the number of active-nosepokes had no significant change (p > 0.05) after saline injection in the reinstatement test, indicating that saline injection did not induce cocaine relapse (Figure 1H). The repeated measures ANOVA of the number of active-nosepokes of the targeted rats showed no significant change (both p > 0.05) after social interaction with unrelapsed partner, indicating that social interaction with unrelapsed partner did not induce relapse of cocaine seeking.
We next explored the effect of different interaction time on the relapse to cocaine seeking, and targeted rats interacted with relapsed partner for 0, 10, 30, or 60 min (Figure 2A). The repeated measures ANOVA showed that the numbers of active-nosepokes of the targeted rats were elevated after 30 min (F1,7 = 9.171, p < 0.05) and 60 min (F1,6 = 7.088, p < 0.05) social interaction with the relapsed partner, but not after 0 min (no social interaction) or 10 min social interaction (Figure 2B, both p > 0.05). The above findings indicated that social interaction required a certain amount of time (no less than 30 min) to produce the facilitation effect on relapse.
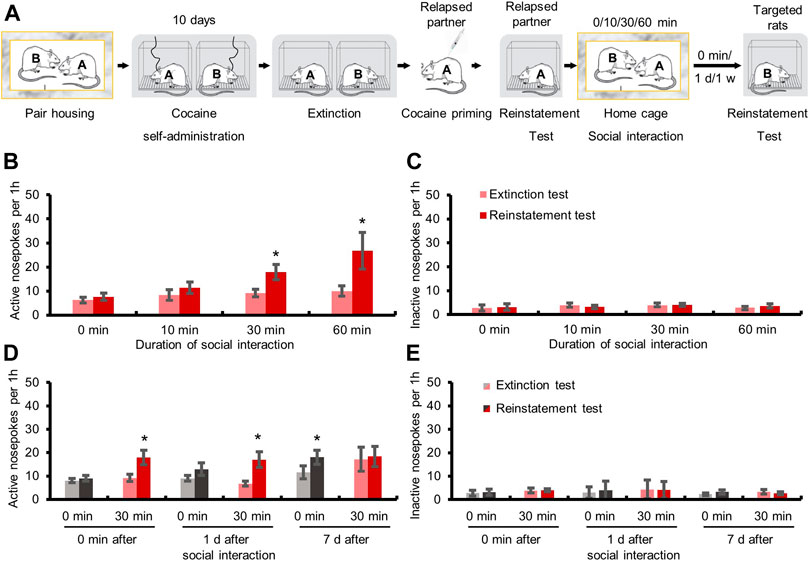
FIGURE 2. Social interaction with relapsed partners for over 30 min triggered relapse to drug-seeking behavior and this effect lasted for at least 1 day. (A) Experimental timeline. (B) Active nosepokes were enhanced after social interaction with relapsed partner for 30 or 60 min. (C) Inactive nosepokes had no change after social interaction (Data are shown as mean ± SEM. *p < 0.05, compared with extinction test. n = 7–9). (D) Active nosepokes were increased immediately or 1 day after 30 min social interaction with relapsed partner. (E) Inactive nosepokes had no change after social interaction (Data are shown as mean ± SEM. *p < 0.05, compared with extinction test. n = 6–9).
Then we further tested how long the facilitation effect of social interaction with relapsed partner on relapse could last (Figure 2A). The repeated measures ANOVA showed that the targeted rats had a higher number of active-nosepokes when tested immediately (F1,7 = 9.171, p < 0.05), or 1 day (F1,5 = 13.164, p < 0.05), but not 7 days (p > 0.05), after 30 min social interaction with relapsed partners, while no significant change was found when the targeted rats which did not interact with relapsed partners were tested immediately or 1 day later (both p > 0.05). Interestingly, when tested 7 days later, the number of active-nosepokes of the targeted rats that did not experience social interaction was increased during the drug seeking test (F1,6 = 6.161, p < 0.05), possibly resulting from spontaneous recovery of drug seeking (Figure 2D). These results demonstrated that the effect of social interaction on cocaine seeking lasted for at least 1 day.
Next, we investigated whether social interaction with relapsed strangers could also induce cocaine relapse (Figures 3A–C). The repeated measures ANOVA with within-subject factors (extinction test and reinstatement test) showed that the numbers of active-nosepokes of the strangers were elevated after cocaine injection (Figure 3D, F1,8 = 13.857, p < 0.05). No significant change was found in the numbers of active-nosepokes of the targeted rats after social interaction with relapsed strangers (Figure 3E, p > 0.05). The above findings indicated that transfer of cocaine relapse occurred during social interaction with relapsed partner rather than relapsed stranger.
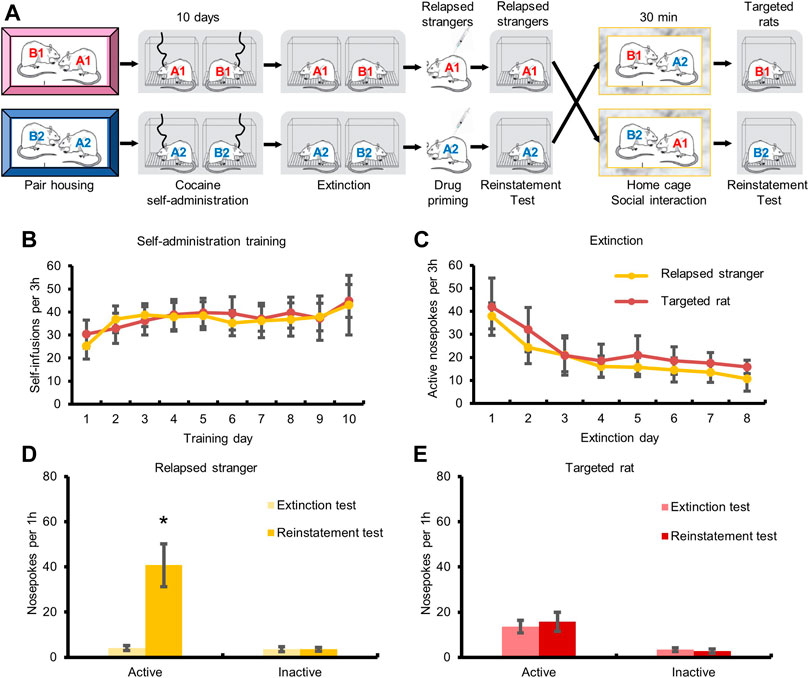
FIGURE 3. Social interaction with relapsed strangers had no effect on drug-seeking behavior. (A) Experimental timeline. (B) Targeted rats and strangers acquired cocaine self-administration training. (C) Drug-seeking behavior of targeted rats and strangers was extinguished. (D) Cocaine injection triggered drug-seeking behavior. (E) Drug-seeking behavior of targeted rats had no change after social interaction with relapsed stranger (Data are shown as mean ± SEM. *p < 0.05, compared with extinction test. n = 9).
Finally, we validated the facilitation effect of social interaction on cocaine relapse through the cocaine-induced conditioned place preference (CPP) model, which is also a widely used animal model to study drug addiction (Figure 4A). The repeated measures ANOVA of CPP scores of relapsed partners with within-subjects factors (baseline test, training test, extinction test, and reinstatement test), showed a significant main effect (Figure 4B, F3,15 = 4.665, p < 0.05). Post hoc tests revealed significant differences between baseline test and training test (p < 0.05), training test and extinction test (p < 0.01), or extinction test and reinstatement test (p < 0.05). Meanwhile, the repeated measures ANOVA of CPP scores of the targeted rats with within-subjects factors (baseline test, training test, extinction test, and reinstatement test), showed a significant main effect (Figure 4B, F3,15 = 14.166, p < 0.01). Post hoc tests revealed significant differences between baseline test and training test (p < 0.01), training test and extinction test (p < 0.01), or extinction test and reinstatement test (p < 0.05). The above results suggested that social interaction with relapsed partners promoted the transfer of cocaine relapse. But if the targeted rats did not interact with relapsed partners, no difference was found between the extinction test and reinstatement test (Figure 4C, p > 0.05).
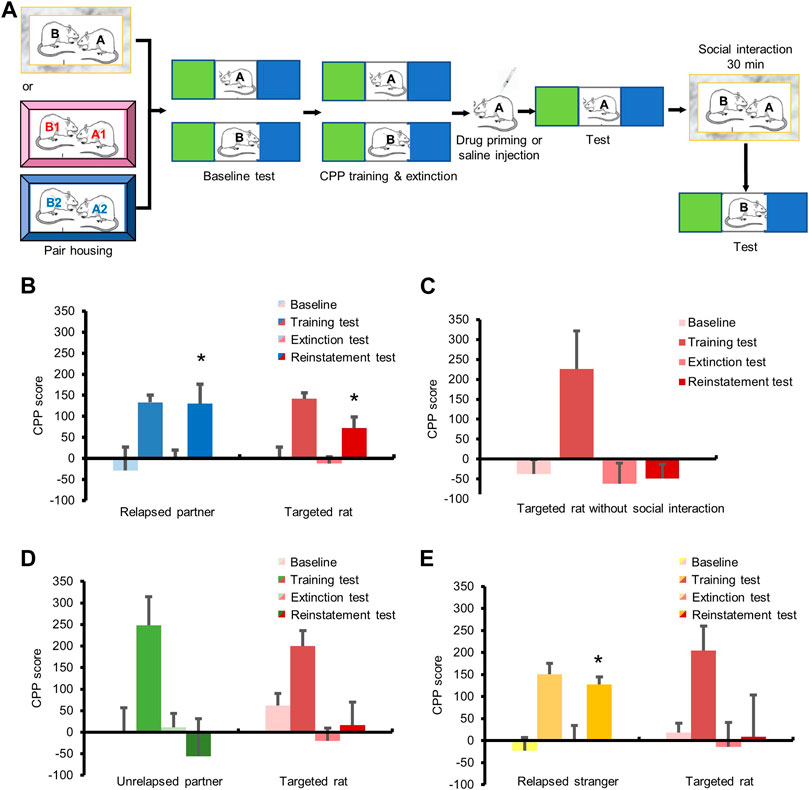
FIGURE 4. Social interaction with relapsed partners triggered relapse in cocaine CPP model. (A) Experimental timeline. (B–E) All rats obtained cocaine CPP training and extinction. (B) Cocaine injection triggered relapse of the partners, and social interaction with relapsed partner enhanced drug seeking of targeted rats. (C) No social interaction had no influence on relapse of targeted rats. (D) Social interaction with unrelapsed partner had no effect on relapse of targeted rats. (E) Social interaction with relapsed stranger had no effect on relapse of targeted rats. (Data are shown as mean ± SEM. *p < 0.05, compared with extinction test. n = 6–7).
For the unrelapsed partners, the repeated measures ANOVA of CPP scores showed a significant main effect (Figure 4D, F3,15 = 3.785, p < 0.05). Post hoc tests revealed significant differences between the baseline test and training test (p < 0.01), or training test and extinction test (p < 0.05), but no difference between the extinction test and reinstatement test (p > 0.05). The repeated measures ANOVA of the CPP scores of targeted rats which interacted with unrelapsed partners revealed a significant main effect (Figure 4D, F3,15 = 9.560, p < 0.01). Post hoc tests revealed significant differences between baseline test and training test (p < 0.05), or training test and extinction test (p < 0.01). No change was found between the extinction test and reinstatement test (p > 0.05), demonstrating that social interaction with unrelapsed partners had no effect on the transfer of cocaine relapse.
A significant main effect was shown for the CPP scores of the relapsed strangers by repeated measures ANOVA (Figure 4E, F3,18 = 14.296, p < 0.01), and post hoc tests revealed significant differences between baseline test and training test (p < 0.01), training test and extinction test (p < 0.01), or extinction test and reinstatement test (p < 0.05). Meanwhile, the repeated measures ANOVA of CPP scores of the targeted rats interacting with relapsed strangers showed a significant main effect (Figure 4E, F3,18 = 4.050, p < 0.05). Post hoc revealed significant differences between baseline test and training test (p < 0.01), or training test and extinction test (p < 0.05), while no change was found between the extinction test and reinstatement test (p > 0.05), indicating that social interaction with relapsed strangers did not affect cocaine seeking.
In the present study, we proposed a novel animal model to explore the effects of social partners on relapse, and investigated the social transfer of drug relapse based on two classic behavioral paradigms, cocaine self-administration and CPP. We found that social interaction with relapsed (cocaine-primed) partners for at least 30 min induced relapse to cocaine seeking behavior, and the effect lasted for over 24 h. In contrast, neither social interaction with relapsed strangers nor with unrelapsed partners had a facilitation effect on cocaine relapse.
Social experiences are important influential factors for drug addiction and relapse (Heilig et al., 2016). In previous studies, many animal models of addiction relapse have been established and validated that drug priming, drug-related cues or context, and stress can induce reinstatement/relapse after extinction (Pohorecky, 2008; Neisewander et al., 2012; Heilig et al., 2016). However, only a few models incorporated social factors, and there remain debates about the role of social factors in relapse. Ribeiro Do Couto et al. (2009) found that social isolation before CPP training or exposure to social defeat stress before cocaine priming promoted relapse to cocaine-induced CPP, whereas exposure to a non-addicted female mouse or brief social interaction with a non-addicted and non-aggressive male mouse before cocaine priming could reduce relapse (Ribeiro Do Couto et al., 2009). Venniro et al. (2018) built a model of choice between drugs and social interaction, and found that access to social interaction with non-addicted rats, as a social reward, could prevent methamphetamine self-administration and relapse. While some studies demonstrated that social interaction with addicted peers promoted the possibility of addiction (Doty and de Wit, 1995; Kirkpatrick and de Wit, 2013), other studies reported the inhibitory effects of social factors on addiction (Deatherage, 1972; Weisinger et al., 1989). For example, compared with rats kept in pairs, rats kept solely got more morphine in the social environment (Alexander et al., 1981; Raz and Berger, 2010). If these isolated rats were allowed to interact with other non-addicted peers before the test, the preference for morphine was also attenuated (Hadaway et al., 1979; Raz and Berger, 2010). Based on the findings above, we speculated that social interaction with non-addicted or unrelapsed peers may prevent relapse, while interaction with addicted or relapsed peers may facilitate relapse. We found that interaction with relapsed partner, but not unrelapsed partner, after extinction promoted cocaine seeking. The present study provides a perspective that relapsed and unrelapsed partners produce different effects on relapse. Our results and previous findings confirmed the social-learning theory (Peitz et al., 2013) which demonstrates that partners of a group affect the behavior of other members.
However, the role of social interaction on addiction and relapse is complex and the state of partners is not the only determining factor. Some research revealed that interaction with non-addicted partners produced a dose-response effect (Wolffgramm, 1990; Wolffgramm and Heyne, 1991). Compared with isolated rats, rats that interacted with members of peer group partly (there was a segregation network between them) was prevented from alcohol seeking, while rats that interacted with partners completely (there was no segregation network between them) presented increased alcohol intake (Wolffgramm, 1990), suggesting there was a dose-effect relationship between interaction degree and alcohol intake. Moreover, the gender of partners also has an effect. A study using prairie voles found that male prairie voles which were pair-housed with other male ones showed more preference to alcohol (Anacker et al., 2011), whereas alcohol preference of those kept with female prairie voles had no change (Hostetler et al., 2012). The above findings reveal that the effect of social interaction on addiction and relapse depends on not only whether the partners got addicted or relapsed but also the interaction degree and gender of partners. Thus, further studies are needed to investigate the effect of social interaction at different degrees or in different communicating ways (i.e., olfactory, auditory, visual, or tactile communication), or with peers having different genders on drug relapse.
Previous research has showed various brain regions responsible for social interaction and provides an insight into the “social brain” (Insel and Fernald, 2004). It includes the brain areas activated during the social cognition tasks, like the regions for social identification, environmental assessment, social motivation, and behavior execution. Both human and animal studies verified the critical effect of the medial prefrontal cortex (mPFC), hippocampus, amygdala, and thalamus on social interaction (Kas et al., 2014), and meanwhile, these brain areas also contribute to cocaine addiction and relapse (Brandon et al., 2007). Notably, EI Rawas et al. (2012) found that similar brain regions were activated by cocaine-induced CPP and social interaction-induced CPP, including prelimbic, infralimbic, orbitofrontal, and cingulate cortex, as well as striatum, central and basolateral amygdala, and ventral tegmental areas, which had been proved to be associated with cocaine conditioned stimuli and social interaction (Thomas et al., 2003; Salchner et al., 2004; Miller and Marshall, 2005). Inhibiting protein synthesis in the mPFC (Marcondes et al., 2020), hippocampus (Garrido Zinn et al., 2016), or amygdala (Gur et al., 2014; Garrido Zinn et al., 2016) impaired the discrimination ability between familiar peers and strangers, suggesting that social interaction with relapsed partners instead of strangers possibly activated these brain regions and retrieved seeking for cocaine. This may be why only interaction with relapsed partners instead of strangers can induce cocaine relapse. Besides, the mirror neuron system is the hub for understanding other’s emotions, intentions, and actions (Rizzolatti et al., 1999; Rizzolatti and Craighero, 2004; Molenberghs et al., 2009). Mirror neurons are widely distributed in the inferior frontal gyrus, primary somatosensory cortex, supplementary motor area, and other cortex (Molenberghs et al., 2009), and a study recruiting patients with lesions in the lateral prefrontal cortex showed deficits in understanding other’s emotion, indicating mirror neurons also exist in the prefrontal cortex (Perry et al., 2017). Therefore, mirror neurons in the prefrontal cortex may play an important role in the social transfer of drug relapse, which needs further investigation.
There are some limitations in our work. First, we established a novel model for the transfer of relapse between partners, but it remains unclear whether it is applicable in other drugs like heroin and nicotine. Furthermore, social interaction may produce similar effects in other disorders such as depression and post-traumatic stress disorder, which also needs further investigation. Second, we did not investigate the neural mechanisms underlying the effects of social interaction on relapse. Future studies need to be conducted to explore how the information is transmitted from the relapsed partners to the targeted rats, and how the brain areas mediating social information processing activate the ones required for addiction and relapse.
In conclusion, we introduced a novel animal model for the social transfer of drug relapse, and found that cocaine relapse could be induced by social interaction with relapsed partner, but not unrelapsed partner or relapsed stranger. Our findings suggest the importance of living in drug-free communities and keeping away from relapsed partners for abstinent drug users, and emphasize the necessity of paying attention to social interaction factors when formulating prevention and treatment strategies for relapse.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Biomedical Ethics Committee for Animal Use and Protection of Peking University.
SM, LL, and YX designed the study. SM, KY, and XL performed the experiments, analyzed the data, and prepared the first draft of the manuscript. WY, KY, YG, ST, YX, PW, YS, JS, and LL revised the manuscript. All the authors have read and approved the final version of the manuscript.
This work was supported in part by the National Natural Science Foundation of China (Nos. 81901352, 81821092, 81871046, 31800897, 82001404 and 81722018) and Clinic Medicine + X Fund of PKUHSC (No. PKU2020LCXQ016).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alexander, B. K., Beyerstein, B. L., Hadaway, P. F., and Coambs, R. B. (1981). Effect of Early and Later colony Housing on Oral Ingestion of Morphine in Rats. Pharmacol. Biochem. Behav. 15 (4), 571–576. doi:10.1016/0091-3057(81)90211-2
Anacker, A. M., Loftis, J. M., Kaur, S., and Ryabinin, A. E. (2011). Prairie Voles as a Novel Model of Socially Facilitated Excessive Drinking. Addict. Biol. 16 (1), 92–107. doi:10.1111/j.1369-1600.2010.00234.x
Bahr, S. J., Hoffmann, J. P., and Yang, X. (2005). Parental and Peer Influences on the Risk of Adolescent Drug Use. J. Prim. Prev. 26 (6), 529–551. doi:10.1007/s10935-005-0014-8
Bathish, R., Best, D., Savic, M., Beckwith, M., Mackenzie, J., and Lubman, D. I. (2017). "Is it Me or Should My Friends Take the Credit?" the Role of Social Networks and Social Identity in Recovery from Addiction. J. Appl. Soc. Psychol. 47 (1), 35–46. doi:10.1111/jasp.12420
Brandon, T. H., Vidrine, J. I., and Litvin, E. B. (2007). Relapse and Relapse Prevention. Annu. Rev. Clin. Psychol. 3, 257–284. doi:10.1146/annurev.clinpsy.3.022806.091455
Caudill, B. D., and Kong, F. H. (2001). Social Approval and Facilitation in Predicting Modeling Effects in Alcohol Consumption. J. Subst. Abuse 13 (4), 425–441. doi:10.1016/s0899-3289(01)00099-2
Caudill, B. D., and Marlatt, G. A. (1975). Modeling Influences in Social Drinking: an Experimental Analogue. J. Consult Clin. Psychol. 43 (3), 405–415. doi:10.1037/h0076689
Davachi, L. (2006). Item, Context and Relational Episodic Encoding in Humans. Curr. Opin. Neurobiol. 16 (6), 693–700. doi:10.1016/j.conb.2006.10.012
Deatherage, G. (1972). Effects of Housing Density on Alcohol Intake in the Rat. Physiol. Behav. 9 (1), 55–57. doi:10.1016/0031-9384(72)90264-8
Doty, P., and de Wit, H. (1995). Effect of Setting on the Reinforcing and Subjective Effects of Ethanol in Social Drinkers. Psychopharmacology (Berl) 118 (1), 19–27. doi:10.1007/BF02245245
Frings, D., Wood, K. V., and Albery, I. P. (2021). New Converts and Seasoned Campaigners: the Role of Social Identity at Different Stages in the Addiction Recovery Journey. Drugs Educ. Prev. Pol., 1–8. doi:10.1080/09687637.2021.1914551
Garrido Zinn, C., Clairis, N., Silva Cavalcante, L. E., Furini, C. R., de Carvalho Myskiw, J., and Izquierdo, I. (2016). Major Neurotransmitter Systems in Dorsal hippocampus and Basolateral Amygdala Control Social Recognition Memory. Proc. Natl. Acad. Sci. U S A. 113 (33), E4914–E4919. doi:10.1073/pnas.1609883113
Goldfarb, E. V., and Sinha, R. (2018). Drug-induced Glucocorticoids and Memory for Substance Use. Trends Neurosci. 41 (11), 853–868. doi:10.1016/j.tins.2018.08.005
Gur, R., Tendler, A., and Wagner, S. (2014). Long-term Social Recognition Memory Is Mediated by Oxytocin-dependent Synaptic Plasticity in the Medial Amygdala. Biol. Psychiatry 76 (5), 377–386. doi:10.1016/j.biopsych.2014.03.022
Hadaway, P. F., Alexander, B. K., Coambs, R. B., and Beyerstein, B. (1979). The Effect of Housing and Gender on Preference for Morphine-Sucrose Solutions in Rats. Psychopharmacology (Berl) 66 (1), 87–91. doi:10.1007/BF00431995
Heilig, M., Epstein, D. H., Nader, M. A., and Shaham, Y. (2016). Time to Connect: Bringing Social Context into Addiction Neuroscience. Nat. Rev. Neurosci. 17 (9), 592–599. doi:10.1038/nrn.2016.67
Hostetler, C. M., Anacker, A. M., Loftis, J. M., and Ryabinin, A. E. (2012). Social Housing and Alcohol Drinking in Male-Female Pairs of Prairie Voles (Microtus Ochrogaster). Psychopharmacology (Berl) 224 (1), 121–132. doi:10.1007/s00213-012-2836-4
Insel, T. R., and Fernald, R. D. (2004). How the Brain Processes Social Information: Searching for the Social Brain. Annu. Rev. Neurosci. 27, 697–722. doi:10.1146/annurev.neuro.27.070203.144148
Kas, M. J., Modi, M. E., Saxe, M. D., and Smith, D. G. (2014). Advancing the Discovery of Medications for Autism Spectrum Disorder Using New Technologies to Reveal Social Brain Circuitry in Rodents. Psychopharmacology (Berl) 231 (6), 1147–1165. doi:10.1007/s00213-014-3464-y
Kelly, J. F., Hoeppner, B., Stout, R. L., and Pagano, M. (2012). Determining the Relative Importance of the Mechanisms of Behavior Change within Alcoholics Anonymous: a Multiple Mediator Analysis. Addiction 107 (2), 289–299. doi:10.1111/j.1360-0443.2011.03593.x
Kirkpatrick, M. G., and de Wit, H. (2013). In the Company of Others: Social Factors Alter Acute Alcohol Effects. Psychopharmacology (Berl) 230 (2), 215–226. doi:10.1007/s00213-013-3147-0
Koordeman, R., Kuntsche, E., Anschutz, D. J., van Baaren, R. B., and Engels, R. C. (2011). Do we Act upon what We See? Direct Effects of Alcohol Cues in Movies on Young Adult’s Alcohol Drinking. Alcohol Alcohol 46 (4), 393–398. doi:10.1093/alcalc/agr028
Larsen, H., Engels, R. C., Souren, P. M., Granic, I., and Overbeek, G. (2010). Peer Influence in a Micro-perspective: Imitation of Alcoholic and Non-alcoholic Beverages. Addict. Behav. 35 (1), 49–52. doi:10.1016/j.addbeh.2009.08.002
Li, L., Lin, C., Wan, D., Zhang, L., and Lai, W. (2012). Concurrent Heroin Use Among Methadone Maintenance Clients in China. Addict. Behav. 37 (3), 264–268. doi:10.1016/j.addbeh.2011.11.004
Lied, E. R., and Marlatt, G. A. (1979). Modeling as a Determinant of Alcohol Consumption: Effect of Subject Sex and Prior Drinking History. Addict. Behav. 4 (1), 47–54. doi:10.1016/0306-4603(79)90020-0
Luo, Y. X., Xue, Y. X., Liu, J. F., Shi, H. S., Jian, M., Han, Y., et al. (2015). A Novel UCS Memory Retrieval-Extinction Procedure to Inhibit Relapse to Drug Seeking. Nat. Commun. 6, 7675. doi:10.1038/ncomms8675
Marcondes, L. A., Nachtigall, E. G., Zanluchi, A., de Carvalho Myskiw, J., Izquierdo, I., and Furini, C. R. G. (2020). Involvement of Medial Prefrontal Cortex NMDA and AMPA/kainate Glutamate Receptors in Social Recognition Memory Consolidation. Neurobiol. Learn. Mem. 168, 107153. doi:10.1016/j.nlm.2019.107153
Miller, C. A., and Marshall, J. F. (2005). Altered Fos Expression in Neural Pathways Underlying Cue-Elicited Drug Seeking in the Rat. Eur. J. Neurosci. 21 (5), 1385–1393. doi:10.1111/j.1460-9568.2005.03974.x
Molenberghs, P., Cunnington, R., and Mattingley, J. B. (2009). Is the Mirror Neuron System Involved in Imitation? A Short Review and Meta-Analysis. Neurosci. Biobehav Rev. 33 (7), 975–980. doi:10.1016/j.neubiorev.2009.03.010
Neisewander, J. L., Peartree, N. A., and Pentkowski, N. S. (2012). Emotional Valence and Context of Social Influences on Drug Abuse-Related Behavior in Animal Models of Social Stress and Prosocial Interaction. Psychopharmacology (Berl) 224 (1), 33–56. doi:10.1007/s00213-012-2853-3
Peitz, G. W., Strickland, J. C., Pitts, E. G., Foley, M., Tonidandel, S., and Smith, M. A. (2013). Peer Influences on Drug Self-Administration: an Econometric Analysis in Socially Housed Rats. Behav. Pharmacol. 24 (2), 114–123. doi:10.1097/FBP.0b013e32835f1719
Perry, A., Saunders, S. N., Stiso, J., Dewar, C., Lubell, J., Meling, T. R., et al. (2017). Effects of Prefrontal Cortex Damage on Emotion Understanding: EEG and Behavioural Evidence. Brain 140 (4), 1086–1099. doi:10.1093/brain/awx031
Pohorecky, L. A. (2008). Psychosocial Stress and Chronic Ethanol Ingestion in Male Rats: Effects on Elevated Plus Maze Behavior and Ultrasonic Vocalizations. Physiol. Behav. 94 (3), 432–447. doi:10.1016/j.physbeh.2008.02.010
Raz, S., and Berger, B. D. (2010). Social Isolation Increases Morphine Intake: Behavioral and Psychopharmacological Aspects. Behav. Pharmacol. 21 (1), 39–46. doi:10.1097/FBP.0b013e32833470bd
Ribeiro Do Couto, B., Aguilar, M. A., Lluch, J., Rodríguez-Arias, M., and Miñarro, J. (2009). Social Experiences Affect Reinstatement of Cocaine-Induced Place Preference in Mice. Psychopharmacology (Berl) 207 (3), 485–498. doi:10.1007/s00213-009-1678-1
Rizzolatti, G., and Craighero, L. (2004). The Mirror-Neuron System. Annu. Rev. Neurosci. 27, 169–192. doi:10.1146/annurev.neuro.27.070203.144230
Rizzolatti, G., Fadiga, L., Fogassi, L., and Gallese, V. (1999). Resonance Behaviors and Mirror Neurons. Arch. Ital. Biol. 137 (2-3), 85–100. doi:10.4449/aib.v137i2.575
Salchner, P., Lubec, G., and Singewald, N. (2004). Decreased Social Interaction in Aged Rats May Not Reflect Changes in Anxiety-Related Behaviour. Behav. Brain Res. 151 (1-2), 1–8. doi:10.1016/j.bbr.2003.07.002
Simons-Morton, B., and Chen, R. S. (2006). Over Time Relationships between Early Adolescent and Peer Substance Use. Addict. Behav. 31 (7), 1211–1223. doi:10.1016/j.addbeh.2005.09.006
Sinha, R. (2011). New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Curr. Psychiatry Rep. 13 (5), 398–405. doi:10.1007/s11920-011-0224-0
Smith, M. A., and Pitts, E. G. (2014). Social Preference and Drug Self-Administration: a Preclinical Model of Social Choice within Peer Groups. Drug Alcohol Depend 135, 140–145. doi:10.1016/j.drugalcdep.2013.12.001
Smith, M. A., Strickland, J. C., Bills, S. E., and Lacy, R. T. (2015). The Effects of a Shared History of Drug Exposure on Social Choice. Behav. Pharmacol. 26 (7 Spec No), 631–635. doi:10.1097/FBP.0000000000000139
Thomas, K. L., Arroyo, M., and Everitt, B. J. (2003). Induction of the Learning and Plasticity-Associated Gene Zif268 Following Exposure to a Discrete Cocaine-Associated Stimulus. Eur. J. Neurosci. 17 (9), 1964–1972. doi:10.1046/j.1460-9568.2003.02617.x
Venniro, M., Zhang, M., Caprioli, D., Hoots, J. K., Golden, S. A., Heins, C., et al. (2018). Volitional Social Interaction Prevents Drug Addiction in Rat Models. Nat. Neurosci. 21 (11), 1520–1529. doi:10.1038/s41593-018-0246-6
Volkow, N. D., and Boyle, M. (2018). Neuroscience of Addiction: Relevance to Prevention and Treatment. Am. J. Psychiatry 175 (8), 729–740. doi:10.1176/appi.ajp.2018.17101174
Walden, B., McGue, M., Lacono, W. G., Burt, S. A., and Elkins, I. (2004). Identifying Shared Environmental Contributions to Early Substance Use: the Respective Roles of Peers and Parents. J. Abnorm Psychol. 113 (3), 440–450. doi:10.1037/0021-843X.113.3.440
Weisinger, R. S., Denton, D. A., and Osborne, P. G. (1989). Voluntary Ethanol Intake of Individually- or Pair-Housed Rats: Effect of ACTH or Dexamethasone Treatment. Pharmacol. Biochem. Behav. 33 (2), 335–341. doi:10.1016/0091-3057(89)90510-8
Wolffgramm, J. (1990). Free Choice Ethanol Intake of Laboratory Rats under Different Social Conditions. Psychopharmacology (Berl) 101 (2), 233–239. doi:10.1007/BF02244132
Wolffgramm, J., and Heyne, A. (1991). Social Behavior, Dominance, and Social Deprivation of Rats Determine Drug Choice. Pharmacol. Biochem. Behav. 38 (2), 389–399. doi:10.1016/0091-3057(91)90297-f
Keywords: social interaction, cocaine, relapse, peer influence, addiction, animal model
Citation: Meng S, Yan W, Liu X, Gong Y, Tian S, Wu P, Sun Y, Shi J, Lu L, Yuan K and Xue Y (2021) Social Interaction With Relapsed Partner Facilitates Cocaine Relapse in Rats. Front. Pharmacol. 12:750397. doi: 10.3389/fphar.2021.750397
Received: 30 July 2021; Accepted: 31 August 2021;
Published: 04 October 2021.
Edited by:
Qi Wang, Southern Medical University, ChinaReviewed by:
Qiu Pingming, Southern Medical University, ChinaCopyright © 2021 Meng, Yan, Liu, Gong, Tian, Wu, Sun, Shi, Lu, Yuan and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanxue Xue, eWFueHVleHVlQGJqbXUuZWR1LmNu; Kai Yuan, eXVhbmthaUBwa3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.