- 1School of Pharmacy, China Medical University, Taichung, Taiwan
- 2Chinese Medicine Research Center, Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, Master Program for Food and Drug Safety, China Medical University, Taichung, Taiwan
- 3Department of Cosmeceutics, China Medical University, Taichung, Taiwan
- 4Department of Chinese Medicine, China Medical University Hospital, Taichung, Taiwan
- 5School of Post-Baccalaureate Chinese Medicine, College of Chinese Medicine, China Medical University, Taichung, Taiwan
- 6Department of Pharmaceutical Sciences, Nihon Pharmaceutical University, Saitama, Japan
- 7Tsuzuki Institute for Traditional Medicine, China Medical University, Taichung, Taiwan
- 8Institute of New Drug Development, China Medical University, Taichung, Taiwan
- 9Department of Food Nutrition and Health Biotechnology, Asia University, Taichung, Taiwan
The increasing interest and demand for skin whitening products globally, particularly in Asia, have necessitated rapid advances in research on skin whitening products used in traditional Chinese medicine (TCM). Herein, we investigated 74 skin whitening prescriptions sold in TCM pharmacies in Taiwan. Commonly used medicinal materials were defined as those with a relative frequency of citation (RFC) > 0.2 and their characteristics were evaluated. Correlation analysis of commonly used medicinal materials was carried out to identify the core component of the medicinal materials. Of the purchased 74 skin whitening prescriptions, 36 were oral prescriptions, 37 were external prescriptions, and one prescription could be used as an oral or external prescription. After analysis, 90 traditional Chinese medicinal materials were obtained. The Apiaceae (10%; 13%) and Leguminosae (9%; 11%) were the main sources of oral and external medicinal materials, respectively. Oral skin whitening prescriptions were found to be mostly warm (46%) and sweet (53%), while external skin whitening prescriptions included cold (43%) and bitter (29%) medicinal materials. Additionally, mainly tonifying and replenishing effects of the materials were noted. Pharmacological analysis indicated that these medicinal materials may promote wound healing, treat inflammatory skin diseases, or anti-hyperpigmentation. According to the Spearman correlation analysis on interactions among medicinal materials with an RFC > 0.2 in the oral skin whitening prescriptions, Paeonia lactiflora Pall. (white) and Atractylodes macrocephala Koidz. showed the highest correlation (confidence score = 0.93), followed by Ziziphus jujuba Mill. (red) and Astragalus propinquus Schischkin (confidence score = 0.91). Seven medicinal materials in external skin whitening prescriptions with an RFC > 0.2, were classified as Taiwan qī bái sàn (an herbal preparation), including Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav., Wolfiporia extensa (Peck) Ginns, Bletilla striata (Thunb.) Rchb. f., Atractylodes macrocephala Koidz., Ampelopsis japonica (Thunb.) Makino, Paeonia lactiflora Pall. (white), and Bombyx mori Linnaeus. Skin whitening prescriptions included multiple traditional Chinese medicinal materials. Despite the long history of use, there is a lack of studies concerning skin whitening products, possibly due to the complex composition of traditional Chinese medicine. Further studies are required to assess the efficacy and safety of these traditional Chinese medicinal materials for inclusion in effective, safe, and functional pharmacological products.
1 Introduction
The global cosmetics market is undergoing an unprecedented boom due to economic development and growing aesthetic needs. Rapidly expanding Asian cosmetic markets, of which China, Japan, South Korea, and India are major consumer countries, are following in the footsteps of European and American countries with the ubiquitous use of cosmetic products, particularly skin whitening products. Moreover, a recent survey noted the increasing prevalence of males using skin whitening products in addition to females (Pillaiyar et al., 2017; Hu et al., 2020).
Skin whitening prescriptions can not only be used to lighten skin tone, but also clinically treat hyperpigmentary disorders by decreasing melanin synthesis (Gillbro and Olsson, 2011). Melanin, one of the important pigments, is synthesized in melanocytes in the basal layer of epidermis and can protect the skin from ultraviolet-induced damage (Brenner and Hearing, 2008). Epidermal melanin content is intimately associated with anthropological origins. As the level of ultraviolet radiation is higher in low-latitude regions, people in these regions have higher melanin content; conversely, melanin content is lower in people at high-latitude regions, hence they have whiter skin (Slominski et al., 2004). Melanogenesis involves the conversion of L-tyrosine to L-dihydroxyphenylalanine (L-DOPA) by tyrosinase before further conversion to L-dopaquinone. Finally, L-dopaquinone undergoes a series of chemical reactions to form melanin (Supplementary Figure S1). Tyrosinase is a rate-limiting enzyme in melanogenesis and is considered an important target for the development of therapies in treating hyperpigmentation (Sonthalia et al., 2016; Ullah et al., 2019; Zaidi et al., 2019).
At present, there are a variety of skin whitening products on the market classified as either “inhibiting melanogenesis” or “inhibiting melanogenesis and promoting melanin removal.” In Taiwan, only 13 skin whitening components (Supplementary Table S1) (Food and Drug Administration, 2014) have been approved for use in cosmetic preparations. While ascorbic acid (vitamin C) is a common component, it is unstable and easily oxidized which limits its direct use. In order to prevent premature degradation, derivatives such as magnesium ascorbyl phosphate, ascorbyl glucoside, and ascorbyl tetraisopalmitate (Balaguer et al., 2008) are often utilized. Caution must be exercised when using various skin whitening components as improper use may lead to dermatitis, erythema, burns, and other skin injuries (Nordin et al., 2021). Thus, manufacturers have begun to seek natural alternatives to develop gentle, hypoallergenic skin whitening products derived from traditional Chinese medicine (TCM).
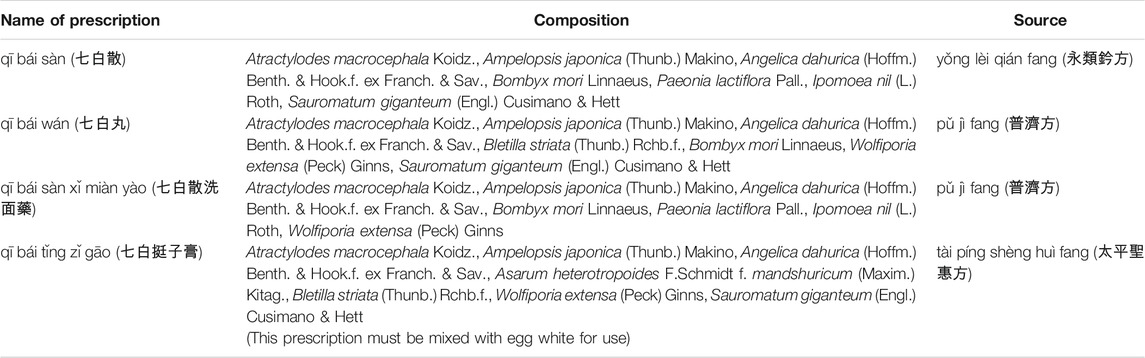
In traditional Chinese medicine books, many words describe dark skin or spots on the face, such as miàn gǎn zèng. In addition, there are ancient descriptions regarding the use of TCM for skin whitening. The Shennong Materia Medica, an extant medicinal text published in 100 B.C., recorded that Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. promotes skin growth and has moisturizing effects. Of additional interest is qī bái sàn, a well-known skin whitening herbal prescription. However, its composition varies among different historical dynasties, geographical regions, and environments. For example, different compositions of qī bái sàn can be found in related prescriptions such as yǒng lèi qián fāng, pǔ jì fāng, and tài píng shèng huì fāng (Table 1). To date, there is still no comprehensive study on skin whitening prescriptions in traditional Chinese medicine pharmacies in Taiwan. Therefore, the aim of this study was to examine the composition of skin whitening prescriptions sold in traditional Chinese medicine pharmacies in Taiwan to understand the usage, methods of preparation, and principles of skin whitening prescriptions in Taiwan.
2 Materials and Methods
2.1 Ethical Review
The research for this study was conducted from March 2020 to April 2021. The study was approved by the Central Regional Research Ethics Committee of China Medical University (CRREC-109-125) (Supplementary Figure S2).
2.2 Research Process
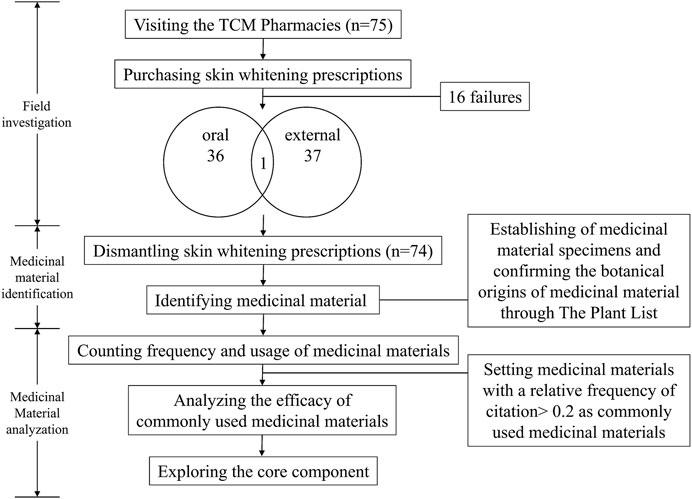
This study complied with the ethnobotanical research guidelines (Rivera et al., 2014; Mullane et al., 2015; Heinrich et al., 2018; Heinrich et al., 2020), and could mainly divided into field investigation, medicinal material identification, and analysis. The complete study methods are shown in the study flowchart (Figure 1).
2.2.1 Field Investigation
Taiwan is an island located at the intersection between Northeast Asia and Southeast Asia and a total area of 36,000 km2 (Tourism Bureau, 2021). The study was conducted over 12 months and we randomly visited the TCM pharmacies that appropriately represented the use of TCM medicine in Taiwan. The number of TCM pharmacies selected was directly proportional to the number of TCM pharmacies in each county and city published by the government (Ministry of Health and Welfare, 2015). A total of 75 TCM pharmacies were visited, included 16 pharmacies were visited but no prescription was obtained. Overall, 74 skin whitening prescriptions were obtained (with 13 TCM pharmacies providing more than one prescription) (Figure 2), including 36 oral prescriptions, 37 external prescriptions, and one prescription which could be used as an oral or external prescription.
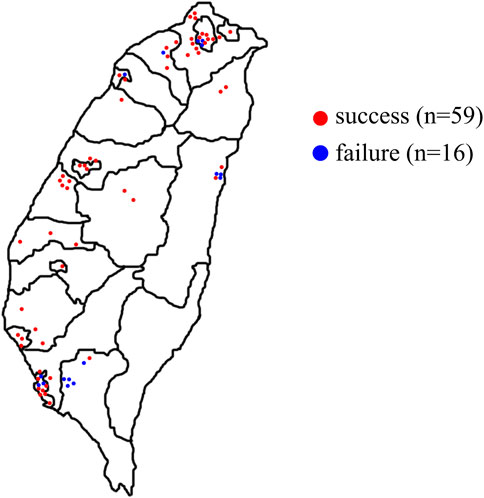
FIGURE 2. Geographical distribution of pharmacies visited for skin whitening prescriptions in Taiwan. All dots were based on latitude and longitude coordinates. Red dots indicated successful pharmacies; blue dots indicated failed pharmacies.
2.2.2 Identification of Medicinal Materials
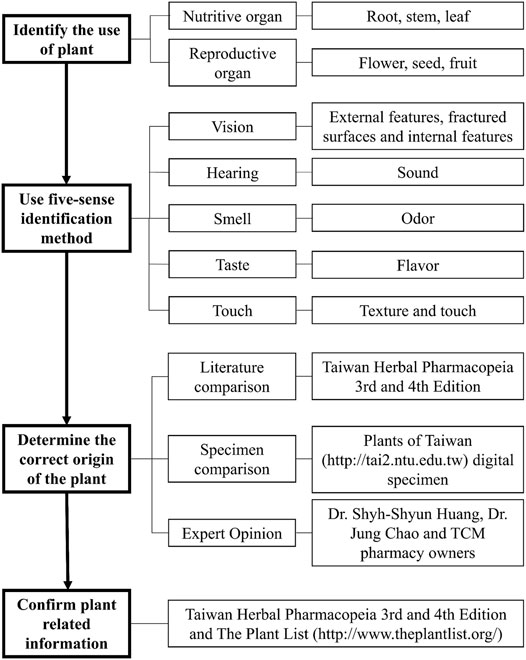
This study examined the purchased medicinal materials and performed the five-sense identification to identify the origins and plant parts of the materials, and compared them with the medicinal material standards to distinguish authentic or misused medicinal materials (Figure 3). We also photographed the materials and recorded the weight of each material. Finally, the materials were numbered and stored in the herbarium of the China Medical University, Taiwan.
2.2.3 Analysis of Medicinal Materials
The various medicinal materials were analyzed and collated based on biological taxonomy, relative frequency of citation (RFC), efficacy of traditional use, and skin-related pharmacological effects. Biological taxonomy included the Scientific name of the crude drug, family, and color. The Plant List (http://www.theplantlist.org/) was used as a source of botanical information. Medicinal materials with RFC > 0.2 were defined as commonly used medicinal materials. The RFC formula was defined as follows (Vitalini et al., 2013; Dixit and Tiwari, 2020; Abbas et al., 2021):
Where
The medicinal materials were indexed against the Taiwan Herbal Pharmacopeia (3rd, 4th edition) (Taiwan Herbal Pharmacopeia 3rd Edition Committee, 2018; Taiwan Herbal Pharmacopeia 4th Edition Committee, 2021), Pharmacopoeia of the People’s Republic of China (Chinese Pharmacopoeia Commission, 2020), and Chinese Materia Medica (State Administration of Traditional Chinese Medicine, 1999). The effects, properties, and flavors of traditional Chinese medicine were cited from the Taiwan Herbal Pharmacopeia (3rd, 4th edition). The PubMed database was systematically searched from Jan 2010 to May 2021 for skin-related pharmacological effects, utilizing keywords such as “skin” and the scientific names of medicinal materials.
2.2.4 Analysis
GraphPad Prism 9.0 (GraphPad Prism version 9.0 for Windows, GraphPad Software, San Diego, California, USA) was used to plot a heat map for Spearman correlation analysis of commonly used medicinal materials used in oral skin whitening prescriptions. The colors of the squares in the heat map were based on the visualization of Spearman correlation matrix of the two medicinal materials. The more intense red hue, the higher the correlation between the two medicinal materials. Conversely, the lighter the color, the lower the correlation between the two medicinal materials (Vacanti, 2019).
3 Results
3.1 Biological Taxonomic Characteristics of Medicinal Materials Used in Skin Whitening Prescriptions
During this study, 74 skin whitening prescriptions were purchased from 59 TCM pharmacies in Taiwan, of which 36 were oral prescriptions, 37 were external prescriptions, and one prescription could be used orally or externally. Oral prescription use method is to add appropriate amount of water to decoct; external prescription use method is to mash the medicinal materials, then add water, honey or milk, and apply to the face. Among the oral and external skin whitening prescriptions, 79 and 56 medicinal materials were found respectively. Overall, a total of 90 medicinal materials were obtained from the 74 prescriptions, and 6 misused medicinal materials were found (Supplementary Table S2). The majority of these medicinal materials were Plantae (93.33%), 3 medicinal materials (3.33%) were obtained from Animalia [Bombyx mori Linnaeus, Crassostrea gigas (Thunberg), and Pteria martensii (Dunker)] and 3 (3.33%) were from Fungi [Tremella fuciformis, Wolfiporia extensa (Peck) Ginns cum pini radix, and Wolfiporia extensa (Peck) Ginns] (Figure 4A).
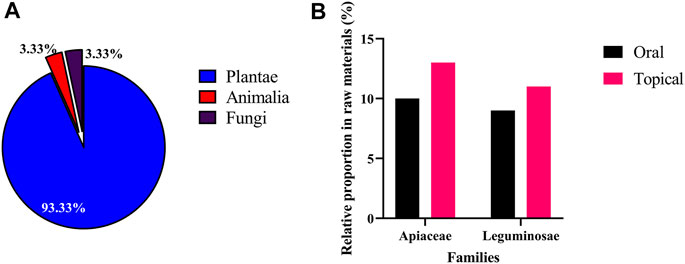
FIGURE 4. Taxonomy of 90 medicinal materials in 74 skin whitening prescriptions. (A) Kingdoms and (B) families.
The most used of medicinal material in oral skin whitening prescriptions was Wolfiporia extensa (Peck) Ginns (RFC = 0.51), followed by Glycyrrhiza uralensis Fisch. and Paeonia lactiflora Pall. (white) (RFC = 0.41), while Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. (RFC = 0.89) was the most commonly used of medicinal material in external skin whitening prescriptions. When classified by family, the most common families in both oral and external skin whitening prescriptions were Apiaceae (10 and 13% respectively) and Leguminosae (9 and 11% respectively) (Figure 4B).
3.2 Analysis of Traditional Efficacy, Skin-Related Pharmacological Effects and Dosage of Commonly Used Medicinal Materials in Skin Whitening Prescriptions
Commonly used medicinal materials were defined as those with RFC > 0.2. Thirteen and seven commonly used medicinal materials were obtained from oral and external skin whitening prescriptions, respectively (Table 2).
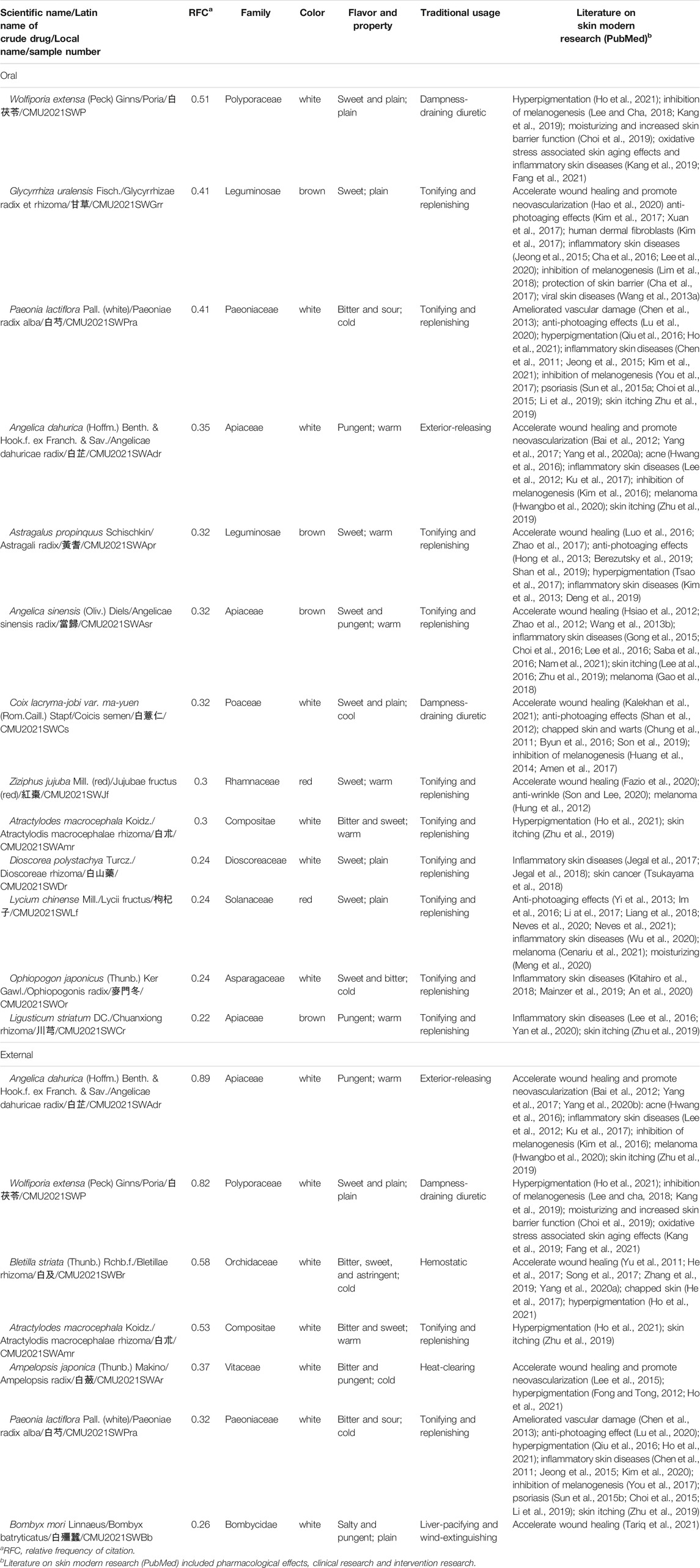
TABLE 2. Medicinal properties and skin-related modern research of commonly used medicinal materials used in skin whitening prescriptions (RFC>0.2).
With regards to the properties (Figure 5A), commonly used medicinal materials used in oral skin whitening prescriptions were mostly warm (46%) and plain (31%), while those used in external were mostly cold (43%), followed by warm (29%) and plain (29%). About the flavors (Figure 5B), commonly used medicinal materials used in oral skin whitening prescriptions were mostly sweet (53%), while those used in external were mostly bitter (29%).
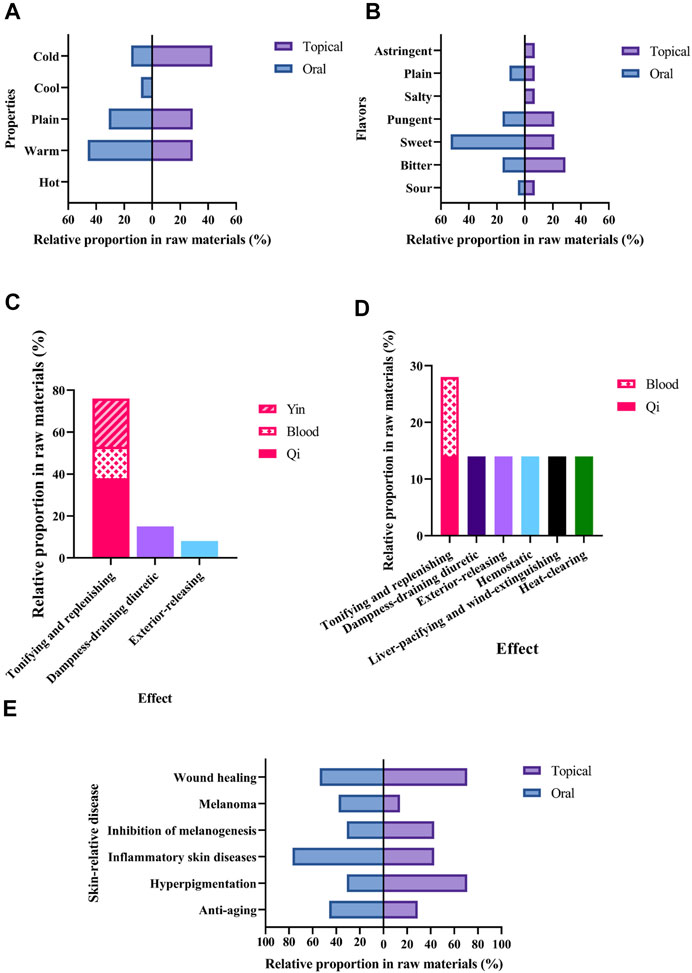
FIGURE 5. Characteristics of medicinal materials with an RFC > 0.2 in skin whitening prescriptions. (A) Properties and (B) Flavors. Histogram of traditional efficacy classifications. (C) oral prescriptions and (D) external prescriptions. (E) Modern research related to skin.
About classification by traditional effect (Figures 5C,D), commonly used medicinal materials used in both oral and external skin whitening prescriptions were mostly tonifying and replenishing. Integrated the modern research related to skin, including pharmacological effects, clinical studies and intervention studies, it is found that most of these medicinal materials could promote wound healing, treat inflammatory skin diseases, or anti-hyperpigmentation (Figure 5E).
The analysis of the various medicinal materials dosage used in prescriptions is presented in Supplementary Table S3. In commonly used oral medicinal materials (Figure 6A), the average dose of Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf was the highest and that Glycyrrhiza uralensis Fisch. was the lowest. Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf showed the largest dose difference across the various TCM pharmacies, while dose differences of the Angelica sinensis (Oliv.) Diels were the smallest. In commonly used external medicinal materials (Figure 6B), the average dosage of Wolfiporia extensa (Peck) Ginns was the highest, while the Bletilla striata (Thunb.) Rchb. f. and Paeonia lactiflora Pall. (white) were the lowest. The dosages of Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. had the largest differences across various TCM pharmacies, while the dosages of the Paeonia lactiflora Pall. (white) exhibited the smallest difference.
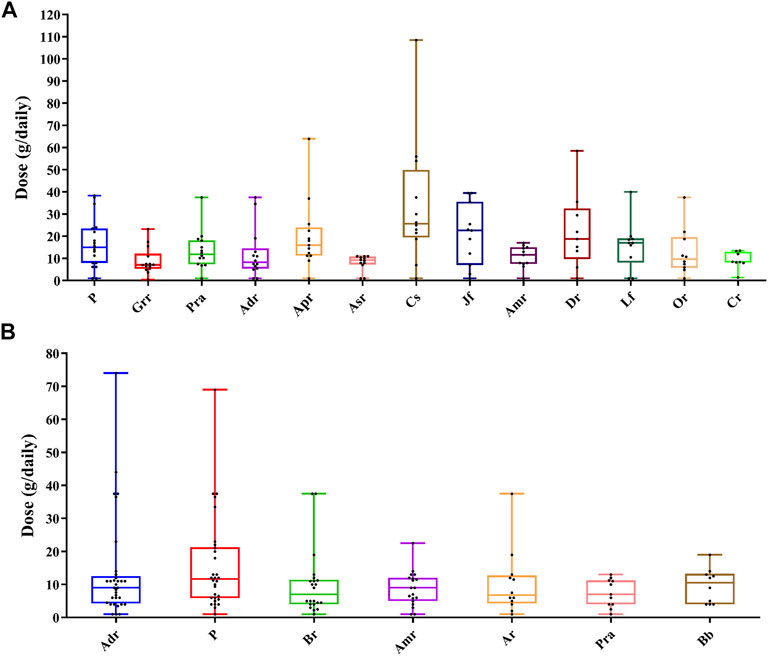
FIGURE 6. Box plots of dose ranges of commonly used medicinal materials. The top line represents the maximum value, and the bottom line represents the minimum value; the bottom of each box represents the first quartile (Q1), the middle line represents the second quartile (Q2), and the top of each box represents the third quartile (Q3). The black dots represent the doses in the collected samples. (A) Oral prescriptions and (B) external prescriptions. Adr, Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav.; Amr, Atractylodes macrocephala Koidz; Apr, Astragalus propinquus Schischkin; Ar, Ampelopsis japonica (Thunb.) Makino; Asr, Angelica sinensis (Oliv.) Diels; Cr, Ligusticum striatum DC.; Cs, Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf; Dr, Dioscorea polystachya Turcz.; Grr, Glycyrrhiza uralensis Fisch.; Jf, Ziziphus jujuba Mill. (red); Lf, Lycium chinense Mill.; Or, Ophiopogon japonicus (Thunb.) Ker Gawl.; P, Wolfiporia extensa (Peck) Ginns; Pra, Paeonia lactiflora Pall. (white).
3.3 Correlation Analysis of Commonly Used Medicinal Materials Used in Oral Skin Whitening Prescriptions
Spearman correlation analysis was performed for commonly used medicinal materials in oral skin whitening prescriptions and a heatmap was plotted (Figure 7A). The highest correlation was detected between Paeonia lactiflora Pall. (white) and Atractylodes macrocephala Koidz. (confidence score = 0.93), followed by the correlation between Ziziphus jujuba Mill. (red) and Astragalus propinquus Schischkin (confidence score = 0.91). In contrast, low correlation was observed between Dioscorea polystachya Turcz. and Glycyrrhiza uralensis Fisch (confidence score = −0.7). Dioscorea polystachya Turcz. also demonstrated a low correlation with Paeonia lactiflora Pall. (white) and Atractylodes macrocephala Koidz. Therefore, Dioscorea polystachya Turcz. is less likely to be present when Glycyrrhiza uralensis Fisch., Paeonia lactiflora Pall. (white), or Atractylodes macrocephala Koidz. are present. When network analysis was performed on medicinal materials with RFC > 0.2 in oral skin whitening prescriptions (Figure 7B) and two medicinal materials with confidence score >0.6 were connected by lines, it was found that Paeonia lactiflora Pall. (white) and Atractylodes macrocephala Koidz. frequently appeared together with Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. or Wolfiporia extensa (Peck) Ginns and Glycyrrhiza uralensis Fisch.; Ziziphus jujuba Mill. (red) and Astragalus propinquus Schischkin were used in combination with Lycium chinense Mill. or Ligusticum striatum DC.; and Angelica sinensis (Oliv.) Diels, Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf, and Dioscorea polystachya Turcz. was a prescription. These combinations could be used as a reference for oral skin whitening prescriptions.
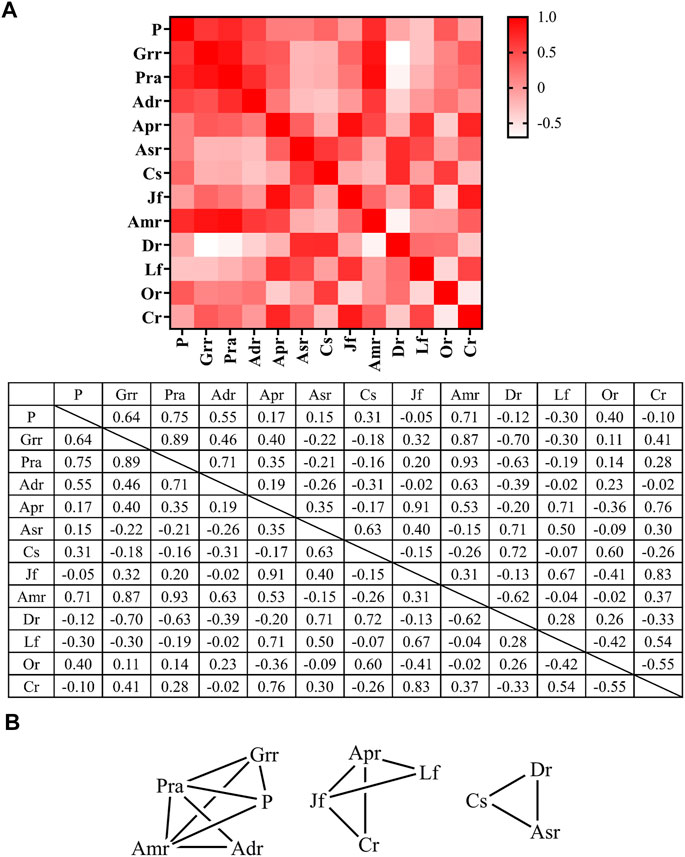
FIGURE 7. Spearman correlation analysis of commonly used medicinal materials used in oral skin whitening prescriptions. (A) Heat map and (B) Network map. Adr, Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav.; Amr, Atractylodes macrocephala Koidz.; Apr, Astragalus propinquus Schischkin; Asr, Angelica sinensis (Oliv.) Diels; Cr, Ligusticum striatum DC.; Cs, Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf; Dr, Dioscorea polystachya Turcz.; Grr, Glycyrrhiza uralensis Fisch.; Jf, Ziziphus jujuba Mill. (red); Lf, Lycium chinense Mill.; Or, Ophiopogon japonicus (Thunb.) Ker Gawl.; P, Wolfiporia extensa (Peck) Ginns; Pra, Paeonia lactiflora Pall. (white).
3.4 Venn Diagram Analysis of Commonly Used Medicinal Materials Used in External Skin Whitening Prescriptions
In this study, medicinal materials with RFC >0.2 in external skin whitening prescriptions were defined as Taiwan qī bái sàn (Figure 8A). Taiwan qī bái sàn consists of Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav., Wolfiporia extensa (Peck) Ginns, Bletilla striata (Thunb.) Rchb. f., Atractylodes macrocephala Koidz., Ampelopsis japonica (Thunb.) Makino, Paeonia lactiflora Pall. (white), and Bombyx mori Linnaeus. Venn diagram analysis of these medicinal materials with qī bái sàn-related prescriptions in yǒng lèi qián fāng, pǔ jì fāng, and tài píng shèng huì fāng found that Taiwan qī bái sàn is the addition and subtraction formula from the qī bái sàn mentioned in ancient books (Figure 8B).
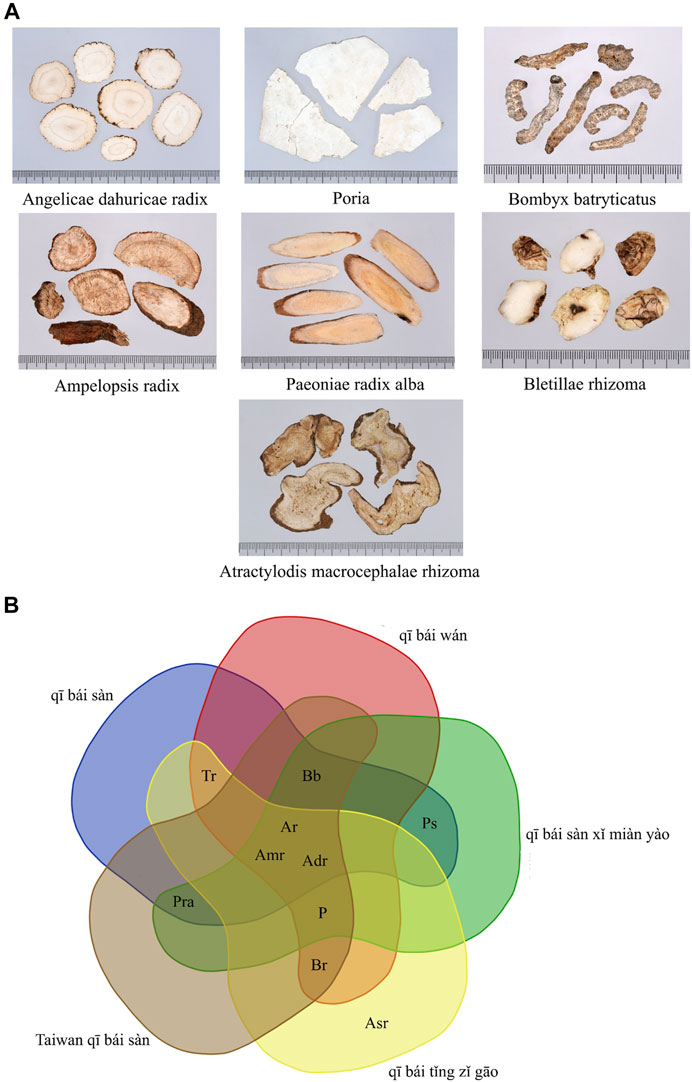
FIGURE 8. (A) Pictures of medicinal materials of Taiwan qī bái sàn. (B) Venn diagram of qī bái sàn-related prescriptions in ancient books and Taiwan qī bái sàn. Adr, Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav.; Amr, Atractylodes macrocephala Koidz.; Ar, Ampelopsis japonica (Thunb.) Makino; Asr, Asarum heterotropoides F. Schmidt f. mandshuricum (Maxim.) Kitag.; Bb, Bombyx mori Linnaeus; Br, Bletilla striata (Thunb.) Rchb. f.; P, Wolfiporia extensa (Peck) Ginns; Pra, Paeonia lactiflora Pall. (white); Ps, Ipomoea nil (L.). Roth; Tr, Sauromatum giganteum (Engl.) Cusimano & Hett.
3.5 Misuse of Medicinal Materials in Skin Whitening Prescriptions
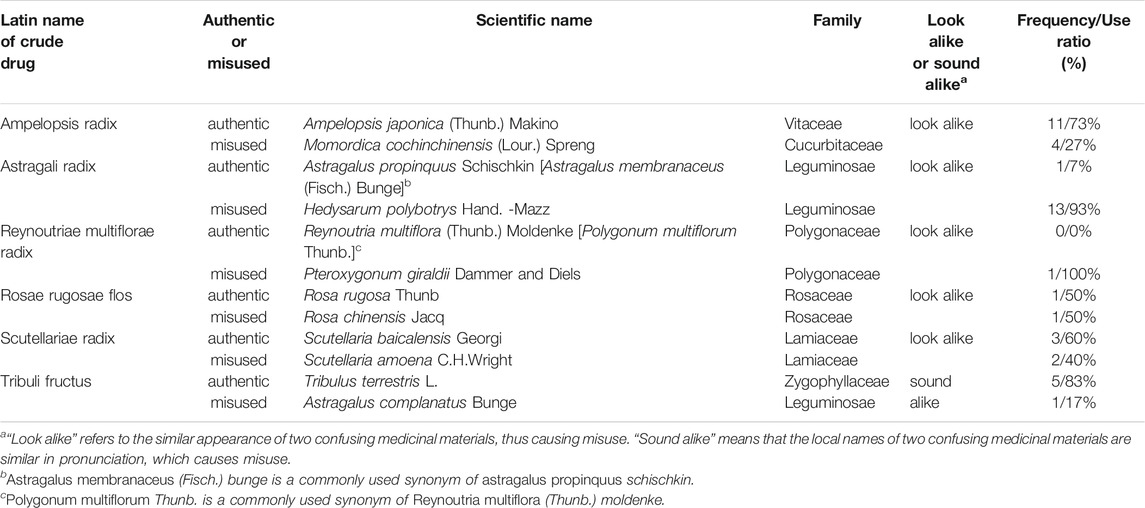
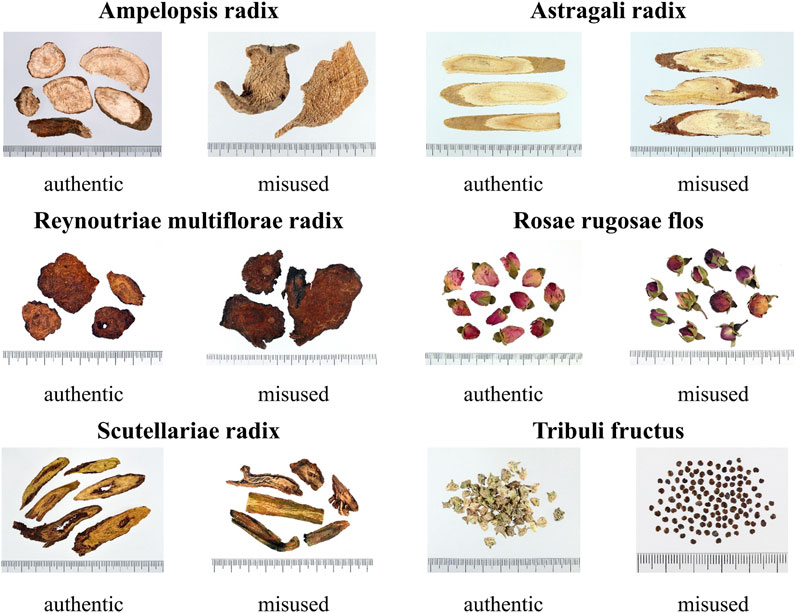
Due to the wide variety of traditional Chinese medicinal materials, some medicinal materials may have the same vernacular name but are composed of different materials, whereas some medicinal materials may have different names but same origin. During integration and analysis of medicinal materials used in skin whitening prescriptions, it was found that Ampelopsis japonica (Thunb.) Makino, Astragalus propinquus Schischkin, Reynoutria multiflora (Thunb.) Moldenke, Rosa rugosa Thunb., Scutellaria baicalensis Georgi, and Tribulus terrestris L. had misused sound alike or look alike medicinal materials (Table 3).
4 Discussion
4.1 Field Investigation Sites
In this study, field investigation was employed to study skin whitening prescriptions sold in TCM pharmacies in Taiwan. Field investigations are mostly used in sociology, geography, or cultural studies and was previously employed to examine the drug treatment habits for certain diseases in some regions, such as traditional Chinese medicine composition used in galactagogues prescriptions (Chao et al., 2020), herbal composition of Qīng-Căo-Chá tea (Huang et al., 2020), and medicinal materials used for hypertension (Baharvand-Ahmadi et al., 2016). Traditional Chinese Medicine, the mainstay of Asian culture, is a form of experience-based therapies, and is a medical care system for diagnosing, preventing, and treating diseases (Xu et al., 2013). Therefore, traditional Chinese pharmacies in Taiwan are important sites for retaining TCM culture.
4.2 Types and Biological Taxonomic Characteristics of Medicinal Materials in Skin Whitening Prescriptions
This study on the composition of skin whitening prescriptions used in Taiwan found that most medicinal materials were from Apiaceae, including Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav., Angelica sinensis (Oliv.) Diels and Ligusticum striatum DC., followed by Leguminosae, including Glycyrrhiza uralensis Fisch. and Astragalus propinquus Schischkin. Apiaceae and Leguminosae plants can inhibit tyrosinase activity, thereby reducing melanogenesis. These plants are rich in phenolic compounds and flavonoids that are proven to have significant antioxidant activity. The previous experiments also showed that they can inhibit matrix metalloproteinases (MMPs), delay skin photoaging, and stimulate keratinocyte and fibroblast migration, which has significant effects on skin regeneration (Tundis et al., 2015; Waqas et al., 2015; Zofia et al., 2020).
Melanin is an important pigment that determines skin, hair, and eye colors, and can be mainly divided into pheomelanin and eumelanin (Zanetti et al., 2001). Melanin synthesis is intimately associated with tyrosinase. Pigmentation is an important photoprotective factor, and its regulatory mechanism is extremely complex and still not completely understood. However, a large volume of data shows that ultraviolet-induced DNA damage and its repair will activate tyrosinase in melanocytes, resulting in melanogenesis (Gilchrest and Eller, 1999; Brenner and Hearing, 2008; Lai et al., 2018). Therefore, inhibition of tyrosinase can inhibit melanogenesis.
4.3 Analysis of Effects and Pharmacology of Commonly Used Medicinal Materials Used in Skin Whitening Prescriptions
TCM has a unique theory where medicinal materials are classified by properties (hot, warm, plain, cool, and cold) and flavors (sour, bitter, sweet, pungent, salty, plain, and astringent). Most TCM materials comprise a combination of flavors (Liao et al., 2008). The 1H-NMR spectrum was used to identify the properties of the medicinal materials, and it was found that their ingredients were very different (Zhang et al., 2020). A previous report showed that warm and hot medicinal materials can regulate the immune system; cold and cool medicinal materials can inhibit cell growth and proliferation (Liang et al., 2013); sweet medicinal materials have supplementation, moderation, and harmonization effects (He et al., 2012), while bitter medicinal materials mostly contain alkaloids with anti-inflammatory effects (Chen et al., 2015). The results of this study found that commonly used medicinal materials used in oral skin whitening prescriptions are mostly warm and sweet while those used in external skin whitening prescriptions are mostly cold and bitter. In combination with previous studies, it can be deduced that oral skin whitening prescriptions mostly focus on immune regulation while external skin whitening prescriptions focus on inflammation alleviation.
4.4 Analysis of Commonly Used Medicinal Materials Used in Oral and External Skin Whitening Prescriptions
Wolfiporia extensa (Peck) Ginns, Paeonia lactiflora Pall. (white), Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav., and Atractylodes macrocephala Koidz. are commonly used medicinal materials used in oral and external skin whitening prescriptions. Wolfiporia extensa (Peck) Ginns regulates tyrosinase activity to inhibit melanogenesis (Lee and Cha, 2018). In past studies had found that Paeonia lactiflora Pall. (white) can be used to treat allergic dermatitis and reduce facial wrinkles. Paeoniflorin in Paeonia lactiflora Pall. (white) can reduce the expression of microphthalmia-associated transcription factor (MITF) and melanogenic enzymes (including tyrosinase, TRP-1, and TRP-2) by regulating the p38 MAPK pathway, thereby inhibiting melanogenesis (Qiu et al., 2016). Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. is a good immunomodulatory agent, and it can significantly increase phagocytosis and the secretion of cytokines by macrophages (Wang et al., 2021). It can also inhibit melanogenesis. It is involved inhibition of tyrosinase synthesis, but it does not inhibit tyrosinase activity (Cho et al., 2006). A study highlighted that the extract of Atractylodes macrocephala Koidz. has immune-enhancing activities (Sun et al., 2015a). Its active ingredients are Atractylenolide I and 14-acetoxy-12-senecioyloxytetradeca-2E, 8E, 10E-trien-4,6-diyn-1-ol. They are both alkaloids that demonstrate anti-inflammatory effects and are tyrosinase inhibitors (Li et al., 2007; Ye et al., 2010).
4.5 Misuse of Medicinal Materials in Skin Whitening Prescriptions
The Taiwan Herbal Pharmacopeia is a codex of management regulations for TCM in Taiwan. The medicinal materials recorded in monographs should comply with the pharmacopeia standards before being manufactured, sold, and dispensed for medical treatment and health care. Medicinal materials recorded in the Taiwan Herbal Pharmacopeia are defined as authentic medicinal materials, while the origins of medicinal materials not included in the pharmacopeia are regarded as misused or fake medicinal materials (Taiwan Herbal Pharmacopeia 3rd Edition Committee, 2018; Taiwan Herbal Pharmacopeia 4th Edition Committee, 2021). Although the government releases information about authentic medicinal materials every year, medicinal materials’ misuse is still commonly seen in markets in Taiwan. Such misused materials were also found in skin whitening prescriptions that were collected in this study, including medicinal materials that look alike, which refers to the similar appearance of two confusing medicinal materials, thus causing misuse, such as Momordica cochinchinensis (Lour.) Spreng. that was used instead of Ampelopsis japonica (Thunb.) Makino, Hedysarum polybotrys Hand-Mazz. that was used instead of Astragalus propinquus Schischkin, Scutellaria amoena C.H.Wright that was used instead of Scutellaria baicalensis Georgi, Pteroxygonum giraldii Dammer & Diels that was used instead of Reynoutria multiflora (Thunb.) Moldenke, and Rosa chinensis Jacq. that was used instead of Rosa rugosa Thunb.; and medicinal materials that sound alike, which means that the local names of two confusing medicinal materials are similar in pronunciation, thus causing misuse, such as Astragalus complanatus Bunge that was used instead of Tribulus terrestris L (Figure 9). Even though misused medicinal materials in skin whitening prescriptions do not seem to cause immediate harm to the human body, there is no way to prove whether they will have adverse interactions with other medicinal materials. Therefore, simple and clear pictures showing that appearance and properties of medicinal materials should be created, and rapid, simple, and convenient identification methods should be developed to disseminate knowledge for identification of misused medicinal materials and remind traditional Chinese medicine users to be cautious and prevent misuse of medicinal materials.
4.6 Limitations and Future Directions
This study had certain limitations that should be addressed in future research. The first limitation is that this study was a field survey study, which only investigated skin whitening prescriptions sold in Chinese medicine stores in Taiwan. The skin whitening prescriptions investigated in this study reflect local usage in Taiwan. Further research will be needed to examine the whitening prescriptions used in traditional Chinese medicine in other regions. The second limitation is that this study only analyzed the core medicinal materials and dosages of the prescriptions but not the efficacy of these botanical drugs. Therefore, animal or cell experiments can be used in future research to verify the effectiveness of the skin whitening medicinal materials or prescriptions. The last limitation is that the Spearman correlation analysis only shows the correlations between prescription drugs and not the drug interactions between them. When discussing prescriptions in future research, we may have to consider both the theoretical significance of traditional Chinese medicine and the practical significance. We should also conduct in vivo and in vitro experiments to further explore the efficacy and the interactions between the medicinal materials.
5 Conclusion
In Asian countries, whiter skin color is synonymous with beauty for women and many Asian women look for natural and without side effects skin whitening products in order to reduce cutaneous pigmentation. This study is the first ethnobotanical survey on skin whitening prescriptions collected from TCM pharmacies in Taiwan. The purpose is to preserve the use of TCM for skin whitening in Taiwan. Although the use of TCM in skin whitening has been widely recorded and many skin whitening medicinal materials were collected from TCM pharmacies in this study, the ingredients of TCM are extremely complex and tends to be affected by many factors, may also be used in misused medicinal materials. Moreover, the efficacy and safety of most medicinal materials have not been scientifically validated. Further studies are needed to support the conversion of traditional skin whitening prescriptions to effective functional products. Thus, the results of this study may provide significant foundational data for subsequent studies The Royal Botanic Gardens, 2013, Li et al., 2017, Institute of Ecology et al., 2021.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the CRREC-109-125. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
C-YK, P-YC, JC, and S-SH performed the field investigation and organized and analyzed the database. C-YK, JC, S-SH, TM, and C-YL contributed conception and design of the study. S-SH and S-YS identified the botanical materials. C-YK, S-SH, JC, S-YS, TM, and C-YL drafted the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. S-SH, JC, S-YS, and H-CC provided guidance for the project and supervised the experiment and manuscript review.
Funding
This research was funded by the Tsuzuki Institute for Traditional Medicine, Grant numbers 108727B8, 109727B8 and 110727B8; China Medical University, Grant numbers CMU108-MF-116 and CMU108-N-22; the Ministry of Science and Technology, Grant number MOST 107-2320-B-039-030-MY3 and 109-2813-C-039-061-B. The China Medical University under the Higher Education Sprout Project and Teaching Practice Research Program, Grant number 1077170A, of the Ministry of Education of Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the herbarium of China Medical University for providing us with space to store the medicinal materials for this study. Also, we would like to thank the 63rd pharmacy students of China Medical University for their assistance in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.736370/full#supplementary-material
References
Abbas, Z., Kousar, S., Aziz, M. A., Pieroni, A., Aldosari, A. A., Bussmann, R. W., et al. (2021). Comparative Assessment of Medicinal Plant Utilization Among Balti and Shina Communities in the Periphery of Deosai National Park, Pakistan. Biology (Basel) 105, 434. doi:10.3390/biology10050434
Amen, Y., Arung, E. T., Afifi, M. S., Halim, A. F., Ashour, A., Fujimoto, R., et al. (2017). Melanogenesis Inhibitors from Coix Lacryma-Jobi Seeds in B16-F10 Melanoma Cells. Nat. Prod. Res. 31, 2712–2718. doi:10.1080/14786419.2017.1292270
An, E. J., Kim, Y., Lee, S. H., Choi, S. H., Chung, W. S., and Jang, H. J. (2020). Ophiopogonin D Ameliorates DNCB-Induced Atopic Dermatitis-like Lesions in BALB/c Mice and TNF-α- Inflamed HaCaT Cell. Biochem. Biophys. Res. Commun. 522, 40–46. doi:10.1016/j.bbrc.2019.10.190
Baharvand-Ahmadi, B., Bahmani, M., Tajeddini, P., Rafieian-Kopaei, M., and Naghdi, N. (2016). An Ethnobotanical Study of Medicinal Plants Administered for the Treatment of Hypertension. J. Ren. Inj Prev 5, 123–128. doi:10.15171/jrip.2016.26
Bai, X. Z., Hu, D. H., Wang, Y. C., Liu, J. Q., Shi, J. H., and Tang, C. W. (2012). Effects of Angelica Dahurica Extract on Biological Behavior of Dermal Fibroblasts. Zhonghua Wai Ke Za Zhi 50, 357–360.
Balaguer, A., Chisvert, A., and Salvador, A. (2008). Environmentally Friendly LC for the Simultaneous Determination of Ascorbic Acid and its Derivatives in Skin-Whitening Cosmetics. J. Sep. Sci. 31, 229–236. doi:10.1002/jssc.200700414
Berezutsky, M. A., Durnova, N. A., and Vlasova, I. A. (2019). Experimental and Clinical Studies of Mechanisms of the Anti-aging Effects of Chemical Compounds in Astragalus Membranaceus (Review). Adv. Gerontol. 32, 702–710. doi:10.1134/S2079057020020046
Brenner, M., and Hearing, V. J. (2008). The Protective Role of Melanin against UV Damage in Human Skin. Photochem. Photobiol. 84, 539–549. doi:10.1111/j.1751-1097.2007.00226.x
Byun, A. R., Kwon, S., and Kim, S. (2016). Administration of Herbal Complexes, Dangguijakyak-San (TJ-23) and Coix Seeds, for Treating Verruca Planae: A Case Report. Explore (NY) 12, 65–67. doi:10.1016/j.explore.2015.10.003
Cenariu, D., Fischer-Fodor, E., Țigu, A. B., Bunea, A., Virág, P., Perde-Schrepler, M., et al. (2021). Zeaxanthin-Rich Extract from Superfood Lycium Barbarum Selectively Modulates the Cellular Adhesion and MAPK Signaling in Melanoma versus Normal Skin Cells In Vitro. Molecules 26, 333. doi:10.3390/molecules26020333
Cha, H. Y., Ahn, S. H., Cheon, J. H., Park, I. S., Kim, J. T., and Kim, K. (2016). Hataedock Treatment Has Preventive Therapeutic Effects in Atopic Dermatitis-Induced NC/Nga Mice under High-Fat Diet Conditions. Evid. Based Complement. Alternat Med. 2016, 1739760. doi:10.1155/2016/1739760
Cha, H. Y., Ahn, S. H., Cheon, J. H., Park, S. Y., and Kim, K. (2017). Hataedock Treatment Has Preventive Therapeutic Effects for Atopic Dermatitis through Skin Barrier protection in Dermatophagoides Farinae-Induced NC/Nga Mice. J. Ethnopharmacol 206, 327–336. doi:10.1016/j.jep.2017.06.001
Chao, J., Ko, C. Y., Lin, C. Y., Tomoji, M., Huang, C. H., Chiang, H. C., et al. (2020). Ethnobotanical Survey of Natural Galactagogues Prescribed in Traditional Chinese Medicine Pharmacies in Taiwan. Front. Pharmacol. 11, 625869. doi:10.3389/fphar.2020.625869
Chen, H., Guo, J., Pang, B., Zhao, L., and Tong, X. (2015). Application of Herbal Medicines with Bitter Flavor and Cold Property on Treating Diabetes Mellitus. Evid. Based Complement. Alternat Med. 2015, 529491. doi:10.1155/2015/529491
Chen, T., Guo, Z. P., Jiao, X. Y., Jia, R. Z., Zhang, Y. H., Li, J. Y., et al. (2011). Peoniflorin Suppresses Tumor Necrosis Factor-α Induced Chemokine Production in Human Dermal Microvascular Endothelial Cells by Blocking Nuclear Factor-Κb and ERK Pathway. Arch. Dermatol. Res. 303, 351–360. doi:10.1007/s00403-010-1116-6
Chen, T., Guo, Z. P., Wang, L., Qin, S., Cao, N., Li, M. M., et al. (2013). Paeoniflorin Suppresses Vascular Damage and the Expression of E-Selectin and ICAM-1 in a Mouse Model of Cutaneous Arthus Reaction. Exp. Dermatol. 22, 453–457. doi:10.1111/exd.12174
Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of the People's Republic of China. China: China Medical Science Press.
Cho, Y. H., Kim, J. H., Park, S. M., Lee, B. C., Pyo, H. B., and Park, H. D. (2006). New Cosmetic Agents for Skin Whitening from Angelica Dahurica. J. Cosmet. Sci. 57, 11–21.
Choi, E., Kang, Y. G., Hwang, S. H., Kim, J. K., Hong, Y. D., Park, W. S., et al. (2019). In Vitro Effects of Dehydrotrametenolic Acid on Skin Barrier Function. Molecules 24, 4583. doi:10.3390/molecules24244583
Choi, M. R., Choi, D. K., Sohn, K. C., Lim, S. K., Kim, D. I., Lee, Y. H., et al. (2015). Inhibitory Effect of Paeonia Lactiflora Pallas Extract (PE) on Poly (I:C)-induced Immune Response of Epidermal Keratinocytes. Int. J. Clin. Exp. Pathol. 8, 5236–5241.
Choi, Y. Y., Kim, M. H., Hong, J., Kim, K., and Yang, W. M. (2016). Effect of Dangguibohyul-Tang, a Mixed Extract of Astragalus Membranaceus and Angelica Sinensis, on Allergic and Inflammatory Skin Reaction Compared with Single Extracts of Astragalus Membranaceus or Angelica Sinensis. Evid. Based Complement. Alternat Med. 2016, 5936354. doi:10.1155/2016/5936354
Chung, C. P., Hsia, S. M., Lee, M. Y., Chen, H. J., Cheng, F., Chan, L. C., et al. (2011). Gastroprotective Activities of Adlay (Coix Lachryma-Jobi L. Var. Ma-Yuen Stapf) on the Growth of the Stomach Cancer AGS Cell Line and Indomethacin-Induced Gastric Ulcers. J. Agric. Food Chem. 59, 6025–6033. doi:10.1021/jf2009556
Deng, G., Chen, W., Wang, P., Zhan, T., Zheng, W., Gu, Z., et al. (2019). Inhibition of NLRP3 Inflammasome-Mediated Pyroptosis in Macrophage by Cycloastragenol Contributes to Amelioration of Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice. Int. Immunopharmacol 74, 105682. doi:10.1016/j.intimp.2019.105682
Dixit, S., and Tiwari, S. (2020). Investigation of Anti-diabetic Plants Used Among the Ethnic Communities of Kanpur Division, India. J. Ethnopharmacol 253, 112639. doi:10.1016/j.jep.2020.112639
Fang, C. L., Paul, C. R., Day, C. H., Chang, R. L., Kuo, C. H., Ho, T. J., et al. (2021). Poria Cocos (Fuling) Targets TGFβ/Smad7 Associated Collagen Accumulation and Enhances Nrf2-Antioxidant Mechanism to Exert Anti-skin Aging Effects in Human Dermal Fibroblasts. Environ. Toxicol. 36, 729–736. doi:10.1002/tox.23075
Fazio, A., La Torre, C., Caroleo, M. C., Caputo, P., Plastina, P., and Cione, E. (2020). Isolation and Purification of Glucans from an Italian Cultivar of Ziziphus Jujuba Mill. And In Vitro Effect on Skin Repair. Molecules 25, 968. doi:10.3390/molecules25040968
Fong, P., and Tong, H. H. (2012). In Silico prediction of the Cosmetic Whitening Effects of Naturally Occurring lead Compounds. Nat. Prod. Commun. 7, 1287–1294. doi:10.1177/1934578x1200701010
Food and Drug Administration, (2014). Know Cosmetic Whitening Ingredients. [Online]. Available: https://www.mohw.gov.tw/cp-3205-21666-1.html (Accessed Jun 17, 2021).
Gao, H. W., Chang, K. F., Huang, X. F., Lin, Y. L., Weng, J. C., Liao, K. W., et al. (2018). Antitumor Effect of N-Butylidenephthalide Encapsulated on B16/F10 Melanoma Cells In Vitro with a Polycationic Liposome Containing PEI and Polyethylene Glycol Complex. Molecules 23, 3224. doi:10.3390/molecules23123224
Gilchrest, B. A., and Eller, M. S. (1999). DNA Photodamage Stimulates Melanogenesis and Other Photoprotective Responses. J. Investig. Dermatol. Symp. Proc. 4, 35–40. doi:10.1038/sj.jidsp.5640178
Gillbro, J. M., and Olsson, M. J. (2011). The Melanogenesis and Mechanisms of Skin-Lightening Agents-Eexisting and New Approaches. Int. J. Cosmet. Sci. 33, 210–221. doi:10.1111/j.1468-2494.2010.00616.x
Gong, P., Lu, Z., Xing, J., Wang, N., and Zhang, Y. (2015). Traditional Chinese Medicine Xuebijing Treatment Is Associated with Decreased Mortality Risk of Patients with Moderate Paraquat Poisoning. PLoS One 10, e0123504. doi:10.1371/journal.pone.0123504
Hao, B., Wang, X., Ma, X., Jin, Y., Fan, W., Laba, C., et al. (2020). Preparation of Complex Microcapsules of Soluble Polysaccharide from Glycyrrhiza Uralensis and its Application in Wound Repair and Scar Inhibition. Int. J. Biol. Macromol 156, 906–917. doi:10.1016/j.ijbiomac.2020.03.121
He, X., Wang, X., Fang, J., Zhao, Z., Huang, L., Guo, H., et al. (2017). Bletilla Striata: Medicinal Uses, Phytochemistry and Pharmacological Activities. J. Ethnopharmacol 195, 20–38. doi:10.1016/j.jep.2016.11.026
He, Y., Zheng, X., Sit, C., Loo, W. T., Wang, Z., Xie, T., et al. (2012). Using Association Rules Mining to Explore Pattern of Chinese Medicinal Formulae (Prescription) in Treating and Preventing Breast Cancer Recurrence and Metastasis. J. Transl Med. 10 (1), S12. doi:10.1186/1479-5876-10-S1-S12
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best Practice in Research - Overcoming Common Challenges in Phytopharmacological Research. J. Ethnopharmacol 246, 112230. doi:10.1016/j.jep.2019.112230
Heinrich, M., Lardos, A., Leonti, M., Weckerle, C., Willcox, M., Applequist, W., et al. (2018). Best Practice in Research: Consensus Statement on Ethnopharmacological Field Studies - ConSEFS. J. Ethnopharmacol 211, 329–339. doi:10.1016/j.jep.2017.08.015
Ho, C. C., Ng, S. C., Chuang, H. L., Chen, J. Y., Wen, S. Y., Kuo, C. H., et al. (2021). Seven Traditional Chinese Herbal Extracts Fermented by Lactobacillus Rhamnosus Provide Anti-pigmentation Effects by Regulating the CREB/MITF/tyrosinase Pathway. Environ. Toxicol. 36, 654–664. doi:10.1002/tox.23069
Hong, M. J., Ko, E. B., Park, S. K., and Chang, M. S. (2013). Inhibitory Effect of Astragalus Membranaceus Root on Matrix Metalloproteinase-1 Collagenase Expression and Procollagen Destruction in Ultraviolet B-Irradiated Human Dermal Fibroblasts by Suppressing Nuclear Factor Kappa-B Activity. J. Pharm. Pharmacol. 65, 142–148. doi:10.1111/j.2042-7158.2012.01570.x
Hsiao, C. Y., Hung, C. Y., Tsai, T. H., and Chak, K. F. (20122012). A Study of the Wound Healing Mechanism of a Traditional Chinese Medicine, Angelica Sinensis, Using a Proteomic Approach. Evid. Based Complement. Alternat Med. 2012, 467531. doi:10.1155/2012/467531
Hu, Y., Zeng, H., Huang, J., Jiang, L., Chen, J., and Zeng, Q. (2020). Traditional Asian Herbs in Skin Whitening: The Current Development and Limitations. Front. Pharmacol. 11, 982. doi:10.3389/fphar.2020.00982
Huang, H. C., Hsieh, W. Y., Niu, Y. L., and Chang, T. M. (2014). Inhibitory Effects of Adlay Extract on Melanin Production and Cellular Oxygen Stress in B16F10 Melanoma Cells. Int. J. Mol. Sci. 15, 16665–16679. doi:10.3390/ijms150916665
Huang, S. S., Chen, T. Y., Deng, J. S., Pao, L. H., Cheng, Y. C., and Chao, J. (2020). An Ethnobotanical Study on Qīng-Căo-Chá Tea in Taiwan. Front. Pharmacol. 11, 931. doi:10.3389/fphar.2020.00931
Hung, C. F., Hsu, B. Y., Chang, S. C., and Chen, B. H. (2012). Antiproliferation of Melanoma Cells by Polysaccharide Isolated from Zizyphus Jujuba. Nutrition 28, 98–105. doi:10.1016/j.nut.2011.05.009
Hwang, Y. L., Im, M., Lee, M. H., Roh, S. S., Choi, B. W., Kim, S. J., et al. (2016). Inhibitory Effect of Imperatorin on Insulin-like Growth Factor-1-Induced Sebum Production in Human Sebocytes Cultured In Vitro. Life Sci. 144, 49–53. doi:10.1016/j.lfs.2015.11.027
Hwangbo, H., Choi, E. O., Kim, M. Y., Kwon, D. H., Ji, S. Y., Lee, H., et al. (2020). Suppression of Tumor Growth and Metastasis by Ethanol Extract of Angelica Dahurica Radix in Murine Melanoma B16F10 Cells. Biosci. Trends 14, 23–34. doi:10.5582/bst.2019.01230
Im, A. R., Lee, H. J., Youn, U. J., Hyun, J. W., and Chae, S. (2016). Orally Administered Betaine Reduces Photodamage Caused by UVB Irradiation through the Regulation of Matrix Metalloproteinase-9 Activity in Hairless Mice. Mol. Med. Rep. 13, 823–828. doi:10.3892/mmr.2015.4613
Institute of Ecology and Evolutionary Biology, NTU (2021). Plants of Taiwan. [Online]. Available: http://tai2.ntu.edu.tw (Accessed May 6, 2021).
Jegal, J., Park, N. J., Bong, S. K., Jegal, H., Kim, S. N., and Yang, M. H. (2017). Dioscorea Quinqueloba Ameliorates Oxazolone- and 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis Symptoms in Murine Models. Nutrients 9, 1324. doi:10.3390/nu9121324
Jegal, J., Park, N. J., Jo, B. G., Bong, S. K., Jegal, H., Yang, M. H., et al. (2018). Anti-Atopic Properties of Gracillin Isolated from Dioscorea Quinqueloba on 2,4-Dinitrochlorobenzene-Induced Skin Lesions in Mice. Nutrients 10, 1205. doi:10.3390/nu10091205
Jeong, S. J., Lim, H. S., Seo, C. S., Kim, J. H., Jin, S. E., Yoo, S. R., et al. (2015). Traditional Herbal Formula Jakyakgamcho-Tang (Paeonia Lactiflora and Glycyrrhiza Uralensis) Impairs Inflammatory Chemokine Production by Inhibiting Activation of STAT1 and NF-Κb in HaCaT Cells. Phytomedicine 22, 326–332. doi:10.1016/j.phymed.2014.12.002
Kalekhan, F., Kudva, A. K., Raghu, S. V., Rao, S., Hegde, S. K., Simon, P., et al. (2021). Traditionally Used Natural Products in Preventing Ionizing Radiation-Induced Dermatitis: First Review on the Clinical Studies. Anticancer Agents Med. Chem. 21. doi:10.2174/1871520621666210405093236
Kang, K. Y., Hwang, Y. H., Lee, S. J., Jang, H. Y., Hong, S. G., Mun, S. K., et al. (2019). Verification of the Functional Antioxidant Activity and Antimelanogenic Properties of Extracts of Poria Cocos Mycelium Fermented with Freeze-Dried Plum Powder. Int. J. Biomater. 2019, 9283207. doi:10.1155/2019/9283207
Kim, J. H., Kim, M. H., Yang, G., Huh, Y., Kim, S. H., and Yang, W. M. (2013). Effects of Topical Application of Astragalus Membranaceus on Allergic Dermatitis. Immunopharmacol Immunotoxicol 35, 151–156. doi:10.3109/08923973.2012.733708
Kim, K. H., Shim, J. S., Kim, H. J., and Son, E. D. (2020). Penta-O-galloyl-β-D-glucose from Paeonia Lactiflora Pall. Root Extract Enhances the Expression of Skin Barrier Genes via EGR3. J. Ethnopharmacol 248, 112337. doi:10.1016/j.jep.2019.112337
Kim, K. J., Xuan, S. H., and Park, S. N. (2017). Licoricidin, an Isoflavonoid Isolated from Glycyrrhiza Uralensis Fisher, Prevents UVA-Induced Photoaging of Human Dermal Fibroblasts. Int. J. Cosmet. Sci. 39, 133–140. doi:10.1111/ics.12357
Kim, M. K., Bang, C. Y., Kim, M. Y., Lee, J. H., Ro, H., Choi, M. S., et al. (2016). Traditional Herbal Prescription LASAP-C Inhibits Melanin Synthesis in B16F10 Melanoma Cells and Zebrafish. BMC Complement. Altern. Med. 16, 223. doi:10.1186/s12906-016-1209-7
Kitahiro, Y., Koike, A., Sonoki, A., Muto, M., Ozaki, K., and Shibano, M. (2018). Anti-inflammatory Activities of Ophiopogonis Radix on Hydrogen Peroxide-Induced Cellular Senescence of normal Human Dermal Fibroblasts. J. Nat. Med. 72, 905–914. doi:10.1007/s11418-018-1223-9
Ku, J. M., Hong, S. H., Kim, H. I., Seo, H. S., Shin, Y. C., and Ko, S. G. (2017). Effects of Angelicae Dahuricae Radix on 2, 4-Dinitrochlorobenzene-Induced Atopic Dermatitis-like Skin Lesions in Mice Model. BMC Complement. Altern. Med. 17, 98. doi:10.1186/s12906-017-1584-8
Lai, X., Wichers, H. J., Soler-Lopez, M., and Dijkstra, B. W. (2018). Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chemistry 24, 47–55. doi:10.1002/chem.201704410
Lee, H., and Cha, H. J. (2018). Poria Cocos Wolf Extracts Represses Pigmentation In Vitro and In Vivo. Cel Mol Biol (Noisy-le-grand) 64, 80–84. doi:10.14715/cmb/2018.64.5.13
Lee, H., Lee, J. K., Ha, H., Lee, M. Y., Seo, C. S., and Shin, H. K. (2012). Angelicae Dahuricae Radix Inhibits Dust Mite Extract-Induced Atopic Dermatitis-like Skin Lesions in NC/Nga Mice. Evid. Based Complement. Alternat Med. 2012, 743075. doi:10.1155/2012/743075
Lee, J., Choi, Y. Y., Kim, M. H., Han, J. M., Lee, J. E., Kim, E. H., et al. (2016). Topical Application of Angelica Sinensis Improves Pruritus and Skin Inflammation in Mice with Atopic Dermatitis-like Symptoms. J. Med. Food 19, 98–105. doi:10.1089/jmf.2015.3489
Lee, J. H., Lim, J. Y., Jo, E. H., Noh, H. M., Park, S., Park, M. C., et al. (2020). Chijabyukpi-Tang Inhibits Pro-inflammatory Cytokines and Chemokines via the Nrf2/HO-1 Signaling Pathway in TNF-Α/ifn-γ-Stimulated HaCaT Cells and Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-like Skin Lesions in Mice. Front. Pharmacol. 11, 1018. doi:10.3389/fphar.2020.01018
Lee, K., Lee, B., Lee, M. H., Kim, B., Chinannai, K. S., Ham, I., et al. (2015). Effect of Ampelopsis Radix on Wound Healing in Scalded Rats. BMC Complement. Altern. Med. 15, 213. doi:10.1186/s12906-015-0751-z
Li, B., He, S., Liu, R., Huang, L., Liu, G., Wang, R., et al. (2019). Total Glucosides of Paeony Attenuates Animal Psoriasis Induced Inflammatory Response through Inhibiting STAT1 and STAT3 Phosphorylation. J. Ethnopharmacol 243, 112121. doi:10.1016/j.jep.2019.112121
Li, C. Q., He, L. C., Dong, H. Y., and Jin, J. Q. (2007). Screening for the Anti-inflammatory Activity of Fractions and Compounds from Atractylodes Macrocephala Koidz. J. Ethnopharmacol 114, 212–217. doi:10.1016/j.jep.2007.08.002
Li, H., Li, Z., Peng, L., Jiang, N., Liu, Q., Zhang, E., et al. (2017). Lycium Barbarum Polysaccharide Protects Human Keratinocytes against UVB-Induced Photo-Damage. Free Radic. Res. 51, 200–210. doi:10.1080/10715762.2017.1294755
Liang, B., Peng, L., Li, R., Li, H., Mo, Z., Dai, X., et al. (2018). Lycium Barbarum Polysaccharide Protects HSF Cells against Ultraviolet-Induced Damage through the Activation of Nrf2. Cell Mol Biol Lett 23, 18. doi:10.1186/s11658-018-0084-2
Liang, F., Li, L., Wang, M., Niu, X., Zhan, J., He, X., et al. (2013). Molecular Network and Chemical Fragment-Based Characteristics of Medicinal Herbs with Cold and Hot Properties from Chinese Medicine. J. Ethnopharmacol 148, 770–779. doi:10.1016/j.jep.2013.04.055
Liao, H., Banbury, L. K., and Leach, D. N. (2008). Antioxidant Activity of 45 Chinese Herbs and the Relationship with Their TCM Characteristics. Evid. Based Complement. Alternat Med. 5 (4), 429–434. doi:10.1093/ecam/nem054
Lim, J. W., Ha, J. H., Jeong, Y. J., and Park, S. N. (2018). Anti-melanogenesis Effect of Dehydroglyasperin C through the Downregulation of MITF via the Reduction of Intracellular cAMP and Acceleration of ERK Activation in B16F1 Melanoma Cells. Pharmacol. Rep. 70, 930–935. doi:10.1016/j.pharep.2018.02.024
Lu, Y. S., Jiang, Y., Yuan, J. P., Jiang, S. B., Yang, Y., Zhu, P. Y., et al. (2020). UVA Induced Oxidative Stress Was Inhibited by Paeoniflorin/Nrf2 Signaling or PLIN2. Front. Pharmacol. 11, 736. doi:10.3389/fphar.2020.00736
Luo, X., Huang, P., Yuan, B., Liu, T., Lan, F., Lu, X., et al. (2016). Astragaloside IV Enhances Diabetic Wound Healing Involving Upregulation of Alternatively Activated Macrophages. Int. Immunopharmacol 35, 22–28. doi:10.1016/j.intimp.2016.03.020
Mainzer, C., Le Guillou, M., Vyumvuhore, R., Chadoutaud, B., Bordes, S., and Closs, B. (2019). Clinical Efficacy of Oligofructans from Ophiopogon Japonicus in Reducing Atopic Dermatitis Flare-Ups in Caucasian Patients. Acta Derm Venereol. 99, 858–864. doi:10.2340/00015555-3224
Meng, H., Li, J., Dong, Y., He, Y., Ren, H., Liu, Y., et al. (2020). Poly Traditional Chinese Medicine Formulation Prepared with Skin Moisturizing Properties. Dermatol. Ther. 33, e14105. doi:10.1111/dth.14105
Ministry of Health and Welfare, (2015). Drug Administration. [Online]. Available: https://dep.mohw.gov.tw/DOS/cp-1729-2939-113.html (Accessed May 6, 2021).
Mullane, K., Enna, S. J., Piette, J., and Williams, M. (2015). Guidelines for Manuscript Submission in the Peer-Reviewed Pharmacological Literature. Biochem. Pharmacol. 97, 225–235. doi:10.1016/j.bcp.2015.06.023
Nam, Y. K., Kim, M. H., Ha, I. J., and Yang, W. M. (2021). Derma-Hc, a New Developed Herbal Formula, Ameliorates Cutaneous Lichenification in Atopic Dermatitis. Int. J. Mol. Sci. 22, 2359. doi:10.3390/ijms22052359
Neves, L. M. G., Parizotto, N. A., Tim, C. R., Floriano, E. M., Lopez, R. F. V., Venâncio, T., et al. (2020). Polysaccharide-rich Hydrogel Formulation Combined with Photobiomodulation Repairs UV-Induced Photodamage in Mice Skin. Wound Repair Regen. 28, 645–655. doi:10.1111/wrr.12826
Neves, L. M. G., Tim, C. R., Floriano, E. M., da Silva de Avó, L. R., Fernandes, J. B., Parizotto, N. A., et al. (2021). Lycium Barbarum Polysaccharide Fraction Associated with Photobiomodulation Protects from Epithelium Thickness and Collagen Fragmentation in a Model of Cutaneous Photodamage. Lasers Med. Sci. 36, 863–870. doi:10.1007/s10103-020-03132-w
Nordin, F. N. M., Aziz, A., Zakaria, Z., and Wan Mohamed Radzi, C. W. J. (2021). A Systematic Review on the Skin Whitening Products and Their Ingredients for Safety, Health Risk, and the Halal Status. J. Cosmet. Dermatol. 20, 1050–1060. doi:10.1111/jocd.13691
Pillaiyar, T., Manickam, M., and Namasivayam, V. (2017). Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 32, 403–425. doi:10.1080/14756366.2016.1256882
Qiu, J., Chen, M., Liu, J., Huang, X., Chen, J., Zhou, L., et al. (2016). The Skin-Depigmenting Potential of Paeonia Lactiflora Root Extract and Paeoniflorin: In Vitro Evaluation Using Reconstructed Pigmented Human Epidermis. Int. J. Cosmet. Sci. 38, 444–451. doi:10.1111/ics.12309
Rivera, D., Allkin, R., Obón, C., Alcaraz, F., Verpoorte, R., and Heinrich, M. (2014). What Is in a Name? the Need for Accurate Scientific Nomenclature for Plants. J. Ethnopharmacol 152, 393–402. doi:10.1016/j.jep.2013.12.022
Saba, E., Lee, C. H., Jeong, da. H., Lee, K., Kim, T. H., Roh, S. S., et al. (2016). Fermented rice Bran Prevents Atopic Dermatitis in DNCB-Treated NC/Nga Mice. J. Biomed. Res. 30, 334–343. doi:10.7555/JBR.30.2016K0001
Shan, H., Zheng, X., and Li, M. (2019). The Effects of Astragalus Membranaceus Active Extracts on Autophagy-Related Diseases. Int. J. Mol. Sci. 20, 1904. doi:10.3390/ijms20081904
Shan, S. J., Xiao, T., Chen, J., Geng, S. L., Li, C. P., Xu, X., et al. (2012). Kanglaite Attenuates UVB-Induced Down-Regulation of Aquaporin-3 in Cultured Human Skin Keratinocytes. Int. J. Mol. Med. 29, 625–629. doi:10.3892/ijmm.2011.873
Slominski, A., Tobin, D. J., Shibahara, S., and Wortsman, J. (2004). Melanin Pigmentation in Mammalian Skin and its Hormonal Regulation. Physiol. Rev. 84, 1155–1228. doi:10.1152/physrev.00044.2003
Son, E. S., Kim, S. H., Kim, Y. O., Lee, Y. E., Kyung, S. Y., Jeong, S. H., et al. (2019). Coix Lacryma-Jobi Var. Ma-Yuen Stapf Sprout Extract Induces Cell Cycle Arrest and Apoptosis in Human Cervical Carcinoma Cells. BMC Complement. Altern. Med. 19, 312. doi:10.1186/s12906-019-2725-z
Son, J., and Lee, S. Y. (2020). Ursonic Acid Exerts Inhibitory Effects on Matrix Metalloproteinases via ERK Signaling Pathway. Chem. Biol. Interact 315, 108910. doi:10.1016/j.cbi.2019.108910
Song, Y., Zeng, R., Hu, L., Maffucci, K. G., Ren, X., and Qu, Y. (2017). In Vivo wound Healing and In Vitro Antioxidant Activities of Bletilla Striata Phenolic Extracts. Biomed. Pharmacother. 93, 451–461. doi:10.1016/j.biopha.2017.06.079
Sonthalia, S., Daulatabad, D., and Sarkar, R. (2016). Glutathione as a Skin Whitening Agent: Facts, Myths, Evidence and Controversies. Indian J. Dermatol. Venereol. Leprol. 82, 262–272. doi:10.4103/0378-6323.179088
State Administration of Traditional Chinese Medicine (1999). Chinese Materia Medica" Editorial Board (1999). Chinese Materia Medica. Shanghai: Shanghai Scientific & Technical Publishers.
Sun, W., Meng, K., Qi, C., Yang, X., Wang, Y., Fan, W., et al. (2015a). Immune-enhancing Activity of Polysaccharides Isolated from Atractylodis Macrocephalae Koidz. Carbohydr. Polym. 126, 91–96. doi:10.1016/j.carbpol.2015.03.034
Sun, Y., Zhang, J., Huo, R., Zhai, T., Li, H., Wu, P., et al. (2015b). Paeoniflorin Inhibits Skin Lesions in Imiquimod-Induced Psoriasis-like Mice by Downregulating Inflammation. Int. Immunopharmacol 24, 392–399. doi:10.1016/j.intimp.2014.12.032
Taiwan Herbal Pharmacopeia 3rd Edition Committee (2018). Taiwan Herbal Pharmacopeia. Republic of China: Ministry of Health and Welfare.
Taiwan Herbal Pharmacopeia 4th Edition Committee (2021). Taiwan Herbal Pharmacopeia. Republic of China: Ministry of Health and Welfare.
Tariq, M., Tahir, H. M., Butt, S. A., Ali, S., Ahmad, A. B., Raza, C., et al. (2021). Silk Derived Formulations for Accelerated Wound Healing in Diabetic Mice. PeerJ 9, e10232. doi:10.7717/peerj.10232
The Royal Botanic Gardens (2013). The Plant List. [Online]. Available: http://www.theplantlist.org/ (Accessed May 7, 2021).
Tourism Bureau (2021). General Information. [Online]. Available: https://www.taiwan.net.tw/m1.aspx?sNo=0027009 (Accessed May 6, 2021).
Tsao, Y. T., Kuo, C. Y., Kuan, Y. D., Lin, H. C., Wu, L. H., and Lee, C. H. (2017). The Extracts of Astragalus Membranaceus Inhibit Melanogenesis through the ERK Signaling Pathway. Int. J. Med. Sci. 14, 1049–1053. doi:10.7150/ijms.20335
Tsukayama, I., Toda, K., Takeda, Y., Mega, T., Tanaka, M., Kawakami, Y., et al. (2018). Preventive Effect of Dioscorea Japonica on Squamous Cell Carcinoma of Mouse Skin Involving Down-Regulation of Prostaglandin E2 Synthetic Pathway. J. Clin. Biochem. Nutr. 62, 139–147. doi:10.3164/jcbn.17-54
Tundis, R., Loizzo, M. R., Bonesi, M., and Menichini, F. (2015). Potential Role of Natural Compounds against Skin Aging. Curr. Med. Chem. 22, 1515–1538. doi:10.2174/0929867322666150227151809
Ullah, S., Park, C., Ikram, M., Kang, D., Lee, S., Yang, J., et al. (2019). Tyrosinase Inhibition and Anti-melanin Generation Effect of Cinnamamide Analogues. Bioorg. Chem. 87, 43–55. doi:10.1016/j.bioorg.2019.03.001
Vacanti, N. M. (2019). The Fundamentals of Constructing and Interpreting Heat Maps. Methods Mol. Biol. 1862, 279–291. doi:10.1007/978-1-4939-8769-6_20
Vitalini, S., Iriti, M., Puricelli, C., Ciuchi, D., Segale, A., and Fico, G. (2013). Traditional Knowledge on Medicinal and Food Plants Used in Val San Giacomo (Sondrio, Italy)--an alpine Ethnobotanical Study. J. Ethnopharmacol 145, 517–529. doi:10.1016/j.jep.2012.11.024
Wang, H., Wang, X., Zhou, L., Zhang, S., An, L., Bao, J., et al. (2021). Structural Characteristics and In Vitro and In Vivo Immunoregulatory Properties of a Gluco-Arabinan from Angelica Dahurica. Int. J. Biol. Macromol 183, 90–100. doi:10.1016/j.ijbiomac.2021.04.077
Wang, J., Chen, X., Wang, W., Zhang, Y., Yang, Z., Jin, Y., et al. (2013a). Glycyrrhizic Acid as the Antiviral Component of Glycyrrhiza Uralensis Fisch. Against Coxsackievirus A16 and Enterovirus 71 of Hand Foot and Mouth Disease. J. Ethnopharmacol 147, 114–121. doi:10.1016/j.jep.2013.02.017
Wang, R., Lechtenberg, M., Sendker, J., Petereit, F., Deters, A., and Hensel, A. (2013b). Wound-healing Plants from TCM: In Vitro Investigations on Selected TCM Plants and Their Influence on Human Dermal Fibroblasts and Keratinocytes. Fitoterapia 84, 308–317. doi:10.1016/j.fitote.2012.12.020
Waqas, M. K., Akhtar, N., Mustafa, R., Jamshaid, M., Khan, H. M., and Murtaza, G. (2015). Dermatological and Cosmeceutical Benefits of Glycine max (Soybean) and its Active Components. Acta Pol. Pharm. 72, 3–11.
Wu, P. Y., Li, T. M., Chen, S. I., Chen, C. J., Chiou, J. S., Lin, M. K., et al. (2020). Complementary Chinese Herbal Medicine Therapy Improves Survival in Patients with Pemphigus: A Retrospective Study from a Taiwan-Based Registry. Front. Pharmacol. 11, 594486. doi:10.3389/fphar.2020.594486
Xu, Q., Bauer, R., Hendry, B. M., Fan, T. P., Zhao, Z., Duez, P., et al. (2013). The Quest for Modernisation of Traditional Chinese Medicine. BMC Complement. Altern. Med. 13, 132. doi:10.1186/1472-6882-13-132
Xuan, S. H., Park, Y. M., Ha, J. H., Jeong, Y. J., and Park, S. N. (2017). The Effect of Dehydroglyasperin C on UVB-Mediated MMPs Expression in Human HaCaT Cells. Pharmacol. Rep. 69, 1224–1231. doi:10.1016/j.pharep.2017.05.012
Yan, N., Tang, Z., Xu, Y., Li, X., and Wang, Q. (2020). Pharmacokinetic Study of Ferulic Acid Following Transdermal or Intragastric Administration in Rats. AAPS PharmSciTech 21, 169. doi:10.1208/s12249-020-01709-w
Yang, L., Han, Z., Chen, C., Li, Z., Yu, S., Qu, Y., et al. (2020a). Novel Probiotic-Bound Oxidized Bletilla Striata Polysaccharide-Chitosan Composite Hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 117, 111265. doi:10.1016/j.msec.2020.111265
Yang, W. T., Ke, C. Y., Wu, W. T., Harn, H. J., Tseng, Y. H., and Lee, R. P. (2017). Effects of Angelica Dahurica and Rheum Officinale Extracts on Excisional Wound Healing in Rats. Evid. Based Complement. Alternat Med. 2017, 1583031. doi:10.1155/2017/1583031
Yang, W. T., Ke, C. Y., Wu, W. T., Tseng, Y. H., and Lee, R. P. (2020b). Antimicrobial and Anti-inflammatory Potential of Angelica Dahurica and Rheum Officinale Extract Accelerates Wound Healing in Staphylococcus Aureus-Infected Wounds. Sci. Rep. 10, 5596. doi:10.1038/s41598-020-62581-z
Ye, Y., Chu, J. H., Wang, H., Xu, H., Chou, G. X., Leung, A. K., et al. (2010). Involvement of P38 MAPK Signaling Pathway in the Anti-melanogenic Effect of San-Bai-tang, a Chinese Herbal Formula, in B16 Cells. J. Ethnopharmacol 132, 533–535. doi:10.1016/j.jep.2010.09.007
Yi, R., Liu, X. M., and Dong, Q. (2013). A Study of Lycium Barbarum Polysaccharides (LBP) Extraction Technology and its Anti-aging Effect. Afr. J. Tradit Complement. Altern. Med. 10, 171–174. doi:10.4314/ajtcam.v10i4.27
You, O. H., Shin, E. A., Lee, H., Kim, J. H., Sim, D. Y., Kim, J. H., et al. (2017). Apoptotic Effect of Astragalin in Melanoma Skin Cancers via Activation of Caspases and Inhibition of Sry-Related HMg-Box Gene 10. Phytother Res. 31, 1614–1620. doi:10.1002/ptr.5895
Yu, L., Nie, X., Pan, H., Ling, S., Zhang, D., and Bian, K. (2011). Diabetes Mellitus Ulcers Treatment with Bletilla Striata Polysaccharide. Zhongguo Zhong Yao Za Zhi 36, 1487–1491. doi:10.4268/cjcmm20111118
Zaidi, K. U., Ali, S. A., Ali, A., and Naaz, I. (2019). Natural Tyrosinase Inhibitors: Role of Herbals in the Treatment of Hyperpigmentary Disorders. Mini Rev. Med. Chem. 19, 796–808. doi:10.2174/1389557519666190116101039
Zanetti, R., Prota, G., Napolitano, A., Martinez, C., Sancho-Garnier, H., Østerlind, A., et al. (2001). Development of an Integrated Method of Skin Phenotype Measurement Using the Melanins. Melanoma Res. 11, 551–557. doi:10.1097/00008390-200112000-00001
Zhang, C., He, Y., Chen, Z., Shi, J., Qu, Y., and Zhang, J. (2019). Effect of Polysaccharides from Bletilla Striata on the Healing of Dermal Wounds in Mice. Evid. Based Complement. Alternat Med. 2019, 9212314. doi:10.1155/2019/9212314
Zhang, J., Guo, W., Li, Q., Sun, F., Xu, X., and Xu, H. (2020). Discriminant Analysis of Traditional Chinese Medicinal Properties Based on Holistic Chemical Profiling by 1H-NMR Spectrometry. Evid. Based Complement. Alternat Med. 2020, 3141340. doi:10.1155/2020/3141340
Zhao, B., Zhang, X., Han, W., Cheng, J., and Qin, Y. (2017). Wound Healing Effect of an Astragalus Membranaceus Polysaccharide and its Mechanism. Mol. Med. Rep. 15, 4077–4083. doi:10.3892/mmr.2017.6488
Zhao, H., Deneau, J., Che, G. O., Li, S., Vagnini, F., Azadi, P., et al. (2012). Angelica Sinensis Isolate SBD.4: Composition, Gene Expression Profiling, Mechanism of Action and Effect on Wounds, in Rats and Humans. Eur. J. Dermatol. 22, 58–67. doi:10.1684/ejd.2011.1599
Zhu, C. S., Nie, A. Z., Zhang, B., and Lin, Z. J. (2019). Medication Rules of Professor Zhang Bing in Treatment of Skin Itching Based on Data Mining. Zhongguo Zhong Yao Za Zhi 44, 597–601. doi:10.19540/j.cnki.cjcmm.20181128.001
Keywords: skin whitening, ethnobotanical, Taiwan, traditional Chinese medicine pharmacy, traditional Taiwanese medicine
Citation: Ko C-Y, Chao J, Chen P-Y, Su S-Y, Maeda T, Lin C-Y, Chiang H-C and Huang S-S (2021) Ethnobotanical Survey on Skin Whitening Prescriptions of Traditional Chinese Medicine in Taiwan. Front. Pharmacol. 12:736370. doi: 10.3389/fphar.2021.736370
Received: 05 July 2021; Accepted: 10 November 2021;
Published: 30 November 2021.
Edited by:
Lie-Fen Shyur, Academia Sinica, TaiwanReviewed by:
Hsing-Yu Chen, Chang Gung Memorial Hospital, TaiwanLinchun Shi, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2021 Ko, Chao, Chen, Su, Maeda, Lin, Chiang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shyh-Shyun Huang, sshuang@mail.cmu.edu.tw
†These authors have contributed equally to this work and share first authorship.
 Chien-Yu Ko
Chien-Yu Ko