
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 03 January 2022
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.738420
 Reza Heidary Moghaddam1
Reza Heidary Moghaddam1 Zeinab Samimi2
Zeinab Samimi2 Sedigheh Asgary3
Sedigheh Asgary3 Pantea Mohammadi4
Pantea Mohammadi4 Soroush Hozeifi5
Soroush Hozeifi5 Fatemeh Hoseinzadeh‐Chahkandak6
Fatemeh Hoseinzadeh‐Chahkandak6 Suowen Xu7*
Suowen Xu7* Mohammad Hosein Farzaei2,8*
Mohammad Hosein Farzaei2,8*Cardiovascular diseases (CVD), as a life-threatening global disease, is receiving worldwide attention. Seeking novel therapeutic strategies and agents is of utmost importance to curb CVD. AMP-activated protein kinase (AMPK) activators derived from natural products are promising agents for cardiovascular drug development owning to regulatory effects on physiological processes and diverse cardiometabolic disorders. In the past decade, different therapeutic agents from natural products and herbal medicines have been explored as good templates of AMPK activators. Hereby, we overviewed the role of AMPK signaling in the cardiovascular system, as well as evidence implicating AMPK activators as potential therapeutic tools. In the present review, efforts have been made to compile and update relevant information from both preclinical and clinical studies, which investigated the role of natural products as AMPK activators in cardiovascular therapeutics.
Cardiovascular diseases (CVD) in most countries are now one of the top concerns of the health care system, and according to a disease statistics report released by the World Health Organization (WHO) in 2019, about 17.9 million people died from CVD (WHO 2021). It is estimated that with the increased prevalence of CVD, the number of expected deaths due to CVD will increase to 24 million per year by 2030. Therefore, the increase in high-risk patients prone to CVD, as well as the high cost of treatment and subsequent debilitating complications, forewarn us of regarding the need for intensive investigation into the pathogenesis of CVD (Afzal 2021).
One of the therapeutic targets for CVD is the AMP-activated protein kinase (AMPK) which is found in most mammalian tissues such as cardiovascular organs (Xu and Si, 2010). In the mid-1990s, Lopaschuck’s Laboratory first investigated the role of AMPK in the heart (Kudo et al., 1995, Kudo et al., 1996). The modifiable major risk factors that lead to CVD are excess weight, dyslipidemia, increased blood pressure, diabetes, and metabolic syndrome, in which AMPK activators may confer benefits (Nellaiappan et al., 2019). Mechanistic research has now discovered signaling pathways that link AMPK to CVD. These routes seem to be interconnected and can be considered as new goals for the design and development of treatment strategies in the future. On the other hand, AMPK deregulation is thought to be related to various cardiovascular disorders (Costantino, Paneni, and Cosentino 2016). Activated AMPK inhibits energy-consuming process and stimulates several metabolic pathways for energy-production and cell survival. AMPK activation in response to cellular energy stress occurs due to the reduction in the ATP/AMP ratio in the cytosol (Hardie et al., 2012). In fact, AMPK is a heterotrimeric serine-threonine kinase, which acts as a metabolic sensor to coordinate catabolic and anabolic pathways in the heart (Arad et al., 2007). In recent years, researchers reported various non-pharmacological and natural compounds based therapeutics from animal studies and considered them in the CVD treatment. Some of natural products have shown promising in vitro and in vivo activities in the AMPK regulation. In this review, we present an overview of mechanisms regulating AMPK in the cardiovascular system; highlighting the role of AMPK signaling in CVD, followed by a description of natural AMPK activators as potential therapeutic tools.
Maintaining energy homeostasis is a fundamental biological pathway that occurs within all living cells. AMPK is a key gatekeeper of energy homeostasis that is activated in response to different stimuli, leading to intracellular decrease in ATP levels. Actually, under cellular stress and/or energy stress, AMPK is activated in response to decreased ATP production or increased ATP consumption (Mihaylova and Shaw, 2011). AMPK exists as a heterotrimer, containing a catalytic subunit (α), and two regulatory subunits (β and γ) (Zhang et al., 2013). Each subunit has multiple isoforms (α1, α2, β1, β2, γ1, γ2, γ3), giving rise to a total of 12 feasible heterotrimer combinations. The kinase activity of α subunit is stimulated more than 100-fold by phosphorylation of a conserved threonine residue located on the kinase activation loop (Thr 172 in α1/α2). The main upstream kinase that is responsible for the phosphorylation of this site was detected as a heterotrimeric complex comprising tumor suppressor kinase LKB1, mouse protein 25 (MO25), and sterile-20-related adaptor (STRAD) (Hardie, 2014). Subsequently, the Ca2+/calmodulin-dependent protein kinase kinases (CaMKKs) (especially CaMKKβ) were discovered as an alternate enzyme playing role in Thr172 phosphorylation in response to the rise in intracellular Ca2+ (Nguyen et al., 2016). Additional studies have proposed that TAK1/MAP3K7 (Transforming growth factor beta-activated kinase 1)/(Mitogen-activated protein kinase 7), a MAPKKK family member, may also phosphorylate Thr172 (Tamargo-Gomez and Marino, 2018). Under conditions of decreased intracellular ATP concentrations, binding of AMP and/or ADP to the γ-regulatory subunit activate this kinase by three complementary mechanisms: promotes phosphorylation of AMPK by the upstream kinases, protects the enzyme against dephosphorylation through conformational changes that inhibits protein phosphatases, as well as causes allosteric activation which is only limited to AMP (Hardie, 2014).
Under conditions of nutrient stress, metabolic checkpoint proteins like AMPK, can inhibit cellular growth. AMPK suppresses mammalian target of rapamycin complex 1 (mTORC1). mTORC1 comprised of the three core subunits (mTOR, Raptor, and mLST8) is a key regulator of ribosomal biogenesis and protein synthesis (Bond, 2016). AMPK controls the mTORC1 through phosphorylation/inactivation of the tumor suppressor TSC2. In addition, findings obtained from lower eukaryotes with TSC2 depletion or TSC2−/− mouse embryonic fibroblasts (MEFs) demonstrated that AMPK can also inhibit mTORC1 pathway through direct phosphorylation of Raptor (Mihaylova and Shaw, 2011). In addition to the control of cell growth, mTORC1 also regulates autophagy, lysosomal degradation of intracellular components sequestered within autophagosome to supply sufficient metabolites in low availability of nutrients or to increase energy demands (Papinski and Kraft, 2016). The process by which TORC1 negatively controls the autophagic machinery was first defined in the yeast. Genetic evaluations of autophagy-deficient mutants result in identifying >30 essential autophagy-related genes (Atg34 and Atg81). The main target is a collection of three proteins including the serine/threonine kinase Atg1and its accessory proteins Atg13 and Atg17. mTORC1 can directly suppress this complex in a nutrient-dependent manner (Alers et al., 2012). Phosphorylation of the Ulk1 (and Ulk2), Atg1 mammalian homologs, moreover their regulatory subunits probably is the mechanism by which mTORC1 can inhibit autophagy in mammals. In addition, AMPK also has the ability to directly phosphorylate Ulk1 complex and induced autophagy induction as well as maintain mitochondrial homeostasis control. Based on the literature, tumor suppressor p53 and the cyclin dependent kinase inhibitor p27 are recruited as other targets of AMPK in growth control (Mihaylova and Shaw, 2011).
AMPK can be regulated by metabolic factors, such as leptin and adiponectin, two well-known adipokines secreted by adipocytes. Through AMPK activation, both hormones inhibit the activity of acetyl coenzyme A carboxylase (ACC) and 3-hydroxy-3-methylgutaryl-coenzyme A (HMG-CoA) reductase, which are responsible for cholesterol and fatty acid synthesis in skeletal muscle and other metabolic tissues (Hardie, 2004; Wang et al., 2018). In addition, hormone sensitive lipase (HSL) and adipocyte triglyceride lipase (ATGL) are the other substrates in adipose tissues which are activated by AMPK (Mihaylova and Shaw, 2011).
Exercise and insulin induce glucose uptake into skeletal muscle through various signaling pathways. Both exercise and insulin can trigger GLUT4 translocation from intracellular vesicles to the cell surface. AS160 and TBC1D1 are two RabGAPs, which have been implicated in this process. AS160 highly expressed in heart, oxidative muscles, and white adipose tissue (WAT). On the other hand, its homologue, TBC1D1 mostly expressed in skeletal muscle, involves exercise-mediated GLUT4 trafficking. RabGAPs control the activity of Rab GTPases, which have been implicated in eukaryotic vesicular trafficking (Stockli et al., 2015). AMPK plays a role in glucose uptake via effects on the AS160 and TBC1D1 (Mihaylova and Shaw 2011).
In addition to the rise in energy expenditure via inducing fatty acid and glucose oxidation, AMPK also seems to control mammalian energy intake through effect on the regions in hypothalamus. For example, AMPK is suppressed in the condition of elevated levels of glucose and insulin, whereas it is stimulated by ghrelin (a gut hormone increasing appetite and food intake) (Hardie, 2004). Generally, AMPK plays a role in the downregulation and upregulation of biosynthetic and catabolic pathways, respectively by acute regulation of the metabolic enzymes and transcriptional changes (Hardie, 2004).
AMPK has been recognized as a key regulator of some transcription factors, co-activators, the histone acetyltransferase p300, histone deacetylase family, and histones themselves. For example, it was reported that AMPK, through phosphorylation of histone H2B, upregulates stress related genes including p21 and cpt1c (Mihaylova and Shaw, 2011). It has recently been proved that AMPK activation affects circadian clock regulation. Mammalian circadian clocks require activators and repressors that regularly control transcription. CLOCK and BMAL1 act as potent stimulators of the expression of Period (Per1, Per2, and Per3) and Cryptochrome genes (Cry1 and Cry2), the encoded proteins of which also inhibit CLOCK: BMAL1. Cry1/2 are targeted for ubiquitination and degradation by ubiquitin ligase complex SCF (SKP1-CUL1-F-box protein) (Huber et al., 2016). AMPK, via phosphorylation of the Cry1, leads to direct binding of the Fbox protein Fbxl3 to Cry1. It has been demonstrated that AMPK can also control the circadian clock by phosphorylation of Casein kinase (Mihaylova and Shaw, 2011).
AMPK also phosphorylates and inactivates the lipogenic transcriptional factors such as carbohydrate-responsive element-binding protein (ChREBP) and sterol regulatory element binding protein -1c (SREBP-1c). These two regulators dictate the expression of major lipogenic enzymes including acetyl-CoA carboxylase (ACC), ATP-citrate lyase (ACLY), acetyl-CoA synthetase (ACS), fatty acid synthase (FAS), stearoyl-CoA desaturase-1 (SCD1), and glycerol-3- phosphate acyltransferase (GPAT) that promote lipogenesis in the liver (Xu et al., 2013). Furthermore, there are observations that phosphorylation of histone acetyltransferase p300 by AMPK can also affect the acetylation and activity of ChREBP (Mihaylova and Shaw, 2011).
Reduction of cellular energy levels during prolonged fasting or intense exercise results in AMPK stimulation and prevention of hepatic gluconeogenesis to some extent through phosphorylation and inactivation of CREB-regulated transcriptional coactivator 2 (CRTC-2) (Altarejos and Montminy, 2011). AMPK also reduces gluconeogenesis through class IIa family of histone deacetylases (IIa HDACs) inactivation leading to reduced de-acetylation and activation of FOXO transcription factor in liver (Mihaylova and Shaw, 2011). It was demonstrated that CRTC-1 is a direct AMPK target interacting with the CREB homologue-1 (CRH-1) transcription factor in vivo. Activating AMPK through reduction of CRTC-1 and CRH-1 activity is responsible for lifespan extension (Mair et al., 2011). Some findings also indicated the involvement of AMPK in the phosphorylation of nuclear receptors HNF4α (NR2A1) and TR4 (NR2C2), zinc-finger protein 692 (ZNF692), and the co-activator PGC-1α. More studies are required to identify exact roles of these events (Mihaylova and Shaw, 2011). It was also reported that AMPK operates in accordance with another metabolic sensor, the NAD+-dependent type III deacetylase SIRT1, thereby regulating the expression of genes responsible for cellular energy metabolism in metabolic tissues. AMPK promotes SIRT1 activity by increment of cellular NAD+ levels, leading to fatty acid oxidation and mitochondrial gene expression. SIRT1 regulates the activity of transcription factors and coregulators including forkhead box class O (FOXO) transcription factors, peroxisome proliferator-activated receptor-gamma (PPARγ), and p53 (Canto et al., 2009).
In addition to many findings reporting AMPK’s role in cell growth and metabolism, some studies have documented that LKB1 and AMPK play critical roles in cell polarity from invertebrates to mammals. For instance, certain epithelial cell polarization in mammalian cell culture and polarity of the cells during critical asymmetric cell divisions in Drosophila are attributed to LKB1 and his orthologs, respectively (Mihaylova and Shaw 2011; Mirouse and Billaud 2011). There are also several studies reporting the role of AMPK in cell polarity. For example, in mammalian MDCK cells, AMPK leads to suitable repolarization and tight junction assembly after calcium switch. Myosin II was recognized as a main downstream target of AMPK. In Drosophila embryo, Myosin II was activated through phosphorylation of its regulatory light chain (referred as MRLCII). However, MRLCII is not targeted as direct substrate of AMPK and it thus becomes important to identify the exact signaling mechanism (Mihaylova and Shaw 2011; Mirouse and Billaud 2011). Besides, CLIP-170 (CLIP1), the microtubule plus end protein, is another substrate of AMPK that affects microtubule assembly (Mihaylova and Shaw 2011).
AMPK is implicated in the regulation of cardiovascular function via coordinating several critical physiological and pathological cellular pathways in the cardiovascular system, which includes both metabolic as well as non-metabolic components of different cell types such as vascular cells, fibroblasts and cardiomyocytes. In the following section, we briefly summarize the important role of AMPK in CVD.
Atherosclerosis is considered as a known chronic inflammatory reaction of artery walls caused by lesions and plaques. Eventually, thrombus formation on atherosclerotic plaques leads to heart attacks and ischemic stroke (Libby et al., 2009; Xu et al, 2021). This disease is related to both increases in low-density lipoprotein cholesterol (LDL-C) and chronic low-grade inflammation, both of which are regulated by AMPK. AMPK regulates proliferation, apoptosis, migration, and autophagy of smooth muscle cells and endothelial cells as well as macrophages. AMPK reduces atherosclerosis progression by inhibition of cell proliferation (via p53 and mTOR) and induction of autophagy (Wang et al., 2017). AMPK arrests cell cycle progression by increasing cells in G0/G1-phase and decreasing cells in S- and G2/M-phase (Igata et al., 2005).
Furthermore, AMPK influences the level of adiponectin, which decreases vascular smooth muscle cell migration induced by insulin-like growth factor-1 (Motobayashi et al., 2009). AMPK affects the antioxidant condition of endothelial cells (Colombo and Moncada 2009) and in vivo, a decrease in AMPK will eventually increases atherosclerosis and ER stress (Dong et al., 2010). Interestingly, AMPK inhibits the macrophage proliferation induced by LDL and atherosclerosis (Ishii et al., 2009).
Hypertension is one of the main risk factors for CVD besides elevated LDL-C and inflammation. The changes in the structure of vessels commonly seen in hypertension is due to vessel remodeling and/or hypertrophy. In hypertensive rats, AMPK is overexpressed as revealed by cDNA microarray (Kurdi et al., 2004). Metformin can activate AMPK and inhibit NF-kB, thus attenuating the expression of adhesion molecules and pro-inflammatory genes induced by cytokines (Hattori et al., 2006), which are crucial for progression of hypertension (Zhou et al., 2010). Indeed, in insulin-sensitive tissues from hypertensive rats, impaired adiponectin-AMPK pathway has been observed (Rodríguez et al., 2008). AMPK causes vasodilation via elevating endothelial nitric oxide by promoting eNOS phosphorylation (Chen et al., 1999), angiotensin-converting enzyme 2 (Zhang et al., 2018), and elevating calcium levels (Schneider et al., 2015).
A high amount of energy generated by the oxidation of different substrates like fatty acids and glucose, is needed for heart to function normally (Rösen et al., 1984; Lloyd et al., 2004). AMPK regulates energetic homeostasis through glycolysis and glucose uptake stimulation, meanwhile attenuating energy consumption (Hue et al., 2002; Dyck and Lopaschuk 2006). AMPK is stimulated throughout low-energy cellular conditions, like myocardial ischemia. Myocardial I/R damage (oxidative and inflammatory damage to the cardiac muscle due to reperfusion after ease of ischemia), is a vital cardiopathic process in which AMPK plays a major role (Gr and Be 2009).
AMPK preserves ischaemic cardiomyocytes, via various processes. On the one hand, AMPK promotes the translocation of glucose transporter type 4 (GLUT4) to the sarcolemmal membrane and boost glucose uptake (Russell et al., 1999). On the other hand, when oxygen supply is restored during reperfusion, extra AMPK stimulation elevates oxidation of fatty acid (Kudo et al., 1995). The effect of AMPK on fatty acid oxidation brings the protective role of AMPK during early reperfusion into question (Dyck and Lopaschuk 2006). Additionally, following the mitochondrial damage in the course of reperfusion, mitochondrial respiratory capacity is maintained by AMPK via opening of the mitochondrial permeability transition pore (mPTP) (Qi and Young 2015).
Interestingly, AMPK was later described as a remarkable cardiac savior against cardiomyocyte apoptosis (Kewalramani et al., 2009). Moreover, in ischaemic heart, AMPK is a vital regulator of autophagy. Autophagy is a survival process to save the substances and energy demand in ischaemic myocardium (Yan et al., 2005; Hamacher-Brady et al., 2007; Nakai et al., 2007; Rothermel and Hill 2007).
Endothelial AMPK might be an important factor in tissues exposed to ischaemic stress. Eventually, It was shown that AMPK is involved in adiponectin mediated pro-angiogenic function (Ouchi et al., 2004). Besides, vascular endothelial growth factor (VEGF) mRNA stability was extended by glucose deprivation (Yun et al., 2005). Metformin administration before, throughout, or following myocardial ischemia has been proved to inhibit ischemia-reperfusion damage and associated adverse left ventricular remodeling (El Messaoudi et al., 2011). In addition, AMPK is neuroprotective against ischaemic stroke (Culmsee et al., 2001; Dasgupta and Milbrandt 2007; Kuramoto et al., 2007).
Left ventricular (LV) remodeling always occur because of cardiac damage after I/R. Cardiac fibrosis, the extracellular matrix (ECM) accumulation in the myocardium, is classified as either reactive interstitial fibrosis or reparative fibrosis, defined by the pathophysiological status (Travers et al., 2016). The inflammatory responses will start quickly after MI and trigger fibrogenesis, leading to the clearance and replacement of damaged cardiac fibroblasts in the injured necrotic part by a solid fibrotic scar (Weber et al., 2013; Daskalopoulos et al., 2014). This is vital and protective for wound healing and viability via preserving myocardial integrity and prevent cardiac rupture development. Within the infarcted area of AMPKα1-deficient mouse hearts, collagen cross-linking and myodifferentiation are remarkably decreased, causing faulty scar collagen maturation, compromised scar contractility, and worsening LV dilatation 30 days post-MI (Noppe et al., 2014). Later, fibrosis will extends to the non-infarcted myocardium, creating myocardial stiffness, diastolic dysfunction, systolic dysfunction, HF and eventual death (Weber et al., 1989; Masci et al., 2014).
Immunosuppressive and anti-inflammatory functions of AMPK have been shown in different cell types and autoimmune/inflammatory diseases (Salt and Palmer 2012). Metformin administration could cause acute AMPK activation (Soraya et al., 2012) and chronic AMPK pre-activation (Soraya et al., 2015) which in turn decreases cardiac remodeling through attenuating infiltration of peripheral neutrophils into the myocardial tissue after MI. On the other hand, during cardiac inflammatory reaction, tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine, was increased. AMPK also works to oppose cardiomyocyte necrosis induced by TNF-α and as well as inflammatory cell infiltration into the ischaemic myocardia (Peng et al., 2009).
Reactive oxygen species (ROS) is a key player in cardiac fibrosis which can be prevented by ROS scavenger treatments (Richter and Kietzmann 2016). SIRT proteins, a group of class III histone deacetylases that play a role in metabolism, decrease cardiac injury/fibrosis induced by ROS via positive feedback loop involving AMPK (Chong et al., 2012). Liu and coworkers has recently demonstrated the effects of anti-inflammatory and anti-oxidative effects of Arctigenin as a natural lignan compound. They found that Arctigenin decreased apoptosis of cardiomyocytes via AMPK/SIRT1 pathway in myocardial I/R Injury (Liu et al., 2020). Interestingly, SIRT3 (Lombard and Zwaans 2014) and SIRT6 (Wang et al., 2016) lower oxidative stress via an AMPK-related pathway as well.
Cardiac hypertrophy, one of the main pathological mechanisms leading to cardiac remodeling. It is described as an increase in gene transcription and protein translation, increased size of cardiomyocytes, and a greater extent of sarcomere organization. Physiological cardiac hypertrophy occurs due to a physiological situation as a compensatory reaction to intense exercise in many athletes. However, pathological hypertrophy occur in response to myocardium mechanical stress, such as volume/pressure overload observed in hypertension or valvular heart disease or myocardial ischemia. In the case of chronic hypertension, pathological hypertrophy turns into abnormal remodeling and dysfunction that eventually promotes heart failure (HF) development (Frey and Olson 2003). Several groups focused on the study of AMPK as a pharmacological target against cardiac hypertrophy and subsequently HF. For instance, an experimental research was conducted to investigate the anti-hypertrophic effect of QF84139 as a novel small molecule (synthesized pyrazine derivative) in phenylephrine-induced hypertrophic model. One notable finding in this study is that QF84139 acts as an effective AMPK activator for the treatment of cardiac hypertrophy (Li et al., 2021). In another recent study (Zhang et al., 2021), Zhang et la have revealed that Bawei Chenxiang Wan as a Tibetan herbal medicine that prevents cardiac hypertrophy in isoprenaline-induced rats by activating AMPK/PPAR-α signaling. AMPK as a key player in metabolic pathways, has a vital function in various cellular processes to protect against cardiac hypertrophy, through regulating energy supply, protein synthesis, autophagy, cytoskeletal network expansion, transcription, ER stress, and microRNA expression (Frey and Olson 2003; Horman et al., 2012).
Cardiac metabolism disturbances is an important factor of hypertrophic process to meed the elevated energy requirements due to increased cardiac workload. Deregulation of cardiac energetics and cardiac dysfunction afterwards could occur as a result of changes in the metabolic status of the cardiomyocytes. AMPK is a key regulator in metabolic processes of the cardiomyocytes (Nascimben et al., 2004; Horman et al., 2012). Therefore AMPK has gained intensive attention as a target in anti-hypertrophic research plans.
An increase in the size of cardiomyocytes and protein synthesis, which cause a thickening of the LV walls (concentric hypertrophy), is the most prominent feature of hypertrophy. AMPK can efficiently inhibit two major mechanism governing protein synthesis. The first one is the eukaryotic elongation factor-2 (eEF2) kinase/eEF2 pathway (Chan et al., 2004) and the other one is (mTOR)/p70 ribosomal protein S6 kinase (p70S6K) axis, the Akt/mammalian target of rapamycin (Zarrinpashneh et al., 2008; Fu et al., 2011). Mice lacking AMPKα2 are largely affected by LV hypertrophy following pressure overload (Zarrinpashneh et al., 2008) or shortly after aortic banding (Zhang et al., 2008). In line with previous observations, AMPK is capable of suppressing cardiac hypertrophy after transverse aortic banding (Li et al., 2014). Moreover, in hypertrophic hearts, AMPK interacts with protein quality control mechanism by promoting the clearance of malfunctioning mitochondria (Li et al., 2015).
Another characteristic of cardiomyocyte hypertrophy is cytoskeletal network expansion, in particular microtubule densiccation, contributing to cardiac contractile dysfunction. AMPKα2 phosphorylates MAP4 (Microtubule-associated protein 4), leading to the decrease in microtubules accumulation and densiccation (Fassett et al., 2013).
AMPK accomplishes its anti-hypertrophic properties via another fascinating mechanism of transcriptional regulation via NFAT pathway (Chan et al., 2008). AMPK inhibit cardiomyocyte hypertrophy, via preventing NFAT translocation to the nucleus. There are also other key regulators involved such as c-myc, the FoxO1/muscle RING-finger protein-1(FoxO1/MuRF1) pathway and the extracellular signal-regulated kinases (ERK) (Esposito et al., 2001; Shibata et al., 2004) and MuRF1 (Arya et al., 2004; Chen et al., 2010). Mitochondria metabolic functions have been shown to be regulated by collaborative actions of c-myc and AMPK (Edmunds et al., 2015).
Autophagy is a remarkable mechanism by which the clearance of unwanted cellular parts and recycles occur (Fimia et al., 2013; Reggiori and Klionsky 2013). Lately, it has been suggested that autophagy has dual roles in cardiac hypertrophy. Constitutive autophagy preserves cardiac function/structure, however extra autophagy activation promotes autophagic cell death and eventually provokes cardiac hypertrophy (Li et al., 2015). Through phosphorylation of the stimulator of autophagy-ULK1, AMPK can promote cardiomyocyte autophagy (Herrero-Martín et al., 2009; Kim et al., 2011).
ER stress is defined as an ER homeostasis imbalance and ER dysfunction. Extra ER stress leads to unfolded/misfolded proteins accumulation and subsequently to cardiac hypertrophy by triggering cell death (Kaufman, 2002; Wang et al., 2017). It has been suggested that AMPK activation indirectly, via protein synthesis inhibition, could preserve heart function by inhibiting ER stress and eventually prevent cardiomyocyte death.
MicroRNAs are crucial regulators in the progression of cardiac hypertrophy. AMPK regulates many microRNAs, such as miR-195, miR-133a, and miR-451 during the course of cardiac hypertrophy. Among all, miR-133a has a key role against cardiac hypertrophy (Care et al., 2007). Adiponectin, a circulating adipose-derived cytokine could activate AMPK, elevates miR-133a level, and finally decreases cardiac hypertrophy induced by Ang II (Li et al., 2016). Some microRNAs are involved in AMPK pathway such as miR-195 (Chen et al., 2012) and miR-451 (Kuwabara et al., 2015), whose expression is elevated in hypertrophic cardiomyocytes, thereby inhibiting the activation of the AMPK/liver kinase B1 (LKB1) signaling axis and amplifying cardiac hypertrophy.
So far, the only AMPK genetic mutations occurs in PRKAG2 (encoding the γ2 isoform of the nucleotide-binding subunit), leading to Wolff–Parkinson–White syndrome (WPWS), a type of heart disease (Arad et al., 2007). Ventricular pre-excitation (a premature excitation of the ventricles, detected by electrocardiogram) is a feature of this syndrome (Gollob et al., 2001). This condition is relatively rare, affecting 0.9–3% of the population (Rosner et al., 1999).
Heart is the preferential tissue where γ2 isoform is highly expressed (Cheung et al., 2000). In fact, the γ subunit mutation in WPWS leads to extra glycogen retention and accumulation in cardiomyocytes and these cells contribute to the accessory pathway formation (named the Bundle of Kent) leading to arrhythmias (Gollob 2003).
One of the critical LV pathology that accompanies diabetes mellitus (DM), is diabetic cardiomyopathy. It can leads to HF if left untreated (Kannel et al., 1974). Studies have shown that AMPK displays anti-fibrotic effects against LV dysfunction in diabetic mouse model of both type 1 and type 2 DM. In db/db mice (a type 2 DM model), reduced AMPK activity, decreased contractile ability and inefficient cardiac metabolism has been described (Daniels 2010). In type 1 DM model (OVE26 mice), AMPK regulates autophagy (Xie et al., 2011). In comparison to wild type littermates, OVE26 mice presented LV dysfunction, lower AMPK function, and attenuated autophagy. Finally, ROS accumulation is a well known etiology for the development of diabetic cardiomyopathy (Seddon et al., 2007). In this regard, AMPK activation in cardiomyocytes prevents glucotoxicity by lowering NOX2-mediated ROS production (Balteau et al., 2014).
Based on the cardiovascular actions of AMPK, natural AMPK activators are of great therapeutic potential. A number of natural products and herbal constituents have been reported to activate AMPK and prevent CVD (summarized in Figure 1).
Resveratrol (3,5,4′-trihydroxy-trans-stilbene), is a natural polyphenolic compound with three hydroxyl groups in its structure. It is widely distributed in edible fruits like berries, pomegranates, grape skin, peanuts, and many others. This polyphenol displays beneficial biological functions in the cardiovascular system due to its anti-inflammatory, anti-atherogenic, hypolipidemic, anti-platelet, and anti-oxidant properties (Cheng et al., 2020). It has been found that resveratrol through AMPK activation improved cardiac function and decreased the risk of CVD development. Several studies have indicated that natural AMPK activators can prevent the development of cardiac hypertrophy and resveratrol as a natural compound can inhibit hypertrophic growth. More recently, treatment with resveratrol has been demonstrated to reverse cardiac hypertrophy in mice following transverse aortic constriction surgery. Resveratrol attenuates phosphatase and tensin homolog (PTEN) degradation by inhibiting proteasome-dependent degradation, thereby leading to the inactivation of AKT/mTOR signaling pathway and activation of AMPK pathways (Chen et al., 2019).
One of the main therapeutic advantages of resveratrol is attributed to its effective antioxidant capacities. Oxidative stress has an important role in the development of hypertension. Resveratrol pretreatment decreases the harmful oxidative consequences of hypertension and cardiac hypertrophy. To test the effect of resveratrol on hypertension, fructose-fed rats were treated with resveratrol (10 mg/kg). Results indicated that resveratrol considerably lowered blood pressure and ROS production, enhanced nitric oxide levels by activating AMPK in the fructose-induced hypertensive rat model (Cheng et al., 2014). Another study in animal models of hypertrophic cardiomyopathy also demonstrated the positive role of resveratrol via increasing the activation of the hierarchical LKB1/AMPK signaling pathway. Blood and cardiac levels of lipid peroxidation byproduct 4-hydroxy-2-nonenal (HNE) is increased during hypertension. HNE inhibits the activation of LKB1 and subsequent AMPK, consistent with this inhibition, mTOR and p70S6 kinase are activated. Resveratrol has been shown to reduce cardiac HNE levels and provide beneficial effects on LV hypertrophy (Dolinsky et al., 2009). According to another study (Dolinsky et al., 2013), Dolinsky et al. showed that resveratrol reduced HNE levels, activated eNOS, and reduced p70S6K activity by activating LKB1 and AMPK in angiotensin II (AngII) -induced hypertensive mice and rats. Resveratrol activates AMPK and blocks the Akt/mTOR/p70S6K pathways which lead to the reduction of smooth muscle cell proliferation and DNA synthesis (Brito et al., 2009).
Researchers have noted that acute treatment with resveratrol (100 μmol/L) can reduce cell growth in rat cardiac myocytes by increasing AMPK and ACC phosphorylation as well as inhibiting Akt activation in the hypertrophic process (Chan et al., 2008). It was observed that resveratrol (30 μmol/L) significantly prevented increases in cardiomyocyte size and protein synthesis in norepinephrine-induced cardiac hypertrophy in adult rats. Moreover, AMPK activation conferred by resveratrol increased the level of nitric oxide (Thandapilly et al., 2011). There was a significant reduction in blood pressure, Ras homolog gene family member A (RhoA) activity and levels of phosphorylated myosin phosphatase-targeting subunit 1 (MYPT1), and myosin light chain (MLC) by resveratrol in AngII-treated mice (Cao et al., 2014).
In a cellular study performed by Hwang et al., potential anti-apoptotic effect of resveratrol (50, 100 μmol/L) was assessed on H9c2 cardiac muscle cells. Results of this investigation suggested that resveratrol acts as a powerful AMPK activator against H2O2-induced cell death and apoptosis (Hwang et al., 2008). AMPK is a pivotal enzyme associated with a wide range of cardiac metabolic pathways such as protein synthesis, glucose metabolism, and fatty acid oxidation, as well as insulin-sensitization. There is also some evidence suggesting the efficacy of resveratrol therapy in diabetic cardiomyopathy. AMPK activation by resveratrol prevented hyperglycemia-induced apoptosis and oxidative stress, by inhibiting NADPH oxidase-derived ROS production in cardiomyocytes (Guo et al., 2015). In addition, in a mouse model of type 2 diabetes mellitus, resveratrol (10 mg/kg) via AMPK activation can decrease disulfide bond A oxidoreductase-like protein (DsbA-L) and adiponectin (APN) levels which are crucial for the prevention of myocardial ischemia/reperfusion injury in diabetes (Yang et al., 2016).
It has been demonstrated that resveratrol (10–100 μmol/L) prevented endothelial dysfunction related to hyperglycemia in HUVECs. Activation of AMPK by resveratrol increased eNOS activity and NO production, also improved vascular function and glycemic control (Xu et al., 2009). Kanamori et al. (2013) have reported that resveratrol (50 mg/kg/day) reduced cardiomyocyte size, heart weight/body weight ratio, promoted autophagy by activating AMPK in postinfarcted LV. AMPK plays a major role in the prevention of HF and cardiac dysfunction. Resveratrol-enriched diet feeding increased SIRT1 expression and AMPK activation, which lead to improved cardiac function in HF (Gu et al., 2014).
Berberine is an eminent component of traditional Chinese medicine. Many studies revealed the cardioprotective effects of berberine is dependent on AMPK activation (Feng et al, 2019). It is suggested that ER stress is the main cause of endothelial dysfunction during hypertension. Berberine (1 μmol/L) significantly suppressed ER stress by activating AMPK in spontaneously hypertensive rats. Moreover, evidence showed that endothelium-dependent contractions are reduced by increasing AMPK phosphorylation in arteries (Liu et al., 2015). The results demonstrated that berberine attenuated Ang II-induced myocardial hypertrophy by regulating LC3 protein and AMPK phosphorylation in vitro conditions (Zeng et al., 2019). The beneficial effects of berberine (0.25–4.0 μmol/L) on mitochondrial dysfunction are suggested to be the result of suppressing doxorubicin-induced cardiac injury via increasing AMPK and reducing AMP/ATP ratio, as well as promoting Bcl-2 protein level (Lv et al., 2012). In line with this observation, AMPK activation by berberine leads to inhibited autophagy and apoptosis in hypoxia-induced myocardial cell injury (Jia et al., 2017).
It has been well established that berberine attenuates vascular inflammation and leads to the suppression of atherogenesis. Treatment with berberine has been found to reduce oxidative stress and atherosclerosis in ApoE−/− mice followed by AMPK-dependent expression of upregulation of uncoupling protein 2 (UCP2) (Wang et al., 2011). In another series of experiments, berberine (5, 10, 20 mg/kg/d) suppressed the formation and accumulation of foam cells by activating the AMPK, SIRT1, and PPAR-γ signaling pathways (Chi et al., 2014). In a recently published study, it has been reported that berberine significantly improved endothelial dysfunction caused by low shear stress-via increasing AMPK phosphorylation. In fact, AMPK inhibited hyaluronidase 2 (Hyal2) pathway and p47phox phosphorylation, also modulated hyaluronic acid synthase 2 (HAS2) activity in HUVECs model (Yang et al., 2019). A further study on endothelial dysfunction has indicated that AMPK activation by berberine increases eNOS activity and decreases the protein expression of NADPH oxidase 4 (NOX4), which elevates NO levels in vascular endothelial cells (Zhang et al., 2013). The ability of berberine in the inhibition of platelet-derived growth factor (PDGF)-induced VSMCs proliferation through activation of AMPK/p53/p21Cip1 pathway has also been demonstrated in vitro (Liang et al., 2008). Berberine-induced activation of AMPK improves cardiac fibrosis, an effect that has been linked to the inhibition of mTOR/p70S6K signaling pathway (Ai et al., 2015).
Current evidence suggests berberine is a widely-used AMPK activator for the treatment of type 2 diabetes with or without CVD (Feng et al., 2019). Berberine treatment for 7 days in insulin-resistant rats (by high fat diet feeding) increased AMP/ATP and ADP/ATP ratios, AKT phosphorylation, and decreased glycogen synthase kinase 3β (GSK3β) through AMPK activation in ischemia–reperfused diabetic rat hearts. This suggests that berberine can be used as a cardioprotective agent against ischemia-reperfusion injury related to diabetes (Chang et al., 2016). In diabetic cardiomyopathic rats, berberine attenuated cardiac hypertrophy through activating both AMPK and AKT activity, and inhibited the expression of GSK3β (Chang et al., 2015). Another important effect of berberine is its ability to promote glucose uptake and glucose consumption in normal or insulin-resistant H9c2 cardiomyocyte cells via AMPK activation (Chang et al., 2013). Berberine (100 nmol/L) attenuated high glucose-induced (50 mmol/L) hypertrophy, decreased LC3-II level, inhibited mitochondrial fission and mTOR, and restored autophagic flux in H9c2 cells by targeting AMPK (Hang et al., 2018).
Ginsenosides, the main bioactive ingredients of ginseng, are a group of saponin compounds with a wide range of biological and therapeutic activities. Ginsenosides (Rg1, Rb1, Rd, Re, and Rg3) are the representative ginsenosides that are most frequently studied. The anti-apoptotic and anti-atherosclerotic effects of Rg1 were investigated in vitro. The levels of autophagy-related proteins such as Atg5, LC3, Beclin1, and p62/SQSMT1 were increased by Rg1 (50 μmol/L). The result demonstrated that Rg1 could suppress apoptosis and promote autophagic flux in macrophages by activation of AMPK/mTOR signaling pathway (Yang et al., 2018). Rg1 (20 mg/kg) is also able to alleviate inflammation and cardiac oxidative stress in diabetic rats, through promoting AMPK/Nrf2/HO-1 signaling pathway (Qin et al., 2019).
Recent findings by Zheng et al. (2020) have shown that Rb1 could inhibit H2O2-induced endothelial dysfunction and increased SIRT1, eNOS, NO production, and suppressed expression of plasminogen activator inhibitor-1 (PAI-1). AMPK activation was also necessary for Rb1 to reduce endothelial aging in this study. Rb1 could ameliorate the autophagy of cardiomyocytes by up-regulating AMPK pathway and reducing levels of p62 and cathepsin B in rat cardiomyocytes under hypoxia conditions (Dai et al., 2019). Sun et al. (Sun et al., 2020) assessed the molecular mechanisms by which Rg3 exerts myocardial protection. The following benefits were observed: promotion of ACC phosphorylation, enhancemeng of autophagy in isoproterenol-induced myocardial infarction (MI), and inhibition of myocardium apoptosis. This myocardial protective effects have also been observed in Re (135 mg/kg) treated rats, in which Re significantly improved cardiac function and LV fibrosis in rat MI model by regulating AMPK/TGF-β1/Smad2/3 signaling (Yu et al., 2020). Hyperlipidemia is considered to be one of the important risk factors for coronary heart disease, such as atherosclerosis, MI, heart attacks. The beneficial and protective effects of Rg3 have been related to the decrease of intracellular cholesterol and triglyceride levels (Lee et al., 2012).
Quercetin, is a flavonoid that has been isolated from a variety of vegetables and fruits including citrus, berries, apples, and tea. There have been numerous studies suggesting that quercetin exhibits anti-inflammatory, antioxidative, cardioprotective, and endothelial protective effects (Chekalina et al., 2018). It has been shown that quercetin (5 and 10 µM) was able to activate AMPK, eNOS, and promote NO production, leading to improved vascular function in cellular model of endothelial dysfunction (Shen et al., 2012). In VSMCs, Kim et al. found that the phosphorylation of AMPK and LKB1 by quercetin (25, 50, 100 µM) regulated the expression of MLC kinase and the phosphorylated myosin light chain (p-MLC), as well as inhibited phenylephrine-induced vasoconstriction (Kim et al., 2018).
Curcumin is a natural polyphenol found in turmeric, which displays beneficial functions including anti-oxidant, anti-inflammatory, anti-angiogenic, anti-thrombotic effects dependent on AMPK activation (Li et al., 2020; Singh et al., 2021). In one study, in vivo and in vitro data indicated that curcumin (200 mg/kg) significantly improved cardiac apoptosis in the hearts of diabetic mice. Activation AMPK and JNK1 by curcumin regulated autophagy and alleviated apoptosis through phosphorylating Bim and Bcl-2 proteins, which lead to the disruption of their interactions with Beclin1 (Yao et al., 2018). A recent study has presented that curcumin-loaded nanoparticles attenuated oxidative stress and inhibited the intracellular ROS increase via AMPK activation in palmitate-induced cardiomyocyte apoptosis (Zhang et al., 2019). Treatment with curcumin (25 μmol/L) suppressed type I and type III collagen synthesis and TGF-β1 production in cultured cardiac fibroblasts, as well as inhibited cardiac fibrosis. Interestingly, one-month administration of dietary curcumin in aged rats restored cerebrovascular endothelium-dependent vasorelaxation. Curcumin also improved AMPK phosphorylation and attenuated ROS production in cultured endothelial cells and cerebral arteries of aged animals. However, after AMPK inhibition, the beneficiary effects of curcumin were not observed, indicating AMPK dependency (Pu et al., 2013). Despite other findings showing that AMPK activation is effective to prevent cardiac fibrosis, Guo et al. reported that curcumin inhibited AMPK/p38 MAPK activity in their study results (Guo et al., 2018), which warrants further study.
Salvianolic acid B is a natural antioxidant compound that is found in Salvia miltiorrhiza (Danshen) roots (Fang et al., 2018; Li et al., 2018). This phenolic acid has been traditionally used for the treatment of CVD. It was found that, in the cultured endothelial cells, treatment of myocardial infarction with salvianolic acid B produced an increase in NO production and activated phosphorylation of Akt and AMPK. Additionally, salvianolic acid B increased cationic amino acid transporters and eNOS expression through the AMPK/PI3K/Akt pathway, leading to stimulated L-arginine uptake (Pan et al., 2011). It has been demonstrated that salvianolic acid B (5, 10, 20 μg/ml) protected HUVECs against H2O2-induced apoptosis via promoting autophagy by AMPK activation and mTOR inhibition (Gao et al., 2019). Yang et al. (2019) suggested that salvianolic acid B can suppress apoptosis and increase cell viability in oxygen-glucose deprivation injury in H9c2 cells through regulating murine double minute 2 (MDM2)/p53 and AMPK signaling pathways.
Crocin, the major bioactive phytochemical component of saffron, can be used as a novel therapeutic agent against CVD. The evidence suggests that crocin pretreatment can promote autophagy during ischemia and reperfusion injury, and reduced myocardial apoptosis, infarct size, and necrosis, accompanied by the activation of AMPK (Zeng et al., 2016). The effect of crocin (10, 20 mg/kg) on diabetic heart dysfunction was evaluated in streptozotocin-induced diabetic rats. Treatment of crocin resulted in a reduction of myocardial apoptosis, as evidenced by increased phosphorylation of myocardial AMPK, normalized levels of autophagy marker proteins, and improved cardiac function in diabetic animals. Therefore, crocin may be a potential therapeutic natural AMPK activator for diabetic cardiomyopathy treatment (Feidantsis et al., 2018).
Naringenin, a naturally-occurring plant flavonoid, is abundantly present in common vegetables and fruits. It is recognized as an effective treatment for CVD (Heidary Moghaddam et al., 2020). Saenz et al. (2018) have been reported that naringenin can prevent foam cell progression and atherosclerosis in human macrophages models. Naringenin treatment (100 µM) increased cholesterol efflux and suppressed macrophage migration via AMPK activation. Due to obvious AMPK and SIRT3 activation capacity, the cardiovascular protective effects of naringenin could involve both targets. A study has shown that naringenin reduces cardiac damage in animal models by activating AMPK-SIRT3 signaling pathway, suggesting that naringenin can be exploited as an effective therapeutic agent for ischemic heart disease (Yu et al., 2019).
In the prior section, the cardioprotective effects of natural AMPK activators are presented. Most of these natural cardioprotective agents have only been evaluated in animal models and in vitro studies. Some of these compounds have entered clinical trials and have demonstrated positive results. In this section, research studies involving the clinical trials of natural AMPK activators are discussed.
In one study, a total of 84 male and female patients with coronary artery disease were used for evaluating the beneficial effects of crocin. Patients were randomly divided into three groups: group 1 received a crocin capsule of 30 mg/day daily; group 2 received a saffron aqueous extract capsule of 30 mg/day daily and group 3 received placebo capsules with similar shapes for 4 weeks. The comparison between groups revealed that there were significantly enhanced expression/activity of SIRT1 and AMPK, also decreased expression of LOX1 and NF-κB in the crocin treated group, compared with the placebo group. In addition, crocin could also reduce serum levels of oxidized low-density lipoprotein and monocyte chemoattractant protein 1 (MCP-1) in these patients. Crocin-treated group also had significant higher efficacy than saffron aqueous extract-treated group (Abedimanesh et al., 2020). Another clinical trial has been launched to evaluate the effectiveness of berberine on galectin-3 expression and macrophage activation in patients with acute coronary syndrome. Galectin-3, as a disease-relevant biomarker, is increased in atherosclerotic lesions and inflammation. In a single-blinded trial, 45 patients were randomly divided into two groups according to 2:1 randomization ratio. One group consisted of 30 patients treated with 300 mg of berberine hydrochloride for 3 months, and the other group consisted of 15 patients who had received only standard therapy. The results indicated that berberine reduces oxidized low-density lipoprotein-induced macrophage activation via decreasing galectin-3 expression by activating AMPK signaling and suppressing NF-κB pathway (Pei et al., 2019).
Berberine has been used to protect cardiomyoblast cells from apoptosis as a preventive and curative treatment of myocardial injury in postoperative patients. The results evidenced that berberine provided a reduction of all inflammatory biomarkers after operation in patients with acute myocardial infarction, as well as inhibiting autophagy and apoptosis in H9C2 cells through the AMPK/mTOR pathway (Qing et al., 2018).
The clinical benefits of resveratrol have been investigated in patients with hypertension and dyslipidemia. Researchers observed that resveratrol leads to reduced endothelial dysfunction by modulation of NO bioavailability in diseased human vessels. The level of tetrahydrobiopterin (BH4) increased after resveratrol treatment, suggesting resveratrol’s effects to increase AMPK and eNOS activation, as well as attenuation of vascular oxidative stress (Carrizzo et al., 2013).
AMPK is the fuel sensor and key target of cardiometabolic homeostasis and diseases. Emerging evidence has suggested AMPK activators from naturally-occuring sources provide notable cardiovascular benefits (Figure 2). AMPK as a sensor of cellular energy also regulate cardiac system bioenergetics and energy metabolism. AMPK activation prevents cardiometabolic disease by its capacity to lower blood pressure, glucolipids, ROS production, and improve NO bioavailability. AMPK activators thus serve as novel potential drugs in gatekeeping cardiovascular health and preventing cardiovascular disease.
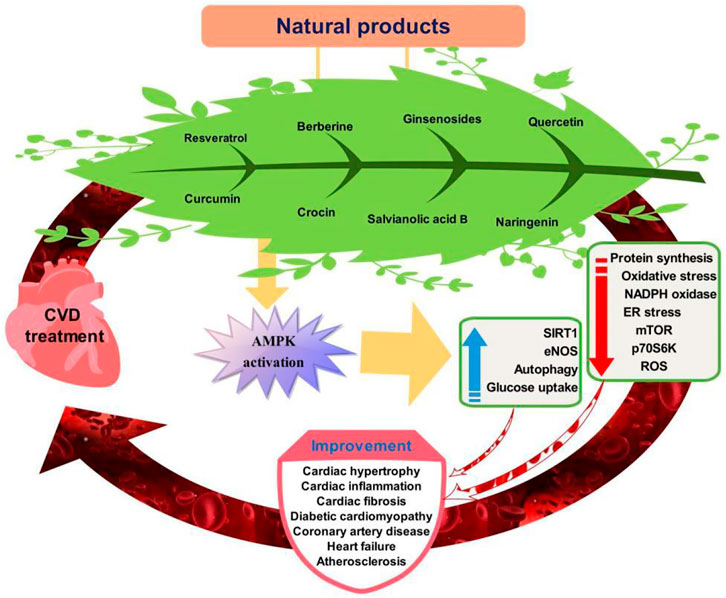
FIGURE 2. Targeting of AMPK signaling pathway by natural products for cardiovascular disease prevention.
However, translational validity regarding the association with natural products, AMPK and CVD tends to be weak. These correlations do not necessarily modify clinical events nor directly address “prevention” in terms of superphysiological or pharmacological doses are used in rodent models or cultured cells. The calculation of human doses in rodents and vice versa is also a critical factor. More research is needed to clarify the exact mechanism of action associated with AMPK activation by natural products as well as to explore the safety and efficacy of natural AMPK activators to treat patients with CVD at therapeutically relevant or human equivalent concentrations and doses.
RM: Writing—original draft, Writing—review and editing. ZS: Writing—original draft, Writing—review and editing. SA: Writing—review and editing. PM: Writing—original draft, Writing—review and editing. SH: Writing—review and editing. FC: Writing—review and editing. SX: Writing—original draft, Writing—review and editing. MF: Writing—original draft, Writing—review and editing.
This study was supported by grants from National Natural Science Foundation of China (Grant Nos. 82070464) and Anhui Provincial Key Research and Development Program (Grant No. 202104j07020051).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abedimanesh, N., Motlagh, B., Abedimanesh, S., Bathaie, S. Z., Separham, A., and Ostadrahimi, A. (2020). Effects of Crocin and Saffron Aqueous Extract on Gene Expression of SIRT1, AMPK, LOX1, NF-Κb, and MCP-1 in Patients with Coronary Artery Disease: A Randomized Placebo-Controlled Clinical Trial. Phytother Res. 34 (5), 1114–1122. doi:10.1002/ptr.6580
Afzal, M. (2021). Recent Updates on Novel Therapeutic Targets of Cardiovascular Diseases. Mol. Cel Biochem 476 (1), 145–155. doi:10.1007/s11010-020-03891-8
Ai, F., Chen, M., Yu, B., Yang, Y., Xu, G., Gui, F., et al. (2015). Berberine Regulates Proliferation, Collagen Synthesis and Cytokine Secretion of Cardiac Fibroblasts via AMPK-mTOR-p70S6K Signaling Pathway. Int. J. Clin. Exp. Pathol. 8 (10), 12509–12516.
Alers, S., Löffler, A. S., Wesselborg, S., and Stork, B. (2012). Role of AMPK-mTOR-Ulk1/2 in the Regulation of Autophagy: Cross Talk, Shortcuts, and Feedbacks. Mol. Cel Biol 32 (1), 2–11. doi:10.1128/MCB.06159-11
Altarejos, J. Y., and Montminy, M. (2011). CREB and the CRTC Co-activators: Sensors for Hormonal and Metabolic Signals. Nat. Rev. Mol. Cel Biol 12 (3), 141–151. doi:10.1038/nrm3072
Arad, M., Seidman, C. E., and Seidman, J. G. (2007). AMP-activated Protein Kinase in the Heart: Role during Health and Disease. Circ. Res. 100 (4), 474–488. doi:10.1161/01.RES.0000258446.23525.37
Arya, R., Kedar, V., Hwang, J. R., McDonough, H., Li, H. H., Taylor, J., et al. (2004). Muscle Ring finger Protein-1 Inhibits PKC{epsilon} Activation and Prevents Cardiomyocyte Hypertrophy. J. Cel Biol 167 (6), 1147–1159. doi:10.1083/jcb.200402033
Balteau, M., Van Steenbergen, A., Timmermans, A. D., Dessy, C., Behets-Wydemans, G., Tajeddine, N., et al. (2014). AMPK Activation by Glucagon-like Peptide-1 Prevents NADPH Oxidase Activation Induced by Hyperglycemia in Adult Cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 307 (8), H1120–H1133. doi:10.1152/ajpheart.00210.2014
Bond, P. (2016). Regulation of mTORC1 by Growth Factors, Energy Status, Amino Acids and Mechanical Stimuli at a Glance. J. Int. Soc. Sports Nutr. 13, 8. doi:10.1186/s12970-016-0118-y
Brito, P. M., Devillard, R., Nègre-Salvayre, A., AlmeidaAlmeida, L. M., Dinis, T. C., Salvayre, R., et al. (2009). Resveratrol Inhibits the mTOR Mitogenic Signaling Evoked by Oxidized LDL in Smooth Muscle Cells. Atherosclerosis 205 (1), 126–134. doi:10.1016/j.atherosclerosis.2008.11.011
Cantó, C., Gerhart-Hines, Z., Feige, J. N., Lagouge, M., Noriega, L., Milne, J. C., et al. (2009). AMPK Regulates Energy Expenditure by Modulating NAD+ Metabolism and SIRT1 Activity. Nature 458 (7241), 1056–1060. doi:10.1038/nature07813
Cao, X., Luo, T., Luo, X., and Tang, Z. (2014). Resveratrol Prevents AngII-Induced Hypertension via AMPK Activation and RhoA/ROCK Suppression in Mice. Hypertens. Res. 37 (9), 803–810. doi:10.1038/hr.2014.90
Carè, A., Catalucci, D., Felicetti, F., Bonci, D., Addario, A., Gallo, P., et al. (2007). MicroRNA-133 Controls Cardiac Hypertrophy. Nat. Med. 13 (5), 613–618. doi:10.1038/nm1582
Carrizzo, A., Puca, A., Damato, A., Marino, M., Franco, E., Pompeo, F., et al. (2013). Resveratrol Improves Vascular Function in Patients with Hypertension and Dyslipidemia by Modulating NO Metabolism. Hypertension 62 (2), 359–366. doi:10.1161/HYPERTENSIONAHA.111.01009
Chan, A. Y., Dolinsky, V. W., Soltys, C. L., Viollet, B., Baksh, S., Light, P. E., et al. (2008). Resveratrol Inhibits Cardiac Hypertrophy via AMP-Activated Protein Kinase and Akt. J. Biol. Chem. 283 (35), 24194–24201. doi:10.1074/jbc.M802869200
Chan, A. Y., Soltys, C. L., YoungYoung, M. E., Proud, C. G., and Dyck, J. R. (2004). Activation of AMP-Activated Protein Kinase Inhibits Protein Synthesis Associated with Hypertrophy in the Cardiac Myocyte. J. Biol. Chem. 279 (31), 32771–32779. doi:10.1074/jbc.M403528200
Chang, W., Li, K., Guan, F., Yao, F., Yu, Y., Zhang, M., et al. (2016). Berberine Pretreatment Confers Cardioprotection against Ischemia-Reperfusion Injury in a Rat Model of Type 2 Diabetes. J. Cardiovasc. Pharmacol. Ther. 21 (5), 486–494. doi:10.1177/1074248415627873
Chang, W., Zhang, M., Li, J., Meng, Z., Wei, S., Du, H., et al. (2013). Berberine Improves Insulin Resistance in Cardiomyocytes via Activation of 5'-adenosine Monophosphate-Activated Protein Kinase. Metabolism 62 (8), 1159–1167. doi:10.1016/j.metabol.2013.02.007
Chang, W., Zhang, M., Meng, Z., Yu, Y., Yao, F., Hatch, G. M., et al. (2015). Berberine Treatment Prevents Cardiac Dysfunction and Remodeling through Activation of 5'-adenosine Monophosphate-Activated Protein Kinase in Type 2 Diabetic Rats and in Palmitate-Induced Hypertrophic H9c2 Cells. Eur. J. Pharmacol. 769, 55–63. doi:10.1016/j.ejphar.2015.10.043
Chekalina, N., Burmak, Y., Petrov, Y., Borisova, Z., Manusha, Y., Kazakov, Y., et al. (2018). Quercetin Reduces the Transcriptional Activity of NF-kB in Stable Coronary Artery Disease. Indian Heart J. 70 (5), 593–597. doi:10.1016/j.ihj.2018.04.006
Chen, B. L., Ma, Y. D., Meng, R. S., Xiong, Z. J., Wang, H. N., Zeng, J. Y., et al. (2010). Activation of AMPK Inhibits Cardiomyocyte Hypertrophy by Modulating of the FOXO1/MuRF1 Signaling Pathway In Vitro. Acta Pharmacol. Sin 31 (7), 798–804. doi:10.1038/aps.2010.73
Chen, C., Zou, L. X., Lin, Q. Y., Yan, X., Bi, H. L., Xie, X., et al. (2019). Resveratrol as a New Inhibitor of Immunoproteasome Prevents PTEN Degradation and Attenuates Cardiac Hypertrophy after Pressure Overload. Redox Biol. 20, 390–401. doi:10.1016/j.redox.2018.10.021
Chen, H., Untiveros, G. M., McKeeMcKee, L. A., Perez, J., Li, J., AntinAntin, P. B., et al. (2012). Micro-RNA-195 and -451 Regulate the LKB1/AMPK Signaling axis by Targeting MO25. PloS one 7 (7), e41574. doi:10.1371/journal.pone.0041574
Chen, Z. P., MitchelhillMitchelhill, K. I., MichellMichell, B. J., Stapleton, D., Rodriguez-Crespo, I., WittersWitters, L. A., et al. (1999). AMP-activated Protein Kinase Phosphorylation of Endothelial NO Synthase. FEBS Lett. 443 (3), 285–289. doi:10.1016/s0014-5793(98)01705-0
Cheng, C. K., Luo, J. Y., Lau, C. W., Chen, Z. Y., Tian, X. Y., and Huang, Y. (2020). Pharmacological Basis and New Insights of Resveratrol Action in the Cardiovascular System. Br. J. Pharmacol. 177 (6), 1258–1277. doi:10.1111/bph.14801
Cheng, P. W., Ho, W. Y., Su, Y. T., Lu, P. J., Chen, B. Z., Cheng, W. H., et al. (2014). Resveratrol Decreases Fructose-Induced Oxidative Stress, Mediated by NADPH Oxidase via an AMPK-dependent Mechanism. Br. J. Pharmacol. 171 (11), 2739–2750. doi:10.1111/bph.12648
Cheung, P. C., Salt, I. P., Davies, S. P., Hardie, D. G., and Carling, D. (2000). Characterization of AMP-Activated Protein Kinase Gamma-Subunit Isoforms and Their Role in AMP Binding. Biochem. J. 346 (3), 659–669. doi:10.1042/bj3460659
Chi, L., Peng, L., Pan, N., Hu, X., and Zhang, Y. (2014). The Anti-atherogenic Effects of Berberine on Foam Cell Formation Are Mediated through the Upregulation of Sirtuin 1. Int. J. Mol. Med. 34 (4), 1087–1093. doi:10.3892/ijmm.2014.1868
Chong, Z. Z., Wang, S., Shang, Y. C., and Maiese, K. (2012). Targeting Cardiovascular Disease with Novel SIRT1 Pathways. Future Cardiol. 8 (1), 89–100. doi:10.2217/fca.11.76
Colombo, S. L., and Moncada, S. (2009). AMPKalpha1 Regulates the Antioxidant Status of Vascular Endothelial Cells. Biochem. J. 421 (2), 163–169. doi:10.1042/BJ20090613
Costantino, S., Paneni, F., and Cosentino, F. (2016). Ageing, Metabolism and Cardiovascular Disease. J. Physiol. 594 (8), 2061–2073. doi:10.1113/JP270538
Culmsee, C., Monnig, J., Kemp, B. E., and Mattson, M. P. (2001). AMP-activated Protein Kinase Is Highly Expressed in Neurons in the Developing Rat Brain and Promotes Neuronal Survival Following Glucose Deprivation. J. Mol. Neurosci. 17 (1), 45–58. doi:10.1385/JMN:17:1:45
Dai, S. N., Hou, A. J., Zhao, S. M., Chen, X. M., Huang, H. T., Chen, B. H., et al. (2019). Ginsenoside Rb1 Ameliorates Autophagy of Hypoxia Cardiomyocytes from Neonatal Rats via AMP-Activated Protein Kinase Pathway. Chin. J. Integr. Med. 25 (7), 521–528. doi:10.1007/s11655-018-3018-y
Daniels, A., van, B. M., Janssen, B. J., Brouns, A. E., Cleutjens, J. P., Roemen, T. H., et al. (2010). Impaired Cardiac Functional reserve in Type 2 Diabetic Db/db Mice Is Associated with Metabolic, but Not Structural, Remodelling. Acta Physiol. (Oxf) 200 (1), 11–22. doi:10.1111/j.1748-1716.2010.02102.x
Dasgupta, B., and Milbrandt, J. (2007). Resveratrol Stimulates AMP Kinase Activity in Neurons. Proc. Natl. Acad. Sci. U S A. 104 (17), 7217–7222. doi:10.1073/pnas.0610068104
Daskalopoulos, E. P., Hermans, K. C. M., van Delft, L., Altara, R., and Blankesteijn, W. M. (2014). “The Role of Inflammation in Myocardial Infarction,“ in Inflammation in Heart Failure (Vol. chapter 3). Academic Press.
Dolinsky, V. W., Chakrabarti, S., Pereira, T. J., Oka, T., Levasseur, J., Beker, D., et al. (2013). Resveratrol Prevents Hypertension and Cardiac Hypertrophy in Hypertensive Rats and Mice. Biochim. Biophys. Acta 1832 (10), 1723–1733. doi:10.1016/j.bbadis.2013.05.018
Dolinsky, V. W., Chan, A. Y., Robillard Frayne, I., Light, P. E., Des Rosiers, C., and Dyck, J. R. (2009). Resveratrol Prevents the Prohypertrophic Effects of Oxidative Stress on LKB1. Circulation 119 (12), 1643–1652. doi:10.1161/CIRCULATIONAHA.108.787440
Dong, Y., Zhang, M., Liang, B., Xie, Z., Zhao, Z., Asfa, S., et al. (2010). Reduction of AMP-Activated Protein Kinase Alpha2 Increases Endoplasmic Reticulum Stress and Atherosclerosis In Vivo. Circulation 121 (6), 792–803. doi:10.1161/CIRCULATIONAHA.109.900928
Dyck, J. R., and Lopaschuk, G. D. (2006). AMPK Alterations in Cardiac Physiology and Pathology: Enemy or Ally. J. Physiol. 574 (1), 95–112. doi:10.1113/jphysiol.2006.109389
Edmunds, L. R., Sharma, L., Wang, H., Kang, A., d'Souza, S., Lu, J., et al. (2015). c-Myc and AMPK Control Cellular Energy Levels by Cooperatively Regulating Mitochondrial Structure and Function. PloS one 10 (7), e0134049. doi:10.1371/journal.pone.0134049
El Messaoudi, S., Rongen, G. A., de Boer, R. A., and Riksen, N. P. (2011). The Cardioprotective Effects of Metformin. Curr. Opin. Lipidol. 22 (6), 445–453. doi:10.1097/MOL.0b013e32834ae1a7
Esposito, G., Prasad, S. V., Rapacciuolo, A., Mao, L., Koch, W. J., and Rockman, H. A. (2001). Cardiac Overexpression of a G(q) Inhibitor Blocks Induction of Extracellular Signal-Regulated Kinase and C-Jun NH(2)-terminal Kinase Activity in In Vivo Pressure Overload. Circulation 103 (10), 1453–1458. doi:10.1161/01.cir.103.10.1453
Fang, J., Little, P. J., and Xu, S. (2018). Atheroprotective Effects and Molecular Targets of Tanshinones Derived from Herbal Medicine Danshen. Med. Res. Rev. 38 (1), 201–228. doi:10.1002/med.21438
Fassett, J. T., Hu, X., Xu, X., Lu, Z., Zhang, P., Chen, Y., et al. (2013). AMPK Attenuates Microtubule Proliferation in Cardiac Hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 304 (5), H749–H758. doi:10.1152/ajpheart.00935.2011
Feidantsis, K., Mellidis, K., Galatou, E., Sinakos, Z., and Lazou, A. (2018). Treatment with Crocin Improves Cardiac Dysfunction by Normalizing Autophagy and Inhibiting Apoptosis in STZ-Induced Diabetic Cardiomyopathy. Nutr. Metab. Cardiovasc. Dis. 28 (9), 952–961. doi:10.1016/j.numecd.2018.06.005
Feng, X., Sureda, A., Jafari, S., Memariani, Z., Tewari, D., Annunziata, G., et al. (2019). Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 9 (7), 1923–1951. doi:10.7150/thno.30787
Fimia, G. M., Kroemer, G., and Piacentini, M. (2013). Molecular Mechanisms of Selective Autophagy. Cell Death Differ 20 (1), 1–2. doi:10.1038/cdd.2012.97
Frey, N., and Olson, E. N. (2003). Cardiac Hypertrophy: the Good, the Bad, and the Ugly. Annu. Rev. Physiol. 65 (1), 45–79. doi:10.1146/annurev.physiol.65.092101.142243
Fu, Y. N., Xiao, H., Ma, X. W., Jiang, S. Y., Xu, M., and Zhang, Y. Y. (2011). Metformin Attenuates Pressure Overload-Induced Cardiac Hypertrophy via AMPK Activation. Acta Pharmacol. Sin 32 (7), 879–887. doi:10.1038/aps.2010.229
Gao, S., Li, S., Li, Q., Zhang, F., Sun, M., Wan, Z., et al. (2019). Protective Effects of Salvianolic Acid B against Hydrogen Peroxide-induced A-poptosis of H-uman U-mbilical V-ein E-ndothelial C-ells and U-nderlying M-echanisms. Int. J. Mol. Med. 44 (2), 457–468. doi:10.3892/ijmm.2019.4227
Gollob, M. H. (2003). Glycogen Storage Disease as a Unifying Mechanism of Disease in the PRKAG2 Cardiac Syndrome. Biochem. Soc. Trans. 31 (1), 228–231. doi:10.1042/bst0310228
Gollob, M. H., Seger, J. J., Gollob, T. N., Tapscott, T., Gonzales, O., Bachinski, L., et al. (2001). Novel PRKAG2 Mutation Responsible for the Genetic Syndrome of Ventricular Preexcitation and Conduction System Disease with Childhood Onset and Absence of Cardiac Hypertrophy. Circulation 104 (25), 3030–3033. doi:10.1161/hc5001.102111
Gu, X. S., Wang, Z. B., Ye, Z., Lei, J. P., Li, L., Su, D. F., et al. (2014). Resveratrol, an Activator of SIRT1, Upregulates AMPK and Improves Cardiac Function in Heart Failure. Genet. Mol. Res. 13 (1), 323–335. doi:10.4238/2014.January.17.17
Guo, S., Meng, X. W., Yang, X. S., Liu, X. F., Ou-Yang, C. H., and Liu, C. (2018). Curcumin Administration Suppresses Collagen Synthesis in the Hearts of Rats with Experimental Diabetes. Acta Pharmacol. Sin 39 (2), 195–204. doi:10.1038/aps.2017.92
Guo, S., Yao, Q., Ke, Z., Chen, H., Wu, J., and Liu, C. (2015). Resveratrol Attenuates High Glucose-Induced Oxidative Stress and Cardiomyocyte Apoptosis through AMPK. Mol. Cel Endocrinol 412, 85–94. doi:10.1016/j.mce.2015.05.034
Hamacher-Brady, A., Brady, N. R., Logue, S. E., Sayen, M. R., Jinno, M., Kirshenbaum, L. A., et al. (2007). Response to Myocardial Ischemia/reperfusion Injury Involves Bnip3 and Autophagy. Cel Death Differ 14 (1), 146–157. doi:10.1038/sj.cdd.4401936
Hang, W., He, B., Chen, J., Xia, L., Wen, B., Liang, T., et al. (2018). Berberine Ameliorates High Glucose-Induced Cardiomyocyte Injury via AMPK Signaling Activation to Stimulate Mitochondrial Biogenesis and Restore Autophagic Flux. Front. Pharmacol. 9, 1121. doi:10.3389/fphar.2018.01121
Hardie, D. G. (2014). AMPK--sensing Energy while Talking to Other Signaling Pathways. Cell Metab 20 (6), 939–952. doi:10.1016/j.cmet.2014.09.013
Hardie, D. G., Ross, F. A., and Hawley, S. A. (2012). AMPK: a Nutrient and Energy Sensor that Maintains Energy Homeostasis. Nat. Rev. Mol. Cel Biol 13 (4), 251–262. doi:10.1038/nrm3311
Hardie, D. G. (2004). The AMP-Activated Protein Kinase Pathway-Nnew Players Upstream and Downstream. J. Cel Sci 117 (Pt 23), 5479–5487. doi:10.1242/jcs.01540
Hattori, Y., Suzuki, K., Hattori, S., and Kasai, K. (2006). Metformin Inhibits Cytokine-Induced Nuclear Factor kappaB Activation via AMP-Activated Protein Kinase Activation in Vascular Endothelial Cells. Hypertension 47 (6), 1183–1188. doi:10.1161/01.HYP.0000221429.94591.72
Heidary Moghaddam, R., Samimi, Z., Moradi, S. Z., Little, P. J., Xu, S., and Farzaei, M. H. (2020). Naringenin and Naringin in Cardiovascular Disease Prevention: A Preclinical Review. Eur. J. Pharmacol. 887, 173535. doi:10.1016/j.ejphar.2020.173535
Herrero‐Martín, G., Høyer‐Hansen, M., García‐García, C., Fumarola, C., Farkas, T., López‐Rivas, A., et al. (2009). TAK1 Activates AMPK‐dependent Cytoprotective Autophagy in TRAIL‐treated Epithelial Cells. EMBO J. 28 (6), 677–685. doi:10.1038/emboj.2009.8
Horman, S., Beauloye, C., Vanoverschelde, J. L., and Bertrand, L. (2012). AMP-activated Protein Kinase in the Control of Cardiac Metabolism and Remodeling. Curr. Heart Fail. Rep. 9 (3), 164–173. doi:10.1007/s11897-012-0102-z
Huber, A. L., Papp, S. J., Chan, A. B., Henriksson, E., Jordan, S. D., Kriebs, A., et al. (2016). CRY2 and FBXL3 Cooperatively Degrade C-MYC. Mol. Cel 64 (4), 774–789. doi:10.1016/j.molcel.2016.10.012
Hue, L., Beauloye, C., Anne-Sophie, M., Marsin, A. S., Bertrand, L., Horman, S., et al. (2002). Insulin and Ischemia Stimulate Glycolysis by Acting on the Same Targets through Different and Opposing Signaling Pathways. J. Mol. Cel Cardiol 34 (9), 1091–1097. doi:10.1006/jmcc.2002.2063
Hwang, J. T., Kwon, D. Y., Park, O. J., and Kim, M. S. (2008). Resveratrol Protects ROS-Induced Cell Death by Activating AMPK in H9c2 Cardiac Muscle Cells. Genes Nutr. 2 (4), 323–326. doi:10.1007/s12263-007-0069-7
Igata, M., Motoshima, H., Tsuruzoe, K., Kojima, K., Matsumura, T., Kondo, T., et al. (2005). Adenosine Monophosphate-Activated Protein Kinase Suppresses Vascular Smooth Muscle Cell Proliferation through the Inhibition of Cell Cycle Progression. Circ. Res. 97 (8), 837–844. doi:10.1161/01.RES.0000185823.73556.06
Ishii, N., Matsumura, T., Kinoshita, H., Motoshima, H., Kojima, K., Tsutsumi, A., et al. (2009). Activation of AMP-Activated Protein Kinase Suppresses Oxidized Low-Density Lipoprotein-Induced Macrophage Proliferation. J. Biol. Chem. 284 (50), 34561–34569. doi:10.1074/jbc.M109.028043
Jia, Z., Lin, L., Huang, S., Zhu, Z., Huang, W., and Huang, Z. (2017). Inhibition of Autophagy by Berberine Enhances the Survival of H9C2 Myocytes Following Hypoxia. Mol. Med. Rep. 16 (2), 1677–1684. doi:10.3892/mmr.2017.6770
Kanamori, H., Takemura, G., Goto, K., Tsujimoto, A., Ogino, A., Takeyama, T., et al. (2013). Resveratrol Reverses Remodeling in Hearts with Large, Old Myocardial Infarctions through Enhanced Autophagy-Activating AMP Kinase Pathway. Am. J. Pathol. 182 (3), 701–713. doi:10.1016/j.ajpath.2012.11.009
Kannel, W. B., Hjortland, M., and Castelli, W. P. (1974). Role of Diabetes in Congestive Heart Failure: the Framingham Study. Am. J. Cardiol. 34 (1), 29–34. doi:10.1016/0002-9149(74)90089-7
Kaufman, R. J. (2002). Orchestrating the Unfolded Protein Response in Health and Disease. J. Clin. Invest. 110 (10), 1389–1398. doi:10.1172/JCI16886
Kewalramani, G., Puthanveetil, P., Wang, F., Kim, M. S., Deppe, S., Abrahani, A., et al. (2009). AMP-activated Protein Kinase Confers protection against TNF-{alpha}-induced Cardiac Cell Death. Cardiovasc. Res. 84 (1), 42–53. doi:10.1093/cvr/cvp166
Kim, J., Kundu, M., Viollet, B., and Guan, K. L. (2011). AMPK and mTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cel Biol 13 (2), 132–141. doi:10.1038/ncb2152
Kim, S. G., Kim, J. R., and Choi, H. C. (2018). Quercetin-Induced AMP-Activated Protein Kinase Activation Attenuates Vasoconstriction through LKB1-AMPK Signaling Pathway. J. Med. Food 21 (2), 146–153. doi:10.1089/jmf.2017.4052
Kudo, N., BarrBarr, A. J., Barr, R. L., Desai, S., and Lopaschuk, G. D. (1995). High Rates of Fatty Acid Oxidation during Reperfusion of Ischemic Hearts Are Associated with a Decrease in Malonyl-CoA Levels Due to an Increase in 5'-AMP-Activated Protein Kinase Inhibition of Acetyl-CoA Carboxylase. J. Biol. Chem. 270 (29), 17513–17520. doi:10.1074/jbc.270.29.17513
Kudo, N., Gillespie, J. G., Kung, L., Witters, L. A., Schulz, R., Clanachan, A. S., et al. (1996). Characterization of 5′ AMP-Activated Protein Kinase Activity in the Heart and its Role in Inhibiting Acetyl-CoA Carboxylase During Reperfusion Following Ischemia. Biochimica. et Biophysica. Acta (BBA)-Lipids and Lipid Metabolism 1301 (1–2), 67–75.
Kuramoto, N., Wilkins, M. E., Fairfax, B. P., Revilla-Sanchez, R., Terunuma, M., Tamaki, K., et al. (2007). Phospho-dependent Functional Modulation of GABA(B) Receptors by the Metabolic Sensor AMP-dependent Protein Kinase. Neuron 53 (2), 233–247. doi:10.1016/j.neuron.2006.12.015
Kurdi, M., Cerutti, C., Randon, J., McGregor, L., and Bricca, G. (2004). Macroarray Analysis in the Hypertrophic Left Ventricle of Renin-dependent Hypertensive Rats: Identification of Target Genes for Renin. J. Renin Angiotensin Aldosterone Syst. 5 (2), 72–78. doi:10.3317/jraas.2004.013
Kuwabara, Y., Horie, T., Baba, O., Watanabe, S., Nishiga, M., Usami, S., et al. (2015). MicroRNA-451 Exacerbates Lipotoxicity in Cardiac Myocytes and High-Fat Diet-Induced Cardiac Hypertrophy in Mice through Suppression of the LKB1/AMPK Pathway. Circ. Res. 116 (2), 279–288. doi:10.1161/CIRCRESAHA.116.304707
Lee, S., Lee, M. S., Kim, C. T., Kim, I. H., and Kim, Y. (2012). Ginsenoside Rg3 Reduces Lipid Accumulation with AMP-Activated Protein Kinase (AMPK) Activation in HepG2 Cells. Int. J. Mol. Sci. 13 (5), 5729–5739. doi:10.3390/ijms13055729
Li, H., Sureda, A., Devkota, H. P., Pittalà, V., Barreca, D., Silva, A. S., et al. (2020). Curcumin, the golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 38, 107343. doi:10.1016/j.biotechadv.2019.01.010
Li, X.-x., Zhang, P., Yang, Y., Wang, J.-j., Zheng, Y.-j., Tan, J.-l., et al. (2021). Small Molecule QF84139 Ameliorates Cardiac Hypertrophy via Activating the AMPK Signaling Pathway. Acta Pharmacol. Sin. doi:10.1038/s41401-021-00678-5
Li, Y., Cai, X., Guan, Y., Wang, L., Wang, S., Li, Y., et al. (2016). Adiponectin Upregulates MiR-133a in Cardiac Hypertrophy through AMPK Activation and Reduced ERK1/2 Phosphorylation. PloS one 11 (2), e0148482. doi:10.1371/journal.pone.0148482
Li, Y., Chen, C., Yao, F., Su, Q., Liu, D., Xue, R., et al. (2014). AMPK Inhibits Cardiac Hypertrophy by Promoting Autophagy via mTORC1. Arch. Biochem. Biophys. 558, 79–86. doi:10.1016/j.abb.2014.06.023
Li, Z., Wang, J., and Yang, X. (2015). Functions of Autophagy in Pathological Cardiac Hypertrophy. Int. J. Biol. Sci. 11 (6), 672–678. doi:10.7150/ijbs.11883
Li, Z. M., Xu, S. W., and Liu, P. Q. (2018). Salvia miltiorrhizaBurge (Danshen): a golden Herbal Medicine in Cardiovascular Therapeutics. Acta Pharmacol. Sin 39 (5), 802–824. doi:10.1038/aps.2017.193
Liang, K. W., Yin, S. C., Ting, C. T., Lin, S. J., Hsueh, C. M., Chen, C. Y., et al. (2008). Berberine Inhibits Platelet-Derived Growth Factor-Induced Growth and Migration Partly through an AMPK-dependent Pathway in Vascular Smooth Muscle Cells. Eur. J. Pharmacol. 590 (1-3), 343–354. doi:10.1016/j.ejphar.2008.06.034
Libby, P., Ridker, P. M., and HanssonHansson, G. K. (2009). Inflammation in Atherosclerosis: from Pathophysiology to Practice. J. Am. Coll. Cardiol. 54 (23), 2129–2138. doi:10.1016/j.jacc.2009.09.009
Liu, C. Y., Zhou, Y., Chen, T., Lei, J. C., and Jiang, X. J. (2020). AMPK/SIRT1 Pathway Is Involved in Arctigenin-Mediated Protective Effects against Myocardial Ischemia-Reperfusion Injury. Front. Pharmacol. 11 (2351), 616813. doi:10.3389/fphar.2020.616813
Liu, L., Liu, J., Huang, Z., Yu, X., Zhang, X., Dou, D., et al. (2015). Berberine Improves Endothelial Function by Inhibiting Endoplasmic Reticulum Stress in the Carotid Arteries of Spontaneously Hypertensive Rats. Biochem. Biophys. Res. Commun. 458 (4), 796–801. doi:10.1016/j.bbrc.2015.02.028
Lloyd, S. G., Wang, P., Zeng, H., and Chatham, J. C. (2004). Impact of Low-Flow Ischemia on Substrate Oxidation and Glycolysis in the Isolated Perfused Rat Heart. Am. J. Physiol. Heart Circ. Physiol. 287 (1), H351–H362. doi:10.1152/ajpheart.00983.2003
Lombard, D. B., and Zwaans, B. M. (2014). SIRT3: as Simple as it Seems. Gerontology 60 (1), 56–64. doi:10.1159/000354382
Lv, X., Yu, X., Wang, Y., Wang, F., Li, H., Wang, Y., et al. (2012). Berberine Inhibits Doxorubicin-Triggered Cardiomyocyte Apoptosis via Attenuating Mitochondrial Dysfunction and Increasing Bcl-2 Expression. PLoS One 7 (10), e47351. doi:10.1371/journal.pone.0047351
Mair, W., Morantte, I., Rodrigues, A. P., Manning, G., Montminy, M., Shaw, R. J., et al. (2011). Lifespan Extension Induced by AMPK and Calcineurin Is Mediated by CRTC-1 and CREB. Nature 470 (7334), 404–408. doi:10.1038/nature09706
Masci, P. G., Doulaptsis, C., Bertella, E., Del Torto, A., Symons, R., Pontone, G., et al. (2014). Incremental Prognostic Value of Myocardial Fibrosis in Patients with Non-ischemic Cardiomyopathy without Congestive Heart Failure. Circ. Heart Fail. 7 (3), 448–456. doi:10.1161/CIRCHEARTFAILURE.113.000996
Mihaylova, M. M., and Shaw, R. J. (2011). The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cel Biol 13 (9), 1016–1023. doi:10.1038/ncb2329
Mirouse, V., and Billaud, M. (2011). The LKB1/AMPK Polarity Pathway. FEBS Lett. 585 (7), 981–985. doi:10.1016/j.febslet.2010.12.025
Motobayashi, Y., Izawa-Ishizawa, Y., Ishizawa, K., Orino, S., Yamaguchi, K., Kawazoe, K., et al. (2009). Adiponectin Inhibits Insulin-like Growth Factor-1-Induced Cell Migration by the Suppression of Extracellular Signal-Regulated Kinase 1/2 Activation, but Not Akt in Vascular Smooth Muscle Cells. Hypertens. Res. 32 (3), 188–193. doi:10.1038/hr.2008.19
Nakai, A., Yamaguchi, O., Takeda, T., Higuchi, Y., Hikoso, S., Taniike, M., et al. (2007). The Role of Autophagy in Cardiomyocytes in the Basal State and in Response to Hemodynamic Stress. Nat. Med. 13 (5), 619–624. doi:10.1038/nm1574
Nascimben, L., IngwallIngwall, J. S., LorellLorell, B. H., Pinz, I., Schultz, V., Tornheim, K., et al. (2004). Mechanisms for Increased Glycolysis in the Hypertrophied Rat Heart. Hypertension 44 (5), 662–667. doi:10.1161/01.HYP.0000144292.69599.0c
Nellaiappan, K., Yerra, V. G., and Kumar, A. (2019). Role of AMPK in Diabetic Cardiovascular Complications: An Overview. Cardiovasc. Hematol. Disord. Drug Targets 19 (1), 5–13. doi:10.2174/1871529X18666180508104929
Nguyen, T. M., Combarnous, Y., Praud, C., Duittoz, A., and Blesbois, E. (2016). Ca2+/Calmodulin-Dependent Protein Kinase Kinases (CaMKKs) Effects on AMP-Activated Protein Kinase (AMPK) Regulation of Chicken Sperm Functions. PLoS One 11 (1), e0147559. doi:10.1371/journal.pone.0147559
Noppe, G., Dufeys, C., Buchlin, P., Marquet, N., Castanares-Zapatero, D., Balteau, M., et al. (2014). Reduced Scar Maturation and Contractility lead to Exaggerated Left Ventricular Dilation after Myocardial Infarction in Mice Lacking AMPKα1. J. Mol. Cel Cardiol 74, 32–43. doi:10.1016/j.yjmcc.2014.04.018
Ouchi, N., Kobayashi, H., Kihara, S., Kumada, M., Sato, K., Inoue, T., et al. (2004). Adiponectin Stimulates Angiogenesis by Promoting Cross-Talk between AMP-Activated Protein Kinase and Akt Signaling in Endothelial Cells. J. Biol. Chem. 279 (2), 1304–1309. doi:10.1074/jbc.M310389200
Pan, C., Lou, L., Huo, Y., Singh, G., Chen, M., Zhang, D., et al. (2011). Salvianolic Acid B and Tanshinone IIA Attenuate Myocardial Ischemia Injury in Mice by NO Production through Multiple Pathways. Ther. Adv. Cardiovasc. Dis. 5 (2), 99–111. doi:10.1177/1753944710396538
Papinski, D., and Kraft, C. (2016). Regulation of Autophagy by Signaling through the Atg1/ULK1 Complex. J. Mol. Biol. 428 (9 Pt A), 1725–1741. doi:10.1016/j.jmb.2016.03.030
Pei, C., Zhang, Y., Wang, P., Zhang, B., Fang, L., Liu, B., et al. (2019). Berberine Alleviates Oxidized Low-Density Lipoprotein-Induced Macrophage Activation by Downregulating Galectin-3 via the NF-Κb and AMPK Signaling Pathways. Phytother Res. 33 (2), 294–308. doi:10.1002/ptr.6217
Peng, W., Zhang, Y., Zhu, W., Cao, C-M., and Xiao, R-P. (2009). AMPK and TNF-α at the Crossroad of Cell Survival and Death in Ischaemic Heart. Cardiovasc Res. 84 (1), 1–3. doi:10.1093/cvr/cvp272
Pu, Y., Zhang, H., Wang, P., Zhao, Y., Li, Q., Wei, X., et al. (2013). Dietary Curcumin Ameliorates Aging-Related Cerebrovascular Dysfunction through the AMPK/uncoupling Protein 2 Pathway. Cell Physiol Biochem 32 (5), 1167–1177. doi:10.1159/000354516
Qi, D., and Young, L. H. (2015). AMPK: Energy Sensor and Survival Mechanism in the Ischemic Heart. Trends Endocrinol. Metab. 26 (8), 422–429. doi:10.1016/j.tem.2015.05.010
Qin, Q., Lin, N., Huang, H., Zhang, X., Cao, X., Wang, Y., et al. (2019). Ginsenoside Rg1 Ameliorates Cardiac Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Diabetes Metab. Syndr. Obes. 12, 1091–1103. doi:10.2147/DMSO.S208989
Qing, Y., Dong, X., Hongli, L., and Yanhui, L. (2018). Berberine Promoted Myocardial protection of Postoperative Patients through Regulating Myocardial Autophagy. Biomed. Pharmacother. 105, 1050–1053. doi:10.1016/j.biopha.2018.06.088
Reggiori, F., and Klionsky, D. J. (2013). Autophagic Processes in Yeast: Mechanism, Machinery and Regulation. Genetics 194 (2), 341–361. doi:10.1534/genetics.112.149013
Richter, K., and Kietzmann, T. (2016). Reactive Oxygen Species and Fibrosis: Further Evidence of a Significant Liaison. Cell Tissue Res 365 (3), 591–605. doi:10.1007/s00441-016-2445-3
Rodríguez, A., Catalán, V., Becerril, S., Gil, M. J., Mugueta, C., Gómez-Ambrosi, J., et al. (2008). Impaired Adiponectin-AMPK Signalling in Insulin-Sensitive Tissues of Hypertensive Rats. Life Sci. 83 (15-16), 540–549. doi:10.1016/j.lfs.2008.07.022
Rösen, P., Adrian, M., Feuerstein, J., and Reinauer, H. (1984). Glycolysis and Glucose Oxidation in the Rat Heart under Nonrecirculating Perfusion Conditions. Basic Res. Cardiol. 79 (3), 307–312. doi:10.1007/BF01908031
Rosner, M. H., Brady, W. J., Kefer, M. P., and Martin, M. L. (1999). Electrocardiography in the Patient with the Wolff-Parkinson-White Syndrome: Diagnostic and Initial Therapeutic Issues. Am. J. Emerg. Med. 17 (7), 705–714. doi:10.1016/s0735-6757(99)90167-5
Rothermel, B. A., and Hill, J. A. (2007). Myocyte Autophagy in Heart Disease: Friend or Foe. Autophagy 3 (6), 632–634. doi:10.4161/auto.4913
Russell, Raymond. R., Bergeron, R., Shulman, G. I., and Young, L. H. (1999). Translocation of Myocardial GLUT-4 and Increased Glucose Uptake through Activation of AMPK by AICAR. Am. J. Physiol. 277 (2), H643–H649. doi:10.1152/ajpheart.1999.277.2.H643
Saenz, J., Santa-María, C., Reyes-Quiroz, M. E., Geniz, I., Jiménez, J., Sobrino, F., et al. (2018). Grapefruit Flavonoid Naringenin Regulates the Expression of LXRα in THP-1 Macrophages by Modulating AMP-Activated Protein Kinase. Mol. Pharm. 15 (5), 1735–1745. doi:10.1021/acs.molpharmaceut.7b00797
Salt, I. P., and Palmer, T. M. (2012). Exploiting the Anti-inflammatory Effects of AMP-Activated Protein Kinase Activation. Expert Opin. Investig. Drugs 21 (8), 1155–1167. doi:10.1517/13543784.2012.696609
Schneider, H., Schubert, K. M., Blodow, S., Kreutz, C. P., Erdogmus, S., Wiedenmann, M., et al. (2015). AMPK Dilates Resistance Arteries via Activation of SERCA and BKCa Channels in Smooth Muscle. Hypertension 66 (1), 108–116. doi:10.1161/HYPERTENSIONAHA.115.05514
Seddon, M., LooiLooi, Y. H., and Shah, A. M. (2007). Oxidative Stress and Redox Signalling in Cardiac Hypertrophy and Heart Failure. Heart 93 (8), 903–907. doi:10.1136/hrt.2005.068270
Shen, Y., Croft, K. D., Hodgson, J. M., Kyle, R., Lee, I. L., Wang, Y., et al. (2012). Quercetin and its Metabolites Improve Vessel Function by Inducing eNOS Activity via Phosphorylation of AMPK. Biochem. Pharmacol. 84 (8), 1036–1044. doi:10.1016/j.bcp.2012.07.016
Shibata, R., Ouchi, N., Ito, M., Kihara, S., Shiojima, I., Pimentel, D. R., et al. (2004). Adiponectin-mediated Modulation of Hypertrophic Signals in the Heart. Nat. Med. 10 (12), 1384–1389. doi:10.1038/nm1137
Singh, L., Sharma, S., Xu, S., Tewari, D., and Fang, J. (2021). Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 26 (13), 4036. doi:10.3390/molecules26134036
Soraya, H., Khorrami, A., Garjani, A., Maleki-Dizaji, N., and Garjani, A. (2012). Acute Treatment with Metformin Improves Cardiac Function Following Isoproterenol Induced Myocardial Infarction in Rats. Pharmacol. Rep. 64 (6), 1476–1484. doi:10.1016/s1734-1140(12)70945-3
Soraya, H., Rameshrad, M., Mokarizadeh, A., and Garjani, A. (2015). Metformin Attenuates Myocardial Remodeling and Neutrophil Recruitment after Myocardial Infarction in Rat. Bioimpacts 5 (1), 3–8. doi:10.15171/bi.2015.02
Steinberg, G. R., and Kemp, B. (2009). amPK in Health and Disease. Physiol. Rev. 89, 1025–1078. doi:10.1152/physrev.00011.2008
Stöckli, J., Meoli, C. C., Hoffman, N. J., Fazakerley, D. J., Pant, H., Cleasby, M. E., et al. (2015). The RabGAP TBC1D1 Plays a central Role in Exercise-Regulated Glucose Metabolism in Skeletal Muscle. Diabetes 64 (6), 1914–1922. doi:10.2337/db13-1489
Sun, G. Z., Meng, F. J., Cai, H. Q., Diao, X. B., Zhang, B., and Bai, X. P. (2020). Ginsenoside Rg3 Protects Heart against Isoproterenol-Induced Myocardial Infarction by Activating AMPK Mediated Autophagy. Cardiovasc. Diagn. Ther. 10 (2), 153–160. doi:10.21037/cdt.2020.01.02
Tamargo-Gómez, I., and Mariño, G. (2018). AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int. J. Mol. Sci. 19 (12), 3812. doi:10.3390/ijms19123812
Thandapilly, S. J., Louis, X. L., Yang, T., Stringer, D. M., Yu, L., Zhang, S., et al. (2011). Resveratrol Prevents Norepinephrine Induced Hypertrophy in Adult Rat Cardiomyocytes, by Activating NO-AMPK Pathway. Eur. J. Pharmacol. 668 (1-2), 217–224. doi:10.1016/j.ejphar.2011.06.042
Travers, J. G., Kamal, F. A., Robbins, J., Yutzey, K. E., and Blaxall, B. C. (2016). Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 118, 1021–1040. doi:10.1161/CIRCRESAHA.115.306565
Wang, J., Ma, A., Zhao, M., and Zhu, H. (2017). AMPK Activation Reduces the Number of Atheromata Macrophages in ApoE Deficient Mice. Atherosclerosis 258, 97–107. doi:10.1016/j.atherosclerosis.2017.01.036
Wang, Q., Liu, S., Zhai, A., Zhang, B., and Tian, G. (2018). AMPK-mediated Regulation of Lipid Metabolism by Phosphorylation. Biol. Pharm. Bull. 41 (7), 985–993. doi:10.1248/bpb.b17-00724
Wang, Q., Zhang, M., Liang, B., Shirwany, N., Zhu, Y., and Zou, M. H. (2011). Activation of AMP-Activated Protein Kinase Is Required for Berberine-Induced Reduction of Atherosclerosis in Mice: the Role of Uncoupling Protein 2. PLoS One 6 (9), e25436. doi:10.1371/journal.pone.0025436
Wang, X. X., Wang, X. L., Tong, M. M., Gan, L., Chen, H., Wu, S. S., et al. (2016). SIRT6 Protects Cardiomyocytes against Ischemia/reperfusion Injury by Augmenting FoxO3α-dependent Antioxidant Defense Mechanisms. Basic Res. Cardiol. 111 (2), 13. doi:10.1007/s00395-016-0531-z
Weber, K. T., Pick, R., Jalil, J. E., Janicki, J. S., Carroll, E. P., and Carroll, Eugenia. P. (1989). Patterns of Myocardial Fibrosis. J. Mol. Cel Cardiol 21 (Suppl. 5), 121–131. doi:10.1016/0022-2828(89)90778-5
Weber, K. T., Sun, Y., BhattacharyaBhattacharya, S. K., Ahokas, R. A., and Gerling, I. C. (2013). Myofibroblast-mediated Mechanisms of Pathological Remodelling of the Heart. Nat. Rev. Cardiol. 10 (1), 15–26. doi:10.1038/nrcardio.2012.158
WHO (2021). Cardiovascular Diseases (CVDs). [online]. Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
Xie, Z., Lau, K., Eby, B., Lozano, P., He, C., Pennington, B., et al. (2011). Improvement of Cardiac Functions by Chronic Metformin Treatment Is Associated with Enhanced Cardiac Autophagy in Diabetic OVE26 Mice. Diabetes 60 (6), 1770–1778. doi:10.2337/db10-0351
Xu, Q., Hao, X., Yang, Q., and Si, L. (2009). Resveratrol Prevents Hyperglycemia-Induced Endothelial Dysfunction via Activation of Adenosine Monophosphate-Activated Protein Kinase. Biochem. Biophys. Res. Commun. 388 (2), 389–394. doi:10.1016/j.bbrc.2009.08.021
Xu, Q., and Si, L.-Y. (2010). Protective Effects of AMP-Activated Protein Kinase in the Cardiovascular System. J. Cell Mol. Med. 14 (11), 2604–2613.
Xu, S., Ilyas, I., Little, P. J., Li, H., Kamato, D., Zheng, X., et al. (2021). Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 73, 924–967. doi:10.1124/pharmrev.120.000096
Xu, X., So, J. S., Park, J. G., and Lee, A. H. (2013). Transcriptional Control of Hepatic Lipid Metabolism by SREBP and ChREBP. Semin. Liver Dis. 33 (4), 301–311. doi:10.1055/s-0033-1358523
Yan, L., Vatner, D. E., Kim, S. J., Ge, H., Masurekar, M., Massover, W. H., et al. (2005). Autophagy in Chronically Ischemic Myocardium. Proc. Natl. Acad. Sci. U S A. 102 (39), 13807–13812. doi:10.1073/pnas.0506843102
Yang, B., Zheng, C., Yu, H., Zhang, R., Zhao, C., and Cai, S. (2019). Cardio-protective Effects of Salvianolic Acid B on Oxygen and Glucose Deprivation (OGD)-treated H9c2 Cells. Artif. Cell Nanomed Biotechnol 47 (1), 2274–2281. doi:10.1080/21691401.2019.1621885
Yang, H., Zhu, L., Gu, Y., Kong, X., Yan Liu, Y., Chen, M., et al. (2019). Berberine Inhibits Low Shear Stress-Induced Glycocalyx Degradation via Modulating AMPK and p47phox/Hyal2 Signal Pathway. Eur. J. Pharmacol. 856, 172413. doi:10.1016/j.ejphar.2019.172413
Yang, P., Ling, L., Sun, W., Yang, J., Zhang, L., Chang, G., et al. (2018). Ginsenoside Rg1 Inhibits Apoptosis by Increasing Autophagy via the AMPK/mTOR Signaling in Serum Deprivation Macrophages. Acta Biochim. Biophys. Sin (Shanghai) 50 (2), 144–155. doi:10.1093/abbs/gmx136
Yang, Q., Wang, H. C., Liu, Y., Gao, C., Sun, L., and Tao, L. (2016). Resveratrol Cardioprotection against Myocardial Ischemia/Reperfusion Injury Involves Upregulation of Adiponectin Levels and Multimerization in Type 2 Diabetic Mice. J. Cardiovasc. Pharmacol. 68 (4), 304–312. doi:10.1097/FJC.0000000000000417
Yao, Q., Ke, Z. Q., Guo, S., Yang, X. S., Zhang, F. X., Liu, X. F., et al. (2018). Curcumin Protects against Diabetic Cardiomyopathy by Promoting Autophagy and Alleviating Apoptosis. J. Mol. Cel Cardiol 124, 26–34. doi:10.1016/j.yjmcc.2018.10.004
Yu, L. M., Dong, X., Xue, X. D., Zhang, J., Li, Z., Wu, H. J., et al. (2019). Naringenin Improves Mitochondrial Function and Reduces Cardiac Damage Following Ischemia-Reperfusion Injury: the Role of the AMPK-SIRT3 Signaling Pathway. Food Funct. 10 (5), 2752–2765. doi:10.1039/c9fo00001a
Yu, Y., Sun, J., Liu, J., Wang, P., and Wang, C. (2020). Ginsenoside Re Preserves Cardiac Function and Ameliorates Left Ventricular Remodeling in a Rat Model of Myocardial Infarction. J. Cardiovasc. Pharmacol. 75 (1), 91–97. doi:10.1097/FJC.0000000000000752
Yun, H., Lee, M., Kim, S. S., and Ha, J. (2005). Glucose Deprivation Increases mRNA Stability of Vascular Endothelial Growth Factor through Activation of AMP-Activated Protein Kinase in DU145 Prostate Carcinoma. J. Biol. Chem. 280 (11), 9963–9972. doi:10.1074/jbc.M412994200
Zarrinpashneh, E., Beauloye, C., Ginion, A., Anne-Catherine, P., Pouleur, A. C., Havaux, X., et al. (2008). AMPKalpha2 Counteracts the Development of Cardiac Hypertrophy Induced by Isoproterenol. Biochem. Biophys. Res. Commun. 376 (4), 677–681. doi:10.1016/j.bbrc.2008.09.057
Zeng, C., Li, H., Fan, Z., Zhong, L., Guo, Z., Guo, Y., et al. (2016). Crocin-Elicited Autophagy Rescues Myocardial Ischemia/Reperfusion Injury via Paradoxical Mechanisms. Am. J. Chin. Med. 44 (3), 515–530. doi:10.1142/S0192415X16500282
Zeng, Z., Pan, Y., Wu, W., Li, L., Wu, Z., Zhang, Y., et al. (2019). Myocardial Hypertrophy Is Improved with Berberine Treatment via Long Non-coding RNA MIAT-Mediated Autophagy. J. Pharm. Pharmacol. 71 (12), 1822–1831. doi:10.1111/jphp.13170
Zhang, J., Dong, J., Martin, M., He, M., Gongol, B., Marin, T. L., et al. (2018). AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 198 (4), 509–520. doi:10.1164/rccm.201712-2570OC
Zhang, J., Wang, Y., Bao, C., Liu, T., Li, S., Huang, J., et al. (2019). Curcumin-loaded PEG-PDLLA N-anoparticles for A-ttenuating P-almitate-induced O-xidative S-tress and C-ardiomyocyte A-poptosis through AMPK P-athway. Int. J. Mol. Med. 44 (2), 672–682. doi:10.3892/ijmm.2019.4228
Zhang, M., Wang, C. M., Li, J., Meng, Z. J., Wei, S. N., Li, J., et al. (2013). Berberine Protects against Palmitate-Induced Endothelial Dysfunction: Involvements of Upregulation of AMPK and eNOS and Downregulation of NOX4. Mediators Inflamm. 2013, 260464. doi:10.1155/2013/260464
Zhang, P., Hu, X., Xu, X., Fassett, J., Zhu, G., Viollet, B., et al. (2008). AMP Activated Protein Kinase-Alpha2 Deficiency Exacerbates Pressure-Overload-Induced Left Ventricular Hypertrophy and Dysfunction in Mice. Hypertension 52 (5), 918–924. doi:10.1161/HYPERTENSIONAHA.108.114702
Zhang, X., Zhang, Z., Wang, P., Han, Y., Liu, L., Li, J., et al. (2021). Bawei Chenxiang Wan Ameliorates Cardiac Hypertrophy by Activating AMPK/PPAR-α Signaling Pathway Improving Energy Metabolism. Front. Pharmacol. 12 (1314), 653901. doi:10.3389/fphar.2021.653901
Zhang, Y. L., Guo, H., Zhang, C. S., Lin, S. Y., Yin, Z., Peng, Y., et al. (2013). AMP as a Low-Energy Charge Signal Autonomously Initiates Assembly of AXIN-AMPK-LKB1 Complex for AMPK Activation. Cel Metab 18 (4), 546–555. doi:10.1016/j.cmet.2013.09.005
Zheng, Z., Wang, M., Cheng, C., Liu, D., Wu, L., Zhu, J., et al. (2020). Ginsenoside Rb1 Reduces H2O2-induced HUVEC D-ysfunction by S-timulating the sirtuin-1/AMPactivated Protein Kinase Pathway. Mol. Med. Rep. 22 (1), 247–256. doi:10.3892/mmr.2020.11096
Keywords: atherosclerosis, cardiovscular disease, AMPK, natural products, drug discovery
Citation: Heidary Moghaddam R, Samimi Z, Asgary S, Mohammadi P, Hozeifi S, Hoseinzadeh‐Chahkandak F, Xu S and Farzaei MH (2022) Natural AMPK Activators in Cardiovascular Disease Prevention. Front. Pharmacol. 12:738420. doi: 10.3389/fphar.2021.738420
Received: 12 July 2021; Accepted: 03 November 2021;
Published: 03 January 2022.
Edited by:
Tim David Hewitson, Royal Melbourne Hospital, AustraliaReviewed by:
John Peter Konhilas, University of Arizona, United StatesCopyright © 2022 Heidary Moghaddam, Samimi, Asgary, Mohammadi, Hozeifi, Hoseinzadeh‐Chahkandak, Xu and Farzaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suowen Xu, c3h1MTk4NEB1c3RjLmVkdS5jbg==; Mohammad Hosein Farzaei, bWguZmFyemFlaUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.