- 1Department of Emergency, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 3State Key Laboratory of Multi-fractions Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Background: Severe pneumonia (SP) has a high mortality rate and is responsible for significant healthcare costs. Chinese herbal injections (CHIs) have been widely used in China as a novel and promising treatment option for SP. Therefore, this study assessed and ranked the effectiveness of CHIs to provide more sights for the selection of SP treatment.
Method: Seven databases were searched from their inception up to April 1, 2021. The methodological quality of included study was evaluated by the Cochrane risk-of-bias tool. Then, a Bayesian network meta-analysis (NMA) was performed by OpenBUGS 3.2.3 and STATA 14.0 software. The surface under the cumulative ranking curve (SUCRA) probability values were applied to rank the examined treatments. A clustering analysis was utilized to compare the effect of CHIs between two different outcomes.
Results: A total of 64 eligible randomized controlled trials (RCTs) involving 5,904 participants were identified for this analysis. Six CHIs including Xuebijing injection (XBJ), Tanreqing injection (TRQ), Reduning injection (RDN), Xiyanping injection (XYP), Shenfu injection (SF), and Shenmai injection (SM) were included. The results of the NMA showed that XBJ [odds ratio (OR) = 0.24, 95% credible interval (CI): 0.19, 0.30], TRQ (OR = 0.22, 95% CI: 0.12, 0.37), RDN (OR = 0.29, 95% CI: 0.04, 0.94), and SM (OR = 0.27, 95% CI: 0.08, 0.63) combined with conventional Western medicine (WM) improved the clinical effective rate more significantly than WM alone. Based on SUCRA values, TRQ + WM (SUCRA: 66.4%) ranked the highest in improving the clinical effective rate, second in four different outcomes, and third in only one. According to the cluster analysis, TRQ + WM exerted a positive effect on improving the efficacy of SP. As for safety, less than 30% (18 RCTs) of the included studies reported adverse drug reactions/adverse drug events (ADRs/ADEs), including 14 RCTs of XBJ, 3 RCTs of TRQ, and 1 RCT of RDN.
Conclusion: In conclusion, the study found that the CHIs as co-adjuvant therapy could be beneficial for patients with SP. TRQ + WM showed an outstanding improvement in patients with SP considering both the clinical effective rate and other outcomes.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42021244587].
Introduction
Pneumonia is a persistent and pervasive burden of disease. A 2016 analysis of mortality trends reveals that pneumonia continues to cause more deaths in the USA than any other infectious disease, with no improvement at all during the preceding 34-year period analyzed (Hansen et al., 2016). Though not fatal, pneumonia can be severe. A fifth of the patients hospitalized for pneumonia need to be admitted to intensive care units (ICU), and a third of those require mechanical ventilation, while 21% of the patients from the community needed admission to the ICU, and 26% of them needed mechanical ventilation (Jain et al., 2015). Severe pneumonia (SP) remains a major cause of mortality and has a high mortality rate of up to 30%–50% (Restrepo et al., 2010; Prina et al., 2015; Lanks et al., 2019). The largest challenge in the next few years will be to diminish this high and unacceptable mortality rate. In addition, due to combined antibiotics, long mechanical ventilation, and hospitalization time, SP is responsible for significant healthcare costs (Welte, 2016).
Currently, therapies for SP mainly depend on antibiotics, mechanical ventilation, and corticosteroid (Metlay et al., 2019). Antibiotic therapy is the backbone of the management of SP. It must be started on an empiric basis and is often a combined therapy, since the causative agent is not identified in a considerable proportion of patients and the delay in the administration of adequate antimicrobials is clearly associated with mortality in patients (Garnacho-Montero et al., 2010). However, adequate initial antibiotics may elevate the risk of antibiotic resistance and the mortality rate (Musher and Thorner, 2014; Aliberti et al., 2019). The severity of pneumonia is determined by interacting processes of immune resistance and tissue resilience such as anti-inflammatory response (Mizgerd, 2017). Corticosteroids could inhibit the expression and action of many cytokines involved in the inflammatory response associated with pneumonia (Torres et al., 2015). However, the use of corticosteroids in clinical practice with SP remains controversial because of the presence of adverse effects (Bi et al., 2016; Wan et al., 2016). Therefore, there is an urgent need for combined and adjunctive therapeutic options to improve outcomes.
In recent years, Chinese herbal injections (CHIs) as adjuvant treatments for SP were widely applied in China (Qi et al., 2011; Lv et al., 2017; Song et al., 2019). Even in the treatment of COVID-19, CHIs displayed more superiority (Guo et al., 2020; Shi et al., 2021). Through clinical medication experience and searching in electronic databases previously, we found that six CHIs including Xuebijing injection (XBJ), Tanreqing injection (TRQ), Reduning injection (RDN), Xiyanping injection (XYP), Shenfu injection (SF), and Shenmai injection (SM) have been widely used in SP because of their remarkable effects. Their efficacy has been evidenced with systematic reviews (Huang et al., 2019; Jin et al., 2020; Zhou et al., 2021). CHIs combined with conventional Western medicine (WM) can greatly improve clinical symptoms and interrupt the vicious cycle of inflammation onset by blocking the uncontrolled release of endogenic inflammatory mediators like IL-6, IL-8, and TNF-α (Diao et al., 2017; Lv et al., 2017; Guo et al., 2020; Luo et al., 2021). However, head-to-head clinical trials comparing the efficacy of the six CHIs are lacking up to now. Without direct evidence, it is difficult to identify the most effective one for patients with SP. As a new method of evidence-based medical statistical methods, the network meta-analysis (NMA) extends principles of a conventional meta-analysis to the evaluation of multiple treatments in a single analysis by combining the direct and indirect evidence (Higgins and Welton, 2015; Shim et al., 2017). Another major value of NMA is that it can rank each CHI according to its effectiveness, which is important for clinicians to make the best treatment choices. Therefore, this study aimed to assess the clinical efficacy and safety of different CHIs combined with WM and provide more evidence for rational selection of CHIs for SP using NMA.
Materials and Methods
Study Registration
This study had been prepared under the guidance of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (in Attachment 1) (Page et al., 2021). The study was prospectively registered on the PROSPERO platform (https://www.crd.york.ac.uk/prospero/) with an assigned registration number CRD42021244587.
Inclusion and Exclusion Criteria
Studies were considered eligible if they met the following criteria: 1) randomized controlled trial (RCT). 2) Adults aged 18 years or older. 3) All included patients were diagnosed with SP according to the “Adult Community-Acquired Pneumonia (CAP) Guidelines for Diagnosis and Treatment” issued by the American Thoracic Society/American Society of Infectious Diseases (AST/IDSA) in 2007 (Mandell et al., 2007) or the “Guidelines for the Diagnosis and Treatment of Community-Acquired Pneumonia in Chinese Adults” developed by the Respiratory Branch of Chinese Medical Association in 2016 (Qu and Cao, 2016). 4) All patients received treatment with WM such as anti-infectives, phlegm reduction medicines, mechanical ventilation, nutritional support, and so on. Based on this, the experimental group received one of the included CHIs, and the control group received another or only WM. The duration of treatment ranged from 7 to 14 days. 5) At least one of the following seven outcomes with clear definitions were evaluated: the clinical effective rate, ICU length of stay, the time of mechanical ventilation, the level of C-reactive protein (CRP), the level of procalcitonin (PCT), leukocyte (WBC), and adverse drug reactions (ADRs)/adverse drug events (ADEs). The clinical effective rate was calculated by the following formula: (number of cured patients + number of improved patients)/total number of patients × 100%. Patients were regarded as cured when their clinical symptoms and the objective indicators disappeared and the patients returned to normal. Patients were regarded as improved when their clinical symptoms and the objective indicators were alleviated. If the clinical symptoms and objective indicators were either unchanged or aggravated, the patients were identified as having an invalid effectiveness status.
Studies were excluded if any of the following criteria were met: 1) If they described data about only a specific population (patients with tumor, pulmonary fibrosis, tuberculosis, using immunosuppressant, secondary respiratory failure in other systems, etc.). 2) The full text was not available or only with abstracts. 3) Data were incorrect, incomplete, or unavailable.
Data Sources and Search Strategy
A comprehensive literature search was performed using the electronic databases of PubMed, the Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Database, and the Chinese Scientific Journal database (VIP) from their inception up to April 1, 2021. The medical subject headings (MeSH) and free text words were used. Language restriction did not exist in this study. Furthermore, we manually searched the reference lists of all retrieved studies. Six different kinds of CHIs were included in this NMA: TRQ, XYP, RDN, XBJ, SF, and SM. Full details of the search strategy are shown in Attachment 2.
Study Selection and Data Extraction
Two researchers (LQ Niu and L Xiao) independently screened the studies according to the inclusion criteria. After checking for duplicate studies, the researchers eliminated reviews and irrelevant studies by reading the titles and abstracts. Then, full texts were read to select studies that meet the pre-specified inclusion criteria. Inconsistencies were resolved by extensive discussion or the third researcher (XZ Liu). A data spreadsheet was developed with Microsoft Excel 2019 to collect relevant information. The information including eligible study characteristics (e.g., first author and year of publication), participant characteristics (e.g., gender, age, and sample), details of interventions (e.g., duration and frequency of drugs), outcome data, and factors to evaluate risk of bias were extracted and entered into the spreadsheet.
Quality Assessment
The methodological quality of each included study was evaluated with Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (Sterne et al., 2019). The domains include the following: 1) randomization process, 2) deviations from intended interventions, 3) missing outcome data, 4) measurement of the outcome, and 5) selection of the reported result. There are some signaling questions required to be answered by “yes (Y),” “probably yes (PY),” “probably no (PN),” “no (N),” or “no information (NI)” for each domain. After that, the risk of bias is categorized into three levels: high risk, some concerns, and low risk. These domain-level judgements will inform an overall risk of bias judgment for the outcome. The quality assessments were performed by two independent reviewers (LQ Niu and L Xiao), and disagreements were resolved by consensus or a third opinion.
Statistical Analysis
OpenBUGS 3.2.3 (Lunn et al., 2009) and STATA 14.0 software (Stata Corporation, College Station, TX, USA) were employed to compute calculations and prepare graphs. For binary outcomes, the combined results were calculated as odds ratios (ORs) with 95% credible intervals (CIs). For continuous outcomes, standardized mean differences (SMD) with 95% CIs were used. When the 95% CIs of ORs did not include one and the 95% CIs of the SMDs did not contain zero, the differences between the groups were considered statistically significant. If there were multi-arm trials, we split them into two-arm trials. Chi-squared test was employed to assess heterogeneity between different studies (Zheng et al., 2019). If with homogeneity (p ≥ 0.1, I2 ≤ 50%), a fixed-effect model was adopted; if with obvious heterogeneity (p < 0.1, I2 > 50%), a random-effect model was applied. If closed loops existed, we employed the inconsistency factor (IF) to examine the consistency between direct and indirect evidence. If 95% CIs of IF values were truncated at zero, it indicated that the two sources are in agreement (Salanti et al., 2011). Due to non-closed loops in this NMA, the assumption of consistency between direct and indirect evidence was not utilized.
The Markov chain Monte Carlo method was performed by using the OpenBUGS software to carry out the NMA. In OpenBUGS software, the number of iterations was set to 300,000, and the first 100,000 iterations were used for the annealing algorithm to eliminate the impact of the initial value. The network graph was constructed using STATA software to show a comparative relationship between different interventions. Surface under the cumulative ranking curve (SUCRA) probability values were applied to rank the examined treatments, and the SUCRA values of 100% and 0% were assigned to the best and worst treatments, respectively (Salanti et al., 2011; Riley et al., 2017). Furthermore, a clustering analysis was utilized to compare the effect of CHIs between two different outcomes. After that, publication bias were reflected by funnel plots (Chaimani et al., 2013; Salanti et al., 2014).
Results
Literature Selection
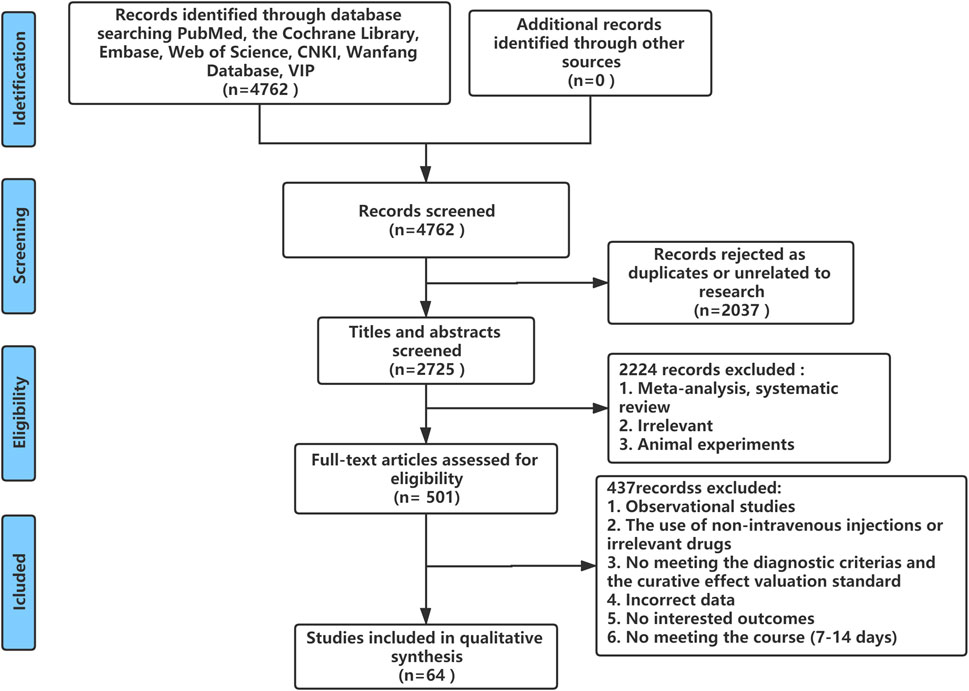
A total of 4,762 studies were identified from the search at first. After removing duplicates, 2,725 remained. By screening titles and abstracts, 2,224 studies were excluded because they were reviews, irrelevant studies, and animal experiments. Afterward, 501 relevant studies were reviewed for eligibility by full-text evaluations. Finally, 64 studies that met the inclusion criteria were included in our Bayesian NMA. Four hundred thirty-seven records were excluded for the following reasons: 1) observational studies (n = 33); 2) the use of non-intravenous injections or irrelevant drugs (n = 63); 3) the disease did not meet the diagnostic criteria or studies did not report the established outcomes (n = 327); 4) incomplete data (n = 6); and 5) duration of therapy was not satisfied (n = 8). The literature selection process is illustrated in Figure 1.
Study Characteristics
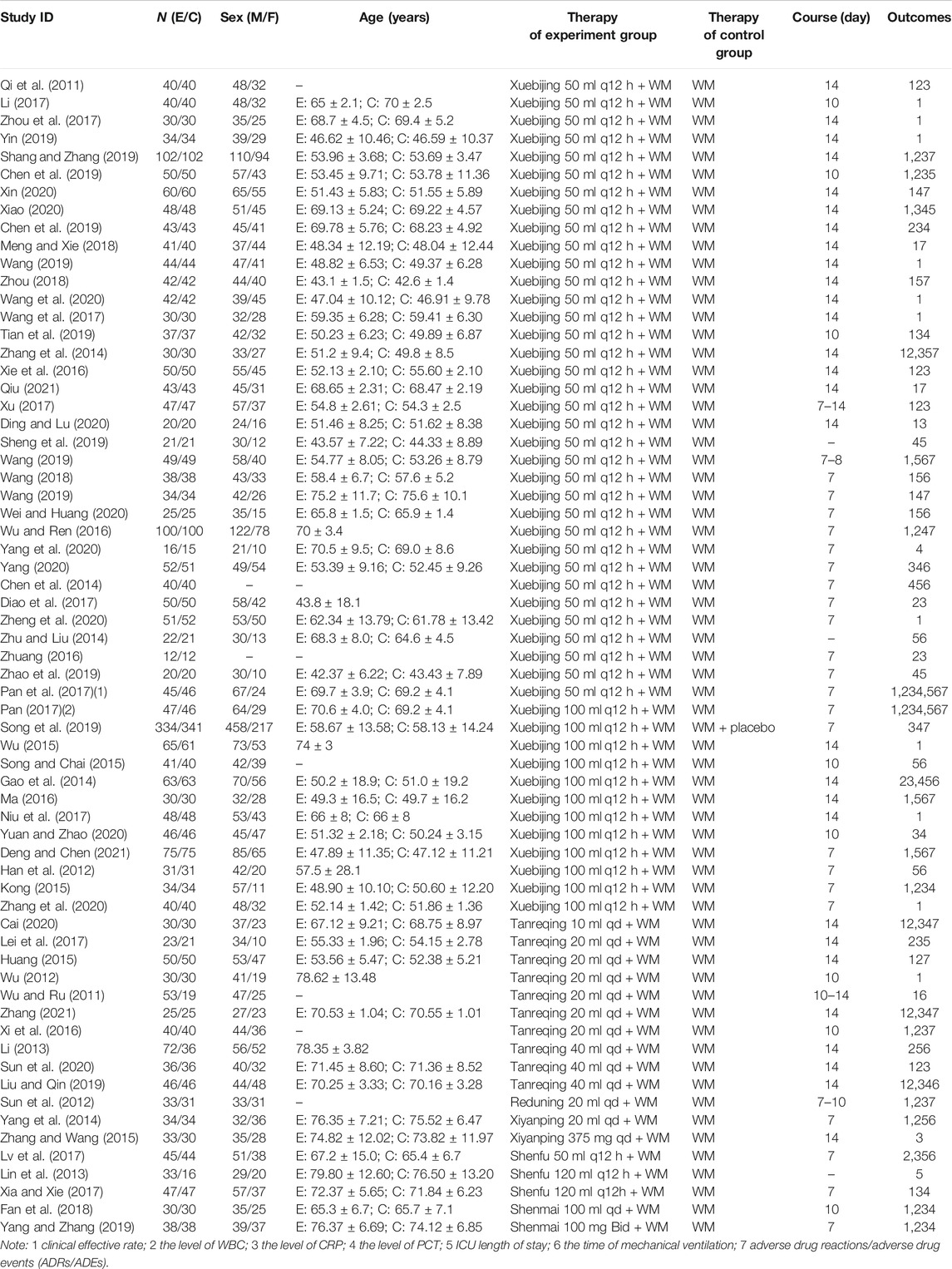
The Bayesian NMA was performed using 64 RCTs with a total of 5,904 adult patients and their sample sizes varying from 24 to 675 participants. All RCTs were conducted in China and published between 2011 and 2021. Six CHIs were incorporated, including XBJ (n = 46), TRQ (n = 10), RDN (n = 1), XYP (n = 2), SF (n = 3), and SM (n = 2). The control groups have been treated with WM such as anti-infectives, apophlegmatisant, mechanical ventilation, vasopressor, and nutritional support. The anti-infectives mainly include fluoroquinolones, β-lactam, carbapenems, and linezolid as monotherapy or combination therapy. On the basis of the control group, the intervention of the experimental group was one of the included CHIs. The duration of treatment ranged from 7 to 14 days. The details of the study characteristics are depicted in Table 1, and the compared connections among each intervention for each outcome are displayed in Figure 2.
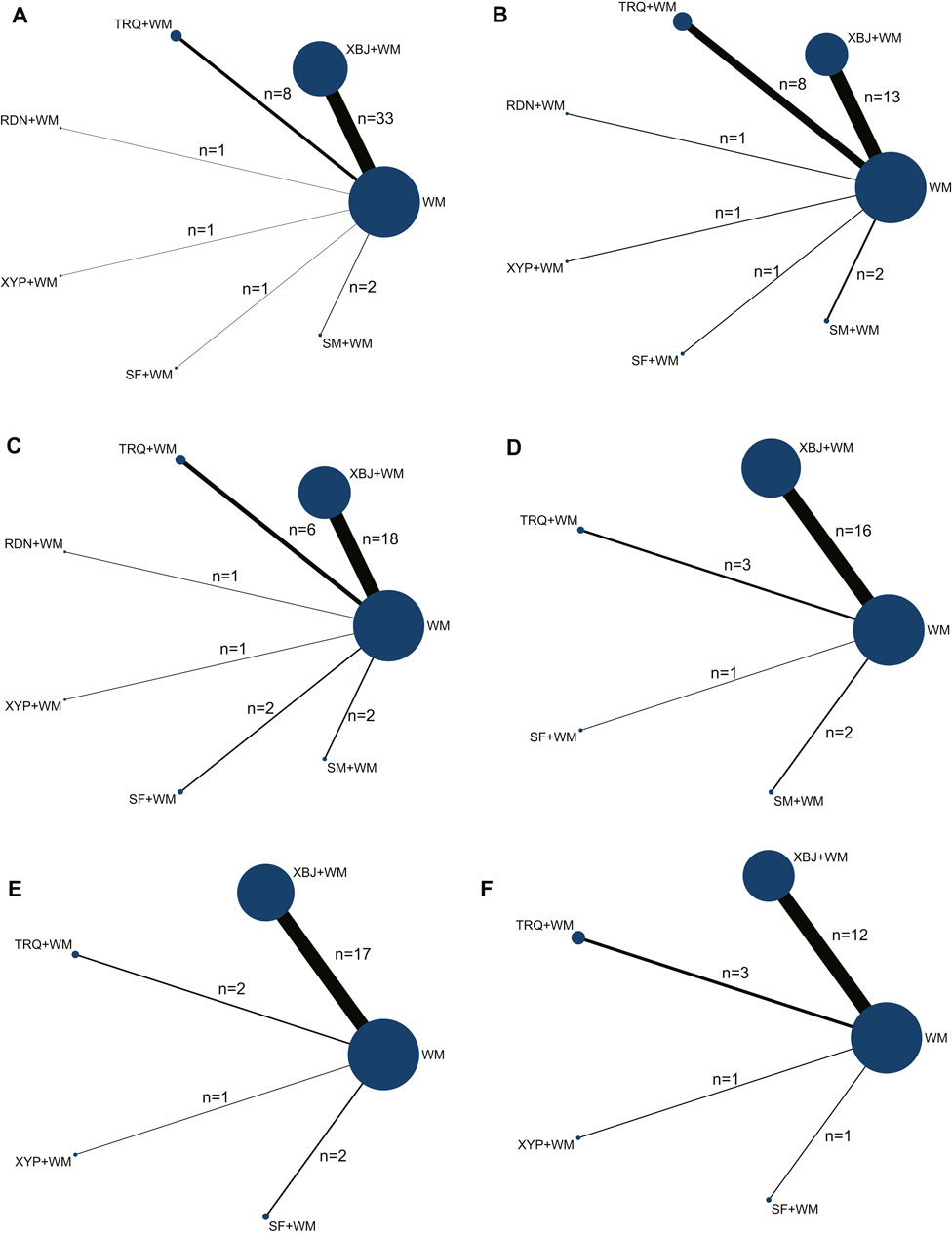
FIGURE 2. Network graph of the different outcomes. (A) Clinical effective rate; (B) the level of leukocyte (WBC); (C) the level of C-reactive protein (CRP); (D) the level of procalcitonin (PCT); (E) intensive care unit (ICU) length of stay; and (F) the time of mechanical ventilation.
Quality Assessments of Studies
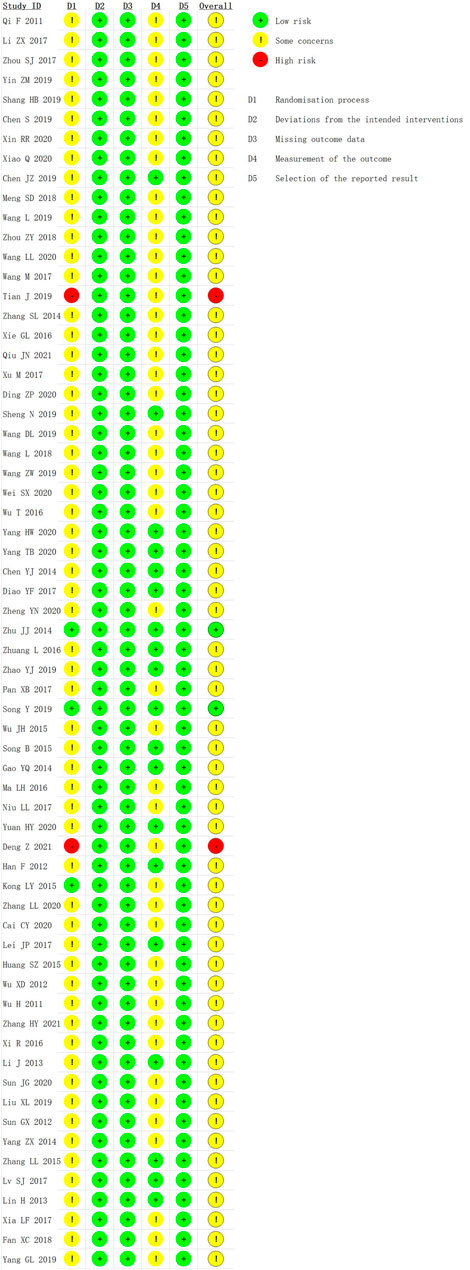
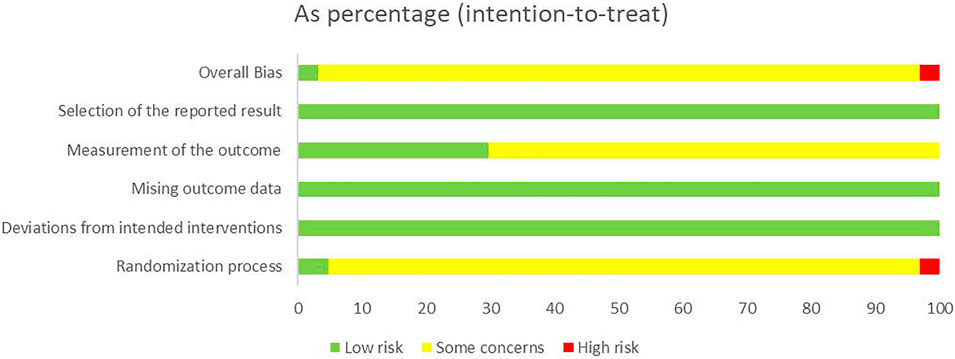
We used the RoB 2 to conduct a quality evaluation. Three studies were assessed as “low risk” (Zhu and Liu, 2014; Kong, 2015; Song et al., 2019) and two studies were “high risk” for the randomization process because of their incorrect method of random sequence generation (Tian et al., 2019; Deng and Chen, 2021). All trials were rated to have low risk of bias for deviations from intended interventions, missing outcome data, and selection of the reported result. Nineteen studies were evaluated as “low risk” in measurement of the outcome (Han et al., 2012; Li, 2013; Lin et al., 2013; Chen et al., 2014; Gao et al., 2014; Zhu and Liu, 2014; Song and Chai, 2015; Zhang and Wang, 2015; Zhuang, 2016; Diao et al., 2017; Lei et al., 2017; Lv et al., 2017; Chen, 2019; Sheng et al., 2019; Song et al., 2019; Zhao et al., 2019; Yang, 2020; Yang et al., 2020; Yuan and Zhao, 2020), and the remaining were assessed as “some concerns.” In general, most studies were classified as “some concerns.” Further details of the risk of bias assessment are shown in Figures 3 and 4. In addition, although blinding was not implemented and applicable for subjects in most studies, the lack of blinding was unlikely to have influenced the assessment of primary outcome indicators.
Outcomes
The Clinical Effective Rate
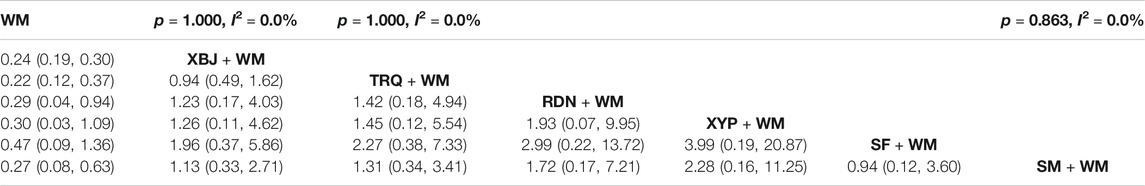
The clinical effective rate was deemed as the primary outcome. A total of 46 RCTs (Qi et al., 2011; Wu and Ru, 2011; Sun et al., 2012; Wu, 2012; Gao et al., 2014; Yang et al., 2014; Zhang et al., 2014; Huang, 2015; Kong, 2015; Wu, 2015; Ma, 2016; Wu and Ren, 2016; Xi et al., 2016; Xie, 2016; Li, 2017; Niu et al., 2017; Pan et al., 2017; Wang et al., 2017; Xia and Xie, 2017; Xu, 2017; Zhou et al., 2017; Fan et al., 2018; Meng and Xie, 2018; Wang, 2018; Zhou, 2018; Chen et al., 2019; Liu and Qin, 2019; Shang and Zhang, 2019; Tian et al., 2019; Wang, 2019; Wang, 2019; Wang et al., 2019; Yang and Zhang, 2019; Yin, 2019; Cai, 2020; Ding and Lu, 2020; Sun et al., 2020; Wang et al., 2020; Wei and Huang, 2020; Xiao et al., 2020; Xin, 2020; Zhang et al., 2020; Zheng et al., 2020; Deng and Chen, 2021; Qiu, 2021; Zhang, 2021) of six CHIs reported the clinical effective rate. In the pairwise meta-analysis, there was no significant heterogeneity in the pooled analysis of all included studies (p = 1.000, I2 = 0.0%). The results of the heterogeneity are shown in Table 2. According to the network of comparisons in Table 2, there were 21 comparisons, and XBJ, TRQ, RDN, and SM combined with WM improved the clinical effective rate more significantly than WM alone (XBJ + WM: OR = 0.24, 95% CI: 0.19, 0.30; TRQ + WM: OR = 0.22, 95% CI: 0.12, 0.37; RDN + WM: OR = 0.29, 95% CI: 0.04, 0.94; and SM + WM: OR = 0.27, 95% CI: 0.08, 0.63). However, the results showed no significant differences between XYP and SF combined with WM than WM alone (XYP + WM: OR = 0.30, 95% CI: 0.03, 1.09 and SF + WM: OR = 0.47, 95% CI: 0.09, 1.36).
In Table 8 and Figure 5, the SUCRA values suggested that TRQ + WM was the optimal treatment, XYP + WM was the second, and RDN + WM was the third.
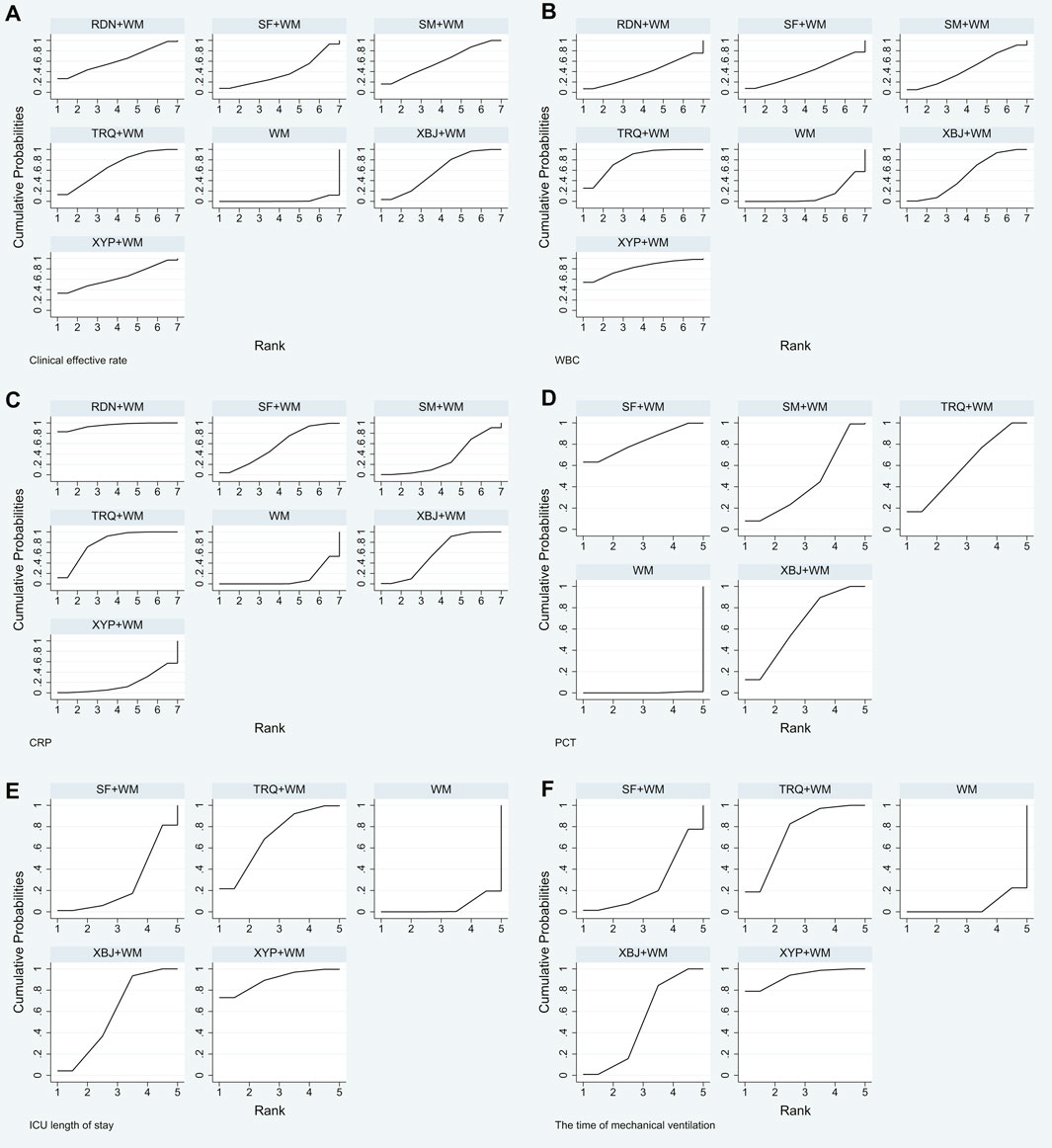
FIGURE 5. Plot of surface under the cumulative ranking curve (SUCRA) for all different outcomes. (A) Clinical effective rate; (B) the level of WBC; (C) the level of CRP; (D) the level of PCT; (E) ICU length of stay; and (F) the time of mechanical ventilation.
The Level of WBC
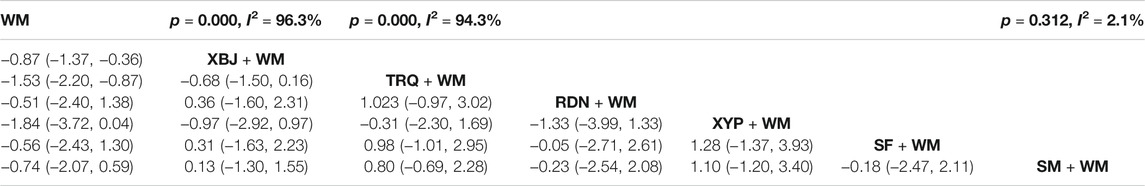
As the other dominating outcome, WBC was estimated in 26 RCTs (Qi et al., 2011; Sun et al., 2012; Li, 2013; Gao et al., 2014; Yang et al., 2014; Zhang et al., 2014; Huang, 2015; Kong, 2015; Wu and Ren, 2016; Xi et al., 2016; Xie, 2016; Zhuang, 2016; Diao et al., 2017; Lei et al., 2017; Lv et al., 2017; Pan et al., 2017; Xu, 2017; Fan et al., 2018; Chen, 2019; Chen et al., 2019; Liu and Qin, 2019; Shang and Zhang, 2019; Yang and Zhang, 2019; Cai, 2020; Sun et al., 2020; Zhang, 2021). The heterogeneity results of the pairwise meta-analysis are shown in Table 3. According to Table 3, the CHIs like XBJ and TRQ combined with WM (XBJ + WM: SMD = −0.87, 95% CI: 1.37, −0.36 and TRQ + WM: SMD = −1.53, 95% CI: 2.20, −0.87) had better clinical effective rate than WM alone, and the differences among the above interventions were statistically significant. However, the rest of the CHIs including RDN, XYP, SF, and SM combined with WM did not perform more outstanding than WM alone. In addition, in terms of Table 3, there were no significant differences between each comparison of different types of CHIs.
Based on the ranking analysis, XYP + WM attained the first rank. TRQ + WM was the second, and XBJ + WM was the third (Table 8 and Figure 5).
The Level of CRP
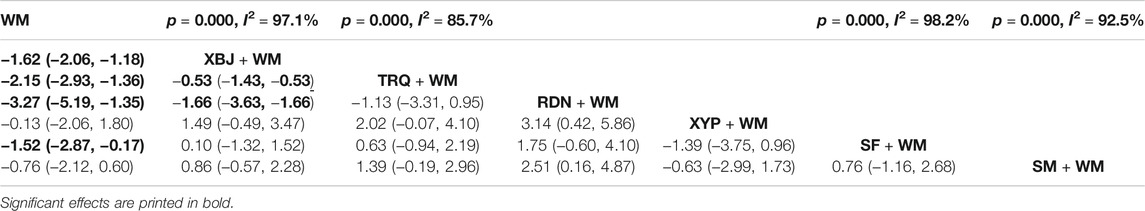
CRP was tested in 30 RCTs (Qi et al., 2011; Sun et al., 2012; Gao et al., 2014; Zhang et al., 2014; Kong, 2015; Zhang and Wang, 2015; Xi et al., 2016; Xie, 2016; Zhuang, 2016; Diao et al., 2017; Lei et al., 2017; Lv et al., 2017; Pan et al., 2017; Xia and Xie, 2017; Xu, 2017; Fan et al., 2018; Chen, 2019; Chen et al., 2019; Liu and Qin, 2019; Shang and Zhang, 2019; Song et al., 2019; Tian et al., 2019; Yang and Zhang, 2019; Cai, 2020; Ding and Lu, 2020; Sun et al., 2020; Xiao et al., 2020; Yang, 2020; Yuan and Zhao, 2020; Zhang, 2021) involved seven interventions. The heterogeneity results of the pairwise meta-analysis are shown in Table 4. Four of them were noticeably better than WM treatment alone for decreasing the level of CRP, as XBJ + WM (SMD = −1.62, 95% CI: 2.06, −1.18), TRQ + WM (SMD = −2.15, 95% CI: 2.93, −1.36), RDN + WM (SMD = −3.72, 95% CI: 5.19, −1.35), and SF + WM (SMD = −1.52, 95% CI: 2.87, −0.17) were remarkable among them compared with WM alone. What is more, based on WM, TRQ and RDN had a more excellent performance in decreasing CRP than XBJ (XBJ + WM vs TRQ + WM: SMD = −0.53, 95% CI: 1.43, −0.53 and XBJ + WM vs RDN + WM: SMD = −1.66, 95% CI: 3.63, −1.66), and the results between the rest of the comparisons of different CHIs showed no significant differences.
The SUCRA mentioned above was also affirmed, and RDN + WM was the best choice, followed by TRQ + WM and XBJ + WM (Table 8 and Figure 5).
The Level of PCT
The potency of decreasing the level of PCT was assessed. The heterogeneity results of the pairwise meta-analysis are shown in Table 5. Four interventions with 22 RCTs (Chen et al., 2014; Gao et al., 2014; Kong, 2015; Wu and Ren, 2016; Pan et al., 2017; Xia and Xie, 2017; Fan et al., 2018; Chen, 2019; Liu and Qin, 2019; Sheng et al., 2019; Song et al., 2019; Tian et al., 2019; Wang, 2019; Yang and Zhang, 2019; Zhao et al., 2019; Cai, 2020; Xiao et al., 2020; Xin, 2020; Yang, 2020; Yang et al., 2020; Yuan and Zhao, 2020; Zhang, 2021) had data in contrast with WM, shown in Table 5. The results revealed that all CHIs involved combined with WM were advantageous in decreasing PCT compared to WM alone (XBJ + WM: SMD = −2.19, 95% CI: 2.67, −1.70; TRQ + WM: SMD = −2.12, 95% CI: 3.27, −0.96; SF + WM: SMD = −2.84, 95% CI: 4.83, −0.86; and SM + WM: SMD = −1.69, 95% CI: 3.11, −0.27), and the differences among the abovementioned combinations were statistically significant.
Treatment ranking based on SUCRA values, from largest to smallest, were as follows: SF + WM, XBJ + WM, TRQ + WM, SM + WM, and WM. The details are depicted in Table 8 and Figure 5.
ICU Length of Stay
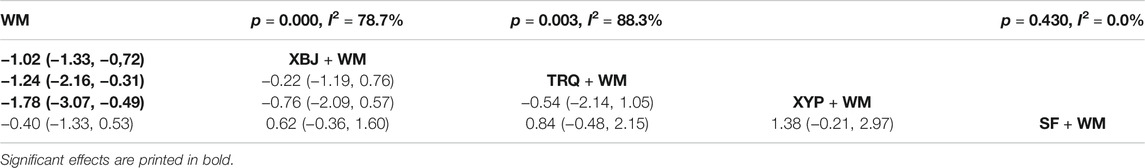
Twenty-two RCTs (Han et al., 2012; Li, 2013; Lin et al., 2013; Chen et al., 2014; Gao et al., 2014; Yang et al., 2014; Zhang et al., 2014; Zhu and Liu, 2014; Song and Chai, 2015; Ma, 2016; Lei et al., 2017; Lv et al., 2017; Pan et al., 2017; Wang, 2018; Zhou, 2018; Chen et al., 2019; Sheng et al., 2019; Wang et al., 2019; Zhao et al., 2019; Wei and Huang, 2020; Xiao et al., 2020; Deng and Chen, 2021) with five treatments reported the ICU length of stay. The heterogeneity results of the pairwise meta-analysis are shown in Table 6. As shown in Table 6, XBJ + WM (SMD = −1.02, 95% CI: 1.33, −0.72), TRQ + WM (SMD = −1.24, 95% CI: 2.16, −0.31), and XYP + WM (SMD = −1.78, 95% CI: 3.07, −0.49) were more effective than WM alone. However, the results showed no significant difference in most cases.
Based on the ranking analysis, XYP + WM attained the first rank. TRQ + WM was the next, and XBJ + WM was the third (Table 8 and Figure 5).
The Time of Mechanical Ventilation
In terms of the time of mechanical ventilation, five treatments with 17 RCTs (Wu and Ru, 2011; Han et al., 2012; Li, 2013; Chen et al., 2014; Gao et al., 2014; Yang et al., 2014; Zhu and Liu, 2014; Song and Chai, 2015; Ma, 2016; Lv et al., 2017; Pan et al., 2017; Wang, 2018; Liu and Qin, 2019; Wang et al., 2019; Wei and Huang, 2020; Yang, 2020; Deng and Chen, 2021) were compared with WM. The heterogeneity results of the pairwise meta-analysis are shown in Table 7. As seen in Table 7, three CHIs combined with WM (XBJ + WM: SMD = −1.72, 95% CI: 2.26, −1.17; TRQ + WM: SMD = −2.42, 95% CI: 3.56, −1.28; and XTP + WM: SMD = −3.41, 95% CI: 5.40, −1.43) had excellent performance in decreasing the time of mechanical ventilation compared to WM alone, and the results were statistically significant.
Results of ranking analysis manifested that XYP + WM was efficacious in decreasing the time of mechanical ventilation. Other beneficial treatments were TRQ + WM and XBJ + WM (Table 8 and Figure 5).
Cluster Analysis
A cluster analysis was performed on the primary outcome and secondary outcomes for the seven treatments. As shown in Figure 6, in terms of the clinical effective rate and the level of WBC, the clinical effective rate and ICU length of stay, and the clinical effective rate and the time of mechanical ventilation, TRQ and XYP combined with WM were similarly superior. According to the clinical effective rate and the level of CRP, TRQ and RDN combined with WM were more beneficial. About the clinical effective rate and the level of PCT, TRQ and XBJ combined with WM were preferred.
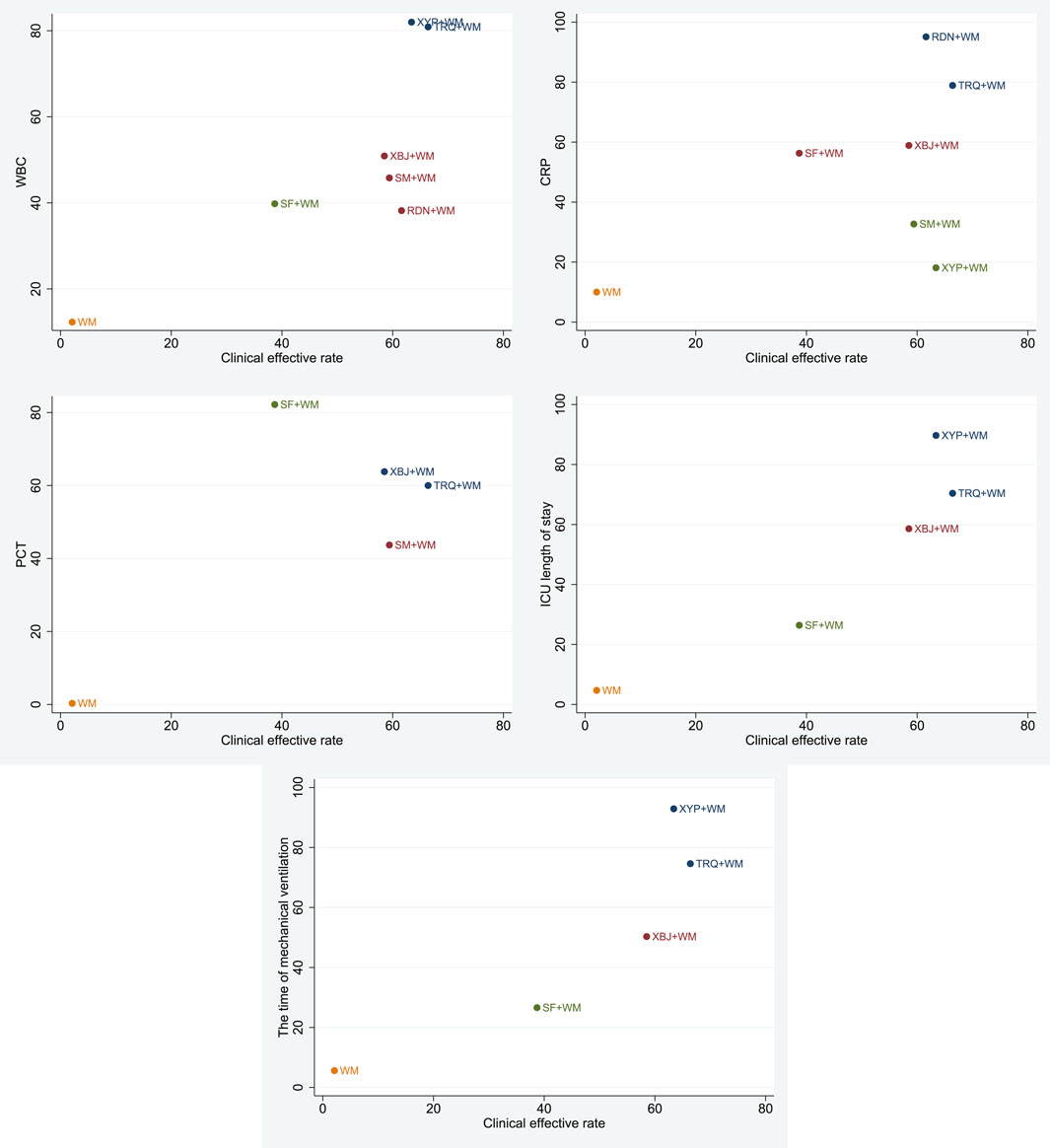
FIGURE 6. Cluster analysis plot for six outcomes. Note: interventions with the same color belong to the same cluster, and interventions located in the upper right corner indicate optimal therapy for two different outcomes.
ADRs/ADEs
Among 64 RCTs, a total of 18 RCTs reported the ADRs/ADEs of interventions. There were 14 RCTs (Gao et al., 2014; Zhang et al., 2014; Ma, 2016; Wu and Ren, 2016; Pan et al., 2017; Meng and Xie, 2018; Zhou, 2018; Shang and Zhang, 2019; Song et al., 2019; Wang, 2019; Wang et al., 2019; Xin, 2020; Deng and Chen, 2021; Qiu, 2021) involving 1,095 participants with XBJ group that reported ADRs, including headache and dizziness (32 cases in 10 RCTs), diarrhea (10 cases in 7 RCTs), nausea and vomiting (4 cases in 3 RCTs), chest discomfort or dyspnea (4 cases in 4 RCTs), itchy skin or rash (9 cases in 6 RCTs), and myelosuppression (4 cases in 4 RCTs). Three RCTs (Huang, 2015; Xi et al., 2016; Zhang, 2021) reported ADRs/ADEs of TRQ, one of the RCTs reported two cases of nausea and vomiting and one case of rash. Another two RCTs did not report ADRs/ADEs. In addition, only one RCT (Sun et al., 2012) mentioned the ADRs of RDN and one case reported thirst. The rest of the included studies did not provide information on any ADRs/ADEs. All of the symptoms were alleviated after corresponding treatment and did not influence the RCTs.
Sensitivity Analysis
To assess the robustness and reliability of the primary outcome results, a sensitivity analysis was conducted based on the results of quality assessments of studies. Two studies were excluded for their high risk of bias (Tian et al., 2019; Deng and Chen, 2021), and the remaining 44 studies conducted a network meta-analysis again. Results did not show relevant deviations compared with the original network meta-analysis. The Bayesian ranking results from high to low for clinical effective rate were TRQ (66.3%), XYP (62.9%), RDN (61.4%), XBJ (59.1%), SF (58.7%), SM (39.5%), and WM (2.0%), respectively. The sensitivity analysis showed that the results of clinical effective rate were robust and reliable.
Funnel Plot Characteristics
Comparison-adjusted funnel plots for different outcomes are displayed in Figure 7. There were three funnel plots of outcomes that were generally symmetrical visually, including the clinical effective rate, ICU length of stay, and the time of mechanical ventilation. Therefore, they had no publication bias. Among the remaining outcomes, the funnel plots were not symmetrically visual, which revealed that there were small sample size and publication bias.
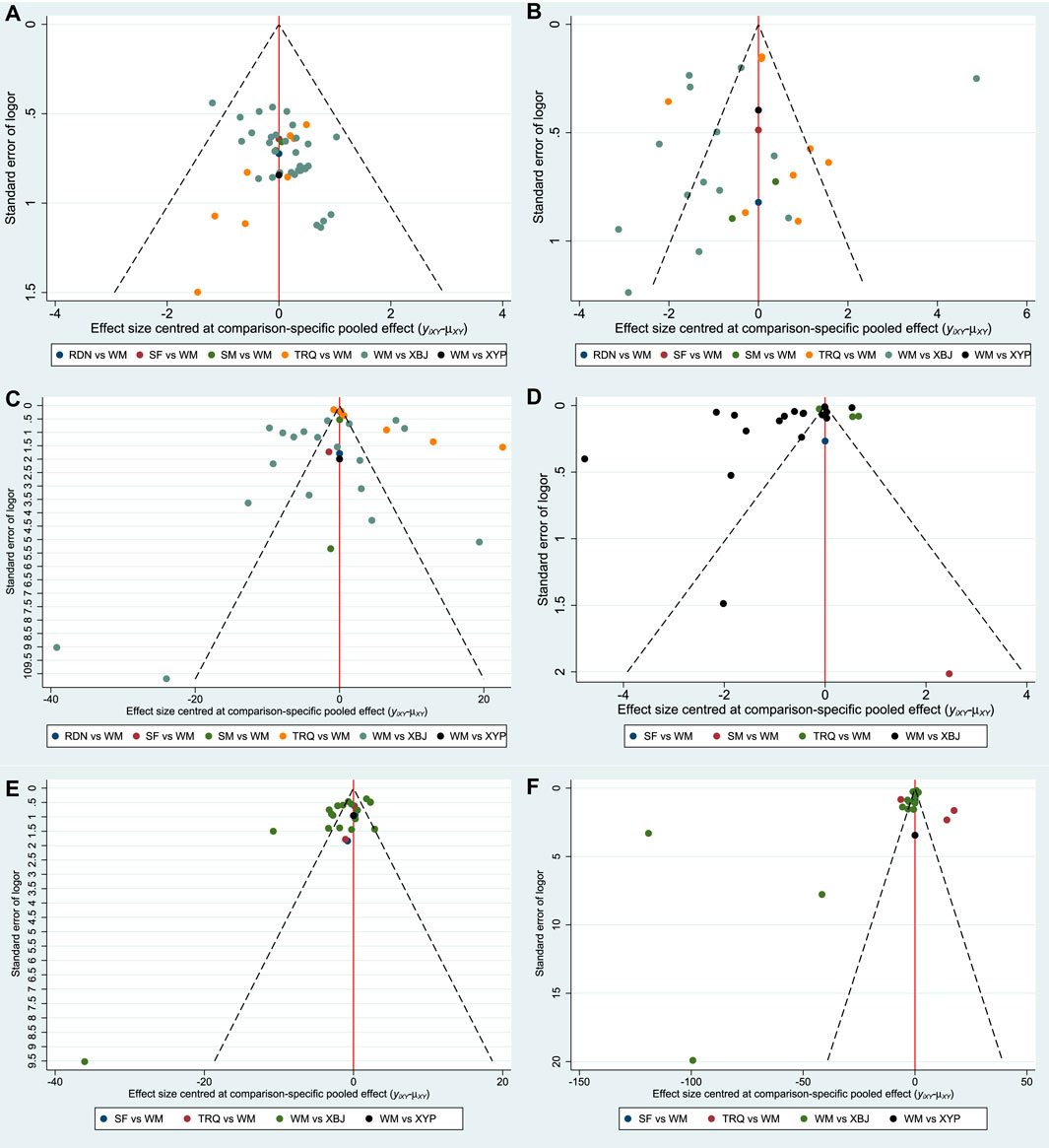
FIGURE 7. Funnel plot. (A) Clinical effective rate; (B) the level of WBC; (C) the level of CRP; (D) the level of PCT; (E) ICU length of stay; and (F) the time of mechanical ventilation.
Discussion
A total of 64 RCTs involving 5,904 participants were included. Six CHIs were identified in the treatment of SP, including XBJ, TRQ, RDN, XYP, SF, and SM. The Mantel-Haenszel random-effects model was used for the meta-analysis because of the heterogeneity. The assumption of consistency between direct and indirect evidence was not utilized due to non-closed loops in this NMA. Six interested outcomes were identified in this network meta-analysis, including clinical effective rate; the time of mechanical ventilation; ICU length of stay; and serum levels of WBC, CRP, and PCT. The results indicated that XBJ, TRQ, RDN, and SM combined with WM had a superior effect than WM alone in terms of the outcome of clinical effective rate. Based on SUCRA values, TRQ combined with WM ranked the highest in improving the clinical effective rate, second in four different outcomes, and third in only one. Similarly, XYP combined with WM ranked second in the clinical effective rate but ranked the highest in three outcomes, which were the level of WBC, ICU length of stay, and the time of mechanical ventilation. What is more, according to the cluster analysis, TRQ and XYP combined with WM were similarly superior in terms of the clinical effective rate and the level of WBC, the clinical effective rate and ICU length of stay, and the clinical effective rate and the time of mechanical ventilation. Therefore, TRQ and XYP combined with WM were worth paying more attention for SP in adults. However, only one RCT of XYP involving 68 participants was included in our study. Taking into account the small sample size, the strength of evidence for this result may be reduced.
As for safety, less than 30% (18 RCTs) of the included studies reported ADRs/ADEs, including 14 RCTs of XBJ, 3 RCTs of TRQ, and 1 RCT of RDN. The ADRs/ADEs mainly included headache and dizziness, nausea and vomiting, diarrhea, chest discomfort or dyspnea, and itchy skin or rash. It is noteworthy that headache and dizziness occurred most frequently among the abovementioned ADRs/ADEs. Though all the ADRs/ADEs were mild and can be relieved by themselves, no RCTs reported the rate of ADRs/ADEs comparing CHIs combined with WM and WM alone. Hence, we could not draw a certain conclusion that combining CHIs with WM will not increase the ADRs/ADEs of the patients. Hopefully, further studies especially clinical trials should pay more attention to these ADRs/ADEs of CHIs, and more studies are needed to determine the safety of CHIs combined with WM for SP.
SP is a complex and refractory disease. The causative pathogen produces an excessive inflammatory response with high levels of anti-inflammatory cytokines. High levels of anti-inflammatory cytokines are initially detected in the plasma and the lungs. These high levels of anti-inflammatory cytokines are associated with ICU admission and mortality (Ramírez et al., 2011). As the present major treatment, adequate initial antibiotics can cause serious drug resistance and did not reduce the mortality rate of patients admitted to the ICU for SP (Garnacho-Montero et al., 2018). Co-adjuvant therapies such as CHIs become more attractive. CHIs combined with WM for SP exhibited a better performance in improving the clinical effective rate and the inflammatory indicators (WBC, CRP, and PCT) and decreasing the ICU length of stay and the time of mechanical ventilation, which may provide the solution for the above problems. Additionally, “Huang di Nei Jing” recorded that “keep healthy, do not be evil.” SP belongs to deficiency in origin and excess in superficiality. Antipyretic CHIs such as TRQ and XYP can relieve fever, become anti-inflammatory, and increase antibiotic sensitivity (Pan, 2015). Restorative CHIs such as SF can lower the pro-inflammatory cytokines and shorten the time of mechanical ventilation, ICU length of stay, the application time of vasoactive drugs, and even the mortality (Zhang and Wang, 2015; Lv et al., 2017). All the above advantages were closely related to the ingredients within them.
TRQ is a traditional Chinese medicine (TCM) consisting of five herbals extracts: Scutellariae Radix, bear bile powder, cornugorais, Lonicerae japonicae flos, and Forsythiae fructus. Based on the theory of traditional Chinese medicine, Scutellariae Radix has a bitter taste and the great effects of clearing heat, drying moisture, and detoxification. Bear bile power has the functions of reducing heat, spasmolysis, and detoxification. Cornugorais could enter the liver meridian and has significant antipyretic and sedative effects. Lonicerae japonicae flos can clear heat and detoxify and dispel the wind. When it is compatible with Forsythiae fructus, its effect will be more significant. In general, TRQ formula clears heat, detoxifies, and removes phlegm according to the traditional Chinese medicine theory. In terms of modern pharmacology, recent in vivo experiments had demonstrated that TRQ had antibacterial and antiviral effects. In some in vitro antibacterial tests, TRQ also showed a strong effect against some bacteria, such as Streptococcus pneumoniae, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), and Haemophilus influenzae, and has markedly strengthened the antibacterial effect of antibiotics (Yang et al., 2018; Wang et al., 2020). Nevertheless, utilizing TRQ had some concerns. As one of the compositions of Tanreqing, bear bile powder is derived from Selenarctos thibetanus Cuvier. In China, the law has prohibited to get bear bile powder from hunting S. thibetanus Cuvier. The bear bile powders used as medicine are mainly derived from artificial feeding bears. What is more, only the farms which have gotten the license of domesticating and breeding wildlife under special state protection are qualified to feed S. thibetanus Cuvier (Author Anonymous, 2004; Author Anonymous, 2013; Author Anonymous, 2015). In addition, the technology of getting bear bile powder is mature and safe by surgical drainage from the bears’ gall bladder. But even so, in consideration of protecting endangered animals and dealing with increased demand of medicine, studying the alternatives is urged. XYP is mainly composed of andrographolide sulfonate. The main active components of SF include ginsenoside and aconite total alkaloids.
There are three advantages that could enhance the credibility of this study. First, to the best of our knowledge, this is the first NMA to compare the effects of different CHIs and rank them for the treatment of SP. Secondly, these results may be helpful to clinicians to make a better choice for the treatment of SP. More importantly, in addition to the clinical phenomena and efficacy, the ICU length of stay, the time of mechanical ventilation, and inflammatory indicators were also analyzed. Inflammatory indicators are more essential to SP because of their relativity to the SP’s pathophysiology. What is more, inflammatory indicators are also relative to the mechanism of drug effect. Critically patients are complex and under many different interventions such as mechanical ventilation. ICU length of stay and the time of mechanical ventilation can not only reflect clinical effects from the side but also reflect economic benefits.
Limitation
This study also has some limitations. First, all RCTs were carried out in China, and the data of clinical studies in other languages were lacking. Second, the qualities of included RCTs in this study were not high. About 58% of RCTs described the method of generating random sequences. Only two RCTs described information of allocation concealment and one RCT accurately set blinding. Third, there was a lack of large sample directly comparing the two injections. The difference among the sample sizes of different injections would also reduce the strength of the evidence for the results. It is necessary to conduct a subgroup analysis based on background diseases, different types of pneumonia, duration of treatment, and Western medicine treatment measure. However, except XBJ injection, the number of included studies for other CHIs was not enough to conduct a subgroup analysis. In addition, it is difficult to conduct a subgroup analysis as the currently included studies could not be accurately categorized based on the above variables. We hope that more RCTs could include only one background disease, one type of pneumonia (CAP, HAP, or VAP), one kind of antibiotics, and a fixed treatment duration in developing the inclusion criteria.
Conclusion
In conclusion, the study found that the CHIs as a co-adjuvant therapy could be beneficial for patients with SP. TRQ + WM had preferable effects in improving the clinical effective rate of SP. XYP + WM was more effective in the perspective of reducing the level of WBC, the ICU length of stay, and the time of mechanical ventilation. So, considering both the clinical effective rate and other outcomes, TRQ + WM showed an outstanding improvement in patients with SP. However, because of the limitations of this study, the results should be verified by more high-quality and large-sample RCTs. Meanwhile, the safety of CHIs should be more monitored and reported.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
LN contributed in the conceptualization of the study; methodology; software; formal analysis; quality assessment; investigation; resources; data curation; writing-original draft preparation, review, and editing; and visualization. LX contributed in the conceptualization of the study, methodology, validation, formal analysis, quality assessment, investigation, data curation, writing-original review and editing, visualization, and project administration. XZ contributed in the investigation, resources, data curation, and software. XuL contributed in the methodology, validation, data curation, quality assessment, writing-original review and editing, and supervision. XiL contributed in the writing-original review and editing, supervision, and project administration. XH contributed in the software; resources; and writing-original draft preparation, review, and editing. MZ contributed in the software; resources; writing-original draft preparation, review, and editing. All data were generated in-house, and no paper mill was used. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the support program of the National Program on Key Basic Research Project (973 Program, no. 2009CB522702) and the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (no. 201928). The funders had no role in study design, data collection, and analysis; report writing; or decision-making for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.743486/full#supplementary-material
References
Author Anonymous, (2004). Law of the People's Republic of China on the Protection of Wildlife. Gaz. Standing Committee Natl. People's Congress People's Republic China (6), 543–546.
Author Anonymous, (2013). Regulations of the People's Republic of China for the Implementation of the Protection of Terrestrial Wildlife, 193–199.
Author Anonymous, (2015). Measures for the Administration of Domestication and Breeding Licenses for Wildlife under Special State Protection. The Bull. State. Counc. People's Republic China (21), 34–35.
Aliberti, S., Cook, G. S., Babu, B. L., Reyes, L. F., A, H. R., H Rodriguez, F., et al. (2019). International Prevalence and Risk Factors Evaluation for Drug-Resistant Streptococcus Pneumoniae Pneumonia. J. Infect. 79 (4), 300–311. doi:10.1016/j.jinf.2019.07.004
Bi, J., Yang, J., Wang, Y., Yao, C., Mei, J., Liu, Y., et al. (2016). Efficacy and Safety of Adjunctive Corticosteroids Therapy for Severe Community-Acquired Pneumonia in Adults: An Updated Systematic Review and Meta-Analysis. PLoS One 11 (11), e0165942. doi:10.1371/journal.pone.0165942
Cai, C. Y. (2020). Clinical Effect of Tanreqing Injection in the Treatment of Severe Pneumonia. Mod. Diagn. Treat. 31 (7), 1028–1030.
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical Tools for Network Meta-Analysis in STATA. PLoS One 8 (10), e76654. doi:10.1371/journal.pone.0076654
Chen, J. Z. (2019). Therapeutic Effect of Xuebijing Injection Combined with Meropenem on Elderly Patients with Severe Pneumonia. Pract. Geriatr. 33 (2), 182–185. doi:10.3969/j.issn.1003-9198.2019.02.019
Chen, S., Liu, H., Lin, T. Y., and Mu, X. J. (2019). Clinical Study of Xuebijing Injection Combined with Piperacillin Sodium and Tazobactam Sodium in the Treatment of Severe Pneumonia. Res. Integr. Tradit. Chin. West. Med. 11 (1), 8–11. doi:10.3969/j.issn.1674-4616.2019.01.003
Chen, Y. J., Gao, Y. Q., and Peng, Y. W. (2014). The Clinical Study of Ulinastatin and Xuebijing on Elderly Patients with Severe Pneumonia. J. Lingnan Emerg. Med. 19 (4), 269–270+273.
Deng, Z., and Chen, W. (2021). The Effect of Xuebijing Injection Combined with Antibiotics in the Treatment of Severe Pneumonia. Chin. J. Clin. Ration Drug Use 14 (8), 71–73.
Diao, Y. F., Zhang, S. J., Zhao, W. Y., Sun, H. T., Zhang, S., and Sun, L. (2017). Effect of Xuebijing Injections on Expression of Interleukin-6 and Tumor Necrosis Factor-α in Patients with Severe Pneumonia. Chin. Tradit. Herb. Med. 48 (6), 1188–1191.
Ding, Z. P., and Lu, Y. Y. (2020). The Clinical Effect of Xuebijing Injection in the Treatment of Severe Pneumonia and its Effect on Patients' Arterial Blood Gas Indexes. Renowned Doct (11), 368–369.
Fan, X. C., Tu, S., and Cao, F. T. (2018). Effect of Shenmai Injection on Inflammatory Factors in Patients with Severe Pneumonia. Hebei Med. (Chin.) 24 (10), 1656–1659. doi:10.3969/j.issn.1006-6233.2018.10.018
Gao, Y. Q., Zhang, H., Liu, M., Zhou, L. J., Sun, G. X., and Ren, Y. J. (2014). Clinical Efficacy and Safety Observation of Xuebijing Injection in Patients with Severe Pneumonia. J. Zhengzhou Univ. 49 (6), 837–840.
Garnacho-Montero, J., Barrero-García, I., Gómez-Prieto, M. G., and Martín-Loeches, I. (2018). Severe Community-Acquired Pneumonia: Current Management and Future Therapeutic Alternatives. Expert Rev. Anti Infect. Ther. 16 (9), 667–677. doi:10.1080/14787210.2018.1512403
Garnacho-Montero, J., García-Cabrera, E., Diaz-Martín, A., Lepe-Jiménez, J. A., Iraurgi-Arcarazo, P., Jiménez-Alvarez, R., et al. (2010). Determinants of Outcome in Patients with Bacteraemic Pneumococcal Pneumonia: Importance of Early Adequate Treatment. Scand. J. Infect. Dis. 42 (3), 185–192. doi:10.3109/00365540903418522
Guo, H., Zheng, J., Huang, G., Xiang, Y., Lang, C., Li, B., et al. (2020). Xuebijing Injection in the Treatment of COVID-19: a Retrospective Case-Control Study. Ann. Palliat. Med. 9 (5), 3235–3248. doi:10.21037/apm-20-1478
Han, F., Zhang, L., and Zhao, S. (2012). The Effect on Immune Function of Xuebijing Injection in Patients with Severe Pneumonia. Chin. Med. Engin. 20 (1), 33+35. doi:10.11569/wcjd.v20.i33.3225
Hansen, V., Oren, E., Dennis, L. K., and Brown, H. E. (2016). Infectious Disease Mortality Trends in the United States, 1980-2014. Jama 316 (20), 2149–2151. doi:10.1001/jama.2016.12423
Higgins, J. P., and Welton, N. J. (2015). Network Meta-Analysis: a Norm for Comparative Effectiveness? Lancet 386 (9994), 628–630. doi:10.1016/s0140-6736(15)61478-7
Huang, S. Z. (2015). The Effect of Integrated Chinese and Western Medicine in Treatment of Severe Pulmonary Infection in ICU. J. Community Med. 13 (4), 24–25.
Huang, X., Duan, X., Zhu, Y., Wang, K., Wu, J., and Tian, X. (2019). Comparative Efficacy of Chinese Herbal Injections for the Treatment of Community-Acquired Pneumonia: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Phytomedicine 63, 153009. doi:10.1016/j.phymed.2019.153009
Jain, S., Self, W. H., Wunderink, R. G., Fakhran, S., Balk, R., Bramley, A. M., et al. (2015). Community-Acquired Pneumonia Requiring Hospitalization Among U.S. Adults. N. Engl. J. Med. 373 (5), 415–427. doi:10.1056/NEJMoa1500245
Jin, L., Xu, Y., and Yuan, H. (2020). Effects of Four Types of Integrated Chinese and Western Medicines for the Treatment of COVID-19 in China: a Network Meta-Analysis. Rev. Assoc. Med. Bras 66 (6), 771–777. doi:10.1590/1806-9282.66.6.771
Kong, L. Y. (2015). The Clinical Efficacy of Xuebijing Injection in Treatment of Severe Community-Acquired Pneumonia in ICU. Tianjin J. Tradit. Chin. Med. 32 (6), 341–343. doi:10.11656/j.issn.1672-1519.2015.06.06
Lanks, C. W., Musani, A. I., and Hsia, D. W. (2019). Community-acquired Pneumonia and Hospital-Acquired Pneumonia. Med. Clin. North. Am. 103 (3), 487–501. doi:10.1016/j.mcna.2018.12.008
Lei, J. P., Zeng, W. L., Wan, Z., and Wu, S. Y. (2017). Clinical Curative Effect of Ulinastatin Combined Tanreqing in Treatment of Severe Pneumonia. Chin. Med. Pharm. 7 (05), 22–25+43. doi:10.3969/j.issn.2095-0616.2017.05.006
Li, J. (2013). Clinical Observation of Tanreqing Injection in Treatment of Severe Pneumonia of Phlegm Obstructing Lung. J. Liaoning Univ. Tradit. Chin. Med. 15 (03), 182–184.
Li, Z. X. (2017). The Clinical Practice Value of Xuebijing Injection Combined with Antibiotics in the Treatment of Severe Pneumonia. Chin. Med. Dev. 32 (S01).
Lin, H., Ma, C. L., Wang, R. H., and Liang, D. Y. (2013). Effect of Shenfu Injection on Blood Lactate and Prognosis in Patients with Severe Pneumonia. J. Emerg. Tradit. Chin. Med. 22 (02), 199–200. doi:10.3969/j.issn.1004-745X.2013.02.012
Liu, X. L., and Qin, Q. Y. (2019). Clinical Efficacy of Tanreqing Injection Combined with Piperacillin Sulbactam in the Treatment of Severe Pneumonia in the Elderly and its Effect on Serum Inflammatory Factors. Inter. Med. Health Guid. News (04), 603–606.
Lunn, D., Spiegelhalter, D., Thomas, A., and Best, N. (2009). The BUGS Project: Evolution, Critique and Future Directions. Stat. Med. 28 (25), 3049–3067. doi:10.1002/sim.3680
Luo, Z., Chen, W., Xiang, M., Wang, H., Xiao, W., Xu, C., et al. (2021). The Preventive Effect of Xuebijing Injection against Cytokine Storm for Severe Patients with COVID-19: A Prospective Randomized Controlled Trial. Eur. J. Integr. Med. 42, 101305. doi:10.1016/j.eujim.2021.101305
Lv, S. J., Lai, D. P., Wei, X., Yan, Q., and Xia, J. M. (2017). The Protective Effect of Shenfu Injection against Elderly Severe Pneumonia. Eur. J. Trauma Emerg. Surg. 43 (5), 711–715. doi:10.1007/s00068-016-0713-2
Ma, L. H. (2016). The Clinical Practice of Xuebijing Injection in the Treatment of Severe Pneumonia. Clin. Med. 2.
Mandell, L. A., Wunderink, R. G., Anzueto, A., Bartlett, J. G., Campbell, G. D., Dean, N. C., et al. (2007). Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 44 (Suppl. 2Suppl 2), S27–S72. doi:10.1086/511159
Meng, S. D., and Xie, B. (2018). Clinical Observation of Xuebijing Injection Combined with Linezolid in Treatment of Severe Pneumonia. Mod. Drugs Clin. 33 (03), 518–522.
Metlay, J. P., Waterer, G. W., Long, A. C., Anzueto, A., Brozek, J., Crothers, K., et al. (2019). Diagnosis and Treatment of Adults with Community-Acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 200 (7), e45–e67. doi:10.1164/rccm.201908-1581ST
Mizgerd, J. P. (2017). Pathogenesis of Severe Pneumonia: Advances and Knowledge Gaps. Curr. Opin. Pulm. Med. 23 (3), 193–197. doi:10.1097/mcp.0000000000000365
Musher, D. M., and Thorner, A. R. (2014). Community-acquired Pneumonia. N. Engl. J. Med. 371 (17), 1619–1628. doi:10.1056/NEJMra1312885
Niu, L. L., Xia, J. H., Zhao, J. R., Chen, M., and Wang, H. F. (2017). The Clinical Observation of Xuebijing Injection in the Treatment of Elderly Severe Pneumonia with Respiratory Failure and its Effect on the Inflammatory Indicators. Shanxi Med. J. 46 (12), 1440–1443. doi:10.3969/j.issn.0253-9926.2017.12.016
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Pan, P. X. (2015). Pharmacological Action and Clinical Application of Tanreqing Injection. Clin. J. Ration Drug Use 8 (17), 174–175.
Pan, X. B., Zhou, J. S., and Zhang, C. Y. (2017). The Effect of Different Doses of Xuebijing Injection Combined with Thymosinα1 in Treatment of Elderly Patients with Severe Pneumonia. Chin. J. Women Child Health Res. 28 (S2), 32–33.
Prina, E., Ranzani, O. T., and Torres, A. (2015). Community-acquired Pneumonia. Lancet 386 (9998), 1097–1108. doi:10.1016/s0140-6736(15)60733-4
Qi, F., Liang, Z. X., She, D. Y., Yan, G. T., and Chen, L. A. (2011). A Clinical Study on the Effects and Mechanism of Xuebijing Injection in Severe Pneumonia Patients. J. Tradit Chin Med. 31 (1), 46–49. doi:10.1016/s0254-6272(11)60011-3
Qiu, J. N. (2021). Effect of Xuebijing Combined with Linezolid on Serological Indexes and Bacterial Clearance Rate in Patients with Severe Pneumonia. Chin. J. Pharm. Econ. 16 (02), 46–48+52. doi:10.12010/j.issn.1673-5846.2021.02.010
Qu, J. M., and Cao, B. (2016). Guidelines for the Diagnosis and Treatment of Community-Acquired Pneumonia in Chinese Adults (2016 Edition). Chin. J. Tuberc. Respir. Dis. 39 (04), 253–279.
Ramírez, P., Ferrer, M., Martí, V., Reyes, S., Martínez, R., Menéndez, R., et al. (2011). Inflammatory Biomarkers and Prediction for Intensive Care Unit Admission in Severe Community-Acquired Pneumonia. Crit. Care Med. 39 (10), 2211–2217. doi:10.1097/CCM.0b013e3182257445
Restrepo, M. I., Mortensen, E. M., Rello, J., Brody, J., and Anzueto, A. (2010). Late Admission to the ICU in Patients with Community-Acquired Pneumonia Is Associated with Higher Mortality. Chest 137 (3), 552–557. doi:10.1378/chest.09-1547
Riley, R. D., Jackson, D., Salanti, G., Burke, D. L., Price, M., Kirkham, J., et al. (2017). Multivariate and Network Meta-Analysis of Multiple Outcomes and Multiple Treatments: Rationale, Concepts, and Examples. Bmj 358, j3932. doi:10.1136/bmj.j3932
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: an Overview and Tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi:10.1016/j.jclinepi.2010.03.016
Salanti, G., Del Giovane, C., Chaimani, A., Caldwell, D. M., and Higgins, J. P. (2014). Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS One 9 (7), e99682. doi:10.1371/journal.pone.0099682
Shang, H. B., and Zhang, C. X. (2019). The Clinical Efficacy of Xuebijing Injection Combined with Ambroxol Hydrochloride in Treatment of Severe Pneumonia. Mod. Med. Health Res. 3 (24), 53–55.
Sheng, N., Zhang, L. L., Lu, C. F., Zhang, Y., Zhou, T., and Zhang, P. R. (2019). Xuebijing Injection in the Treatment of Severe Pneumonia. J. Trop. Med. 19 (03), 357–359+363. doi:10.3969/j.issn.1672-3619.2019.03.024
Shi, N., Guo, L., Liu, B., Bian, Y., Chen, R., Chen, S., et al. (2021). Efficacy and Safety of Chinese Herbal Medicine versus Lopinavir-Ritonavir in Adult Patients with Coronavirus Disease 2019: A Non-randomized Controlled Trial. Phytomedicine 81, 153367. doi:10.1016/j.phymed.2020.153367
Shim, S., Yoon, B. H., Shin, I. S., and Bae, J. M. (2017). Network Meta-Analysis: Application and Practice Using Stata. Epidemiol. Health 39, e2017047. doi:10.4178/epih.e2017047
Song, B., and Chai, Y. F. (2015). The Clinical Efficacy Study of Xuebijing Injection in the Treatment of Severe Pneumonia. Jilin Med. 36 (14), 3101–3102. doi:10.3969/j.issn.1004-0412.2015.14.105
Song, Y., Yao, C., Yao, Y., Han, H., Zhao, X., Yu, K., et al. (2019). XueBiJing Injection versus Placebo for Critically Ill Patients with Severe Community-Acquired Pneumonia: A Randomized Controlled Trial. Crit. Care Med. 47 (9), e735–e743. doi:10.1097/CCM.0000000000003842
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a Revised Tool for Assessing Risk of Bias in Randomised Trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Sun, G. X., Zu, Y. N., and Li, G. Y. (2012). Study on Adjuvant Therapy of Reduning Injection for Severe Pneumonia. Prac. Pharm. Clin. Remed. 15 (07), 439–440. doi:10.3969/j.issn.1673-0070.2012.07.024
Sun, J. G., Li, C., Zheng, C. L., Sun, A. H., Ai, Y. Y., Pan, H. Y., et al. (2020). Clinical Observation of Tanreqing Injection Combined with Western Medicine in the Treatment of Severe Pneumonia. Beijing J. Tradit. Chin. Med. 39 (01), 66–69.
Tian, J., Yang, S., Song, D. D., and Han, X. H. (2019). Progress in Clinical Efficacy of Xuebijing in Treatment of Severe Pneumonia. J. Clin. Pulm. Med. 24 (05), 865–868+873. doi:10.3969/j.issn.1009-6663.2019.05.022
Torres, A., Sibila, O., Ferrer, M., Polverino, E., Menendez, R., Mensa, J., et al. (2015). Effect of Corticosteroids on Treatment Failure Among Hospitalized Patients with Severe Community-Acquired Pneumonia and High Inflammatory Response: a Randomized Clinical Trial. Jama 313 (7), 677–686. doi:10.1001/jama.2015.88
Wan, Y. D., Sun, T. W., Liu, Z. Q., Zhang, S. G., Wang, L. X., and Kan, Q. C. (2016). Efficacy and Safety of Corticosteroids for Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis. Chest 149 (1), 209–219. doi:10.1378/chest.15-1733
Wang, D. L., Zhao, L. D., Li, L. J., and Zhou, M. J. (2019). Effects of Xuebijing Combined Cefoperazone Sulbactam Sodium and Levofloxacin on Immune Function, Coagulation Function and Therapeutic Effect in Patients with Severe Community Acquired Pneumonia. Chin. Hosp. Pharm. J. 39 (15), 1561–1565.
Wang, L. (2019). Clinical Effect Observation of Xuebijing Injection Combined with Linezolid in the Treatment of Severe Pneumonia. J. Chin. Presc. Drug 17 (04), 83–84. doi:10.3969/j.issn.1671-945X.2019.04.054
Wang, L. (2018). Clinical Efficacy of Xuebijing Injection in Treatment of Severe Pneumonia and its Effect on Blood Gas and Serum Inflammatory Cytokines. J. Clin. Pulm. Med. 23 (06), 1101–1104. doi:10.3969/j.issn.1009-6663.2018.06.033
Wang, L. L., Weng, F. Z., and Yan, J. (2020). The Clinical Study of Xuebijing Injection as an Adjuvant Therapy in Treatment of Severe Pneumonia and its Effects on Pulmonary Function and Bacterial Clearance Rate. J. Chin. Prescription Drug 18 (01), 96–97. doi:10.3969/j.issn.1671-945X.2020.01.054
Wang, L., Tao, Y. L., and Chen, W. S. (2020). Research Progress on Chemical Constituents, Pharmacological Effects and Clinical Application of Tanreqing Injection. Chin. Tradit. Herbal Drugs 51 (12), 3318–3328. doi:10.7501/j.issn.0253-2670.2020.12.026
Wang, M., Wu, K. W., and Wang, Q. (2017). The Effect of Xuebijing Injection on Inflammatory Factors and Prognosis in Patients with Severe Pneumonia Combined with Respiratory Failure. J. Clin. Prac. Med. 21 (11), 1338–1340.
Wang, Z. W. (2019). Therapeutic Evaluation of Biapenem Combined with Xuebijing Injection in Treatment of Elderly Patients with Severe Hospital Acquired Pneumonia. Hubei J. Tradit. Chin. Med. 41 (03), 7–10.
Wei, S. X., and Huang, Y. M. (2020). Discussion on the Role of Xuebijing Injection in the Treatment of Severe Pneumonia. J. Chin. Presc. Drug 18 (07), 122–123. doi:10.3969/j.issn.1671-945X.2020.07.066
Welte, T. (2016). Severe Pneumonia in the Intensive Care Unit. Med. Klin Intensivmed Notfmed 111 (4), 279–289. doi:10.1007/s00063-016-0165-9
Wu, H., and Ru, H. Y. (2011). The Clinical Efficacy Observation of Tanreqing Injection in the Treatment of Severe Pneumonia. Guiding. J. Tradit. Chin. Med. Pharm. 17 (10), 43–44. doi:10.3969/j.issn.1672-951X.2011.10.019
Wu, J. H. (2015). Efficacy of Xuebijing Injection in Treatment of Elderly Patients with Severe Pneumonia and Respiratory Failure and its Inflammatory Factors. Med. Recap. 21 (22), 4166–4168. doi:10.3969/j.issn.1006-2084.2015.22.051
Wu, T., and Ren, C. F. (2016). The Curative Effect Analysis of Xuebijing and Antibiotics Used in Combination to Treat Elderly Patients with Community Acquired Pneumonia. All Health 10 (13), 297–298.
Wu, X. D. (2012). Clinical observation of Tanreqing injection combined with de escalation therapy of antibiotics in elderly patients with severe pneumonia. J. Clin. Pulm. Med. 17 (11), 1981–1982. doi:10.3969/j.issn.1009-6663.2012.11.018
Xi, R., Shi, X. L., Qu, N. N., and Wang, L. N. (2016). Clinical Observation of Severe Pneumonia Treated with Tanreqing and Antibiotics. World J. Integr. Tradit. Chin. West. Med. 11 (07), 956–958.
Xia, L. F., and Xie, Z. X. (2017). Clinical Observation of Early Application of Shenfu Injection Combined with Yanhuning Injection on Elderly Patients with Severe Community-Acquired Pneumonia and its Effect on Serum Related Indexes and Coagulation Related Indexes. Hebei J. Tradit. Chin. Med. 39 (12), 1801–1805+1811. doi:10.3969/j.issn.1002-2619.2017.12.009
Xiao, Q., Yu, D., Yan, D. F., and Zhu, C. Y. (2020). Clinical Value Effect Evaluation of Xuebijing Injection Combined with Meropenem in Treatment of Elderly Severe Pneumonia and Effect on Serum Inflammatory Cytokines. Chin. J. Ration Drug Use 17 (11), 47–51. doi:10.3969/j.issn.2096-3327.2020.11.012
Xie, G. L. (2016). The Clinical Effect of 100 Cases with Severe Pneumonia Treated with Xuebijing Injection Combined with Thymosin. Clin. Med. 36 (03), 47–48.
Xin, R. R. (2020). Clinical Study on Xuebijing Injection Combined with Moxifloxacin in Treatment of Severe Pneumonia. Mod. Drugs Clin. 35 (05), 863–867.
Xu, M. (2017). Clinical Efficacy of Xuebijing Injection Combined with Antimicrobial Drugs in Treatment of Severe Pneumonia in ICU. Jiangxi Med. J. 52 (06), 509–510. doi:10.3969/j.issn.1006-2238.2017.06.014
Yang, G. L., and Zhang, Z. H. (2019). Clinical Effect Observation of Low Dose Hydrocortisone Combined with Shenmai Injection in the Treatment of Severe Pneumonia. Clin. J. Med. Offic. 47 (06), 636–637.
Yang, H. W., Shi, F., Yu, Q. P., Ye, X. W., Liu, S., Luo, Y., et al. (2020). Effect of Xuebijing on Serum Soluble Myeloid Cell Trigger Receptor-1 (sTREM-1) Level and CPIS Score in Elderly Patients with Severe Pneumonia. Jiangxi Med. J. 55 (08), 1008–1011+1041. doi:10.3969/j.issn.1006-2238.2020.08.012
Yang, T. B. (2020). The Efficacy on Arterial Blood Gas Indexes and Serum TNF-α, PCT, CRP Level of Patients with Severe Pneumonia Treated with Xuebijing Injection Combined with Bronchoalveolar Lavage. J. Mod. Med. Health Res. 4 (11), 79–81.
Yang, W., Liu, J., Blažeković, B., Sun, Y., Ma, S., Ren, C., et al. (2018). In Vitro antibacterial Effects of Tanreqing Injection Combined with Vancomycin or Linezolid against Methicillin-Resistant Staphylococcus aureus. BMC Complement Altern. Med. 18 (1), 169. doi:10.1186/s12906-018-2231-8
Yang, Z. X., Fan, T. B., and Li, J. (2014). Xiyanping Injection for Severe Pneumonia with Syndrome of Phlegm-Heat Obstructing Lung. Beijing J. Tradit. Chin. Med. 33 (12), 894–896.
Yin, Z. M. (2019). The Clinical Observation of Xuebijing Injection Combined with Linezolid in the Treatment of Severe Pneumonia. Spec. Health (20), 62–63. doi:10.3969/j.issn.2095-6851.2019.20.093
Yuan, H. Y., and Zhao, B. (2020). The Efficacy on Arterial Blood Gas Indexes and Serum Inflammatory Factors of Patients with Severe Pneumonia Treated with Xuebijing Injection Combined with Bronchoalveolar Lavage. J. Mod. Med. Health Res. 4 (15), 91–93.
Zhang, H. Y. (2021). Clinical Study of Tanreqing Injection Combined with Piperacillin Sulbactam in the Treatment of Senile Severe Pneumonia. Res. Integr. Tradit. Chin. West. Med. 13 (01), 5–7. doi:10.3969/j.issn.1674-4616.2021.01.002
Zhang, L. L., and Wang, G. L. (2015). Clinical Observation of Xiyanping Injection in Treatment of Elderly Severe Pneumonia. J. Emerg. Chin. Tradit. Med. 24 (12), 2289–2290. doi:10.3969/j.issn.1004-745X.2015.12.082
Zhang, L. L., Wang, Y. J., and Yuan, Y. (2020). Clinical Efficacy Observation of Xuebijing Injection Combined with Antimicrobial Drugs in the Treatment of Severe Pneumonia in ICU. Oriental Medicated Diet (12), 119.
Zhang, S. L., Meng, X. H., Zhang, H. W., Hao, Y. Y., and Huang, F. Y. (2014). The Clinical Efficacy of Xuebijing Injection Combined with Thymopentin in Treatment of Severe Pneumonia. Mod. J. Integr. Tradit. Chin. West. Med. 23 (30), 3346–3347+3350. doi:10.3969/j.issn.1008-8849.2014.30.015
Zhao, Y. J., Yin, G. H., Liu, Z., and Li, L. D. (2019). Application of Xuebijing in the Treatment of Severe Pneumonia. Chin. Heal. Stand. Management 10 (21), 93–95. doi:10.3969/j.issn.1674-9316.2019.21.039
Zheng, H., Chen, Q., Chen, M., Wu, X., She, T. W., Li, J., et al. (2019). Nonpharmacological Conservative Treatments for Chronic Functional Constipation: A Systematic Review and Network Meta-Analysis. Neurogastroenterol Motil. 31 (1), e13441. doi:10.1111/nmo.13441
Zheng, Y. N., He, F., Sha, H. W., Zhang, X., and Yuan, W. W. (2020). Effect of Xuebijing Injection Combined with Biapenem on Pulmonary Function in Patients with Severe Pneumonia. Prog. Mod. Biomed. 20 (23), 4561–4564+4592.
Zhou, D., Xie, L., Shi, X., Liu, F., Wu, S., Zhang, S., et al. (2021). A Meta-Analysis of the Clinical Efficacy of Tanreqing Injection Combined with Antibiotics vs Antibiotics Alone for Treating Pulmonary Infection Secondary to Intracerebral Hemorrhage. Medicine (Baltimore) 100 (11), e24905. doi:10.1097/md.0000000000024905
Zhou, S. J., Wang, G., and Ye, J. R. (2017). Clinical Study of Ambroxol Hydrochloride Combined with Xuebijing Injection for Treatment of Elderly Patients with Severe Pneumonia. Chin. J. Tradit. Chin. Med. West. Med. Crit. Care 24 (5).
Zhou, Z. Y. (2018). The Clinical Efficacy of Xuebijing Injection Combined with Conventional Western Medicine in Treatment of Severe Pneumonia. World Latest Med. Inf. 18 (81), 87+89.
Zhu, J. J., and Liu, L. J. (2014). The Effect on Inflammatory Cytokines in Serum and Bronchoalveolar Lavage Fluid of Xuebijing Injection in Treatment of Severe Pneumonia. Jiangsu Med. J. 40 (10), 1187–1189.
Keywords: network meta-analysis, severe pneumonia, Chinese herbal injections, combination therapy, antibiotic
Citation: Niu L, Xiao L, Zhang X, Liu X, Liu X, Huang X and Zhang M (2022) Comparative Efficacy of Chinese Herbal Injections for Treating Severe Pneumonia: A Systematic Review and Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 12:743486. doi: 10.3389/fphar.2021.743486
Received: 19 July 2021; Accepted: 01 December 2021;
Published: 10 January 2022.
Edited by:
Fang-Rong Chang, Kaohsiung Medical University, TaiwanCopyright © 2022 Niu, Xiao, Zhang, Liu, Liu, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinqiao Liu, MTM4MjExNTAxMTJAMTYzLmNvbQ==; Lu Xiao, eGxqaHkxOTg3QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Liqing Niu
Liqing Niu Lu Xiao1,2,3*†
Lu Xiao1,2,3*† Xuemin Zhang
Xuemin Zhang