- 1Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium
- 2Department of Pediatrics, KU Leuven, Leuven, Belgium
Objectives: Cystic fibrosis transmembrane conductance regulator (CFTR) modulators, Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor), have substantially improved patients’ lives yet significantly burden healthcare budgets. This analysis aims to compare pricing and reimbursement of aforementioned cystic fibrosis medicines, across European countries.
Methods: Clinical trial registries, national databases, health technology assessment reports and grey literature of Austria, Belgium, Denmark, France, Germany, Ireland, Poland, Spain, Sweden, Switzerland, Netherlands, the United Kingdom were consulted. Publicly available prices, reimbursement statuses, economic evaluations, budget impact analyses and managed entry agreements of CFTR modulators were examined. Results: In Belgium, lowest list prices were observed for Kalydeco® (ivacaftor) and Symkevi® (tezacaftor/ivacaftor) at €417 per defined daily dose (DDD) and €372 per average daily dose (ADD), respectively. Whereas, Switzerland had the lowest price for Orkambi® (lumacaftor/ivacaftor) listed at €309 per DDD. Spain had the highest prices for Kalydeco® (ivacaftor) and Symkevi® (tezacaftor/ivacaftor) at €850 per DDD and €761 per ADD, whereas Orkambi® (lumacaftor/ivacaftor) was most expensive in Poland at €983 per DDD. However, list prices were subject to confidential discounts and likely varied from actual costs. In all countries, these treatments were deemed not to be cost-effective. The annual budget impact of the CFTR modulators varied between countries and depended on factors such as local product prices, size of target population, scope of costs and discounting. However, all modulators were fully reimbursed in ten of the evaluated countries except for Sweden and Poland that, respectively, granted reimbursement to one and none of the therapies. Managed entry agreements were confidential but commonly adopted to address financial uncertainties.
Conclusion: Discrepancies concerning prices, reimbursement and access were detected for Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) across European countries.
Introduction
Cystic fibrosis (CF) is a rare condition affecting more than 48,000 individuals in Europe. With an occurrence of 1 in 2000–3,000, it is also the continent with the highest incidence of CF (European Cystic Fibrosis Society, 2020) (Farrell, 2008; Bell et al., 2020). Over time, technological advancements such as preconception carrier screening have led to a decline in incidence rates in some countries or regions (Lopes-Pacheco, 2016; Bell et al., 2020). However, newborn screening, improved care and clinical awareness have contributed to decreased pediatric mortality, a stable and a continuously growing CF adult population, now exceeding the pediatric population (Burgel et al., 2015; Lopes-Pacheco, 2016; Balfour-Lynn and King, 2020; Bell et al., 2020).
Inheritance of the disease is autosomal recessive and caused by errors in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (Rafeeq and Murad, 2017; Bell et al., 2020). Over 2000 CFTR mutations have been identified and are grouped into six classes based on the protein defect (Rafeeq and Murad, 2017). Class I mutations result in no functional CFTR and include nonsense mutations, splice mutations or deletions (De Boeck et al., 2014; Rafeeq and Murad, 2017). In Class II, characterized by the most common heterozygous or homozygous F508del mutation affecting 85% of people with CF (PWCF) in Europe, the CFTR protein is misfolded and unable to reach the cell surface (De Boeck et al., 2014; Rafeeq and Murad, 2017). Gating mutations, typically describing G551D, S549R or V520F alterations that prevent opening of the CFTR channel, are categorized in Class III (De Boeck et al., 2014; Rafeeq and Murad, 2017). Class IV describes impairment of CFTR regulation by faulty channel conformation e.g. D1152H or R117H mutations (De Boeck et al., 2014; Rafeeq and Murad, 2017). Splicing mutations of Class V, such as 3,849+10 kb C → T, result in insufficient CFTR channels and Class VI mutations cause increased degradation of the unstable protein (De Boeck et al., 2014; Rafeeq and Murad, 2017).
A dysfunctional CFTR protein generates a chloride and bicarbonate ionic imbalance while increasing influx of sodium and water (Morrison et al., 2019). This disrupts the natural pH and alters the apical liquid layer of epithelial cells and digestive fluids into accumulating thick mucus or ‘mucoviscidosis’. This phenotypically manifests into persistent obstruction and inflammation of organs such as the lungs and gastrointestinal tract (NICE, 2017; Rafeeq and Murad, 2017; Morrison et al., 2019; Bell et al., 2020). Further complications can lead to deterioration of vital organs and death.
However, innovative therapies have increased life expectancy of PWCF to above 40 years (Lopes-Pacheco, 2016; Lopes-Pacheco, 2019). CFTR modulators have revolutionized the treatment of CF from symptomatic therapy, consisting of antibiotics, bronchodilators and mucolytic medicines, to mechanism-targeting therapies (Lopes-Pacheco, 2016; Cystic Fibrosis Foundation, 2021b). Currently, four modulators, developed by Vertex Pharmaceuticals, Inc., are authorized in the European Union, namely: Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor), Symkevi®/Symdeko® (tezacaftor/ivacaftor) and Kaftrio®/Trikafta® (ivacaftor/tezacaftor/elexacaftor) (European Medicines Agency, 2021b; European Medicines Agency, 2021c; European Medicines Agency, 2021d; European Medicines Agency, 2021a). Kalydeco® (ivacaftor), the first CFTR potentiator introduced in 2012, is used in infants aged 4 months or older (European Medicines Agency, 2021b). Its active substance, ivacaftor extends the opening of the CFTR channel gate and increases activity of defective protein (Rafeeq and Murad, 2017; Lopes-Pacheco, 2019). Subsequently, Orkambi® (lumacaftor/ivacaftor) was launched as a combination therapy for patients 2 years and older, with a homozygous F508del mutation, and contains both ivacaftor and lumacaftor (Lopes-Pacheco, 2016; Lopes-Pacheco, 2019; European Medicines Agency, 2021c). The latter corrects the misfolding of the CFTR protein and, in combination with potentiator Kalydeco® (ivacaftor), facilitates chloride secretion. Symkevi® (tezacaftor/ivacaftor) is indicated in patients aged 6 years and older with the F508del mutation, homozygous or heterozygous with a residual function mutation (Lopes-Pacheco, 2019; European Medicines Agency, 2021d). This therapy combines ivacaftor and tezacaftor and has clinically improved tolerability and pharmacokinetics than its predecessor Orkambi® (lumacaftor/ivacaftor). Most recently, Kaftrio® (ivacaftor/tezacaftor/elexacaftor) was approved for patients, 12 years or older homozygous or heterozygous with a minimal function mutation for the F508del mutation (Lopes-Pacheco, 2019; European Medicines Agency, 2021a). It is a triple combination therapy containing ivacaftor, tezacaftor and a third corrector, elexacaftor proven to be more efficacious than Symkevi® (tezacaftor/ivacaftor). All these therapies, except for Orkambi® (lumacaftor/ivacaftor), of which orphan designation was withdrawn at market authorization upon request of the company, are designated as orphan medicinal products (OMP). Symkevi® (tezacaftor/ivacaftor) and Kaftrio® (ivacaftor/tezacaftor/elexacaftor) are used in combination with Kalydeco® (ivacaftor) in therapy (European Medicines Agency, 2021b; European Medicines Agency, 2021d; European Medicines Agency, 2021a).
Moreover, for each indication of Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor), phase 3 clinical trials reported improved pulmonary functions, expressed in lung clearance index (LCI 2.5) and percentage predicted forced expiratory volume in one second (ppFEV1), compared to placebos (Table 1) (EU Clinical Trials Register, 2015b; EU Clinical Trials Register, 2015c; EU Clinical Trials Register, 2015a; EU Clinical Trials Register, 2017c; EU Clinical Trials Register, 2017b; EU Clinical Trials Register, 2017a; EU Clinical Trials Register, 2018). However, statistically significant difference was only achieved in 3,849 + 10 KB C→T or D1152H CFTR mutations, G551D and Non-G551D CFTR gating mutations for Kalydeco® (ivacaftor), in homozygous F508del CFTR mutations for Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) (EU Clinical Trials Register, 2015c; EU Clinical Trials Register, 2015a; EU Clinical Trials Register, 2017c; EU Clinical Trials Register, 2017b).
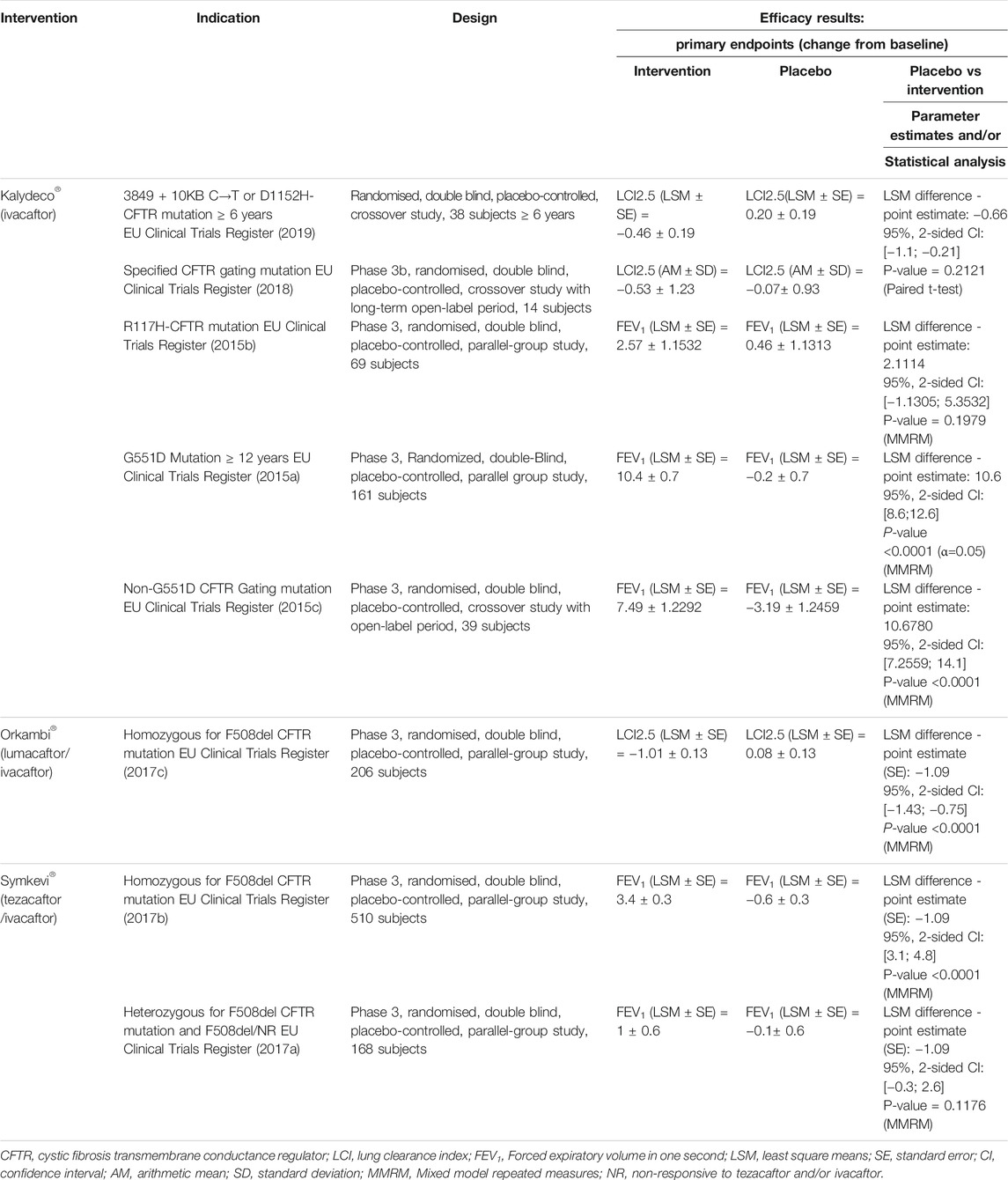
TABLE 1. Description of design and efficacy results of the pivotal trials in each indication of Kalydeco®, Orkambi® and Symkevi®.
Although Orkambi® (lumacaftor/ivacaftor) and Symkevi® have, moderately, while Kalydeco® (ivacaftor) and Kaftrio® (ivacaftor/tezacaftor/elexacaftor) have, greatly, improved quality of life for many patients, access to these medicines is not always guaranteed due to their associated high cost and burden on healthcare budgets (Chevreul et al., 2016; Lopes-Pacheco, 2019). After the adoption of CFTR modulators, a significantly higher expenditure was observed in Europe: a recent study reviewed a database of PWCF and showed that only the four percent of PWCF who were on CFTR modulators caused an increase of 27.5% in CF pharmaceutical spending (Chevreul et al., 2016). This is expected to augment further as the market uptake will grow when all eligible PWCF receive CFTR protein-targeting medicines. Additionally, new CFTR-modulators from Vertex and other companies such as Abbvie and Eloxx Pharmaceuticals are in the pipeline (Cystic Fibrosis Foundation, 2021a; Lopes-Pacheco, 2019). To illustrate, Germany noted an expenditure of €159 million in 2016 and estimates this amount to triple to €594 million if all patients would receive these modulators (Frey et al., 2019).
To inform reimbursement decisions of new medicines, many European jurisdictions perform health technology assessment (HTA) (Morel et al., 2013). For rare disease therapies such as these CFTR modulators, however, high uncertainty on medicine performance exists due to the limited and genetically heterogeneous population as well as adoption of surrogate endpoints in clinical settings (Kent et al., 2014; McLeod et al., 2020). To allow market access of Vertex’ products while accounting for clinical uncertainties and high costs, some healthcare authorities closed mutual agreements with the manufacturer (Morel et al., 2013).
In this study, we aim to comparatively analyze publicly accessible list prices, reimbursement decisions, economic evaluations, budget impact analyses (BIAs), managed entry agreements (MEAs) and multinational collaborations of Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) in European countries.
Methods
We conducted a comparative analysis of list prices, reimbursement statuses, economic evaluations, BIAs and MEAs of Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi®(tezacaftor/ivacaftor) in selected European countries. Kaftrio® (ivacaftor/tezacaftor/elexacaftor) was not included in the analysis due to limited information availability as it was recently authorized. Twelve countries were selected based on publicly accessible data and consisted of Austria, Belgium, Denmark, France, Germany, Ireland, Poland, Spain, Sweden, Switzerland, the Netherlands, the United Kingdom. If information was confidential or not available for a specific country, the country was not analyzed further.
Official list prices and reimbursement status of the CFTR modulators were recovered from public sources and grey literature, namely, medicinal products databases, formularies and/or pharmaceutical registries and government-specific healthcare or reimbursement databases. The latter comprised of Belgian National Institute for Health and Disability Insurance (NIHDI) and Belgisch Centrum voor Farmacotherapeutische Informatie (BCFI), German Gemeinsamer Bundesausschuss (G-BA) and Rote Liste, Swedish Tandvårds-och läkemedelsförmånsverket (TLV), Scottish Medicines Consortium (SMC), English National Health Service (NHS) and National Institute for Health and Care Excellence (NICE), Dutch Geneesmiddelenvergoedingssysteem (GVS), French Ministère des Affaires Sociale et de la Santé and Centre National Hospitalier d'Information sur le Médicament (CNHIM), Danish Lægemiddelstyrelsen, Austrian Österreichische Sozialversicherung (SV), Irish Health Service Executive (HSE) and Swiss Federal Office of Public Health (FOPH). Additional information on reimbursement status was collected from parliamentary reports and the company’s press releases.
To conduct a comparison between countries, we converted list prices to prices per defined daily dose (DDD) which represents the assumed average maintenance dose per day for a medicine used for its main indication in adults (World Health Organization, 2021). If no DDD of the CFTR modulator was available for a specific dose, instead, we converted list price to price per average daily dose (ADD) as indicated in the package leaflet. For Kalydeco’s® (ivacaftor) dose of 150 mg, a DDD of 0.3g was specified (WHO, 2020). Only for Orkambi’s® (lumacaftor/ivacaftor) tablet dose of 200 mg/125 mg, a DDD of four tablets was stated (WHO, 2021). For the other tablet dose of 100 mg/125 mg of Orkambi® (lumacaftor/ivacaftor), we adopted an ADD of four tablets instead (European Medicines Agency, 2015). For Symkevi® (tezacaftor/ivacaftor), no DDD was released thus the ADD was defined as one 100mg/150 mg Symkevi® (tezacaftor/ivacaftor) tablet combined with one 150 mg Kalydeco® (ivacaftor) tablet. (European Medicines Agency, 2018). List prices comprised of pharmacist fee and value added tax (VAT). If the price was listed without pharmacist fee, it was specified, or without tax, it was recalculated with the VAT rate on prescription-only medicines from the corresponding country (Bundesverband der Pharmazeutischen Industrie (BPI), 2020). Currencies were subsequently converted to 2021 € with Belgium as the target country using the ‘CCEMG - EPPI-Centre Cost Converter’ online tool (The Campbell and Cochrane Economics Methods Group and the Evidence for CCEMG, 2021). It was assumed that original data related to the year of the data source. Finally, prices were rounded to the unit.
To compare economic evaluations, we considered following design parameters; model, perspective, comparator, time horizon, costs and discounting. The incremental cost-effectiveness ratio (ICER) or cost per quality-adjusted life years (QALY) and sensitivity analyses were also included.
Furthermore, publicly available BIAs were reviewed on their design including perspective, time horizon, target population (size), costs, discounting and uncertainty. The results of BIAs were also reported. This information was gathered from reimbursement applications or health technology appraisal reports from the respective agencies.
We determined whether a financial or performance-based MEA, between the company and national healthcare payers for Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) existed for reimbursement in some European countries. Lastly, the impact of multinational collaborations on market access to PWCF was assessed by reviewing literature. To that end, Pubmed, ISPOR, national healthcare payers’ websites and the company’s official website were consulted.
Results
List Prices
List prices for Kalydeco® (ivacaftor) ranged from €417 to €850 per DDD in Belgium and Spain, respectively (see Figure 1). For Orkambi® (lumacaftor/ivacaftor), the lowest price was at €309 per DDD in Switzerland and the highest price was at €983 per DDD in Poland. Symkevi® (tezacaftor/ivacaftor) prices varied between €372 per ADD in Belgium and €761 per ADD in Spain. No price for Kalydeco® (ivacaftor) and Symkevi® (tezacaftor/ivacaftor) was available for Poland. Except for in Denmark, Kalydeco® (ivacaftor) was considerably higher priced than Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor). Compared to Orkambi® (lumacaftor/ivacaftor), Symkevi® (tezacaftor/ivacaftor) was more expensive apart from in Belgium, Demark, Germany and France where both treatments’ prices were similar.
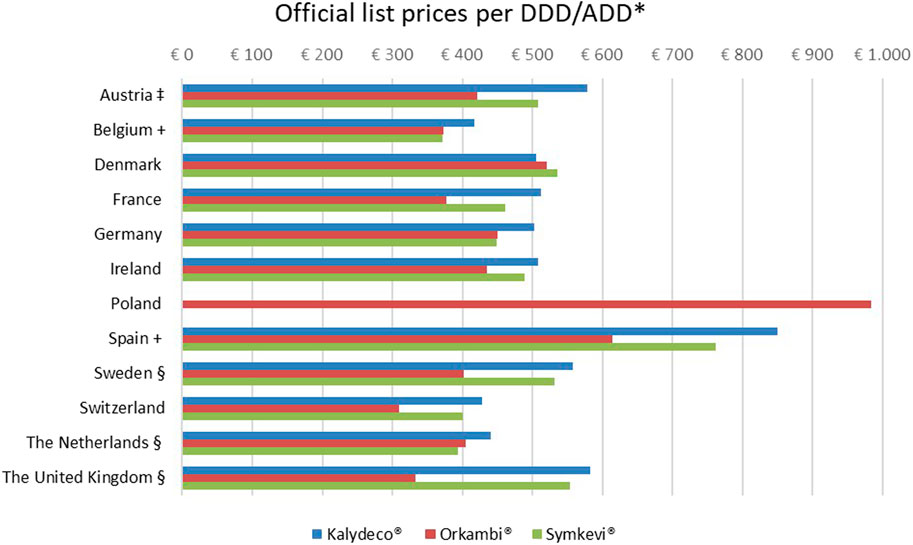
FIGURE 1. Official list prices per DDD/ADD of Kalydeco®, Orkambi® and Symkevi®. DDD, Defined Daily Dose. ADD, Average Daily Dose. *Consulted on September 22, 2021, VAT included and expressed in 2021 €. ‡10% VAT rate included manually. +Tablet form of Orkambi®: 100/125 mg instead of 200/125 mg §pharmacist fee excluded. References: Austria (Österreichische Sozialversicherung, 2021). Belgium (RIZIV, 2021a; RIZIV, 2021c; RIZIV, 2021b). Denmark (Danish Medicines Agency, 2021c; Danish Medicines Agency, 2021b; Danish Medicines Agency, 2021a). France (Theriaque CNHIM - Centre National Hospitalier d'Information sur le Médicament, 2021a; Theriaque CNHIM - Centre National Hospitalier d'Information sur le Médicament, 2021b; Thériaque CNHIM - Centre National Hospitalier d'Information sur le Médicament, 2021). Germany (Rote Liste, 2021c; Rote Liste, 2021a; Rote Liste, 2021b). Ireland (Health Service Executive (HSE), 2021). Poland (medycyna praktyczna, 2021). Spain (Vademecum, 2021a; Vademecum, 2021c; Vademecum, 2021b). Sweden (Tandvårds-och läkemedelsförmånsverket, 2021c; Tandvårds- och läkemedelsförmånsverket, 2021b; Tandvårds- och läkemedelsförmånsverket, 2021a). Switzerland (Office fédéral de la santé publique, 2021; Open Drug Database, 2021b; Open Drug Database, 2021c; Open Drug Database, 2021a). Netherlands (Zorginstituut Nederland, 2021b; Zorginstituut Nederland, 2021c; Zorginstituut Nederland, 2021d. The United Kingdom (NICE British National Formulary, 2021b; NICE British National Formulary, 2021c; NICE British National Formulary, 2021a).
Reimbursement Status
The three CFTR modulators were fully reimbursed in ten out of 12 examined countries except from Sweden and Poland (see Table 2). In France, healthcare authorities decided to officially reimburse Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) at a partial rate of 65%, however, PLWCF were exempt from any out-of-pocket costs through the long-lasting illness (ALD) scheme (Haute Autorité de Santé, 2014, Haute Autorité de Santé, 2019; Haute Autorité de Santé, 2020c, APM News, 2021; L’assurance maladie (ameli), 2021; Vaincre la mucoviscidose, 2021). A reimbursement decision for Symkevi® (tezacaftor/ivacaftor) was reached in 2021, 1 year after the positive reimbursement advice in May of 2020 (Haute Autorité de Santé HAS, 2020c; Vertex Pharmaceuticals Incorporated, 2021). Furthermore, in Switzerland, Kalydeco® (ivacaftor) reimbursement is conditioned by particular clinical modifications (Bundesamt für Gesundheit BAG, 2015). The Swedish TLV negatively decided on reimbursement of Kalydeco® (ivacaftor) and Symkevi® (tezacaftor/ivacaftor) (Tandvårds-och läkemedelsförmånsverket TLV, 2019). In Poland, no CFTR modulator is currently reimbursed and an official administrative decision after negative advice from the Economic Commission is awaited (Miłkowski, 2020; Munyama et al., 2020).
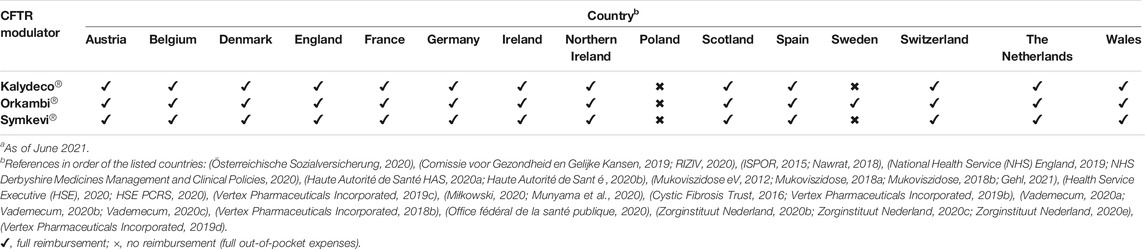
TABLE 2. Reimbursement status of Kalydeco®, Orkambi® and Symkevi® in specific European countries.a
Economic Evaluations
Tables 3–5 show the company’s and/or health authorities’ economic evaluations per investigated country, in terms of design and cost-effectiveness (ICER) for Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor), respectively.
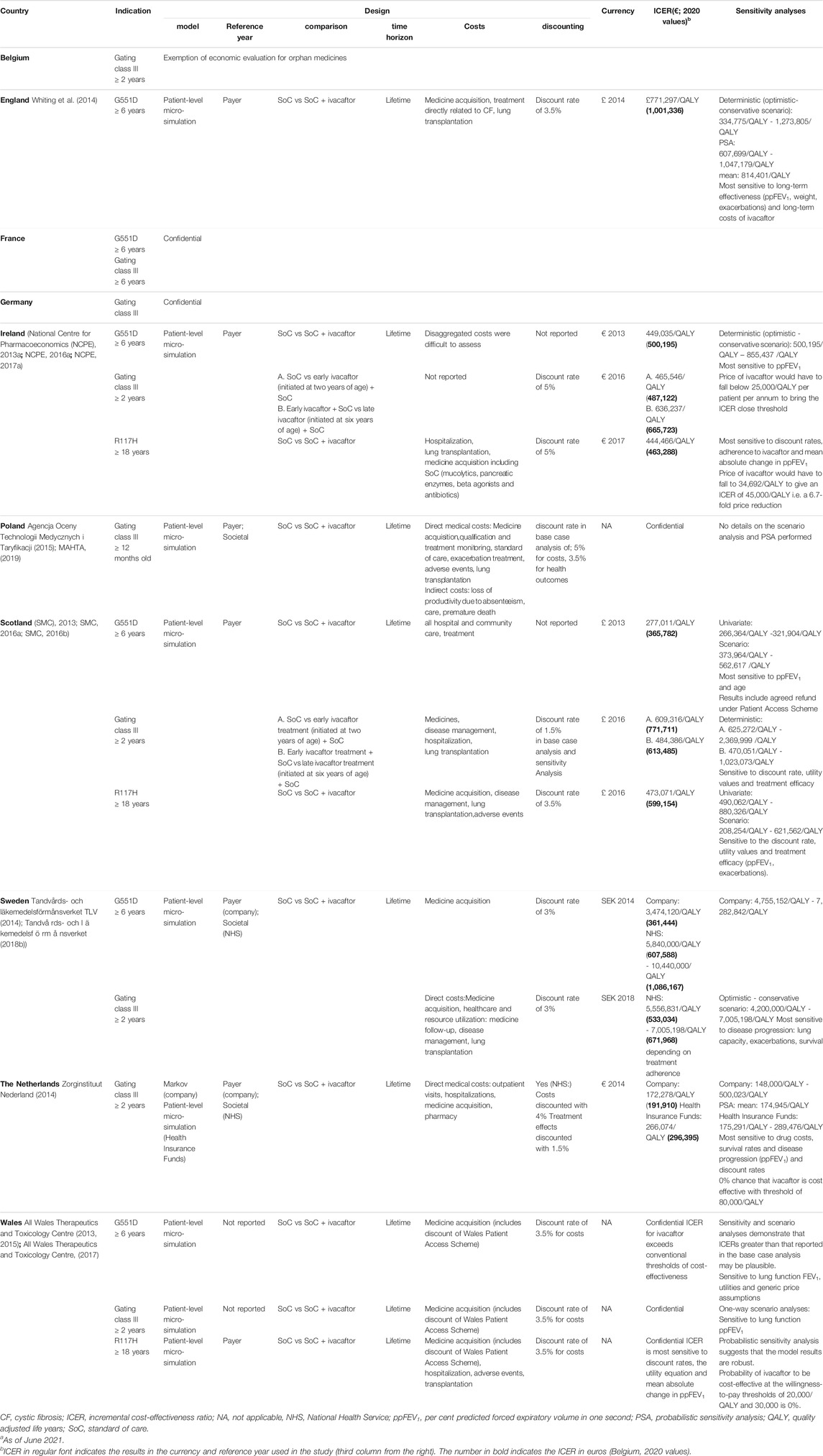
TABLE 3. Overview of the economic evaluation of different European countries for Kalydeco® (ivacaftor).a
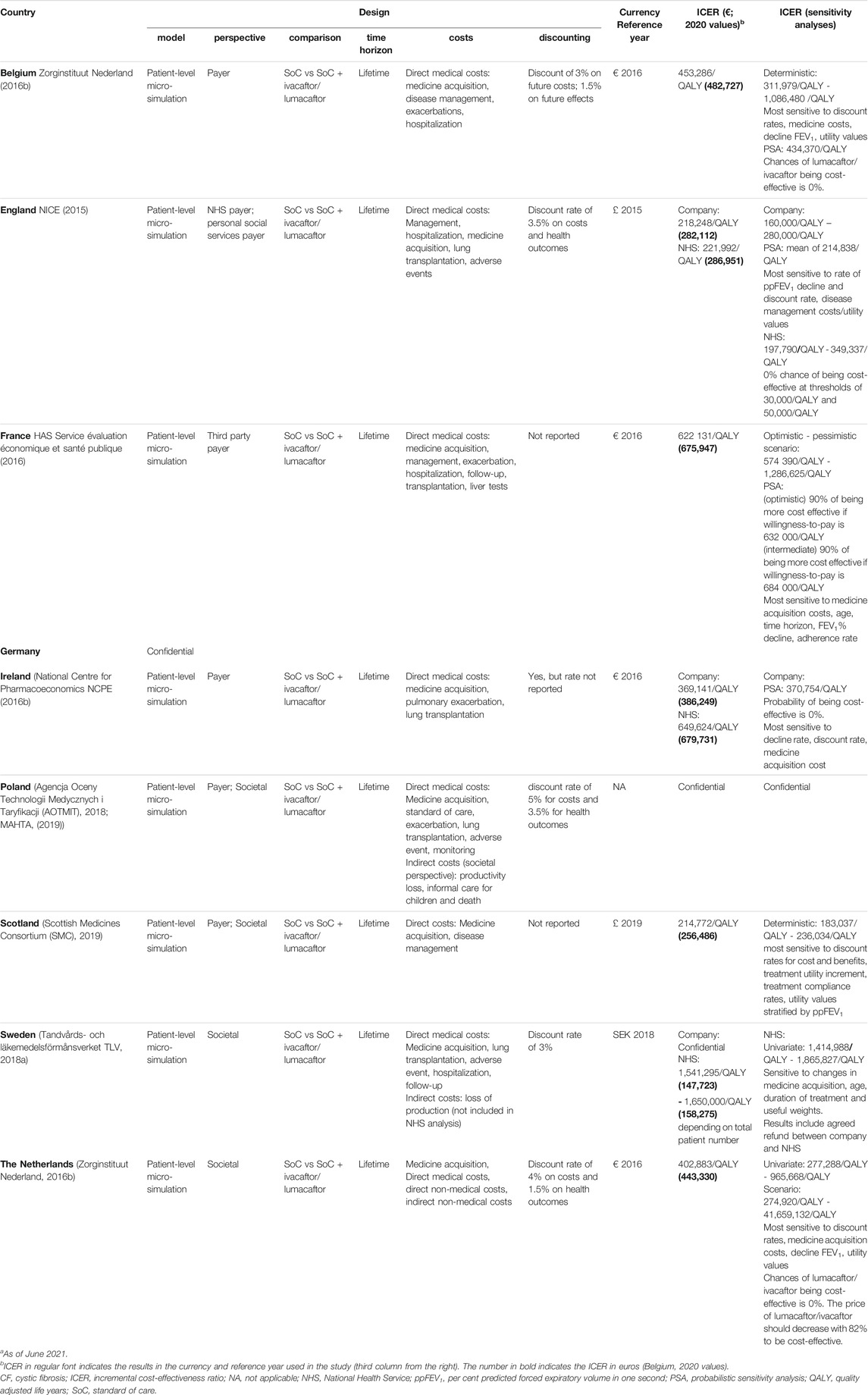
TABLE 4. Overview of the economic evaluation of different European countries for Orkambi® (lumacaftor/ivacaftor).a
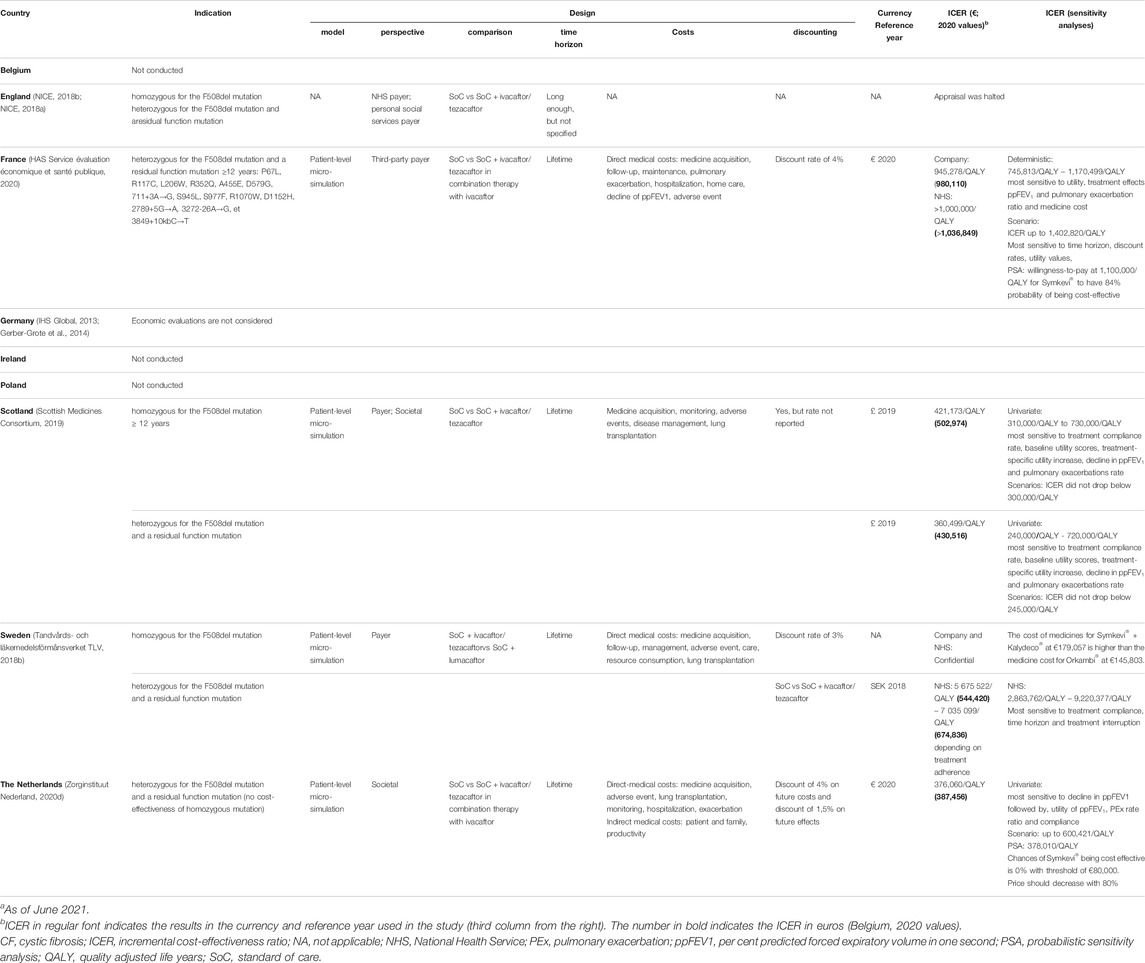
TABLE 5. Overview of the economic evaluation of different European countries for Symkevi® (tezacaftor/ivacaftor).a
Design
For Kalydeco® (ivacaftor) an economic evaluation was provided by the company and/or the country-specific healthcare authorities in the indications of G551D in children above six, gating class III children above two and/or R117H mutations in adults over 18 (see Table 3). A patient-level micro-simulation model, payer perspective, ivacaftor plus standard of care with standard of care only comparison and a lifetime horizon were adopted in most countries. However, differences were detected for: the Netherlands where the company carried out an evaluation based on a Markov model and the health authorities adopted a societal perspective; Poland and Sweden for which, respectively, the company and health authority adopted a societal perspective next to the payer’s perspective; Scotland and Sweden, for which early ivacaftor treatment (initiated at 2 years of age) was additionally compared to standard of care and late ivacaftor treatment (initiated at 6 years of age); Wales, for which the perspective in G551D and gating class III mutations indications were not reported. Adjustments to the economic evaluation design made by local health authorities were claimed to be more adaptive to their population’s characteristics. Reported costs often included medicine costs but also direct condition-related costs and indirect non-medical costs assessed in Poland. Furthermore, a discount rate for costs and/or health outcomes and sensitivity analyses, scenario or probabilistic, were generally considered.
In all countries, Orkambi® (lumacaftor/ivacaftor) was evaluated for people homozygous for the F508del CFTR mutation (see Table 4). In every economic evaluation, a patient-level micro-simulation model, third-party payer perspective and/or societal perspective was adopted. The treatment combined with the standard of care was compared to standard of care only. A lifetime horizon and medicine costs but also direct medical costs were generally considered. Poland and the Netherlands were the sole countries to also include indirect costs in their evaluation. When reported, a discount rate was applied to costs and health outcomes.
Symkevi® (tezacaftor/ivacaftor) was evaluated in its indication either in people homozygous for the F508del mutation and/or heterozygous for the F508del mutation with residual function mutation (see Table 5). Again, third-party payer perspective and/or societal perspective was adopted while the intervention, tezacaftor/ivacaftor combination therapy with ivacaftor, plus standard of care was compared to either standard of care only or standard of care and lumacaftor in the case of Sweden. Lifetime was generally considered as a time horizon. Furthermore, medicine costs, direct medical costs, and additionally, for the Netherlands, indirect costs were integrated in the economic evaluations. A discount rate on costs and/or outcomes was also applied.
Cost-Effectiveness (Incremental Cost-Effectiveness Ratio)
Kalydeco® (Ivacaftor)
The ICERs varied greatly per indication and across the analyzed countries (see Table 3). An ICER of €1M per QALY in the G551D indication was predicted for England. The company provided ICERs for G551D, gating class III (initiated at two or 6 years of age) and R117H mutations: for Ireland these were, respectively, €500K per QALY, €487K per QALY or €666K per QALY and €463K per QALY whereas for Scotland, values were, respectively, €366K per QALY, €772K per QALY or €613K per QALY and €599K per QALY. For Sweden, in the indication of G551D, the company estimated an ICER of €361K per QALY whereas their health authority adjusted this value to an ICER ranging between €608K and €1.1M per QALY and reported an ICER between €533K and €672K per QALY in the gating class III indication. Likewise, for gating class III mutations in the Netherlands, the company provided an ICER of €192K per QALY that was adjusted by their health insurance fund to a value of €296K per QALY. The ICER values predicted by local health authorities were generally higher, more accurate and less varying. Both deterministic and probabilistic sensitivity analyses showed that ICERs were, generally, most sensitive to treatment efficacy measurements, costs of ivacaftor, discount rates, age and utility values. France, Poland and Wales did not publicly disclose their ICER estimations.
Orkambi® (Lumacaftor/Ivacaftor)
The company predicted an ICER of €483K per QALY for Belgium, €282K per QALY for England, €676K per QALY for France, €386K per QALY for Ireland, €256K per QALY for Scotland and €443K per QALY for the Netherlands (see Table 4). English and Irish health authorities corrected the company’s predicted ICER to €287K per QALY and €680K per QALY, respectively. The Swedish health authority predicted an ICER between €148K and €158K per QALY while the company’s ICER was confidential. The ICERs, after correction by health authorities were estimated to be significantly higher than the company’s predictions. For England and Ireland, this meant an ICER that was approximately €5,000 per QALY and €300,000 per QALY, respectively, higher than the company’s predicted ICERs. For countries like England, Ireland and the Netherlands, that rely on an ICER threshold for reference, Orkambi® (lumacaftor/ivacaftor) had zero percent chances of being cost-effective. In France, Orkambi® (lumacaftor/ivacaftor) had a 90% probability of being cost-effective if the willingness-to-pay would at least be €632K per QALY while the Netherlands reported that the price of Orkambi® (lumacaftor/ivacaftor) should be reduced with about 82% to be deemed cost-effective. Deterministic and probabilistic sensitivity analyses indicated that ICER values were most sensitive to medicine costs, age, time horizon, treatment outcomes, adherence, utility values and discounting. No cost-effectiveness estimate was publicly available for Poland.
Symkevi® (Tezacaftor/Ivacaftor)
The company reports an ICER for France of €980K per QALY in the heterozygous indication whereas, an ICER of €503K per QALY for homozygotes and of €431K per QALY for heterozygotes was predicted for Scotland (see Table 5). For the Netherlands, an ICER of €387K per QALY in the heterozygous indication was estimated. However, health authorities in France believed the ICER prediction of the company to be an underestimation and claimed a more accurate ICER, specific to its population characteristics, to be above €1M per QALY. For Sweden, only an ICER value estimated to be between €544K and €675K per QALY for heterozygotes was reported by their health authority. Moreover, for homozygotes, Symkevi® (tezacaftor/ivacaftor) treatment cost was valued to be approximately €34,000 more expensive than that of Orkambi® (lumacaftor/ivacaftor). ICER ranges estimated by the health authorities were generally higher and depicted a smaller variation between the values. For all listed ICERs, a deterministic analysis was performed which showed highest sensitivity for utility values, treatment effects, medicine costs, adherence and discount rates. Probabilistic sensitivity analysis for France showed that the willingness-to-pay should be set at €1.1M for Symkevi® (tezacaftor/ivacaftor) to have an 84% probability of being cost-effective. For the Netherlands a price decrease of 80% would be required as Symkevi® (tezacaftor/ivacaftor) would have zero percent chances of being cost-effective considering the current price and threshold. England, Germany, Ireland and Poland had no public data on cost-effectiveness available.
Country-Specific Outcomes
Belgium does not consider cost-effectiveness for orphan medicines (Denis et al., 2009). In their analysis for Orkambi® (lumacaftor/ivacaftor), jointly assessed with the Netherlands, the Dutch ICER threshold of €80,000 per QALY was used as a reference and Orkambi® (lumacaftor/ivacaftor) was deemed not cost-effective.
Data on economic evaluations by the French HTA Agency (CEESP) were available for Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor), although France generally does not consider cost-effectiveness for reimbursement (Denis et al., 2009; Haute Autorité de Santé HAS, 2014; Haute Autorité de Santé, 2019; Haute Autorité de Santé, 2020c). For Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor), CEESP reported significant clinical uncertainties considering long term efficacy on FEV1% and pulmonary exacerbations and required a significant reduction in price for the interventions to be deemed cost-effective.
The Dutch Healthcare Institute adopts a threshold to determine cost-effectiveness and inform their Health Minister on reimbursement (Denis et al., 2009). The price of Kalydeco® (ivacaftor) would have to be reduced by 82% for the treatment to bring the ICER below the thresholds and be deemed cost-effective. Orkambi® (lumacaftor/ivacaftor) was given negative advice for reimbursement due to failed cost-effectiveness, insufficient clinically proven effect, lack of long-term data on lung function but also a limited patient eligibility (Zorginstituut Nederland, 2016a; Zorginstituut Nederland, 2019a). Symkevi® (tezacaftor/ivacaftor) was negatively advised for heterozygotes but positively advised for homozygotes with the condition that the price would not be set higher than Orkambi® (lumacaftor/ivacaftor)’s price given that it has a similar therapeutic value (Zorginstituut Nederland, 2019b; Zorginstituut Nederland, 2020a).
In Sweden, cost-effectiveness is flexible, influenced by disease severity and usually determined based on a range of €35,000 to €100,000 per QALY (Denis et al., 2009). However, cost-effectiveness is not a primary criterium and no official threshold is defined. Kalydeco® (ivacaftor)’s and Symkevi® (tezacaftor/ivacaftor)’s costs were not deemed reasonable compared to their clinical benefit and therefore not funded for any of their indication (Tandvårds-och läkemedelsförmånsverket TLV, 2019). In contrast, Orkambi® (lumacaftor/ivacaftor) was funded with the requirement to register specific effect parameters and a reduced cost.
For England, Kalydeco® (ivacaftor) was shown not to be cost-effective unless a discount would be agreed and ICER would fall within the increased ultra-orphan medicines threshold margin of £100,000 to £300,000 per QALY (Whiting et al., 2014; NHS England, 2015; National Institute for Health and Care Excellence (NICE), 2017; Kelly et al., 2018). Orkambi® (lumacaftor/ivacaftor) had zero percent chance of being cost-effective compared to the standard of care at their official threshold of £30,000 per QALY and was given a negative recommendation.
The Scottish Medicines Consortium (SMC) does not specify a formal ICER cut off but NHS’ threshold of £20,000 per QALY is often used as a reference (Scottish Medicines Consortium, 2021a). In some cases, a higher cost per QALY may be accepted and additional factors are assessed to determine value for money (Denis et al., 2009). SMC did not advise Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) nor Symkevi® (tezacaftor/ivacaftor) for reimbursement within NHS Scotland because of insufficient justification of the cost in relation to the health benefit and a lack of robust economic and clinical analysis (Scottish Medicines Consortium (SMC), 2013; SMC, 2016a; SMC, 2016b; Kelly et al., 2018; Scottish Medicines Consortium, 2019).
Ireland considered incremental cost-effectiveness with a threshold of €45,000 per QALY in their economic evaluation (National Centre for Pharmacoeconomics (NCPE), 2017b). NCPE suggested significant price reductions for Kalydeco® (ivacaftor) and Orkambi® (lumacaftor/ivacaftor) as acquisition costs were very high; no cost-effectiveness was proven and long-term clinical data was absent (National Centre for Pharmacoeconomics (NCPE), 2013b; NCPE, 2016c). Symkevi® (tezacaftor/ivacaftor) was not subject to HTA.
In Poland, cost-effectiveness with an ICER threshold of three times their GDP per capita of that year, is considered (Kolasa et al., 2018). The Polish HTA Agency (AOTMiT) gave a negative recommendation for Kalydeco® (ivacaftor) and Orkambi® (lumacaftor/ivacaftor) because of insufficient clinical evidence, poor quality data and cost in relation to the benefit being insufficiently justified (Agencja Oceny Technologii Medycznych i Taryfikacji, 2015; Agencja Oceny Technologii Medycznych i Taryfikacji (AOTMIT), 2018). No HTA report for was currently available for Symkevi® (tezacaftor/ivacaftor) although a reimbursement application was filed in February of 2021 (Oddech Zycia, 2021).
In Wales, the English NHS threshold of £100,000 to £300,000 per QALY for the economic evaluation of ultra-orphan medicines was adopted (Denis et al., 2009; National Institute for Health and Care Excellence (NICE), 2017). Kalydeco® (ivacaftor) was negatively recommended by their health technology assessment body (AWMSGs) as issues surrounding cost-effectiveness and clinical uncertainties were defined (Drakeford, 2013; All Wales Medicines Strategy Group (AWMSG), 2019).
Budget Impact Analyses
Kalydeco® (Ivacaftor)
For most countries a BIA was provided in the indication of gating class III mutations and/or G551D mutations (see Table 6). In addition, England, Scotland and Wales also analyzed the budget impact in the indication of R117H mutations. In terms of design, payer’s perspective was adopted in all cases, except for Wales that did not report their perspective. Budget impact results were depicted over an annual, 3-year, 5-year and/or life time horizon. Population size varied per country and depending on the indication. An open population was considered in Ireland, Scotland and Wales, in the indications G551D and R117H mutations. In Scotland, market uptake of Kalydeco® (ivacaftor) for the gating class III and for G551D mutations were estimated to be 100 and 90%, respectively. For the Netherlands a market expansion with a treatment uptake of 100% was predicted. Medicine only costs were considered in Belgium, Poland, Sweden and the Netherlands, disaggregated costs were not reported for Ireland and all other countries considered costs beyond medication costs. Discount rates were generally not adopted or confidential, except for England and for Ireland in the indications of gating class III and R117H mutations. Overall, detailed information on handling uncertainty was confidential, however, England, Poland and the Netherlands performed deterministic sensitivity analyses and Wales performed a probabilistic sensitivity analysis of patient number and disease management costs. Some countries reported one gross or net budget impact per indication, while others disaggregated their estimates and stated the first and last year budget impact over the chosen horizon.
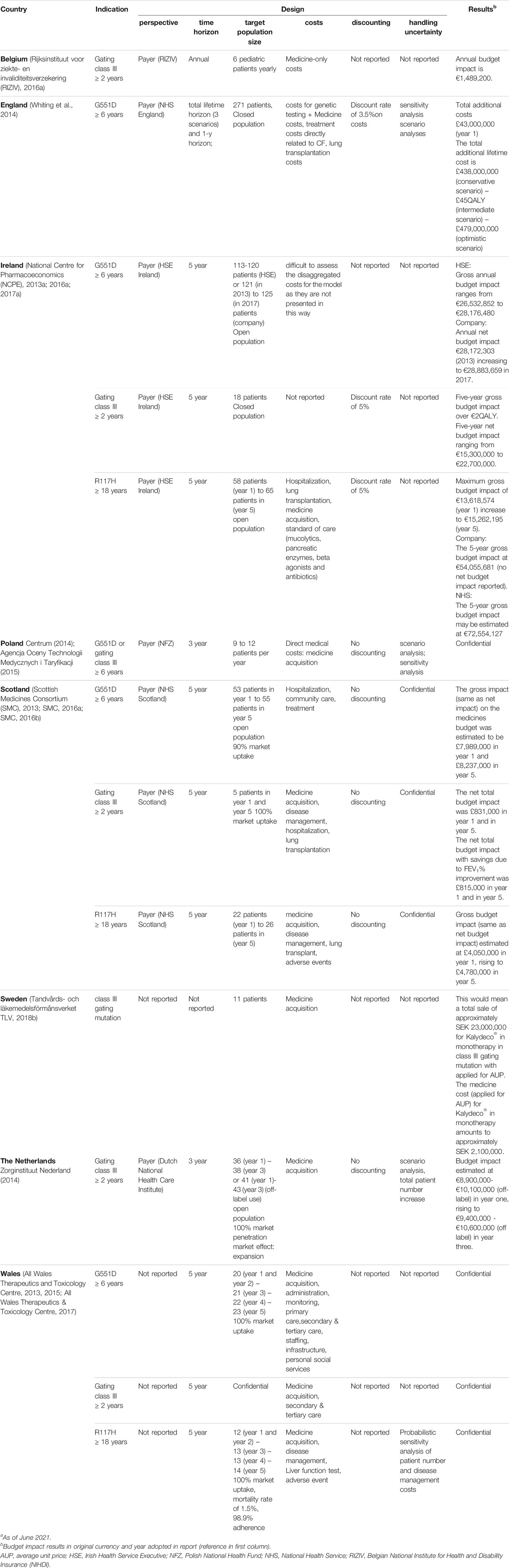
TABLE 6. Overview of budget impact analyses of different European countries for Kalydeco®.a
Orkambi® (Lumacaftor/Ivacaftor)
A BIA was conducted for patients homozygous for the F508del mutation in all selected countries (see Table 7). Calculations were done from the perspective of the payer and the chosen time frame differed, from a 1-year to a 3-year and a 5-year horizon, respectively in Poland, Belgium and the remaining countries. An open population was only considered in Scotland, where patient number dynamically changed with discontinuation and in England, where they accounted for adherence and a yearly incremental market uptake. Other countries considered a closed population. Additionally, Belgium and the Netherlands reported a possible larger population size, in case Orkambi® (lumacaftor/ivacaftor) would expand the current treatment market and be entirely adopted by all ages. Direct medical costs beyond medicine-associated costs, such as hospitalization and adverse events costs, were included in the analyses of England and Ireland only. No information on discount rates was publicly released. Only Poland reported on the use of sensitivity analysis and patient number influencing the potential budget impact. Belgium, the Netherlands and England disaggregated budget impact results and reported yearly amounts. In England, both budget estimates of the company and the national health service were reported, with the latter being slightly higher. One total budget impact estimation over the analyzed time horizon was reported for England, Ireland and Poland.
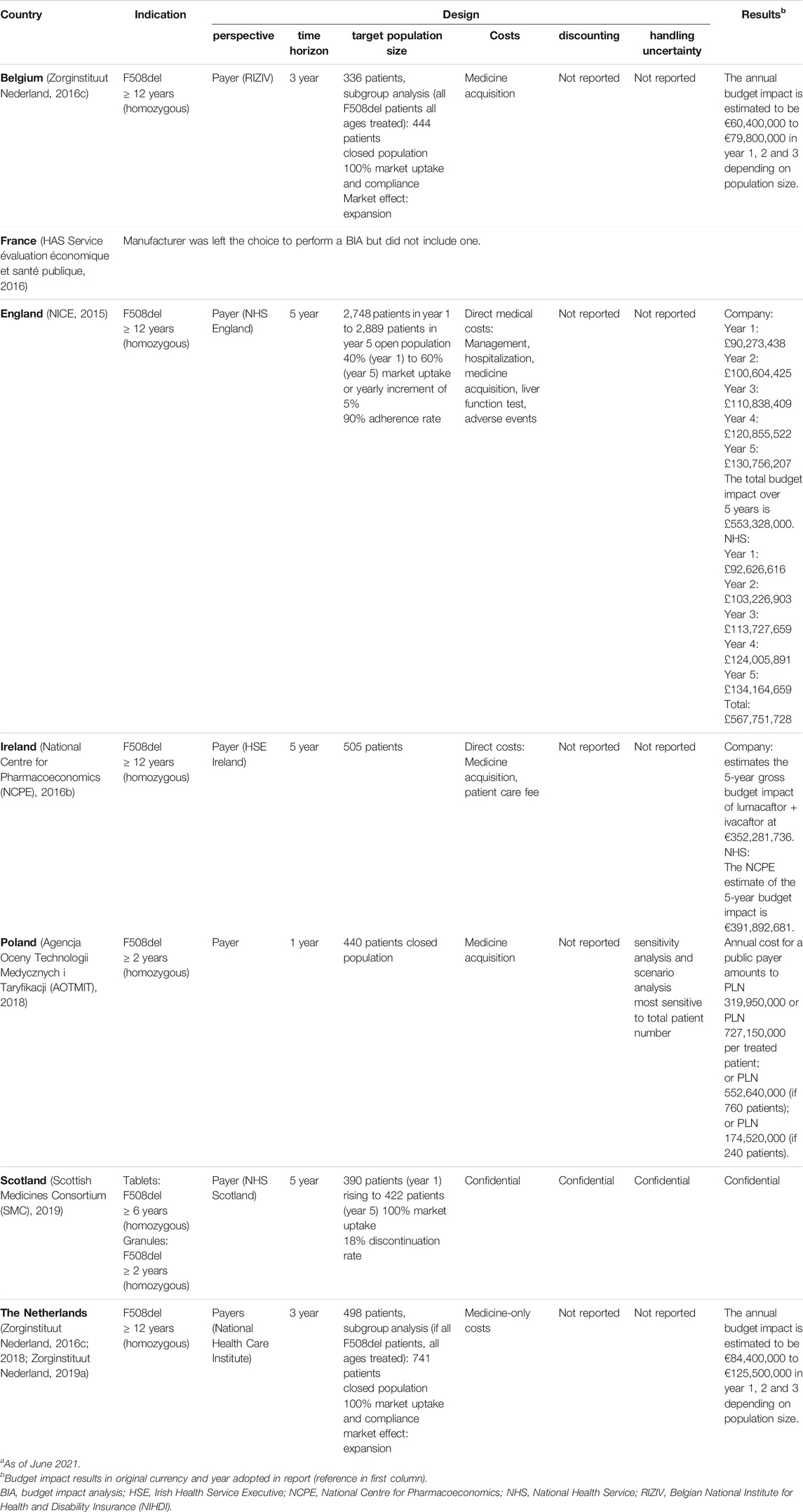
TABLE 7. Overview of budget impact analyses of different European countries for Orkambi®.a
Symkevi® (Tezacaftor/Ivacaftor)
BIAs for Symkevi® (tezacaftor/ivacaftor) were performed in the indication of homozygous F508del and/or heterozygous F508del with residual CFTR function mutation (see Table 8). For all countries the payer’s perspective was adopted to estimate the impact while time horizons included 3-year horizons for the Netherlands and Sweden, a 5-year horizon for Scotland and a lifetime horizon in the case of Sweden. An open population was considered in France and Scotland with the latter country also reporting a 100% market uptake while changes in population size incur partly due to discontinuation. Sweden and the Netherlands studied a closed population. Netherlands predicted market expansion and an alternative population size in case of full market uptake of Symkevi® (tezacaftor/ivacaftor) across all ages in the heterozygous indication. With respect to the scope of costs, medicine-only costs were generally considered while direct medical costs beyond medicine-costs, such as follow-up and maintenance costs, were reported in France only. Discount rates were generally not reported and uncertainty in the analyses for France and the Netherlands was addressed by scenarios. The latter, particularly for the Netherlands, was done by alternating treatment compliance rate. Budget impact results were generally confidential, only Sweden and the Netherlands published one total annual estimate over their respective time horizons.
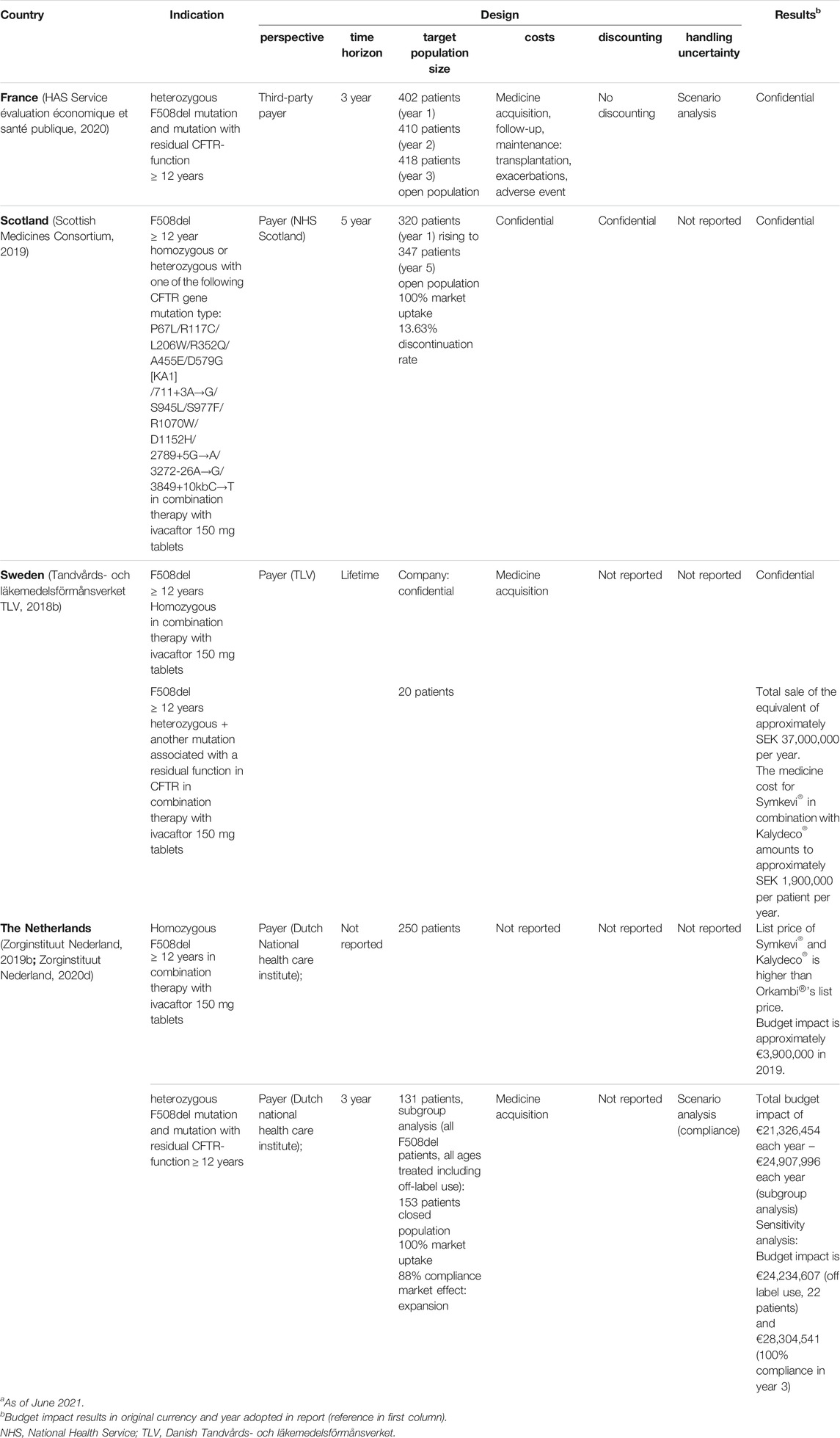
TABLE 8. Overview of budget impact analyses of different European countries for Symkevi®.a
Managed Entry Agreements
To have CF products reimbursed, the company and some European countries have set up a unique portfolio-deal agreement (Bruce, 2018; Vertex Pharmaceuticals Incorporated, 2018c). The concept was introduced to pay for the company’s CF products considered expensive, not cost-effective and clinically uncertain in many jurisdictions. These portfolio deals aim to facilitate entry of the company’s current products and those in the pipeline for the treatment of CF, while mitigating potential risks for their reimbursement (Rawson, 2018). To that end, a confidential discounted price based on caps, is agreed upon and, in many instances, this contract is coupled with the collection of data concerning clinical uncertainties.
The Republic of Ireland pioneered in 2017, as the first market to establish this portfolio approach for Kalydeco® (ivacaftor) and Orkambi® (lumacaftor/ivacaftor) and the company’s future CF products (Vertex Pharmaceuticals Incorporated, 2017; Vertex Pharmaceuticals Incorporated, 2018c). This agreement, with HSE, formed the blueprint for similar subsequent contracts between the company and Swedish TLV and county councils but also the Danish pharmaceutical and procurement body, Amgros (Nawrat, 2018; Vertex Pharmaceuticals Incorporated, 2018c; Vertex Pharmaceuticals Incorporated, 2018b; Vertex Pharmaceuticals Incorporated, 2018a). A recent study claims that the agreements in Sweden are mostly cost-sharing to address affordability whereas clinical uncertainties usually remain unsolved (Andersson et al., 2020). In Denmark, the price caps in the agreement are linked to the number of patients adopting the treatments (Bruce, 2018). In 2019, the company managed to bring its portfolio approach to England, Northern Ireland and Wales (Vertex Pharmaceuticals Incorporated, 2019c; National Institute for Health and Care Excellence (NICE), 2021). This agreement is performance based and supersedes any previous agreement between the company and NICE (National Institute for Health and Care Excellence (NICE), 2021). Under this deal, the company is required to deliver answers to clinical uncertainties that arose after health technology appraisal. These a priori defined elements and data are collected in the UK CF registry, that is monitored by NICE and funded by the company.
In other markets where reimbursement of the CF products exists, the company has agreed on other proposals.
Switzerland reached an agreement for the eligible population of Orkambi® (lumacaftor/ivacaftor) and Symkevi®/Symdeko® (tezacaftor/ivacaftor) along with any future extension by age for Symkevi®/Symdeko® (tezacaftor/ivacaftor) (Carvalho, 2020; Plüss, 2020; Vertex Pharmaceuticals Incorporated, 2020). These medicines were added to the Swiss medicine specialties list and are reimbursed by health insurance. This deal could also facilitate future market entry of Kaftrio®/Trikafta® (ivacaftor/tezacaftor/elexacaftor) for which an application has been filed with Swissmedic.
In Scotland, a 5-year interim deal for Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) was realized in 2019, requiring to collect real-world evidence and to resubmit the medicines to the Scottish Medicines Consortium during the contract period (Vertex Pharmaceuticals Incorporated, 2019b; Cystic Fibrosis Trust, 2019). In 2020, a deal for the triple-therapy, Kaftrio® (ivacaftor/tezacaftor/elexacaftor) was reached even before market authorization in Europe (Cystic Fibrosis Trust, 2020).
In 2016, a pay-for-performance agreement was set up between the company and NIHDI due to remaining concerns about high budget impact and effectiveness, in terms of disease progression, survival rates and hospitalization rates (Comissie voor Gezondheid en Gelijke Kansen, 2019; Comissie voor Gezondheid en Gelijke Kansen, 2021). This allowed for a 3-year temporary inclusion of Kalydeco® (ivacaftor) on the Belgian reimbursement list. In return, the company was required to collect data and resolve established clinical uncertainties (Fair Healthdata, 2015; Sectoraal Comité, 2017). To account for the budgetary risks, a yearly amount based on profits and number of treated patients was refunded to NIHDI (Rijksinstituut voor ziekte-en invaliditeitsverzekering (RIZIV, 2016a; RIZIV, 2016b). Since the end of the agreement, it has been amended, renewed and is still ongoing (Rijksinstituut voor ziekte-en invaliditeitsverzekering (RIZIV, 2019). An agreement for the reimbursement of cystic fibrosis medicines, Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor), was reached in March of 2021 (Mucovereniging, 2021; Vlaamse Radio-en Televisieomroeporganisatie (VRT), 2021). That same month, the company applied for reimbursement of their most recent innovative therapy, Kaftrio® (ivacaftor/tezacaftor/elexacaftor).
In the Netherlands, although not cost-effective, Orkambi® (lumacaftor/ivacaftor) was added to their reimbursement list (van Rijn, 2016). Currently, a confidential price-agreement with conditions is set up between the company and the government for all three modulators (Zorginstituut Nederland, 2019a; Zorginstituut Nederland, 2019b; Zorginstituut Nederland, 2021a). Likewise, for Orkambi® (lumacaftor/ivacaftor), a straight reimbursement deal was achieved in Austria but also Kalydeco® (ivacaftor) and Symkevi® (tezacaftor/ivacaftor) are found on their specialty list (Pinto, 2018; Rawson, 2018; Vertex Pharmaceuticals Incorporated, 2018a; Österreichische Sozialversicherung, 2020).
Both Kalydeco® (ivacaftor) and Orkambi® (lumacaftor/ivacaftor) are reimbursed in France (Vertex Pharmaceuticals Incorporated, 2019). Kalydeco® (ivacaftor) was given a positive decision after reimbursement application (Haute Autorité de Santé HAS, 2014). For 4 years, Orkambi® (lumacaftor/ivacaftor) was available to a set of patients through a temporary use authorization (ATU) until a price agreement was achieved (Association Gregory Le Marchal - Vaincre la Mucoviscidose, 2019). Recently, Symkevi® (tezacaftor/ivacaftor) price negotiations were finalized and the medicine was added to the reimbursement list (Journal Officiel de le République Française, 2021; La Voix Du Nord, 2021; Vertex Pharmaceuticals Incorporated, 2021).
Since the market authorization of the cystic fibrosis medicines by the European Commission, the modulators are available in Germany (GKV-Spitzenverband, 2012; GKV-Spitzenverband, 2015; Vertex Pharmaceuticals Incorporated, 2016; GKV-Spitzenverband, 2018a). However, a reimbursement agreement between the German National Association of Statutory Health Insurance Funds (GKV) and the company was founded on the obligation of the pharmaceutical company to automatically report the CF modulators’ price and product information through electronic data transmission, in accordance with legal Section 131 (4) SGB V (GKV-Spitzenverband, 2018b).
In Spain, managed entry of Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) in combination with Kalydeco® (ivacaftor) was obtained by establishing a mixed model of financing (Rivera, 2019). Spanish health authorities agreed on the company’s proposal for a cap on spending combined with pay-for-performance reflecting clinical uncertainty (Vertex Pharmaceuticals Incorporated, 2019a; Grubert, 2019).
International Collaborations
Several countries were hesitant to adopt Orkambi® (lumacaftor/ivacaftor) due to its high price and clinical uncertainties. To mitigate these uncertainties, Belgium and the Netherlands performed a joint price negotiation as part of the Beneluxa initiative in 2015 (O'Donnell, 2015; Paun, 2018; Rawson, 2018; Beneluxa Initiative on Pharmaceutical Policy, 2021). The negotiation was a pilot study of a larger international collaboration, additionally involving Luxembourg, Austria and Ireland, which was set up to jointly assess highly priced and innovative medicines often intended for a small population (De Block, 2015). In the case of Orkambi® (lumacaftor/ivacaftor), Belgian and Dutch negotiations resulted in a negative decision to reimburse the medicine as no agreement could be established (Allen, 2017). The Ministers of Health deemed the medicine to be overpriced and not cost-effective (van Rijn, 2016; De Block, 2017). A price reduction of 82% was requested to make Orkambi® (lumacaftor/ivacaftor) cost-effective. Ultimately, Netherlands managed to strike a deal with the company alone. Another 4 years was needed for Orkambi® (lumacaftor/ivacaftor) to be reimbursed and available in Belgium (Zorginstituut Nederland, 2017; Mucovereniging, 2021).
Discussion
Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) were the first treatments authorized in the European Union to target the underlying mechanism of the dysfunctional CFTR protein in cystic fibrosis (Lopes-Pacheco, 2016; Rafeeq and Murad, 2017; Lopes-Pacheco, 2019). With these treatments and the latest Kaftrio® (ivacaftor/tezacaftor/elexacaftor), about 90% of PWCF are able to be treated, however access to these CFTR modulators in EU Member States is challenged because of the associated high costs and constricted healthcare budgets (Schneider et al., 2017; Rawson, 2018).
Prices and Efficacy
Although, prices are closely relatable in some countries, our findings show that Kalydeco® (ivacaftor) is generally the most expensive CFTR modulator, followed by Symkevi® (tezacaftor/ivacaftor) and, lastly, by Orkambi® (lumacaftor/ivacaftor). This price relation may be reflective of the effectiveness of the modulators, as Kalydeco® (ivacaftor) has proven to be of highest clinical added value. At the launch of Kalydeco® (ivacaftor) unmet medical need for PWCF was high and no alternative was available. The modulator showed significant lung improvement in gating mutations but was indicated for a small population. (Lopes-Pacheco, 2019; European Medicines Agency, 2021b). More recently, an observational study confirmed the ability of treatment with Kalydeco® (ivacaftor) to be disease modifying (Bessonova et al., 2018). With the introduction of Orkambi® (lumacaftor/ivacaftor), the most common mutation in PWCF was able to be treated and clinical studies showed moderate lung function amelioration, however the tolerability of this treatment in patients with low baseline lung function was poor and interactions with other medication had been reported. With Symkevi® (tezacaftor/ivacaftor) a more extensive population is able to be treated with comparable but fewer side effects than Orkambi® (lumacaftor/ivacaftor) (Lopes-Pacheco, 2019). Health authorities might have had greater negotiation power and might have been stricter on price depending on clinical added value and unmet medical need with the second and third generation of medicines, namely Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor).
Furthermore, our results highlight apparent intra-variability when it comes to pricing of the same medicine in different European countries. As price-setting and HTA in Europe is determined nationally by the member states, price differences are inevitable (Young et al., 2017). It should also be noted that reported list prices may differ from the actual paid price subject to a discount determined in a confidential contract with the company. This discount differs among countries and is dependent on factors such as the country’s negotiation power and use of external reference pricing (Rémuzat et al., 2015). Additionally, prices for individuals may vary from these averages, as dosage differs according to weight and age group. Underlying price differences may also be influenced by the included pharmacist fees, wholesale quotas and the national VAT on prescription-only medicines.
Economic Evaluations
Overall, Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) were considered not cost-effective in the studied countries. HTA bodies unanimously reported clinical uncertainties on long-term lung function and requested a price reduction of the modulators for the ICER to fall below the adopted threshold or comply with their cost-effectiveness requirements. Some countries (Netherlands, England, Sweden, France) questioned the accuracy of the ICER values determined by the company and requested additional data to support their outcome or delivered a recalculated ICER value showing the company’s initial ICERs to be a considerable underestimation. Apparent discrepancies between ICER values internationally could be explained by differences in methodological guidelines for economic evaluations (Hay et al., 2010). The chosen simulation model, patient-level microsimulation or Markov, but also differences in perspective, payer or society, could affect the outcome (Schuller et al., 2015). Furthermore, differing ICERs could be influenced by the source for retrieval, from clinical trial or country-specific data, of input values such as QALYs or costs, particularities in healthcare system and the applied discount rate.
Cost-effectiveness might also be influenced by the approach adopted for the assessment of these CFTR modulators. In Poland and Ireland, Kalydeco® (ivacaftor) and Symkevi® (tezacaftor/ivacaftor) were assessed in their general HTA process for medicines and under the same criteria and threshold as non-orphan medicines (Caban et al., 2016; National Centre for Pharmacoeconomics (NCPE), 2017b; Vaithyanathan et al., 2018; Malinowski et al., 2019). It was shown that with these conditions, orphan medicines are most likely not to be cost-effective due to typical characteristics of a small population and limited clinical data availability. Some states, such as England, Scotland and Wales, established a HTA process specific to orphan medicines and others, like Sweden and the Netherlands, rely on a process dependent on disease severity, which allowed Kalydeco® (ivacaftor) and Symkevi® (tezacaftor/ivacaftor) to be measured against a higher or more flexible ICER threshold (Denis et al., 2009; NHS England, 2015; National Institute for Health and Care Excellence (NICE), 2017; Kelly et al., 2018; Tandvårds-och läkemedelsförmånsverket TLV, 2019). Other countries like Belgium, France and Germany do not rely on ICER values to determine the value of (orphan) medicines (Denis et al., 2009). In Belgium, the company was exempt of delivering a cost-effectiveness analysis and only a BIA for both orphan medicines was requested. In France, the CF modulators were evaluated according to their clinical added value or service medical rendu (SMR) and similarly in Germany, the assessment was based on additional medical benefit (Denis et al., 2009; IHS Global, 2013; Gerber-Grote et al., 2014; Haute Autorité de Santé HAS, 2014; Haute Autorité de Sant é , 2019; Haute Autorité de Sant é , 2020c).
Budget Impact Analyses
Budget impacts varied amongst countries and were dependent on the country’s CF patient number, medication prices, included or excluded treatment-related costs and discounting. Comparison of budget impact between countries and interventions was complicated as results were reported over varying time horizons and numerical outcomes were not depicted in a consistent form. Some countries such as England and Germany, considered a budget impact threshold (Ollendorf et al., 2017; Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG), 2020; IQWIG, 2021). In England this meant that commercial discussions were mandatory for reimbursement of Kalydeco® (ivacaftor) and Orkambi® (lumacaftor/ivacaftor). Germany did not release information on their budget impact calculations, however benefit reassessment by their health authority, G-BA, meant that both orphan medicines Kalydeco® (ivacaftor) and Symkevi® (tezacaftor/ivacaftor) breached their €50 million budget impact benchmark (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG), 2020; IQWIG, 2021). Overall, countries unanimously considered budget impact to be high. The accuracy of the budget estimates is not guaranteed as analyses did not always methodologically adhere to BIA guidelines, information was missing and some parameters were unspecified (Sullivan et al., 2014). Lack of transparency due to confidentiality also prevents insight into the actual budget impact. Assuring the methodological quality of future BIA could allow a more in-depth analysis and better informing of decision-makers on affordability (Abdallah et al., 2021).
Managed Entry Agreements
The reimbursement of the CF products was possible through MEAs between specific countries and the company. HTA reports emphasized not only the need to reduce prices substantially to increase affordability but also to address uncertainty around long-term clinical efficacy. To resolve uncertainties, some countries conditioned the reimbursement by requiring the company to monitor medicine administration and collect data on agreed efficacy measures in a register. Conventionally, these agreements are temporary, revised periodically and put in place for one product. In this case, the company pioneered with their portfolio-deal agreement for all their current and future CF products. The impact of this type of agreement on affordability and evidence collection is still uncertain, but arguably, agreeing to reimburse all future CF products without a rigorous HTA might have critical implications in the future.
Cross-Border Collaboration
Although the Netherlands and Belgium joined forces in price negotiations for reimbursement as a pioneer project under the Beneluxa initiative, an agreement could not be reached (Allen, 2017). To accommodate a seamless market access process for high cost and innovative medicines in the future, efforts towards information sharing and joint assessment such as done by Beneluxa and the International Horizon Scanning Initiative, should be maintained and further developed (Natsis, 2019; Beneluxa Initiative on Pharmaceutical Policy, 2021). Expansion in terms of number of countries participating in such initiatives should be further encouraged, as coalitions for negotiations with pharmaceutical companies have proven to be successful (Government of the Netherlands, 2018; Sheet, 2019). Moreover, to circumvent intricacies relating to various HTA processes amongst countries, performing assessments aggregately in an independent, joint network such as EUnetHTA could promote a more streamlined process. In turn, this could equip countries with more reliable, transparent and qualitative information to accurately perform their national HTA and increase their bargaining power with companies (O’Mahony, 2019; European Network For Health Technology Assessment, 2021).
Our study shows that despite failed cost-effectiveness, high budget impact and negative recommendations, Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) are reimbursed in the majority of analyzed countries. MEAs and portfolio deals allowed for the adoption of these CF medicines but also other decision criteria such as equity and equal access, disease severity, innovation, patients’ and clinicians’ views, patient advocacy, media attention but also prevalence seem to have played a role in final reimbursement decisions (Denis et al., 2009; Drakeford, 2013; National Institute for Health and Care Excellence (NICE), 2017; Ollendorf et al., 2017; Kelly et al., 2018; Tandvårds-och läkemedelsförmånsverket TLV, 2019; Andersson et al., 2020; Smith and Barry, 2020; Scottish Medicines Consortium, 2021b).
Strengths and Limitations
Our study sheds light on the market access of CFTR modulators in European countries based on a comprehensive analysis of pricing information, economic evaluations, BIAs, MEAs and reimbursement decisions. However, our findings were limited by public availability of data and confidentiality of reports. Depicted prices are facial prices and do not reflect the actual medicine price with discount. Critical information on cost-effectiveness and budget impact was often blacked-out or assessment reports were incomplete. Thus, the selection of countries in this study was based on availability of HTA documents. Little insight of MEAs was possible, therefore, details on considered clinical uncertainties and their influence on the final agreed discount is unknown.
Conclusion
This study shows that the CFTR modulators Kalydeco® (ivacaftor), Orkambi® (lumacaftor/ivacaftor) and Symkevi® (tezacaftor/ivacaftor) are generally considered to be expensive, not cost-effective and with a high budget impact in selected European countries. Reimbursement of these medicines was dependent on the ability of respective countries to form an agreement with the company. Even though most analyzed countries offered full reimbursement of treatments, some only selectively reimbursed certain treatments (Sweden) or none at all (Poland). Our findings point to unequal access, differential pricing and delayed availability of cystic fibrosis modulators in Europe.
Author Contributions
KA and SS contributed to conception and design of the study. KA acquired the necessary data for the review that was analysed and interpreted by all authors. KA drafted the manuscript under supervision of KDB, MD and SS. All authors critically revised the manuscript, read and approved the submitted version. Funding was obtained by SS.
Funding
This research was funded by the Research Foundation of Flanders (FWO, Fonds voor Wetenschappelijk Onderzoek) grant G0B9819N.
Conflict of Interest
SS has previously conducted research about market access of orphan medicines sponsored by the Belgian Health Care Knowledge Centre and by Genzyme (now Sanofi), and he has participated in an orphan medicine roundtable sponsored by Celgene. SS is a member of the ISPOR Rare Disease Special Interest Group’s Challenges in Research and Health Technology Assessment of Rare Disease Technologies Working Group, the International Working Group on Orphan Drugs, and the Innoval Working Group on Ultra-Rare Disorders. KDB has been principal investigator in several clinical trials conducted by Vertex Pharmaceuticals and for which her institution has received funding. She or her institution has received fees for participation in advisory boards, satellite symposia or specific projects.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
With special consideration to Sevengul Car, Enid Froeyen, Jordi Gombert and Sofie Vannuffelen for initiating this study.
References
Abdallah, K., Huys, I., Claes, K., and Simoens, S. (2021). Methodological Quality Assessment of Budget Impact Analyses for Orphan Drugs: A Systematic Review. Front. Pharmacol. 12, 630949. doi:10.3389/fphar.2021.630949
Agencja Oceny Technologii Medycznych i Taryfikacji (AOTMIT) (2018). Rekomendacja nr 105/2018 z dnia 9 listopada 2018 r. London: AOTMIT.
Agencja Oceny Technologii Medycznych i Taryfikacji (2015). Rekomendacja nr 54/2015 z dnia 22 czerwca 2015 r. London: AOTMIT.
All Wales Medicines Strategy Group (AWMSG) (2019). Correspondence Ref/CJP/rml/P-05-797 [Online]. Available at: https://business.senedd.wales/documents/s93802/07.08.19%20Correspondence%20-%20All%20Wales%20Medicines%20Strategy%20Group%20to%20Chair.pdf (Accessed June 30, 2021).
All Wales Therapeutics & Toxicology Centre (2017). AWMSG Secretariat Assessment Report. Ivacaftor: Kalydeco®. 150 mg film-coated tablets. Reference number:2680.
All Wales Therapeutics and Toxicology Centre (2013). AWMSG Secretariat Assessment Report 150 mg film-coated tablets. Ivacaftor: Kalydeco®. Reference number: 772.
All Wales Therapeutics and Toxicology Centre (2015). AWMSG Secretariat Assessment. Report. Ivacaftor: Kalydeco®. 150 mg film-coated tablets. Reference number: 2294.
Allen, O. (2017). Beneluxa collaboration joint pricing negotiations fail [Online]. Available at: https://www.allenovery.com/en-gb/global/blogs/life-science/beneluxa-collaboration-joint-pricing-negotiations-fail (Accessed June 20, 2021).
Andersson, E., Svensson, J., Persson, U., and Lindgren, P. (2020). Risk sharing in managed entry agreements-A review of the Swedish experience. Health Policy 124 (4), 404–410. doi:10.1016/j.healthpol.2020.02.002
APM News (2021). Mucoviscidose: publication de la prise en charge de Kaftrio* et Symkevi* [Online]. Available at: https://www.apmnews.com/freestory/10/369866/mucoviscidose-publication-de-la-prise-en-charge-de-kaftrio-et-symkevi (Accessed September 24, 2021).
Association Gregory Le Marchal - Vaincre la Mucoviscidose (2019). Accord en vue sur le prix d’Orkambi® 4 ans après l’AMM : entre soulagement et inquiétude!. [Online]. Available at: https://www.vaincrelamuco.org/sites/default/files/20191112_cp_vaincre_la_muco_accord_orkambi.pdf (Accessed June 17, 2021).
Balfour-Lynn, I. M., and King, J. A. (2020). CFTR modulator therapies - Effect on life expectancy in people with cystic fibrosis. Paediatric Respir. Rev., 2020. doi:10.1016/j.prrv.2020.05.002
Bell, S. C., Mall, M. A., Gutierrez, H., Macek, M., Madge, S., Davies, J. C., et al. (2020). The future of cystic fibrosis care: a global perspective. Lancet Respir. Med. 8 (1), 65–124. doi:10.1016/S2213-2600(19)30337-6
Beneluxa Initiative on Pharmaceutical Policy (2021). Beneluxa Initiative: Rationale, Vision, Areas of Cooperation, History and Participating Countries [Online]. Available at: https://beneluxa.org/collaboration (Accessed June 20, 2021).
Bessonova, L., Volkova, N., Higgins, M., Bengtsson, L., Tian, S., Simard, C., et al. (2018). Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax 73 (8), 731–740. doi:10.1136/thoraxjnl-2017-210394
Bruce, F. (2018). Vertex Extends Portfolio Pricing and Reimbursement Approach To Denmark [Online]. Available at: https://pink.pharmaintelligence.informa.com/PS124039/Vertex-Extends-Portfolio-Pricing-and-Reimbursement-Approach-To-Denmark (Accessed May 7, 2021).
Bundesamt für Gesundheit BAG (2015). Kalydeco, Vertex Pharmaceuticals (Switzerland) Sàrl Erweiterung der Limitation in der Spezialitätenliste per 1. November 2015[Online]. Available at: file:///C:/Users/u0129868/Downloads/Kalydeco%20Modifications%20de%20limitation%2001.11.2015.pdf (Accessed September 22, 2021).
Bundesverband der Pharmazeutischen Industrie (BPI) (2020). Value Added Tax. (VAT) rate on prescription-only drugs in Europe in 2020, by country* [Graph]. In Statista. [Online]. Available at: https://www-statista-com.kuleuven.e-bronnen.be/statistics/458957/vat-rate-on-prescription-only-drugs-in-europe/ (Accessed September 22, 2021).
Burgel, P. R., Bellis, G., Olesen, H. V., Viviani, L., Zolin, A., Blasi, F., et al. (2015). Future trends in cystic fibrosis demography in 34 European countries. Eur. Respir. J. 46, 133–141. doi:10.1183/09031936.00196314
Caban, A., Lach, S., Rémuzat, C., and Toumi, M. (2016). Access To Orphan Drugs In Poland - Is Change In Health Technology Assessment Approach Required. Value in Health 19 (7), A442–A443. doi:10.1016/j.jval.2016.09.559
Carvalho, J. (2020). Use of Vertex’s Orkambi and Symdeko To Be Reimbursed in Switzerland. [Online]. Available at: https://cysticfibrosisnewstoday.com/2020/04/23/vertex-enters-agreement-swiss-authorities-for-orkambi-symdeko-reimbursement-program/ (Accessed May 7, 2021).
CCEMGThe Campbell and Cochrane Economics Methods Group and the Evidence for Policy and Practice Information and Coordinating Centre (2021). CCEMG – EPPI-Centre Cost Converter [Online]. Available at: https://eppi.ioe.ac.uk/costconversion/default.aspx (Accessed June 23, 2021).
Centrum, H. T. A. (2014). Ocena konsekwencji finansowych dla płatnika publicznego decyzji o finansowaniu stosowania produktu Kalydeco® (iwakaftor) w leczeniu pacjentów z mukowiscydozą w ramach programu lekowego w warunkach polskich – analiza wplywu na system ochrony zdrowia.
Chevreul, K., Michel, M., Brigham, K. B., López-Bastida, J., Linertová, R., Oliva-Moreno, J., et al. (2016). Social/economic costs and health-related quality of life in patients with cystic fibrosis in Europe. Eur. J. Health Econ. 17 (Suppl. 1), 7–18. doi:10.1007/s10198-016-0781-6
Comissie voor Gezondheid en Gelijke Kansen (2019). Openbare Commissievergadering - 12 NOVEMBER 2019 Voormiddag. [Online]. Available at: https://www.dekamer.be/doc/CCRI/html/55/ic048x.html (Accessed November 25, 2020).
Comissie voor Gezondheid en Gelijke Kansen (2021). Openbare Commissievergadering - DINSDAG 5 JANUARI 2021. Voormiddag[Online]. Available at: https://www.dekamer.be/doc/CCRI/html/55/ic321x.html (Accessed June 23, 2021).
Cystic Fibrosis Foundation (2021a). Drug development pipeline [Online]. Available at: https://www.cff.org/trials/pipeline (Accessed March 4, 2021).
Cystic Fibrosis Foundation (2021b). Treatments and Therapies/Medications [Online]. Available at: https://www.cff.org/Life-With-CF/Treatments-and-Therapies/Medications/ (Accessed March 4, 2021).
Cystic Fibrosis Trust (2019). Breakthrough on Orkambi and Symkevi in Scotland. [Online]. Available at: https://www.cysticfibrosis.org.uk/news/breakthrough-on-orkambi-and-symkevi-in-scotland (Accessed May 7, 2021).
Cystic Fibrosis Trust (2020). Scotland agrees deal for life-saving triple therapy [Online]. Available at: https://www.cysticfibrosis.org.uk/news/scotland-agrees-deal-for-lifesaving-triple-therapy (Accessed May 7, 2021).
Cystic Fibrosis Trust (2016). Scotland leads the way with Kalydeco for younger children. [Online]. Available at: https://www.cysticfibrosis.org.uk/news/scotland-leads-the-way-with-kalydeco-for-younger-children (Accessed).
Danish Medicines Agency (2021a). Medicinpriser.dek Symkevi [Online]. Available at: https://www.medicinpriser.dk/Default.aspx?id=15&vnr=396604 (Accessed September 22, 2021).
Danish Medicines Agency (2021b). Medicinpriser.dk Kalydeco [Online]. Available at: https://www.medicinpriser.dk/Default.aspx?id=15&vnr=492666 (Accessed September 22, 2021).
Danish Medicines Agency (2021c). Medicinpriser.dk Orkambi [Online]. Available at: https://www.medicinpriser.dk/Default.aspx?id=15&vnr=418179 (Accessed September 22, 2021).
De Block, M. (2017). Prijsonderhandelingen Geneesmiddel Voor Mucoviscidose Beëindigd [Online]. Available at: https://www.maggiedeblock.be/prijsonderhandelingen-geneesmiddel-voor-cystische-fibrose-beeindigd/ (Accessed June 20, 2021).
De Block, M. (2015). TERUGBETALING WEESGENEESMIDDELEN: NEDERLAND EN BELGIË STAPPEN SAMEN NAAR FARMASECTOR. [Online]. Available at: https://www.maggiedeblock.be/terugbetaling-weesgeneesmiddelen-nederland-en-belgie-stappen-samen-naar-pharmasector/ (Accessed June 20, 2021).
De Boeck, K., Zolin, A., Cuppens, H., Olesen, H. V., and Viviani, L. (2014). The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J. Cyst Fibros 13 (4), 403–409. doi:10.1016/j.jcf.2013.12.003
Denis, A., Simoens, S., Fostier, C., Mergaert, L., and Cleemput, I. (2009). Beleid voor zeldzame ziekten en weesgeneesmiddelen - KCE reports 112A. [Online]. Brussel: Federaal Kenniscentrum voor de Gezondheidszorg (KCE). Available at: https://kce.fgov.be/sites/default/files/atoms/files/d20091027330.pdf (Accessed).
Drakeford, M. (2013). Written Statement - Access to new medicines: availability of ivacaftor (Kalydeco) within NHS Wales for the treatment of cystic fibrosis. [Online]. Available at: https://gov.wales/written-statement-access-new-medicines-availability-ivacaftor-kalydeco-within-nhs-wales-treatment (Accessed June 30, 2021).
EU Clinical Trials Register (2015a). EudraCT Number 2008-007416-15 [Online]. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2008-007416-15/results (Accessed March 5, 2021).
EU Clinical Trials Register (2015b). EudraCT Number 2012-000387-19 [Online]. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-000387-19/results (Accessed March 5, 2021).
EU Clinical Trials Register (2015c). EudraCT Number 2012-000388-26 [Online]. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-000388-26/results (Accessed March 5, 2021).
EU Clinical Trials Register (2017a). EudraCT Number 2014-004787-37 Results [Online]. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-004787-37/results (Accessed March 5, 2021).
EU Clinical Trials Register (2017b). EudraCT Number 2014-004837-13 Results [Online]. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-004837-13/results (Accessed March 5, 2021).
EU Clinical Trials Register (2017c). EudraCT Number 2015-000543-16 Results [Online]. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-000543-16/results (Accessed March 5, 2021).
EU Clinical Trials Register (2018). EudraCT number 2015-001267-39 Results [Online]. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-001267-39/results (Accessed March 5, 2021).
EU Clinical Trials Register (2019). EudraCT Number 2017-000457-39 Results [Online]. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-000457-39/results (Accessed March 5, 2021).
European Cystic Fibrosis Society (2020). ECFS Patient Registry General Information and Introduction [Online]. Available at: https://www.ecfs.eu/ecfspr (Accessed April 17, 2020).
European Medicines Agency (2021a). Kaftrio [Online]. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/kaftrio (Accessed March 4, 2021).
European Medicines Agency (2021b). Kalydeco [Online]. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/kalydeco (Accessed March 4, 2021).
European Medicines Agency (2021c). Orkambi [Online]. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/orkambi (Accessed March 4, 2021).
European Medicines Agency (2015). Orkambi, INN-lumacaftor & ivacaftor: EPAR: Product Information. [Online]. Available at: https://www.ema.europa.eu/en/documents/product-information/orkambi-epar-product-information_en.pdf (Accessed February 17, 2021).
European Medicines Agency (2021d). Symkevi [Online]. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/symkevi (Accessed March 4, 2021).
European Medicines Agency (2018). Symkevi, INN-tezacaftor/ivacaftor: EPAR - Product Information [Online]. Available at: https://www.ema.europa.eu/en/documents/product-information/symkevi-epar-product-information_en.pdf (Accessed February 17, 2021).
European Network For Health Technology Assessment (2021). About EUnetHTA. Vision, Mission, and Values. [Online]. Available at: https://www.eunethta.eu/about-eunethta/mission-vision-and-values/ (Accessed September 24, 2021).
Fair Healthdata (2015). Managed Entry Agreement - Evaluation of the new medication Kalydeco to treat cystic fibrosis [Online]. Available at: https://fair.healthdata.be/dataset/b562f5a1-baa4-4581-92aa-5b5cdb3ab743 (Accessed June 23, 2021).
Farrell, P. M. (2008). The prevalence of cystic fibrosis in the European Union. J. Cyst Fibros 7 (5), 450–453. doi:10.1016/j.jcf.2008.03.007
Frey, S., Stargardt, T., Schneider, U., and Schreyögg, J. (2019). The Economic Burden of Cystic Fibrosis in Germany from a Payer Perspective. Pharmacoeconomics 37 (8), 1029–1039. doi:10.1007/s40273-019-00797-2
Gehl, B. (2021). Mukoviszidose (zystische Fibrose). [Online]. Available at: https://www.mooci.org/krankheiten/mukoviszidose-zystische-fibrose/ (Accessed September 22, 2021).
Gerber-Grote, A., Sandmann, F. G., Zhou, M., ten Thoren, C., Schwalm, A., Weigel, C., et al. (2014). Decision making in Germany: Is health economic evaluation as a supporting tool a sleeping beauty. Z. Evid. Fortbild Qual. Gesundhwes 108 (7), 390–396. doi:10.1016/j.zefq.2014.06.018
GKV-Spitzenverband (2012). Erstattungsbetragsverhandlungen nach § 130b SGB V - Ivacaftor. [Online]. Available at: https://www.gkv-spitzenverband.de/krankenversicherung/arzneimittel/verhandlungen_nach_amnog/ebv_130b/wirkstoff_67640.jsp (Accessed June 17, 2021).
GKV-Spitzenverband (2015). Erstattungsbetragsverhandlungen nach § 130b SGB V - Lumacaftor./Ivacaftor[Online]. Available at: https://www.gkv-spitzenverband.de/krankenversicherung/arzneimittel/verhandlungen_nach_amnog/ebv_130b/wirkstoff_489409.jsp (Accessed June 17, 2021).
GKV-Spitzenverband (2018a). Erstattungsbetragsverhandlungen nach § 130b SGB V - Tezacaftor./Ivacaftor[Online]. Available at: https://www.gkv-spitzenverband.de/krankenversicherung/arzneimittel/verhandlungen_nach_amnog/ebv_130b/wirkstoff_967617.jsp (Accessed June 17, 2021).
GKV-Spitzenverband (2018b). Rahmenvertrag nach § 131 SGB V. [Online]. Available at: https://www.gkv-spitzenverband.de/media/dokumente/krankenversicherung_1/arzneimittel/rahmenvertraege/pharmazeutische_unternehmer/2018-03-26_Arzneimittel_Rahmenvertrag_131_SGB_V.pdf (Accessed June 17, 2021).
Government of the Netherlands (2018). Positive outcome of joint reimbursement negotiations on Spinraza [Online]. Available at: https://www.government.nl/ministries/ministry-of-health-welfare-and-sport/news/2018/07/12/positive-outcome-of-joint-reimbursement-negotiations-on-spinraza (Accessed July 21, 2021).
Grubert, N. (2019). Implications of landmark cystic fibrosis pricing agreements for the wider industry [Online]. Available at: https://www.linkedin.com/pulse/implications-landmark-cystic-fibrosis-pricing-wider-industry-grubert/ (Accessed June 17, 2021).
HAS Service évaluation économique et santé publique (2016). Avis d'efficience ORKAMBI (lumacaftor/ivacaftor) Vertex Pharmaceuticals.
HAS Service évaluation économique et santé publique (2020). Avis d’efficience SYMKEVI (Vertex Pharmaceuticals).
Haute Autorité de Santé, H. A. S. (2020a). Base de données publique des médicaments Fiche info: KALYDECO 150 mg. comprimé pelliculé[Online]. Available at: http://base-donnees-publique.medicaments.gouv.fr/extrait.php?specid=65036318 (Accessed).
Haute Autorité de Santé, H. A. S. (2020b). Base de données publique des médicaments Fiche info: ORKAMBI 200 mg/125 mg. comprimé pelliculé[Online]. Available at: http://base-donnees-publique.medicaments.gouv.fr/extrait.php?specid=66352529# (Accessed).
Haute Autorité de Santé, H. A. S. (2019). COMMISSION DE LA TRANSPARENCE Avis du 18 septembre 2019 sur l'Orkambi [Online]. Available at: https://www.has-sante.fr/upload/docs/evamed/CT-17846_ORKAMBI_PIC_INS_Avis2_CT17846.pdf (Accessed March 17, 2021).
Haute Autorité de Santé, H. A. S. (2014). TRANSPARENCY COMMITTEE Opinion 5 November 2014. on Kalydeco[Online]. Available at: https://www.has-sante.fr/upload/docs/application/pdf/2015-09/kalydeco_en_ct13972_val.pdf (Accessed March 17, 2021).
Haute Autorité de Santé, H. A. S. (2020c). Transparency Committee Opinion on Symkevi with Kalydeco [Online]. Available at: https://www.has-sante.fr/upload/docs/application/pdf/2020-09/symkevi_summary_ct18280_18279.pdf (Accessed July 4, 2021).
Hay, J. W., Smeeding, J., Carroll, N. V., Drummond, M., Garrison, L. P., Mansley, E. C., et al. (2010). Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report--Part I. Value Health 13 (1), 3–7. doi:10.1111/j.1524-4733.2009.00663.x
Health Service Executive (HSE) (2021). List of High Tech Items as at 1st February 2021 [Online]. Available at: https://www.hse.ie/eng/staff/pcrs/online-services/august-2020-high-tech-list.pdf (Accessed September 22, 2021).
Health Service Executive (HSE) (2020). List of high tech items [Online]. Available at: https://www.hse.ie/eng/staff/pcrs/online-services/high-tech-list-mar-2020.pdf (Accessed November 20, 2020).
HSE PCRS (2020). Reimbursable Items - Medicines and Aids provided. [Online]. Available at: https://www.sspcrs.ie/libr/html/monthlyproductupdate.pdf (Accessed November 25, 2020).
IHS Global (2013). G-BA defends position of Germany as innovation-friendly market as Kalydeco receives mixed verdict. [Online]. Available at: https://ihsmarkit.com/country-industry-forecasting.html?ID=1065976094 (Accessed June 27, 2021).
Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG) (2020). Tezacaftor/ivacaftor (combination with ivacaftor; cystic fibrosis, 12 years and older, F508del mutation, homozygous) – Benefit assessment according to §35a Social Code. Book V[Online]. Available at: https://www.iqwig.de/download/a20-54_tezacaftor-ivacaftor_extract-of-dossier-assessment_v1-0.pdf?rev=186215 (Accessed June 27, 2021).
Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG) (2021). Tezacaftor/ivacaftor (combination with ivacaftor; cystic fibrosis, 6 to 11 years, F508del mutation, heterozygous) – Benefit assessment according to §35a Social Code. Book V[Online]. Available at: https://www.iqwig.de/download/a20-108_tezacaftor-ivacaftor_extract-of-dossier-assessment_v1-0.pdf?rev=207745 (Accessed June 27, 2021).
ISPOR (2015). Denmark – Pharmaceuticals Global Health Technology Assessment Road Map TRADE CHANNELS FOR MARKET ENTRY [Online]. Available at: https://tools.ispor.org/htaroadmaps/Denmark.asp (Accessed).
Journal Officiel de le République Française (2021). Arrêté du 2 juillet 2021 modifiant la liste des spécialités pharmaceutiques remboursables aux assurés sociaux - ANNEXES SYMKEVI.
Kelly, K., von Wilamowitz-Moellendorff, C., Curry, A., and Ralston, S. (2018). PND99 Cystic fibrosis therapies: key drivers behind differing HTA decisions. Value in Health (21), S3. doi:10.1016/j.jval.2018.09.2065
Kent, L., Reix, P., Innes, J. A., Zielen, S., Le Bourgeois, M., Braggion, C., et al. (2014). Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J. Cyst Fibros 13 (2), 123–138. doi:10.1016/j.jcf.2013.09.005
Kolasa, K., Zwolinski, K. M., Zah, V., Kaló, Z., and Lewandowski, T. (2018). Revealed preferences towards the appraisal of orphan drugs in Poland - multi criteria decision analysis. Orphanet J. Rare Dis. 13 (1), 67. doi:10.1186/s13023-018-0803-9
L'assurance maladie(ameli) (2021). Les affections de longue durée (ALD). [Online]. Available at: https://www.ameli.fr/assure/droits-demarches/maladie-accident-hospitalisation/affection-longue-duree-ald/affection-longue-duree-ald (Accessed September 22, 2021).
La Voix Du Nord (2021). Mucoviscidose : deux nouveaux traitements, Kaftrio et Symkevi. désormais remboursés[Online]. Available at: https://www.lavoixdunord.fr/1037077/article/2021-06-28/mucoviscidose-deux-nouveaux-traitements-kaftrio-et-symkevi-desormais-rembourses (Accessed July 4, 2021).
Lopes-Pacheco, M. (2016). CFTR Modulators: Shedding Light on Precision Medicine for Cystic Fibrosis. Front. Pharmacol. 7, 275. doi:10.3389/fphar.2016.00275
Lopes-Pacheco, M. (2019). CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 10, 1662. doi:10.3389/fphar.2019.01662
Mahta, Sp. z. o. o. (2019). Kalydeco® (iwakaftor) w leczeniu mukowiscydozy u chorych w wieku 12 miesięcy i starszych. z jedną z następujących mutacji bramkowania genu CFTR (klasy III): G551D G1244E, G1349D–G178R. G551S, S1251N, S1255P, S549N lub S549R – analiza ekonomiczna.
Mahta, Sp. z. o. o. (2019). Orkambi® (lumakaftor + iwakaftor) w leczeniu mukowiscydozy u chorych z homozygotyczną mutacją F508del genu CFTR.
Malinowski, K. P., Kawalec, P., Trąbka, W., Czech, M., Petrova, G., Manova, M., et al. (2019). Reimbursement Legislations and Decision Making for Orphan Drugs in Central and Eastern European Countries. Front. Pharmacol. 10, 487. doi:10.3389/fphar.2019.00487
McLeod, C., Wood, J., Schultz, A., Norman, R., Smith, S., Blyth, C. C., et al. (2020). Outcomes and endpoints reported in studies of pulmonary exacerbations in people with cystic fibrosis: A systematic review. J. Cyst Fibros 19 (6), 858–867. doi:10.1016/j.jcf.2020.08.015
medycyna praktyczna (2021). Orkambi (iwakaftor + lumakaftor) - tabletki powlekane. [Online]. Available at: https://www.mp.pl/pacjent/leki/lek/98596,Orkambi-tabletki-powlekane (Accessed September 22, 2021).
Miłkowski, M. (2020). Interpelacja nr 1708 do ministra zdrowia w sprawie braku refundacji leków przyczynowych na mukowiscydozę. w tym Orkambi i Kalydeco [Online]. Available at: http://sejm.gov.pl/Sejm9.nsf/interpelacja.xsp?typ=INT&nr=1708 (Accessed).
Morel, T., Arickx, F., Befrits, G., Siviero, P., van der Meijden, C., Xoxi, E., et al. (2013). Reconciling uncertainty of costs and outcomes with the need for access to orphan medicinal products: a comparative study of managed entry agreements across seven European countries. Orphanet J. Rare Dis. 8, 198. doi:10.1186/1750-1172-8-198
Morrison, C. B., Markovetz, M. R., and Ehre, C. (2019). Mucus, mucins, and cystic fibrosis. Pediatr. Pulmonol 54 (Suppl. 3), S84–S96. doi:10.1002/ppul.24530
Mucovereniging (2021). Belangenverdediging niewe geneesmiddelen [Online]. Available at: https://www.muco.be/nl/wat-doen-we/belangenverdediging/nieuwe-geneesmiddelen/ (Accessed June 17, 2021).
Mukoviszidose, e. V.Ivacaftor (Kalydeco®; VX-770) approved in Germany [Online] (2012). Available at: https://www.muko.info/einzelansicht/ivacaftor-kalydecor-vx-770-in-deutschland-zugelassen (Accessed September 22, 2021).
Mukoviszidose, e. V. (2018a). G-BA certifies additional benefits for Lumacaftor/Ivacaftor also for children between 6 and 11 years. [Online]. Available at: https://www.muko.info/einzelansicht/g-ba-bescheinigt-zusatznutzen-fuer-lumacaftorivacaftor-auch-fuer-kinder-zwischen-6-und-11-jahren (Accessed September 22, 2021).
Mukoviszidose, e. V. (2018b). New CFTR modulator approved in Europe. [Online]. Available at: https://www.muko.info/einzelansicht/neuer-cftr-modulator-in-europa-zugelassen (Accessed September 22, 2021).
Munyama, K., Czernow, Z., Sibińska, K., Niemczyk, M., and Sługocki, W. (2020). Interpelacja nr 1708 - do ministra zdrowia - w sprawie braku refundacji leków przyczynowych na mukowiscydozę. w tym Orkambi i Kalydeco[Online]. Available at: http://sejm.gov.pl/Sejm9.nsf/InterpelacjaTresc.xsp?key=BLFJVU (Accessed).
National Centre for Pharmacoeconomics (NCPE) (2013a). Cost-effectiveness of Ivacaftor (Kalydeco™) for the treatment of cystic fibrosis in patients age 6 years and older who have the G551D mutation.
National Centre for Pharmacoeconomics (NCPE) (2013b). Ivacaftor. Kalydeco®)[Online]. Available at: http://www.ncpe.ie/drugs/ivacaftor-kaldeco/ (Accessed June 29, 2021).
National Centre for Pharmacoeconomics (NCPE) (2016a). Cost-effectiveness of Ivacaftor (Kalydeco) for children with cystic fibrosis aged 2 years and older and weighing less than 25kg who have one of the following gating (class III) mutations in the CFTR gene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R.
National Centre for Pharmacoeconomics (NCPE) (2016b). Cost-effectiveness of Lumacaftor/Ivacaftor (Orkambi) for cystic fibrosis in patients aged 12 years and older who are homozygous for the F508del mutation in the CFTR gene.
National Centre for Pharmacoeconomics (NCPE) (2016c). Lumacaftor/ivacaftor (Orkambi®)[Online]. Available at: http://www.ncpe.ie/drugs/lumacaftorivacaftor-orkambi/ (Accessed June 29, 2021).
National Centre for Pharmacoeconomics (NCPE) (2017a). Cost-effectiveness of Ivacaftor (Kalydeco®) for the treatment of cystic fibrosis in patients aged 18 years and older who have an R117H mutation in the CFTR gene.
National Centre for Pharmacoeconomics (NCPE) (2017b). Processes and Criteria Used by the NCPE when evaluating Orphan Drugs [Online]. Available at: https://data.oireachtas.ie/ie/oireachtas/committee/dail/32/joint_committee_on_health/submissions/2017/2017-07-12_opening-statement-national-centre-for-pharmacoeconomics-ncpe_en.pdf (Accessed June 29, 2021).
National Institute for Health and Care Excellence (NICE) (2017). Changes to NICE drug appraisals: what you need to know[Online]. Available at: https://www.nice.org.uk/news/feature/changes-to-nice-drug-appraisals-what-you-need-to-know (Accessed June 27, 2021).
National Health Service (NHS) England (2019). NHS England concludes wide-ranging deal for cystic fibrosis drugs [Online]. Available at: https://www.england.nhs.uk/2019/10/nhs-england-concludes-wide-ranging-deal-for-cystic-fibrosis-drugs/ (Accessed).
National Institute for Health and Care Excellence (NICE) (2021). Data collection agreement of Kaftrio, Symkevi, Orkambi and Kalydeco for treating cystic fibrosis patients. [Online]. Available at: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/data-collection-agreement#9-ownership-of-the-data (Accessed May 7, 2021).
Natsis, Y. (2019). BeNeLuxA et al.: the best is yet to come [Online]. Available at: https://epha.org/wp-content/uploads/2020/01/epha-beneluxa-et-al-the-best-is-yet-to-come.pdf (Accessed July 21, 2021).
Nawrat, A. (2018). Vertex’s cystic fibrosis drugs become publicly available in Denmark [Online]. Available at: https://www.pharmaceutical-technology.com/news/vertexs-cf-drugs-denmark-amgros/ (Accessed).
NHS Derbyshire Medicines Management and Clinical Policies (2020). Medicines Not Reimbursed Through National Prices and Directly Commissioned by Nhs England. [Online]. Available at: http://www.derbyshiremedicinesmanagement.nhs.uk/assets/High_cost_drugs/NHS-England-drugs-list-v15-2020-2021.pdf (Accessed).
NHS England (2015). Clinical Commissioning Policy: Ivacaftor for Cystic Fibrosis. (named mutations)[Online]. Available at: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/10/a01pc-ivacftr-cystic-fibrosis.pdf (Accessed June 27, 2021).
NICE British National Formulary (2021a). Kalydeco_medicinal forms [Online]. Available at: https://bnf.nice.org.uk/medicinal-forms/ivacaftor.html (Accessed September 21, 2021).
NICE British National Formulary (2021b). Orkambi_medicinal forms [Online]. Available at: https://bnf.nice.org.uk/medicinal-forms/lumacaftor-with-ivacaftor.html (Accessed September 21, 2021).
NICE British National Formulary (2021c). Symkevi_medicinal forms [Online]. Available at: https://bnf.nice.org.uk/medicinal-forms/tezacaftor-with-ivacaftor.html (Accessed September 21, 2021).
NICE (2018a). Single Technology Appraisal: Tezacaftor and ivacaftor combination therapy for treating cystic fibrosiswith the F508del. mutation/Final scope[Online]. Available at: https://www.nice.org.uk/guidance/gid-ta10277/documents/final-scope (Accessed).
NICE (2018b). Tezacaftor and ivacaftor combination therapy for treating cystic fibrosis with the F508del mutation. [ID1303] In development [GID-TA10277][Online]. Available at: https://www.nice.org.uk/guidance/indevelopment/gid-ta10277 (Accessed).
O'Donnell, P. (2015). Belgium, Netherlands team up to take on pharma over prices [Online]. Available at: https://www.politico.eu/article/belgium-netherlands-team-up-to-take-on-pharma-over-prices/ (Accessed June 20, 2021).
Office fédéral de la santé publique (2020). Préparations par principe actif [Online]. Available at: http://www.xn--spezialittenliste-yqb.ch/ShowPreparations.aspx?searchType=SUBSTANCE (Accessed November 24, 2020).
Office fédéral de la santé publique (2021). Préparations par principe actif[Online]. Available at: http://www.xn--spezialittenliste-yqb.ch/ShowPreparations.aspx?searchType=SUBSTANCE (Accessed September 22, 2021).
Ollendorf, D. A., Chapman, R., and Pearson, S. D. (2017). Assessing the Effectiveness and Value of Drugs for Rare Conditions. Boston: Institute for Clinical and Economic Review.
O’Mahony, J. F. (2019). Beneluxa: What are the Prospects for Collective Bargaining on Pharmaceutical Prices Given Diverse Health Technology Assessment Processes. PharmacoEconomics 37 (5), 627–630. doi:10.1007/s40273-019-00781-w
Open Drug Database (2021a). Preparation Kalydeco [Online]. Available at: https://ch.oddb.org/fr/gcc/search/zone/drugs/search_query/kalydeco? (Accessed September 22, 2021).
Open Drug Database (2021b). Preparation Orkambi [Online]. Available at: https://ch.oddb.org/fr/gcc/search/zone/drugs/search_query/orkambi? (Accessed September 22, 2021).
Open Drug Database (2021c). Preparation Symdeko [Online]. Available at: https://ch.oddb.org/fr/gcc/search/zone/drugs/search_query/symdeko/search_type/st_oddb?#best_result (Accessed September 22, 2021).
Paun, C. (2018). Europe struggles to face down Big Pharma. [Online]. Available at: https://www.politico.eu/article/drug-pricing-big-pharma-even-facing-big-pharma-together-countries-still-struggle-to-haggle/ (Accessed June 20, 2021).
Pinto, D. (2018). Agreement Readies Denmark, Austria for Approval of Vertex CF Therapies [Online]. Available at: https://cysticfibrosisnewstoday.com/2018/10/03/agreement-readies-denmark-austria-approval-vertex-cf-therapies/ (Accessed June 17, 2021).
Plüss, J. D. (2020). Swiss deal on cystic fibrosis drugs could change price negotiations. [Online]. Available at: https://www.swissinfo.ch/eng/patient-pressure_swiss-deal-on-cystic-fibrosis-drugs-could-change-drug-price-negotiations/45705128 (Accessed May 7, 2021).
Rafeeq, M. M., and Murad, H. A. S. (2017). Cystic fibrosis: current therapeutic targets and future approaches. J. Transl Med. 15 (1), 84. doi:10.1186/s12967-017-1193-9
Rawson, J. (2018). Portfolio access agreements – Lessons learnt from Vertex Pharma’s Orkambi. [Online]. Available at: https://partners4access.com/portfolio-access-agreements-lessons-learnt-from-vertex-pharmas-orkambi/ (Accessed June 17, 2021).
Rémuzat, C., Urbinati, D., Mzoughi, O., El Hammi, E., Belgaied, W., and Toumi, M. (2015). Overview of external reference pricing systems in Europe. J. Mark Access Health Pol., 3. doi:10.3402/jmahp.v3.27675
Rijksinstituut voor ziekte- en invaliditeitsverzekering (RIZIV) (2016a). AANVRAAG TOT OPNAME - WEESGENEESMIDDEL KALYDECO GEMOTIVEERD DEFINITIEF VOORSTEL Dossier, 1223–1224.
Rijksinstituut voor ziekte- en invaliditeitsverzekering (RIZIV) (2016b). Overeenkomst met aanvrager voor tijdelijke inschrijving van Kalydeco. Onthaal: RIZIV.
Rijksinstituut voor ziekte- en invaliditeitsverzekering (RIZIV) (2019). Overeenkomst met aanvrager voor tijdelijke inschrijving van Kalydeco. Onthaal: RIZIV.
Rivera, M. (2019). Orkambi: la CIPM acordó en julio un. “modelo mixto” de financiación [Online]. Available at: https://www.redaccionmedica.com/secciones/industria/orkambi-la-cipm-acordo-en-julio-un-modelo-mixto-de-financiacion-7310 (Accessed June 17, 2021).
RIZIV (2020). Vergoedbare geneesmiddelen _Kalydeco 150mg [Online]. Available at: https://ondpanon.riziv.fgov.be/SSPWebApplicationPublic/nl/Public/ProductSearch (Accessed November 20, 2020).
RIZIV (2021a). Vergoedbare geneesmiddelen _Kalydeco 150mg. [Online]. Available at: https://ondpanon.riziv.fgov.be/SSPWebApplicationPublic/nl/Public/ProductSearch (Accessed September 22, 2021).
RIZIV (2021b). Vergoedbare geneesmiddelen Orkambi. [Online]. Available at: https://ondpanon.riziv.fgov.be/SSPWebApplicationPublic/nl/Public/ProductSearch (Accessed September 22, 2021).
RIZIV (2021c). Vergoedbare geneesmiddelen Symkevi. [Online]. Available at: https://ondpanon.riziv.fgov.be/SSPWebApplicationPublic/nl/Public/ProductSearch (Accessed September 22, 2021).
Rote Liste (2021a). Kalydeco® 150 mg Filmtabletten. [Online]. Available at: https://www.rote-liste.de/suche/praep/27475-0/Kalydeco%C2%AE%2075%20mg%2F-150%C2%A0mg%20Filmtabletten (Accessed September 22, 2021).
Rote Liste (2021b). Orkambi® 200 mg/125 mg. Filmtabletten[Online]. Available at: https://www.rote-liste.de/suche/praep/27476-0/Orkambi%C2%AE%20100%20mg%2F125%20mg%2F-200%20mg%2F125%20mg%20Filmtabletten (Accessed September 22, 2021).
Rote Liste (2021c). Symkevi® 100 mg/150 mg. Filmtabletten[Online]. Available at: https://www.rote-liste.de/suche/praep/27060-0/Symkevi%C2%AE%20100%20mg%2F150%20mg%20Filmtabletten (Accessed September 22, 2021).
Schneider, E. K., Reyes-Ortega, F., Li, J., and Velkov, T. (2017). Can Cystic Fibrosis Patients Finally Catch a Breath with Lumacaftor/Ivacaftor. Clin. Pharmacol. Ther. 101 (1), 130–141. doi:10.1002/cpt.548
Schuller, Y., Hollak, C. E., and Biegstraaten, M. (2015). The quality of economic evaluations of ultra-orphan drugs in Europe - a systematic review. Orphanet J. Rare Dis. 10 (1), 92. doi:10.1186/s13023-015-0305-y
Scottish Medicines Consortium (2021a). A guide to Quality Adjusted Life Years. (QALYs)[Online]. Available at: http://www.scottishmedicines.org.uk/media/2839/guide-to-qalys.pdf (Accessed June 28, 2021).
Scottish Medicines Consortium (SMC) (2016a). Detailed advice for use of Kalydeco in Scotland (class III gating mutations).
Scottish Medicines Consortium (SMC) (2016b). Detailed advice for use of Kalydeco in Scotland (R117H mutation).
Scottish Medicines Consortium (SMC) (2013). Detailed advice for use of kalydeco in Scotland (G551D mutation).
Scottish Medicines Consortium (SMC) (2019). lumacaftor and ivacaftor 200mg/125mg, 100mg/125mg film-coated tablets and 100mg/125mg,150mg/188mg granules in sachets. (Orkambi®).
Scottish Medicines Consortium (2019). tezacaftor and ivacaftor 100mg/150mg. film-coated tablets (Symkevi®)[Online]. Available at: https://www.scottishmedicines.org.uk/media/4643/tezacaftor-ivacaftor-symkevi-final-july-2019-for-website.pdf. (Accessed).
Sectoraal Comité (2017). BERAADSLAGING NR. 16/063. VERWERKING VAN GECODEERDE PERSOONSGEGEVENS VOOR HET GENEESMIDDEL KALYDECO[Online]. Available at: https://www.ehealth.fgov.be/ehealthplatform/file/view/64bb6fa1bf3f5496f63bb2f2c980d0f445a93dab?filename=16-063-n092-geneesmiddel_kalydeco-gewijzigd_op_16_mei_2017.pdf (Accessed June 23, 2021).
Sheet, P. (2019). After Canada, Sweden Shows Interest In BeNeLuxA Joint Horizon Scanning. [Online]. Available at: https://pink.pharmaintelligence.informa.com/PS140799/After-Canada-Sweden-Shows-Interest-In-BeNeLuxA-Joint-Horizon-Scanning (Accessed July 21, 2021).
Smith, A., and Barry, M. (2020). Utilisation, expenditure and cost-effectiveness of cystic fibrosis drugs in Ireland: a retrospective analysis of a national pharmacy claims database. BMJ Open 10, e040806. doi: doi:doi:10.1136/bmjopen-2020-040806
Sozialversicherung, Ö. (2021). Arzneispezialitäten [Online]. Available at: https://www.sozialversicherung.at/oeko/views/index.xhtml (Accessed September 22, 2021).
Sozialversicherung, Ö. (2020). Arzneispezialitäten [Online]. Available at: https://www.sozialversicherung.at/oeko/views/index.xhtml (Accessed November 24, 2020).
Sullivan, S. D., Mauskopf, J. A., Augustovski, F., Jaime Caro, J., Lee, K. M., Minchin, M., et al. (2014). Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 17 (1), 5–14. doi:10.1016/j.jval.2013.08.2291
Tandvårds- och läkemedelsförmånsverket (2014). Kalydeco (ivakaftor) Hälsoekonomiskt kunskapsunderlag Begränsad utvärdering. Stockholm: TVM.
Tandvårds- och läkemedelsförmånsverket (2021a). Kalydeco ingår i läkemedelsförmånerna. [Online]. Available at: https://www.tlv.se/download/18.4f95c55f16ae8a2c70c798cb/1559026548407/bes190523_kalydeco.pdf (Accessed September 22, 2021).
Tandvårds- och läkemedelsförmånsverket (2021b). Orkambi ingår i läkemedelsförmånerna. [Online]. Available at: https://www.tlv.se/download/18.500ea4181641067957a31b99/1529412747506/bes180614_orkambi.pdf (Accessed September 22, 2021).
Tandvårds- och läkemedelsförmånsverket (2021c). Symkevi ingår inte i högkostnadsskyddet. [Online]. Available at: https://www.tlv.se/download/18.129f584917976cd4f813d4ab/1621591355478/bes210520_symkevi.pdf (Accessed September 22, 2021).
Tandvårds- och läkemedelsförmånsverket (2019). Symkevi och Kalydeco ingår inte i högkostnadsskyddet. [Online]. Available at: https://www.tlv.se/beslut/beslut-lakemedel/avslag-och-uteslutningar/arkiv/2019-05-24-symkevi-och-kalydeco-ingar-inte-i-hogkostnadsskyddet.html (Accessed July 4, 2021).
Tandvårds- och läkemedelsförmånsverket (2018a). Stockholm: TVM.Underlag för beslut om subvention - Nyansökan Nämnden för läkemedelsförmåner Orkambi (lumakaftor + ivakaftor).
Tandvårds- och läkemedelsförmånsverket (2018b). Underlag för beslut om subvention - Nyansökan Nämnden för läkemedelsförmåner Symkevi (tezakaftor/ivakaftor) och Kalydeco (ivakaftor). Stockholm: TVM.
Theriaque CNHIM - Centre National Hospitalier d'Information sur le Médicament (2021). Prix medicaments virtuels Ivacaftor 150 mg [Online]. Available at: https://www.theriaque.org/apps/monographie/view/prix_comp.php?id=28184&sp (Accessed September 22, 2021).
Theriaque CNHIM - Centre National Hospitalier d'Information sur le Médicament (2021a). Prix medicaments virtuels Orkambi 200mg/125mg [Online]. Available at: https://www.theriaque.org/apps/monographie/view/prix_comp.php?id=32408&sp (Accessed September 22, 2021).
Theriaque CNHIM - Centre National Hospitalier d'Information sur le Médicament (2021b). Prix medicaments virtuels Symkevi 100mg/50mg [Online]. Available at: https://www.theriaque.org/apps/monographie/view/prix_comp.php?id=37975&sp (Accessed September 22, 2021).
Vademecum (2020a). KALYDECO Comp. recub. con película 150 mg. [Online]. Available at: https://www.vademecum.es/medicamento-kalydeco_42004 (Accessed November 25, 2020).
Vademecum (2020b). ORKAMBI Comp. recub. con película 100/125 mg. [Online]. Available at: https://www.vademecum.es/medicamento-orkambi+comp.+recub.+con+pelicula+100%2F125+mg_48234 (Accessed November 25, 2020).
Vademecum (2020c). SYMKEVI Comp. recub. con película 100 mg/150 mg. [Online]. Available at: https://www.vademecum.es/medicamento-symkevi_48247 (Accessed November 25, 2020).
Vademecum (2021a). KALYDECO Comp. recub. con película 150 mg. [Online]. Available at: https://www.vademecum.es/medicamento-kalydeco_42004 (Accessed September 22, 2021).
Vademecum (2021b). ORKAMBI Comp. recub. con película 100/125 mg. [Online]. Available at: https://www.vademecum.es/medicamento-orkambi+comp.+recub.+con+pelicula+100%2F125+mg_48234 (Accessed September 22, 2021).
Vademecum (2021c). SYMKEVI Comp. recub. con película 100 mg/150 mg. [Online]. Available at: https://www.vademecum.es/medicamento-symkevi_48247 (Accessed September 22, 2021).
Vaincre la mucoviscidose (2021). Prise en charge des soins. [Online]. Available at: https://www.vaincrelamuco.org/face-la-mucoviscidose/aider-accompagner/droits-et-demarches/prise-en-charge-des-soins (Accessed September 22, 2021).
Vaithyanathan, G., Dekkers, R., and van Engen, A. (2018). Php297 - Changes In Ultra-orphan Drug Assessments In Poland. Value in Health 21, S201. doi:10.1016/j.jval.2018.09.1191
van Rijn, M. J. (2016). Brief aan Parlement Vergoedingsbesluit Orkambi. [Online]. Available at: https://www.vbb.com/media/Insights_Articles/Brief_aan_Parlement_Vergoedingsbesluit_Orkambi_TK.pd.pdf (Accessed June 20, 2021).
Vertex Pharmaceuticals Incorporated (2019). Vertex annonce un accord pour le remboursement d’ORKAMBI®. (lumacaftor/ivacaftor) en France [Online]. Available at: https://www.vaincrelamuco.org/sites/default/files/20191120_vertex_orkambi_cp.pdf (Accessed June 17, 2021).
Vertex Pharmaceuticals Incorporated (2019a). Spanish Government Approves National Reimbursement of ORKAMBI® (lumacaftor/ivacaftor) and SYMKEVI® (tezacaftor/ivacaftor) in Combination With KALYDECO® (ivacaftor)[Online]. Available at: https://investors.vrtx.com/news-releases/news-release-details/spanish-government-approves-national-reimbursement-orkambir (Accessed).
Vertex Pharmaceuticals Incorporated (2019b). Vertex Announces New Access Agreement with Scottish Government for ORKAMBI®. (lumacaftor/ivacaftor) and SYMKEVI® (tezacaftor/ivacaftor)[Online]. Available at: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-new-access-agreement-scottish-government (Accessed).
Vertex Pharmaceuticals Incorporated (2019c). Vertex Confirms Northern Ireland Offer Accepted for Cystic Fibrosis Medicines. [Online]. Available at: https://investors.vrtx.com/news-releases/news-release-details/vertex-confirms-northern-ireland-offer-accepted-cystic-fibrosis (Accessed).
Vertex Pharmaceuticals Incorporated (2019d). Vertex Confirms Wales Offer Accepted for Access to All Licensed Cystic Fibrosis Medicines. [Online]. Available at: https://investors.vrtx.com/news-releases/news-release-details/vertex-confirms-wales-offer-accepted-access-all-licensed-cystic (Accessed).
Vertex Pharmaceuticals Incorporated (2018a). Vertex Announces Access Contract in Denmark for Current and Future Cystic Fibrosis Medicines. [Online]. Available at: https://www.businesswire.com/news/home/20181001005495/en/Vertex-Announces-Access-Contract-in-Denmark-for-Current-and-Future-Cystic-Fibrosis-Medicines (Accessed June 17, 2021).
Vertex Pharmaceuticals Incorporated (2018b). Vertex Announces Long-Term Access Agreement in Sweden for Cystic Fibrosis Medicine ORKAMBI®. (lumacaftor/ivacaftor)[Online]. Available at: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-long-term-access-agreement-sweden-cystic (Accessed).
Vertex Pharmaceuticals Incorporated (2018c). Vertex Submission to Health and Social Care Committee Summary of Vertex Engagement Appendix 3c.
Vertex Pharmaceuticals Incorporated (2016). Vertex Announces German Reimbursement Agreement for ORKAMBI® (Lumacaftor/Ivacaftor), the First Medicine to Treat the Underlying Cause of Cystic Fibrosis in People Ages 12 and Older with Two Copies of the F508del Mutation [Online]. Available at: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-german-reimbursement-agreement-orkambir (Accessed March 17, 2021).
Vertex Pharmaceuticals Incorporated (2017). Vertex Announces Long-Term Reimbursement Agreement with the Republic of Ireland for ORKAMBI® (lumacaftor/ivacaftor). KALYDECO® (ivacaftor) and Future Cystic Fibrosis Medicines[Online]. Available at: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-long-term-reimbursement-agreement-republic (Accessed May 7, 2021).
Vertex Pharmaceuticals Incorporated (2020). Vertex Announces Innovative Reimbursement Agreement in Switzerland for ORKAMBI®. (lumacaftor/ivacaftor) and SYMDEKO® (tezacaftor/ivacaftor and ivacaftor) for Eligible Cystic Fibrosis Patients[Online]. Available at: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-innovative-reimbursement-agreement-switzerland (Accessed May 7, 2021).
Vertex Pharmaceuticals Incorporated (2021). Vertex Announces National Reimbursement Agreement in France for KAFTRIO®. (ivacaftor/tezacaftor/elexacaftor) and SYMKEVI® (tezacaftor/ivacaftor) for Eligible Cystic Fibrosis Patients[Online]. Available at: https://www.businesswire.com/news/home/20210628005507/en/Vertex-Announces-National-Reimbursement-Agreement-in-France-for-KAFTRIO%C2%AE-ivacaftortezacaftorelexacaftor-and-SYMKEVI%C2%AE-tezacaftorivacaftor-for-Eligible-Cystic-Fibrosis-Patients (Accessed July 4, 2021).
Vlaamse Radio- en Televisieomroeporganisatie (VRT) (2021). Overheid gaat peperdure mucomedicijnen terugbetalen. Vandenbroucke: “Gratis voor meer dan 600 patiënten”[Online]. Available at: https://www.vrt.be/vrtnws/nl/2021/02/26/overheid-gaat-peperdure-mucomedicijnen-terugbetalen-minister-va/ (Accessed June 17, 2021).
Whiting, P., Al, M., Burgers, L., Westwood, M., Ryder, S., Hoogendoorn, M., et al. (2014). Ivacaftor for the treatment of patients with cystic fibrosis and the G551D mutation: a systematic review and cost-effectiveness analysis. Health Technol. Assess. 18 (18), 1–106. doi:10.3310/hta18180
WHO Collaborating Centre for Drug Statistics Methodology (2020). ATC/DDD Index of Other respiratory system products. [Online]. Available at: https://www.whocc.no/atc_ddd_index/?code=R07AX02 (Accessed February 17, 2021).
WHO Collaborating Centre for Drug Statistics Methodology (2021). List of DDDs combined products. [Online]. Available at: https://www.whocc.no/ddd/list_of_ddds_combined_products/ (Accessed February 17, 2021).
World Health Organization (2021). Daily Defined Dose (DDD) [Online]. Available at: https://www.who.int/tools/atc-ddd-toolkit/about-ddd (Accessed March 5, 2021).
Young, K. E., Soussi, I., and Toumi, M. (2017). The perverse impact of external reference pricing (ERP): a comparison of orphan drugs affordability in 12 European countries. A call for policy change. J. Mark Access Health Pol. 5 (1), 1369817. doi:10.1080/20016689.2017.1369817
Zorginstituut Nederland (2016a). GVS-advies. lumacaftor/ivacaftor: Orkambi®. bij cystische fibrose (CF) bij patiënten van 12 jaar en ouder die homozygoot zijn voor de F508del-mutatie in het CFTR-gen [Online]. Available at: https://www.zorginstituutnederland.nl/publicaties/adviezen/2016/05/13/gvs-advies-lumacaftor-ivacaftor-orkambi-bij-cystische-fibrose-cf-bij-patienten-van-12-jaar-en-ouder-die-homozygoot-zijn-voor-de-f508del-mutatie-in-het-cftr-gen (Accessed June 28, 2021).
Zorginstituut Nederland (2016b). GVS-advies. lumacaftor/ivacaftor: Orkambi®. bij cystische fibrose (CF) bij patiënten van 12 jaar en ouder die homozygoot zijn voor de F508del-mutatie in het CFTR-gen (herbeoordeling).
Zorginstituut Nederland (2017). Orkambi®: van beoordeling tot vergoeding [Online]. Available at: https://www.zorginstituutnederland.nl/publicaties/publicatie/2017/10/26/orkambi-van-beoordeling-tot-vergoeding (Accessed June 20, 2021).
Zorginstituut Nederland (2018). Marginale toetsing nieuwe dosering lumacaftor/ivacaftor (Orkambi®). lumacaftor/ivacaftor: Orkambi®.
Zorginstituut Nederland (2019a). GVS-advies lumacaftor/ivacaftor (Orkambi®) uitbreiding bijlage 2 voorwaarde voor toepassing bij kinderen. van 2 t/m 5 jaar.
Zorginstituut Nederland (2019b). GVS-advies tezacaftor/ivacaftor (Symkevi®) met ivacaftor monopreparaat (Kalydeco®) bij cystische fibrose (CF) bij patiënten van 12 jaar en ouder die homozygoot zijn voor de F508del-mutatie in het CFTR-gen.
Zorginstituut Nederland (2020a). GVS-advies tezacaftor/ivacaftor. (Symkevi®) in combinatie met ivacaftor monopreparaat (Kalydeco®) bij de behandeling van cystische fibrose[Online]. Available at: https://www.zorginstituutnederland.nl/publicaties/adviezen/2020/08/11/gvs-advies-tezacaftor-ivacaftor-symkevi-in-combinatie-met-ivacaftor-monopreparaat-kalydeco-bij-de-behandeling-van-cystische-fibrose (Accessed June 28, 2021).
Zorginstituut Nederland (2020b). Kalydeco Tablet Filmomhuld 150mg [Online]. Available at: https://www.medicijnkosten.nl/medicijn?artikel=KALYDECO+TABLET+FILMOMHULD+150MG&id=e545a4a5f66f2ce3019dc93bc187a754 (Accessed November 20, 2020).
Zorginstituut Nederland (2020c). Orkambi Tablet Filmomhuld 200/125mg [Online]. Available at: https://www.medicijnkosten.nl/medicijn?artikel=ORKAMBI+TABLET+FILMOMHULD+200%2F125MG&id=8384f161a133797ad7e1520c382e236f (Accessed November 20, 2020).
Zorginstituut Nederland (2020d). Pakketadvies ivacaftor/tezacaftor in combinatie met ivacaftor voor de behandeling van patiënten met cystische fibrose.
Zorginstituut Nederland (2020e). SYMKEVI TABLET FILMOMHULD 100/150MG [Online]. Available at: https://www.medicijnkosten.nl/medicijn?artikel=SYMKEVI+TABLET+FILMOMHULD+100%2F150MG&id=ee0823380852b71236684bed222fcfd2 (Accessed November 20, 2020).
Zorginstituut Nederland (2021a). GVS-advies ivacaftor (Kalydeco®) uitbreiding bijlage 2-voorwaarden [Online]. Available at: https://www.zorginstituutnederland.nl/publicaties/adviezen/2021/05/25/gvs-advies-ivacaftor-kalydeco-uitbreiding-bijlage-2 (Accessed June 28, 2021).
Zorginstituut Nederland (2021b). KALYDECO TABLET FILMOMHULD 150MG [Online]. Available at: https://www.medicijnkosten.nl/medicijn?artikel=KALYDECO+TABLET+FILMOMHULD+150MG&id=e545a4a5f66f2ce3019dc93bc187a754 (Accessed September 22, 2021).
Zorginstituut Nederland (2021c). Orkambi Tablet Filmomhuld 200/125mg [Online]. Available at: https://www.medicijnkosten.nl/medicijn?artikel=ORKAMBI+TABLET+FILMOMHULD+200%2F125MG&id=8384f161a133797ad7e1520c382e236f (Accessed September 22, 2021).
Zorginstituut Nederland (2021d). Symkevi Tablet Filmomhuld 100/150mg [Online]. Available at: https://www.medicijnkosten.nl/medicijn?artikel=SYMKEVI+TABLET+FILMOMHULD+100%2F150MG&id=ee0823380852b71236684bed222fcfd2 (Accessed September 22, 2021).
Zycia, O. (2021). Dwa wnioski o refundację leków przyczynowych w Polsce: Symkevi i Kaftrio. [Online]. Available at: https://oddechzycia.pl/aktualnosci/dwa-wnioski-o-refundacje-lekow-przyczynowych-w-polsce-symkevi-i-kaftrio/ (Accessed June 29, 2021).
Keywords: cystic fibrosis, gene modulators, pricing, reimburesement, comparative analaysis
Citation: Abdallah K, De Boeck K, Dooms M and Simoens S (2021) A Comparative Analysis of Pricing and Reimbursement of Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Europe. Front. Pharmacol. 12:746710. doi: 10.3389/fphar.2021.746710
Received: 24 July 2021; Accepted: 08 October 2021;
Published: 08 November 2021.
Edited by:
Michael Thiede, IUBH University of Applied Sciences, GermanyReviewed by:
Lutz Naehrlich, Justus-Liebig-University Giessen, GermanyAidan Hollis, University of Calgary, Canada
Copyright © 2021 Abdallah, De Boeck, Dooms and Simoens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khadidja Abdallah, a2hhZGlkamEuYWJkYWxsYWhAa3VsZXV2ZW4uYmU=
 Khadidja Abdallah
Khadidja Abdallah Kris De Boeck2
Kris De Boeck2 Marc Dooms
Marc Dooms