
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 14 October 2021
Sec. Drugs Outcomes Research and Policies
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.741671
This article is part of the Research Topic Social and Administrative Policy in Healthcare and Pharmacy Practice View all 20 articles
Background: Pharmaceutical expenditure has been increasing worldwide. Many countries have attempted to contain the increase through collective bargaining, including in China. In 2015, the Chinese government introduced a new policy to empower regional governments to reduce pharmaceutical prices through its existing tendering system which enables a lower price for products with higher procurement volumes. Xiangyang municipality in Hubei province took a lead in piloting this initiative.
Objectives: This study aimed to evaluate the effects of the volume-price contract initiative on pharmaceutical price procured by the public hospitals in Xiangyang.
Methods: A retrospective comparative design was adopted. The price of cardiovascular medicines (349 products under 164 International Nonproprietary Names) procured by the public hospitals in Xiangyang was compared with those procured in Yichang municipality in Hubei. A total of 15,921 procurement records over the period from January 2017 to December 2018 were examined (Xiangyang started the volume-price contract initiative in January 2018). Generalized linear regression models with a difference-in-differences approach which could reflect the differences between the two cities between January 2018 and December 2018 were established to test the effects of the volume-price contract initiative on pharmaceutical prices.
Results: On average, the procurement price for cardiovascular medicines adjusted by defined daily dosage in Xiangyang dropped by 41.51%, compared with a 0.22% decrease in Yichang. The difference-in-differences results showed that the volume-price contract initiative resulted in a 36.24% drop (p = 0.006) in the price (30.23% for the original brands, p = 0.008), in addition to the therapeutic competition effect (31.61% reduction in the price, p = 0.002). The top 100 domestic suppliers were highly responsive to the initiative (82.80% drop in the price, p = 0.001).
Conclusion: The volume-price contract initiative has the potential to bring down the price of pharmaceutical supplies. Higher responses from the domestic suppliers are evident.
Pharmaceuticals account for a profound share of total health expenditure, ranging from an average of 19.7% in high-income countries to 30.4% in low-income countries (Ye et al., 2011). This proportion was around 14.17% in European countries in 2018 (Eurostat, 2021). A rapid growth in pharmaceutical spending is a worldwide concern. The Intercontinental Medical Statistics (IMS) estimated that pharmaceutical expenditure has been increasing at a speed significantly higher than that of global economic growth. From 2010 to 2015, there was an annual growth of 6.2% in global spending on medicines, rising from $US887 billion to $US1069 billion (IMS, 2015). It is projected to exceed $US1.1 trillion in 2024 (IQVIA, 2020).
Soaring pharmaceutical expenditure imposes a great burden on government budgets, which has triggered a range of policy, regulatory and managerial interventions. Collective purchasing has been used as a tool worldwide to lower the price of pharmaceutical supplies (Dylst et al., 2011; Maniadakis et al., 2018). It forces suppliers to compete for the right to become a dominant supplier in certain markets in line with some strictly predefined criteria (Maniadakis et al., 2018). A purchaser can increase its bargaining power by widening the network of collective purchasing. South Africa, for example, introduced a national tendering system for pharmaceuticals in 1982. Empirical evidence showed that the price of pharmaceutical supplies covered by the tendering system dropped by an average of 40% or more between 2003 and 2016 (Wouters et al., 2019). In Mexico, a commission to purchase antiretrovirals and other medicines achieved a cost saving of $US52.1–121.8 million in its first 4 years since inception in 2008 (Gomez-Dantes et al., 2012; Adesina et al., 2013). Cost savings were also found through collective purchasing at the subnational levels, such as the Intermunicipal Health Consortium in Brazil (de Amaral and Blatt, 2011) and the hospital networks in Serbia (Milovanovic et al., 2004) and Brazil (Sigulem and Zucchi, 2009). Collective tendering in European countries has been proved to enhance competition, resulting in reduced prices in pharmaceutical supplies (Dylst et al., 2011; van Woerkom et al., 2012; Vogler et al., 2017; Jensen et al., 2020). In China, rising pharmaceutical expenditure has attracted a great deal of policy attention over the past decade. From 2010 to 2017, pharmaceutical expenses as a proportion of health expenditure in China declined from 41.6% to 34.4% (National Health Commission, 2019). However, it remained at a high level in comparison with OECD (Organization for Economic Cooperation and Development) countries (18.2% in 2010 and 16.1% in 2017) (OECD, 2020). The actual pharmaceutical spending over the same period increased from 883.59 billion yuan ($US129.45 billion) to 1820.30 billion yuan ($US266.67 billion) (National Health Commission, 2019).
Traditionally, pharmaceutical policy debates in China were centered around caps in pharmaceutical prices and mark-up margins allowed for health providers (Hasan et al., 2019; Liu et al., 2019). The Chinese government categorized pharmaceutical products into two groups. Group A are mainly prescription medicines while Group B are mainly over-the-counter medicines (National Development and Reform Commission, 2005). The National Development and Reform Commission (NDRC) imposed a price cap for Group A medicines based on the declared costs from the manufacturers. The provincial governments imposed a price cap for Group B medicines under the guiding prices developed by the NDRC (National Development and Reform Commission, 2005; Hasan et al., 2019). Between 1997 and 2013, over 30 mandatory regulations mostly related to price caps on medicines were announced. Empirical studies showed that these policies were not as effective as anticipated. Pharmaceutical manufacturers could easily evade price caps by registering their products as innovative new drugs through some minor modifications such as dosage forms (Wu et al., 2015; Hu and Mossialos, 2016). This is not unique to China (Vernaz et al., 2013). Meanwhile, the 15% mark-up rule for health institutions provided perverse incentives for medical doctors to prescribe more expensive medicines such as injections and traditional Chinese medicines (Meng et al., 2005; Zeng et al., 2014; Zeng et al., 2015; Hu and Mossialos, 2016). Eventually, the NDRC abolished the price cap policy in 2015 (Hu and Mossialos, 2016). Markups for health institutions on sales of medicines have been officially removed since 2017 throughout the country (Tang et al., 2018). As a result, there are high expectations that centralized tendering and collective purchasing which is gradually developing towards volume-price contract initiative will play a significant role in curtailing the pricing inflation of pharmaceutical products (Hu and Mossialos, 2016).
The exploration of centralized tendering and collective procurement of medicines in China dates back to the 1990s. But it was not until 2009 that it became a nationwide province-based governmental practice. The centralized procurement arrangements started with essential medicines for primary care and were gradually extended to pharmaceutical procurements for public hospitals. Each provincial government has its own online platform, supporting tendering, contracting, purchasing, and distribution of pharmaceutical products (Cai, 2017). Since 2010, each tender has been required to submit two separate bidding documents (“two-envelope”) demonstrating its bidding price and quality of products and services, respectively (Hu and Mossialos, 2016). The winners were supposed to go with the suppliers with the highest composite score of the two envelopes (although usually the lowest price won) (Hu et al., 2019). In some provinces, only one supplier would be contracted to supply certain medicines, while in other provinces, two or more suppliers could be contracted (Hu and Mossialos, 2016). It was estimated that the price of essential medicines for primary care decreased by an average of 25% and even over 50% in some provinces between 2009 and 2010 (Hu, 2013).
Despite the overall drop in prices, the procurement system was criticized for its lack of capacity to link price with volumes of purchased medicines (Fu et al., 2015). The tendering systems overseen by the provincial governments were only responsible for identifying contracted suppliers and settling the prices of pharmaceutical products. No procurement volumes were announced specifically in the tendering. It was up to each individual health institution to make monthly purchase orders and to settle on delivered prices through a “second bargaining” with the suppliers. In addition, there was a lack of supervision over the procurement contracts signed between the health institutions and the suppliers. As a result, pharmaceutical suppliers were placed in a financial dilemma since they could not properly establish an offer based on an accurate estimation of the market share (Tang et al., 2017). Some awarded suppliers would simply not deliver purchase orders if their bidding price was deemed too low to cover the costs. This was particularly common for the lowest-priced generic medicines (Dylst et al., 2013; Fang et al., 2013). Meanwhile, suppliers were likely to manage the risk of market uncertainty through inflating prices, especially for high-priced products (Jiang et al., 2014).
In 2015, the central government issued two policy documents, instructing provincial governments to rationalize procurement prices by attaching procurement volumes to prices in procurement contracts (General Office of the State Council, 2015; National Health and Family Planning Commission, 2015). In practice, it is up to each provincial government to decide the scope for the volume-price contract initiative. Provincial governments continue to set up the highest prices allowed for the included pharmaceutical products. However, health institutions are grouped (selected or all-inclusive at a municipal level or across several municipalities) to bargain for further lower prices for a collective volume of purchase orders. The procurement procedures have to be carried out on the provincial online procurement platform (Li et al., 2018).
Hubei started to pilot the volume-price contract initiative in 2016 in three municipalities: Wuhan, Xiangyang and Ezhou. The municipal governments were authorized to develop their own pharmaceutical catalogues covered in the initiative. However, the procurement volume of each pharmaceutical product had to be justified with reference to its consumption in the previous year (Government of Hubei Province, 2016). For each pharmaceutical product defined by molecule structure, formulation and strength, no more than two suppliers could be awarded. The government used this strategy to make the tendering more attractive (less suppliers and less competition) to those who were willing to reduce price (Government of Hubei Province, 2016). In 2017, the Hubei government issued policy instructions on the volume-based procurement procedure as a condition to sign volume-price contracts (Health Commission of Hubei Province, 2017). This study aimed to evaluate the price effect of the volume-price contract initiative on pharmaceutical supplies to public hospitals in Xiangyang municipality of Hubei province. The findings would be helpful for both researchers and policy makers since such empirical evidence is still lacking as far as we know.
A retrospective comparative study was conducted in Hubei province. The volume-price contract initiative implemented in Xiangyang municipality was evaluated, with Yichang municipality serving as the control.
Hubei covers an area of 185,900 km2 and has about 59.02 million residents. Its per capita annual disposable income reached 31,889 yuan ($US4672) for urban and 13,812 yuan ($US 2023) for rural residents in 2017, 87.62% and 102.83% of the national average, respectively (Bureau of Statistics of Hubei Province, 2018a). About 5.89% of GDP (gross domestic product) was spent on health (192.472 billion yuan, or $US28.200 billion) in 2016. There were approximately 2.50 registered physicians, 3.10 nurses, and 6.37 hospital beds per 1,000 people across 36,357 health care institutions in the province in 2017 (National Health Commission of China, 2018).
Xiangyang occupies a comparable land size (19,728 km2) as Yichang (21,084 km2), but with more dense dwelling. The GDP in Xiangyang ranked second among all municipalities in Hubei in 2017, while Yichang ranked third (Bureau of Statistics of Hubei Province, 2018a). However, the disposable income per capita of all residents in Xiangyang ($US3520) was slightly lower than that of Yichang ($US3543) in 2017. Xiangyang had more health resources and spent more on health compared with Yichang (Table 1) (Bureau of Statistics of Hubei Province, 2018c; b).
Yichang was selected as a control group through a comprehensive assessment of all 17 municipalities in Hubei using an unweighted TOPSIS (technique for order performance by similarity to ideal solution) method (Tang et al., 2016) (Table 1 in the Supplementary Material). Yichang had the closest match (TOPSIS score) with Xiangyang considering eight matching variables: GDP, per capita GDP, population size, per capita disposable income, number of health institutions, number of hospital beds, number of licensed (assistant) doctors, and number of skilled health workers (Table 1). These indicators can reflect the level of economic and health system development (Bureau of Statistics of Hubei Province, 2018a; c; b).
Supplementary material Table S1 Results of (unweighted) TOPSIS ranking.
A retrospective comparative with a difference-in-differences approach was adopted. A total of 15,921 procurement records for cardiovascular medicines over the period from January 2017 to December 2018 were examined. Cardiovascular medicines were chosen in this study since it is a key area with the growing prevalence of cardiovascular diseases and multiple medicines relevant to the treatment of cardiovascular diseases (Mirsafaei et al., 2020; Wei et al., 2020). In addition, they accounted for a large proportion of procurements for specialized medicines, and the volume-price contract initiative for cardiovascular medicines was mature and had a clear cut implementation in January 2018 (Centralized Pharmaceuticals Tendering and Procurement Center of Xiangyang, 2017). In contrast, Yichang, the control group, had not introduced the volume-price contract system over the entire study period. This enabled us to compare the procurement records in the two municipalities before and after the new initiative.
Data came from the Hubei Medical Procurement Administrative Procurement System (HMPAPS). Eligible records were identified using the anatomical therapeutic chemical (ATC) classification and coding system. We restricted the study sample to cardiovascular medicines with an ATC code C. A total of 15,921 procurement records over the study period for cardiovascular medicines were extracted, covering 164 International Nonproprietary Names (INN) and 349 products. These medicines were procured for the 35 public hospitals in the two municipalities: 21 in Xiangyang and 14 in Yichang. Medication needs depended on the local population and their health profiles. The local governments and medical institutions were delegated with the power to select the medicines in line with their local clinical needs. Data items extracted included: procurement serial number, hospital name, time of procurement, INN, formulations, strength, package size, procurement price per package (CNY, Chinese Yuan), procurement volume (packages), procurement cost (CNY), and suppliers of different medicines. We further classified the pharmaceutical products into subgroups according to their brand (original brand and generic) and administration routes (injectable and oral). The suppliers were categorized by ownership (domestic, joint venture, foreign-owned) and ranking of financial outputs (Southern Medicine Economic Research Institute of National Medical Products Administration, 2018; 2019).
The Xiangyang municipality (intervention group) introduced the volume-price contract initiative through a staggered approach, starting with several proton pump inhibitors (digestive medicines) at the beginning of 2017, followed by antimicrobial medicines and patent Chinese medicines in August 2017. Lessons learnt from the two stages of implementation were fed into the final stage of implementation in January 2018, targeting a broad range of specialized medicines including cardiovascular medicines. Procurement of the relevant medicines from all of the 21 public hospitals in Xiangyang were pooled for a better bargaining price based on the large pooled volume. Tendering was organized with a promised volume for each procured medicine calculated based on the clinical needs for six or more months (Health Commission of Hubei Province, 2017). The price of each procured medicine (with specified INN, dosage form, strength, pack size) under the volume-price contract was fixed for all of the covered local health institutions, which lasted for 1 year. In the volume-price contract system, the tendering and procurement procedures were integrated.
Over the study period, the Yichang municipality (control group) maintained its existing procurement system similar to that of Xiangyang prior to the introduction of the volume-price contract system. The provincial government organized the tendering and determined the awarded suppliers without guaranteed procurement volumes. Each individual hospital then conducted its own “second bargaining” for the price of purchase orders (Health and Family Planning Commission of Hubei Province, 2014). Under such a system, the governments were only responsible for identifying suppliers through tendering, leaving the actual procurements in the hands of individual health institutions.
Price change was the primary interest of this study, which was the major policy goal of the volume-price contract initiative. The unit price of each procured medicine was calculated based on its defined daily dosage (DDD) defined by World Health Organization in 2018 (WHO Collaborating Centre for Drug Statistics Methodology, 2018) in absolute monetary terms (CNY).
Generalized linear regression models with a difference-in-differences approach were established to determine price (non-normal distribution) changes associated with the volume-price contract initiative:
where Y indicates the unit price of procured medicines, i indicates a specific cardiovascular product, j represents the public hospital, and t indicates the month (24-month periods).
Several indicators were calculated to measure competition (
We established modelling for the entire sample, as well as modelling for the subsamples categorized by ownership of suppliers and characteristics of medicines in line with the Hedonic model (Rosen, 1974). The estimation of standard errors in the modeling for the sample were clustered at the level of cardiovascular products (procurement serial numbers).
Modified Park tests and Box-Cox tests were used to estimate family distribution and link function of the outcome indicator (unit price), respectively. Log link and Gamma distribution were applied in the modelling for the entire sample and most subsamples, except for the subsample containing foreign-owned suppliers only and the subsample containing original brands only, for which log link and inverse Gaussian distribution were applied. In addition, square root link and Gamma distribution were applied in the modelling for the subsample containing oral medicines only (Table 2).
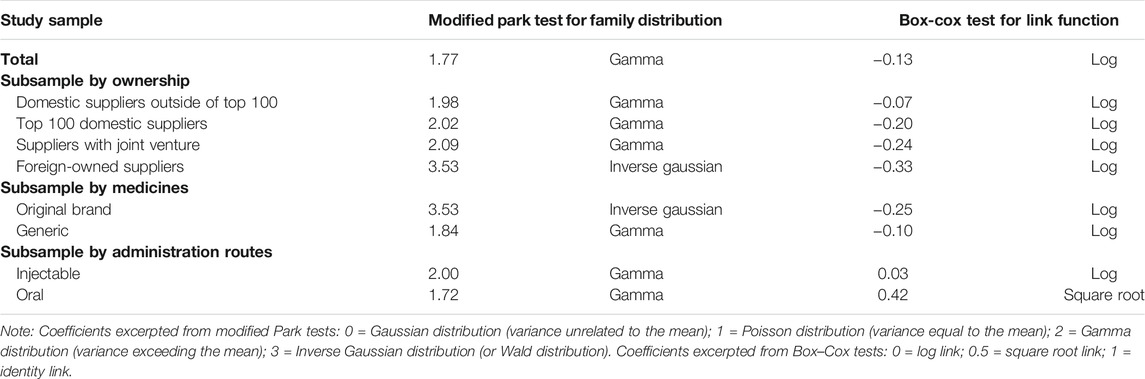
TABLE 2. Estimation of link function (Park tests) and family distribution (Box–Cox tests) of unit price of pharmaceutical products.
Table 3 shows changes to the procured cardiovascular medicines before and after the new initiative in the intervention group (Xiangyang) and the control group (Yichang). There were no obvious changes in the number of INNs and type of products in the control group. But the type of products dropped by 38.86%, from 229 before the initiative to 140 after the initiative; and the number of INNs dropped by 26.98%, from 126 down to 92 in the intervention group. Meanwhile, the monthly average number of both generic (changing from 1.86 to 1.09) and therapeutic (changing from 6.63 to 3.84) competitors per product also declined in the intervention group, compared with an increase in the control group (changing from 2.29 to 2.41 for generic competitors and from 7.99 to 8.71 for therapeutic competitors). Overall, the share of different medicines (generic vs original brands; oral vs injectable) and suppliers (by ownership and financial outputs) remained unchanged: the vast majority of the market was occupied by generic and oral medicines and domestic suppliers.
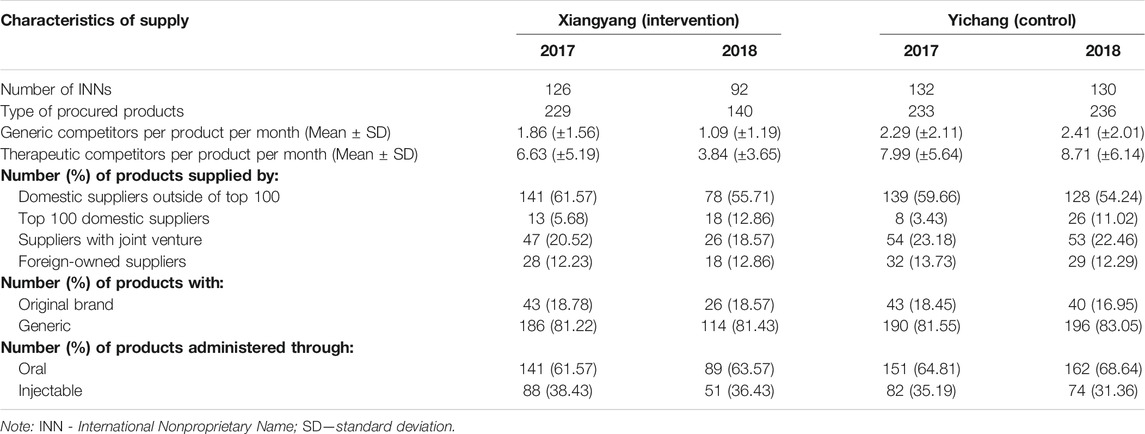
TABLE 3. Supply of cardiovascular medicines in the participating municipalities over the study period (2017–2018).
The median price (DDD adjusted) of procured cardiovascular medicines decreased by 41.51% in the intervention group (Xiangyang) after the initiative, down from 5.30 Yuan in 2017 to 3.10 Yuan in 2018, compared with a 0.22% decrease (from 4.49 Yuan to 4.48 Yuan) in the control group (Yichang). The median unit price hovered at a high level prior to the introduction of the volume-price contract system in the intervention group. The new initiative resulted in a dramatic drop in the unit price from the third month to the fifth month after the introduction of the volume-price contract system, followed by a levelling off of around two and three Yuan. Over the study period, no significant changes in the median unit price were observed in the control group. The median unit price in the control group was lower prior to the new initiative but higher post the new initiative in comparison with the intervention group (Figure 1).
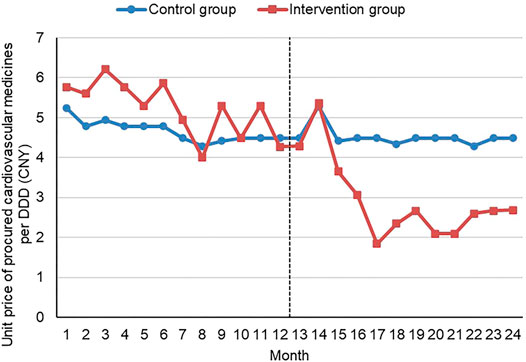
FIGURE 1. Median unit price per DDD of procured cardiovascular medicines by month Note: CNY—Chinese Yuan; DDD—defined daily dosage
Considering the log link applied in the modelling, we made some transformations of the coefficients (Zhang et al., 2017).
The results of the difference-in-differences regression analyses showed that the volume-price contract initiative was associated with a 36.24% reduction (p = 0.006) in the unit price (DDD adjusted) of the procured cardiovascular medicines after adjustment for variations in other variables. In addition, therapeutic competition was associated with a 31.61% reduction (p = 0.002) in the unit price. No significant effects of generic competition were found (Table 4).
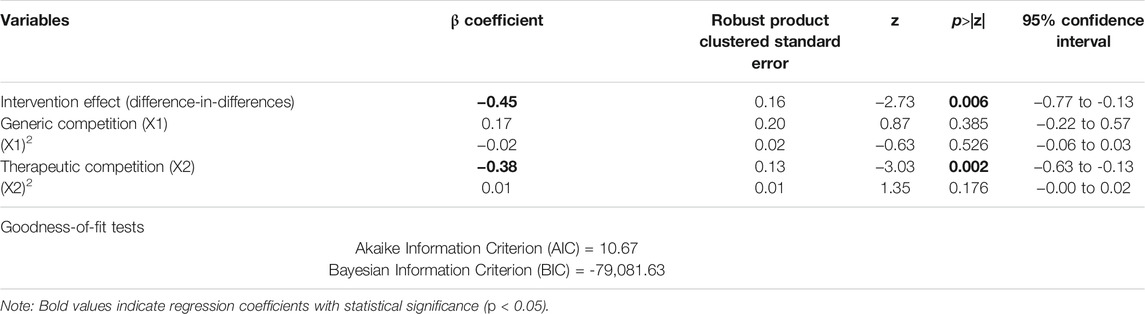
TABLE 4. Generalized linear regression model on unit price of all procured cardiovascular medicines with difference-in-differences analyses.
The subgroup difference-in-differences analyses revealed that the intervention effects on the unit price of procured cardiovascular medicines were statistically significant for those with an original brand and those supplied by the top 100 domestic suppliers. The unit price from the top 100 domestic suppliers dropped by 82.80% (p = 0.001), while the unit price of those with an original brand dropped by 30.23% (p = 0.008) as a result of the volume-price contract arrangements. No significant intervention effects were observed for the generic medicines and other suppliers. No significant intervention effects were observed in subgroups of medicines categorized by administration routes (Table 5).
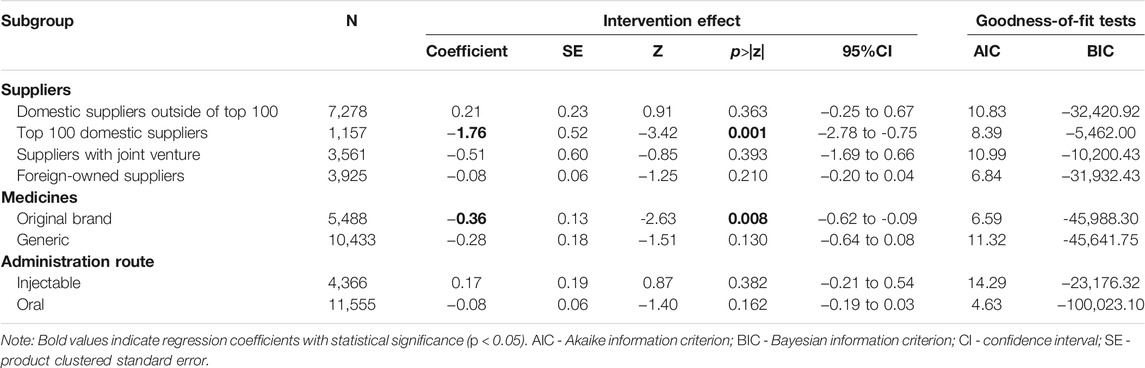
TABLE 5. Subgroup difference-in-differences analyses (generalized linear regression) on unit price of procured cardiovascular medicines.
Our study examined the impact of the volume-price contract initiative on the unit price of procured cardiovascular medicines through a natural experimental design involving 15,921 procurement records for 35 hospitals over a 2-year period. The generalized linear regression model with a difference-in-differences approach revealed that the volume-price contract arrangements contributed to a 36.24% drop in the unit price of procured cardiovascular medicines and a 31.61% drop in the unit price resulting from therapeutic competition after adjustment for variations in other variables. The medicines with an original brand and those supplied by the top 100 domestic suppliers were particularly sensitive to the new initiative, with a 30.23% and 82.80% drop in unit pricing, respectively.
The results indicate that the volume-price contract initiative offers an additional tool to reduce the unit price of medicines on top of the competition mechanism. Collective tendering and purchasing has been a common practice in most countries worldwide to source affordable medicines. The rationale lies in the theory of economies of scale (Li and Bai, 2019). With a large volume, the marginal cost for increasing production drops, which can result in a lowered average unit cost. Furthermore, a promised purchase volume brings certainty, which can help suppliers avoid or reduce some administrative and transaction costs. In the past, the awarded tenderers had to conduct market research, negotiate with individual health institutions, and promote their products in competition with other suppliers to win a purchase order. These costs, in particular the marketing costs, could be very high and had to be factored into consideration in the price setting (Ge, 2020; Huang and Tao, 2020). The new procurement arrangement now offered the awarded tenderers assurance of a large pooled purchase volume, giving them costing advantages in manufacturing and distributing the contracted products. This may even generate a flow-over effect on the surrounding regions through intensified price competition (Li and Bai, 2019), although we did not observe such a phenomenon in our study.
It is important to note that the impact of the volume-price contract initiative varies by supplier. The foreign-owned and joint ventures and the smaller domestic suppliers in this study were found to be less responsive to the new initiative in price setting than the top 100 domestic suppliers. The underlying reasons are not very clear. For small suppliers, their production capacity is limited, which may prevent them from participating in the large volume-based tendering. Unlikely their large counterparts, small suppliers do not have the advantage of economies of scale and may have limited space to cut costs. In addition, small domestic suppliers are most likely to be local. There may be a lack of incentives for them to reduce price under the protectionism of local governments (Wu et al., 2014).
Another interesting finding of this study is that generic medicines are less responsive in price setting to the volume-price contract system than those with an original brand. Generic medicines are always priced lower than their original-brand counterparts in the pharmaceutical retail market. The price gap between generic medicines and original brands, including cardiovascular medicines, is quite big in China (Zeng, 2013), which gives the original brands more room for price reduction. Indeed, most generic medicines are produced by small manufacturers in China. They tend to enter the retail market with low prices. The availability of lower-priced competitors can drive down the price of the original brands (Chapman et al., 2019). However, the original brands do not always engage in price competition with the generic medicines in China. They have occupied a large market share and are able to maintain higher prices due to longstanding concerns from the public about the quality of generic medicines. The perceived difference in the quality of medicines has weakened the competition effect between generic medicines and original brands (Chen and Rao, 2019).
The findings of this study have several policy implications. First, the effect of the volume-price contract initiative is effective in bringing down price only when the procurement volume is large enough. This imposes a serious challenge to the procurement of generic medicines as there are large numbers of suppliers but each occupies a small market share. The municipality-wide procurement volume may not be big enough to incentivize suppliers to cut the price of already lower-priced generic medicines. A higher (provincial or even national) level of pooled procurement arrangement can increase the procurement volume and create a competitive market. This may also encourage large manufacturers to produce generic medicines. In recent years, the national government in China has encouraged 11 provinces/regions to organize volume-based procurement for some generic medicines (Tang et al., 2019). Second, the medicines with a brand name are very responsive in price setting to the volume-price contract system, which can bring benefits in driving the quality improvement of generic medicines as their price gaps are shrinking. In 2016, the State Council of China released policy guidelines for establishing efficacy equivalence of generic medicines with an aim to resume consumer confidence in generic medicines through strengthened quality assurance mechanisms (The State Council of the People’s Republic of China, 2016).
To our knowledge, this is the first study of its kind in China to examine the impact of the volume-price contract system on the unit price of procured cardiovascular medicines. It provides additional evidence to the existing literature that advocates for collective tendering and purchasing of medicines based on volume and price. Data used in this study were extracted from the tendering platform, which had a large sample size and avoided sampling bias.
There are some limitations in this study. First, we could not exclude the potential impact of heterogeneity of medicines although the study was limited to cardiovascular medicines. The quality and efficacy information of the procured medicines was absent, preventing us from assessing the impacts of the new procurement system comprehensively apart from the unit price. The potential impact of the new supply arrangement on clinical services and patient care outcomes is unknown. However, this may not be an issue since the quality gap between generic medicines and originator brands is being gradually narrowed as seen in countries including China (Davit et al., 2009; Corrao et al., 2014; Jackevicius et al., 2016; The State Council of the People’s Republic of China, 2016). In addition, there is little difference in effectiveness or safety of different medicines for cardiovascular diseases (Wei et al., 2020). Future studies should take a patient perspective and cover a wider range of medicines as the new supply arrangement may have differing effects on the supply of different medicines. Second, each individual transaction was treated as a unit of analysis without consideration of the duration of contract (because of the lack of variations) and how previous contracts informed subsequent procurement from the same supplier (because of data unavailability). Third, although China’s pharmaceutical supply system has been improved substantially by the strong regulations from the government (Yan et al., 2018), there is still problem of fragmentation in the regulatory, which exacerbates the lack of transparency in the pharmaceutical system (Hu and Mossialos, 2016). In addition, China’s pharmaceutical market has been characterized by dispersion and low concentration, which leads to uneven pricing problems of pharmaceuticals (Hu and Mossialos, 2016). Thus, the generalization of the conclusions to other settings should be conducted with caution.
In conclusion, the volume-price contract initiative is effective in reducing the unit price of procured cardiovascular medicines. The effect remained significant after adjustment for the competition effects. However, the impacts of the new initiative vary by medicine and supplier. The cardiovascular medicines with an original brand and the top 100 domestic suppliers were more responsive to the new initiative than others. Increasing procurement volumes may further enhance the impact of the volume-price contract system. But local protectionism can create a great barrier for cross-region collaborations.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Conception and design: ZL, CL, KZ, and YT. Collection and assembly of data: ZL, KZ, and YT. Statistical analysis: ZL, CL, and YT. Interpretation: ZL, CL, KZ, JL, and YT. Manuscript preparation: ZL, CL and YT. Manuscript review: ZL, CL, KZ, JL, and YT.
This study was supported by the National Natural Science Foundation of China, Grant number 71704058. The funding body played no role in the study design; the collection, analysis, and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study was supported by the National Natural Science Foundation of China, Grant number 71704058. The authors are also grateful to the staff in Hubei Public Resource Trading Center for their kind help in data collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.741671/full#supplementary-material
Adesina, A., Wirtz, V. J., and Dratler, S. (2013). Reforming Antiretroviral price Negotiations and Public Procurement: the Mexican Experience. Health Policy Plan 28 (1), 1–10. doi:10.1093/heapol/czs015
Amaral, S. M., and Blatt, C. R. (2011). Municipal Consortia for Medicine Procurement: Impact on the Stock-Out and Budget. Rev. Saude Publica 45 (4), 799–801. doi:10.1590/s0034-89102011005000016
Bureau of Statistics of Hubei Province (2018a). Hubei Statistic Year Book 2018. [Online]. Available: http://tjj.hubei.gov.cn/tjsj/sjkscx/tjnj/qstjnj/(Accessed Sept 14, 2020).
Bureau of Statistics of Hubei Province (2018b). Xiangyang Statistic Year Book 2018. [Online]. Available: http://tjj.hubei.gov.cn/tjsj/sjkscx/tjnj/gsztj/xys/(Accessed Nov 13, 2020).
Bureau of Statistics of Hubei Province (2018c). Yichang Statistic Year Book 2018. [Online]. Available: http://tjj.hubei.gov.cn/tjsj/sjkscx/tjnj/gsztj/ycs/(Accessed Nov 13, 2020).
Cai, X. (2017). The Evolution of the Chinese Centralized Drug Procurement and its Logical Relationship with Medical Insurance Payment. Chin. J. Health Pol. 10 (06), 6–12. doi:10.3969/j.issn.1674-2982.2017.06.002
Centralized Pharmaceuticals Tendering and Procurement Center of Xiangyang (2017). Announcement of the Volume-Based Procurement of Specialized Medications in Urban Public Hospitals in Xiangyang in 2017. [Online]. Available: http://yxcg.jyzx.xiangyang.gov.cn/new/show10601.html (Accessed Nov 12, 2020).
Chapman, S. R., Aladul, M. I., and Fitzpatrick, R. W. (2019). Lost Cost Savings to the NHS in England Due to the Delayed Entry of Multiple Generic Low-Dose Transdermal Buprenorphine: a Case Scenario Analysis. BMJ Open 9 (8), e026817. doi:10.1136/bmjopen-2018-026817
Chen, H., and Rao, Y. (2019). Practice and Critical Thinking on the Pharmaceutical Procurement with Target Quantity in New Era. China J. Pharm. Econ. 14 (07), 19–26. doi:10.12010/j.issn.1673-5846.2019.07.004
Corrao, G., Soranna, D., Merlino, L., and Mancia, G. (2014). Similarity between Generic and Brand-Name Antihypertensive Drugs for Primary Prevention of Cardiovascular Disease: Evidence from a Large Population-Based Study. Eur. J. Clin. Invest. 44 (10), 933–939. doi:10.1111/eci.12326
Davit, B. M., Nwakama, P. E., Buehler, G. J., Conner, D. P., Haidar, S. H., Patel, D. T., et al. (2009). Comparing Generic and Innovator Drugs: A Review of 12 Years of Bioequivalence Data from the United States Food and Drug Administration. Ann. Pharmacother. 43 (10), 1583–1597. doi:10.1345/aph.1M141
Dylst, P., Vulto, A., Godman, B., and Simoens, S. (2013). Generic Medicines: Solutions for a Sustainable Drug Market?. Appl. Health Econ. Health Pol. 11 (5), 437–443. doi:10.1007/s40258-013-0043-z
Dylst, P., Vulto, A., and Simoens, S. (2011). Tendering for Outpatient Prescription Pharmaceuticals: What Can Be Learned from Current Practices in Europe?. Health Policy 101 (2), 146–152. doi:10.1016/j.healthpol.2011.03.004
Eurostat (2021). Health Care Expenditure by Function. [Online]. Available: https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (Accessed Sept 16, 2021).
Fang, Y., Wagner, A. K., Yang, S., Jiang, M., Zhang, F., and Ross-Degnan, D. (2013). Access to Affordable Medicines after Health Reform: Evidence from Two Cross-Sectional Surveys in Shaanxi Province, Western China. Lancet Glob. Health 1 (4), E227–E237. doi:10.1016/S2214-109X(13)70072-X
Fu, H., Chen, X., Zhang, X., and He, C. (2015). The Analysis of Key Problems and Countermeasure about Drug Centralized Procurement. Health Econ. Res. (09), 7–9. doi:10.14055/j.cnki.33-1056/f.20150909.017
Ge, P. (2020). The Mechanism of the "4+7 Recruitment Model" in Reducing Drug Prices. Tech. Econ. Guide 28 (10), 189+187.
General Office of the State Council (2015). Guidance on Improving Centralized Procurement of Pharmaceuticals in Public Hospitals. [Online]. Available: http://www.gov.cn/zhengce/content/2015-02/28/content_9502.htm (Accessed Sept 24, 2021).
Gómez-Dantés, O., Wirtz, V. J., Reich, M. R., Terrazas, P., and Ortiz, M. (2012). A New Entity for the Negotiation of Public Procurement Prices for Patented Medicines in Mexico. Bull. World Health Organ. 90 (10), 788–792. doi:10.2471/BLT.12.106633
Government of Hubei Province (2016). Notice on Further Deepening the Pilot Work of Medical and Health System Reform in Hubei Province. [Online]. Available: http://www.hubei.gov.cn/zfwj/ezbf/201608/t20160817_1713379.shtml (Accessed Nov 12, 2020).
Hasan, S. S., Kow, C. S., Dawoud, D., Mohamed, O., Baines, D., and Babar, Z. U. (2019). Pharmaceutical Policy Reforms to Regulate Drug Prices in the Asia Pacific Region: The Case of Australia, China, India, Malaysia, New Zealand, and South Korea. Value Health Reg. Issues 18, 18–23. doi:10.1016/j.vhri.2018.08.007
Health and Family Planning Commission of Hubei Province (2014). Notice on Printing and Distributing "the Administrative Measures for Centralized Procurement of Medicines for Medical Institutions in Hubei Province (For Trial Implementation)". [Online]. Available: http://wjw.hubei.gov.cn/zfxxgk/zc/gkwj/202009/t20200928_2933551.shtml (Accessed Mar 23, 2021).
Health Commission of Hubei Province (2017). Notice on Comprehensively Promoting the Volume-Based Procurement of Drugs in Public Hospitals. [Online]. Available: http://wh.eliansun.com/complaint/show12644.html (Accessed Nov 12, 2020).
Hu, J., and Mossialos, E. (2016). Pharmaceutical Pricing and Reimbursement in China: When the Whole Is Less Than the Sum of its Parts. Health Policy 120 (5), 519–534. doi:10.1016/j.healthpol.2016.03.014
Hu, S., Chen, C., Yuan, S., Xue, F., Shi, L., Fang, Y., et al. (2019). The Effects of a New Public Medicine Procurement Policy on Medicine Price in Shaanxi Province, Western China: An Interrupted Time Series Analysis. Front. Pharmacol. 10, 950. doi:10.3389/fphar.2019.00950
Hu, S. (2013). Essential Medicine Policy in China: Pros and Cons. J. Med. Econ. 16 (2), 289–294. doi:10.3111/13696998.2012.751176
Huang, Y., and Tao, L. (2020). The Influence of Drug Centralized Group Procurement Policy to Chinese Pharmaceutical Industry–An Analysis Based on Perspective of Industrial Economics. China Health Insurance 137 (02), 64–67. doi:10.19546/j.issn.1674-3830.2020.2.014
IMS (2015). Global Medicines Use in 2020: Outlook and Implications. [Online]. Available: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-medicines-use-in-2020 (Accessed Nov 10, 2020).
IQVIA (2020). Global Medicine Spending and Usage Trends: Outlook to 2024. [Online]. Available: https://www.iqvia.com/insights/the-iqvia-institute/reports/global-medicine-spending-and-usage-trends (Accessed Jan 20, 2021).
Jackevicius, C. A., Tu, J. V., Krumholz, H. M., Austin, P. C., Ross, J. S., Stukel, T. A., et al. (2016). Comparative Effectiveness of Generic Atorvastatin and Lipitor® in Patients Hospitalized with an Acute Coronary Syndrome. J. Am. Heart Assoc. 5 (4), e003350. doi:10.1161/jaha.116.003350
Jensen, T. B., Kim, S. C., Jimenez-Solem, E., Bartels, D., Christensen, H. R., and Andersen, J. T. (2020). Shift from Adalimumab Originator to Biosimilars in Denmark. JAMA Intern. Med. 180 (6), 902–903. doi:10.1001/jamainternmed.2020.0338
Jiang, C., Fu, J., and Li, Y. (2014). Exploring the Reason for the System of High Medicine Price. Chin. Health Econ. 33 (04), 20–22. doi:10.7664/CHE20140406
Li, C., Liu, Y., Wang, W., Cui, D., Zhang, Y., and Yin, X. (2018). Review and Prospect of Drug's Centralized Purchasing System in China. Chin. Hosp. Manag. 38 (09), 17–19+23.
Li, D., and Bai, X. (2019). The General Mechanism of Reducing the Price of Medicines by Quantity Purchase and the Analysis of “4+7 Recruitment Mode”. Health Econ. Res. 36 (08), 10–12. doi:10.14055/j.cnki.33-1056/f.2019.08.003
Liu, J., Wang, L., Liu, C., and Zhang, X. (2017). Impact of price Deregulation Policy on the Affordability of Essential Medicines for Women's Health: a Panel Data Analysis. Expert Rev. Pharmacoecon Outcomes Res. 17 (6), 625–631. doi:10.1080/14737167.2017.1330151
Liu, Y., Zhang, J., Cheng, T. C. E., Ru, Y., and Hua, G. (2019). The Impacts of Drug Price Regulations in China. J. Syst. Sci. Syst. Eng. 28 (6), 674–693. doi:10.1007/s11518-019-5431-y
Maniadakis, N., Holtorf, A. P., Otávio Corrêa, J., Gialama, F., and Wijaya, K. (2018). Shaping Pharmaceutical Tenders for Effectiveness and Sustainability in Countries with Expanding Healthcare Coverage. Appl. Health Econ. Health Pol. 16 (5), 591–607. doi:10.1007/s40258-018-0405-7
Meng, Q., Cheng, G., Silver, L., Sun, X., Rehnberg, C., and Tomson, G. (2005). The Impact of China's Retail Drug price Control Policy on Hospital Expenditures: a Case Study in Two Shandong Hospitals. Health Policy Plan 20 (3), 185–196. doi:10.1093/heapol/czi018
Milovanovic, D. R., Pavlovic, R., Folic, M., and Jankovic, S. M. (2004). Public Drug Procurement: the Lessons from a Drug Tender in a Teaching Hospital of a Transition Country. Eur. J. Clin. Pharmacol. 60 (3), 149–153. doi:10.1007/s00228-004-0736-1
Mirsafaei, L., Reiner, Ž., Shafabakhsh, R., and Asemi, Z. (2020). Molecular and Biological Functions of Quercetin as a Natural Solution for Cardiovascular Disease Prevention and Treatment. Plant Foods Hum. Nutr. 75 (3), 307–315. doi:10.1007/s11130-020-00832-0
National Development and Reform Commission (2005). Notice on Printing and Distributing the List of Drugs Priced by the National Development and Reform Commission. [Online]. Available: https://www.ndrc.gov.cn/xwdt/xwfb/200507/t20050714_958325.html (Accessed Sept 11, 2020).
National Health and Family Planning Commission (2015). Notice on Implementing the Improvement of Guidance on Centralized Procurement of Pharmaceuticals in Public Hospitals. [Online]. Available: http://www.nhc.gov.cn/yaozs/s3573/201506/36a74780403d4eed96ca93b665620941.shtml (Accessed Sept 24, 2021).
National Health Commission (2019). China Health Statistical Digest. Beijing: Peking Union Medical College Press.
National Health Commission of China (2018). China Health Statistics Yearbook. [Online]. Available: http://www.stats.gov.cn/tjsj/ndsj/2018/indexch.htm (Accessed Nov 13, 2020).
OECD (2020). Pharmaceutical Spending (Indicator). [Online]. Available: https://www.oecd-ilibrary.org/content/data/998febf6-en (Accessed Oct 26, 2020).
Rosen, S. (1974). Hedonic Prices and Implicit Markets: Product Differentiation in Pure Competition. J. Polit. Economy 82 (1), 34–55. doi:10.2307/183089910.1086/260169
Sigulem, F., and Zucchi, P. (2009). E-procurement in the Brazilian Healthcare System: the Impact of Joint Drug Purchases by a Hospital Network. Rev. Panam Salud Publica 26 (5), 429–434. doi:10.1590/S1020-49892009001100007
Southern Medicine Economic Research Institute of National Medical Products Administration (2018). Top 100 Pharmaceutical Industries in China in 2017. [Online]. Available: http://www.yyjjb.com.cn/yyjjb/201809/20180930120712712_1274.shtml (Accessed Mar 14, 2021).
Southern Medicine Economic Research Institute of National Medical Products Administration (2019). Top 100 Pharmaceutical Industries in China in 2018. [Online]. Available: http://www.jksb.com.cn/index.php?m=wap&a=show&catid=22&id=138333 (Accessed Mar 14, 2021).
Spinks, J., Chen, G., and Donovan, L. (2013). Does Generic Entry Lower the Prices Paid for Pharmaceuticals in Australia? A Comparison before and after the Introduction of the Mandatory price-reduction Policy. Aust. Health Rev. 37 (5), 675–681. doi:10.1071/AH13024
Tang, M., He, J., Chen, M., Cong, L., Xu, Y., Yang, Y., et al. (2019). "4+7" City Drug Volume-Based Purchasing and Using Pilot Program in China and its Impact. Drug Discov. Ther. 13 (6), 365–369. doi:10.5582/ddt.2019.01093
Tang, W., Xie, J., Lu, Y., Liu, Q., Malone, D., and Ma, A. (2018). Effects on the Medical Revenue of Comprehensive Pricing Reform in Chinese Urban Public Hospitals after Removing Drug Markups: Case of Nanjing. J. Med. Econ. 21 (4), 326–339. doi:10.1080/13696998.2017.1405817
Tang, Y., Liu, C., and Zhang, X. (2017). Delivery of Essential Medicines to Primary Care Institutions and its Association with Procurement Volume and Price: A Case Study in Hubei Province, China. Appl. Health Econ. Health Pol. 15 (1), 57–64. doi:10.1007/s40258-016-0276-8
Tang, Y., Liu, C., and Zhang, X. (2016). Public Reporting as a Prescriptions Quality Improvement Measure in Primary Care Settings in China: Variations in Effects Associated with Diagnoses. Sci. Rep. 6, 39361. doi:10.1038/srep39361
The State Council of the People's Republic of China (2016). Suggestions on Implementation of Evaluation of Quality and Efficacy Consistency of Generic Drugs. [Online]. Available: http://www.gov.cn/zhengce/content/2016-03/05/content_5049364.htm (Accessed Mar 14, 2021).
Vernaz, N., Haller, G., Girardin, F., Huttner, B., Combescure, C., Dayer, P., et al. (2013). Patented Drug Extension Strategies on Healthcare Spending: A Cost-Evaluation Analysis. Plos Med. 10 (6), e1001460. doi:10.1371/journal.pmed.1001460
Vogler, S., Paris, V., Ferrario, A., Wirtz, V. J., de Joncheere, K., Schneider, P., et al. (2017). How Can Pricing and Reimbursement Policies Improve Affordable Access to Medicines? Lessons Learned from European Countries. Appl. Health Econ. Health Pol. 15 (3), 307–321. doi:10.1007/s40258-016-0300-z
Wei, J., Galaviz, K. I., Kowalski, A. J., Magee, M. J., Haw, J. S., Narayan, K. M. V., et al. (2020). Comparison of Cardiovascular Events Among Users of Different Classes of Antihypertension Medications: A Systematic Review and Network Meta-Analysis. JAMA Netw. Open 3 (2), e1921618. doi:10.1001/jamanetworkopen.2019.21618
WHO Collaborating Centre for Drug Statistics Methodology (2018). DDD: Definition and General Considerations. [Online]. Available: https://www.whocc.no/ddd/definition_and_general_considera/(Accessed Sept 11, 2019).
Woerkom, Mv., Piepenbrink, H., Godman, B., Metz, Jd., Campbell, S., Bennie, M., et al. (2012). Ongoing Measures to Enhance the Efficiency of Prescribing of Proton Pump Inhibitors and Statins in The Netherlands: Influence and Future Implications. J. Comp. Eff. Res. 1 (6), 527–538. doi:10.2217/cer.12.52
Wouters, O. J., Sandberg, D. M., Pillay, A., and Kanavos, P. G. (2019). The Impact of Pharmaceutical Tendering on Prices and Market Concentration in South Africa over a 14-year Period. Soc. Sci. Med. 220, 362–370. doi:10.1016/j.socscimed.2018.11.029
Wu, B., Zhang, Q., and Qiao, X. (2015). Effects of Pharmaceutical price Regulation: China's Evidence between 1997 and 2008. J. Asia Pac. Economy 20 (2), 290–329. doi:10.1080/13547860.2014.964968
Wu, J., Xu, J., Liu, G., and Wu, J. (2014). Pharmaceutical Pricing: an Empirical Study of Market Competition in Chinese Hospitals. Pharmacoeconomics 32 (3), 293–303. doi:10.1007/s40273-013-0099-5
Yan, S., Xu, D., and Ouyang, Z. (2018). Effects of New Healthcare Reform on Pharmaceutical Supply System of China. China Med. Herald 15 (35), 142–145.
Ye, L., Hernandez, P., Abegunde, D., and Edejer, T. (2011). The World Medicines Situation 2011. Geneva: World Health Organization.
Zeng, W. (2013). A price and Use Comparison of Generic versus Originator Cardiovascular Medicines: a Hospital Study in Chongqing, China. BMC Health Serv. Res. 13, 390. doi:10.1186/1472-6963-13-390
Zeng, W., Finlayson, A. E., Shankar, S., de Bruyn, W., and Godman, B. (2015). Prescribing Efficiency of Proton Pump Inhibitors in China: Influence and Future Directions. BMC Health Serv. Res. 15, 11. doi:10.1186/s12913-014-0638-6
Zeng, W., Zhen, J., Feng, M., Campbell, S. M., Finlayson, A. E., and Godman, B. (2014). Analysis of the Influence of Recent Reforms in China: Cardiovascular and Cerebrovascular Medicines as a Case History to Provide Future Direction. J. Comp. Eff. Res. 3 (4), 371–386. doi:10.2217/cer.14.28
Zhang, Y., Ma, Q., Chen, Y., and Gao, H. (2017). Effects of Public Hospital Reform on Inpatient Expenditures in Rural China. Health Econ. 26 (4), 421–430. doi:10.1002/hec.3320
Keywords: volume-price contract, collective procurement, competition, pharmaceutical, China, price
Citation: Li Z, Liu C, Zuo K, Liu J and Tang Y (2021) Effects of Volume-Price Contracts on Pharmaceutical Prices: A Retrospective Comparative Study of Public Hospitals in Hubei of China. Front. Pharmacol. 12:741671. doi: 10.3389/fphar.2021.741671
Received: 15 July 2021; Accepted: 30 September 2021;
Published: 14 October 2021.
Edited by:
Kingston Rajiah, International Medical University, MalaysiaReviewed by:
Brian Godman, University of Strathclyde, United KingdomCopyright © 2021 Li, Liu, Zuo, Liu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuqing Tang, ZHJfdHlxQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.