- 1Department of Endocrinology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Clinical department of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Food is people’s primal want. A reasonable diet and healthy food not only provide nutrients for human growth but also contribute to disease prevention and treatment, while following an unhealthy diet can lead to an increased risk of many diseases, especially metabolic disorders, such as diabetes. Nature is enriched with different food sources, and it seems that purely natural products are more in line with the current concept of health, which enhance the formation of the notion that “Food/Diet Supplements from Natural Sources as a Medicine.” As a delicious fruit, the medicinal values such as anticancer, antibacterial, antioxidation, and antiglycating properties of lychee have been found. Lychee (Litchi in Chinese) is a subtropical fruit plant belonging to the family Sapindaceae. It has been widely cultivated in warm climates worldwide, particularly in China, for thousands of years. In recent years, various phytochemical components such as quercetin, procyanidin A2, and (2R)-naringenin-7-O-(3-O-αL-rhamnopyranosyl-β-D-glucopyranoside) have been identified in a lychee seed, which may lend a lychee seed as a relatively safe and inexpensive adjuvant treatment for diabetes and diabetic complications. In fact, accumulating evidence has shown that lychee seed, lychee seed extracts, and related compounds have promising antihyperglycemic activities, including improving insulin resistance, anti-inflammatory effect, lipid regulation, neuroprotection, antineurotoxic effect, and renoprotection effect. In this review, we summarized publications on antiglycemic effects and mechanisms of lychee seed, lychee seed extracts, and related compounds, which included their efficacies as a cure for diabetes and diabetic complications in cells, animals, and humans, attempting to obtain a robust evidence basis for the clinical application and value of lychee seed.
Introduction
Diabetes is a severe, long-term (or chronic) disease in the world, defined as a blood glucose profile higher than normal, due to a disturbed insulin secretion or a disturbed insulin effect or usually both (Petersmann et al., 2019). Based on the most recent data issued by the International Diabetes Federation (IDF) (Saeedi et al., 2019), the number of adults aged 20–79 years globally with diabetes has reached nearly 463.0 million in 2019. It is estimated that the number will rise to 578.4 million by 2030, and 700.2 million by 2045, which means that the global diabetes epidemic markedly increases at an incredible speed among populations. Obviously, it has become a significant global public health problem. Individuals with diabetes are more prone to develop complications such as retinopathy, nephropathy, coronary artery disease, peripheral arterial disease, and stroke, contributing to higher mortality rates (Ford, 2011; Tandon et al., 2012). Thus, the prevention and treatment of diabetes hold considerable importance. Currently, the main treatments for diabetes include insulin injection, oral diabetes medications, and pancreatic islet transplantation (Ryan et al., 2005; Doyle-Delgado et al., 2020). However, the available treatments only delay the progress of the disease rather than curing it, leading to the lengthy and costly therapy, and comprise side effects, which impart a heavy economic and psychological burden on patients.
Food is the first necessity of people. Poor diet is associated with a higher risk of many diseases (GBD 2017 Diet Collaborators, 2019), especially diabetes (Srour et al., 2020), while some healthy food is reported to improve glycemic control (Reynolds et al., 2020). Studies supported a positive association between dietary intake of momordica charantia and blood sugar reduction (Kibiti and Afolayan, 2015). Buckwheat also had effects on reducing serum glucose concentrations in diabetic rats (Kawa et al., 2003). Consequently, the notion that “Food/Diet Supplements from Natural Sources as a Medicine” has become popular and appealing among diabetic patients. In China, herbs in nature with homology of medicine and food have been widely studied. Lychee, a fruit tree belonging to family Sapindaceae, originating from China, is widely cultivated in warm climates in many regions around the world and is botanically related to Litchi chinensis Sonn (Jiang, 2003; Yang et al., 2006; Huang et al., 2014). Due to the high nutrients and savory flavor as well as the attractive red, lychee is widely favored by humans. Lychee seed, the dried mature seed of lychee, an ancient traditional drug–food homologous herbal medicine, was used to smooth Qi, dispel cold, alleviate polydipsia, and relieve pain in China (Kilari and Putta, 2016). Accumulating research studies recently focused on the antidiabetic activity of lychee seed, although the underlying mechanisms of action have not been studied thoroughly. A summary of antidiabetic studies of lychee seed will be helpful for offering a reference basis for deeper investigations and clinical use of this natural herb medicine (Table 1). We examined the electronic resources with the PubMed, EMBASE, Web of Science, and China National Knowledge Infrastructure based on the information limited to English and Chinese literatures up till Jun 2021.

TABLE 1. The antihyperglycemic activity and the mechanisms of lychee seed in clinical trials, in vitro, and in vivo studies.
Botanical Descriptions of Lychee
Lychee (Litchi chinensis Sonn.) is a subtropical medium evergreen dome-shaped tree with a glossy grayish stem belonging to the family Sapindaceae. It generally grows to less than 10 m in height, rarely up to 15 m or more. The pinnate leaves which consist of 4–8 pairs of elliptic or lanceolate, long acuminate, glabrous leaflets are leathery, 5–7 cm long, and 2–4 cm broad. The yellowish-white flower is small in size with a tetramerous calyx. Terminal inflorescence is about 5–30 cm long with multibranched panicles and slender pedicels. The fruit is ellipsoidal or nearly round in shape, estimated at 2.5 cm in diameter and clothed with a coarse thicker ring or pericarp with strawberry to red in color at maturity. Inside the pericarp is lychee aril that is milky-white and semitransparent with a sweet, juicy, and delicious taste. A seed with a smooth and glossy surface is brown or reddish brown in color and elliptic to ovate in shape covered by a fleshy aril. The size of the seed varies greatly between 1 and 2 cm in length, as shown in Figure 1.

FIGURE 1. Lychee fruit, lychee aril, and lychee seed (color version of these figures is available at https://699pic.com/tupian-500450779.html?bindPhone=1, https://item.m.jd.com/product/53077629044.html?).
The History of Lychee Seed
Lychee seed, the dry mature seed of Litchi chinensis Sonn, is known to have a remarkable medicinal value in ancient China. The oldest available Chinese written source which described the application of lychee seed is Ben Cao Yan Yi traced back to the Song dynasty (AD 1116). In ancient Chinese medical practices, lychee seed was always used for hernia, orchitis, ulcers, and intestinal troubles. Diabetes-related symptoms were known as “Xiaoke” (emaciation and thirst) in the ancient Chinese medical literature (Tong et al., 2012). In Compendium of Materia Medica (Ben Cao Gang Mu) written by Li Shizhen (from 1518 to 1593 AD) during the Ming dynasty, the lychee seed was warm in nature, sweet in taste, and could act as a beneficial agent in thirst-quenching. Numerous Chinese patent medicines such as Jinlida granule (Tian et al., 2018) and Jiangtangtongmai tablets (Su et al., 2017) approved by the Chinese Food and Drug Administration, containing lychee seed, were clinically used for the treatment of diabetes.
Potential Bioactive Compounds of Lychee Seed Responsible for Hypoglycemic Activities
Lychee seed is thought to improve glycemic control via various bioactive compounds with great pharmaceutical and biomedical potential. The flavanones, flavonols, proanthocyanidins, and dihydrochalcone fractions of lychee seed are the most investigated for their hypoglycemic activities. With the continuous optimization of the lychee seed extraction process, a number of monomers have been successfully identified and isolated. Here, several single isolated compounds including (2R)-naringenin-7-O-(3-O-αL-rhamnopyranosyl-β-d-glucopyranoside) (Ren et al., 2011), (2S)-Pinocembrin-7-O-(6-O-a-l-rhamnopyranosyl-β-d-glucopyranoside) (Ren et al., 2011), quercetin (Ren et al., 2013), procyanidin A1 (Xiong et al., 2020), procyanidin A2 (Choi et al., 2017), and phlorhizin (Ren et al., 2013) exhibiting potential beneficial effects on regulating glycemia are prominently described in Table 2.
Pharmacology
Improving Insulin Resistance
Insulin has a pivotal function in ensuring the homeostasis of energy metabolism through a coordination of the storage and utilization of fuel molecules in insulin-targeted organs (Castan-Laurell et al., 2012). Insulin resistance (IR) is a pathological condition defined by the inability of insulin to stimulate glucose disposal and is considered as a key player in the development of type 2 diabetes mellitus (Brown and Walker, 2016). Although the precise pathophysiology of IR in diabetes has not yet been delineated, inflammatory response, oxidative stress, insulin receptor mutations, endoplasmic reticulum stress, and mitochondrial dysfunction are currently regarded as the possible underlying mechanisms (Yaribeygi et al., 2019). Consequently, numerous genes such as INS, AKT1, IL-6, TP53, TNF, VEGFA, MAPK3, EGFR, EGF, and SRC have been revealed to be associated with the development of IR (Gao et al., 2020). The relatively prominent signaling pathways involved in the formation of IR are the pathways of insulin resistance, adipocytokine, insulin, PI3K-Akt, ERK, AMPK, and HIF-1 (Ozaki et al., 2016; Huang et al., 2018; Gao et al., 2020). In the glucose tolerance test, intragastric administration of a lychee seed water extractant remarkably decreased hyperinsulinemia and potentiated insulin sensitivity (Guo et al., 2003a). Another study indicated that lychee seed extracts could increase the quality of life of streptozotocin (STZ) combined with a high-fat diet–induced type 2 diabetes rats. Compared to the control group, the insulin resistance index in the lychee seed extract group was dramatically reduced, which in turn increased the insulin sensitivity index progressively (Man et al., 2016). The PI3K/AKT/mTOR signaling pathway makes essential contribution to the occurrence of IR. Activation of the PI3K/AKT/mTOR signaling pathway could improve insulin-induced glucose (Yin et al., 2017; Han et al., 2020). Lychee seed extracts significantly improved IR in a type 2 diabetes mouse model by elevating the expression levels of PI3K, AKT, and mTOR to trigger the PI3K/AKT/mTOR signaling pathway (Man et al., 2017). Recently, growing evidence has shown that microRNAs as crucial regulators of gene expression perform a critical role in the development of IR (Honardoost et al., 2014; Wen et al., 2014; Xihua et al., 2019). One study showed that the microRNA expression changed significantly in db/db mouse administered extract of lychee seed (0.015 g/d, i.g.) (Zhang et al., 2013). In addition, abundant studies have demonstrated that endoplasmic reticulum stress–induced pancreatic β-cell destruction is one of the vital mechanisms of progression for both insulin-dependent diabetes and non–insulin-dependent diabetes (Cnop et al., 2017). Endoplasmic reticulum stress can not only directly damage the insulin signaling pathway but also further promote IR in a variety of ways (Ozcan and Tabas, 2012; Dong et al., 2017). Experiments in vitro have confirmed that lychee semen effective constituents can significantly reduce the mRNA expression of glucose regulatory protein 78 (Grp78) (Liao et al., 2014; Li et al., 2015) which contributes to endoplasmic reticulum stress and the activation of unfolded protein response (UPR). Elevated pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) and leptin levels have been demonstrated to be closely associated with IR (Ayina et al., 2016; Alzamil, 2020). In addition, plasma free fatty acid (FFA) is viewed as a potential factor to IR and disrupts insulin secretion (Bergman and Ader, 2000). Lychee seed extracts could improve insulin sensitivity by reducing the levels of TNF-α, hyper-leptinemia, and FFA in diabetic rats (Guo et al., 2004).
Antioxidant Effect
Oxidative stress is induced by an imbalance between the production of free radicals and the antioxidant mechanisms, which is a well-known contributor to the pathogenesis and progression of diabetes via several molecular mechanisms, such as β-cell dysfunction and defects of the normal insulin signaling pathways (Yaribeygi et al., 2020). In addition, the excessive production of reactive oxygen species (ROS) inside the cell occupies a pivotal role in the onset of oxidative stress (Zhang et al., 2016). The body produces excess ROS, which is known to enhance nuclear factor (NF)-κB activity (Zheng et al., 2015), β-cell maturation, and apoptosis increase. In a 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, the total flavonoids of lychee seed showed a potent antioxidant activity (Ren et al., 2017). Moreover, lychee seed extracts could significantly accelerate the clearance of O2− in the cerebrum of mice with diabetes induced by alloxan (Li et al., 2006). Malondialdehyde (MDA), an oxidative stress marker, is produced when ROS within cells oxidize unsaturated fatty acids (Kwiecien et al., 2014). Several animal studies revealed that lychee seed extracts remarkably improved the activity of superoxide dismutases (SODs) which was the central antioxidant defense system against O2− (Fukai and Ushio-Fukai, 2011) and decreased MDA in animal models of diabetic rats (Pan et al., 1999; Guo et al., 2003b; Guo et al., 2004).
Anti-Inflammatory Effect
The relationship between inflammation and diabetes has received extensive attention. It is believed that diabetes is a chronic inflammatory state (Wellen and Hotamisligil, 2005). Indeed, recent studies have emphasized and found substantial evidence that many inflammatory cytokines such as transforming growth factor beta 1 (TGF-β1) (Herder et al., 1984-2002; Olivieri et al., 2010; Shi et al., 2018), monocyte chemotactic peptide 1 (MCP-1) (Reddy et al., 2017), and macrophage migration-inhibitory factor (MIF) (Sánchez-Zamora and Rodriguez-Sosa, 2014; Abu El-Asrar et al., 2019) are reported to be responsible for the pathogenesis of the development of diabetes or diabetes complications. The currently available medical therapy mainly targets the underlying etiology. Hence, inhibition of excessive inflammatory responses might provide a potentially promising candidate for future therapeutics of diabetes. Lychee seed extracts could alleviate the inflammation reaction in rats with impaired glucose tolerance, which was associated with the downregulation expression of TGF-β1, MCP-1, and MIF (Qi, 2017). NF-κB is central to inflammatory responses and is tightly linked to various inflammatory diseases. Lychee seed extracts directly affected the mRNA levels of NF-κB, which prevented diabetes (Man et al., 2016). An experiment in diabetic nephropathy mice models has revealed that the saponin of lychee seed could delay the diabetic kidney inflammation development through inhibiting the expression of MCP-1 and intercellular cell adhesion molecule-1 (ICAM-1) protein in the kidney tissue, reducing the content of pro-inflammatory cytokines including interleukin-1β (IL-1β) and interleukin-6 (IL-6) in the serum (Qin, 2017).
Lipid Regulation
Glucose and lipid metabolism are intrinsically related to one another in many aspects. Diabetic dyslipidemia is common in individuals with diabetes (Athyros et al., 2018). The pathophysiological mechanism of diabetic dyslipidemia is highly complex and multifactorial, yet accepted as a preponderant contributor in the occurrence of diabetic dyslipidemia is IR with an attendant increase in free fatty acid flux into the liver (Mooradian, 2009). The most predominant clinical presentation of the interaction is marked by elevated triglycerides (TGs), decreased high-density lipoprotein cholesterol (HDL-C), and predominance of small-dense low-density lipoprotein (LDL) (Parhofer, 2015). The saponin of lychee seed affected the lipid metabolism in dexamethasone (DX)-induced insulin-resistant rats by lowering the content of total cholesterol (TC), TG, and low-density lipoprotein cholesterol (LDL-C) (Guo et al., 2003b). Simultaneously, the potential lipid-modifying effect of lychee seed extracts was also demonstrated by the other two animal studies (Pan et al., 1999; Li et al., 2015).
Kidney Protection Effect
Diabetic kidney disease (DKD), a severe microvascular complication of diabetes, is the primary cause of end-stage renal failure and the single strongest predictor of mortality in diabetic patients (Reidy et al., 2014; Thomas et al., 2016). Strict glycemic management dramatically reduces DKD morbidity, which suggests that metabolic disorders resulting from hyperglycemia, including changes in energy utilization and mitochondrial damage, exert a critical role in the disease progression (Reidy et al., 2014). Presently, multifactorial management of DKD primarily includes diet therapy, glucose-lowering therapy, lipid control, and preserving renal function (Selby and Taal, 2020). Despite various therapeutic strategies, the morbidity and mortality of DKD remain high throughout the world. Traditional Chinese herbal medicine can possess antidiabetic effects and improve renal function on DKD obviously. Research showed that saponin of lychee seed could reduce the blood glucose and ameliorate pathological damage and kidney lesions of diabetic nephropathy model rats through repressing the expression of inflammatory factors and attenuating inflammatory responses in kidney tissue (Qin, 2017). Out of many cytokines implicated in fibrosis, transforming growth factor-β1 (TGF-β1), fibronectin (FN), and collagen IV (Col IV) promoting extracellular matrix (ECM) accumulation are the most notorious (Downer et al., 1988; Chen et al., 2017). In the rat mesangial cells induced by high glucose and tumor necrosis factor-α (TNF-α), the total flavonoids of lychee seed distinctly decreased the protein expression of TGF-β1, FN, and Col IV, which indicated the total flavone might improve the diabetic nephropathy fibrosis process (Liu, 2016; Liu et al., 2016). In addition, the saponin of the lychee seed was confirmed to obviously reduce the content of IL-6 and IL-1β secreted by human glomerular mesangial cells (HMC) and decrease the secretion of ECM to slow down the sclerosis process of glomerulus (Zhang, 2016).
Neuroprotection and Cognitive Function Improvement
Cognitive dysfunction is considered as a serious and common comorbidity or even a complication of diabetes (Biessels and Despa, 2018). They share common biological mechanisms including deficits in insulin signaling, neuroinflammatory pathways, mitochondrial (Mt) metabolism, the sirtuin-peroxisome proliferator-activated receptor-gamma coactivator 1α (SIRT-PGC-1α) axis, and Tau signaling (Zilliox et al., 2016). An animal test showed that the lychee seed extract fluid could protect the nervous system by significantly improving the transmit function of the cholinergic nervous system in the cerebrum of mice with diabetes induced by alloxan and accelerating the clearance of O2− (Li et al., 2006). In another research, compared with the model group, amyloid beta (Aβ) and Tau deposition of the experimental rats in the medium- and high-dose lychee seed extract administration groups [1.39 and 2.78 g/(kg.d)] were significantly decreased (Zeng et al., 2016). Similarly, investigators have found that lychee seed extracts consisting of numerous ingredients such as adenosine, 5-hydroxymethyluridine, and 4-p-coumaroylquinic acid dramatically protected against neuronal damage and prevented the decline in the cognitive function through lowering serum glucose, ameliorating IR, and suppressing the aggregation of Aβ, Tau protein, and advanced glycation end products (AGEs) in the hippocampus of type 2 diabetes rats (Tang et al., 2018), while further study demonstrated that polyphenols derived from lychee seed inhibited hyperphosphorylated Tau through improving IR via upregulating IRS-1/PI3K/Akt and downregulating GSK-3β (Xiong et al., 2020).
Based on studies on diabetes and diabetic complication intervention with lychee seed in vivo and in vitro, the underlying hypoglycemic mechanisms of lychee seed are summarized in Figure 2.
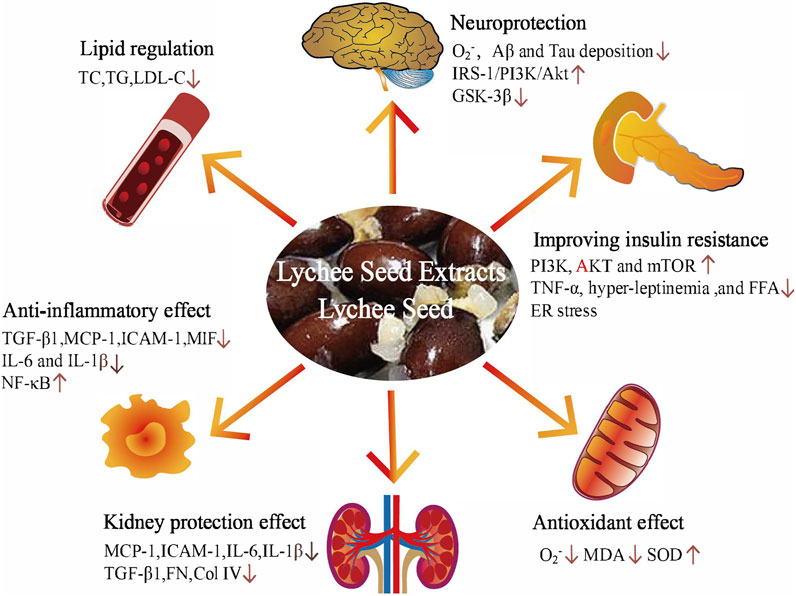
FIGURE 2. The underlying mechanism of hypoglycemic activity of lychee seed and lychee seed extracts. Amyloid beta (Aβ); tumor necrosis factor-α (TNF-α); free fatty acids (FFAs); endoplasmic reticulum (ER); malondialdehyde (MDA); superoxide dismutase (SOD); monocyte chemotactic protein 1 (MCP-1); intercellular cell adhesion molecule-1 (ICAM-1); interleukin-6 (IL-6); interleukin-1 (IL-1β); transforming growth factor-β1 (TGF-β1); fibronectin (FN); collagen IV (Col IV); macrophage migration-inhibitory factor (MIF); nuclear factor-κB (NF-κB); cholesterol (TC); triglyceride (TG); and low-density lipoprotein cholesterol (LDL-C).
Conclusion and Perspective
The rising prevalence and financial burden of diabetes and its complications have made it one of the greatest health threats facing the 21st century. Although significant advances have been made toward a long-term therapeutic approach to treat diabetes, it is tough to control the blood glucose level precisely, and the use of oral hypoglycemic agents comes with many limitations, including side effects (gastrointestinal intolerance and myocardial events) (Nissen and Wolski, 2007; McCreight et al., 2016). Lychee seed as a natural source showed antidiabetic effects from lowering blood glucose to alleviating diabetic complications. Its beneficial effects have also been validated by several clinical observations (Zhang and Teng, 1985; Shen, 1991). Through the literature review, the underlying mechanisms, improving insulin resistance, antioxidant effect, anti-inflammatory effects, lipid regulation, kidney protection effect, and neuroprotection and cognitive function improvement of lychee seed in treating diabetes are also worth investigating. For further research of lychee seed within this field, several issues should be considered. An in vitro research showed that saponin of lychee seed had no effect on glycometabolism in an insulin resistance model of pepatocellular carcinoma (HepG2) cells (Qin, 2017), which may be related to the site of drug action. The impact of saponin of lychee seed on improving IR may not be effected in hepatocytes but in other peripheral tissues such as muscle and fat. Thus, the corresponding site of action of lychee seed needs to be explicitly investigated. Most of the elucidation of the antidiabetic mechanisms scratches only at the surface, and researchers need to probe deeper into analyzing the detailed molecular mechanisms of the effects of lychee seed intervention. Consequently, comprehensive and much more robust evidence is desperately needed. As outlined in the above review, although some clinical studies show positive results in the treatment of diabetes, large, double-blind, randomized, placebo-controlled, multicenter clinical trials are needed.
In conclusion, lychee seed might be developed as a multi-target agent and prescribed as a useful adjuvant to the current treatment for diabetes and especially diabetic complications. Despite the enormous therapeutic potential, further comprehensive investigation from bench to clinical reasearch is warranted.
Author Contributions
FL designed the study and is the corresponding author. YZ, DJ, and XA drafted the manuscript and figure. LD and YD drafted the table. All authors approved the final version of the manuscript.
Funding
This work was supported by the 2015 Traditional Chinese Medicine Scientific Research (No. 201507001-11).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu El-Asrar, A. M., Ahmad, A., Siddiquei, M. M., De Zutter, A., Allegaert, E., Gikandi, P. W., et al. (2019). The Proinflammatory and Proangiogenic Macrophage Migration Inhibitory Factor Is a Potential Regulator in Proliferative Diabetic Retinopathy. Front. Immunol. 10, 2752. doi:10.3389/fimmu.2019.02752
Alzamil, H. (2020). Elevated Serum TNF-α Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J. Obes. 2020, 5076858. doi:10.1155/2020/5076858
Athyros, V. G., Doumas, M., Imprialos, K. P., Stavropoulos, K., Georgianou, E., Katsimardou, A., et al. (2018). Diabetes and Lipid Metabolism. Hormones (Athens) 17 (1), 61–67. doi:10.1007/s42000-018-0014-8
Ayina, C. N., Noubiap, J. J., Etoundi Ngoa, L. S., Boudou, P., Gautier, J. F., Mengnjo, M. K., et al. (2016). Association of Serum Leptin and Adiponectin with Anthropomorphic Indices of Obesity, Blood Lipids and Insulin Resistance in a Sub-saharan African Population. Lipids Health Dis. 15, 96. doi:10.1186/s12944-016-0264-x
Bergman, R. N., and Ader, M. (2000). Free Fatty Acids and Pathogenesis of Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 11 (9), 351–356. doi:10.1016/s1043-2760(00)00323-4
Biessels, G. J., and Despa, F. (2018). Cognitive Decline and Dementia in Diabetes Mellitus: Mechanisms and Clinical Implications. Nat. Rev. Endocrinol. 14, 591–604. doi:10.1038/s41574-018-0048-7
Brown, A. E., and Walker, M. (2016). Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 18 (8), 75. doi:10.1007/s11886-016-0755-4
Castan-Laurell, I., Dray, C., Knauf, C., Kunduzova, O., and Valet, P. (2012). Apelin, a Promising Target for Type 2 Diabetes Treatment? Trends Endocrinol. Metab. 23 (5), 234–241. doi:10.1016/j.tem.2012.02.005
Chen, H. G., Guo, H. J., Qin, Y., Zhang, S. Q., Liang, H. J., and Li, C. G. (2006). Interventional Effect of Litchi Nucleus Extract Fluid on Blood Glucose ,blood Lipid and Other Correlated Indexes of Diabetic Model Mice. Chin. J. Clin. Rehabil. (07), 79–81. doi:10.3321/j.issn:1673-8225.2006.07.040
Chen, L., Huang, R. Y., Zhong, W. Q., Huang, X. Q., Liu, X. Y., Tan, Z. J., et al. (2008). Diabetes Mellitus Model Building in Mice and the Efficacy and Biochemical Indices of the Litchi Nucleus Extract Fluid on it. Contemp. Med. (03), 27–29.
Chen, Z., Xie, X., Huang, J., Gong, W., Zhu, X., Chen, Q., et al. (2017). Connexin43 Regulates High Glucose-Induced Expression of Fibronectin, ICAM-1 and TGF-Β1 via Nrf2/ARE Pathway in Glomerular Mesangial Cells. Free Radic. Biol. Med. 102, 77–86. doi:10.1016/j.freeradbiomed.2016.11.015
Choi, S.-A., Lee, J. E., Kyung, M. J., Youn, J. H., Oh, J. B., and Whang, W. K. (2017). Anti-diabetic Functional Food with Wasted Litchi Seed and Standard of Quality Control. Appl. Biol. Chem. 60 (2), 197–204. doi:10.1007/s13765-017-0269-9
Cnop, M., Toivonen, S., Igoillo-Esteve, M., and Salpea, P. (2017). Endoplasmic Reticulum Stress and eIF2α Phosphorylation: The Achilles Heel of Pancreatic β Cells. Mol. Metab. 6 (9), 1024–1039. doi:10.1016/j.molmet.2017.06.001
Dong, Y., Fernandes, C., Liu, Y., Wu, Y., Wu, H., Brophy, M. L., et al. (2017). Role of Endoplasmic Reticulum Stress Signalling in Diabetic Endothelial Dysfunction and Atherosclerosis. Diab Vasc. Dis. Res. 14 (1), 14–23. doi:10.1177/1479164116666762
Downer, G., Phan, S. H., and Wiggins, R. C. (1988). Analysis of Renal Fibrosis in a Rabbit Model of Crescentic Nephritis. J. Clin. Invest. 82 (3), 998–1006. doi:10.1172/jci113710
Doyle-Delgado, K., Chamberlain, J. J., Shubrook, J. H., Skolnik, N., and Trujillo, J. (2020). Pharmacologic Approaches to Glycemic Treatment of Type 2 Diabetes: Synopsis of the 2020 American Diabetes Association's Standards of Medical Care in Diabetes Clinical Guideline. Ann. Intern. Med. 173 (10), 813–821. doi:10.7326/m20-2470
Ford, E. S. (2011). Trends in the Risk for Coronary Heart Disease Among Adults with Diagnosed Diabetes in the U.S.: Findings from the National Health and Nutrition Examination Survey, 1999-2008. Diabetes Care 34 (6), 1337–1343. doi:10.2337/dc10-2251
Fukai, T., and Ushio-Fukai, M. (2011). Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 15 (6), 1583–1606. doi:10.1089/ars.2011.3999
Gao, P., Hu, Y., Wang, J., Ni, Y., Zhu, Z., Wang, H., et al. (2020). Underlying Mechanism of Insulin Resistance: A Bioinformatics Analysis Based on Validated Related-Genes from Public Disease Databases. Med. Sci. Monit. 26, e924334. doi:10.12659/msm.924334
Guo, H. Y., Huang, B. C., Huang, L. P., Huang, J. L., Li, P. L., Wang, Y. W., et al. (2013). Effect of Shutangbao and Litchi Seed on Alloxan Induced Diabetic Mice. J. Pract. Diabetology 9 (06), 28–31. doi:CNKI:SUN:LNSY.0.2013-06-015.
Guo, J., Li, L., Pan, J., Qiu, G., Li, A., Huang, G., et al. (2004). [Pharmacological Mechanism of Semen Litchi on Antagonizing Insulin Resistance in Rats with Type 2 Diabetes]. Zhong Yao Cai 27 (06), 435–438. doi:10.13863/j.issn1001-4454.2004.06.022
Guo, J. W., Liao, H. F., Pan, J. Q., Ye, B. B., Liao, X. Z., Xie, W. J., et al. (2003). Effects of Saponin of Litchi Seed on Blood Glucose and Lipid in Dexamethasone-Induced Insulin Resistant Rats. Guangdong Pharm. J. (05), 32–35. doi:10.3969/j.issn.1674-229X.2003.05.015
Guo, J. W., pan, J. Q., Qiu, G. Q., Li, A. H., Xiao, L. Y., and Han, C. (2003). Effects of Litchi Seed on Enhancing Insulin Sensitivity in Type 2 Diabetic-Insulin Resistant Rats. Chin. J. New Drug (07), 526–529. doi:10.3321/j.issn:1003-3734.2003.07.009
Guo, S. M., Zheng, Y. L., Zhu, H., and Liu, Z. J. (1999). Study on Pharmacodynamics of Folk Chinese Herbal Medicine in the Treatment of Diabetes, Res. Traditional Chin. Med. (03), 3–5.
GBD 2017 Diet Collaborators Health Effects of Dietary Risks in 195 Countries, 1990-2017: a Systematic Analysis for the Global Burden of Disease Study 2017, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–1972. doi:doi:10.1016/s0140-6736(19)30041-8
Han, N., Fang, H. Y., Jiang, J. X., and Xu, Q. (2020). Downregulation of microRNA-873 Attenuates Insulin Resistance and Myocardial Injury in Rats with Gestational Diabetes Mellitus by Upregulating IGFBP2. Am. J. Physiol. Endocrinol. Metab. 318 (5), E723–e35. doi:10.1152/ajpendo.00555.2018
Herder, C., Zierer, A., Koenig, W., Roden, M., Meisinger, C., and Thorand, B. (1984-20022009). Transforming Growth Factor-Beta1 and Incident Type 2 Diabetes: Results from the MONICA/KORA Case-Cohort Study, 1984-2002. Diabetes Care 32 (10), 1921–1923. doi:10.2337/dc09-0476
Honardoost, M., Sarookhani, M. R., Arefian, E., and Soleimani, M. (2014). Insulin Resistance Associated Genes and miRNAs. Appl. Biochem. Biotechnol. 174 (1), 63–80. doi:10.1007/s12010-014-1014-z
Huang, F., Zhang, R., Yi, Y., Tang, X., Zhang, M., Su, D., et al. (2014). Comparison of Physicochemical Properties and Immunomodulatory Activity of Polysaccharides from Fresh and Dried Litchi Pulp. Molecules 19 (4), 3909–3925. doi:10.3390/molecules19043909
Huang, X., Liu, G., Guo, J., and Su, Z. (2018). The PI3K/AKT Pathway in Obesity and Type 2 Diabetes. Int. J. Biol. Sci. 14 (11), 1483–1496. doi:10.7150/ijbs.27173
Jiang, Y. (2003). Postharvest Biology and Technology of Litchi Fruit. Int. J. Food Agric. Environ. 1, 76–81.
Jiang, Z. G., Ren, K., Lin, Z., Xu, D. D., Pan, Z., and Gao, Q. P. (2011). Study on Hypoglycemic Activity of Litchi Semen Effective Constituents. J. Changchun Univ. Chin. Med. 27 (01), 14–16. doi:10.13463/j.cnki.cczyy.2011.01.066
Jiang, Z. G. (2011). Researches on Chemical Constituents and Hypoglycemic Activity of Litchi Seeds [master Thesis]. Changchun University Of Chinese Medicine.
Kawa, J. M., Taylor, C. G., and Przybylski, R. (2003). Buckwheat Concentrate Reduces Serum Glucose in Streptozotocin-Diabetic Rats. J. Agric. Food Chem. 51 (25), 7287–7291. doi:10.1021/jf0302153
Kibiti, C. M., and Afolayan, A. J. (2015). Herbal Therapy: A Review of Emerging Pharmacological Tools in the Management of Diabetes Mellitus in Africa. Pharmacogn Mag. 11 (Suppl. 2), S258–S274. doi:10.4103/0973-1296.166046
Kilari, E. K., and Putta, S. (2016). Biological and Phytopharmacological Descriptions of Litchi Chinensis. Pharmacogn Rev. 10 (19), 60–65. doi:10.4103/0973-7847.176548
Kuang, L. X., Luo, M. L., Liu, Y. H., and Xie, C. Y. (1997). Hypoglycemic Effect of Semen Litch on normal Blood Glucose Levels Mice and Diabetic Model Mice by Alloxan. Chin. J. Hosp. Pharm. (06), 16–17+47. CNKI:SUN:ZGYZ.0.1997-06-008.
Kwiecien, S., Jasnos, K., Magierowski, M., Sliwowski, Z., Pajdo, R., Brzozowski, B., et al. (2014). Lipid Peroxidation, Reactive Oxygen Species and Antioxidative Factors in the Pathogenesis of Gastric Mucosal Lesions and Mechanism of protection against Oxidative Stress - Induced Gastric Injury. J. Physiol. Pharmacol. 65 (5), 613–622.
Li, C. G., Zhang, S. Q., Wang, S. Y., Cui, M. M., Zhou, M. E., Qin, Q., et al. (2006). Intervention of Litchi Nucleus Extract Fluid in the Blood Biochemical Indexes of Model Mice with Diabetes Mellitus Induced by Alloxan. Chin. J. Clin. Rehabil. 10 (31), 61–63. doi:10.3321/j.issn:1673-8225.2006.31.027
Li, C. Q., Liao, X. B., Li, X. H., Guo, J. W., Qu, X. L., and Li, L. M. (2015). [Effect and Mechanism of Litchi Semen Effective Constituents on Insulin Resistance in Rats with Type 2 Diabetes Mellitus]. Zhong Yao Cai 38 (7), 1466–1471. doi:10.13863/j.issn1001-4454.2015.07.032
Li, F. X., Pan, M., Nong, N. Y., Huang, M. P., Li, L., Liang, H. Y., et al. (2008). Model Establishment of Diabetes Mellitus Mice and Experimental Study Litchi Nucleus Extract Fluid on its Therapy. Contemp. Med. (03), 15–17.
Liang, D., Li, W. C., Q, D. J., Qin, Y. J., Huang, Q. A., and Zhang, S. Q. (2009). Establishment of Diabetic Mouse Model and Study of Hypoglycemic Effects of Litchi Nucleus Extract Fluid. Contemp. Med. 15 (18), 41–42. doi:10.3969/j.issn.1009-4393.2009.18.028
Liao, X. B., Li, C. Q., Li, X. H., Guo, J. W., Yuan, D. S., and Qu, X. L. (2014). Effect and Mechanism of Litchi Semen Effective Constituents on Insulin Resistance in 3T3-L1 Adipocytes. J. Chin. Med. Mater. 37 (07), 1247–1250. doi:10.13863/j.issn1001-4454.2014.07.035
Liu, M., Pan, Z., Nan, Z., Dai, D. X., and Wang, Y. H. (2016). Total Flavonoids of Lichi on High Sugar Combination TNF-α Induced Protein Expression of FN and Col Ⅳ in Rat Mesangial Cells. Jilin J. Tradit Chin. Med. 36 (02), 181–183. doi:10.13463/j.cnki.jlzyy.2016.02.023
Liu, M. (2016). The Effects of Total Flavonoids of Litchi on Proliferation and Mechanism in Rat Mesangial Cells Induced by High Glucose Combined with TNF -α [master Thesis], Changchun University of Chinese Medicine.
Lou, Z. M., Tian, J. X., Wang, W. X., Yuan, H., Luo, D. J., Yang, Y. H., et al. (2007). Effect of Total Saponin Extract from Litchi Core on the Blood Glucose Levels of Diabetic Mice. Zhejiang Med. J. (06), 548–549+605. doi:10.3969/j.issn.1006-2785.2007.06.011
Man, S., Ma, J., Yao, J., Cui, J., Wang, C., Li, Y., et al. (2017). Systemic Perturbations of Key Metabolites in Type 2 Diabetic Rats Treated by Polyphenol Extracts from Litchi Chinensis Seeds. J. Agric. Food Chem. 65 (35), 7698–7704. doi:10.1021/acs.jafc.7b02206
Man, S. L., WAng, Y., Ma, J., Wang, Y., and Ma, L. (2019). Mechanism of Litchi Semen Active Ingredients in Inhibiting Cell Uptake of Insulin. J. Tianjing Univ. Sci. Tech. 34 (02), 6–11. doi:10.13364/j.issn.1672-6510.20170294
Man, S., Ma, J., Wang, C., Li, Y., Gao, W., and Lu, F. (2016). Chemical Composition and Hypoglycaemic Effect of Polyphenol Extracts from Litchi Chinensis Seeds. J. Funct. Foods 22, 313–324. doi:10.1016/j.jff.2016.01.032
McCreight, L. J., Bailey, C. J., and Pearson, E. R. (2016). Metformin and the Gastrointestinal Tract. Diabetologia 59 (3), 426–435. doi:10.1007/s00125-015-3844-9
Mooradian, A. D. (2009). Dyslipidemia in Type 2 Diabetes Mellitus. Nat. Clin. Pract. Endocrinol. Metab. 5 (3), 150–159. doi:10.1038/ncpendmet1066
Nissen, S. E., and Wolski, K. (2007). Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N. Engl. J. Med. 356 (24), 2457–2471. doi:10.1056/NEJMoa072761
Olivieri, A., De Angelis, S., Dionisi, S., D'Annunzio, G., Locatelli, M., Marinaro, M., et al. (2010). Serum Transforming Growth Factor β1 during Diabetes Development in Non-obese Diabetic Mice and Humans. Clin. Exp. Immunol. 162 (3), 407–414. doi:10.1111/j.1365-2249.2010.04253.x
Ozaki, K. I., Awazu, M., Tamiya, M., Iwasaki, Y., Harada, A., Kugisaki, S., et al. (2016). Targeting the ERK Signaling Pathway as a Potential Treatment for Insulin Resistance and Type 2 Diabetes. Am. J. Physiol. Endocrinol. Metab. 310 (8), E643–e51. doi:10.1152/ajpendo.00445.2015
Ozcan, L., and Tabas, I. (2012). Role of Endoplasmic Reticulum Stress in Metabolic Disease and Other Disorders. Annu. Rev. Med. 63, 317–328. doi:10.1146/annurev-med-043010-144749
Pan, J. Q., Liu, H. C., LIu, G. N., Hu, Y. L., Yang, X. Q., Chen, L. X., et al. (1999). Experimental Research of Litchi Seed on Hypoglycaemic, Lipid-Regulating, and Antioxidant Effects. Guangdong Pharm. J. (01), 47–50.
Parhofer, K. G. (2015). Interaction between Glucose and Lipid Metabolism: More Than Diabetic Dyslipidemia. Diabetes Metab. J. 39 (5), 353–362. doi:10.4093/dmj.2015.39.5.353
Petersmann, A., Müller-Wieland, D., Müller, U. A., Landgraf, R., Nauck, M., Freckmann, G., et al. (2019). Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 127 (S 01), S1–s7. doi:10.1055/a-1018-9078
Qi, Y. Q. (2017). Effect of Semen Litchi Effective Constituents on Regulation of Inflammation Factors in Rats with Pre-diabetes-impaired Glucose, master Thesis. Guangzhou University of Chinese Medicine.
Qin, N. (2017). Study on the Mechanism of the Effect of Litchi Saponin on Diabetic Nephropathy, master Thesis. Changchun University of Chinese Medicine.
Reddy, S., Amutha, A., Rajalakshmi, R., Bhaskaran, R., Monickaraj, F., Rangasamy, S., et al. (2017). Association of Increased Levels of MCP-1 and Cathepsin-D in Young Onset Type 2 Diabetes Patients (T2DM-Y) with Severity of Diabetic Retinopathy. J. Diabetes Complications 31 (5), 804–809. doi:10.1016/j.jdiacomp.2017.02.017
Reidy, K., Kang, H. M., Hostetter, T., and Susztak, K. (2014). Molecular Mechanisms of Diabetic Kidney Disease. J. Clin. Invest. 124 (6), 2333–2340. doi:10.1172/JCI72271
Ren, S., Duo-Duo, X. U., Gao, Y., Yu-Ting, M. A., and Gao, Q. P. (2013). Flavonoids from Litchi(Litchi Chinensis Sonn.) Seeds and Their Inhibitory Activities on α-Glucosidase. Chem. Res. Chin. Universities 29 (004), 682–685. doi:10.1007/s40242-013-3030-x
Ren, S. (2011). Researches on Chemical Constituents and Hypoglycemic Activity of Litchi (Litchi Chinensis Sonn.) Seeds, [PhD Thesis], Changchun University of Chinese Medicine.
Ren, S., Zhao, Y., Wang, Z., Zhu, H. Y., and Zhang, J. (2017). Optimization of Extraction and Separation Process of Total Flavonoids from Lychee Seed and Evaluation In Vitro Hypoglycemic Activity. Lishizhen Med. Materia Med. Res. 28 (12), 2834–2837. CNKI:SUN:SZGY.0.2017-12-007.
Ren, S., Xu, D., Pan, Z., Gao, Y., Jiang, Z., and Gao, Q. (2011). Two Flavanone Compounds from Litchi (Litchi Chinensis Sonn.) Seeds, One Previously Unreported, and Appraisal of Their α-glucosidase Inhibitory Activities. Food Chem. 127 (4), 1760–1763. doi:10.1016/j.foodchem.2011.02.054
Reynolds, A. N., Akerman, A. P., and Mann, J. (2020). Dietary Fibre and Whole Grains in Diabetes Management: Systematic Review and Meta-Analyses. Plos Med. 17 (3), e1003053. doi:10.1371/journal.pmed.1003053
Ryan, E. A., Paty, B. W., Senior, P. A., Bigam, D., Alfadhli, E., Kneteman, N. M., et al. (2005). Five-year Follow-Up after Clinical Islet Transplantation. Diabetes 54 (7), 2060–2069. doi:10.2337/diabetes.54.7.2060
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., et al. (2019). Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 157, 107843. doi:10.1016/j.diabres.2019.107843
Sánchez-Zamora, Y. I., and Rodriguez-Sosa, M. (2014). The Role of MIF in Type 1 and Type 2 Diabetes Mellitus. J. Diabetes Res. 2014, 804519. doi:10.1155/2014/804519
Selby, N. M., and Taal, M. W. (2020). An Updated Overview of Diabetic Nephropathy: Diagnosis, Prognosis, Treatment Goals and Latest Guidelines. Diabetes Obes. Metab. 2 (Suppl. 1), 3–15. doi:10.1111/dom.14007
Shen, M. F. (1991). Clinical Study on the Treatment of Diabetes Mellitus with Lychee Seed Tablets. Chin. Traditional Patent Med. (11), 24. doi:10.1002/sim.6908
Shen, W. Y., Gu, C. F., Yang, W., and Hu, M. J. (1986). Effects of Litchi Seed on Alloxan Induced Diabetic Mice. Chin. J. Mod. Appl. Pharm. (04), 8–9. doi:10.13748/j.cnki.issn1007-7693.1986.04.003
Shi, G. J., Shi, G. R., Zhou, J. Y., Zhang, W. J., Gao, C. Y., Jiang, Y. P., et al. (2018). Involvement of Growth Factors in Diabetes Mellitus and its Complications: A General Review. Biomed. Pharmacother. 101, 510–527. doi:10.1016/j.biopha.2018.02.105
Srour, B., Fezeu, L. K., Kesse-Guyot, E., Allès, B., Debras, C., Druesne-Pecollo, N., et al. (2020). Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern. Med. 180 (2), 283–291. doi:10.1001/jamainternmed.2019.5942
Su, L., Wang, X. L., Yin, C. C., and Jia, Y. M. (2017). Effect of Jiangtangtongmai Tablets Combined with Insulin Injection on Coagulation Status and Microvascular Complications in Patients with Type 2 Diabetes Mellitus. Pharmacol. Clin. Chin. Materia Med. 33 (06), 159–162. doi:10.13412/j.cnki.zyyl.2017.06.043
Tandon, N., Ali, M. K., and Narayan, K. M. (2012). Pharmacologic Prevention of Microvascular and Macrovascular Complications in Diabetes Mellitus: Implications of the Results of Recent Clinical Trials in Type 2 Diabetes. Am. J. Cardiovasc. Drugs 12 (1), 7–22. doi:10.2165/11594650-000000000-00000
Tang, Y., Yu, C., Wu, J., Chen, H., Zeng, Y., Wang, X., et al. (2018). Lychee Seed Extract Protects against Neuronal Injury and Improves Cognitive Function in Rats with Type II Diabetes Mellitus with Cognitive Impairment. Int. J. Mol. Med. 41 (1), 251–263. doi:10.3892/ijmm.2017.3245
Thomas, M. C., Cooper, M. E., and Zimmet, P. (2016). Changing Epidemiology of Type 2 Diabetes Mellitus and Associated Chronic Kidney Disease. Nat. Rev. Nephrol. 12 (2), 73–81. doi:10.1038/nrneph.2015.173
Tian, J., Lian, F., Yang, L., and Tong, X. (2018). Evaluation of the Chinese Herbal Medicine Jinlida in Type 2 Diabetes Patients Based on Stratification: Results of Subgroup Analysis from a 12-week Trial. J. Diabetes 10 (2), 112–120. doi:10.1111/1753-0407.12559
Tong, X. L., Dong, L., Chen, L., and Zhen, Z. (2012). Treatment of Diabetes Using Traditional Chinese Medicine: Past, Present and Future. Am. J. Chin. Med. 40 (5), 877–886. doi:10.1142/s0192415x12500656
Wellen, K. E., and Hotamisligil, G. S. (2005). Inflammation, Stress, and Diabetes. J. Clin. Invest. 115 (5), 1111–1119. doi:10.1172/jci25102
Wen, F., Yang, Y., Jin, D., Sun, J., Yu, X., and Yang, Z. (2014). MiRNA-145 Is Involved in the Development of Resistin-Induced Insulin Resistance in HepG2 Cells. Biochem. Biophys. Res. Commun. 445 (2), 517–523. doi:10.1016/j.bbrc.2014.02.034
Wu, J., Xu, Y., Liu, X., Chen, M., Zhu, B., Wang, H., et al. (2020). Isolation and Structural Characterization of a Non-competitive α-glucosidase Inhibitory Polysaccharide from the Seeds of Litchi Chinensis Sonn. Int. J. Biol. Macromol 154, 1105–1115. doi:10.1016/j.ijbiomac.2019.11.170
Wu, Q. H., Liang, S. M., Li, Y. H., Han, J., Zhao, M. H., Jian, Z. H., et al. (1991). Screening Study of TCM Simple and Proved Recipes on Diabetes. J. Guangzhou Univ. Traditional Chin. Med. (Z1), 218–223. doi:CNKI:SUN:REST.0.1991-Z1-040.
Xiao, Z. J., Guo, J. W., and Xu, F. (2015). Effect of Litchi Saponin and Litchi Flavones on Insulin Resistance in HepG2 Cells. J. Pharm. Pract. 33 (04), 316–318+50. doi:10.3969/j.issn.1006-0111.2015.04.007
Xihua, L., Shengjie, T., Weiwei, G., Matro, E., Tingting, T., Lin, L., et al. (2019). Circulating miR-143-3p Inhibition Protects against Insulin Resistance in Metabolic Syndrome via Targeting of the Insulin-like Growth Factor 2 Receptor. Transl Res. 205, 33–43. doi:10.1016/j.trsl.2018.09.006
Xiong, R., Wang, X. L., Wu, J. M., Tang, Y., Qiu, W. Q., Shen, X., et al. (2020). Polyphenols Isolated from Lychee Seed Inhibit Alzheimer's Disease-Associated Tau through Improving Insulin Resistance via the IRS-1/PI3K/Akt/GSK-3β Pathway. J. Ethnopharmacol 251, 112548. doi:10.1016/j.jep.2020.112548
Yang, B., Wang, J., Zhao, M., Liu, Y., Wang, W., and Jiang, Y. (2006). Identification of Polysaccharides from Pericarp Tissues of Litchi (Litchi Chinensis Sonn.) Fruit in Relation to Their Antioxidant Activities. Carbohydr. Res. 341 (5), 634–638. doi:10.1016/j.carres.2006.01.004
Yaribeygi, H., Farrokhi, F. R., Butler, A. E., and Sahebkar, A. (2019). Insulin Resistance: Review of the Underlying Molecular Mechanisms. J. Cel Physiol 234 (6), 8152–8161. doi:10.1002/jcp.27603
Yaribeygi, H., Sathyapalan, T., Atkin, S. L., and Sahebkar, A. (2020). Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid Med. Cel Longev 2020, 8609213. doi:10.1155/2020/8609213
Yin, X., Xu, Z., Zhang, Z., Li, L., Pan, Q., Zheng, F., et al. (2017). Association of PI3K/AKT/mTOR Pathway Genetic Variants with Type 2 Diabetes Mellitus in Chinese. Diabetes Res. Clin. Pract. 128, 127–135. doi:10.1016/j.diabres.2017.04.002
Yuan, H., Tian, J. X., Zhang, Y. M., Shen, L., Yu, H. Y., Yin, H. P., et al. (2006). Study on the Hypoglycemic Activity of Saponin Extracted from Litchi Seed. J. Hangzhou Teach. College(Medical edition) (01), 6–7+10.
Yuan, H. (2010). Effect of Polysaccharides Extracted from Litchi Seeds Ondiabetic Mice Challenged by Alloxan. Health Res. 30 (04), 252–255+61. doi:10.19890/j.cnki.issn1674-6449.2010.04.003
Zeng, Y., Bao, C. R., Tang, Y., Liu, J., Qin, D. L., Chen, H. X., et al. (2016). Effect of Litchi Seed Extract on Cognitive Impairment in Type 2 Diabetic Rats. Chin. Traditional Patent Med. 38 (03), 672–676. doi:10.3969/j.issn.1001-1528.2016.03.042
Zhang, H., and Teng, Y. (1985). Effect of Li Ren (Semen Litchi) Antidiabetes Pills in 45 Cases of Diabetes Mellitus, J. Traditional Chin. Med. (02), 40–41.
Zhang, H. N. (2016).Study on Expression of Induction SOCS-1 and Relevant Inflammatory Factor with Angiotensin II & Intervention Effect of Saponins of Litchi Seed, [master Thesis], Changchun University of Chinese Medicine.
Zhang, J., Wang, X., Vikash, V., Ye, Q., Wu, D., Liu, Y., et al. (2016). ROS and ROS-Mediated Cellular Signaling. Oxid Med. Cel Longev 2016, 4350965. doi:10.1155/2016/4350965
Zhang, T., Guo, J. Y., Qiao, M. M., Yang, Y., Chen, L., Li, C. M., et al. (2013). The Potential Mechanism of Litchi Nucleus Extracts on Decreasing Blood Glucose Metabolism via Serum miRNAs Expression Pattern in Db/db Mouse. J. Wenzhou Med. Coll. 43 (10), 631–635+41. doi:10.3969/j.issn.1000-2138.2013.10.01
Zhang, Y. Q., Zheng, W., Liu, K. Q., Qu, W. D., Feng, C. X., and Xu, D. D. (2020). Study on Optimization of Water Extraction-Ethanol Precipitation Technology of Polysaccharide from Litchi Chinensis Seed and its Inhibitory Activity to α-glucosidase. China Pharm. 31 (16), 1995–2000. doi:10.6039/j.issn.1001-0408.2020.16.14
Zheng, S., Zhao, M., Ren, Y., Wu, Y., and Yang, J. (2015). Sesamin Suppresses STZ Induced INS-1 Cell Apoptosis through Inhibition of NF-Κb Activation and Regulation of Bcl-2 Family Protein Expression. Eur. J. Pharmacol. 750, 52–58. doi:10.1016/j.ejphar.2015.01.031
Zhong, S. S., Deng, Z. J., Li, C. Q., Sha, C. W., Guo, J. W., Li, L. M., et al. (2015). Inhibition of Semen Litchi Effective Fractions on α-Glucosidase. Pharm. Today 25 (09), 617–619.
Zhou, Y. (2016). Screening Activities of Traditional Chinese Medicines for Treating Diabetes and Faeces Metabonomics Study of Type 2 Diabetic Rats, master Thesis. Jilin University.
Keywords: lychee seed, diet supplements, diabetes, pharmacological mechanisms, effect
Citation: Zhang Y, Jin D, An X, Duan L, Duan Y and Lian F (2021) Lychee Seed as a Potential Hypoglycemic Agent, and Exploration of its Underlying Mechanisms. Front. Pharmacol. 12:737803. doi: 10.3389/fphar.2021.737803
Received: 07 July 2021; Accepted: 06 September 2021;
Published: 08 October 2021.
Edited by:
Michał Tomczyk, Medical University of Bialystok, PolandReviewed by:
Ana Clara Aprotosoaie, Grigore T. Popa University of Medicine and Pharmacy, RomaniaMikhail Olugbemiro Nafiu, University of Ilorin, Nigeria
Copyright © 2021 Zhang, Jin, An, Duan, Duan and Lian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengmei Lian, bGZtNTY1QHNvaHUuY29t
†These authors have contributed equally to this work and share first authorship
 Yuehong Zhang
Yuehong Zhang De Jin
De Jin Xuedong An
Xuedong An Liyun Duan1
Liyun Duan1 Fengmei Lian
Fengmei Lian